Ultrasonic-Assisted Fabrication of TiO2-Based Composite Photocatalysts for Enhanced Photocatalysis of Organic Pollutants: A Review
Abstract
1. Introduction
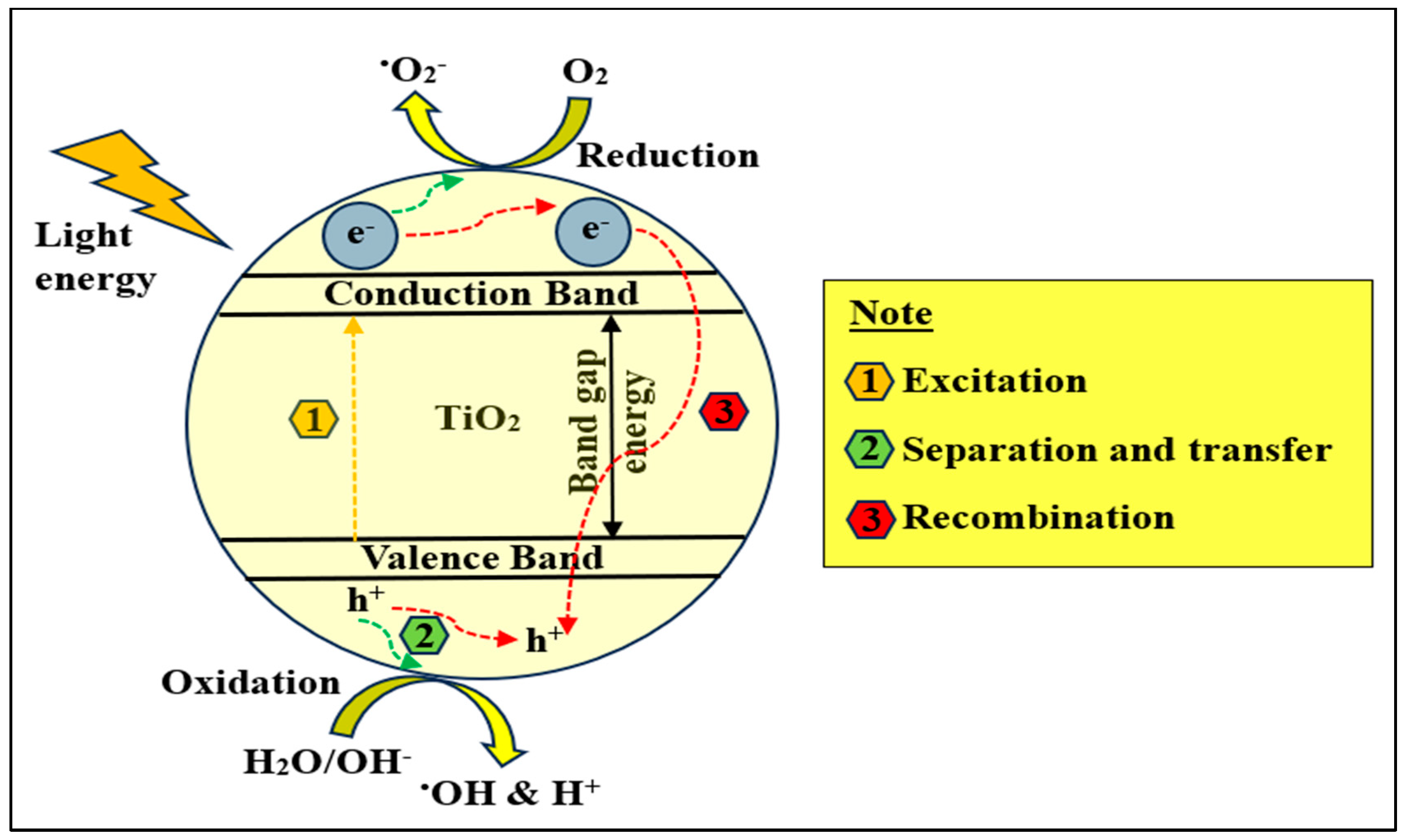
2. Basic Photocatalysis Principles
TiO2
3. TiO2-Based Nanocomposite Photocatalysts
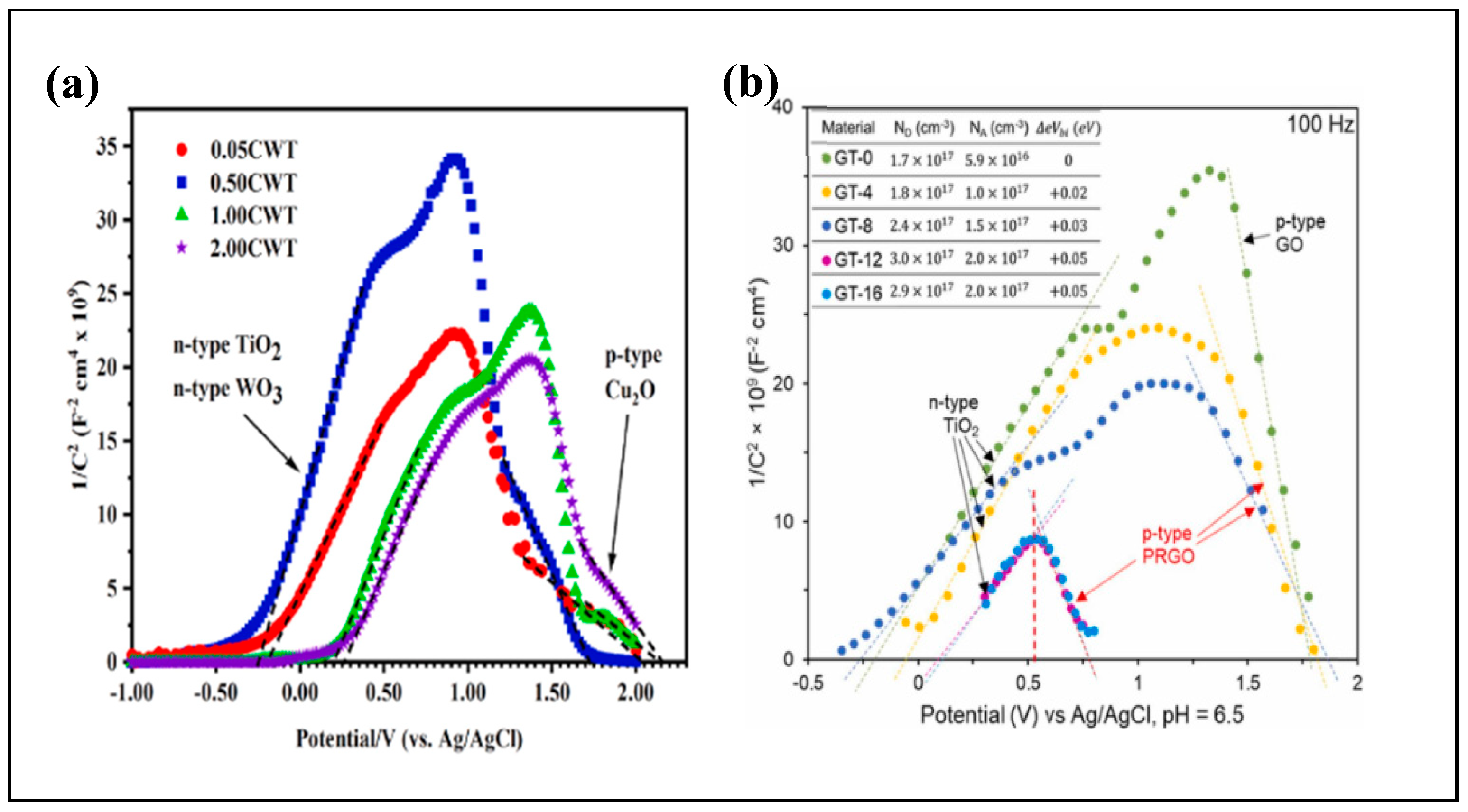
3.1. Formation of Heterojunction
3.2. Type II Heterojunction
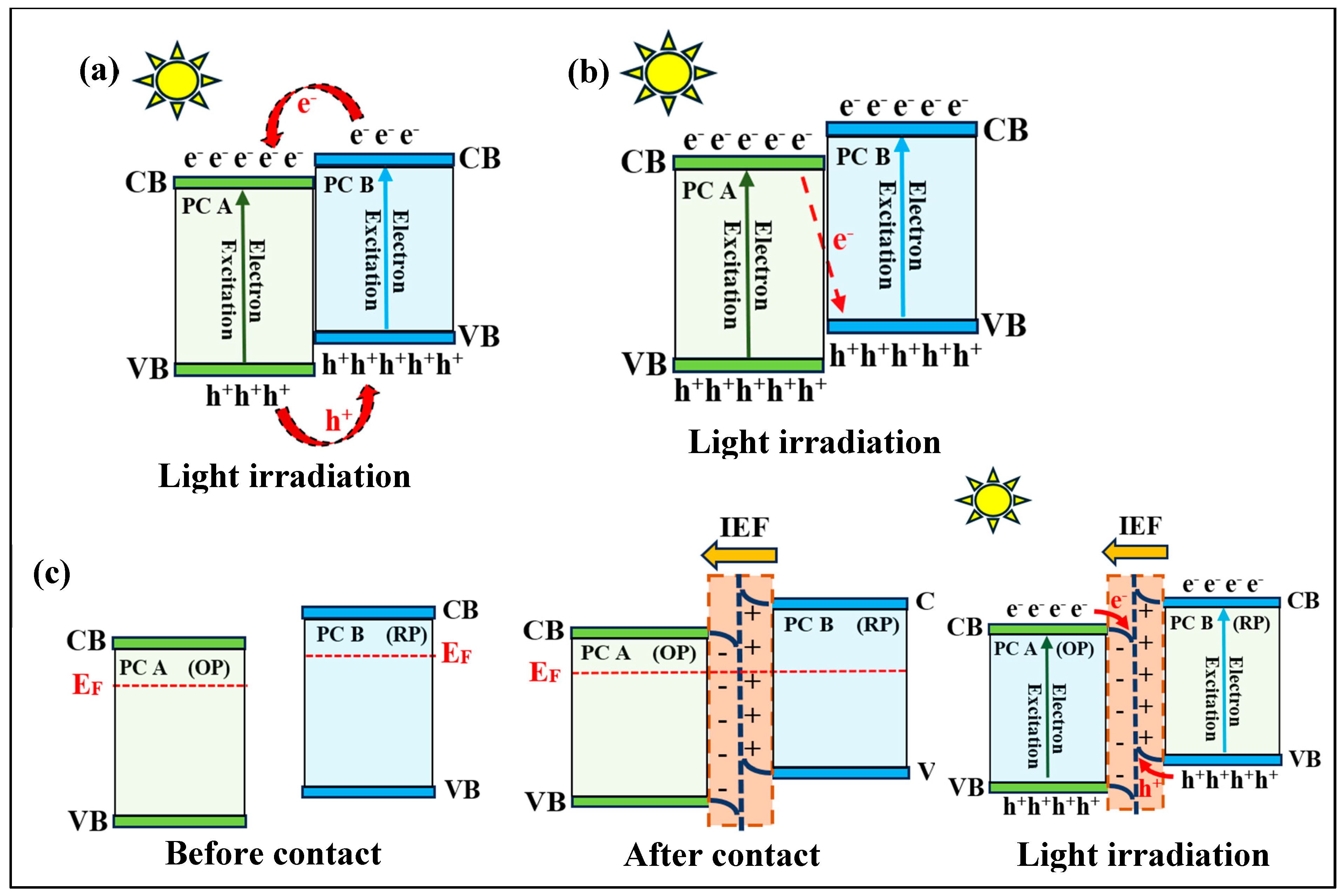
3.3. Z-Scheme Heterojunction
3.4. S-Scheme Heterojunction
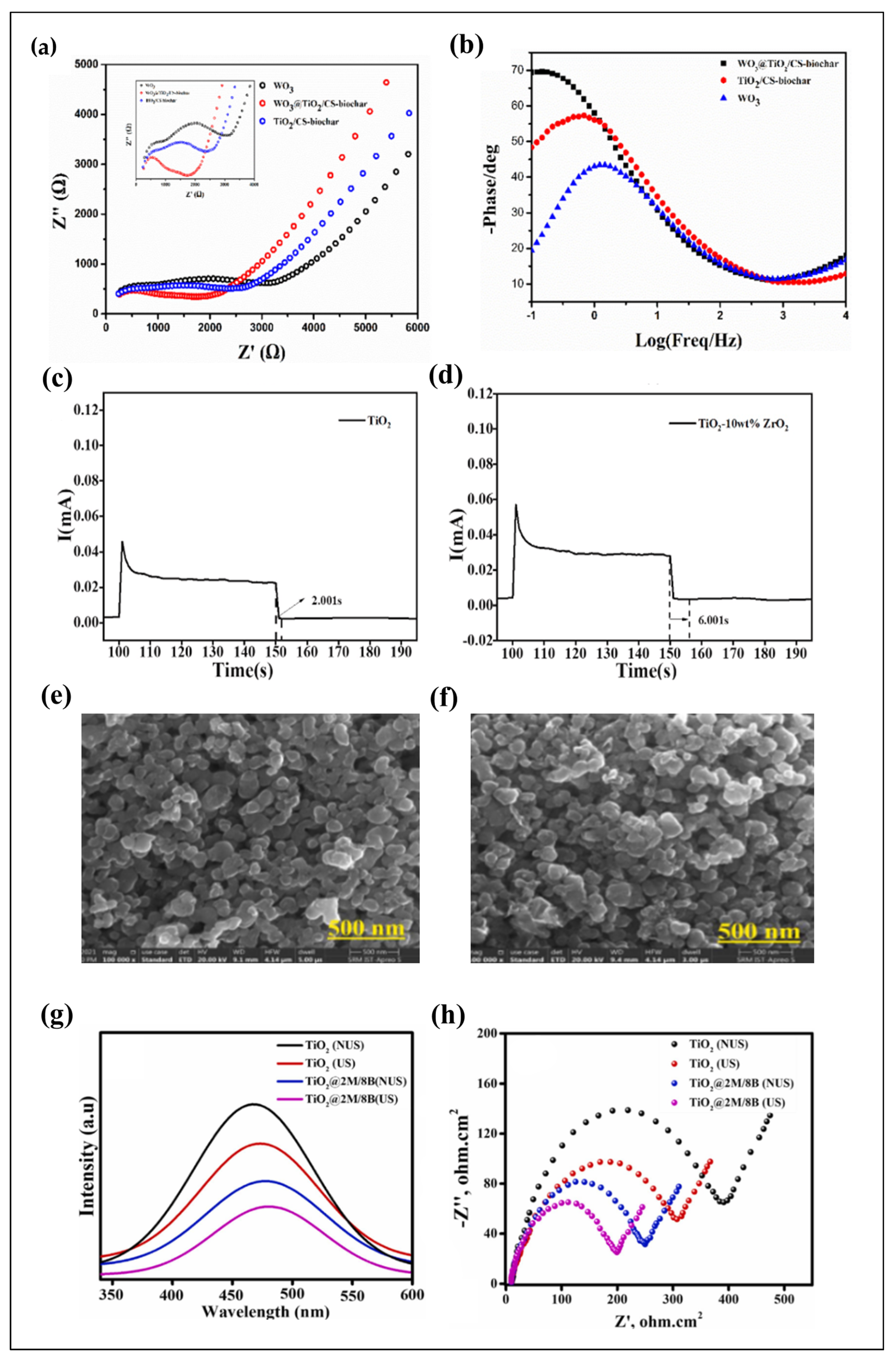
4. Ultrasonic-Assisted Fabrication of TiO2-Based Nanocomposite Photocatalysts
4.1. Parameters of Ultrasonication
4.1.1. Time
4.1.2. Power or Intensity
4.1.3. Frequency
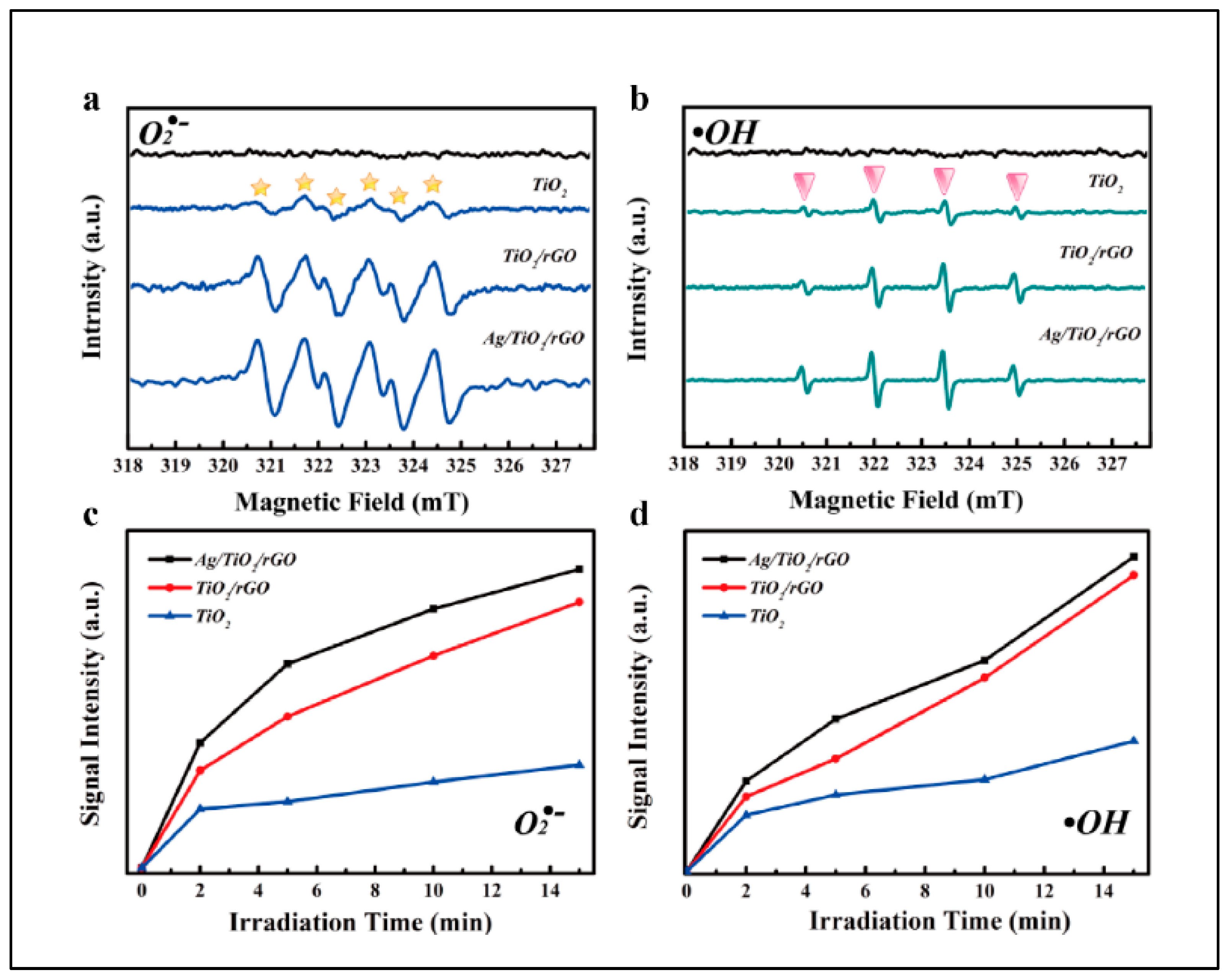
5. Photocatalytic Activity of TiO2-Based Nanocomposite Photocatalysts
Factors Affecting the Photodegradation Performance
6. Conclusions and Future Perspectives of Titanium Dioxide (TiO2)-Based Photocatalysts
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNESCO World Water Assesment Programme; Connor, R. The United Nations World Water Development Report 2024: Water for Prosperity and Peace; SIDALC: Turrialba, Costa Rica, 2024. [Google Scholar]
- Dawood, A.F.; Khalil, M.A.A.K. Removal of basic fuchsine dye using (TiO2/MWCNTs) nanomaterial. Mater. Today Proc. 2022, 49, 2888–2897. [Google Scholar] [CrossRef]
- Madkhali, N.; Prasad, C.; Malkappa, K.; Choi, H.Y.; Govinda, V.; Bahadur, I.; Abumousa, R.A. Recent update on photocatalytic degradation of pollutants in waste water using TiO2-based heterostructured materials. Results Eng. 2023, 17, 100920. [Google Scholar] [CrossRef]
- Vakili, A.; Zinatizadeh, A.A.; Rahimi, Z.; Zinadini, S.; Mohammadi, P.; Azizi, S.; Karami, A.; Abdulgader, M. The impact of activation temperature and time on the characteristics and performance of agricultural waste-based activated carbons for removing dye and residual COD from wastewater. J. Clean. Prod. 2023, 382, 134899. [Google Scholar] [CrossRef]
- Tsai, C.-G.; Tseng, W.J. Preparation of TiN–TiO2 composite nanoparticles for organic dye adsorption and photocatalysis. Ceram. Int. 2020, 46, 14529–14535. [Google Scholar] [CrossRef]
- Ali, A.; Shoeb, M.; Li, B.; Khan, M.A. Photocatalytic degradation of antibiotic drug and dye pollutants under visible-light irradiation by reduced graphene oxide decorated MoO3/TiO2 nanocomposite. Mater. Sci. Semicond. Process. 2022, 150, 106974. [Google Scholar] [CrossRef]
- Ren, X.; Chen, R.; Ding, S.; Fu, N. Preparation and photocatalytic performance of a magnetically recyclable ZnFe2O4@TiO2@Ag2O p-n/Z-type tandem heterojunction photocatalyst: Degradation pathway and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130604. [Google Scholar] [CrossRef]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.; Feng, W.; Wu, X. Environmental and health impact of unrecovered API from pharmaceutical manufacturing wastes: A review of contemporary treatment, recycling and management strategies. Sustain. Chem. Pharm. 2022, 30, 100865. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galban-Malag, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Kumar, R.; Kumar, A.; Shankar, R.; Khan, N.A.; Gupta, K.N.; Ram, M.; Arya, R.K. Pharmaceutical waste-water treatment via advanced oxidation based integrated processes: An engineering and economic perspective. J. Water Process Eng. 2023, 54, 103977. [Google Scholar] [CrossRef]
- Rasouli, K.; Alamdari, A.; Sabbaghi, S. Ultrasonic-assisted synthesis of α-Fe2O3@TiO2 photocatalyst: Optimization of effective factors in the fabrication of photocatalyst and removal of non-biodegradable cefixime via response surface methodology-central composite design. Sep. Purif. Technol. 2023, 307, 122799. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U.; Jassal, V. Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: A review. J. Environ. Manag. 2017, 190, 208–222. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Khan, M.S.; Othmani, A.; Khanday, W.A.; Gökkuş, Ö.; Osagie, C.; Ahmaruzzaman, M.; Mishra, S.R.; et al. Sustainable remediation technologies for removal of pesticides as organic micro-pollutants from water environments: A review. Appl. Surf. Sci. Adv. 2024, 19, 100558. [Google Scholar] [CrossRef]
- Swathy, K.; Vivekanandhan, P.; Yuvaraj, A.; Sarayut, P.; Kim, J.S.; Krutmuang, P. Biodegradation of pesticide in agricultural soil employing entomopathogenic fungi: Current state of the art and future perspectives. Heliyon 2024, 10, e23406. [Google Scholar] [CrossRef]
- Dhiman, N.; Chaudhary, S.; Singh, A.; Chauhan, A.; Kumar, R. Sustainable degradation of pharmaceutical waste using different fungal strains: Enzyme induction, kinetics and isotherm studies. Environ. Technol. Innov. 2022, 25, 102156. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Adsorption and removal of seven antibiotic compounds present in water with the use of biochar derived from the pyrolysis of organic waste feedstocks. J. Environ. Chem. Eng. 2021, 9, 105868. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, X.; Du, R.; Yang, Y.; Rong, C.; Qin, Y.; Li, Y.-Y. A review on anaerobic membrane bioreactors for enhanced valorization of urban organic wastes: Achievements, limitations, energy balance and future perspectives. Sci. Total Environ. 2022, 820, 153284. [Google Scholar] [CrossRef]
- Yang, M.; Watson, J.; Wang, Z.; Si, B.; Jiang, W.; Zhou, B.; Zhang, Y. Understanding and design of two-stage fermentation: A perspective of interspecies electron transfer. Renew. Sustain. Energy Rev. 2022, 168, 112891. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, J.; Qiu, X.; Deng, X.; Ren, X.; Ge, W.; Yuan, H. Coagulation performance and floc properties for synchronous removal of reactive dye and polyethylene terephthalate microplastics. Process Saf. Environ. Prot. 2022, 165, 66–76. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Lu, X.; Sheng, J.; Zheng, X.; Xiao, X. Flocculation of combined contaminants of dye and heavy metal by nano-chitosan flocculants. J. Environ. Manag. 2021, 299, 113589. [Google Scholar] [CrossRef]
- Peng, C.; Chen, L.; Wu, X.; Wei, X.; Tehrim, A.; Dai, M.; Xu, S. Identification of adsorption or degradation mechanism for the removal of different ionic dyes with iron-carbon micro-electrolysis process. J. Environ. Chem. Eng. 2021, 9, 105690. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Sun, H.; Feng, Y.; Lu, Y.; Zhao, Y.; Chen, L. A novel macro-meso-micro intimate heterogeneous structure constructed 3DOM TiO2/rGO/TiN composite for efficiently solar photocatalysis degradation of organic dye pollutants. Appl. Surf. Sci. 2023, 621, 156774. [Google Scholar] [CrossRef]
- Manivannan, R.; Ryu, J.; Son, Y.-A. Photo discoloration of eosin yellow dye under visible light using TiO2@TPPS nanocomposite synthesized via ultrasonic assisted method. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125601. [Google Scholar] [CrossRef]
- Kalikeri, S.; Kodialbail, V.S. Visible light active Bismuth ferrite embedded TiO2 nanocomposite structures for dye mineralization by photocatalysis-A strategy to harness solar energy for remediation of water contaminated with mixture of dyes. Surf. Interfaces 2023, 36, 102492. [Google Scholar] [CrossRef]
- Xu, H.; Hao, Z.; Feng, W.; Wang, T.; Fu, X. The floating photocatalytic spheres loaded with weak light-driven TiO2-based catalysts for photodegrading tetracycline in seawater. Mater. Sci. Semicond. Process. 2022, 144, 106610. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Mohammed, J.; Suleiman, A.B.; Ndikilar, C.E.; Sa’id, R.S.; Muhammad, I. Insights into hybrid TiO2-g-C3N4 heterostructure composite decorated with rGO sheet: A highly efficient photocatalyst for boosted solar fuel (hydrogen) generation. Chem. Phys. Impact 2023, 6, 100157. [Google Scholar] [CrossRef]
