Abstract
Multinary metal oxides are widely applied in energy storage and conversion, heterogeneous catalysis and environmental technologies, but their wide band gaps, low intrinsic electronic conductivity and limited density of active sites severely restrict their practical efficiency. This review examines non-metallic doping via the substitutional, interstitial or mixed incorporation of light elements such as B, C, N, F, P and S as a versatile strategy to overcome these fundamental limitations. We begin by outlining the primary synthesis methodologies for doped oxides, such as sol–gel, chemical vapor deposition, and hydrothermal routes, followed by a critical discussion of the multi-technique characterization framework required to verify successful dopant incorporation and elucidate its structural and electronic consequences. We focus on the fundamental principles of how doping parameters—such as mode, element type, and concentration—can be tuned to regulate material properties. The key mechanisms for performance enhancement, including synergistic lattice reconstruction, defect engineering, and electronic structure modulation, are emphasized. Significant advancements are highlighted in applications like energy storage, fuel cells, water splitting, and CO2 reduction. Finally, we assess current challenges, such as the precise control of doping sites and long-term stability, and offer perspectives on the rational design of next-generation oxide materials.
1. Introduction
Multinary metal oxides, as highly promising functional materials, are widely used in energy storage and conversion, heterogeneous catalysis, and gas sensing [1,2]. Their unique crystal structures and tunable physicochemical properties make them indispensable in emerging low-carbon energy technologies, ranging from lithium-ion batteries to solid oxide fuel cells and photocatalytic systems. However, their inherent low electronic conductivity, limited catalytic activity, and poor environmental adaptability severely restrict their practical performance, hindering the large-scale application of devices based on these materials. From the perspective of electronic properties, multinary metal oxides, such as spinel-type nickel ferrite (NiFe2O4) and perovskite-type lanthanum ferrite (LaFeO3) are wide band gap semiconductors or insulators, with a band gap typically exceeding 2 eV. Their valence bands are mainly composed of O 2p orbitals, while the conduction bands originate from the d orbitals of metal cations. This wide band gap characteristic limits the thermal excitation of charge carriers at room temperature, as only a negligible number of electrons can overcome the band gap to participate in charge transport. Additionally, the stable d-electron configurations of metal cations further hinder electron transfer between cations, resulting in extremely low intrinsic electronic conductivity (typically <10−5 S cm−1 for undoped samples) [3]. This poor conductivity severely restricts ion/electron transport kinetics in energy conversion and storage devices, leading to high polarization resistance and low energy efficiency.
In terms of catalytic performance, the activity of these oxides largely depends on surface defect sites such as oxygen vacancies or specific high-valent metal cations. During crystal growth, to minimize surface energy, the material tends to form a complete lattice structure, leading to a relatively low density of such active sites. Consequently, the utilization efficiency of bulk cations remains below 10% in many systems, as most metal cations in the bulk lattice cannot participate in surface catalytic reactions. Furthermore, mismatched adsorption energies between surface sites and reaction intermediates (e.g., hydroxyl ion (OH−), hydroperoxyl ion (OOH−)) often result in slow reaction kinetics [4].
Non-metallic doping, achieved by incorporating light elements such as B, C, N, F, P, and S, provides an effective solution to the aforementioned issues. Unlike metal doping, which often introduces structural complexity or increases material cost, non-metallic dopants, which have small atomic radii and adjustable valence states, can be precisely incorporated into the oxide lattice through various preparatory strategies. Depending on the type of doping element and the mode of incorporation such as substitutional, interstitial, or mixed, doping can induce lattice distortion such as expansion or contraction, regulate the band structure such as narrowing band gaps or introducing mid-gap states, and generate oxygen vacancies, and all these effects collectively enhance the material’s conductivity, catalytic activity, and stability.
This review provides a comprehensive examination of the progress, challenges, and future directions in the non-metallic doping of multinary metal oxides. We will begin by classifying the key structural characteristics of representative oxide families. Subsequently, we will discuss the primary synthesis routes and the systematic characterization techniques essential for verifying successful doping and understanding its effects. A central focus will be placed on elucidating how different doping modes and element types regulate lattice distortion, defect generation, and electronic structure. By correlating these fundamental modifications with performance gains, this review aims to outline the current state of the art and provide insights toward the rational design and scalable synthesis of next-generation materials for a low-carbon energy future. It establishes a unified theoretical framework and standardized characterization paradigm for non-metallic doping research, serving as a critical bridge between scattered studies and guiding subsequent explorations in related fields. Moreover, as sustainable energy technologies become increasingly pivotal, the tailored regulation of multinary metal oxides via non-metallic doping holds immense potential to address core performance bottlenecks in energy storage and conversion systems.
2. Classification and Structural Characteristics of Multinary Metal Oxides
Multinary metal oxides exhibit a rich variety of crystal structures and corresponding physicochemical properties, which are fundamentally determined by their cation arrangements, oxygen coordination environments, and tunable stoichiometry. Understanding the structural characteristics of these oxides is essential for tailoring their functionalities in energy conversion, storage, and catalysis. Representative structural archetypes include spinel, perovskite, ilmenite, and polyoxometalate clusters, while alternative classification schemes based on composition and electronic properties further enrich their categorization.
2.1. Spinel Structure
Spinel oxides adopt the general chemical formula AB2O4, where A and B represent metal cations and O denotes oxygen anions. Their crystal lattice contains two key cationic sites: tetrahedral and octahedral interstitial sites. According to the distribution of cations in these two sites, spinels are generally classified into two typical configurations [5].
Normal spinel: With the general formula Atetra(B2)octaO4 [6], divalent metal cations typically occupy tetrahedral sites, whereas trivalent cations reside in octahedral sites. Representative examples include zinc ferrite (ZnFe2O4) [7] (Figure 1a) and magnesium cobaltite (MgCo2O4)) [8].
Inverse spinel: With the general formula Btetra(AB)octaO4, a characteristic cation exchange occurs between A and B sites. B cations occupy both tetrahedral and octahedral sites, while A cations are exclusively located at octahedral sites. Typical examples are nickel ferrite (NiFe2O4) [9] and zinc ferrite (ZnFe2O4) [7] in its inverse configuration (Figure 1b).
2.2. Perovskite Structure
Perovskite oxides have the general formula ABO3 [10] (Figure 1c), with representative examples such as lanthanum ferrite (LaFeO3) and strontium titanate (Sr2TiO4). In this structure, A sites are usually occupied by alkaline earth or rare earth metals with 12-fold coordination, and B sites by transition metals with 6-fold coordination [11]. Perovskites are particularly attractive because of their low cost, flexible composition [12], and wide application range [13]. Such structural tunability enables precise modulation of electronic and catalytic properties through element substitution and defect engineering.
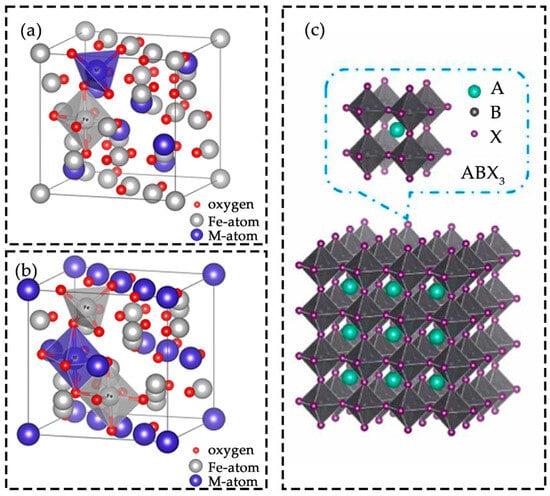
Figure 1.
Crystal structures of (a) normal spinel; (b) inverse spinel. Reprinted with permission from Ref. [6]. Copyright 2016 Copyright J. Photon. Energy; (c) perovskites. Reprinted with permission from Ref. [10]. Copyright 2024 Copyright Nano Energy.
2.3. Other
2.3.1. Other Representative Structures
The ilmenite framework represents an important ceramic structure that occurs in diverse multicomponent oxides and offers extensive possibilities for structural modification and functional applications. Materials such as FeTiO3 [14], NiTiO3 [14], MgTiO3 [15], and β-CdSnO3 [16] adopt this structure, and ceramic powders based on this framework can be synthesized by liquid-phase mixing methods [17]. Transition-metal doping can be used to tailor the structural properties of NiTiO3; complex substitution at Ti sites of MgTiO3 ceramics allows control of their microwave dielectric properties; and N-doped MgTiO3 can be synthesized while retaining the ilmenite lattice. These strategies provide valuable guidance for tuning the performance of ilmenite-structured materials. Applications include N-doped MgTiO3 as a visible-light photocatalyst for O2 evolution, Ni3Mn3Ti6O18 thin-film photoanodes for solar water splitting, and β-CdSnO3 as an ethanol-sensing material. The microwave dielectric properties of MgTiO3 ceramics are also an important research direction, highlighting the ilmenite structure’s potential in photocatalysis, energy conversion, gas sensing, and dielectric applications.
Polymetallic oxygen clusters (POMs) exhibit clear structural classification, favorable morphological characteristics, and excellent integration and hybridization capabilities. Typical structural types include Anderson-type polyoxometalates [18]; among them, phosphomolybdic acid is not only a key representative but can also participate in chemical polymerization of polyaniline and polypyrrole [19]. When employed as catalysts in redox cycle studies, POMs display highly uniform morphologies that underpin stable catalytic performance [18]. Their structures enable excellent integration and hybridization: for example, POMs can be encapsulated in mesoporous or microporous thorium-based porphyrin metal–organic frameworks to construct composite materials with matched size and structural stability [19]; they can also form in situ hybrid organic–inorganic materials during the chemical polymerization of polyaniline and polypyrrole [19], broadening their functional applications.
2.3.2. Other Classification Criteria
Beyond the above structural prototypes, multinary metal oxides can also be classified by their composition, stoichiometry, and electrical properties.
Based on composition and stoichiometry, multinary systems can form oxides with varying stoichiometric ratios [20]. For instance, the ternary transition-metal oxide lithium nickel manganese cobalt oxide (LiNi0.5Mn0.3Co0.2O2), composed of nickel (Ni), manganese (Mn), cobalt (Co), and oxygen (O) in specific proportions, is widely used as a cathode material in lithium-ion batteries [20]. Variations in the Ni, Mn, and Co ratios significantly affect electrochemical performance: excessively high Ni content can cause lattice collapse during cycling; increasing Mn improves structural stability but reduces electronic conductivity; and Co helps balance electronic conduction and structural stability. Similarly, in non-stoichiometric systems such as strontium cobaltite (SrCoO3−δ), oxygen defect content can be tuned via composition regulation, providing a natural structural basis for defect engineering and non-metallic doping [21].
Based on electrical properties, multinary oxides can be categorized as conductors, semiconductors, or insulators. This classification is closely linked to their suitability for electronic devices and energy storage. Most undoped spinels and perovskites are wide-band-gap semiconductors or insulators with extremely low electronic conductivity at room temperature (typically <10−5 S cm−1). Certain transition-metal perovskites such as lanthanum cobaltite (LaCoO3) can achieve electronic conduction through valence-state changes in metal cations (Co3+/Co4+ redox couple) at high temperatures, exhibiting semiconductor–metal transition behavior. Moreover, non-metallic doping, such as F doping of Sr2TiO4, can convert insulators into semiconductors and even induce semi-metallic characteristics by adjusting the doping concentration, thereby broadening their applications in photocatalysis and electrocatalysis.
3. Non-Metallic Doping
3.1. Doping Methods
3.1.1. Sol–Gel Method
The sol–gel method is a widely used technique for the preparation of doped metal oxide materials. This process involves dissolving metal ions and nonmetallic sources, such as ammonium dihydrogen phosphate (NH4H2PO4), which serves as a phosphorus source, in an appropriate solvent to form a homogeneous solution. The key advantage of the sol–gel method lies in its ability to achieve uniform mixing of the components, ensuring that the nonmetallic element is well-dispersed within the metal oxide matrix. This uniform distribution is critical for optimizing the desired properties of the final material at both macro and micro scales.
Once the homogeneous solution is prepared, the process continues with gelation, followed by drying and calcination steps. During gelation, the solution undergoes a transformation into a gel-like phase, which facilitates further manipulation and stabilization of the material. Drying removes excess solvent, and calcination at high temperatures allows the formation of the final doped product. Through this series of steps, the sol–gel method ensures that the doped material has a consistent composition and phase structure, with the nonmetallic element (such as phosphorus) uniformly integrated into the metal oxide lattice.
For example, Figure 2a illustrates the preparation of phosphorus-doped LaFeO3 (P-LaFeO3) [22]. via the sol–gel method. In this case, phosphorus is atomically dispersed in the LaFeO3 lattice, which significantly influences the material’s properties. The calcination process not only ensures the incorporation of phosphorus into the LaFeO3 matrix but also allows for the production of doped particles with controllable particle sizes, providing flexibility in tailoring the material’s characteristics for specific applications. This method’s ability to control particle size and achieve homogeneous doping is particularly valuable for enhancing the performance of materials in various applications, such as catalysis and energy storage.
3.1.2. Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition (CVD) is a versatile technique primarily applied in the preparation of thin films, where nonmetallic elements are introduced into a host matrix through the diffusion and reaction of gaseous precursors at elevated temperatures. The essential mechanism of this process involves the volatilization of a nonmetallic source, followed by its gaseous diffusion and subsequent reaction with the surface or lattice of the target material. By precisely controlling the experimental parameters, such as temperature, atmosphere, and precursor-to-matrix ratios, CVD allows for effective incorporation of dopants, thereby tailoring the physicochemical properties of the resulting materials.
The process can be adapted depending on the type of nonmetallic dopant and host matrix. For instance, sulfur is placed in the upstream region of the gas inlet and CoMn2O4 is placed in the downstream region relative to sulfur. Under an argon-protected atmosphere, sulfur is volatilized upon heating and then transported by the argon flow to CoMn2O4 where it reacts with CoMn2O4, and the sulfur-CoMn2O4 composite is finally prepared. Air is isolated by argon throughout the entire process which prevents side reactions between the reactants and components in the air and ensures the successful preparation of the target product (Figure 2b) [23].
Phosphorus doping provides another example of CVD versatility. Using sodium hypophosphite monohydrate (NaH2PO2·H2O) as the phosphorus precursor, the matrix and phosphorus source (in a mass ratio of 1:10) are placed together in a ceramic boat. The system is heated under an argon atmosphere at a ramp rate of 2 °C/min to temperatures ranging from 300 to 600 °C, which are then maintained for 3 h. During this process, decomposition of the phosphorus precursor generates gaseous phosphorus-containing species that react with the matrix. By tuning the calcination temperature, the reaction extent can be effectively controlled, leading to samples with different phosphorus doping levels [24].
Nitrogen doping has also been realized through CVD using ammonia (NH3) as the nitrogen source. In this case, strontium iron molybdate (Sr2Fe1.5Mo0.5O6-δ, SFMO) powders are exposed to a continuous ammonia flow (20 mL/min) at 350 °C, 450 °C, and 550 °C for 6 h, respectively. High-temperature decomposition of NH3 generates reactive nitrogen species that diffuse into the matrix, producing nitrogen-doped SFMON series samples [25].
Overall, CVD offers a controllable and effective route for introducing nonmetallic dopants into oxide matrices. By systematically adjusting the process parameters such as temperature, precursor-to-matrix ratios, and gas atmosphere, it is possible to regulate doping levels and thereby fine-tune the structural and functional properties of the resulting materials.
3.1.3. Hydrothermal Method
The hydrothermal method is an effective approach for synthesizing doped metal oxide materials, relying on reactions carried out in a high-temperature, high-pressure aqueous environment. In this process, metal salts, nonmetallic sources, and mineralizers are introduced into a sealed reaction kettle, where controlled reaction conditions such as temperature, pressure, and duration facilitate the incorporation of dopants into the target matrix. The aqueous environment not only enhances the solubility of reactants but also promotes efficient ion exchange and redox reactions, enabling uniform doping and crystal growth.
This method is particularly suited for enabling reactions between nonmetallic sources (e.g., sulfur, nitrogen) and metal salts under hydrothermal conditions. For example, thiourea can serve as a sulfur source for the preparation of sulfur-doped copper ferrite (S-CuFe2O4), where the sulfur species released from thiourea decomposition react with copper and iron salts to yield the doped product (Figure 2c) [26]. Similarly, urea has been widely employed as a nitrogen precursor; upon thermal decomposition under hydrothermal conditions, urea releases ammonia (NH3), which interacts with transition metal salts to produce nitrogen-doped manganese cobaltite (N-MnCo2O4) [27].
Overall, the hydrothermal method offers several advantages for nonmetallic doping, including mild reaction conditions compared to traditional solid-state methods, tunable reaction parameters, and the ability to produce well-crystallized doped products with controlled morphologies and compositions. These features make it a powerful strategy for tailoring the structural and functional properties of multinary metal oxides.
3.1.4. Electrospinning Annealing Method
The electrospinning annealing method is a powerful approach for fabricating nanofibrous structures with tailored compositions and morphologies. In this process, a precursor solution containing metal salts and a nonmetallic dopant source is electrospun into continuous polymer-based fibers, which are subsequently subjected to controlled annealing to remove the polymer and crystallize the desired doped oxide phase. This strategy enables the uniform distribution of dopants at the molecular level while simultaneously achieving fibrous architectures with high aspect ratios.
