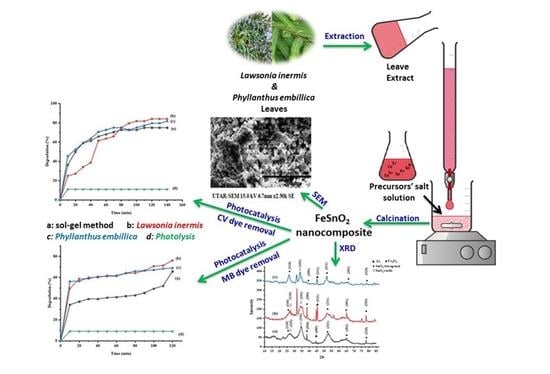
The Impact of Different Green Synthetic Routes on the Photocatalytic Potential of FeSnO2 for the Removal of Methylene Blue and Crystal Violet Dyes under Natural Sunlight Exposure
Abstract
1. Introduction
2. Results and Discussion
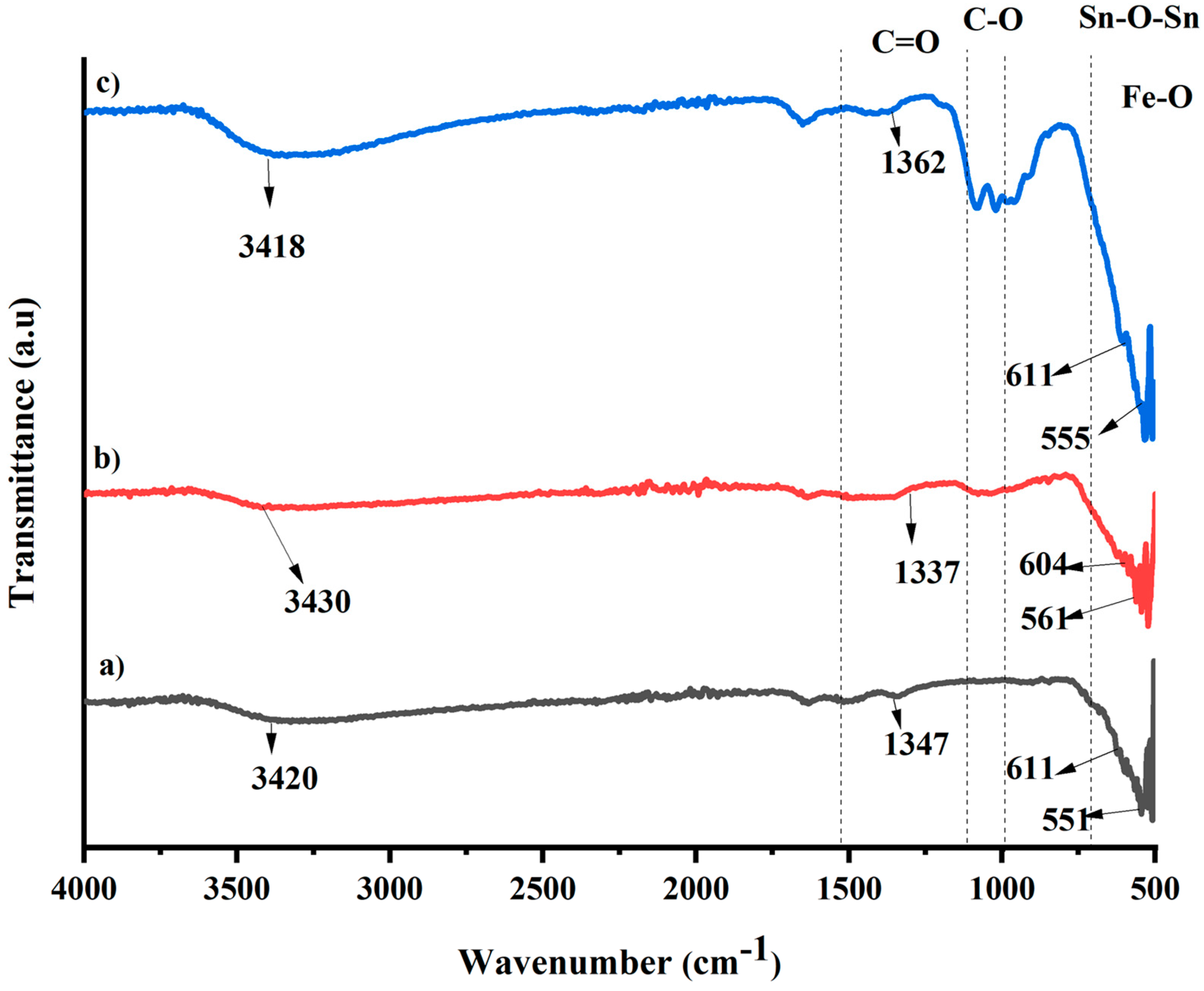
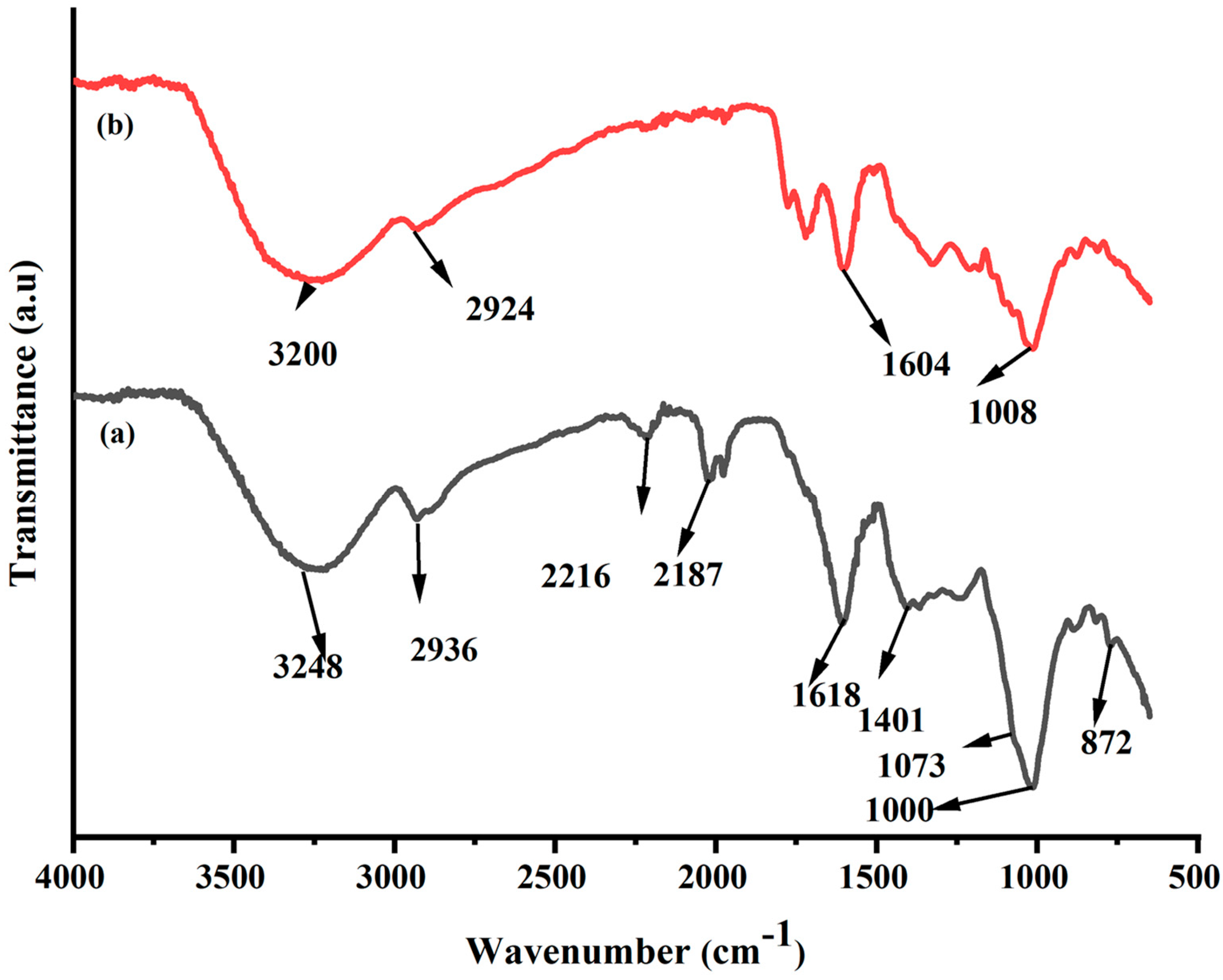
2.1. FT-IR Analysis
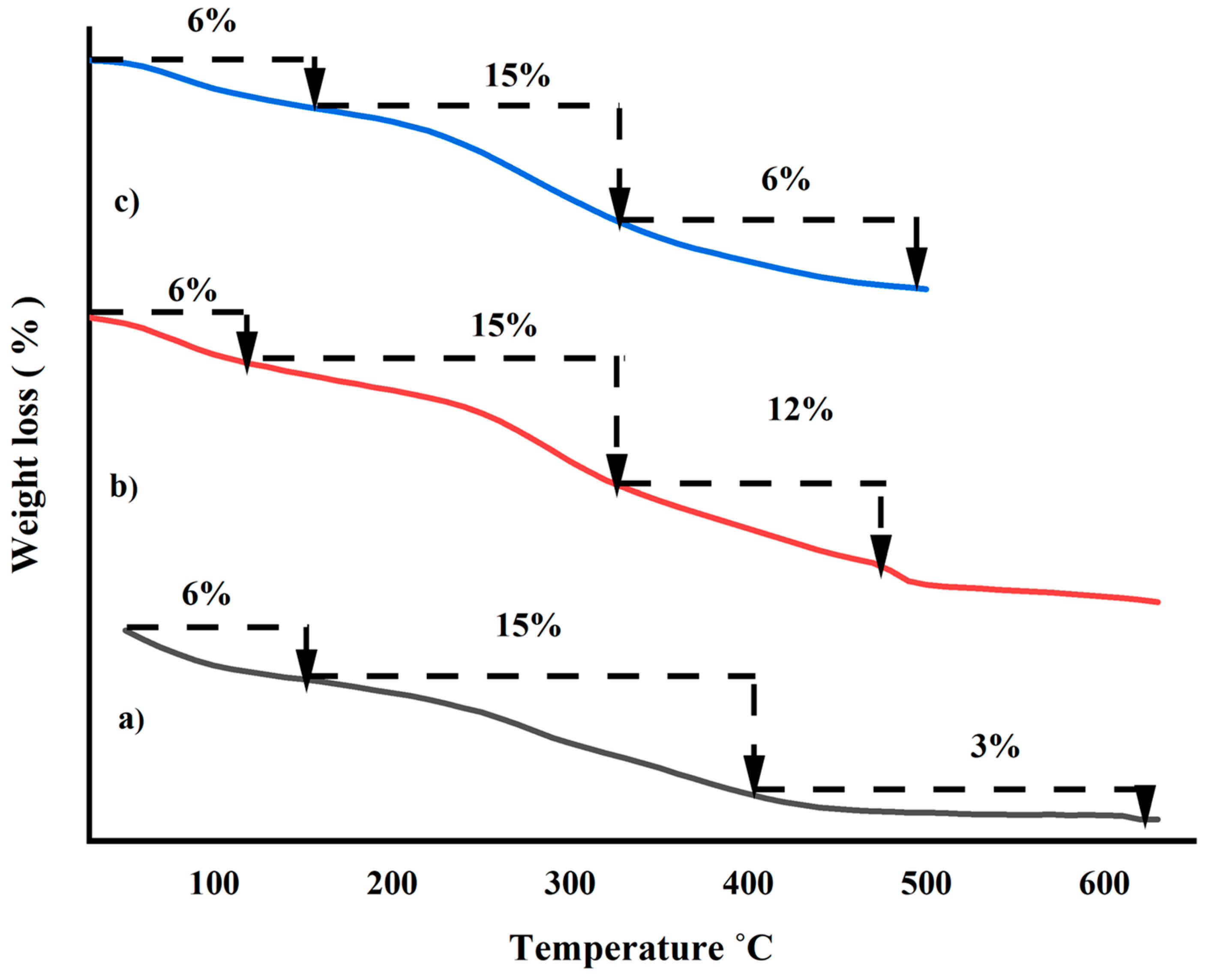
2.2. Thermogravimetric/Differential Scanning Calorimetric Analysis
2.3. X-ray Diffraction Analysis and Phase Determination
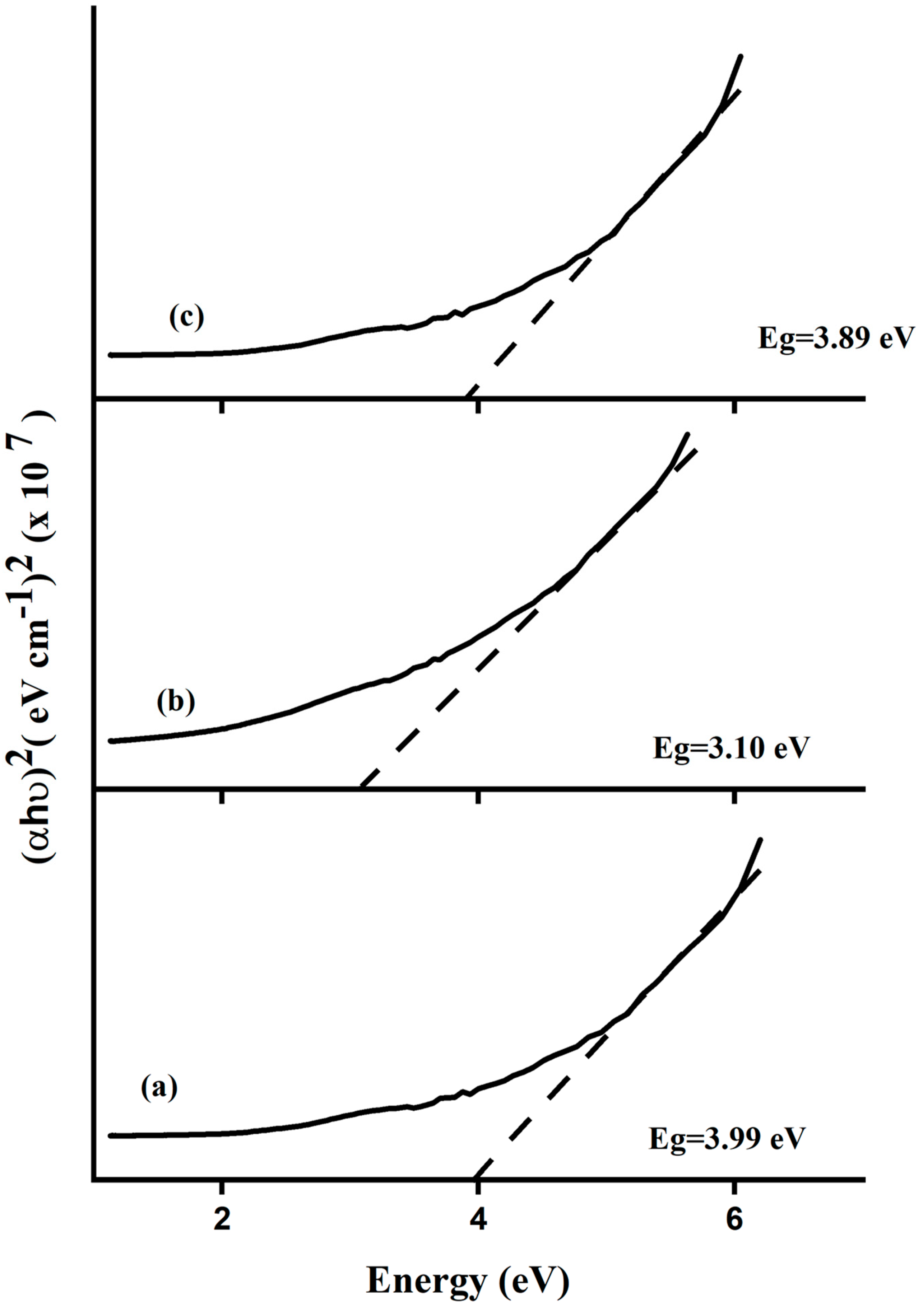
2.4. Optical Studies
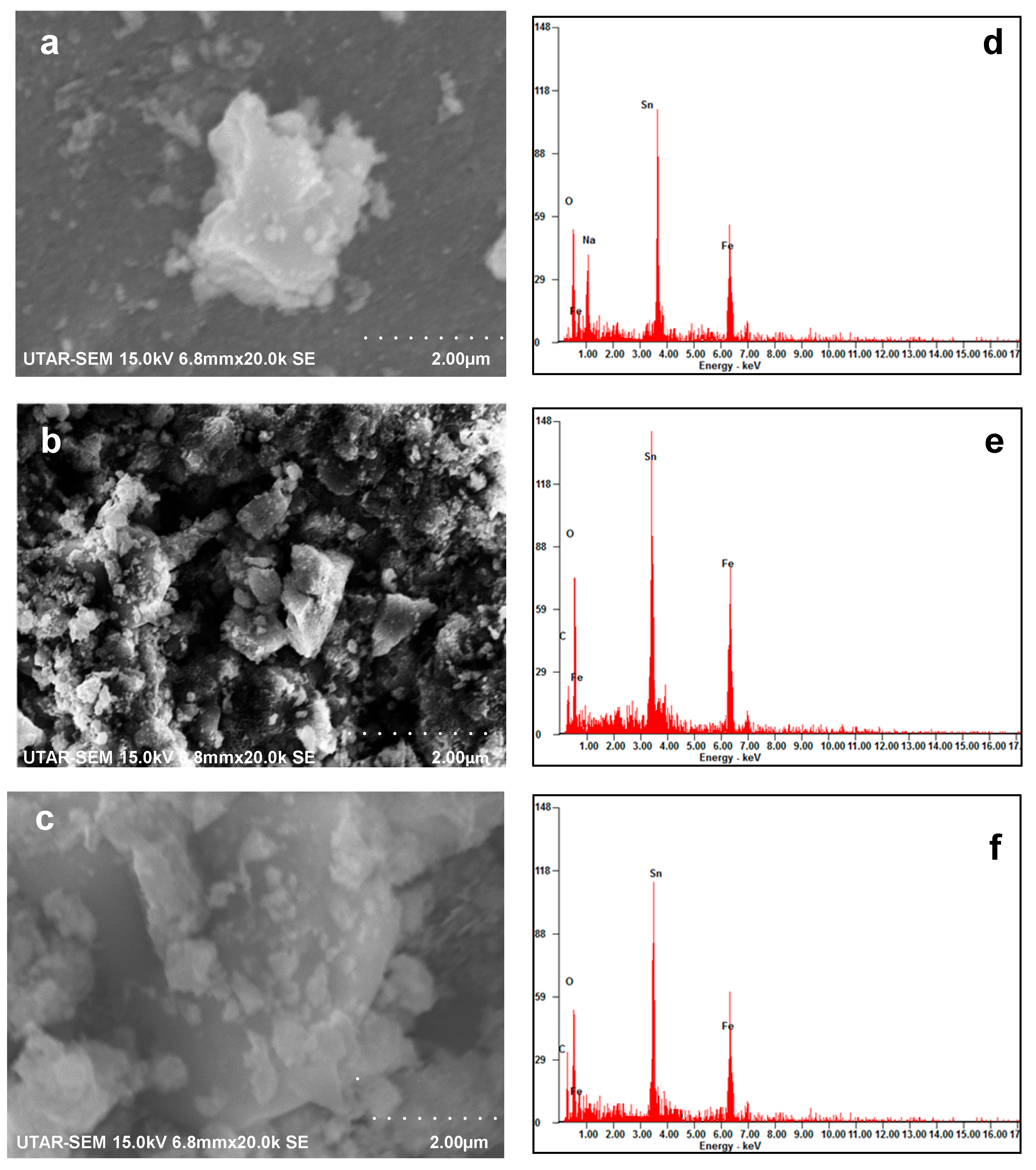
2.5. SEM and EDX Analyses
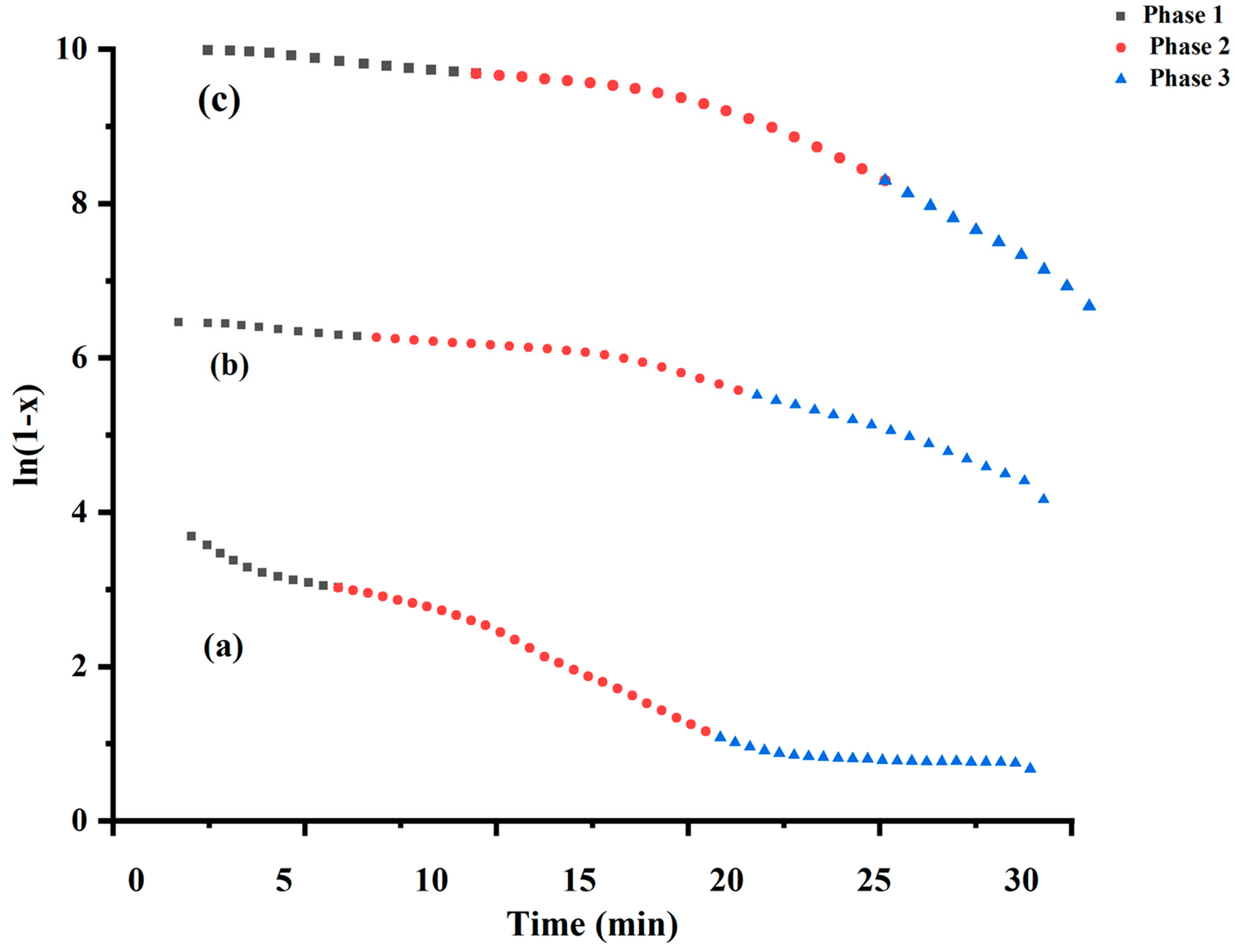
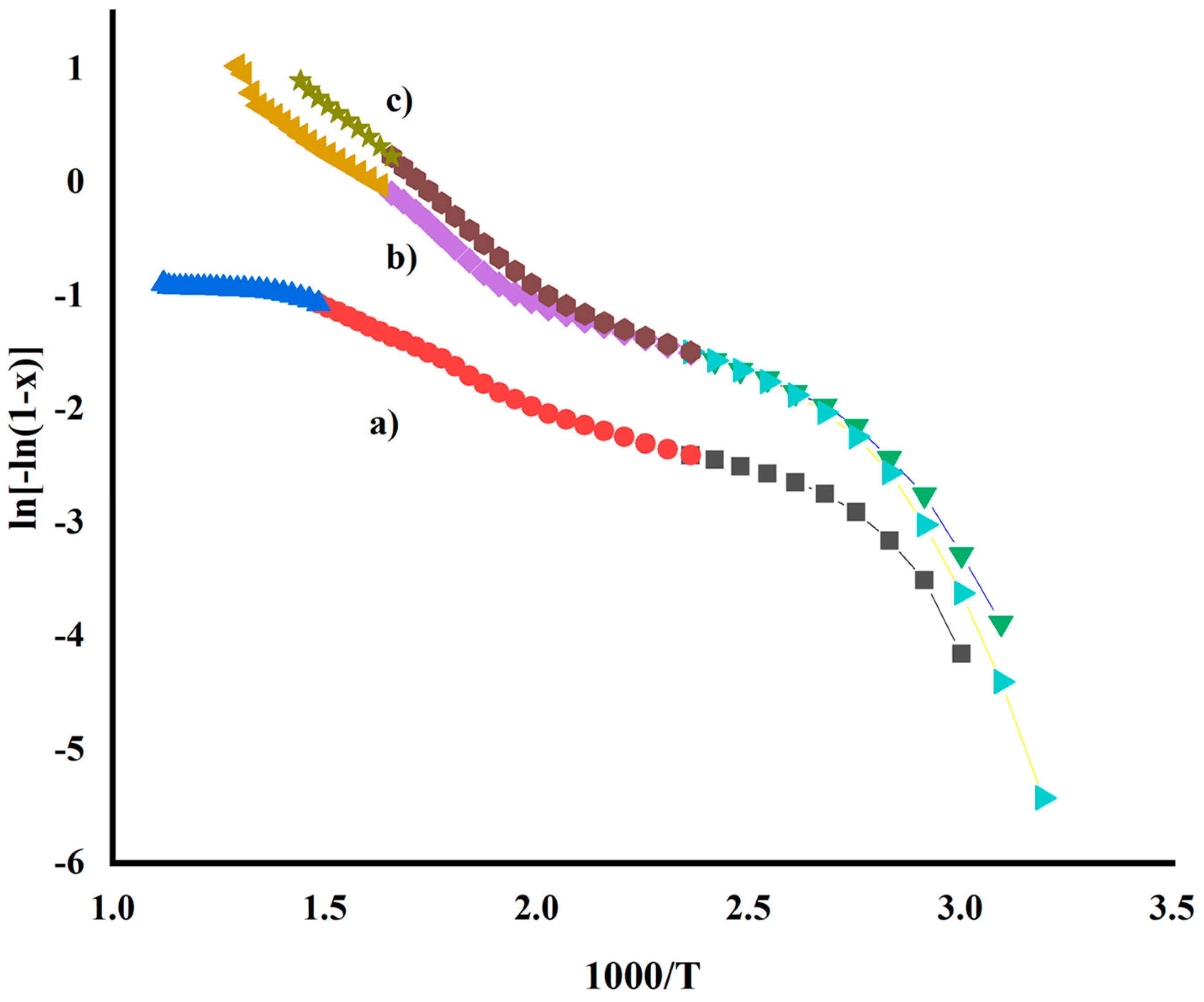
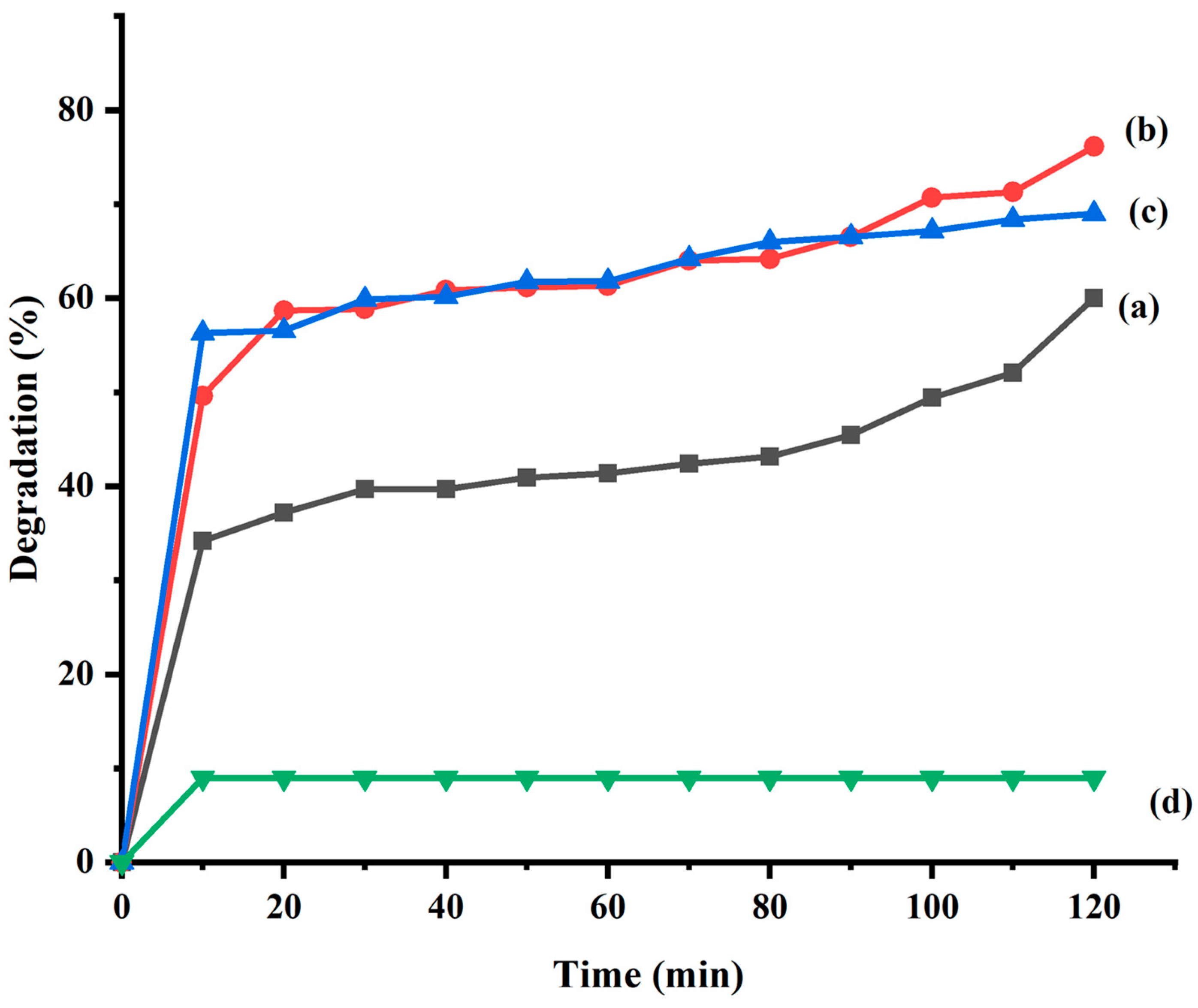
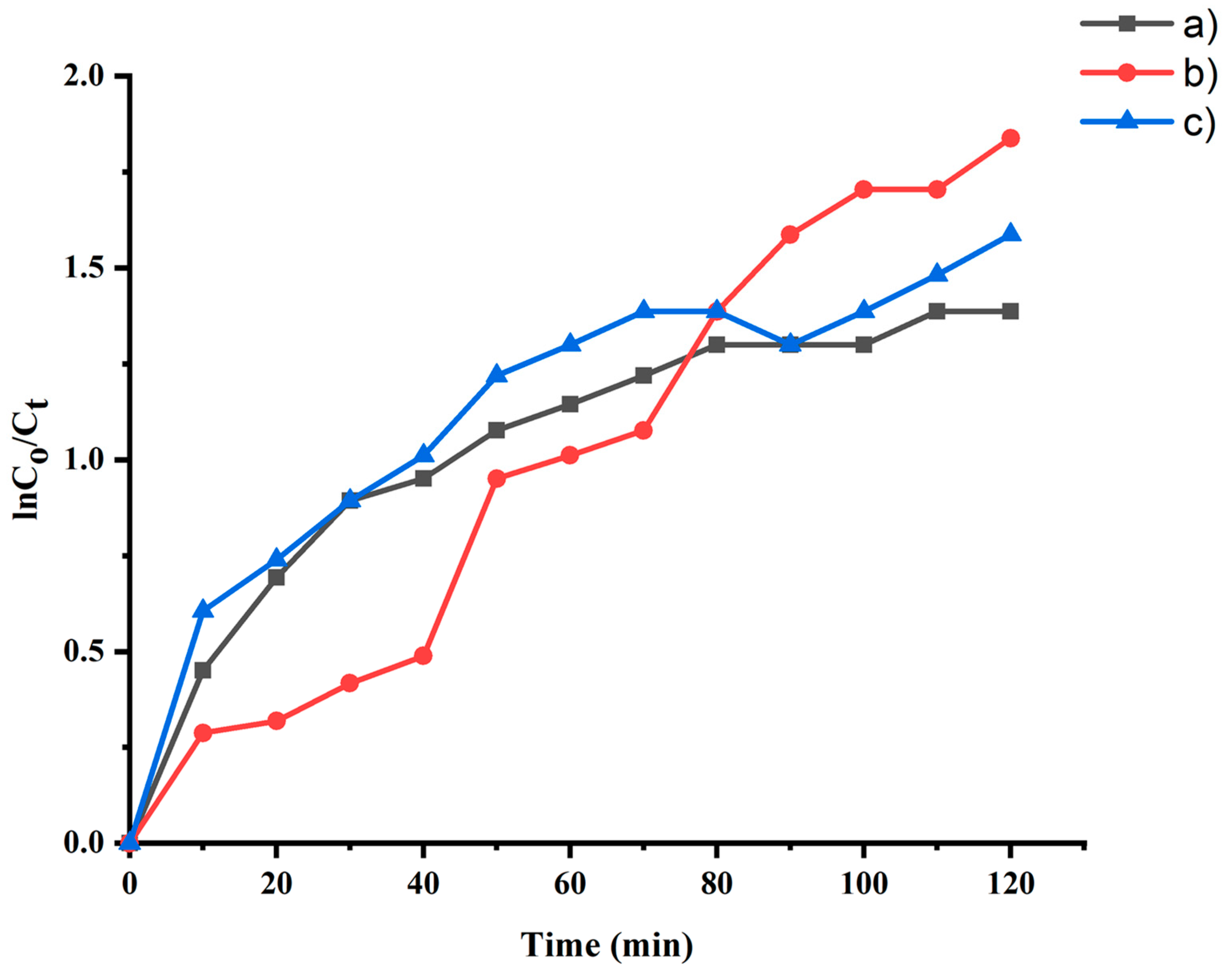
2.6. Photocatalytic Studies
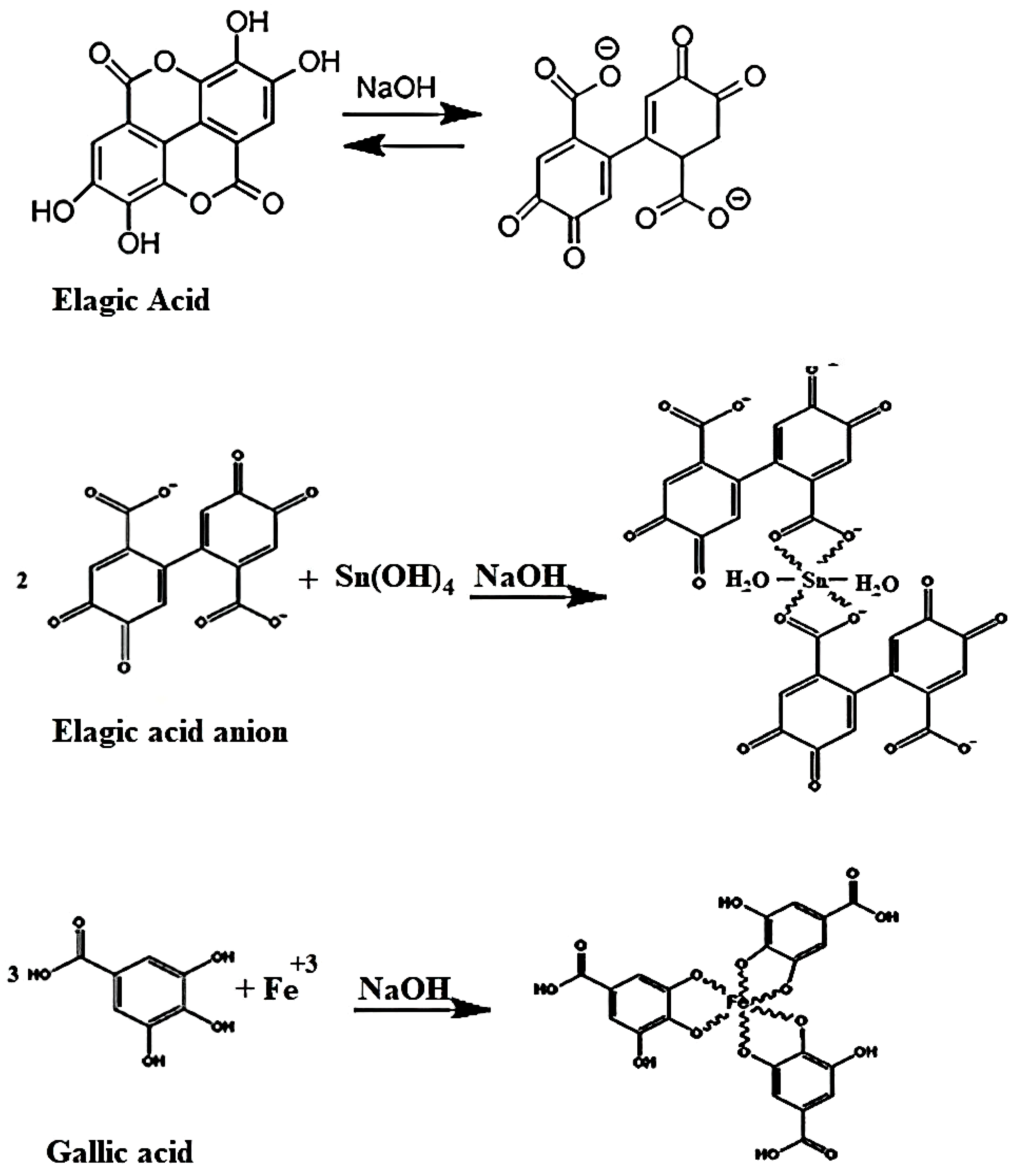
2.7. Mechanism of the Green Synthesis of FeSnO2 Nanocomposites
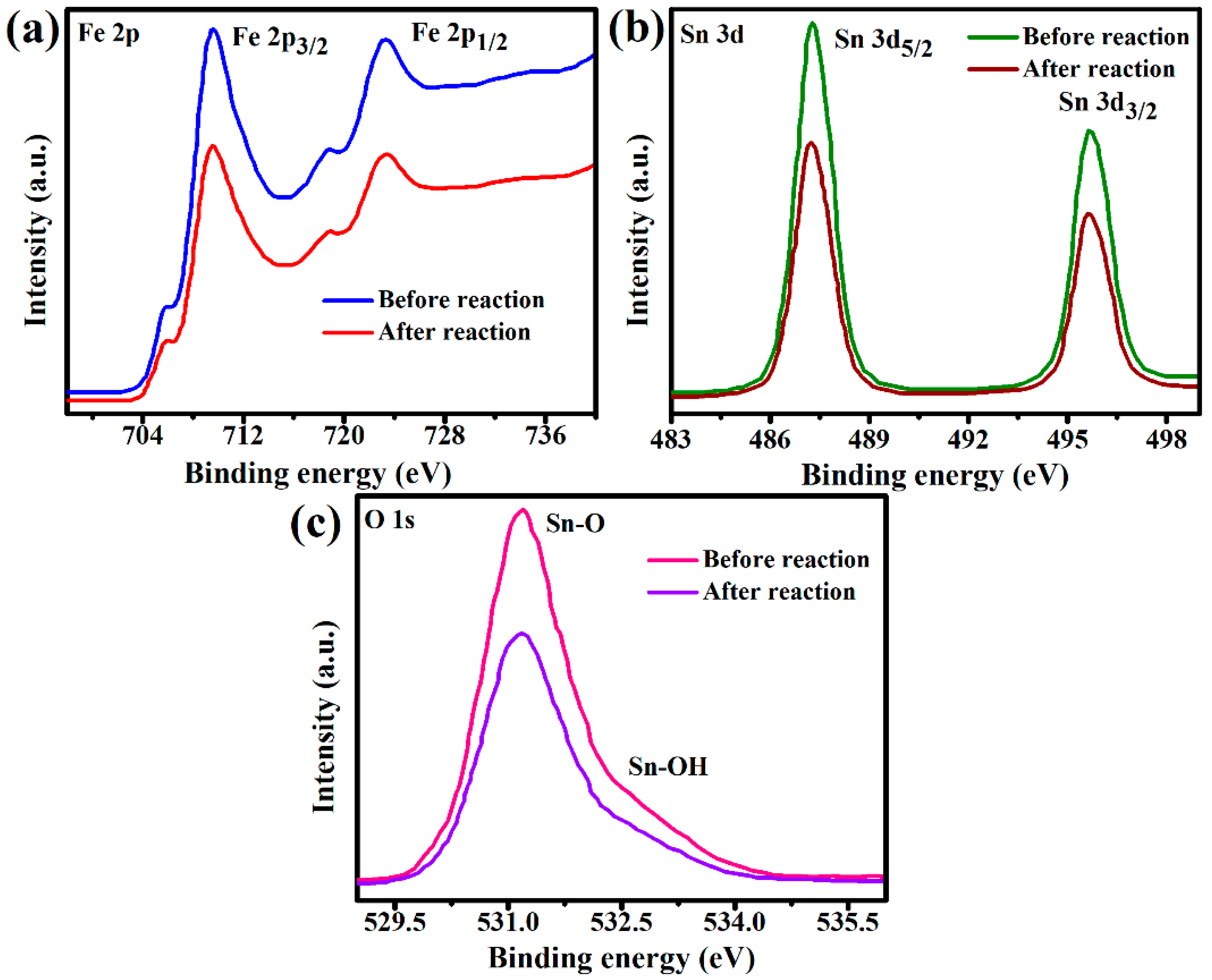
2.8. XPS Analysis
3. Experimental Details
3.1. Chemicals and Reagents
3.2. Preparation of Plant Extract
3.3. Synthesis of FeSnO2 Nanocomposite
3.3.1. FeSnO2 Nanocomposite Synthesis via Sol-Gel Technique
3.3.2. Green Route for the Synthesis of FeSnO2 Nanocomposite
3.4. Characterization Techniques
3.5. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies—A critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Šafařı, I.; Šafařı, M. Detection of low concentrations of malachite green and crystal violet in water. Water Res. 2002, 36, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Fosso-Kankeu, E.; Waanders, F.; Geldenhuys, M. Impact of nanoparticles shape and dye property on the photocatalytic degradation activity of TiO2. Int. J. Sci. Res. 2016, 5, 528–535. [Google Scholar]
- Roy, M.; Saha, R. Dyes and their removal technologies from wastewater: A critical review. Int. J. Health Policy Manag. 2015, 2, 160–166. [Google Scholar]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A.; Bahmanrokh, G.; Navasery, M.; Vaziri, P. Visible light-induced degradation of methylene blue in the presence of photocatalytic ZnS and CdS nanoparticles. Int. J. Mol. Sci. 2012, 13, 12242–12258. [Google Scholar] [CrossRef]
