BiOI/Magnetic Nanocomposites Derived from Mine Tailings for Photocatalytic Degradation of Phenolic Compounds (Caffeic Acid) in Winery Wastewater
Abstract
1. Introduction
2. Results and Discussion
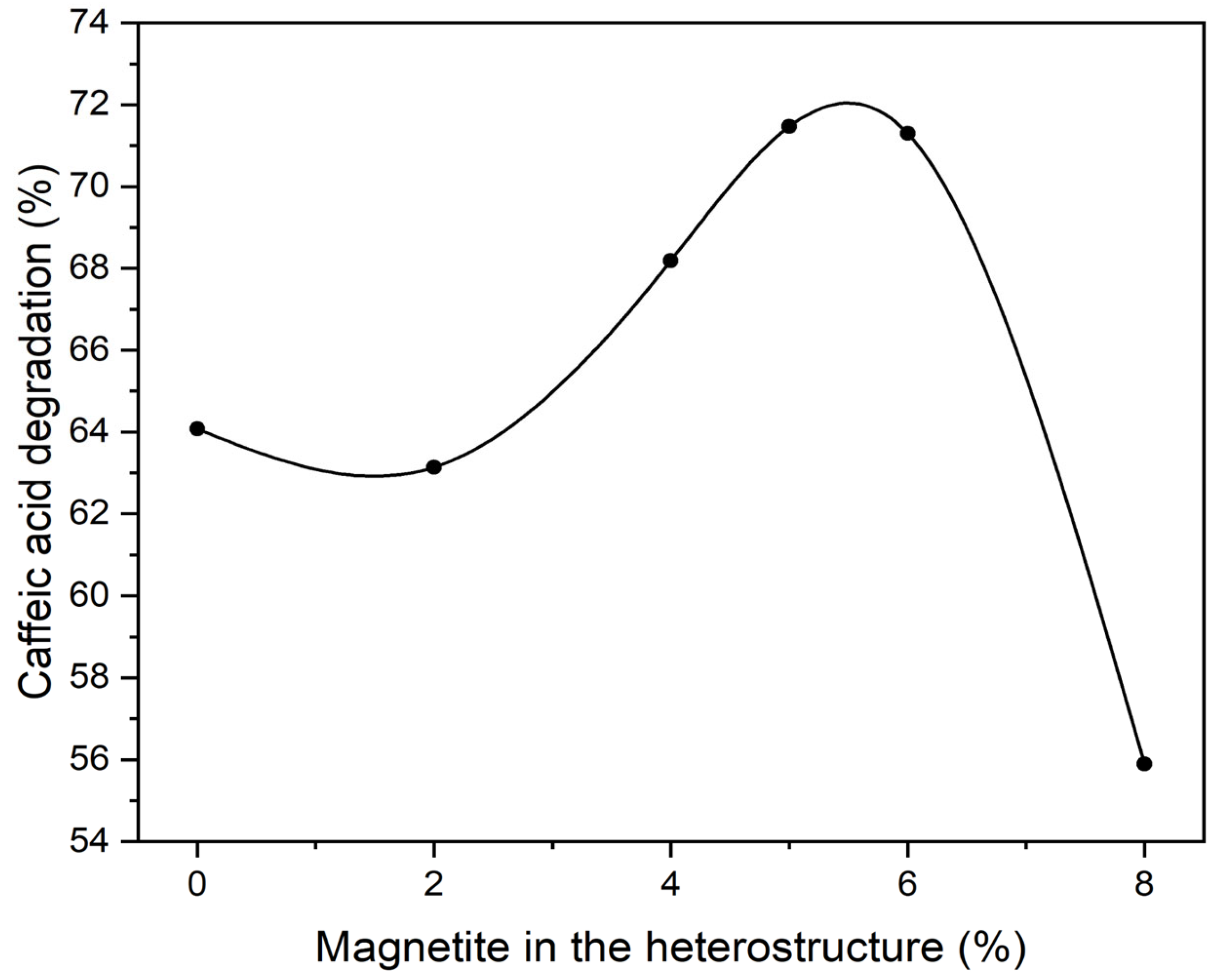
2.1. Determining the Optimal Ratio of Magnetite in the BiOI/Magnetite (Fe3O4) Heterostructure
