Abstract
An efficient and operationally simple method for the synthesis of α-acyloxy ketones through the readily available 2-methylimidazole-promoted reaction of α-hydroxy ketones and anhydrides is developed. In the reaction, the anhydrides act as both a substrate and a solvent. The new method features good substrate tolerance, mild reaction conditions, readily accessible starting materials, and excellent yields, providing facile and green access to the targets. Importantly, the reaction also avoids the use of reagents with pungent odors, such as pyridine, in traditional esterification, which may promote the development of organocatalysis using nitrogen-containing heterocyclic compounds as catalysts.
1. Introduction
As a kind of significant structural motif simultaneously possessing both the ketone and O-acyl groups adjacent to each other, α-acyloxy ketone compounds are extensively present in a large number of natural products and biological molecules [1,2]. α-acyloxy ketone is also the core unit of some drug molecules (Figure 1), such as Taxol cortisone acetate [3,4], and these molecules containing the moiety of α-acyloxy ketone usually show good anti-cancer, anti-inflammatory, and antibacterial activities [5,6,7].
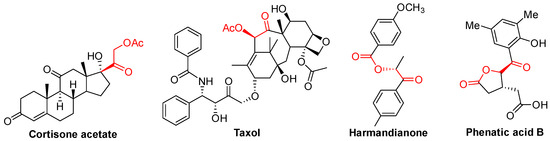
Figure 1.
Some typical bioactive compounds (or drugs) containing the unit of α-acyloxy ketone.
Moreover, due to the existence of multiple functionalization sites, the α-acyloxy ketone compounds can serve as versatile intermediates via various transformations, such as cyclization, deoxygenation of ester group, Favorskii rearrangement, etc. [8,9,10]. Therefore, the efficient synthesis of α-acyloxy ketones has always been one of the research focuses of scientific researchers [11,12].
As reported before, oxidative coupling reactions between ketones and metal acetates, e.g., Mn(OAc)3, Pb(OAc)4, are the main approach to access α-acyloxy ketones [13,14]. However, these methods suffer from certain disadvantages, such as excessive by-products, poor selectivity, and the use of toxic metal oxidants (Scheme 1a).
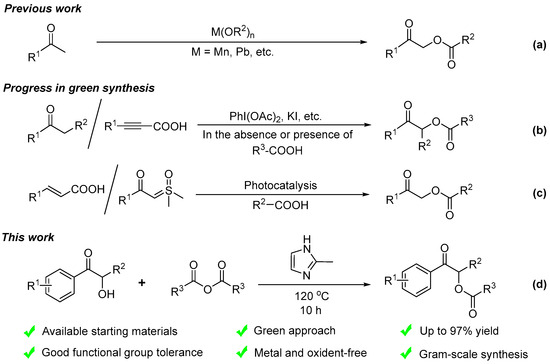
Scheme 1.
Serial synthesis reactions for α-acyloxy ketone compounds.
With the development of green synthetic chemistry, the exploration of efficient synthesis methods of α-acyloxy ketones that meet the requirements of green chemistry and sustainable chemistry has attracted much attention [15,16,17]. For example, researchers began to use low-toxic, cheap, and easily available iodine reagents [18,19,20,21] (Scheme 1b), and green and efficient photocatalysis methods were developed to synthesize α-acyloxy ketones [22,23] (Scheme 1c).
Although various new methods are constantly being reported, it is still necessary to explore a green synthesis method for α-acyloxy ketones from economical and readily available raw materials through simple operation with excellent yield.
At the same time, many benzoin-type compounds, namely α-hydroxy ketones, are common intermediates in organic synthesis and medicinal chemistry [24,25]. Thus, based on our continuous interest in green chemistry [26,27], especially green synthetic chemistry [28,29,30], herein, we hope to disclose a facile and efficient method for the synthesis of α-acyloxy ketones.
The reaction uses α-hydroxy ketones and anhydrides as raw materials and the cheap and readily available 2-methylimidazole as a basic catalyst (Scheme 1d). Among the reaction systems, the anhydrides can also be used as a solvent. This new approach does not involve any metal catalyst and does not require any oxidant or reductant. It is easy to operate and has excellent yield. Importantly, the reaction also avoids the use of reagents with pungent odors, such as pyridine, as a catalyst in traditional anhydride-based esterification reactions, which is conducive to promoting the development of organocatalysis.
2. Results
2.1. Optimization of Reaction Conditions
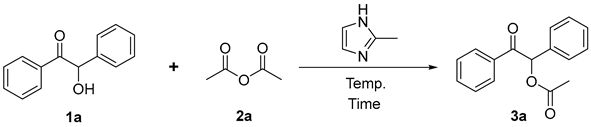
As an important heterocyclic compound, 2-methylimidazole is stable, cheap, and easy to obtain and can be used as a synthon in various synthesis reactions [31,32]. Moreover, it can also be used as a catalyst or additive to effectively promote the reaction [33,34]. Thus, using the reaction of 2-hydroxy-2-phenylacetophenone (1a) and acetic anhydride (2a) as a model under the promotion of 2-methylimidazole, we systematically explored various factors, such as the type and amount of base, reaction time and reaction temperature to optimize the best conditions. The results are shown in Table 1.

Table 1.
Optimization of reaction conditions [a].
First, we examined the amount of base 2-methylimidazole (Entries 1–6). The results show that 2.0 equiv. of 2-methylimidazole can give the best yield (90%, Entry 4). However, further increasing the amount of 2-methylimidazole, does not further increase the yield (Entries 5–6).
Second, we investigated the reaction time (Entries 7–9). It can be found that increasing the reaction time does not increase the yield. However, decreasing the reaction time from 12 h to 10 h is beneficial for improving the yield (Entry 7 vs. Entry 4). It is a pity that, when the reaction time is further reduced to 8 h, the yield is decreased slightly.
In addition, suitably lowering the reaction temperature is beneficial for increasing the yield of 3a (Entry 10 vs. Entry 7, Entry 13 vs. Entry 2). Obviously, too low temperatures are not beneficial for the reaction, e.g., Entry 12. Thus, the suitable temperature should be 120 °C (Entry 10). It is worth noting that acetic anhydride 2a acts as both a substrate and a solvent herein, and its boiling point is 140 °C, so the effect of increasing temperature on the yield is not explored.
Furthermore, we also examined the amount of acetic anhydride 2a (in a sense, it is the ratio of the dosage between reactants). As shown in Entries 14–16, it can be found that neither increasing nor decreasing the amount of 2a may increase the yield (Entries 14–16 vs. Entry 10).
Therefore, the optimal conditions we obtained are as follows: 1a (0.3 mmol), 2a (3 mL), and 2-methylimidazole (0.6 mmol) as the catalyst at 120 °C for 10 h. At this time, the yield is the highest and up to 94% (Entry 10).
2.2. Scope of Substrates
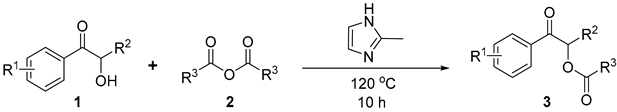
Under the optimized conditions, we systematically investigated the substrate tolerance using different α-hydroxy ketones 1 and anhydrides 2. The results are presented in Table 2.

Table 2.
Substrate scope of various α-hydroxy ketones 1 anhydrides 2 [a,b].
