Abstract
As key intermediate products in petroleum and chemical units, C4 hydrocarbons can be converted to ethene and propylene. While C4 olefins can be cracked into ethene and propylene on acid catalysts, such reactions with C4 paraffins are difficult under these conditions. In this study, a bifunctional metal–acid catalyst, BDHC, was prepared for catalytic dehydrogenation and catalytic cracking, using ZSM-5 zeolite for cracking active groups and Fe2O3 and Cr2O3 for dehydrogenation active groups. In the catalyzed reaction, C4 paraffins are converted to C4 olefins, which are subsequently cracked into ethene and propylene. The BDHC catalyst’s high relative crystallinity and large specific surface area and pore volume promote adsorption of reactant molecules. Moreover, the appropriate acid content suppresses side pathways and produces more ethene and propylene. Under optimized conditions, the ethene yield was 11.20%, the propylene yield was 27.51%, and the sum of the ethene and propylene yields was 38.71%.
1. Introduction
C4 hydrocarbons, specifically C4 paraffins and olefins, are critical intermediates in various processes, such as catalytic cracking, steam cracking, and oil field recovery. Their significance lies in their utility as feedstocks for manufacturing a wide range of industrial products. In recent years, due to the fast-paced expansion of the coal chemical industry, methanol-to-olefin plants have also generated a significant quantity of C4 hydrocarbons [1]. However, the full use of C4 hydrocarbons is limited. Most C4 hydrocarbons are used as industrial or civil fuel, with less than 40% used to produce gasoline blend constituents, such as alkylated oil and methyl tertiary-butyl ether (MTBE), or other chemical goods, such as rubber, resin, and fiber. Driven by the rapid expansion of downstream products, ethene and propylene consumption has increased annually, resulting in a tight supply-and-demand situation [2]. Converting C4 hydrocarbons to ethene and propylene via rational processing can enhance the utilization rate of C4 hydrocarbons while mitigating the imbalance between the availability of and requirement for ethene and propylene, thus also enhancing the economic efficiency of refineries [3].
Numerous companies worldwide have developed procedures for deep catalytic cracking to generate ethene and propylene from C4 olefins. These procedures include the OCP process developed by UOP and Atofina, the Propylur process by Lurgi, the MOI process by Mobil, the Superflex process by KBR, the Omega Plant process by Asahi Kasei, and the OCC process by SINOPEC Shanghai Research Institute of Petrochemicals [4]. These well-established technologies use moderate reaction conditions and result in over 50% yields for ethene and propylene compared to C4 olefins [5]. However, when dealing with C4 hydrocarbons containing high levels of C4 paraffins, conversion of the paraffins becomes problematic [6,7]. In most cases, these processes use fixed-bed reactors, which do not allow for continuous reaction and regeneration [8]. Ethene and propylene production through catalytic cracking of C4 paraffins remains in the laboratory stage. Progress has been made in improving the yields of these components by selecting appropriate zeolite materials, modifying catalysts, and optimizing reaction conditions [9]. However, the overall ethene and propylene yields remain relatively low [10]. Low-carbon alkane dehydrogenation technology has matured, with the Oleflex process by UOP and Catofin by CB&I Lummus being widely used in industrial applications globally [11]. However, low-carbon alkane dehydrogenation technology poses challenges [12,13]. On the one hand, it can only generate certain olefins, such as isobutene through isobutane dehydrogenation, and converting butene to ethene and propylene can be problematic [14,15,16]. On the other hand, low-carbon alkane dehydrogenation technology often relies on costly precious-metal catalysts [17,18,19,20].
Because of its unique pore structure, ZSM-5 zeolite has been widely utilized in diverse catalytic reactions [21,22]. Adding a small quantity of Fe increases the acidity of the ZSM-5 zeolite [23,24], which not only enhances the transformation of C4 hydrocarbons, especially C4 paraffins, but also improves the selectivity for ethene and propylene [25,26,27,28]. Cr-based catalysts are the most commonly used non-precious-metal catalysts for low-carbon alkane dehydrogenation technology [29,30]. On a catalyst carrier, Cr2O3 displays significant dehydrogenation activity, which results in the activation of alkane molecules adsorbed on the catalyst surface, creating Cr-C and O-H bonds [31,32]. After that, the H on the β-site is transferred to the active Cr site, forming Cr-H and C=C bonds. Ultimately, the catalysts release hydrogen with olefins [33,34].
Composite catalysts combining catalytic cracking and catalytic dehydrogenation are a promising approach to converting C4 hydrocarbons to ethene and propylene. In this study, a bifunctional metal–acid catalyst, BDHC, was prepared for catalytic dehydrogenation and catalytic cracking, and suitable reaction conditions were investigated in a fixed fluidized bed reactor.
2. Results
2.1. Catalyst Properties
As shown in Table 1, BDHC not only consisted of Al2O3, SiO2, and P2O5—the basic composition of MFI zeolite—but also contained minute quantities of Fe2O3 and Cr2O3, which play a key role in dehydrogenation.

Table 1.
Composition of catalysts.
Table 2 shows that, compared to RZSM, BDHC exhibited greater relative crystallinity, as well as a larger specific surface area and pore volume, allowing for greater adsorption of reactant molecules and consequently enhancing the reaction; the micropore volume was similar between the two catalysts.

Table 2.
Physical properties of catalysts.
Figure 1 shows the X-ray diffraction (XRD) spectra of the two catalysts; it can be observed that BDHC and RZSM exhibit well-defined diffraction peaks, which are characteristic of the MFI topology and located in the 2θ ranges of 5°~10° and 20°~25°. No obvious impurity phase was detected, which suggests that the addition of the metal elements did not alter the pore structure of ZSM-5 zeolite.
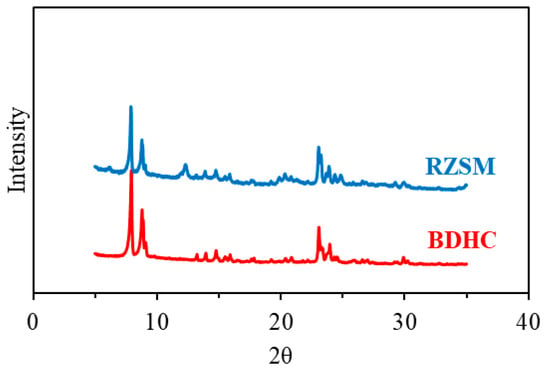
Figure 1.
