Abstract
Photocatalysis is an advanced oxidation process that is an environmentally friendly option and one of the most critical technologies in green chemistry today. This work studied the upscaling of photocatalysis as a suitable process for wastewater treatment to remove emerging pollutants. For this purpose, unsupported and supported TiO2 photocatalysts were tested in the photodegradation of ciprofloxacin as a model molecule of an emerging wastewater component, using visible, UV radiation, and solar light. The suitability of TiO2 as a photocatalyst to decompose ciprofloxacin was confirmed in batch photoreactor under Visible and UV radiation, with degradation rates up to 90% after 30 min of irradiation and low adsorption values. TiO2 as a photocatalyst coated in glass support material at the packed bed photoreactor showed good photoactivity for emergent contaminants degradation (95%) under solar radiation. It has been possible to verify that the photocatalytic reactor system constitutes a viable process for eliminating emerging contaminants through environmentally sustainable treatments. Our results corroborate the possibility of degrading emerging contaminants by solar radiation using a packed bed photoreactor, providing a more effective option from a practical and economical point of view for wastewater effluent treatments.
1. Introduction
According to the World Health Organization (WHO), over 2000 million people used a contaminated drinking water source in 2020. Moreover, climate change and population growth are expected to exacerbate this dramatic situation. On 23 October 2000, the European Parliament and Council adopted the EU Water Framework Directive (WFD—2000/60/EC), establishing a community action in water policy. In this context, at the beginning of 2022 (19 January), the EU Commission updated the “watch list” for emerging water pollutants [1,2,3,4]. The study of emerging contaminants, including pharmaceutical compounds, has become one of the biggest concerns in current environmental research. Pharmaceutical and personal care products are organic micropollutants present in wastewater and the aquatic environment due to their wide use in human and veterinary medicine.
Conventional wastewater purification treatments are inefficient for the complete removal of emerging contaminants. The composition profile and levels of concentration found in wastewater vary depending on the country and consumption of pharmaceutical and personal care products. The lethal effects suggest a low ecotoxicological risk, although considering sublethal and chronic effects the situation is more serious. Aquatic organisms are exposed to low concentrations continuously during their life cycle, observing adverse associated effects (e.g., on growth and reproduction, morphological alterations, and genotoxicity, among others). Although these pollutants do not yet pose an immediate risk to humans, are being incorporated into lists of drinking water quality standards.
Fluoroquinolones (FQs) are a class of broad-spectrum antibiotics that are commonly used in both human and veterinary medicine. They inhibit key bacterial enzymes that are involved with unwinding the DNA helix for replication and transcription. FQs are widely used in Europe and are not readily biodegradable by microorganisms [5]. They are not completely metabolized in the human body and approximately 20–90% of FQs ingested are excreted in their pharmacologically active forms, which leads to significant loads being discharged into domestic sewage [6].
Furthermore, conventional wastewater treatment in wastewater treatment plants generally results in prolonged exposure of wastewater-borne bacteria to higher concentrations of emergent pollutants than those present in treated effluents because of the extended biomass solid retention times at which plants usually operate. Extended exposure of bacterial communities to minimal inhibitory concentrations levels of some emergent pollutants (antibacterial compounds) can result in a bad environment for developing wastewater treatment.
Ciprofloxacin (CIP), a second-generation FQ and one of the most prescribed drugs in the world is a broad-spectrum antibiotic (effective against gram-positive and gram-negative bacteria) and it has been regularly found in wastewater at levels that could induce bacterial resistance. Therefore, there are some incentives for removing CIP, as well as other FQs and pharmaceuticals, from wastewater. To achieve this objective some treatment technologies capable of selectively and efficiently eliminating emergent pollutants must be implemented. Advanced oxidation processes are viable treatment methods for degrading or completely removing CIP and other emergent pollutants from drinking water and wastewater.
Removing these new water pollutants means a challenge to traditional wastewater treatments [7,8,9,10]. Thus, it is mandatory to develop and study modern and more efficient water cleaning technologies that ensures the removal of these water pollutants, mineralizing them, and keeping the quality of drinking water and aquatic environments for future generations [11,12].
In recent years, photocatalysis has become a promising approach to decomposing water pollutants [13,14,15,16,17,18]. This fact is based on several factors, such as its low cost, easy operation without side products, and promising efficiency in converting pollutants into CO2 and H2O [19,20]. This advanced oxidation process (AOP) consists of the degradation of the pollutants through the reaction with a hydroxyl radical (·OH), generated in the presence of a light source, water, and a photo-active catalyst [21,22]. Figure 1 shows a simplified scheme of the photocatalytic reaction mechanism between the hydroxyl radical and pollutants [23]. Photocatalytic degradation starts with the activation and migration of electrons from the valence band to the conduction band when the semiconductor material, acting as a photocatalyst, is illuminated under the same energy as the bandgap of the photocatalytic material. In the second stage, the positive holes in the valence band generate hydroxyl radicals from the water molecules. Due to their high oxidizing properties, these radicals decompose the organic molecules (normally pollutants in wastewater). In addition, the excited electrons in the valence band react with dissolved oxygen species, able to decompose any substance adsorbed on the photocatalytic surface [24]. Similar to other catalytic processes with environmental purposes, the catalyst plays the most significant role [25].