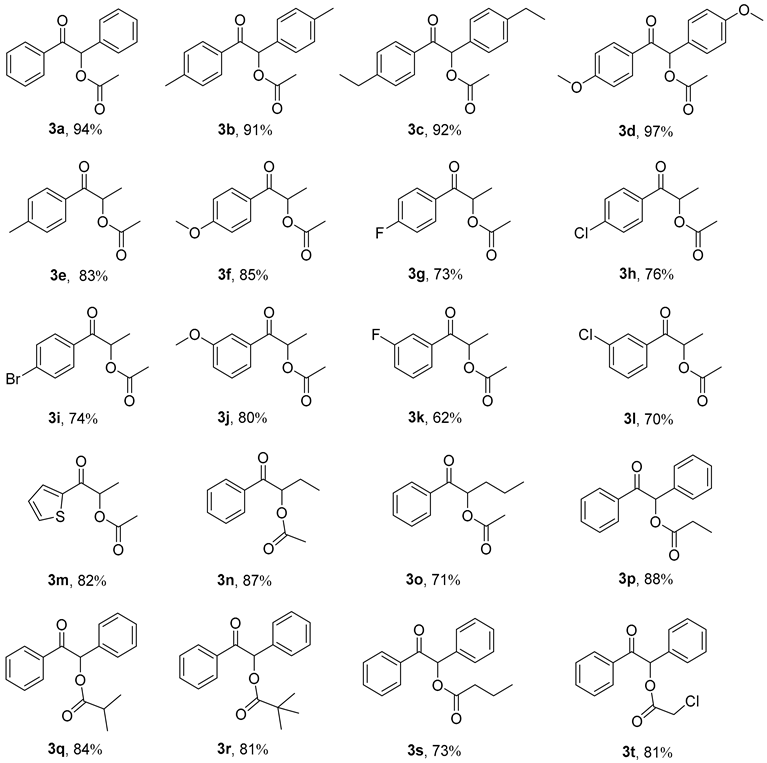
First, we examined the influence of different α-hydroxy ketones 1 on the reaction yield. Pleasingly, many α-hydroxy ketones 1 can be successfully employed with excellent yields (3a–3o).In particular, as shown in Table 2, when the two side groups of α-hydroxy ketones are both aryls, the yield is usually more than 90% (3a–3d, 91–97%).
When the two side groups of hydroxyketone substrates 1 are different, the yield of the corresponding product is slightly decreased (3e–3o, 62–87%). Interestingly, among them, the α-hydroxy ketone 1, which contains an electron-donating group, exhibits a higher yield than the one with an electron-withdrawing group, such as 3f (85%) vs. 3h (76%), 3j (80%) vs. 3l (70%). Perhaps the remote electron-donating groups in the substrates 1 are beneficial for enhancing the attacking activity of hydroxyl groups in this esterification reaction. It is worth noting that heteroaryl substituted α-hydroxy ketones 1 can also give the corresponding products in good yields, such as 3m (82%).
On the other hand, the influence of different anhydrides 2 on the reaction yield was also explored (Table 2). As anticipated, different substrates 2 can also react in this process to produce corresponding products in good yields (3p–3t, 73–88%). Among them, with the growth of the anhydride carbon chain, the yield will gradually decrease, such as 3a (94%) vs. 3p (88%) vs. 3s (73%).
Similarly, due to the steric effect, when using anhydride containing a large steric hindrance group as substrate 2, the yield will also decrease, such as 3p (88%) vs. 3q (84%) vs. 3r (81%).
It is worth noting that, even if an alkaline catalyst 2-methylimidazole is used in the reaction, the halo-substituted anhydride substrates 2 can also yield the corresponding products in good yields, such as 3t (81%).
2.3. Structural Characterization Analysis
The results demonstrate that the NMR spectral data for the 20 compounds are consistent with the simulated values. For example, the 1H NMR spectra of these compounds exhibit a peak with the integration as one unit near 5.90 ppm, corresponding to the methyne hydrogen in the product. Similarly, in the 13C NMR spectra, there is a peak near 71.0 ppm, which corresponds to the carbon in this methyne.
In addition, product 3, containing fluorine atoms, will lead to the splitting of the peaks of some carbon atoms in the 13C NMR spectra. Taking 3g as an example, the peaks of six carbon atoms are found to be split, and the splitting rule is in line with the reported [35,36], J1 ≈ 255.0 Hz > J2 ≈ 22.5 Hz > J3 ≈ 10.0 Hz > J4 ≈ 3.0 Hz, and the corresponding chemical shift of the fluorine atoms can be found in 19F NMR spectra as anticipated.
On the other hand, the molecular weights of the newly synthesized compound in products 3 in this experiment were obtained by high-resolution mass spectrometry (HR-MS). For example, the theoretical calculated value of [M − H]− for 3b is 309.1496, while the actual test value is 309.1492 (Figure S1), which is within the allowable range of error.
In addition, we conducted X-ray single-crystal diffraction experiments on compound 3q. The crystallographic data are provided in Table S1 [37], with no A-type or B-type errors, confirming that 3q has the expected structure.
Obviously, the X-ray single-crystal diffraction results of compound 3q in Figure 2 disclose that the molecule contains two benzene rings. Moreover, the ketone and O-acyl groups are adjacent to each other in this molecule, which is fully consistent with the structure of 3q.
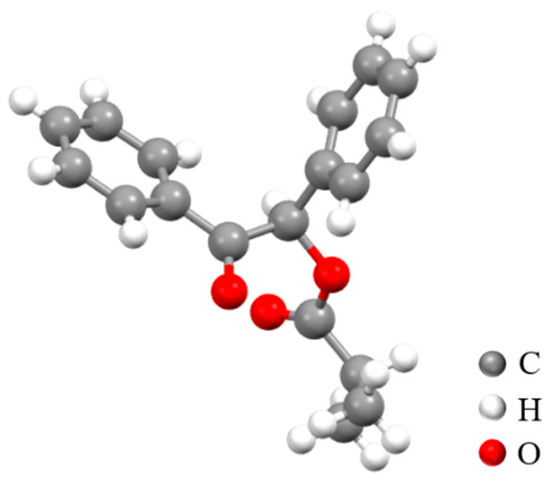
Figure 2.
The crystal structure of 3q (CCDC: 2376760).
2.4. Mechanism Investigation
Due to the special heteroaromatic structure simultaneously containing two nitrogen atoms in the imidazole ring, imidazole compounds can act as nucleophiles and as an acid/base catalyst. Therefore, the organic catalytic applications of imidazole compounds have attracted widespread attention [38,39]. Based on the results of the control experiments and the reported literature [39,40,41], a possible mechanism is proposed as Scheme 2.
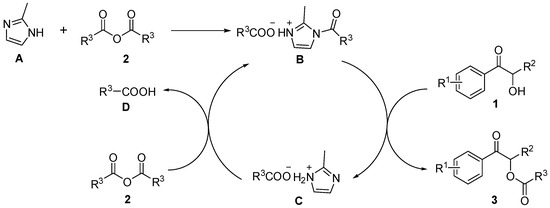
Scheme 2.
Proposed mechanism.
In this reaction, anhydride 2 first reacts with the catalyst 2-methylimidazole (which is labeled as A in Scheme 2) to form acyl imidazole carboxylate B in situ [40]. Subsequently, the intermediate B reacts with α-hydroxy ketone 1 to generate the target product 3 and imidazole carboxylate C after proton transfer [39]. In the end, the intermediate C reacts with the remaining anhydride 2 in the system to obtain the corresponding carboxylic acid D and regenerate intermediate B, which will continue to participate in the reaction and complete the catalytic cycle [41,42].
