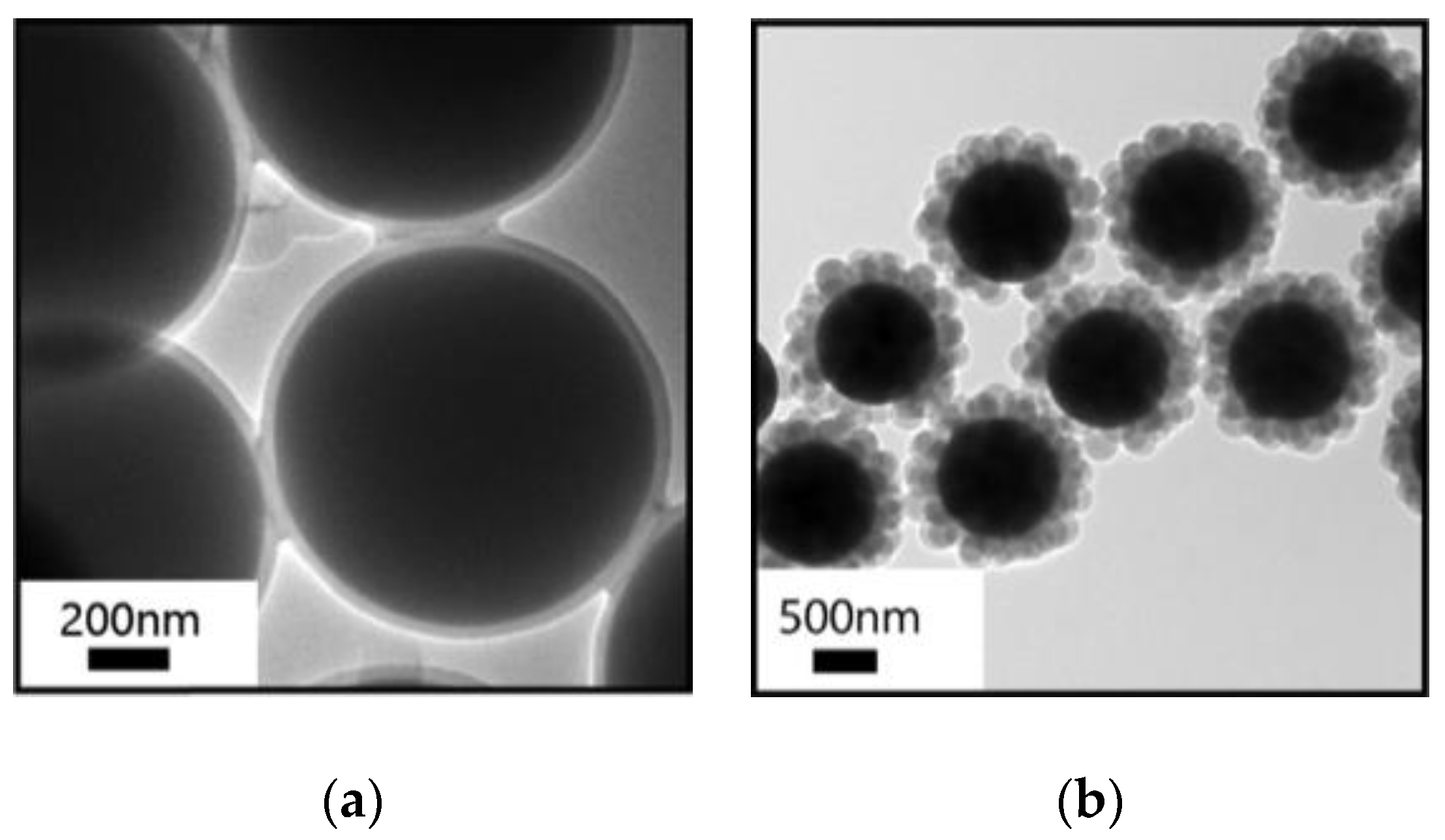
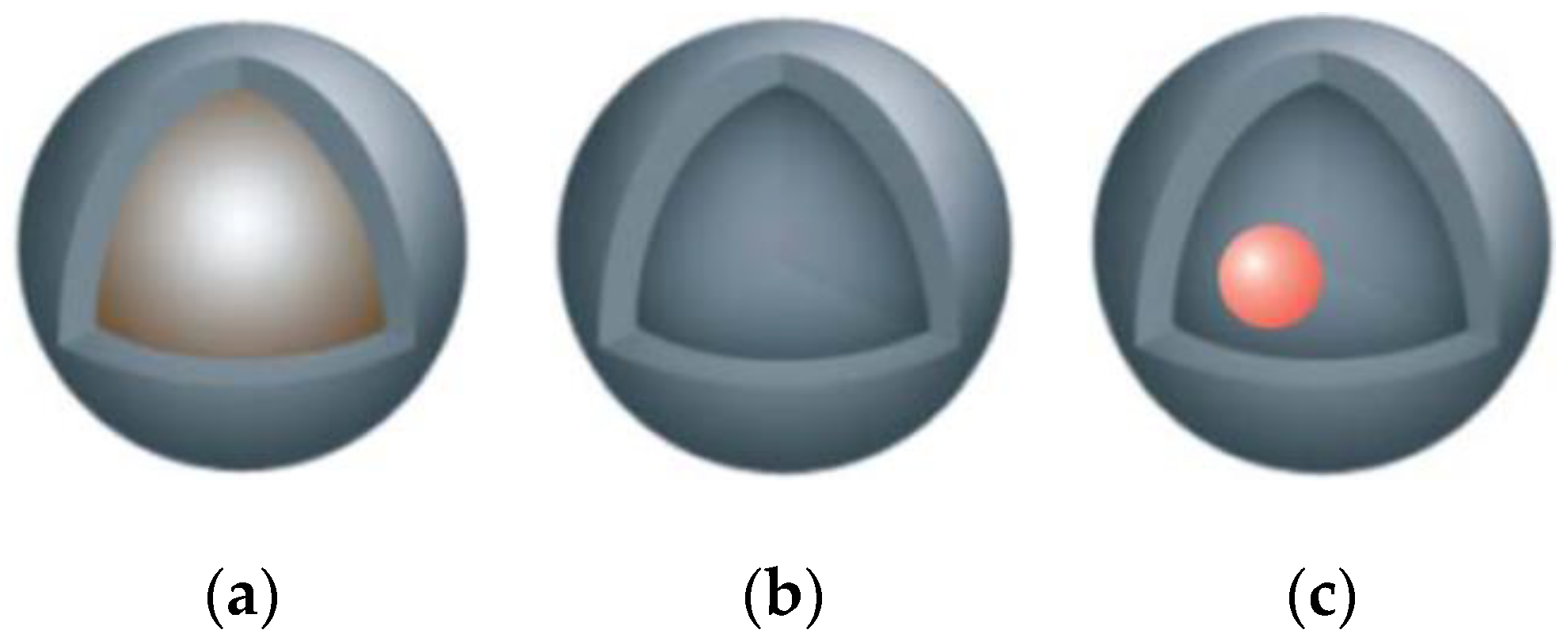
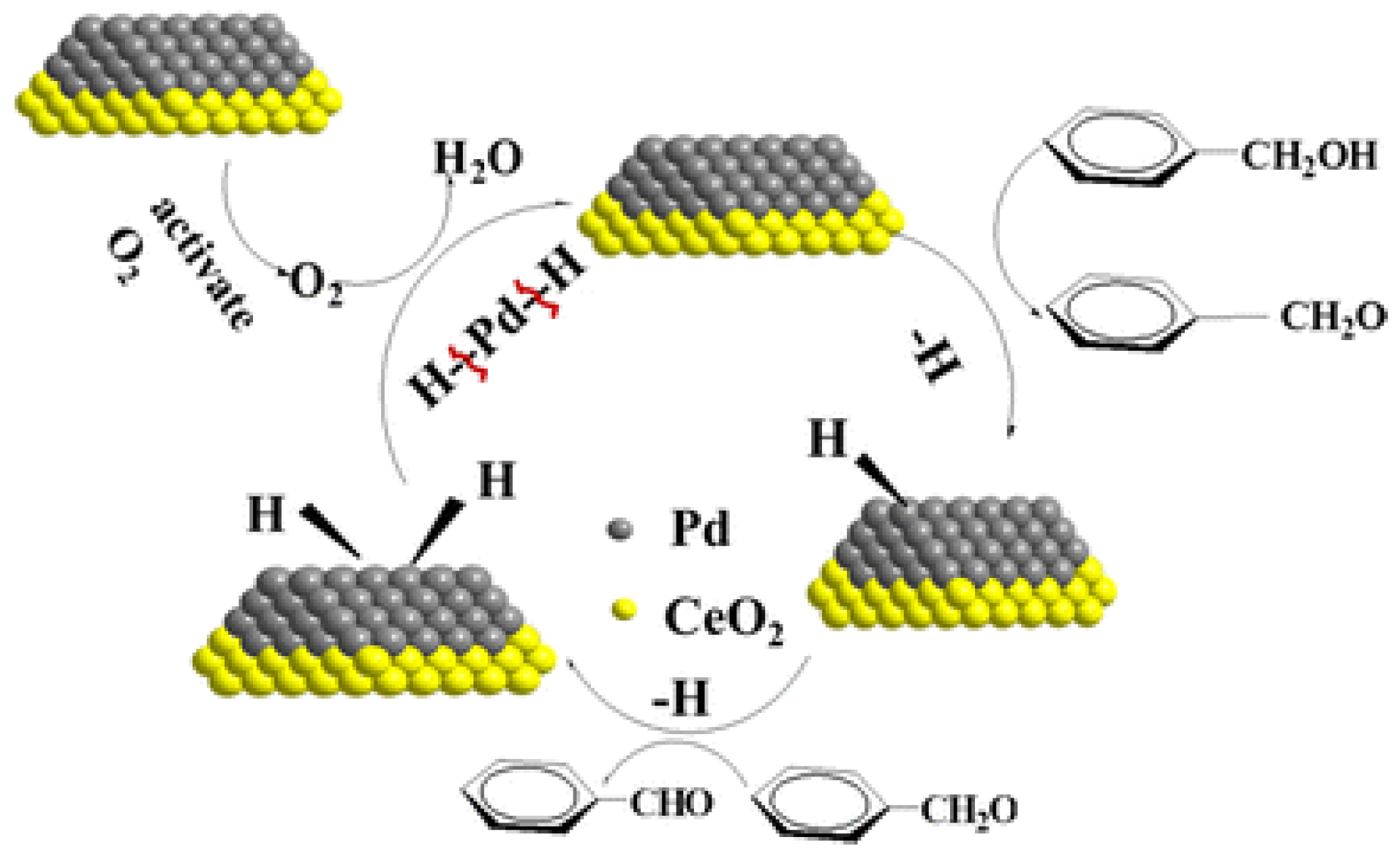
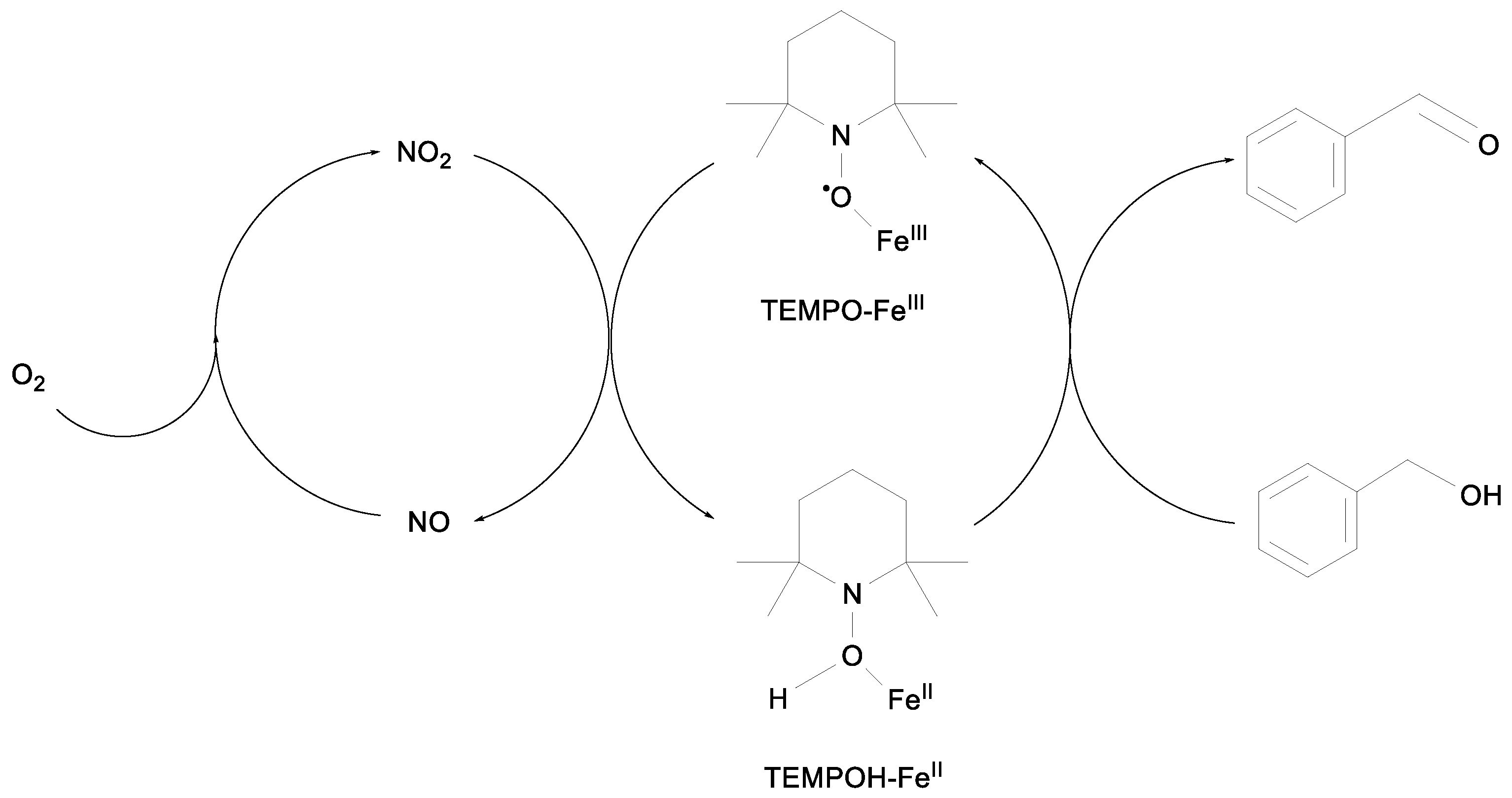
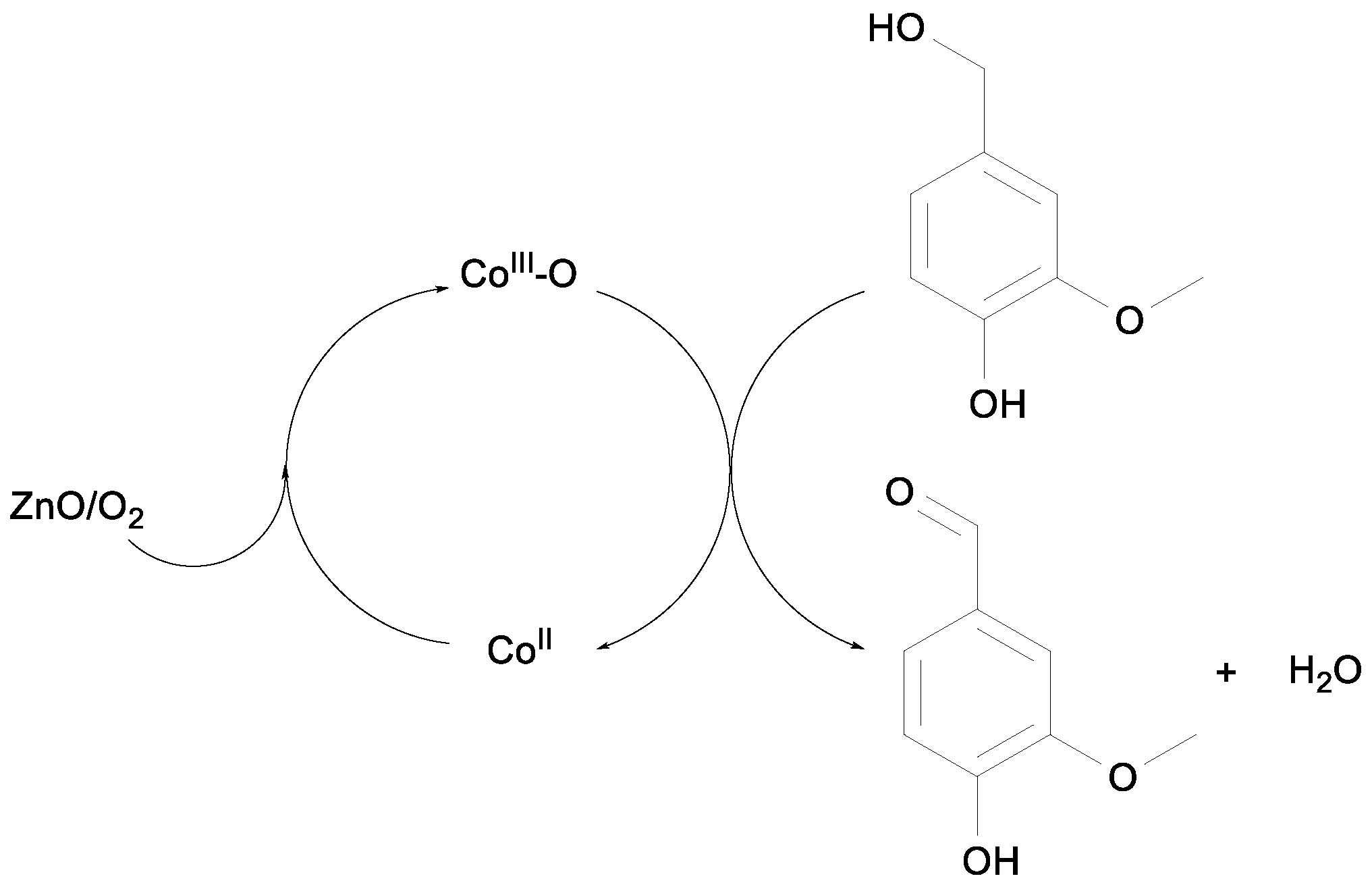
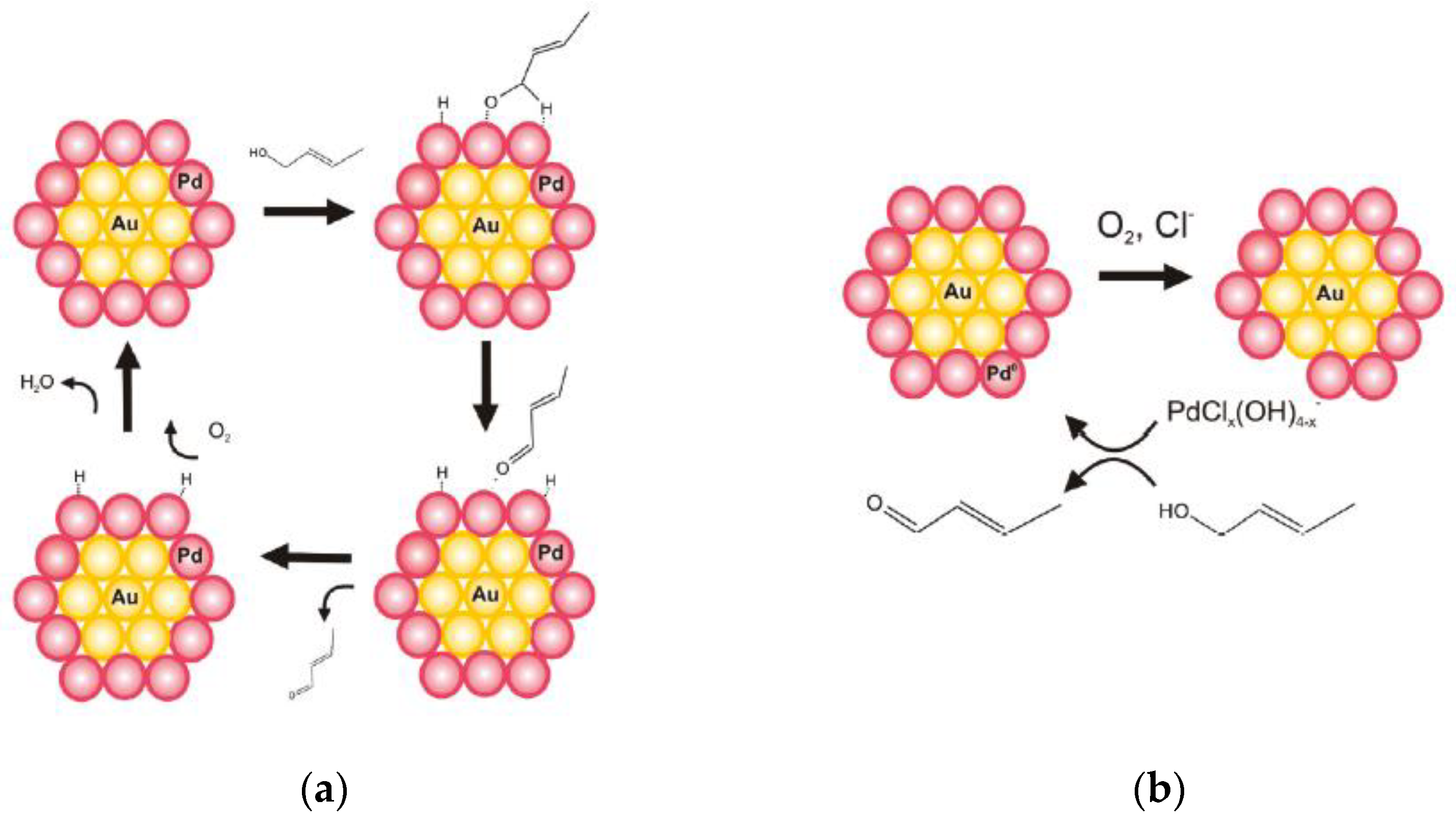
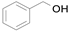
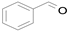
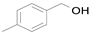
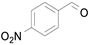
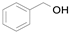
