Abstract
In recent years, one of the major problems facing humanity has been the contamination of the environment by various organic pollutants, with some of them exhibiting environmental persistence or pseudo-persistence. For this reason, it is necessary today, more than ever, to find new and effective methods for degrading these persistent pollutants. Transition metal selenides (TMSes) have emerged as a versatile and promising class of catalysts for the degradation of organic pollutants through various advanced oxidation processes (AOPs). The widespread use of these materials lies in the desirable characteristics they offer, such as unique electronic structures, narrow band gaps, high electrical conductivity, and multi-valent redox behavior. This review comprehensively examines recent progress in the design, synthesis, and application of these TMSes—including both single- and composite systems, such as TMSes/g-C3N4, TMSes/TiO2, and heterojunctions. The catalytic performance of these systems is being highlighted, regarding the degradation of organic pollutants such as dyes, pharmaceuticals, antibiotics, personal care products, etc. Further analysis of the mechanistic insights, structure–activity relationships, and operational parameter effects are critically discussed. Emerging trends, such as hybrid AOPs combining photocatalysis with PMS or electro-activation, and the challenges of stability, scalability, and real wastewater applicability are explored in depth. Finally, future directions emphasize the integration of multifunctional activation methods for the degradation of organic pollutants. This review aims to provide a comprehensive analysis and pave the way for the utilization of TMSe catalysts in sustainable and efficient wastewater remediation technologies.
1. Introduction
One of the most important environmental problems nowadays is the lack and deterioration of water quality [1]. Modern production activities, the increasing needs of a growing population, and rising standards have led to increased demand for water of high quality [2]. But with more industrial processes, agricultural discharges, and municipal waste, the entry of organic contaminants into water bodies is ongoing, with some of them being pharmaceuticals [3,4], dyes [5,6], pesticides [7], personal care products [4,8], and several others [1]. Although in the last two decades the issue of water resource protection has been of greater importance, the need to tackle current threats to water contamination and deterioration is of vital priority. For this reason, the scientific community has turned its attention to efforts for finding new and effective ways of water remediation, with the main ones being adsorption [9,10,11,12], membrane separation [13,14], ion exchange [15], chemical precipitation [16], and others. However, such conventional decontamination methods often fail to remove or degrade organic pollutants completely from water bodies, thereby leading to the environmental accumulation of these, resulting in contamination of aquatic media, aquatic life damage, and harmful effects on organisms of all environmental compartments [1,17,18,19,20]. Thus, considerable attention has been paid to advanced oxidation processes (AOPs) as emerging and more effective decontamination technologies.
Since traditional water and wastewater treatment processes are often inadequate in eliminating the described pollutants, heterogeneous catalysis has emerged as a promising approach to degrade recalcitrant pollutants by catalyzing oxidation and reduction reactions. AOPs represent among the most effective decontamination technologies used in the past decade to degrade these persistent pollutants from environmental matrices. It is a family of methods, based on the formation and use of so-called reactive oxygen species (ROS), that induce the oxidation of the complex and toxic organic molecules listed above, occasionally inducing the process to their complete mineralization [21,22,23,24]. Their wide-scale usage by the scientific community for decontamination of environmental matrices, and, in particular, of aqueous matrices, relies mostly on the multitude of advantages it has over other decontamination technologies [25].
AOPs can be categorized into homogeneous and heterogeneous systems. While in homogeneous catalysis, catalysts operate in the same phase as the reactants and often show high reaction rates, they face significant drawbacks, such as difficulty in separation, catalyst recovery, and poor long-term stability. In contrast, heterogeneous catalysis, where the catalyst is in a different phase (a solid interacting with aqueous-phase pollutants), offers notable advantages, including ease of recovery, structural stability, and reusability. These characteristics make heterogeneous systems particularly desirable for industrial-scale applications. Among the various heterogeneous AOPs, interest from the scientific community has been placed in the use of a catalyst to initiate radical-driven processes for pollutant degradation. One of the most extensively studied AOPs is photocatalysis, where semiconductors are activated by light to produce electron–hole pairs [26,27] which can interact with surface-adsorbed species and lead to the formation of ROS, such as hydroxyl radicals (•OH), superoxide radicals (O2•−), and singlet oxygen (1O2), that degrade pollutants into harmless products such as CO2 and H2O [28,29]. Another category, chemical AOPs, is based on the direct chemical activation of oxidants and relies on surface-active catalysts (generally transition metals) to initiate ROS formation. The effectiveness of these methods depends mainly on the types of ROS formed through the activation of oxidants. For instance, sulfate radical-based advanced oxidation processes (SR-AOPs) are based on the activation of oxidants, such as persulfate (PS or PDS, S2O82−) and peroxymonosulfate (PMS, HSO5−), and lead to the formation of ROS, such as sulfate radicals and hydroxyl radicals [30,31,32,33,34,35]. Additionally, electrochemical AOPs (E-AOPs) rely on the creation of an electrochemical system to generate in situ highly reactive species in order to degrade or convert organic pollutants into smaller and non-toxic molecules [36,37]. Sonocatalytic AOPs, driven by ultrasonic cavitation, take advantage of the energy of soundwaves of frequencies of 300–1000 kHz to induce chemical reactions by exploiting the phenomenon of acoustic cavitation, i.e., the rapid formation, growth, and collapse of cavitation bubbles in a medium (solid, liquid, or gas), generating extreme local temperatures and pressures with the end result being the formation of ROS through a series of chemical reactions from collapsing of cavitation bubbles [38,39].
1.1. Fundamental Aspects of Selenide-Based Catalysts
As mentioned earlier, the efficiency of heterogeneous AOPs is closely linked to the use of appropriate catalysts, which play a crucial role in the formation of the ROS needed to drive the system toward pollutant degradation. Transition metal-based catalysts such as iron [40,41,42,43,44], cobalt [45,46,47,48,49,50], nickel [51,52,53,54], and copper compounds [55,56,57,58,59,60] have been widely investigated in the literature as promising candidates for application in AOPs as decontamination technologies. However, growing attention in recent years has been given to a particular class of catalysts: transition metal chalcogenides (TMCs) and, more specifically, transition metal selenides (TMSes).
Transition metal chalcogenides (TMCs) are a group of compounds characterized by the general chemical formula MX, with M being a transition metal atom and X a chalcogen atom. Chalcogens are in group 16 of the periodic table and include oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). Chalcogens exhibit different metallic properties. Descending the periodic table, the atomic number increases, leading to a transition from nonmetals (O and S) to metalloids (Se and Te) and, finally, metals (Po). Therefore, chalcogens can be used to synthesize metal catalysts, resulting in metal chalcogenides with different properties depending, each time, on the combination of the transition metal atom and the chalcogen atom [61,62,63,64,65,66,67].
TMSes are a subgroup of TMCs where selenium is the chalcogen. This class of compounds is identified by the simple chemical formula MSe and can be further classified into stoichiometric categories, which cover the materials with the chemical formulas MSe, M2Se, MSe2, and MxSey, and a non-stoichiometric category, which covers the materials with the chemical formula M1−xSe [68]. The crystal structure of metal selenides (MSes) can be classified into layered (groups IVB-VIIB of the periodic table) and non-layered structures (group VIII of the periodic table) [69]. The structure of metal selenides plays a crucial role in their catalytic properties. Stoichiometry and the crystal phase of MSes can significantly affect their bandgap and thermal stability [70].
On the other hand, the crystal structure of transition metal diselenides (TMDSes, MSe2) can be classified into layered and pyrite structures. Generally, when M is a transition metal from groups IV–VI of the periodic table, the favored structure is the layered one, while, in the case where the transition metal M is from groups VII–VIII of the periodic table, the favored structure is the pyrite-type crystal [71]. There are notable differences between these two structures; particularly, layered structures display significant anisotropies in their electrical, chemical, mechanical, and thermal properties [72,73], unlike the cubic symmetry of pyrite structures. Additionally, the bonding of Se-M differs in these two structures, as Van der Waals interactions are observed in the layered structure, while, in pyrite structures, covalent interactions prevail [71]. As indicated by Figure 1, these materials exist in various crystalline phases, the primary ones being trigonal, hexagonal, and rhombohedral [74].
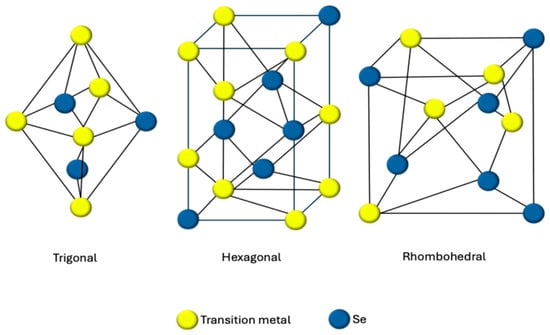
Figure 1.
Schematic illustration of the primary crystalline phases of TMSes (trigonal, hexagonal, and rhombohedral).
The electronic structure and crystal phase of TMSes depend not only on the number of electrons in the d-orbital but also on the coordination environment of the transition metals. The gradual filling of non-bonding d-bands in transition metals from groups IVB to VIIB and VIII of the periodic table affects the electronic properties of the TMSes [73,75,76,77]. For example, materials that have fully occupied d-orbitals, such as MoSe2, have semiconductor properties, while materials that have partially filled d-orbitals, such as NbSe2, have metallic properties [78,79]. The coordination environment also affects the preferred structure of the synthesized TMSes, with the octahedral structure formed in cases where the M position of the ΤMSEs is occupied by transition metals of group IVB, triagonal prismatic and octahedral when occupied by transition metals of group VB, triagonal prismatic when occupied by transition metals of group VIB, distorted octahedral when occupied by transition metals of group VIIB, and octahedral when occupied by transition metals of group VIIB [69].
As a result, TMSes exhibit a diverse range of electronic, structural, and catalytic properties, rendering them important in a wide range of applications. In electrocatalysis, for example, for hydrogen evolution reactions (HER) and oxygen reduction reaction (ORR) in water splitting, CoSe2 is one of the most studied transition metal selenides [80]; Swesi et al. [81] reported the superiority of NiSe2 over different nickel-based selenides in water-splitting applications, and MoSe2 is one of the most affordable and effective electrocatalysts in HER [82]. In energy storage applications, due to their energy density and charge-carrying properties, TMSes have replaced numerous expensive conventional electrodes in batteries, photovoltaics, supercapacitors, etc. [83,84]. Additionally, copper, vanadium, and molybdenum-based selenides, due to their high surface areas, active surface sites, and quick carrier mobility, have been utilized in sensors, exhibiting exceptional detection limits and range capabilities [85,86,87,88,89]. Furthermore, TMSes with tunable bandgap energy and charge carrier separation are effective photocatalysts for environmental remediation and pollutant degradation.
To fully exploit the properties of TMSes for the applications mentioned, it is crucial to carefully consider the synthetic methods employed to ensure the final materials possess the desired characteristics, such as morphology, phase, etc. In recent years, hydrothermal synthesis has been the most important and simple method for the preparation of TMSes. Hydrothermal synthesis is used for controlling the synthesis of a variety of nanostructured selenides, such as MoSe2 [90,91], FeSe2 [92,93], CoSe2 [94,95], NiSe2 [96,97], CdSe [98,99], CuSe [100], and ZnSe [101], by adding metal sources, selenium precursors, and reducing agents (hydrazine hydrate or NaBH4), usually in an autoclave, and reacting under high temperature and pressure conditions. By varying parameters such as temperature, reaction time, pH, and precursor compounds, the final products exhibit distinct morphological characteristics. Solvothermal synthesis differs from hydrothermal synthesis as it utilizes an organic solvent instead of water. This alteration permits reactions that cannot occur under standard conditions. Another synthetic method that is mainly used for layered TMSes in electrochemical storage applications and catalysis is liquid phase exfoliation (LPE) [73,102]. Exfoliation of the TMSes is carried out in order to overcome the Van der Waals forces between the layers of the TMSes so that the final monolayer or few-layer structured materials can retain the properties, and new properties emerge due to confinement effects [103,104]. This synthetic method is one of the most effective methods for preparing two-dimensional materials by blending TMSes with suitable solvents and polymers [74]. Electrodeposition is also a synthetic method for TMSes that are used in energy storage applications and electrocatalysis [105,106,107,108,109,110]. Important influences on the properties and morphological characteristics of the final product are parameters such as the concentration of the precursors, the potential of the electric field, the deposition time, the temperature, and the pH of the electrolyte [81,108,111]. Chemical vapor deposition (CVD) is a simple, controllable, and widespread technique for synthesizing TMSe films or three-dimensional structures through reactions between Se vapor carried by gas and metal substrates or precursors within a high vacuum quartz tube [74,112,113]. These methods represent some of the primary approaches for synthesizing TMSes, and the desired properties must be meticulously considered based on the intended application to select the optimal synthetic route, as this choice profoundly impacts the properties and morphological characteristics of the final product.
1.2. Scope of the Review
Taking into consideration all the properties of TMSes mentioned above, such as unique electronic structure, tunable band gap, and high catalytic activity, it is evident that TMSes can also be used in decontamination technologies for the degradation of organic pollutants, mainly in aqueous substrates. Previous reviews of TMSes have primarily focused on energy-related applications such as electrocatalysis for HER and OER (oxygen evolution reaction) [74,114,115,116,117,118,119], supercapacitors [120,121,122,123,124], solar cells [125], and rechargeable batteries [126,127,128,129]. While these reviews have comprehensively analyzed the electronic structures, synthetic strategies, and energy-related functionalities of these materials, only limited attention has been given to their role in environmental catalysis, which is restricted to photocatalytic degradation [69], and the discussion regarding TMSe-based composite catalysts has been fragmented. To date, no systematic review regarding TMSe-based catalysts for pollutant removal through AOPs has been presented. Therefore, this work aims to fill this gap and provide a comprehensive analysis of the use of TMSEs and their composites in AOPs, shedding light on the synthetic methods used to fabricate the catalysts with the desired characteristics, and analyzing the mechanisms that contribute to the degradation efficiency, as well as their stability and imminent use for large-scale environmental applications.
2. Classification of Selenide-Based Catalysts Used for the Degradation of Organic Pollutants
2.1. Single-Metal Selenide Catalysts
Regarding the use of single metal selenide catalysts, the first research study comes from Durairaj et al., 2019 [130], who aimed to study the performance of a new selenide-based catalyst for the degradation of a dye, Reactive Red 120 (RR120), and a phenolic compound, 4-nitrophenol, using sulfate radical-based technologies. For this reason, they employed a one-pot hydrothermal synthesis for the growth of a high-purity hierarchical Cu2Se nanostructure film on a Cu foil, with copper having a dual role, acting as a substrate as well as a source of copper for the reaction. They chose copper as the transition metal because it has been reported as an effective activator of persulfate [131,132] and also because copper selenides can give a plethora of nanostructures, such as nanorods, nanowires, nanospheres, and hexagonal nano powders [133].
After conducting degradation experiments of RR120 in the presence of PMS alone and in the presence of Cu2Se alone, it was observed that neither was capable of leading to the degradation of the dye on its own. However, this was not the case when both PMS and Cu2Se were present simultaneously. The Cu2Se/PMS system resulted in the complete degradation of RR120 in just 2 h, proving that Cu2Se is an excellent activator of PMS for the degradation of organic pollutants. With the help of appropriate scavengers (TBA for •OH and MeOH for •OH and SO4•−), insight was gained into the types of ROS contributing to the degradation process, indicating the predominance of SO4•− in the degradation of RR120 by the Cu2Se/PMS system, while also presenting the possible mechanism of PMS activation by Cu2Se (Figure 2 demonstrates the general activation mechanism of PMS, which is also described by Reactions (1)–(8)).
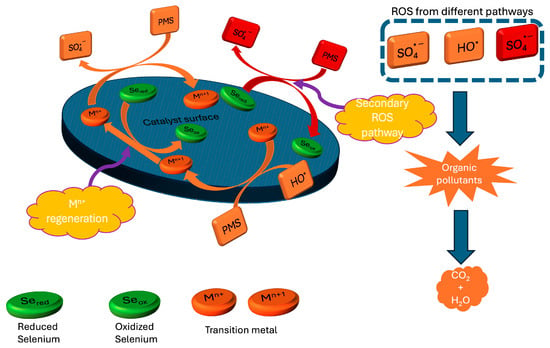
Figure 2.
General activation mechanism of PMS by transition metals in heterogeneous catalysis.
Radical pathway:
Mn+ + HSO5− → M(n + 1)+ + SO4•− + HO−
M(n + 1)+ + HSO5− → Mn+ + SO5•− + H+
SO4•− + H2O → SO42− + HO• + H+
SO4•− + HO− → SO42− + HO•
Secondary pathway (Se-assisted):
Se(red) + HSO5− → Se(ox) + SO4•− + HO−
Se(ox) + HSO5− → Se(red) + SO5•− + H+
Mn+ regeneration pathway:
M(n+1)+ + Se(red) → Mn+ + Se(ox) + SO42−
M(n + 1)+ + SO4•− → Mn+ + SO42−
The influence of factors, such as PMS concentration and pH, on the degradation process of the RR120 dye was also studied, as the activation of PMS by metal catalysts is a pH-dependent reaction [134]. An increase in PMS concentration from 0.01 mM to 1 mM led to a significant increase in dye degradation, which was attributed to the presence of a greater number of ROS contributing to the degradation process. Regarding the effect that pH had on the degradation of RR120 by the Cu2Se/PMS system, although the PMS activation could occur over a wide pH range (2–12), the authors observed that maximum system performance, with the maximum reaction rate constant, occurred at pH = 10, while the minimum reaction rate constant occurred at acidic pH. Regarding the stability of Cu2Se nanostructure film for its use in SR-AOPS, it was used for four consecutive catalytic degradation cycles of RR120, with its activity, however, decreasing, as degradation rates were 99.5% (1st cycle) ⟶ 82.2% (2nd cycle) ⟶ 79.8% (3rd cycle) ⟶ 61.2% (4th cycle) for the same reaction time (2 h). In addition, the oxidation degradation pathway via MS was studied in this article, presenting the degradation of RR120 into smaller fragments that are finally mineralized to CO2 and H2O. Also, the degradation of 4-nitrophenol by the Cu2Se/PMS system was studied, with the results being encouraging as an 80% degradation rate occurred over three hours, while, with the help of XRD, the crystalline stability of the Cu2Se catalyst was confirmed compared to the fresh Cu2Se catalyst. Finally, the research team presented another application of Cu2Se, electrocatalytic HER activity, and even showed greater electrocatalytic HER activity compared to pure Cu foil, which was attributed to higher charge transfer efficiency, and reported that even after 500 cycles, there was no decrease in its effectiveness.
Fang et al., 2020 [135] studied the use of FeSe2 to activate various oxidants (PMS, PS, and H2O2) for organic pollutants degradation. As in the previous case, the hydrothermal method was used to synthesize a highly crystalline FeSe2 without any impurities. Initially, to test the activation ability of PMS by FeSe2, 2,4,4′-Trichlorobiphenyl (PCB28) degradation experiments were performed. The results were particularly encouraging as FeSe2 was able to activate PMS and thus the FeSe2/PMS system led to 95% degradation of PCB28 in 3 h (Table 1). Following this, factors such as the concentration of oxidant and different FeSe2 loadings were evaluated. A positive correlation was found between the increase in catalyst loading (0.1 g/L ⟶ 2.0 g/L), PMS concentration (0.5 mM ⟶ 1.0 mM), and PCB28 degradation. However, a further increase in the oxidant concentration (1.0 mM ⟶ 5.0 mM), combined with the limited amount of the catalyst, acted in an inhibitive way due to the reaction of the produced active species with the excess PMS. Using EPR and appropriate quenching agents (EtOH and TBA), they observed the formation of •OH and SO4•− from PMS activation; however, the dominant role in PCB28 degradation was attributed to •OH. Additionally, by using NaF to determine whether aquatic or surface-bound radicals were the dominant species, as they usually coexist during PMS activation [136], the authors concluded that surface-bound •OH contributed to the degradation process. Regarding the mechanism of PMS activation and the formation of the active species leading to PCB28 degradation, the authors reported a synergy between Se and Fe species, as Se species (acting as electron donors) can reduce Fe(III) to Fe(II) on the surface of FeSe2, as the dominant pathway for ROS generation. The presence of natural organic matter (NOM), specifically humic acid (HA) and inorganic ions (CO32−, NO3−, and Cl−), had little or no effect on PCB28 degradation, indicating that the FeSe2/PMS system can be used in environmental samples. Concerning the reuse of the FeSe2 catalyst, encouraging results were observed due to its increased stability and low Fe leaching after five consecutive runs, while, in the case of the washing of the catalyst in order to remove the intermediate degradation byproducts from its surface, its activity returns to its original levels. Finally, as shown by Table 1 data, FeSe2 catalyst was effectively used to degrade other pollutants and activate other oxidants (PS and H2O2), but the results were clearly better in the case of PMS activation.