It is worth noting that we have also tried other common nitrogen-containing heterocyclic compounds as the catalyst for this reaction, and the results of the control experiments shown in Scheme 3 can also provide good evidence for the above mechanism.
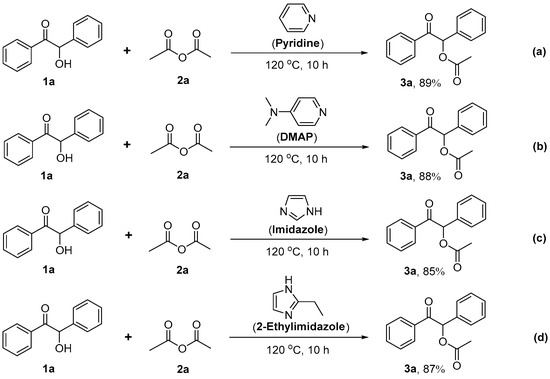
Scheme 3.
Control experiments (the amount of each catalyst used is 2.0 equiv.).
For example, some traditional basic catalysts with the structure of nitrogen-containing heterocycles, e.g., pyridine (which only contains a nitrogen atom in its structure, Scheme 3a, though it is more effective than other catalyst systems for the same reaction reactants [43,44]), 4-dimethylaminopyridine (DMAP, which contains two nitrogen atoms but only a nitrogen atom in its ring structure, Scheme 3b), are not as effective as 2-methylimidazole because they lack the efficient recycling process of imidazole carboxylates shown in Scheme 2.
For other imidazole-type basic catalysts with the structure of nitrogen-containing heterocycles, e.g., imidazole (Scheme 3c) and 2-ethyl imidazole (Scheme 3d), it also can be found that the yield of 3a is significantly reduced. This may be caused by the electron-donating effect of the methyl on 2-methylimidazole. Compared with simple imidazole, the presence of methyl can stabilize the positive charge on the nitrogen atom and is more conducive to the formation of intermediates B and C in Scheme 2.
However, for 2-ethylimidazole, when compared with 2-methylimidazole, although the ethyl group also has an electron-donating effect, its large steric hindrance makes it slightly difficult to form intermediate B in Scheme 2, so the effect of 2-ethylimidazole as a catalyst is not as good as 2-methylimidazole.
It should be noted that 2-methylimidazole plays two roles in the reaction. It can not only react with the anhydride to generate intermediate B to promote the reaction (Scheme 2) but also act as a base to continuously neutralize the carboxylic acid generated during the reaction. Therefore, the amount of 2-methylimidazole has a significant effect on the yield of the reaction, which is consistent with the results of condition optimization.
In short, compared with other traditional nitrogen-containing heterocyclic organic catalysts (typically, e.g., pyridine and DMAP), 2-methylimidazole has a better catalytic effect on this esterification reaction due to its interesting reaction mechanism shown in Scheme 2.
2.5. Practicability of Gram-Scale Reaction
Since α-acyloxy ketones are common synthetic intermediates widely used in the fields of organic synthesis and biomedicine [45,46,47], to explore the potential for industrial application, a gram-scale reaction of 2-hydroxy-2-phenylacetophenone (1a) and acetic anhydride (2a) was investigated (Scheme 4).
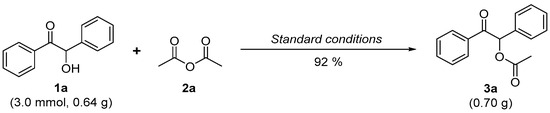
Scheme 4.
Gram-scale reaction of 3a.
As shown in Scheme 4, when the reaction scale is increased to ten times, the yield of 3a is decreased by only 2% (92% vs. 94%). Therefore, the method has good practicability and is beneficial to avoid using traditional odorous pyridine as a catalyst for the esterification of anhydrides and alcohols in gram-scale synthesis.
Organocatalysis is an important part of organic synthetic chemistry [48,49]. Organic catalysts are widely used in synthetic reactions due to their good tolerance and excellent catalytic performance [50,51,52], especially some phosphorus [53], sulfur [54], and nitrogen [55] containing organic catalysts have developed recently. Among them, nitrogen-containing organic catalysts are relatively easy to obtain and have been given more attention in different applications, such as [3 + 2] cycloaddition [56], esterification reaction [57], oxidation of aldehydes [58], etc.
Although the development of nitrogen-containing organic catalysts has made great progress, there are still some disadvantages of nitrogen-containing organic catalysts, such as the limited types and relatively complex structures. Therefore, the development of nitrogen-containing organic catalysts with a simple structure and excellent catalytic ability, such as the synthetic reaction using the odorless, cheap, and easy-to-post-process 2-methylimidazole as a catalyst proposed in this paper, is of great significance.
3. Materials and Methods
3.1. General Information
1H, 13C, 19F NMR spectra were collected on a Bruker AVANCE NEO-600 MHz, 150 MHz and 564 MHz in Guangzhou, China in CDCl3 using tetramethylsilane (TMS) as an internal standard 19F NMR testing were recorded without proton decoupling.
Mass spectra were recorded on a Thermo Scientific ISQ gas chromatograph-mass spectrometer in Guangzhou, China. High-resolution mass spectra (HR-MS) were obtained with a Thermo Finnigan MAT 95XP mass spectrometer in Wuhan, China. Melting point (m.p.) was measured with a INESA Physico-Optical Instrument Co., Ltd. WRS-1B melting point instrument in Guangzhou, China. Single-crystal X-ray analysis was obtained using Agilent Gemini E in Xiamen, China. Reactions were monitored using thin-layer chromatography (TLC) and visualized with UV light at 254 nm.
All reagents and solvents were purchased from commercial sources and used without further purification. Different α-hydroxy ketones 1 were synthesized according to the literature procedure [59,60], except for 2-hydroxy-2-phenylacetophenone (1a) and 2-hydroxy-1,2-bis(4-methoxyphenyl)ethan-1-one (1d).
3.2. Experimental Procedure for α-Hydroxy Ketones 1
For symmetrical substrates 1 (e.g., 1b–1c), as shown inScheme 5, according to the literature [59], the mixture of aldehyde (1.0 mmol), triethylamine (0.3 mmol), 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride (0.05 mmol) and ethanol (10 mL) was stirred at 80 °C for 16 h.
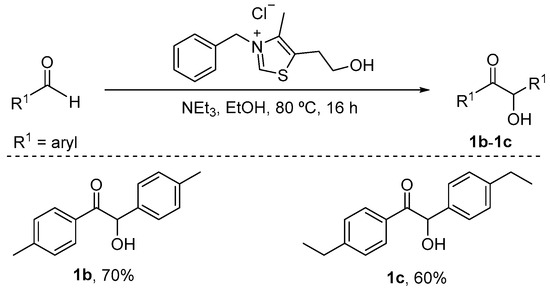
Scheme 5.
Synthesis route of α-hydroxy ketones 1b–1c.
After completion, the solvent was removed under reduced pressure. Subsequently, EtOAc (15 mL) was added to the reaction mixture. The organic layers were extracted with the saturated sodium chloride solution (3 × 15 mL). Then, the organic layer was dried over anhydrous Na2SO4.
Finally, the solvent was removed by reduced pressure. The crude products were purified by column chromatography on silica gel to afford the desired products 1b–1c.
For unsymmetrical substrates 1 (e.g., 1e–1o), as shown inScheme 6, according to the literature [60], the mixture of propiophenone compounds (1.0 mmol), I2 (40 mol%), and DMSO (2 mL) was stirred at 60 °C for 24 h.