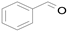
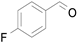
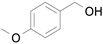
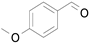
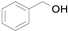
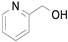
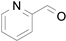
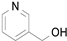
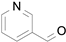
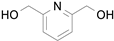
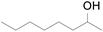
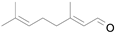
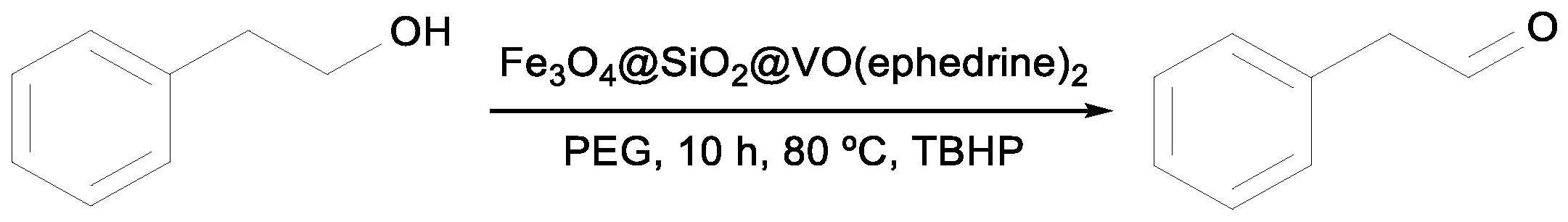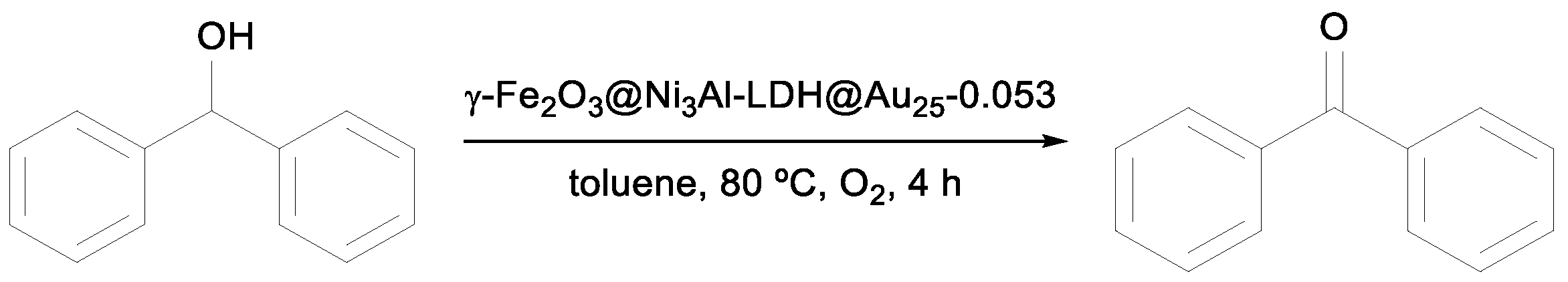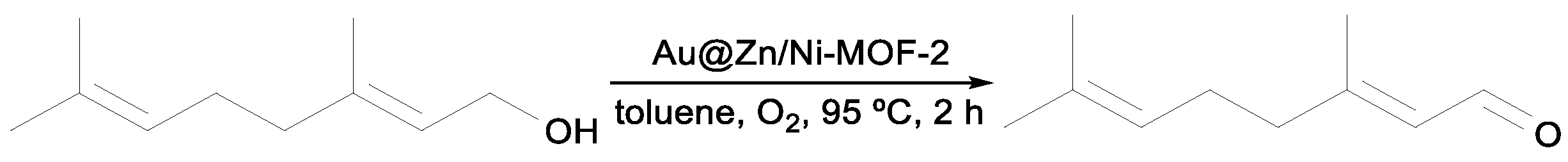2.1. Benzylic Alcohols
In 2015, Wang et al. synthesized the Au@Pd bimetallic core–shell supported on SiO
2 and investigated it as a catalyst for the solvent-free selective aerobic oxidation of benzyl alcohol (
Scheme 1) [
32]. The thickness of the Pd layer controlled by atomic layer deposition (ALD) at a rate of ~0.8 nm per Pd ALD cycle affects the catalytic activity. The total yield increases and the benzaldehyde selectivity decreases with the number of cumulative Pd ALD cycles reaching the maximum turnover frequency (TOF) of 2.76 × 10
4 h
−1, at the optimized Pd shell thickness of 0.6–0.8 nm obtained after 8 Pd ALD cycles (Au@8Pd/SiO
2) (entry 1,
Table 1) [
32].