For example, Figure 2d illustrates the preparation of boron-doped LaCoO3 heterojunctions using this method. In this case, boric acid (H3BO3) is introduced into the precursor solution as the boron source, followed by the addition of polyvinylpyrrolidone (PVP). The mixture is stirred to ensure molecular-level dispersion of boron within the precursor. During the subsequent electrospinning process, nanofibers are formed, and upon annealing, the polymer template is removed while crystalline boron-doped LaCoO3 heterojunctions are obtained [28].
The electrospinning annealing method effectively balances specific surface area and charge transfer efficiency. The fibrous morphology provides a large surface area and abundant active sites, while the intimate dopant distribution enhances charge transport across the heterojunction. These advantages make the technique particularly suitable for applications in catalysis, energy conversion, and sensing, where both surface reactivity and charge transfer kinetics are critical.
3.1.5. Impregnation Post Treatment Method
The impregnation post treatment method introduces nonmetallic dopants into oxide matrices through liquid-phase reactions with nonmetallic sources, followed by subsequent heat treatment. This approach enables the incorporation of target elements into the material lattice while preserving the overall structural framework. Depending on the dopant element (e.g., boron, fluorine) and matrix characteristics, the reaction pathway and structural evolution may differ, offering flexibility in material modification strategies.
For instance, boron doping in manganese ferrite (B-MnFe2O4) has been achieved via this method. After calcination in air, the material is immersed in an alkaline sodium borohydride (NaBH4) solution to prepare amorphous nanowires composed of Mn, Fe, O, and carbon (C), denoted as MFOC. In this process, NaBH4 plays a dual role as both a reducing agent and a boron source. The highly reducible hydrogen ion (H−) scavenges oxygen, thereby creating oxygen vacancies, while simultaneously driving lattice reconstruction. This facilitates the partial replacement of oxygen species within the MnFe2O4 spinel structure by boron species, ultimately yielding effectively boron-doped MFOC nanowires.
Fluorine doping of copper bismuth oxide (CuBi2O4, CBO) nanorod arrays has also been demonstrated using this approach. Pre-synthesized CBO nanorod arrays are immersed in a 1 mg mL−1 sodium fluoride (NaF) aqueous solution for 2 h, during which fluorine ions (F−) adhere to the material surface and penetrate the near-surface lattice through liquid-phase adsorption. The treated sample is then annealed at 300 °C for 1 h in air. This mild heat treatment promotes ion exchange between F− and oxygen ions in the CBO lattice, resulting in the formation of fluorine-doped CuBi2O4 (F-CBO) nanorod arrays while retaining the original one-dimensional morphology (Figure 2e).
Overall, the impregnation post treatment method offers several advantages, including operational simplicity, precise control over doping, and preservation of the matrix’s morphological integrity. By avoiding complex precursor preparation and employing straightforward liquid-phase infiltration coupled with mild annealing, this technique provides an efficient pathway for the rapid and targeted modification of functional materials.
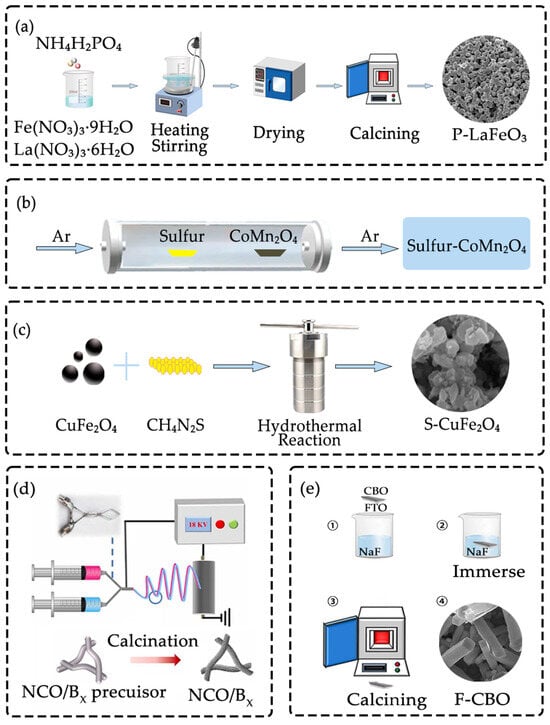
Figure 2.
(a) Preparation of P-LaFeO3 via sol–gel method. Adapted with permission from Ref. [22]. Copyright 2025 Copyright Chem. Eng. J.; (b) Preparation of S-CoMn2O4 via CVD. Adapted with permission from Ref. [23]. Copyright 2023 Copyright Appl. Surf. Sci.; (c) Preparation of S-CuFe2O4 via hydrothermal method. Adapted with permission from Ref. [26]. Copyright 2023 Copyright Sci. Rep.; (d) Preparation of B-LaCoO3 via electrospinning. Adapted with permission from Ref. [28]. Copyright 2023 Copyright J. Alloys Compd.; (e) Preparation of F-CuBi2O4 via impregnation method. Adapted with permission from Ref. [29]. Copyright 2022 Copyright J. Catal.
3.2. Systematic Characterization Techniques for Non-Metallic Doping
Verifying the success of non-metallic doping is a systematic process that requires multiple characterization techniques to construct a complete evidence chain from different dimensions. These techniques complement each other to address core questions such as whether the doped element is incorporated into the matrix, how it exists, what changes it induces, and how it ultimately enhances performance.
3.2.1. Characterization of Elemental Presence, Chemical States, and Distribution
Confirming the successful incorporation of nonmetallic dopants requires comprehensive characterization of their presence, chemical states, and spatial distribution. Among the available techniques, X-ray Photoelectron Spectroscopy (XPS) is the most direct and powerful tool. XPS not only detects doped elements with high sensitivity but also reveals their chemical states (e.g., substitutional versus interstitial incorporation) and provides insights into electronic structure variations [5,8,28,30,31].
The versatility of XPS has been demonstrated across diverse research fields. In lithium-ion battery studies, XPS has been employed to confirm nitrogen doping in nitrogen-doped carbon-coated lithium titanate (NC_LTO). The analysis verified the presence of C–N bonds, indicating that nitrogen forms a conductive network with carbon, thereby enhancing material conductivity and directly supporting the excellent rate performance and cycling stability of NC_LTO [32]. In photoelectrochemistry, XPS analysis of fluorine-doped CuBi2O4 (F-CuBi2O4) nanorod arrays showed that the F 1s spectrum confirmed the introduction of F−. These results correlate with optimized band edge positions and improved photogenerated carrier separation efficiency, explaining the superior H2O2 production performance [29]. Similarly, in catalysis, XPS characterization of nitrogen-doped MnCo2O4 (N-MnCo2O4) activating peroxymonosulfate (PMS) demonstrated that the N 1s spectrum elucidated nitrogen coordination configurations, while the O 1s spectrum confirmed increased oxygen vacancy content. This evidence directly established the pathway of nonmetallic doping leading to defect enrichment, providing mechanistic support for subsequent catalytic and kinetic analyses [27]. For sulfur-doped CoMn2O4 (S-CoMn2O4) in Fenton-like reactions for Rhodamine B (Rh.B) degradation, XPS confirmed sulfur incorporation.
Complementary to XPS, Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy coupled with Energy Dispersive Spectroscopy (TEM-EDS mapping) provide essential information on elemental distribution at the microscale, ruling out elemental segregation or secondary phase formation [7,23,27,28,32]. For example, in phosphorus-doped MnCoO4, SEM revealed that phosphorus doping optimized particle sintering behavior, producing denser interparticle connections and a porous structure that increased the specific surface area. TEM-EDS mapping showed uniform distribution of Mn, Co, O, and P without evidence of segregation or secondary phases. High-resolution TEM further revealed that phosphorus doping expanded the lattice spacing of the (111) plane (0.485 nm versus 0.480 nm in undoped MnCoO4), confirming that phosphorus incorporation induced oxygen vacancies and lattice distortion. These findings provided strong microstructural evidence supporting the doping-induced improvements in electronic structure and catalytic activity [8].
Morphological transformations caused by nonmetallic doping have also been captured through TEM imaging. In boron-doped carbon nanotubes (BCNTs), TEM observations indicated that pristine CNTs and BCNTs with different doping levels maintained diameters of 25 nm and wall thicknesses of 10 nm. However, their morphologies evolved from quasi-straight to bamboo-like, and eventually to twisted structures with increasing boron content, a phenomenon attributed to the slightly longer B–C bond compared to the C–C bond. Residual Fe catalyst particles encapsulated within the nanotubes were also observed, highlighting the complexity of nanotube preparation and structural modification [33].
Together, these complementary characterization techniques provide compelling, multiscale evidence of successful nonmetallic doping. XPS elucidates the elemental presence, chemical states, and defect formation, while SEM and TEM-EDS mapping confirm uniform elemental distribution and microstructural optimization. Such integrated characterization approaches establish the critical link between dopant incorporation, defect engineering, and enhanced material performance.
3.2.2. Characterization of Effects on Crystal Structure and Defects
After confirming elemental incorporation, it is essential to analyze the impact of nonmetallic doping on the host crystal structure and defect states. Such investigations elucidate the structure–performance correlation, providing fundamental insights into doping-induced property modulation.
X-ray diffraction (XRD) remains the most fundamental characterization method to probe structural changes. It enables the identification of lattice expansion, contraction, or distortion through diffraction peak shifting and broadening, while confirming the absence of impurity phases [21,22,23,34]. For instance, in phosphorus-doped SrFeO3−δ cathodes for solid oxide fuel cells (SOFCs), XRD results revealed significant peak shifts and systematic variations in lattice parameters after P doping, directly confirming successful lattice incorporation. These modifications optimized oxygen ion transport channels and proton conductivity, laying the structural foundation for enhanced oxygen reduction reaction (ORR) activity and long-term SOFC stability [35]. Similarly, in sulfur-doped CuFe2O4 for arsenic removal, XRD analysis confirmed phase purity and detected slight peak shifts, correlating subtle lattice regulation with improved isothermal adsorption capacity and kinetic parameters. This clarified how fine structural adjustments optimize the surface chemical environment and adsorption active sites [26]. In another case, fluorine-doped Ruddlesden–Popper Sr2TiO4 showed lattice regulation that preserved its layered structure while significantly increasing oxygen vacancy concentrations and defect accessibility, thus improving photocatalytic water-splitting activity for hydrogen evolution [36].
High-resolution transmission electron microscopy (HRTEM) provides direct atomic-level evidence to complement XRD results, enabling visualization of lattice distortions and defect formation [37]. HRTEM of phosphorus-doped CoS2–MoS2 hollow spheres confirmed the coexistence of CoS2, MoS2, and MoO2 lattice fringes without the appearance of secondary phosphide phases, proving atomic-level P incorporation rather than independent phase formation [38]. In nitrogen-doped three-dimensional graphene (3DNG), HRTEM revealed that N-3DNG exhibited a predominantly amorphous structure with locally ordered regions, consisting of 4–5 graphene layers [39].
Selected area electron diffraction (SAED) is an indispensable supplementary tool that verifies whether doping alters crystal symmetry, crystallinity, or induces disorder [40,41]. Subtle changes in lattice constants can manifest as shifts or splitting of diffraction spots. For instance, SAED analysis of sulfur-doped TiO2 nanowires (S-TiO2 NWs) and N,S-co-doped TiO2 NWs, supported by HRTEM, confirmed their single-crystalline nature and growth along the [001] direction, with an interplanar spacing of 0.35 nm corresponding to the {001} anatase facet [42]. Similarly, SAED characterization confirmed the cubic symmetry of oxydifluoride (TiOF2) and its transformation to phase-pure anatase TiO2 after calcination under H2S atmosphere, verifying sulfur incorporation while retaining structural integrity. This structural confirmation provided essential support for subsequent photocatalytic performance analysis [43].
Raman spectroscopy serves as a powerful complementary tool due to its high sensitivity to local structures and lattice defects [44,45]. In nitrogen-doped graphene, Raman spectra showed characteristic G and 2D bands while exhibiting stronger D bands due to doping-induced disorder, though the relatively low ID/IG ratio (0.06–0.25) indicated that high crystallinity was preserved. In boron-doped graphene, characteristic D (1350 cm−1) and G (1590 cm−1) bands were observed, with consistently higher ID/IG ratios compared to undoped graphene, demonstrating that boron substitution introduced additional lattice defects [46].
Electron paramagnetic resonance (EPR)/electron spin resonance (ESR) is a highly sensitive and authoritative technique for directly detecting paramagnetic defects such as oxygen vacancies, typically evidenced by a signal at g ≈ 2.002. This method not only confirms doping-induced defect states but also complements XRD, XPS, Raman, and electron microscopy, collectively establishing a robust structure–property understanding [8,22]. For example, EPR analysis of oxygen-doped porous ultrathin g-C3N4 nanosheets (PUOCNs) under visible light detected 5,5-dimethyl-1-pyrrolin-N-oxide (DMPO) adduct signals, confirming the generation of reactive oxygen radicals that drive photocatalytic pollutant degradation [47]. In Mn/P co-doped biochar (Mn/P-C) systems activating peroxymonosulfate (PMS), EPR identified SO4−, O2−, and 1O2 species with intensities significantly higher than control systems, thereby verifying both radical and non-radical oxidation pathways for tetracycline hydrochloride degradation [48]. Conversely, in fluorine-doped TiO2, EPR revealed a markedly weaker signal at g = 1.99 compared to undoped TiO2, demonstrating that fluorine effectively suppresses oxygen vacancy concentration [49].
Together, these complementary techniques such as XRD, HRTEM, SAED, Raman, and EPR provide a comprehensive and multiscale toolkit for unraveling the effects of nonmetallic doping on crystal structure and defects. The integrated use of these methods establishes a direct connection between doping, structural modifications, and defect evolution, thereby clarifying the mechanisms underpinning enhanced material performance.
3.2.3. Characterization of Regulation of Electronic Structure and Optical Properties
Following confirmation of structural incorporation and defect regulation, it is essential to investigate how non-metallic doping modifies the intrinsic electronic structure and optical properties of host materials. These properties directly determine light–matter interactions, charge transport behavior, and ultimately device performance.
UV–Visible diffuse reflectance spectroscopy (UV–Vis DRS) is one of the most widely used and direct techniques for probing doping-induced changes in electronic structure, particularly in relation to the optical band gap and absorption edge [50,51]. Redshifts in absorption edges or band gap narrowing provide clear evidence for enhanced light absorption arising from non-metallic incorporation. For example, C, N, and S-doped TiO2 exhibited significant redshifts in their absorption edges compared with pristine TiO2, thereby extending absorption into the visible-light region. Tauc plot analysis revealed pronounced band gap narrowing from 3.33 eV (pristine TiO2) to as low as 2.18 eV for the C/N/S co-doped sample (CNST), with single-element doped samples showing intermediate values [52]. Similarly, in N-doped and N/Ag co-doped ZnO, pure ZnO absorbed mainly in the UV region (356 nm, Eg = 3.20 eV), whereas N doping redshifted the edge to 371 nm (Eg = 3.16 eV). With N/Ag co-doping, the band gap further narrowed to 3.09 eV and a new broad absorption band at 450 nm appeared, attributed to Ag surface plasmon resonance (SPR), thereby significantly improving visible-light absorption [53]. In another case, nitrogen-doped TiO2 exhibited both an absorption edge redshift and a new absorption band in the 400–550 nm region, providing strong evidence for band gap narrowing and visible-light response [54].
Photoluminescence (PL) and time-resolved photoluminescence (TRPL) are highly sensitive probes of photogenerated carrier dynamics. They allow indirect assessment of electron–hole separation efficiency by monitoring changes in fluorescence intensity and carrier lifetimes. In non-metallic doped semiconductors, reduced PL intensity or prolonged lifetimes signify suppressed recombination and enhanced charge separation [55]. For instance, PL spectra of C, N, S-doped biphasic TiO2 showed similar excitonic peaks (470 nm) to undoped samples, but with much lower intensity, indicating more efficient carrier separation. This conclusion was further corroborated by transient absorption spectroscopy, supporting improved photocatalytic reduction in o-dinitrobenzene [56]. Likewise, PL studies on ZnO, N–ZnO, and N/Ag–ZnO revealed that while all samples exhibited near-band-edge (390 nm) and defect-related (470 nm) emissions, doped samples, especially N/Ag–ZnO, showed significantly reduced PL intensity. This synergistic suppression of recombination explained their superior photocatalytic degradation of Rh.B [53].
X-ray Absorption Fine Structure (XAFS) provides atomic-level insight into the local coordination and electronic environment of doped atoms [57]. In nitrogen-doped edge-functionalized graphene oxide (N–f-GrO), C, O, and N K-edge analyses confirmed successful N incorporation, removal of oxygen groups, and the emergence of new electronic states (288.1 eV, 530.07 eV, and 400 eV). These results highlighted modifications in the local electronic environment and electron transition behavior, with sharper characteristic peaks confirming stronger local ordering [58]. Similarly, in fluorine/nitrogen-doped graphdiyne oxide-modified SnO2 electron transport layers (FGDYO–SnO2), Sn L3-edge XAFS revealed an increased Sn oxidation state and reduced oxygen vacancies, while in situ Pb L3-edge analysis detected Pb–F bonds at the SnO2–PbI2 interface. These findings demonstrated enhanced interfacial interaction, suppression of PbI2 crystallization, and stabilization of perovskite lattice order, which are critical for high-performance optoelectronic devices [59].