- Chau, J.H.F.; Lee, K.M.; Pang, Y.L.; Abdullah, B.; Juan, J.C.; Leo, B.F.; Lai, C.W. Photodegradation assessment of RB5 dye by utilizing WO3/TiO2 nanocomposite: A cytotoxicity study. Environ. Sci. Pollut. Res. 2022, 29, 22372–22390. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, J.; Ma, B.; Yang, Z.; Zhang, T.; Wang, X. Recyclable, hierarchical hollow photocatalyst TiO2@SiO2 composite microsphere realized by raspberry-like SiO2. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125112. [Google Scholar] [CrossRef]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F.; Juan, J.C.; Johan, M.R. Advanced photocatalytic degradation of acetaminophen using Cu2O/WO3/TiO2 ternary composite under solar irradiation. Catal. Commun. 2022, 163, 106396. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon nanofiber Composite: An efficient Visible-light photocatalyst obtained from electrospinning and hydrothermal methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.-L.; Wang, Y.-T.; Ma, Z.; Yang, T.-Y.; Zhang, T.; Zhang, Y.-H. In-situ synthesized porphyrin polymer/TiO2 composites as high-performance Z-scheme photocatalysts for CO2 conversion. J. Colloid Interface Sci. 2021, 596, 342–351. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Chandrasekaran, A. Recent advances in TiO2/ZnS-based binary and ternary photocatalysts for the degradation of organic pollutants. Sci. Total Environ. 2023, 868, 161525. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Aslam, S.; Noor, A.E.; Jini, D.; Majeed, S.; Velusamy, P.; Alothman, A.A.; Alshgari, R.A.; Saleh Mushab, M.S.; et al. Hydrothermally synthesized Ag-TiO2 nanofibers (NFs) for photocatalytic dye degradation and antibacterial activity. Chemosphere 2023, 321, 138077. [Google Scholar] [CrossRef]
- Divya, G.; Jaishree, G.; Siva Rao, T.; Prasanna Chippada, M.L.V.; Divya Lakshmi, K.V.; Sai Supriya, S. Improved catalytic efficiency by N-doped TiO2 via sol gel under microwave irradiation: Dual applications in degradation of dye and microbes. Hybrid Adv. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Liu, S.; Yang, W.; Chen, Y.; Zhang, Y. Synthesis of TiO2/MOF-801(Zr) by a wet impregnation at room temperature for highly efficient photocatalytic reduction of Cr(VI). Solid State Sci. 2022, 129, 106912. [Google Scholar] [CrossRef]
- Zikriya, M.; Nadaf, Y.F.; Bharathy, P.V.; Renuka, C.G. Luminescent characterization of rare earth Dy3+ ion doped TiO2 prepared by simple chemical co-precipitation method. J. Rare Earths 2019, 37, 24–31. [Google Scholar] [CrossRef]
- Wei, X.; Cai, H.; Feng, Q.; Liu, Z.; Ma, D.; Chen, K.; Huang, Y. Synthesis of co-existing phases Sn-TiO2 aerogel by ultrasonic-assisted sol-gel method without calcination. Mater. Lett. 2018, 228, 379–383. [Google Scholar] [CrossRef]
- Patil, S.M.; Deshmukh, S.P.; More, K.V.; Shevale, V.B.; Mullani, S.B.; Dhodamani, A.G.; Delekar, S.D. Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Mater. Chem. Phys. 2019, 225, 247–255. [Google Scholar] [CrossRef]
- Sekar, R.; Sivasamy, R.; Ricardo, B.; Manidurai, P. Ultrasonically synthesized TiO2/ZnS nanocomposites to improve the efficiency of dye sensitized solar cells. Mater. Sci. Semicond. Process. 2021, 132, 105917. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Nguyen, V.-H.; Boonupara, T.; Phan Thi, L.-A.; Watcharenwong, A.; Sumitsawan, S.; Udomkun, P. Challenges and effectiveness of nanotechnology-based photocatalysis for pesticides-contaminated water: A review. Environ. Res. 2022, 212, 113336. [Google Scholar] [CrossRef]
- Swedha, M.; Balasurya, S.; Syed, A.; Das, A.; Sudheer Khan, S. Continuous photocatalysis via Z-scheme based nanocatalyst system for environmental remediation of pharmaceutically active compound: Modification, reaction site, defect engineering and challenges on the nanocatalyst. J. Mol. Liq. 2022, 353, 118745. [Google Scholar] [CrossRef]
- Zhuang, Q.; Chen, H.; Zhang, C.; Cheng, S.; Dong, W.; Xie, A. Rapid chromium reduction by metal-free organic polymer photocatalysis via molecular engineering. J. Hazard. Mater. 2022, 434, 128938. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Rajabzadeh-Khosroshahi, M.; Eshaghi, M.M.; Rahmani, E.; Motasadizadeh, H.; Arshad, R.; Rahdar, A.; Pandey, S. TiO2-based nanocomposites for cancer diagnosis and therapy: A comprehensive review. J. Drug Deliv. Sci. Technol. 2023, 82, 104370. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, S.; Zhang, L.; Watanabe, S. Mesoporous single crystal titanium oxide microparticles for enhanced visible light photodegradation. Opt. Mater. 2022, 127, 112297. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Yuju, S.; Xiujuan, T.; Dongsheng, S.; Zhiruo, Z.; Meizhen, W. A review of tungsten trioxide (WO3)-based materials for antibiotics removal via photocatalysis. Ecotoxicol. Environ. Saf. 2023, 259, 114988. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, Z.; Zhu, H.; Yan, G. Nanoflower-like BiOBr/TiO2 p-n heterojunction composites for enhanced photodegradation of formaldehyde and dyes. Inorg. Chem. Commun. 2022, 146, 110167. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, M.; Liu, W.; Sun, W.; Meng, X.; Zheng, Y. Ultrasonically synthesized N-TiO2/Ti3C2 composites: Enhancing sonophotocatalytic activity for pollutant degradation and nitrogen fixation. Sep. Purif. Technol. 2021, 276, 119287. [Google Scholar] [CrossRef]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F.; Juan, J.C.; Lee, K.M.; Qian, X.; Badruddin, I.A.; Zai, J. Direct Z-scheme Cu2O/WO3/TiO2 nanocomposite as a potential supercapacitor electrode and an effective visible-light-driven photocatalyst. J. Environ. Manag. 2024, 363, 121332. [Google Scholar] [CrossRef]
- Qiu, P.; Xiong, J.; Lu, M.; Liu, L.; Li, W.; Wen, Z.; Li, W.; Chen, R.; Cheng, G. Integrated p-n/Schottky junctions for efficient photocatalytic hydrogen evolution upon Cu@TiO2-Cu2O ternary hybrids with steering charge transfer. J. Colloid Interface Sci. 2022, 622, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Debnath, K.; Majumder, T.; Mondal, S.P. Photoelectrochemical study of hydrothermally grown vertically aligned rutile TiO2 nanorods. Chem. Phys. 2022, 561, 111609. [Google Scholar] [CrossRef]
- Tai, X.H.; Lai, C.W.; Yang, T.C.K.; Johan, M.R.; Lee, K.M.; Chen, C.-Y.; Juan, J.C. Highly effective removal of volatile organic pollutants with p-n heterojunction photoreduced graphene oxide-TiO2 photocatalyst. J. Environ. Chem. Eng. 2022, 10, 107304. [Google Scholar] [CrossRef]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F.; Juan, J.C.; Lee, K.M.; Badruddin, I.A.; Kumar, A.; Sharma, G. Study of Calcination Temperature Influence on Physicochemical Properties and Photodegradation Performance of Cu2O/WO3/TiO2. Catalysts 2025, 15, 601. [Google Scholar] [CrossRef]
- Hao, L.; Teng, D.; Guo, X.; Wu, B.; Wan, J.; Zhang, J.; Yang, J.-H. Construction of TiO2/BiOCl S-Scheme heterojunction and photocatalytic degradation of norfloxacin. J. Photochem. Photobiol. A Chem. 2023, 444, 115004. [Google Scholar] [CrossRef]
- Deng, A.; Sun, Y.; Gao, Z.; Yang, S.; Liu, Y.; He, H.; Zhang, J.; Liu, S.; Sun, H.; Wang, S. Internal electric field in carbon nitride-based heterojunctions for photocatalysis. Nano Energy 2023, 108, 108228. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.-Y.; Fang, B.; Yang, H. Z-scheme systems: From fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, G.; Xie, L.; Wu, P.; Liu, H.; Wang, J.; Xie, Y.; Chen, J.; Lu, C.-Z. Promoting photocatalytic H2 evolution through interfacial charge separation on the direct Z-scheme ZnIn2S4/ZrO2 heterojunction. Int. J. Hydrog. Energy 2023, 48, 32782–32796. [Google Scholar] [CrossRef]
- Li, G.; Cai, Y.; Wang, X.; Zhang, L.; Xie, Q.; Chen, P.; Li, C.; Sun, J.; Li, T.; Dong, L. Direct Z-scheme heterojunction rutile-TiO2/g-C3N4 catalyst constructed by solid grinding method for photocatalysis degradation. Chem. Phys. 2022, 559, 111558. [Google Scholar] [CrossRef]
- El Sharkawy, H.M.; Abo El-Khair, M.A.; Morshedy, A.S. Construction of SnS2@SnO2 nanocomposite Z-scheme heterojunction for dual-functional photocatalysis: Green hydrogen generation and crystal violet degradation. Int. J. Hydrog. Energy 2025, 137, 471–486. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Yue, X.; Cheng, L.; Fan, J.; Xiang, Q. 2D/2D BiVO4/CsPbBr3 S-scheme heterojunction for photocatalytic CO2 reduction: Insights into structure regulation and Fermi level modulation. Appl. Catal. B Environ. 2022, 304, 120979. [Google Scholar] [CrossRef]