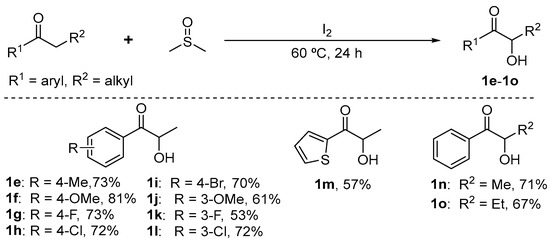
Scheme 6.
Synthesis route of α-hydroxy ketones 1e–1o.
After cooling down to room temperature, the reaction mixture was diluted with EtOAc (10 mL) and washed with 0.1 mol/L Na2S2O3 (5 mL) aqueous solution, extracted with ethyl acetate (3 × 5 mL). Then, the organic layers were dried over anhydrous Na2SO4.
Finally, the solvent was removed by reduced pressure. The crude products were purified by column chromatography on silica gel to afford the desired products 1e–1o.
3.3. Experimental Procedure for Compounds 3a–3t
As shown in Scheme 7, α-acyloxy ketones 3a–3t were successfully synthesized. The mixture of α-hydroxy ketones 1a–1o (0.30 mmol, 1.0 equiv.), 2-methylimidazole (0.60 mmol, 2.0 equiv.) and anhydrides 2a–2f (3 mL) under air atmosphere was stirred at 100 °C for 10 h.
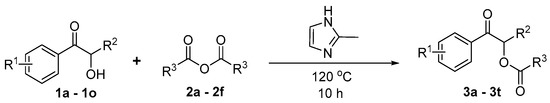
Scheme 7.
Synthesis route for compounds 3a–3t.
After the completion of the reaction, the solvent was removed under reduced pressure. Subsequently, the water (15 mL) was added to the reaction mixture and extracted with dichloromethane (3 × 10 mL). The combined organic layers were extracted with the saturated sodium chloride solution (25 mL). Then, the organic layer was dried over anhydrous Na2SO4.
Finally, the solvent was removed by reduced pressure. The crude products were purified by column chromatography on silica gel to afford the desired products 3a–3t.
3.4. Characterization Data for All Products 3a–3t
We characterized the synthesized compounds 3a–3t by NMR, melting point, etc., and the results are listed in the following.
(1) 2-Oxo-1,2-diphenylethyl acetate (3a), white solid; m.p.: 71–73 °C (71.6 mg, 94%) (90% [45]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.19 (s, 3H, CH3), 6.87 (s, 1H, CH), 7.33–7.39 (m, 5H, ArH), 7.46 (d, J = 7.8 Hz, 2H, ArH), 7.49 (t, J = 7.2 Hz, 1H, ArH), 7.93 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 20.9, 77.8, 128.7, 128.8, 128.9, 129.2, 129.4, 133.7, 170.5, 193.8.
(2) 2-Oxo-1,2-di-p-tolylethyl acetate (3b), colorless oil (77.0 mg, 91%) (88% [45]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.20 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.35 (s, 3H, CH3), 6.83 (s, 1H, CH), 7.17 (d, J = 7.8 Hz, 2H, ArH), 7.19 (d, J = 8.4 Hz, 2H, ArH), 7.35 (d, J = 7.8 Hz, 2H, ArH), 7.84 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.0, 21.3, 21.8, 77.6, 128.8, 129.0, 129.4, 129.9, 131.0, 132.2, 139.4, 144.5, 170.6, 193.4.
(3) 1,2-Bis(4-ethylphenyl)-2-oxoethyl acetate (3c), colorless oil (85.6 mg, 92%); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.20 (t, J = 7.8 Hz, 3H, CH3), 1.21 (t, J = 7.2 Hz, 3H, CH3), 2.20 (s, 3H, CH3), 2.62 (q, J = 7.8 Hz, 2H, CH3), 2.66 (q, J = 7.2 Hz, 2H, CH3), 6.84 (s, 1H, CH), 7.20 (d, J = 7.8 Hz, 2H, ArH), 7.22 (d, J = 8.4 Hz, 2H, ArH), 7.39 (d, J = 7.8 Hz, 2H, ArH), 7.88 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 15.1, 15.4, 21.0, 28.7, 29.1, 77.6, 128.3, 128.8, 128.9, 129.2, 131.2, 132.4, 145.7, 150.6, 170.6, 193.4; ESI-HRMS, m/z: Calcd for C20H21O3 [M − H]−: 309.1496, Found: 309.1492.
(4) 1,2-Bis(4-methoxyphenyl)-2-oxoethyl acetate (3d), colorless oil (91.2 mg, 82%) (66% [61]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.18 (s, 3H, CH3), 3.76 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 6.80 (s, 1H, CH), 6.83 (d, J = 9.0 Hz, 2H, ArH), 6.88 (d, J = 9.0 Hz, 2H, ArH), 7.38 (d, J = 9.0 Hz, 2H, ArH), 7.92 (d, J = 9.0 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.0, 55.4, 55.5, 77.1, 113.9, 114.6, 126.2, 127.6, 130.3, 131.2, 160.4, 163.8, 170.7, 192.2.
(5) 1-Oxo-1-(p-tolyl)propan-2-yl acetate (3e), colorless oil (51.3 mg, 83%) (83% [61]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.50 (d, J = 7.2 Hz, 3H, CH3), 2.12 (s, 3H, CH3), 2.39 (s, 3H, CH3), 5.94 (q, J = 7.2 Hz, 1H, CH), 7.25 (d, J = 7.8 Hz, 2H, ArH), 7.83 (d, J = 7.8 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.3, 20.8, 21.8, 71.4, 128.6, 129.5, 131.9, 144.6, 170.4, 196.4.
(6) 1-(4-Methoxyphenyl)-1-oxopropan-2-yl acetate (3f), colorless oil (56.6 mg, 85%) (73% [61]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.52 (d, J = 7.2 Hz, 3H, CH3), 2.14 (s, 3H, CH3), 3.86 (s, 3H, CH3), 5.94 (q, J = 7.2 Hz, 1H, CH), 6.95 (d, J = 9.0 Hz, 2H, ArH), 7.93 (d, J = 9.0 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.4, 20.8, 55.5, 71.2, 114.0, 127.2, 130.8, 163.9, 170.4, 195.3.
(7) 1-(4-Fluorophenyl)-1-oxopropan-2-yl acetate (3g), colorless oil (46.0 mg, 73%) (80% [62]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.52 (d, J = 7.2 Hz, 3H, CH3), 2.14 (s, 3H, CH3), 5.91 (q, J = 7.2 Hz, 1H, CH), 7.14–7.16 (m, 2H, ArH), 7.96–7.99 (m, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.2, 20.9, 71.2, 116.1 (d, J = 21.0 Hz), 130.9 (d, J = 3.0 Hz), 131.3 (d, J = 9.0 Hz), 166.1 (d, J = 255.0 Hz), 170.5, 195.5; 19F NMR (564 MHz, CDCl3), δ, ppm: −104.0.
(8) 1-(4-Chlorophenyl)-1-oxopropan-2-yl acetate (3h), colorless oil (51.5 mg, 76%) (90% [61]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.51 (d, J = 6.6 Hz, 3H, CH3), 2.13 (s, 3H, CH3), 5.88 (q, J = 7.2 Hz, 1H, CH), 7.44 (d, J = 8.4 Hz, 2H, ArH), 7.87 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.1, 20.8, 71.4, 129.3, 130.0, 132.8, 140.2, 170.5, 195.9.