- Panimalar, S.; Uthrakumar, R.; Selvi, E.T.; Gomathy, P.; Inmozhi, C.; Kaviyarasu, K.; Kennedy, J. Studies of MnO2/g-C3N4 hetrostructure efficient of visible light photocatalyst for pollutants degradation by sol-gel technique. J. Surf. Interfaces 2020, 20, 100512. [Google Scholar] [CrossRef]
- Chikhale, L.; Shaikh, F.; Mulla, I.; Suryavanshi, S. Synthesis, structural and optical properties of Fe-doped nanocrystalline SnO2. J. Mater. Sci. Mater. Electron. 2017, 28, 12063–12069. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Bu, X.; Wu, Q.; Hang, Z.; Dong, Z.; Wu, X. Facile one-step synthesis of TiO2/Ag/SnO2 ternary heterostructures with enhanced visible light photocatalytic activity. Sci. Rep. 2018, 8, 10532. [Google Scholar] [CrossRef]
- Divya, J.; Pramothkumar, A.; Hilary, H.; Jayanthi, P.J. Impact of copper (Cu) and iron (Fe) co-doping on structural, optical, magnetic and electrical properties of tin oxide (SnO2) nanoparticles for optoelectronics applications. J. Mater. Sci. Mater. 2021, 32, 16775–16785. [Google Scholar] [CrossRef]
- Wei, K.; Gu, X.Y.; Chen, E.Z.; Wang, Y.Q.; Dai, Z.; Zhu, Z.R.; Kang, S.Q.; Wang, A.C.; Gao, X.P.; Sun, G.Z.; et al. Dissymmetric interface design of SnO2/TiO2 side-by-side bi-component nanofibers as photoanodes for dye sensitized solar cells: Facilitated electron transport and enhanced carrier separation. J. Colloid Interface Sci. 2021, 583, 24–32. [Google Scholar] [CrossRef]
- Tennakone, K.; Bandara, J.; Bandaranayake, P.K.M.; Kumara, G.R.A.; Konno, A. Enhanced efficiency of a dye-sensitized solar cell made from MgO-coated nanocrystalline SnO2. Jpn. J. Appl. Phys. 2001, 40, L732. [Google Scholar] [CrossRef]
- Lee, J.S.; Sim, S.K.; Min, B.; Cho, K.; Kim, S.W.; Kim, S. Structural and optoelectronic properties of SnO2 nanowires synthesized from ball-milled SnO2 powders. J. Cryst. Growth 2004, 267, 145–149. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Hu, Q.; Zhang, D.; Ma, Z.; Cheng, Z.; Xu, J. Morphology and size effect of Pd nanocrystals on formaldehyde and hydrogen sensing performance of SnO2 based gas sensor. J. Alloys Compd. 2022, 906, 163765. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, Q.; Ou, L.H.; Du, H.L.; Long, Y.; Zhen, W.J. Solid-Phase Synthesis of Non-metal (S, N)-Doped Tin Oxide Nanopowders at Room Temperature and its Photodegradation Properties for Wastewater of Biomass Treatment. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2748–2762. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–993. [Google Scholar] [CrossRef]
