Abstract
This study transformed discarded courgette biomass into biochar (BC) via pyrolysis at 500 °C and employed it as an activator of potassium periodate (PI) for atrazine (ATZ) degradation. Characterization analyses confirmed that the synthesized BC possessed a porous structure, a high carbon content (76.13%), crystalline SiO2, KCl, and CaCO3 phases, as well as abundant oxygen-containing functional groups (–OH, C=O, C=C, –COOH), which are favorable for catalytic activation. The point of zero charge of 4.25 indicates that the BC surface carries a suitable charge distribution, promoting effective electrostatic interactions under near-neutral pH conditions. Under optimal operating conditions (neutral pH, [ATZ]o = 7.3 mg/L, [PI]o = 2.7 mM, [BC]o = 0.55 g/L, and 25 ± 0.5 °C), the system achieved 99.35% ATZ removal (first-order kinetic rate constant = 0.0601 min−1) and 64.23% TOC mineralization within 60 min. Quenching tests confirmed iodate radicals and singlet oxygen as the primary species, with hydroxyl and superoxide radicals playing secondary roles. The proposed mechanism suggests that electron transfer from oxygen-containing groups on the BC surface activates PI, leading to the generation of reactive oxygen species that facilitate ATZ degradation via synergistic radical and non-radical pathways. The BC catalyst exhibited strong recyclability, with only ~9% efficiency loss after five cycles. The BC/PI system also demonstrated high removal of tetracycline (79.54%) and bisphenol A (85.6%) within 60 min and complete Congo red dye degradation in just 30 min. Application to real industrial wastewater achieved 72.77% ATZ removal, 53.02% mineralization, and a treatment cost of 1.2173 $/m3, demonstrating the practicality and scalability of the BC/PI system for sustainable advanced wastewater treatment.
1. Introduction
Atrazine (ATZ), a commonly used herbicide, presents major environmental and health concerns due to its long-lasting presence in aquatic systems and its risk of polluting water supplies [1]. As a common agricultural chemical, ATZ is often found in surface water and groundwater, where it can accumulate and affect both aquatic life and human health [2]. Environmental issues include its harmful effects on marine life and its impact on ecosystem balance. In humans, long-term exposure to atrazine has been connected to various health risks, such as hormonal imbalances, reproductive problems, and cancer [3,4]. Given these concerns, it is crucial to remove ATZ from wastewater before it is discharged into water bodies. Conventional treatment methods for ATZ removal, including biological, physical, and chemical processes, are often insufficient due to their limitations. Biological treatments, such as activated sludge processes, use microorganisms to degrade pollutants [5]. However, ATZ’s chemical stability and resistance to biodegradation make these methods ineffective, with ATZ potentially accumulating in the sludge [6]. Physical methods, such as adsorption, can capture ATZ but are hindered by the high material costs and the need for frequent regeneration. Membrane filtration, such as reverse osmosis, is also energy-intensive and generates large volumes of wastewater, making it impractical for large-scale applications [7]. Chemical treatments, such as chlorination, can degrade ATZ but are costly, require specialized equipment, and may produce harmful by-products [8]. These conventional methods often fail to offer an efficient and cost-effective solution for ATZ removal, underscoring the need for alternative, economical, and sustainable approaches [9].
Recently, advanced oxidation processes (AOPs) have emerged as impactful methods for removing refractory organic micropollutants from aqueous solutions [10]. These processes rely on generating reactive oxygen species (ROS) using oxidants, e.g., hydrogen peroxide (H2O2), peracetic acid, peroxymonosulfate (PMS), persulfate (PS), and periodate (PI, IO4−) [11,12,13]. Among these oxidants, PI exhibits superior oxidation capability owing to its longer and weaker I–O bond (≈1.78 Å), compared to the O–O bonds in PS (1.497 Å), PMS (1.453 Å), and H2O2 (1.457 Å; bond energy ≈ 213 kJ/mol) [12,14]. This structural feature facilitates easier bond cleavage and enhances the formation of highly reactive radicals such as iodate radicals (•IO3) and periodate radicals (•IO4). These radicals possess high redox potentials and longer lifetimes (up to 10−3 s) relative to hydroxyl radicals (•OH; lifetime ≈ 10−9 s) and sulfate radicals (lifetime ≈ 10−6 s), allowing for more sustained oxidation activity in aqueous systems [15]. These characteristics reduce spatial crowding around the central iodine atom, allowing PI to be safely stored and transported over extended periods without significant degradation or reactivity loss, making it easier for the molecule to interact with activators and generate ROS [16,17]. Gaber et al. [12] studied the degradation of tetracycline using a green-synthesized magnetite-supported biochar and reported a comparative evaluation of PI and other common oxidants. The PI-based system exhibited the highest oxidation performance among the tested oxidants, achieving a degradation efficiency of 83.4% with a corresponding pseudo-first-order rate constant of 0.0419 min−1. In contrast, the systems utilizing PS, PMS, and H2O2 achieved lower degradation efficiencies of 70.23, 61.19, and 39.27%, with respective rate constants of 0.0244, 0.019, and 0.0095 min−1. Elmitwalli et al. [18] compared the oxidation performance of different oxidants, including PI, PS, and H2O2, in the degradation of sulfamethazine using mulukhiyah stalks-derived biochar as a green activator. Among the tested systems, the PI-based system demonstrated the highest degradation efficiency, achieving nearly complete pollutant removal (below the detection limit) within 100 min, whereas the systems utilizing PS and H2O2 achieved lower degradation efficiencies of approximately 76 and 51%, respectively, under identical conditions.
Although PI can oxidize certain organic pollutants, its relatively slow reaction kinetics and moderate oxidation potential (+1.6 V vs. NHE) hinder its effectiveness in decomposing stable compounds. This limitation reduces its practicality in wastewater treatment applications, where rapid degradation is essential, and leads to higher operational costs [19]. To overcome these challenges, research has focused on activation techniques that enhance the reactivity of PI by generating highly oxidative ROS, including •IO3 radicals, superoxide radicals (O2•−), •OH radicals, and non-radical singlet oxygen (1O2). Several techniques have been developed to activate PI and enhance its oxidative capacity for pollutant degradation [20,21]. Common activation methods include ultraviolet light irradiation, transition metal catalysis, ultrasound, freezing, and the use of chemical activators like hydroxylamine and alkalis [18,22]. While these techniques improve the reactivity of periodate, they come with limitations. For instance, UV light activation can be energy-intensive and may require specialized equipment, while transition metal catalysis may result in metal ion leaching, leading to secondary contamination. Ultrasound and freezing methods also face challenges such as high operational costs and limited scalability [23,24]. Therefore, there is an urgent need to innovate environmentally friendly and economical activators for PI to enhance the degradation of persistent organic micropollutants.
Carbon-based materials such as carbon nanotubes (CNTs) and graphene oxide (GO) have been investigated as potential activators for PI because of their abundance of oxygen-rich functional groups and large surface area [14,19]. These characteristics enable the successful activation of oxidants such as PI and the production of ROS, promoting the effective breakdown of organic pollutants [25,26]. However, research on PI activation with carbonaceous compounds is limited, and the underlying mechanisms of the degradation process are not yet fully comprehended, warranting further investigation. Moreover, the practical application of these materials is often hindered by the complexity and high cost of their synthesis. The production of GO and CNTs, for instance, typically requires costly chemical precursors (such as graphite flakes or hydrocarbon gases), involves multiple oxidation and purification steps, and demands significant energy input during processing. These factors collectively result in elevated production costs and increased environmental footprints, restricting the large-scale feasibility of such materials [27,28]. Biochar presents several advantages over other carbonaceous materials when employed as a catalyst for PI activation. One of the primary benefits of biochar lies in its economic and environmental sustainability. Biochar is typically produced via the pyrolysis of abundant and low-cost biomass waste (e.g., agricultural residues, food waste, or forestry by-products), which not only provides a value-added route for waste utilization but also reduces overall production expenses [6,29]. Although the pyrolysis process consumes energy, its cost remains considerably lower than that associated with the synthesis of advanced carbon materials.
Furthermore, biochar exhibits a highly porous structure and abundant oxygen-containing surface functional groups that facilitate electron transfer to PI, generating ROS with strong oxidative potential. Its high adsorption capacity also enables the accumulation of organic pollutants near active sites, enhancing degradation efficiency. Therefore, biochar offers a practical, cost-effective, and sustainable alternative to engineered carbon materials for large-scale PI-based oxidation systems [30,31].
In this study, biochar derived from food waste in the form of discarded courgette biomass (including flesh, seeds, and peduncles) was synthesized through pyrolysis under a nitrogen atmosphere. The resulting courgette-derived BC was evaluated as a PI activator to degrade ATZ. To the best of our knowledge, this study represents the application of the courgette-based biochar in water treatment that has not been previously fabricated in the literature. Due to its high moisture and organic matter content, improper management of courgette food waste not only results in leachate pollution but also leads to a significant loss of natural resources [32]. This study introduces a sustainable and low-cost PI activator that promotes resource recovery and supports circular waste management, while mitigating the environmental impacts of conventional food waste disposal. The synthesized BC was comprehensively characterized using advanced techniques to elucidate its structural, morphological, and surface chemical properties, thereby providing deeper insight into its potential catalytic activity. Preliminary control experiments were performed to clarify the specific role of the courgette-derived BC in activating PI and its synergistic effect on ATZ degradation. Additionally, the catalytic performance of BC toward PI activation was compared with other typical oxidants (PMS, PS, and H2O2) to determine the most efficient system for further investigations. In all experiments, the pseudo-first-order kinetic model was applied to determine the corresponding rate constants, which allowed a quantitative assessment of the ATZ catalytic efficiency. Further, the possible formation of toxic iodinated disinfection by-products (I-DBPs) during the degradation of ATZ in the BC/PI process was assessed. The effects of solution pH and temperature on ATZ degradation efficiency were individually investigated. In addition, key operational parameters, such as catalyst dosage, initial pollutant concentration, and PI concentration, were then optimized using response surface methodology (RSM) combined with a central composite design (CCD) to ensure a statistically robust evaluation of the system. On the other hand, the reusability of the catalyst was assessed over five successive cycles to evaluate its potential for long-term application in practical wastewater treatment processes. Quenching experiments were performed to identify the predominant ROS responsible for ATZ degradation in the BC/PI system, and the corresponding degradation mechanism was subsequently proposed. Furthermore, the efficiency of the BC/PI system was evaluated in different water matrices, and the influence of common coexisting constituents, such as natural organic matter (NOM) and various inorganic ions, was investigated to assess its performance under real environmental conditions. Moreover, the degradation pathways of ATZ were elucidated through the identification of its generated intermediates. In addition, the toxicity of ATZ and its degradation products was assessed to assess potential environmental risks. The universality of the system’s catalytic performance was further examined using representative model organic micropollutants. Finally, the scalability and practical feasibility of the BC/PI system were assessed using real agrochemical industrial wastewater containing ATZ, and the overall treatment cost was estimated to evaluate its economic viability for large-scale implementation.
2. Results and Discussion
2.1. Characterization of the Synthesized Biochar
Figure 1a presents the SEM micrograph of the synthesized catalyst. The surface structure of the BC appears rough and irregular, which is likely linked to its high carbon content [6]. Additionally, the observed porous structure of the prepared BC is presumably a result of gas evolution during high-temperature pyrolysis [19]. The EDS pattern of the produced BC is shown in Figure 1b. The results revealed that carbon (C) and potassium (K) were the predominant elements in the prepared BC, with mass fractions of 76.13 and 11.58%, respectively. In addition, minor quantities of oxygen (O), phosphorus (P), magnesium (Mg), calcium (Ca), chlorine (Cl), and iron (Fe) were detected, with respective mass fractions of 5.02, 3.04, 1.8, 1.29, 1.1, and 0.04%. These findings confirm that the synthesized BC is a carbon-rich material.
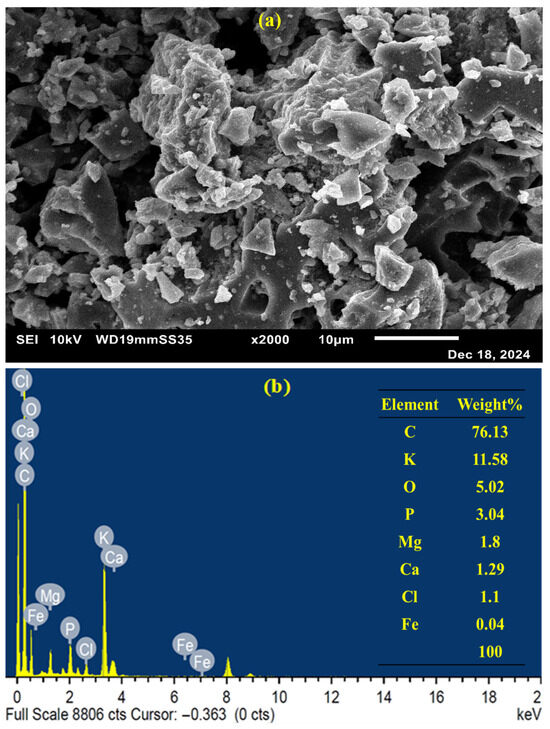
Figure 1.
(a) SEM image and (b) EDS pattern of the synthesized BC before treatment.
The broad FTIR survey of the catalyst before treatment is presented in Figure 2. The infrared peak at 3385.11 cm−1 was linked to the vibrations of the hydroxyl functional group (O–H) [16,19]. The characteristic bands observed at 1575.75 and 1389.98 cm−1 were assigned to the carbonyl group (C=O) and C=C group, respectively [16,33]. The absorption peak located at 1061.2 cm−1 corresponded to the carbon-oxygen bond of the carboxyl group (–COOH) [6,18]. The 563.4–872.87 cm−1 spectral range was matched with the C–H bond with different vibration modes [18,33]. These findings suggest that the catalyst is abundant in oxygen-containing functional groups. These functional groups likely enhance the synthesized BC’s ability to act as an activator for electron-transfer mediators and facilitate the decomposition of periodate [25]. A concise summary of the FTIR bands for the synthesized BC, along with a comparison to previously reported FTIR data from the literature and their associated functional groups, is provided in Table S1.
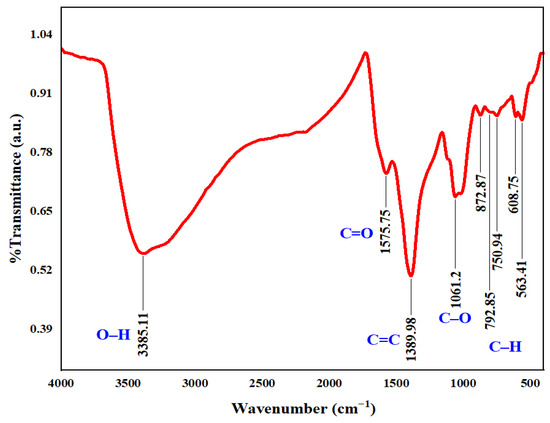
Figure 2.
FTIR full spectrum of the raw courgette-derive biochar.
The XRD diffraction peaks of the synthesized BC correspond well with those of quartz (SiO2), potassium chloride (KCl), and calcite (CaCO3), as identified from their respective reference cards: ICSD No. 01-076-0941, ICSD No. 01-075-0296, and JCPDS No. 00-001-0837 (Figure 3). The complete list of diffraction peaks for the synthesized BC, along with the standard diffraction peaks and associated Miller indices (hkl) for SiO2, KCl, and CaCO3, is provided in Table S2. The diffraction peaks observed at 2θ° = 21.9°, 25.02°, 31.98°, 35.96°, 46.42°, 53.48°, and 58.64° were ascribed to the (101), (110), (102), (200), (113), (203), and (310) planes of SiO2, respectively. On the other hand, the diffraction peaks at 2θ° = 24.46°, 28.18°,40.88°, 50°, 66.24°, and 73.58° were well matched with the (111), (200), (220), (222), (420), and (422) planes of KCl, respectively. Further, the peaks located at 2θ° = 23.04°, 29.52°, 43.28°, 57.62°, and 62.62° were attributed to (012), (104), (202), (122), and (125) crystallographic planes of CaCO3, respectively. The characteristic peaks of carbon were absent due to carbon’s amorphous nature and overlapping with other diffraction peaks [33]. Gaber et al. [33] attributed the diffraction peaks of spinach-stalk biochar to SiO2, KCl, and CaCO3. Elmitwalli et al. [18] identified similar peaks in biochars from mulukhiyah stalks and potato peels. The presence of these crystalline mineral phases can be explained by the natural composition of the courgette biomass. Silicon dioxide arises from phytoliths, which are amorphous silica bodies accumulated in plant tissues during growth and commonly retained in biochar after pyrolysis [34]. Further, CaCO3 originates from calcium absorbed by plants from soil and fertilizers, where Ca is an essential macronutrient; during thermal treatment, calcium compounds crystallize as CaCO3 [35]. Similarly, the presence of KCl is attributed to the high potassium content of the vegetable biomass, since K is a key nutrient in plants and can form crystalline salts during pyrolysis [36]. It is noteworthy that although XRD clearly revealed the crystalline phases of SiO2, elemental Si was not detected in the EDS spectrum (Figure 1b). This discrepancy arises because of its relatively low concentration. Similar findings were reported by Ngernyen et al. [37], who reported that XRD patterns of both pristine and magnetic biochar revealed crystalline minerals such as SiO2, KCl, and CaCO3. However, the Si signal in EDS remained undetectable because of its low abundance.
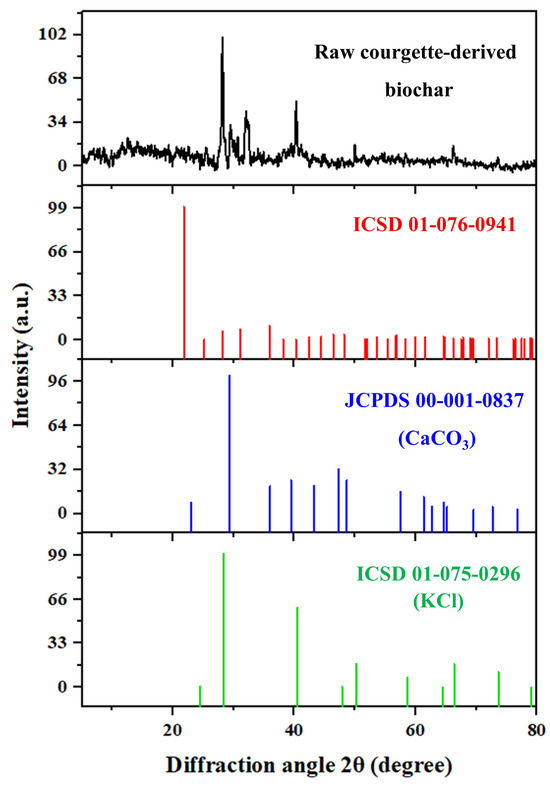
Figure 3.
XRD pattern of the raw courgette-derive biochar, with reference diffraction peaks corresponding to SiO2, CaCO3, and KCl phases.