- Nie, C.; Wang, X.; Lu, P.; Zhu, Y.; Li, X.; Tang, H. Advancements in S-scheme heterojunction materials for photocatalytic environmental remediation. J. Mater. Sci. Technol. 2023, 169, 182–198. [Google Scholar] [CrossRef]
- Yang, S.; Lu, Q.; Wang, F.; Zhi, Y.; Chen, J.; Wang, Y.; Zhang, H.; Yin, H.; Sun, P.; Cao, W. S-scheme SnO/TiO2 heterojunction with high hole mobility for boosting photocatalytic degradation of gaseous benzene. Chem. Eng. J. 2023, 478, 147345. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, X.; Nie, Y.; Yang, D.; Xiang, G. 2D/2D Janus BiTeCl/GeSe vdW heterostructure as a robust high-performance S-scheme photocatalyst for water splitting. Appl. Surf. Sci. 2023, 635, 157694. [Google Scholar] [CrossRef]
- Xiao, Y.; Abulizi, A.; Okitsu, K.; Ren, T. Facile fabrication of SnO2 modified hierarchical BiOI S-scheme heterojunction photocatalyst with efficient activity for carbon dioxide reduction. J. Ind. Eng. Chem. 2023, 125, 317–324. [Google Scholar] [CrossRef]
- Huong, V.H.; Nguyen, V.-C.; Ha, M.N.; Pham, D.V.; Nguyen, T.B.; Ma, Y.-R.; Ngac, A.B.; Loan, T.T. A S-scheme heterojunction Fe-doped TiO2/SnO2 with rich oxygen vacancies for photo-Fenton degradation of Rhodamine B under visible light illumination. Opt. Mater. 2023, 140, 113864. [Google Scholar] [CrossRef]
- Rezaei, M.M.; Seyed Dorraji, M.S.; Hosseini, S.F.; Rasoulifard, M.H. S-scheme heterojunction of MoO3 nanobelts and MoS2 nanoflowers for photocatalytic degradation. Sci. Rep. 2025, 15, 10789. [Google Scholar] [CrossRef]
- Salehi Ghalehsefid, E.; Ghorbani Jahani, Z.; Aliabadi, A.; Ghodrati, M.; Khamesan, A.; Parsaei-Khomami, A.; Mousavi, M.; Hosseini, M.-A.; Ghasemi, J.B.; Li, X. TiO2 nanotube/ZnIn2S4 nanoflower composite with step-scheme heterojunction for efficient photocatalytic H2O2 production and organic dye degradation. J. Environ. Chem. Eng. 2023, 11, 110160. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Dai, J.; Songsiriritthigul, P.; Oo, T.Z.; Zaw, M.; Lwin, N.W.; Aung, S.H.; Chen, F. Defect engineering in 0D/2D TiO2/g-C3N4 heterojunction for boosting photocatalytic degradation of tetracycline in a tetracycline/Cu2+ combined system. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132624. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Su, B.; Ma, J. Two-step construction of WO3@TiO2/CS-biochar S-scheme heterojunction and its synergic adsorption/photocatalytic removal performance for organic dye and antibiotic. Diam. Relat. Mater. 2023, 131, 109560. [Google Scholar] [CrossRef]
- Song, C. Enhancing photocatalytic degradation of hydrolyzed polyacrylamide in oilfield wastewater using BiVO4/TiO2 heterostructure nano-photocatalyst under visible light irradiation. Int. J. Electrochem. Sci. 2023, 18, 100363. [Google Scholar] [CrossRef]
- Wang, R.; Cao, J.; Liu, J.; Zhang, Y. Synthesis of CuO@TiO2 nanocomposite and its photocatalytic and electrochemical properties. Application for treatment of azo dyes in industrial wastewater. Int. J. Electrochem. Sci. 2023, 18, 100316. [Google Scholar] [CrossRef]
- Wang, F.; Pan, K.; Wei, S.; Ren, Y.; Zhu, H.; Wu, H.-H.; Zhang, Q. Solvothermal preparation and characterization of ordered-mesoporous ZrO2/TiO2 composites for photocatalytic degradation of organic dyes. Ceram. Int. 2021, 47, 7632–7641. [Google Scholar] [CrossRef]
- Raj, M.G.; Vijayakumar, E.; Preetha, R.; Narendran, M.G.; Neppolian, B.; Bosco, A.J. Implanting TiO2 @MoS2/BiVO4 nanocomposites via sonochemically assisted photoinduced charge carriers promotes highly efficient photocatalytic removal of tetracycline. J. Alloys Compd. 2022, 929, 167252. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Z.; Guo, J.; Shen, R.; Xu, F. Magnetic nanoscaled Fe3O4-CeO2-TiO2 composite: UV-Fenton reaction to degrade AO-7 dye. Inorg. Chem. Commun. 2023, 149, 110389. [Google Scholar] [CrossRef]
- Mahamud, M.; Taddesse, A.M.; Bogale, Y.; Bezu, Z. Zeolite supported CdS/TiO2/CeO2 composite: Synthesis, characterization and photocatalytic activity for methylene blue dye degradation. Mater. Res. Bull. 2023, 161, 112176. [Google Scholar] [CrossRef]
- Gajera, R.; Patel, R.V.; Yadav, A.; Labhasetwar, P.K. Adsorption of cationic and anionic dyes on photocatalytic flyash/TiO2 modified chitosan biopolymer composite. J. Water Process Eng. 2022, 49, 102993. [Google Scholar] [CrossRef]
- Yavuz, C.; Ela, S.E. Fabrication of g-C3N4-reinforced CdS nanosphere-decorated TiO2 nanotablet composite material for photocatalytic hydrogen production and dye-sensitized solar cell application. J. Alloys Compd. 2023, 936, 168209. [Google Scholar] [CrossRef]
- Bae, J.-h.; Do, S.-b.; Cho, S.-h.; Lee, K.-m.; Lee, S.-E.; Kim, T.-O. TiO2 treatment using ultrasonication for bubble cavitation generation and efficiency assessment of a dye-sensitized solar cell. Ultrason. Sonochem. 2022, 83, 105933. [Google Scholar] [CrossRef]
- Sun, H.; Qin, P.; Liang, Y.; Yang, Y.; Zhang, J.; Guo, J.; Hu, X.; Jiang, Y.; Zhou, Y.; Luo, L.; et al. Sonochemically assisted the synthesis and catalytic application of bismuth-based photocatalyst: A mini review. Ultrason. Sonochem. 2023, 100, 106600. [Google Scholar] [CrossRef]
- Kale, D.P.; Deshmukh, S.P.; Shirsath, S.R.; Bhanvase, B.A. Sonochemical preparation of multifunctional rGO-ZnS-TiO2 ternary nanocomposite and its application for CV dye removal. Optik 2020, 208, 164532. [Google Scholar] [CrossRef]
- Potle, V.D.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K. Sonochemical preparation of ternary rGO-ZnO-TiO2 nanocomposite photocatalyst for efficient degradation of crystal violet dye. Optik 2020, 208, 164555. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Lu, C.; Zou, J.; Ruan, M.; Yin, Y.; Qing, M.; Song, Q. Enhancement of catalytic activity in NH3-SCR reaction by promoting dispersibility of CuCe/TiO2-ZrO2 with ultrasonic treatment. Ultrason. Sonochem. 2021, 72, 105466. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, Z.; Morsali, A. Amine-Functionalized Metal-Organic Frameworks: From Synthetic Design to Scrutiny in Application. Coord. Chem. Rev. 2022, 459, 214445. [Google Scholar] [CrossRef]
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.; Wang, D.; Zhou, W.; Gu, L.; Wu, H.; Liu, G.; Zhao, Y. Integrating Suitable Linkage of Covalent Organic Frameworks into Covalently Bridged Inorganic/Organic Hybrids toward Efficient Photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862–4871. [Google Scholar] [CrossRef]
- Wang, P.; Zong, L.; Guan, Z.; Li, Q.; Yang, J. PtNi Alloy Cocatalyst Modification of Eosin Y-Sensitized g-C3N4/GO Hybrid for Efficient Visible-Light Photocatalytic Hydrogen Evolution. Nanoscale Res. Lett. 2018, 13, 33. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, Z.; Dong, C.; Wang, Z.; Yao, S.; Gao, H.; Liu, Z.; Liu, Y.; Yang, B.; Zhang, H. Ultrathin BiOX (X = Cl, Br, I) Nanosheets with Exposed {001} Facets for Photocatalysis. ACS Appl. Nano Mater. 2020, 3, 1981–1991. [Google Scholar] [CrossRef]
- Purkayastha, M.D.; Sil, S.; Singh, N.; Ray, P.P.; Darbha, G.K.; Bhattacharyya, S.; Mallick, A.I.; Majumder, T.P. Sonochemical synthesis of nanospherical TiO2 within graphene oxide nanosheets and its application as a photocatalyst and a Schottky diode. FlatChem 2020, 22, 100180. [Google Scholar] [CrossRef]
- Huang, Y.; Qiu, S.; Lian, H.; Xu, J. Rearrangement of α-terpineol by using PTA/TiO2 catalyst synthesized by ultrasonic-assisted impregnation method. Tetrahedron 2022, 108, 132659. [Google Scholar] [CrossRef]
- Wittawat, R.; Rittipun, R.; Jarasfah, M.; Nattaporn, B. Synthesis of ZnO/TiO2 spherical particles for blue light screening by ultrasonic spray pyrolysis. Mater. Today Commun. 2020, 24, 101126. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.-L.; Yang, T.C.K.; Chen, J.-N.; Chen, S.-W. Effect of ultrasound-induced hydroxylation and exfoliation on P90–TiO2/g-C3N4 hybrids with enhanced optoelectronic properties for visible-light photocatalysis and electrochemical sensing. Ceram. Int. 2020, 46, 18002–18018. [Google Scholar] [CrossRef]