- Messaadi, C.; Ghrib, T.; Jalali, J.; Ghrib, M.; Alyami, A.A.; Gaidi, M.; Silvan, M.M.; Ezzaouia, H. Synthesis and characterization of SnO2-TiO2 nanocomposites photocatalysts. Curr. Nanosci. 2019, 15, 398–406. [Google Scholar] [CrossRef]
- Zhang, J.; Rahman, Z.U.; Zheng, Y.; Zhu, C.; Tian, M.; Wang, D. Nanoflower like SnO2-TiO2 nanotubes composite photoelectrode for efficient photocathodic protection of 304 stainless steel. Appl. Surf. Sci. 2018, 457, 516–521. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Hussein, H.S.; ALOthman, Z.A.; ALanazi, A.G.; Alsaiari, N.S.; Khalid, A. Green synthesis of Fe–Cu bimetallic supported on alginate-limestone nanocomposite for the removal of drugs from contaminated water. Polymers 2023, 15, 1221. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Rizvi, Z.R.; Shahzad; Farrukh, M.A. Neodymium oxide nanoparticles synthesis using phytochemicals of leaf extracts of different plants as reducing and capping agents: Growth mechanism, optical, structural and catalytic properties. J. Chin. Chem. Soc. 2022, 69, 462–475. [Google Scholar] [CrossRef]
- Wanakai, S.I.; Kareru, P.G.; Makhanu, D.S.; Madivoli, E.S.; Maina, E.G.; Nyabola, A.O. Catalytic degradation of methylene blue by iron nanoparticles synthesized using Galinsoga parviflora, Conyza bonariensis and Bidens pilosa leaf extracts. SN Appl. Sci. 2019, 1, 1148. [Google Scholar] [CrossRef]
- Iram, F.; Farrukh, M. Transferring Nanotechnology Concept towards Business Perspectives; Daya Publishing House: New Delhi, India, 2015; pp. 165–183. [Google Scholar]
- Fresno, F.; Tudela, D.; Coronado, J.M.; Fernández-García, M.; Hungría, A.B.; Soria, J. Influence of Sn4+ on the structural and electronic properties of Ti1−x Snx O2 nanoparticles used as photocatalysts. Phys. Chem. Chem. Phys. 2006, 8, 2421–2430. [Google Scholar] [CrossRef]
- Jain, V.; Shah, D.; Sonani, N.; Dhakara, S.; Patel, N. Pharmacognostical and preliminary phytochemical investigation of Lawsonia inermis L. leaf. Rom. J. Biol.-Plant Biol. 2010, 55, 127–133. [Google Scholar]
