Abstract
Phytoremediation is considered as a green alternative for remediating metal-contaminated soil and water, yet further efforts are needed to minimise secondary pollution after phytoremediation. This study investigates a cost-effective and sustainable method to synthesise carbon quantum dot supported on zinc oxide (CQD-ZnO) composites using extracted zinc (Zn) from post-phytoremediated plants, plant extracts, and CQDs derived from water hyacinth (Eichhornia crassipes) for the sonocatalytic degradation of malachite green. The CQD-ZnO materials were characterised by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), Brunauer–Emmett–Teller (BET) surface analysis, and ultraviolet–visible (UV–Vis) spectroscopy to confirm their crystalline structure, morphology, functional groups, surface area, and optical properties. The composites exhibited disaggregation of agglomerates, high crystallinity, and increased carbon content due to the addition of CQDs containing phenolic functional groups (e.g., polyphenols, flavonoids) from the plant extract. The highest sonocatalytic degradation efficiency (84.52%) was achieved after 90 min of treating 10 ppm malachite green using 1 g/L of the CQD-ZnO composite at a natural pH, with 300 W ultrasonic power at 25 kHz. This study paves the way for the development of environmentally friendly, high-performance sonocatalysts from post-phytoremediated plants for wastewater treatment applications.
1. Introduction
Phytoremediation has emerged as an environmentally friendly and cost-effective bioremediation method that employs terrestrial or aquatic plants to remove pollutants from heavy metal-contaminated soil and water environments [1]. The bioaccumulation of heavy metal ions takes place in different parts of the plant, including the roots, stems, leaves, flowers, and fruits [2]. Durairaj [3] reported that Eichhornia crassipes can remove up to 88.3% of zinc from the electroplating industry’s wastewater with an optimum contact time of 22 days. Besides heavy metal removal, phytoremediation also offers advantages such as promoting biodiversity, reducing erosion, minimising energy consumption, and improving soil structure and fertility [4,5]. However, secondary contamination may arise from improper biomass management during the post-phytoremediation process. As the biomass decomposes, the accumulated heavy metals leach back into the surrounding soils or water sources, posing environmental and health risks to ecosystems and the food chain [6]. Hence, phytoremediated plants are classified as special waste and require a specific disposal method that leads to additional disposal costs [4].
The conventional disposal method of biomass, such as incineration and composting, poses a risk of generating secondary pollution [7]. In this context, metal recovery from plant biomass provides a sustainable solution to convert the metal-contaminated biomass waste into high-value nanoparticles, aligning with the circular economy principles. Green synthesis of metal oxide nanoparticles has been recognised for its reduced toxicity, low energy consumption, and biocompatibility, in comparison with the chemical-mediated synthesis of metal oxide nanoparticles, which use hazardous reagents and is energy-intensive [8]. Plant-mediated synthesis of the nanoparticles uses the bottom-up approach, and constructs nanoparticles by integrating the simpler, atomic-level substances into nanoscale particles [9]. Phytochemicals present in the plant extracts, such as flavonoids, alkaloids, terpenoids, and phenolic compounds, serve as natural reducing and stabilising agents, facilitating the synthesis of metal oxide nanoparticles [10]. Green synthesis employs sustainable plant resources, aligning closely with green chemistry principles. In addition to green synthesis, various advanced degradation technologies have been developed to address the limitations of conventional wastewater treatment methods; one such technique is the catalytic degradation approach reported by [11], which demonstrates alternative pathways for efficient detoxification of chlorinated organic pollutants.
Zinc oxide (ZnO) is a well-known semiconductor material with promising applications in solar-driven catalysis, water treatment, and air remediation [12]. It is a promising alternative to titanium dioxide (TiO2) photocatalysts due to its nontoxicity, distinct photoelectric characteristics, strong catalytic activity, and electron transfer capabilities [13,14,15]. However, ZnO has certain drawbacks that hinder its broader application. Its wide band gap (3.37 eV) restricts light absorption to the ultraviolet (UV) region, which accounts for only around 3–5% of the solar spectrum [16]. Moreover, the rapid recombination of the electron–hole pair and the occurrence of photocorrosion in the aqueous environment further limit its photocatalytic activity [17]. To overcome these issues, various modifications to ZnO nanoparticles have been developed, including surface modification, crystallinity enhancement, sensitiser integration, and doping or co-doping with metals and non-metals to improve its photocatalytic performance [18].
Among the modification strategies, the coupling of ZnO with carbon quantum dots (CQDs) has gained considerable attention from many researchers due to its unique properties. CQDs are nanoscale carbon-based materials, typically less than 10 nm in size [19]. CQDs have high biocompatibility, exceptional photoluminescence and electron transfer properties and ease of surface modification, allowing them to stand out in the medicine, electronics, and energy fields [20]. The introduction of CQDs in ZnO enhances light absorption, increases light utilisation, and helps in the separation of photogenerated charges [13]. The CQDs function as electron acceptors, prolonging charge carrier separation and thereby promoting photocatalytic efficiency [21]. In a study by Bazazi et al. [22], the incorporation of 20% CQDs into ZnO nanoparticles resulted in a significant enhancement in Rhodamine B (RhB) dye degradation, achieving an efficiency of 88% compared to just 37% with pure ZnO nanoparticles. This notable improvement clearly demonstrates the synergistic effect of CQDs on the photocatalytic performance of ZnO-based nanoparticles, highlighting their promising applications in environmental remediation.
In this study, ZnO catalysts were synthesised using phytoremediated plant biomass as a zinc precursor. The ZnO nanoparticles were further modified with CQDs extracted from accumulator plants to improve their sonocatalytic performance. To the best of our knowledge, a limited variety of plant extracts have been explored so far, and no studies have specifically focused on the Eichhornia crassipes-derived biosynthesis of ZnO nanoparticles coupled with CQDs. Comprehensive characterisation of the material was carried out to assess the surface morphology, chemical composition, functional group, crystallinity, surface area, optical–electronic properties, and sonocatalytic performance of the developed composites. This study examines the distinct characteristics of the biosynthesised CQD-ZnO composites and investigates their sonocatalytic activity across varying concentrations of CQD solution.
2. Results and Discussion
2.1. Characterisation of Biosynthesised ZnO
2.1.1. XRD
Figure 1a shows the XRD spectra of the biosynthesised ZnO particles with different ratios of nitric acid-digested Zn-containing solution to plant extract in the range of 2θ from 5° to 80°. The XRD patterns for the biosynthesised ZnO particles exhibited numerous intense and sharp diffraction peaks. The peaks could be observed at 2θ = 31.8°, 34.4°, 36.4°, 47.6°, 56.8°, 62.9°, and 67.8° across all ratios of biosynthesised ZnO particles. The presence of these peaks corresponded to the (100), (002), (101), (102), (110), (103), and (112) indices, respectively. Similar results were also reported in other studies of biosynthesised ZnO using Commelina beghalensis [23]. The observed planes are also consistent with the standard JCPDS data file (Card No. 89-0510) [24], which suggested the biosynthesised ZnO had a hexagonal wurtzite structure [25]. When decreasing the ratio of extracted Zn to plant extract, the intensity of these peaks was noticeably reduced. The reduction in intensity could be attributed to the increasing phytochemical content possessed by the biosynthesised ZnO particles [23]. This phenomenon occurred due to the higher concentration of plant extract compared to Zn during the biosynthesis process, which limited the crystallisation of the ZnO nanoparticles [26].