The nitrogen adsorption–desorption isotherm of the courgette-derived biochar (Figure 4a) exhibited a Type III isotherm according to the International Union of Pure and Applied Chemistry (IUPAC) classification, confirming the presence of internal pores [38]. The corresponding pore size distribution is shown in Figure 4b. The synthesized BC exhibited a modest SBET of 13.041 m2/g with a total pore volume of 0.1516 cm3/g. The average pore diameter obtained from the BET (slit model) was 23.249 nm, while the BJH average and median pore diameters were 58.938 and 52.532 nm, respectively. These values indicate a combination of mesopores (2–50 nm) and macropores (>50 nm), resulting in a hierarchically porous structure [39]. The coexistence of meso- and macropores enhances mass transfer and facilitates the diffusion of larger organic molecules during catalytic processes [40]. Zhang et al. [41] reported that biochars with modest surface areas within the range of single digits to about 10–20 m2/g can achieve excellent performance in catalytic applications. This activity is primarily attributed to their well-developed pore connectivity, surface heterogeneity, and the abundance of functional groups that promote electron transfer and active radical generation.
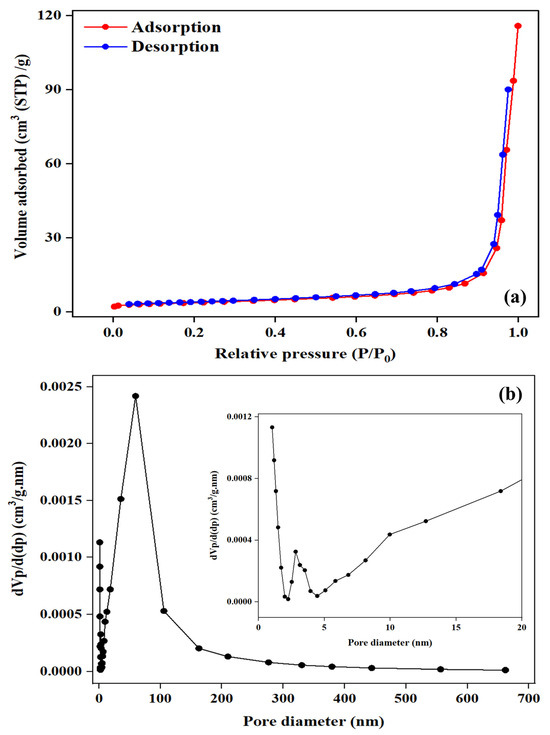
Figure 4.
(a) BET adsorption–desorption isotherm of the courgette-derived BC and (b) BJH pore size distribution profile (inset: enlarged view of the 1–20 nm range).
The pHpzc of the synthesized BC was quantified as 4.25 (Figure S1), highlighting the positive charge on the BC surface at pH < 4.25 and a negative charge at pH higher than this threshold. ATZ has a pKa of 1.68, meaning its amine group is protonated and positively charged at pH values below 1.68. Above this pH, ATZ exists chiefly in its neutral state [42]. At pH < 1.68, both ATZ and the BC surface are positively charged, resulting in electrostatic repulsion that hinders adsorption. In the pH range between 1.68 and 4.25, ATZ remains neutral while the BC surface is positively charged. Although electrostatic interactions are minimal in this range, adsorption may still occur through van der Waals forces and hydrophobic interactions [43,44]. At pH values above 4.25, the BC surface becomes negatively charged, while ATZ remains neutral. In this scenario, although direct electrostatic attraction is absent, adsorption may still be facilitated by polar interactions or hydrogen bonding [45].
2.2. Preliminary Control Experiments
2.2.1. Synergistic Effect of BC on PI Activation
The catalytic performance of the synthesized BC in activating PI was evaluated by comparing three reaction systems: BC alone, PI alone, and the combined BC/PI system, to evaluate the biochar synergistic activation effects. The experiments were carried out for 60 min at an initial ATZ concentration of 10 mg/L, a BC dosage of 0.5 g/L, a PI concentration of 2 mM, ambient temperature, and neutral pH. As shown in Figure 5a, the single BC system achieved an ATZ reduction of only 15.25% due to its modest surface area at 1000 rpm. The decrease in mixing speed to 200, 400, 600, and 800 rpm attained removal ratios of 6.3, 8.4, 9.1, and 10.3%, respectively, whereas the increase in mixing speed above 1000 rpm only achieved 15.6% removal efficiency. Therefore, 1000 rpm was determined as the optimal mixing speed due to its ability to attain sufficient interaction between the biochar and ATZ. In the sole PI system, 41.91% ATZ removal was achieved, primarily attributed to PI oxidation. The combined presence of BC and PI within the BC/PI system led to a marked improvement in ATZ removal percentage (74.1%). The limited ATZ removal and minor BC adsorption capacity (3.05 mg/g) in the single BC system demonstrated the slight role of self-adsorption in ATZ elimination. This can be ascribed to the lack of charge-charge interactions among the negatively charged BC (pHpzc = 4.25) and the neutral ATZ molecules (pKa = 1.68) under the experimental conditions (pH = 7) [46]. Nonetheless, the polar or hydrogen bonding interactions, along with the high porosity of the synthesized BC, likely mitigate this limitation. These attributes enhance the availability of adsorption sites and extend the contact duration, thereby promoting efficient mass transfer [47]. The modest degradation of ATZ observed when using PI alone suggests that the generated ROS were insufficient to drive the degradation process effectively which may be due to the relatively lower redox potential of PI (+1.6 V vs. NHE) [11]. Further, the PI consumption in the case of sole PI without an activator was limited, where the concentration of PI decreased from 2 mM to 1.8 mM, and iodate ion concentration was around 0.2 mM, which indicated the limited generation of iodate radicals and the modest degradation efficiency in this system. The enhanced catalytic efficiency of the BC/PI system is closely correlated with the physicochemical characteristics of the synthesized biochar and the production of ROS through PI activation at the active sites of BC. The SEM analysis revealed a rough and irregular surface with a hierarchically porous architecture (Figure 1a), which facilitates mass transfer and provides abundant contact sites for PI and ATZ molecules. The moderate surface area (13.041 m2/g) and the coexistence of meso- and macropores (23–59 nm) promote diffusion of reactants and the retention of reactive species, thereby accelerating oxidation. The EDS and XRD analyses demonstrated the presence of surface minerals such as CaCO3, KCl, and SiO2 (Figure 1b and Figure 3), which introduce electron-rich or positively charged sites that can facilitate electron transfer and enhance PI adsorption. Particularly, Ca2+ species on the BC surface improve the electrostatic affinity toward the negatively charged PI anions, thereby promoting its activation [6,12]. Moreover, the FTIR spectra (Figure 2) confirmed the presence of abundant oxygenated functional groups (–OH, –COOH, and C=O), which act as active redox centers for electron donation to PI. These groups facilitate the cleavage of the I–O bond in PI (bond length ≈ 1.78 Å), leading to the generation of multiple ROS, including •IO3, •OH, O2• −, and 1O2, as illustrated in Equations (1)–(6) [18]. The amorphous carbon matrix and surface heterogeneity further enhance charge transfer efficiency between BC and PI, supporting continuous ROS regeneration. The findings mentioned above underscore the indispensable roles of both BC and PI in the BC/PI oxidative system for ATZ degradation.
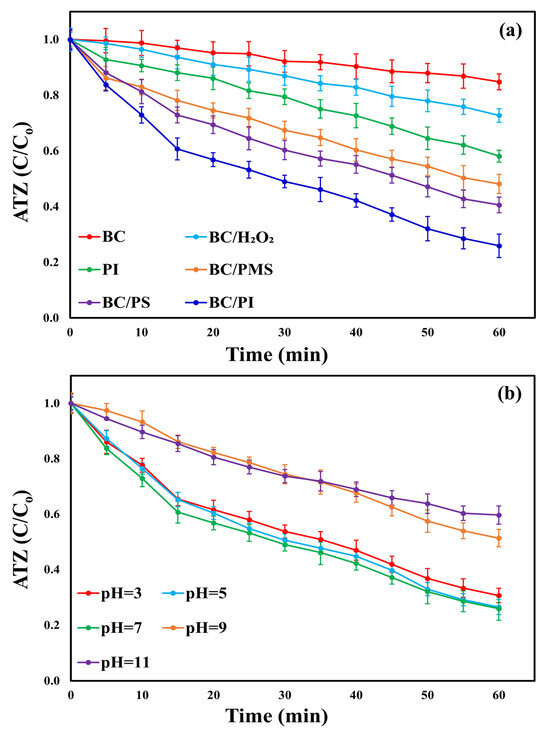
Figure 5.
(a) ATZ deterioration performance across different processes (Settings: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C) and (b) ATZ degradation at different pH values using the BC/PI system (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, and T = 25 ± 0.5 °C).
Additionally, the kinetic constants for pseudo-first-order reactions were determined to assess the effectiveness of BC in facilitating ATZ removal. The K values for the three systems (individual BC, sole PI, and combined BC/PI) were 0.0026 (R2 = 0.9942) min−1, 0.0085 (R2 = 0.9964) min−1, and 0.023 (R2 = 0.9934) min−1, respectively, as presented in Figure S2. The higher kinetic rate constants observed in the BC-catalyzed PI process, compared to the individual PI and BC systems, highlight the strong catalytic activity of BC toward PI, underscore the significant synergistic effect between PI and BC in the ATZ oxidative degradation, and validate the system’s oxidative efficiency in degrading various organic micropollutants. Moreover, the BC/PI system exhibited an ATZ degradation rate constant comparable to pollutants’ degradation rate constants reported in earlier biochar-based PI activation studies [26,28]. This observation underscores the comparable catalytic proficiency of the synthesized BC in facilitating PI activation.
Further, for a TOC level of 4.16 mg/L, the TOC mineralization efficiencies in the sole BC, single PI, and combined BC/PI systems were 6.92, 28.49, and 50.58%, respectively, as shown in Figure S3. These outcomes demonstrate that the BC-activated PI system achieved significantly greater mineralization compared to either PI or BC alone. The high TOC mineralization for the BC/PI degradation system could be owing to the generated reactive species that can attack ATZ and mineralize to simpler by-products and/or harmless compounds (e.g., CO2, H2O) as shown in Section 2.10. However, the lower TOC mineralization ratio, relative to the ATZ degradation percentage in the BC-catalyzed PI system, is likely due to ATZ intermediates that could generate during the degradation process, which were not fully mineralized [6,48]. This observation aligns with the findings by Naddeo et al. [49] and Li et al. [50], who reported lower TOC mineralization ratios than degradation percentages of organic pollutants in PI activation systems. Nevertheless, the current study exhibits a higher TOC mineralization ratio compared to previously reported PI-based AOPs. However, this cannot be considered as a superiority over other reports due to the different conditions in the previous PI-based AOPs, such as the pollutant concentration, PI dose, catalyst dose, and initial TOC concentration. For instance, Xiong et al. [10] achieved only 18.5% TOC removal despite 83.5% tetracycline degradation in the CuFeS2/PI system at PI dose of 0.8 mM, catalyst dose of 0.3 g/L, and pollutant concentration of 50 mg/L, while Li et al. [11] observed 45% TOC mineralization (Initial TOC 17.86 mg/L) alongside 82% decolorization of acid orange 7 dye in a granular activated carbon-PI activated system at PI dose of 10 mM and catalyst dose of 1 g/L.
Furthermore, the synergistic ratio of the BC-activated PI system for ATZ degradation was determined to be 2.07 (>1), indicating a pronounced synergistic interaction between BC and PI. This result confirms the enhanced performance of the combined BC/PI system over the individual use of BC or PI in improving PI activation and accelerating ATZ degradation [3,21].
2.2.2. Catalytic Activity of BC with Various Oxidants
The catalytic performance of the synthesized BC was assessed in conjunction with commonly used oxidizing agents in AOPs, including PMS, PS, and H2O2. The experiments were conducted under identical conditions with an initial ATZ concentration of 10 mg/L, a BC dosage of 0.5 g/L, an oxidant concentration of 2 mM, ambient temperature, and neutral pH. The ATZ degradation ratios for the BC-catalyzed PI, PS, PMS, and H2O2 treatment processes were 74.1%, 59.42%, 51.86%, and 27.28%, respectively (Figure 5a). The corresponding K values were 0.023 (R2 = 0.9934) min−1, 0.0156 (R2 = 0.9943) min−1, 0.0126 (R2 = 0.9931) min−1, and 0.005 (R2 = 0.9971) min−1, as presented in Figure S2. These results underscore the synthesized BC’s high efficiency in activating the tested oxidants and degrading ATZ, highlighting its broad catalytic potential. Additionally, the findings establish the relative oxidation capability of the investigated oxidants by the synthesized BC as follows: PI > PS > PMS > H2O2, consistent with previous BC-catalyzed AOPs [18,27]. The BC/H2O2 system exhibited the lowest ATZ degradation efficiency among the tested degradation processes, likely due to the strong O–O bond in H2O2, with a bond strength of approximately 213 kJ/mol, which hinders its cleavage by the synthesized BC [51]. The primary reactive species identified in the BC/H2O2 system were •OH radicals, while SO4• − radicals were predominant in the BC-activated PS and PMS systems, as shown in Figures S4–S6. In these experiments, specific radical scavengers (50 mM) were used to confirm the roles of different reactive oxygen species: tert-butanol for •OH, furfuryl alcohol for 1O2, and chloroform for O2• − [52,53]. Methanol was employed to simultaneously quench both SO4• − and •OH [54]. The reduced oxidation potential and half-life of •OH compared to SO4• − may explain the superior ATZ degradation efficiency observed in the BC-catalyzed PS and PMS systems relative to the BC/H2O2 system [53]. Additionally, the ATZ removal efficiency in the BC-catalyzed PS process surpassed that of the BC-catalyzed PMS process, potentially due to the weaker O–O bond in PS (d = 1.497 Å) compared to PMS (d = 1.453 Å). The weaker O–O bond in PS facilitates its activation and cleavage, enabling a more effective generation of ROS than PMS [24]. The consumption ratios of H2O2, PMS, PS, and PI in the BC-catalyzed AOPs were 23.53%, 52.02%, 65.63%, and 83.62%, respectively, as shown in Figure S7. PI exhibited the highest consumption ratio among the investigated oxidants, indicating the synthesized BC’s strongest affinity for PI. This affinity facilitated the generation of a greater quantity of ROS, resulting in enhanced oxidation performance and higher ATZ removal efficiency [27]. Furthermore, the long I–O bond length in the PI molecule (1.78 Å) contributes to its easier activation and cleavage, promoting ROS production [24]. The effective interaction between PI and the biochar could be further due to the structure of the biochar, where it has positive calcium ions on the surface that can improve the affinity of PI towards the biochar’s surface. Consequently, PI proved to be the most effective tested oxidant, with the BC/PI system demonstrating superior oxidation performance compared to the other oxidation methods. Therefore, the BC/PI system was selected for subsequent experiments.
2.3. Assessment of Iodinated By-Product Formation
A critical challenge in implementing PI activation processes for water treatment lies in the potential generation of I-DBPs during the degradation process. Toxic I-DBPs, such as hypoiodous acid (HOI), iodide ions (I−), molecular iodine (I2), and triiodide ion (I3−), are of particular concern due to their high reactivity and potential to form secondary toxic compounds. These species can participate in halogenation reactions with NOM or pollutants, generating organoiodine compounds that are more toxic, persistent, and less biodegradable. Prolonged exposure to such I-DBPs has been associated with adverse effects on human health, including thyroid dysfunction, cytotoxicity, genotoxicity, and potential carcinogenicity. Therefore, their formation during PI-based AOPs must be carefully avoided to ensure both environmental safety and public health protection [15,55]. In this study, the potential generation of harmful iodine-containing species throughout ATZ degradation in the BC/PI system was evaluated by monitoring the concentrations of IO4− and IO3− ions under the following conditions: an initial ATZ concentration of 10 mg/L, a BC dosage of 0.5 g/L, a PI concentration of 2 mM, ambient temperature, and neutral pH (Figure S8). The results demonstrated that during ATZ degradation, IO4− was gradually consumed, accompanied by a corresponding increase in IO3− concentration, indicating a nearly complete stoichiometric conversion of IO4− to IO3−. This observation implies that the formation of potentially hazardous reactive iodine species did not occur. Instead, the process predominantly generated IO3−, a stable and nontoxic iodine species typically present in iodized salt. Further, this supports the conversion of most of PI ions to iodate radicals which explains the high degradation efficiency in the BC/PI system. These outcomes affirm the sustainability and safety of the proposed treatment process, highlighting its suitability for real-world water purification applications without posing risks to public health or the environment. Similar results were noted in prior PI-based AOP studies [17,22,23].
2.4. Effect of pH
The pH value significantly influences the distribution of reactive species and thereby affects pollutant degradation [56]. A sequence of tests was employed at different pH levels to investigate the impact of pH on ATZ breakdown ratios and kinetic rate constants within the BC/PI process, as shown in Figure 5b and Figure S9, respectively. The experiments were performed for 60 min under the following conditions: ([ATZ]o = 10 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.5 g/L, and [PI]o = 2 mM). ATZ degradation ratios were 69.35, 73.53, 74.1, 48.67, and 40.33% at pH levels of 3, 5, 7, 9, and 11, respectively. The associated rate constants were 0.0201 (R2 = 0.9948) min−1, 0.022 (R2 = 0.996) min−1, 0.023 (R2 = 0.9934) min−1, 0.0106 (R2 = 0.9938) min−1, and 0.0092 (R2 = 0.9964) min−1.
The findings demonstrate that ATZ degradation in the BC/PI system is more efficient under neutral and acidic conditions, while significant inhibition is observed under alkaline conditions. This behavior may be due to the reduced reduction potential of the PI species in basic solutions (+0.7 V vs. NHE) compared to its higher potential in acidic solutions (+1.6 V vs. NHE) [11]. Furthermore, under strongly acidic conditions, hydrogen ions (H+) interact with unpaired electrons on the synthesized BC surface, generating hydrogen atoms (H•), which subsequently react with IO4− to produce IO3•, as described in Equations (7) and (8) [57]. However, the generated H• can also scavenge IO3•, reducing it to IO3−, as outlined in Equation (9) [11]. In contrast, the decline in ATZ degradation efficiency and reaction rate constants in alkaline conditions is attributed to the formation of hydrogen diperiodate ions (H2I2O104−), which is the dominant PI species at pH > 8. This species exhibits lower reactivity and reduced redox potential compared to IO4− [20,58]. These findings align with previous PI based-AOPs [16,18]. The ATZ removal ratios in acidic and neutral solutions showed no significant difference. As a result, subsequent investigations were carried out under neutral pH to reduce the cost associated with pH adjustments.