- Mohamed, T.S.; Refahy, L.A.-G.; Romeih, M.H.; Hamed, M.M.; Ahmed, H.O.; El-Hashash, M.A.-A. Chromatographic Isolation and Characterization of Certain Bioactive Chemical Ingredients of Phyllanthus emblica Extracts and Assessment of Their Potentials as Antiviral and Anticancer Agents. Egypt. J. Chem. 2022, 65, 179–192. [Google Scholar] [CrossRef]
- Gupta, A.; Bonde, S.R.; Gaikwad, S.; Ingle, A.; Gade, A.K.; Rai, M. Lawsonia inermis-mediated synthesis of silver nanoparticles: Activity against human pathogenic fungi and bacteria with special reference to formulation of an antimicrobial nanogel. IET Nanobiotechnol. 2014, 8, 172–178. [Google Scholar] [CrossRef]
- Masum, M.M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Naseem, T.; Farrukh, M.A. Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J. Chem. 2015, 2015, 912342. [Google Scholar] [CrossRef]
- Oda, Y.; Nakashima, S.; Kondo, E.; Nakamura, S.; Yano, M.; Kubota, C.; Masumoto, Y.; Hirao, M.; Ogawa, Y.; Matsuda, H. Comparison of lawsone contents among Lawsonia inermis plant parts and neurite outgrowth accelerators from branches. J. Nat. Med. 2018, 72, 890–896. [Google Scholar] [CrossRef]
- Elangovan, M.; Dhanarajan, M.; Elangovan, I.; Elangovan, M. Determination of bioactive compounds from the petroleum ether leaf extract of moringa oleifera and Phyllanthus emblica using GC-MS analysis. World J. Pharm. Res 2015, 4, 1284–1298. [Google Scholar]
- Huda, A.; Handoko, C.T.; Bustan, M.D.; Yudono, B.; Gulo, F. New route in the synthesis of Tin (II) oxide micro-sheets and its thermal transformation. Mater. Lett. 2018, 211, 293–295. [Google Scholar] [CrossRef]
- Perveen, H.; Farrukh, M.A.; Khaleeq-ur-Rahman, M.; Munir, B.; Tahir, M.A. Synthesis, structural properties and catalytic activity of MgO-SnO2 nanocatalysts. Russ. J. Phys. Chem. A 2015, 89, 99–107. [Google Scholar] [CrossRef]
- Wagini, N.H.; Soliman, A.S.; Abbas, M.S.; Hanafy, Y.A.; Badawy, E.-S.M. Phytochemical analysis of Nigerian and Egyptian henna (Lawsonia inermis L.) leaves using TLC, FTIR and GCMS. Plant 2014, 2, 27–32. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Uddin Khandaker, M.; Modafer, Y.; Almukhlifi, H.A.; Bazaid, A.S.; Aldarhami, A.; Alanazi, A.M.; Jefri, O.A.; Uddin, M.M.; et al. Biologically Reduced Zinc Oxide Nanosheets Using Phyllanthus emblica Plant Extract for Antibacterial and Dye Degradation Studies. J. Chem. 2023, 2023, 3971686. [Google Scholar] [CrossRef]