X-ray diffraction spectra of the catalysts.
As shown in Figure 2, the N2 adsorption–desorption isotherms of BDHC and RZSM both exhibited type IV physisorption, typical of micro- and mesoporous structures.
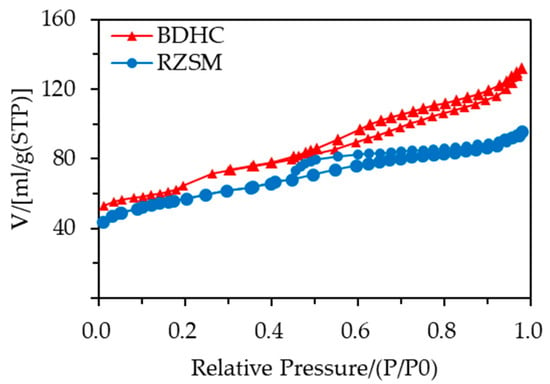
Figure 2.
Nitrogen adsorption–desorption isotherms of catalysts.
Figure 3 presents the ammonia temperature-programmed desorption (NH3-TPD) curves of the two catalysts. Both catalysts exhibited characteristic peaks at 200 °C and 350 °C, suggesting the existence of weak and strong acid sites. However, BDHC showed smaller characteristic peaks than RZSM, implying that BDHC had fewer total and strong acid sites than RZSM.
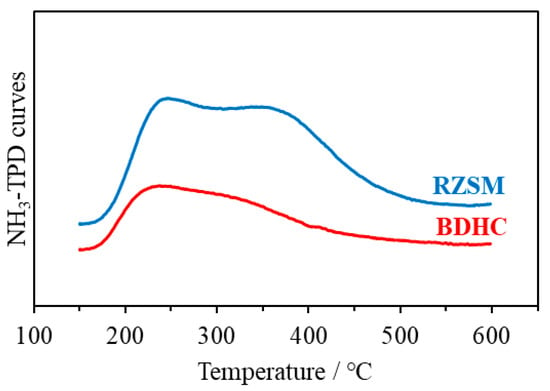
Figure 3.
NH3-TPD curves of BDHC and RZSM.
From the pyridine IR acidity of the two catalysts, as shown in Table 3, it is evident that at 200 °C, BDHC had fewer total Brønsted and Lewis acid sites than RZSM, and at 350 °C, BDHC had fewer strong Brønsted and strong Lewis acid sites than RZSM.

Table 3.
Pyridine IR acidity of BDHC and RZSM.
2.2. Comparison Between BDHC and RZSM
The catalytic dehydrogenative cracking performance of BDHC and RZSM was investigated at T = 620 °C, WHSV = 1.6 h−1, C/O = 25, and a feedstock volumetric dilution ratio (D) of 5 in a fixed fluidized bed reactor (FFB) plant. As shown in Table 4, the conversion of C4 hydrocarbons on BDHC was 59.21%, which was significantly higher than that on RZSM, indicating that BDHC had a higher capacity to convert C4 hydrocarbons. The ethene and propylene yields of BDHC were higher than those of RZSM as well, and the sum of ethene and propylene yields reached 38.21%, which was more than 10% higher than that obtained with RZSM. The hydrogen yield of BDHC was 0.39%, much higher than RZSM’s 0.29%, indicating that BDHC had stronger dehydrogenation activity and was able to promote dehydrogenation of C4 paraffins. However, although more ethene and propylene can be generated, more by-products, such as methane, ethane, and coke, will also be produced.

Table 4.
Product distributions of BDHC and RZSM.
2.3. Reaction Conditions
The effects of reaction temperature (T), weight hourly space velocity (WHSV), catalyst–oil mass ratio (C/O), and feedstock volume dilution ratio (D) were investigated using BDHC as a catalyst, and the optimized reaction conditions were obtained.
2.3.1. Reaction Temperature (T)
Figure 4 presents the influence of the reaction temperature (T). Figure 4A,B demonstrate that conversion of C4 hydrocarbons increased continuously with increasing reaction temperature, and the growth rate decreased beyond a reaction temperature of 620 °C. Ethene and propylene yields displayed similar trends, initially increasing and then tending to level off. As shown in Figure 4C, as the reaction temperature increased, the hydrogen yield increased rapidly and then tended to level off and decreased slightly; the methane yield increased quickly, and the coke yield increased slightly and then rose rapidly when the reaction temperature exceeded 620 °C. As demonstrated in Figure 4D, the molar ratio of methane to (ethene + propylene) (mr) increased continuously, which also indicates that increasing the reaction temperature has a stronger promoting effect on the thermal cracking reaction than other reactions.
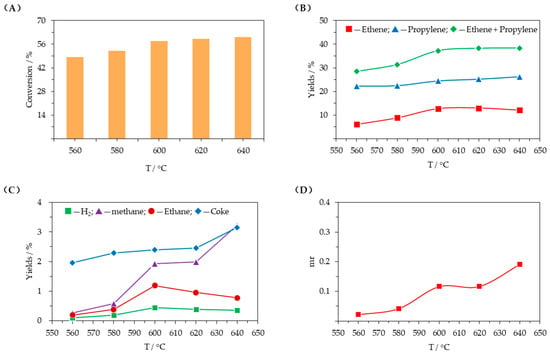
Figure 4.
Influence of the reaction temperature (T = 560–640 °C, WHSV = 1.6 h−1, C/O = 25, D = 5). (A) conversion of C4 hydrocarbons; (B) ethene and propylene yields; (C) yields of other products; (D) molar ratio of methane to (ethene + propylene) (mr).