Beyond experimental techniques, density functional theory (DFT) calculations are indispensable for elucidating doping effects at the electronic level. They not only predict band gap modifications but also explain experimental observations by linking orbital interactions to carrier dynamics [60]. For instance, FLAPW-based DFT studies revealed that only substitutional nitrogen doping in TiO2 leads to favorable N 2p–O 2p orbital hybridization and band gap narrowing, avoiding trap-state formation associated with interstitial configurations. This explained the redshift in absorption edges and improved photocatalytic activity observed experimentally [54]. Likewise, VASP-based calculations on B, N, P, and Si-doped graphene-like C8 structures indicated that stable doping configurations can effectively open narrow band gaps (0.05–1.47 eV) while retaining high carrier mobility, with Si-doping showing the highest carrier mobility (16,975 cm2V−1·s−1). These results provided theoretical justification for their application in flexible optoelectronic devices [61].
In summary, the combined use of UV–Vis DRS, PL/TRPL, XAFS, and DFT builds a comprehensive picture of how non-metallic doping regulates electronic structure and optical properties. While spectroscopic techniques directly capture band gap evolution, light absorption, and charge dynamics, XAFS unveils local electronic environments, and DFT complements experiments with mechanistic insights. Together, they reveal how doping-induced structural modifications translate into enhanced visible-light utilization, efficient charge separation, and improved functional performance.
3.2.4. Verification of Functional Performance
After comprehensive structural, electronic, and optical characterization, the ultimate validation of non-metallic doping lies in the functional performance of the material. This step completes the transition from fundamental research to practical application, linking modifications at the atomic and electronic levels to macroscopic performance improvements.
Electrochemical tests are central to performance verification, with Linear Sweep Voltammetry (LSV) serving as a primary technique for evaluating electrocatalytic activity. LSV does not directly probe the material’s structural features, but it provides an intuitive measure of catalytic performance through parameters such as onset potential, current density, and overpotential, thereby reflecting the practical impact of doping on reactions such as hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and oxygen reduction reaction (ORR) [21,23,51,62]. Complementing LSV, Electrochemical Impedance Spectroscopy (EIS) quantifies charge transfer kinetics at the electrode–electrolyte interface, with reduced charge transfer resistance (Rct) indicating enhanced electronic conductivity and carrier mobility induced by doping. Together, LSV and EIS establish a robust framework to verify doping-induced performance enhancements.
For instance, phosphorus-doped LaFeO3−δ exhibited improved bifunctional oxygen catalytic activity, with LSV revealing enhanced ORR half-wave potential and reduced OER overpotential. EIS analysis confirmed faster charge transfer kinetics, collectively demonstrating the effectiveness of phosphorus doping in enhancing electrocatalytic performance. In the case of N-doped graphene (N-graphene), LSV measurements showed a more positive onset potential, higher half-wave potential, and increased limiting current density for ORR in alkaline fuel cells, surpassing even Pt benchmarks. The material underwent a four-electron reaction pathway, demonstrated excellent operational stability, and resisted methanol crossover, confirming the high efficiency of N-graphene as a metal-free electrocatalyst [63,64].
Similarly, phosphorus-doped MoS2/rGO composites displayed significantly reduced overpotential (172.8 mV at 10 mA·cm−2) and a Tafel slope of 70.7 mV·dec−1, indicating enhanced HER activity [65]. EIS measurements further supported these findings, showing a decrease in Rct from 59.6 Ω (undoped) to 22.3 Ω (P-doped), highlighting the optimization of interfacial charge transfer and improved reaction kinetics [62].
Beyond electrocatalysis, EIS also verifies photocatalytic performance. For example, BiFeO3@B-rGO nanocomposites exhibited a markedly smaller impedance radius compared with pristine BiFeO3 or B-rGO, indicating suppressed electron–hole recombination and enhanced surface electron transport, which translated into improved photocatalytic activity [66]. Similarly, carbon-doped K2Ta2O6 (C-KTO) demonstrated lower semicircle radius in Nyquist plots compared with S-doped and undoped counterparts, confirming more efficient photogenerated carrier separation and higher photocatalytic degradation activity towards Methylene Blue (MB), Methyl Orange (MO), and 4-Chlorophenol (4-CP) [67].
The integration of multiple characterization techniques provides a holistic verification loop. For example, in TiO2-XNX, XRD confirmed anatase–rutile mixed phase formation, XPS identified nitrogen functional states (substitutional β-N at 396 eV and chemisorbed γ-N2 at 400/402 eV), UV–Vis spectroscopy showed redshifted absorption edges, and photocatalytic tests demonstrated superior visible-light activity, hydrophilicity, and stability compared with undoped TiO2. Collectively, these results confirm the critical role of nitrogen doping in enhancing TiO2 photocatalysis [54].
For N-graphene, nitrogen content and functional states such as pyridinic, pyrrolic, graphitic, and pyridinic N oxide altered local charge distribution, creating positively charged adjacent carbon sites that serve as active centers for electrocatalysis. Raman spectroscopy showed increased D-band intensity (1348 cm−1), indicating defect formation and sp2 carbon reorganization, which enhanced electron transport. Electrochemical testing revealed a kinetic current of 65 μA for ORR, significantly higher than pristine graphene (1 μA), with reduced overpotential and high selectivity toward ORR and H2O2 reduction. The onset potential for H2O2 reduction shifted from −130 mV (pristine) to 0 mV, with an expanded linear detection range (10−5–2.8 mM), directly demonstrating that nitrogen doping effectively regulates chemical states and structural defects to boost electrocatalytic performance [68].
In summary, functional verification via LSV, EIS, and complementary photocatalytic or electrochemical tests provides definitive evidence that non-metallic doping enhances material performance. When integrated with structural, electronic, and optical characterizations, these results establish a complete cause–effect relationship linking atomic-level modifications to macroscopic functionality.
In summary, Table 1 presents the multidimensional characterization system for verifying Non-metallic doping. The successful verification of Non-metallic doping relies on a multi-technique combination strategy, progressing from elemental, structural, and electronic levels to performance, forming a logically rigorous and evidence based comprehensive system.

Table 1.
Multi-Dimensional Characterization System for Non-Metallic Doping Verification.
4. Regulation of Non-Metallic Doping
The effect of Non-metallic doping is regulated by multiple factors, among which Non-metallic doping modes, element types, doping concentrations, and co-doping synergistic effects are core variables. These factors ultimately determine the extent of material performance improvement by affecting the degree of lattice reconstruction, defect generation efficiency, and electron rearrangement effect.
4.1. Doping Modes and Structural Effects
Nonmetallic atoms incorporate into the lattice of multinary metal oxides primarily through two modes: substitutional doping and interstitial doping. Substitutional doping is the dominant pathway, which can be further divided into substitution of anionic oxygen sites and substitution of cationic B sites.
4.1.1. Oxygen Site Substitution
Oxygen site substitution represents one of the fundamental strategies for non-metallic doping in multinary metal oxides, wherein non-metallic atoms directly replace oxygen anionic sites within the crystal lattice. As illustrated in Figure 3a, the undoped ABO3 perovskite structure exhibits a complete and well-ordered lattice arrangement. In contrast, Figure 3b depicts the O-site substitution scenario, where non-metallic atoms (yellow) occupy oxygen sites (red) within the lattice, introducing local lattice distortions and modifying the electronic environment.
A representative example is nitrogen-doped Sr2Fe1.5Mo0.5O6-δ (SFMON), in which nitrogen atoms substitute oxygen sites to achieve non-metallic doping [25]. Other notable cases include fluorine substitution for O2− in Sr2TiO4 [36] and sulfur substitution in CuFe2O4 [26]. In cerium copper cobalt oxide (CeCu0.5Co0.5O3), boron partially substitutes for lattice oxygen, reducing the lattice constant, disrupting long-range order, inhibiting grain growth, and forming a more uniform solid solution. These structural changes increase the density of active sites and improve catalytic performance [34].
The underlying mechanism of oxygen site substitution lies in the differences in atomic radius and electronegativity between the dopant and oxygen, which induce lattice expansion or contraction and can also modify the local electronic structure. Critically, this substitution generates non-metal-driven oxygen vacancies (Ov), which are widely recognized as the primary active centers for enhancing catalytic activity [51]. For instance, phosphorus doping in perovskite LaFeO3 induces lattice engineering that promotes the formation of Ov [22]. In essence, O-site doping enhances material activity by altering the anionic sublattice, generating and regulating oxygen vacancies, and thereby improving both electronic and catalytic properties [8].
4.1.2. Cationic B Site Substitution
In contrast to anionic site regulation, B-site substitution primarily targets the transition metal cation sites in multicomponent oxides, such as the B-sites in spinel or perovskite structures [30,34]. This approach modulates the local chemical environment of B-sites through the introduction of non-metallic dopants. For instance, boron (B) doping has been used to regulate the octahedral sites in MnFe2O4 spinel, enabling efficient seawater splitting [30]. This form of doping, also referred to as lattice B-doping, can induce ferromagnetism in certain catalysts [34], thereby enhancing their activity in degradation reactions. Overall, B-site regulation primarily affects material functionality by altering the geometry of the cationic sublattice and modifying the distribution and activity of active sites [30].
As illustrated in Figure 3c, cationic B-site substitution in perovskites can be achieved via non-metallic doping. Representative examples include:
- Substitution of Fe3+ by P5+ in LaFeO3 [24],
- Substitution of Co3+ by P5+ in LaCoO3 perovskite [28], and
- Substitution of Co3+ by P5+ in MnCo2O4 spinel [8].
The differences in ionic radius and valence between the non-metallic dopants and B-site metal ions lead to slight lattice parameter adjustments without disrupting the host structure. For example, after P substitutes Co3+ in LaCoO3, XRD refinement reveals minimal changes in lattice parameters, and the rhombohedral structure is retained. Likewise, P substitution of Fe3+ in LaFeO3 preserves the orthorhombic perovskite framework. In addition, B-site substitution by non-metallic elements triggers a charge compensation mechanism due to valence differences, simultaneously inducing variations in the metal ion valence state and generating oxygen vacancies).
This targeted modulation of B-sites thus provides a powerful approach to tailor the electronic, structural, and functional properties of multinary metal oxides for enhanced catalytic, electrochemical, and photocatalytic performance.
4.1.3. Interstitial Doping
Non-metallic doping is widely recognized as a robust strategy for modulating the properties of transition metals and their compounds [62,69]. Unlike conventional substitutional doping, interstitial doping does not require the dopant to replace lattice O atoms or B-site cations; instead, dopants can occupy lattice interstitial sites or interact via surface effects [32,70]. This flexibility allows interstitial doping to uniquely influence the structural, electronic, and catalytic behavior of metal oxides.
Interstitial doping is particularly suited for non-metallic elements with small atomic radii, which can readily embed into lattice interstices due to their size advantage. For instance, Figure 3d illustrates the incorporation of non-metallic atoms into the interstitial sites of metal oxide lattices. A representative example is boron-doped sodium manganese nickel oxide (Na0.95Mn0.6Ni0.4O2) [71], where B3+ ions (ionic radius 0.23 Å) preferentially occupy tetrahedral interstitial sites rather than substituting octahedrally coordinated transition metal sites. Similarly, phosphorus and boron can be doped into the tetrahedral interstitial sites of Li7Mn8B9O10 via mechanochemical doping, existing as PO43− and BO45− tetrahedral configurations, respectively [72].
The primary functional effect of interstitial doping lies in its ability to introduce defect sites or point defects [55,60], distinguishing it from conventional O-site or B-site substitution. These defects act as localized centers that modulate electron transport kinetics, typically slowing carrier recombination and enhancing the separation of photogenerated charges. Consequently, interstitially doped materials often exhibit improved electronic, optical, and catalytic performance due to defect-mediated regulation of carrier dynamics.
In summary, interstitial doping offers a complementary pathway to substitutional strategies, providing unique control over lattice defects, electronic transport, and local chemical environments, particularly in systems where the dopant’s small size and tetrahedral coordination preference facilitate defect engineering.
4.1.4. Mixed Doping
Mixed doping strategies combine two distinct doping modes, where one mode predominates and the other serves as a complementary mechanism, without disrupting the host phase structure. This approach allows precise tuning of both the lattice and electronic environment, leveraging the advantages of each doping type. As illustrated in Figure 3e,f, mixed doping can adopt different configurations. Figure 3e depicts a scenario dominated by interstitial doping coupled with oxygen-site substitution, while Figure 3f shows interstitial doping acting as a supplement to cationic B-site substitution.
A representative example is boron doping in NiCo2O4/LaCoO3 heterojunctions, where B primarily substitutes Co in LaCoO3, and interstitial B occupies the interface as a secondary doping mode [28]. This dual doping not only finely regulates the valence states of metal cations while increasing the Co2+/Ni2+ ratio but also optimizes the local electronic environment via interfacial interstitial sites, ultimately enhancing the activation efficiency of peroxymonosulfate (PMS).
Another example is nitrogen-doped MnCo2O4 [27], where substitutional nitrogen (Ns) replaces lattice O2− or occupies oxygen vacancies (Ov), while interstitial nitrogen (Ni) inserts into lattice interstices. Both doping modes coexist at the atomic scale, preserving the integrity of the spinel host structure and enabling synergistic optimization of performance through dual-site regulation.
In summary, mixed doping provides a versatile strategy to simultaneously exploit substitutional and interstitial effects, achieving enhanced control over cation valence, lattice defects, and local electronic structure, while maintaining the structural stability of multinary metal oxides.
4.1.5. Surface Confined Doping
Surface confined doping represents a distinct non-metallic doping strategy that selectively modifies the material surface, functioning primarily as a surface engineering approach rather than altering the bulk lattice. This strategy allows precise tuning of surface chemistry and electronic properties while preserving the structural integrity of the host material.
A typical example is phosphorus doping in nickel manganese oxide (NiMn2O4) via chemical vapor deposition (CVD) [73]. In this case, P selectively substitutes specific surface sites, forming P=O and P–O bonds, without disrupting the spinel framework. The underlying mechanism involves the construction of phosphorus-oxygen functional groups on the surface, which enhances the adsorption of dichloromethane (DCM) molecules and optimizes surface charge transfer, thereby significantly improving electrocatalytic dechlorination performance.
Similarly, in sulfur-doped CoMn2O4 spinel [23], FTIR and XPS analyses reveal that sulfur exists as sulfate ions (SO42−) anchored on the surface via S=O bonds. Unlike conventional lattice substitution, this surface modification induces oxygen vacancies and regulates metal valence states indirectly, synergistically enhancing electron transfer and increasing the exposure of active sites.
Collectively, surface confined doping offers a novel pathway for catalyst design, emphasizing the optimization of surface functionalities rather than bulk lattice modification. This approach enables efficient control over adsorption, charge transfer, and catalytic activity, providing an attractive alternative to traditional substitutional or interstitial doping strategies.
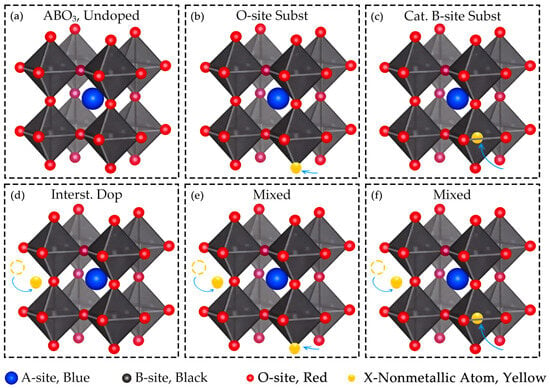
Figure 3.
Various configurations of non-metallic doping in perovskite structures. (a) Displays the pristine perovskite structure; (b) Presents oxygen site substitution via non-metallic doping in perovskite; (c) Shows cationic B-site substitution with non-metallic doping in perovskite; (d) Depicts interstitial non-metallic doping in perovskite; (e) Demonstrates mixed non-metallic doping in perovskite, integrating interstitial doping and oxygen site substitution; (f) Exhibits mixed non-metallic doping in perovskite, combining interstitial doping and cationic B-site substitution. The legend indicates that blue spheres represent A-site cations, black spheres denote B-site cations, red spheres stand for O-site anions, and yellow spheres signify non-metallic dopant atoms (X).
4.2. Type of Nonmetallic Element
The selection of non-metallic dopants plays a pivotal role in tailoring the structural, electronic, and catalytic properties of multinary metal oxides, and represents one of the core directions in functional material research. Different nonmetallic elements impart distinct effects, both at the lattice and surface levels, thereby modulating light absorption, charge transport, and catalytic activity.