(9) 1-(4-Bromophenyl)-1-oxopropan-2-yl acetate (3i), colorless oil (59.9 mg, 74%) (68% [63]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.50 (d, J = 6.6 Hz, 3H, CH3), 2.12 (s, 3H, CH3), 5.87 (q, J = 7.2 Hz, 1H, CH), 7.61 (d, J = 8.4 Hz, 2H, ArH), 7.89 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.1, 20.8, 71.4, 128.9, 130.0, 132.2, 133.2, 170.5, 196.1.
(10) 1-(3-Methoxyphenyl)-1-oxopropan-2-yl acetate (3j), colorless oil (53.3 mg, 80%); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.51 (d, J = 6.6 Hz, 3H, CH3), 2.13 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 5.92 (q, J = 7.2 Hz, 1H, CH), 7.11–7.13 (m, 1H, ArH), 7.35–7.39 (m, 1H, ArH), 7.45 (s, 1H, ArH), 7.50 (d, J = 7.8 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.3, 20.8, 55.6, 71.6, 112.9, 120.2, 121.0, 129.9, 135.8, 160.0, 170.5, 196.8; ; ESI-HRMS, m/z: Calcd for C12H15O4 [M + H]+: 223.0965, Found: 223.0963.
(11) 1-(3-Fluorophenyl)-1-oxopropan-2-yl acetate (3k), colorless oil (39.1 mg, 62%) (97% [64]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.52 (d, J = 7.2 Hz, 3H, CH3), 2.13 (s, 3H, CH3), 5.87 (q, J = 7.2 Hz, 1H, CH), 7.27–7.30 (m, 1H, ArH), 7.44–7.47 (m, 1H, ArH), 7.61–7.63 (m, 1H, ArH), 7.71 (d, J = 7.8 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.1, 20.8, 71.6, 115.4 (d, J = 22.5 Hz), 120.8 (d, J = 21.0 Hz), 124.2 (d, J = 3.0 Hz), 130.6 (d, J = 7.5 Hz), 136.5 (d, J = 6.0 Hz), 163.0 (d, J = 247.5 Hz), 170.5, 195.9; 19F NMR (564 MHz, CDCl3), δ, ppm: −111.3.
(12) 1-(3-Chlorophenyl)-1-oxopropan-2-yl acetate (3l), colorless oil (47.5 mg, 70%) (75% [65]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.51 (d, J = 6.6 Hz, 3H, CH3), 2.13 (s, 3H, CH3), 5.86 (q, J = 7.2 Hz, 1H, CH), 7.40–7.43 (m, 1H, ArH), 7.55 (d, J = 7.8 Hz, 1H, ArH), 7.79 (d, J = 7.8 Hz, 1H, ArH), 7.90 (s, 1H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.1, 20.8, 71.5, 126.7, 128.6, 130.2, 133.6, 135.3, 136.1, 170.5, 195.9.
(13) 1-Oxo-1-(thiophen-2-yl)propan-2-yl acetate (3m), colorless oil (48.7 mg, 82%) (92% [64]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.56 (d, J = 7.2 Hz, 3H, CH3), 2.14 (s, 3H, CH3), 5.73 (q, J = 7.2 Hz, 1H, CH), 7.14–7.16 (m, 1H, ArH), 7.69 (d, J = 4.8 Hz, 1H, ArH), 7.80 (d, J = 4.8 Hz, 1H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 17.8, 20.9, 72.4, 128.4, 132.8, 134.6, 140.6, 170.4, 189.9.
(14) 1-Oxo-1-phenylbutan-2-yl acetate (3n), colorless oil (53.8 mg, 87%) (91% [62]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.01 (t, J = 7.8 Hz, 3H, CH3), 1.81–1.97 (m, 2H, CH2), 2.15 (s, 3H, CH3), 5.80–5.82 (m, 1H, CH), 7.45–7.48 (m, 2H, ArH), 7.57 (t, J = 7.2 Hz, 1H, ArH), 7.93 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 10.0, 20.8, 24.9, 76.5, 128.5, 128.9, 133.6, 135.0, 170.8, 196.7.
(15) 1-Oxo-1-phenylbutan-2-yl acetate (3o), colorless oil (46.9 mg, 71%) (77% [62]); 1H NMR (600 MHz, CDCl3), δ, ppm: 0.93 (t, J = 7.8 Hz, 3H, CH3), 1.45–1.50 (m, 2H, CH2), 1.79–1.83 (m, 2H, CH2), 2.13 (s, 3H, CH3), 5.85–5.87 (m, 1H, CH), 7.44–7.47 (m, 2H, ArH), 7.56 (t, J = 7.2 Hz, 1H, ArH), 7.92 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 13.8, 18.9, 20.7, 33.5, 75.2, 128.4, 128.8, 133.6, 134.8, 170.7, 196.8.
(16) 2-Oxo-1,2-diphenylethyl propionate (3p), colorless oil (70.8 mg, 88%) (99%, [66]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.19 (t, J = 7.8 Hz, 3H, CH3), 2.44–2.58 (m, 2H, CH2), 6.87 (s, 1H, CH), 7.32–7.41 (m, 5H, ArH), 7.47 (d, J = 7.8 Hz, 2H, ArH), 7.51 (t, J = 7.2 Hz, 1H, ArH), 7.94 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 9.1, 27.5, 77.6, 128.7, 128.8, 128.9, 129.2, 129.4, 133.6, 133.8, 134.8, 174.1, 194.1.
(17) 2-Oxo-1,2-diphenylethyl isobutyrate (3q), white solid; m.p.: 75–77 °C (white solid; m.p.: 80–81 °C [66]) (71.0 mg, 84%); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.22 (d, J = 7.2 Hz, 3H, CH3), 1.38 (d, J = 7.2 Hz, 3H, CH3), 2.70–2.77 (m, 1H, CH), 6.86 (s, 1H, CH), 7.33–7.41 (m, 5H, ArH), 7.48–7.51 (m, 3H, ArH), 7.95 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 18.8, 19.1, 33.9, 77.4, 128.6, 128.7, 128.9, 129.1, 129.3, 133.5, 133.8, 134.8, 176.7, 194.1.
(18) 2-Oxo-1,2-diphenylethyl pivalate (3r), white solid; m.p.: 107–109 °C (m.p.: 110–111 °C [47]) (71.9 mg, 81%); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.29 (s, 9H, 3CH3), 6.80 (s, 1H, CH), 7.33–7.42 (m, 5H, ArH), 7.47 (d, J = 7.8 Hz, 2H, ArH), 7.51 (t, J = 7.2 Hz, 1H, ArH), 7.94 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 27.2, 38.9, 77.4, 128.4, 128.7, 128.9, 129.1, 129.2, 133.5, 134.0, 135.0, 178.2, 194.4.
(19) 2-Oxo-1,2-diphenylethyl butyrate (3s), colorless oil (61.8 mg, 73%) (91% [67]); 1H NMR (600 MHz, CDCl3), δ, ppm: 0.98 (t, J = 7.2 Hz, 3H, CH3), 1.69–1.75 (m, 2H, CH2), 2.40–2.51 (m, 2H, CH2), 6.86 (s, 1H, CH), 7.34–7.41 (m, 5H, ArH), 7.46 (d, J = 7.2 Hz, 2H, ArH), 7.51 (t, J = 7.8 Hz, 1H, ArH), 7.94 (d, J = 7.8 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 13.8, 18.5, 36.0, 77.5, 128.7, 128.9, 129.2, 129.4, 133.6, 133.8, 134.9, 173.3, 194.1.