Dehydrogenation and cracking are both endothermic reactions. Increasing the reaction temperature is conducive to shifting the chemical equilibrium to the right, gradually accelerating the reaction rate, and converting more C4 hydrocarbons, so the conversion rate continuously improves. When the reaction temperature increases beyond a certain threshold, the growth rate of the chemical equilibrium constant decreases, and accordingly, so does the growth rate of C4 hydrocarbons conversion. The growth rate of the chemical equilibrium constant of the catalytic dehydrogenation reaction decreases as the reaction temperature increases past a certain threshold, while the proportion of thermal cracking increases quickly and continuously, exceeding that of catalytic cracking. Large amounts of ethene can be produced by both catalytic dehydrogenative cracking and thermal cracking, but propylene comes mainly from catalytic dehydrogenative cracking. As the reaction temperature increases, the rate of thermal cracking increases more rapidly, resulting in higher ethene and propylene yields. When the reaction temperature exceeds 620 °C, further conversion of C4 hydrocarbons is limited, and the growth rates of ethene and propylene yields gradually slow down. However, the methane and coke yields continue to increase rapidly. A high reaction temperature is necessary to obtain high ethene and propylene yields. However, high temperature requires more energy and promotes thermal cracking, which would generate more methane and coke. Thus, 620 °C is selected as the upper temperature limit.
2.3.2. Weight Hourly Space Velocity (WHSV)
Figure 5 presents the influence of weight hourly space velocity (WHSV). Figure 5A,B show that C4 hydrocarbons conversion and ethene yield decreased as WHSV increases, while propylene yield gradually increased. The sum of ethene and propylene yields first increased and then decreased, reaching its peak value when WHSV was 2.4 h−1. From Figure 5C,D, it can be observed that as the WHSV increased, hydrogen, methane, ethane, and coke yields all decreased, with the trend slowing down after WHSV exceeded 2.4 h−1; the molar ratio of methane to (ethene + propylene) (mr) followed a similar decreasing trend, indicating that catalytic dehydrogenation and thermal cracking were both suppressed.
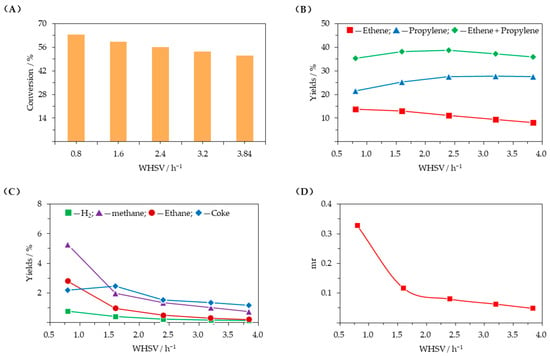
Figure 5.
Influence of weight hourly space velocity (T = 620 °C, WHSV = 0.8–3.8 h−1, C/O = 25, D = 5). (A) conversion of C4 hydrocarbons; (B) ethene and propylene yields; (C) yields of other products; (D) molar ratio of methane to (ethene + propylene) (mr).
Increasing WHSV means that the residence time of C4 hydrocarbons in the catalyst bed decreases, as does the number of C4 hydrocarbons molecules adsorbed in the catalyst channels; this influences the interaction between reactant molecules and metal and acid sites, inhibiting the dehydrogenation of C4 paraffins and the subsequent C4 olefin cracking. This will reduce C4 hydrocarbons conversion and decrease hydrogen, methane, ethane, ethene and coke yields. Instead, a large amount of propylene is produced, and since its secondary conversion is inhibited, the propylene yield increases. Therefore, to balance the total yield of target products while reducing the formation of by-products, a WHSV of 2.4 h−1 is appropriate.
2.3.3. Catalyst–Oil Mass Ratio (C/O)
Figure 6 presents the influence of the catalyst–oil mass ratio (C/O). As shown in Figure 6A–C, C4 hydrocarbons conversion and product yields all increased with increasing C/O, but when C/O exceeded 25, the increasing trend slowed down, except for hydrogen and coke yields, which increased rapidly. From Figure 6D, it can be observed that when C/O exceeded 25, the molar ratio of methane to (ethene + propylene) (mr) increased rapidly as well.
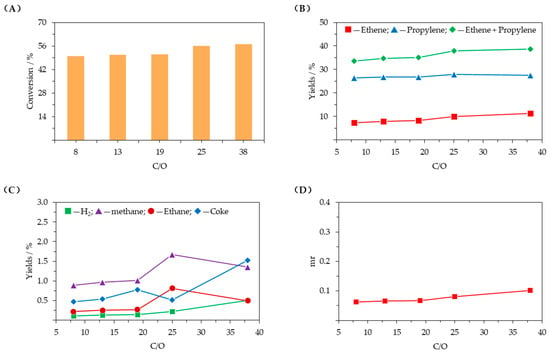
Figure 6.
Influence of catalyst–oil mass ratio (T = 620 °C, WHSV = 2.4 h−1, C/O = 5–38, D = 5). (A) conversion of C4 hydrocarbons; (B) ethene and propylene yields; (C) yields of other products; (D) molar ratio of methane to (ethene + propylene) (mr).
Higher C/O means that C4 hydrocarbons contact more catalyst per unit time—more reactant molecules are adsorbed on metal and acid sites, which promotes dehydrogenation and cracking and thus increases C4 hydrocarbons conversion and ethene and propylene production. When C/O reaches 25, reactant molecules no longer require more catalyst for the reaction, and further increasing C/O has little impact on the C4 hydrocarbons conversion rate and ethene and propylene yields. This is because more catalyst will carry more heat, which promotes side reactions such as thermal cracking, leading to a rapid increase in the yields of hydrogen and coke. Considering the catalyst cost and yields of ethene, propylene, and other products, C/O should not be too high.
2.3.4. Feedstock Volume Dilution Ratio (D)
Figure 7 presents the influence of the feedstock volume dilution ratio (D). As shown in Figure 7A,B, as D increased, C4 hydrocarbons conversion and propylene yield initially increased and then tended to level off, while ethene yield did not change significantly. In addition, Figure 7C,D show that other product yields and the molar ratio of methane to (ethene + propylene) (mr) decreased quickly with increasing D.