- Seeharaj, P.; Kongmun, P.; Paiplod, P.; Prakobmit, S.; Sriwong, C.; Kim-Lohsoontorn, P.; Vittayakorn, N. Ultrasonically-assisted surface modified TiO2/rGO/CeO2 heterojunction photocatalysts for conversion of CO2 to methanol and ethanol. Ultrason. Sonochem. 2019, 58, 104657. [Google Scholar] [CrossRef]
- Tian, W.-J.; Chen, H.-J.; Zhao, L.; Zheng, L.-L.; Hu, Z.-N.; Yang, Y.-L. Preparation of adsorptive TiO2/SiO2 core-shell nanospheres by ultrasonic-coupled alkali leaching process: Synergy of adsorption and photocatalysis towards 2,4-dinitrophenol removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132530. [Google Scholar] [CrossRef]
- Chen, R.; Ding, S.; Wang, B.; Ren, X. Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity. Coatings 2022, 12, 1407. [Google Scholar] [CrossRef]
- Mahammed Shaheer, A.R.; Thangavel, N.; Rajan, R.; Abraham, D.A.; Vinoth, R.; Sunaja Devi, K.R.; Shankar, M.V.; Neppolian, B. Sonochemical assisted impregnation of Bi2WO6 on TiO2 nanorod to form Z-scheme heterojunction for enhanced photocatalytic H2 production. Adv. Powder Technol. 2021, 32, 4734–4743. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Yang, H.; Xiong, Y.; Liu, C.; Han, Y.; Yu, Z.-Z.; Li, X. One-Step In Situ Synthesis of a Reduced Graphene Oxide-Based Hybrid Hydrogel for Highly Efficient Water Evaporation and Comprehensive Wastewater Treatment. ACS Appl. Mater. Interfaces 2025, 17, 46046–46058. [Google Scholar] [CrossRef]
- Galedari, M.; Mehdipour Ghazi, M.; Mirmasoomi, S.R. Novel visible-driven Ag2O/Fe2O3/TiO2 nano sized hetero-structured photocatalyst: Synthesis, characterization and photo-degradation of tetracycline. Chem. Eng. Res. Des. 2021, 170, 248–255. [Google Scholar] [CrossRef]
- Jonda, E.; Sarman, M.; Bezdicka, P.; Kormunda, M.; Ecorchard, P.; Murafa, N.; Michalska, M. Surface modification of nano-TiO2 with Cu nanoparticles. Ceram. Int. 2025, in press. [Google Scholar] [CrossRef]
- Qi, Y.; Shen, Y.; Zhao, S.; Jiang, X.; Ma, R.; Cui, B.; Zhao, Q.; Wei, D. Degradation of multiple xanthates using highly efficient visible light-responsive BiOBr-TiO2 composite photocatalysts. J. Ind. Eng. Chem. 2023, 132, 461–473. [Google Scholar] [CrossRef]
- He, Z.; Zhang, M.; Yu, H.; Li, Z.; Deng, B.; Ren, H. Multifunctional BiPO4/TiO2/rGO composite for enhancement removal of malachite green and levofloxacin via adsorption and visible-light photocatalysis synergy. Mater. Sci. Semicond. Process. 2024, 169, 107891. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Benaissa, M.; Hamdy, M.S.; Ahmed, M.A.; Glal, M. Synthesis of an efficient, and recyclable mesoporous BiVO4/TiO2 direct Z-scheme heterojunction by sonochemical route for photocatalytic hydrogen production and photodegradation of rhodamine B dye in the visible region. Opt. Mater. 2021, 114, 110761. [Google Scholar] [CrossRef]
- Bahal, M.; Kaur, N.; Sharotri, N.; Sud, D. Investigations on Amphoteric Chitosan/TiO2 Bionanocomposites for Application in Visible Light Induced Photocatalytic Degradation. Adv. Polym. Technol. 2019, 2019, 2345631. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A.; Alghamdi, Y.G.; Al-Khathami, N.D.; Mohamed, R.M. Co3O4 Nanoparticles Accommodated Mesoporous TiO2 framework as an Excellent Photocatalyst with Enhanced Photocatalytic Properties. Opt. Mater. 2022, 131, 112643. [Google Scholar] [CrossRef]
- Kaviyarasan, K.; Vinoth, V.; Sivasankar, T.; Asiri, A.M.; Wu, J.J.; Anandan, S. Photocatalytic and photoelectrocatalytic performance of sonochemically synthesized Cu2O@TiO2 heterojunction nanocomposites. Ultrason. Sonochem. 2019, 51, 223–229. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Chen, Y.; Fan, H.; et al. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloys Compd. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Nodehi, R.N.; Yaghmaeian, K.; Jaafari, J.; Niari, M.H.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Azari, A.; Shariatifar, N. Catalytic decomposition of 2-chlorophenol using an ultrasonic-assisted Fe3O4–TiO2@MWCNT system: Influence factors, pathway and mechanism study. J. Colloid Interface Sci. 2018, 512, 172–189. [Google Scholar] [CrossRef]
- Wang, J.; Ren, P.; Du, Y.; Zhao, X.; Chen, Z.; Pei, L.; Jin, Y. Construction of tubular g-C3N4/TiO2 S-scheme photocatalyst for high-efficiency degradation of organic pollutants under visible light. J. Alloys Compd. 2023, 947, 169659. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Kane, A.; Chafiq, L.; Dalhatou, S.; Bonnet, P.; Nasr, M.; Gaillard, N.; Dikdim, J.M.D.; Monier, G.; Assadi, A.A.; Zeghioud, H. g-C3N4/TiO2 S-scheme heterojunction photocatalyst with enhanced photocatalytic Carbamazepine degradation and mineralization. J. Photochem. Photobiol. A Chem. 2022, 430, 113971. [Google Scholar] [CrossRef]
- Alsalme, A.; Galal, A.H.; El-Sherbeny, E.F.; Soltan, A.; Abdel-Messih, M.F.; Ahmed, M.A. Fabrication of S-scheme TiO2/g-C3N4 nanocomposites for generation of hydrogen gas and removal of fluorescein dye. Diam. Relat. Mater. 2022, 122, 108819. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ahamad, T.; Ahmad, N.; Khan, M.Z. Removal enhancement of acid navy blue dye by GO-TiO2 nanocomposites synthesized using sonication method. Mater. Chem. Phys. 2019, 238, 121906. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochem. 2020, 61, 104849. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-y.; Li, K.-k.; Jia, J.; Zhang, Y.-t. Coal-based graphene as a promoter of TiO2 catalytic activity for the photocatalytic degradation of organic dyes. New Carbon Mater. 2022, 37, 1172–1180. [Google Scholar] [CrossRef]
- Lacerda Fernandes, Í.; Pereira Barbosa, D.; Botelho de Oliveira, S.; Antônio da Silva, V.; Henrique Sousa, M.; Montero-Muñoz, M.; Coaquira, J.A.H. Synthesis and characterization of the MNP@SiO2@TiO2 nanocomposite showing strong photocatalytic activity against methylene blue dye. Appl. Surf. Sci. 2022, 580, 152195. [Google Scholar] [CrossRef]
- AbdulKareem, E.A.; Mahmoud, Z.H.; Khadom, A.A. Sunlight assisted photocatalytic mineralization of organic pollutants over rGO impregnated TiO2 nanocomposite: Theoretical and experimental study. Case Stud. Chem. Environ. Eng. 2023, 8, 100446. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Kale, D.P.; Kar, S.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K.; Sonawane, S.H. Ultrasound assisted preparation of rGO/TiO2 nanocomposite for effective photocatalytic degradation of methylene blue under sunlight. Nano-Struct. Nano-Objects 2020, 21, 100407. [Google Scholar] [CrossRef]
- Balta, Z.; Simsek, E.B. Insights into the photocatalytic behavior of carbon-rich shungite-based WO3/TiO2 catalysts for enhanced dye and pharmaceutical degradation. New Carbon Mater. 2020, 35, 371–383. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Cho, M.H.; Yoon, T. Fabrication of binary SnO2/TiO2 nanocomposites under a sonication-assisted approach: Tuning of band-gap and water depollution applications under visible light irradiation. Ceram. Int. 2021, 47, 15073–15081. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Lü, C.; Yin, C.; Xu, G.; Qin, X.; Wu, W. Dual S-scheme SnO2/TiO2(A)/TiO2(R) nanowires for visible-light-driven naphthalene complete degradation. J. Ind. Eng. Chem. 2024, 129, 474–487. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Hu, J.; Zhang, Q.; Gong, L.; Wei, X. Preparation of SnS2/TiO2 by a thermo-solvent ultrasonic method and its high photo-catalytic performance for decontamination under visible light. J. Environ. Chem. Eng. 2020, 8, 104121. [Google Scholar] [CrossRef]
- Sedaghati, N.; Habibi-Yangjeh, A.; Pirhashemi, M.; Asadzadeh-Khaneghah, S.; Ghosh, S. Integration of BiOI and Ag3PO4 nanoparticles onto oxygen vacancy rich-TiO2 for efficient visible-light photocatalytic decontaminations. J. Photochem. Photobiol. A Chem. 2020, 400, 112659. [Google Scholar] [CrossRef]