(20) 2-Oxo-1,2-diphenylethyl 2-chloroacetate (3t), colorless oil (70.0 mg, 81%) (95% [68]); 1H NMR (600 MHz, CDCl3), δ, ppm: 4.19–4.29 (dd, J1 = 42.6 Hz, J2 = 15.0 Hz, 2H, CH2), 6.94 (s, 1H, CH), 7.37–7.41 (m, 5H, ArH), 7.47 (d, J = 7.8 Hz, 2H, ArH), 7.52 (t, J = 7.2 Hz, 1H, ArH), 7.92 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 40.8, 79.2, 128.8, 128.9, 129.0, 129.4, 129.8, 133.0, 133.8, 134.3, 167.0, 192.8.
The detailed 1H, 13C, and 19F NMR spectra for all compounds 3a–3t are provided in the Supplementary Materials.
4. Conclusions
In summary, we successfully developed an oxyacyloxylation of α-hydroxy ketones and anhydrides mediated by 2-methylimidazole. This reaction can efficiently give a series of α-acyloxy ketone derivatives without any metal catalyst, oxidants, or reductants. In the reaction, the anhydrides can act as both a substrate and a solvent. At the same time, this reaction also avoids the use of reagents with pungent odors, such as pyridine, in traditional esterification reactions. Thus, it meets the requirements for the development of green chemistry in many fields. More importantly, the discovery of this new research result is also conducive to promoting the development of organocatalysis using nitrogen-containing heterocyclic compounds as catalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14110811/s1, and contains the 1H, 13C, and 19F NMR spectra for all compounds 3a–3t.
Author Contributions
Conceptualization, Z.-Y.W.; methodology, S.-W.Y. and Z.-H.L.; formal analysis, S.-W.Y. and M.-X.L.; data curation, Y.Z. and W.-X.Y.; writing-original draft preparation, S.-W.Y.; writing-review and editing, S.-W.Y., J.-Y.X. and Z.-Y.W.; project administration, Z.-Y.W.; funding acquisition, Z.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Guangdong Basic and Applied Basic Research Foundation (no. 2021A1515012342).
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Materials published online.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, J.C.T.; De La Peña, R.; Tocol, C.; Sattely, E.S. Reconstitution of early paclitaxel biosynthetic network. Nat. Commun. 2024, 15, 1419. [Google Scholar] [CrossRef] [PubMed]
- Li, C.K.; Yin, X.X.; Wang, S.; Sui, S.Y.; Liu, J.M.; Sun, X.C.; Di, J.M.; Chen, R.D.; Chen, D.W.; Han, Y.T.; et al. A Cytochrome P450 enzyme catalyses oxetane ring formation in paclitaxel biosynthesis. Angew. Chem. Int. Ed. 2024, 63, e202407070. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, J.N.; Shi, J.H.; Cao, S.T.; Luo, J.M.; Zheng, T.T.; Wang, M. iTRAQ-based quantitative proteomic analysis of arthrobacter simplex in response to cortisone acetate and its mutants with improved A1-dehydrogenation efficiency. J. Agric. Food Chem. 2023, 71, 6376–6388. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, Y.; Su, Z.; Xiong, L.Y.; Wang, P.P.; Lei, W.; Yan, X.; Ma, D.W.; Zhao, G.P.; Zhou, Z.H. Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol. Nat. Commun. 2024, 15, 2339. [Google Scholar] [CrossRef]
- Keskar, K.; Zepeda-Velazquez, C.; Dokuburra, C.B.; Jenkins, H.A.; McNulty, J. The synthesis of densely functionalised α-acyloxy enaminals and enaminones via a novel homogeneous silver(I) catalysed rearrangement. Chem. Commun. 2019, 55, 10868–10871. [Google Scholar] [CrossRef]
- Kong, J.; Lacroix, C.; Bournaud, C.; Yamashita, Y.; Kobayashi, S.; Vo-Thanh, G. Enantioselective acyl-transfer/protonation reactions with designed chiral thiourea-iminophosphorane catalysts. Adv. Synth. Catal. 2024, 366, 1101–1106. [Google Scholar] [CrossRef]
- Kwon, S.; Meng, F.F.; Tamam, H.; Gadalla, H.H.; Wang, J.P.; Dong, B.Y.; Jannasch, A.S.H.; Ratliff, T.L.; Yeo, Y. Systemic delivery of paclitaxel by Find-Me nanoparticles activates antitumor immunity and eliminates tumors. ACS Nano 2024, 18, 3681–3698. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Baire, B. An unprecedented (semi) Favorskii rearrangement. Evidence for the 2-(acyloxy)cyclopropanones. Org. Lett. 2018, 20, 1748–1751. [Google Scholar] [CrossRef]
- Prasad, P.K.; Reddi, R.N.; Arumugam, S. Recent methods for the synthesis of α-acyloxy ketones. Org. Biomol. Chem. 2018, 16, 9334–9348. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, J.D.; Cheng, J.P. Diazaphosphinyl radical-catalyzed deoxygenation of α-carboxy ketones: A new protocol for chemo-selective C-O bond scission via mechanism regulation. Chem. Sci. 2020, 11, 8476–8481. [Google Scholar] [CrossRef]
- Jafarpour, F.; Azizzade, M.; Golpazir-Sorkheh, Y.; Navid, H.; Rajai-Daryasarei, S. Divergent synthesis of α-aroyloxy ketones and indenones: A controlled domino radical reaction for di- and trifunctionalization of alkynes. J. Org. Chem. 2020, 85, 8287–8294. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Guo, Y.H.; Li, Z.Y.; Jiang, H.F.; Qi, C.R. Silver-catalyzed coupling of ethynylbenziodoxolones with CO2 and amines to afford O-β-oxoalkyl carbamates. Org. Lett. 2024, 26, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Tanyeli, C.; Iyigün, C. Manganese(III) acetate based oxidation of substituted α′-position on cyclic α,β-unsaturated ketones. Tetrahedron 2003, 59, 7135–7139. [Google Scholar] [CrossRef]
- White, J.D.; Jeffrey, S.C. Synthesis of the northern sector (C8-C19) of rapamycin via Chan rearrangement and oxidation of an α-acyloxyacetate. Tetrahedron 2009, 65, 6642–6647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onodera, K.; Takashima, R.; Suzuki, Y. Selective synthesis of acylated cross-benzoins from acylals and aldehydes via N-heterocyclic carbene catalysis. Org. Lett. 2021, 23, 4197–4202. [Google Scholar] [CrossRef]