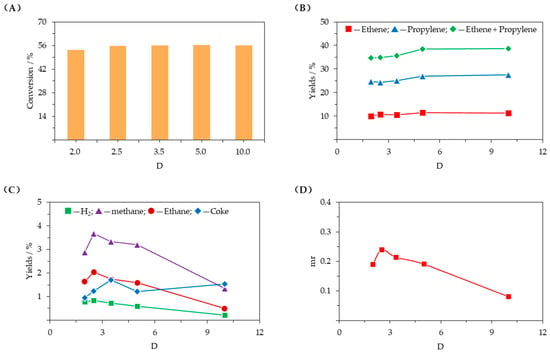
Figure 7.
Influence of feedstock volume dilution ratio (T = 620 °C, WHSV = 2.4 h−1, C/O = 25, D = 2–10). (A) conversion of C4 hydrocarbons; (B) ethene and propylene yields; (C) yields of other products; (D) molar ratio of methane to (ethene + propylene) (mr).
C4 hydrocarbons mostly dehydrogenate and crack into products with fewer carbons, increasing the number of molecules in the system. Therefore, decreasing the feed hydrocarbon partial pressure helps shift the chemical equilibrium to the right. Increasing D can reduce the feed hydrocarbon partial pressure, which can promote the conversion of C4 hydrocarbons and produce more propylene. However, due to the 620 °C reaction temperature, ethene can be produced not only by catalytic dehydrogenation and catalytic cracking but also by thermal cracking, which results in the ethene yield changing less with D. Even though decreasing the feed hydrocarbon partial pressure is beneficial for dehydrogenation and thermal cracking, catalytic cracking is more sensitive to the change in D, which increases the yields of other products and decreases mr. Since a relatively high D requires large amounts of diluent gases, such as nitrogen or water vapor, which significantly increases material and energy consumption during the reaction process, an appropriate dilution ratio is 5.
Based on the above experimental results, the appropriate conditions were a reaction temperature (T) of 620 °C, weight hourly space velocity (WHSV) of 2.4 h−1, catalyst–oil mass ratio (C/O) of 25, and feedstock volume dilution ratio (D) of 5, which resulted in C4 hydrocarbons conversion of 56.14%, ethene yield of 11.20%, propylene yield of 27.51%, and a combined ethene and propylene yield of 38.71%. Table 5 compares the results obtained in this study with those reported in references [6,10,26,35,36,37] on the production of ethene and propylene from C4 hydrocarbons (C4 paraffins or C4 olefins). The comparison reveals significant discrepancies in ethene and propylene yields. These discrepancies are mainly due to differences in the catalysts, experimental devices, and reaction conditions used.

Table 5.
Comparison of product distributions between this paper and references.
3. Discussion
As illustrated in Figure 8, ZSM-5 zeolite has an MFI topology, and its channel structure is zigzag-shaped. The pore diameters are 0.51 × 0.55 nm in a-axis and 0.53 × 0.56 nm in b-axis, which are bigger than the molecular dynamic diameters of C4 hydrocarbons (iso-butane: 0.51 nm; n-butane: 0.43 nm; iso-butene: 0.48 nm; 1-butene: 0.45 nm). The reactant molecules of C4 hydrocarbons can enter the channels of ZSM-5 zeolite and adsorb onto Brønsted and Lewis acid active sites. Subsequently, protonation reactions convert C4 paraffins and C4 olefins into carbocations, and then ethene and propylene are generated through series reactions, including direct cracking and oligomerization–cracking. As shown in Table 6, the activation energy for the cracking reactions of n-butane and iso-butane is much higher than that of 1-butene and iso-butene. Therefore, under the same conditions, 1-butene and iso-butene are prone to cracking reactions, while n-butane and iso-butane are difficult to convert. If C4 paraffins can be converted to C4 olefins, more ethene and propylene can be obtained. The bifunctional metal–acid catalyst (BDHC) prepared in this study was combined with Fe2O3 and Cr2O3 for dehydrogenation active groups and ZSM-5 zeolite for cracking active groups, as shown in Figure 9. Fe2O3 and Cr2O3 can improve the conversion of C4 paraffins, and ZSM-5 zeolite is able to enhance the catalytic cracking of C4 olefins. C4 paraffins on metals can be converted to C4 olefins, which can then be cracked into ethene and propylene in ZSM-5 zeolite channels, thus promoting the conversion of C4 hydrocarbons and increasing ethene and propylene yields. In addition, BDHC had high relative crystallinity and a large specific surface area and pore volume, promoting adsorption of reactant molecules and conversion of C4 hydrocarbons. This can also be seen from the comparison of the reaction results between the BDHC and RZSM catalysts. The conversion of C4 hydrocarbons is 59.21% on the BDHC catalyst but only 40.00% on the RZSM catalyst. Therefore, it can be proved that trace amounts of Fe2O3 and Cr2O3 can increase the conversion rate of C4 hydrocarbons by promoting the conversion of C4 paraffins—that is, they have a significant effect.
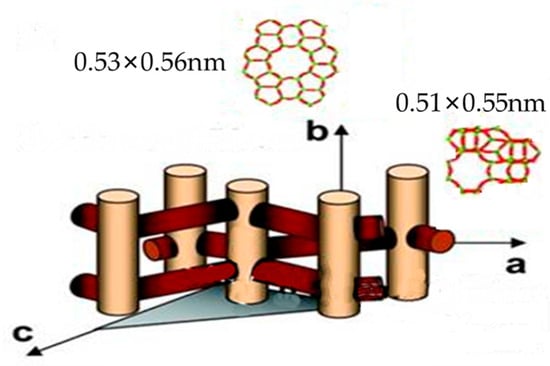
Figure 8.
Channel structure of ZSM-5 zeolite.

Table 6.
Activation energy of C4 paraffins and C4 olefins.
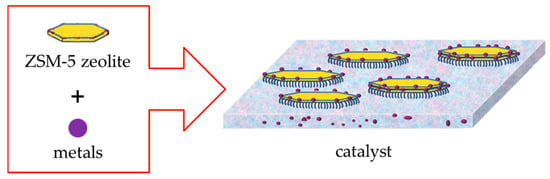
Figure 9.
