Abstract
Annually, the food industry generates large amounts of waste and by-products, causing serious problems in their management and final disposal. In particular, by-products are mainly recovered as livestock feed. A most appealing strategy to valorize them has herein been investigated, through polyhydroxyalkanoate (PHA) production. In this view, a stream rich in volatile fatty acids deriving from the acidogenic fermentation of reground pasta (RP), a farinaceous food-industry by-product, was used as a carbon source for PHA production with a phototrophic purple bacteria (PPB) consortium. PPB are very versatile organisms that present a unique metabolism allowing them to adapt to a variety of environmental conditions. The PPB-PHA enrichment phase was performed in a lab-scale semi-continuous photo-bioreactor under a permanent carbon feast regime, with organic loading rate (OLR) increments from 14 to 19 mmolC/Ld. The results showed that the fermented RP solution composition (with 23.4% of HV precursors on a COD basis) was suitable for the PHBHV copolymer production, with the PPB consortium being capable of reaching a very high content in the hydroxyvalerate (HV) monomer, with a maximum of 60% (gHV/gPHA). Regarding the PHA accumulation stage where the light intensity was increased up to 20.2 W/L, a further increase in the culture PHA content by 76% after 12 h was obtained. Overall, these results open the possibility of valorizing food-industry by-products through the development of a biocatalytic process for PHA production with PPB, thus making the overall approach more sustainable from a green perspective.
1. Introduction
Food-industry by-products are the residual materials generated during the processing, production, or manufacturing of food and beverages. These by-products often result from the extraction of primary food components, such as peels, seeds, or pulp, and can include materials that are not intended for human consumption [,]. One of the main problems in their management concerns their improper disposal, increasing their accumulation in the environment, disrupting natural processes, and leading to habitat degradation. As a consequence, the decomposition of organic by-products in landfills contributes to greenhouse gas emissions and also leads to the depletion of available space [,].
While traditionally viewed as waste, these compounds can show a range of challenges and opportunities for sustainable and responsible practices that have gained increasing attention in recent years []. Hence, initiatives such as promoting sustainable alternatives and innovative solutions are crucial to mitigating this environmental impact. As an example, innovative technologies can convert by-products into valuable resources, such as carboxylic acids, bioenergy, fertilizers, or animal feed [,,,,]. In recent years, attempts have been made to valorize food by-products through the production of bioplastics, such as polyhydroxyalkanoates (PHAs), that are fully biodegradable biopolymers suitable for a wide range of industrial applications as food packaging, molded goods, paper coatings, non-woven fabrics, adhesives, films, performance additives, and medical equipment [,], thus leading both plastics and the food industry towards more environmentally sustainable practices. This is possible through the acidogenic fermentation of such by-products and wastes, by which a solution rich in volatile fatty acids (VFAs), direct precursors for PHA production with mixed microbial cultures (MMCs), can be obtained [,,].
PHA production from by-products or wastes is being widely tested by exploiting aerobic organisms, including both pure cultures and MMCs [,,,,]. In this study, the possibility of producing PHAs, and consequently valorizing a farinaceous by-product (reground pasta), has been investigated in a lab-scale semi-continuous system by using a phototrophic mixed culture (PMC) enriched in phototrophic purple bacteria (PBB). PPB present unique metabolic versatility for recovering resources, operating anaerobically, and enabling high carbon recovery efficiency from waste streams. This efficiency is achieved by minimizing carbon dissipation through oxidative metabolism []. In addition, depending on the conditions in which PPB grow (CO2 availability, carbon and phosphorus excess, reduced sulfur), three other classes of storage compounds can be produced by PPB besides PHAs: glycogen, polyphosphate (poly-P), and zero-valence sulfur []. A broad potential industrial application of these polymers is possible. As an example, glycogen can be used as a substrate for alcoholic fermentation processes or for anaerobic digestion []; poly-P can be used as fertilizers or as additives in the food industry, and sulfur recovery from biogas desulphurization can be obtained [,]. Regarding PPB-PHA production, in this study, an alternative approach to the carbon feast/famine (FF) selection strategy commonly applied for MMC was explored. This method involves cultivating PPB in a permanent carbon feast regime (PF), maintaining a constant external carbon supply. In a light-anaerobic-stress environment, these bacteria can utilize ATP generated from photosynthesis to assimilate external carbon. Subsequently, cellular metabolic processes, such as substrate uptake and growth, result in the generation of reduced molecules (e.g., NADH, NADPH), which need to be oxidized to maintain cellular homeostasis. In the absence of external electron acceptors, cells activate internal mechanisms to oxidize these reduced molecules.
The PF strategy aims to select organisms based on their ability to internally regulate the cell’s reducing power through PHA formation, which necessitates the reduction of its precursors during polymer formation. Consequently, organisms capable of dissipating reducing power through PHA production will be favored, and thus high PHA content and carbon recovery can be achieved. The primary advantage of this regime over the FF strategy lies in the potential for simultaneous and continuous growth and PHA accumulation throughout the cycle, eliminating unproductive periods characteristic of the extended famine phase in FF regimes. Furthermore, the capability of this consortium for wastewater treatments by removing and recovering carbon for microbial growth is well known [,]. Unfortunately, the use of PBB is currently still a niche, as the associated costs make their use uneconomic compared to other existing technologies []. One of the major drawbacks of phototrophic processes remains the high light demand, and to date, most studies employing PPB have been performed in artificially illuminated systems. The utilization of synthetic media is also a crucial bottleneck in the process. Indeed, the substrate costs, e.g., 372–755 USD·t−1 for acetic acid and 3900–4400 USD·t−1 for malic acid [,], limit the economic large-scale utilization of PPB to the generation of very-high-value products. The future challenge in PHA production by PBB is focused on increasing the economic feasibility of the whole process flow.
For this reason, the latest research activities on the PPB culture focused on replacing synthetic feedstocks with cheaper waste streams rich in organic and nutrient matter [,] and supporting the light energy demands with natural sunlight illumination, where the upscaling of the phototrophic polyhydroxyalkanoate (PHA) production technology in a pilot-scale system operated in outdoor conditions was performed []. Along this line, the present study focused on the assessment of the feasibility of integrating the valorization of farinaceous food-industry by-products (e.g., reground pasta) with the phototrophic PHA production technology, and since the direct selection of PPB with real fermented solutions is not widely researched, this opens a novel route for valorization of food-industry by-products via PPB technology.
2. Results
2.1. RP Fermented Solution
The PPB selection reactor was fed with the solution obtained from the acidogenic fermentation of reground pasta, as an external organic C source. The fermented solution was characterized by 72% of the total COD (chemical oxygen demand) being in the soluble form, so a large proportion of the substrate supplied to the PPB semi-continuous system was more readily available for consumption by the bacteria (Table 1). The soluble fraction contained volatile fatty acids (VFAs) such as acetic, propionic, and butyric acids, with a smaller presence of valeric acid, and these corresponded to 42% and 58% of total and soluble COD, respectively. The remaining fraction (~55% of total COD) not converted into fermented products or residual free sugars could be linked to the residual polysaccharides (not analyzed here) due to incomplete upstream feedstock fermentation and RP particles still left over after centrifugation.