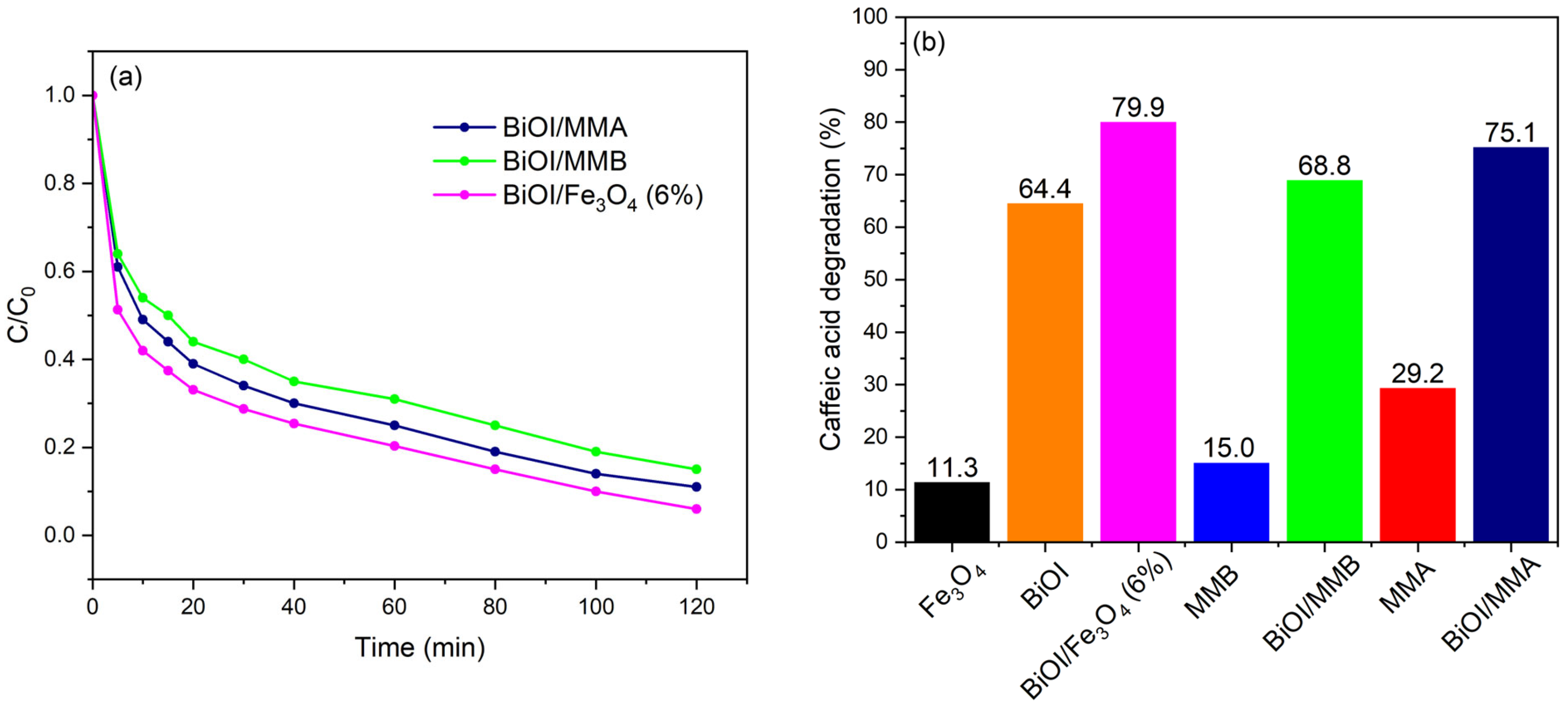
2.2. Photocatalytic Evaluation of the BiOI/Magnetic Material (MM) Heterostructures
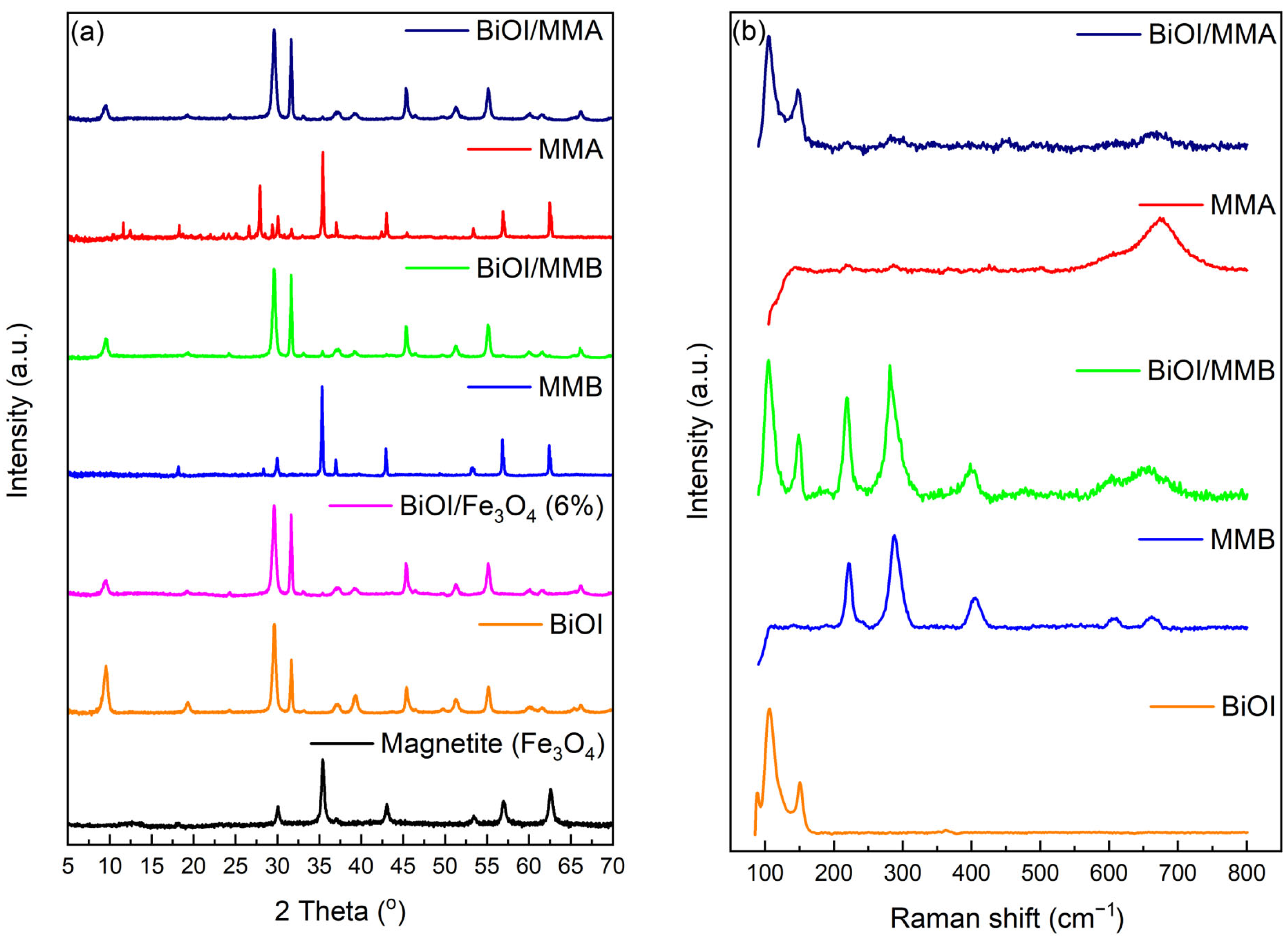
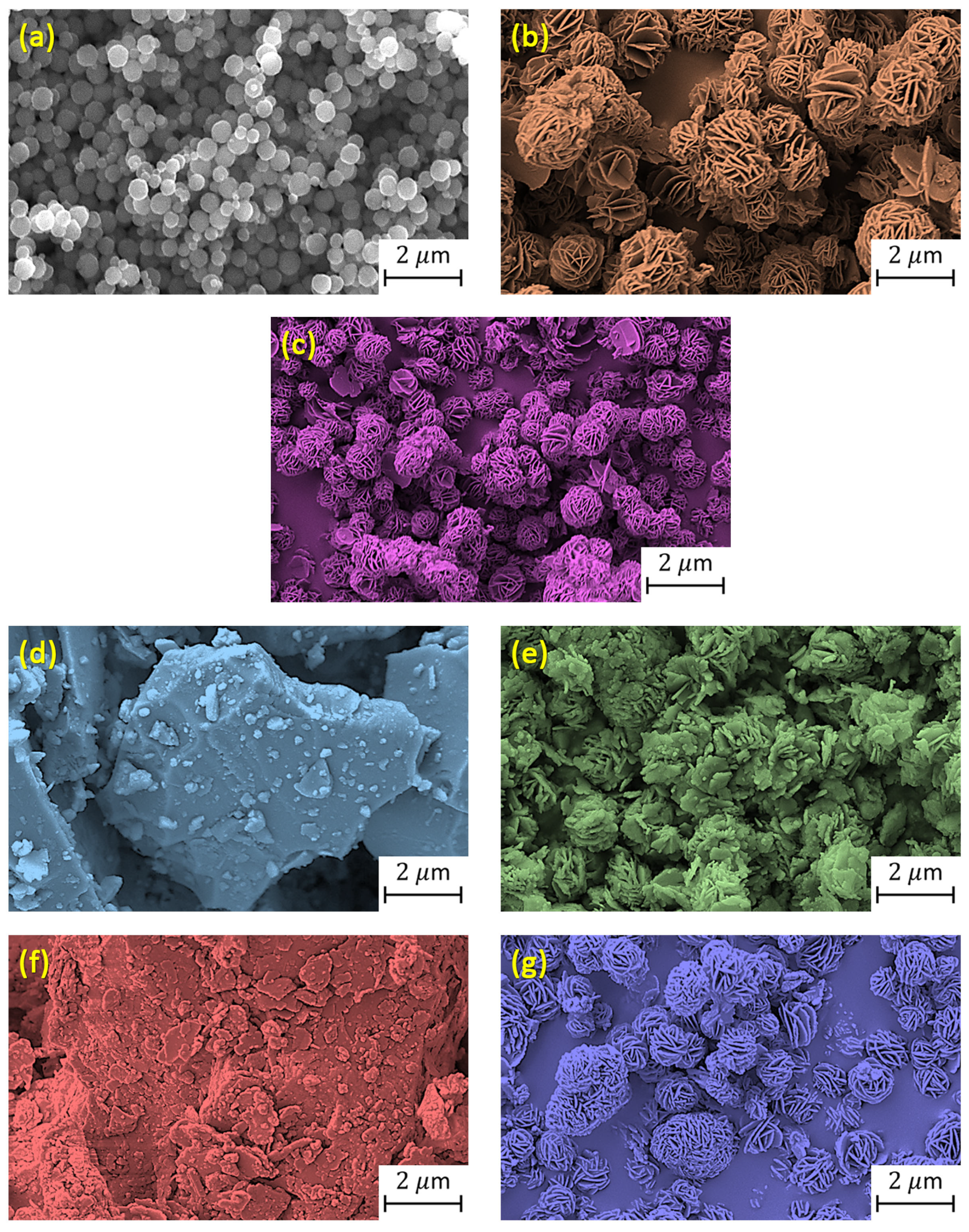
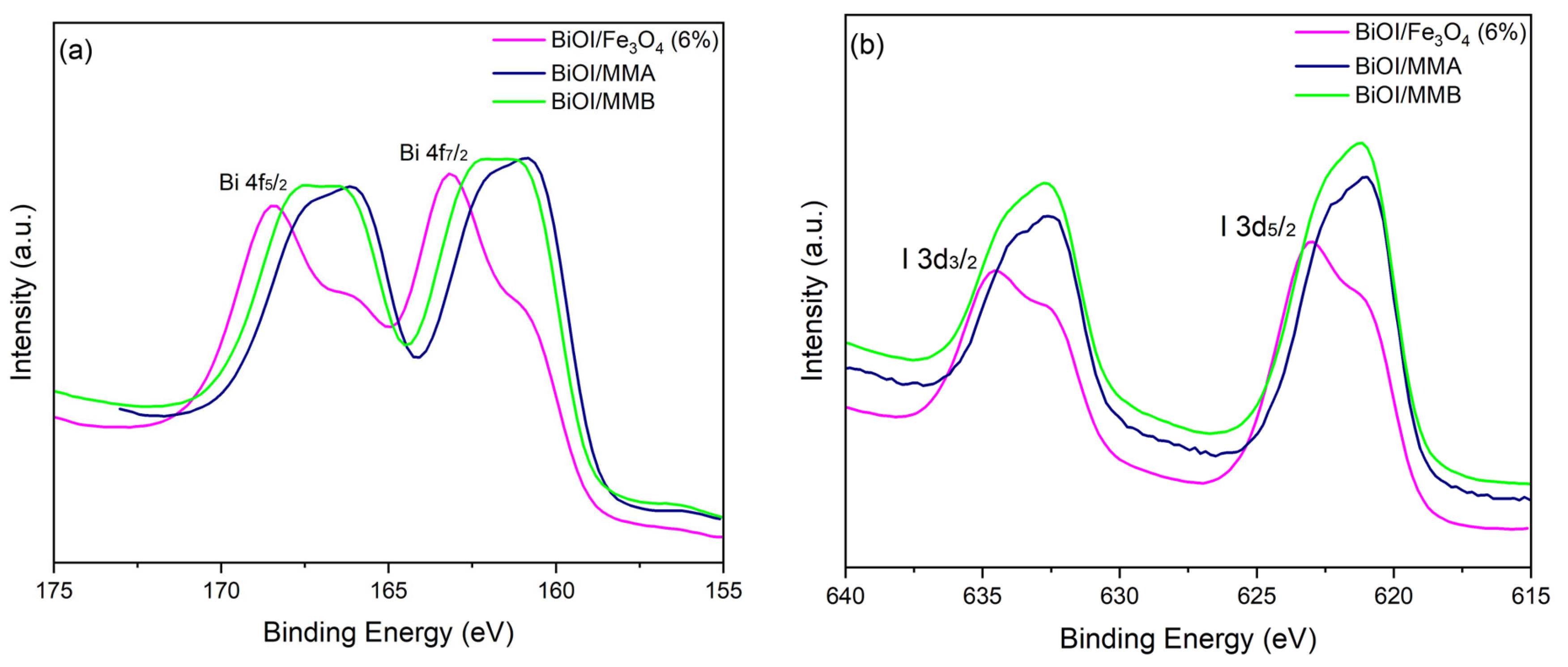
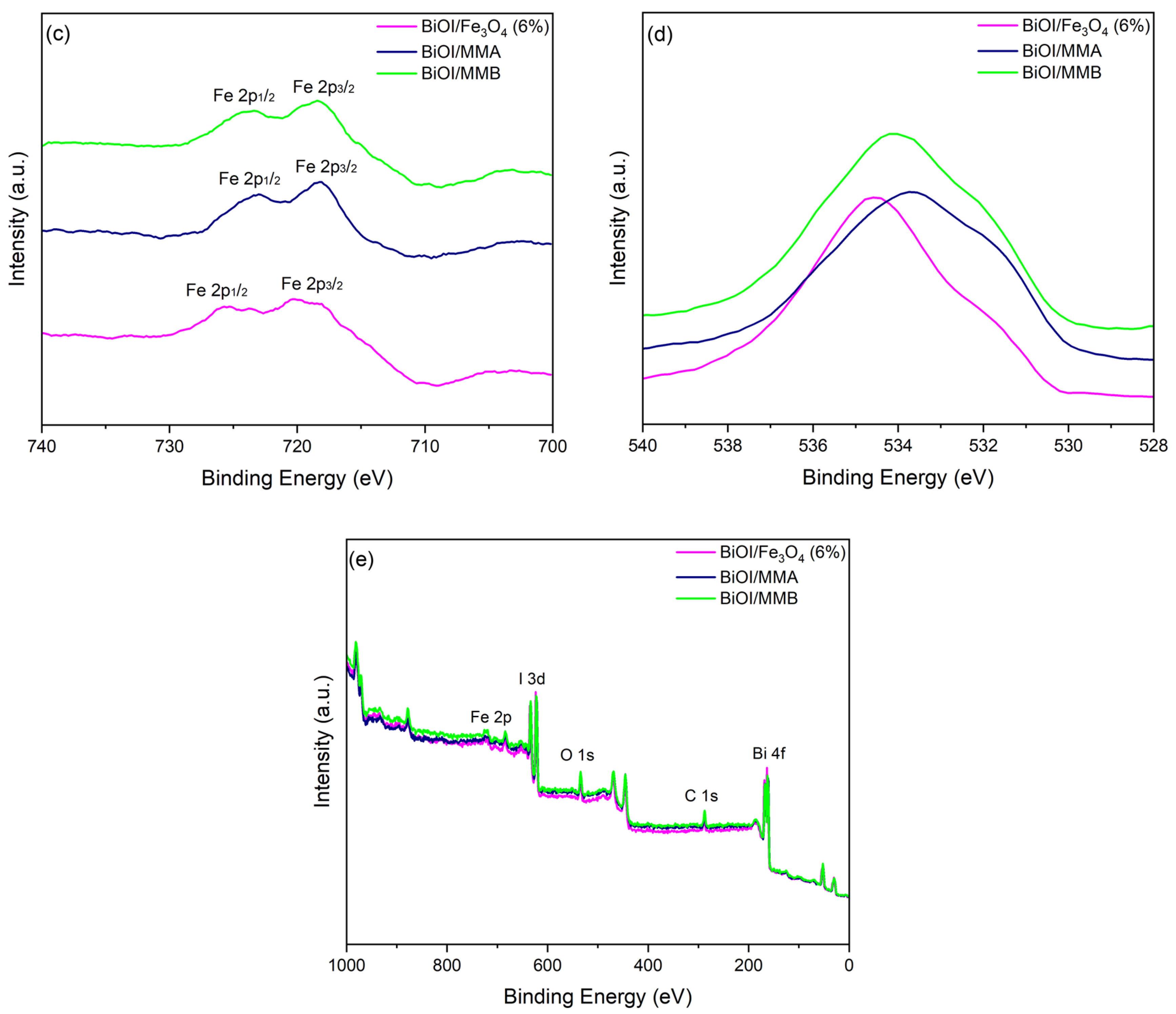