Bifunctional metal–acid catalyst.
In dehydrogenative cracking of C4 hydrocarbons, catalytic dehydrogenation converts C4 paraffins to C4 olefins, which then undergo catalytic cracking to generate ethene and propylene [38,39]. Catalytic dehydrogenation and cracking occur simultaneously because both C4 paraffins and C4 olefins exist in feedstocks. Catalytic cracking of C4 olefins involves two different reaction paths, direct cracking and oligomerization–cracking, with both ethene and propylene being produced, although oligomerization–cracking reactions are more likely to produce propylene [35].
Figure 10 shows the reaction pathway of the catalytic dehydrogenative cracking of C4 paraffins. In Figure 10a, C4 paraffins first undergo specific interactions with the metal active sites (Fe2+ and Cr2+) on zeolites. This interaction breaks the C-H bonds in the C4 paraffin molecules, prompting hydrogen atoms to transfer to the metal sites, thereby forming the H-M2+(OH)-H intermediate. In the structure of this intermediate, the metal ion (M2+) bridges two hydrogen atoms through a hydroxyl group (-OH), serving as a key transition state of the reaction. Subsequently, the intermediate undergoes a further dehydrogenation reaction: under the catalytic action of the metal sites, the two hydrogen atoms combine, detach from the intermediate structure, and generate hydrogen gas (H2). Meanwhile, due to charge rearrangement, the C4 alkyl fragment that has lost hydrogen atoms forms highly reactive C4 carbocations. C4 carbocations bound to acid sites can undergo direct cracking and oligomerization–cracking. In Figure 10b, the carbocation is the second carbon; when it undergoes β-scission, propylene and methane are produced. The molar ratio of propylene to methane is nearly 1. In Figure 10c, the carbocation is the first carbon; when it undergoes β-scission, two ethenes are obtained. Anyway, direct cracking, also called the monomolecular mechanism, can generate more ethene and propylene [40,41]. In addition, C4 carbocations bound to acid sites can also be converted to C8 carbocations through oligomerization, which subsequently creates more opportunities for cracking. In Figure 10d, the carbocation is the second carbon; the C8 carbocation undergoes β-scission twice, yielding one ethene and two propylenes. Of course, because there are many different isomers of C8 carbocations, which can undergo cracking, isomerization, hydrogen–transformation, polymerization, and so on, the products are complex [36,37]. To obtain more ethene and propylene, these side reactions, including aromatization and polymerization, need to be suppressed, which can be achieved by modifying catalysts [42].
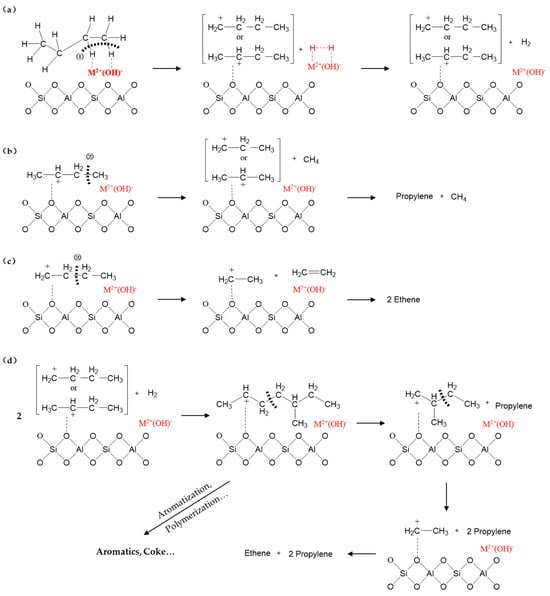
Figure 10.
The reaction pathway of the catalytic dehydrogenative cracking of C4 paraffins. (a) Catalytic dehydrogenative cracking of C4 paraffins; (b,c) direct cracking of C4 carbocations; (d) oligomerization–cracking of C4 carbocations.
BDHC contains both dehydrogenation active groups (Fe2O3 and Cr2O3) and cracking active groups (ZSM-5), so it has both catalytic dehydrogenation and catalytic cracking activities [43]. However, it can be observed from Table 3 that BDHC has significantly fewer acid sites and thus weaker catalytic cracking activity than RZSM, and the proportion of the oligomerization–cracking reaction of C4 olefins is lower than that obtained with RZSM. The characteristics of BDHC provide advantages in C4 hydrocarbons conversion and ethene and propylene production.
4. Materials and Methods
4.1. Materials
The C4 hydrocarbons utilized in this study were procured from Beijing Haipu Beifen Gas Co., Ltd. (Beijing, China), as shown in Table 7. The mass fraction of C4 paraffins, including iso-butane and n-butane, was 20.46% in this feedstock.

Table 7.
Mass proportions of individual constituents within C4 hydrocarbons.
4.2. Catalysts
The BDHC catalyst was prepared in a small laboratory pilot plant, and the RZSM catalyst was purchased from Qilu Branch of Sinopec Catalyst Co., Ltd. (Zibo, China). Before testing and analysis, both catalysts were hydrothermally aged at 800 °C for 6 h with 100% steam.
The samples’ chemical compositions were determined using a Rigaku 3013 X-ray fluorescence spectrometer (XRF) (Tokyo, Japan). Moreover, the samples’ crystalline phases were determined using an X-ray diffractometer from PA Nalytical (Almelo, The Netherlands). The specific surface area and pore volume of the samples were calculated using the BET method on an ASAP 24,000 adsorbent apparatus from Micromeritics (Norcross, GA, USA).
The Brønsted and Lewis acid sites of the samples were identified through the use of pyridine as a probe molecule on a Fourier infrared spectrometer from Bio-Rad (Hercules, CA, USA). After pyridine desorption at 200 °C and 350 °C, the concentrations of the total and strong Lewis/Brønsted acid sites were obtained from the integrated absorbance of the respective bands. The concentrations of Brønsted acid and Lewis acids were determined by the infrared absorption bands of pyridine at 1540 cm−1 and 1453 cm−1, respectively. The molar extinction coefficients were based on the work of C. A. Emeis et al. [44].