For example, in C and Fe co-doped LaCoO3, XRD analysis shows that the (104) characteristic diffraction peak shifts to a lower 2θ angle, indicating lattice distortion induced by C substituting lattice oxygen and Fe incorporation. FTIR confirms the presence of new characteristic vibrations at 1117 cm−1 (C–O–C), 668 cm−1 (Co–C–O), and 444 cm−1 (Co–C), while XPS further verifies the formation of Co–C bonds at 282.8 eV and the presence of Fe3+. UV–Vis DRS demonstrates that co-doping enhances visible light absorption, with the absorption edge redshifted from 422 nm (Fe single-doped) to 471 nm and the band gap reduced from 2.94 eV to 2.63 eV. This enhancement is attributed to the synergistic effect of C narrowing the band gap and Fe introducing mid-gap energy levels. Consequently, C and Fe co-doped LaCoO3 exhibits superior photocatalytic CO2 reduction activity compared with single-doped or undoped samples [50].
As shown in Figure 4a [24], phosphorus doping in LaFeO3−δ (LFP-5) provides another clear demonstration of nonmetallic element incorporation. Polarization curves show more positive onset potentials, higher half-wave potentials, and larger kinetic current densities compared with pristine LaFeO3−δ and conductive acetylene black. Figure 4b shows the High-Angle Annular Dark-Field STEM (HAADF-STEM) and elemental mapping reveal that La, Fe, and O are uniformly distributed, while P is predominantly enriched in the surface layer, directly confirming successful doping and optimization of electron transport and catalytic active sites.
Similarly, as shown in Figure 4c [74], sulfur incorporation into M,S-Fe3O4 (M = Ni, Co, Mn) is confirmed via EDS elemental mapping, showing uniform dispersion of S and metal cations, which supports the formation of a homogeneous doped structure.
For nitrogen doping, as shown in Figure 4d [25], XRD patterns of (SFMONs), indicate that the (110) peak shifts to a lower 2θ angle while retaining the cubic Pm-3m structure. This shift is consistent with lattice expansion due to the partial substitution of O2− by N3− (N3− 1.71 Å > O2− 1.40 Å). Figure 4e shows EDS mapping confirms uniform distribution of N alongside Sr, Fe, Mo, and O, verifying successful incorporation and providing a foundation for enhanced HER activity.
As shown in Figure 4f [36], fluorine doping in Sr2TiO4−XFX also illustrates lattice modifications. At low doping levels (x ≤ 0.05), XRD peaks shift to higher 2θ angles and peak intensities increase, indicating lattice contraction due to the smaller ionic radius of F− (0.133 nm vs. O2− 0.140 nm) and improved crystallinity. Even when minor impurity phases appear at x = 0.1, successful F incorporation at lower concentrations is evident.
As shown in Figure 4g [75], Boron doping in LaMnO3(B-LMO) induces morphological and structural changes, as SEM images show a transition from a dense structure to a porous morphology. BET measurements confirm increased surface area and decreased pore size, indirectly demonstrating successful B incorporation and lattice reconstruction.
The effects of different nonmetallic elements can vary widely within the same host material. In SrTiO3, monodoping with B, C, N, F, P, or S produces distinct outcomes [76]. Boron, fluorine, and phosphorus p-orbitals show minimal hybridization with O 2p orbitals, leaving the band gap nearly unchanged. Carbon and nitrogen exhibit weak hybridization, slightly narrowing the band gap, with visible light absorption arising from isolated energy levels. In contrast, S 3p orbitals strongly hybridize with O 2p orbitals, broadening the valence band and significantly reducing the band gap. This leads to a 120 nm redshift of the absorption edge and increased absorption intensity, making sulfur doping particularly advantageous for high-efficiency photocatalysis.
Collectively, these studies underscore that the type of nonmetallic dopant critically determines the structural modulation, electronic properties, and functional performance of multinary metal oxides, highlighting the importance of rational dopant selection in the design of advanced functional materials.
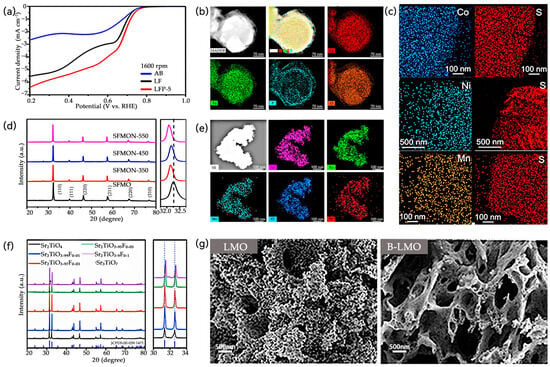
Figure 4.
(a) LSV curves of LF, LFP, and conductive acetylene black (AB) on RDE at 1600 rpm with a scan rate of 10 mVs−1; (b) The HAADF-STEM image and corresponding EDX element mapping images of LFP-5. Adapted with permission from Ref. [24]. Copyright 2018 Copyright Nano Energy; (c) Partial figures of HAADF-STEM-EDS mapping of Ni,S-Fe3O4/IF (Interconnected Framework), Co,S-Fe3O4/IF and Mn,S-Fe3O4/IF. Adapted with permission from Ref. [74]. Copyright 2024 Copyright Appl. Catal. B Environ.; (d) Left: XRD patterns of the SFMO, SFMON-350, SFMON-450, and SFMON-550. Right: Magnified XRD patterns in the 2θ range of 31.7–32.7 degrees; (e) energy-dispersive X-ray spectroscopy element mapping of SFMON-450. Adapted with permission from Ref. [25]. Copyright 2021 Copyright Mater. Today Energy; (f) XRD patterns of Sr2TiO4−XFX (X = 0, 0.01, 0.03, 0.05 and 0.1). FESEM images and the corresponding particle size histograms of Sr2TiO4, Sr2TiO3.99F0.01, Sr2TiO3.97F0.03, and Sr2TiO3.95F0.05. Adapted with permission from Ref. [36]. Copyright 2022 Copyright Mater. Today Energy; (g) SEM results of LMO and B-LMO. Adapted with permission from Ref. [75]. Copyright 2025 Copyright Ceram. Int.
4.3. Doping Concentration
The concentration of nonmetallic dopants is a critical factor in determining the structural, electronic, and functional properties of multinary metal oxides. Doping concentration directly affects microstructure, electronic configuration, and electrochemical behavior, with both insufficient and excessive doping negatively impacting performance.
For instance, in phosphorus-doped ternary transition metal oxides (Co–Ni–Mo systems), NaH2PO2·H2O was used as the phosphorus source to synthesize P-Co0.21Ni0.79MoO4−n via gas–solid phase phosphidation at 400 °C under argon. Different dosages of the phosphorus source (0.2 g, 0.4 g, 0.8 g) were used to regulate the doping amount. These dosages refer to the doping amounts of the precursor rather than the actual final doping contents, and their effects were systematically analyzed. As shown in Figure 5a–d [77], SEM images reveal that at low phosphorus content (P-Co0.21Ni0.79MoO4−n, 0.2 g), the nanosheets exhibit poor surface roughness, insufficient P incorporation, and limited electroactive sites. At the optimal dosage (P-Co0.21Ni0.79MoO4−n, 0.4 g), a porous three-dimensional hierarchical array is formed with uniform P distribution, promoting electrolyte penetration and providing abundant reaction sites. Conversely, an excessive dosage (P-Co0.21Ni0.79MoO4−n, 0.8 g) leads to agglomeration or clogging of the nanosheets, which destroys the effective morphology.
XRD analysis confirms interstitial P incorporation across all dosages without the formation of impurity phases. As the P content increases, diffraction peaks shift to lower 2θ angles due to lattice expansion induced by the larger ionic radius of P relative to O. At the electronic structure level, optimal P doping results in positive shifts in Co 2p, Ni 2p, and Mo 3d binding energies, with Mo present in low oxidation states (Mo4+/Mo5+), enhancing electrochemical activity. The P 2p XPS spectra further verify stable anionic incorporation. Electrochemical measurements show that P-Co0.21Ni0.79MoO4 at 0.4 g dosage exhibits a specific capacitance of 1127.5 F g−1 at 0.5 A g−1, retains 547.1 F g−1 at 20 A g−1, and has a low charge transfer resistance of 0.4 Ω·cm2. An asymmetric supercapacitor with activated carbon achieves an energy density of 49.2 Wh kg−1 at a power density of 747.7 W/kg and maintains 91% capacitance after 5000 cycles. These results demonstrate that 0.4 g represents the optimal P dosage, balancing active site availability and structural integrity. Insufficient doping limits performance, while excessive doping causes structural degradation.
Doping temperature is a key factor affecting doping content and another important parameter that regulates nonmetallic doping concentration and site distribution. In N-doped La2Ti2O7(N-LTO) nanocrystals, Wang showed that nitridation temperature affects N content, site occupation, and band structure. As shown in Figure 5e [78], XPS analysis indicates that untreated LTO exhibits a single N 1s peak (398.05 eV), while NH3-treated samples display three peaks corresponding to N-Ti-N (395–396 eV, substitutional), N-Ti-O (398–399 eV, low electron density), and interstitial N (400 eV), confirming successful multi-site doping. Photocatalytic tests reveal that performance initially improves with temperature, peaking at 650 °C. At 600 °C, total N content is 3.53 at%, dominated by N-Ti-O sites, leading to weak visible light absorption and low NO conversion. At 650 °C, total N content increases to 4.79 at% with balanced site distribution, redshifting the absorption edge to 453 nm and achieving a high specific surface area (44 m2 g−1), which synergistically reduces carrier recombination and maximizes photocatalytic activity. At higher temperatures (700–750 °C), excessive N–Ti–O or N-Ti-N doping creates deep traps and oxygen vacancies, increasing recombination rates and reducing photocatalytic efficiency.
4.4. Co-Doping with Other Elements
Co-doping of metals and nonmetals has emerged as an effective strategy for tuning the electrocatalytic performance of metal oxides by synergistically modifying their electronic structure, surface properties, and mass transfer characteristics. A representative example involves Fe3O4-based electrodes co-doped with different metals (Ni, Co, Mn) and sulfur (denoted as M,S-Fe3O4/IF). Electrochemical impedance spectroscopy (EIS) measurements, as shown in Figure 5f [74], indicate that the Ni,S-Fe3O4/IF electrode exhibits the lowest charge transfer resistance (Rct ≈ 2.7 Ω), reflecting the fastest electron transfer kinetics during the oxygen evolution reaction (OER).
Multi-current step chronopotentiometry further demonstrates that M,S-Fe3O4/IF electrodes respond rapidly and reversibly across varying current densities. Such behavior reflects the preservation of mechanical robustness, electronic conductivity, and efficient mass transport during the OER process. These attributes stem from the cooperative effect of metal and nonmetal co-doping, which concurrently (i) modulates the electronic structure of the host lattice to enhance charge-carrier mobility, (ii) accelerates interfacial charge transfer, and (iii) facilitates reactant diffusion toward catalytically active sites. The integration of these factors establishes a highly favorable reaction environment, thereby delivering performance superior to that of single-doped or pristine counterparts.
Beyond experimental electrochemical analysis, co-doping strategies have also been explored theoretically. Zhou et al. employed first-principles calculations to investigate the impact of simultaneous metal and nonmetal doping (F, N, S with metals Cr) on the electronic structure and optical properties of BiFeO3, as shown in Figure 5g [79]. Computational results, including the dielectric function, absorption coefficient, refractive index, and extinction coefficient, reveal that co-doping modifies the optical response of the material. For instance, variations in the absorption edge and refractive index among different co-doped systems indicate that dopants can tailor light–matter interactions and electronic transitions. These simulations provide insight into the mechanistic basis for co-doping effects, complementing experimental findings and highlighting the potential of metal–nonmetal co-doping for multifunctional material design.
In summary doping concentration is a key determinant of performance which requires careful optimization of source dosage temperature and processing time [80] and metal-nonmetal co-doping leverages synergistic effects these factors together enable balancing structural integrity dopant distribution and electronic optical properties reducing charge transfer resistance improving mass transport and ultimately maximizing functional efficiency such as electrocatalytic photocatalytic and photophysical performance in nonmetallic-doped or multinary metal oxide systems.
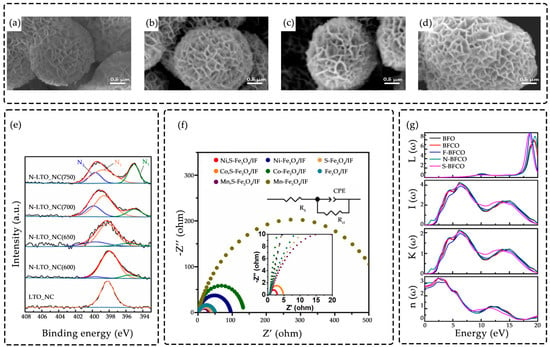
Figure 5.
(a) SEM images of Co0.21Ni0.79MoO4; (b) P-Co0.21Ni0.79MoO4-1; (c) P-Co0.21Ni0.79MoO4-2 and (d) P-Co0.21Ni0.79MoO4-3. The samples P-Co0.21Ni0.79MoO4, P-Co0.21Ni0.79MoO4-1, P-Co0.21Ni0.79MoO4-2, and P-Co0.21Ni0.79MoO4-3 correspond to doping with 0 g, 0.2 g, 0.4 g, and 0.8 g of the phosphorus precursor, respectively. Adapted with permission from Ref. [77]. Copyright 2020 Copyright J. Energy Storage; (e) High resolution N 1s XPS spectra of LTO_NC before and after heat treatment in NH3 gas flow at different temperatures without Ar+ sputtering(N1,N-Ti-N, green; N2,N-Ti-O, orange; N3, interstitial N atoms in O-N-Ti, blue). Adapted with permission from Ref. [78]. Copyright 2021 Copyright Chem. Eng. J.; (f) Nyquist plots of various catalysts of Ni,S-Fe3O4/IF, Co,S-Fe3O4/IF, Ni-Fe3O4/IF, Co-Fe3O4/IF, S-Fe3O4/IF, Fe3O4/IF. Adapted with permission from Ref. [74]. Copyright 2024 Copyright Appl. Catal. B Environ.; (g) L(ω), I(ω), K(ω) and n(ω) of BFO, BFCO(Cr-BFO) and X-doped BFCO. Adapted with permission from Ref. [79]. Copyright 2019 Copyright Phys. Lett. A.
5. Mechanisms of Structure and Electronic State Regulation by Non-Metallic Doping
During non-metal doping of multicomponent oxides, charge compensation and variation in bonding character the primary microscopic effects. Charge compensation is the most fundamental initial consequence. When a high-valence non-metal cation substitutes a low-valence B-site cation, or when a low-valence non-metal anion replaces O2−, lattice charge neutrality is disrupted. The resulting imbalance is compensated mainly through two routes. The first and most prevalent route is the formation of oxygen vacancies; when a low-valence non-metal occupies an oxygen site, it withdraws electrons from neighboring oxygen atoms and thereby generates oxygen vacancies. The second route operates when a high-valence non-metal substitutes a metal cation; in this case a high-valence metal ion is reduced to a lower oxidation state or a low-valence metal ion is oxidized to a higher state, and the two pathways often act cooperatively to preserve overall electroneutrality. For example, In P-doped LaFeO3, the signal at g = 2.003 in electron paramagnetic resonance increases markedly, directly evidencing a sharp rise in the concentration of paramagnetic oxygen vacancies. X-ray photoelectron spectra show that as the phosphorus content rises the Fe2+/Fe3+ ratio grows from 0.32 to 0.57 [22]. When boron is introduced into the spinel/perovskite heterojunction oxide NiCo2O4/LaCoO3, the small B3+ ion (radius 0.20 Å) replaces Co3+ (0.54 Å) and induces lattice distortion that weakens Co–O bonds, promotes lattice-oxygen release and creates abundant oxygen vacancies. Simultaneously, the electron-donating character of boron reduces Co3+ and Ni3+ to Co2+ and Ni2+ [28]. In layered perovskite Sr2TiO4, co-doping with non-metal nitrogen and metal niobium places N3− on O2− sites and Nb5+ on Ti4+ sites; the substitution of N3− for O2− introduces a charge imbalance that must be compensated [81].
5.1. Lattice Distortion
Incorporation of non-metal atoms into the lattice produces expansion or contraction that is governed by the ionic radius mismatch between the dopant and the host atom, by the charge-compensation pathway, and by the accompanying reconstruction of chemical bonding.
A radius mismatch distorts the lattice and leads to measurable dimensional changes. In sulfur-doped CuFe2O4, the larger S2− ion replaces O2− and shifts X-ray diffraction peaks to lower angles, indicating an increased interplanar spacing [82]. Similarly, when N3−, which is also larger than O2−, is introduced into Sr2Fe1.5Mo0.5O6-δ, the (110) reflection moves to lower angles and the unit cell expands [25]. Conversely, boron incorporation into LaMnO3 shrinks the lattice; the smaller B3+ ion substitutes for Mn3+ and shortens Mn–O bond lengths, so the structure contracts [75].