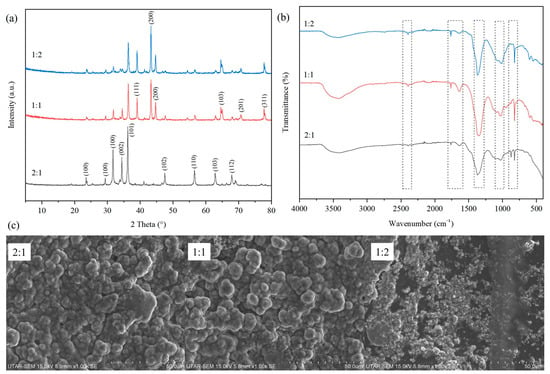
Figure 1.
(a) XRD patterns, (b) FTIR spectra, and (c) SEM images of biosynthesised ZnO with different ratios of extracted Zn to plant extract (2:1, 1:1 and 1:2).
On the other hand, the peaks at 39.1°, 43.4°, 64.5°, 70.7°, and 77.7°, assigned to planes (111), (200), (103), (201), and (311), which were in accordance with JCPDS card No. 36-1451, were notably recorded only for biosynthesised ZnO with extracted Zn-to-plant extract ratios of 1:1 or 1:2, which is attributed to the hexagonal wurtzite structure of ZnO and confirms the successful formation of crystalline ZnO in the composite. This phenomenon could be due to the excessive amount of plant extract used during the ZnO biosynthesis process which yielded large amount of residual phytochemical content. Based on the XRD characterisation results, the plant extract had a significant effect on the crystalline structure of the particles as well as the intensity of the peaks.
2.1.2. FTIR
Figure 1b shows the FTIR analysis of the biosynthesised ZnO particles with different ratios of extracted Zn to plant extract in the wavenumber range from 400 to 4000 cm−1. A broad absorption band was observed across all samples between 3200 cm−1 and 3640 cm−1, which corresponded to the stretching vibrations of O−H bonds due to the moisture content [27]. According to Neamah et al. [28], this region of O−H bonds could also be associated with the presence of phenolic compounds originating from the plant extract. Based on the results obtained, as the amount of plant extract increased, the transmittance intensity of the O–H band also increased due to a greater number of phenolic compounds present within the samples.
The peaks could be observed at 2400 cm−1, 1760 cm−1, 1630 cm−1, 1370 cm−1, 1045 cm−1, 876 cm−1, and 824 cm−1. The small absorption peak at 2400 cm−1 across all biosynthesised ZnO samples may correspond to the asymmetric stretching of atmospheric CO2 [29]. In addition, a sharp absorption peak at 1760 cm−1 was attributed to the presence of the C=O bond [30]. The C=O bonds were closely related to stabilising agents, such as polyphenols and flavonoids present within the plant extract [28]. Hence, the peak at 1760 cm−1 was found to be increasing in intensity as the ratio of extracted Zn to plant extract increased. The stretching at 1630 cm−1 was due to the C=C and C=O stretching with N–H bending in the primary amine, related to the proteins present in the plant extract [31]. On the other hand, the significant sharp absorption peak at 1370 cm−1 was attributed to the C-H bending [32]. In addition, the medium–intense absorption peak at 1045 cm−1 was attributed to the stretching vibrations of saturated primary alcohol C–O [33]. Next, the minor sharp absorption peak at 824 cm−1 was the result of C–H bond-stretching vibrations from the aromatic rings [34].
The FTIR spectra showed the participation of plant extract-derived functional groups such as aliphatic amines, nitrile groups, carboxylic compounds, and amino acids during the biosynthesis of ZnO particles, which could act as natural capping or stabilising agents, while the biomolecules such as polyphenols and proteins acted as reducing agents during the bioreduction process [35,36].
2.1.3. SEM
Figure 1c shows the surface morphologies of the biosynthesised ZnO particles with different ratios of extracted Zn to plant extract. Based on the SEM images, the particles of biosynthesised ZnO were generally agglomerated and possessed an irregular shape. This phenomenon is common in the biosynthesis of metal oxide particles, where the affinity between the particles causes them to stick together and form asymmetrical clusters during the biosynthesis process [37]. The sizes of the irregularly shaped particles were also inconsistent across the biosynthesised ZnO samples with different ratios of extracted Zn to plant extract. For example, the ZnO particles were agglomerated in different bulk sizes in Figure 1c. Meanwhile, the clusters of agglomerated ZnO particles were seemingly less bulky in size and the granular ZnO particles were also smaller in size as the ratio of extracted Zn to plant extract increased. This finding is in line with the result reported by Bopape et al. [23], whereby the higher number of phytochemicals present in the plant extract could result in the biosynthesis of smaller and more rounded ZnO particles. As the amount of plant extract added to biosynthesise ZnO was increased, the average size of the agglomerated ZnO particles tended to decrease, as shown in Figure 1c. This phenomenon might be due to the plant extract present on the surface of biosynthesised ZnO particles, which worked as a capping agent to help minimise the aggregation effect [38].
2.2. Characterisation Carbon Quantum Dot-Zinc Oxide Particles
2.2.1. SEM
Figure 2a–g illustrates the SEM images of biosynthesised ZnO, solvothermal ZnO and CQD-ZnO composites using various volumes of CQD solutions. The SEM images revealed that the solvothermal ZnO possessed a long cylindrical shape with a flower-like structure. These flower-shaped clusters of rod-like ZnO were also reported by Das et al. [39] during the microwave, hydrothermal, and microwave-assisted hydrothermal ZnO preparation method. According to Zhao et al. [40], the average diameter of the CQDs synthesised from water hyacinth via the hydrothermal method was 2.44 ± 0.57 nm, and they were uniformly dispersed without apparent aggregation. Based on Figure 2a–e, when CQDs were incorporated into the biosynthesised ZnO to fabricate CQD-ZnO composites, the aggregation effect imposed on the biosynthesised ZnO was reduced significantly. The hydrothermal method of incorporating CQD into the biosynthesised ZnO resulted in the transformation of the irregular shape of biosynthesised ZnO into a hexagonal shape [41].
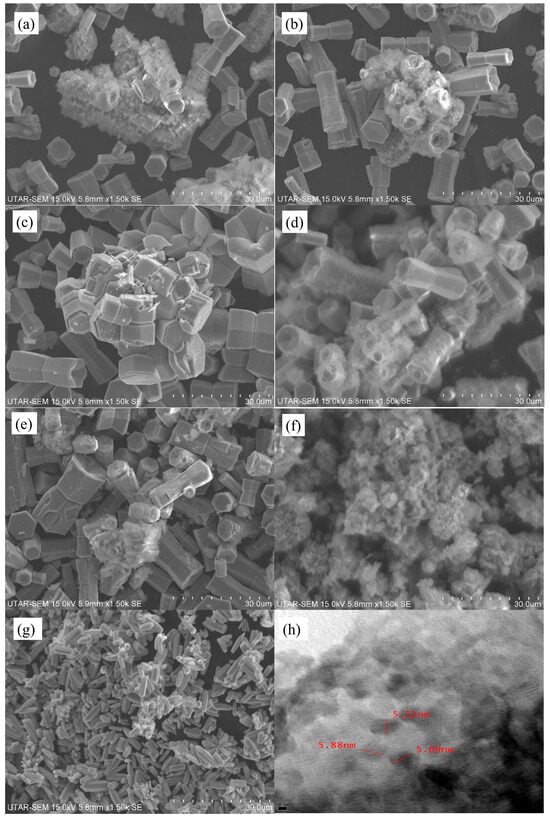
Figure 2.