The acid content of the samples was measured using ammonia temperature-programmed desorption (NH3-TPD) on an Autochem Ⅱ 2920 desorbed instrument from Mack (Norcross, GA, USA).
4.3. Test Equipment
A fixed fluidized bed reactor (FFB) plant was used for the evaluation, which was designed by Sinopec Research Institute of Petroleum Processing Co., Ltd. (Beijing, China) and manufactured by Shanghai Meryer Experimental Equipment Co., Ltd. (Shanghai, China). It consisted of a pre-heater, a reactor, the 1st condenser, the 2nd and 3rd condensers, and a gas collector (Figure 11). Prior to the test, a certain amount of catalyst was loaded into the reactor, and the reactor temperature was increased to a set value while maintaining the catalyst in a fluidized state. The process is as follows: The raw material is preheated and mixed with high-temperature water vapor and then enters the bottom of the fluidized reactor through the feed nozzle to react on the hot catalyst. After the reaction, water vapor enters the reactor for vapor extraction, and the reaction oil and gas enter the multi-stage condensation-and-separation system for separation to obtain gas products and liquid products. At the end of the reaction, the reactor is automatically heated to the regeneration temperature, while oxygen is fed into the reactor to burn the coke on the catalyst, and the regenerated flue gas is measured by an infrared analysis meter to measure the real-time CO2 concentration. The coke yield is calculated according to the flue gas flow integral.
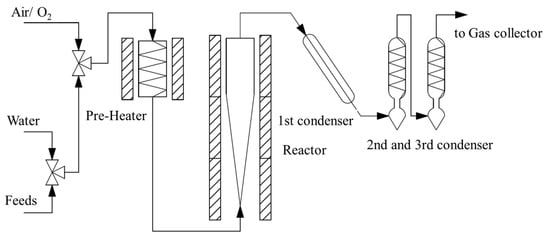
Figure 11.
Schematic flow diagram of fixed fluidized bed reactor (FFB) plant.
4.4. Analysis
The volumetric percentage of each component in the vapor was determined using an Agilent 6890 gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and thermal conductivity detector (TCD). The weight of the liquid product was obtained through weighing. The Agilent 7890 gas chromatograph (Santa Clara, CA, USA) was employed to simulate real boiling point distillation and determine the distillation range of liquid products. Gasoline referred to the C5–221 °C fraction, diesel to the 222–330 °C fraction, and heavy oil to fractions > 331 °C. The mass percentage of each fraction could also be obtained. For the gasoline, PIONA analysis was performed, and a hydrocarbon composition in the C5–C12 range was obtained.
The conversion rate (x) of C4 hydrocarbons was calculated using Equation (1), and the yield of products (y) was calculated using Equation (2).
where m represents the mass flow of feedstocks, while m0 and mi represent the mass flows of C4 hydrocarbons and other products in the output, respectively.
The BDHC catalyst used in this study is bifunctional, promoting catalytic dehydrogenation and catalytic cracking, but the thermal cracking reaction cannot be ignored because the reaction temperature exceeded 600 °C [45]. In order to explore the comprehensive performance of the catalyst, the conversion rate was used to measure the ability of the catalyst to convert C4 hydrocarbons; the hydrogen yield was used to measure the catalytic dehydrogenation performance; the ethene and propylene yields were used to examine the catalytic cracking performance of the catalyst; and the molar ratio of methane to (ethene + propylene) (mr) in the product was used to measure the relative strength of thermal cracking versus catalytic cracking in the reaction.
5. Conclusions
A bifunctional metal–acid catalyst, BDHC, was prepared for catalytic dehydrogenation and catalytic cracking, using ZSM-5 zeolite for cracking active groups and Fe2O3 and Cr2O3 for dehydrogenation active groups. In the catalyzed reaction, C4 paraffins are converted to C4 olefins, which are then cracked into ethene and propylene. Compared to RZSM, which lacked dehydrogenation active sites, BDHC had greater relative crystallinity, specific surface area, and pore volume, promoting adsorption of reactant molecules and conversion of C4 hydrocarbons, thus producing more ethene and propylene. After a series of investigation, the optimized parameters were determined to be a reaction temperature (T) of 620 °C, weight hourly space velocity (WHSV) of 2.4 h−1, catalyst–oil mass ratio (C/O) of 25, and feedstock volume dilution ratio (D) of 5, resulting in a C4 hydrocarbons conversion of 56.14%, ethene yield of 11.20%, propylene yield of 27.51%, and a combined ethene and propylene yield of 38.71%.