2.3. Characterizing Materials
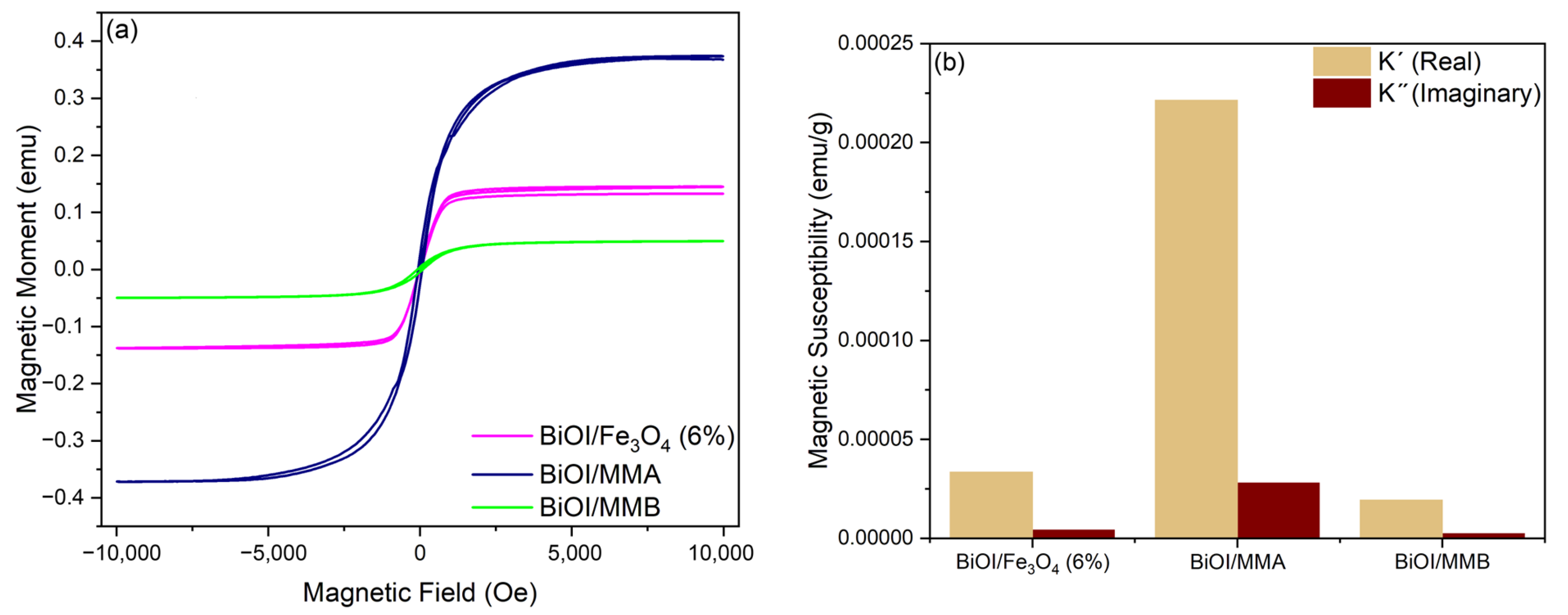
2.4. Magnetic Properties
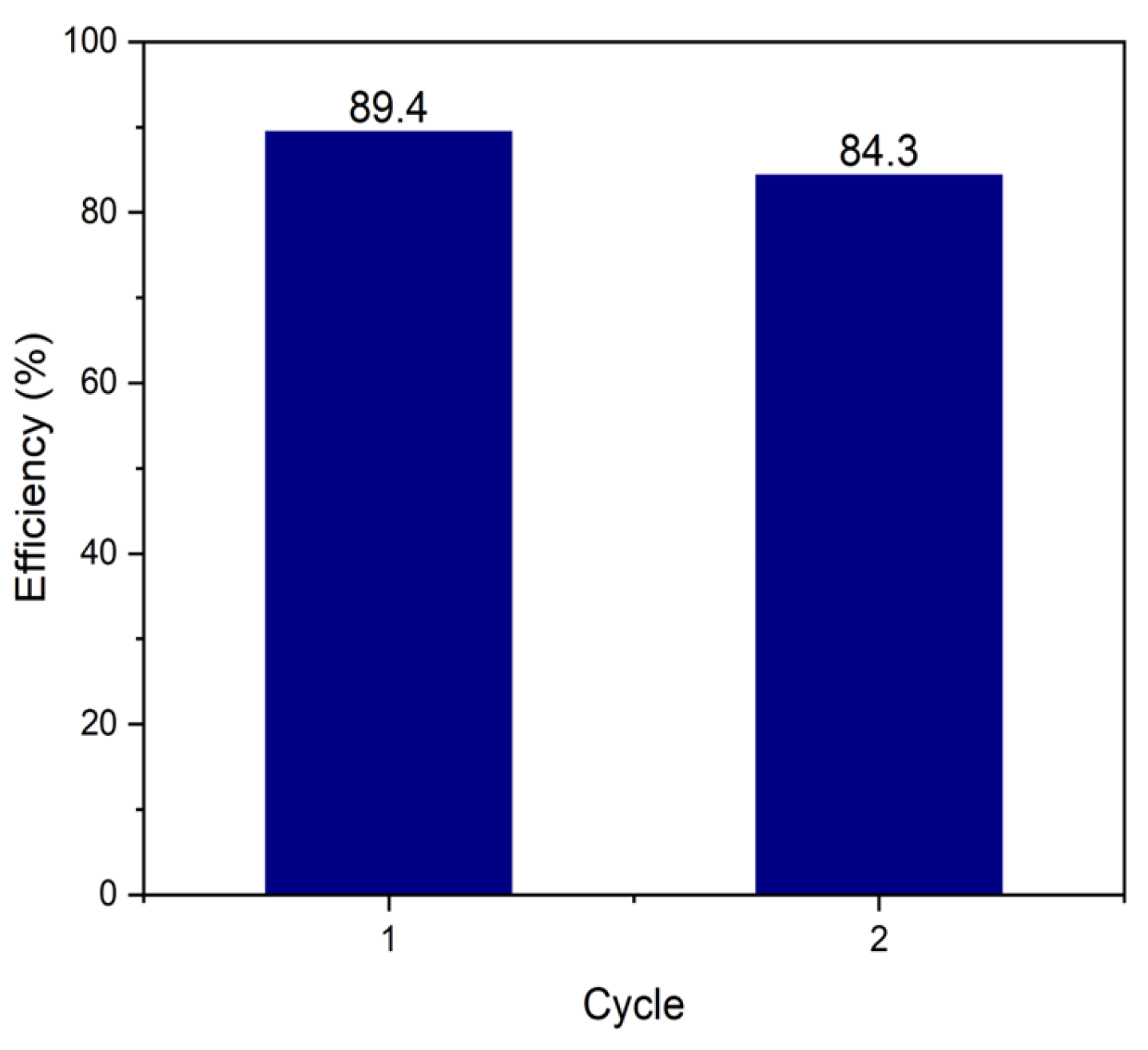
2.5. Reutilization of the BiOI/MMA Heterostructure
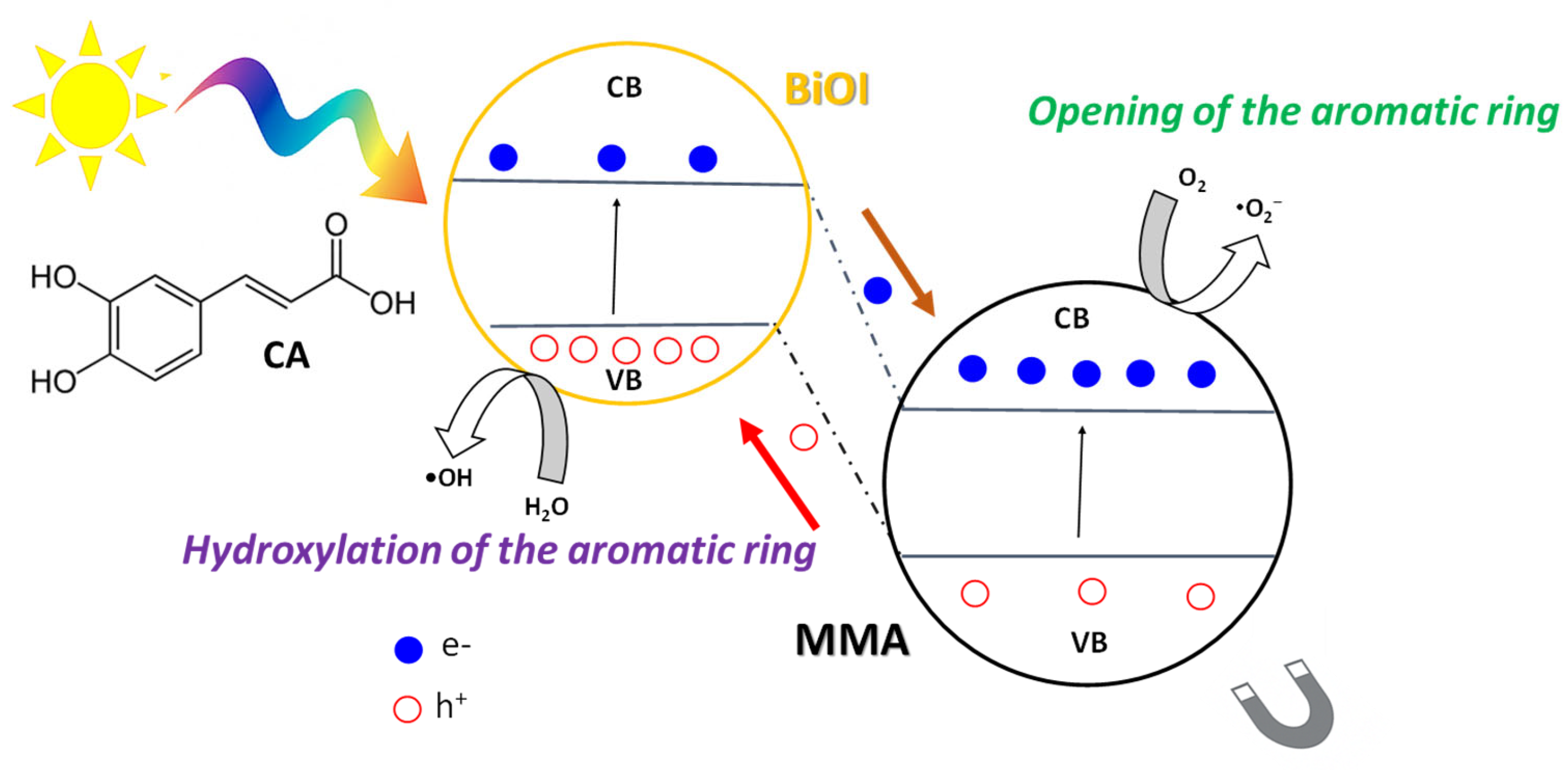
2.6. Proposed Photocatalytic Mechanism
- I.
- Hydroxylation of the aromatic ring, mainly caused by h+ and, to a lesser degree, •OH.
- II.
- Opening of the aromatic ring, favored by h+ and •O2−.
- III.
- Formation of short chain carboxylic acids (maleic, oxalic and acetic acids) and partial oxidation intermediates.
- IV.
- Final mineralization to CO2 to H2O.