Oxygen-vacancy formation relaxes the lattice. In P-doped LaFeO3, the smaller P5+ ion replaces Fe3+ and would normally shrink the unit cell, yet the simultaneous creation of numerous oxygen vacancies releases part of the atomic constraints and produces a slight expansion; the cell volume increases from 242.97 to 243.53 Å3 [22]. Similarly, when nitrogen is introduced into MnCo2O4, the departure of lattice oxygen generates vacancies that loosen the network, shift diffraction peaks to lower angles and enlarge the interplanar spacing [27].
Charge-compensation-induced valence changes and oxygen vacancies also distort the lattice. In iodine-double-site-doped Ba0.5Sr0.5(Co0.8Fe0.2)0.9O3−δ (BSCF), iodine occupies both B and O sites, Co3+ is partially reduced to Co2+, and the combined radius change and charge redistribution expand the lattice parameter from 3.953 to 3.966 Å [83]. In P-doped MnCo2O4, P5+ substitutes for cobalt, Co3+ reduces to the larger Co2+, and the accompanying oxygen vacancies increase the parameter from 8.258 to 8.262 Å [8].
Electronegativity differences alter the covalent and ionic characters of metal-non-metal bonds, modify bond lengths and strengths, induce phase transitions, and change lattice parameters.
An increase in covalent bond length together with a bond strength decrease leads to lattice expansion. In S2−-doped CuFe2O4, the M–S bond is longer than the M–O bond although S2− is smaller than O2−, so the lattice expands. When sulfur substitutes for O2− in SrCoO3−δ as S2−, the structure transforms from a hexagonal phase to a more symmetric cubic perovskite [82]. The electronegativity of sulfur is 2.58, significantly lower than that of oxygen at 3.44, so the Coulomb attraction between the metal cation Co3+ and the anion weakens and internal stress is reduced. Charge compensation occurs through the formation of oxygen vacancies and partial reduction of Co3+ to Co2+, and the oxygen vacancies further relax the lattice [21]. Undoped SrCoO3−δ adopts a hexagonal phase. When non-metal phosphorus is introduced as P5+ that replaces Co3+, X-ray diffraction shows that the hexagonal reflections disappear completely and a pure tetragonal perovskite forms. This change is attributed to the strong covalent P–O bond that forms after P5+ substitutes for Co3+; the directionality and stability of this bond suppress the interlayer sliding characteristic of the hexagonal phase and drive the lattice toward a denser, moderately symmetric tetragonal structure [51].
Lattice expansion suppresses agglomeration and enlarges the specific surface area. When iodine occupies dual sites in BSCF, the expanded lattice offers additional room for atom growth, while the newly formed Co–I bond weakens inter-particle attraction. Consequently, particle size decreases, dispersion improves, agglomeration is avoided, the specific surface area increases slightly, and more active sites are exposed [83]. In N-doped MnCo2O4, the interplanar spacing increases because N3− replaces O2−. The enlarged lattice hinders diffusion of Mn and Co atoms toward the particle surface, so the morphology changes from rod-like agglomerates to separated irregular grains. Although the specific surface area becomes smaller, surface roughness rises and the density of catalytically active sites increases [27].
Lattice contraction promotes densification and refines particle size distribution. Upon fluorine incorporation into Sr2TiO4, the smaller F− ion replaces O2−, X-ray diffraction peaks shift to higher 2θ values, and atomic packing becomes tighter. As the fluorine content increases, particles grow uniformly without noticeable agglomeration, balancing specific surface area and structural stability [36].
5.2. Crystallinity
Most non-metal dopants disrupt the long-range periodic order of the crystal and lower crystallinity. The underlying origin is that the foreign atoms introduce lattice distortion, oxygen vacancies, or impurity phases that hinder regular atomic packing. In nitrogen-doped MnCo2O4, M–N and M–N–O bonds form and trigger local electron redistribution, while the departure of lattice oxygen creates oxygen vacancies. These combined effects destroy lattice periodicity and reduce crystallinity [27].
Some non-metal dopants suppress grain growth by generating oxygen vacancies that impede atomic migration, thereby increasing the fraction of small crystallites and lowering overall crystallinity. In P-doped LaFeO3 the orthorhombic symmetry is retained, yet the replacement of Fe3+ by P5+ creates abundant oxygen vacancies. These vacancies block atomic diffusion toward grain surfaces, while the covalent character of P–O bonds restricts the freedom of lattice atoms to rearrange [22,24]. Consequently, grain growth is hindered and crystallinity decreases as the phosphorus content rises. When the boron content in LaMnO3 is progressively increased, the diffraction peak intensities first remain stable at low doping levels; at high boron loadings; however, excess boron can no longer be accommodated in the lattice and local amorphous regions form. This leads to a sudden drop in peak intensity, a marked increase in peak width, structural collapse, and complete loss of crystallinity [75]. Similarly, sulfur incorporation into CuFe2O4 at low levels preserves the cubic spinel structure with only a slight crystallinity reduction, yet at higher sulfur contents characteristic reflections of a CuS impurity phase appear and the intensity of the main phase decreases by 30%. The presence of this secondary phase not only dilutes the proportion of the host crystal but also disrupts the orderly growth of CuFe2O4 grains, further depressing crystallinity [82]. Surface functional groups can exert an additional influence. After sulfur functionalization, CoMn2O4 retains its body-centered cubic framework, yet the M–O–S linkages formed between sulfate and surface Co/Mn atoms hinder regular atomic packing at the surface and suppress grain growth. The resulting grain refinement lowers the degree of crystallization [23].
Moderate non-metal doping can preserve or even enhance crystallinity by filling intrinsic lattice defects, suppressing secondary phases, or promoting an order–order phase transition, provided the dopant size and valence match the host lattice. When the ionic radius of the non-metal closely resembles that of the atom it replaces, the dopant heals existing vacancies and leads to more regular atomic packing. In Sr2TiO4, an appropriate fluorine content maintains the tetragonal structure because F− is almost the same size as O2−; by filling oxygen-vacancy defects it reduces disordered regions, while the stronger covalency of F–O bonds relative to Ti–O bonds stiffens the lattice, favors regular stacking, enlarges grain size and markedly increases crystallinity [36]. Similarly, nitrogen incorporation into Sr2Fe1.5Mo0.5O6-δ introduces N3− ions whose radius matches that of O2−; the substitution introduces no appreciable distortion and, through charge compensation that oxidizes Fe3+ to Fe4+, stabilizes the framework and heals vacancy-related disorder [25]. The additional presence of nitrogen promotes long-range ordering of Fe and Mo atoms, gives sharper lattice fringes and lowers the fraction of disordered grain boundaries, thereby raising crystallinity.
Some non-metal dopants convert a low-symmetry, poorly crystallized phase into a higher-symmetry, better ordered one. Sulfur substitution for O2− in SrCoO3−δ weakens Co–O Coulomb attraction and drives a hexagonal-to-cubic perovskite transition; the cubic phase possesses higher symmetry, denser atomic packing, and the sulfur simultaneously suppresses SrCO3 impurity formation, so both phase purity and crystallinity improve. The gain in crystallinity is accompanied by particle regularization and reduced agglomeration [21]. In P-doped SrCoO3−δ, the undoped material adopts a hexagonal structure that gives weak, split reflections and poor crystallinity. When P5+ replaces Co3+, strong, directional P–O bonds form and convert edge-shared CoO6 octahedra of the hexagonal phase into corner-shared octahedra of a tetragonal perovskite, creating a more stable, ordered framework. The particle size distribution narrows from 1–5 µm to 2–3 µm, agglomeration is absent, and the regular surface lowers Sr-ion leaching. The P–O network simultaneously inhibits Sr loss and secondary-phase precipitation, further consolidating the crystallinity of the tetragonal host and meeting the long-term stability requirements of DSSC cathodes [51].
Tuning crystallinity by non-metal doping essentially balances defect activity and structural stability through the synergy between element–lattice compatibility and dopant content, offering a controllable microscopic route for property optimization of multicomponent oxides.
The degree of crystallinity directly governs grain-growth perfection, thereby altering surface regularity and defect distribution. Lower crystallinity refines grains and increases surface defects and roughness. In N-doped MnCo2O4, reflection intensities weaken and broaden, crystallinity drops, lattice fringes become more disordered, the number of defects rises, grain size shrinks, and the surface develops a porous, rough morphology enriched in defects [27]. Similarly, as the boron content in LaMnO3 increases, peaks broaden, crystallinity decreases, grain size falls from 38 nm, and extensive oxygen vacancies generated by lattice distortion blur particle edges, raise roughness, and create additional active sites [75].
5.3. Electronic Structure Reconfiguration
Non-metal doping modulates electronic structure through four convergent pathways: adjustment of lattice parameters, induction of oxygen vacancies, reconstruction of ion valence states, and optimization of bonding character. These changes operate across four interlinked dimensions—band structure, electron-cloud distribution, carrier characteristics, and defect-state energy levels—ultimately aligning the material with the functional demands of catalysis, energy storage, and related applications. Lattice parameters govern the space and manner in which grains grow, determining atomic packing density and thereby controlling both the rate of grain growth and the final particle size.
Non-metal doping directly tailors band-gap width and band-edge positions by modifying atomic-orbital hybridization and introducing impurity states, thereby serving as the primary route for extending the optical response. In N-doped Sr2Fe1.5Mo0.5O6-δ, replacement of O2− by N3− promotes mixing between N 2p and O 2p orbitals together with lattice expansion. This hybridization increases the overlap of Fe 3d and O 2p orbitals, narrows the band gap from a higher to a lower value, and enhances electron delocalization. As a result, the electron-transfer resistance for H+ reduction during the hydrogen evolution reaction decreases and the overpotential is significantly reduced [25]. In dual-site doped BSCF, simultaneous incorporation of I− at O sites and I5+ at B sites enables I 5p orbitals to mix with Co 3d and Fe 3d states, pushing the Co 3d and Fe 3d bands closer to the Fermi level and narrowing the band gap. The narrowed gap lowers the energy barrier of the rate-determining step of the oxygen evolution reaction [83]. In a defect-dominated narrowing scenario, P-doped LaFeO3 shows that substitution of Fe3+ by P5+ creates numerous oxygen vacancies which introduce defect levels inside the forbidden gap. The band gap decreases from a higher to a lower value and the valence-band maximum shifts downward as the defect states are filled, improving the separation efficiency of photogenerated electron–hole pairs. Consequently, the conversion of methane in chemical-looping partial oxidation increases from a low to a markedly higher value [22].
Non-metal doping adjusts the positions of the conduction-band minimum and valence-band maximum so that the material meets the thermodynamic potential requirements of specific reactions. For photocatalytic hydrogen evolution, the conduction-band minimum must lie below the H+/H2 redox potential. In F-doped Sr2TiO4, substitution of O2− by F− contracts the lattice and weakens the overlap between Ti 3d and O 2p orbitals. The conduction-band potential shifts from −0.39 eV to −0.43 eV, strengthening the driving force for electron reduction in water, while the band gap narrows slightly from 3.32 eV to 3.20 eV. Consequently, the photocatalytic hydrogen evolution rate increases by 44% relative to the undoped material [36].
Non-metal doping enhances catalytic activity by modulating the electron density of metallic active sites and shifting the d-band center, thereby optimizing the adsorption strength of reaction intermediates. In N-doped MnCo2O4, the Mn 2p and Co 2p peaks exhibit a blue shift, indicating increased electron density around the metal centers [27]. After sulfur incorporation into CuBi2O4, Cu–S bonds form; the electron-donating nature of sulfur raises the electron density at Cu sites. Meanwhile, changes in metal valence, for example, from Co3+ to Co2+, alter the d-orbital electron occupancy and shift the d-band center, preventing either too strong or too weak intermediate adsorption [84]. In dual-site iodine-doped BSCF, iodine cations donate electrons to cobalt through Co–O–I bridges, partially reducing Co3+ to Co2+. The orbital filling of Co increases from 1.0 to 1.2, and the d-band center moves downward from −1.8 eV to −2.0 eV, leading to lower OER overpotential and a reduced Tafel slope [83].
Non-metal doping improves charge-utilization efficiency by accelerating carrier separation and transport, thereby suppressing electron-hole recombination. This improvement originates from defect-state trapping and lattice-order adjustment. In N-doped MnCo2O4, nitrogen incorporation raises the oxygen-vacancy concentration; these vacancies trap photogenerated electrons and prevent their recombination with holes. Photoluminescence intensity consequently decreases, evidencing higher electron-transfer efficiency [27]. Similarly, when B3+ substitutes for Mn3+ in LaMnO3, B–O bonds form and weaken the covalency of Mn–O bonds, inducing oxygen vacancies that act as electron-capture centers. The reduced photoluminescence intensity indicates diminished recombination.
Non-metal doping generates oxygen vacancies and new bond defects that create localized states within the band gap. These states act as both carrier traps and electron transport channels, further optimizing electronic structure. In S-doped CuBi2O4, sulfur incorporation increases the vacancy-to-lattice-oxygen ratio, producing defect levels just above the valence-band maximum [84]. In B-doped LaCoO3, substitution of Co3+ by B3+ forms B–O bonds whose covalency introduces interfacial defect states. These levels extend electron delocalization and reduce the charge-transfer resistance across the NiCo2O4/LaCoO3 interface [28].
Incorporation of non-metal dopants into multicomponent oxide lattices triggers charge compensation that generates oxygen vacancies, alters cation valences, and modifies chemical bonding. These changes produce microstructural distortions manifested as variations in crystallinity and lattice parameters, while simultaneously inducing electronic rearrangements that encompass band structure, electron-cloud distribution, carrier density, and defect-state energy levels. The synergy between structural and electronic perturbations governs the optimization of macroscopic properties. Any single macroscopic performance metric requires the cooperative tuning of multiple microscopic variables; consequently, microstructural reconstruction inevitably refines mesoscale characteristics that ultimately determine the macroscopic response. Non-metal doping is therefore essential for enhancing multicomponent oxide performance, because it exploits charge compensation to regulate band structure, carrier concentration, and surface activity, thereby overcoming intrinsic performance limits.
6. Application Fields of Non-Metallic Doping
6.1. Field of Energy
Energy science covers several key technological domains, among which battery technology, supercapacitor technology, and catalysis technology constitute the main research directions. In the field of battery technology, typical systems include lithium-ion batteries (LIBs), zinc-air batteries (ZABs), and solid oxide fuel cells. Catalysis technology is further subdivided into electrocatalysis and photocatalysis. This classification reflects the principal research scope of energy conversion and storage technologies and highlights the importance of different technical paths in the energy field. Multinary metal oxides, thanks to their tunable composition and crystal structure, show great application potential in energy conversion and storage. Their functionality can be precisely designed to suit specific applications. For example, NiCo2O4 is used for photocatalytic CO2 reduction to CO [85], Li4Ti5O12 serves as an anode material in lithium-ion batteries, and NiCo2O4 is also a typical material for zinc-ion batteries. Perovskite-type oxides such as LaNiO3, LaMnO3, SrFeO3−δ, and SrCo0.8Fe0.2O3−δ are widely used as cathode materials in solid oxide fuel cells due to their adjustable oxygen mobility and electronic conductivity. In photocatalysis, materials like Bi2WO6 and NiCo2O4 are employed in light-driven reactions, while in electrocatalysis, NiCo2O4, LaMnO3, and CoFe2O4 exhibit good catalytic activity. Particularly noteworthy is the recent emergence of high-entropy spinel oxides such as (NiCoMnCrFe)3O4, which demonstrate excellent activity and stability in anodic catalysis for hydrogen production from seawater electrolysis, offering a new direction for the application of high-entropy materials in energy catalysis [86].
In the energy field, non-metal doping tailors the microscopic structure of materials and thereby markedly improves their macroscopic performance. Within lithium-ion battery technology, multicomponent oxide Li4Ti5O12 (LTO) serves as the main host; non-metal doping optimizes lattice structure, electronic states and interfacial characteristics, leading to greatly enhanced electrochemical performance that contrasts sharply with that of undoped LTO. Iodine [87], nitrogen [88] and fluorine [89] incorporation each raise the specific capacity at high rate, lower the charge-transfer resistance and increase the Li+ diffusion coefficient. Fluorine doping in particular produces a thinner and more uniform surface film. These combined benefits improve the specific capacity, high-rate tolerance and cycling stability of LTO. Co-doping with sulfur and cobalt enlarges the specific surface area and introduces additional mesopores that facilitate mass transport; the sulfur-cobalt co-doped material delivers a higher specific capacity at 50 charge-discharge rate (C-rate) [90]. The improvements originate from the synergistic regulation offered by non-metal dopants, which widen Li+ diffusion channels, induce the reduction of Ti4+ to Ti3+ to raise electron concentration, and establish Ti–I, Ti–N–O and Ti-S bonds that enhance electronic transport, lower impedance and strengthen interfacial films. Undoped LTO, in contrast, suffers from narrow diffusion channels, poor electronic conductivity and extensive side reactions, so its macroscopic performance is clearly inferior. Fluorine-doped Li4Ti5O12 that is further modified by a carbon-nitrogen double coating through polyaniline pyrolysis exhibits lattice expansion caused by the substitution of O2− by F−. The enlarged lattice increases Li+ diffusion to 2.82 × 10−14 cm2s−1, 17-fold higher than that of pristine LTO, and delivers a discharge specific capacity of 165.38 milliampere hours per gram at 0.5 C-rate while retaining 93.51 percent of that capacity after 150 cycles at 1 C-rate [70]. These modifications also reduce the risk of lithium dendrite formation that is often encountered with graphite anodes. Carbon-doped titanium oxide of the form TiCXOY has been applied in lithium-ion cells. Relative to the undoped oxide, carbon insertion lowers the oxygen content, partially reduces Ti and introduces both Ti3+ ions and oxygen vacancies, leading to higher electronic conductivity and improved rate capability [91].