Table 1.
Reground pasta fermented solution characterization.
2.2. Operation of the Bioreactor with Phototrophic Purple Bacteria
During the 30 days of operation under the permanent carbon feast regime, the biomass concentration of the culture slightly oscillated from 1.5 ± 0.2 g X/L at the beginning of the reactor operation to 1.7 ± 0.1 g X/L at the end of the working days (Figure 1). The specific light intensity of the culture also varied as a direct consequence of the biomass oscillations ranging from 1.5 ± 0.1 to 1.2 ± 0.1 g W/gX (Figure 1). The slight fluctuation in the biomass concentration indicates that the system worked under stable conditions, with similar values compared with the PPB culture operated under a PF regime with acetate as a feedstock as described by Fradinho et al. [].
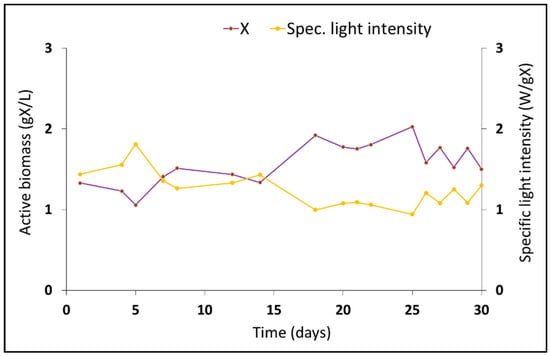
Figure 1.
Characterization of the PPB semi-continuous reactor during the working period: evolution of the biomass concentration and specific light intensity of the phototrophic purple bacteria culture.
Figure 2a shows the VFA profile during the reactor working period with an increase in the applied organic load rate (OLR) values. Despite the recognized influence of VFAs on cells’ PHA content, there was not a complementary effect on the PHA content profile (Figure 2b) with a maximum of 8.3% over the 30 days. However, slight fluctuations in PHA levels could be correlated to the availability of volatile fatty acids. For instance, a drop in PHA was noted on day 13 with a minimum content of 1.76% gPHA/gVSS, after several days of low VFA availability in the culture broth, while an increase in PHA was observed with the rise in the applied OLR in stage 2. Similar patterns were observed between stage 2 and stage 3. Notably, values of the intracellular polymers content remained stable in stage 3, around 3.2 ± 0.2% (wt/wt) and 4.1 ± 0.2% (wt/wt) for glycogen and PHA, respectively, likely due to the increased availability of VFAs in the reactor.
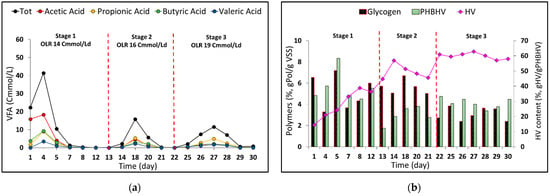
Figure 2.
Characterization of the PPB semi-continuous reactor during the working period: (a) trend of the VFA concentration; (b) evolution of the PPB polymer content (PHA and glycogen) and the composition of PHBHV in terms of HV content.
This can be explained by considering that despite the low/zero VFA concentration on some days of the reactor operation, the PPB culture was being continuously fed during the working days (0.9, 1.0, and 1.2 gsolCOD/Ld during stages 1, 2, and 3, respectively) and, in fact, was permanently consuming the VFAs present in the influent solution (which were 58% of the soluble COD) as well as consuming the remaining soluble COD (Supplementary Figure S1). This is in line with the study of Almeida et al. [], where during some days of system operation in a PF regime (continuously fed with fermented domestic wastewater), the culture had low VFA availability and yet retained the PHA storage capacity, which was especially noticeable upon OLR increments.
Furthermore, a possible consequence of the VFA content oscillation concerned the abundance of bacteriochlorophyll pigments (PPB photosynthetic pigments). Indeed, a decrease in bacteriochlorophyll (Bchl) content was found during the days when the presence of VFAs in the reaction broth was very low (days 14 and 21). It is possible that PPB were making use of their highly flexible metabolism, turning down photoheterotrophic growth due to low VFA availability and favoring chemotrophic growth on the remaining soluble COD fraction. At higher OLR operation, Bchl content increased with increased VFA availability. Nevertheless, during the operation days, BChl values were always higher than chlorophyll (Chl) values, indicating that even with a low CODVFAs/totCOD ratio fed to the reactor, the culture was highly enriched in PPB (Supplementary Figure S2).
Regarding the PHA composition in terms of HV monomer content, a substantial increase was observed from 14.4% (wt/wt) up to almost 60% in the last 7 days of operation. This can be likely explained by the lower amount of HB acid precursors present in the RP fermented solution (18.4% on total COD basis) in relation to the amount in the synthetic mixture fed to the inoculum reactor (70%), bringing an increase in the HV content. Furthermore, this was also confirmed by the average values of the specific substrate uptake rates of propionate (0.10 ± 0.01 Cmol/CmolX d) and valerate (0.050 ± 0.004 Cmol/CmolX d), which were higher than the obtained values for acetate and butyrate (0.06 ± 0.01 and 0.030 ± 0.002 Cmolacids/CmolX d, respectively), during the whole working period. This behavior demonstrated how the PPB culture was able to adapt to the different compositions of the reactor-fed substrate, achieving greater stability in polymer composition during stage 3. This result is an important finding, as it suggests that it is possible to tune the PHA composition in PPB by appropriately modifying the VFA composition of the substrate.
To clarify the metabolism occurring during the PPB operation, three cycles were monitored, and the culture behavior on day 18 is shown in Figure 3. During a cycle of the selection reactor, it can be observed that the culture grew, and the total amount of VFAs present in the RP solution was almost linearly consumed (Figure 3a) but was not exhausted, with soluble COD also being present as previously mentioned, maintaining the PPB in a feast regime. The average value of the VFA uptake rate from three selection reactor cycles was 0.23 ± 0.02 Cmol VFA/CmolX d, and the phosphate and nitrogen consumption rates were equal to 1.7 ± 0.4 mgP/gX d and 97.0 ± 11.7 mgN/gX d. Unlike the carbon source, nutrients (Figure 3b) were mostly consumed (by considering the daily supply—PLR and NLR reported in Section 4.2), with a consumption equal to 2.38 ± 0.1 mgP/Ld and 144.6 ± 2.5 mgN/Ld, in agreement with the known capability of PPB to remove nutrients, showing the potential for the integration of this process within a strategy of nutrient resource recovery.