This pH-dependent behavior can be rationalized by the interplay between the structural and surface properties of the synthesized biochar and the chemical speciation of PI. The synthesized BC possesses a pHpzc of 4.25, meaning that its surface carries a positive charge under acidic conditions and becomes negatively charged at higher pH. At pH ≤ 7, the positively charged BC surface electrostatically attracts the negatively charged IO4− species, promoting their adsorption and subsequent activation on active sites enriched with oxygen-containing functional groups (–OH, –COOH, C=O) as confirmed by FTIR analysis (Figure 2). These groups facilitate electron transfer from the biochar to PI, promoting the generation of ROS such as •IO3, •OH, O2• −, and 1O2, which drive ATZ oxidation. On the other hand, the presence of calcium on the biochar surface, as evidenced by the XRD pattern (Figure 3) and EDS spectrum (Figure 1a), can promote interactions between the negatively charged PI anions and the biochar surface under acidic and neutral conditions, while the degradation efficiency declined at alkaline conditions owing to the competition between PI and hydroxyl ions on the biochar’s surface. The enhancement of the interaction between the biochar and PI at neutral and acidic conditions can improve the generation of reactive species, thereby ameliorating the degradation performance.
2.5. Influence of Temperature
The removal efficiency of ATZ was assessed across different values of reaction temperatures (25, 35, 45, 55, 65, and 75 °C) in the BC/PI system under the following conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and a treatment period of 60 min. As illustrated in Figure 6a, ATZ degradation increased slightly with temperature, achieving efficiencies of 74.1, 76.73, 77.92, 80.08, 83.7, and 86.06% at 25, 35, 45, 55, 65, and 75 °C. The corresponding reaction rates increased from 0.023 (R2 = 0.9934) min−1 at 25 °C to 0.0247 (R2 = 0.9925) min−1, 0.0256 (R2 = 0.9922) min−1, 0.0273 (R2 = 0.9916) min−1, 0.0303 (R2 = 0.991) min−1, and 0.0326 (R2 = 0.9907) min−1 at 25, 35, and 45 °C, respectively (Figure S10). This increase in ATZ removal and kinetic rate constants with temperature is likely due to thermal activation and increased molecular motion, which facilitates PI activation, generates more ROS, and improves ATZ degradation [19]. Similar results were documented by Chen et al. [14], who observed increased tetracycline removal efficiency and K values with rising temperatures in a system using nitrogen-doped carbon nanotubes encapsulating cobalt (Co@NCNTs) for PI activation. The enhancement in ATZ degradation with temperature can be attributed not only to thermal activation but also to the intrinsic structural and compositional characteristics of the biochar catalyst. The courgette-derived BC possesses a rough and irregular surface with a hierarchical pore structure comprising both mesopores and macropores. Such textural features facilitate efficient diffusion and adsorption of ATZ molecules, ensuring that temperature-induced molecular motion translates effectively into enhanced surface interactions [59]. Moreover, the BC surface is rich in oxygen-containing functional groups, which serve as active sites for electron transfer and radical generation during PI activation. These functional moieties likely become more reactive at elevated temperatures, promoting faster electron exchange and thereby enhancing the formation of ROS, which are primarily responsible for ATZ oxidation [6,12]. In addition, the mineral phases identified in the XRD analysis (SiO2, KCl, and CaCO3) may contribute synergistically to the catalytic process. Calcium carbonate can facilitate surface redox reactions by providing active sites that promote electron exchange and enhance interfacial charge transfer, while SiO2 provides structural stability and enhances surface roughness, thereby increasing the available reaction interface. The presence of KCl also promotes ionic conductivity, which may accelerate the charge transfer processes during PI activation [60,61,62]. Collectively, these physicochemical features explain the observed temperature-dependent enhancement in catalytic performance, beyond simple kinetic acceleration due to increased molecular motion. The Arrhenius equation (Equation (10)) was implemented to determine the activation energy (Ea) for the oxidative degradation of ATZ in the BC/PI system [63]. As shown in Figure S11, the Ea for ATZ removal in the BC/PI process was calculated as 5.93 kJ/mol. This value is significantly less than the Ea results reported for ATZ degradation in previously studied AOPs (Table S3). For instance, the heat/PS system reported by Ji et al. [2] exhibited an Ea of 141 kJ/mol, while the heat/PS system reported by Jiang et al. [1] showed a similarly high Ea of 102 kJ/mol. In comparison, biochar/nanoscale zero-valent iron (nZVI)/PS systems, such as soybean stalk biochar/nZVI/PS and soybean straw-derived biochar/sulfurized nZVI/PS, showed activation energies of 83.34 and 47.63 kJ/mol, respectively [64,65]. The remarkably low Ea obtained in this study underscores the superior energy efficiency of the BC/PI system and reflects the intrinsic reactivity of the biochar surface. The presence of abundant surface defects, heteroatoms, and redox-active mineral phases facilitates spontaneous PI activation with minimal external energy input. Consequently, the BC/PI system demonstrates a highly favorable thermodynamic profile for ATZ oxidation, confirming that the unique structural and chemical attributes of the courgette-derived BC play a pivotal role in achieving efficient catalytic performance even under mild conditions.
where K is the kinetic rate constant (s−1), T indicates the reaction temperature (Kelvin), R indicates the molar gas constant (R = 8.3145 J/mol.K), and A is the pre-exponential factor.
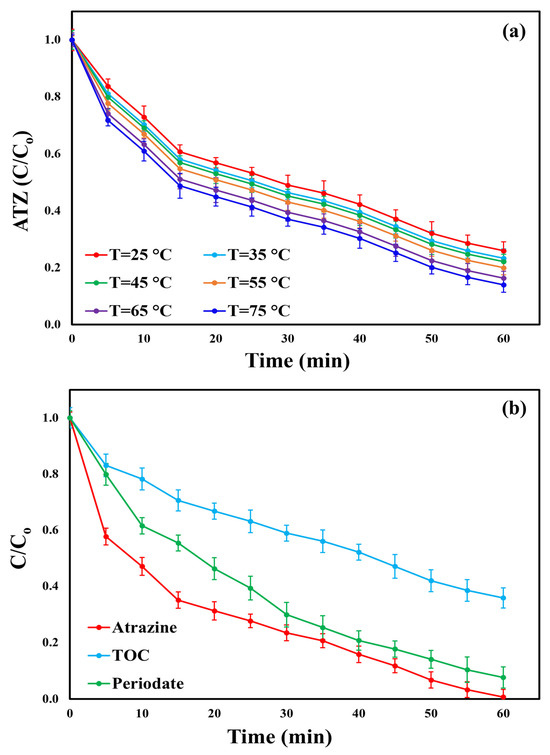
Figure 6.
(a) ATZ degradation at different temperatures using the BC/PI system (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, and pH = 7) and (b) ATZ degradation, TOC mineralization, and PI consumption ratios utilizing the BC/PI system under the optimal settings (pH = 7, [ATZ]o = 7.3 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM).
Given the minor variations in removal efficiency with increasing temperature, subsequent experiments were performed at 25 °C to represent environmentally relevant and energy-efficient operational conditions.
2.6. Model Generation
2.6.1. Statistical Analysis
Equation (11) represents the quadratic model relating ATZ removal efficiency to the ATZ starting concentration (X, mg/L), PI initial concentration (Y, mM), and the BC dosage (Z, g/L). The high R2 value of 0.9494 indicates a strong correlation and demonstrates the model’s effectiveness in capturing the influence of operating parameters on ATZ removal. However, the obtained model is restricted to the ranges of operating parameters in Table 1, as the model was developed based on the experiments in Table 2, and this model cannot describe the prediction of an experiment outside this range.
ATZ removal efficiency = 52.5 − 5.21 X − 2.1 Y + 123.4 Z + 0.331 X2 + 6.04 Y2 + 7.1 Z2 − 0.917 XY − 4.19 XZ − 4.4 YZ

Table 1.
Settings of the practical design.

Table 2.
Experimental and predicted ATZ removal efficiencies under varying operational conditions.
The measured ATZ removal ratios and the corresponding removal percentages calculated from the polynomial equation across the 20 experiments are summarized in Table 2. The minimal deviation between the measured and calculated ATZ removal percentages validates the reliability and accuracy of the model. The ANOVA was conducted using Minitab® 22 to verify the statistical adequacy, predictive reliability, and overall significance of the developed response surface model, the results of which are summarized in Table 3. The R2 value of 94.94% and the adjusted R2 of 90.38%, both approaching unity, demonstrate the strong reliability and predictive capability of the regression model. Additionally, the minimal difference between the two coefficients (4.56%) reflects excellent agreement between the predicted and experimental values, confirming that the fitted polynomial model provides a statistically sound representation of the system. This consistency validates the model’s adequacy in describing the effects of the experimental variables on ATZ removal efficiency within the BC/PI system. Furthermore, the high R2 value indicates that approximately 94.94% of the total variation in ATZ removal efficiency is captured by the model, with only about 5% remaining unexplained, thereby reinforcing its robustness. The model’s overall statistical significance was further supported by a high F-value (20.83) and a very low p-value (<0.05), confirming the strong correlation between the observed and predicted responses (Table 2). In addition, most of the model terms exhibited p-values below 0.05, signifying that they exerted statistically significant influences on the response. Among the examined operational parameters, the BC dose had the most substantial impact on ATZ removal efficiency. This is reflected by the highest F-value of 83.84. The initial ATZ concentration ranked second in significance, showing a notable influence with an F-value of 51.49. In contrast, the initial PI concentration had the least effect on ATZ removal efficiency. This is evident from the lowest F-value of 38.53, as depicted by the ANOVA results.

Table 3.
The ANOVA results of the RSM-CCD model.
2.6.2. Validation of the Model
The optimal operating values of the independent parameters were identified using the response optimizer function in the Minitab® 22 statistical software, with the objective of maximizing the ATZ removal efficiency as the target response. The resulting optimal values were 7.3 mg/L for [ATZ]o, 2.7 mM for [PI]o, and 0.55 g/L for [BC]o, as outlined in Table 4. These optimized conditions were subsequently employed in the following experimental investigations.

Table 4.
Data derived from the RSM-CCD model.
To further validate the reliability of the generated model, an additional investigation was conducted utilizing the BC/PI process under the optimum conditions (pH = 7, [pollutant]o = 7.3 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM) for a 60 min process time. The measured ATZ degradation efficiency of 99.35% (Figure 6b) was closely aligned with the calculated value of 99.19%, as presented in Table 4. This strong agreement reinforces the model’s precision and applicability. The pseudo-first-order kinetic rate constant of ATZ removal was 0.0601 (R2 = 0.9462) min−1 (Figure S12). Additionally, the TOC mineralization ratio of ATZ reached only 64.23% (Figure 6b). Complete mineralization could not be attained because of the formation of ATZ intermediates, which maintain a high total organic load in solution and require extended time for generating additional ROS to further degrade these by-products [18]. The PI consumption ratio of 92.38% (Figure 6b) demonstrates the efficient activation of PI within the BC/PI system. This activation generates ROS, which is crucial in promoting ATZ breakdown and facilitating TOC mineralization. To demonstrate the catalytic efficiency of the courgette-derived BC, a performance comparison between the synthesized catalyst and previously reported materials used for PI activation in the degradation of various organic pollutants has been conducted. As explained in Table 5, the comparison indicates that the courgette-derived BC exhibits comparable or even superior catalytic performance, underscoring its potential as a sustainable and efficient activator for PI-based oxidation processes.

Table 5.
Performance comparison of courgette-derived biochar with previously reported materials for PI activation in the degradation of various organic pollutants.
Furthermore, a comparison of the BC/PI system developed in this study with previously reported AOPs for ATZ degradation was carried out to demonstrate its superior efficiency and practical applicability. As summarized in Table 6, the BC/PI system achieved a 99.35% ATZ removal efficiency within 60 min under the conditions ([PI]o = 2.7 mM, [ATZ]o = 7.3 mg/L, pH = 7, [BC]o = 0.55 g/L, and T = 25 ± 0.5 °C). On the other hand, the PS activation system using spinach-derived biochar achieved a slightly higher degradation ratio of 99.8%; however, it required 120 min to reach this efficiency, which is double the time required by the BC/PI system [6]. Similarly, the photocatalysis system utilizing graphene-promoted graphitic carbon nitride (g-C3N4) nanosheets achieved complete degradation (100%), but required 300 min, highlighting the BC/PI system’s significant advantage in degradation speed [67]. Other methods, such as the PMS activation process employing copper cobalt oxide (CuCo2O4) nanoparticles, exhibited a comparable removal of 99% within 30 min [9]. However, experimental settings like the slightly acidic pH of 6.8 and the specific catalyst used, may limit its applicability. In contrast, The BC/PI system operates under a neutral pH, which is more environmentally friendly and versatile for real-world applications. In comparison to less effective AOPs, the Fenton-like process using nZVI achieved only a 50% ATZ removal ratio within 120 min [8]. Additionally, the electrooxidation/H2O2 system demonstrated a lower removal efficiency of 45.42% in 90 min [68]. These methods not only required more time but also exhibited reduced degradation performance, underscoring the superior efficiency and kinetics of the BC/PI system. Furthermore, while the ozone oxidation process employing cerium dioxide (CeO2) achieved rapid degradation with a degradation percentage of 85.5% in just 10.0 min, its overall efficiency was lower than that of the BC-catalyzed PI process [4]. The suggested process combination of high efficiency, reasonable kinetics, and compatibility with neutral pH conditions highlights its potential as a promising and practical approach for ATZ degradation.

Table 6.
Performance comparison of the BC/PI system with other AOPs for ATZ degradation.
2.6.3. Effects of Independent Parameters
The effects of the investigated parameters in the RSM-CCD model (ATZ concentration, PI concentration, and BC initial dose) on ATZ degradation efficiency are depicted in Figure 7. The results revealed that increasing BC dosage up to the optimal level of 0.55 g/L enhanced ATZ removal efficiency. Increasing the initial BC dose enhances the BC surface area, thereby expanding the active sites available for ATZ adsorption and PI activation. As a result, the generation of ROS is intensified, significantly promoting the oxidative degradation of ATZ [29]. Moreover, increasing the BC dose increases the number of oxygen-containing functional groups in the reaction solution, which in turn facilitates the generation of additional ROS [69]. However, exceeding the optimum dosage led to a decline in ATZ removal efficiency, likely due to the agglomeration of BC particles. Such aggregation diminishes the beneficial effect of overdosing by reducing the active surface area and, consequently, limits the generation of ROS, thereby weakening the system’s oxidative performance [25].
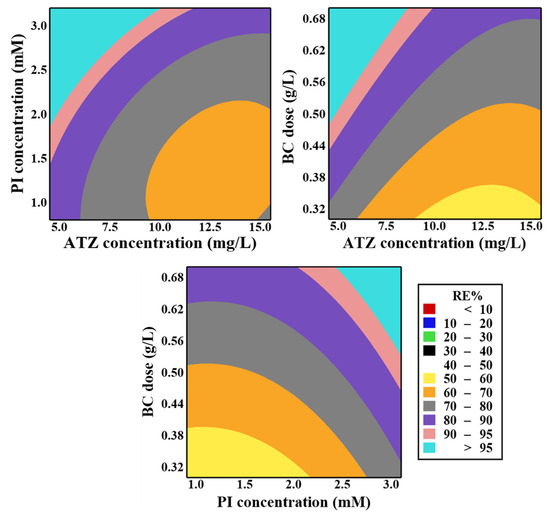
Figure 7.
Contour curves of the impacts of [ATZ]o, [BC]o, and [PI]o on ATZ degradation ratio employing the BC-catalyzed PI process.
At lower ATZ concentrations (<7.3 mg/L), its degradation efficiency improved because the number of generated ROS is sufficient relative to the fewer ATZ molecules. This ensures more effective interactions and facilitates the degradation process [33]. Conversely, when the ATZ concentration surpassed 7.3 mg/L, its degradation efficiency declined. This decrease can be attributed to the increased number of ATZ molecules, which demand a greater quantity of ROS to achieve effective degradation [6]. Additionally, increased ATZ concentrations lead to greater production of its by-products, which compete with ATZ for active sites, thereby reducing the ATZ removal efficiency [19]. Moreover, increasing the initial concentration of ATZ may result in its buildup on the reactive locations of the BC, potentially hindering the generation of ROS [59].
Elevating the PI concentration up to its optimal concentration of 2.7 mM improved the efficiency of ATZ removal. This improvement can be attributed to the accelerated catalytic oxidation that is facilitated by the elevated number of IO4− ions in the reaction solution, which promotes the generation of ROS, thereby increasing ATZ degradation [30]. Nevertheless, when the PI concentration was oversupplied (>optimal level), the ATZ removal efficiency declined. Excess IO4− may compete with ATZ and its intermediates for reactive species, forming less reactive by-products. It reacts with •OH and •IO3, diverting them from degradation pathways and reducing their availability for ATZ oxidation (Equations (12) and (13)) [70,71]. Additionally, excess IO4− promotes the formation of stable compounds such as dimeric iodate (I2O6) and dimeric periodate (I2O8), which do not contribute to oxidative reactions (Equations (14) and (15)) [18]. Furthermore, at elevated PI concentrations, the active sites on the BC surface become insufficient to achieve complete PI activation, resulting in decreased ATZ removal efficiency [17].
2.7. Recyclability of the Synthesized Biochar
Recycling experiments were conducted for five successive runs under the optimum conditions to evaluate the reusability of the synthesized biochar. BC particles were collected after each cycle by vacuum filtration with a 0.22 μm membrane filter and centrifuged at 3000 rpm for 20 min, after which the supernatant was discarded. The catalyst powder was thoroughly washed and centrifuged multiple times with ultrapure water and ethanol until the supernatants became colorless, ensuring the removal of any adsorbed pollutant particles. This process ensured the removal of any adsorbed pollutant particles. The cleaned BC powder was subsequently dehydrated at 60 °C for 24 h. For each reuse cycle, the dried powder was introduced into a freshly prepared ATZ degradation solution [29,48]. The yielded ATZ removal efficiencies across the successive runs were 99.35, 97.5, 95.33, 92.03, and 90.27%, respectively, corresponding to an overall reduction of approximately 9% in ATZ removal efficiency, as shown in Figure 8a. In comparison to previous studies on biochar-based PI activation, He et al. [16] observed a 17.5% decline in diclofenac sodium removal efficiency after five repeated cycles (80 min) employing the UV/sewage sludge-derived biochar/PI system. Additionally, Seo et al. [31] reported a 20% reduction in organic pollutant degradation efficiency after five consecutive runs (60 min) utilizing the seaweed-derived biochar/PI system. In contrast, the limited decrease in ATZ removal efficiency in the current study highlights the superior stability and reusability of the synthesized BC. This high reusability likely results from the wealth of functional groups on the BC surface that facilitate strong interactions with PI and support repeated cycles of effective electron transfer without significant breakdown of the BC’s structure. These groups also provide reactive sites that help retain PI molecules, thus enabling efficient degradation of contaminants over multiple uses. Additionally, the porous structure of BC enhances its ability to adsorb both PI and the target pollutant, maximizing the contact area and interaction frequency necessary for catalytic reactions [17,29]. However, minor declines in ATZ degradation efficiency over multiple cycles may be attributed to the gradual loss of BC particles during the recovery process [71]. As depicted in Figure S13, the mass of BC introduced into the fresh ATZ solution exhibited a gradual decline over successive run. Initially set at 0.55 g/L, the BC mass decreased to 0.5336, 0.51876, 0.4969, and 0.4803 g/L, corresponding to catalyst mass loss percentages of 2.98, 5.71, 9.66, and 12.67% after the second, third, fourth, and fifth runs, respectively. Additionally, blockage of the active sites throughout the repeated runs by ATZ or its generated by-products could decrease the ATZ removal ratios across the cycles [18]. The degradation kinetics were evaluated across the five consecutive runs, yielding reaction rate constants of 0.0601 (R2 = 0.9462) min−1 for the 1st run, 0.0516 (R2 = 0.9797) min−1 for the 2nd run, 0.046 (R2 = 0.9874) min−1 for the 3rd run, 0.0402 (R2 = 0.99) min−1 for the 4th run, and 0.0377 (R2 = 0.9903) min−1 and for the 5th run (Figure S14). These rates align well with the observed ATZ removal efficiencies across the runs. The high R2 values demonstrate strong correlations, confirming consistent degradation performance throughout the cycles. Furthermore, SEM, EDS, and XRD analyses of the BC particles after five consecutive degradation cycles were conducted (Figure 8b,c). No significant changes were observed in the XRD diffraction peaks, EDS composition, or SEM morphology of BC after degradation, indicating that the catalyst maintained its stability across the five runs. Thus, the system demonstrated excellent reusability, with only minor performance losses mainly due to catalyst recovery limitations rather than intrinsic deactivation. The decreased porosity in SEM images and the reduced intensities of XRD peaks confirmed ATZ adsorption on the BC surface.