- Boudraa, R.; Talantikite-Touati, D.; Souici, A.; Djermoune, A.; Saidani, A.; Fendi, K.; Amrane, A.; Bollinger, J.-C.; Nguyen Tran, H.; Hadadi, A.; et al. Optical and photocatalytic properties of TiO2–Bi2O3–CuO supported on natural zeolite for removing Safranin-O dye from water and wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114845. [Google Scholar] [CrossRef]
- Bathula, C.; Rabani, I.; Sekar, S.; Youi, H.-K.; Choy, J.-Y.; Kadam, A.; Shretha, N.K.; Seo, Y.-S.; Kim, H.-S. Enhanced removal of organic dye by activated carbon decorated TiO2 nanoparticles from Mentha Aquatica leaves via ultrasonic approach. Ceram. Int. 2021, 47, 8732–8739. [Google Scholar] [CrossRef]
- Akhter, P.; Ali, F.; Ali, A.; Hussain, M. TiO2 decorated CNTs nanocomposite for efficient photocatalytic degradation of methylene blue. Diam. Relat. Mater. 2024, 141, 110702. [Google Scholar] [CrossRef]
- Manivannan, R.; Ryu, J.; Son, Y.-A. Controlled ultrasonic synthesis of TiO2@C3N4 nanocomposites with porphyrin as a solid-state electron mediator: A promising material for pollutant discoloration under visible light. Ceram. Int. 2021, 47, 14399–14407. [Google Scholar] [CrossRef]
- He, X.; Meng, X.; Sun, J.; Yuan, Z.; He, Y.; Chen, S. Synthesis of TiO2@Fe2O3 Nanocomposites as effective Photocatalyst for degradation of p-nitophenol in oilfield wastewater. Int. J. Electrochem. Sci. 2022, 17, 221179. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P. Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chem. Eng. J. 2020, 384, 123245. [Google Scholar] [CrossRef]
- Tian, M.; Wang, J.; Sun, R.; Lu, D.; Li, N.; Liu, T.; Yao, M.; Zhang, G.; Li, L. Facile synthesis of rod-like TiO2-based composite loaded with g-C3N4 for efficient removal of high-chroma organic pollutants based on adsorption-photocatalysis mechanism. Inorg. Chem. Commun. 2022, 141, 109517. [Google Scholar] [CrossRef]
- Chi, N.; Xu, W. Synthesis of TiO2/g-C3N4 Hybrid Photocatalyst and its Application for Degradation of Chlorophenol as Organic Water Pollutant. Int. J. Electrochem. Sci. 2022, 17, 220929. [Google Scholar] [CrossRef]
- Toghan, A.; Abd El-Lateef, H.M.; Taha, K.K.; Modwi, A. Mesoporous TiO2@g-C3N4 composite: Construction, characterization, and boosting indigo carmine dye destruction. Diam. Relat. Mater. 2021, 118, 108491. [Google Scholar] [CrossRef]
- Izzataddini, A.; Romdoni, Y.; Helmiyati; Novi Marantika, R.; Amir, Z.; Kadja, G.T.M.; Jiwanti, P.K.; Khalil, M.; Mohamed Jan, B. Enhancement of visible light organic dyes photodegradation using TiO2 (001)/Graphene oxide nanocomposite. Inorg. Chem. Commun. 2023, 157, 111379. [Google Scholar] [CrossRef]
- Uma, K.; Singaravelu, C.M.; Kavinkumar, V.; Jothivenkatachalam, K.; Lin, J.-H. Ultrasonically modified P25-TiO2 /In2O3 heterostructured nanoparticles: An efficient dual- responsive photocatalyst for solution and gas phase reactions. J. Taiwan Inst. Chem. Eng. 2021, 125, 257–266. [Google Scholar] [CrossRef]
- Ardani, M.R.; Pang, A.L.; Pal, U.; Haniff, M.A.S.M.; Ismail, A.G.; Hamzah, A.A.; Khanday, W.A.; Ahmadipour, M. Ultrasonic-assisted of TiO2-MWCNT nanocomposite with advanced photocatalytic efficiency for elimination of dye pollutions. Diam. Relat. Mater. 2023, 137, 110066. [Google Scholar] [CrossRef]
- Karamifar, M.; Sabbaghi, S.; Mohtaram, M.S.; Rasouli, K.; Mohsenzadeh, M.; Kamyab, H.; Derakhshandeh, A.; Dolatshah, L.; Moradi, H.; Chelliapan, S. Ultrasonic-assisted synthesis of TiO2/MWCNT/Pani nanocomposite: Photocatalyst characterization and optimization of efficient variables in the degradation of benzene via RSM-CCD. Powder Technol. 2024, 432, 119176. [Google Scholar] [CrossRef]
- Wafi, M.A.E.; Ahmed, M.A.; Abdel-Samad, H.S.; Medien, H.A.A. Exceptional removal of methylene blue and p-aminophenol dye over novel TiO2/RGO nanocomposites by tandem adsorption-photocatalytic processes. Mater. Sci. Energy Technol. 2022, 5, 217–231. [Google Scholar] [CrossRef]
- Moghni, N.; Boutoumi, H.; Khalaf, H.; Makaoui, N.; Colón, G. Enhanced photocatalytic activity of TiO2/WO3 nanocomposite from sonochemical-microwave assisted synthesis for the photodegradation of ciprofloxacin and oxytetracycline antibiotics under UV and sunlight. J. Photochem. Photobiol. A Chem. 2022, 428, 113848. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Y.; Yu, Y.; Shen, K.; Hu, K.; Zhou, J.; Wu, M.; Liu, S.; Wang, Z. Effect of synthesis process steps on the structure and photocatalytic performance of ZnO–TiO2 composites. Ceram. Int. 2024, 50, 3168–3175. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, L.; Zhang, L.; Yu, B.; Yang, C.; Zhou, L.; Lou, B. Optimizing the dewatering performance of zinc smelting iron Slag: Investigating the influence of ultrasonic Time, ultrasonic Power, and Liquid-Solid ratio using response surface methodology. Ultrason. Sonochem. 2024, 103, 106797. [Google Scholar] [CrossRef]
- Abrinaei, F.; Aghabeygi, S. Optimization on preparation conditions to improve the nonlinear optical response of ZnO/TiO2/ZrO2 ternary nanocomposites under continuous-wave laser irradiation. Optik 2022, 255, 168720. [Google Scholar] [CrossRef]
- Kumar, S.; Sinhmar, P.S.; Gogate, P.R. Ultrasound assisted improved synthesis of TiO2 catalyst and subsequent evaluation for isomerization of alpha pinene. Chem. Eng. Process. Process Intensif. 2021, 169, 108591. [Google Scholar] [CrossRef]
- Tiple, A.; Sinhmar, P.S.; Gogate, P.R. Improved direct synthesis of TiO2 catalyst using sonication and its application for the desulfurization of thiophene. Ultrason. Sonochem. 2021, 73, 105547. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Monje, A.; Zitzumbo-Guzmán, R.; Bañuelos-Díaz, J.A.; Zaragoza-Contreras, E.A. Ultrasonic dispersion and activation of TiO2 nanoparticles and its effect on bacterial inhibition in EVA films. Mater. Chem. Phys. 2019, 235, 121760. [Google Scholar] [CrossRef]
- Elavarasan, M.; Uma, K.; Yang, T.C.K. Photocatalytic oxidation of ethanol using ultrasonic modified TiO2; an in-situ diffuse reflectance infrared spectroscopy study. Results Phys. 2019, 13, 102237. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Z.; Wang, F.; Chi, Y.; Wang, Z.; Tofil, S.; Yao, J. Grain refinement and Laves phase dispersion by high-intensity ultrasonic vibration in laser cladding of Inconel 718. J. Mater. Res. Technol. 2024, 30, 8563–8575. [Google Scholar] [CrossRef]
- Qayyum, A.; Giannakoudakis, D.A.; LaGrow, A.P.; Bondarchuk, O.; Łomot, D.; Colmenares, J.C. High-frequency sonication for the synthesis of nanocluster-decorated titania nanorods: Making a better photocatalyst for the selective oxidation of monoaromatic alcohol. Catal. Commun. 2022, 163, 106406. [Google Scholar] [CrossRef]
- Wood, R.J.; Lee, J.; Bussemaker, M.J. A parametric review of sonochemistry: Control and augmentation of sonochemical activity in aqueous solutions. Ultrason. Sonochem. 2017, 38, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Bandeira, C.R.P.P.; Dória, A.R.; Cruz Ribeiro, J.Y.; Prado, L.R.; Anjos de Jesus, R.; Carvalho Andrade, H.M.; Souza de Santana Castro, R.; Romanholo Ferreira, L.F.; Egues, S.M.S.; Figueiredo, R.T. Tuning the sound frequency in the audible region during the synthesis of the precursor TiO2: Evaluation of the sound effect on the structure and photoactivity relationship. Mater. Chem. Phys. 2021, 265, 124521. [Google Scholar] [CrossRef]
- Oliveira, A.C.d.M.; de Jesus, R.A.; Bilal, M.; Iqbal, H.M.N.; Bharagava, R.N.; Yerga, R.M.N.; Ferreira, L.F.R.; Egues, S.M.; Figueiredo, R.T. Influence of sound and calcination temperature on the fabrication of TiO2-based photocatalysts and their photoactivity for H2 production. Mol. Catal. 2022, 529, 112523. [Google Scholar] [CrossRef]
- Dong, H.; Wang, P.; Yang, Z.; Li, R.; Xu, X.; Shen, J. Dual improvement in curcumin encapsulation efficiency and lyophilized complex dispersibility through ultrasound regulation of curcumin–protein assembly. Ultrason. Sonochem. 2022, 90, 106188. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Tran, T.T.V.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zhang, S.; Zhang, L.; Cui, Y.; Shi, H. Ultrasound assisted TiO2@Fe3O4 nanocomposites photocatalytic degradation of organic pollutants in potato starch processing wastewater. Sep. Purif. Technol. 2024, 332, 125799. [Google Scholar] [CrossRef]