- Segueni, L.; Rahal, A.; Benhaoua, B.; Allag, N.; Benhaoua, A.; Aida, M. Iron doping SnO2 thin films prepared by spray with moving nozzle: Structural, morphological, optical, and type conductivities. Dig. J. Nanomater. Biostruct. 2019, 14, 923–934. [Google Scholar]
- Badger, R.M. The relation between internuclear distances and the force constants of diatomic molecules. Phys. Rev. 1935, 48, 284. [Google Scholar] [CrossRef]
- Kaya, S.; Chamorro, E.; Petrov, D.; Kaya, C. New insights from the relation between lattice energy and bond stretching force constant in simple ionic compounds. Polyhedron 2017, 123, 411–418. [Google Scholar] [CrossRef]
- Kaupp, M.; Danovich, D.; Shaik, S. Chemistry is about energy and its changes: A critique of bond-length/bond-strength correlations. Coord. Chem. Rev. 2017, 344, 355–362. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, M.; Liu, Z.; Sun, D.; Vardeny, Z.V.; Liu, F. Structural, electronic, and magnetic properties of tris (8-hydroxyquinoline) iron (III) molecules and their magnetic coupling with ferromagnetic surface: First-principles study. J. Condens. Matter Phys. 2016, 28, 176004. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Beheshtian, J.; Firooz, A.A. Synthesis of undoped and Fe nanoparticles doped SnO2 nanostructure: Study of structural, optical and electrocatalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 7568–7574. [Google Scholar] [CrossRef]
- Bashir, M.; Ali, S.; Farrukh, M.A. Green synthesis of Fe2O3 nanoparticles from orange peel extract and a study of its antibacterial activity. J. Korean Phys. Soc. 2020, 76, 848–854. [Google Scholar] [CrossRef]
- Eslami, S.; Ebrahimzadeh, M.A.; Biparva, P. Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron-overloaded mice, a thalassemia model. RSC Adv. 2018, 8, 26144–26155. [Google Scholar] [CrossRef] [PubMed]
- Ibarguen, C.A.; Mosquera, A.; Parra, R.; Castro, M.; Rodríguez-Páez, J. Synthesis of SnO2 nanoparticles through the controlled precipitation route. Mater. Chem. Phys. 2007, 101, 433–440. [Google Scholar] [CrossRef]
- Preethi, T.; Senthil, K.; Pachamuthu, M.P.; Balakrishnaraja, R.; Sundaravel, B.; Geetha, N.; Bellucci, S. Effect of Fe Doping on Photocatalytic Dye-Degradation and Antibacterial Activity of SnO2 Nanoparticles. Adsorp. Sci. Technol. 2022, 2022, 9334079. [Google Scholar] [CrossRef]