Dong et al., 2021 [137] studied the application of another TMDSe, commercially available MoSe2, in light-driven peroxymonosulfate activation and degradation of pharmaceuticals, presenting a comprehensive in-depth study. Their interest in this particular TMDSe mainly lies in its features, such as low band gap, crystalline structure, excellent electrochemical properties, and thermal stability [138]. The influence of various factors on the catalytic activity of the MoSe2/PMS system was first studied for the degradation of a commonly detected emerging contaminant, carbamazepine (CBZ). Regarding the pH and temperature of visible-light PMS activation, it was observed that maximum degradation is achieved at a temperature of 25 °C and pH = 4. Factors such as catalyst dosage and PMS significantly affected the system, with an increase in MoSe2 concentration up to a certain point (0.1 g/L ⟶ 0.3 g/L) leading to an increase in catalytic activity, while a further increase (0.3 g/L ⟶ 0.5 g/L) was inhibitory, as it would have weakened the light absorption. Moreover, an increase in PMS concentration (0.3 mM ⟶ 1.8 mM) also positively influenced CBZ degradation.

Table 1.
Overview of the degradation of various organic pollutants by simple selenide catalyst. All experiments were carried out under ambient temperature conditions, unless otherwise explicitly reported in the corresponding references.
Table 1.
Overview of the degradation of various organic pollutants by simple selenide catalyst. All experiments were carried out under ambient temperature conditions, unless otherwise explicitly reported in the corresponding references.
| Catalyst | Synthesis Method | Pollutant | Catalyst (g L−1) | Pollutant (mg L−1) | Oxidant (mM) | % Degradation (Time) | TOC Removal | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cu2Se | One-pot hydrothermal | RR120 | - | - | 1 (PMS) | 99.5% (2 h) | - | [130] |
| 4-Nitrophenol | - | - | 1 (PMS) | 80% (3 h) | - | |||
| FeSe2 | Hydrothermal | SMX | 0.5 | 20 | 1 (PMS) | 100% (2 h) | 51.2% (2 h) | [135] |
| PFOA | 0.5 | 10 | 5 (PMS) | 100% (3 h) | 20.3% (2 h) | |||
| BPA | 0.5 | 20 | 1 (PMS) | 100% (2 h) | 58.9% (2 h) | |||
| CTC | 0.5 | 20 | 1 (PMS) | 100% (2 h) | 46.3% (2 h) | |||
| FeSe2 | Hydrothermal | PCB28 | 0.5 | 1 | 1 (PS) | 92% (4 h) | - | [135] |
| 0.5 | 1 | 1 (H2O2) | 46% (4 h) | - | ||||
| BPA | 0.5 | 20 | 1 (PS) | 93% (4 h) | - | |||
| 0.5 | 20 | 1 (H2O2) | 95% (4 h) | - | ||||
| FeSe2 | Hydrothermal | PCB28 | 0.5 | 1 | 1 (PMS) | 95% (3 h) | - | [135] |
| FeSe2 (FS-1) | Wet chemical | CR | 2.5 | ~10 | H2O2 | 84.28% (28 h) | - | [139] |
| FeSe2 (FS-2) | CR | 2.5 | ~10 | H2O2 | 90.94% (28 h) | - | ||
| FeSe2 (FS-3) | CR | 2.5 | ~10 | H2O2 | 89.74% (28 h) | - | ||
| FeSe2 (FS-1) | Wet chemical | MB | 2.5 | ~11 | H2O2 | 33.4% (28 h) | - | [139] |
| FeSe2 (FS-2) | MB | 2.5 | ~11 | H2O2 | 93.3% (28 h) | - | ||
| FeSe2 (FS-3) | MB | 2.5 | ~11 | H2O2 | 25.5% (28 h) | - | ||
| CdSe | Solvothermal | MB | - | - | - | 75% (3 h) | - | [140] |
| CdSe | Hydrothermal | MB | - | - | - | 80% (3 h) | - | [141] |
| MoSe2 | Purchased | CBZ | 0.3 | 2 | 1.2 (PMS) | 100% (30 min) | 60% (30 min) | [137] |
| IBU | 0.3 | 2 | 1.2 (PMS) | 38% (30 min) | - | |||
| BZP | 0.3 | 2 | 1.2 (PMS) | 100% (10 min) | 80% (30 min) |
RR120: Reactive Red 120, SMX: Sulfamethoxazole, PFOA: Perfluorooctanoic acid, BPA: Bisphenol A, CTC: Chlortetracycline, PCB28: 2,4,4′-Trichlorobiphenyl, CR: Congo red, MB: Methylene blue, CBZ: Carbamazepine, IBU: Ibuprofen, BZP: Benzophenone-3.
The use of visible light in either the PMS system or the MoSe2/PMS system significantly enhanced the degradation of CBZ (PMS + visible light > PMS and MoSe2/PMS + visible light > MoSe2/PMS), concluding that photo-generated electrons participate in the activation of PMS (Figure 3 and Reactions (9)–(16)). To determine whether MoSe2/PMS/visible light can be used in real sewage treatment, given that it contains a plethora of cations (Na+, Mg2+, K+, and Ca2+) and anions (PO43−, SO42−, NO3−, and Cl−), the effect of these on CBZ degradation was studied. Both the presence of the above anions and cations inhibited the degradation of CBZ, with the cations, due to electrostatic interactions, being adsorbed onto the negatively charged surface of MoSe2 [142], while the anions competed for the reactive species [132]. An important factor in the implementation of this system is its stability and reuse. The results were particularly encouraging as not only the stability of the crystalline structure of MoSe2 was confirmed after its implementation, but it was also capable of being used for five consecutive catalytic degradation cycles of CBZ, although a gradual decrease in system performance was observed, which was attributed to the transformation of Μο4+ to Μο6+/Μο5+. Additionally, 0.52 ppm of leached Mo ions, and also the release of a small amount of H2Se into the aqueous solution, were observed.
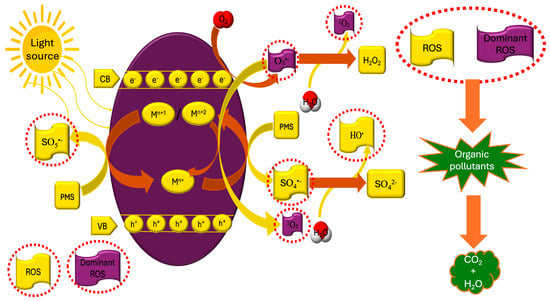
Figure 3.
Photocatalytic activation of PMS by TMSes.
In order to shed light on the type of reactive species formed that led to the degradation of CBZ, suitable scavengers (MeOH for •OH and SO4•−, isopropanol for •OH, p-benzoquinone for O2•−, tryptophan for 1O2, and Μο6+ for electrons) were used and reported the predominance, initially, of O2•−, which were formed by the reaction of photo-generated electrons and charge carrier (electrons and holes) with oxygen, and then 1O2 from the transformation of O2•−. In addition, based on their results and on the literature regarding the degradation pathway of CBZ [143], they also reported two possible pathways, with the first involving conversion of CBZ to iminostilbene, and then carrying out carboxylation and ketonization, and the second one involving ring-opening, cyclization, and dihydroxylation of hydroxylated CBZ intermediates.
Photoexcitation of the catalyst: MSe + hv → e− + h+
Superoxide formation: O2 + e− → O2•−
Metal-assisted PMS activation: Mn+ + HSO5− → M(n + 1)+/M(n + 2)+ + SO4•− + HO−
Metal regeneration pathway: M(n + 1)+/M(n + 2)+ + e− → Mn+
Alternative PMS activation: M(n + 1)+/M(n + 2)+ + HSO5− → Mn+ + SO5•− + H+
Secondary ROS production: SO4•− + H2O → SO42− + HO• + H+
Singlet oxygen formation: 2O2•− + 2H2O → H2O2 + 2HO− + 1O2
Metal-assisted formation of singlet oxygen: M(n+2)+ + O2•− → Mn+ + 1O2
Finally, MoSe2 as a co-catalyst in Fe(III)/PMS or Fe(II)/PMS systems was also investigated, on the grounds that oxidation between Mo4+ ions and Fe3+ ions could enhance the degradation efficiency and reduce the iron sludge. The results were positive in terms of the degradation of IBU and BZP, but not CBZ, by the Fe(II)/MoSe2/PMS system, compared to the Fe(II)/PMS system. Similar results were observed in the case of the Fe(IIΙ)/MoSe2/PMS system compared to the Fe(III)/PMS system, i.e., enhancing the degradation of IBU and BZP, but not CBZ.
Another study was presented by Karuppasamy et al. [139], who investigated the effect of precursors on the synthesis of FeSe2 catalysts for the purpose of utilizing the electrocatalytic oxidative process through electro-Fenton treatment under UV light for the detoxification of the organic dye pollutants Congo red (CR) and methylene blue (MB). The synthesis of FeSe2 was carried out by a wet chemical process and used three different precursors as Se sources [Se powder (FS-1), H2SeO3 (FS-2), and SeO2 (FS-3)]. The results were quite interesting because, based on XRD and Raman, the diffraction patterns of the synthesized nanostructured FS-1, FS-2, and FS-3 were identical and exhibited an orthorhombic ferroselite structure; however, there were significant differences in the morphology and purity of these catalysts. Specifically, the FS-1 catalyst exhibited agglomerated microgranular particles with a specific surface area of 37.50 m2g−1, the FS-2 catalyst displayed a combination of nano-flower and nano-stick-like morphologies with a specific surface area of 74.68 m2g−1, and the FS-3 catalyst consisted of irregular nanoflakes with a specific surface area of 57.01 m2g−1. They also demonstrated significant differences in their catalytic activity regarding dye removal (CR and MB) via electro-Fenton degradation. Specifically, the catalytic activity in CR detoxification followed the trend FS-2 > FS-3 > FS-1, while in MB detoxification it followed the trend FS-2 > FS-1 > FS-3, as shown in the data in Table 1. The superiority of FS-2 in the effective degradation of these dyes over other catalysts was attributed to better oxygen-donating properties, high surface area, and higher catalytic nature, while they also reported that the formation of hydroxyl radicals (on the catalyst surface and solution) from redox pair Fe(II)/Fe(III) played an important role in the degradation mechanism of the FeSe2/H2O2 system.
Two examples of CdSe used for photocatalytic degradation of MB dye were originally presented by Venci et al., 2022 [140]. In the first case, the effect of temperature on the solvothermal synthesis of CdSe was studied with a view to its subsequent application in the photocatalytic degradation of MB [140]. In the second case, the effect of different molar concentrations of Se and Cd precursors on the hydrothermal synthesis of CdSe was examined [141].
In researching the impact of synthesis temperature, solvothermal synthesis was performed at three different temperatures: 100 °C, 150 °C, and 180 °C. Based on XRD, the diffraction patterns of the synthesized CdSe were identical and presented a wurtzite type of hexagonal structure. As the temperature increased, there was a corresponding increase in the intensities of the peaks of the diffraction patterns. A positive correlation was also found in the composition temperature and particle size, as a gradual increase in temperature led to an increase in particle size [19.32 nm (100 °C) < 26.31 nm (150 °C) < 31.26 nm (180 °C)], while a gradual decrease was observed in band gap energy [2.23 eV (100 °C) < 2.21 eV (150 °C) < 2.18 eV (180 °C)]. Consequently, a gradual increase in synthesis temperature led to a CdSe of larger particle size and smaller band gap energy. Finally, its photocatalytic activity was confirmed by the degradation of MB from the produced superoxide and hydroxyl radicals, and it was carried out at a rate of 75% in 3 h.
In the second case, Venci et al., 2022 [141] kept the temperature constant at 180 °C and studied the effect of different molar concentrations of Se and Cd precursors (Cd precursor:Se precursor, 0.1:0.05 and 0.01:0.005) on the hydrothermal composition of the CdSe. Essentially, the Cd precursor/Se precursor ratio remained stable in both cases, i.e., 1:2; however, some differences appeared in the end products. Initially, as in their previous study, both diffraction patterns were identified and exhibited the same wurtzite-type hexagonal structure previously reported. In the case of 0.1:0.05, a clustered rod-like structure with particle sizes of 24 nm and a band gap energy of 2.32 eV was observed, while in the case of 0.01:0.005, a flower-like clustered rod structure with particle sizes of 18.5 nm and a band gap energy of 2.36 eV was observed. Therefore, a decrease in concentrations of Se and Cd precursors, even if the ratio remained stable, led to a decrease in particle size and an increase in band gap energy, which is in line with previous studies. It was further noted that the synthesized CdSe nanoparticles exhibited a quantum confinement effect due to their higher band gap energy compared to bulk CdSe and small size. Finally, the CdSe with the lower molar concentration, i.e., 0.01:0.005, was selected due to the lower rate of electron–hole pair recombination and enhanced photocatalytic activity and exhibited a degradation efficiency of MB of 80% in 3 h, and, in this case, as in the previous one, the produced superoxide and hydroxyl radicals played a key role in the degradation efficiency.
The studies presented above focus on the use of these catalysts as either PMS or PS activators in SR-AOPs or as photocatalysts for the degradation of organic pollutants, by the generation of various ROS such as singlet oxygen, sulfate, superoxide, and hydroxyl radicals. In most cases, degradation percentage is greater than 90% under optimized conditions (usually catalyst dosage: 0.5–2.5 g/L and oxidant: ~1 mM), with reaction time required in most cases ≥ 2 h. Regarding the stability and reusability of these catalysts, reusability is often demonstrated in 4–5 cycles with a gradual decrease in some cases, due to issues such as phase instability and metal ion leaching.
2.2. Composite and Heterostructure Selenide Catalysts
In photocatalysis, graphitic carbon nitride (g-C3N4) and TiO2 are two of the most prevalent semiconductors used where composite materials are introduced [144,145,146,147,148,149,150,151,152,153,154]. This is more due to the multifunctionality of properties they exhibit that enhance the photocatalytic activity of pristine materials [155,156]. Nonetheless, while g-C3N4 and TiO2 are individually able to generate ROS when irradiated, they possess inherently dissimilar physicochemical properties. In TiO2’s case, the existence of different physicochemical characteristics depends on the crystal phase, size, and shape of the particles [157]. For example, anatase is an n-type semiconductor material with band gap energy (~3.2 eV), high crystallinity, and chemical stability with higher photocatalytic activity, and rutile possesses slightly lower band gap energy (~3.0 eV) but higher thermodynamic stability and stronger UV absorption, while brookite, as the least investigated TiO2 phase, exhibits unique electronic behavior when it forms efficient heterojunctions with the rutile and anatase TiO2 crystalline phases, leading to increased charge carrier separation and redox efficiency [158,159,160]. However, TiO2’s limited visible-light absorption and the tendency for rapid recombination of photogenerated charge carriers restrict its photocatalytic efficiency under solar irradiation [161,162]. In contrast, g-C3N4 is a metal-free polymeric semiconductor material with reduced band-gap energy of approximately 2.7 eV and visible-light absorbing characteristics. It can easily be synthesized from relatively cheap precursors, such as melamine or urea, and exhibits good chemical stability [155,163]. However, pristine g-C3N4 possesses low surface area and fast electron–hole recombination, which are detrimental to its photocatalytic performance [156]. Given these features, the following sections will highlight the reported studies in the literature regarding composite TMSe-based catalysts and, particularly, those coupled with g-C3N4 and TiO2.
2.2.1. TMSes/g-C3N4
Composite TMSe systems for the degradation of organic pollutants represent a relatively new approach due to the combined benefits they offer. In particular, in photocatalytic processes, g-C3N4 with a typical 2D structure has gained the interest of the scientific community due to the multitude of advantages it provides, such as photochemical stability, non-toxicity, ease of cost-effective synthesis, eco-friendly nature, abundance of raw materials, and suitable band gap value [164,165,166,167,168]. Nevertheless, bulk g-C3N4 exhibits insufficient photocatalytic activity owing to low visible light absorption and rapid carrier recombination [164,169]. For this reason, composite g-C3N4 systems are being employed to address these drawbacks in photocatalytic degradation of organic pollutants.
The use of the CoSe2/g-C3N4 system was introduced by two different research groups for the degradation of organic pollutants. Specifically, Jia et al., 2023 [170] studied the effect of various mass contents of CoSe2 on binary heterojunction photocatalyst CoSe2/g-C3N4 (nanosheets) in photocatalytic activities such as HER and the degradation of tetracycline hydrochloride (TCH). A solvothermal-type method was employed for the synthesis of CoSe2/g-C3N4 with different mass contents of CoSe2 [%x CoSe2/g-C3N4 (nanosheets), where x = 1.1, 3.2, 5.4, 7.5 and 9.3 wt%], and it was observed that CoSe2 exhibits a spherical structure while in CoSe2/g-C3N4, and there was a homogeneous distribution of CoSe2 on the surface of the g-C3N4 nanosheets. Amongst them, the photocatalyst 7.5% CoSe2/g-C3N4 was used in the study of the photocatalytic degradation of TCH, where it showed enhanced activity compared to CoSe2 and g-C3N4 as 86.2% degradation was achieved in one hour, while CoSe2 and g-C3N4 achieved a degradation efficiency of 26.7% and 75.1%, respectively, in the same period. During the process of TCH degradation, a series of reactions, such as dehydration, dihydroxylation, and others, were observed, leading to the formation of ten intermediate products that can further be mineralized into CO2 and H2O. Finally, to elucidate the enhancement of the photocatalytic activity of the 7.5% CoSe2/g-C3N4, appropriate scavengers were used, and determined that O2•− were the predominant ROS.
Dileepkumar et al., 2022 [171] hydrothermally synthesized CoSe2 nanorods and then attempted, through a hydrothermal step, again to graft these onto g-C3N4 nanosheets. The synthesis was successful, and they subsequently examined the photocatalytic efficiency of the CoSe2-gC3N4 system regarding the degradation of BPA. Without changing any parameters, they observed that the CoSe2-gC3N4 system exhibited increased photocatalytic activity, as it was able to achieve almost 93% degradation within 3.5 h, with the photocatalytic activity following the trend CoSe2-gC3N4 > CoSe2 > gC3N4. The same trend was followed regarding the mineralization efficiency of BPA, i.e., CoSe2-gC3N4 (90.31%) > CoSe2 (41.65%) > gC3N4 (35.12%), exhibiting the superiority of the composite CoSe2-gC3N4. The influence of various factors (pH, catalyst, and BPA dosage) on the photocatalytic activity of the CoSe2-gC3N4 system was studied and a change was observed in various pH (3–12), with the maximum effectiveness occurring in acidic conditions, while a decisive role in the performance of the system played the electrostatic interactions between CoSe2-gC3N4 surface and BPA [172,173]. A positive correlation was found between the increase in catalyst dosage, up to a certain point (30 mg L−1), and the degradation efficiency, while an increase beyond this had no significant effect on the system, which was probably attributed to the fact that in higher amounts of catalyst, the solution becomes darker, thus stopping the passive light and inhibiting the degradation. An increase in BPA concentration inhibited degradation due to the limited number of active centers on the surface of the catalyst that could interact with the pollutant. In addition, CoSe2-gC3N4 was effectively used for five consecutive catalytic cycles, with performance reduced to a very small degree (93%, 1st cycle ⟶ 86%, 5th cycle), while, with regard to the photocatalytic degradation mechanism of BPA, with the use of appropriate quenching agents (KI for photogenerated holes, NaN3 for 1O2, and MeOH for HO•), it was stated that the largest contribution came not from the photogenerated holes or 1O2, but rather from HO•. Finally, the authors reported its successful implementation in HER and OER activities and its effective use in supercapacitor applications, highlighting its successful application in both remediation technologies.