In zinc-based battery systems, non-metal-doped multicomponent oxides deliver markedly improved electrochemical performance through tailored crystal structures, activated surface sites and accelerated electron transport. Relative to the undoped reference, sulfur-doped NiCo2O4 (S-NCO) offers a higher specific capacity and excellent cyclability in alkaline zinc cells; it retains its capacity over 4000 cycles with coulombic efficiency close to 100%, an advance that originates from a conductive phase and an enlarged specific surface area [92]. Fluorine-substituted LaCoO3 (F-LaCoO3), employed as the cathode in zinc-air batteries, exhibits a lower oxygen-evolution over-potential together with increased open-circuit voltage and specific capacity, benefits that are traced to additional oxygen vacancies and a higher Co3+ fraction [93]. Nitrogen-doped LaMnO3 (LMNO) likewise delivers a higher peak power density and specific capacity, and it remains stable for 500 cycles whereas the undoped analog fades rapidly. Phosphorus-doped MnCo2O4, in which P5+ occupies Co sites, generates abundant oxygen vacancies and raises the Co2+ fraction, thereby boosting oxygen-reduction activity [94]. The specific surface area expands from 6.88 to 17.25 m2 g−1, the cell achieves a peak power density of 152 mW cm−2 and steady operation is maintained for 120 h, offering an inexpensive yet high-performance cathode for zinc-air batteries [8] (Figure 6a). Undoped oxides, handicapped by scarce active sites, poor conductivity and extensive interfacial side reactions, clearly lag behind. Through lattice engineering, active-species optimization and impedance reduction, non-metal doping collectively upgrades capacity, cycle life and energy efficiency in zinc batteries.
In the solid oxide fuel cell field, non-metal-doped multicomponent oxides enhance electrochemical performance by tailoring lattice structure, active sites, and interface reaction kinetics. Compared with the undoped reference, interstitial fluorine-substituted SrFeO3−δ maintains a stable Ruddlesden-Popper structure and produces anisotropic lattice expansion. The increased Fe4+ content and higher proportion of active oxygen species significantly reduce interfacial resistance, leading to a higher single-cell peak power density at 700 degrees Celsius and excellent thermal cycling stability [95]. Fluorine-substituted La0.5Ba0.5FeO3−δ (LBF) and La0.5Ba0.5Cu0.5Fe0.5O3−δ (LBCF), exhibit greatly reduced BaCO3 precipitation and markedly improved oxygen exchange kinetics, resulting in noticeably higher power densities [96]. Phosphorus-substituted LaFeO3−δ, the field of Chemical Looping Partial Oxidation of Methane (CLPOM), generates P–O bonds and a small amount of Fe4+, enlarges specific surface area, markedly raises the ORR half-wave potential, lowers the OER overpotential and Tafel slope, and simultaneously decreases charge-transfer resistance [24]. Phosphorus substitution of SrFeO3−δ cathodes increases oxygen vacancy concentration and oxygen diffusion, reducing polarization resistance to 0.08 Ω·cm2, which is 42.86% lower than that of the undoped material, and decreases the thermal expansion coefficient to 18.17 × 10−6 K−1, 32.7% lower. The single-cell power density reaches 749.36 mW cm−2, surpassing reported Fe and Co based cathodes, while maintaining low polarization resistance from 650–800 °C [97]. Undoped multicomponent oxides display clearly inferior performance because of sluggish ORR kinetics, poor degradation resistance, and insufficient active sites. Non-metal dopants such as fluorine and phosphorus jointly achieve an overall breakthrough in SOFC performance through lattice optimization, active species regulation, and impedance reduction.
In the supercapacitor field, non-metal-doped multicomponent oxides significantly enhance energy-storage performance by tailoring crystal structure, electronic conduction, and surface active sites. Compared with undoped samples, phosphorus-doped MnCo2O4 nanotube arrays maintain a high specific capacitance while exhibiting superior rate capability and cycling stability. Their equivalent series resistance and charge-transfer resistance are both lower than those of the undoped counterpart [98]. Carbon-doped CoFe2O4/Fe2O3 composites deliver higher specific capacitance, lower charge-transfer resistance, and better redox reversibility [99]. These improvements originate from lattice distortion, increased specific surface area, and optimized electronic structure induced by non-metal doping, which jointly promote notable advances in specific capacitance, rate tolerance, and cycling stability of supercapacitors.
In electro-catalytic energy conversion, non-metal-doped multicomponent oxides significantly advance catalytic performance by tailoring active sites, electron transport, and the adsorption energetics of reaction intermediates. Relative to their undoped counterparts, phosphorus–sulfur co-doped NiMoO4 exhibits lower over-potentials for both hydrogen and oxygen evolution in overall water splitting, a reduced full-cell voltage, smaller charge-transfer resistance, and prolonged stability [100]. Boron-substituted CoFe2O4 displays decreased over-potentials and Tafel slopes for the oxygen and hydrogen evolution reactions; the accompanying generation of oxygen vacancies and mild lattice expansion markedly accelerates reaction kinetics [101]. Sulfur-doped LaNiO3, employed as a cathode in lithium–oxygen cells, presents higher oxygen-vacancy and Ni3+ concentrations together with an enlarged surface area, leading to reduced ORR/OER over-potentials, increased discharge capacity, and extended cycle life [102]. Undoped oxides, handicapped by scarce active sites, poor electronic conductivity, and high reaction barriers, deliver inferior activity. Through systematic optimization of lattice structure, valence states, and oxygen-vacancy content, non-metal doping effectively lowers impedance and over-potential, enabling a comprehensive breakthrough in electro-catalytic performance.
In energy-oriented photocatalysis, non-metal-doped multicomponent oxides improve macroscopic performance by tailoring band structure, photogenerated carrier separation efficiency and surface active sites. For photoelectrochemical water oxidation, nitrogen-substituted BiVO4 lowers the over-potential. The undoped oxide requires 1.44 V, whereas surface nitrogen incorporation reduces the value to 0.93 V under a single-site adsorption-evolution mechanism and to 1.16 V under a dual-site bridge mechanism. Density-functional theory shows that the proton adsorption energy on nitrogen is 0.52 eV lower than on oxygen and that the water-splitting barrier on vanadium is 0.43 eV lower than on bismuth, while a superoxide-like O–O dimer is stabilized [103]. Relative to undoped Bi2WO6, boron-substituted Bi2WO6 retains a pure orthorhombic phase, forms B–O bonds and exhibits a red-shift in characteristic diffraction peaks, a larger specific surface area and a mesoporous structure that favors mass transport [104]. Photocatalytic carbon dioxide reduction is markedly enhanced by carbon and iron co-substituted LaCoO3. Carbon occupies oxygen sites and creates Co–C bonds that generate C–O–C and C–O–H groups, introducing micro-strain that is amplified by iron substitution. The absorption edge shifts from 422 nm, corresponding to a 2.94 eV gap, to 471 nm, corresponding to a 2.63 eV gap, thereby extending visible-light harvesting. Iron introduces 3d mid-gap levels, while carbon, through Co-C bonds, traps electrons and strongly suppresses carrier recombination. Methane selectivity reaches 82%, 3.5 times higher than that of the undoped oxide [50]. Undoped multicomponent oxides suffer from wide band gaps, high recombination rates and scarce surface sites, so their photocatalytic activity is clearly inferior. Non-metal doping lowers reaction barriers, preserves crystal integrity, optimizes active species and improves porosity, leading to a synergistic enhancement of photocatalytic efficiency and stability.
Non-metal-doped multicomponent oxides continue to demonstrate versatility beyond the previously discussed areas. In chemical-looping partial oxidation of methane, phosphorus-substituted LaFeO3 regulates the redox cycle of the oxygen carrier. The dopant lowers the oxygen-vacancy formation energy to 3.65 eV and raises the vacancy content to 86.54% while the Fe2+/Fe3+ ratio stabilizes at 0.52. At 850 °C, methane conversion reaches 89.83% with a syngas yield of 5.14 millimoles per gram, and the material retains stable performance over twenty cycles [22] (Figure 6b). For alkaline water electrolysis, phosphorus-substituted CoMoO4 undergoes a phase transition that yields a porous morphology. The presence of Mo5+ and Co–P bonds confirms successful incorporation of phosphorus and a modified electronic environment around the metals. The resulting lower charge-transfer resistance and higher conductivity reduce the hydrogen-evolution over-potential to 138 mV, only slightly above that of commercial Pt/C, while the Tafel slope decreases and the Faradaic efficiency reaches 96.8%. The overall hydrogen-evolution activity surpasses that of the undoped oxide and outperforms numerous reported transition-metal oxide catalysts [105]. Phosphorus-substituted SrCo0.8Fe0.2O3−δ cathodes (SCFP), for the field of proton ceramic fuel cells (PCFCs), generate additional oxygen vacancies and enhance proton conduction; under humid air at 650 °C, the area-specific resistance is 0.24 Ω cm2, 29.4% lower than that of the undoped parent [35] (Figure 6c). In direct seawater electrolysis, boron-substituted MnFe2O4 addresses activity and stability bottlenecks. The lower electronegativity of boron relative to oxygen leads to electron donation from boron to transition-metal sites through B–Mn/Fe bonds, increases Fe 3d–O 2p orbital overlap, and lowers the energy barrier of the rate-determining oxygen-evolution step from 5.52 eV to 2.92 eV. Coupling boron incorporation with NaBH4 reduction introduces further oxygen vacancies, suppresses chlorine evolution and hypochlorous acid formation, and improves both intrinsic activity and corrosion resistance, rendering the material a promising catalyst for efficient and stable seawater splitting [30].
Table 2 concisely collates the core information of non-metallic doping elements, corresponding multinary metal oxides, and their targeted energy applications, providing a clear reference for the practical development of related materials. In summary, non-metallic doping provides a versatile and effective strategy for enhancing ionic/electronic transport, introducing defects, and tuning catalytic active sites, thereby enabling high-performance energy storage and conversion materials. Its implementation supports industrialization and cost reduction in next-generation energy devices.
Figure 6.
(a) Schematic diagram of Phosphorus-Doped MnCo2O4 Spinel Catalyst for Zinc-Air Batteries. Adapted with permission from Ref. [8]. Copyright 2025 Copyright ACS Appl. Mater. Interfaces; (b) Schematic diagram of the principle of CL-POM. Adapted with permission from Ref. [22]. Copyright 2025 Copyright Chem. Eng. J.; (c) Distribution of Relaxation Times (DRT) plots of SCFP and SCF electrodes. Adapted with permission from Ref. [35]. Copyright 2024 Copyright Ceram. Int.

Table 2.
Applications of non-metallic doping of multinary metal oxide in the energy field.
Table 2.
Applications of non-metallic doping of multinary metal oxide in the energy field.
| Doping Element | Material | Application |
|---|---|---|
| B | MnFe2O4 | Seawater Electrolysis |
| CoFe2O4 | HER, OER | |
| C | CoFe2O4/Fe2O3 | Hydrogen Production |
| C,Fe | LaCoO3 | Photocatalytic CO2 Reduction |
| N | Li4Ti5O12 | LIBs |
| LaMnO3 | SFOF | |
| BiVO4 | OER | |
| F | Li4Ti5O12 | LIBs |
| SrFeO3−δ | Electrocatalytic | |
| La0.5Ba0.5FeO3−δ, La0.5Ba0.5Cu0.5FeO3−δ | SOFC | |
| LaCoO3 | ZIBs | |
| P | LaFeO3−δ | Metal-Air Battery |
| SrCo0.8Fe0.2O3−δ | PCFCs | |
| LaFeO3 | CLPOM | |
| SrFeO3−δ | SOFC | |
| MnCo2O4 | Supercapacitor | |
| CoMoO4 | Hydrogen Production | |
| NiMoO4 | HER, OER | |
| S | LaNiO3 | ORR, OER |
| NiCo2O4 | ZIBs | |
| S,Co | Li4Ti5O12 | LIBs |
| I | Li4Ti5O12 | LIBs |
Data compiled from source [22,24,30,35,50,87,88,89,90,92,93,94,95,96,97,98,99,100,101,102,103,105].
6.2. Non-Metal Doping Strategies and Composite Engineering for Energy Material Performance Enhancement
Non-metal doping functions as a versatile and flexible materials modification strategy and presently occupies an important position in modern materials science. Implementation routes are diverse and cover direct insertion of non-metal elements into a target host, indirect tuning of carbon coatings through non-metal-doped carbon, and construction of novel composites that integrate doped species. These approaches effectively adjust electrical, catalytic and physicochemical properties while opening new avenues for the development of high-performance energy materials. Consequently, non-metal-doping technology plays a pivotal role in driving innovation and performance advances in energy-related materials.
Non-metal-doped carbon coatings enhance the performance of multicomponent oxides by optimizing the conductive network, stabilizing the interface and increasing the number of active sites. In lithium-ion battery anodes, nitrogen-doped carbon-coated Li4Ti5O12 (NC-LTO) delivers higher discharge capacities and better cycle stability than both pristine LTO and conventional carbon-coated LTO (C-LTO) at various rates. NC-LTO retains the spinel structure, exhibits uniform particle size and contains a uniform carbon layer with a moderate nitrogen content. These features collectively provide superior high-rate capability and long-term cycling performance [32]. In oxygen evolution electrocatalysis, nitrogen-doped carbon-coated NiCo2O4 nanorods (NiCo2O4@NC) show lower over-potential, a smaller Tafel slope, reduced charge-transfer resistance and enlarged electrochemical surface area compared with bare NiCo2O4. The catalyst maintains stable performance after prolonged cycling and its increased surface area further confirms high activity, making NiCo2O4@NC an efficient OER material [106]. When fluorine and nitrogen co-doped carbon nanotubes (F@N-CNT) are used as a conductive additive for LTO electrodes, the composite outperforms conventional carbon black. The F@N-CNT modified electrode delivers higher capacity at 30 C-rate and retains excellent performance at lower rates, while cyclic stability is clearly improved. Covalent C-F bonds are formed and charge-transfer resistance is markedly reduced. These structural characteristics jointly contribute to the outstanding rate performance and cycling stability of the F@N-CNT coated LTO composite. Uncoated or undoped-carbon-coated samples suffer from an incomplete conductive network, extensive interfacial side reactions and a scarcity of active sites, leading to obviously inferior macroscopic performance [107]. Non-metal-doped carbon coatings achieve a synergistic breakthrough by stabilizing the crystal phase, introducing beneficial dopant bonds and active species, lowering impedance and increasing capacity.
Creating a composite between a non-metal-doped oxide and a second phase represents an effective route for enhancing electrocatalytic reactions relevant to energy conversion. In one example, nitrogen is introduced into MoO2 and the resulting N-MoO2 is coupled with metallic copper to yield an alkaline hydrogen evolution catalyst. Relative to bare MoO2 and bare Cu, the N-MoO2/Cu heterostructure exhibits superior activity. Nitrogen incorporation generates new active sites and perturbs the electronic framework, leading to higher intrinsic HER kinetics. Shifts in Mo 3d and Cu 2p binding energies indicate electron migration from N-MoO2 toward Cu, establishing an interface that accelerates charge transfer. Intimate contact between MoO2and Cu creates a morphology that exposes abundant active sites. The electrochemical surface area of N-MoO2/Cu exceeds that of all reference samples, while its charge-transfer resistance and Tafel slope are markedly lower, confirming faster reaction kinetics [108]. A related strategy employs nitrogen-doped NiCo2S4/CoO to build multilayer hollow heterojunction microspheres for the oxygen evolution reaction in zinc-air batteries. Relative to undoped NiCo2S4/CoO and to conventional RuO2, the N-doped hollow spheres deliver significantly enhanced performance. Nitrogen incorporation modifies the electronic structure and increases the density of active sites. Changes in the oxidation states of Ni and Co reveal the formation of metal-nitrogen bonds that promote electron transfer. Electron microscopy images show a multilayer hollow architecture that provides a large surface area and numerous active sites while facilitating charge migration. The Nyquist plot of N-NiCo2S4/CoO displays the smallest arc, indicating high electrical conductivity. Polarization curves place its OER over-potential well below that of reference catalysts, and long-term cycling tests confirm excellent durability [109]. These studies demonstrate that coupling non-metal-doped oxides with complementary phases offers a powerful approach to tune electronic structure, increase active-site density and optimize architecture, leading to substantial advances in electrocatalytic activity and stability.