SEM images of (a) 2 mL CQD-ZnO, (b) 6 mL CQD-ZnO, (c) 10 mL CQD-ZnO, (d) 14 mL CQD-ZnO, (e) 18 mL CQD-ZnO, (f) Bio ZnO, (g) Solvo ZnO, and (h) TEM image of 14 mL CQD-ZnO.
Based on Figure 2d, the CQDs were deposited on the surface of biosynthesised ZnO, forming rough surfaces on the biosynthesised ZnO. As the added CQD solution increased, the hexagon-shaped biosynthesised ZnO particles were distributed well, as shown in Figure 2e. It was revealed that the CQD solution resulted in an electrostatic force of attraction and wrapped around the ZnO surface by refilling or depleting the CQD valence band [42]. Nevertheless, the deposition of CQD on biosynthesised ZnO particles did not alter the crystallinity of the ZnO-CQD composites, which was in line with the XRD results obtained earlier [13]. Figure 2h illustrates the TEM image of the CQD-ZnO composite. It was suggested that the CQD-ZnO composite exhibited an agglomerated structure and was covered with tiny black dots. The CQDs appeared as black dots with an average diameter ranging from 5.22 to 5.88 nm and were dispersed evenly on the surface of the composite without displaying much aggregation. This finding aligns well with the findings reported by Liu et al. [43], and it suggests that the CQDs were successfully incorporated into the biosynthesised ZnO.
2.2.2. BET
Figure 3a represents the nitrogen adsorption–desorption isotherms of biosynthesised ZnO, solvothermal ZnO, and CQD-ZnO composites using various volumes of CQD solutions. As shown in the isotherms, the adsorbed quantity of nitrogen was initially increased at low relative pressure due to the formation of a monolayer on the surface of the samples. After that, the formation of multilayers at higher relative pressure led to a continuous increment in the adsorbed quantity of nitrogen. All samples exhibited a clear hysteresis loop at relative pressure (P/P0) of 0.85 to 1.0, indicating the existence of a mesoporous structure [13]. According to Siddique et al. [44], the mesoporous structure is the ideal characteristic of a catalyst as it enables the adsorption of reactants due to its high surface area and also allows the entry of reactants into the pores for adsorption onto the active sites on the inside of the catalyst.
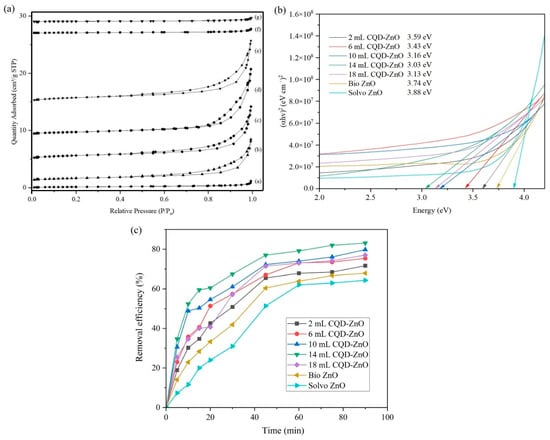
Figure 3.
(a) Nitrogen adsorption–desorption isotherms, (b) Tauc plots, and (c) catalytic removal efficiency of MG by biosynthesised ZnO, solvothermal ZnO, and CQD-ZnO composites using various volumes of CQD solutions (initial dye concentration: 10 ppm; catalyst loading: 1 g/L; solution pH: natural; ultrasonic power: 300 W; ultrasonic frequency: 25 kHz; solution temperature: room temperature).
Table 1 represents the specific surface area and pore volume of biosynthesised ZnO, solvothermal ZnO, and CQD-ZnO composites using various volumes of CQD solutions. Based on the results, the 14 mL CQD-ZnO composite possessed the highest values of specific surface area and pore volume among all the other samples. On the other hand, solvothermal ZnO exhibited the lowest specific surface area and pore volume. The specific surface area and pore volume values of biosynthesised ZnO were higher than those of solvothermal ZnO, but the incorporation of different amounts of CQD into the biosynthesised ZnO particles further increased the values. According to Mohamed Isa et al. [45], a higher specific surface area and pore volume would be favourable for the catalytic performance of a catalyst, as this would translate into more available active sites for the following reaction to take place. The specific surface area and pore volume increased as the amount of CQD was increased; this phenomenon is also in agreement with the results obtained by Thyda et al. [46]. This can be attributed to the introduction of CQD suppressing the recombination of photoexcited electron–hole pairs, hence inhibiting the accumulation of biosynthesised ZnO particles [42].

Table 1.
BET surface area and pore volume of biosynthesised ZnO, solvothermal ZnO, and CQD-ZnO composites using various volumes of CQD solutions.
2.2.3. UV-Vis DRS
Figure 3b represents the Tauc plot of the biosynthesised ZnO, solvothermal ZnO and CQD-ZnO composites using various volumes of CQD solutions. Based on the results obtained, the band gap energies of 2 mL, 6 mL, 10 mL, 14 mL, and 18 mL CQD-ZnO composites, biosynthesised ZnO, as well as solvothermal ZnO were around 3.59 eV, 3.43 eV, 3.16 eV, 3.03 eV, 3.13 eV, 3.74 eV, and 3.88 eV, respectively. The band gap energies of biosynthesised ZnO particles were lower than those of the solvothermal ZnO particles, which suggests that the introduction of plant extract—as an alternative to the conventional chemical-based reducing agent—reduced the conduction band and uplifted the valence band [47]. This helped to narrow the band gap energy of ZnO particles and may have enhanced the sonocatalytic performance of the biosynthesised ZnO. The reduction in band gap energy could also be attributed to the lattice disorder caused by the presence of phytochemicals and phenolic compounds within the plant extract. On the other hand, the incorporation of CQD onto biosynthesised ZnO to synthesise CQD-ZnO composites further reduced the band gap energy. The findings were in good agreement with Xu et al. [13], where the introduction of CQD in ZnO led to the lowering of the band gap energy, and the activation energy could be utilised more efficiently. However, as the amount of CQD added during the synthesis of CQD-ZnO composites was increased beyond 14 mL, the band gap energy of the resulting composite began to increase slightly due to the excessive incorporation of CQD, potentially leading to the agglomeration of CQD within the composite and consequently reducing its light absorption capacity [48]. In short, the band gap energy of the 14 mL CQD-ZnO composite displayed the lowest band gap energy among the synthesised CQD-ZnO composites.
2.2.4. Surface Morphology
Figure 2a–g illustrates the SEM images of biosynthesised ZnO, solvothermal ZnO, and CQD-ZnO composites using various volumes of CQD solutions. The SEM images revealed that the solvothermal ZnO possessed a long cylindrical shape with a flower-like structure. These flower-shaped clusters of rod-like ZnO were also reported by Das et al. [39] during the microwave, hydrothermal, and microwave-assisted hydrothermal ZnO preparation method.