In 2020, Luo et al. reported the Au/SH-MON@m-SiO
2 core–shell which consists of mesoporous organosilica functionalized with thiol groups (shell) coating gold nanoparticles housed as an internal part of the catalyst (core). This catalyst displays cooperative properties, i.e., while the mesopores of the shell facilitate access and release of reactants and products, and the internal pores of the organosilica provide a hydrophobic microenvironment [
33]. The catalytic activity of this core–shell was tested for the aerobic oxidation of benzyl alcohol and, although the reported conversion was not impressive (possibly due to the low gold content of 0.91 wt% Au), the selectivity towards benzaldehyde is remarkable (99%, entry 2,
Table 1). These core–shell structures showed rather low stability during the recycling process. A significant loss of activity is already observed at the end of the third cycle (ca. 41% compared to the first cycle) while maintaining a high selectivity towards benzaldehyde (99%). These results have been attributed to the degradation of the mesoporous silica shell and the aggregation of the gold nanoparticles, which affect the active sites and, thus, compromise their catalytic activity [
33].
At the same time, Hammond-Pereira et al. aerobically oxidized benzyl alcohol in the presence of silica-encapsulated gold core@shell nanoparticles, Au@m-SiO
2, under alkaline conditions using K
2CO
3. The substrate conversion of 60.4% was obtained after 1 h of reaction under solvent-free conditions, and a TOF value of 1.28 × 10
4 h
−1 was registered, indicating a rather high activity [
34]. The selectivity for aldehyde (ca. 75%) (entry 3,
Table 1) is attributed to the mesoporous shell of the AuNPs which seems to inhibit the formation of larger species. The addition of potassium carbonate has a distinct effect on the conversion and selectivity of the reaction, i.e., it favors the substrate conversion from 17.3 to 60.4% but decreases the selectivity from 98.7 to 75.0% [
34].
Zhang et al. reported the successful preparation of a Pd/ZDC@m-SiO
2 core–shell structure, which consists of Pd nanoparticles (PdNPs) supported on ZIF-8 derived porous carbon (ZDC) as a core and mesoporous silica as a shell, and its application as a catalyst in the aerobic oxidation of benzyl alcohol. Excellent catalytic performance with high conversion and selectivity was observed (entry 4,
Table 1). The high stability of this core–shell was confirmed by reusing it for eight cycles without loss of activity and was attributed to its structural configuration which prevents the sintering of PdNPs during the calcination process as well as their leaching during catalytic tests [
35].
Lian et al. synthesized the homogeneous spherical hematite core–shell (Fe
2O
3@Fe
2O
3) through a one-pot surfactant-free solvothermal treatment of FeCl
3.6H
2O and investigated its catalytic activity in the peroxidative oxidation (H
2O
2 30% aq. sol.) of benzyl alcohol achieving 42% conversion and 95% selectivity towards benzaldehyde after 12 h of reaction (entry 5,
Table 1) [
36]. Other catalysts such as microparticles of α-Fe
2O
3, γ-Fe
2O
3, and Fe
3O
4 (1 mol% Fe) have been tested under the same reaction conditions. The selectivities towards benzaldehyde remained of the same order for the core–shell (92–94%), but the benzyl alcohol conversion was found to be ca. 5 times lower (7–9%), demonstrating the superiority of the core–shell structure. The core–shell hematite catalyst Fe
2O
3@Fe
2O
3 proved to be very promising since it allowed the combination of high catalytic activity with high stability since the catalytic performance remained the same after 5 catalytic cycles (41% yield and 95% selectivity for benzaldehyde) [
36].
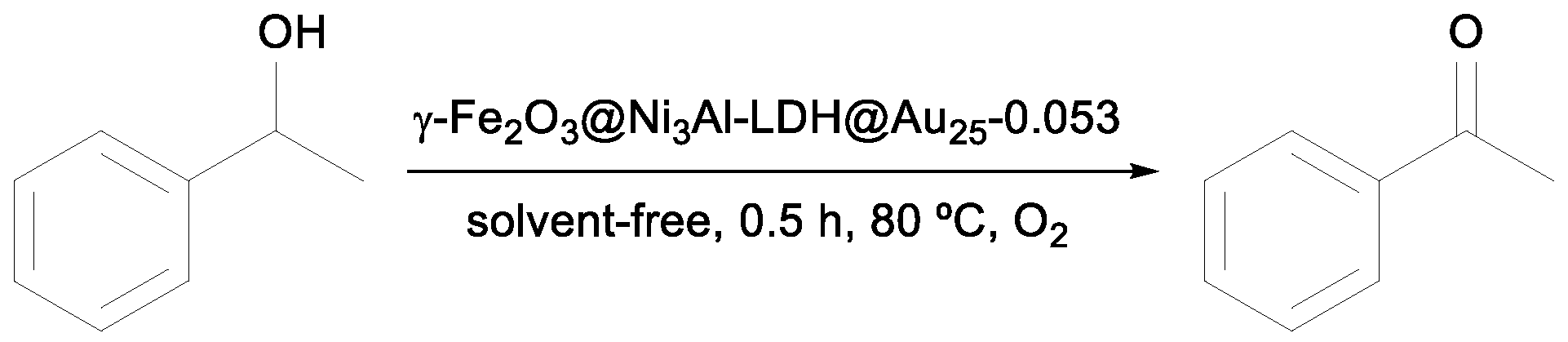
Yin et al. screened the catalytic activity of the hierarchical hollow nanostructured core–shell γ-Fe
2O
3@Ni
3Al-LDH@Au
25-0.053 in the aerobic oxidation of benzyl alcohol [
37] and showed an excellent performance with almost complete conversion observed after 1.5 h (entry 6,
Table 1). Comparing only the percentage yields presented in
Table 1, this catalyst and the Pd/ZDC@m-SiO
2 synthesized by Zhang et al. [
35] showed the best catalytic conversion of benzyl alcohol under specific reaction conditions [
37].
Zhang et al. reported the synthesis of core–shell microspheres using Fe
3O
4 as a core coated with layers of poly(allylamine hydrochloride)-Pd(II) (PAH) and poly(sodium 4-styrenesulfonate) PSS and by an outer layer of palladium nanoparticles as the shell, denoted here as Fe
3O
4@PAH@PSS@Pd [
38]. This catalyst was used in the peroxidative oxidation reaction (H
2O
2 30% aq. sol.) of benzyl alcohol with an alcohol conversion of 95% and selectivity towards benzaldehyde of 90% (entry 7,
Table 1). The microspheres could be reused for three more cycles without significant loss of activity [
38].