- Javaid, A.; Farrukh, M.A. Comparison of photocatalytic and antibacterial activities of allotropes of graphene doped Sm2O3 nanocomposites: Optical, thermal, and structural studies. J. Chin. Chem. Soc. 2023, 70, 32–45. [Google Scholar] [CrossRef]
- Ayed, L.; Cheriaa, J.; Laadhari, N.; Cheref, A.; Bakhrouf, A. Biodegradation of crystal violet by an isolated Bacillus sp. Ann. Microbiol. 2009, 59, 267–272. [Google Scholar] [CrossRef]
- Iamsaard, S.; Arun, S.; Burawat, J.; Sukhorum, W.; Wattanathorn, J.; Nualkaew, S.; Sripanidkulchai, B. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. J. Zhejiang Univ. Sci. B 2014, 15, 405–408. [Google Scholar] [CrossRef]
- Liu, Y.; RamaRao, N.; Miller, T.; Hadjipanayis, G.; Teplyakov, A.V. Controlling physical properties of iron nanoparticles during assembly by “click chemistry”. J. Phys. Chem. C 2013, 117, 19974–19983. [Google Scholar] [CrossRef]
- Chaudhary, G.; Goyal, S.; Poonia, P. Lawsonia inermis Linnaeus: A phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 91–98. [Google Scholar]
- Khan, K.H. Roles of Emblica officinalis in medicine—A review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- De Siqueira Leite, K.C.; Garcia, L.F.; Sanz, G.; Colmati, F.; de Souza, A.R.; da Costa Batista, D.; Luque, R. Electrochemical characterization of a novel nimesulide anti-inflammatory drug analog: LQFM-091. J. Electroanal. Chem. 2018, 818, 92–96. [Google Scholar] [CrossRef]
- López Alarcón, C.I.; Pérez Cruz, F.; Villamena, F.; Zapata Torres, G.; Das, A.; Headley, C.; Quezada, E.; Olea Azar, C. Selected hydroxycoumarins as antioxidants in cells: Physicochemical and reactive oxygen species scavenging studies. J. Phys. Org. Chem. 2013, 26, 773–783. [Google Scholar]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Al-Saeedi, S.I.; Areej, A.; Qamar, M.T.; Alhujaily, A.; Iqbal, S.; Alotaibi, M.T.; Aslam, M.; Qayyum, M.A.; Bahadur, A.; Awwad, N.S. Isotherm and kinetic studies for the adsorption of methylene blue onto a novel Mn3O4-Bi2O3 composite and their antifungal performance. Front. Environ. Sci. 2023, 11, 500. [Google Scholar] [CrossRef]





| Samples | Fe-O | Sn-O | ||
|---|---|---|---|---|
| Calculated | Literature | Calculated | Literature | |
| FeSnO2 synthesized via the sol-gel method | 2.28 | 1.92–1.99 [39] | 2.43 | 2.3 [40] |