2.3. Evaluation of Catalytic Performance of CQD-ZnO Composites
Figure 3c represents the catalytic removal efficiency of MG by biosynthesised ZnO, solvothermal ZnO, and CQD-ZnO composites prepared using various volumes of CQD solutions. The catalyst-free sonolysis results revealed that the dye concentration remained unchanged after 90 min of ultrasound irradiation without catalyst, indicating that sonolysis alone contributed negligibly to dye removal. Based on the results obtained, the 14 mL CQD-ZnO composite yielded the highest removal efficiency of 84.52% while the solvothermal ZnO exhibited the lowest removal efficiency of 62.16%. The incorporation of CQD into the biosynthesised ZnO led to an increase in its catalytic performance, as evident in Figure 3c. The biosynthesised ZnO yielded an MG removal efficiency of 71.47%, which was slightly better than solvothermal ZnO, but lower than all the other CQD-ZnO composites. Increasing the amount of CQD added during the synthesis of CQD-ZnO composites from 2 mL to 14 mL resulted in a proportional improvement from 71.83% to 84.52%, before showing a slight plunge to about 78.98% when the dosage of CQD used was 18 mL. This trend was also reported by Tran et al. [49], where the sudden drop in removal efficiency could be attributed to the agglomeration of a high amount of CQD, which resulted in low energy absorption. The finding can be supported by the UV−Vis DRS results, where the band gap energy increased beyond 14 mL CQD and decreased its sonocatalytic performance. In short, the incorporation of an appropriate amount of CQD was able to further improve the catalytic performance of the biosynthesised ZnO. In this context, the 14 mL CQD-ZnO composite synthesised through the addition of 14 mL of CQD solution was deemed to possess the highest potential in improving the removal of MG pollutant.
A direct comparison with previously reported ZnO–CQD systems indicates that the present green-synthesised CQD-ZnO exhibits competitive sonocatalytic performance while offering distinct practical advantages, as summarised in Table 2. CQD-ZnO composites prepared via hydrothermal or modified wet chemical routes have achieved high removal efficiencies (97–99%), though these results were obtained under prolonged light irradiation (120–180 min) and with varying light sources (UV, visible, or xenon lamps). For instance, ball-milled/hydrothermal CQD-ZnO and ZnO/N-CQD composites have demonstrated 88% (120 min, UV) and 80% (30 min, visible) degradation efficiencies for other dyes, respectively. In contrast, the green-synthesised CQD-ZnO in this study achieved 84.52% removal of 10 ppm malachite green under ultrasonic irradiation within 90 min.

Table 2.
Comparison of the catalytic efficiency of CQD-ZnO in this study with previously reported ZnO–CQD composites and their derivatives.
This comparison highlights the effective pollutant removal capability of sonocatalysis, an activation pathway that enhances mass transfer and radical generation through cavitation. Moreover, the present work employs an environmentally benign, green synthesis route for CQD preparation that avoids the use of harsh chemicals typically involved in wet chemical or hydrothermal methods. Therefore, although the absolute removal efficiency is slightly lower than that of the best light-driven systems, the integration of green synthesis, sonocatalytic activation, a relatively shorter reaction time, and the successful degradation of malachite green collectively position this method as a novel and practically attractive approach for sustainable dye remediation.
The enhanced sonocatalytic performance can be attributed to the synergistic effect of CQD-ZnO nanocomposites. The mechanism of the nanocomposites is depicted in Figure 4. Sonocatalysis is based on the heat energy and sonoluminescence induced by the ultrasonic cavitation phenomenon [53]. The introduction of an ultrasonic wave caused the formation and growth of air bubbles. Once the bubbles collapsed, the energy was released in the form of heat and light [54]. Sonication affected the dispersion and activated the CQD-ZnO sonocatalyst when the energy released was equal to or higher than the band gap energy of ZnO [54,55]. The electron transfer from ZnO to the conduction band (CB) of the CQD upon exposure to sonication left a hole (h+) in the valence band (VB) of ZnO, as shown in Equation (1) [42].
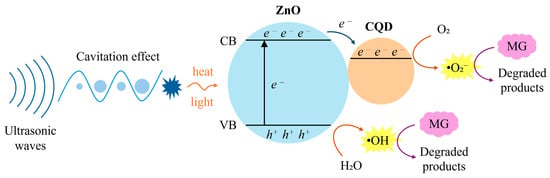
Figure 4.
Schematic diagram of sonocatalytic mechanism of CQD-ZnO.
Then, Equation (2) shows that the sonoexcited electrons accumulated on CQDs reduce the absorbed oxygen to superoxide radicals (•O2−). In Equation (3), the holes migrate to the surface of the catalyst, where they oxidise water molecules and generate hydroxyl radicals (•OH) [50].
The superoxide radicals (•O2−) undergo protonation with the water molecules to form hydrogen peroxide radicals (•HO2), which react further with water to produce hydrogen peroxide (H2O2), as shown in Equations (4) and (5). Hydrogen peroxide acts as a supplementary source to dissociate into the hydroxyl radicals (•OH), as in Equation (6), further accelerating the degradation [53].
Lastly, Equation (7) shows that the hydroxyl radicals (•OH) subsequently react with the organic pollutants, leading to the mineralisation of MG into CO2 and H2O [53].
Hence, the findings obtained from the 2 mL to 14 mL CQD solutions validated the theory that the integration of CQD into ZnO nanoparticles enhanced the sonocatalytic degradation efficiency by minimising the recombination rate of electron–hole pairs. However, excessive CQD loading at 18 mL resulted in a decline in degradation efficiency, as the surplus CQD acts as a recombination centre of electrons, thereby reducing the generation of reactive oxidising species for the degradation of MG [48].
It is noteworthy that the present study focused primarily on the comprehensive characterisation and catalytic performance of CQD-ZnO under controlled experimental conditions. To maintain consistency across the tested parameters, factors such as solution pH, pollutant type, and concentration were not included in this investigation. However, it is recognised that these parameters could significantly affect the catalytic efficiency and degradation mechanisms. Therefore, future studies will systematically explore these variables, which will provide a more holistic understanding of the catalyst’s performance in diverse wastewater treatment environments.
3. Experimental Section
3.1. Chemicals and Reagents
Zinc nitrate hexahydrate (Zn(NO3)2•6H2O), nitric acid (HNO3), and absolute ethanol (C2H5OH) (purity ≥ 99.5%) were purchased from Merck (Darmstadt, Germany). Malachite green (purity ≥ 99%) was purchased from Friendemann Schmidt (Selangor, Malaysia). All chemicals were used as received without any purification, and all aqueous solutions were prepared by using distilled water.
3.2. Extraction of Zn from Post-Phytoremediation Plant and Plant Extract Preparation
The extraction of Zn from the post-phytoremediation plant and plant extract preparation methods were adapted from research performed by Barbaroux et al. [56]. The plant chosen for this study was Eichhornia crassipes (water hyacinth), as obtained from our previous study [57]. Firstly, the plant biomass was harvested from a local Zn-contaminated site, washed, then oven-dried at 90 °C overnight. 1 g of the finely ground plant biomass was then placed inside a furnace at a temperature of 500 °C for 2 h to undergo calcination. The calcined plant biomass was then mixed with 10 mL of HNO3 and left in a fume hood for overnight digestion. The pre-digested calcined plant biomass was then heated to a temperature of 120 °C for 4 h, filtered and diluted to 50 mL using distilled water. 5 g of the remaining dried, uncalcined plant biomass was then heated in 500 mL of distilled water at a temperature of 60 °C for an hour and then filtered to obtain the plant extract solution.