- Sakkani, N.; Jha, D.K.; Whatley, E.; Zhao, J.C.G. Visible light-assisted organocatalytic α-acyloxylation of ketones using carboxylic acids and N-halosuccinimides. Chem. Commun. 2022, 58, 11308–11311. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, S.K. Metal-free synthesis of α-acyloxy ketones from carboxylic acids and sulfoxonium ylides. Org. Biomol. Chem. 2023, 21, 8819–8822. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, G.S.; Yang, Y.A.; Jiang, J.S.; Feng, Z.M.; Zhang, P.C. 1,2-Dibromoethane and KI mediated α-acyloxylation of ketones with carboxylic acids. Chin. Chem. Lett. 2020, 31, 711–714. [Google Scholar] [CrossRef]
- Wu, J.W.; Shi, H.W.; Liu, J.G.; Wang, R.Y.; Zhou, J.; Xu, X.L.; Xu, H.J. Electrochemical oxidative C(sp3)-H/O-H cross-coupling for the synthesis of α-acyloxyketones. Org. Chem. Front. 2023, 10, 2459–2464. [Google Scholar] [CrossRef]
- Gao, T.Y.; Yang, Y.W.; Hu, L.Z.; Luo, D.; Zhang, X.H.; Xiong, Y. Metal-free PhI(OAc)2-oxidized decarboxylation of propiolic acids towards synthesis of α-acetoxy ketones and insights into general decarboxylation with DFT calculations. Org. Biomol. Chem. 2023, 21, 1457–1462. [Google Scholar] [CrossRef]
- Chen, X.; Xin, Y.C.; Zhao, Z.W.; Hou, Y.J.; Wang, X.X.; Xia, W.J.; Li, Y.M. Decarboxylative oxyacyloxylation of propiolic acids: Construction of alkynyl-containing α-acyloxy ketones. J. Org. Chem. 2021, 86, 8216–8225. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Peng, Y.Z.; Wang, Y.J.; Bao, X.G. Construction of a-acyloxy ketones via photoredox-catalyzed O-H insertion of sulfoxonium ylides with carboxylic acids. Org. Lett. 2023, 25, 6613–6617. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mondal, S.; Midya, S.P.; Das, S.; Mondal, S.; Ghosh, P. Merging photocatalytic C-O cross-coupling for α-oxycarbonyl-β-ketones: Esterification of carboxylic acids via a decarboxylative pathway. Org. Lett. 2023, 25, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Zade, V.M.; Gangnale, L.D.; Athawale, P.R.; Reddy, D.S. Direct deoxygenation of α-hydroxy and α,β-dihydroxy ketones using a silyl lithium reagent. J. Org. Chem. 2023, 88, 14227–14235. [Google Scholar] [CrossRef] [PubMed]
- Casajus, H.; Lagarde, A.; Nauton, L.; Ocal, N.; Leremboure, M.; Fessner, W.D.; Duguet, N.; Charmantray, F.; Hecquet, L. Cleavage of aliphatic α-hydroxy ketones by evolved transketolase from geobacillus stearothermophilus. ACS Catal. 2022, 12, 3566–3576. [Google Scholar] [CrossRef]
- Chen, S.-H.; Jiang, K.; Liang, Y.-H.; He, J.-P.; Xu, B.-J.; Chen, Z.-H.; Wang, Z.-Y. Fine-tuning benzazole-based probe for the ultrasensitive detection of Hg2+ in water samples and seaweed samples. Food Chem. 2023, 428, 136800. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Huang, Y.; Chen, S.-H.; Yu, S.-W.; Chen, Z.-J.; Li, Z.-H.; Zeng, Y.; Chen, N.; Cao, L.; Wang, Z.-Y. The first specific probe for pyrrolidine with multifunction by the interaction mechanism of atomic economic reaction. iScience 2024, 27, 110024. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Yang, K.; Chen, X.-Y.; Arulkumar, M.; Wang, N.; Chen, S.-H.; Wang, Z.-Y. A 3,4-dihalo-2(5H)-furanone initiated ring-opening reaction of DABCO in the absence of a metal catalyst and additive and its application in a one-pot two-step reaction. Green Chem. 2019, 21, 3782–3788. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. 1,1-Diphenylvinylsulfide as functional AIEgen derived from the aggregation-caused-quenching molecule 1,1-diphenylvinylsulfide through simple thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef]
- Yu, S.-W.; Chen, Z.-J.; Chen, Z.-H.; Chen, S.-H.; Yang, K.; Xu, W.-J.; Wang, Z.-Y. Trace water in a BF3·OEt2 system: A facile access to sulfinyl alkenylsulfones from alkynes and sodium sulfinates. Org. Biomol. Chem. 2023, 21, 7776–7781. [Google Scholar] [CrossRef]
- Passia, M.T.; Demaerel, J.; Amer, M.M.; Drichel, A.; Zimmer, S.; Bolm, C. Acid-mediated imidazole-to-fluorine exchange for the synthesis of sulfonyl and sulfonimidoyl fluorides. Org. Lett. 2022, 24, 8802–8805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Yu, S.-W.; Xu, W.-J.; Li, M.-X.; Zeng, Y.; Deng, S.-W.; Lin, J.-Y.; Wang, Z.-Y. Synthesis of novel trisubstituted olefin-type probe molecules containing N-heterocycles and their application in detection of malononitrile. Organics 2024, 5, 46–58. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Liao, Y.T.; Kang, T.C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016, 18, 5957–5961. [Google Scholar] [CrossRef]
- Wu, C.P.; Sun, C.; Ren, K.X.; Yang, F.L.; Du, Y.X.; Gu, X.X.; Wang, Q.H.; Lai, C. 2-Methyl imidazole electrolyte additive enabling ultra-stable Zn anode. Chem. Eng. J. 2023, 452, 139465. [Google Scholar] [CrossRef]
- Dorel, R.; Boehm, P.; Schwinger, D.P.; Hartwig, J.F. Copper-mediated fluorination of aryl trisiloxanes with nucleophilic fluoride. Chem. Eur. J. 2020, 26, 1759–1762. [Google Scholar] [CrossRef]
- Spiller, T.E.; Donabauer, K.; Brooks, A.F.; Witek, J.A.; Bowden, G.D.; Scott, P.J.H.; Sanford, M.S. Room-temperature photochemical copper-mediated fluorination of aryl iodides. Org. Lett. 2024, 26, 6433–6437. [Google Scholar] [CrossRef]
- CCDC 2376760 (for 3q) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 10 August 2024).