7. Conclusion Challenges and Future Perspectives
This review systematically studies the research progress of non-metallic doping in multinary metal oxides and clearly identifies this strategy as a core approach to address the intrinsic limitations of multinary metal oxides, including wide band gaps, low electronic conductivity, and insufficient density of active sites. The crystal structures of multinary metal oxides provide structural platforms for doping modification and also determine the selectivity and regulatory potential of doping sites. Mature preparation techniques such as sol–gel, chemical vapor deposition, hydrothermal synthesis, electrospinning annealing, and impregnation post treatment enable the controllable introduction of non-metallic dopants; meanwhile, a multi-dimensional characterization system consisting of XPS, XRD, EPR, and other techniques forms an evidence chain for verifying elemental existence, structural defects, and electronic properties, providing systematic evidence for doping effects. Through multiple modes, including O site substitution, B site substitution, interstitial doping, mixed doping, non-metallic doping induces lattice reconstruction, e.g., phase transition of S-doped SrCoO3−δ, defect engineering, e.g., oxygen vacancy introduction via P doping, and electronic structure modulation, e.g., S 3p O 2p orbital hybridization in SrTiO3, which synergistically enhance the conductivity, catalytic activity, and stability of materials. Relevant breakthroughs have been validated in application scenarios such as energy storage, e.g., F-doped Li4Ti5O12 with a 17-fold increase in lithium ion diffusion coefficient, and catalysis, e.g., P-doped SrFeO3−δ reduced the polarization resistance of solid oxide fuel cells by 42.86%, laying a solid foundation for the development of low-carbon energy materials.
In summary, this review systematically consolidates the advancements, mechanisms, and applications of non-metallic doping in multinary metal oxides, offering invaluable theoretical and experimental guidance for the development of high-performance energy materials. Its integration of structural regulation, electronic modulation, and performance verification not only enriches the fundamental understanding of non-metallic doping but also provides a replicable research template for other functional oxide systems. As the global pursuit of carbon neutrality accelerates, non-metallic doping will remain a cost-effective and versatile strategy to unlock the intrinsic potential of multinary metal oxides—driving innovations in batteries, fuel cells, electrocatalysis, and photocatalysis. Despite existing challenges in precise doping control and long-term stability, the continuous refinement of synthesis techniques and deepening of mechanism research will propel these doped materials to play an irreplaceable role in building a sustainable, low-carbon energy ecosystem. Non-metallic doping in multinary metal oxides faces significant challenges, primarily reflected in the precise control of doping sites and ratios. These oxides possess complex crystal lattice structures including A-sites, B-sites, and O-sites, which makes it difficult to achieve the precise occupancy of dopant atoms at target sites. Maintaining structural integrity and catalytic activity during long-term service remains a bottleneck in energy storage and conversion systems.
Author Contributions
Conceptualization, Y.K. and Z.W.; methodology, Z.W.; validation, Z.W.; investigation, Z.W.; resources, Z.W.; data curation, Z.W.; writing—original draft preparation, Z.W.; writing—review and editing, J.G.; visualization, J.G.; supervision, Y.K. and J.G.; project administration, Y.K.; funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (NSFC, Grant No. 22379153), Ningbo Key Research and Development Project (2023Z147), Future Network Special Program of CAS (181GJHZ2024074FN), and Ningbo 3315 Program.
Data Availability Statement
The data supporting the conclusions of this review are summarized from published works. Please refer to the original works for further information. No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bui, T.H.; Shin, J.H. Perovskite Materials for Sensing Applications: Recent Advances and Challenges. Microchem. J. 2023, 191, 108924. [Google Scholar] [CrossRef]
- Yang, W.-D.; Zhao, R.-D.; Guo, F.-Y.; Xiang, J.; Loy, S.; Liu, L.; Dai, J.-Y.; Wu, F.-F. Interface Engineering of Hybrid ZnCo2O4@Ni2.5Mo6S6.7 Structures for Flexible Energy Storage and Alkaline Water Splitting. Chem. Eng. J. 2023, 454, 140458. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, S.; Yan, Y.; Yang, Y.; Luther, J.M.; Wei, S.-H.; Parilla, P.; Zhu, K. Electronic Structure and Optical Properties of α-CH3NH3PbBr3 Perovskite Single Crystal. J. Phys. Chem. Lett. 2015, 6, 4304–4308. [Google Scholar] [CrossRef]
- Zhuang, G.; Chen, Y.; Zhuang, Z.; Yu, Y.; Yu, J. Oxygen Vacancies in Metal Oxides: Recent Progress towards Advanced Catalyst Design. Sci. China Mater. 2020, 63, 2089–2118. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, X.; Wu, X.; Feng, S.; Huang, K. Synthesis and Advantages of Spinel-Type Composites. Mater. Chem. Front. 2023, 7, 5288–5308. [Google Scholar] [CrossRef]
- Taffa, D.H.; Dillert, R.; Ulpe, A.C.; Bauerfeind, K.C.L.; Bredow, T.; Bahnemann, D.W.; Wark, M. Photoelectrochemical and Theoretical Investigations of Spinel Type Ferrites (MxFe3−xO4) for Water Splitting: A Mini-Review. J. Photonics Energy 2016, 7, 012009. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-Type Materials Used for Gas Sensing: A Review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Yan, J.; Yi, X.; Zhao, W.; Zheng, Y. Nonmetallic Phosphorus-Driven Oxygen Vacancy and Electronic Structure Modulation Enhancing Bifunctional Catalytic Activity of Cobalt-Based Spinel Air Electrode for Rechargeable Zn–Air Batteries. ACS Appl. Mater. Interfaces 2025, 17, 47057–47069. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Yang, Y.; Li, C.; Shi, Z.; Feng, S. Manipulation of Electrochemical Surface Reconstruction on Spinel Oxides for Boosted Water Oxidation Reaction. ACS Catal. 2025, 15, 8361–8389. [Google Scholar] [CrossRef]
- Wen, J.; Rong, K.; Jiang, L.; Wen, C.; Wu, B.; Sa, B.; Qiu, Y.; Ahuja, R. Copper-Based Perovskites and Perovskite-like Halides: A Review from the Perspective of Molecular Level. Nano Energy 2024, 128, 109802. [Google Scholar] [CrossRef]
- Grabowska, E. Selected Perovskite Oxides: Characterization, Preparation and Photocatalytic Properties—A Review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Chuangchote, S.; Roongraung, K. Perovskite Materials for Hydrogen Production. In Materials for Hydrogen Production, Conversion, and Storage; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 539–573. ISBN 978-1-119-82958-4. [Google Scholar]
- Liu, D.; Zhou, P.; Bai, H.; Ai, H.; Du, X.; Chen, M.; Liu, D.; Ip, W.F.; Lo, K.H.; Kwok, C.T.; et al. Development of Perovskite Oxide-Based Electrocatalysts for Oxygen Evolution Reaction. Small 2021, 17, 2101605. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sawada, S. The Study on Substances Having the Ilmenite Structure I. Physical Properties of Synthesized FeTiO3 and NiTiO3 Ceramics. J. Phys. Soc. Jpn. 1956, 11, 496–501. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, E.S. Dependence of Microwave Dielectric Properties on the Complex Substitution for Ti-Site of MgTiO3 Ceramics. Ceram. Int. 2017, 43, S326–S333. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Y.; Zhang, R. Ilmenite Structure-Type β-CdSnO3 Used as an Ethanol Sensing Material. Mater. Lett. 1995, 23, 69–71. [Google Scholar] [CrossRef]
- Ermawati, F.U. The Use of Liquid Mixing Method to Prepare Ilmenite Structure-Based Ceramic Powders. J. Phys. Conf. Ser. 2020, 1491, 012004. [Google Scholar] [CrossRef]
- Hall, J.N.; Kropf, A.J.; Delferro, M.; Bollini, P. Kinetic and X-Ray Absorption Spectroscopic Analysis of Catalytic Redox Cycles over Highly Uniform Polymetal Oxo Clusters. ACS Catal. 2023, 13, 5406–5427. [Google Scholar] [CrossRef]
- Huang, Z.-W.; Hu, K.-Q.; Mei, L.; Wang, D.-G.; Wang, J.-Y.; Wu, W.-S.; Chai, Z.-F.; Shi, W.-Q. Encapsulation of Polymetallic Oxygen Clusters in a Mesoporous/Microporous Thorium-Based Porphyrin Metal–Organic Framework for Enhanced Photocatalytic CO2 Reduction. Inorg. Chem. 2022, 61, 3368–3373. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x= 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) Cathode Material for Lithium-Ion Batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, M.; Li, J.; Lu, Y.; Wang, L.; Wang, Z.; Zhang, X.; Ding, X. Modulation of Electronic Structure and Oxygen Vacancies of Perovskites SrCoO3−δ by Sulfur Doping Enables Highly Active and Stable Oxygen Evolution Reaction. Electrochim. Acta 2021, 390, 138872. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, J.; Lu, Y.; Li, S.; Han, S.; Liu, C.; Ma, Z. Non-Metallic Phosphorus-Induced Lattice Engineering in LaFeO3 Perovskite: Oxygen Vacancy Modulation for Enhanced Methane Conversion in Chemical Looping Partial Oxidation. Chem. Eng. J. 2025, 520, 165963. [Google Scholar] [CrossRef]
- Cai, F.; Sun, C.; Sun, Z.; Lai, Y.; Ding, H. Sulfur-Functionalized CoMn2O4 as a Fenton-like Catalyst for the Efficient Rhodamine B Degradation. Appl. Surf. Sci. 2023, 623, 157044. [Google Scholar] [CrossRef]
- Li, Z.; Lv, L.; Wang, J.; Ao, X.; Ruan, Y.; Zha, D.; Hong, G.; Wu, Q.; Lan, Y.; Wang, C.; et al. Engineering Phosphorus-Doped LaFeO3−δ Perovskite Oxide as Robust Bifunctional Oxygen Electrocatalysts in Alkaline Solutions. Nano Energy 2018, 47, 199–209. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Wang, X.; Jia, L.; Jiang, H.; Huang, M.; Toghan, A. Nitrogen-Doped Sr2Fe1.5Mo0.5O6-δ Perovskite as an Efficient and Stable Catalyst for Hydrogen Evolution Reaction. Mater. Today Energy 2021, 20, 100695. [Google Scholar] [CrossRef]
- Aslam, A.; Abid, M.Z.; Rafiq, K.; Rauf, A.; Hussain, E. Tunable Sulphur Doping on CuFe2O4 Nanostructures for the Selective Elimination of Organic Dyes from Water. Sci. Rep. 2023, 13, 6306. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xing, Y.; Liu, H.; Jin, X.; Kou, B.; Ni, G. Electronic Structure Modulation and Peroxymonosulfate Activation Mechanism of N-Doped MnCo2O4: A Study on the Efficient Catalytic Degradation of Sulfamethoxazole. Appl. Surf. Sci. 2025, 685, 161976. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Dual Strategies to Construct Electrospun NiCo2O4/Boron-Doped LaCoO3 Heterojunction Catalysts to Activate Peroxymonosulfate for Organic Pollutant Degradation. J. Alloys Compd. 2023, 968, 172184. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Hu, X.; Wu, X.; Chen, W.; Zhou, S.; Li, J.; Qi, C.; Ma, D.-K. Fluorine-Doped CuBi2O4 Nanorod Arrays for Enhanced Photoelectrochemical Oxygen Reduction Reaction toward H2O2 Production. J. Catal. 2022, 410, 339–346. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Zhao, Q.; Abudula, A.; Ma, Y.; Yan, K.; Guan, G. Tuning Octahedron Sites in MnFe2O4 Spinel by Boron Doping for Highly Efficient Seawater Splitting. Appl. Catal. B Environ. 2023, 330, 122577. [Google Scholar] [CrossRef]
- Kavitha, S.; Kurian, M. Acetylation of Glycerol for Triacetin Production over Metal/Non-Metal Doped Cobalt Ferrite Nanocatalysts. Braz. J. Chem. Eng. 2024, 41, 399–407. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Zhang, X.; Wang, J.; Nie, P.; Che, Q.; Ding, B. Nitrogen-Doped Carbon Coated Li4Ti5O12 Nanocomposite: Superior Anode Materials for Rechargeable Lithium Ion Batteries. J. Power Sources 2013, 221, 122–127. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Niu, B.; Wang, L.; Li, M.; Yao, W.; Zang, K.; Zhou, L.; Hu, X.; Zheng, Y. Lattice B-Doping Evolved Ferromagnetic Perovskite-like Catalyst for Enhancing Persulfate-Based Degradation of Norfloxacin. J. Hazard. Mater. 2022, 425, 127949. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Di, H.; Yang, M.; Yang, G.; Wang, W.; Ran, R.; Zhou, W. High-Performance Phosphorus-Doped SrCo0.8Fe0.2O3−δ Cathode for Protonic Ceramic Fuel Cells. Ceram. Int. 2024, 50, 40409–40416. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-Metal Fluorine Doping in Ruddlesden–Popper Perovskite Oxide Enables High-Efficiency Photocatalytic Water Splitting for Hydrogen Production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Środoń, J.; Andreoli, C.; Elsass, F.; Robert, M. Direct High-Resolution Transmission Electron Microscopic Measurement of Expandability of Mixed-Layer Illite/Smectite in Bentonite Rock. Clays Clay Miner. 1990, 38, 373–379. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, H.; Dai, X.; Nie, F.; Ren, Z.; Yin, X.; Gan, Y.; Wu, B.; Cao, Y.; Zhang, X. Phosphorus-Doping Induced Electronic Modulation of CoS2–MoS2 Hollow Spheres on MoO2 Film-Mo Foil for Synergistically Boosting Alkaline Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 33388–33396. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, H.; Xiao, Y.; Hu, H.; Cai, Y.; Liang, Y.; Sun, L.; Liu, Y.; Zheng, M. Three-Dimensional Nitrogen-Doped Graphene as Binder-Free Electrode Materials for Supercapacitors with High Volumetric Capacitance and the Synergistic Effect between Nitrogen Configuration and Supercapacitive Performance. Electrochim. Acta 2016, 218, 32–40. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect Engineering in Photocatalytic Materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Xie, S.; Sun, W.; Sun, J.; Wan, X.; Zhang, J. Apparent Symmetry Rising Induced by Crystallization Inhibition in Ternary Co-Crystallization-Driven Self-Assembly. Nat. Commun. 2023, 14, 6496. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Wu, H. Development of High Efficient Visible Light-Driven N, S-Codoped TiO2 Nanowires Photocatalysts. Appl. Surf. Sci. 2015, 328, 335–343. [Google Scholar] [CrossRef]
- Wen, C.Z.; Hu, Q.H.; Guo, Y.N.; Gong, X.Q.; Qiao, S.Z.; Yang, H.G. From Titanium Oxydifluoride (TiOF2) to Titania (TiO2): Phase Transition and Non-Metal Doping with Enhanced Photocatalytic Hydrogen (H2) Evolution Properties. Chem. Commun. 2011, 47, 6138–6140. [Google Scholar] [CrossRef]
- Chaitra, U. Impact of Defect Sites on the Raman Scattering Properties of Nitrogen Doped ZnO Thin Films. Inorg. Chem. Commun. 2021, 131, 108784. [Google Scholar] [CrossRef]