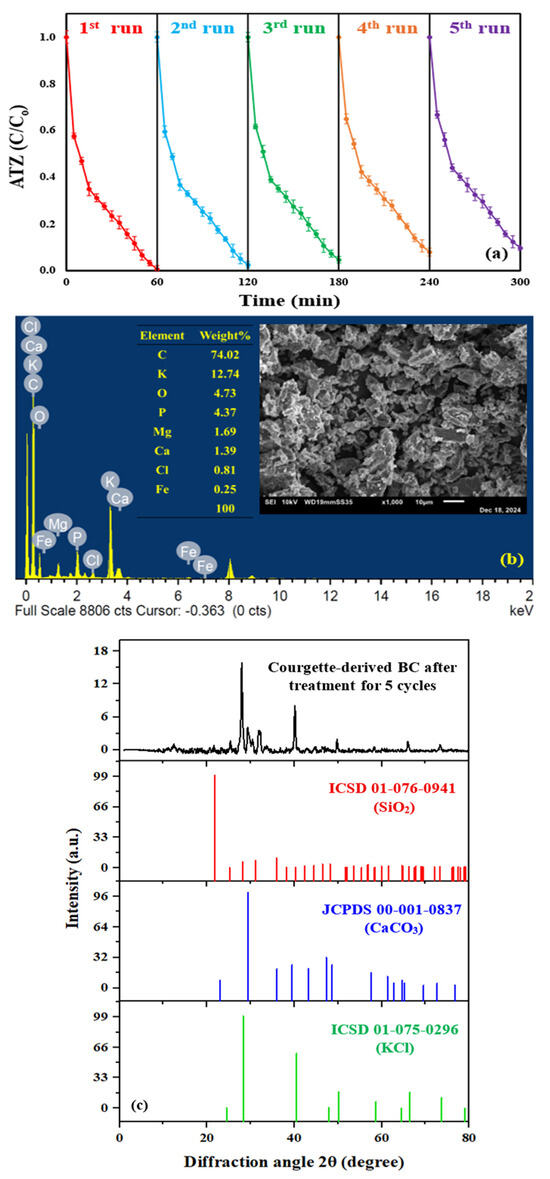
Figure 8.
(a) ATZ degradation ratios using the BC/PI system in five successive runs (pH = 7, [Pollutant]o = 7.3 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM), (b) EDS pattern of the synthesized BC after treatment for five successive cycles (inset: SEM image), and (c) XRD pattern of the courgette-derive biochar after treatment.
2.8. Identification of Dominant ROS in the BC/PI System
To elucidate the relative contribution of ROS to ATZ degradation within the proposed system, quenching experiments were conducted under the optimum reaction conditions. Excess amounts (50 mM) of several scavenging agents were added to the ATZ reaction mixture prior to initiating the reaction to perform quenching tests. The scavengers used included potassium iodide, chloroform, tert-butanol (kinetic rate: 7.6 × 108 M−1S−1), furfuryl alcohol (Kinetic rate: 1.2 × 108 M−1S−1), and phenol, targeting surface-bound radicals, O2• −, •OH, 1O2, and (•OH and •IO3), respectively [52,53]. As shown in Figure 9a, ATZ degradation was slightly reduced from 99.35% for the blank sample (without scavengers’ additions) to 90.44% with potassium iodide, indicating limited involvement of surface-bound radicals in the degradation reaction. Chloroform and tert-butanol had stronger inhibitory effects, reducing ATZ degradation to 81.8 and 75.94%, respectively, suggesting minor roles for O2• − and •OH. The most substantial decreases occurred with furfuryl alcohol and phenol, reducing ATZ degradation ratios to 59.88 and 40.56%, respectively, highlighting the major roles of •IO3 and 1O2 in PI activation. Kinetic studies further supported these findings, as degradation rate constants declined from 0.0601 (R2 = 0.9857) min−1 for the control to 0.038 (R2 = 0.9857) min−1, 0.0289 (R2 = 0.9857) min−1, 0.0244 (R2 = 0.9857) min−1, 0.0153 (R2 = 0.9857) min−1, and 0.0094 (R2 = 0.9857) min−1 with potassium iodide, chloroform, tert-butanol, furfuryl alcohol, and phenol, respectively, as presented in Figure S15. Previous studies on catalytic PI-activated systems for the degradation of organic pollutants have reported comparable outcomes [22,72]. In future work, electron spin resonance (ESR) measurements and chemical probe analyses will be integrated with scavenging experiments to provide further confirmation of the degradation mechanism.
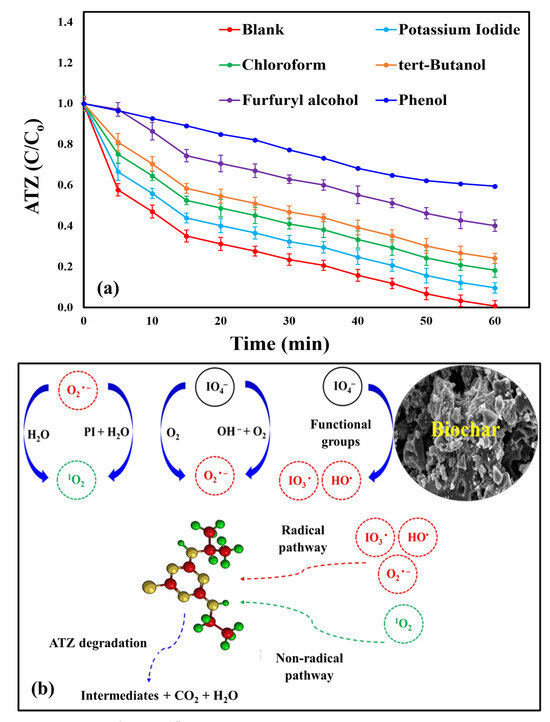
Figure 9.
(a) ATZ degradation efficiency using the BC/PI process with various scavengers (Conditions: pH = 7, [ATZ]o = 7.3 mg/L, T = 25 ± 0.5 °C, [quencher]o = 50 mM, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM) and (b) proposed ATZ degradation mechanism by the BC/PI system.
2.9. Possible ATZ Degradation Mechanism
The structural and surface properties of the courgette-derived BC play a vital role in facilitating electron transfer from the BC surface to PI ions during the activation process. The partially graphitized carbon structure and interconnected porous morphology observed in the SEM image (Figure 1a) facilitate efficient electron mobility across the BC surface, promoting rapid charge transfer during PI activation [12]. Moreover, the EDS analysis (Figure 1b) revealed a significant presence of potassium, which facilitates electron transfer within the BC/PI system. Potassium can interact with the surface oxygen groups, improving charge delocalization. Further, its electrostatic effect enhances polarization between the BC and PI ions, thereby accelerating electron transfer within the BC/PI system [54]. Additionally, FTIR analysis (Figure 2) confirmed the presence of abundant oxygen-containing functional groups (–OH, C=O, and C–O), which serve as redox-active sites capable of donating electrons to PI ions. This electron donation promotes the reduction in PI and the efficient generation of various ROS, thereby achieving superior ATZ degradation performance [6]. Electron transfer from the biochar to periodate ions initiates their activation and subsequent decomposition, leading to the formation of •IO3 and •OH radicals that facilitate pollutant degradation, Equations (1) and (2) [17,71]. Further, O2• − could be produced through the reaction of dissolved oxygen (O2), IO4−, and hydroxyl ions (OH−) or the reaction between OH− and IO4− only, as shown in Equations (3) and (4) [18]. Moreover, 1O2 can be generated through the interaction between O2• − and water molecules (H2O), and IO4− or the reaction of O2• −, H2O only, as depicted in Equations (5) and (6) [18]. Ultimately, the generated ROS could degrade ATZ into CO2, H2O, and intermediate products. Figure 9b provides an overview of the ATZ decomposition mechanism in the BC-catalyzed PI process.
2.10. Proposed ATZ Degradation Pathways
The m/z peaks of the intermediates formed during ATZ degradation in the BC/PI system are shown in Figure S16, and the corresponding compound details are summarized in Table S4. Based on the identified degradation intermediates, the possible ATZ degradation pathways were proposed, as illustrated in Figure 10. The formation of product P6 (m/z = 198) is attributed to the ROS-induced dechlorination of ATZ (P7, m/z = 216), while isomerization of P6 produced the by-product P5 (m/z = 196) [73,74]. Side-chain oxidation of ATZ generated the intermediate P4 (m/z = 146) [2]. The by-product P2 (m/z = 128) formed from either side-chain oxidation of P6 or ROS attack and dechlorination of P4 [75]. Ring cleavage of P2 and P5 by-products produced the intermediates P3 (m/z = 133) and P1 (m/z = 74), respectively [5]. Successive radical attacks on the generated by-products could lead to their mineralization to CO2 and H2O [6,76]. The evaluation of the toxicity of the generated by-products was performed using the toxicity estimation software tool (TEST) (version 5.1.2) towards Daphnia magna and fathead minnow by estimating the half-lethal concentration (LC50) as shown in Table S5. The results in Table S5 indicate that the generated intermediates have lower toxicity compared to ATZ, except for P4 in the case of Daphnia magna. The reduction in toxicity demonstrates the potential of the degradation system to convert the toxic organic pollutants to intermediates with limited toxicity.
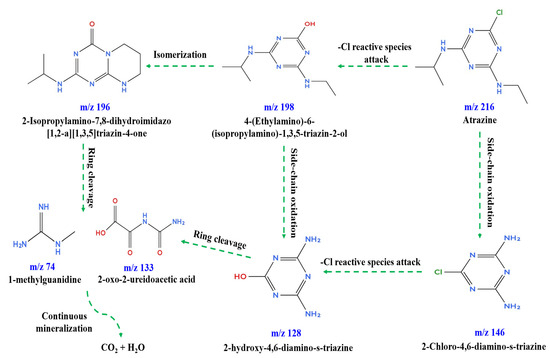
Figure 10.
Proposed ATZ degradation pathways.
2.11. Environmental Significance and Practical Application
2.11.1. Effect of Coexisting Water Components
Real wastewater typically contains a complex mixture of inorganic ions and organic substances that can interfere with the catalytic degradation of organic pollutants [77]. To simulate real treatment conditions, several natural water matrices, including ultrapure water, tap water, seawater, lake water, and drain water, were employed as reaction media for the degradation of ATZ in the BC/PI system. The sampling locations of the collected wastewater are provided in Text S1, and their corresponding physicochemical properties are summarized in Table S6. All experiments were conducted for 60 min at pH 7 and a reaction temperature of 25 ± 0.5 °C. A volume of 100 mL from each water matrix was used as the reaction medium in place of ultrapure water (control). Each sample was spiked with identical concentrations of ATZ (10 mg/L), catalyst (0.5 g/L), and PI (2 mM) to initiate the degradation process. In comparison with a 99.35% ATZ removal ratio in ultrapure water, degradation ratios declined to 96.09, 77.8, 65.15, and 44.6% for tap water, river water, seawater, and drain water, respectively (Figure 11a). Correspondingly, reaction rate constants decreased from 0.0601 (R2 = 0.9462) min−1 in ultrapure water to 0.0478 (R2 = 0.9857) min−1, 0.0257 (R2 = 0.9917) min−1, 0.0179 (R2 = 0.9964) min−1, and 0.0094 (R2 = 0.9967) min−1, respectively, as illustrated in Figure S17. The reduced ATZ degradation observed in these real water matrices is likely due to the inhibitory effects of coexisting dissolved organic matter, inorganic ions (reflected by TDS and electrical conductivity), and turbidity [78]. Dissolved organics can compete with ATZ for ROS, reducing the availability of ROS for ATZ degradation [79]. Furthermore, inorganic ions may react with ROS to produce radicals with lower oxidation potential, decreasing the system’s overall reactivity [53]. Additionally, organic compounds and inorganic ions may block active sites on the BC surface, slowing down ROS generation and consequently reducing ATZ degradation [5]. Suspended particulate matter responsible for turbidity can scavenge or physically adsorb ROS and pollutants. It may also obstruct active sites on the BC surface, limiting the number of available catalytic sites. Furthermore, particulate matter can hinder effective interactions between the organic pollutant, catalyst, and oxidant. These combined effects ultimately lead to a reduction in the apparent degradation rate [80]. A statistical comparison between the measured matrix properties and removal efficiencies reveals a very strong inverse relationship with dissolved organic content: as TOC increases across the matrices (ultrapure 4.16 → tap 18.27 → river 52.06 → seawater 71.35 → drainage 123.28 mg/L), ATZ removal declines almost monotonically. This trend aligns with the competitive scavenging behavior of ROS by dissolved organic matter. High ionic strength is further implicated in the reduced reactivity observed for seawater and drainage samples. Seawater exhibits very high conductivity and TDS (1618.27 μS/cm and 2815.65 mg/L, respectively), while drainage water also shows elevated values (523.52 μS/cm; 939.87 mg/L). These mechanisms likely explain the pronounced decrease in rate constants and overall removal in seawater and drainage matrices relative to ultrapure water. In addition, turbidity exhibited a clear negative correlation with degradation performance. Water matrices with higher turbidity levels, such as drainage (29.49 NTU) and river water (16.26 NTU), showed significantly lower rate constants and removal efficiencies. The strong inverse relationship observed in our dataset indicates that turbidity plays a major inhibitory role in the degradation process. By contrast, pH differences among matrices in our study are small (ranging from 6.57 to 8.43) and show no clear systematic relationship with removal efficiency in this dataset. While pH can modulate radical speciation and catalyst surface charge, the dominant inhibitory factors here appear to be dissolved organic matter and matrix ionic/particulate load. Taken together, these findings indicate that TOC content and turbidity are the main factors responsible for the reduced ATZ removal efficiency in real water matrices, while elevated TDS and conductivity further contribute to inhibition in saline or highly mineralized waters. Despite these inhibitory factors, the BC/PI system exhibited notable resilience when tested with real water samples, highlighting its potential applicability for tertiary treatment processes in real wastewater management. However, to enhance the performance of the BC/PI system in complex real water environments, it is essential to apply appropriate pre-treatment methods (e.g., coagulation, flocculation, filtration, or adsorption) or implement process optimization strategies (e.g., increasing oxidant/catalyst dosage, extending contact time, or employing staged treatment).
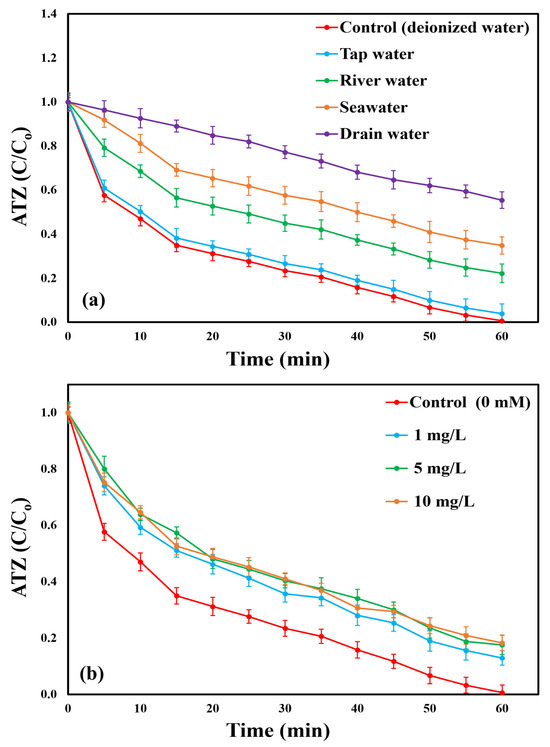
Figure 11.
(a) ATZ degradation ratios utilizing the BC/PI system with different water matrices and (b) ATZ degradation ratios using the BC/PI system at different HA acid concentrations (Conditions: pH = 7, [ATZ]o = 7.3 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM).
Coexisting water components such as NOM, inorganic anions, and inorganic cations can inhibit the catalytic breakdown of organic pollutants in natural water bodies [81,82]. Therefore, the effects of representative substances frequently present in real aquatic environments on the oxidative degradation of ATZ were investigated. Small amounts of the tested substances according to the examined concentration were introduced into the reaction solution before initiating the degradation process. HA, representing NOM, was tested at 1, 5, and 10 mg/L doses. The tested inorganic anions, including Cl−, SO42−, NO3−, CO32−, HCO3−, HPO42−, and H2PO42− ions, were examined at concentrations of 1, 10, and 100 mM. Further, the effects of common cations, including Na+, K+, Mg2+, Cu2+, and Fe2+ ions, were assessed at a concentration of 10 mM.
Figure 11b and Figure S18 explain the effect of HA on ATZ degradation efficiency and kinetic rate constants in the BC/PI system, respectively. The addition of a small concentration of HA (1 mg/L) reduced the ATZ removal efficiency to 87.09%. This represents a decrease of approximately 12% compared to the control (sample without additions), which achieved a removal efficiency of 99.35%. At an increased HA concentration of 5 mg/L, ATZ removal efficiency declined further to 82.47%, showing an additional 5% reduction. At 10 mg/L HA, a negligible decrease (<1%) was observed, resulting in an ATZ removal efficiency of 81.75%. The increase in HA concentration to 5 mg/L and 10 mg/L did not adversely decrease the degradation efficiency due to the saturation of the biochar’s surface by HA at specific concentration of HA. Thus, adding higher concentrations did not show significant inhibition confirming that the inhibition in the case of HA was mainly due to the catalyst surface interaction with HA. The adsorption of hydroxyl and carboxyl groups from HA onto the BC surface could block its’ reactive locations, diminishing ATZ removal efficiency [26,83]. Additionally, the competition between ATZ and HA for ROS consumption might slightly inhibit the degradation efficiency [3,84]. The pseudo-first-order rate constants were 0.0335 (R2 = 0.9929) min−1, 0.0293 (R2 = 0.9929) min−1, and 0.0291 (R2 = 0.9914) min−1 for 1, 5, and 10 mg HA/L, respectively, compared to 0.0601 (R2 = 0.9462) min−1 in the control. Yang et al. [19] obtained similar results using nitrogen and magnesium codoped biochar to degrade bensulfuron methyl herbicide in a PI activation system.