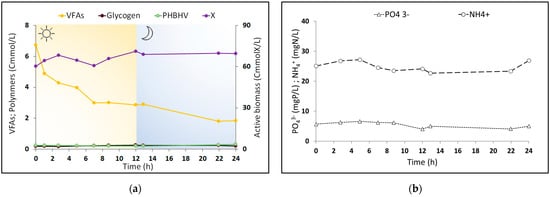
Figure 3.
Culture performance during a typical 24 h cycle of the semi-continuous reactor on day 18 in a permanent feast regime: (a) VFA consumption profile and polymers transformation; (b) nutrient consumption profile.
PHA had only a marginal production (2.62 ± 0.13% wt/wt), where most of the consumed VFAs were used for cell growth, with a biomass concentration equal to 66.3 ± 1.2 CmmolX/L. Regarding glycogen, no substantial variations were observed in its content (2.34 ± 0.10% wt/wt). In fact, the PPB presented a growth yield on VFAs contained in the RP fermented solution of 0.84 ± 0.07 Cmol X/CmolVFAs, while the PHA and glycogen production yield was only 0.02 ± 0.001 and 0.016 ± 0.001 Cmol polymers/CmolVFAs respectively. This suggested the carbon source taken up was required more for biomass growth and cell maintenance processes, decreasing the carbon availability for polymer storage. Nevertheless, the PPB were characterized by a higher global carbon recovery, (Y~0.88 Cmol/Cmol VFAs) with a continuous VFA consumption during the entire cycle.
2.3. PPB Microbial Characterization
Throughout the bioreactor operation, changes in the culture’s composition were observed. The sludge was arranged in small flocs aggregated with a nucleus of the residual presence of algae (as supported by the low Chl detected—Figure S2), surrounded by numerous bacteria (Supplementary Figure S3a). Also, Nile Blue staining permitted the observation of the internal PHA granules, where some bacterial morphological groups, as rod- and cocci-shaped bacteria, appeared to accumulate the polymer granules (Figure S3b). In relation to the bacterial community present in the reactor, FISH analyses indicated a prevalence of Alphaproteobacteria groups followed by Betaproteobacteria with a smaller presence of rod-shaped Rhodpseudomonas. All the other FISH probes that were tested did not show a positive signal (Supplementary Table S1).
Regarding the analysis of the gene amplicon sequences targeting the bacterial 16S rRNA gene variable region 1–3, combined with taxonomic classification, these allowed the estimation of the abundance of different microbial groups for the PPB community. Table 2 summarizes the main taxonomic groups identified for the culture selected with the RP fermented mixture. Despite some occasional occurrence of organisms from other diverse phyla (Actinobacteria and Firmicutes—Supplementary Table S2), the sequencing results in Table S2 show that the majority of the microorganisms were members of the phylum Proteobacteria. Within this phylum, the identified populations belonged mostly to the Alphaproteobacteria class, in line with the FISH analyses, with a greater incidence of the bacterial genera Blastochloris and Rhodomicrobium (purple non-sulfur bacteria—PNSB [], characteristic morphology of this genus showed in Figure S3c) that were particularly dominant in the last day of operation.

Table 2.
Heatmap of the most abundant taxonomic groups (≥0.3% of abundance in at least one sample) identified in the PPB semi-continuous reactor fed with RP fermented solution  less abundant to more abundant. Here, P indicates the phylum, C the class, and G the genus of bacteria.
less abundant to more abundant. Here, P indicates the phylum, C the class, and G the genus of bacteria.
2.4. PPB-PHA Accumulation Capacity
To evaluate the PPB-PHA accumulation capacity, an accumulation test of 12 h was performed with continuous illumination, during which the fermented solution was supplied to bacteria in small pulses to simultaneously prevent carbon depletion and substrate inhibition (Figure 4). The results indicated a continuous PHBHV accumulation during both pulses, where a PHBHV concentration of 10.2 Cmmol PHA/L was achieved, which corresponded to an accumulation of 10% g PHA/g VSS, starting from an initial content of 3.8%. Regarding the PHA maximum specific production rate, a value equal to 0.35 ± 0.1 Cmol PHA/Cmol X d was reached, with the specific substrate uptake rate equal to 1.09 Cmol VFAs/Cmol X d, demonstrating that the culture became faster in the substrate consumption by increasing the light intensity. During the accumulation test, a higher glycogen accumulation yield of 0.55 ± 0.12 Cmol Glycogen/Cmol VFAs (18.5% gGlycogen/gVSS) than YPHA/S (0.32 ± 0.08 Cmol PHA/Cmol VFAs) was detected. The growth yield was equal to 0.12 ± 0.04 Cmol X/Cmol VFAs. This aspect again reflected the high carbon recovery yield of the PPB culture (~0.99 Cmol/Cmol VFAs) which, in anaerobic conditions, can dissipate excess of reducing power from the taken-up VFAs through both PHA production and CO2 fixation via the Calvin–Benson–Bassam (CBB) cycle, thus promoting glycogen storage []. However, the consumption of more reduced acids (butyric and valeric) occurred less during the accumulation test, confirming the preference of PPB for consuming shorter-chain acids rather than medium-chain ones []. This also means that probably the glycogen accumulation was triggered more by the consumption of residual sugars present in the RP solution than through the CO2 fixation. The lower valeric consumption was also reflected in the PHA composition, where HV content decreased during the test (Supplementary Figure S4), while with the replenishment of acetic acid with the second pulse, there was a preferential HB accumulation, thus obtaining a composition equal to 60 HB/40 HV (%, wt/wt) at the end of the test.
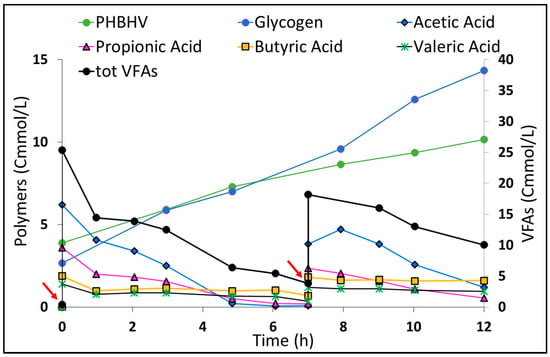
Figure 4.
Accumulation batch test under constant illuminated conditions, light intensity of 20.2 W/L, with the addition of multiple pulses of fermented solution: evolution of the PPB polymer contents (PHA and glycogen) and the VFA trend, where red arrows indicate the fermented solution pulses.
Considering the yields in terms of COD, values of 0.31 ± 0.08 gCOD PHA/gCOD VFAs, 0.34 ± 0.13 gCOD Gly/gCOD VFAs, and 0.11 ± 0.04 gCOD X/gCOD VFAs were reached for PHA, glycogen, and growth, respectively. The total yield of about 0.77, lower than the total calculated on a C-mol basis, could be attributed to the activation of the nitrogenase enzyme, by which PPB can balance the redox power through the production of hydrogen []. With ammonia concentrations higher than ~20 mgN/L, an inhibition of this enzyme can occur, resulting in low hydrogen yields []. During the conducted accumulation test, an initial ammonia concentration of 38.0 mgN/L was observed, but during the test, a gradually more favorable ammonia concentration for activating this enzyme complex was identified (Supplementary Figure S5), thus probably resulting in partial COD conversion into H2 (not measured here).