| FeSnO2 synthesized via the green method using Lawsonia inermis leaf extract | 2.29 | 2.42 | ||
| FeSnO2 synthesized via the green method using Phyllanthus embillica leaf extract | 2.28 | 2.43 | ||
| Samples | Phase | Temp (K) | Rate Constant (k) (min−1) | Half-Life (min) | Ea (Jmol−1 × 103) | ΔH (Jmol−1 × 103) | ΔS (Jmol−1 K−1) | ΔG (Jmol−1 × 103) |
|---|---|---|---|---|---|---|---|---|
| Uncalcined FeSnO2 from sol-gel | 1 | 423 | 0.017 | 39.10 | 20.15 | 16.63 | −263.23 | 16.90 |
| 2 | 673 | 0.021 | 32.48 | 13.44 | 7.84 | −296.77 | 8.14 | |
| 3 | 893 | 0.004 | 170.68 | 2.77 | 4.64 | −323.49 | 4.32 | |
| Uncalcined FeSnO2 using Lawsonia inermis leaf extract | 1 | 393 | 0.041 | 16.61 | 36.08 | 32.81 | −206.03 | 33.01 |
| 2 | 593 | 0.063 | 10.94 | 14.34 | 9.413 | −282.96 | 9.69 | |
| 3 | 753 | 0.159 | 4.35 | 14.88 | 14.88 | −272.56 | 15.15 | |
| Uncalcined FeSnO2 using Phyllanthus embillica leaf extract | 1 | 423 | 0.042 | 16.50 | 36.29 | 32.78 | −210.22 | 32.99 |
| 2 | 603 | 0.112 | 6.18 | 21.01 | 15.99 | −265.69 | 16.26 | |
| 3 | 693 | 0.260 | 2.66 | 19.41 | 19.41 | −259.48 | 19.67 |
| Samples | Crystallite Size (nm) SizeWH (nm) SizeSEM nm | Strain ε | Band Gap (eV) | Dislocation Density (δ) (nm)−2 | SSA (m2g−1) | ||
|---|---|---|---|---|---|---|---|
| Schrrer W-H | |||||||
| FeSnO2 synthesized via the sol-gel method | 29.49 | 20.85 | 1.2 × 10−2 | 3.99 | 1.15 × 10−3 | 27.45 | |
| FeSnO2 synthesized via the green method using Lawsonia inermis leaf extract | 14.54 | 11.30 | 4.2 × 10−3 | 3.10 | 4.73 × 10−3 | 55.68 | |
| FeSnO2 synthesized via the green method using Phyllanthus embillica leaf extract | 20.43 | 14.86 | 9.5 × 10−3 | 3.89 | 2.39 × 10−3 | 39.63 | |
| Sr. No | Samples | Band Gap (eV) | Degradation Efficiency (%) | Time (min) | Dye Used | Reference |
|---|---|---|---|---|---|---|
| 1 | 4% Fe doped SnO2 | 2.65 | 87.2 | 200 | Methyl orange | [45] |
| 2 | Fe doped TiO2- SnO2 | - | 15.24 | 60 | Methylene blue | [23] |
| 3 | FeSnO2 (sol-gel) | 3.89 | 66 | 120 | Methylene blue | This study |
| 4 | FeSnO2 (Lawsonia inermis leaves) | 3.10 | 76 | 120 | Methylene blue | This study |
| 5 | FeSnO2 (Phyllanthus embillica leave) | 3.99 | 69 | 120 | Methylene blue | This study |
| 6 | FeSnO2 (sol-gel) | 3.89 | 75 | 120 | Crystal violet | This study |
| 7 | FeSnO2 (Lawsonia inermis leaves) | 3.01 | 84 | 120 | Crystal violet | This study |