The synthesis of Fe
3O
4@SiO
2@Au@Pd core–shell and its catalytic activity in the aerobic oxidation reaction of benzyl alcohol was reported by Silva et al. [
39]. The optimized Au:Pd molar ratio of 10:1 resulted in the 87.7% conversion of benzyl alcohol with the 86.4% selectivity towards benzaldehyde (entry 8,
Table 1). The stability of Fe
3O
4@SiO
2@Au@Pd was also evaluated, but the results showed fluctuations in the conversion between cycles. This effect was attributed to the presence of amino groups in Fe
3O
4@SiO
2 used for a better impregnation of gold in the core–shell which could interact with the reaction products. Meanwhile, at the end of the sixth cycle, the conversion was 50.3%, and the selectivity towards benzaldehyde increased to 92.5% [
39].
More recently, Silva et al. calcinated the Fe
3O
4@SiO
2@Au@Pd core–shell and observed that the thermal treatment altered the structure of the nanocomposite, leading to an increase in particle size, metal segregation, and oxidation of palladium to PdO. The treated material showed increased catalytic activity (4-fold) for the aerobic oxidation of benzyl alcohol at 100 °C under 6 bar O
2 with a maximum activity for an Au:Pd molar ratio of 1:2 [
45].
In 2019, Lv et al. reported the SiO
2@Co
3O
4@Au@m-SiO
2 core–shell [
40] while Yuan et al. synthesized the hollow Co
3O
4@Au@m-SiO
2 core–shell [
41], both being applied in the aerobic oxidation reaction of benzyl alcohol. After optimizing the reaction parameters such as the amount of catalyst, temperature, oxygen flow rate, and reaction time, a conversion of 58% and a selectivity towards benzaldehyde of 82% were obtained for the former catalyst after 6 h reaction at 160 °C and a 60 mL/min O
2 flow rate, whereas for the latter core–shell, a conversion and a selectivity of 55 and 84%, respectively, were achieved after 5 h reaction at 140 °C and a 50 mL/min O
2 flow rate (entries 9 and 10,
Table 1). Both catalysts demonstrated a good resistance maintaining the catalytic performance after 5 cycles [
40,
41].
In 2020, Wu et al. synthesized polymeric ionic liquid microspheres (PILM) (core) and coated them with a layer of Pd nanoparticles (PdNPs) covered with a layer of CeO
2 (shell) forming the PILM@Pd@CeO
2 core–shell [
42]. The supported PdNPs were uniformly distributed on the surface of the polymer microspheres with aggregation being avoided through the addition of a CeO
2 layer. The core–shell was used for the aerobic oxidation reaction of benzyl alcohol; under optimized reaction conditions in terms of reaction parameters such as the amount of an alkaline additive (K
2CO
3), temperature (160 °C), and oxygen flow rate (60 mL/min), an alcohol conversion of 48%, a selectivity towards benzaldehyde of 98%, and a TOF of 6.33 × 10
2 h
−1 were achieved (entry 11,
Table 1). They also tested the stability of the catalyst using recycling tests and found that these core–shell structures maintained their catalytic performance after five cycles [
42].
In 2023, Su et al. synthesized the spherical core–shell PILM@Au@Al(OH)
3 with the polymeric ionic liquid microspheres (PILM) as the core coated with gold nanoparticles and covered with an aluminum hydroxide layer. This material was used as a catalyst in the peroxidative oxidation reaction (H
2O
2, 30% aq. sol.) of benzyl alcohol. The optimized reaction conditions are 30 mg of catalyst, 2.2 wt% of Au content, 80 °C, H
2O
2:benzyl alcohol molar ratio of 1.25:1, and 6 h of the reaction time. Under these conditions, a conversion of 76% and a selectivity towards benzaldehyde of 92% were obtained. The effect of recycling the catalyst was also studied. It was found that after five cycles, the core–shell was stable and did not show significant loss in conversion and selectivity [
46].
Meaney et al. synthesized the PAM@SiO
2@Au core–shell which consisted of poly(acrylamide) with deposited SiO
2 layer (shell) and gold nanoparticles (AuNPs) on the surface. It was then applied in the oxidation of benzyl alcohol and showed a maximum conversion of ca. 40% and a selectivity towards benzoic acid of 90% after 20 h of reaction (entry 12,
Table 1). The effect of the reaction time on the product yields was analyzed, and benzoic acid was already detected as the main product after 8 h. The presence of a strong base (NaOH) promoted the formation of benzoic acid as the main product [
43].
Lu et al. synthesized the gold–microgel nanocomposite PS@PNIPA/Au core–shell consisting of a spherical polystyrene core coated with a poly(
N-isopropylacrylamide) (PNIPA) shell cross-linked by
N,
N′-methylenebisacrylamid [
44]. This material was subsequently used as a support for the immobilization of gold nanoparticles and applied as a catalyst for the aerobic oxidation reaction of benzyl alcohol under mild conditions (entry 13,
Table 1). Green chemistry principles were considered using water as the solvent and air as the oxidant at room temperature (25 °C). The thermosensitive character of the core–shell microgels is responsible for the temperature effect on the catalytic activity of the gold–microgel composite. In fact, the TOF value of 1.59 × 10
2 h
−1 was achieved at 25 °C, whereas increasing the temperature to 40 °C resulted in the higher TOF value of 2.20 × 10
3 h
−1. Thus, the catalytic activity varies with the polarity and volume of the gold–microgel composites, since at lower temperatures, the thermosensitive microgel network is hydrophilic and swells in water, while at higher temperatures it becomes hydrophobic and shrinks, becoming oil soluble and favoring the transport of hydrophobic benzyl alcohol to the Au nanoparticle surface [
44].
Regarding the yields obtained by the different core–shell in the benzyl alcohol oxidation reaction shown in
Table 1, it appears that the lowest yields (40–51%) are obtained in the presence of monometallic core–shells (PAM@SiO
2@Au, Fe
2O
3@Fe
2O
3, and Au/SH-MON@m-SiO
2—entries 12, 5, and 2, respectively,
Table 1) whereas the polymetallic core–shells (γ-Fe
2O
3@Ni
3Al-LDH@Au25-0.053, Pd/ZDC@m-SiO
2, Fe
3O
4@PAH@PSS@Pd, and Au@8Pd/SiO
2—entries 6, 4, 7, and 1, respectively,
Table 1) are more effective and show the highest yields (91–99%), probably due to their synergistic properties.
Several publications reported the application of different core–shell type catalysts in the oxidation of substituted benzyl alcohols as well as the effect of the different group’s nature and position.