3. Discussion
3.1. PPB Culture Performance
In this section, the performance of the PPB culture obtained in the present study is compared with literature studies performed in lab-scale reactors under PF or FF operation, either with synthetic or fermented feedstocks. In Table 3, the main kinetic and stoichiometric parameters obtained during the reactor operation and during the accumulation tests are reported.

Table 3.
Summary of the operating conditions of the PHA storage PPB investigated in this study and corresponding biomass and PHA production performance compared with the literature.
Regarding the average value of the substrate uptake rate, it was lower than the values obtained in the other studies reported here. In the study of Almeida et al. [], when a PF regime and a fermented solution were adopted, the substrate uptake rate was almost doubled (0.48 ± 0.05 Cmol S/Cmol Xd) compared to the specific substrate uptake rate obtained here (0.23 ± 0.02 Cmol S/Cmol Xd), while the biomass yield was twice (0.84 CmolX/Cmol S) the value present in the PF of Almeida et al. (0.42 CmolX/Cmol S). Accordingly, the PPB selected in this study turned the substrate consumption more towards microbial growth rather than polymer accumulation, reflecting an evidently lower accumulation PHA yield. Even more, when compared with the feast and famine strategy adopted by Almeida et al. [], the substrate uptake rate and the PHA accumulation yield of this work were much lower. This can be explained by the fact that FF usually promotes high specific substrate uptake rates compared with a PF regime, but these high carbon consumption rates occur for a few hours due to the short period of the feast phase [].
However, it must be considered that the specific rate defined here was only on the acid component of the fermented solution, which was 42% of the total COD. Considering that there was no accumulation of soluble COD fed to the reactor in the system, the specific substrate uptake rate could have been around 0.45 gCOD S/gCOD Xd, very close to the value obtained by Fradinho et al. [], equal to 0.61 gCOD S/gCOD Xd, where a PF regime was used and a synthetic acetate solution was used as feedstock. Even though the two systems worked differently, the performance of the selected culture in the latter is very similar to the performance obtained during the semi-continuous reactor operation of this work, including in terms of PHA accumulation yield and PHA content, thus suggesting the achievement of a good performance. During the accumulation test, the microbial culture selected here diverged from the results of Fradinho in 2016 [], with lower PHA accumulation probably due to competing pathways for reducing power, with a maximum PHA content of just 10% (gPHA/gVSS). This performance can be explained by considering that in the work of Fradinho (2016) [], the applied external carbon source consisted only of acetate, which is the best precursor for PPB-PHA production, while in this study, the VFA components also included propionic, butyric, and valeric acids, thus requiring further CO2 fixation for substrate uptake to balance redox potential, having glycogen production in parallel with the PHA accumulation. If we compare the PPB process with aerobic MMC operated under an FF regime and also using a fermented solution rich in reduced VFAs, obtained from fruit waste [], the PHA content obtained here deviates further. Indeed, in the work of Silva and colleagues (2022) [], a PHA content of 30.3 ± 1.6% (wt/wt) in the selection reactor, reaching a content of up to 71% during the accumulation stage, was obtained. In aerobic MMCs, the take-up of more reduced VFAs and their storage as PHAs are possible and favored due to the oxidative operating conditions that allow overcoming redox limitations. However, ATP production in aerobic MMC is dependent on substrate oxidation, which results in higher carbon losses as CO2 in comparison to the anaerobic PPB mixed cultures, with the latter enabling higher carbon recovery yields.
Moreover, the type of carbon source applied here, with a low CODVFAs/totCOD ratio, compromised the PHA storage yield, in line with the previous finding, where non-VFA COD components present, as complex sugars, can be consumed by PPB but not for PHA production, negatively affecting its storage []. Indeed, when analyzing the accumulation tests performed by Almeida in 2021, it can be seen the best performance was achieved when the non-sugar-containing feedstock was used. When comparing the accumulation test with sugar-containing feedstock to the findings of this work, a similar behavior was observed. However, the greater PHA storage capacity in the study of Almeida et al. (2021) [] can be related to its feedstock exhibiting a higher ratio of acid on a C-mol basis (72%) compared to the ratio observed in the fermented solution used here (38.8% C-molVFAs/C-mol).
These findings suggested that to enhance the system in the PHA production, an operational adjustment to higher light availabilities during the accumulation stage is useful for incrementing metabolic activity and leading to higher polymer content. Furthermore, to select PPB with higher PHA accumulation capacity under anaerobic conditions, and to improve the valorization of the food-industry by-products, strict control of the upstream fermentation process should be mandatory to guarantee a fermented influent with high content in terms of VFAs (preferably acetate) and without sugars.
3.2. PPB Microbial Characteristics
The results in terms of microbial characterization obtained in this work showed that phototrophic mixed cultures can evolve and change in response to the operating conditions being varied, being capable of contributing to PHA production. Indeed, from the beginning of reactor operation, the abundance of bacterial groups varied, with Blastochloris becoming more abundant at the end of the working period (47% abundance). In a previous study, generally, the presence of this genus indicated a lower substrate concentration and/or shorter contact times with the substrate []. Also, the presence of transient illumination periods is another factor that can influence the bacterial community composition. An increase in Blastochloris has also been reported for an acetate-fed PMC that was operated under a substrate-competing environment [].
Another important factor that can impact the microbial culture composition is the applied OLR. Indeed, as observed in a previous study under low OLR in a PF regime and with fermented domestic wastewater solution as substrate, the culture became highly enriched in Rhodopseudomonas (84% abundance), with a moderate presence of Rhizobium (11.2%) and a minor presence of Rhodobacter (1.3%) []. In the present study, a moderate presence of Rhizobium and Rhodobacter was also detected with enrichment in Rhodopseudomonas (5.9%) at the end of the 30 days of operation. For Rhodomicrobium, a decrease in abundance from 84.8% to 37.4% was detected. This genus can grow in diverse photoautotrophic and photoheterotrophic conditions, performing nitrogen fixation []. This could suggest that in this work, the increase in the Blastochloris genus and the decrease in Rhodomicrobium may be favored under lower VFA availability since there were days in which the VFAs/solCOD ratio detected in the reactor was low, possibly triggering a shift in the metabolic pathways used by the PPB consortium, favoring one genus over another. Hence, the PHA content obtained in this work (around 10% gPHA/gVSS during the accumulation test, Table 3) was likely due to organisms within the genera Rhodopseudomonas, Rhodomicrobium, and/or Blastochloris which managed to prevail and store PHA under the transient light condition and at low applied OLR.