In a study conducted by Shen et al., 2019 [174], the photocatalytic activity of a series of different wt% (1, 3, 5, 10) ratios of β-FeSe/g-C3N4 towards Rhodamine B (RhB) was investigated. The authors aimed to enhance the catalytic activity of g-C3N4 through its coupling with tetragonal phase FeSe (β-FeSe) nanorods, which exhibit an excellent ability in capturing electrons, thus addressing the problem of recombination of the photogenerated carriers (electrons and holes). The synthesis of the x% wt β-FeSe/g-C3N4 was carried out via a solid state reaction, and reported that 3%wt β-FeSe/g-C3N4 exhibited the highest decolorization efficiency of approximately 45% in 3 h; however, it was noted that a higher β-FeSe content acted in an inhibitive way, as the excess of β-FeSe found on the surface of g-C3N4 reduces the absorption of light, thus reducing its photocatalytic activity. The use of the oxidant H2O2 in the 3%wt β-FeSe/g-C3N4 system was also examined in order to enhance its photocatalytic activity, resulting in a significant increase as a 100% degradation achieved in just 60 min, which was attributed to the formation of HO• from either the photolysis of the H2O2 or the photogenerated electrons that were captured by H2O2. Lastly, upon reusing the catalyst in the system 3%wt β-FeSe/g-C3N4/ H2O2, encouraging results were observed as it could be effectively used for four consecutive cycles without any performance decline.
A similar and more recent study was presented by Hadadi et al., 2023 [175] who investigated the effects of different mass contents of CdSe (1%, 2.5%, 5%, and 10%) in g-C3N4/CdSe composites. The synthesis of these composites was performed using a pulsed laser process, a method that is relatively simple, fast, eco-friendly, and does not require additional post purification in order to achieve the dispersion of CdSe nanoparticles on the polymeric surface of g-C3N4. It was observed that, with the increase in CdSe mass loading at g-C3N4/CdSe composites, the absorption of g-C3N4/CdSe composites not only shifts towards a longer wavelength region but also extends in the visible region, compared to g-C3N4. Furthermore, photoluminescence spectra clearly demonstrated reduced recombination of the photogenerated carriers. These attributes enhanced the photocatalytic activity of g-C3N4/CdSe composites in terms of degradation of MB and RhB dyes, with the order of photocatalytic activity being g-C3N4/CdSe 1% < g-C3N4 < g-C3N4/CdSe 5% < g-C3N4/CdSe 2.5% and g-C3N4 < g-C3N4/CdSe 5% < g-C3N4/CdSe 1% < g-C3N4/CdSe 2.5% in the case of RhB and MB, respectively. There were significant differences in the order of the catalytic activity towards the degradation of these two dyes; however, in both cases the highest rates of degradation were observed from g-C3N4/CdSe 2.5% composite. The CdSe/g-C3N4 system was reported to operate via a type-II heterojunction charge transfer, consistent with the band alignment of CdSe and g-C3N4. The reduced photoluminescence intensity of the composites further confirmed efficient electron–hole separation within this configuration. The structural stability and the maintenance of its photocatalytic activity over three consecutive catalytic cycles was confirmed, as there was a minor decrease compared to the first cycle, while the formation of superoxide radicals and the h+ in the VB of g-C3N4 were reported on the mechanism that led to the degradation of these dyes. Finally, the application of g-C3N4/CdSe 2.5% as an electrocatalyst in HER was also highlighted, with the results being particularly encouraging as it exhibited enhanced activity compared to bulk g-C3N4.
A number of studies have been presented regarding the use of zinc selenide paired with graphitic carbon nitride, although different approaches have been adopted in each case. The first article was published by Zhao et al., 2018 [176] and reported the enhancement of bulk g-C3N4’s photocatalytic activity through the deposition of ZnSe quantum dots (QDs) and nano-Ag on its surface, thus synthesizing a novel semiconductor. In order to study the effect that the synthesis of these composites had on the photocatalytic activity, comparative photocatalytic degradation experiments of the antibiotic Ceftriaxone (CTX) were performed by the bulk g-C3N4, ZnSe QDs, x% ZnSe/g-C3N4 (x = 3, 5, 9), x% Ag/g-C3N4 (x = 3, 5, 7, 9 mg of Ag and 100 mg of g-C3N4), and x% ZnSe-Ag/g-C3N4 (x = 1, 3, 5, 7, 9, 11 mg of ZnSe and 100 mg of 7% Ag/g-C3N4). What has been observed regarding the photocatalytic degradation of CTX is, as shown by Table 2, that the lowest rate of degradation occurs in the case of ZnSe QDs with g-C3N4 following, and, thereafter, each stage of modification of g-C3N4 implies enhanced photocatalytic activity. In particular, the dispersion of Ag nanoparticles on the surface of g-C3N4 led to higher degradation rates of CTX, with 7% Ag/g-C3N4 being the most active, and dispersion of ZnSe QDs on the surface of g-C3N4 exhibited even greater impact on the photocatalytic degradation of CTX, with 7% ZnSe/g-C3N4 being the most active. The dispersion of 7% ZnSe QDs on the surface of 7% Ag/g-C3N4 exhibited the highest catalytic efficiency. Therefore, the photocatalytic activity, in terms of CTX degradation, followed the trend ZnSe < g-C3N4 < x% Ag/g-C3N4 < x% ZnSe/g-C3N4 < x% ZnSe-Ag/g-C3N4. The predominance of the photocatalytic system 7% ZnSe-Ag/g-C3N4 was mainly attributed to factors, such as larger surface area, that lead to higher adsorbability and better efficiency in the separation of photogenerated carriers (electrons and holes). Positive results were also found regarding the stability and reuse of the catalyst, as its activity remained unchanged after three catalytic cycles, and no morphological difference was observed between the initial 7%ZnSe -Ag/g-C3N4 and final 7% ZnSe-Ag/g-C3N4. Ιn addition, to propose a possible mechanism of CTX degradation, appropriate quenching agents (EDTA-2Na for h+, isopropanol for •OH, p-benzoquinone for O2•−, and K2Cr2O7 for electrons) were used, and the authors observed the contribution of different active species in each system and, in particular,, in the case of 7% Ag/g-C3N4, h+, electrons and O2•− played an important role, while in the case of 5% ZnSe/g-C3N4 and 7% ZnSe-Ag/g-C3N4, h+ and •OH were the dominant species, with the possible mechanism given by Reactions (17)–(21).
ZnSe-Ag/g-C3N4 + hv → h+ + e−
h+ + H2O → •OH + H+
e− + O2 → O2•−
2H+ + 2O2•− → O2 + H2O2
H2O2 → 2•OH

Table 2.
Overview of the degradation of various organic pollutants by TMSes/g-C3N4 composite catalysts. All experiments were carried out under ambient temperature conditions, unless otherwise explicitly reported in the corresponding references.
In addition, Ehsan et al., 2020 [177] studied the photocatalytic degradation of CR dye by g-C3N4, ZnSe, and ZnSe/g-C3N4 composite. The synthesis of the hexagonal ZnSe structure was carried out with the help of the hydrothermal method, and a similar method was employed in order to achieve a complete decoration of high-intensity ZnSe nanocrystals on the surface of g-C3N4. It was observed that the highest photocatalytic degradation was presented by composite ZnSe/g-C3N4 as a 95.69% degradation rate was achieved in just 60 min, while, at the same time, ZnSe showed a 60.97% rate, and g-C3N4 a 52.93% rate, of degradation of CR. The enhancement of the photocatalytic activity of composite ZnSe/g-C3N4 compared to bulk g-C3N4 and ZnSe was mainly attributed, as in the previously mentioned cases, to efficient separation of photogenerated carriers, which plays a key role in the photocatalytic activity of a system. The successful use of the ZnSe/g-C3N4 composite for three consecutive catalytic cycles was also reported, after cleaning with propanol in order to remove any adsorbed degradation products from the surface of the catalyst and release its active centers. However, a gradual decrease was observed after each cycle [5% decrease (2nd cycle) ⟶ 9% decrease (3rd cycle)]. The authors, after reporting that alignment of the energy levels of the composite semiconductor played an important role in the degradation mechanism, as the ZnSe/g-C3N4 composite was described as a type-II heterojunction, where electrons transferred from the CB of ZnSe to that of g-C3N4 and promoted O2•− generation, which was confirmed with the use of appropriate quenching agents such as TBA for •OH, XTT for O2•− and NaN3 for 1O2, proving that O2•− had the key role.
While previous research by Ehsan et al., 2020 [177] focused on the degradation mechanism of CR dye by ZnSe/g-C3N4, and the study presented by Zhao et al., 2018 [176] emphasized the effect of different ZnSe QDs deposition rates on the surface of g-C3N4, Bigdeli Tabar et al., 2023 [178] aimed to tackle the common issues arising from the synthesis and use of type-II heterostructure ZnSe/g-C3N4 nanocomposites, such as the involvement of only photogenerated electrons in the degradation processes or the recombination of photogenerated carriers. They succeeded in synthesizing a composite ZnSe/g-C3N4 with varying g-C3N4 concentrations, i.e., ZnSe/g-C3N4 x% (x = 5, 10, 15 wt%), which, in addition to ZnSe and g-C3N4, featured independently formed Se nanoparticles. The interesting aspect of this research, concerning the synthesis of ZnSe/g-C3N4, is that while initially ZnSe exhibited a mixed morphology of nanoparticles, nanofibers, and polyhedral nanostructures, the addition of g-C3N4 affected the formation of each phase. At low g-C3N4 concentrations, specifically in ZnSe/g-C3N4 10%, a uniform morphology of nanoparticles was observed, which consisted of g-C3N4 sheets, ZnSe nanostructures, and Se nanoparticles, whereas in ZnSe/g-C3N4 15% a layered morphology was observed. Consequently, the synthesis of this composite positively impacted the photocatalytic activity in terms of MB degradation, as over 120 min (as shown in Table 2), with pristine g-C3N4, 55% degradation was achieved, while 75% was achieved with pristine ZnSe. In contrast, in the case of ZnSe/g-C3N4 x%, the results showed differences as an increase in g-C3N4 loading (5% ⟶ 10%) resulted in enhanced photocatalytic activity, with ZnSe/g-C3N4 10% demonstrating the highest activity, as MB degradation rate of 97% was achieved in 120 min, while further increase in g-C3N4 concentration (15%) had an inhibiting effect, resulting in a degradation rate lower than that of pristine ZnSe and g-C3N4. Furthermore, a comparison was made between ZnSe/g-C3N4 10% and pristine ZnSe regarding their stability and reuse. What was observed, after four consecutive catalytic cycles, was the predominance of ZnSe/g-C3N4 10% over pristine ZnSe, as the photocatalytic activity of ZnSe/g-C3N4 10% remained stable, while in the case of pristine ZnSe, a decrease of 32% was observed in the fourth catalytic cycle. Furthermore, ZnSe/g-C3N4 10%, unlike pristine ZnSe, exhibited excellent morphological stability, as the phase of ZnSe/g-C3N4 10% remained stable due to the protective g-C3N4 shell, thus highlighting its potential application as an industrial photocatalyst. Finally, regarding the mechanism of degradation of MB by ZnSe/g-C3N4 10%, it appears that the presence of Se nanoparticles plays a decisive role in its enhanced photocatalytic activity (Figure 4), and, as they act as mediators, the photogenerated electrons of Se nanoparticles can recombine with the photogenerated holes of ZnSe and g-C3N4, thereby reducing the recombination rate of photogenerated carriers in the ZnSe/g-C3N4 10% composite.
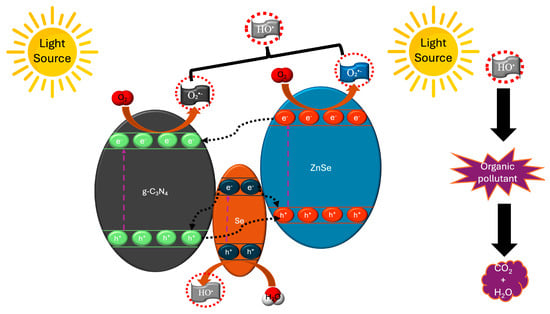
Figure 4.
Schematic illustration of the type-II heterostructure of ZnSe/g-C3N4 with Se as a mediator, based on ZnSe/Se/g-C3N4 [178]. “Reprinted/adapted with permission from Ref. [178]. Copyright 2023, Elsevier B.V.”.
Two different research groups presented studies on the use of nickel selenides in combination with g-C3N4 to degrade organic pollutants through photocatalytic processes. Significant differences, however, are observed both in the synthesis and use of nickel selenide/g-C3N4 composites as well as in the areas they focused on. Specifically, the first research published by Chen et al., 2021 [179] focused on solvothermal synthesis of x% NiSe/g-C3N4 (x = 1, 3, 6, 9, 15 wt%), so that in situ incorporation of NiSe nanodots onto g-C3N4 could lead to a composite photocatalyst with enhanced light absorption and separation of photogenerated carriers. What was first observed is that different loadings of NiSe co-catalyst on the g-C3N4 surface significantly affected the activity of the x% NiSe/g-C3N4 system, relative to photocatalytic hydrogen production performance. All NiSe/g-C3N4 composites with different NiSe loadings showed enhanced photocatalytic activity compared to NiSe and g-C3N4, with activity following the trend NiSe < g-C3N4 < 1% NiSe/g-C3N4 < 15% NiSe/g-C3N4 < 9% NiSe/g-C3N4 < 6% NiSe/g-C3N4 < 3% NiSe/g-C3N4 and with the optimal photocatalyst appearing to be 3% NiSe/g-C3N4. Taking these results into account, the further experiments that followed used only the 3% NiSe/g-C3N4 photocatalyst from composites, as they are the most effective. A study of its photocatalytic activity concerning the degradation of oxytetracycline (OTC) and methyl orange dye (MO) was also carried out, and what was observed in both cases was the enhanced photocatalytic activity of 3% NiSe/g-C3N4 against NiSe and g-C3N4, as in the first case a degradation rate of 98.68% was achieved in 60 min, while in the second case 92.25% after 5 min (Table 2). The deposition of NiSe nanodots played an important role and positively influenced the photocatalytic activity of the system, specifically indicating that its metallic character could facilitate both transport and separation of photogenerated carriers. In addition, with the use of suitable scavengers, the role of O2•− as the dominant active species in the degradation mechanism was reported. Regarding the stability and reuse of 3% NiSe/g-C3N4, the results were particularly encouraging due to its successful use for five consecutive catalytic degradation cycles of MO and OTC, with a very small reduction in activity, which, however, was probably attributed to the recovery method of the photocatalyst.
The second research study, published by Poiyamozhi et al., 2024 [180], mentioned the ultrasonic-assisted hydrothermal synthesis of a series of x% g-C3N4/NiSe2 (x = 10, 20, 30 wt%) and, in contrast to the previous study, focused on the effect of different rates of g-C3N4 decorated NiSe2 on the photocatalytic degradation of Reactive Blue 5 (RB 5) and Reactive Violet 5 (RV 5) dyes. It was observed that the structural characteristics of x% g-C3N4/NiSe2 are such that the hexagonal structure of NiSe2 is linked to the g-C3N4 nanosheets, maintaining the structural stability of NiSe2. Meanwhile, an increase in g-C3N4 concentration led to increased intensity of the XRD peaks, which has been linked in earlier research to a larger surface area and, consequently, increased photocatalytic activity [184,185]. What was observed during the use of x% g-C3N4/NiSe2 in the photocatalytic degradation of RB 5 and RV 5 is that an increase in g-C3N4 concentration had a positive effect, with the order of catalytic activity in both cases following the trend NiSe2 < g-C3N4 < 10% g-C3N4/NiSe2 < 20% g-C3N4/NiSe2 < 30% g-C3N4/NiSe2, an improvement which is mainly attributed to the effect of the incorporation of g-C3N4 as it enhances conductivity, light absorption in the visible region, reduces band gap energy, and increases the surface area. The degradation rates of RB 5 and RV 5 from 30% g-C3N4/NiSe2, under the influence of natural sunlight (during the month of July), were 86.4% and 89.4% in 150 min, respectively. The stability and reuse of 30% g-C3N4/NiSe2 were also positive, maintaining its initial activity, almost unchanged, after five catalytic cycles, while structural changes were observed in the sixth cycle. Additionally, to demonstrate the successful application of this photocatalyst for a number of organic pollutants, independent of their charge characteristics, the 30% g-C3N4/NiSe2 system was used to degrade the cationic dye Crystal Violet (CV), achieving a degradation rate of 93.1%, and the anionic dye CR with degradation rate of 94.4%. Finally, in order to shed light on the degradation mechanism, suitable scavengers (triethanolamine for h+, 1,4-benzoquinone for O2•− and isopropanol for •OH) were used and the contribution of both •OH and h+ was noted.
A co-precipitation method has been employed for the synthesis of a type-II SnSe/g-C3N4 x% (x = 5, 10, 15 wt%) heterostructure with different g-C3N4 concentrations by Saray et al., 2019 [181] for the purpose of applying these composites to the photocatalytic degradation of MB dye. The optimum g-C3N4 loading that produced a final product with high thermal stability and crystallinity was wt% = 10. The lowest photocatalytic activity was presented by pristine g-C3N4 while the highest photocatalytic activity was exhibited by SnSe/g-C3N4 10%, achieving complete degradation of MB within 25 min. The order of activity followed the trend g-C3N4 < SnSe/g-C3N4 5% < SnSe/g-C3N4 15% < SnSe < SnSe/g-C3N4 10%. This enhanced photocatalytic activity was mainly attributed to factors such as higher specific surface area and efficient separation of the photogenerated charge carriers by the formation of this type-II p(SnSe)-n(g-C3N4) heterostructure. However, a dye degradation rate in the absence of light was also reported for all the above systems, in this case being SnSe < SnSe/g-C3N4 5% < g-C3N4 < SnSe/g-C3N4 10% < SnSe/g-C3N4 15%, attributed to surface adsorption due to their porous structures. A comparison was made between pristine SnSe and SnSe/g-C3N4 10% in terms of reuse and stability over six consecutive catalytic cycles, revealing the predominance of the SnSe/g-C3N4 10% composite, as its photocatalytic activity remained almost constant even in the sixth catalytic cycle, in contrast to pristine SnSe, where a significant decrease in its photocatalytic activity was noted in the sixth cycle. This decrease was mainly attributed to the existence of extra phases, such as Se, SnO2, and SnSe2, due to the creation of free selenium ions and the conversion of Se2+ to Se4+, which was not observed in the case of SnSe/g-C3N4 10% composite as SnSe nanoparticles were protected by a shell of g-C3N4. Concerning the study of the photocatalytic degradation mechanism of MB, in the case of pristine SnSe, O2•− played a decisive role, while in the case of SnSe/g-C3N4 10%, photogenerated h+ were the primary reactive species.
Mohan et al., 2022 [182] made efforts to synthesize several different TMSe nanoflowers to apply them to photocatalytic water-splitting reactions and photocatalytic RhB degradation. With the help of the hydrothermal method, they synthesized zinc and iron selenides such as ZnSe, FeSe2, ZnFeSe, and Zn/FeSe2, where ZnSe and FeSe2 nanoparticles exhibited a nanoflower structure. In the case of ZnFeSe, FeSe nanoflowers are decorated with zinc metal nanodots, while in the case of Zn/FeSe2, the zinc nanodots are invisible due to their homogenous mixing with iron atoms. It was observed that FeSe2 and Zn/FeSe2 showed a wider spectrum of light absorption compared to ZnSe and ZnFeSe. Taking into account the BET surface area of the above photocatalysts, as it has been reported to have a direct effect on photocatalytic activity [186], it was noted that the larger surface area was observed in the case of Zn/FeSe2 (probably due to the addition of zinc metal nanodots on petals of the FeSe2 nanoflowers), following the trend ZnFeSe < FeSe2 < ZnSe < Zn/FeSe2. Regarding photocatalytic HER activities, the range of activity followed the trend ZnSe < ZnFeSe < FeSe2 < Zn/FeSe2, with the predominance of Zn/FeSe2 being mainly attributed to factors such as wider absorption spectrum and larger surface area mentioned previously, as well as effective interfacial charge separation. Therefore, to further enhance the photocatalytic activity of Zn/FeSe2, they synthesized g-C3N4-Zn/FeSe2 composite and the result was its superiority over Zn/FeSe2 in both hydrogen and oxygen production, with the largest difference, however, occurring in the latter case. The photocatalytic degradation of the RhB dye by g-C3N4-Zn/FeSe2 composite was also studied, showing higher activity than both g-C3N4 and Zn/FeSe2, with the order of photocatalytic activity in this case being Zn/FeSe2 (65% in 60 min) < g-C3N4-Zn/FeSe2 (98% in 60 min), which indicates that the contribution of g-C3N4 had a positive effect. Finally, g-C3N4-Zn/FeSe2 was effectively used in both HER and OER and degradation of RhB for five consecutive catalytic cycles, maintaining its activity unchanged.