The impact of varying concentrations of inorganic ions on ATZ degradation in the BC/PI system is illustrated in Figure 12, while kinetic rate constants are presented in Figure S20. A summary of ATZ degradation ratios and K values at different concentrations of the tested inorganic anions is shown in Table S7. The strong inhibitory effect of NO3− on ATZ degradation was evident, with ATZ removal efficiency decreasing to 89.58% at a NO3− concentration of 1 mM and continuing to decline as NO3− concentration increased. A similar trend was reported in a study by Chen et al. [14], who utilized N-doped carbon nanotubes encapsulating cobalt as a PI activator for the removal of tetracycline from aqueous solutions. Although SO42− and H2PO4− anions exhibited a significant inhibitory effect on the system, ATZ removal efficiency remained above 85% even at high concentrations (100 mM). Xu et al. [24] reported a similar inhibitory effect of H2PO4− (5 mM) on the degradation of acid orange 7 dye in a visible light/PI-activated system. Conversely, Eslami et al. [21] observed a negligible SO42− inhibition in para-nitrophenol removal using a simultaneous UV and ultrasound system for PI activation.
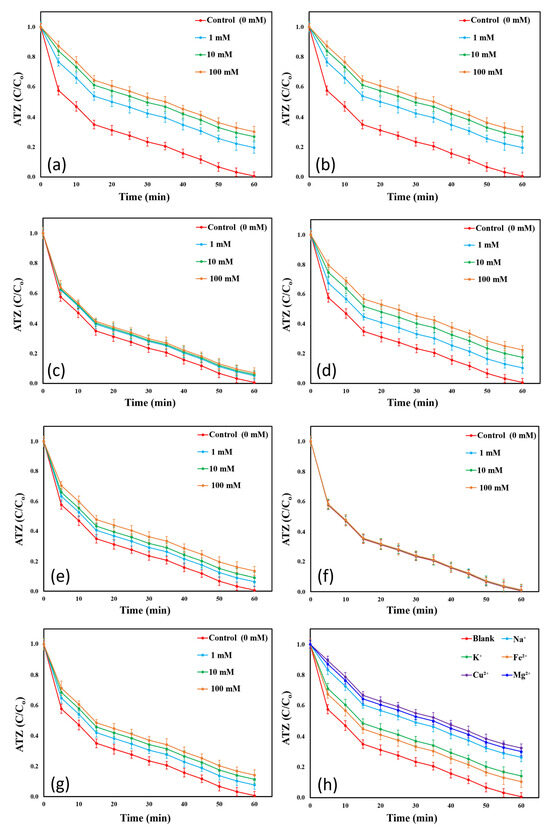
Figure 12.
Impacts of various present inorganic ions on ATZ removal in the BC-catalyzed PI system: (a) HCO3−, (b) CO32−, (c) Cl−, (d) NO3−, (e) SO42−, (f) HPO42−, (g) H2PO4−, and (h) different cations at 10 mM (Conditions: pH = 7, [ATZ]o = 7.3 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM).
The BC/PI system demonstrated strong adaptability to Cl− and HPO42−. At a concentration of 1 mM Cl−, the ATZ removal ratio declined by only 4.5%. Increasing the Cl− concentration to 10 and 100 mM resulted in minimal further decreases in ATZ degradation. Xu et al. [24] noted a slight inhibitory effect of Cl− at a concentration of 5 mM on the degradation of azo dye, in a PI-activated system. ATZ removal ratios decreased by only 0.7% to an HPO42− concentration of 100 mM, making it the anion with the lowest inhibitory effect on ATZ degradation in the BC/PI system. Similarly, Wang et al. [17] reported an insignificant effect of HPO42− on sulfisoxazole degradation in a PI-based process. Bicarbonate ions exhibited the highest inhibition effect on ATZ removal in the BC/PI system. Upon adding HCO3−, the ATZ degradation efficiency decreased to 74.34%, 67.59%, and 62.32% at HCO3− concentrations of 1, 10, and 100 mM, respectively, with corresponding kinetic constants of 0.0233 (R2 = 0.9928) min−1, 0.0192 (R2 = 0.9955) min−1, and 0.0162 (R2 = 0.9973) min−1. Xiong et al. [10] reported that, among various anions tested, HCO3− exhibited the strongest inhibitory effect on the degradation of tetracycline hydrochloride, in a CuFeS2/PI system. The inhibition effect of 100 mM CO32− resulted in an ATZ degradation ratio of 69.77% and a K value of 0.0204 (R2 = 0.9946) min−1. These findings align with previous studies by Elmitwalli et al. [18] and Xiong et al. [10]. The observed decline in ATZ removal efficiency after the addition of various inorganic components can result from several contributing factors. Adsorption of inorganic compounds on the catalyst surface can form an inorganic layer that reduces or blocks active sites. This blockage impedes the diffusion of PI and ATZ, diminishing its removal efficiency [19,85]. Additionally, inorganic ions might compete with ATZ for the generated ROS, thus reducing the ATZ degradation percentage [86,87]. Further, HCO3− introduction into the reaction solution increased the pH, which is less favorable in the BC/PI system because it promotes the formation of less ROS, which reduces the system’s overall efficiency [20,58]. Furthermore, certain inorganic anions, including Cl−, CO32−, HCO3−, and NO3−, exhibit quenching effects on some of the generated ROS, leading to the formation of radicals with lower oxidation potential. These low-reactivity radicals can partially deplete the ROS, thereby reducing the ATZ degradation [18,81]. Chloride ions can react with OH• and IO3•, forming less reactive chlorine species such as OHCl−, Cl•, and Cl2• −, as shown in Equations (16)–(20) [65,66]. Hydroxyl radicals may be scavenged by NO3−, resulting in the formation of nitrate radicals (NO3•) with a lower oxidation potential, as indicated in Equation (21) [88,89]. Bicarbonate ions are potent scavengers of OH• and IO3•. Their reaction with these species results in the formation of carbonate radicals (CO3• −), which exhibit a substantially lower oxidation potential, as illustrated in Equations (22) and (23) [28,90]. Similarly, CO32− can react with IO3• to form CO3• −, as shown in Equation (24) [91]. In addition, CO32− can quench OH•, resulting in the release of carbon dioxide (CO2), as illustrated in Equations (25) and (26) [92,93]. Notably, the negligible inhibitory impact of certain coexisting inorganic ions on the oxidative performance of the BC/PI system suggests that these ions were either easily oxidized by the BC/PI system or did not substantially interfere with the activity of the primary reactive species [17,89].
The addition of various cations at a concentration of 10 mM exerts different levels of inhibition on ATZ removal in the BC/PI system, as explained in Figure 12h. Among these, Cu2+ and Mg2+ displayed the highest inhibitory effects, resulting in ATZ removal efficiencies of 67.67 and 69.88%, respectively, after 60 min. In contrast, Fe2+showed the least inhibition, achieving an ATZ removal ratio of 89.5%. Na+ and K+ exhibited moderate inhibitory effects. Correspondingly, the kinetic rate constants were 0.0192 (R2 = 0.9955) min−1, 0.0202 (R2 = 0.9946) min−1,0.0228 (R2 = 0.9931) min−1, 0.0326 (R2 = 0.9905) min−1, and 0.0367 (R2 = 0.9903) min−1 after the addition of 10 mM of Cu2+, Mg2+, Na+, K+, and Fe2+, respectively, as shown in Figure S19h. Table S8 summarizes ATZ degradation percentages and kinetic rate constants following the introduction of various cations. The differences in inhibitory behavior after the addition of various cations can be explained by considering the surface chemistry and charge characteristics of the synthesized biochar. The pHpzc of the courgette-derived BC was determined to be 4.25 (Figure S1), indicating that at the reaction pH (7), the BC surface carries a net negative charge. This negative surface readily attracts positively charged cations through electrostatic interactions. Divalent cations such as Cu2+and Mg2+, owing to their higher charge density and stronger electrostatic interactions, can more effectively adsorb onto the negatively charged surface of BC at pH 7. This suggests that the complexation between Cu2+ and Mg2+ and the biochar’s surface is likely the primary mechanism responsible for the observed inhibitory effects. These cations preferentially bind to oxygen-containing functional groups, including O–H, C=O, and –COOH sites, as confirmed by the FTIR spectrum (Figure 2). Such strong adsorption can alter the surface charge distribution of BC and block reactive sites that are otherwise available for the adsorption and activation of PI and atrazine ATZ molecules [94]. Consequently, this competitive occupation hinders electron transfer between BC and PI, suppressing the generation of ROS. Furthermore, Cu2+ can undergo redox interactions with ROS, acting as a radical scavenger. For instance, Cu2+ can react with O2• − and •OH radicals to form less reactive species (e.g., Cu+ or Cu–O complexes), which decreases the steady-state concentration of ROS in solution. Mg2+, although not redox-active, can strongly coordinate with surface oxygen atoms or water molecules, forming stable hydration layers that limit the contact between PI and active sites. As a result, both cations significantly inhibit the catalytic activation of PI and the subsequent oxidative degradation of ATZ [95,96]. In contrast, monovalent cations such as Na+ and K+ exhibit weaker electrostatic attraction toward the negatively charged BC surface because of their lower charge density and single positive charge compared with divalent cations. Their smaller ionic strength and relatively larger hydration shells reduce their ability to approach and adsorb firmly onto the active oxygen-containing sites of the biochar. Consequently, these cations form weaker outer-sphere complexes rather than strong inner-sphere bonds, leading to less blockage of catalytic sites and minimal interference with the adsorption of PI or ATZ molecules. As a result, the influence of Na+ and K+ on electron transfer processes and ROS generation is less pronounced, producing only a mild inhibitory effect on ATZ degradation compared with divalent cations such as Cu2+ and Mg2+. Interestingly, Fe2+ displayed the least inhibitory effect, which can be attributed to its redox-active nature. Unlike other cations, in the BC/PI system, Fe2+ may accelerate the decomposition of PI by donating electrons, leading to the formation of highly reactive species such as O2• −, •OH, and •IO3 radicals. Moreover, the presence of Fe2+ can facilitate interfacial electron transfer between the oxygenated functional groups on biochar and PI, thereby sustaining ROS production and enhancing degradation efficiency [97].
In conclusion, the BC/PI system demonstrated excellent oxidation performance in the presence of common inorganic ions such as K+, Fe2+, H2PO4−, SO42−, Cl−, and HPO4−2, underscoring its promise for practical water treatment applications. However, its oxidative efficiency is adversely affected by the presence of other typical constituents, including NOM, HCO3−, CO32−, and NO3−. This finding highlights the necessity of removing these interfering substances from wastewater before employing the BC/PI system.
2.11.2. Universality of the BC/PI System
To examine the universality of the proposed catalytic system, three additional model micropollutants (tetracycline, bisphenol A, and Congo red) were introduced under the same experimental conditions. These compounds were selected to represent different classes of environmentally relevant contaminants: (i) tetracycline, a broad-spectrum antibiotic extensively used in human and veterinary medicine; (ii) bisphenol A, a widely studied endocrine-disrupting chemical derived from industrial activities; and (iii) Congo red, a synthetic azo dye commonly associated with textile effluents [48,82,98]. Compared with ATZ, these pollutants vary substantially in molecular structure, functional groups, charge properties, and environmental significance, thereby providing a broader assessment of the catalyst’s applicability. Solutions of these pollutants were employed as reaction solutions in place of the ATZ solution, after which the catalyst and oxidant were added to initiate the degradation process. The experiments were conducted under the optimum conditions for a 60 min reaction period. The degradation efficiencies of the tested pollutants and the reaction kinetic curves are presented in Figure 13a and Figure S20, respectively. Compared to ATZ with a 99.35% removal ratio and 0.0601 (R2 = 0.9462) min−1 rate constant, Congo red dye was fully removed within 30 min, with a rate constant of 0.1041 (R2 = 0.9835) min−1. The removal efficiencies and rate constants for tetracycline and bisphenol A were 79.54% (K = 0.0309 min−1, R2 = 0.9771) and 85.6% (K = 0.032 min−1, R2 = 0.9971), respectively. Additionally, the mineralization rates for ATZ, Congo red, tetracycline, and bisphenol A were 64.23, 82.32, 39.87, and 51.71%, respectively, as depicted in Figure S21. The relatively lower TOC mineralization efficiencies, compared to the pollutants’ degradation efficiencies, may be ascribed to the generation of intermediate products with increased resistance to decomposition compared to the original compounds [23]. The lowest removal efficiency was observed for tetracycline (79.54%), which remains relatively high given that the experimental conditions were optimized for ATZ degradation. These results demonstrate that the BC/PI system effectively degrades structurally diverse organic micropollutants beyond ATZ. The high degradation and mineralization efficiencies across antibiotics, industrial chemicals, and synthetic dyes highlight its broad-spectrum applicability and confirm its potential as a universal, robust catalyst for treating heterogeneous micropollutants in aquatic environments.
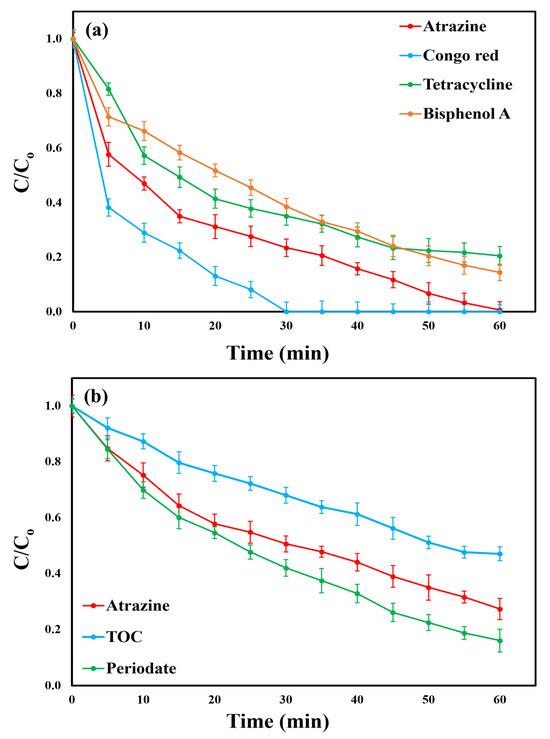
Figure 13.
(a) Degradation efficiency of various organic micropollutants using the BC/PI system and (pH = 7, [pollutant]o = 7.3 mg/L, T = 25 ± 0.5 °C, [BC]o = 0.55 g/L, and [PI]o = 2.7 mM) and (b) ATZ degradation, TOC mineralization, and PI consumption ratios utilizing the BC/PI process in actual agrochemical effluent.
2.11.3. Application of the BC/PI System in Treating Industrial Wastewater
The treatment of real industrial agrochemical wastewater in the BC/PI system was evaluated under the optimum conditions. The characteristics of the real effluent are presented in Table S6. The initial concentrations of ATZ and TOC were 184.57 mg/L and 510.36 mg/L, respectively. ATZ and TOC removal percentages reached 72.77 and 53.02%, respectively (Figure 13b). The ATZ degradation reaction rate constant was 0.0217 (R2 = 0.9941) min−1 (Figure S22). The reduced TOC mineralization ratio in comparison to the ATZ degradation percentage can likely be because of the generation of ATZ by-products during the breakdown process, which were not fully mineralized [29,33]. As illustrated in Figure 13b and Figure S12, when ultrapure water was used as the reaction medium under the optimal conditions (pH = 7, [ATZ]o = 7.3 mg/L, [BC]o = 0.55 g/L, T = 25 ± 0.5 °C, and [PI]o = 2.7 mM) for 60 min, the ATZ degradation ratio, TOC mineralization efficiency, and ATZ degradation rate constant were found to be 99.35%, 64.23%, and 0.0601 (R2 = 0.9462) min−1, respectively. The reduction in ATZ removal efficiency, TOC mineralization, and the ATZ degradation rate constant observed in the real wastewater sample compared to ultrapure water is likely associated with the existence of various pollutants in the actual sample. These pollutants decompose into smaller by-products, which contribute to maintaining the overall organic load, block active sites on the BC surface, and compete with ATZ for ROS, thereby diminishing the overall treatment efficiency [6,21]. Additionally, in the real wastewater sample, the initial ATZ and TOC levels were greater than those of the ultrapure water, and the optimization of operating parameters was based on the ultrapure water conditions [53]. Nevertheless, achieving 72.77% ATZ removal and 53.06% TOC mineralization in real wastewater highlights the BC/PI system’s effectiveness in actual conditions, supporting its application in industrial wastewater treatment plants. Moreover, the 84% consumption ratio of PI (Figure 13b) demonstrates the effectiveness of the BC/PI system in activating PI within the real sample, thereby generating ROS capable of degrading ATZ and contributing to the mineralization of TOC. On the other hand, the total treatment cost for the real wastewater sample processed using the BC/PI system was calculated, as detailed in Text S2. This total cost, comprising both annual amortization and operating costs, was estimated to be 1.2173 $/m3. The affordability of the BC/PI system highlights its viability as a scalable and practical solution for industrial wastewater treatment.
3. Materials and Methods
3.1. Chemicals and Raw Water Samples
3.2. Biochar Synthesis and Characterization
Biomass materials were collected from courgette food waste, including flesh, seeds, and peduncles, as illustrated in Figure S23. The biomass was crushed into smaller pieces, carefully rinsed with ultrapure water, and then dried in a thermal chamber (BINDER, ED53, Camarillo, CA, USA) at 105 °C overnight. The dried samples were then subjected to pyrolysis in a high-temperature kiln (AMF 25 N, Asahi-Rika (ASH) Co., Chiba, Japan) at 500 ± 0.5 °C for 120 min in N2 atmosphere. The pyrolysis process was conducted at a thermal speed of 10 °C/min, with a 6 L/h N2 delivery velocity. After pyrolysis, the resulting powder was cooled to ambient temperature (T = 25 ± 0.5 °C) in a desiccator and subjected to repeated washing with 1 M HCl to remove residual inorganic impurities. Subsequently, the material was rinsed several times with ultrapure water until the filtrate reached a neutral pH (pH = 7) to confirm the complete elimination of residual acid. Several previous biochar synthesis studies applied these purification procedures to ensure effective removal of impurities prior to drying and storage [99,100]. The purified powder was subsequently dried at 105 ± 0.5 °C for 24 h. The final courgette-derived BC was crushed and screened through a 0.149 mm screen mesh to remove oversized grains and stored under dry conditions for later use. The BC catalyst’s point of zero charge (pHpzc) was determined by conducting the powder blending approach, commonly referred to as the pH drift technique, as described in detail in our earlier study [32]. Additionally, the synthesized BC was characterized using various advanced analytical techniques. Scanning electron microscopy (SEM) was employed to explore the catalyst morphology, while energy-dispersive spectroscopy (EDS) was used to analyze the chemical composition and elemental distribution. The crystalline structure and lattice parameters of the catalyst were assessed through X-ray diffraction (XRD). Additionally, the chemical groups present on the BC were identified using Fourier-transform infrared spectroscopy (FTIR). The specific surface area (SBET) was determined using the Brunauer–Emmett–Teller (BET) model, while the pore size distribution and total pore volume were evaluated using the Barrett–Joyner–Halenda (BJH) method. Details of the characterization devices and techniques applied in the current study are presented in Text S3.