3. Materials and Methods
3.1. Sample Preparation
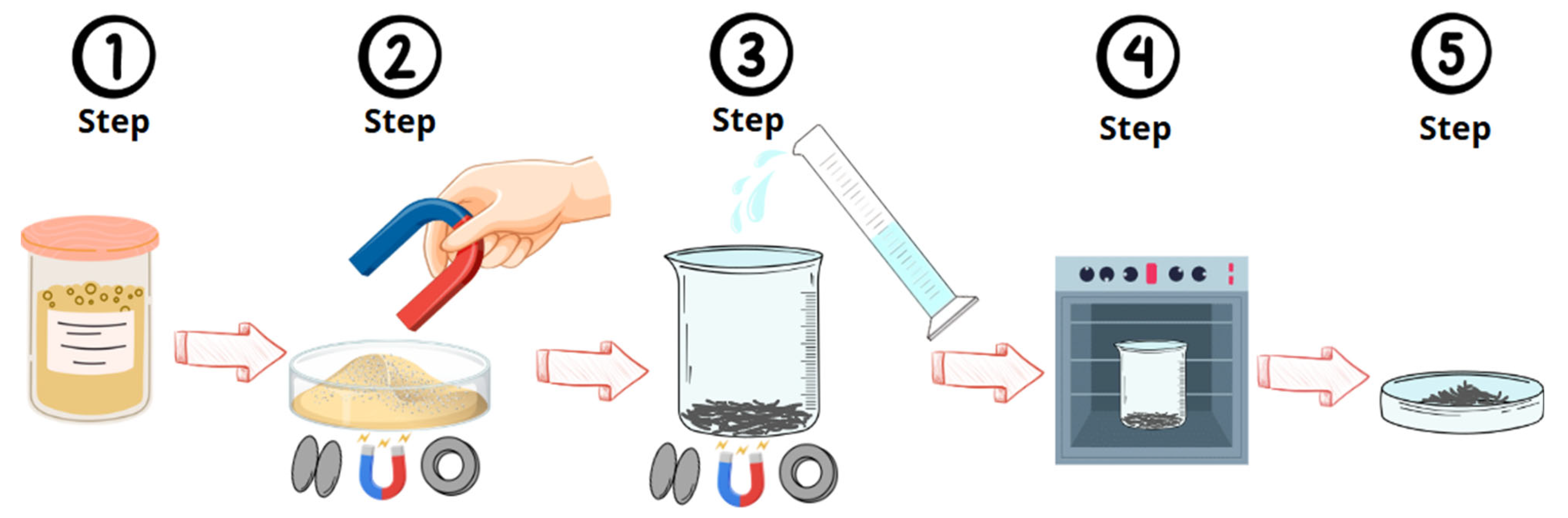
3.2. Extracting Magnetic Material from Mine Tailings
3.3. Synthesizing Magnetite
3.4. Optimizing the Amount of Magnetite in the Photocatalyst
3.5. Synthesizing BiOI/Magnetic Material (BiOI/MM)
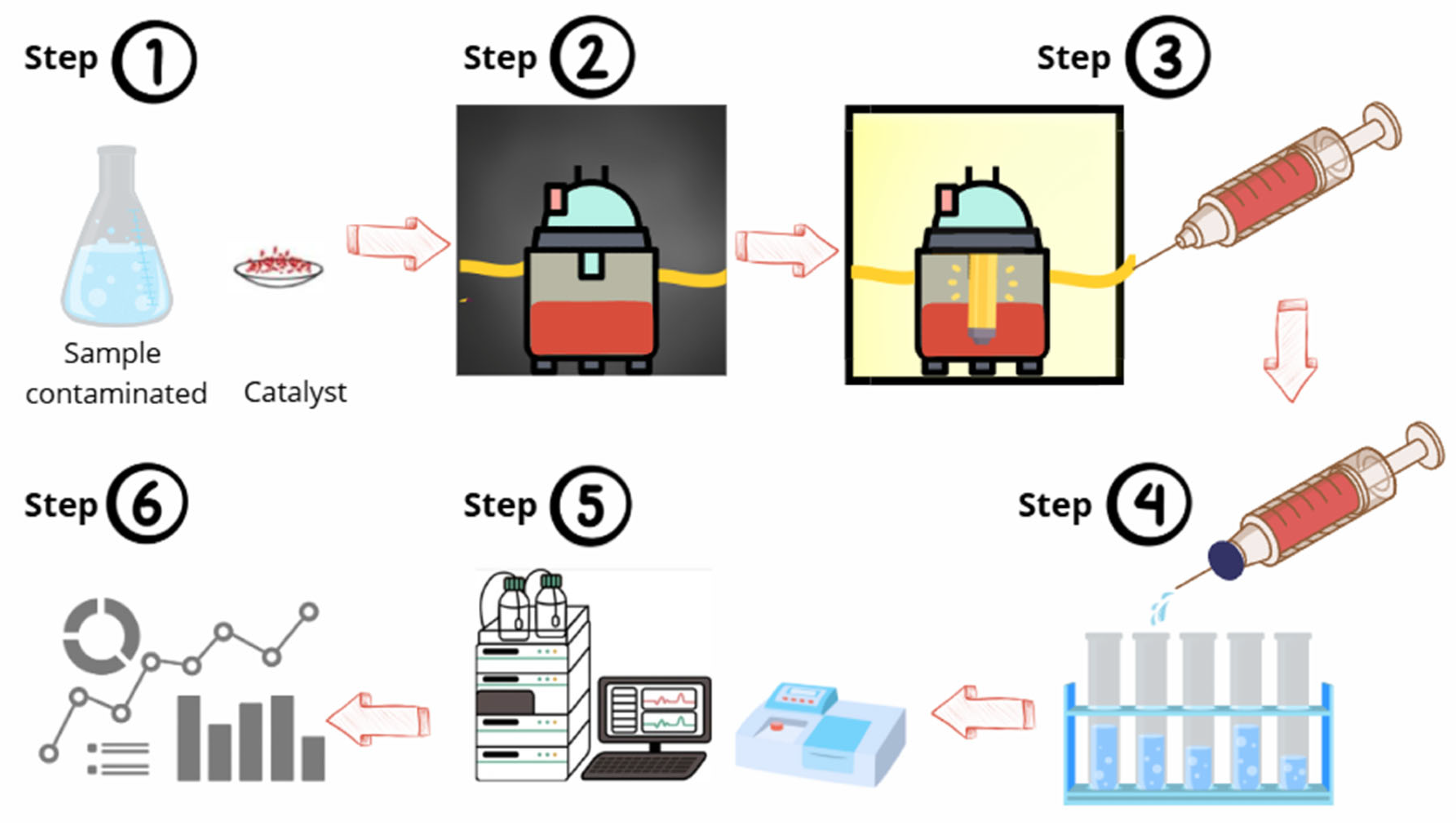
3.6. Determining Photocatalytic Efficiencies
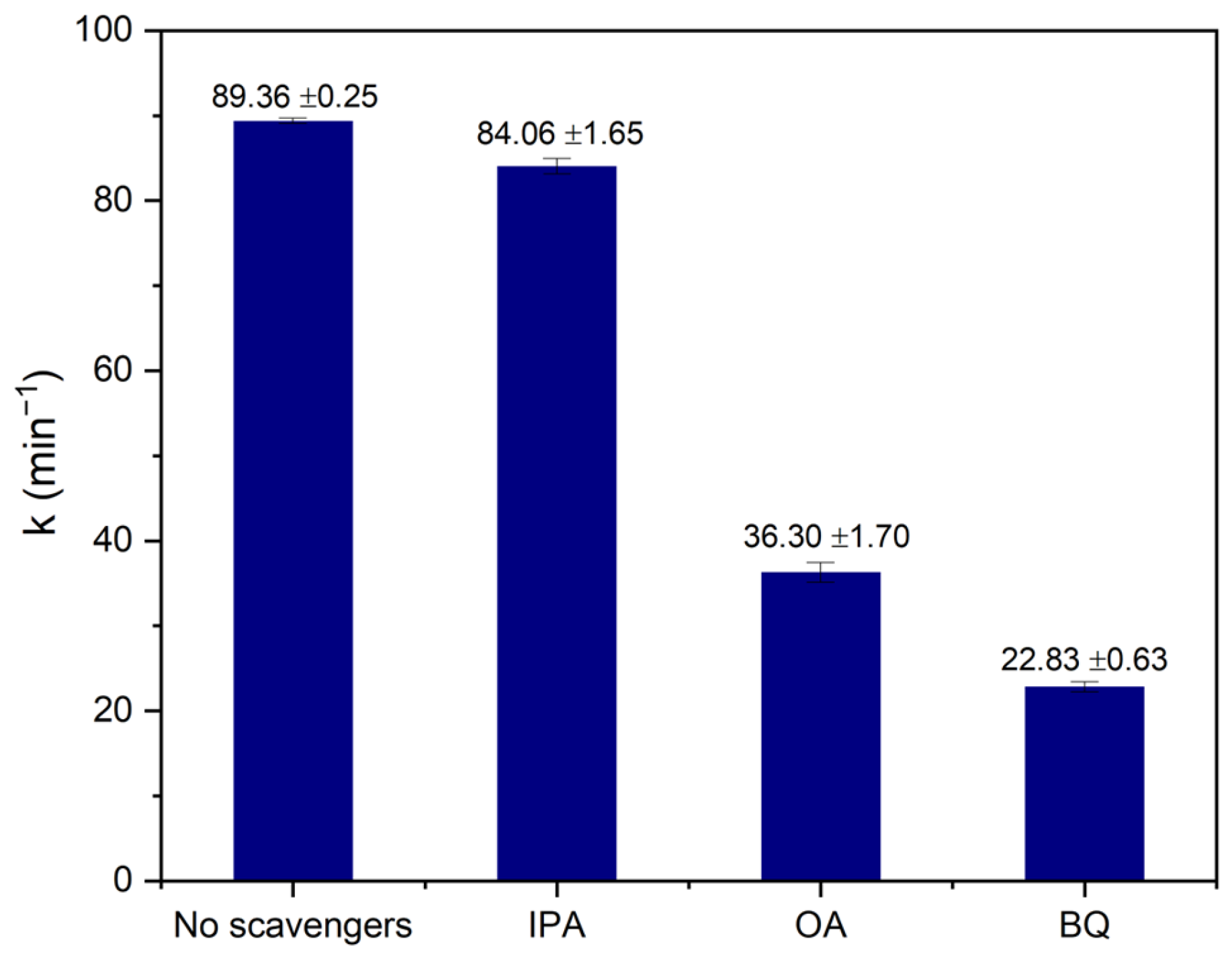
3.7. Experiments with Reactive Species Scavengers
3.8. Characterizing the Synthesized Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater Using Biological Approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Hübner, U.; Spahr, S.; Lutze, H.; Wieland, A.; Rüting, S.; Gernjak, W.; Wenk, J. Advanced Oxidation Processes for Water and Wastewater Treatment—Guidance for Systematic Future Research. Heliyon 2024, 10, e30402. [Google Scholar] [CrossRef]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced Oxidation Process for the Treatment of Industrial Wastewater: A Review on Strategies, Mechanisms, Bottlenecks and Prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef]
- Muscetta, M.; Ganguly, P.; Clarizia, L. Solar-Powered Photocatalysis in Water Purification: Applications and Commercialization Challenges. J. Environ. Chem. Eng. 2024, 12, 113073. [Google Scholar] [CrossRef]
- Mera, A.C.; Rodríguez, C.A.; Meléndrez, M.F.; Valdés, H. Synthesis and Characterization of BiOI Microspheres under Standardized Conditions. J. Mater. Sci. 2017, 52, 944–954. [Google Scholar] [CrossRef]
- Hao, R.; Xiao, X.; Zuo, X.; Nan, J.; Zhang, W. Efficient Adsorption and Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride Using Mesoporous BiOI Microspheres. J. Hazard. Mater. 2012, 209–210, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Amrillah, T. Potential Application of Bismuth Oxyiodide (BiOI) When It Meets Light. Nanoscale 2024, 16, 5079–5106. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sudhaik, A.; Raizada, P.; Shandilya, P.; Sharma, R.; Hosseini-Bandegharaei, A. Photocatalytic Performance and Quick Recovery of BiOI/Fe3O4@graphene Oxide Ternary Photocatalyst for Photodegradation of 2,4-Dintirophenol under Visible Light. Mater. Today Chem. 2019, 12, 85–95. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Zhang, P.; Lou, X.W. Design of Heterostructured Hollow Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Mater. 2019, 31, 1900281. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Wahab, R.; Al-Khedhairy, A.A.; Khan, A.; Rahman, F. Magnetically Separable and Visible Light-Driven Photocatalytic Activity of Graphene Oxide Based α-Fe2O3 Nanocomposite. Mater. Chem. Phys. 2024, 316, 129111. [Google Scholar] [CrossRef]
- Li, X.; Niu, C.; Huang, D.; Wang, X.; Zhang, X.; Zeng, G.; Niu, Q. Preparation of Magnetically Separable Fe3O4/BiOI Nanocomposites and Its Visible Photocatalytic Activity. Appl. Surf. Sci. 2013, 286, 40–46. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, L.-G.; Yuan, Y.-P.; Zhu, Y.-J.; Xia, J.; Zhu, J.-F. Facile Fabrication of Magnetically Separable Graphitic Carbon Nitride Photocatalysts with Enhanced Photocatalytic Activity under Visible Light. J. Mater. Chem. A 2013, 1, 3008–3015. [Google Scholar] [CrossRef]
- Ali, A.; Amin, M.; Tahir, M.; Ali, S.S.; Hussain, A.; Ahmad, I.; Mahmood, A.; Farooq, M.U.; Farid, M.A. G-C3N4/Fe3O4 Composites Synthesized via Solid-State Reaction and Photocatalytic Activity Evaluation of Methyl Blue Degradation under Visible Light Irradiation. Front. Mater. 2023, 10, 1180646. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Z.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Novel Ti3C2/Bi@BiOI Nanosheets with Gradient Oxygen Vacancies for the Enhancement of Spatial Charge Separation and Photocatalytic Performance: The Roles of Reactive Oxygen and Iodine Species. Chem. Eng. J. 2021, 426, 130764. [Google Scholar] [CrossRef]