The effect of different loadings of g-C3N4 on the photocatalytic activity of CuxSey was studied by Nouri et al., 2020 [183] to enhance the visible-light photocatalytic performance of CuxSey by the synergistic action of g-C3N4. The synthesis of these materials was carried out using a simple and cost-effective co-precipitation method. Initially, in the case of CuxSey, a mixture of hexagonal CuSe, tetragonal Cu3Se2, orthorhombic Cu2Se, and hexagonal Se phases was observed. The synthesis of these type-II p(CuxSey)/n(g-C3N4) x% (x = 5, 10, 15 wt%) materials with the introduction of the different concentrations of g-C3N4 did not affect the morphology of these materials nor the different phases of CuxSey; however, at its highest loading, i.e., 15%, a new CuSe2 phase appeared that was not present in other materials. A study of the photocatalytic activity of the above materials for the degradation of MB dye was conducted, and it was observed that all CuxSey/g-C3N4 x% composites exhibited enhanced activity compared to pristine CuxSey and g-C3N4, with activity following the order g-C3N4 < CuxSey < CuxSey/g-C3N4 15% < CuxSey/g-C3N4 10% < CuxSey/g-C3N4 5%, where CuxSey/g-C3N4 5% achieved complete MB degradation in just 60 min. A study and comparison of the stability and reuse of CuxSey/g-C3N4 5% and pristine CuxSey were carried out, and it was observed that, in the case of CuxSey/g-C3N4 5%, the photocatalytic activity remained nearly unchanged even after five consecutive cycles, while a significant decrease (approximately 20%) was observed in the case of pristine CuxSey for the same number of cycles. Additionally, a significant change was noted in the CuxSey/g-C3N4 5% phase after the photocatalytic process, with the presence of additional Se phases, reinforcing their hypothesis that Se ions on the surface of CuxSey react with g-C3N4, acting as a bridge between p-type CuxSey and n-type g-C3N4. To illuminate the reasons why CuxSey/g-C3N4 5% exhibited enhanced photocatalytic activity, a study of the degradation mechanism was conducted. It was observed that factors such as higher efficiency of charge transfer, effective electron–hole separation, and longer lifetime of electron–hole pair enhanced the activity of the system. Furthermore, using appropriate scavengers, it was noted that HO• played a dominant role in the degradation process, whereas, in the case of pristine CuxSey, O2•− were the dominant reactive species.
Based on the studies presented in this section, it is evident that the formation of TMSes/g-C3N4 composites leads to photocatalysts with enhanced photocatalytic activity compared to pristine TMSes and g-C3N4. This enhanced activity is mainly attributed to the formation of efficient heterojunctions (such as type-II or Z-scheme), featuring improved charge carrier recombination, expanded visible light absorption, and appropriate redox potentials. A key observation is that, in cases regarding the study of TMSe loading, optimized TMSe loading (~3–15 wt%) leads to the enhanced ROS formation, such as HO• and O2•−, while also noting that excessive TMSe loadings may inhibit performance due to the blockage of active sites. In all cases presented, degradation rates of ≥90% were reported under optimized conditions (catalyst dosage: 0.1–1 g/L and pollutant concentration: 10–30 mg/L) within 5–150 min, and times apparently improved compared to the cases of single-metal selenides discussed in the previous section. TMSes/g-C3N4 composites also exhibited good stability for about 5–6 photocatalytic cycles.
2.2.2. TMSes/TiO2
TiO2 has been studied extensively in the past as an efficient photocatalyst due to its numerous advantages, such as its high stability, non-toxicity, porous structure, and large surface area [187,188,189]. However, many obstacles exist to its practical use, primarily due to the high band gap energy (3.2 eV) and faster recombination of the photogenerated carriers, characteristics that negatively affect its photocatalytic activity [190,191]. For this reason, various strategies have been developed to overcome these problems, such as the modification of TiO2 with other semiconductors, which leads to the formation of nanocomposite heterostructures. In this category, we will mention the cases in which transition metal selenides paired with TiO2 have been used to degrade organic pollutants.
The first research study comes from Li et al., 2012 [192] who, through a facile and environmentally friendly photo-assisted chemical bath deposition, synthesized intriguing rosalike CuSe crystals on TiO2 nanotube arrays. The study includes the in situ conversion of Cu2O nanowires preloaded on TiO2 nanotube arrays into CuSe rosalike crystals under visible light irradiation. Thus, the synthesis of a p-n Cuse/TiO2 heterojunction was achieved, which featured enhanced charge separation and visible light absorption and significantly reduced charge recombination rate, compared to the unmodified TiO2 nanotube arrays. A study was conducted on the influence of various parameters on the synthetic process to understand the formation of rosalike hierarchical CuSe nanostructures. Regarding deposition time, it was observed that increasing time facilitated the formation of rosalike nanostructures, as initially nanoparticles (2 min of chemical deposition) were observed, then there was coexistence of nanoparticles and hexagonal nanoplates (5 min of chemical deposition), and finally, further increase in time led to the self-organization of the existing nanoplates to a rosalike structure. Regarding the effect of temperature on the synthetic process, it was observed that 50 °C was the optimal temperature for the formation of rosalike CuSe, as, at a lower temperature (30 °C), irregular grains were observed, while at higher temperatures (70 °C) hexagons and particles appeared. Light irradiation also had a significant impact, as, in the absence of this, the formation of these rosalike structures was not observed. However, stirring conditions revealed that synthesis with stirring led to rosalike CuSe with crumpled nanosheets, while without stirring led to rosalike CuSe with hexagonal sheets. Finally, a study of the photocatalytic activity of CuSe/TiO2 evaluated through the degradation of anthracene-9-carboxylic acid (ACA) and demonstrated complete degradation within 70 min under simulated solar irradiation. The enhanced activity was attributed to a reduced recombination rate and increased life of photo-generated carriers, while the reuse of CuSe/TiO2 was also reported for ten consecutive catalytic cycles, maintaining its almost constant performance.
Xu et al., 2019 [193] reported the in situ synthesis of TiO2/CdSe nanocomposite using poly(acrylic acid) (PAA), as a ligand of CdSe, by a high internal phase emulsion (HIPE) method. This approach achieved a better dispersion of TiO2 around the CdSe, with the TiO2/CdSe heterostructure displaying a strong interaction between TiO2 nanosheets and CdSe quantum dots, a greater specific surface area, and efficient charge separation. The study of the photocatalytic activity of TiO2/CdSe heterostructure for MB degradation was carried out due to features such as a larger specific surface area, enhanced light absorption, and effective charge separation. The TiO2/CdSe composite showed enhanced photocatalytic activity compared to TiO2 and CdSe in both UV light irradiation and simulated sunlight irradiation, with the degradation rate of MB being 98.5% and 90.5% within 5 h, respectively (Table 3). As shown by the data in Table 3, although under UV a higher rate of degradation was observed compared to simulated sunlight, in the latter case the enhanced photocatalytic activity was markedly greater compared to TiO2 and CdSe, indicating that the introduction of CdSe quantum dots (which are capable of absorbing in the visible light region) had a positive effect on TiO2, which functions only in the UV region. Considering the reuse and stability of TiO2/CdSe composite, its effective use for three consecutive catalytic cycles under UV and simulated solar light was reported, with its photocatalytic activity remaining almost constant. Regarding the MB degradation mechanism from the TiO2/CdSe composite, the reaction of MB molecules with h+ and reactive species such as O2•− and HO• was reported.

Table 3.
Overview of the degradation of various organic pollutants by TMSes/TiO2 catalysts. All experiments were carried out under ambient temperature conditions, unless otherwise explicitly reported in the corresponding references.
To find a greener alternative to hazardous Cd and Pb semiconductors, Warkhade et al., 2019 [194] utilized CoSe for the hydrothermal synthesis of TiO2/CoSe nanocomposite, resulting in a final product by random deposition of spherical CoSe particles in TiO2 nanorods. Compared to RhB degradation rates from bare TiO2 and CoSe, photocatalytic degradation from TiO2/CoSe nanocomposite was clearly enhanced, with the order of activity being TiO2 < CoSe < TiO2/CoSe, with the corresponding degradation rates for bare TiO2, a nd the CoSe and TiO2/CoSe nanocomposite being 15%, 18%, and 99.86% at 90 min. It appears that the integration of CoSe on TiO2 nanorods had a positive effect in the case of the photocatalytic degradation of MB by TiO2/CoSe, as a degradation rate of approximately 98% was achieved in 90 min. The results were also encouraging in terms of stability and reuse of nanocomposites, as not only was it successfully used for six consecutive catalytic degradation cycles of RhB (with a 6% reduction), but based on XRD and SEM data, no change in the structure and morphology of TiO2/CoSe was observed. Factors such as the concentration of photocatalyst and dye appear to have influenced photocatalytic degradation as in the first case, an increase in concentration of 0.1 g L−1 to 0.5 g L−1 led to an increase in activity, with a further increase (0.5 g L−1 ⟶ 0.7 g L−1) inhibiting function, possibly due to an inability to absorb light due to aggregation and precipitation of the catalyst. A similar trend was observed in the case of an increase in RhB concentration, i.e., initially it worked positively, but a concentration greater than 0.06 g L−1 functioned in an inhibiting way due to the difficulty of absorbing light from the catalyst caused by the opacity of the solution. The authors concluded that the optimal degradation conditions were 0.5 g L−1 for the concentration of the catalyst and 0.06 g L−1 for the dye concentration. Finally, regarding the possible mechanism that led to the degradation of RhB, the enhanced photocatalytic activity of nanocomposite is due to improved charge separation and increased light absorption of the type-II heterojunction (Figure 5), while appropriate scavengers reported the primary involvement of O2•− and, to a lesser extent, the involvement of h+, e−, and HO•.
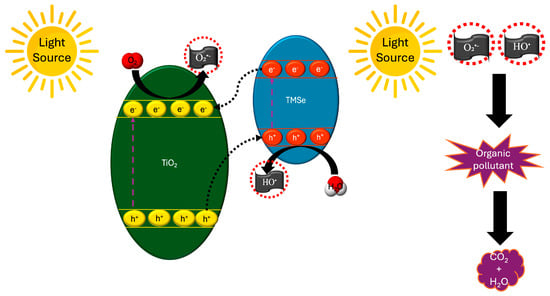
Figure 5.
Schematic illustration of the photocatalytic mechanism of a type-II heterostructure of TiO2/TMSe for organic pollutant degradation.
A relatively recent study presented by Khan et al., 2023 [195] examined the wet-chemical synthesis of FeSe2-TiO2 nanocomposites with different wt% combinations, namely 25% FeSe2—75% TiO2 (FT-1) and 50% FeSe2–50% TiO2 (FT-2). In this way, they managed to synthesize composites with high purity and crystallinity, which morphologically exhibited the combined characteristics of pure FeSe2 and TiO2. They investigated their possible application both in photocatalytic degradation processes and in energy storage applications. First, what they observed in the RhB degradation study is that both composites (FT-1 and FT-2) showed apparently enhanced photocatalytic activity compared to pure FeSe2 and TiO2, with FT-1 demonstrating the best activity. Within 60 min, RhB degradation occurred at a rate of approximately 98%, whereas for FT-2, pure FeSe2 and TiO2, 90 min was required for 96%, 100 min for 91%, and 180 min for 94%, respectively. As in previous studies, the enhanced photocatalytic activity of composites is mainly due to factors such as a greater number of active centers, higher efficiency of charge transfer, and effective electron–hole separation. FT-1, as the most active photocatalyst, was effectively used for four catalytic cycles, with activity showing a minimal reduction. Finally, their application as supercapacitor electrode materials was studied, with the FT-1 displaying the highest capacity, followed by the FT-2, FeSe2, and TiO2.
The hydrothermal synthesis of TiO2/XAC/MoSe2 (x = 0.25, 0.50, 0.75, 1.00) was presented by Ahmad et al., 2023 [196] based on the fact that, on the one hand, carbon materials, such as activated carbon (AC), are often used to modify TiO2 due to desirable properties such as excellent electrical conductivity, large surface area, and porous structure [197,198,199], while on the other hand, MoSe2 has been proven in the literature as an excellent co-catalyst due to its low cost, narrow band gap, and its 2D layer structure [200,201,202]. The photocatalytic activity of TiO2/XAC/MoSe2 was studied in terms of the degradation of MB. A positive correlation was found between activated carbon loading and MB degradation rate as the maximum activity was observed from TiO2/1.00AC/MoSe2 with a degradation rate of 83% in 150 min, with the order of activity being TiO2 < TiO2/0.25AC/MoSe2 < TiO2/0.50AC/MoSe2 < TiO2/0.75AC/MoSe2 < TiO2/1.00AC/MoSe2 (Table 3). All TiO2/XAC/MoSe2 composites exhibited increased photocatalytic activity compared to pure TiO2, mainly due to enhanced light absorption caused by the presence of activated carbon and a reduction in band gap energy of TiO2 due to 2D MoSe2. Parameters that can affect the photocatalytic activity of the system have been studied, such as the concentration of the catalyst and dye, pH, and others. In the case of the catalyst concentration, an optimal concentration (0.10 g L−1) was found, and any further increase was detrimental due to catalyst aggregation and an increase in solution opacity, resulting in reduced light absorption. A negative effect was observed from the increase in dye concentration due to the limited number of active centers on the surface of the photocatalyst, while, relative to the effect of pH, decreased activity occurred at an acidic pH range (2–4) and increased activity occurred at pH = 7–10, as MB is a cationic dye that is adsorbed on the negatively charged active surface of the photocatalyst in basic medium, due to electrostatic attraction. Finally, a study of the possible degradation mechanism of MB by the TiO2/1.00AC/MoSe2 system was carried out, with MoSe2 sheets acting as active reaction sites, offering a reduced charge recombination rate and effective separation of the photogenerated carriers, while MB degradation involves first adsorption on the surface of the catalyst and then formation of active species such as O2•− and HO• by reduction in the molecular oxygen from electrons in the CB of MoSe2 and from the oxidation of water molecules by the h+ of the VB of MoSe2, respectively.
The research presented in this section highlights the formation of type-II heterojunctions between TiO2 (wide bandgap) and TMSe (narrow bandgap), mainly synthesized via hydrothermal or sol–gel method. A key observation is that TMSe acts as a visible-light sensitizer and electron donor, while TiO2 serves as a stable support and electron acceptor. The synthesis of TMSes/TiO2 composites results in materials with enhanced visible light absorption and reduced charge recombination rates. This category of photocatalysts exhibited degradation rates > 85% under optimized conditions (catalyst dosage: 0.1–1 g/L and pollutant concentration: 10–60 mg/L) and retained their stability and photocatalytic activity over multiple catalytic cycles (≥4). In such composites, as in the case of TMSes/g-C3N4 composites, enhanced photocatalytic activity compared to either pristine TMSes or pristine TiO2 was demonstrated. Ιt is clear, however, that the number of studies conducted on the use of TMSes/TiO2 composites is lower than the number of TMSes/g-C3N4 composites presented in the previous section, which is likely due to the superior characteristics of g-C3N4 compared to TiO2 for creating composite catalysts, such as strong interface interactions, easy modifications, better band alignment, etc.
3. Miscellaneous Composite and Heterostructure Selenide Catalysts
In this category, we will refer to TMSe composite catalysts that have been studied in the processes of degrading organic pollutants through AOPs and do not fall into the aforementioned categories.
3.1. Fe-Selenide-Based Catalysts
A thorough investigation was presented by Zhang et al., 2022 [203] who synthesized a novel MoS2@FeSe2@CFS via a two-step solvothermal method. The first step involves in situ growth of FeSe2 on the surface of a non-woven carbon fiber sheet (CFS), while the second step involves in situ growth of MoS2. A study of its catalytic activity was conducted regarding the degradation of MB in the presence of H2O2, with the results being particularly interesting as a comparison was made between MoS2@CFS, FeSe2@CFS, and FeSe2@MoS2@CFS, to investigate the effect of each component and loading order. The results of this comparison displayed a range of activity following the trend CFS < FeSe2@CFS < FeSe2@MoS2@CFS < MoS2@CFS < MoS2@FeSe2@CFS, with corresponding degradation rates shown in Table 4. The superiority and enhanced Fenton catalytic performance of MoS2@FeSe2@CFS, due to its high surface area and adsorption capacity, facilitates effective mass transfer and MB adsorption, as it was observed that within 5 min, the degradation of MB was performed at a rate of 99.5%. It appeared that loading order on the surface of CFS had a significant effect, with the clearly reduced catalytic activity of the FeSe2@MoS2@CFS observed as the loss of a significant amount of Fe ions from the surface of the heterogeneous Fenton catalyst occurred. In addition, the superiority of MoS2@FeSe2@CFS was also observed in a comparison made between a composite (Fe3O4@FeSe2@CFS) that includes a conventional Fenton-like catalyst (Fe3O4) but also demonstrates its superiority over other conventional Fenton-like catalysts. The study examined the effect of parameters, such as pH, temperature, MB, and H2O2 concentration, and FeSe2@MoS2@CFS dosage in order to find optimal conditions. An increase in temperature led to a slight increase in catalytic activity, while alkaline pH conditions acted detrimentally, probably due to precipitation of Fe(OH)3, resulting in a weakening of the Fe(III)/Fe(II) redox cycle, but also in the formation of FeO2+. Increasing parameters such as H2O2 concentration and FeSe2@MoS2@CFS dosage worked positively in the degradation of MB due to increased production of hydroxyl radicals on the catalytic performance of FeSe2@MoS2@CFS in Fe(III)/Fe(II) cycle, while increasing MB concentration beyond a certain point (40 mg L−1) had a negative effect due to increased adsorption of MB intermediates on the catalyst surface. The successful use of MoS2@FeSe2@CFS for five consecutive catalytic cycles was also reported, maintaining activity at high levels, but leached Fe and Mo ions, with the highest amounts being 0.27 mg L−1 and 0.94 mg L−1, respectively, were observed. Regarding the degradation mechanism of MB, Fe(II) activates H2O2 to form hydroxyl radicals, Mo(IV) reacts with H2O2 to form Mo(VI) and hydroxyl radicals, and S acts as a cocatalyst in the Fe(III)/Fe(II) cycle, which is indeed enhanced by the deposition of FeSe2 and MoS2 on the surface of CFS and leads to the degradation of MB through two degradation pathways that coexist simultaneously. Using appropriate quenchers, the main contribution of HO• is highlighted in the degradation of MB and, to a lesser extent, that of O2•− and 1O2. Furthermore, the catalyst’s effectiveness was demonstrated in a lab-scale continuous flow reactor, maintaining high MB removal efficiency across multiple cycles and revealing results for potential scalable wastewater treatment applications.

Table 4.
Overview of the degradation of various organic pollutants by miscellaneous TMSe composite catalysts. All experiments were carried out under ambient temperature conditions, unless otherwise explicitly reported in the corresponding references.