3.3. Experimental Setup
Batch degradation experiments were carried out in 250 mL Erlenmeyer flasks containing 100 mL of freshly prepared ATZ solution at the desired concentration. Each flask was placed on a magnetic hotplate stirrer (MSH–20D, Daihan Scientific, Hanoi City, Vietnam) set to maintain a constant stirring speed of 1000 rpm. Unless otherwise stated, reactions were carried out at room temperature and neutral pH for a duration of 60 min. The pH of the solution was adjusted using small aliquots of 0.1 M sodium hydroxide (NaOH) and sulfuric acid (H2SO4). A multiparameter instrument (HQ440d, HACH, Loveland, CO, USA) equipped with a pH electrode (PHC301, HACH, Loveland, CO, USA) was utilized to monitor and confirm the pH of the solution. The reaction temperature was controlled using a hotplate and monitored periodically with a digital thermometer to ensure stable thermal conditions. Predetermined quantities of the catalyst and oxidant were added to the pollutant solution to initiate the degradation reaction. All tests were performed three times, and the error bars in the figures indicate the corresponding standard deviations.
Aliquots (3 mL) from the reaction solution were withdrawn at 5 min intervals using disposable syringes. Each aliquot was immediately quenched by spiking 100 µL of a 1 M sodium thiosulfate (Na2S2O3) reagent in a centrifuge tube to terminate the oxidation reaction and prevent further degradation of ATZ [29]. Then they were centrifuged at 3000 rpm for 5 min (EBA 20, Hettich, Buford, GA, USA) and subjected to filtration through a 0.22 μm membrane filter to retain the activator particles. A specified volume of the filtered sample was transferred to the analysis vial for immediate analysis or stored at 4 °C for no longer than 24 h for future measurements. Removal efficiencies of pollutants, total organic carbon (TOC) mineralization ratios, and oxidant consumption ratios during the degradation processes were calculated using Equation (27) [3]. The kinetics of the degradation reactions were assessed using the pseudo-first-order kinetic model, as expressed in Equation (28) [93]. The correlation coefficient (R2) values of the kinetic models were evaluated to verify their predictive reliability and goodness of fit. On the other hand, the biochar adsorptive capacity (Q, mg/g) was calculated from Equation (29) [3].
where Co refers to the influent concentration, C denotes the final or residual concentration after the degradation reaction, K indicates the kinetic rate constant (min−1), t (min) is the degradation period, V is the initial volume of the reaction solution (L), and W refers to the mass of the catalyst used (g).
3.4. Experimental Procedures
Preliminary control experiments were conducted for two basic reasons. First, to elucidate the specific role of the courgette-derived BC in PI activation, as well as its synergistic contribution to ATZ degradation. Second, to compare the catalytic activity of BC toward PI activation with that observed in systems employing other common oxidants used in AOPs (PMS, PS, and H2O2) to identify the system achieving the highest ATZ degradation efficiency for subsequent experiments. The role of the synthesized BC in PI activation involved a comparison among three systems: (i) BC alone, to examine the adsorption of ATZ onto the biochar surface and within its pores; (ii) PI alone, to evaluate non-catalytic oxidation; and (iii) the combined BC/PI system, to assess the synergistic catalytic effect. The tests were conducted over 60 min under the following conditions: an initial ATZ concentration of 10 mg/L, a BC dosage of 0.5 g/L, a PI concentration of 2 mM, ambient temperature, and neutral pH. Additionally, the synergistic ratio was calculated to quantitatively evaluate the contribution of BC to PI activation, as presented in Equation (30) [93]. A synergistic ratio exceeding unity denotes a positive synergistic interaction between BC and PI, while values equal to or below one indicate merely additive or antagonistic effects [101].
where KBC/PI is the reaction rate constant when both BC and PI are utilized simultaneously, KPI represents the pseudo-first-order kinetic constant for the process employing PI alone, and KBC denotes the rate constant for the system using only the BC catalyst. All k values are expressed in min−1. To compare the catalytic activity of the courgette-derived BC with different oxidants, identical initial concentrations (2 mM) of each oxidant were introduced into separate ATZ reaction flasks under identical operational conditions. The removal efficiency of ATZ and the corresponding oxidant consumption ratios were evaluated to determine the most efficient BC-catalyzed system for use in subsequent experiments. Moreover, the influence of pH (3–11) and temperature (25–75 °C) on the catalytic efficiency of the selected system was systematically examined. Additionally, RSM coupled with a CCD was applied to optimize and assess the effects of the synthesized BC dosage, initial PI concentration, and ATZ concentration on the degradation performance within the BC/PI system. The reusability and structural stability of the courgette-derived BC were further evaluated over five consecutive degradation cycles. To elucidate the underlying degradation mechanism, radical scavenging experiments were conducted to identify the dominant reactive species contributing to ATZ degradation. Specific scavengers were used, including potassium iodide, chloroform, tert-butanol, furfuryl alcohol, and phenol, which selectively targeted surface-bound radicals, O2• −, •OH, 1O2, •IO3, respectively [52,53]. On the other hand, the degradation intermediates of ATZ in the BC/PI system were identified, and the corresponding degradation pathways were proposed. Further, the toxicity of ATZ and its transformation products was systematically assessed to evaluate the potential environmental implications of the treatment process. In addition, the influence of different water matrices, including ultrapure water, tap water, seawater, lake water, and drain water, was investigated to assess the effects of NOM and dissolved inorganic ions on the system’s catalytic efficiency. Furthermore, the effects of typical coexisting constituents were systematically examined within the BC/PI system. These included humic acid (HA) as a representative of NOM, along with various inorganic anions and cations. The investigated anions comprised chloride (Cl−), sulfate (SO42−), nitrate (NO3−), carbonate (CO32−), bicarbonate (HCO3−), and hydrogen phosphate (HPO42−) ions, while the examined cations included sodium (Na+), potassium (K+), magnesium (Mg2+), copper (Cu2+), and ferrous (Fe2+) ions. To evaluate the universality of the BC/PI system, its catalytic performance was further tested using representative model micropollutants. Tetracycline was selected as a widely used antibiotic. Bisphenol A was chosen as an industrial endocrine-disrupting compound. Congo red was included as a synthetic azo dye commonly detected in textile effluents. Finally, the scalability and practical feasibility of the BC/PI system were evaluated using real agrochemical industrial wastewater contaminated with ATZ. This investigation aimed to determine the system’s performance in complex water matrices containing various organic and inorganic constituents that may influence catalytic activity. Additionally, the overall treatment cost for processing the actual wastewater sample using the BC/PI system was estimated to evaluate the economic viability of the proposed approach for large-scale applications. The detailed experimental flowchart is presented in Figure S24.
3.5. Design of Experiments and Statistical Analysis
RSM was applied to evaluate the effects of selected independent factors (initial oxidant concentration and initial catalyst dosage) on the target response (ATZ removal efficiency) and to identify the optimal operational conditions of these factors. By enabling the assessment of both individual and interactive effects among variables, RSM provides notable advantages, including reduced resource utilization, lower experimental costs, and shorter optimization time. Moreover, it enhances process robustness and reliability by attaining statistically significant outcomes with a limited number of experimental runs [6,48]. Before performing the RSM analysis, an experimental design must be defined to determine the arrangement and levels of the independent variables. In this study, a CCD was adopted as an efficient alternative to the full factorial design. This approach enables the modeling of higher-order response surfaces while considerably reducing the total number of experiments required [70]. Each factor was investigated at five coded levels (−2, −1, 0, +1, +2), where the central point (0) represented the midpoint of the examined range. The complete parameter ranges and corresponding coded levels are presented in Table 1, which includes ATZ concentrations (5–15 mg/L), BC dosages (0.3–0.7 g/L), and oxidant concentrations (1–3 mM).
The experimental design, model development, parameter optimization, statistical evaluation, and generation of response surface plots were performed using Minitab® software (version 22). To enhance model reliability, quantify experimental variability, and verify reproducibility, the central point was repeated six times [102]. Following the input of the selected variables and their corresponding levels, the software generated a design matrix comprising 20 experimental runs (Table 2), representing all possible combinations of the independent factors. In a CCD, the total number of runs (N) is determined using the following equation (Equation (31)) [103]:
where F is the number of factors, and nc is the number of center points. For the present study, with three factors (F = 3), the design included 8 factorial points (23), 6 axial points (2 × 3), and 6 center points (nc = 6), yielding a total of 20 experimental runs. The center points are replicated to estimate experimental error, thereby improving the precision of the quadratic model and enhancing the statistical reliability of the results by allowing accurate assessment of model adequacy [104]. Therefore, the experimental conditions listed in Table 2 for runs 15–20 correspond to the center points and are deliberately identical. Additionally, a polynomial model was constructed to describe the linear, quadratic, and interaction effects of the studied parameters on the response variable as reported by Moeinzadeh et al. [105]. This model facilitated both the prediction of ATZ removal efficiency and the statistical fitting of experimental data [106]. Optimization of the developed empirical model constitutes a pivotal phase of this research. In the present study, the optimization procedure was conducted to determine the conditions that yield the maximum response efficiency. Concurrently, regression analysis and analysis of variance (ANOVA) were employed to assess the suitability and statistical soundness of the proposed model. The fitness and significance of model parameters were examined using the R2 value, Fisher’s tests (F-values), and the corresponding p-values. In this framework, model terms exhibiting a high F-value coupled with a p-value ≤ 0.05 were considered statistically significant [44]. Furthermore, two-dimensional contour plots were utilized to visualize and interpret the interaction effects among the independent variables.
The concentrations of ATZ, tetracycline, bisphenol A, IO4−, and iodate ions (IO3−) were measured employing high-performance liquid chromatography (HPLC, LC–2040C, Shimadzu, Kyoto, Japan) fitted with a diode array detector and autosampler. The chromatographic separation was achieved using an Agilent ZORBAX Eclipse XDB-C18 column (Agilent Technologies, Santa Clara, CA, USA) (4.6 × 250 mm, 5 μm). Details of the chromatography conditions (mobile phase composition, flow rate, injection volume, retention time, column temperature, and detection wavelengths) are provided in Table S9. The concentrations of IO4− and IO3− were quantified by generating calibration curves from standard solutions of KIO4 and NaIO3, respectively, prepared within the concentration range of 0–500 mM [23,107]. UV-Vis spectrophotometer (Evolution 350, Thermo Fisher Scientific, Waltham, MA, USA) was utilized to quantify the concentration of Congo red dye at a wavelength of 498 nm [98]. The PS concentration was measured using the potassium iodide colorimetric approach, as outlined in Text S4. Residual H2O2 concentrations in the BC-activated H2O2 system were determined using the HACH Method 10290, which is adapted from the Standard Methods for the Examination of Water and Wastewater, Method 4500-PAA (peracetic acid) [108]. The detailed method procedures are described in Text S4. A TOC analyzer (TOC-L CPH/CPN, Shimadzu, Japan) was employed to analyze the TOC concentrations and assess the mineralization extent. The by-products generated during ATZ degradation in the BC/PI system were identified using a liquid chromatography–mass spectrometry (LC–MS, Shimadzu). The analysis was conducted with a mobile phase composed of 95% methanol and 5% ultrapure water, at a flow rate of 0.4 mL min−1, and the column temperature was maintained at 30 °C [6]. The electrical conductivity was measured using a measurement device (HQ440d, HACH, Loveland, CO, USA) equipped with a conductivity probe (CDC401, HACH, Loveland, CO, USA). Turbidity was measured using the Turb 430T turbidity meter (WTW, Ann Arbor, MI, USA). Total dissolved solids (TDS) were quantified following the Standard Method 2540–C [108].
4. Conclusions
This study reports the use of courgette-derived BC as an efficient, low-cost, and sustainable activator of PI for the degradation of ATZ. Advanced characterization analyses confirmed the biochar’s strong potential as an efficient activator in catalytic oxidation processes. The results revealed its carbon-rich nature and the presence of abundant oxygen-containing functional groups (–OH, C=O, C=C, and –COOH), which enhance electron transfer and facilitate PI activation. Additionally, the BC exhibited a hierarchically porous structure composed of meso- and macropores, promoting efficient mass transfer during catalytic reactions. The combined BC/PI system exhibited significantly higher ATZ removal and TOC mineralization efficiencies than either BC or PI alone, achieving a synergistic ratio greater than one and a markedly enhanced kinetic rate constant. These results clearly confirm a strong synergistic interaction between the courgette-derived biochar and periodate in the degradation of atrazine. The BC/PI system exhibited the highest ATZ degradation efficiency and kinetic rate constant among all tested oxidants (PS, PMS, and H2O2), confirming its superior performance. Additionally, no harmful iodine derivatives were found in the BC-catalyzed PI process indicating the system’s potential to function with minimal risk of secondary contamination and confirming its environmental compatibility and safety for practical applications. The degradation efficiency of ATZ in the BC/PI system was strongly influenced by pH. The system exhibited superior catalytic activity under acidic to neutral conditions but declined notably in alkaline media. As no significant difference was observed between acidic and neutral conditions, neutral pH was selected as optimal to reduce the cost associated with pH adjustments. The system achieved efficient ATZ degradation under ambient temperature, with only about a 12% increase in ATZ removal efficiency observed between 25 °C and 75 °C, demonstrating its strong catalytic performance even under mild thermal conditions and strong adaptability to environmental variations. Moreover, the BC/PI system exhibited a remarkably lower activation energy (5.93 kJ/mol) than those reported for other AOPs, confirming its high energy efficiency. The generated quadratic model effectively described the relationship between ATZ removal efficiency and the main operational variables (initial ATZ concentration, initial PI concentration, and catalyst dose), exhibiting high R2 value of 0.9494 and adjusted R2 value of 0.9038, which confirms its robustness and predictive reliability. The minimal deviation between the experimental and predicted values further validated the model’s accuracy. The optimum conditions obtained from the RSM were [ATZ]o = 7.3 mg/L, [PI]o = 2.7 mM, and [BC]o = 0.55 g/L at pH 7 and 25 °C. Under these optimum conditions, the BC/PI system achieved an ATZ degradation efficiency of 99.35% and a TOC mineralization of 64.23% after 60 min of reaction. The synthesized BC exhibited excellent reusability, maintaining high ATZ removal efficiencies of 99.35, 97.5, 95.33, 92.03, and 90.27% over five consecutive cycles, corresponding to a minor overall decline of approximately 9%. The SEM, EDS, and XRD analyses confirmed that the BC retained its morphology, elemental composition, and crystallinity after multiple runs, demonstrating the system’s structural stability and consistent catalytic performance. Quenching experiments confirmed that iodate radicals and singlet oxygen were the predominant ROS responsible for ATZ degradation in the BC/PI system, while hydroxyl radicals and superoxide radicals contributed to a lesser extent. The proposed mechanism suggests that PI activation was primarily driven by electron transfer from the surface of the courgette-derived BC to PI ions. This process was facilitated by the catalyst’s porous structure, abundant surface oxygen-containing functional groups, and potassium-enriched composition, which promoted the generation of diverse ROS. The degradation pathway analysis revealed that ATZ underwent a series of oxidative transformations, including dechlorination, side-chain oxidation, and ring cleavage, driven by ROS in the BC/PI system. The detected intermediates indicate progressive breakdown of ATZ into smaller and less complex molecules, ultimately leading to complete mineralization into CO2 and H2O. These findings confirm that the BC/PI process effectively promotes deep oxidation of ATZ through multiple ROS-mediated pathways. The toxicity assessment using the TEST software indicated that the degradation intermediates exhibited lower toxicity toward Daphnia magna and fathead minnow compared to ATZ, highlighting the system’s effectiveness in transforming toxic pollutants into less harmful by-products. ATZ removal declined from 99.35% in ultrapure water to 96.09, 77.8, 65.15, and 44.6% in tap, river, seawater, and drain water, respectively. The reduced performance is mainly attributed to dissolved organic matter and turbidity, which scavenge ROS and block active sites on the BC surface. High ionic strength and conductivity in saline waters further suppressed catalytic reactivity. Nevertheless, the BC/PI system maintained considerable degradation efficiency under realistic conditions, confirming its robustness for complex water matrices. These findings highlight the system’s potential for real wastewater applications, which could be further improved through pre-treatment or operational optimization. The proposed system exhibited strong tolerance toward Cl−, SO42−, NO3− anions, which showed only slight effects on ATZ degradation. In contrast, CO32−, HCO3−, HPO42−, and H2PO42− significantly inhibited the process by scavenging reactive species and blocking active sites on the catalyst surface. The inhibitory effects of cations followed the order Cu2+ > Mg2+ > Na+ ≈ K+ > Fe2+. Divalent cations (Cu2+ and Mg2+) showed stronger adsorption on the negatively charged biochar surface, thereby blocking active sites and hindering electron transfer. In contrast, Fe2+ caused the least inhibition due to its redox-active nature, which promotes continuous ROS generation. This result underscores the importance of eliminating such interfering species from wastewater prior to applying the BC/PI system. The BC/PI system demonstrated strong universality by effectively degrading various organic micropollutants beyond ATZ, including tetracycline, bisphenol A, and Congo red. Despite differences in molecular structures and functional groups, all pollutants showed high degradation efficiencies. Congo red was completely removed within 30 min, while tetracycline and bisphenol A achieved 79.54 and 85.6% removal, respectively. These results confirm that the courgette-derived BC/PI system possesses broad-spectrum catalytic activity and strong potential as a versatile and robust treatment process for diverse micropollutants in aquatic environments. The system demonstrated strong applicability in treating real industrial ATZ-contaminated agrochemical wastewater, achieving 72.77% ATZ removal and 53.06% TOC mineralization within 60 min under the optimum conditions. Although the degradation efficiency was lower than that in ultrapure water due to competing pollutants and higher organic loads, the system still showed effective PI activation (84% consumption) and stable catalytic performance. Furthermore, the total treatment cost of 1.2173 $/m3 highlights the BC/PI system as an efficient, affordable, and scalable option for industrial wastewater treatment applications. Future studies could focus on further enhancing the BC/PI system’s performance in complex water matrices by exploring pre-treatment methods or process optimization strategies to mitigate the inhibitory effects of coexisting ions and organic matter. Additionally, ESR measurements and chemical probe analyses could be integrated with scavenging experiments to provide further confirmation of the degradation mechanism. Investigations into scaling up the system for continuous-flow treatment, assessing long-term operational stability, and catalyst regeneration would also support practical industrial applications, while expanding tests to a wider range of emerging contaminants could confirm its versatility and broad-spectrum applicability. Additionally, exploring the incorporation of dopants, such as iron or magnesium, into the synthesized BC could further enhance its catalytic performance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15111049/s1, Text S1: Chemicals and raw water samples; Text S2: Cost estimation; Text S3: Characterization techniques of the synthesized biochar; Text S4: Quantifying PS and H2O2 concentrations; Figure S1: Point of zero charge of the synthesized BC; Figure S2: Kinetic curves for ATZ degradation across various systems (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S3: TOC mineralization ratios (%) in the sole BC, sole PI, and BC/PI systems (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S4: ATZ degradation efficiency in the BC/H2O2 system with different quenchers (50 mM) under the conditions ([ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [H2O2]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S5: ATZ degradation efficiency in the BC/PMS system with different quenchers (50 mM) under the conditions ([ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PMS]o = 2 mM, and pH = 7); Figure S6: ATZ degradation efficiency in the BC/PS system with different quenchers (50 mM) under the conditions ([ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PS]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S7: Consumption ratios (%) of PI, PS, PMS, and H2O2 in the BC/PI, BC/PS, BC/PMS, and BC/H2O2, respectively; Figure S8: Variation of iodine species in BC/PI system (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S9: Kinetic curves for ATZ degradation in the BC/PI system at different pH values; Figure S10: Kinetic curves for ATZ degradation in the BC/PI system at different temperatures; Figure S11: The activation energy of the catalytic degradation of ATZ using the BC/PI system; Figure S12: Pseudo-first-order kinetic rate constant for ATZ degradation using the BC/PI system under the optimum conditions (Conditions: [ATZ]o = 7.3 mg/L, [BC]o = 0.55 g/L, [PI]o = 2.7 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S13: Weight loss of the BC catalyst using the BC/PI system in five consecutive runs; Figure S14: ATZ degradation kinetic curves in the BC/PI system in five successive runs (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S15: ATZ degradation kinetic curves in the BC/PI system with different quenchers (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S16: The m/z peaks of the ATZ-generated intermediates; Figure S17: ATZ degradation kinetic curves in the BC/PI system with different water matrices (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S18: ATZ degradation kinetic curves in the BC/PI system at different HA concentrations (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S19: ATZ degradation kinetic curves in the BC/PI system with different anions and cations: (a) HCO3−, (b) CO32−, (c) Cl−, (d) NO3−, (e) SO42−, (f) HPO4−2, (g) H2PO4−, and (h) different cations at 10 mM (Conditions: [ATZ]o = 7.3 mg/L, [BC]o = 0.55 g/L, [PI]o = 2.7 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S20: Degradation kinetic curves in the BC/PI system with different organic micropollutants (Conditions: [ATZ]o = 10 mg/L, [BC]o = 0.5 g/L, [PI]o = 2 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S21: TOC mineralization ratios (%) of various organic micropollutants using the BC/PI system (Conditions: [Pollutant]o = 7.3 mg/L, [BC]o = 0.55 g/L, [PI]o = 2.7 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S22: Pseudo-first-order kinetic rate constant for ATZ degradation using the BC/PI system with real agrochemical wastewater under the optimal conditions ([BC]o = 0.55 g/L, [PI]o = 2.7 mM, pH = 7, and T = 25 ± 0.5 °C); Figure S23: Courgette biomass constituents; Figure S24: The experimental flowchart; Table S1: FTIR peak positions of the synthesized BC before treatment with corresponding functional groups based on literature; Table S2: XRD diffraction peaks (2θ°) of the synthesized BC before and after treatment with corresponding miller indices (hkl) from reference cards; Table S3: Comparison of Ea values for ATZ degradation in different AOPs; Table S4: Details of the transformation products of ATZ after degradation using the BC/PI system; Table S5: Toxicity evaluation of ATZ and its by-products; Table S6: Physicochemical characteristics of raw water samples; Table S7: ATZ removal efficiencies and reaction rate constants after the addition of various anions at different concentrations; Table S8: ATZ removal efficiencies and reaction rate constants after adding various cations (10 mM); Table S9: HPLC conditions for the analysis of different compounds; references [109,110,111] are cited in the supplementary material.