- Qian, D.; Zhong, S.; Wang, S.; Lai, Y.; Yang, N.; Jiang, W. Promotion of Phenol Photodegradation Based on Novel Self-Assembled Magnetic Bismuth Oxyiodide Core–Shell Microspheres. RSC Adv. 2017, 7, 36653–36661. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Dong, X.; Lin, C.; Wen, X.; Yan, Q. Synthesis and Characterization of Fe3O4/BiOI n-p Heterojunction Magnetic Photocatalysts. Appl. Surf. Sci. 2018, 455, 742–747. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, F.; Li, Y. A Review on the Effects of TiO2 Surface Point Defects on CO2 Photoreduction with H2O. J. Mater. 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Du, N.; Song, S.; Hou, W. Synthesis, Characterization, and Visible-Light Photocatalytic Activity of BiOI Hierarchical Flower-like Microspheres. RSC Adv. 2014, 4, 31393–31399. [Google Scholar] [CrossRef]
- Khairudin, K.; Abu Bakar, N.F.; Osman, M.S. Magnetically Recyclable Flake-like BiOI-Fe3O4 Microswimmers for Fast and Efficient Degradation of Microplastics. J. Environ. Chem. Eng. 2022, 10, 108275. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Dutta, V.; Chauhan, A.; Verma, R.; Gopalkrishnan, C.; Nguyen, V.-H. Recent Trends in Bi-Based Nanomaterials: Challenges, Fabrication, Enhancement Techniques, and Environmental Applications. Beilstein J. Nanotechnol. 2022, 13, 1316–1336. [Google Scholar] [CrossRef]
- Adhikari, S.; Mandal, S.; Kim, D.-H. Recent Development Strategies for Bismuth-Driven Materials in Sustainable Energy Systems and Environmental Restoration. Small 2023, 19, 2206003. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on Nanoscale Bi-Based Photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, S.A.; Inbanathan, S.S.R.; Modarres, M.; Kumar, R.; Algadi, H.; Ibrahim, A.A.; Wendelbo, R.; Packiaraj, R.; Alhamami, M.A.M.; et al. Enhanced Sunlight-Driven Photocatalytic, Supercapacitor and Antibacterial Applications Based on Graphene Oxide and Magnetite-Graphene Oxide Nanocomposites. Ceram. Int. 2022, 48, 29349–29358. [Google Scholar] [CrossRef]
- Abbadi, A.; Mucsi, G. A Review on Complex Utilization of Mine Tailings: Recovery of Rare Earth Elements and Residue Valorization. J. Environ. Chem. Eng. 2024, 12, 113118. [Google Scholar] [CrossRef]
- Tian, Y.; Olivetti, E.A. Strategies for Industrial Residue Valorization through Metal Recovery, Use in Construction, or CO2 Mineralization. Waste Manag. 2025, 203, 114824. [Google Scholar] [CrossRef] [PubMed]
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards Mine Tailings Valorization: Recovery of Critical Materials from Chilean Mine Tailings. J. Clean. Prod. 2020, 263, 121555. [Google Scholar] [CrossRef]
- Guadarrama Guzmán, P.; Fernández Villagómez, G.; Alarcón Herrera, M.T. Assessment of Risk to Health Caused by the Exposure to Mining Waste in Durango, Mexico. Ing. Investig. Y Tecnol. 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Lam, E.J.; Montofré, I.L.; Álvarez, F.A.; Gaete, N.F.; Poblete, D.A.; Rojas, R.J. Methodology to Prioritize Chilean Tailings Selection, According to Their Potential Risks. Int. J. Environ. Res. Public Health 2020, 17, 3948. [Google Scholar] [CrossRef]
- Catastro Nacional de Depósitos de Relaves 2021; Servicio Nacional de Geología y Minería: Santiago, Chile, 2021.