In subsequent research by Xiao et al., 2022 [204], the hydrothermal synthesis of FeSe2/Fe3O4 with different molar ratios of 0.1, 0.2, and 0.3, i.e., 0.1 FeSe2/Fe3O4, 0.2 FeSe2/Fe3O4, and 0.3 FeSe2/Fe3O4, was implemented for the degradation of tetracycline hydrochloride (TC-HCl) under visible light and visible light + PMS. The catalytic activity of FeSe2/Fe3O4 composites, as well as pure FeSe2 and Fe3O4, is given by the series of FeSe2 < Fe3O4 < 0.3 FeSe2/Fe3O4 < 0.1 FeSe2/Fe3O4 < 0.2 FeSe2/Fe3O4 with the obviously enhanced catalytic activity of all composites compared to pure FeSe2 and Fe3O4, likely due to their higher specific surface areas, pore volumes, and unique pore structure, which provide more adsorption sites. Among the composites with different molar ratios, 0.2 FeSe2/Fe3O4 proved to be the most active, and the study of catalytic activity under visible light and visible light + PMS focused solely on this composite. In the absence of catalyst, the visible light + PMS system has led to degradation of TC-HCl at a rate of 23.1% in 60 min, while the most active catalyst, 0.2 FeSe2/Fe3O4, achieved a percentage degradation of 87.8% in the same time frame, an activity which was greater than the system 0.2 FeSe2/Fe3O4 + visible light (28.6% in 60 min) and 0.2 FeSe2/Fe3O4 + PMS (69.3% in 60 min). The catalytic order followed the trend FeSe2 < Fe3O4 < 0.2 FeSe2/Fe3O4. The superiority of the 0.2 FeSe2/Fe3O4 + visible light + PMS system is attributed to the conversion of Fe(III) to Fe(II) by light absorption, promoting the Fe(III)/Fe(II) redox cycle and activating PMS. A study of the influence of various factors on the degradation process of TC-HCl revealed that, as observed in other previously mentioned studies, an optimal catalyst and PMS dosage was found. In the first case, an increase beyond this value inhibited performance due to aggregation and turbidity of the solution, while in the second case, a decrease in reactive species occurred due to a reduced number of active centers and the self-quenching of sulfate and hydroxyl radicals. The optimal pH value for the 0.2 FeSe2/Fe3O4 + visible light + PMS system was pH = 5, while the pH = 7–11 negatively affected TCH degradation. On the reuse and stability of the catalyst, encouraging results were obtained, demonstrating its effective use for five consecutive cycles, with the degradation efficiency on the fifth cycle being 78.65% in 60 min. Regarding the degradation mechanism, the dominant roles of O2•−, SO4•−, and HO•, along with the synergistic action of Fe and Se on PMS activation, were evident.
A multivalent Cu2−xSe/FeSe2 Z-type heterojunction with different Fe:Cu molar ratios, i.e., Fe:Cu = 2.5% (CFS1), Fe:Cu = 5% (CFS2), Fe:Cu = 7.5% (CFS3), and Fe:Cu = 10% (CFS4), was obtained by a hydrothermal method and its photoelectrochemical, electrocatalytic, and photocatalytic performance was studied by Chen et al., 2023 [205]. This efficient heterojunction was constructed using 0D Cu2−xSe nanoparticles and 1D FeSe2 nanorods with multi-valence states such as Cu+, Cu2+, Fe+, Fe2+, etc., increasing carrier transport ability and improving redox ability, thus enhancing its catalytic activity. Photoelectrochemical studies revealed that all CFS composites exhibited higher performance than that of pure Cu2−xSe and FeSe2, with Fe:Cu molar ratio playing a key role, and CFS3 exhibiting the highest photocurrent density. Electrocatalytic hydrogen evolution reaction tests demonstrated improved performance compared to pure Cu2−xSe and FeSe2, with CFS2 excelling against the rest. Then, the photocatalytic activity through degradation of the antibiotic TC-HCl was studied, and it was observed that both CFS composites and pure Cu2−xSe and FeSe2 demonstrated a certain adsorption capacity (2.1–13.2%) after 1 h of dark treatment, that could work positively due to better photocatalyst and TC-HCl contact, thus enhancing photocatalytic activity. The result was that all CFS composites showed higher photocatalytic activity compared to pure Cu2−xSe and FeSe2, with the order following the trend FeSe2 < Cu2−xSe < CFS2 < CFS1 < CFS4 < CFS3, while CFS3, as the most active, was effectively used for five consecutive catalytic cycles, maintaining 87% activity in the fifth catalytic cycle. A positive correlation was found between CFS3 concentration and catalytic activity, while still under research to shed light on the degradation mechanism of TC-HCl, the formation of O2•− was the dominant ROS that led to the degradation of TC-HCl, with authors still reporting that Cu2−xSe/FeSe2 Z-type heterojunction could also improve the separation efficiency of the photogenerated carriers and enhance the redox capacity.
The next two surveys are presented by Chen et al., 2022 [206] and Xiong et al., 2022 [207] who conducted the hydrothermal synthesis novel Z-type multidimensional FeSe2/CuSe and 1D/2D FeSe2/SnSe heterojunctions, respectively. Initially, in the case of Chen et al., 2022 [206], the successful deposition of FeSe2 nanorods on the surface of CuSe hexagonal nanosheets was observed, with the final FeSe2/CuSe heterojunction displaying enhanced visible light absorption, interface contact, and higher specific surface area. Photocatalytic tests among FeSe2/CuSe with different Fe:Cu molar ratios, i.e., Fe:Cu = 1% (FCS1), Fe:Cu = 2.5% (FCS2), Fe:Cu = 5% (FCS3), and Fe:Cu = 7.5% (FCS4), and pure FeSe2 and CuSe on the photocatalytic degradation of the dye MB and the antibiotic Levofloxacin (LEV) were conducted. The activity in terms of MB degradation follows the series FeSe2 < CuSe < FCS4 < FCS1 < FCS2 < FCS3, while in LEV degradation it follows the series CuSe < FeSe2 < FCS1 < FCS2 < FCS4 < FCS3, with the superiority of all composites over pure FeSe2 and CuSe being evident. FCS3, as the most active photocatalyst, was used for five consecutive catalytic cycles while maintaining degradation at constant levels. Photoelectrochemical studies also revealed the superiority of FCS heterojunctions due to improved efficiency of carrier separation and reduced internal recombination, indicating its effective application in electrocatalysis. Finally, the formation of O2•− and the maintenance of maximum redox activity in Z-type multidimensional FeSe2/CuSe were mentioned.
In the case of Xiong et al., 2022 [207], the deposition of FeSe2 nanorods on the surface of SnSe nanosheets led to the formation of 1D/2D FeSe2/SnSe heterojunction, and the photocatalytic activity of heterojunctions with different Fe:Sn molar ratios, i.e., Fe:Cu = 1% (FSS1), Fe:Cu = 2.5% (FSS2), Fe:Cu = 5% (FSS3), and Fe:Cu = 7.5% (FSS4), and pure FeSe2 and SnSe was assessed in terms of MB degradation. What was observed also in this case, as in that of Chen et al., 2022 [206], is the predominance of heterojunctions over pure FeSe2 and SnSe, with the order of activity following the trend FeSe2 < SnSe < FSS1 < FSS2 < FSS4 < FSS3. As the most active, FSS3 was effectively used for five consecutive catalytic cycles, maintaining structural stability and performance at constant levels. Furthermore, an optimal Fe:Sn molar ratio was found, which led to a heterojunction with enhanced photocatalytic activity due to improved light absorption, efficient charge carrier separation, reduced internal resistance, and effective interfacial bonding of Sn-Se-Fe. Finally, the positive effect that the deposition of FeSe2 nanorods on SnSe nanosheets had on photoelectrochemical activities was noted.
3.2. Other Selenide-Based Catalysts
In the study presented by Cheng et al., 2022 [208], a novel 0D/2D ZnSe/SnSe heterojunction photocatalyst was synthesized through a two-step hydrothermal method. In this way, 0D ZnSe nanoparticles were uniformly decorated on the surface of 2D SnSe nanosheets, constructing a 0D/2D heterojunction with broad visible light absorption, strong redox potentials, and intimate interfacial contact that leads to rapid charge separation and improved carrier transmission. Photocatalytic degradation experiments were conducted using MB and LEV from ZnSe/SnSe with different Zn:Sn molar ratios, i.e., Zn:Sn = 1% (ZSS1), Zn:Sn = 2.5% (ZSS2), Zn:Sn = 5% (ZSS3), and Zn:Sn = 7.5% (ZSS4), and demonstrated the superiority of heterojunction photocatalysts as the order of activity in terms of the degradation of ΜΒ follows the trend SnSe < ZnSe < ZSS4 < ZSS3 < ZSS1< ZSS2. As shown, there is an optimal Zn:Sn molar ratio, i.e., Zn:Sn = 2.5%, for the photocatalytic activity of ZSS, as excessive ZnSe reduces the carrier’s transmission efficiency. ZSS4, as the most active photocatalyst, was used for five consecutive catalytic cycles to degrade MB, consistently maintaining it at high levels. However, there was a slight increase in the third catalytic cycle, compared to the first, which was probably attributed to surface activation of the photocatalyst after illumination. In addition, the enhanced activity of ZSS2 against pure ZnSe and SnSe was also demonstrated in the case where LEV degradation was studied (Table 4). Photoelectrochemical experiments were also conducted and demonstrated the superiority of ZSS2 as it exhibited high carrier mobility and low internal resistance. In the study, carried out in order to shed light on the mechanism that led to the degradation of MB, the authors reported the production of hydroxyl radicals, as the primary ROS, from the oxidative reaction between photo-induced holes and water.
A study published by Du et al., 2022 [209] mentioned the two-step solvothermal synthesis of a novel dual functional Z-scheme CdSe/Se/BiOBr photocatalyst for H2O2 production and Ciprofloxacin (CIP), a common fluoroquinolone antibiotic, degradation. The interest in this study was the resulting flower-like structure (composed of numerous nanosheets) of the composite, which provided high specific surface area and abundant active sites, while the selenium doping provided enhanced light absorption and efficient charge carrier separation. Under visible light irradiation, photocatalytic H2O2 production was conducted using different weight ratios of CdSe/Se:BiOBr, i.e., CdSe/Se:BiOBr = 0.1 (CSB-0.1), CdSe/Se:BiOBr = 1 (CSB-1), CdSe/Se:BiOBr = 2 (CSB-2), and CdSe/Se:BiOBr = 5 (CSB-5). The authors concluded that CSB-2 was the best catalyst, as it exhibited the highest H2O2 production. The superiority of this photocatalyst over others was demonstrated, and its effective use for four cycles of H2O2 production was also mentioned, with the proposed mechanism that led to its production. Photocatalytic experiments of the different weight ratios of CdSe/Se:BiOBr in the CdSe/Se/BiOBr composite were conducted on the degradation of CIP, in order to test their photocatalytic activity. The order of activity followed the trend CdSe/Se < BiOBr < CSB-0.1 < CSB-5 < CSB-1< CSB-2 with the corresponding degradation rates shown in Table 4. As shown, all CSB composites exhibited higher photocatalytic degradation compared to bare CdSe/Se and BiOBr, with CSB-2 being the most active. Enhanced photocatalytic activity was also observed in the degradation of Triclosan (TCS) and phenol, with the corresponding degradation rates of 97.2% in 100 min and 90.1% in 3 min. The reuse of CSB-2 was reported for four photocatalytic CIP degradation cycles, observing a gradual decrease after each cycle; however, even after the fourth cycle, degradation reached 86% in 30 min. In addition, its photocatalytic activity was tested in various water matrices, showing satisfactory results and highlighting its potential application for water decontamination technologies. Finally, concerning the mechanism that led to CIP degradation, HO• and h+ played significant roles with a general photocatalytic mechanism of a Z-scheme heterostructure being depicted in Figure 6.
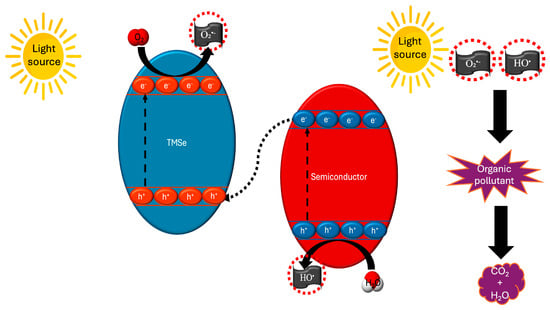
Figure 6.
Schematic illustration of the general photocatalytic mechanism of a Z-scheme heterostructure of TMSe/semiconductor for organic pollutant degradation.
A novel hollow cubic skeleton nanoflower cathode catalyst MoSe2/CNC (cubic nanocage), that was reported by Fan et al., 2025 [210], was synthesized through a co-hydrothermal method, and its catalytic activity was studied for the electro-activated PMS degradation of norfloxacin (NOR), a representative antibiotic pollutant. The synthesis of the MoSe2/CNC involved the use of CoFe Prussian blue analogs (CoFePBA) as precursor for the CNC, which was eventually decorated with MoSe2 nanosheets. The MoSe2/CNC provided high specific surface area and numerous active sites, enhancing electrical conductivity and catalytic performance. The electro-activated PMS system by the MoSe2/CNC electrode achieved 92.7% degradation of NOR in 120 min, while it was also reported to be used for six catalytic cycles, observing a small decrease (6.4%) during the sixth cycle. A study of the effect of various factors, such as catalyst dosage, PMS concentration, pH, current intensity, and precursors ratio (Co:Fe and MoSe2:CNC), was conducted in order to find optimal conditions. The optimization of Co:Fe ratio led to a higher surface area, allowing for more decoration of MoSe2, thereby increasing Mo sites, and the MoSe2:CNC optimization improved NOR degradation through the synergic effect between MoSe2 and CNC. A positive correlation was found between current intensity and NOR degradation as it enhanced PMS activation by electron transfer and redox cycles of Co(II)/Co(III), Fe(II)/Fe(III), and Mo(IV)/Mo(V)/Mo(VI). An increase in PMS dosage led to enhanced degradation, although beyond 0.5 mM, it had a negative effect as the excess of PMS could quench the sulfate radicals; however, the system still exhibited effective NOR degradation over a wide range of pH. Finally, regarding the degradation mechanism, although the formation of SO4•−, HO•, and 1O2 was reported, O2•− was identified as the dominant ROS, which was confirmed with radical quenching experiments and EPR tests. DFT and intermediate analysis revealed that the degradation pathway of NOR could occur in three potential pathways, including piperazine ring opening (Pathway I), defluorination and hydroxylation (Pathway II), and cleavage of C-N bond connecting the piperazine and benzene ring (Pathway III). Finally, toxicity assessments were conducted, including seed germination experiments, and confirmed that the intermediates exhibited significantly lower toxicity.
Novel polycationic selenides (PCS) were synthesized by hydrothermal method, and its applications as efficient solar-light-driven photocatalysts for the degradation of hazardous organic dyes Crystal Violet (CV) and Eosin-Y (EY) were presented by Nawaz et al., 2023 [211]. Specifically, the authors focused on the hydrothermal synthesis of a quaternary FeBiCoSe4 composite, as it has been reported that this category of selenides exhibits a multitude of desirable characteristics for electrocatalytic and photocatalytic applications, such as high surface area, reduced recombination of charge carriers, and maximum redox coupling [213]. The PCS exhibited a spherical morphology with a rough surface, providing numerous active sites. Photocatalytic experiments for the degradation of CV and EY were conducted under optimal conditions, while also the effect of parameters, such as pH, catalyst dosage, dye concentration, and irradiation time, was studied. A positive correlation was found between an increase in irradiation time and dye degradation, due to the availability of active sites on the surface of the photocatalyst, and an optimal catalyst dosage was reported, while further increase over this limit led to reduced photocatalytic degradation due to increased turbidity and lower production of electron–hole pairs, and while a similar effect was observed in the case of dye concentration, as an increase beyond the optimal limit led to decreased degradation as a greater number of dye molecules were adsorbed on the surface of the photocatalyst, resulting in lower production of electron–hole pairs. On the effect of pH, EY, as an ionic dye, exhibited high degradation at pH = 5 due to strong electrostatic interactions with the catalyst, while CV, as a cationic dye, exhibited high degradation at pH = 11. Therefore, under the optimal conditions, PCS showed degradation rates of 99.47% and 99.31% in 60 min for CV and EY, respectively. Recyclability and stability of the PCS was also studied during three consecutive cycles, maintaining performance at high levels, even though a slight decrease was observed due to the blocking of the active centers.
The study presented by Sajjadi et al., 2017 [212] introduced a 3-step sonochemical-hydrothermal synthesis of CdSe/GQDs (graphene quantum dots) for the efficient sonocatalytic degradation of MB. CdSe nanoparticles were first prepared, then GQDs were generated from citric acid, and, finally, CdSe was coated with GQDs. GQD coating on CdSe led to enhanced dispersion, preventing agglomeration of CdSe nanoparticles and improving electron transmission. The sonocatalytic performance of unmodified CdSe nanoparticles and CdSe/GQDs was studied regarding the MB degradation and revealed that the sonodegradation efficiency for sonolysis, CdSe nanoparticles, and CdSe/GQDs nanocatalyst were 13.8%, 33.6%, and 99% in 90 min, respectively. The low generation of hydroxyl radicals in sonolysis is significantly improved with the use of CdSe/GQDs, and it increased the number of cavitation bubbles and enhanced mass transfer. The effect of main operational parameters (catalyst dosage, MB concentration, pH, and ultrasonic bath power) on the sonocatalytic performance was studied and revealed that maximum activity was observed at pH = 9, where more hydroxyl radicals were generated. A positive correlation was found in the increase in ultrasonic bath power, as higher power led to improved generation of hydroxyl radicals, while an increase in catalyst dosage up to 1 g L−1 led to increased degradation efficiency, while further increase had an inhibiting effect due to nanoparticle aggregation, thus reducing active sites and generation of hydroxyl radicals. A negative effect was also observed with the increase in MB concentration, possibly due to the reduced number of surface-active centers, increased adsorbed MB molecules, and absorption of UV light by these molecules. In addition, the effect of different GQD amounts was studied (1.5, 2.5, and 3.5 wt%), and it was observed that an increase beyond the optimal value (2.5 wt%) inhibited the process due to the electron–hole recombination. Further experiments showed that the addition of oxidants such as PS and H2O2 further improved the degradation of MB. Finally, the excellent reusability of CdSe/GQDs was demonstrated, as it was used effectively for five cycles, with minimal activity loss, and with the help of GC-MS, and the intermediates were identified, and a possible pathway was suggested.
In summary, selenide composite catalysts exhibited superior catalytic performance due to enhanced charge carrier separation, broader light absorption, and synergistic redox activity between different active centers. Main synthesis paths include hydrothermal or solvothermal routes, used in a plethora of AOPs, such as photocatalysis, SR-AOPs, sonocatalysis, etc, leading to the generation of a variety of ROS that contribute to organic pollutant degradation. Optimal degradation performance of a wide range of organic pollutants is achieved under near neutral pH (6–8), with catalyst dosage of 0.2–1.0 g/L and pollutant concentration of 20–80 mg/L, and reusability of 4–6 catalytic cycles is being observed, while maintaining their catalytic activity. Overall, composite selenides represent a robust class of materials for AOPs, offering a balance between high activity, structural stability, and practical applicability.
4. Current Trends and Future Research Needs
As revealed, the use of transition metal selenides in processes of degradation of organic pollutants from water has garnered significant interest from the scientific community in recent years. Their application extends beyond just one degradation process, such as photocatalysis, which is the most studied for these materials based on Figure 7, and encompasses a wide range of AOPs.
Figure 7.
Pie diagram showing the number of papers in this review regarding (a) the different AOPs that were utilized for the degradation of organic pollutants and (b) the different categories of organic pollutants.
In recent years, trends have shifted towards simpler and eco-friendly synthetic methods, such as hydrothermal or solvothermal methods, due to their simple process, easy parameterization of synthesis conditions (time, temperature, and selection of precursors), and better control of morphology and electronic structures of TMSes, characteristics that greatly affect the catalytic activity of final materials.
A particularly innovative and promising development in the field is the hybridization of photocatalysis with other AOPs, such as sonocatalysis, electrocatalysis, and PMS/PS activation, in order to enhance the degradation efficiency of the system. With the implementation of these hybrid AOPs, an enhanced catalytic efficiency is observed due to the flexibility under varied environmental conditions and the exploitation of multiple ROS generation pathways.
As highlighted in Figure 7, many of the studies referenced in this review article primarily focus on the catalytic activity of TMSes concerning dye degradation. However, wastewater streams typically contain complex and persistent organic pollutants such as pharmaceuticals, pesticides, industrial solvents, personal care products, etc. In addition, a comparative study of the toxicity of the intermediates to the parent compound is carried out in very few studies in order to shed light on the efficiency of the degradation process in terms of the toxicity assessment. Therefore, future research should concentrate on studying the toxicity of intermediates, understanding their formation through degradation pathways, and expanding the scope of targeted pollutants to assess the environmental safety of treated effluents.