Author Contributions
M.M.G.: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing—original draft. M.A.R.: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, and Writing—review and editing. H.S.: Formal analysis, Investigation, Methodology, Resources, Visualization, Software, and Writing—review and editing. M.S.: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, and Writing—review and editing. A.M.A.: Investigation, Project administration, Resources, Visualization, Validation, and Writing—review and editing. M.E.: Conceptualization, Investigation, Methodology, Visualization, Software, Supervision, Validation, and Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Data Availability Statement
All data will be made available as published and as requested through the corresponding authors’ contacts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jiang, C.; Yang, Y.; Zhang, L.; Lu, D.; Lu, L.; Yang, X.; Cai, T. Degradation of Atrazine, Simazine and Ametryn in an Arable Soil Using Thermal-Activated Persulfate Oxidation Process: Optimization, Kinetics, and Degradation Pathway. J. Hazard. Mater. 2020, 400, 123201. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J.; Zhou, Q. Heat-Activated Persulfate Oxidation of Atrazine: Implications for Remediation of Groundwater Contaminated by Herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Chen, X.; Hu, C.; Hong, F.; Fang, Y.; Yuan, X.; Tian, H.; Huang, Y. Activation of Persulfate with Hydrodynamic Cavitation in the Removal of Atrazine: Regulating the Concentration of OH and SO4− and the Degradation Mechanism. J. Water Process Eng. 2024, 65, 105828. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, T.; Liu, S.; Sun, S.; Wang, Y.; Liu, X.; Wang, J.; Liu, R. Catalytic Ozonation of Atrazine Enhanced by Mesoporous CeO2: Morphology, Performance and Intermediates. Water 2022, 14, 3431. [Google Scholar] [CrossRef]
- Teng, X.; Li, J.; Wang, J.; Liu, J.; Ge, X.; Gu, T. Effective Degradation of Atrazine in Wastewater by Three-Dimensional Electrochemical System Using Fly Ash-Red Mud Particle Electrode: Mechanism and Pathway. Sep. Purif. Technol. 2021, 267, 118661. [Google Scholar] [CrossRef]
- El-Bestawy, E.A.; Gaber, M.; Shokry, H.; Samy, M. Effective Degradation of Atrazine by Spinach-Derived Biochar via Persulfate Activation System: Process Optimization, Mechanism, Degradation Pathway and Application in Real Wastewater. Environ. Res. 2023, 229, 115987. [Google Scholar] [CrossRef]
- Jia, W.; Wang, H.; Wu, Q.; Sun, L.; Si, Q.; Zhao, Q.; Wu, Y.; Ren, N.; Guo, W. Insight into Chinese Medicine Residue Biochar Combined with Ultrasound for Persulfate Activation in Atrazine Degradation: Acanthopanax Senticosus Precursors, Synergistic Effects and Toxicity Assessment. Sci. Total Environ. 2023, 880, 163054. [Google Scholar] [CrossRef]
- Plaza, J.; Arencibia, A.; López-Muñoz, M.J. Evaluation of NZVI for the Degradation of Atrazine in Heterogeneous Fenton-like Systems at Circumneutral PH. J. Environ. Chem. Eng. 2021, 9, 106641. [Google Scholar] [CrossRef]
- Yin, H.; Li, J.; Yan, H.; Cai, H.; Wan, Y.; Yao, G.; Guo, Y.; Lai, B. Activation of Peroxymonosulfate by CuCo2O4 Nano-Particles towards Long-Lasting Removal of Atrazine. Water Reuse 2021, 11, 542–559. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, X.; Liu, Y.; Li, W.; He, Y.; Deng, Y.; Lin, Z.; Zhou, Y. Activation of Periodate by Chalcopyrite for Efficient Degradation of Tetracycline Hydrochloride. Sep. Purif. Technol. 2024, 333, 125813. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Qi, C.; Lin, C. Activation of Periodate by Granular Activated Carbon for Acid Orange 7 Decolorization. J. Taiwan Inst. Chem. Eng. 2016, 68, 211–217. [Google Scholar] [CrossRef]
- Gaber, M.M.; Toghan, A.; Shokry, H.; Samy, M. Efficient Oxidative Degradation of Organic Pollutants in Real Industrial Effluents Using a Green-Synthesized Magnetite Supported on Biochar Catalyst. RSC Adv. 2025, 15, 31522–31538. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; You, X.; Li, Y.; Lin, H.; Chen, J.P. A Novel Bimetallic Fe-Cu-CNT Catalyst for Effective Catalytic Wet Peroxide Oxidation: Reaction Optimization and Mechanism Investigation. Chem. Eng. J. 2024, 479, 147320. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Zhang, X.; Tang, W.; He, J.; Yang, Y. ZIF-67/Melamine-Derived Cobalt Encapsulated in N-Doped CNTs for Periodate Activation toward Tetracycline Degradation. J. Water Process Eng. 2024, 65, 105879. [Google Scholar] [CrossRef]
- Sukhatskiy, Y.; Shepida, M.; Sozanskyi, M.; Znak, Z.; Gogate, P.R. Periodate-Based Advanced Oxidation Processes for Wastewater Treatment: A Review. Sep. Purif. Technol. 2023, 304, 122305. [Google Scholar] [CrossRef]
- He, L.; Lv, L.; Pillai, S.C.; Wang, H.; Xue, J.; Ma, Y.; Liu, Y.; Chen, Y.; Wu, L.; Zhang, Z.; et al. Efficient Degradation of Diclofenac Sodium by Periodate Activation Using Fe/Cu Bimetallic Modified Sewage Sludge Biochar/UV System. Sci. Total Environ. 2021, 783, 146974. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Meng, S.; Zhou, C.; Xiong, Z.; He, C.; Liu, Y.; Zhang, H.; Zhou, P.; Lai, B. Metal-Free Carbon Nanotube Boosted Periodate Oxidation towards Organic Pollutants: Identification of Active Sites and Activation Mechanism. Sep. Purif. Technol. 2025, 354, 129186. [Google Scholar] [CrossRef]
- Elmitwalli, T.; Fouad, M.; Mossad, M.; Samy, M. Periodate Activation by Mulukhiyah Stalks and Potato Peels-Derived Biochars for the Efficient Degradation of Sulfamethazine. J. Environ. Chem. Eng. 2024, 12, 112101. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, Z.; Xu, G.; Yu, Y. Nitrogen and Magnesium Codoped Biochar Activates Periodate to Remediate Bensulfuron Methyl-Contaminated Water at Low Temperature: Performance, Mechanisms, and Phytotoxicity. J. Hazard. Mater. 2024, 480, 135803. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, H.Y.; Choi, J.; Nam, I.H.; Lee, S.; Lee, S.; Kim, J.H.; Lee, C.; Lee, J. Oxidizing Capacity of Periodate Activated with Iron-Based Bimetallic Nanoparticles. Environ. Sci. Technol. 2014, 48, 8086–8093. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Mehdipour, F.; Feizi, R.; Ghanbari, F.; Lin, K.Y.A.; Bagheri, A.; Madihi-Bidgoli, S. Periodate Activation by Concurrent Utilization of UV and US for the Degradation of Para-Nitrophenol in Water: A Synergistic Approach. Korean J. Chem. Eng. 2023, 40, 882–891. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, C.; Lou, Y.; Yu, X.; Feng, M. Promoting Selective Water Decontamination via Boosting Activation of Periodate by Nanostructured Ru-Supported Co3O4 Catalysts. J. Hazard. Mater. 2023, 442, 130058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, S.; Ye, C.; Ou, R.; Zeng, H.; Yu, X.; Feng, M. Sunlight-Activated Periodate Oxidation: A Novel and Versatile Strategy for Highly Efficient Water Decontamination. Chem. Eng. J. 2023, 451, 138642. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Q.; He, J.; Liu, F.; Yan, X.; Xu, Y. Visible Light-Assisted Periodate Activation Using Carbon Nitride for the Efficient Elimination of Acid Orange 7. Separations 2024, 11, 274. [Google Scholar] [CrossRef]
- Zhang, X.; Verbist, M.; Kamali, M.; Xue, Y.; Liu, Y.; Jin, P.; Costa, M.E.V.; Appels, L.; Cabooter, D.; Dewil, R. Activation of Periodate with Pinewood Biochar-CuO Composite for the Removal of Recalcitrant Organic Pollutants—Mechanisms and Degradation Products. Chem. Eng. J. 2023, 465, 142916. [Google Scholar] [CrossRef]
- He, L.; Yang, S.; Shen, S.; Ma, Y.; Chen, Y.; Xue, J.; Wang, J.; Zheng, L.; Wu, L.; Zhang, Z.; et al. Novel Insights into the Mechanism of Periodate Activation by Heterogeneous Ultrasonic-Enhanced Sludge Biochar: Relevance for Efficient Degradation of Levofloxacin. J. Hazard. Mater. 2022, 434, 128860. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, Z.; Chen, K.; Ding, D.; Yang, S.; Cai, T. Applying a Novel Advanced Oxidation Process of Biochar Activated Periodate for the Efficient Degradation of Bisphenol A: Two Nonradical Pathways. Chem. Eng. J. 2023, 453, 139889. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Chen, Y.; Shen, S.; Xue, J.; Ma, Y.; Zheng, L.; Wu, L.; Zhang, Z.; Yang, L. Iron-Manganese Oxide Loaded Sludge Biochar as a Novel Periodate Activator for Thiacloprid Efficient Degradation over a Wide PH Range. Sep. Purif. Technol. 2022, 288, 120703. [Google Scholar] [CrossRef]
- Gaber, M.M.; Samy, M.; Shokry, H. Effective Degradation of Synthetic Micropollutants and Real Textile Wastewater via a Visible Light-Activated Persulfate System Using Novel Spinach Leaf-Derived Biochar. Environ. Sci. Pollut. Res. 2024, 31, 25163–25181. [Google Scholar] [CrossRef]
- He, L.; Yang, S.; Yang, L.; Shen, S.; Li, Y.; Kong, D.; Chen, Z.; Yang, S.; Wang, J.; Wu, L.; et al. Ball Milling-Assisted Preparation of Sludge Biochar as a Novel Periodate Activator for Nonradical Degradation of Sulfamethoxazole: Insight into the Mechanism of Enhanced Electron Transfer. Environ. Pollut. 2023, 316, 120620. [Google Scholar] [CrossRef]
- Seo, G.; Narendra Kumar, A.V.; Shin, W.S. Periodate Activation and PH Regulation Using Ball-Milled Metal-Oxide-Mineral-Biochar Composite for Removal of Antibiotics from Contaminated Water. Environ. Res. 2024, 260, 119611. [Google Scholar] [CrossRef]
- Gu, Y.; Cai, F.; Zhu, Z.; Dai, Z.; Chen, C.; Liu, G. Improving the Methane Production from Zucchini Stem by Response Surface Methodology and Different Pretreatments. Ind. Crops Prod. 2020, 150, 112402. [Google Scholar] [CrossRef]
- Gaber, M.M.; Shokry, H.; Samy, M.; El-Bestawy, E.A. Green Approach for Fabricating Hybrids of Food Waste-Derived Biochar/Zinc Oxide for Effective Degradation of Bromothymol Blue Dye in a Photocatalysis/Persulfate Activation System. Chemosphere 2024, 364, 143245. [Google Scholar] [CrossRef]
- Anjum, M.; Prakash, N.B. Production of Phytolith and PhytOC and Distribution of Extractable Si Pools in Aerobic Rice as Influenced by Different Si Sources. Front. Plant Sci. 2023, 14, 1146416. [Google Scholar] [CrossRef]
- Maaoui, A.; Chagtmi, R.; Apicella, B.; Cerciello, F.; Senneca, O.; Trabelsi, A.B.H. Calcium-Rich Biochar Derived from Cactus Feedstock and Its Efficient Adsorption Properties for Industrial Dye. Appl. Sci. 2025, 15, 894. [Google Scholar] [CrossRef]
- Rajput, N.A.; Laghari, M.; Mangio, H.-U.-R.; Soothar, R.K. Enhanced Phosphorus and Potassium Recovery through Co-Pyrolysis of Nutrient-Rich Biomass Feedstocks for Engineered Biochar Production. Bioresour. Technol. Rep. 2024, 26, 101818. [Google Scholar] [CrossRef]
- Ngernyen, Y.; Petsri, D.; Sribanthao, K.; Kongpennit, K.; Pinijnam, P.; Pedsakul, R.; Hunt, A.J. Adsorption of the Non-Steroidal Anti-Inflammatory Drug (Ibuprofen) onto Biochar and Magnetic Biochar Prepared from Chrysanthemum Waste of the Beverage Industry. RSC Adv. 2023, 13, 14712–14728. [Google Scholar] [CrossRef]
- Bläker, C.; Muthmann, J.; Pasel, C.; Bathen, D. Characterization of Activated Carbon Adsorbents—State of the Art and Novel Approaches. ChemBioEng Rev. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- Gao, J.; Li, Z.; Tao, X.; Li, X.; Zhu, K. A Comprehensive Study of Multiscale Pore Structural Characteristics in Deep-Buried Coals of Different Ranks. Sci. Rep. 2025, 15, 8299. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhuang, Y.; Liu, J.; Zhang, X. Three-Dimensionally Ordered Macroporous ZSM-5 Zeolite Supported High-Dispersed Ni2P: Highly Active Catalyst for Quinoline Hydrodenitrogenation. Chem. Eng. J. 2024, 480, 148236. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, W.; Peng, H.; Pan, B.; Xing, B. Functional Biochar and Its Balanced Design. ACS Environ. Au 2022, 2, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Urseler, N.; Bachetti, R.; Biolé, F.; Morgante, V.; Morgante, C. Atrazine Pollution in Groundwater and Raw Bovine Milk: Water Quality, Bioaccumulation and Human Risk Assessment. Sci. Total Environ. 2022, 852, 158498. [Google Scholar] [CrossRef]
- Bayati, M.; Numaan, M.; Kadhem, A.; Salahshoor, Z.; Qasim, S.; Deng, H.; Lin, J.; Yan, Z.; Lin, C.H.; de Fidalgo Cortalezzi, M. Adsorption of Atrazine by Laser Induced Graphitic Material: An Efficient, Scalable and Green Alternative for Pollution Abatement. J. Environ. Chem. Eng. 2020, 8, 104407. [Google Scholar] [CrossRef]
- Gaber, M.M.; Shokry, H.; Hassanin, A.H.; Awad, S.; Samy, M.; Elkady, M. Novel Palm Peat Lignocellulosic Adsorbent Derived from Agricultural Residues for Efficient Methylene Blue Dye Removal from Textile Wastewater. Appl. Water Sci. 2025, 15, 32. [Google Scholar] [CrossRef]
- Chen, G.C.; Shan, X.Q.; Zhou, Y.Q.; Shen, X.E.; Huang, H.L.; Khan, S.U. Adsorption Kinetics, Isotherms and Thermodynamics of Atrazine on Surface Oxidized Multiwalled Carbon Nanotubes. J. Hazard. Mater. 2009, 169, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of Adsorption and Functionalization of Biochar for Pesticides: A Review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- do Nascimento, C.T.; Vieira, M.G.A.; Scheufele, F.B.; Palú, F.; da Silva, E.A.; Borba, C.E. Adsorption of Atrazine from Aqueous Systems on Chemically Activated Biochar Produced from Corn Straw. J. Environ. Chem. Eng. 2022, 10, 107039. [Google Scholar] [CrossRef]
- Gaber, M.M.; Samy, M.; El-Bestawy, E.A.; Shokry, H. Effective Degradation of Tetracycline and Real Pharmaceutical Wastewater Using Novel Nanocomposites of Biosynthesized ZnO and Carbonized Toner Powder. Chemosphere 2024, 352, 141448. [Google Scholar] [CrossRef]
- Naddeo, V.; Belgiorno, V.; Kassinos, D.; Mantzavinos, D.; Meric, S. Ultrasonic Degradation, Mineralization and Detoxification of Diclofenac in Water: Optimization of Operating Parameters. Ultrason. Sonochemistry 2010, 17, 179–185. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Qi, C.; Zhang, H.; Ma, J. Enhanced Activation of Periodate by Iodine-Doped Granular Activated Carbon for Organic Contaminant Degradation. Chemosphere 2017, 181, 609–618. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-Catalyzed Sulfate Radical-Based Advanced Oxidation: A Review on Heterogeneous Catalysts and Applications. Appl. Catal. B 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Huang, J.; Ding, Y.; Zong, Y.; Wu, D.; Jiang, J.; Jiang, X. Activation of Periodate by Self-Recycled Ru(III)/TiO2 for Selective Oxidation of Aqueous Organic Pollutants: Essential Role of Homogeneous Ru(V)=O Species. Chem. Eng. J. 2023, 473, 145012. [Google Scholar] [CrossRef]
- Gaber, M.M.; Samy, M.; Azam, A.; Shokry, H. Remediation of Paracetamol-Contaminated Water by Novel Burned Hookah Charcoal Residues via Persulfate Activation Under Visible Light: Optimization, Mechanism, and Real Pharmaceutical Wastewater Application. J. Environ. Chem. Eng. 2024, 12, 114399. [Google Scholar] [CrossRef]