Regarding Propionicimonas and Clostridium sensu stricto 12 genera, in a minor presence (Table S1), these are facultative anaerobes that present better growth under anaerobic conditions and are able to ferment glucose into acetate, propionate, and butyrate []; thus, their growth was likely favored by the presence of residual sugar in the fermented solution.
Overall, this study allowed the pinpointing of microbial groups that exhibit higher PHA accumulation potential, alongside discerning optimal operational conditions to maximize their prevalence and PHA storage. This could pave the way for refining strategies aimed at bolstering PHA production in PPB systems, thus also simplifying the downstream separation of PHAs from the biomass. Therefore, environmental and cost–benefit analyses for determining the economic viability of scaling up this process for commercial production will be performed in future works.
4. Materials and Methods
4.1. Fermented Solution as a Carbon Source
The fermented solution fed to the PPB reactor was obtained from the acidogenic fermentation of a farinaceous industry by-product, i.e., reground pasta (RP), performed in an SBR of 1.1 L working volume. RP was chosen from our previous study, where the acidogenic fermentation potential of 9 different food by-products was investigated through batch tests []. Briefly, the acidogenic SBR was fed with an RP solution (34.0 gCODRP/L corresponding to 6.8 gCODRP/Ld) for a total of 97 working days, by imposing the pH equal to 5.50 ± 0.01 through a control system, with a hydraulic retention time (HRT) and sludge retention time (SRT) of 4 days. Periodically the effluent downstream of the process was centrifuged to remove the residual biomass and RP particles. Afterward, to preserve the characteristics of the fermented solution, it was placed in a refrigerator at 4 °C under mixing conditions and fed for 30 working days to the PPB semi-continuous system.
4.2. Operation of PPB-PHA Selection Reactor
The inoculum used in this work was obtained from a lab-scale photo-bioreactor containing a photosynthetic consortium already enriched in purple bacteria, operated at the same regime used here, but using a synthetic VFA mixture as feedstock (39% acetic acid, 18% propionic acid, 31% butyric acid, and 12% valeric acid on COD basis) and containing a biomass concentration of 1.5 g VSS/L. The PPB selective reactor operation under light/dark periods was performed in a volume of 3.8 L reactor with 24-h cycles, where in the first 12 h of the cycle, the reactor was under light conditions illuminated externally by a tungsten lamp (60 W) and internally by an infrared lamp at a light intensity of 192 W/m2 and of 112 W/m2, respectively, which corresponded to a total volumetric intensity of 1.91 W/L of culture broth. In the last 12 h of the cycle, the reactor was under dark conditions. This volumetric light intensity was chosen to increase the selective pressure, allowing an increase in the polymer storage in subsequent PHA accumulation stages upon a surplus of light intensity, greater than that required for growth []. In addition, an obscuring black foil (UV-Vis filter from LEE Filters (Andover, UK), model 299 1.2ND) was placed around the photo-bioreactor in front of the external lamps to shield the visible component of the light, avoiding the growth of visible-absorbing chlorophyll-containing organisms (microalgae and cyanobacteria).
The fermented solution, described in Section 2.1, was used as a carbon source, continuously fed to the reactor during the 30 days of operation, having a concentration of 288 Cmmol/L (10.5 gCODvfa/L) in terms of acetic, propionic, butyric, and valeric acids. The total flow rate fed to the system was 633 mL/d, divided into two feeding components consisting of the fermented solution and a mineral medium, taking place via two different peristaltic pumps. The mineral medium contained 3.8 g NH4Cl, 2.2 g NaCl, 0.8 g MgCl2·6H2O, 0.2 g MgSO4·7H2O, 0.2 g CaCl2·2H2O, 1.0 g K2HPO4, 61.2 g KH2PO4, 26.6 mL iron citrate, and 5.4 mL trace element solution per liter. The C/N/P ratio was maintained constant by adopting the general formula for anaerobic biomass grown under carbon-limited conditions []: CH1.8O0.5N0.2S0.002P0.02.
However, during the semi-continuous reactor operation, the OLR was adjusted to the organic carbon demand of the culture by varying the carbon inlet flow rate (decreasing the mineral medium component, thus not varying the total flow), thus avoiding the depletion of the external C source. Considering only the VFA component of the feedstock, the applied OLR increased from 14 mmolCVFAs/Ld (0.53 gCODVFAs/Ld) up to 16 and 19 (0.60 and 0.71 gCODVFAs/Ld, respectively), and these three operating conditions were identified as stage 1, stage 2, and stage 3 of the reactor operation, respectively. The average values of the phosphate and nitrogen loading rates (PLR and NLR) during the whole reactor working period were 2.89 ± 0.13 mgP/L d and 148.66 ± 0.13 mgN/L d, respectively. In terms of soluble COD fed to the reactor (0.9–1.2 gCODsol/Ld), the values align with the OLR utilized by Fradinho et al. [], set at 1.3 gCOD/Ld with a 24-h light cycle. However, given the halved illumination period in this study (12 h), the OLR was nearly halved as well. Generally, these lower values are appropriate for the slower kinetics of photosynthetic microorganisms compared with the MMC aerobic systems [,,].
The discharge was carried out by means of a level purge, using a drainage siphon to guarantee anaerobic conditions, resulting in an HRT and SRT of 6 days. The temperature was maintained at around 30.0 ± 1.0 °C through a thermostatic bath, and the pH was maintained at 7.5 ± 0.1 through a system of control with 0.66 M HCl.
4.3. PHA Accumulation Test
After 3 SRTs of stable biomass concentration, the storage performance of the biomass selected in a semi-continuous reactor system was evaluated by batch accumulation tests in a closed and anaerobic 0.5 L working volume reactor, mixed by a magnetic stirrer at the same temperature and pH as the selective reactor. The tests were performed under tungsten lamp illumination and a light intensity of 20.2 W/L. The test started by collecting 0.5 L from the enrichment reactor before the beginning of the light period. Afterward, the collected mixed liquor and the batch reactor headspace were sparged with nitrogen gas to ensure an oxygen-free atmosphere within the accumulation batch. Two pulses of RP fermented solution (25 and 15 Cmmol/L on total VFA basis) were added throughout the test to maintain an excess of the C source and thus prevent carbon depletion. The tests were conducted under nutrient (nitrogen and phosphorus)-limiting conditions by not adding mineral solution to the collected mixed liquor. However, microbial growth was not completely inhibited as the fermented solution had residual concentrations of nutrients (around 10 mg/L of phosphate and 38 mg/L of nitrogen). The mixed liquor was sampled at the beginning of each test, to assess the metabolic activity of biomass, and every hour, thus obtaining a complete characterization of the accumulation test.