Zhao et al. tested the catalytic activity of Ru@SQ (Ru nanoparticles coated with disodium anthraquinone-2,6-disulfonate) in the aerobic oxidation of benzyl alcohol and its
p-methyl and
p-chlorinated derivatives and found that the conversion under the same conditions is independent of the substrate (
Table 2) [
47]. The high performance and selectivity towards the aldehyde demonstrated by the Ru@SQ core–shell may be related to the fact that the ruthenium nanoparticles (RuNPs) are surrounded by a uniform organic layer, which could prevent the over-oxidation of the respective benzaldehydes to acids [
47].
Sholjaei et al. optimized the reaction conditions for the oxidation of benzyl alcohol in the presence of the core–shell type catalyst RuO
2@ZrO
2 [
48]. Different temperatures (25 and 80 °C), amount of catalyst (10, 20, and 30 mg), type of oxidant (TBHP, H
2O
2, and O
2), and type of solvent (acetonitrile, toluene, and dichloromethane) were tested. Quite good conversions and selectivities towards benzaldehyde were achieved (80 and 87.5%, respectively, entry 1,
Table 3). It should be noted that the authors managed to carry out three cycles without significant loss of activity, maintaining the conversion of benzyl alcohol at the level of 80%. Subsequently, the same catalyst was applied to different substituted benzyl alcohols (
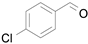
Table 3), the
p-chlorobenzyl alcohol being found to be the most active substrate in terms of product yield, selectivity, and reaction time (entry 2,
Table 3) [
48].
Rostami et al. reported the application of the magnetic Fe
3O
4@SiO
2 core–shell supported on an oxo-vanadium ephedrine complex in the peroxidative oxidation (TBHP 70% aq. sol.) of benzyl alcohol [
49]. Different reaction conditions (i.e., dichloromethane, acetonitrile, water, or polyethylene glycol (PEG) as a solvent, room temperature or 80 °C, amount of the catalyst of 30, 40, or 50 mg) were investigated. After optimization of the reaction conditions, an excellent yield of 98% integrated with a high selectivity for benzaldehyde (>99) was reached after 3 h (entry 1,
Table 4). The catalyst was easily recovered and reused until the sixth cycle without significant loss of activity. This catalyst was also tested for the oxidation of other benzylic alcohols, i.e., those with electron donating (entries 4 and 5,
Table 4) and electron withdrawing (entries 2 and 3,
Table 4) groups. The latter type of alcohol was found to have lower activity. Interestingly, the yield of the corresponding aldehyde upon oxidation of the more sterically hindered benzylic alcohol substituted with the methyl group at the
ortho position reached 97% (entry 5,
Table 4). However, a much longer reaction time (47 h) was required in this case [
49].
Ghalavand et al. reported the design of magnetic nanoparticles copper(I) ion complexes with 1,2,3-triazole bridged immobilized on poly(acrylic acid) denoted as the Fe
3O
4@MAPTMS@PAA@Triazole@Cu(I) composite and its catalytic activity for the peroxidative oxidation (H
2O
2 30% aq. sol.) of benzyl alcohol in acetonitrile [
50]. The parameters of temperature and Cu(I) content were optimized, and a yield of 85% of benzaldehyde was obtained after 5 h of reaction (entry 1,
Table 5). Due to the magnetic properties of Fe
3O
4, the catalyst can be easily separated. Its stability has been evaluated in recovery experiments which show that this composite material retains its catalytic activity during up to three cycles. In addition, a hot filtration test showed a low leaching of iron into the solution confirming the heterogeneous nature of the catalyst. The catalytic activity of this composite was extended to substituted benzylic alcohols carrying the electron donor groups (entries 3 and 4,
Table 5) or the electron acceptor groups (entries 2, 5, and 6,
Table 5). It was found that alcohols of the latter group are oxidized in a shorter period of time (2 h, 60 °C) compared to those containing the donor groups (5 h, 60 °C or 3 h, 85 °C, entries 3 and 4,
Table 5). The di-substituted substrate, 2,4-dichlorobenzyl alcohol, gives much higher yields of the reaction product (2,4-dichlorobenzaldehyde) than the p-mono-substituted 4-chlorobenzyl alcohol under the same conditions (entries 2 and 6,
Table 5) [
50].
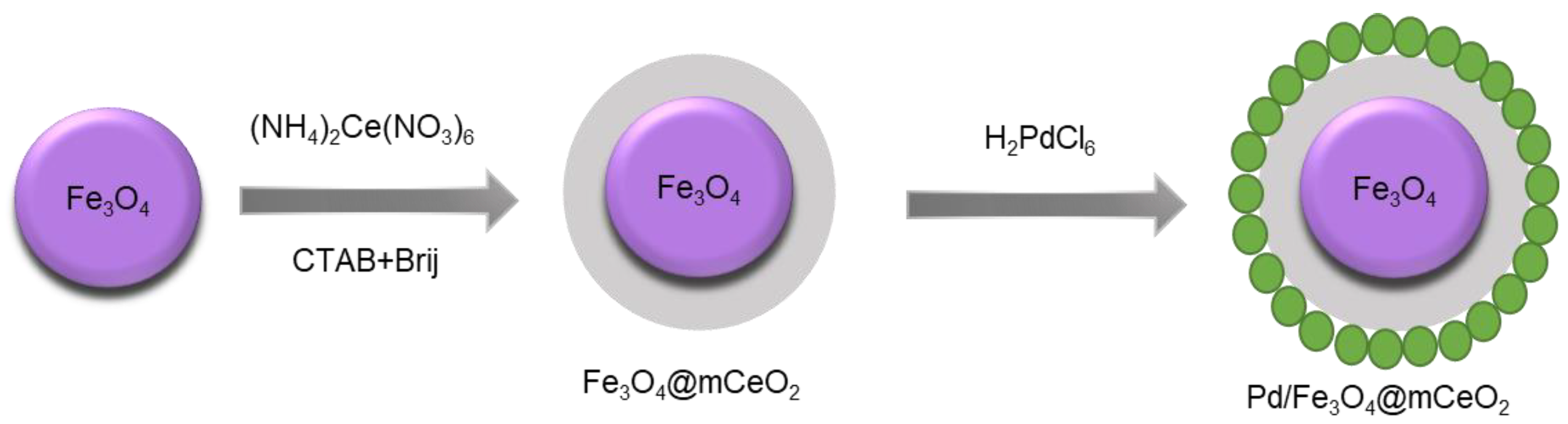
Kong et al. tested the catalytic performance of a Pd/Fe
3O
4@mCeO
2 core–shell in the aerobic oxidation of benzyl alcohol to benzaldehyde at normal pressure. The multifunctional composite was prepared using a layer-by-layer method (
Figure 4) [
51]. Under optimized conditions (2.6 wt% Pd content, CeO
2 support, and temperature 100 °C), the core–shell nanostructured palladium catalyst was able to convert 80.5% of benzyl alcohol to benzaldehyde with 94.8% selectivity after 7 h (entry 1,
Table 6). Being heterogeneous and magnetic, the catalyst was easily separated from the reaction mixture under an external magnetic field. This is a significant advantage of this system, as conventional separation techniques result in high catalyst losses. The catalyst could be reused and showed high efficiency even after seven catalytic cycles. Pd supported on CeO
2 or TiO
2 resulted in better catalytic activity compared to the SiO
2 or C supports. This effect can be explained by the larger oxygen defects in the former supports, which increased the metal/support synergy and, at the same time activating O
2 more efficiently for the aerobic oxidation of benzyl alcohol. It was found that the introduction of an electron withdrawing group (Cl, NO
2) at the
para-position of the alcohol molecule significantly decreased both the product yield and the reaction selectivity (entries 2 and 5,
Table 6), whereas the introduction of electron donor groups (Me, MeO,
tBu) did not significantly affect these parameters compared to the unsubstituted benzyl alcohol (entries 1, 3, 4, and 6,
Table 6) [
51]. However, the increased electronic density in the benzene ring of the aromatic alcohol favors its oxidation to the corresponding aldehyde [
51].