- Gaber, M.M.; Rashid, N.; Alzahrani, A.; Alanazi, F. Utilization of Incense Stick Residues as a Sustainable Catalyst for Efficient Peroxydisulfate Activation in the Oxidative Degradation of Atrazine from Real Industrial Effluents. J. Environ. Chem. Eng. 2025, 13, 116031. [Google Scholar] [CrossRef]
- Zong, Y.; Shao, Y.; Zeng, Y.; Shao, B.; Xu, L.; Zhao, Z.; Liu, W.; Wu, D. Enhanced Oxidation of Organic Contaminants by Iron(II)-Activated Periodate: The Significance of High-Valent Iron-Oxo Species. Environ. Sci. Technol. 2021, 55, 7634–7642. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Dong, Z.Y.; Dong, Z.Y.; Wu, Y.H.; Ji, S.J.; Hu, L.L.; Yang, X.Y.; Liu, H.; Xu, B. Insight into the Activation of Periodate by Mn(II) for Rapid Degradation of Sulfadiazine: Performance and Mechanisms. Sep. Purif. Technol. 2024, 342, 127023. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient Reductive Dechlorination of Monochloroacetic Acid by Sulfite/UV Process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Singlet-Oxygen Generation in Alkaline Periodate Solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research Status, Trends, and Mechanisms of Biochar Adsorption for Wastewater Treatment: A Scientometric Review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Xiao, P.; Yi, X.; Wu, M.; Wang, X.; Zhu, S.; Gao, B.; Liu, Y.; Zhou, H. Catalytic Performance and Periodate Activation Mechanism of Anaerobic Sewage Sludge-Derived Biochar. J. Hazard. Mater. 2022, 424, 127692. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Ma, S.; Zhan, S.; Wang, J.; Gao, B.; Tang, X.; Zhu, Q.; Xu, S.; Zhuang, X. A Green Strategy for Porous Biochar Fabrication with Superior Capacity for Peroxydisulfate Activation to Degrade Sulfadiazine: The Cooperative Role of C-Sp 3 and Specific Surface Area. Biochar 2023, 5, 24. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.; Zhong, Y.; Guan, Y.; Chen, Q.; Du, W.; Liu, G. Biological Calcium Carbonate Enhanced the Ability of Biochar to Passivate Antimony and Lead in Soil. Environ. Sci. Process. Impacts 2023, 25, 1365–1373. [Google Scholar] [CrossRef]
- Peng, H.; Xu, L.; Zhang, W.; Liu, F.; Lu, X.; Lu, W.; Danish, M.; Lin, K. Different Kinds of Persulfate Activation with Base for the Oxidation and Mechanism of BDE209 in a Spiked Soil System. Sci. Total Environ. 2017, 574, 307–313. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Y.; Jiang, S.; Wang, Y.; Li, H.; Han, W.; Qu, J.; Wang, L.; Hu, Y. Graphene-like Carbon Sheet-Supported NZVI for Efficient Atrazine Oxidation Degradation by Persulfate Activation. Chem. Eng. J. 2021, 403, 126309. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, S.; Li, H.; Zhang, R.; Jiang, Z.; Zhang, Y. A Stable Biochar Supported S-NZVI to Activate Persulfate for Effective Dichlorination of Atrazine. Chem. Eng. J. 2022, 431, 133937. [Google Scholar] [CrossRef]
- Xu, S.; Wei, H.; Li, X.; Chen, L.; Song, T. Treatment of Tetracycline in an Aqueous Solution with an Iron–Biochar/Periodate System: Influencing Factors and Mechanisms. Water Sci. Technol. 2024, 89, 3344–3356. [Google Scholar] [CrossRef] [PubMed]
- Altendji, K.; Hamoudi, S. Efficient Photocatalytic Degradation of Aqueous Atrazine over Graphene-Promoted g-C3N4 Nanosheets. Catalysts 2023, 13, 1265. [Google Scholar] [CrossRef]
- Komtchou, S.; Dirany, A.; Drogui, P.; Robert, D.; Lafrance, P. Removal of Atrazine and Its By-Products from Water Using Electrochemical Advanced Oxidation Processes. Water Res. 2017, 125, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Mensah, K.; Samy, M.; Ezz, H.; Elkady, M.; Shokry, H. Utilization of Iron Waste from Steel Industries in Persulfate Activation for Effective Degradation of Dye Solutions. J. Environ. Manag. 2022, 314, 115108. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; El-Fakharany, E.M.; Mensah, K.; Amer, K.; Li, J.; Leung, D.Y.C. Efficient Degradation of Polyethylene (PE) Plastics by a Novel Vacuum Ultraviolet-Activated Periodate System: Operating Parameters, By-Products, and Toxicity. J. Environ. Chem. Eng. 2024, 12, 114848. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, P.; Zhou, H.; Huang, B.; Sun, M.; He, C.S.; Zhang, H.; Ao, Z.; Liu, W.; Lai, B. Removal of Phenols by Highly Active Periodate on Carbon Nanotubes: A Mechanistic Investigation. Environ. Sci. Technol. 2023, 57, 10804–10815. [Google Scholar] [CrossRef]
- Ling, C.; Wu, S.; Han, J.; Dong, T.; Zhu, C.; Li, X.; Xu, L.; Zhang, Y.; Zhou, M.; Pan, Y. Sulfide-Modified Zero-Valent Iron Activated Periodate for Sulfadiazine Removal: Performance and Dominant Routine of Reactive Species Production. Water Res. 2022, 220, 118676. [Google Scholar] [CrossRef]
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of Atrazine by ZnxCu1−xFe2O4 Nanomaterial-Catalyzed Sulfite under UV–Vis Light Irradiation: Green Strategy to Generate SO4−. Appl. Catal. B 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Khan, H.M.; Hapeshi, E.; Fatta-Kassinos, D.; Dionysiou, D.D. Kinetic and Mechanism Investigation on the Photochemical Degradation of Atrazine with Activated H2O2, S2O82− and HSO5−. Chem. Eng. J. 2014, 252, 393–403. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Yao, G.; Zhang, Y.; Li, X.; Lai, B. Improving the Degradation of Atrazine in the Three-Dimensional (3D) Electrochemical Process Using CuFe2O4 as Both Particle Electrode and Catalyst for Persulfate Activation. Chem. Eng. J. 2019, 361, 1317–1332. [Google Scholar] [CrossRef]
- El-monem, H.A.; Mahanna, H.; El-Halwany, M.; Samy, M. Photo-Thermal Activation of Persulfate for the Efficient Degradation of Synthetic and Real Industrial Wastewaters: System Optimization and Cost Estimation. Environ. Sci. Pollut. Res. 2024, 31, 24153–24162. [Google Scholar] [CrossRef]
- Belghit, A.; Merouani, S.; Dehane, A. Exploring the Intensifying Effect of Periodate/Hydroxylamine Oxidation Process for the Effective Mineralization of Textile Dyes Effluents with Consideration of Environmental Matrices. Asia-Pac. J. Chem. Eng. 2024, 19, e2981. [Google Scholar] [CrossRef]
- Samy, M.; Gar Alalm, M.; Fujii, M.; Ibrahim, M.G. Doping of Ni in MIL-125(Ti) for Enhanced Photocatalytic Degradation of Carbofuran: Reusability of Coated Plates and Effect of Different Water Matrices. J. Water Process Eng. 2021, 44, 102449. [Google Scholar] [CrossRef]
- Sheng, J.; Guo, S.; Yuan, C.; Nie, X.; Cui, P.; Jiang, H. Degradation Bensulfuron-Methyl by Magnetic CoFe Alloy@N-Doped Graphitized Carbon Derived from CoFe2O4 Activated by Peroxymonosulfate. Chem. Eng. J. 2023, 466, 143158. [Google Scholar] [CrossRef]
- Lu, H.; Wang, X.; Cong, Q.; Chen, X.; Li, Q.; Li, X.; Zhong, S.; Deng, H.; Yan, B. Research Progress on the Degradation of Organic Pollutants in Water by Activated Persulfate Using Biochar-Loaded Nano Zero-Valent Iron. Molecules 2024, 29, 1130. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Z.; Pan, Y.; Zhang, Y.; Gong, H.; Mei, X.; Qiao, W.; Gan, L. Effect of Lignocellulosic Biomass Composition on the Performance of Biochar for the Activation of Peroxymonosulfate to Degrade Diclofenac. Sep. Purif. Technol. 2023, 311, 123312. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, W.; Lin, X.; Wang, Y.; Lu, X.; Yu, Y.; Gu, H.; Heng, S.; Zhang, H.; Ma, J. Periodate Activation with Stable MgMn2O4 Spinel for Bisphenol A Removal: Radical and Non-Radical Pathways. Chem. Eng. J. 2023, 459, 141574. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, X.; Shangguan, Z.; Qu, J.; Zhang, W. A Low-Cost Magnetic Catalyst (MnFe2O4) for Ciprofloxacin Degradation via Periodate Activation: The Synergistic Effect of Mn and Fe. Chem. Eng. J. 2023, 464, 142562. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Q.; Du, J.; Wang, H.; Li, X.; Ren, N. Enhanced Removal of Sulfadiazine by Sulfidated ZVI Activated Persulfate Process: Performance, Mechanisms and Degradation Pathways. Chem. Eng. J. 2020, 388, 124303. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Tong, L.; Liu, N.; Wang, C.; Hu, Q. N-Doped Biochar-Catalyzed Dechlorination of Carbon Tetrachloride in Sulfide-Containing Aqueous Solutions: Performances, Mechanisms and Pathways. Water Res. 2022, 223, 119006. [Google Scholar] [CrossRef]
- Song, T.; Wang, Z.; Jiang, Y.; Yang, S.; Deng, Q. Research Progress on the Degradation of Organic Pollutants in Wastewater via Ultrasound/Periodate Systems: A Review. Molecules 2024, 29, 2562. [Google Scholar] [CrossRef]
- He, L.; Yang, C.; Ding, J.; Lu, M.Y.; Chen, C.X.; Wang, G.Y.; Jiang, J.Q.; Ding, L.; Liu, G.S.; Ren, N.Q.; et al. Fe, N-Doped Carbonaceous Catalyst Activating Periodate for Micropollutant Removal: Significant Role of Electron Transfer. Appl. Catal. B 2022, 303, 120880. [Google Scholar] [CrossRef]
- Ghanbari, F.; Yaghoot-Nezhad, A.; Wacławek, S.; Lin, K.Y.A.; Rodríguez-Chueca, J.; Mehdipour, F. Comparative Investigation of Acetaminophen Degradation in Aqueous Solution by UV/Chlorine and UV/H2O2 Processes: Kinetics and Toxicity Assessment, Process Feasibility and Products Identification. Chemosphere 2021, 285, 131455. [Google Scholar] [CrossRef]
- Du, J.; Xiao, G.; Xi, Y.; Zhu, X.; Su, F.; Kim, S.H. Periodate Activation with Manganese Oxides for Sulfanilamide Degradation. Water Res. 2020, 169, 115278. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Hayati, F.; Isari, A.A.; Anvaripour, B.; Fattahi, M.; Kakavandi, B. Ultrasound-Assisted Photocatalytic Degradation of Sulfadiazine Using MgO@CNT Heterojunction Composite: Effective Factors, Pathway and Biodegradability Studies. Chem. Eng. J. 2020, 381, 122636. [Google Scholar] [CrossRef]
- Peng, F.; Yin, R.; Liao, Y.; Xie, X.; Sun, J.; Xia, D.; He, C. Kinetics and Mechanisms of Enhanced Degradation of Ibuprofen by Piezo-Catalytic Activation of Persulfate. Chem. Eng. J. 2020, 392, 123818. [Google Scholar] [CrossRef]
- Khajeh, M.; Amin, M.M.; Fatehizadeh, A.; Aminabhavi, T.M. Synergetic Degradation of Atenolol by Hydrodynamic Cavitation Coupled with Sodium Persulfate as Zero-Waste Discharge Process: Effect of Coexisting Anions. Chem. Eng. J. 2021, 416, 129163. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, Y.; Xiong, H.Q.; Wu, F.; Lv, T.X.; Fang, Y.C.; Xiang, H. The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions. Sustainability 2023, 15, 6711. [Google Scholar] [CrossRef]
- Richaud, A.; Méndez, F.; Barba-Behrens, N.; Florian, P.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Electrophilic Modulation of the Superoxide Anion Radical Scavenging Ability of Copper(II) Complexes with 4-Methyl Imidazole. J. Phys. Chem. A 2021, 125, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, Y.; Ling, Y.; Chu, X.; Cheng, Y.; Min, F. Effects of Mg(II) Doping Amount on the Hydration Characteristics of Kaolinite Surface: Molecular Dynamics Simulations and Experiments. Surf. Interfaces 2024, 45, 103869. [Google Scholar] [CrossRef]
- Da, Y.; Gu, X.; Wang, Y.; Lu, X.; Xie, G.; Zhang, L.; Liu, S.; Zhao, X.; Tian, J.; Xiao, Y.; et al. Electron Transfer Enhancing Fe2+/Fe3+ Cycle by Persistent Free Radicals (PFRs) Contained Biochars to Active H2O2 for PNP Degradation. Surf. Interfaces 2025, 72, 107344. [Google Scholar] [CrossRef]
- Moeinzadeh, R.; Azizi, N.; Hekmati, M.; Qomi, M.; Esmaeili, D. ZnONPs/Covalent Triazine Frameworks Nanocomposite as High-Performance Photocatalysts for Degradation of Congo Red under Visible Light. Mater. Chem. Phys. 2023, 307, 128122. [Google Scholar] [CrossRef]
- Biswas, B.; Rahman, T.; Sakhakarmy, M.; Jahromi, H.; Eisa, M.; Baltrusaitis, J.; Lamba, J.; Torbert, A.; Adhikari, S. Phosphorus Adsorption Using Chemical and Metal Chloride Activated Biochars: Isotherms, Kinetics and Mechanism Study. Heliyon 2023, 9, e19830. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, S.; Zhang, B.; Chen, W.; An, M.; Yang, Z.; Gao, H.; Qin, S. Zinc and Sulfur Functionalized Biochar as a Peroxydisulfate Activator via Deferred Ultraviolet Irradiation for Tetracycline Removal. RSC Adv. 2024, 14, 5648–5664. [Google Scholar] [CrossRef]
- Babaei, S.; Rokhbakhsh-Zamin, F.; Ahmadian, M.; Kalantar-Neyestanaki, D.; Kazemipour, N. Photo-Fenton like Catalyst System with CuCOFe2O4@AC Nanoparticles for Ciprofloxacin Removal from Aqueous Solutions and Effluent Toxicity Assessment by Escherichia Coli and Enterococcus Faecalis Bacteria. Appl. Water Sci. 2024, 14, 199. [Google Scholar] [CrossRef]
- Sarrai, A.E.; Hanini, S.; Merzouk, N.K.; Tassalit, D.; Szabó, T.; Hernádi, K.; Nagy, L. Using Central Composite Experimental Design to Optimize the Degradation of Tylosin from Aqueous Solution by Photo-Fenton Reaction. Materials 2016, 9, 428. [Google Scholar] [CrossRef]
- Momen, S.B.; Siadat, S.D.; Akbari, N.; Ranjbar, B.; Khajeh, K. Applying Central Composite Design and Response Surface Methodology to Optimize Growth and Biomass Production of Haemophilus Influenzae Type B. Jundishapur J. Microbiol. 2016, 9, e25246. [Google Scholar] [CrossRef]
- Iwundu, M.P.; Oko, E.T. Design Efficiency and Optimal Values of Replicated Central Composite Designs with Full Factorial Portions. Afr. J. Math. Stat. Stud. 2021, 4, 89–117. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M. Effective Photocatalytic Degradation of Sulfamethazine by CNTs/LaVO4 in Suspension and Dip Coating Modes. Sep. Purif. Technol. 2020, 235, 116138. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F. Photocatalytic Degradation of Sulfamethoxazole by Hierarchical Magnetic ZnO@g-C3N4: RSM Optimization, Kinetic Study, Reaction Pathway and Toxicity Evaluation. J. Hazard. Mater. 2018, 359, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, F.; Choi, W. Production of Reactive Oxygen Species by the Reaction of Periodate and Hydroxylamine for Rapid Removal of Organic Pollutants and Waterborne Bacteria. Environ. Sci. Technol. 2020, 54, 6427–6437. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Waste Water, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, CO, USA; Water Environment Federation (WEF): Alexandria, VA, USA, 2017. [Google Scholar]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline Degradation by Persulfate Activated with Magnetic γ-Fe2O3/CeO2 Catalyst: Performance, Activation Mechanism and Degradation Pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Nasr, M. Artificial Intelligence, Regression Model, and Cost Estimation for Removal of Chlorothalonil Pesticide by Activated Carbon Prepared from Casuarina Charcoal. Sustain. Environ. Res. 2018, 28, 101–110. [Google Scholar] [CrossRef]
- Elhafeez, M.S.A. Evaluation of Analytical Method for Determination of Tetracyclines in Their Dosage Forms by HPLC Using Different Types of Chromatographic Stationary Phases (RP-C8 and RP-C18). Egypt. J. Anim. Health 2021, 1, 1–10. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).