4.4. Analytical Methods
Total/volatile solid and total/volatile suspended solid (TS and VS, TSS and VSS) concentrations were measured according to Standard Methods []. Ammonia and phosphate concentrations were measured by colorimetry using a segmented flow analyzer (Skalar, Breda, The Netherlands) measured at 880 nm []. The total and soluble chemical oxygen demand (totCOD and solCOD) were measured by digestion of the total and filtered (0.45 um) sample, respectively, with dichromate at 150 °C according to Standard Methods []. Lactate, acetic, propionic, iso-butyric, butyric, iso-valeric, valeric, and caproic acids were determined by high-performance liquid chromatography (HPLC) using a chromatograph equipped with an Agilent Metacarb 87H column (Santa Clara, CA, USA) and two detectors connected in line, one ultraviolet detector (UV) and a refractive index detector (RI). A solution of 0.01 N of H2SO4 was used as a mobile phase with a flow rate of 0.5 mL/min and a 30 °C operating temperature. The detection wavelength was set at 210 nm []. All samples were analyzed after centrifugation at 13.000 rpm for 3 min and filtration through a 0.2 µm membrane. The sum of the fermented products was expressed as the chemical oxygen demand (COD) by using the corresponding conversion factor for each acid []. Total and soluble sugars (in terms of free sugar content) were determined using the Dubois method [], and the absorbance values were measured by a spectrophotometer at 490 nm. Glycogen determination was performed through digestion, for two hours at 100 °C, of the samples after the addition of 2 mL of a 0.66 M solution of HCL. Afterward, the samples were filtered through a 0.2 µm membrane and analyzed using the same HPLC used for VFAs. PHA determination was performed by gas chromatography using a method reported elsewhere []. Then, the obtained samples were injected into a gas chromatograph coupled to a flame ionization detector (GC-FID Varian CP-3800, Varian, Palo Alto, CA, USA) and equipped with a ZBWax-Plus column. Helium was the carrier gas at a flow rate of 1 mL/min, and heptadecane was used as the internal standard for the gas chromatography analysis. The stoichiometry conversion numbers used to express PHA concentration in terms of COD were 1.67 g COD/g HB and 1.92 g COD/g HV. Bacteriochlorophyll a + b (Bchl a + b) and chlorophyll a (Chl a) pigments were also determined by the method reported here []. The absorption spectra of the pigments in ethanol were performed using a Camspec MA09T spectrophotometer (Spectronic Camspec Ltd., Leeds, UK), determining the absorbance value at a wavelength of 664 nm and 773 nm for chlorophyll and bacteriochlorophyll, respectively.
4.5. Microbial and Metagenomic Analysys
Microbial communities coming from the PPB semi-continuous reactor were examined under a microscope (Olympus BX51 epifluorescence microscope, Tokyo, Japan) for morphological observation, visualization of PHA granules (through Nile Blue staining), and bacterial community analysis by fluorescence in situ hybridization (FISH). Nile Blue staining was performed on wet biomass according to Bengtsson et al. (2008) [], thus determining the presence of intracellular PHA granules in the bacteria cytoplasm. Regarding FISH analysis, sludge samples were fixed with paraformaldehyde as described by Nielsen et al. []. The following specific oligonucleotide probes were employed: ALF969 for Alphaproteobacteria, BET42a for Betaproteobacteria, GAM42a for Gammaproteobacteria, Delta425a for DeltaProteobacteria, LGC354 for Firmicutes, DSBAC 357 for Desulfobacteriaceae, DSB706 for Desulfobulbaceae, DSV687 for Desulfobulbaceae, and TM7305 and TM7905 to identify filament morphotype and cocci, respectively []. For the identification of purple bacteria, the following probes were also employed: GRb was used for Rhodobacter and Roseobacter; Rhodopseud for Rhodopseudomonas; within Betaproteobacteria, RHC439 was used for Rhodocyclus.
Regarding the metagenomic analyses, samples were analyzed by extracting DNA from all organisms [] using gene amplicon sequencing targeting bacterial 16S rRNA gene variable region 1–3 (bV13-A) in combination with taxonomic classification against the MiDAS 2.1.3 database, prepared by a custom protocol according to Caporaso et al. [] using the specific primers [27F] AGAGTTTGATCCTGGCTCAG and [534R] ATTACCGCGGCTGCTGG []. DNA concentration was measured using a Qubit dsDNA HS Assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Gel electrophoresis using Tapestation 2200 and D1000/High sensitivity D1000 screen tapes (Agilent, Santa Clara, CA, USA) was used to validate the product size and purity of a subset of sequencing libraries.
4.6. Calculations
To describe and characterize the process, the following equations were used: The biomass PHA content was calculated as a percentage of volatile suspended solids (VSSs) on a mass basis (%PHA = 100 × g PHA/g VSS), where VSSs include active biomass (X), PHA, and glycogen. Active biomass was calculated by subtracting PHA and glycogen from VSS.
The composition of the intracellular polymer was calculated as a percentage of HB and HV on a mass basis (%HB = 100 × g HB/g PHA; %HV = 100 × g HV/g PHA), where PHA includes HB and HV monomers.
The maximum specific substrate uptake rate, in terms of total carboxylic acids, or even for each acid (acetate, propionate, butyrate, and valerate) present in the RP fermented solution (−qS in Cmol S/Cmol X d), the phosphate and nitrogen specific consumption rates (in Cmol P/Cmol X d and Cmol N/Cmol X d), and the maximum specific PHA production rate (qP in Cmol PHA/Cmol X d) were determined through a mass balance for the semi-continuous reactor, and by adjusting a linear regression line to the experimental concentrations determined over time and dividing the slope of the fitting at time zero by the concentration of active biomass at that point for the accumulation test.
The yields of PHA per substrate consumed (YPHA/S in Cmol PHA/Cmol S) were calculated by dividing the amount of PHA formed by the amount of acids consumed; the same calculation was performed for the glycogen yield (Y Gly/S in Cmol Gly/Cmol S). The specific light intensity of the culture (W/g X) was calculated by dividing the volumetric intensity of the culture broth (1.91 W/L) by the active biomass concentration (g X/L). “S” indicates the VFAs of the fermented solution.