| 8 | FeSnO2 (Phyllanthus embillica leaves) | 3.99 | 81 | 120 | Crystal violet | This study |
| Phyto Chemicals | Chemical Formula | Structural Formula | Redox Potential (V) | Reference |
|---|---|---|---|---|
| Scopoltin | C10H8 O4 | 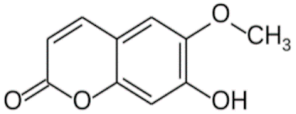 | 0.737 | [52,53] |
| Ascorbic Acid | C6H8O6 | 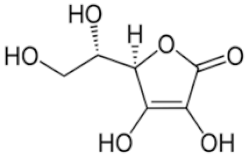 | 0.015 | [54] |
| Ellagic acid | C14H6O8 | 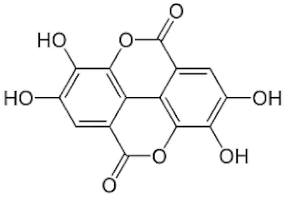 | 0.187 | [54] |
| Gallic Acid | C7H6O5 | 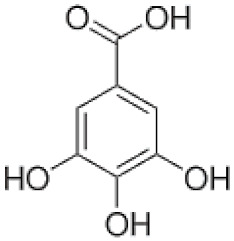 | 0.267 | [21] |
| Sample Code | Synthesis of FeSnO2 Nanocomposite Using | Method |
|---|---|---|
| (a) | NaOH | sol-gel |
| (b) | Lawsonia inermis leaf extract | Green synthesis |
| (c) | Phyllanthus embilica leaf extract | Green synthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaukat, A.; Farrukh, M.A.; Chong, K.-K.; Nawaz, R.; Qamar, M.T.; Iqbal, S.; Awwad, N.S.; Ibrahium, H.A. The Impact of Different Green Synthetic Routes on the Photocatalytic Potential of FeSnO2 for the Removal of Methylene Blue and Crystal Violet Dyes under Natural Sunlight Exposure. Catalysts 2023, 13, 1135. https://doi.org/10.3390/catal13071135
Shaukat A, Farrukh MA, Chong K-K, Nawaz R, Qamar MT, Iqbal S, Awwad NS, Ibrahium HA. The Impact of Different Green Synthetic Routes on the Photocatalytic Potential of FeSnO2 for the Removal of Methylene Blue and Crystal Violet Dyes under Natural Sunlight Exposure. Catalysts. 2023; 13(7):1135. https://doi.org/10.3390/catal13071135
Chicago/Turabian StyleShaukat, Arifa, Muhammad Akhyar Farrukh, Kok-Keong Chong, Rabia Nawaz, Muhammad Tariq Qamar, Shahid Iqbal, Nasser S. Awwad, and Hala A. Ibrahium. 2023. "The Impact of Different Green Synthetic Routes on the Photocatalytic Potential of FeSnO2 for the Removal of Methylene Blue and Crystal Violet Dyes under Natural Sunlight Exposure" Catalysts 13, no. 7: 1135. https://doi.org/10.3390/catal13071135
APA StyleShaukat, A., Farrukh, M. A., Chong, K.-K., Nawaz, R., Qamar, M. T., Iqbal, S., Awwad, N. S., & Ibrahium, H. A. (2023). The Impact of Different Green Synthetic Routes on the Photocatalytic Potential of FeSnO2 for the Removal of Methylene Blue and Crystal Violet Dyes under Natural Sunlight Exposure. Catalysts, 13(7), 1135. https://doi.org/10.3390/catal13071135