3.3. Biosynthesis of ZnO Nanoparticles
ZnO nanoparticles were biosynthesised according to the study by Hassan et al. [58] with slight modifications. Firstly, 40 mL of the Zn-containing nitric acid-digested solution was reacted with 20 mL of plant extract, resulting in an extracted Zn-to-plant extract ratio of 2:1. The mixture was then stirred at 350 rpm at an elevated temperature of 60 °C for an hour. Next, the mixture was dried in an oven overnight and then calcined at 500 °C in a furnace for 2 h. The solid white biosynthesised ZnO was collected and ground to fine powder using mortar and pestle. The biosynthesis process was then repeated to produce ZnO with different ratios of extracted Zn to plant extract of 1:1 and 1:2. The biosynthesised ZnO nanoparticles with different ratios were then subjected to FTIR, XRD, and SEM characterisation to determine the optimum ratio for subsequent studies.
3.4. Synthesis of Carbon Quantum Dot-Zinc Oxide Composites
The biosynthesised ZnO with the most optimum ratio of extracted Zn to plant extract was selected to be incorporated with CQD. The CQD was fabricated from the same plant via the solvothermal method based on the study by Hak et al. [59] with some modifications. Firstly, the fresh plant was oven-dried at 90 °C overnight. The dried plant biomasses were then ground using a mortar and sieved through a 3.18 mm sieve. 5 g of the dried plant powder was mixed with 120 mL of distilled water and stirred at 400 rpm for 30 min at room temperature. Next, the mixture was transferred to a Teflon tube and sealed inside a stainless-steel autoclave. The autoclave was then heated at 180 °C overnight and cooled down naturally. The mixture was then filtered to remove insoluble particulates. The yellowish-brown filtrate solution was dried at 100 °C overnight and then diluted with distilled water.
The carbon quantum dot-zinc oxide composites (CQD-ZnO) were prepared by modifying the procedure in the report by Xu et al. [13]. In this process, 0.1 g of previously biosynthesised ZnO with optimum ratio was dispersed with a certain amount of CQD solution (2, 6, 10, 14, and 18 mL) in a mixture containing 14 mL of distilled water and 6 mL of absolute ethanol. The mixture was then transferred to a Teflon tube inside a stainless-steel autoclave and heated to a temperature of 140 °C for 4 h via solvothermal method. After the solvothermal process, the solid sample was collected through centrifugation and washed multiple times with ethanol and water. The obtained CQD-ZnO composites were labelled 2, 6, 10, 14, and 18 mL CQD-ZnO, corresponding to the volume of CQD solutions added. Additionally, the solvothermal ZnO was prepared via the same method by replacing the biosynthesised ZnO with Zn(NO3)2•6H2O without any addition of CQD solution.
3.5. Characterisation Studies
The biosynthesised CQD-ZnO composites, biosynthesised and solvothermal ZnO nanoparticles were characterised to understand their optical and physicochemical properties. Scanning electron microscopy (SEM, Hitachi, S-3400 N, Tokyo, Japan) was used to analyse the surface morphology of the nanoparticles, while a Transmission Electron Microscope (TEM, Philips, Tecnai 20, Hillsboro, OR, USA) was used to provide magnified images of the internal structure of the sample. Brunauer–Emmett–Teller (BET, Micromerities 3Flex, Norcross, GA, USA) was used to determine the physical properties of the samples including pore size distribution and specific surface area. Meanwhile, UV–Vis diffuse reflection spectroscopy (UV−Vis DRS, Shimadzu, UV 3600, Kyoto, Japan) measured the band gap energies of the synthesised nanoparticles. The spectra were recorded with barium sulphate as the reference and the wavelength range of the optical reflectance curve was between 300 nm and 800 nm. Equation (8) shows the Tauc relation used to evaluate the band gap energy, of the sample.
where
optical absorption coefficient
Planck’s constant (6.63 × 10−34 J s)
frequency of light
X-ray diffraction (XRD, Shimadzu, XRD-6000, Kyoto, Japan) technique was used to obtain the phase information and crystalline nature of the samples. The various modes of vibrations were identified to determine the presence of functional groups in the nanoparticles using Fourier transform infrared spectroscopy (FTIR, Nicolet, IS10 system, Waltham, MA, USA).
3.6. Sonocatalytic Activity of Carbon Quantum Dot-Zinc Oxide Composites Using Various Volumes of CQD Solutions for Malachite Green Degradation
The sonocatalytic activity of the synthesised CQD-ZnO composites was assessed through the degradation of malachite green (MG) under ultrasound irradiation. The sonocatalytic performance evaluation was carried out using an Elma Transsonic series TI-H-5 ultrasonic bath (Singen, Germany) operated at 25 kHz and 300 W. Synthetic dye wastewater containing 10 ppm MG was prepared in a 250 mL beaker, and 1 g/L of CQD-ZnO composites was dispersed as the catalyst. For comparison, the biosynthesised ZnO with an optimum ratio of extracted Zn to plant extract and solvothermal ZnO were also added as catalysts under identical conditions. The ultrasonic irradiation commenced immediately, and 5 mL aliquots were collected at 10 min intervals over a total reaction time of 90 min. Each sample was centrifuged to separate the catalysts before liquid sample analysis. Control experiments were conducted to differentiate between adsorption, sonolysis, and true sonocatalytic degradation. Before each run, the suspension was stirred in the dark for 90 min to achieve adsorption–desorption equilibrium between the dye molecules and catalyst surface. A catalyst-free sonolysis test was also performed under identical ultrasonic conditions to evaluate the effect of the ultrasound alone. All experiments were conducted in triplicate under identical conditions to ensure data reproducibility, and the corresponding standard deviations are presented in the Supplementary Information. A single-beam UV−Vis spectrophotometer (Agilent, Cary 100, Santa Clara, CA, USA) was used to measure the concentration of dye samples collected at a maximum absorbance wavelength of 617 nm. The dye removal efficiency was evaluated using Equation (9), where C0 is the initial dye concentration and Ct is the dye concentration at time t.
The overall synthesis workflow and experimental arrangement is presented as Figure 5, which summarises the main steps involved in the preparation process, including precursor solution preparation, mixing with the carbon quantum dots, drying, and subsequent thermal treatment to obtain the CQD-ZnO composite.