5. Conclusions
This study explored the potential of selecting a PHA-accumulating phototrophic mixed culture enriched in PPB under a permanent feast regime operation by using a farinaceous by-product fermented solution as carbon feedstock. The findings suggested that, even with a low ratio of CODvfas/totCOD of the applied feedstock (42% gCODVFAs/gCODtotal), the culture was highly enriched in PPB with a trace number of fermentative bacteria, obtaining a very high carbon recovery during the accumulation stage through balancing the reducing power into bacterial growth and PHA and glycogen storage. The results showed that when using this fermented solution, during the accumulation stage, a preference in the storage of glycogen occurred (likely via CO2 fixation and solCOD consumption), obtaining a content equal to 18.5% (gGlycogen/gVSS) against 10.0% of PHA (gPHA/gVSS), but demonstrating the ability of bacteria to increase the initial PHA content when exposed to higher light availability. However, a high HV content was achieved (maximum 60% wt/wt in the reactor and 40% in the accumulation test), showing the good adaptation of the PPB culture to the substrate composition, thus suggesting the possibility of tuning the polymer composition through feedstock adjustments. With the system studied here, very high yields of carbon recovery were achieved, thus demonstrating the possibility of valorizing food-industry by-products by PPB-PHA production. This result ascertains that the selection of PHA-accumulating phototrophic cultures is not limited to the chosen strategy alone, but also is highly dependent on the type of substrate fed to the reactor.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal14040239/s1: Figure S1: Characterization of the PPB semi-continuous reactor during the working period: soluble COD and total VFA concentration trends. Figure S2: The PPB semi-continuous reactor during the working period: pigment trend of bacteriochlorophyll and chlorophyll during the working period. Figure S3: Microscopic images of the selected PPB cultures in the semi-continuous reactor. Figure S4: Accumulation batch test under constant illuminated conditions, evolution of the nutrient concentration. Figure S5: Accumulation batch test under constant illuminated conditions, evolution of the composition of PHA in terms of HB-HV content. Table S1: Heatmap of the identified taxonomic groups (≥0.1% of abundance in at least one sample) identified in the PPB semi-continuous reactor fed with RP fermented solution. Table S2: FISH results for the PPB system fed with the RP fermented solution under the PF regime on the last day of operation.
Author Contributions
Conceptualization, M.V. and J.F.; validation, J.F.; formal analysis, A.M. and M.P.; investigation, A.M. and M.P.; data curation, A.M. and J.F.; writing—original draft preparation, A.M.; writing—review and editing, J.F. and M.V.; visualization, A.M.; supervision, J.F.; funding acquisition, M.V. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Usable Packaging project, which has received funding from the Bio-based Industries Joint Undertaking (JU) under the European Union’s Horizon 2020 research and innovation program under grant agreement No. 836884. The JU receives support from the European Union’s Horizon 2020 research and innovation program and the Bio-based Industries Consortium. This research was also financed by national funds from FCT (Fundação para a Ciência e a Tecnologia), I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences (UCIBIO) and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy (i4HB). M.P. acknowledges the financial support of FCT (Fundação para a Ciência e a Tecnologia) through the Ph.D. grant DFA/BD/05659/2020.
Data Availability Statement
The data presented in this study are available upon reasonable request.
Acknowledgments
The authors would like to give special thanks to Maria Reis (Chemistry Department, NOVA School of Science and Technology) for her valuable discussions of the experimental results and contribution to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Keijser, T. Financial collateral arrangements in the European Union: Current state and the way forward. Unif. Law Rev. 2017, 22, 258–300. [Google Scholar] [CrossRef]
- Tiwari, A.; Khawas, R. Food Waste and Agro By-Products: A Step towards Food Sustainability. In Innovation in the Food Sector through the Valorization of Food and Agro-Food By-Products; InTech Open: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Marchetti, A.; Salvatori, G.; Tayou Nguemna, L.; Grumi, M.; Ozan Basar, A.; Pardo-Figuerez, M.; Lagaron, J.M.; Prieto, C.; Marcoaldi, C.; Villano, M.; et al. Developing Bioplastics from Agro-Industrial Wastes for Applications in Food Packaging. In Developing Circular Agricultural Production Systems; Burleigh Dodds Science Publishing: London, UK, 2024; pp. 273–316. [Google Scholar]
- Sahai, S.; Sharma, C.; Singh, S.K.; Gupta, P.K. Assessment of Trace Gases, Carbon and Nitrogen Emissions from Field Burning of Agricultural Residues in India. Nutr. Cycl. Agroecosyst. 2011, 89, 143–157. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Galanakis, C.M. Sustainable Applications for the Valorization of Cereal Processing By-Products. Foods 2022, 11, 241. [Google Scholar] [CrossRef]
- Gottardo, M.; Bolzonella, D.; Adele Tuci, G.; Valentino, F.; Majone, M.; Pavan, P.; Battista, F. Producing Volatile Fatty Acids and Polyhydroxyalkanoates from Foods By-Products and Waste: A Review. Bioresour. Technol. 2022, 361, 127716. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From Sewage Sludge and Agri-Food Waste to VFA: Individual Acid Production Potential and up-Scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef] [PubMed]
- De Donno Novelli, L.; Moreno Sayavedra, S.; Rene, E.R. Polyhydroxyalkanoate (PHA) Production via Resource Recovery from Industrial Waste Streams: A Review of Techniques and Perspectives. Bioresour. Technol. 2021, 331, 124985. [Google Scholar] [CrossRef] [PubMed]
- Szacherska, K.; Oleskowicz-Popiel, P.; Ciesielski, S.; Mozejko-Ciesielska, J. Volatile Fatty Acids as Carbon Sources for Polyhydroxyalkanoates Production. Polymers 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Salvatori, G.; Astolfi, M.L.; Fabiani, M.; Fradinho, J.; Reis, M.A.M.; Gianico, A.; Bolzonella, D.; Villano, M. Evaluation of the Acidogenic Fermentation Potential of Food Industry By-Products. Biochem. Eng. J. 2023, 199, 109029. [Google Scholar] [CrossRef]
- Marzulli, F.; Musivand, S.; Arengi, M.; De Caprariis, B.; De Filippis, P.; Marchetti, A.; Majone, M.; Villano, M. Coupled Biological and Thermochemical Process for Plastic Waste Conversion Into Biopolymers. Chem. Eng. Trans. 2023, 100, 2023. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Bengtsson, S.; Pisco, A.R.; Reis, M.A.M.; Lemos, P.C. Production of Polyhydroxyalkanoates from Fermented Sugar Cane Molasses by a Mixed Culture Enriched in Glycogen Accumulating Organisms. J. Biotechnol. 2010, 145, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Matos, M.; Pereira, B.; Ralo, C.; Pequito, D.; Marques, N.; Carvalho, G.; Reis, M.A.M. An Integrated Process for Mixed Culture Production of 3-Hydroxyhexanoate-Rich Polyhydroxyalkanoates from Fruit Waste. Chem. Eng. J. 2022, 427, 131908. [Google Scholar] [CrossRef]