Mechanistic studies concerning the degradation pathways and the ROS generation are gaining momentum, as they significantly influence the catalytic activity of the system. However, topics such as the stability of the TMSes, particularly metal leaching and structural stability, which are key considerations for their practical implementation on wastewater treatment technologies, remain underdeveloped, and future studies should explore these areas to assess the true viability of TMSes in water decontamination technologies.
A direct comparison of catalytic performance among different TMSe-based materials is challenging because reported studies often employ different reaction conditions, such as pollutants, concentrations, light sources, etc. For direct comparison, it is essential that future works adopt standardized model pollutants and/or quantitative ROS determination methods. Such harmonization would allow more reliable cross-study comparisons and facilitate the rational design of more effective TMSe-based catalysts.
5. Conclusions
In conclusion, transition metal selenide-based catalysts have emerged as a dynamic and versatile class of materials for the degradation of organic pollutants through AOPs. Desirable characteristics such as unique electronic structures, tunable band gaps, high surface reactivity, and ability to facilitate multi-electron redox reactions under visible light or alternative activation routes (electrochemical, ultrasonic), render them highly attractive candidates for water decontamination technologies. The strong foundations for the utilization of these TMSes in AOPs were first established by the synthesis and use of simple selenide catalysts (FeSe2, MoSe2, etc.), with the help of which the scientific community understands the advantages and disadvantages of these materials. With this knowledge, and in order to overcome the obstacles typically presented in the use of standalone catalysts, new paths have been drawn, mainly concerning the synthesis of multi-metallic, composite systems integrating TMSes with g-C3N4 or TiO2 and heterostructured TMSe systems, materials that exhibit enhanced catalytic activity against the pristine ones, primarily due to the synergistic properties between different cationic centers and enhanced charge carrier separation dynamics.
Transition metal selenide (TMSe)-based catalysts have been reported to generate a variety of ROS such as •OH, O2•−, SO4•−, and 1O2, depending each time on the activation pathway and the nature of the composite system (e.g., TMSe/g-C3N4, TMSe/TiO2). The degradation ability of these ROS is strongly dependent on both the structure of the pollutant and the catalytic environment. Generally, •OH and SO4•− exhibit the highest oxidation potential and are capable of degradation of a wide spectrum of organic pollutants, while O2•− and 1O2 typically play a complementary role, facilitating selective oxidation pathways and improving overall efficiency through synergistic effects. Thus, the type and relative contribution of each ROS in TMSe-based AOPs are case-dependent, governed by the catalyst design, operational conditions, and the pollutant’s molecular structure.
Furthermore, innovations in green synthesis techniques, surface engineering, and bandgap tuning have further pushed the performance boundaries. Overall, the most critical structural properties of TMSe-based catalysts that govern pollutant degradation efficiency include high specific surface area, suitable band gap energy (Eg) for visible-light activation, optimized charge carrier separation, and strong interfacial contact in composites. Among the various synthesis approaches, hydrothermal/solvothermal methods and post-synthetic heterojunction synthesis have proven to be most effective in tailoring these beneficial features, thereby enhancing the catalytic performance of TMSe-based systems.
Despite this development in the use of TMSes in organic pollutant degradation processes, the studies that have been presented in this review article that focus on the stability of those TMSes were reported as successful for at least five consecutive catalytic cycles; however, few studies analyze catalyst deactivation, leaching behavior, changes in crystallinity, or phase transformation that can occur over these multiple cycles. An interesting approach, as it can lead to faster degradation rates, improved efficiency, and multiple activation pathways (such as light excitation + sulfate radical generation/electrochemical processes), lies in the combination of photocatalysis with other AOPs, and should be further explored due to the multitude of advantages that it offers compared to conventional AOPs. It is, therefore, necessary to adequately answer these unanswered questions in the future so that TMSes can transition from laboratory studies to real-world decontamination technologies and be applied in real-world wastewater systems. The integration of hybrid AOPs, in combination with a complete mechanistic study of degradation pathways, toxicity levels of intermediates, the effect of various parameters on the degradation process, the stability and reuse of TMSes, and, finally, the scalable synthesis of TMSes, could be the key to unlocking the next stage of efficient and sustainable real-world pollutant degradation systems.
Author Contributions
Conceptualization, I.K.; methodology, D.M.; formal analysis, D.M.; investigation, D.M.; resources, I.K.; writing—original draft preparation, D.M.; writing—review and editing, I.K.; visualization, D.M.; supervision, I.K.; funding acquisition, D.M. and I.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 5th Call for HFRI PhD Fellowships (Fellowship Number: 83599).
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Activated carbon |
| ACA | Anthracene-9-carboxylic acid |
| BPA | Bisphenol A |
| BZP | Benzophenone-3 |
| CB | Conduction band |
| CBZ | Carbamazepine |
| CFS | Carbon fiber sheet |
| CIP | Ciprofloxacin |
| CNC | Carbon nanocage |
| CoFePBA | CoFe Prussian blue analogs |
| CR | Congo red |
| CTC | Chlortetracycline |
| CTX | Ceftriaxone |
| CV | Crystal violet |
| CVD | Chemical vapor deposition |
| E-AOPs | Electrochemical advanced oxidation processes |
| EY | Eosin-Y |
| GQDs | Graphene quantum dots |
| HA | Humic acid |
| HER | Hydrogen evolution reaction |
| HIPE | High internal phase emulsion |
| IBU | Ibuprofen |
| LEV | Levofloxacin |
| LPE | liquid phase exfoliation |
| MB | Methylene blue |
| MO | Methyl orange |
| MSes | Metal selenides |
| NOM | Natural organic matter |
| NOR | Norfloxacin |
| OER | Oxygen evolution reaction |
| ORR | Oxygen reduction reaction |
| OTC | Oxytetracycline |
| PAA | Poly(acrylic acid |
| PCB28 | 2,4,4′-Trichlorobiphenyl |
| PCS | Polycationic selenides |
| PFOA | Perfluorooctanoic acid |
| PMS | Peroxymonosulfate |
| PS | Persulfate |
| QDs | Quantum dots |
| RB 5 | Reactive Blue 5 |
| RhB | Rhodamine B |
| ROS | Reactive oxygen species |
| RR120 | Reactive Red 120 |
| RV 5 | Reactive violet 5 |
| SMX | Sulfamethoxazole |
| SR-AOPs | Sulftate-radical advanced oxidation processes |
| TC-HCl | Tetracycline hydrochloride |
| TCH | Tetracycline hydrochloride |
| TCS | Triclosan |
| TMCs | Transition metal chalcogenides |
| TMDSes | Transition metal dichalcogenides |
| TMSes | Transition metal selenides |
| UV | Ultraviolet |
| VB | Valence band |
References
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- McMichael, S.; Waso, M.; Reyneke, B.; Khan, W.; Byrne, J.A.; Fernandez-Ibanez, P. Electrochemically Assisted Photocatalysis for the Disinfection of Rainwater under Solar Irradiation. Appl. Catal. B Environ. 2021, 281, 119485. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-Steroidal Anti-Inflammatory Drugs in the Environment: Where Were We and How Far We Have Come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological Effects, Environmental Fate and Risks of Pharmaceutical and Personal Care Products in the Water Environment: A Review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef]
- Heryanto, H.; Tahir, D. Trends, Mechanisms, and the Role of Advanced Oxidation Processes in Mitigating Azo, Triarylmethane, and Anthraquinone Dye Pollution: A Bibliometric Analysis. Inorg. Chem. Commun. 2025, 175, 114104. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Terreros, G.; Cifuentes-Cabello, C.; D’Espessailles, A.; Munoz, F. Impact of Pesticide Exposure on Auditory Health: Mechanisms, Efferent System Disruption, and Public Health Implications. Toxicology 2025, 512, 154071. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Z.; Yao, B.; Zhou, Y. Occurrence, Bioaccumulation, Fate, and Risk Assessment of Emerging Pollutants in Aquatic Environments: A Review. Sci. Total Environ. 2024, 923, 171388. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, J.; Xiong, W.; Yang, Z.; Sun, S.; Jia, M.; Xu, Z. In-Situ Growing of Metal-Organic Frameworks on Three-Dimensional Iron Network as an Efficient Adsorbent for Antibiotics Removal. Chem. Eng. J. 2020, 392, 124844. [Google Scholar] [CrossRef]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of Nitroimidazole Antibiotics from Water by Adsorption over Metal–Organic Frameworks Modified with Urea or Melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously Efficient Adsorption and Photocatalytic Degradation of Tetracycline by Fe-Based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Jin, X.; Guo, J.; Hossain, M.F.; Lu, J.; Lu, Q.; Zhou, Y.; Zhou, Y. Recent Advances in the Removal and Recovery of Phosphorus from Aqueous Solution by Metal-Based Adsorbents: A Review. Resour. Conserv. Recycl. 2024, 204, 107464. [Google Scholar] [CrossRef]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J. Removal of Phenolic Compounds from Industrial Waste Water Based on Membrane-Based Technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of Heavy Metals from Water Sources in the Developing World Using Low-Cost Materials: A Review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, S.; Xia, L.; Wang, Z.; Suo, N.; Chen, H.; Long, Y.; Zhou, B.; Yu, Y. In-Situ Ion Exchange Electrocatalysis Biological Coupling (i-IEEBC) for Simultaneously Enhanced Degradation of Organic Pollutants and Heavy Metals in Electroplating Wastewater. J. Hazard. Mater. 2019, 364, 562–570. [Google Scholar] [CrossRef]
- Hao, J.; Ji, L.; Li, C.; Hu, C.; Wu, K. Rapid, Efficient and Economic Removal of Organic Dyes and Heavy Metals from Wastewater by Zinc-Induced in-Situ Reduction and Precipitation of Graphene Oxide. J. Taiwan Inst. Chem. Eng. 2018, 88, 137–145. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Patel, N.; Srivastav, A.L.; Patel, A.; Singh, A.; Singh, S.K.; Chaudhary, V.K.; Singh, P.K.; Bhunia, B. Nitrate Contamination in Water Resources, Human Health Risks and Its Remediation through Adsorption: A Focused Review. Environ. Sci. Pollut. Res. 2022, 29, 69137–69152. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, B.; Wang, Z.; Tang, L.; Ji, C.; Zhao, C.; Feng, L.; Feng, Y. Homogeneous/Heterogeneous Metal-Catalyzed Persulfate Oxidation Technology for Organic Pollutants Elimination: A Review. J. Environ. Chem. Eng. 2023, 11, 109586. [Google Scholar] [CrossRef]
- Huang, C.P.; Dong, C.; Tang, Z. Advanced Chemical Oxidation: Its Present Role and Potential Future in Hazardous Waste Treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Machulek, A., Jr.; Oliveira, S.C.; Osugi, M.E.; Ferreira, V.S.; Quina, F.H.; Dantas, R.F.; Oliveira, S.L.; Casagrande, G.A.; Anaissi, F.J.; Silva, V.O.; et al. Application of Different Advanced Oxidation Processes for the Degradation of Organic Pollutants. In Organic Pollutants—Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A Comprehensive Review on Reactive Oxygen Species (ROS) in Advanced Oxidation Processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Ma, D.; Yang, Y.; Liu, B.; Xie, G.; Chen, C.; Ren, N.; Xing, D. Zero-Valent Iron and Biochar Composite with High Specific Surface Area via K2FeO4 Fabrication Enhances Sulfadiazine Removal by Persulfate Activation. Chem. Eng. J. 2021, 408, 127992. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Jin, X.; Tatit, U.L.; Luo, X.; Zhou, Y.; Zhou, Y. Strategies to Enhance the Catalytic Activity of MoS2 in Advanced Oxidation Processes: A Review. Sep. Purif. Technol. 2025, 356, 129841. [Google Scholar] [CrossRef]
- Huo, X.; Yang, Y.; Niu, Q.; Zhu, Y.; Zeng, G.; Lai, C.; Yi, H.; Li, M.; An, Z.; Huang, D.; et al. A Direct Z-Scheme Oxygen Vacant BWO/Oxygen-Enriched Graphitic Carbon Nitride Polymer Heterojunction with Enhanced Photocatalytic Activity. Chem. Eng. J. 2021, 403, 126363. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; Vilar, V.J.P. Strategies for the Intensification of Photocatalytic Oxidation Processes towards Air Streams Decontamination: A Review. Chem. Eng. J. 2020, 391, 123531. [Google Scholar] [CrossRef]
- Lai, C.; An, N.; Li, B.; Zhang, M.; Yi, H.; Liu, S.; Qin, L.; Liu, X.; Li, L.; Fu, Y.; et al. Future Roadmap on Nonmetal-Based 2D Ultrathin Nanomaterials for Photocatalysis. Chem. Eng. J. 2021, 406, 126780. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate Radical Anion Oxidation of Diclofenac and Sulfamethoxazole for Water Decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Zhang, B.T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate Radical and Its Application in Decontamination Technologies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1756–1800. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbk, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of Peroxodisulfate and Peroxymonosulfate by Ultrasound with Different Frequencies: Impact on Ibuprofen Removal Efficient, Cost Estimation and Energy Analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems. Catalysts 2020, 10, 1299. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the Electrochemical Conversion/Combustion of Organic Pollutants for Waste Water Treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods: A General Review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli, G.; Belen, R.K.; Apikyan, G. Ultrasound as a Catalyzer of Aqueous Reaction Systems: The State of the Art and Environmental Applications. Appl. Catal. B Environ. 2001, 29, 167–176. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced Oxidation Processes for the Treatment of Contaminants of Emerging Concern. In Contaminants of Emerging Concern in Water and Wastewater: Advanced Treatment Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 299–365. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Wang, H.; Miao, S.; Deng, Z.; Yin, L. Activation of Peroxymonosulfate by Fe Based Metal Organic Framework for Selective Degradation of Phenol in Water. J. Water Process Eng. 2024, 68, 106454. [Google Scholar] [CrossRef]
- Li, F.; Yuan, C.; Niu, Y.; Sheng, J.; Wang, X.; Shi, Y.; Jiang, H. Cobalt/Iron Bimetallic Oxide Coated with Graphitized Nitrogen-Doped Carbon (Fe2O3-CoO@NC) Derived from Cobalt/Iron Solid Complex as Peroxymonosulfate (PMS) Activator for Efficient Bensulfuron-Methyl Degradation. Environ. Res. 2024, 263, 120249. [Google Scholar] [CrossRef]
- Peng, H.; Gu, H.; Xu, Z.; Xiong, G.; Gao, P.; Wang, S.; Li, X.; Li, F. Degradation Mechanisms and Toxicity Determination of Bisphenol A by FeOx-Activated Peroxydisulfate under Ultraviolet Light. Environ. Technol. 2024, 46, 13–24. [Google Scholar] [CrossRef]
- Ge, Y.; Zhong, X.; Wang, K.; Huang, L.; Zheng, Z.; Wu, C.; Fang, S.; Lin, X.; Lin, J. CoFe2O4-Catalytic Ceramic Membrane for Efficient Carbamazepine Removal via Peroxymonosulfate Activation. Sep. Purif. Technol. 2025, 357, 129876. [Google Scholar] [CrossRef]
- Gao, D.; Wang, H.; Lu, Q.; Ye, C.; Cai, J.; Wang, L. Enhanced PMS Activation Efficiency and SMX Degradation Performance via Fe(II)/Fe(III) Cycling with Efficient CCF@MoS2@GA-Fe Catalyst. Environ. Res. 2025, 274, 121299. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, S.B.; Zhao, F.; Safaei, Z.; Srivastava, V.; Ramasamy, D.L.; Iftekhar, S.; Kalliola, S.; Sillanpää, M. Degradation and Mineralization of Phenol in Aqueous Medium by Heterogeneous Monopersulfate Activation on Nanostructured Cobalt Based-Perovskite Catalysts ACoO3 (A = La, Ba, Sr and Ce): Characterization, Kinetics and Mechanism Study. Appl. Catal. B Environ. 2017, 215, 60–73. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient Degradation of Atrazine by LaCoO3/Al2O3 Catalyzed Peroxymonosulfate: Performance, Degradation Intermediates and Mechanism. Chem. Eng. J. 2019, 372, 796–808. [Google Scholar] [CrossRef]
- Yamada, Y.; Yano, K.; Hong, D.; Fukuzumi, S. LaCoO 3 Acting as an Efficient and Robust Catalyst for Photocatalytic Water Oxidation with Persulfate. Phys. Chem. Chem. Phys. 2012, 14, 5753–5760. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Guo, Y.; Zhang, Y.; Xu, B.; Qi, F. LaCoO3 Perovskite Oxide Activation of Peroxymonosulfate for Aqueous 2-Phenyl-5-Sulfobenzimidazole Degradation: Effect of Synthetic Method and the Reaction Mechanism. Chem. Eng. J. 2016, 304, 897–907. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, L.; Wang, Y.; Qiao, H.; Zhao, Y.; Li, D.; Bu, Y. Accelerated Peroxymonosulfate Activation over Defective Perovskite with Anchored Cobalt Nanoparticles for Organic Contaminant Removal. Chem. Eng. J. 2024, 496, 153712. [Google Scholar] [CrossRef]
- Niu, J.; He, T.; Cheng, J. In-Situ Synthesis of Carbon-Coated Co3O4 Nanowire for Efficient Activation of Peroxymonosulfate While Reducing Ion Leaching through Protection and Secondary Enrichment. Chem. Eng. J. 2024, 500, 157301. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Ye, L.; Li, X.; Lv, S.W. A Newly-Designed Free-Standing NiCo2O4 Nanosheet Array as Effective Mediator to Activate Peroxymonosulfate for Rapid Degradation of Emerging Organic Pollutant with High Concentration. Chemosphere 2022, 307, 136073. [Google Scholar] [CrossRef]