- Bartasyte, A.; Chaix-Pluchery, O.; Kreisel, J.; Santiso, J.; Margueron, S.; Boudard, M.; Jimenez, C.; Abrutis, A.; Weiss, F. Raman Spectroscopy and X-Ray Diffraction Studies of Stress Effects in PbTiO/Sub 3/Thin Films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 2623–2631. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-Free Synthesis of 2D Porous Ultrathin Nonmetal-Doped g-C3N4 Nanosheets with Highly Efficient Photocatalytic H2 Evolution from Water under Visible Light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Cui, K.; Liu, Y.; Lv, L. Mn and P Co-Doped Biochar Catalyst for Persulfate Efficient Degradation of Tetracycline hydrochloride: Process and Mechanism. J. Environ. Chem. Eng. 2024, 12, 114228. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Huang, Y.; Zhou, Z.; Shen, S. Reduction of Oxygen Vacancy and Enhanced Efficiency of Perovskite Solar Cell by Doping Fluorine into TiO2. J. Alloys Compd. 2016, 681, 191–196. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Fang, W. Enhanced Visible-Light Active C and Fe Co-Doped LaCoO3 for Reduction of Carbon Dioxide. Catal. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Liu, Y.; Zhong, Y.; Xu, X.; Sun, Y.; Wang, J.; Zhou, W.; Shao, Z. An Intrinsically Conductive Phosphorus-Doped Perovskite Oxide as a New Cathode for High-Performance Dye-Sensitized Solar Cells by Providing Internal Conducting Pathways. Sol. RRL 2019, 3, 1900108. [Google Scholar] [CrossRef]
- Helmy, E.T.; Nemr, A.E.; Arafa, E.; Eldafrawy, S.; Mousa, M. Photocatalytic Degradation of Textile Dyeing Wastewater under Visible Light Irradiation Using Green Synthesized Mesoporous Non-Metal-Doped TiO2. Bull. Mater. Sci. 2021, 44, 30. [Google Scholar] [CrossRef]
- Naik, S.S.; Lee, S.J.; Yeon, S.; Yu, Y.; Choi, M.Y. Pulsed Laser-Assisted Synthesis of Metal and Nonmetal-Codoped ZnO for Efficient Photocatalytic Degradation of Rhodamine B under Solar Light Irradiation. Chemosphere 2021, 274, 129782. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Goswami, T.; Yadav, D.K.; Bhatt, H.; Kaur, G.; Shukla, A.; Babu, K.J.; Ghosh, H.N. Defect-Mediated Slow Carrier Recombination and Broad Photoluminescence in Non-Metal-Doped ZnIn2S4 Nanosheets for Enhanced Photocatalytic Activity. J. Phys. Chem. Lett. 2021, 12, 5000–5008. [Google Scholar] [CrossRef]
- El-Hosainy, H.M.; El-Sheikh, S.M.; Ismail, A.A.; Hakki, A.; Dillert, R.; Killa, H.M.; Ibrahim, I.A.; Bahnemann, D.W. Highly Selective Photocatalytic Reduction of O-Dinitrobenzene to o-Phenylenediamine over Non-Metal-Doped TiO2 under Simulated Solar Light Irradiation. Catalysts 2018, 8, 641. [Google Scholar] [CrossRef]
- Hoy-Benítez, J.A.; Colina-Ruiz, R.A.; Lezama-Pacheco, J.S.; Mustre de León, J.; Espinosa-Faller, F.J. Local Atomic Structure and Lattice Defect Analysis in Heavily Co-Doped ZnS Thin Films Using X-Ray Absorption Fine Structure Spectroscopy. J. Phys. Chem. Solids 2020, 136, 109154. [Google Scholar] [CrossRef]
- Avashthi, G.; Maktedar, S.S.; Singh, M. Scrutinizing XAFS Spectroscopy and Biocompatibility of N-Doped Edge-Functionalized Graphene Oxide. Acta Crystallogr. Sect. Found. Adv. 2017, 73, C690. [Google Scholar] [CrossRef]
- Wang, D.; Guo, X.; Zhang, G.; Liu, Y.; Liu, S.; Zhang, Z.; Chai, Y.; Chen, Y.; Zhang, J.; Sun, B. SnO2 Electron Transport Layer Modified by F/N-Doped Graphdiyne and in Situ XRD and in Situ XAFS Exploration on Its Effect on Perovskite Active Layer. Nano Today 2023, 50, 101852. [Google Scholar] [CrossRef]
- Wen, J.; Li, N.; Shi, Q.; Wu, H.; Feng, X.; Wang, C.; Zhang, J. First-Principles Calculations to Investigate Electronic Structures and Magnetic Regulation of Non-Metallic Elements Doped BP with Point Defects. J. Mol. Graph. Model. 2023, 118, 108370. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; He, X.; Wei, Q.; Zhang, J.; Wei, B.; Li, M. Effects of Non-Metal Doping in Graphene-like Structure: Engineering Narrow Bandgaps and Reserved Carrier Mobility. J. Alloys Compd. 2025, 1035, 181578. [Google Scholar] [CrossRef]
- Fan, C.; Zang, Z.; Zhang, X. Non-Metal Doping Regulation in Transition Metal and Their Compounds for Electrocatalytic Water Splitting. Int. J. Hydrogen Energy 2024, 56, 1273–1283. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; He, S.; Tjiu, W.W.; Pan, J.; Xia, Y.-Y.; Liu, T. Nitrogen-Doped Graphene Nanoribbons as Efficient Metal-Free Electrocatalysts for Oxygen Reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, B.; Yang, Y.; Zhao, M.; Fang, Y.; Cui, Y.; Tian, J. Enhanced Electrocatalytic Performance of P-Doped MoS2/rGO Composites for Hydrogen Evolution Reactions. Molecules 2025, 30, 1205. [Google Scholar] [CrossRef]
- Preetha, R.; Govinda raj, M.; Vijayakumar, E.; Narendran, M.G.; Varathan, E.; Neppolian, B.; Jeyapaul, U.; Bosco, A.J. Promoting Photocatalytic Interaction of Boron Doped Reduced Graphene Oxide Supported BiFeO3 Nanocomposite for Visible-Light-Induced Organic Pollutant Degradation. J. Alloys Compd. 2022, 904, 164038. [Google Scholar] [CrossRef]
- Angineni, R.; Venkataswamy, P.; Veldurthi, N.K.; Ramaswamy, K.; Sudheera, M.; Vithal, M. Photocatalytic Degradation Studies of Carbon and Sulfur-Doped K2Ta2O6. J. Mater. Sci. Mater. Electron. 2023, 34, 633. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-Doped Graphene and Its Electrochemical Applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Han, Z.; Wang, Z.; Huang, X.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Nonmetal Doping as a Robust Route for Boosting the Hydrogen Evolution of Metal-Based Electrocatalysts. Chem.–Eur. J. 2020, 26, 3930–3942. [Google Scholar] [CrossRef]
- Noerochim, L.; Wibowo, A.T.; Widyastuti; Subhan, A.; Prihandoko, B.; Caesarendra, W. Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries 2022, 8, 5. [Google Scholar] [CrossRef]
- Sun, N.; Zeng, Z.; Zhang, L.; Li, L.; Song, C.; Yang, J. Boron-Doping P2/O3 Biphasic Na0.95Mn0.6Ni0.4O2 for High Performance Cathode Materials of Sodium-Ion Batteries. J. Energy Storage 2025, 135, 118379. [Google Scholar] [CrossRef]
- Mahara, Y.; Oka, H.; Nonaka, T.; Kosaka, S.; Takahashi, N.T.; Kondo, Y.; Makimura, Y. Activation and Stabilization of Mn-Based Positive Electrode Materials by Doping Nonmetallic Elements. Adv. Energy Mater. 2023, 13, 2301843. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, S.; Li, X.; Shi, J.; Mao, Y.; Wang, M.; Tan, F. P-Doped NiMn2O4 Hollow Tubular Nanofiber Spinel Composites for Electrocatalytic Dechlorination. ACS Appl. Nano Mater. 2024, 7, 1713–1722. [Google Scholar] [CrossRef]
- Liu, H.-J.; Zhang, S.; Fan, R.-Y.; Liu, B.; Lv, R.-Q.; Chai, Y.-M.; Dong, B. Activated M,S Co-Doping (M = Ni, Co, Mn) Inverse Spinel Oxides with Mixed Mechanisms for Water Oxidation. Appl. Catal. B Environ. 2024, 343, 123567. [Google Scholar] [CrossRef]
- Yuan, B.; Lai, F.; Yan, R.; Yu, Q.; Wang, T.; Gao, H.; Hao, R. Nonmetallic Boron-Doped LaMnO3 with Synergistic Enhancement in Oxidation Activity and Sulfur Resistance for Elemental Mercury Removal. Ceram. Int. 2025, 51, 43626–43636. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.; Jing, Y.; Yao, Y.; Ma, J.; Sun, J. Effect of Non-Metal Elements (B, C, N, F, P, S) Mono-Doping as Anions on Electronic Structure of SrTiO3–ScienceDirect. Comput. Mater. Sci. 2013, 79, 69–74. [Google Scholar] [CrossRef]
- Xing, T.; Ouyang, Y.; Chen, Y.; Zheng, L.; Wu, C.; Wang, X. P-Doped Ternary Transition Metal Oxide as Electrode Material of Asymmetric Supercapacitor. J. Energy Storage 2020, 28, 101248. [Google Scholar] [CrossRef]
- Wang, J.; Asakura, Y.; Hasegawa, T.; Yin, S. High-Concentration N-Doped La2Ti2O7 Nanocrystals: Effects of Nano-Structuration and Doping Sites on Enhancing the Photocatalytic Activity. Chem. Eng. J. 2021, 423, 130220. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, Y.; Wang, Z.; Mao, W.; Weng, Y.; Zhang, J.; Pu, Y.; Yang, J.; Li, X. Effect of Non-Metallic X(X=F, N, S) and Cr Co-Doping on Properties of BiFeO3: A First-Principles Study. Phys. Lett. A 2019, 383, 383–388. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Time-Dependent Evolution of the Nitrogen Configurations in N-Doped Graphene Films. RSC Adv. 2016, 6, 106914–106920. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Zhao, Y.; Zeng, X. Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light. Int. J. Mol. Sci. 2022, 23, 10927. [Google Scholar] [CrossRef]
- Quyyum, U.; Rafiq, K.; Abid, M.Z.; Ahmad, F.; Rauf, A.; Hussain, E. Tunable Sulphur Doping in CuFe2O4 for the Efficient Removal of Arsenic through Arsenomolybdate Complex Adsorption: Kinetics, Isothermal and Mechanistic Studies. Environ. Sci. Water Res. Technol. 2023, 9, 1147–1160. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Zhao, S.-N.; Wu, Y.; Liu, C.; Huang, J.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; et al. A Dual-Site Doping Strategy for Developing Efficient Perovskite Oxide Electrocatalysts towards Oxygen Evolution Reaction. Nano Energy 2022, 99, 107344. [Google Scholar] [CrossRef]
- Bu, X.; Li, J.; Wang, J.; Li, Y.; Zhang, G. Boosting Charge Transfer Promotes Photocatalytic Peroxymonosulfate Activation of S-Doped CuBi2O4 Nanorods for Ciprofloxacin Degradation: Key Role of Ov–Cu–S and Mechanism Insight. Chem. Eng. J. 2024, 494, 153075. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, M.; Qin, J.; Zhou, H.; Ding, Z. Reinforced Photocatalytic Reduction of CO2 to CO by a Ternary Metal Oxide NiCo2O4. Phys. Chem. Chem. Phys. 2015, 17, 16040–16046. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xiang, X.; Liu, Y.; Yang, X.; Shi, N.; Xu, D.; Zhou, C.; Han, M.; Bao, J.; Huang, W. Defect-Rich High-Entropy Spinel Oxide as an Efficient and Robust Oxygen Evolution Catalyst for Seawater Electrolysis. SusMat 2025, 5, e70010. [Google Scholar] [CrossRef]
- Noerochim, L.; Prabowo, R.S.; Widyastuti, W.; Susanti, D.; Subhan, A.; Idris, N.H. Enhanced High-Rate Capability of Iodide-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries 2023, 9, 38. [Google Scholar] [CrossRef]
- Ballal, R.S.; Kalubarme, R.S.; Jayswal, M.S.; Chothe, U.P.; Kulkarni, M.V.; Kale, B.B. N-Doped Lithium Titanium Oxide for High Power Li-Ion Rechargeable Batteries with Excellent Cyclability. Inorg. Chem. Commun. 2024, 161, 112013. [Google Scholar] [CrossRef]
- Zhu, S.; Du, C.; Zhang, J.; Zhang, J. The Effects of Fluorine Doping on the Structure, Interface and Electrochemical Properties of Lithium Titanate. Ceram. Int. 2024, 50, 25095–25102. [Google Scholar] [CrossRef]
- Bai, L.; Pan, B.; Song, F.; Chen, Q. Co/S Co-Doped Li4Ti5O12 as Lithium-Ion Batteries Anode for High-Rate. J. Energy Storage 2023, 73, 109155. [Google Scholar] [CrossRef]
- Huth, N. New Synthetic Pathways for Carbon-Doped Oxides for Battery and Related Applications. Ph.D. Thesis, The University of St Andrews, St. Andrews, Scotland, 2024. [Google Scholar]
- Liu, D.; Xu, W.; Zheng, D.; Wang, Y.; Wang, F.; Zhou, L.; Nie, Z.; Lu, X. Sulfur Doped NiCo2O4 Nanosheets as Advanced Cathode for Flexible Alkaline Zn Batteries. J. Power Sources 2023, 571, 233088. [Google Scholar] [CrossRef]
- Ran, J.; Wang, L.; Si, M.; Liang, X.; Gao, D. Tailoring Spin State of Perovskite Oxides by Fluorine Atom Doping for Efficient Oxygen Electrocatalysis. Small Weinh. Bergstr. Ger. 2023, 19, e2206367. [Google Scholar] [CrossRef]
- Mondal, S.; Sarkar, S.; Riyaz, M.; Kar, M.; Fortuin, A.C.; Vashishth, S.; Das, R.; Eswaramoorthy, M.; Kramer, D.; Peter, S.C. Nitrogen Doping-Induced Structural Distortion in LaMnO3 Enhances Oxygen Reduction and Oxygen Evolution Reactions. ACS Energy Lett. 2024, 9, 3440–3447. [Google Scholar] [CrossRef]
- Huan, D.; Zhang, L.; Zhu, K.; Li, X.; Peng, R.; Ding, D.; Xia, C. Improving Ruddlesden-Popper Electrocatalysts through Interstitial Fluorination-Driven Rearrangements of Local Coordination Environment. Sustain. Mater. Technol. 2023, 38, e00754. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; He, C.; Wang, Y.; Ni, J.; Ni, C. Unraveling the Microstructure and Defect Chemistry in Fluorine-Doped Cathode for a Protonic Solid Oxide Fuel Cell. Chem. Eng. J. 2025, 505, 159774. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, C.; Zhang, Z.; Xia, B.; Zhang, H.; Ding, J.; Zhang, L.; Zhang, W.; Lang, X.; Cai, K. Non-Metal Doping Enhsances Oxygen Reduction Kinetics and CO2 Tolerance of SrFeO3−δ Perovskite as High-Performance Cathodes for Solid Oxide Fuel Cells. Fuel 2024, 378, 132917. [Google Scholar] [CrossRef]
- Feng, W.; Pu, W.; Zheng, Y.; Wu, H.; Li, L.; Wei, X. Excellent Rate Capability Supercapacitor Electrodes with Highly Hydroxyl Ion Adsorption Capacity Enabled by P-Doped MnCo2O4 Nanotube Arrays. Appl. Surf. Sci. 2022, 599, 153908. [Google Scholar] [CrossRef]
- Farooq, A.; Khalil, S.; Basha, B.; Habib, A.; Al-Buriahi, M.S.; Warsi, M.F.; Yousaf, S.; Shahid, M. Electrochemical Investigation of C-Doped CoFe2O4/Fe2O3 Nanostructures for Efficient Electrochemical Water Splitting. Int. J. Hydrogen Energy 2024, 51, 1318–1332. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Chen, H.; Yang, Y.; Wang, D.; Shang, H.; Zhang, B. Phosphorus and Sulfur Co-Doped Nickel Molybdate with Rich-Oxygen Vacancies for Efficient Water Splitting. J. Colloid Interface Sci. 2025, 677, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wen, B.; Dong, Q.; Wang, P.; Lyu, X. Boron Doped CoFe2O4 with Enriched Oxygen Vacancy as Bifunctional Electrocatalysts for Overall Water Splitting. Int. J. Hydrogen Energy 2025, 105, 556–564. [Google Scholar] [CrossRef]
- Li, R.; Long, J.; Li, M.; Du, D.; Ren, L.; Zhou, B.; Zhao, C.; Xu, H.; Wen, X.; Zeng, T.; et al. Sulfur-Doped LaNiO3 Perovskite Oxides with Enriched Anionic Vacancies and Manipulated Orbital Occupancy Facilitating Oxygen Electrode Reactions in Lithium-Oxygen Batteries. Mater. Today Chem. 2022, 24, 100889. [Google Scholar] [CrossRef]
- Liang, Q.; Ouhbi, H.; Österbacka, N.; Ambrosio, F.; Wiktor, J. Defect Engineering in BiVO4 Photoanodes: The Synergistic Role of Nitrogen Doping and Oxygen Vacancy for Oxygen Evolution Reaction. J. Phys. Energy 2025, 7, 045030. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, C.; Chen, P.; Chu, X.; Zhu, L. Enhanced Photocatalytic Performance of Boron Doped Bi2WO6 Nanosheets under Simulated Solar Light Irradiation. J. Hazard. Mater. 2013, 254–255, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, N.; Liao, L.; Luo, Y.; Wang, S.; Cao, F.; Zhou, W.; Huang, D.; Chen, H. Doping β-CoMoO4 Nanoplates with Phosphorus for Efficient Hydrogen Evolution Reaction in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 37038–37045. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhang, M.; Li, M.; Li, M.; Li, J.; Zhi, L. Phosphorus-Doped Nickel Cobalt Oxide (NiCo2O4) Wrapped in 3D Hierarchical Hollow N-Doped Carbon Nanoflowers as Highly Efficient Bifunctional Electrocatalysts for Overall Water Splitting. J. Colloid Interface Sci. 2024, 668, 243–251. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kang, S.H.; Roh, K.C.; An, G.-H. Enhancing Lithium Titanate Anode Performance through Surface Modification with Fluorine and Nitrogen Co-Doped Carbon Nanotubes. J. Alloys Compd. 2024, 1004, 175768. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Wen, C.; Dong, L.; Tan, C.; Li, B.; Fan, M.; He, H.; Chen, Z. Nitrogen-Doped MoO2/Cu Heterojunction Electrocatalyst for Highly Efficient Alkaline Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2024, 66, 103–109. [Google Scholar] [CrossRef]
- He, B.; Song, J.-J.; Li, X.-Y.; Xu, C.-Y.; Li, Y.-B.; Tang, Y.-W.; Hao, Q.-L.; Liu, H.-K.; Su, Z. A Nitrogen-Doped NiCo2S4/CoO Hollow Multi-Layered Heterostructure Microsphere for Efficient Oxygen Evolution in Zn–Air Batteries. Nanoscale 2021, 13, 810–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).