- Zeng, S.; Song, F.; Lu, P.; He, Q.; Zhang, D. Improving PHA Production in a SBR of Coupling PHA-Storing Microorganism Enrichment and PHA Accumulation by Feed-on-Demand Control. AMB Express 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA Production by Mixed Cultures and Renewable Waste Materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Betancourth, C.; Echeverri, S.; Rodriguez-Gonzalez, C.; Wist, J.; Combariza, M.Y.; Sanabria, J. Enhancement of PHA Production by a Mixed Microbial Culture Using VFA Obtained from the Fermentation of Wastewater from Yeast Industry. Fermentation 2022, 8, 180. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Batstone, D.J.; Grassino, M.; Vlaeminck, S.E.; Puyol, D.; Verstraete, W.; Kleerebezem, R.; Oehmen, A.; Ghimire, A.; Pikaar, I.; et al. Purple Phototrophic Bacteria for Resource Recovery: Challenges and Opportunities. Biotechnol. Adv. 2020, 43, 107567. [Google Scholar] [CrossRef]
- Comer, A.D.; Abraham, J.P.; Steiner, A.J.; Korosh, T.C.; Markley, A.L.; Pfleger, B.F. Enhancing Photosynthetic Production of Glycogen-Rich Biomass for Use as a Fermentation Feedstock. Front. Energy Res. 2020, 8, 93. [Google Scholar] [CrossRef]
- Vainshtein, M.B.; Gogotova, G.I.; Heinritz, N.-J. Removal of H2S by the Purple Sulphur Bacterium Ectothiorhodospira Shaposhnikovii. World J. Microbiol. Biotechnol. 1994, 10, 110–111. [Google Scholar] [CrossRef]
- Hülsen, T.; Barry, E.M.; Lu, Y.; Puyol, D.; Keller, J.; Batstone, D.J. Domestic Wastewater Treatment with Purple Phototrophic Bacteria Using a Novel Continuous Photo Anaerobic Membrane Bioreactor. Water Res. 2016, 100, 486–495. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; Zheng, Z.; Meng, F.; Du, T.; He, S. Bio-Conversion of Photosynthetic Bacteria from Non-Toxic Wastewater to Realize Wastewater Treatment and Bioresource Recovery: A Review. Bioresour. Technol. 2019, 278, 383–399. [Google Scholar] [CrossRef]
- Mondala, A.H. Direct Fungal Fermentation of Lignocellulosic Biomass into Itaconic, Fumaric, and Malic Acids: Current and Future Prospects. J. Ind. Microbiol. Biotechnol. 2015, 42, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Moscoviz, R.; Trably, E.; Bernet, N.; Carrère, H. The Environmental Biorefinery: State-of-the-Art on the Production of Hydrogen and Value-Added Biomolecules in Mixed-Culture Fermentation. Green Chem. 2018, 20, 3159–3179. [Google Scholar] [CrossRef]
- Batstone, D.J.; Hülsen, T.; Mehta, C.M.; Keller, J. Platforms for Energy and Nutrient Recovery from Domestic Wastewater: A Review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef]
- Almeida, J.R.; Serrano, E.; Fernandez, M.; Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Polyhydroxyalkanoates Production from Fermented Domestic Wastewater Using Phototrophic Mixed Cultures. Water Res. 2021, 197, 117101. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; León, E.S.; Rogalla, F.; Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Polyhydroxyalkanoates Production in Purple Phototrophic Bacteria Ponds: A Breakthrough in Outdoor Pilot-Scale Operation. Sci. Total Environ. 2024, 912, 168899. [Google Scholar] [CrossRef]
- Fradinho, J.C.; Reis, M.A.M.; Oehmen, A. Beyond Feast and Famine: Selecting a PHA Accumulating Photosynthetic Mixed Culture in a Permanent Feast Regime. Water Res. 2016, 105, 421–428. [Google Scholar] [CrossRef]
- Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Suzuki, K.; Ludwig, W.; Whitman, W.B. (Eds.) Bergey’s Manual® of Systematic Bacteriology; Springer: New York, NY, USA, 2012; ISBN 978-0-387-95043-3. [Google Scholar]
- Makowka, A.; Nichelmann, L.; Schulze, D.; Spengler, K.; Wittmann, C.; Forchhammer, K.; Gutekunst, K. Glycolytic Shunts Replenish the Calvin–Benson–Bassham Cycle as Anaplerotic Reactions in Cyanobacteria. Mol. Plant 2020, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.; Stevens, B.; Ebrahimi, S.; Alloul, A.; Vlaeminck, S.E.; Weissbrodt, D.G. Enrichment and Aggregation of Purple Non-Sulfur Bacteria in a Mixed-Culture Sequencing-Batch Photobioreactor for Biological Nutrient Removal From Wastewater. Front. Bioeng. Biotechnol. 2020, 8, 557234. [Google Scholar] [CrossRef]
- Stephanopoulos, G.N.; Aristidou, A.A.; Nielsen, J. Metabolic Engineering: Principles and Methodologies; Academic Press: Cambridge, MA, USA, 1998; ISBN 0126662606. [Google Scholar]
- Marchetti, A.; Lorini, L.; Salvatori, G.; Gianico, A.; Majone, M.; Villano, M. Polyhydroxyalkanoates Production by Mixed Microbial Cultures in Sequencing Batch Reactors Operated Under Different Feeding Conditions. Chem. Eng. Trans. 2022, 93, 163–168. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) Production by a Mixed Microbial Culture Using Sugar Molasses: Effect of the Influent Substrate Concentration on Culture Selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Majone, M.; Vallini, G.; Di Gregorio, S.; Beccari, M. Effect of the Applied Organic Load Rate on Biodegradable Polymer Production by Mixed Microbial Cultures in a Sequencing Batch Reactor. Biotechnol. Bioeng. 2006, 93, 76–88. [Google Scholar] [CrossRef]
- APHA. Washington DC. Standard methods for the examination of water and wastewater. Part 5. Am. J. Public Health 1995, 85, P.164–P.203. [Google Scholar] [CrossRef]
- Oliveira, C.S.S.; Silva, C.E.; Carvalho, G.; Reis, M.A. Strategies for Efficiently Selecting PHA Producing Mixed Microbial Cultures Using Complex Feedstocks: Feast and Famine Regime and Uncoupled Carbon and Nitrogen Availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Matsuo, T.; Wentzel, M.C.; Marais, G.V.R. Wastewater and Biomass Characterization for the Activated Sludge Model No. 2: Biological Phosphorus Removal. Water Sci. Technol. 1995, 31, 13–23. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of Polyhydroxyalkanoates by Activated Sludge Treating a Paper Mill Wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Nguyen, H.T.T.; Mcilroy, S.J.; Mielczarek, A.T.; Seviour, R. Identification of Polyphosphate-Accumulating and Glycogen-Accumulating Organisms by FISH. In FISH Handbook for Biological Wastewater Treatment; IWA Publishing: London, UK, 2009; pp. 25–31. [Google Scholar]
- Hugenholtz, P.; Tyson, G.W.; Webb, R.I.; Wagner, A.M.; Blackall, L.L. Investigation of Candidate Division TM7, a Recently Recognized Major Lineage of the Domain Bacteria, with No Known Pure-Culture Representatives. Appl. Environ. Microbiol. 2001, 67, 411–419. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Jumpstart Consortium Human Microbiome Project Data Generation Working Group. Evaluation of 16S RDNA-Based Community Profiling for Human Microbiome Research. PLoS ONE 2012, 7, e39315. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).