- Pan, X.; Pu, J.; Zhang, L.; Gong, X.; Luo, X.; Fan, L. Bimetallic Iron-Nickel Phosphide as Efficient Peroxymonosulfate Activator for Tetracycline Hydrochloride Degradation: Performance and Mechanism. Environ. Res. 2024, 249, 118362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Zhang, X.; Wang, S. Highly Efficient Targeted Adsorption and Catalytic Degradation of Ciprofloxacin by a Novel Molecularly Imprinted Bimetallic MOFs Catalyst for Persulfate Activation. Chemosphere 2024, 357, 141894. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Pan, S.; Lu, J.; Asif, A.H.; Cui, S.; Wang, S.; Sun, H. Ruthenium Decorated Nickel-Iron Layered Double Hydroxides (NiFe-LDH) for Promoting Peroxymonosulfate Activation and Atrazine Degradation. J. Environ. Chem. Eng. 2024, 12, 112996. [Google Scholar] [CrossRef]
- Fu, C.; Yi, X.; Liu, Y.; Zhou, H. Cu2+ Activated Persulfate for Sulfamethazine Degradation. Chemosphere 2020, 257, 127294. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Ma, J.; Pang, S.; Zhang, T. A Review on Advanced Oxidation Processes Homogeneously Initiated by Copper(II). Chem. Eng. J. 2022, 427, 131721. [Google Scholar] [CrossRef]
- Peng, J.; Chang, Y.; Xu, L.; Zhang, Y.; Wang, T.; Wang, X.; Liu, H.; Yan, G.; Gao, S.; Liu, D.; et al. Insights into the Enhanced Removal of Sulfamethoxazole via Peroxymonosulfate Activation Catalyzed by Bimetallic (Co/Cu) Doped Graphitic Carbon Nitride: Reaction Kinetics, Mechanisms, and Pathways. Chem. Eng. J. 2023, 476, 146692. [Google Scholar] [CrossRef]
- Okpara, E.C.; Wojuola, O.B.; Quadri, T.W.; Banks, C.E. An Overview of Advanced Oxidation Processes Using Copper-Based Catalytic Degradation of Organic Pollutants in Water. Appl. Mater. Today 2024, 36, 102053. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Shan, Y.; Yang, S.; Wu, L.; Shi, T. Degradation of Ciprofloxacin by Magnetic CuS/MnFe2O4 Catalysts Efficiently Activated Peroxymonosulfate. J. Taiwan Inst. Chem. Eng. 2024, 161, 105533. [Google Scholar] [CrossRef]
- Liu, J.; Lai, Z.; Wang, K.; Wu, J.; Yang, S.; Liu, Z.; Meng, G.; Guo, X. Template-Based Fabrication of Copper Oxide for Persulfate Activation: Investigating Non-Radical Mechanisms in Efficient Bisphenol a Degradation. Korean J. Chem. Eng. 2025, 42, 843–855. [Google Scholar] [CrossRef]
- Eisa, T.; Abdelkareem, M.A.; Jadhav, D.A.; Mohamed, H.O.; Sayed, E.T.; Olabi, A.G.; Castaño, P.; Chae, K.J. Critical Review on the Synthesis, Characterization, and Application of Highly Efficient Metal Chalcogenide Catalysts for Fuel Cells. Prog. Energy Combust. Sci. 2023, 94, 101044. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.J.; Jie, J.S.; Meng, X.M.; Zapien, J.A.; Lee, S.T. Homoepitaxial Growth and Lasing Properties of ZnS Nanowire and Nanoribbon Arrays. Adv. Mater. 2006, 18, 1527–1532. [Google Scholar] [CrossRef]
- Radovanovic, P.V.; Barrelet, C.J.; Gradečak, S.; Qian, F.; Lieber, C.M. General Synthesis of Manganese-Doped II-VI and III-V Semiconductor Nanowires. Nano Lett. 2005, 5, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Wang, Z.L. Growth of Anisotropic One-Dimensional ZnS Nanostructures. J. Mater. Chem. 2006, 16, 3898–3905. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Rozati, S.M. Electrocatalytic Properties of CoS2/MoS2/RGO as a Non-Noble Dual Metal Electrocatalyst: The Investigation of Hydrogen Evolution and Methanol Oxidation. J. Phys. Chem. Solids 2019, 135, 109103. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Bahaa, A.; Eisa, T.; Alawadhi, H.; Al-Asheh, S.; Chae, K.J.; Olabi, A.G. Synthesis and Performance Evaluation of Various Metal Chalcogenides as Active Anodes for Direct Urea Fuel Cells. Renew. Sustain. Energy Rev. 2021, 150, 111470. [Google Scholar] [CrossRef]
- Sarkar, S.; Patel, S.; Sampath, S. Efficient Oxygen Reduction Activity on Layered Palladium Phosphosulphide and Its Application in Alkaline Fuel Cells. J. Power Sources 2020, 445, 227280. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Synthesis and Characterization of a Nickel Selenide Series via a Hydrothermal Process. Superlattices Microstruct. 2014, 65, 79–90. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Transition Metal Selenides and Diselenides: Hydrothermal Fabrication, Investigation of Morphology, Particle Size and and Their Applications in Photocatalyst. Adv. Colloid Interface Sci. 2021, 287, 102321. [Google Scholar] [CrossRef]
- Schäfer, A.; Kollwitz, M.; Ahlrichs, R. Electronic Excitation Energies in Copper Selenide Clusters. J. Chem. Phys. 1996, 104, 7113–7121. [Google Scholar] [CrossRef]
- Verble, J.L.; Humphrey, F.M. Infrared and Raman Spectra of MnS2. Solid State Commun. 1974, 15, 1693–1697. [Google Scholar] [CrossRef]
- Wilson, J.A.; Di Salvo, F.J.; Mahajan, S. Charge-Density Waves and Superlattices in the Metallic Layered Transition Metal Dichalcogenides. Adv. Phys. 1975, 24, 117–201. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Peng, X.; Yan, Y.; Jin, X.; Huang, C.; Jin, W.; Gao, B.; Chu, P.K. Recent Advance and Prospectives of Electrocatalysts Based on Transition Metal Selenides for Efficient Water Splitting. Nano Energy 2020, 78, 105234. [Google Scholar] [CrossRef]
- Keszler, D.A.; Ibers, J.A.; Maoyu, S.; Jiaxi, L. New Ternary and Quaternary Transition-Metal Selenides: Syntheses and Characterization. J. Solid State Chem. 1985, 57, 68–81. [Google Scholar] [CrossRef]
- Wilson, J.A.; Yoffe, A.D. The Transition Metal Dichalcogenides Discussion and Interpretation of the Observed Optical, Electrical and Structural Properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Lu, T.; Dong, S.; Zhang, C.; Zhang, L.; Cui, G. Fabrication of Transition Metal Selenides and Their Applications in Energy Storage. Coord. Chem. Rev. 2017, 332, 75–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Zhao, H.; Du, Y. MoSe2 Nanosheets Grown on Carbon Cloth with Superior Electrochemical Performance as Flexible Electrode for Sodium Ion Batteries. RSC Adv. 2015, 6, 1440–1444. [Google Scholar] [CrossRef]
- Wickramaratne, D.; Khmelevskyi, S.; Agterberg, D.F.; Mazin, I.I. Ising Superconductivity and Magnetism in NbSe2. Phys. Rev. X 2020, 10, 041003. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Yan, Y.; Cao, J.; Li, X.; Zhou, S.; Peng, Z.; Zeng, J. Engineering the Electrical Conductivity of Lamellar Silver-Doped Cobalt(II) Selenide Nanobelts for Enhanced Oxygen Evolution. Angew. Chem. 2017, 129, 334–338. [Google Scholar] [CrossRef]
- Swesi, A.T.; Masud, J.; Nath, M. Nickel Selenide as a High-Efficiency Catalyst for Oxygen Evolution Reaction. Energy Environ. Sci. 2016, 9, 1771–1782. [Google Scholar] [CrossRef]
- Gao, D.; Xia, B.; Wang, Y.; Xiao, W.; Xi, P.; Xue, D.; Ding, J. Dual-Native Vacancy Activated Basal Plane and Conductivity of MoSe2 with High-Efficiency Hydrogen Evolution Reaction. Small 2018, 14, 1704150. [Google Scholar] [CrossRef] [PubMed]
- Rajaramanan, T.; Shanmugaratnam, S.; Gurunanthanan, V.; Yohi, S.; Velauthapillai, D.; Ravirajan, P.; Senthilnanthanan, M. Cost Effective Solvothermal Method to Synthesize Zn-Doped TiO2 Nanomaterials for Photovoltaic and Photocatalytic Degradation Applications. Catalysts 2021, 11, 690. [Google Scholar] [CrossRef]
- Pirashanthan, A.; Murugathas, T.; Mariappan, K.; Ravirajan, P.; Velauthapillai, D.; Yohi, S. A Multifunctional Ruthenium Based Dye for Hybrid Nanocrystalline Titanium Dioxide/Poly(3-Hexylthiophene) Solar Cells. Mater. Lett. 2020, 274, 127997. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Antonatos, N.; Luxa, J.; Děkanovský, L.; Ashtiani, S.; Fomekong, R.L.; Sofer, Z. Two-Dimensional VSe2 Nanoflakes as a Promising Sensing Electrocatalyst for Nitrobenzene Determination in Water Samples. Electrochim. Acta 2024, 475, 143653. [Google Scholar] [CrossRef]
- Baek, J.; Yin, D.; Liu, N.; Omkaram, I.; Jung, C.; Im, H.; Hong, S.; Kim, S.M.; Hong, Y.K.; Hur, J.; et al. A Highly Sensitive Chemical Gas Detecting Transistor Based on Highly Crystalline CVD-Grown MoSe2 Films. Nano Res. 2017, 10, 1861–1871. [Google Scholar] [CrossRef]
- Malavekar, D.B.; Jadhav, S.B.; Kale, S.B.; Patil, U.M.; Lokhande, C.D. Surface Modification of Copper Selenide for Reliable Non-Enzymatic Glucose Sensing. Mater. Today Sustain. 2022, 20, 100215. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, J.; Zhang, W.; Hu, N.; Wang, J.; Huang, L.; Wang, R.; Suo, Y.; Wang, J. Monolithic Copper Selenide Submicron Particulate Film/Copper Foam Anode Catalyst for Ultrasensitive Electrochemical Glucose Sensing in Human Blood Serum. J. Mater. Chem. B 2018, 6, 718–724. [Google Scholar] [CrossRef]
- Umapathi, S.; Singh, H.; Masud, J.; Nath, M. Nanostructured Copper Selenide as an Ultrasensitive and Selective Non-Enzymatic Glucose Sensor. Mater. Adv. 2021, 2, 927–932. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Lei, J.; Dong, W.; Wang, J. Integrated 3D MoSe2@Ni0.85Se Nanowire Network with Synergistic Cooperation as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Medium. Electrochim. Acta 2017, 246, 712–719. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Huang, Y.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal Synthesis of N-Doped RGO/MoSe2 Composites and Enhanced Electro-Catalytic Hydrogen Evolution. J. Mater. Sci. 2017, 52, 13561–13571. [Google Scholar] [CrossRef]
- Nitsche, F.; Goltz, T.; Klauss, H.H.; Isaeva, A.; Müller, U.; Schnelle, W.; Simon, P.; Doert, T.; Ruck, M. Room-Temperature Synthesis, Hydrothermal Recrystallization, and Properties of Metastable Stoichiometric FeSe. Inorg. Chem. 2012, 51, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, X.; Che, G.; Fan, W.; Liu, C. Controlled Hydrothermal Synthesis and Magnetic Properties of Three-Dimensional FeSe2 Rod Clusters and Microspheres. Chem. Eng. J. 2013, 215–216, 508–516. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically Oriented Cobalt Selenide/NiFe Layered-Double-Hydroxide Nanosheets Supported on Exfoliated Graphene Foil: An Efficient 3D Electrode for Overall Water Splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef]
- Gao, M.R.; Liang, J.X.; Zheng, Y.R.; Xu, Y.F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.H. An Efficient Molybdenum Disulfide/Cobalt Diselenide Hybrid Catalyst for Electrochemical Hydrogen Generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef]
- Moloto, N.; Moloto, M.J.; Coville, N.J.; Sinha Ray, S. Optical and Structural Characterization of Nickel Selenide Nanoparticles Synthesized by Simple Methods. J. Cryst. Growth 2009, 311, 3924–3932. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Sobhani, A. Effect of Nickel Salt Precursors on Morphology, Size, Optical Property and Type of Products (NiSe or Se) in Hydrothermal Method. Opt. Mater. 2013, 35, 904–909. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Synthesis and Characterization of CdSe Nanostructures by Using a New Selenium Source: Effect of Hydrothermal Preparation Conditions. Mater. Res. Bull. 2014, 53, 7–14. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Chen, S.; Liang, B. Hydrothermal Synthesis of CdSe Hierarchical Dendrites Using Ionic Liquid as Template. Mater. Lett. 2012, 66, 264–266. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. A New Simple Route for the Preparation of Nanosized Copper Selenides under Different Conditions. Ceram. Int. 2014, 40, 8173–8182. [Google Scholar] [CrossRef]
- Gong, H.; Huang, H.; Wang, M.; Liu, K. Characterization and Growth Mechanism of ZnSe Microspheres Prepared by Hydrothermal Synthesis. Ceram. Int. 2007, 33, 1381–1384. [Google Scholar] [CrossRef]
- Zhu, C.; Gao, D.; Ding, J.; Chao, D.; Wang, J. TMD-Based Highly Efficient Electrocatalysts Developed by Combined Computational and Experimental Approaches. Chem. Soc. Rev. 2018, 47, 4332–4356. [Google Scholar] [CrossRef]
- Zeng, H.; Dai, J.; Yao, W.; Xiao, D.; Cui, X. Valley Polarization in MoS2 Monolayers by Optical Pumping. Nat. Nanotechnol. 2012, 7, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wang, G.; Han, W.; Ye, H.; Zhu, C.; Shi, J.; Niu, Q.; Tan, P.; Wang, E.; Liu, B.; et al. Valley-Selective Circular Dichroism of Monolayer Molybdenum Disulphide. Nat. Commun. 2012, 3, 887. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Y.A.; Ivanou, D.K.; Streltsov, E.A. Electrodeposition of PbSe onto N-Si(100) Wafers. Electrochim. Acta 2008, 53, 5051–5057. [Google Scholar] [CrossRef]
- Mahalingam, T.; Thanikaikarasan, S.; Dhanasekaran, V.; Kathalingam, A.; Velumani, S.; Rhee, J.K. Preparation and Characterization of MnSe Thin Films. Mater. Sci. Eng. B 2010, 174, 257–262. [Google Scholar] [CrossRef]
- Erenturk, B.; Gurbuz, S.; Corbett, R.E.; Claiborne, S.A.M.; Krizan, J.; Venkataraman, D.; Carter, K.R. Formation of Crystalline Cadmium Selenide Nanowires. Chem. Mater. 2011, 23, 3371–3376. [Google Scholar] [CrossRef]
- Demura, S.; Okazaki, H.; Ozaki, T.; Hara, H.; Kawasaki, Y.; Deguchi, K.; Watanabe, T.; Denholme, S.J.; Mizuguchi, Y.; Yamaguchi, T.; et al. Electrodeposition as a New Route to Synthesize Superconducting FeSe. Solid State Commun. 2013, 154, 40–42. [Google Scholar] [CrossRef]
- Delphine, S.M.; Jayachandran, M.; Sanjeeviraja, C. Pulsed Electrodeposition and Characterisation of Tungsten Diselenide Thin Films. Mater. Chem. Phys. 2003, 81, 78–83. [Google Scholar] [CrossRef]
- Damien, D.; Anil, A.; Chatterjee, D.; Shaijumon, M.M. Direct Deposition of MoSe2 Nanocrystals onto Conducting Substrates: Towards Ultra-Efficient Electrocatalysts for Hydrogen Evolution. J. Mater. Chem. A 2017, 5, 13364–13372. [Google Scholar] [CrossRef]
- Moysiadou, A.; Koutsikou, R.; Bouroushian, M. Pulse Electrodeposition of Copper Selenides from Acidic Aqueous Baths. Mater. Lett. 2015, 139, 112–115. [Google Scholar] [CrossRef]
- Xi, X.; Zhao, L.; Wang, Z.; Berger, H.; Forró, L.; Shan, J.; Mak, K.F. Strongly Enhanced Charge-Density-Wave Order in Monolayer NbSe2. Nat. Nanotechnol. 2015, 10, 765–769. [Google Scholar] [CrossRef]
- Wang, K.; Xi, D.; Zhou, C.; Shi, Z.; Xia, H.; Liu, G.; Qiao, G. CoSe2 Necklace-like Nanowires Supported by Carbon Fiber Paper: A 3D Integrated Electrode for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 9415–9420. [Google Scholar] [CrossRef]
- Wu, Z. Transition Metal Selenides for Oxygen Evolution Reaction. Energy Technol. 2024, 12, 2301574. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Gao, W.; Mao, Z.; Hu, E.; Gao, X.; Zhang, J.; Chen, Z. Strategies to Enhance the Electrocatalytic Behavior of Metal Selenides for Hydrogen Evolution Reaction: A Review. Int. J. Hydrogen Energy 2023, 48, 36722–36749. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, S.; Zheng, Y.; Rong, X.; Bai, M.; Tang, Y.; Ma, T.; Cheng, C.; Zhao, C. Emerging Electrocatalytic Activities in Transition Metal Selenides: Synthesis, Electronic Modulation, and Structure-Performance Correlations. Chem. Eng. J. 2023, 451, 138514. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Abdukayum, A.; Xie, X.; Gao, S.; Liu, X.; Zhang, L.; Liu, Q.; Hu, G. Recent Advances in Selenide-Based Electrocatalysts for Hydrogen/Oxygen Evolution Reaction: From Mechanism and Synthesis to Application. Mater. Today Energy 2024, 44, 101641. [Google Scholar] [CrossRef]
- Zeng, C.; Dai, L.; Jin, Y.; Liu, J.; Zhang, Q.; Wang, H. Design Strategies toward Transition Metal Selenide-Based Catalysts for Electrochemical Water Splitting. Sustain. Energy Fuels 2021, 5, 1347–1365. [Google Scholar] [CrossRef]
- Feng, W.; Pang, W.; Xu, Y.; Guo, A.; Gao, X.; Qiu, X.; Chen, W. Transition Metal Selenides for Electrocatalytic Hydrogen Evolution Reaction. ChemElectroChem 2020, 7, 31–54. [Google Scholar] [CrossRef]
- Ahmed, S.; Gondal, M.A.; Alzahrani, A.S.; Almessiere, M.A. Critical Review on Transition Metal Selenides/Graphene Composite as Futuristic Electrode Material for High Performance Supercapacitors. J. Energy Storage 2023, 74, 109214. [Google Scholar] [CrossRef]
- Sajjad, M.; Amin, M.; Javed, M.S.; Imran, M.; Hu, W.; Mao, Z.; Lu, W. Recent Trends in Transition Metal Diselenides (XSe2: X = Ni, Mn, Co) and Their Composites for High Energy Faradic Supercapacitors. J. Energy Storage 2021, 43, 103176. [Google Scholar] [CrossRef]
- Ali Khan, B.; Haider, F.; Zhang, T.; Zahra, S. Advances in Graphene-Transition Metal Selenides Hybrid Materials for High-Performance Supercapacitors: A Review. Chem. Rec. 2025, 25, e202500037. [Google Scholar] [CrossRef]
- Yadav, M.S.; Kour, S.; Sharma, A.L. A Critical Review on Various Bimetallic Chalcogenides (Sulfides, Selenides, and Tellurides) as Efficient Electrode Materials for Advanced Supercapacitors. Mater. Today Commun. 2024, 41, 110520. [Google Scholar] [CrossRef]
- Tang, G.; Liang, J.; Wu, W. Transition Metal Selenides for Supercapacitors. Adv. Funct. Mater. 2024, 34, 2310399. [Google Scholar] [CrossRef]
- Tapa, A.R.; Xiang, W.; Zhao, X. Metal Chalcogenides (MxEy; E = S, Se, and Te) as Counter Electrodes for Dye–Sensitized Solar Cells: An Overview and Guidelines. Adv. Energy Sustain. Res. 2021, 2, 2100056. [Google Scholar] [CrossRef]
- Pan, J.; Wang, X.; Li, H.; Cui, Z.; Chen, H.; Nie, J.; Gou, H.; Yu, D.; Chen, C.; Liu, Y. The Conversion-Type Selenides as Potential High-Energy Cathode Materials for Mg-Based Batteries: A Review. ACS Sustain. Chem. Eng. 2022, 10, 14980–15006. [Google Scholar] [CrossRef]
- Wang, H.; Deng, N.; Wang, S.; Wang, X.; Li, Y.; Zeng, Q.; Luo, S.; Cui, X.; Cheng, B.; Kang, W. Advanced Preparation and Application of Transition Metal Selenides in Lithium–Sulfur Batteries: A Review. J. Mater. Chem. A Mater. 2022, 10, 23433–23466. [Google Scholar] [CrossRef]
- Ni, W.; Li, X.; Shi, L.-Y.; Ma, J. Research Progress on ZnSe and ZnTe Anodes for Rechargeable Batteries. Nanoscale 2022, 14, 9609–9635. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Peng, J.; Lai, J.; Zhang, H.; Yang, J. Transition Metal Selenide-Based Anodes for Advanced Sodium-Ion Batteries: Electronic Structure Manipulation and Heterojunction Construction Aspect. Molecules 2024, 29, 3083. [Google Scholar] [CrossRef]
- Durairaj, A.; Sakthivel, T.; Ramanathan, S.; Obadiah, A.; Vasanthkumar, S. Hierarchical Cu2Se Nanostructures Film for Peroxymonosulfate Activation and Electrocatalytic Hydrogen Evolution. J. Taiwan Inst. Chem. Eng. 2019, 99, 66–73. [Google Scholar] [CrossRef]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of Carbamazepine by Fe(II)-Activated Persulfate Process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef]