- Rauwerdink, A.; Kazlauskas, R.J. How the same core catalytic machinery catalyzes 17 different reactions: The serine-histidine-aspartate catalytic triad of α/β -hydrolase fold enzymes. ACS Catal. 2015, 5, 6153–6176. [Google Scholar] [CrossRef]
- Raum, H.N.; Modig, K.; Akke, M.; Weininger, U. Proton transfer kinetics in histidine side chains determined by pH-dependent multi-nuclear NMR relaxation. J. Am. Chem. Soc. 2024, 146, 22284–22294. [Google Scholar] [CrossRef]
- Nowrouzi, N.; Alizadeh, S.Z. In situ generated acylimidazolium acetate as an efficient catalyst and acylating agent for the acetylation of alcohols, phenols, and amines at ambient temperature. Chin. J. Catal. 2013, 34, 1787–1790. [Google Scholar] [CrossRef]
- Hajjami, M.; Ghorbani-Choghamarani, A.; Norouzi, M. An efficient and facile procedure for synthesis of acetates from alcohols catalyzed by poly(4-vinylpyridinium tribromide). Chin. J. Catal. 2012, 33, 1661–1664. [Google Scholar] [CrossRef]
- Peixoto, D.; Figueiredo, M.; Gawande, M.B.; Corvo, M.C.; Vanhoenacker, G.; Afonso, C.A.M.; Ferreira, L.M.; Branco, P.S. Developments in the reactivity of 2-methylimidazolium salts. J. Org. Chem. 2017, 82, 6232–6241. [Google Scholar] [CrossRef] [PubMed]
- Jammi, S.; Rout, L.; Saha, P.; Akkilagunta, V.K.; Sanyasi, S.; Punniyamurthy, T. Synthesis, structure and application of chiral copper(II) coordination polymers for asymmetric acylation. Inorg. Chem. 2008, 47, 5093–5098. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Ghaemy, M.; Zeraatpisheh, F. Iodine supported on 3-aminopropyl silica gel as efficient catalyst for acetylation of alcohols under solvent-free conditions. Chin. J. Chem. 2009, 27, 347–352. [Google Scholar] [CrossRef]
- Speckmeier, E.; Padié, C.; Zeitler, K. Visible light mediated reductive cleavage of C-O bonds accessing α-substituted aryl ketones. Org. Lett. 2015, 17, 4818–4821. [Google Scholar] [CrossRef]
- Chen, J.Z.; Zhang, Z.F.; Liu, D.L.; Zhang, W.B. Palladium-catalyzed chemo- and enantioselective C-O bond cleavage of α-acyloxy ketones by hydrogenolysis. Angew. Chem. Int. Ed. 2016, 55, 8444–8447. [Google Scholar] [CrossRef]
- Speckmeier, E.; Zeitler, K. Desyl and phenacyl as versatile, photocatalytically cleavable protecting groups: A classic approach in a different (visible) light. ACS Catal. 2017, 7, 6821–6826. [Google Scholar] [CrossRef]
- Zhu, H.; Manchado, A.; Omar Farah, A.; McKay, A.P.; Cordes, D.B.; Cheong, P.H.-Y.; Kasten, K.; Smith, A.D. Isothiourea- catalysed acylative dynamic kinetic resolution of tetra-substituted morpholinone and benzoxazinone lactols. Angew. Chem. Int. Ed. 2024, 63, e202402908. [Google Scholar] [CrossRef]
- Iino, Y.; Matsushima, Y.; Nakashima, K.; Hirashima, S.I.; Miura, T. Organocatalyzed synthesis of γ-alkenyl butenolides via asymmetric direct vinylogous conjugate addition-elimination of substituted furanone derivatives to β-phenylsulfonylenones. J. Org. Chem. 2024, 89, 11789–11795. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Panger, J.L.; Lupi, M.; Denmark, S.E. Organoselenium-catalyzed enantioselective synthesis of 2-oxa- zolidinones from alkenes. Org. Lett. 2024, 26, 6703–6708. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Sha, Q. B(C6F5)3-catalyzed multicomponent reactions of 2,3-diketoesters, amines, allenes, and nucleophiles: Synthesis of 2α-functionalized pyrroles. J. Org. Chem. 2024, 89, 12286–12297. [Google Scholar] [CrossRef]
- Alahyen, I.; Taillier, C.; Lhoste, J.; Dalla, V.; Comesse, S. N-Benzyloxyacrylamides as bisnucleophiles in an organocatalyzed domino aza-Michael/Morita-Baylis-Hillman sequence. Org. Lett. 2024, 26, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhong, L.J.; Lv, G.F.; Li, Y.; Li, J.H. Photocatalytic dual decarboxylative alkenylation mediated by triphenylphosphine and sodium iodide. Org. Biomol. Chem. 2020, 18, 5589–5593. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Gao, G.C.; Cheng, J.D.; Li, J.T.; Chen, X.S.; Chen, X.M.; Zhang, D.H.; Li, H.Q.; Cai, X.H.; Huang, B.B. Photocatalytic organosulfur reagent-promoted selective mono-(deutero)hydrodechlorination. Green Chem. 2024, 26, 5167–5172. [Google Scholar] [CrossRef]
- Kuan, J.Y.; Chen, J.H.; Han, J.L. Switchable synthesis of tritylone alcohols and 2-benzoylbenzoate esters from spiroindane-1,3-diones. J. Org. Chem. 2024, 89, 12360–12369. [Google Scholar] [CrossRef] [PubMed]
- Dhami, A.; Kumar, A.; Hisana, K.N.; Mohanan, K. Organocatalyzed, three-component construction of cyanopyrazoles using diazoacetonitrile. Adv. Synth. Catal. 2024, 366, 2551–2556. [Google Scholar] [CrossRef]
- Agrawal, S.; Majhi, P.; Goodfellow, A.; Tak, R.; Cordes, D.; McKay, A.; Kasten, K.; Buehl, M.; Smith, A.D. Enantioselective synthesis of tetra-substituted 3-hydroxyphthalide esters by isothiourea-catalysed acylative dynamic kinetic resolution. Angew. Chem. Int. Ed. 2024, 63, e202402909. [Google Scholar] [CrossRef]
- Dai, P.F.; Qu, J.P.; Kang, Y.B. Organocatalyzed aerobic oxidation of aldehydes to acids. Org. Lett. 2019, 21, 1393–1396. [Google Scholar] [CrossRef]
- Onida, K.; Haddleton, A.J.; Norsic, S.; Boisson, C.; D’Agosto, F.; Duguet, N. Organocatalytic synthesis of substituted vinylene carbonates. Adv. Synth. Catal. 2021, 363, 5129–5137. [Google Scholar] [CrossRef]
- Liang, Y.F.; Wu, K.; Song, S.; Li, X.Y.; Huang, X.Q.; Jiao, N. I2- or NBS-catalyzed highly efficient α-hydroxylation of ketones with dimethyl sulfoxide. Org. Lett. 2015, 17, 876–879. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, H.H. Study on the α-acetoxylation of sp3-C-H bonds adjacent to carbonyl of arones. Chin. J. Org. Chem. 2016, 36, 826–829. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, P.F. Sulfur-mediated difunctionalization of internal and terminal alkynes for the synthesis of α-acetoxy ketones. Tetrahedron Lett. 2020, 61, 151707. [Google Scholar] [CrossRef]
- Nagano, T.; Jia, Z.H.; Li, X.S.; Yan, M.; Lu, G.; Chan, A.S.C.; Hayashi, T. Redox catalysis of halide ion for formal cross-dehydrogenative coupling: Bromide ion-catalyzed direct oxidative α-acetoxylation of ketones. Chem. Lett. 2010, 39, 929–931. [Google Scholar] [CrossRef]
- Hokamp, T.; Wirth, T. Hypervalent iodine(III)-catalysed enantioselective α-acetoxylation of ketones. Chem. Eur. J. 2020, 26, 10417–10421. [Google Scholar] [CrossRef] [PubMed]
- Nestl, B.M.; Bodlenner, A.; Stuermer, R.; Hauer, B.; Kroutil, W.; Faber, K. Biocatalytic racemization of synthetically important functionalized α-hydroxyketones using microbial cells. Tetrahedron-Asymmetry 2007, 18, 1465–1474. [Google Scholar] [CrossRef]
- Pei, W.W.; Li, S.H.; Nie, X.P.; Li, Y.W.; Pei, J.; Chen, B.Z.; Wu, J.; Ye, X.L. Convenient syntheses of 2-alkyl(aryl)-4,5- diphenyloxazoles and 2-alkyl(aryl)-4-phenyloxazoles. Synthesis 1998, 1998, 1298–1304. [Google Scholar] [CrossRef]
- Agrawal, S.; Martinez-Castro, E.; Marcos, R.; Martín-Matute, B. Readily available ruthenium complex for efficient dynamic kinetic resolution of aromatic α-hydroxy ketones. Org. Lett. 2014, 16, 2256–2259. [Google Scholar] [CrossRef]
- Loner, C.M.; Luzzio, F.A.; Demuth, D.R. Preparation of azidoaryl- and azidoallryloxazoles for click chemistry. Tetrahedron Lett. 2012, 53, 5641–5644. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).