Author Contributions
W.M.: Investigation, writing of the original draft of the manuscript, and analysis of the data. G.Z.: Collection of references, preparation of figures, analysis of the data, and writing—review and editing. Q.Y. and J.Y.: Analysis of the data, editing, and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (Fund No. 2021YFA1501304) and a research grant from Sinopec Research Institute of Petroleum Processing Co., Ltd. (Fund No. R18045).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Authors Wenming Ma, Genquan Zhu, Qimin Yuan was employed by the company Sinopec Research Institute of Petroleum Processing Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| C/O | catalyst–oil mass ratio |
| D | feedstock volume dilution ratio |
| mr | molar ratio of methane to (ethene + propylene) |
| Pi | feed hydrocarbon partial pressure |
| T | reaction temperature |
| WHSV | weight hourly space velocity |
References
- Wakui, K.; Satoh, K.; Sawada, G.; Shiozawa, K.; Matano, K.; Suzuki, K.; Hayakawa, T.; Yoshimura, Y.; Murata, K.; Mizukami, F. Dehydrogenative Cracking of N-Butane Using Double–Stage Reaction. Appl. Catal. A Gen. 2002, 230, 195–202. [Google Scholar] [CrossRef]
- Ma, W.; Cheng, X.; Zhu, G.; Xie, C. DCC-Plus Process and Flexibility of Its Products. Acta Pet. Sin. (Pet. Process. Sect.) 2019, 35, 217–223. [Google Scholar]
- Pan, R.; Jia, M.; Li, Y.; Li, X.; Dou, T. In Situ Delamination of Ferrierite Zeolite and Its Performance in the Catalytic Cracking of C4 Hydrocarbons. Chin. J. Chem. Eng. 2014, 22, 1237–1242. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Z.; Xu, C.; Zhong, S.; Bai, E. Advances in Catalytic Cracking of C4 Olefin to Propylene. Chem. Ind. Eng. Process 2005, 24, 1347–1351. [Google Scholar]
- Wang, X.; Zhao, Z.; Xu, C.; Duan, A.; Zhang, L.; Jiang, G. Research Advances in the Catalytic Cracking of C4/C5 Hydrocarbons to Light Olefins. Chin. J. Appl. Chem. 2007, 24, 1225–1231. [Google Scholar]
- Ji, D.; Wang, B.; Qian, G.; Gao, Q.; Lü, G.; Yan, L.; Suo, J. A Highly Efficient Catalytic C4 Alkane Cracking over Zeolite ZSM-23. Catal. Commun. 2005, 6, 297–300. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, T.Y.; Lee, C.H.; Kang, S.; Kim, J.W.; Jeong, M.; Lee, S.C.; Kim, J.C. Hybrid Catalysts in a Double-Layered Bed Reactor for the Production of C2–C4 Paraffin Hydrocarbons. Catal. Commun. 2019, 127, 29–33. [Google Scholar] [CrossRef]
- Eng, C.; Miller, R.; Orriss, R. Producing Propylene. Hydrocarb. Eng. 2004, 7, 9. [Google Scholar]
- Agarwal, N.; Sanchez-Castillo, M.A.; Cortright, R.D.; Madon, R.J.; Dumesic, J.A. Catalytic Cracking of Isobutane and 2-Methylhexane over USY Zeolite: Identification of Kinetically Significant Reaction Steps. Ind. Eng. Chem. Res. 2002, 41, 4016–4027. [Google Scholar] [CrossRef]
- Guo, T.; Gao, J.; Zhang, Q. Overgrowth of ZSM-5 on Kaoline and Its Cayalytic Cracking Properities for N-Butane to Light Olefins. Ind. Catal. 2011, 19, 33–36. [Google Scholar]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Hou, W.; Lin, K.; Zhang, X.; Xu, B.; Wang, Y.; Lu, X.; Gao, Y.; Ma, R.; Fu, Y.; Zhu, W. Highly Stable and Selective Pt/TS-1 Catalysts for the Efficient Nonoxidative Dehydrogenation of Propane. Chem. Eng. J. 2023, 474, 145648. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Zhang, Q.; Kondratenko, V.A.; Jiang, G.; Sun, H.; Han, S.; Wang, Y.; Cui, G.; Zhou, M.; et al. Molecularly Defined Approach for Preparation of Ultrasmall Pt-Sn Species for Efficient Dehydrogenation of Propane to Propene. J. Catal. 2023, 418, 290–299. [Google Scholar] [CrossRef]
- Monai, M.; Gambino, M.; Wannakao, S.; Weckhuysen, B.M. Propane to Olefins Tandem Catalysis: A Selective Route Towards Light Olefins Production. Chem. Soc. Rev. 2021, 50, 11503–11529. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z. Dynamic Modeling of CATOFIN® Fixed-Bed Iso-Butane Dehydrogenation Reactor for Operational Optimization. Int. J. Chem. React. Eng. 2016, 14, 491–515. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Cueto, J.; Alonso-Doncel, M.D.M.; Kubů, M.; Čejka, J.; Serrano, D.P.; García-Muñoz, R.A. Propane Dehydrogenation over Pt and Ga-Containing MFI Zeolites with Modified Acidity and Textural Properties. Catal. Today 2024, 427, 114437. [Google Scholar] [CrossRef]
- Chen, S.; Luo, R.; Zhao, Z.; Pei, C.; Xu, Y.; Lu, Z.; Zhao, C.; Song, H.; Gong, J. Concerted Oxygen Diffusion Across Heterogeneous Oxide Interfaces for Intensified Propane Dehydrogenation. Nat. Commun. 2023, 14, 2620. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X.; Wang, Q.; Xiaoyue, W.; Zhou, C.; Yang, Y. Recent Progress in Heterogeneous Metal and Metal Oxide Catalysts for Direct Dehydrogenation of Ethane and Propane. Chem. Soc. Rev. 2021, 50, 5590–5630. [Google Scholar] [CrossRef]
- Li, C.; Wang, G. Dehydrogenation of Light Alkanes to Mono-Olefins. Chem. Soc. Rev. 2021, 50, 4359–4381. [Google Scholar] [CrossRef]
- Nawaz, Z.; Chu, Y.; Yang, W.; Tang, X.; Wang, Y.; Wei, F. Study of Propane Dehydrogenation to Propylene in an Integrated Fluidized Bed Reactor Using Pt-Sn/Al-SAPO-34 Novel Catalyst. Ind. Eng. Chem. Res. 2010, 49, 4614–4619. [Google Scholar] [CrossRef]