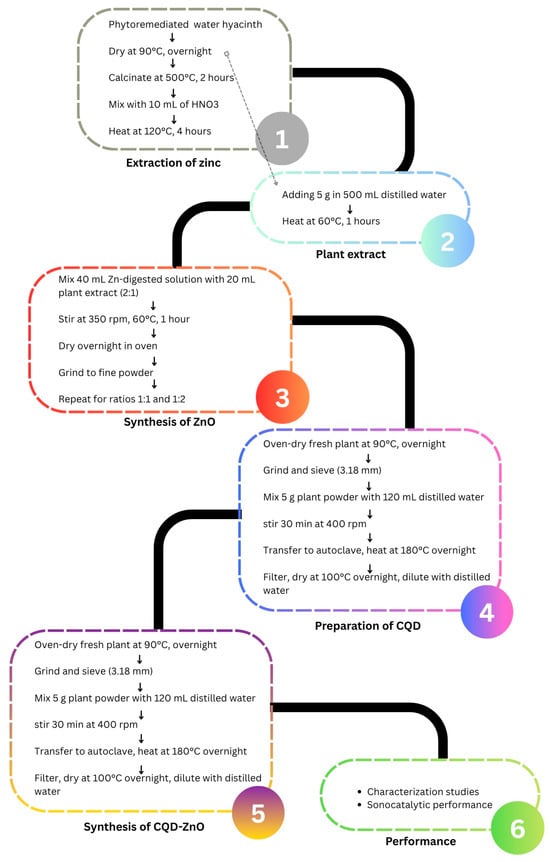
Figure 5.
Schematic illustration of the synthesis process of the CQD-ZnO composite.
4. Conclusions
In summary, ZnO nanoparticles were successfully biosynthesised using zinc extracted from water hyacinth and plant extract derived from the same biomass. The optimised 14 mL CQD-ZnO composite achieved the highest sonocatalytic degradation efficiency of 84.52% for malachite green under ultrasonic irradiation, outperforming both biosynthesised ZnO and solvothermal ZnO. This demonstrates a clear synergistic effect between CQDs and ZnO, promoting enhanced electron–hole separation and improved degradation activity. Beyond its laboratory performance, this study highlights a sustainable approach to transforming phytoremediated biomass into high-value catalytic material for wastewater treatment. The biosynthesis route aligns with the circular economy and green chemistry principles by recovering metals from contaminated biomass and reducing secondary waste. Future research should focus on evaluating the influence of environmental factors such as pH, salinity, temperature, and catalyst dosage, as well as testing the composite’s durability and reusability under real wastewater conditions. These efforts will be essential to assess the scalability and practical feasibility of CQD-ZnO composites for industrial-scale water purification applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15111051/s1, Table S1: Summary of the catalytic removal efficiency of MG by biosynthesised ZnO and solvothermal ZnO and CQD–ZnO composites using various volume of CQD solutions (initial dye concentration: 10 ppm; catalyst loading: 1 g/L; solution pH: natural; ultrasonic power: 300 W; ultrasonic frequency: 25 kHz; solution temperature: room temperature).
Author Contributions
Conceptualization, writing—review and editing, Y.L.P.; writing—original draft preparation, H.W.T.; methodology and resources, S.L.; visualisation and validation, J.W.T.; data curation, W.C.C.; data curation and visualisation, S.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education (MoHE) Malaysia that provided the Fundamental Research Grant Scheme (FRGS/1/2022/TK05/UTAR/02/34) and the Universiti Tunku Abdul Rahman (UTAR) Research Fund (IPSR/RMC/UTARRF/2020-C2/P01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the authors.
Acknowledgments
The research was supported by the Ministry of Higher Education (MoHE), through the Fundamental Research Grant Scheme (FRGS/1/2022/TK05/UTAR/02/34) and the Universiti Tunku Abdul Rahman (UTAR) Research Fund (IPSR/RMC/UTARRF/2020-C2/P01). This research was also supported in part by the Kurita Overseas Research Grant (21Pmy076) provided by the Kurita Water and Environ-ment Foundation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bayuo, J.; Rwiza, M.J.; Choi, J.W.; Njau, K.N.; Mtei, K.M. Recent and Sustainable Advances in Phytoremediation of Heavy Metals from Wastewater Using Aquatic Plant Species: Green Approach. J. Environ. Manag. 2024, 370, 122523. [Google Scholar] [CrossRef]
- Sharma, S.; Dadhwal, R.; Banerjee, R. Nanoparticle Assisted Phytoremediation: An Eco-Friendly Approach for Removal of Heavy Metals from the Environment. J. Environ. Sci. 2025, 159, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, S. Sorption Capacity of Eichhornia crassipes (Mart.) Solms for Zinc Removal from Electroplating Industry Wastewater. Environ. Sci. Pollut. Res. 2024, 31, 30849–30866. [Google Scholar] [CrossRef] [PubMed]
- Ranauda, M.A.; Prigioniero, A.; Ortega, M.L.; Gizzi, G.; Fosso, E.; Maisto, M.; Zuzolo, D.; Tartaglia, M.; Guarino, C. Poaceae in Phytoremediation: A Systematic Review of Current Knowledge, Research Trends and Insights into Useful Plant Traits. J. Hazard. Mater. 2025, 496, 139225. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Akter, R.; Rahman, M.M.; Kurasaki, M. Phytoremediation: Background, Principle, and Application, Plant Species Used for Phytoremediation. Handb. Environ. Chem. 2022, 115, 199–224. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Ouvrard, S.; Sirguey, C.; Qiu, R.; Wu, B. Environmental Remediation Potential of a Pioneer Plant (Miscanthus sp.) from Abandoned Mine into Biochar: Heavy Metal Stabilization and Environmental Application. J. Environ. Manag. 2024, 366, 121751. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A Critical Review on the Phytoremediation of Heavy Metals from Environment: Performance and Challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Zango, Z.U.; Ibnaouf, K.H.; Garba, A.; Aldaghri, O.; Wadi, I.A.; Hosseini-Bandegharaei, A.; Baigenzhenov, O. Advances in Green Synthesis, Modification Strategies, and Photocatalytic Application of Metal Oxide Nanoparticles for Organic Pollutants Degradation: A Comprehensive and in-Depth Review. J. Mol. Liq. 2025, 428, 127497. [Google Scholar] [CrossRef]
- de Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Costa, J.A.S.; da Silva, C.M.P.; Iqbal, H.M.N.; Ferreira, L.F.R. Metal/Metal Oxide Nanoparticles: A Revolution in the Biosynthesis and Medical Applications. Nano-Struct. Nano-Objects 2024, 37, 101071. [Google Scholar] [CrossRef]
- Balaji, S.; Pandian, M.S.; Ganesamoorthy, R.; Karchiyappan, T. Green Synthesis of Metal Oxide Nanoparticles Using Plant Extracts: A Sustainable Approach to Combat Antimicrobial Resistance. Environ. Nanotechnol. Monit. Manag. 2025, 23, 101066. [Google Scholar] [CrossRef]
- Ye, F.; Liu, Y.; Lv, Q.; Gao, B.; Xia, J.; Li, X.; Dou, M.; Zhao, K.; Ahmad, M.; Xiao, Z.; et al. Unveiling the Mechanism of Efficient Detoxification by Pd Species in Chlorinated Pollutant Degradation. Chin. Chem. Lett. 2025, 111136. [Google Scholar] [CrossRef]