- Davies, K.R.; Cherif, Y.; Pazhani, G.P.; Anantharaj, S.; Azzi, H.; Terashima, C.; Fujishima, A.; Pitchaimuthu, S. The upsurge of photocatalysts in antibiotic micropollutants treatment: Materials design, recovery, toxicity and bioanalysis. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100437. [Google Scholar] [CrossRef]
- Hou, Y.; Pu, S.; Shi, Q.; Mandal, S.; Ma, H.; Xue, S.; Cai, G.; Bai, Y. Ultrasonic impregnation assisted in-situ photoreduction deposition synthesis of Ag/TiO2/rGO ternary composites with synergistic enhanced photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2019, 104, 139–150. [Google Scholar] [CrossRef]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Zamani, M.R.; Aminabhavi, T.M. Photocatalytic activity of visible-light-driven L-Proline-TiO2/BiOBr nanostructured materials for dyes degradation: The role of generated reactive species. J. Environ. Manag. 2023, 326, 116691. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.B.; Baek, S.K.; Yun, Y.D.; Jung, S.H.; Cho, S.W.; Ahn, C.H.; Cho, H.K. Compositionally graded SnO2/TiO2 bi-layered compounds with dramatically enhanced charge transport efficiency for self-driven water purification applications. J. Alloys Compd. 2019, 776, 839–849. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kanimozhi, K.; Arularasu, M.V.; Ramalingam, G.; Kaviyarasu, K. Self-cleaning mechanism of synthesized SnO2/TiO2 nanostructure for photocatalytic activity application for waste water treatment. Surf. Interfaces 2019, 17, 100346. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Thompson-Yusuff, K.A.; Adeniyi, O.D.; Olutoye, M.A. Siliceous termite hill supported ZnO-TiO2 as a solar light responsive photocatalyst: Synthesis, characterization and performance in degradation of methylene blue dye. Surf. Interfaces 2022, 34, 102360. [Google Scholar] [CrossRef]
- Rajput, R.B.; Jamble, S.N.; Kale, R.B. A review on TiO2/SnO2 heterostructures as a photocatalyst for the degradation of dyes and organic pollutants. J. Environ. Manag. 2022, 307, 114533. [Google Scholar] [CrossRef]
- Narzary, S.; Alamelu, K.; Raja, V.; Jaffar Ali, B.M. Visible light active, magnetically retrievable Fe3O4@SiO2@g-C3N4/TiO2 nanocomposite as efficient photocatalyst for removal of dye pollutants. J. Environ. Chem. Eng. 2020, 8, 104373. [Google Scholar] [CrossRef]
- Huang, J.; Jing, H.-X.; Li, N.; Li, L.-X.; Jiao, W.-Z. Fabrication of magnetically recyclable SnO2-TiO2/CoFe2O4 hollow core-shell photocatalyst: Improving photocatalytic efficiency under visible light irradiation. J. Solid State Chem. 2019, 271, 103–109. [Google Scholar] [CrossRef]
- Duta, A.; Andronic, L.; Enesca, A. The influence of low irradiance and electrolytes on the mineralization efficiency of organic pollutants using the Vis-active photocatalytic tandem CuInS2/TiO2/SnO2. Catal. Today 2018, 300, 18–27. [Google Scholar] [CrossRef]
- Moradi, S.; Farhadian, M.; Nazar, A.R.S.; Moghadam, M. Application of Bi2WO6/N-TiO2 catalyst immobilized on FTO in a tray photoreactor for textile color degradation from aqueous solutions: Effects of mineral salts. J. Mol. Liq. 2023, 377, 121520. [Google Scholar] [CrossRef]
- Kumar, A.; Raorane, C.J.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Raj, V.; Kim, S.C. Synthesis of TiO2, TiO2/PAni, TiO2/PAni/GO nanocomposites and photodegradation of anionic dyes Rose Bengal and thymol blue in visible light. Environ. Res. 2023, 216, 114741. [Google Scholar] [CrossRef] [PubMed]
| Photocatalyst Composite | Preparation Technique | Optimum Ultrasonication Parameters | Band Gap (eV) of Composite | Surface Area (m2/g) | Experimental Conditions | Photocatalysis Performance (%; min−1 for k) | Ref. |
|---|---|---|---|---|---|---|---|
| rGO/MXene-TiO2/polyaniline | Thermal assisted ultrasonication | Time: 30 min Power: N/A Freq.: N/A | 2.99 | TiO2: N/A Composite: N/A | Dosage: 0.20 g/L [Methyl orange]: 10 mg/L [Methylene blue]: 10 mg/L [Rhodamine B]: 10 mg/L [Norfloxacin]: 10 mg/L [Tetracycline]: 10 mg/L Time: 60 min pH: N/A Light: Solar Volume: 50 mL | Methyl orange TiO2: N/A Composite: 98.40; Methylene blue TiO2: N/A Composite: 99.10; Rhodamine B TiO2: N/A Composite: 99.80; Norfloxacin TiO2: N/A Composite: 87.80; Tetracycline TiO2: N/A Composite: 95.10 | [101] |
| Ag2O/TiO2/Fe2O3 | Impregnation assisted ultrasonication | Time: 45 min Power: N/A Freq.: 28 kHz | 2.18 | TiO2: N/A Composite: N/A | Dosage: 0.75 g/L [Tetracycline]: 15 mg/L Time: 90 min pH: N/A Light: Visible (60 W) Volume: 300 mL | TiO2: N/A Composite: 90 | [102] |
| WO3/TiO2 | Ultrasonic-assisted solvothermal | Time: 30 min Power: N/A Freq.: N/A | 2.80 to 3.10 | TiO2: 46.49 Composite: 41.73 to 48.20 | Dosage: 0.25 g/L [Reactive Black 5]: 30 mg/L Time: 60 min pH: 3 Light: Visible (150 W) Volume: 80 mL | TiO2: N/A Composite: 92; k = 0.044 | [28] |
| Cu2O/WO3/TiO2 | Ultrasonic-assisted hydrothermal | Time: 60 min Power: N/A Freq.: N/A | 2.70 | TiO2: N/A Composite: N/A | Dosage: 0.25 g/L [Acetaminophen]: 1 mg/L Time: 60 min pH: N/A Light: Visible (150 W) Volume: 80 mL | TiO2: k = 0.024 Composite: k = 0.044 | [30] |
| Cu/TiO2 | Low temperature chemical process with ultrasonication | Time: 60 min Power: 280 W Freq.: N/A | 3.11 to 3.23 | TiO2: N/A Composite: N/A | Dosage: 0.30 g/L [Acid orange 7]: N/A [Rhodamine B]: N/A Time: 120 min pH: N/A Light: UV; UV and Visible; Visible Volume: N/A | Acid orange 7 98 Rhodamine B 99 | [103] |
| Cu2O/WO3/TiO2 | Ultrasonic-assisted solvothermal | Time: 60 min Power: N/A Freq.: N/A | 2.35 to 2.90 | TiO2: N/A Composite: 35.77 | Dosage: 0.13 g/L [Reactive Black 5]: 30 mg/L Time: 120 min pH: 5 Light: Visible (150 W) Volume: 80 mL | TiO2: N/A Composite: k = 0.023 | [53] |
| BiOBr/TiO2 | Ultrasonic-assisted hydrothermal and water bath precipitation | Time: 5 min Power: N/A Freq.: N/A | N/A | TiO2: 46.49 Composite: 42.13 | Dosage: 0.20 g/L [Sodium ethyl-xanthate]: 20 mg/L Time: 40 min pH: N/A Light: Visible (400 W) Volume: 50 mL | TiO2: N/A Composite: 93.96; k = 0.065 | [104] |
| BiPO4/TiO2/rGO | Ultrasonic-assisted hydrothermal | Time: N/A Power: N/A Freq.: N/A | 1.78 | TiO2: N/A Composite: N/A | Dosage: 0.67 g/L [Malachite green]: 20 mg/L [Levofloxacin]: 20 mg/L Time: Until complete degradation of malachite green and levofloxacin pH: N/A Light: Visible (300 W) Volume: 60 mL | Malachite green TiO2: N/A Composite: 100 (45 min); k = 0.091 Levofloxacin TiO2: N/A Composite: 94 (120 min); k = 0.024 | [105] |
| BiVO4/TiO2 | Ultrasonic-assisted solvothermal | Time: N/A Power: N/A Freq.: N/A | 2.69 | TiO2: N/A Composite: N/A | Dosage: 5 g/L [hydrolyzed polyacrylamide]: 20 mg/L Time: Until complete degradation of hydrolyzed polyacrylamide pH: N/A Light: Visible (35 W) Volume: 200 mL | TiO2: 100 (115 min) Composite: 100 (25 min) | [76] |
| BiVO4/TiO2 | Ultrasonication | Time: 120 min Power: N/A Freq.: 80 kHz | 2.72 | TiO2: 97.70 Composite: 59.30 | Dosage: 1 g/L [Rhodamine b]: N/A Time: 120 min pH: N/A Light: Visible (300 W) Volume: 100 mL | TiO2: k = 0.002 Composite: k = 0.021 | [106] |
| chitosan/TiO2 | Ultrasonication | Time: 30 min Power: N/A Freq.: 40 kHz | N/A | TiO2: N/A Composite: N/A | Dosage: 1 g/L [Malachite green]: 10 mg/L Time: 240 min pH: 7 Light: Visible (15 W) Volume: 100 mL | TiO2: N/A Composite: 91.94; k = 0.007 | [107] |
| Co3O4/TiO2 | Ultrasonic-assisted impregnation | Time: N/A Power: N/A Freq.: N/A | 2.35 | TiO2: 200 Composite: 184 | Dosage: 1 g/L [Ciprofloxacin]: 10 mg/L Time: 60 min pH: N/A Light: Visible (300 W) Volume: 50 mL | TiO2: k = 0.001 Composite: 100; k = 0.016 | [108] |
| CuO/TiO2 | Ultrasonication | Time: 15 min Power: N/A Freq.: N/A | 2.81 | TiO2: N/A Composite: N/A | Dosage: 2.40 g/L [Acid yellow 36]: 30 mg/L Time: Until complete degradation of acid yellow 36 pH: N/A Light: UV-visible (350 W) Volume: 250 mL | TiO2: 100 (100 min) Composite: 100 (45 min) | [77] |
| Cu2O/TiO2 | Ultrasonication | Time: 15 min Power: 100 W Freq.: 20 kHz | 2.12 | TiO2: N/A Composite: N/A | Dosage: N/A [Methyl orange]: N/A Time: 240 min pH: N/A Light: Visible Volume: N/A | TiO2: N/A Composite: k = 0.223 | [109] |