- Cacciuttolo, C.; Cano, D. Environmental Impact Assessment of Mine Tailings Spill Considering Metallurgical Processes of Gold and Copper Mining: Case Studies in the Andean Countries of Chile and Peru. Water 2022, 14, 3057. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Quiroz, I.A.; Magni, C.R.; Yáñez, M.A.; Martínez, E.E. Long-Term Effects of Copper Mine Tailings on Surrounding Soils and Sclerophyllous Vegetation in Central Chile. Water Air Soil Pollut. 2022, 233, 288. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of Heavy Metal Contaminated Soils by Biochar: Mechanisms, Potential Risks and Applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of Rare Earth Elements Minerals from Iron Oxide–Silicate Rich Tailings—Part 1: Magnetic Separation. Miner. Eng. 2019, 136, 50–61. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Li, K.; Wen, J.; Xing, S.; Ma, Z.; Wu, Y. Heterogeneous Photo-Fenton Degradation of Organic Pollutants with Amorphous Fe-Zn-Oxide/Hydrochar under Visible Light Irradiation. Sep. Purif. Technol. 2017, 188, 105–111. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of Iron-Based Materials in Heterogeneous Advanced Oxidation Processes for Wastewater Treatment: A Review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Pham, T.-H.; Lee, B.-K.; Kim, J. Improved Adsorption Properties of a Nano Zeolite Adsorbent toward Toxic Nitrophenols. Process Saf. Environ. Prot. 2016, 104, 314–322. [Google Scholar] [CrossRef]
- Ladeia Ramos, R.; Rezende Moreira, V.; Santos Amaral, M.C. Phenolic Compounds in Water: Review of Occurrence, Risk, and Retention by Membrane Technology. J. Environ. Manag. 2024, 351, 119772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, C.; Feng, C.; Ren, Y.; Hu, Y.; Yan, B.; Wu, C. The Occurrence and Fate of Phenolic Compounds in a Coking Wastewater Treatment Plant. Water Sci. Technol. 2013, 68, 433–440. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.J.; Gimeno, O.; Frades, J. Treatment of Olive Oil Mill Wastewater by Fenton’s Reagent. J. Agric. Food Chem. 2001, 49, 1873–1880. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The Anaerobic Co-Digestion of Food Waste and Cattle Manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- McHenry, M.E.; Laughlin, D.E. Structure and Chemistry of Ferrites and Related Oxide Systems. In Modern Ferrites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 21–45. ISBN 978-1-118-97149-9. [Google Scholar]
- Zhang, Q.; Yu, L.; Xu, C.; Zhao, J.; Pan, H.; Chen, M.; Xu, Q.; Diao, G. Preparation of Highly Efficient and Magnetically Recyclable Fe3O4@C@Ru Nanocomposite for the Photocatalytic Degradation of Methylene Blue in Visible Light. Appl. Surf. Sci. 2019, 483, 241–251. [Google Scholar] [CrossRef]
- Jalandhara, D.; Kumar, S.; Kumar, S.; Rekha, M.M.; Sharma, S.V.; Kaushal, S. BiFeO3 as a Next-Generation Photocatalyst: Bridging Material Design with Environmental Remediation. ChemPhysChem 2025, 26, e202401092. [Google Scholar] [CrossRef]
- Zaharieva, J.; Tsvetkov, M.; Georgieva, M.; Tzankov, D.; Milanova, M. “Core/Shell” Nanocomposites as Photocatalysts for the Degradation of the Water Pollutants Malachite Green and Rhodamine B. Int. J. Mol. Sci. 2024, 25, 6755. [Google Scholar] [CrossRef]
- Samarasinghe, L.V.; Muthukumaran, S.; Baskaran, K. Recent Advances in Visible Light-Activated Photocatalysts for Degradation of Dyes: A Comprehensive Review. Chemosphere 2024, 349, 140818. [Google Scholar] [CrossRef] [PubMed]
- Shebanova, O.N.; Lazor, P. Raman Study of Magnetite (Fe3O4): Laser-Induced Thermal Effects and Oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Chang, H.S.W.; Chiou, C.-C.; Chen, Y.-W.; Sheen, S.R. Synthesis, Characterization, and Magnetic Properties of Fe3O4Thin Films Prepared via a Sol–Gel Method. J. Solid State Chem. 1997, 128, 87–92. [Google Scholar] [CrossRef]
- Sparavigna, A.C. Raman Spectroscopy of the Iron Oxides in the Form of Minerals, Particles and Nanoparticles. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Zhao, L.; Zhao, S. Core/Shell Fe3O4/BiOI Nanoparticles with High Photocatalytic Activity and Stability. J. Nanoparticle Res. 2016, 18, 318. [Google Scholar] [CrossRef]
- Chang, M.-J.; Wang, H.; Liu, J.; Du, H.-L.; Li, H.-L. Facile Synthesis of Fe3O4@BiOI Core/Shell Nanostructures by Magnetic-Assisted Successive Ionic Layer Adsorption and Reaction for Catalytic Application. J. Nanosci. Nanotechnol. 2017, 17, 3759–3764. [Google Scholar] [CrossRef]
- Ahmed, S.; Inayat, S.; Javed, I. Synthesis Methods and Characterization of Iron Oxide Nanoparticles: A Biomedical Perspective. Synth. Sinter. 2025, 5, 109–128. [Google Scholar] [CrossRef]
- Ali, F.M.; Hmadeh, M.; O’Brien, P.G.; Perovic, D.D.; Ozin, G.A. Photocatalytic Properties of All Four Polymorphs of Nanostructured Iron Oxyhydroxides. ChemNanoMat 2016, 2, 1047–1054. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Luo, Y.; Yu, J.; Meng, T.; Li, Z.; Tian, L.; Liu, Y.; Yang, H. Morphology-Controlled Synthesis of BiOI: Adsorption–Photocatalytic Synergistic Removal of Aqueous Cr(VI). J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Alam, K.M.; Kumar, P.; Kar, P.; Thakur, U.K.; Zeng, S.; Cui, K.; Shankar, K. Enhanced Charge Separation in G-C3N4–BiOI Heterostructures for Visible Light Driven Photoelectrochemical Water Splitting. Nanoscale Adv. 2019, 1, 1460–1471. [Google Scholar] [CrossRef]
- Ahghari, M.R.; Amiri-khamakani, Z.; Maleki, A. Synthesis and Characterization of Se Doped Fe3O4 Nanoparticles for Catalytic and Biological Properties. Sci. Rep. 2023, 13, 1007. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.-D. Facile Synthesis of Nanostructured BiOI Microspheres with High Visible Light-Induced Photocatalytic Activity. J. Mater. Chem. 2010, 20, 5866–5870. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Li, D.; Liu, R.; Liu, S. Facile Synthesis of Flower-like BiOI Hierarchical Spheres at Room Temperature with High Visible-Light Photocatalytic Activity. Mater. Sci. Eng. B 2015, 193, 112–120. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhu, Q. Structural and Electronic Properties of BiOX (X = F, Cl, Br, I) Considering Bi 5f States. Comput. Mater. Sci. 2009, 46, 1076–1084. [Google Scholar] [CrossRef]
- Ge, S.; Zhao, K.; Zhang, L. Microstructure-Dependent Photoelectrochemical and Photocatalytic Properties of BiOI. J. Nanoparticle Res. 2012, 14, 1015. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Maxisch, T.; Ceder, G. Oxidation Energies of Transition Metal Oxides within the GGA+U Framework. Phys. Rev. B 2006, 73, 195107. [Google Scholar] [CrossRef]
- Liu, H.; Cao, W.-R.; Su, Y.; Chen, Z.; Wang, Y. Bismuth Oxyiodide–Graphene Nanocomposites with High Visible Light Photocatalytic Activity. J. Colloid Interface Sci. 2013, 398, 161–167. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L. Generalized One-Pot Synthesis, Characterization, and Photocatalytic Activity of Hierarchical BiOX (X = Cl, Br, I) Nanoplate Microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Zhongtian, F.; Chengshuo, H.; Xin, Z.; Zhongxue, F. Study on Preparation and Recovery of Magnetic BiOI/rGO/Fe3O4 Composite Photocatalyst. Results Phys. 2020, 16, 102931. [Google Scholar] [CrossRef]
- Kumar, R.; Gogoi, R.; Sharma, K.; Singh, A.; Siril, P.F. Facile Synthesis of Z-Scheme Fe-nPPy/BiOI Nanocomposites for Enhanced Visible Light Driven Photocatalytic Activity. Environ. Sci. Adv. 2024, 3, 85–96. [Google Scholar] [CrossRef]
- Zhu, G.; Hojamberdiev, M.; Zhang, S.; Din, S.T.U.; Yang, W. Enhancing Visible-Light-Induced Photocatalytic Activity of BiOI Microspheres for NO Removal by Synchronous Coupling with Bi Metal and Graphene. Appl. Surf. Sci. 2019, 467–468, 968–978. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed Synthesis and Surface Engineering Strategies of Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef]
- Sultana, S.; Mansingh, S.; Parida, K.M. Facile Synthesis of CeO2 Nanosheets Decorated upon BiOI Microplate: A Surface Oxygen Vacancy Promoted Z-Scheme-Based 2D-2D Nanocomposite Photocatalyst with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2018, 122, 808–819. [Google Scholar] [CrossRef]