- Nugroho, D.; Wannakan, K.; Nanan, S.; Benchawattananon, R. The Synthesis of Carbon Dots//Zincoxide (CDs/ZnO-H400) by Using Hydrothermal Methods for Degradation of Ofloxacin Antibiotics and Reactive Red Azo Dye (RR141). Sci. Rep. 2024, 14, 2455. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Lu, Y.N.; Tao, F.F.; Liang, P.F.; Zhang, P.A. ZnO Nanoparticles Modified by Carbon Quantum Dots for the Photocatalytic Removal of Synthetic Pigment Pollutants. ACS Omega 2023, 8, 7845–7857. [Google Scholar] [CrossRef] [PubMed]
- Coccia, F.; Mascitti, A.; Rastelli, G.; d’Alessandro, N.; Tonucci, L. Sustainable Photocatalytic Reduction of Maleic Acid: Enhancing CuxO/ZnO Stability with Polydopamine. Appl. Sci. 2025, 15, 1631. [Google Scholar] [CrossRef]
- Aina, A.R.N.; Patel, H.; Aich, S.; Roy, B.; Samanta, N.S.; Pal, B. Recent Advances in ZnO Based Photocatalysts for Industrial Dye Degradation. Discov. Appl. Sci. 2025, 7, 977. [Google Scholar] [CrossRef]
- Shalahuddin Al Ja’farawy, M.; Kusumandari; Purwanto, A.; Widiyandari, H. Carbon Quantum Dots Supported Zinc Oxide (ZnO/CQDs) Efficient Photocatalyst for Organic Pollutant Degradation—A Systematic Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100681. [Google Scholar] [CrossRef]
- Meenakshi, G.; Sivasamy, A. Enhanced Photocatalytic Activities of CeO2@ZnO Core-Shell Nanostar Particles through Delayed Electron Hole Recombination Process. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128920. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Iqbal Rokon, M.Z.; Rahim, M.A.; Hossain, M.I.; Islam, M.S.; Ali, M.R.; Bacchu, M.S.; Waizumi, H.; Komeda, T.; Hossain Khan, M.Z. Enhanced Photocatalytic Activity of Cu and Ni-Doped ZnO Nanostructures: A Comparative Study of Methyl Orange Dye Degradation in Aqueous Solution. Heliyon 2023, 9, e16506. [Google Scholar] [CrossRef]
- Kamaruzaman, N.A.N.; Fadzil, N.I.; Alli, Y.A.; Tan, T.; Abdullah, J.; Rashid, S.A. Optimized Synthesis and Characterization of Red-Shifted Nitrogen-Doped Carbon Quantum Dots for Highly Sensitive Detection of Fe3+ Ions in Aqueous Media. Sens. Actuators A Phys. 2025, 394, 116895. [Google Scholar] [CrossRef]
- Krishna Saraswat, S.; Ahmed Mustafa, M.; Kamil Ghadir, G.; Kaur, M.; Guamán Lozada, D.F.; Hasen shuhata alubiady, M.; Muzahem Al-Ani, A.; Alshahrani, M.Y.; Kadhem Abid, M.; Salih Jumaa, S.; et al. Carbon Quantum Dots: A Comprehensive Review of Green Synthesis, Characterization and Investigation Their Applications in Bioimaging. Inorg. Chem. Commun. 2024, 162, 112279. [Google Scholar] [CrossRef]
- Roostaee, M.; Ranjbar-Karimi, R. Emerging Trends in Carbon Quantum Dots: Synthesis, Characterization, and Environmental Photodegradation Applications. Mater. Sci. Semicond. Process 2025, 188, 109212. [Google Scholar] [CrossRef]
- Bazazi, S.; Jodeyri, S.; Hosseini, S.P.; Arsalani, N.; Rashidzadeh, B.; Fathalipour, S.; Seidi, F.; Hashemi, E. Ball Mill-Hydrothermal Method for One-Step Synthesis of Zinc Oxide/Carbon Quantum Dot (ZnO-CQD) Nanocomposites as Photocatalyst for Degradation of Organic Pollutants. J. Photochem. Photobiol. A Chem. 2023, 445, 115096. [Google Scholar] [CrossRef]
- Bopape, D.A.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of ZnO: Effect of Plant Concentration on the Morphology, Optical Properties and Photodegradation of Dyes and Antibiotics in Wastewater. Optik 2022, 251, 168459. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Dosen, A.; Blanton, T.N. PDF-5+: A Comprehensive Powder Diffraction FileTM for Materials Characterization. Powder Diffr. 2024, 39, 47–59. [Google Scholar] [CrossRef]
- Menon, P.S.; Anjana, M.P.; Jose, A.K.; Kunjumon, J.; PA, A.; Chandran, S.; George, M.; Vinitha, G.; Sajan, D. The Role of Defects on Linear and Nonlinear Optical Properties of Pristine and Nickel Doped Zinc Oxide Nanoparticles. Surf. Interfaces 2022, 34, 102393. [Google Scholar] [CrossRef]
- Vera, J.; Herrera, W.; Hermosilla, E.; Díaz, M.; Parada, J.; Seabra, A.B.; Tortella, G.; Pesenti, H.; Ciudad, G.; Rubilar, O. Antioxidant Activity as an Indicator of the Efficiency of Plant Extract-Mediated Synthesis of Zinc Oxide Nanoparticles. Antioxidants 2023, 12, 784. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Jing, G.; El Zein, B.; Jaithon, T.; Ruangtong, J.; T-Thienprasert, J.; T-Thienprasert, N.P. Effects of Waste-Derived ZnO Nanoparticles against Growth of Plant Pathogenic Bacteria and Epidermoid Carcinoma Cells. Crystals 2022, 12, 779. [Google Scholar] [CrossRef]
- Neamah, S.A.; Albukhaty, S.; Falih, I.Q.; Dewir, Y.H.; Mahood, H.B. Biosynthesis of Zinc Oxide Nanoparticles Using Capparis Spinosa L. Fruit Extract: Characterization, Biocompatibility, and Antioxidant Activity. Appl. Sci. 2023, 13, 6604. [Google Scholar] [CrossRef]
- Habtoor, S.S.; Basri, H.B.; Zaini, M.; Rahmawati, A.; Shah, T. Ex Situ Synthesis and Characterization of Chitosan-ZnO Nanocomposites Using ZnO Nanoparticles Prepared by the Precipitation Method. AIMS Mater. Sci. 2025, 12, 686–702. [Google Scholar] [CrossRef]
- Albarakaty, F.M.; Alzaban, M.I.; Alharbi, N.K.; Bagrwan, F.S.; Abd El-Aziz, A.R.M.; Mahmoud, M.A. Zinc Oxide Nanoparticles, Biosynthesis, Characterization and Their Potent Photocatalytic Degradation, and Antioxidant Activities. J. King Saud. Univ. Sci. 2023, 35, 102434. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Wijesinghe, U.; Thiripuranathar, G.; Menaa, F.; Iqbal, H.; Razzaq, A.; Almukhlifi, H. Green Synthesis, Structural Characterization and Photocatalytic Applications of ZnO Nanoconjugates Using Heliotropium Indicum. Catalysts 2021, 11, 831. [Google Scholar] [CrossRef]
- Kalaivani, M.; Ravi, S. Green Synthesis of ZnO NPs and CdO-ZnO Nanocomposites Using Aqueous Extract of Water Hyacinth (Eichhornia crassipes) Characterization, Structural and Nano-Fertilizer Using Application. Indian J. Sci. Technol. 2023, 16, 1918–1926. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-Inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Parveen, S.; Riyazur Rahman, F.; Thulasi Krishnan, S.; Kalaiarasi, G.; Dinesh, A.; Srimathi Priya, L.; Gnanasekaran, L.; Santhamoorthy, M.; Ayyar, M.; Santhoshkumar, S. Green Synthesis of Metal Oxide Nanoparticles via Plant Extracts for Biological Applications: A Review. Trends Sci. 2025, 22, 9592. [Google Scholar] [CrossRef]