| α-Fe2O3/TiO2 | Ultrasonication followed by wet impregnation | Time: 8 min Power: 70 W Freq.: N/A | 2.80 | TiO2: N/A Composite: 106.34 | Dosage: 0.01 g/L [Cefixime]: 20.50 mg/L Time: 103 min pH: 4.76 Light: Visible Volume: 100 mL | TiO2: N/A Composite: 98.80; k = 0.057 | [12] |
| Fe3O4/SiO2/ZnO/ZnS | Ultrasonic-assisted in situ surface sulfidation | Time: 120 min Power: N/A Freq.: N/A | 2.69 | TiO2: N/A Composite: 43.50 | Dosage: 0.50 g/L [Oxytetracycline]: 10 mg/L [Tetracycline]: 10 mg/L Time: 90 min (UV light); 180 min (visible light) pH: N/A Light: UV (250 W); visible (300 W) Volume: 50 mL | Oxytetracycline (UV light) TiO2: N/A Composite: 99.10 Tetracycline (visible light) TiO2: N/A Composite: 80.90 | [110] |
| Fe3O4/TiO2/MWCNT | Ultrasonic-assisted wet impregnation | Time: 30 min Power: 300 W Freq.: 40 kHz | 1.75 | TiO2: 28 Composite: 181.42 | Dosage: 0.40 g/L [2-chlorophenol]: 2 mg/L Time: 15 min pH: 5 Light: UV-visible (125 W) Volume: 200 mL | TiO2: N/A Composite: 86 | [111] |
| g-C3N4/TiO2 | Ultrasonic-assisted hydrothermal | Time: N/A Power: N/A Freq.: N/A | 2.88 | TiO2: N/A Composite: N/A | Dosage: 0.40 g/L [Methylene blue]: 20 mg/L [Tetracycline]: 20 mg/L [Congo red]: 20 mg/L [Eosin y]: 20 mg/L Time: Different reaction time pH: N/A Light: Visible Volume: 50 mL | Methylene blue TiO2: 68.90; k = 0.019 (60 min) Composite: 96.60; k = 0.055 (60 min) Tetracycline TiO2: 73.60; k = 0.044 (30 min) Composite: 100; k = 0.315 (30 min) Congo red TiO2: N/A Composite: 100 (60 min) Eosin y TiO2: N/A Composite: 87.70 (10 min) | [112] |
| g-C3N4/TiO2 | Ultrasonication | Time: N/A Power: N/A Freq.: N/A | 2.98 | TiO2: 50.19 Composite: 49.37 | Dosage: 1 g/L [Tetracycline]: 50 mg/L Time: 50 min pH: N/A Light: UV (300 W) Volume: 50 mL | TiO2: N/A Composite: 96.53; k = 0.057 | [113] |
| g-C3N4/TiO2 | Ultrasonic-assisted wet impregnation | Time: 30 min Power: N/A Freq.: N/A | 2.99 | TiO2: 125.57 Composite: 80.65 | Dosage: N/A [Carbamazepine]: 10 mg/L Time: 360 min pH: N/A Light: UV (24 W) Volume: N/A | TiO2: N/A Composite: 71.41; k = 0.003 | [114] |
| g-C3N4/TiO2 | Ultrasonication | Time: 60 min Power: 200 W Freq.: N/A | 2.79 | TiO2: 156 Composite: 43 | Dosage: 1 g/L [Fluorescein dye]: concentration of 5 × 10−5 Time: 120 min pH: N/A Light: Visible (350 W) Volume: 100 mL | TiO2: 45; k = 0.002 Composite: 92; k = 0.025 | [115] |
| GO/TiO2 | Ultrasonication | Time: 60 min Power: N/A Freq.: N/A | 3.56 | TiO2: N/A Composite: N/A | Dosage: N/A [Acid navy blue]: N/A Time: 90 min pH: N/A Light: UV (125 W) Volume: N/A | TiO2: N/A Composite: 95; k = 0.042 | [116] |
| GO/TiO2 | Ultrasonic-assisted hydrothermal | Time: 30 min Power: 200 W Freq.: 40 kHz | 2.72 | TiO2: N/A Composite: N/A | Dosage: 4 g/L [Salicylic acid]: 1 mM Time: 60 min pH: N/A Light: Sunlight Volume: 250 mL | TiO2: N/A Composite: 57; k = 0.001 | [117] |
| Graphene/TiO2 | Ultrasonic-assisted hydrothermal | Time: 30 min Power: N/A Freq.: N/A | 2.45 | TiO2: 29.61 Composite: 35.89 | Dosage: N/A [Rhodamine b]: 20 mg/L [Methyl orange]: 30 mg/L Time: 120 min pH: N/A Light: Visible (300 W) Volume: 50 mL | Rhodamine b TiO2: k = 0.003 Composite: k = 0.013 Methyl orange TiO2: k = 0.003 Composite: k = 0.015 | [118] |
| MNP/SiO2/TiO2 | Ultrasonic-assisted solvothermal followed by sol–gel | Time: N/A Power: N/A Freq.: N/A | N/A | TiO2: 54 Composite: 167 | Dosage: 0.56 g/L [Methylene blue]: 3.50 mg/L Time: 120 min pH: 7 Light: UV (80 W) Volume: 50 mL | TiO2: k = 0.028 Composite: k = 0.060 | [119] |
| rGO/TiO2 | Photolysis followed by ultrasonication | Time: 60 min Power: N/A Freq.: N/A | 2.70 | TiO2: N/A Composite: N/A | Dosage: 0.10 g/L [Congo red]: 10 mg/L [Trichloroacetic acid]: 10 mg/L Time: 100 min pH: N/A Light: Direct sunlight Volume: 100 mL | Congo red TiO2: k = 0.010 Composite: k = 0.026 Trichloroacetic acid TiO2: k = 0.001 Composite: k = 0.026 | [120] |
| rGO/TiO2 | Ultrasonication | Time: 30 min Power: N/A Freq.: N/A | N/A | TiO2: N/A Composite: N/A | Dosage: 2 g/L [Methylene blue]: 20 mg/L Time: 30 min pH: 13.20 Light: Sunlight (between 11 am and 3 pm) Volume: 100 mL | TiO2: N/A Composite: 91.30 | [121] |
| rGO/ZnO/TiO2 | Ultrasonication | Time: 60 min Power: N/A Freq.: N/A | N/A | TiO2: N/A Composite: N/A | Dosage: 0.10 g/L [Crystal violet]: 50 mg/L Time: 20 min pH: 6.50 Light: UV Volume: 100 mL | TiO2: N/A Composite: 87.06 | [87] |
| rGO/ZnS/TiO2 | Ultrasonication | Time: 30 min Power: 240 W Freq.: 22 kHz | Composite showed a red shift of absorbance compared to others | TiO2: N/A Composite: N/A | Dosage: 0.40 g/L [Crystal violet]: 50 mg/L Time: 50 min pH: N/A Light: UV Volume: 100 mL | TiO2: N/A Composite: 97 | [86] |
| Shungite/WO3/TiO2 | Ultrasonic-solvothermal | Time: 60 min Power: N/A Freq.: N/A | 2.83 | TiO2: N/A Composite: N/A | Dosage: 0.20 g/L [Orange II]: 10 mg/L Time: 120 min pH: N/A Light: UV Volume: 50 mL | TiO2: N/A Composite: 93.40; k = 0.036 | [122] |
| SnO2/TiO2 | Ultrasonic-assisted impregnation | Time: N/A Power: N/A Freq.: N/A | 2.92 | TiO2: N/A Composite: N/A | Dosage: 0.20 g/L [Tetracycline]: 10 mg/L [Methylene blue]: N/A [Congo red]: N/A Time: 40 min (tetracycline); 135 min (methylene blue); 100 min (congo red) pH: N/A Light: Visible Volume: 20 mL | Tetracycline TiO2: N/A Composite: 98.76; k = 0.086 Methylene blue TiO2: N/A Composite: 99 Congo red TiO2: N/A Composite: 90 | [123] |
| SnO2/TiO2(A)/TiO2(R) | Ultrasonic-assisted alkaline hydrothermal | Time: 150 min Power: N/A Freq.: N/A | 2.72 | TiO2: N/A Composite: 78.50 | Dosage: N/A [Naphthalene]: 30 mg/L Time: 220 min pH: N/A Light: Visible (300 W) Volume: N/A | TiO2: 24.40; k = 0.001 Composite: 100; k = 0.038 | [124] |
| SnS2/TiO2 | Thermosolvent ultrasonication | Time: 240 min Power: N/A Freq.: 15 kHz | Absorption wavelength of 647 nm | TiO2: N/A Composite: 5.65 | Dosage: 0.50 g/L [Methyl orange]: 20 mg/L [Methyl blue]: 5 mg/L [Phenol]: 10 mg/L Time: 60 min pH: N/A Light: Visible (300 W) Volume: 400 mL | Methyl orange TiO2: N/A Composite: k = 0.009 Methyl blue TiO2: N/A Composite: k = 0.015 Phenol TiO2: N/A Composite: k = 0.003 | [125] |
| TiO2/BiOI/Ag3PO4 | Ultrasonication | Time: 10 min Power: N/A Freq.: N/A | N/A | TiO2: 48.10 Composite: 94.20 | Dosage: 0.40 g/L [Rhodamine b]: ~5 mg/L Time: 60 min pH: N/A Light: Visible (50 W) Volume: 250 mL | TiO2: N/A Composite: 99.30; k = 0.061 | [126] |
| TiO2/Bi2O3/CuO/zeolite | Ultrasonic-assisted wet impregnation | Time: 30 min Power: N/A Freq.: 50 kHz | 1.73 | TiO2: N/A Composite: 16.85 | Dosage: 0.40 g/L [Safranin-O]: 16 mg/L Time: 300 min pH: N/A Light: Sunlight Volume: 200 mL | TiO2: N/A Composite: 94.10 | [127] |
| TiO2/Carbon | Ultrasonication | Time: N/A Power: N/A Freq.: N/A | 2.77 | TiO2: 27.07 Composite: 49.27 | Dosage: 0.026 wt% [Rhodamine b]: N/A Time: 75 min pH: N/A Light: UV (400 W) Volume: 100 mL | TiO2: 54 Composite: 94 | [128] |
| TiO2/carbon nanotubes | Ultrasonic-assisted hydrothermal | Time: N/A Power: N/A Freq.: N/A | N/A | TiO2: N/A Composite: N/A | Dosage: 2 g/L [Methylene blue]: 30 mg/L Time: 180 min pH: 6.30 Light: Visible Volume: 100 mL | TiO2: N/A Composite: 84; k = 0.030 | [129] |
| TiO2/C3N4/TCP | Ultrasonication | Time: 30 min Power: 100 W Freq.: 20 kHz | Absorption wavelength between 400 and 700 nm | TiO2: N/A Composite: N/A | Dosage: 1 g/L [Rhodamine b]: 12 mg/L Time: 60 min pH: N/A Light: Visible (300 W) Volume: 10 mL | TiO2: 51.70 Composite: 97.70 | [130] |
| TiO2/Fe2O3 | Ultrasonic-assisted impregnation | Time: 60 min Power: N/A Freq.: N/A | 2.63 | TiO2: N/A Composite: N/A | Dosage: 0.003 g/L [p-nitrophenol]: 50 mg/L Time: Until complete degradation of p-nitrophenol pH: N/A Light: Solar (300 W) Volume: 300 mL | TiO2: 100% (70 min) Composite: 100% (60 min) | [131] |
| TiO2/g-C3N4 | Ultrasonic-assisted calcination | Time: N/A Power: 500 W Freq.: 40 kHz | 2.34 | TiO2: N/A Composite: N/A | Dosage: 0.50 g/L [Acetaminophen]: 5 mg/L Time: 30 min pH: N/A Light: Visible (300 W) Volume: 100 mL | TiO2: N/A Composite: 99.30 | [132] |