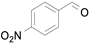
After the successful synthesis of the Fe
3O
4@P4VP@FeCl
3 core–shell by introducing the catalytically active FeCl
3 moiety into the Fe
3O
4@P4VP (P4VP-poly(4-vinylpyridine)) core (4.36 wt% Fe content) through the interaction between P4VP and FeCl
3 (
Figure 5), Li et al. investigated its ability as a catalyst for the aerobic oxidation of benzyl alcohol [
52]. Reactions were carried out using 2 mol% (molar percentage based on iron) of Fe
3O
4@P4VP@FeCl
3, 0.1 mmol NaNO
2, 0.2 mmol of 2,2,6,6-tetramethylpiperidinyl-1-oxy (TEMPO), and 1 mmol of benzyl alcohol in acetonitrile for 12 h at 60 °C under 1 atm O
2. Using this catalytic system, it was possible to combine an excellent conversion with a high selectivity, both around 99% (entry 1,
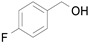
Table 7). In agreement with the previous study, the introduction of an electron withdrawing group (F) in the
para-position of the aromatic ring was unfavorable for the oxidation, while the presence of electron donor substituents (Me, MeO) had no significant effect on the yield and selectivity (entries 2–4,
Table 7). It should also be noted that reactions carried out in the presence of Fe
3O
4 and Fe
3O
4@P4VP failed, demonstrating that the FeCl
3 moiety was an active part of the catalyst [
52].
Lang et al. synthesized the structured spherical nanocomposite consisting of a core of γ-Fe
2O
3 and a shell of mesoporous silica and applied it as a catalyst in the oxidation of benzyl alcohols. The unsubstituted benzyl alcohol showed the best performance in this reaction with a conversion and selectivity of more than 90% (entry 1,
Table 8). The substituted benzyl alcohols either with the donor group Me or with the acceptor group NO
2 give much lower product yields, but the conversion of
p-nitrobenzyl alcohol is very selective (98%, entries 2 and 3,
Table 8). In the recycling experiments, the catalyst showed some loss of activity after three cycles, but the selectivity remained above 80% [
53].
Qin et al. synthesized the hollow cubic Au@Zn/Ni-MOF-2 core–shell nanoreactor and applied it to different substrates, namely benzyl alcohol and derivatives (
Table 9), in toluene at 95 °C and using air as the green oxidant. This core–shell showed excellent catalytic performance towards benzyl alcohol after 2 h of reaction (entry 1,
Table 9). The catalyst was reused after recovery in five cycles without loss of catalytic activity (yield and selectivity). The improved stability of the hollow core–shell Au@Zn/Ni-MOF-2 compared to pure AuNPs highlights the synergistic core–shell effect. Substituted alcohols have a significantly lower activity towards oxidation. For the
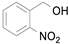
o-nitrobenzyl alcohol, where the substituent is close to the oxidizing hydroxylic group, a product yield of only 8% was obtained. Such an inactivation may be due to steric hindrance imposed by the
ortho NO
2 substituent (entries 2–4,
Table 9) [
54].
In 2022, Ardakani et al. immobilized the dioxo-molybdenum (VI) complex with unsymmetrical Schiff base on the CoFe
2O
4@SiO
2 core–shell and named it CoFe
2O
4@SiO
2@[MoO
2(salenac-OH)] (where salenac-OH is [9-(2′,4′-dihydroxyphenyl)-5,8-diaza-4-methylnone-2,4,8-trienato)](-2)). This composite was used as a catalyst in various peroxidative oxidations of alcohols (TBHP, 70% aq. sol.). The reaction conditions, including the type of solvent, the amount of catalyst, and the type and amount of oxidant, were optimized for the benzyl alcohol. A yield of 90%, a selectivity of 98%, and a TON of 66.7 were recorded after 2 h (entry 1,
Table 10). Due to the magnetic properties of the catalyst, it was removed using an external magnet. A reduction of about 10% in the reaction yield was observed after four cycles of the catalyst recovery. This effect may be related to the leaching of Mo. The substrates with the electron donor substituents showed higher activity (entries 2–6,
Table 10) [
55].
In 2023, Hou et al. applied the Fe
3O
4@Cu
3(BTC)
2 core–shell in the aerobic oxidation of benzyl alcohols studying the effect of different substituents. This composite shows a higher catalytic activity in the presence of electron donating groups (MeO) compared to the electron withdrawing groups (F) (entries 2 and 3,
Table 11). This material also shows excellent catalytic activity for the unsubstituted benzyl alcohol (entry 1,
Table 11) [
56].
Recently, Dabiri et al. synthesized the Fe
3O
4@PDA/Pd@N-RGO core–shell which consists of a magnetite core coated with polydopamine (PDA), to which palladium nanoparticles are subsequently immobilized to this set. This structured composite is surrounded by a nitrogen-doped reduced graphene oxide (N-RGO) layer. The catalytic activity of this material was investigated in the aerobic oxidation of alcohols. Benzyl alcohol was used to optimize parameters such as solvent type, the base, the Pd content, and temperature. The yield of 94% and the selectivity towards benzaldehyde of 99% were obtained for the optimized conditions (entry 1,
Table 12). The catalyst was stable after five recovery cycles, with no significant losses, but the yield decreased from 94% to 87%. The effect of different substituents was also investigated. The substrates with the electron acceptor groups (F and NO
2) showed lower conversions, although the
p-chlorobenzylic alcohol was as active as the unsubstituted substrate and the alcohols with the electron donor groups (
Table 12) [
57].