- Diao, Z.H.; Wei-Qian; Guo, P.-R.; Kong, L.J.; Pu, S.Y. Photo-Assisted Degradation of Bisphenol A by a Novel FeS2@SiO2 Microspheres Activated Persulphate Process: Synergistic Effect, Pathway and Mechanism. Chem. Eng. J. 2018, 349, 683–693. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, K.; Srivastava, O.N. Template Free-Solvothermaly Synthesized Copper Selenide (CuSe, Cu2−xSe, β-Cu2Se and Cu2Se) Hexagonal Nanoplates from Different Precursors at Low Temperature. J. Cryst. Growth 2010, 312, 2804–2813. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-Mediated Activation of Peroxymonosulfate and Sulfate Radical Attack on Phenolic Compounds. Implications of Chloride Ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Zhang, T.; Cui, H.; Dionysiou, D.D.; Liu, C.; Gao, J.; Wang, Y.; Zhou, D. Synergy between Iron and Selenide on FeSe2(111) Surface Driving Peroxymonosulfate Activation for Efficient Degradation of Pollutants. Environ. Sci. Technol. 2020, 54, 15489–15498. [Google Scholar] [CrossRef]
- Fang, G.D.; Dionysiou, D.D.; Al-Abed, S.R.; Zhou, D.M. Superoxide Radical Driving the Activation of Persulfate by Magnetite Nanoparticles: Implications for the Degradation of PCBs. Appl. Catal. B Environ. 2013, 129, 325–332. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Ye, Z.; He, J.; Zheng, Z.; Gong, X.; Zhang, J.; Lo, I.M.C. Superoxide Radicals Dominated Visible Light Driven Peroxymonosulfate Activation Using Molybdenum Selenide (MoSe2) for Boosting Catalytic Degradation of Pharmaceuticals and Personal Care Products. Appl. Catal. B Environ. 2021, 296, 120223. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Lo, K.; Matthews, T.S.; Li, J.; Grossman, J.C.; Wu, J. Thermally Driven Crossover from Indirect toward Direct Bandgap in 2D Semiconductors: MoSe2 versus MoS2. Nano Lett. 2012, 12, 5576–5580. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Santhoshkumar, P.; Hussain, T.; Vikraman, D.; Yim, C.J.; Hussain, S.; Shanmugam, P.; Alfantazi, A.; Manickam, S.; Kim, H.S. Influence of Selenium Precursors on the Formation of Iron Selenide Nanostructures (FeSe2): Efficient Electro-Fenton Catalysts for Detoxification of Harmful Organic Dyestuffs. Chemosphere 2021, 272, 129639. [Google Scholar] [CrossRef]
- Venci, X.; George, A.; Raj, A.D.; Irudayaraj, A.A.; Pazhanivel, T.; Josephine, R.L.; Sundaram, S.J.; Kaviyarasu, K.; Raja, A.; Al-Mekhlafi, F.A.; et al. Photocatalytic Degradation Effect of CdSe Nanoparticles for Textile Wastewater Effluents at Low Cost and Proves to Be Efficient Method. Environ. Res. 2022, 213, 113595. [Google Scholar] [CrossRef]
- Venci, X.; George, A.; Raj, A.D.; Irudayaraj, A.A.; Josephine, R.L.; Sundaram, S.J.; Kaviyarasu, K. Self-Assembly of CdSe 3D Urchins and Their Photocatalytic Response. Environ. Res. 2022, 214, 113804. [Google Scholar] [CrossRef]
- Fung, C.S.L.; Khan, M.; Kumar, A.; Lo, I.M.C. Visible-Light-Driven Photocatalytic Removal of PPCPs Using Magnetically Separable Bismuth Oxybromo-Iodide Solid Solutions: Mechanisms, Pathways, and Reusability in Real Sewage. Sep. Purif. Technol. 2019, 216, 102–114. [Google Scholar] [CrossRef]
- Li, J.; Dodgen, L.; Ye, Q.; Gan, J. Degradation Kinetics and Metabolites of Carbamazepine in Soil. Environ. Sci. Technol. 2013, 47, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon Nitride Based Nanocomposites: A Review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Stancu, A.G.; Râpă, M.; Popa, C.L.; Donțu, S.I.; Matei, E.; Covaliu-Mirelă, C.I. Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis. Molecules 2025, 30, 3186. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent Progress in g-C3N4, TiO2 and ZnO Based Photocatalysts for Dye Degradation: Strategies to Improve Photocatalytic Activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef] [PubMed]
- Maridevaru, M.C.; Sorrentino, A.; Aljafari, B.; Anandan, S. Composites for Aqueous-Mediated Heterogeneously Catalyzed Degradation and Mineralization of Water Pollutants on TiO2—A Review. J. Compos. Sci. 2022, 6, 350. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic Degradation of Sulfamethoxazole Using TiO2-Based Materials—Perspectives for the Development of a Sustainable Water Treatment Technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Gilani, M.R.H.S.; Maaz, M.; Akhter, P.; Hussain, M.; Jeong, K.-E.; Kwon, E.E.; Bae, S.; Park, Y.-K. Advancements in TiO2-Based Photocatalysis for Environmental Remediation: Strategies for Enhancing Visible-Light-Driven Activity. Chemosphere 2024, 349, 140703. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled Adsorption and Photocatalysis of g-C3N4 Based Composites: Material Synthesis, Mechanism, and Environmental Applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Bairamis, F.; Rapti, I.; Konstantinou, I. g-C3N4 Based Z-Scheme Photocatalysts for Environmental Pollutants Removal. Curr. Opin. Green Sustain. Chem. 2023, 40, 100749. [Google Scholar] [CrossRef]
- Prasad, C.; Madkhali, N.; Govinda, V.; Choi, H.Y.; Bahadur, I.; Sangaraju, S. Recent Progress on the Development of g-C3N4 Based Composite Material and Their Photocatalytic Application of CO2 Reductions. J. Environ. Chem. Eng. 2023, 11, 109727. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Ren, J.-T.; Gao, L.-J.; Yuan, Z.-Y. Engineering of g-C3N4-Based Composites for Photocatalytic and Electrocatalytic Water Splitting: Recent Progress, Challenges and Perspective. Coord. Chem. Rev. 2024, 507, 215752. [Google Scholar] [CrossRef]
- Bi, J.; Zhu, Z.; Li, T.; Lv, Z. Research Progress on g-C3N4-Based Materials for Efficient Tetracyclines Photodegradation in Wastewater: A Review. J. Water Process Eng. 2024, 66, 105941. [Google Scholar] [CrossRef]
- Singh, R.; Chauhan, M.; Garg, P.; Sharma, B.; Attri, P.; Sharma, R.K.; Sharma, D.; Chaudhary, G.R. A Critical Review on Visible Light Active Graphitic Carbon Nitride (g-CN) Based Photocatalyst for Environment Remediation Application: A Sustainable Approach. J. Clean. Prod. 2023, 427, 138855. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Xu, G.; Wu, M. Graphitic Carbon Nitride Based Nanocomposites for the Photocatalysis of Organic Contaminants under Visible Irradiation: Progress, Limitations and Future Directions. Sci. Total Environ. 2018, 633, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Roškarič, M.; Žerjav, G.; Zavašnik, J.; Finšgar, M.; Pintar, A. Effect of TiO2 Morphology on the Properties and Photocatalytic Activity of g-C3N4/TiO2 Nanocomposites Under Visible-Light Illumination. Molecules 2025, 30, 460. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on Magnetically Separable Graphitic Carbon Nitride-Based Nanocomposites as Promising Visible-Light-Driven Photocatalysts. J. Mater. Sci. Mater. Electron. 2017, 29, 1719–1747. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef]
- Hussain, A.; Tahir, M.; Yang, W.; Ji, R.; Zheng, K.; Umer, M.; Ahmad, S.M.; Boota, M.; Iftikhar, A.; Raza, A.; et al. Synergic Effect among Activated Carbon/Sulphur-Assisted Graphitic Carbon Nitride for Enhanced Photocatalytic Activity. Diam. Relat. Mater. 2023, 135, 109836. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Dou, Q.; Chen, Q.; Wang, X.; Hu, C.; Paul, R. Defect Engineering in Polymeric Carbon Nitride with Accordion Structure for Efficient Photocatalytic CO2 Reduction and H2 Production. Chem. Eng. J. 2022, 450, 138425. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 Based Noble-Metal-Free Photocatalyst Systems: A Review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, T.; Li, K.; Zhang, J.; Wan, J.; Zhang, Y. Cobaltous Selenide/g-C3N4 Heterojunction Photocatalyst Based on Double-Electron Migration Mechanism Promotes Hydrogen Production and Tetracycline Hydrochloride Degradation. Int. J. Hydrogen Energy 2023, 48, 3901–3915. [Google Scholar] [CrossRef]
- Dileepkumar, V.G.; Balaji, K.R.; Vishwanatha, R.; Basavaraja, B.M.; Ashoka, S.; Al-Akraa, I.M.; Santosh, M.S.; Rtimi, S. CoSe2 Grafted on 2D gC3N4: A Promising Material for Wastewater Treatment, Electrocatalysis and Energy Storage. Chem. Eng. J. 2022, 446, 137023. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T.; Mohamed, A.R. Surface Charge Modification via Protonation of Graphitic Carbon Nitride (g-C3N4) for Electrostatic Self-Assembly Construction of 2D/2D Reduced Graphene Oxide (RGO)/g-C3N4 Nanostructures toward Enhanced Photocatalytic Reduction of Carbon Dioxide to Methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Wang, K.; Song, S.; Zhang, Q.; Jin, Y.; Zhang, Q. Fabrication of Protonated g-C3N4 Nanosheets as Promising Proton Conductive Materials. Chem. Commun. 2019, 55, 7414–7417. [Google Scholar] [CrossRef]
- Shen, S.; Zhong, W.; Wang, Z.; Lin, Z.; Feng, S. β-FeSe Nanorods Composited g-C3N4 with Enhanced Photocatalytic Efficiency. R. Soc. Open Sci. 2019, 6, 181886. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, N.A.; Baig, U.; Gondal, M.A.; Mohamed, M.J.S.; Dastageer, M.A. Pulsed Laser Induced Synthesis of Graphitic Carbon Nitride-Cadmium Selenide Nanocomposite for Photo-Catalytic Degradation of Organic Dyes, and Electro-Catalytic Hydrogen Evolution Reaction. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130711. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Shi, H.; Wang, Y.; Ren, Y.; Liu, E.; Zhang, X.; Fan, J.; Hu, X. Photocatalytic Activity Enhanced by Synergistic Effects of Nano-Silver and ZnSe Quantum Dots Co-Loaded with Bulk g-C3N4 for Ceftriaxone Sodium Degradation in Aquatic Environment. Chem. Eng. J. 2018, 353, 56–68. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Shafiq, M.; Hamid, S.; Shafiee, A.; Usman, M.; Khan, I.; Ashiq, M.N.; Arfan, M. Reactive Oxygen Species: New Insights into Photocatalytic Pollutant Degradation over g-C3N4/ZnSe Nanocomposite. Appl. Surf. Sci. 2020, 532, 147418. [Google Scholar] [CrossRef]
- Bigdeli Tabar, M.; Azimi, H.; Yousefi, R. The Se Role as a Mediator to Enhance the Photocatalytic Performance of the ZnSe/g-C3N4 Nanocomposites. Appl. Surf. Sci. 2023, 622, 156912. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Chen, F.; Shi, H. Metallic NiSe Cocatalyst Decorated g-C3N4 with Enhanced Photocatalytic Activity. Chem. Eng. J. 2021, 413, 127474. [Google Scholar] [CrossRef]
- Poiyamozhi, M.V.V.T.; Thivya, J. Design and Fabrication of Graphitic Carbon Nitride Incorporated Nickel Selenide Hybrid Composite for Effective Degradation of Organic Pollutants under UV and Natural Sunlight Irradiations. Diam. Relat. Mater. 2024, 142, 110726. [Google Scholar] [CrossRef]
- Saray, A.M.; Yousefi, R.; Zare-Dehnavi, N.; Jamali-Sheini, F. Improvement Visible-Light Photocatalytic Performance of Single-Crystalline SnSe1±x NPs toward Degradation of Organic Pollutants. Solid State Sci. 2019, 98, 106044. [Google Scholar] [CrossRef]
- Mohan, H.; Ha, G.H.; Oh, H.S.; Kim, G.; Shin, T. Zinc Iron Selenide Nanoflowers Anchored g-C3N4 as Advanced Catalyst for Photocatalytic Water Splitting and Dye Degradation. Chemosphere 2022, 307, 135937. [Google Scholar] [CrossRef]
- Nouri, M.; Zare-Dehnavi, N.; Jamali-Sheini, F.; Yousefi, R. Synthesis and Characterization of Type-II p(CuxSey)/n(g-C3N4) Heterojunction with Enhanced Visible-Light Photocatalytic Performance for Degradation of Dye Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124656. [Google Scholar] [CrossRef]
- Zeng, J.H.; Jin, B.B.; Wang, Y.F. Facet Enhanced Photocatalytic Effect with Uniform Single-Crystalline Zinc Oxide Nanodisks. Chem. Phys. Lett. 2009, 472, 90–95. [Google Scholar] [CrossRef]
- Durairasan, M.; Karthik, P.S.; Balaji, J.; Rajeshkanna, B. Enhanced Visible Light Photocatalytic Performance of WSe2/CNT Hybrid Photocatalysts That Were Synthesized by a Facile Hydrothermal Route. Ionics 2021, 27, 2151–2158. [Google Scholar] [CrossRef]
- Ali, Z.; Asif, M.; Huang, X.; Tang, T.; Hou, Y. Hierarchically Porous Fe2CoSe4 Binary-Metal Selenide for Extraordinary Rate Performance and Durable Anode of Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1802745. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/Graphene-Based Nanocomposites for Water Treatment: A Brief Overview of Charge Carrier Transfer, Antimicrobial and Photocatalytic Performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced Nitrogen Doping in TiO2 Nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Yang, L.; Liu, C.; Chen, Y.; Meng, D. Photo-Assisted Synthesis of Rosalike CuSe Hierarchical Nanostructures on TiO2 Nanotubes with Remarkable Photocatalytic Performance. Electrochim. Acta 2012, 83, 394–401. [Google Scholar] [CrossRef]
- Xu, D.; Liu, B.; Zou, W.; Wang, H.; Zhang, C. In Situ Synthesis of TiO2 Nanosheets@CdSe Nanocomposites and the Improved Photocatalytic Performance on Removal of Methylene Blue. Appl. Surf. Sci. 2019, 487, 91–100. [Google Scholar] [CrossRef]
- Warkhade, S.K.; Zodape, S.P.; Pratap, U.R.; Wankhade, A.V. Rutile TiO2/CoSe Nanocomposite: An Efficient Photocatalyst for Photodegradation of Model Organic Dyes under Visible Light Irradiation. J. Mol. Liq. 2019, 279, 434–443. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.U.; Tahir, K.; Liu, J.; Zaki, M.E.A.; Almarhoon, Z.M.; Alanazi, A.A.; Althagafi, T.M.; Al-Saeedi, S.I.; Mohamedbakr, H.G. FeSe2/TiO2 Heterostructure as an Efficient Photocatalyst and Their Electrochemical Energy Storage Applications. Mater. Chem. Phys. 2023, 303, 127793. [Google Scholar] [CrossRef]
- Ahmad, S.; Tahir, M.; Kamal, G.; Zhang, X.; Nazir, S.; Tahir, M.; Jiang, B.; Safdar, M. TiO2/Activated Carbon/2D Selenides Composite Photocatalysts for Industrial Wastewater Treatment. Water 2023, 15, 1788. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Akbarzadeh Pivehzhani, O.; Johan, M.R. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef]
- Yan, Y.; Kuang, W.; Shi, L.; Ye, X.; Yang, Y.; Xie, X.; Shi, Q.; Tan, S. Carbon Quantum Dot-Decorated TiO2 for Fast and Sustainable Antibacterial Properties under Visible-Light. J. Alloys Compd. 2019, 777, 234–243. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous Nanomaterials for the Enhancement of TiO2 Photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Xie, T.; Liu, Y.; Wang, H.; Wu, Z. Layered MoSe2/Bi2WO6 Composite with P-N Heterojunctions as a Promising Visible-Light Induced Photocatalyst. Appl. Surf. Sci. 2018, 444, 320–329. [Google Scholar] [CrossRef]
- Rivera, P.; Schaibley, J.R.; Jones, A.M.; Ross, J.S.; Wu, S.; Aivazian, G.; Klement, P.; Seyler, K.; Clark, G.; Ghimire, N.J.; et al. Observation of Long-Lived Interlayer Excitons in Monolayer MoSe2–WSe2 Heterostructures. Nat. Commun. 2015, 6, 6242. [Google Scholar] [CrossRef] [PubMed]
- Coehoorn, R.; Haas, C.; Dijkstra, J.; Flipse, C.J.F.; de Groot, R.A.; Wold, A. Electronic Structure of MoSe2, MoS2, and WSe2. I. Band-Structure Calculations and Photoelectron Spectroscopy. Phys. Rev. B 1987, 35, 6195–6202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Ran, L.; Wang, X.; Zou, B.; Zhou, L. Mechanism and Performance Evaluation of FeSe2 and MoS2-Loaded Carbon Fiber Sheet as a Novel Heterogeneous Fenton-like Catalyst. Appl. Surf. Sci. 2022, 598, 153860. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, J.; Liu, D.; Liu, W.; Li, L.; Liu, X.; Sun, Y. Efficient Degradation of Tetracycline Hydrochloride by Peroxymonosulfate Activated by Composite Materials FeSe2/Fe3O4 under Visible Light. Chem. Phys. Lett. 2022, 805, 139944. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Zhang, J.; Lin, J.; Yang, S.; Xiong, X.; Qin, H.; Xi, J.; Kong, Z.; Song, L. Cu2−xSe/FeSe2 Z-Type Heterojunction Demonstrate Versatile Boosting Photoelectrochemical, Electrocatalytic and Photocatalytic Properties. J. Alloys Compd. 2023, 947, 169496. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Xiong, X.; Lin, J.; Yang, S.; Xi, J.; Kong, Z. A Novel Z-Type Multidimensional FeSe2/CuSe Heterojunction Photocatalyst with High Photocatalytic and Photoelectrochemical Performance. Int. J. Hydrogen Energy 2022, 47, 28879–28893. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, J.; Cheng, Y.; Chen, C.; Zeng, J.; Xi, J.; Kong, Z.; Yuan, Y.-J.; Ji, Z. Excellent Photocatalytic and Photoelectrochemical Activities from 1D/2D FeSe2/SnSe Heterojunction Photocatalysts Constructed by FeSe2 Nanorods and SnSe Nanosheets. Int. J. Hydrogen Energy 2022, 47, 7189–7201. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, H.; Zhang, J.; Xiong, X.; Chen, C.; Zeng, J.; Xi, J.; Yuan, Y.-J.; Ji, Z. Novel 0D/2D ZnSe/SnSe Heterojunction Photocatalysts Exhibiting Enhanced Photocatalytic and Photoelectrochemical Activities. J. Alloys Compd. 2022, 897, 163123. [Google Scholar] [CrossRef]
- Du, C.; Nie, S.; Zhang, C.; Wang, T.; Wang, S.; Zhang, J.; Yu, C.; Lu, Z.; Dong, S.; Feng, J.; et al. Dual-Functional Z-Scheme CdSe/Se/BiOBr Photocatalyst: Generation of Hydrogen Peroxide and Efficient Degradation of Ciprofloxacin. J. Colloid Interface Sci. 2022, 606, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, J.; Song, P. Electro-Activated Peroxymonosulfate Based on Hollow Cubic Skeleton Nanoflower Cathode Catalyst MoSe2/Cubic Nanocage (CNC) for Enhanced Degradation of Norfloxacin: Performance, Mechanism, Degradation Pathways and Toxicity. J. Colloid Interface Sci. 2025, 681, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Atif, M.; Khan, A.; Siddique, M.; Ali, N.; Naz, F.; Bilal, M.; Kim, T.H.; Momotko, M.; Haq, H.U.; et al. Solar Light Driven Degradation of Textile Dye Contaminants for Wastewater Treatment—Studies of Novel Polycationic Selenide Photocatalyst and Process Optimization by Response Surface Methodology Desirability Factor. Chemosphere 2023, 328, 138476. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Kamali, M. Sonocatalytic Degradation of Methylene Blue by a Novel Graphene Quantum Dots Anchored CdSe Nanocatalyst. Ultrason. Sonochem. 2017, 39, 676–685. [Google Scholar] [CrossRef]
- Sheebha, I.; Venugopal, V.; James, J.; Maheskumar, V.; Sakunthala, A.; Vidhya, B. Comparative Studies on Hierarchical Flower like Cu2XSnS4[X= Zn, Ni, Mn & Co] Quaternary Semiconductor for Electrocatalytic and Photocatalytic Applications. Int. J. Hydrogen Energy 2020, 45, 8139–8150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).