| TiO2/g-C3N4 | Ultrasonic-assisted alkali hydrothermal | Time: N/A Power: N/A Freq.: N/A | 3.00 | TiO2: N/A Composite: 140.73 | Dosage: 1 g/L [Methylene blue]: 60 mg/L Time: 240 min pH: N/A Light: Visible Volume: 50 mL | TiO2: N/A Composite: 97 | [133] |
| TiO2/g-C3N4 | Ultrasonic-assisted hydrothermal | Time: N/A Power: N/A Freq.: N/A | 2.88 | TiO2: N/A Composite: N/A | Dosage: 0.20 g/L [2-Chlorophenol]: 25 mg/L Time: Until complete degradation of 2-chlorophenol pH: N/A Light: UV-visible (250 W) Volume: 100 mL | TiO2: 100% (110 min) Composite: 100% (65 min) | [134] |
| TiO2/g-C3N4 | Ultrasonication | Time: 45 min Power: N/A Freq.: N/A | 2.97 | TiO2: 111 Composite: 155 | Dosage: 0.50 g/L [Indigo carmine]: 50 mg/L Time: 60 min pH: N/A Light: Visible Volume: 100 mL | TiO2: 24; k = 0.004 Composite: 98; k = 0.030 | [135] |
| TiO2/g-C3N4 | Ultrasonic-assisted hydrothermal | Time: N/A Power: N/A Freq.: N/A | 2.68 | TiO2: N/A Composite: 58.26 | Dosage: 0.20 g/L [Tetracycline]: 50 mg/L Time: 120 min pH: N/A Light: Visible (300 W) Volume: 100 mL | TiO2: N/A Composite: k = 0.008 | [74] |
| P90-TiO2/g-C3N4 | Ultrasonication | Time: 480 min Power: 150 W Freq.: 37 kHz | 2.53 | TiO2: N/A Composite: N/A | Dosage: 0.50 g/L (sunset yellow FCF); 1 g/L (phenol) [Sunset yellow FCF]: 10 mg/L [Phenol]: 30 mg/L Time: 5 min (sunset yellow FCF); 120 min (phenol) pH: N/A Light: Visible Volume: 100 mL | Sunset yellow FCF TiO2: k = 0.089 Composite: 98.80, k = 0.837 Phenol TiO2: k = 0.004 Composite: 99.35; k = 0.036 | [96] |
| TiO2/GO | Ultrasonication | Time: 60 min Power: N/A Freq.: N/A | Overlapping of the absorption edge of TiO2/GO with GO | TiO2: 49 Composite: 92 | Dosage: 0.10 g/L [Congo red]: 5 mg/L [Methyl orange]: 5 mg/L Time: 30 min pH: N/A Light: UV Volume: 100 mL | Congo red TiO2: N/A Composite: k = 0.065 Methyl orange TiO2: N/A Composite: k = 0.063 | [93] |
| TiO2/GO | Ultrasonication | Time: N/A Power: N/A Freq.: N/A | 3.13 | TiO2: N/A Composite: N/A | Dosage: 0.50 g/L [Methylene blue]: 0.01 mM [Crystal violet]: 0.01 mM Time: 10 min pH: N/A Light: Visible (12 W) Volume: 50 mL | Methylene blue TiO2: k = 0.135 Composite: 95; k = 0.325 Crystal violet TiO2: k = 0.162 Composite: 99; k = 0.494 | [136] |
| TiO2/In2O3 | Ultrasonic-assisted sol–gel | Time: N/A Power: 50 W Freq.: N/A | 2.92 | TiO2: N/A Composite: N/A | Dosage: 0.25 g/L [Acid blue]: 10 mg/L Time: 90 min pH: N/A Light: UV-visible (350 W) Volume: 100 mL | TiO2: k = 0.030 Composite: 98; k = 0.040 | [137] |
| TiO2/MoS2/BiVO4 | Ultrasonic-assisted hydrothermal | Time: 120 min Power: 750 W Freq.: 20 kHz | 2.84 | TiO2: 52.30 Composite: 132.70 | Dosage: 1 g/L [Tetracycline]: 20 mg/L Time: 90 min pH: N/A Light: Visible (300 W) Volume: 40 mL | TiO2: 52.30 Composite: 90.35 | [79] |
| TiO2/MWCNT | Ultrasonic-assisted sol–gel | Time: 60 min Power: N/A Freq.: 20 kHz | 2.75 | TiO2: 76.34 Composite: 194.35 | Dosage: 0.25 g/L [Methylene blue]: 5 mg/L [Rhodamine b]: 5 mg/L Time: 60 min pH: N/A Light: UV Volume: 200 mL | Methylene blue TiO2: 62.63; k = 0.019 Composite: 92.36; k = 0.051 Rhodamine b TiO2: 71.56; k = 0.022 Composite: 94.13; k = 0.053 | [138] |
| TiO2/MWCNT/Pani | Ultrasonic-assisted in situ polymerization | Time: N/A Power: N/A Freq.: N/A | 2.90 | TiO2: 120.05 Composite: 78.69 | Dosage: 1.50 g/L [Benzene]: 700 mg/L Time: 80 min pH: 6 Light: Visible (500 W) Volume: 10 mL | TiO2: N/A Composite: 84.92 | [139] |
| TiO2/RGO | Ultrasonic-assisted hydrothermal | Time: 60 min Power: N/A Freq.: N/A | 3.05 | TiO2: 41.20 Composite: 77.20 | Dosage: 1 g/L [Methylene blue]: 6.40 mg/L Time: 60 min pH: N/A Light: UV (6 W); sunlight (1pm and 3 pm in Cairo; 30.39w/m2) Volume: 50 mL | UV light TiO2: 28.01 Composite: 85.76 Sunlight TiO2: N/A Composite: 81.00 | [140] |
| TiO2/SiO2 | Ultrasonication coupled with alkali leaching | Time: 120 min Power: 160 W Freq.: N/A | Absorption wavelength of 763 nm | TiO2: N/A Composite: N/A | Dosage: 0.20 g/L [2,4-dinitrophenol]: 20 mg/L Time: 120 min pH: N/A Light: Xenon (150 W) Volume: 100 mL | TiO2: N/A Composite: k = 0.080 | [98] |
| TiO2/TPPS | Ultrasonication | Time: 30 min Power: 100 W Freq.: 20 kHz | 2.87 | TiO2: N/A Composite: N/A | Dosage: 0.10 g/L [Eosin yellow]: 10 mg/L Time: 50 min pH: N/A Light: Visible Volume: 100 mL | TiO2: k = 0.028 Composite: 99; k = 0.090 | [24] |
| TiO2/WO3 | Ultrasonication and microwave | Time: 60 min Power: 750 W Freq.: 20 kHz | 2.73 | TiO2: 260 Composite: 235 | Dosage: 0.50 g/L [Ciprofloxacin]: 20 mg/L [Oxytetracycline]: 20 mg/L Time: 45 min (UV light); 120 min (sunlight) for ciprofloxacin 60 min (UV light); 120 min (daylight) for oxytetracycline pH: N/A Light: UVA (20 W); sunlight Volume: 200 mL | Ciprofloxacin TiO2: 100; k = 0.039 (UV light) 45; k = 0.005 (sunlight) Composite: 100; k = 0.133 (UV light) 96; k = 0.034 (sunlight) Oxytetracycline TiO2: 91; k = 0.041 (UV light) 55; k = 0.007 (sunlight) Composite: 100; k = 0.082 (UV light) 97; k = 0.028 (sunlight) | [141] |
| TiO2/WO3 | Ultrasonic-assisted sol–gel | Time: 10 min Power: 22 kHz Freq.: 190 W | 2.60 | TiO2: N/A Composite: 65.39 | Dosage: 0.50 g/L [Congo red]: 20 mg/L [Methyl red]: 20 mg/L Time: 120 min pH: N/A Light: Visible (250 W) Volume: 100 mL | Congo red TiO2: N/A Composite: 95 Methyl red TiO2: N/A Composite: 100 | [39] |
| TiO2/ZnIn2S4 | Ultrasonic-assisted hydrothermal | Time: 60 min Power: N/A Freq.: N/A | Absorption wavelength of 436 nm | TiO2: N/A Composite: N/A | Dosage: N/A [Rhodamine b]: N/A Time: 120 min pH: N/A Light: Visible (300 W) Volume: N/A | TiO2: 37.40; k = 0.004 Composite: 90.20; k = 0.033 | [73] |
| TiO2/ZnO | Ultrasonic-assisted precipitation | Time: 10 min Power: N/A Freq.: N/A | Around 2.30 | TiO2: N/A Composite: N/A | Dosage: 4 g/L [Methyl orange]: 30 mg/L Time: N/A pH: N/A Light: UV (15 W) Volume: 20 mL | TiO2: 36.20 Composite: 58.70 | [142] |
| Zeolite/CdS/TiO2/CeO2 | Ultrasonic-assisted impregnation | Time: N/A Power: N/A Freq.: N/A | 2.10 | TiO2: N/A Composite: N/A | Dosage: 1.50 g/L [Methylene blue]: 10 mg/L Time: 120 min pH: 8 Light: Visible (85 W) Volume: 100 mL | TiO2: 28 Composite: 99.90 | [81] |
| ZnFe2O4/TiO2 | Solvothermal followed by ultrasonication | Time: 90 min Power: N/A Freq.: N/A | 2.03 | TiO2: 12.73 Composite: 54.81 | Dosage: 0.30 g/L [Rhodamine b]: 10 mg/L Time: 60 min pH: N/A Light: UV (500 W) Volume: N/A | TiO2: 94.90; k = 0.045 Composite: 97.30; k = 0.062 | [99] |
| ZnFe2O4/TiO2/Ag2O | Solvothermal, followed by ultrasonication and chemical precipitation | Time: 90 min Power: N/A Freq.: N/A | Absorption wavelength of 702 nm | TiO2: N/A Composite: 120.90 | Dosage: 0.30 g/L [Rhodamine b]: 10 mg/L Time: 40 min pH: N/A Light: UV (500 W) Volume: 500 mL | TiO2: N/A Composite: 98.40; k = 0.094 | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, J.H.F.; Kong, E.D.H.; Chia, J.C.; Lai, C.W.; Juan, J.C.; Li, Y.; Xiang, P.; Badruddin, I.A.; Kumar, A. Ultrasonic-Assisted Fabrication of TiO2-Based Composite Photocatalysts for Enhanced Photocatalysis of Organic Pollutants: A Review. Catalysts 2025, 15, 1010. https://doi.org/10.3390/catal15111010
Chau JHF, Kong EDH, Chia JC, Lai CW, Juan JC, Li Y, Xiang P, Badruddin IA, Kumar A. Ultrasonic-Assisted Fabrication of TiO2-Based Composite Photocatalysts for Enhanced Photocatalysis of Organic Pollutants: A Review. Catalysts. 2025; 15(11):1010. https://doi.org/10.3390/catal15111010
Chicago/Turabian StyleChau, Jenny Hui Foong, Ethan Dern Huang Kong, Jing Chang Chia, Chin Wei Lai, Joon Ching Juan, Yue Li, Ping Xiang, Irfan Anjum Badruddin, and Amit Kumar. 2025. "Ultrasonic-Assisted Fabrication of TiO2-Based Composite Photocatalysts for Enhanced Photocatalysis of Organic Pollutants: A Review" Catalysts 15, no. 11: 1010. https://doi.org/10.3390/catal15111010
APA StyleChau, J. H. F., Kong, E. D. H., Chia, J. C., Lai, C. W., Juan, J. C., Li, Y., Xiang, P., Badruddin, I. A., & Kumar, A. (2025). Ultrasonic-Assisted Fabrication of TiO2-Based Composite Photocatalysts for Enhanced Photocatalysis of Organic Pollutants: A Review. Catalysts, 15(11), 1010. https://doi.org/10.3390/catal15111010