- Su, G.; Liu, L.; Kuang, Q.; Liu, X.; Dong, W.; Niu, M.; Tang, A.; Xue, J. Enhanced Visible-Light Photocatalytic Activity and Recyclability of Magnetic Core-Shell Fe3O4@SiO2@BiFeO3–Sepiolite Microspheres for Organic Pollutants Degradation. J. Mol. Liq. 2021, 335, 116566. [Google Scholar] [CrossRef]
- Jiang, W.; Loh, H.; Low, B.Q.L.; Zhu, H.; Low, J.; Heng, J.Z.X.; Tang, K.Y.; Li, Z.; Loh, X.J.; Ye, E.; et al. Role of Oxygen Vacancy in Metal Oxides for Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2023, 321, 122079. [Google Scholar] [CrossRef]
- Ou, X.; Liu, X.; Liu, W.; Rong, W.; Li, J.; Lin, Z. Surface Defects Enhance the Adsorption Affinity and Selectivity of Mg(OH)2 towards As(V) and Cr(VI) Oxyanions: A Combined Theoretical and Experimental Study. Environ. Sci. Nano 2018, 5, 2570–2578. [Google Scholar] [CrossRef]
- Parkinson, G.S.; Diebold, U. Adsorption on Metal Oxide Surfaces. In Surface and Interface Science; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 793–817. ISBN 978-3-527-68058-0. [Google Scholar]
- Bagus, P.S.; Ilton, E.S.; Nelin, C.J. The Interpretation of XPS Spectra: Insights into Materials Properties. Surf. Sci. Rep. 2013, 68, 273–304. [Google Scholar] [CrossRef]
- Fernández-Afonso, Y.; Asín, L.; Beola, L.; Moros, M.; de la Fuente, J.M.; Fratila, R.M.; Grazú, V.; Gutiérrez, L. Iron Speciation in Animal Tissues Using AC Magnetic Susceptibility Measurements: Quantification of Magnetic Nanoparticles, Ferritin, and Other Iron-Containing Species. ACS Appl. Bio Mater. 2022, 5, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Riahi, K.; van de Loosdrecht, M.M.; Alic, L.; ten Haken, B. Assessment of Differential Magnetic Susceptibility in Nanoparticles: Effects of Changes in Viscosity and Immobilisation. J. Magn. Magn. Mater. 2020, 514, 167238. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic Characterization of Iron Oxide Nanoparticles for Biomedical Applications. In Biomedical Nanotechnology: Methods and Protocols; Petrosko, S.H., Day, E.S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 47–71. ISBN 978-1-4939-6840-4. [Google Scholar]
- Wang, X.; Cabrera, D.; Yang, Y.; Telling, N. Probing Magnetization Dynamics of Iron Oxide Nanoparticles Using a Point-Probe Magneto-Optical Method. Front. Nanotechnol. 2023, 5, 1214313. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient Decomposition of Organic Compounds and Reaction Mechanism with BiOI Photocatalyst under Visible Light Irradiation. J. Mol. Catal. A Chem. 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Xia, Y.-M.; Zhang, J.-H.; Xia, M.; Zhao, Y.; Chu, S.-P.; Gao, W.-W. Peony-like Magnetic Graphene Oxide/Fe3O4/BiOI Nanoflower as a Novel Photocatalyst for Enhanced Photocatalytic Degradation of Rhodamine B and Methylene Blue Dyes. J. Mater. Sci: Mater. Electron. 2020, 31, 1996–2009. [Google Scholar] [CrossRef]
- Gholizadeh Khasevani, S.; Gholami, M.R. Evaluation of the Reaction Mechanism for Photocatalytic Degradation of Organic Pollutants with MIL-88A/BiOI Structure under Visible Light Irradiation. Res. Chem. Intermed. 2019, 45, 1341–1356. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Zhou, Q.; Wu, L.; Li, W.; Ren, R.; Lv, Y. Visible-Light Photocatalytic Performance, Recovery and Degradation Mechanism of Ternary Magnetic Fe3O4/BiOBr/BiOI Composite. RSC Adv. 2019, 9, 23545–23553. [Google Scholar] [CrossRef]
- Gallegos-Alcaíno, A.; Barría, G.P.; Moreno, Y.; Fernández, I.; Poblete, R.; Maureira-Cortés, H.; Figueroa Alvarado, A.C.; Hernández, C.B.; Flores, J. Nanostructured Magnetite Coated with BiOI Semiconductor: Readiness Level in Advanced Solar Photocatalytic Applications for the Remediation of Phenolic Compounds in Wastewater from the Wine and Pisco Industry. Appl. Sci. 2024, 14, 9898. [Google Scholar] [CrossRef]
- Xia, Y.; He, Z.; Su, J.; Tang, B.; Hu, K.; Lu, Y.; Sun, S.; Li, X. Fabrication of Magnetically Separable NiFe2O4/BiOI Nanocomposites with Enhanced Photocatalytic Performance under Visible-Light Irradiation. RSC Adv. 2018, 8, 4284–4294. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, X.; Si, Y.; Gao, J.; Du, H.; Pei, S.; Zhang, X.; Yan, Q. Enhanced Photocatalytic Performance of ZnFe2O4/BiOI Hybrid for the Degradation of Methyl Orange. J. Mater. Sci: Mater. Electron. 2019, 30, 8055–8063. [Google Scholar] [CrossRef]
- Zamarreño, R.; Díaz, F. Recuperación de metales económicamente importantes desde relaves mineros abandonados, usando biolixiviación en columnas de fase inversa, de bajo costo y ambientalmente sostenible. Av. En Cienc. E Ing. 2021, 12, 31–42. [Google Scholar]
- Zamarreño-Bastías, R.; Mera, A.C. Recuperación de compuestos de hierro y soluciones de cobalto de relaves mineros. Techno Rev. Int. Technol. Sci. Soc. Rev./Rev. Int. Tecnol. Cienc. Soc. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Zamarreño, R.A.; Gonzalez, P.N.; Hanshing, E.X.; Amar, G.A.; Pizarro, C.M. Evaluación del riesgo ambiental por la presencia de mercurio en relaves mineros dentro de la ciudad de Andacollo, Chile. Av. Cienc. Ing. 2013, 4, 75–83. [Google Scholar]
| Material | Specific Surface (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) | Eg Value (eV) |
|---|---|---|---|---|
| BiOI | 16.3 | 0.31 | 1.98 | |
| Magnetite (Fe3O4) | 19 | 23.0 | 0.13 | 1.47 |
| BiOI/Fe3O4 (6%) | 15 | 17.0 | 0.067 | 1.80 |
| MMA | 1 | - | 0.006 | 2.00 |
| MMB | 1 | - | 0.001 | 2.00 |
| BiOI/MMA | 13 | 19.5 | 0.064 | 1.98 |
| BiOI/MMB | 11 | 18.6 | 0.052 | 2.00 |
| Sample | Peak (eV) | Assignation | Peak (eV) | Assignation | Peak (eV) | Assignation |
|---|---|---|---|---|---|---|
| BiOI/Fe3O4 (6%) | 529.7 | O2− in Bi-O or Fe-O | 531.0 | OH− surface/defects | 532.3 | Surface water/organics |
| BiOI/MMA | 529.8 | O2− in Bi-O or Fe-O | 531.0 | OH− surface/defects | 532.2 | Surface water/organics |
| BiOI/MMB | 529.6 | O2− in Bi-O or Fe-O | 531.0 | OH− surface/defects | 532.2 | Surface water/organics |
| Material | Magnetic Composition | Target Contaminant | Eg eV | Photocatalytic Efficiency (%) | Irradiation Time (min) | Reuse (N° of Cycles) | Photocatalytic Loss (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| BiOI/MMA | MMA (Tailing A) | CA | 1.98 | 89.4 | 120 | 2 | 5.10 | This work |
| BiOI/Fe3O4 | Fe3O4 | CA | 1.97 | 77.2 | 180 | 0 | --- | [95] |
| BiOI/Fe3O4 | Fe3O4 | Phenol | 1.78 | 90.0 | 300 | 5 | 16.0 | [22] |
| BiOI/rGO/Fe3O4 | Fe3O4 | RhB | 1.40 | 95.9 | 480 | 10 | 13.6 | [77] |
| NiFe2O4/BiOI | NiFe2O4 | RhB CV MB | 1.84 | 91–96 | 120 | 5 | 8.00 | [96] |
| ZnFe2O4/BiOI | ZnFe2O4 | MO | 1.87 | 81.2 | 60 | --- | --- | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro, V.A.; Zamorano, C.V.; Araya Vera, C.; Mera, A.C.; Zamarreño Bastias, R.; Alvarez, A.A. BiOI/Magnetic Nanocomposites Derived from Mine Tailings for Photocatalytic Degradation of Phenolic Compounds (Caffeic Acid) in Winery Wastewater. Catalysts 2025, 15, 937. https://doi.org/10.3390/catal15100937
Alfaro VA, Zamorano CV, Araya Vera C, Mera AC, Zamarreño Bastias R, Alvarez AA. BiOI/Magnetic Nanocomposites Derived from Mine Tailings for Photocatalytic Degradation of Phenolic Compounds (Caffeic Acid) in Winery Wastewater. Catalysts. 2025; 15(10):937. https://doi.org/10.3390/catal15100937
Chicago/Turabian StyleAlfaro, Valeria Araya, Celeste Vega Zamorano, Claudia Araya Vera, Adriana C. Mera, Ricardo Zamarreño Bastias, and Alexander Alfonso Alvarez. 2025. "BiOI/Magnetic Nanocomposites Derived from Mine Tailings for Photocatalytic Degradation of Phenolic Compounds (Caffeic Acid) in Winery Wastewater" Catalysts 15, no. 10: 937. https://doi.org/10.3390/catal15100937
APA StyleAlfaro, V. A., Zamorano, C. V., Araya Vera, C., Mera, A. C., Zamarreño Bastias, R., & Alvarez, A. A. (2025). BiOI/Magnetic Nanocomposites Derived from Mine Tailings for Photocatalytic Degradation of Phenolic Compounds (Caffeic Acid) in Winery Wastewater. Catalysts, 15(10), 937. https://doi.org/10.3390/catal15100937