- Mier, D.; Aguayo, A.T.; Gamero, M.; Gayubo, A.G.; Bilbao, J. Kinetic Modeling of N-Butane Cracking on HZSM-5 Zeolite Catalyst. Ind. Eng. Chem. Res. 2010, 49, 8415–8423. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic Cracking of Hydrocarbons over Modified ZSM-5 Zeolites to Produce Light Olefins: A Review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Xie, Z.; Jin, W.; Yang, W.; Chen, Q.; Tang, Y. Effect of Phosphorus on HZSM-5 Catalyst for C4-Olefin Cracking Reactions to Produce Propylene. J. Catal. 2007, 248, 29–37. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Zhang, Y.; Xie, Z.; Yue, Y.; Chen, Q.; Tang, Y. Synthesis of ZSM-48 Zeolites and Their Catalytic Performance in C4-Olefin Cracking Reactions. Appl. Catal. A Gen. 2006, 299, 167–174. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Chen, Z.; Li, Z.; Yang, Q.; Hu, L.; Jiang, G.; Xu, C.; Wang, Y.; Zhao, Z. Hierarchical ZSM-5 Zeolites with Tunable Sizes of Building Blocks for Efficient Catalytic Cracking of I-Butane. Ind. Eng. Chem. Res. 2018, 57, 10327–10335. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Duan, A.; Zhang, P. CrHZSM-5 Zeolites–Highly Efficient Catalysts for Catalytic Cracking of Isobutane to Produce Light Olefins. Catal. Lett. 2006, 109, 65–70. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Duan, A.; Wang, X.; Zhang, P. Catalytic Cracking of Isobutane over HZSM-5, FeHZSM-5 and CrHZSM-5 Catalysts with Different SiO2/Al2O3 Ratios. J. Porous Mater. 2008, 15, 213–220. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Zhang, P.; Duan, A. FeHZSM-5 Molecular Sieves – Highly Active Catalysts for Catalytic Cracking of Isobutane to Produce Ethene and Propylene. Catal. Commun. 2006, 7, 199–203. [Google Scholar] [CrossRef]
- Gascón, J.; Téllez, C.; Herguido, J.; Menéndez, M. Propane Dehydrogenation over a Cr2O3/Al2O3 Catalyst: Transient Kinetic Modeling of Propene and Coke Formation. Appl. Catal. A Gen. 2003, 248, 105–116. [Google Scholar] [CrossRef]
- Vernikovskaya, N.V.; Savin, I.G.; Kashkin, V.N.; Pakhomov, N.A.; Ermakova, A.; Molchanov, V.V.; Nemykina, E.I.; Parahin, O.A. Dehydrogenation of Propane–Isobutane Mixture in a Fluidized Bed Reactor over Cr2O3/Al2O3 Catalyst: Experimental Studies and Mathematical Modelling. Chem. Eng. J. 2011, 176–177, 158–164. [Google Scholar] [CrossRef]
- Jin, D.; Xu, H.; Zhu, J.; Cheng, D. Activation of Cr2O3 for Propane Dehydrogenation by Doping with Pt Single-Atom Promotor. Mol. Catal. 2023, 551, 113624. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Mashkin, M.Y.; Baybursky, V.L.; Pribytkov, P.V.; Murashova, E.V.; Kalmykov, K.B.; Shesterkina, A.A.; Kapustin, G.I.; Tkachenko, O.P.; Dunaev, S.F. Effect of Chromium Precursor on the Catalytic Behavior of Chromium Oxide Catalysts in Oxidative Propane and Isobutane Dehydrogenation with Carbon Dioxide. Catalysts 2025, 15, 226. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Z.; Cai, L.; Tian, X.; Chu, W.; Yang, W. Effect of Calcination Atmosphere on the Structure and Catalytic Behavior of Cr2O3/Al2O3 Catalysts for Dehydrogenation of Propane. Ind. Eng. Chem. Res. 2022, 61, 16479–16488. [Google Scholar] [CrossRef]
- Olsbye, U.; Virnovskaia, A.; Prytz, Ø.; Tinnemans, S.J.; Weckhuysen, B.M. Mechanistic Insight in the Ethane Dehydrogenation Reaction over Cr/Al2O3 Catalysts. Catal. Lett. 2005, 103, 143–148. [Google Scholar] [CrossRef]
- Krannila, H.; Haag, W.O.; Gates, B.C. Monomolecular and Bimolecular Mechanisms of Paraffin Cracking: N-Butane Cracking Catalyzed by HZSM-5. J. Catal. 1992, 135, 115–124. [Google Scholar] [CrossRef]
- Iwase, Y.; Sakamoto, Y.; Shiga, A.; Miyaji, A.; Motokura, K.; Koyama, T.; Baba, T. Shape-Selective Catalysis Determined by the Volume of a Zeolite Cavity and the Reaction Mechanism for Propylene Production by the Conversion of Butene Using a Proton-Exchanged Zeolite. J. Phys. Chem. C 2012, 116, 5182–5196. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid Strength Controlled Reaction Pathways for the Catalytic Cracking of 1-Butene to Propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Wakui, K.; Satoh, K.; Sawada, G.; Shiozawa, K.; Matano, K.; Suzuki, K.; Hayakawa, T.; Yoshimura, Y.; Murata, K.; Mizukami, F. Cracking of N-Butane over Alkaline Earth-Containing HZSM-5 Catalysts. Catal. Lett. 2002, 84, 259–264. [Google Scholar] [CrossRef]
- Wakui, K.; Satoh, K.; Sawada, G.; Shiozawa, K.; Matano, K.; Suzuki, K.; Hayakawa, T.; Yoshimura, Y.; Murata, K.; Mizukami, F. Dehydrogenative Cracking of N-Butane over Modified HZSM-5 Catalysts. Catal. Lett. 2002, 81, 83–88. [Google Scholar] [CrossRef]
- Chen, C.; Rangarajan, S.; Hill, I.M.; Bhan, A. Kinetics and Thermochemistry of C4–C6 Olefin Cracking on H-ZSM-5. Acs Catal. 2014, 4, 2319–2327. [Google Scholar] [CrossRef]
- Miyaji, A.; Sakamoto, Y.; Iwase, Y.; Yashima, T.; Koide, R.; Motokura, K.; Baba, T. Selective Production of Ethene and Propylene via Monomolecular Cracking of Pentene over Proton-Exchanged Zeolites: Pentene Cracking Mechanism Determined by Spatial Volume of Zeolite Cavity. J. Catal. 2013, 302, 101–114. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Banerjee, S.; Panjala, D. Product Distribution in the Aromatization of Dilute Ethene over H-GaAlMFI Zeolite: Effect of Space Velocity. Microporous Mesoporous Mater. 2002, 51, 203–210. [Google Scholar] [CrossRef]
- Hakuli, A.; Kytökivi, A.; Krause, A.O.I. Dehydrogenation of I-Butane on CrOx/Al2O3 Catalysts Prepared by ALE and Impregnation Techniques. Appl. Catal. A Gen. 2000, 190, 219–232. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, X.; Xu, C.; Gao, J. Secondary Cracking of Gasoline and Diesel from Heavy Oil Catalytic Pyrolysis. Chin. J. Chem. Eng. 2007, 15, 309–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).