- Haji, B.S.; Barzinjy, A.A.; Abbas, A.O.; Kaygili, O.; Mousa, M.S. Green Synthesis of ZnO Nanoparticles Using Citrullus lanatus Fruit Extract and Their Potential for Microwave Absorption. Nano-Struct. Nano-Objects 2025, 43, 101502. [Google Scholar] [CrossRef]
- Rana, G.; Dhiman, V.K.; Ali, S.K.; Chauhan, A.; Jabir, M.S.; Ghotekar, S. Emerging Developments in Plant-Based Metal Nanomaterials for Diverse Versatile Applications—A Review. Results Chem. 2025, 15, 102231. [Google Scholar] [CrossRef]
- Das, A.; Mathan Kumar, P.; Bhagavathiachari, M.; Nair, R.G. Shape Selective Flower-like ZnO Nanostructures Prepared via Structure-Directing Reagent Free Methods for Efficient Photocatalytic Performance. Mater. Sci. Eng. B 2021, 269, 115149. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Q.; Cao, J.; Qian, C.; Ye, J.; Xu, S.; Zhang, Y.; Li, Y. Facile and Green Synthesis of Highly Fluorescent Carbon Quantum Dots from Water Hyacinth for the Detection of Ferric Iron and Cellular Imaging. Nanomaterials 2022, 12, 1528. [Google Scholar] [CrossRef]
- Ejsmont, A.; Goscianska, J. Hydrothermal Synthesis of ZnO Superstructures with Controlled Morphology via Temperature and PH Optimization. Materials 2023, 16, 1641. [Google Scholar] [CrossRef]
- Na, G.; Kang, J.W. Green Synthesis of ZnO/CQD Nanocomposite Using Chestnut Shell and Evaluating Its Photocatalytic Antimicrobial Activity under Visible Light. Food Res. Int. 2025, 205, 115948. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Cui, L.; Wang, P.; Zhang, Z.; Chen, L.; Wang, Z. Microwave-Assisted Synthesis of CQDs/ZnO Hollow Microspheres for Complete NOx Oxidation under Visible Light. J. Environ. Sci. 2026, 159, 1–9. [Google Scholar] [CrossRef]
- Siddique, M.; Fayaz, N.; Saeed, M. Synthesis, Characterization, Photocatalytic Activity and Gas Sensing Properties of Zinc Doped Manganese Oxide Nanoparticles. Phys. B Condens. Matter 2021, 602, 412504. [Google Scholar] [CrossRef]
- Mohamed Isa, E.D.; Shameli, K.; Ch’ng, H.J.; Che Jusoh, N.W.; Hazan, R. Photocatalytic Degradation of Selected Pharmaceuticals Using Green Fabricated Zinc Oxide Nanoparticles. Adv. Powder Technol. 2021, 32, 2398–2409. [Google Scholar] [CrossRef]
- Thyda, L.; Joseph, J.K.; Naresh, K.; Dasi, G.; Suneetha, S.; Thangavel, R.; Jayalakshmi, V.; Amaladass, P.; Thangaraju, K. Green Synthesis of Carbon Quantum Dots Derived from Mango-Leaves (M−CQDs): M−CQDs/ZnO Nanorods Heterostructure Thin Films for Efficient Self-Powered UV Photodetector Applications. Appl. Surf. Sci. 2025, 685, 162032. [Google Scholar] [CrossRef]
- Halder, A.; Mohan, G.R.; Matheshwaran, S.; Jha, S.K. Green Synthesis of Neem (Azadirachta indica) Functionalized Zinc Oxide with Enhanced Antimicrobial Properties. Next Mater. 2025, 8, 100725. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Uchida, J.; Katsurao, T.; Sakabe, H.; Ohtani, B.; Sasaki, K. Efficient Photocatalytic Degradation of Emerging Ciprofloxacin under Visible Light Irradiation Using BiOBr/Carbon Quantum Dot/Saponite Composite. Environ. Res. 2022, 212, 113635. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.P.H.; Pham, M.T.; Wang, Y.F.; Chang, T.C.; You, S.J. Tuning Visible Light-Driven Photocatalytic NO Removal: Insights from Glucose-Derived CQDs/ZnO Nanorods Composite. J. Environ. Chem. Eng. 2023, 11, 111561. [Google Scholar] [CrossRef]
- Toma, E.E.; Stoian, G.; Cojocaru, B.; Parvulescu, V.I.; Coman, S.M. ZnO/CQDs Nanocomposites for Visible Light Photodegradation of Organic Pollutants. Catalysts 2022, 12, 952. [Google Scholar] [CrossRef]
- Mohamed, W.A.A.; Abd El-Gawad, H.H.; Mekkey, S.D.; Galal, H.R.; Labib, A.A. Zinc Oxide Quantum Dots: Confinement Size, Photophysical and Tunning Optical Properties Effect on Photodecontamination of Industrial Organic Pollutants. Opt. Mater. 2021, 118, 111242. [Google Scholar] [CrossRef]
- Widiyandari, H.; Prilita, O.; Al Ja’farawy, M.S.; Nurosyid, F.; Arutanti, O.; Astuti, Y.; Mufti, N. Nitrogen-Doped Carbon Quantum Dots Supported Zinc Oxide (ZnO/N-CQD) Nanoflower Photocatalyst for Methylene Blue Photodegradation. Results Eng. 2023, 17, 100814. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, H. Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light. Molecules 2023, 28, 3706. [Google Scholar] [CrossRef]
- Rodríguez-Flores, T.; Hernández-Pérez, I.; de la Huerta-Hernández, G.E.; Suárez-Parra, R.; Haro-Pérez, C. Sonocatalytic Degradation of RB-5 Dye Using ZnO Nanoparticles Doped with Transition Metals. Environ. Sci. Pollut. Res. 2025, 32, 783–797. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Abramova, A.V.; Cravotto, G. Sonochemical Processes for the Degradation of Antibiotics in Aqueous Solutions: A Review. Ultrason. Sonochem. 2021, 74, 105566. [Google Scholar] [CrossRef]
- Barbaroux, R.; Meunier, N.; Mercier, G.; Taillard, V.; Morel, J.L.; Simonnot, M.O.; Blais, J.F. Chemical Leaching of Nickel from the Seeds of the Metal Hyperaccumulator Plant Alyssum Murale. Hydrometallurgy 2009, 100, 10–14. [Google Scholar] [CrossRef]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C.; Lai, C.W.; Abdullah, A.Z. Exploring the Potential of Utilizing Aquatic Macrophytes for Enhanced Phytoremediation of Zinc in Artificial Wastewater: Characteristics and Parameter Studies. Sustainability 2023, 15, 15170. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elkady, M.F.; El-Sayed, E.M.; Mahmoud, I.M. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Green and Chemical Synthesis Techniques for Phenol Decontamination. Int. J. Nanoelectron. Mater. 2018, 11, 179–194. [Google Scholar]
- Hak, C.H.; Leong, K.H.; Chin, Y.H.; Saravanan, P.; Tan, S.T.; Chong, W.C.; Sim, L.C. Water Hyacinth Derived Carbon Quantum Dots and G-C3N4 Composites for Sunlight Driven Photodegradation of 2,4-Dichlorophenol. SN Appl. Sci. 2020, 2, 1030. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).