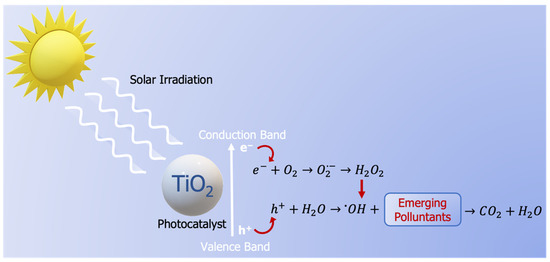
Figure 1.
Mechanism schema of photocatalytic degradation of organic pollutants into CO2 and H2O in a TiO2 catalyst.
In photocatalysis, metal-transition oxides such as TiO2, ZnO, Fe2O3, SnO, and V2O5 have been pointed as the most photo-active materials due to their physicochemical properties, together with high chemical stability and non-toxicity [26,27,28,29,30]. TiO2, with a bandgap of 3.2 eV, is one of the most studied photocatalysts in the last years, with a broad range of organic compounds such as hydrocarbons, and aromatic compounds, among others. However, despite its significant activity, there are several limitations of this material, such as low surface area and poor sensitivity under solar irradiation [31,32,33,34], necessary to overcome by implementing new operational strategies. In this context, the immobilization of the TiO2 over support by a hydrothermal method seems to be the most feasible one. This way allows for the reusing of the catalytic materials from the treated effluent stream and the continuous operation, which are critical factors from an industrial point of view and scale-up [35,36]. Nevertheless, most of the current photocatalytic tests are only performed in batch photoreactors [37,38,39,40,41,42]. This experiment stage is the first step of catalyst testing. However, it is mandatory to develop an accessible and valuable packed bed reactor system that allows scaling and studying the possible industrial applications of photocatalytic materials.
This study builds upon our previous investigations in the field of photocatalysis [23,43,44,45] aiming to advance the understanding of removing emerging water pollutants. To achieve this objective, we have developed a novel and optimized packed bed photoreactor. For this purpose, we impregnated TiO2 in a glass of several geometries using artificial UV/visible light and natural sunlight to treat polluted water with ciprofloxacin (an emergent pollutant in wastewater) in a laboratory and pilot plant scale. Our results show the viability of the packed bed reactor proposed to test photocatalytic materials to decompose emerging water pollutants, supporting the upscaling of photocatalysis at the industrial level.
2. Results and Discussion
2.1. Adsorption Properties of the Photocatalytic Material
As mentioned in the references of this field of study [18], water pollutants can be adsorbed on the surface of some materials with adsorbent properties, decreasing its content in the wastewater. However, it is necessary to distinguish between the adsorption phenomenon and the photocatalytic activity of the material to corroborate the total degradation (mineralization to CO2 + H2O) of the pollutant. Figure 2 shows the pollutant decrease in the absence of any light source in the unsupported (i.e., batch photoreactor) and supported TiO2 (i.e., Raschig cylinders and rings) photocatalytic materials.
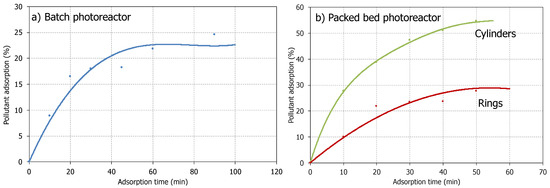
Figure 2.
Pollutant adsorption capacity in the absence of a light source. (a) Batch photoreactor (photocatalytic material as TiO2 suspension); (b) Packed bed photoreactor (photocatalytic bed configured by TiO2 supported-Raschig cylinders or TiO2 supported- rings.
From the data in Figure 2, it is clear that there is a decrease in ciprofloxacin content in wastewater due to the adsorption effect on the catalytic surfaces. The higher values were found when the packed bed photoreactor was tested, particularly with the glass cylinders, which offer a higher surface available to support the photocatalytic active material.
The extent of adsorption of the emergent contaminant onto the photocatalytic material does not directly correlate with pollutant degradation, as the adsorbed molecules may persist on the catalytic surface without undergoing degradation. In this way, an FT-IR analysis of the material surface would reveal the presence of the pollutant adsorbed [45]. A comparison between the emergent pollutant content decrease in wastewater during photocatalytic degradation by activation with the light of the semiconductor photocatalytic material might allow distinction between both phenomena; being the complete degradation of the contaminant the desired phenomenon.
2.2. Degradation by Photolysis
Before evaluating the photocatalytic activity of the studied material, it is also necessary to determine the photolytic effect of the light sources on the ciprofloxacin molecule. This series of experiments are considered blank runs, simulating the illumination operating conditions of the photocatalytic tests but without any photocatalyst addition. Figure 3 shows the contaminant degradation by photolysis under UV and visible radiation in the batch photoreactor and under natural solar light in the packed bed photoreactor.
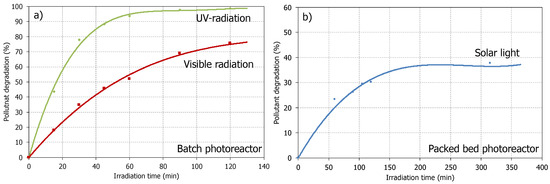
Figure 3.
Pollutant degradation by photolysis. (a) Batch photoreactor; (b) Packed bed photoreactor.
At the laboratory scale, UV-radiation (Hg-lamp) seems to be more efficient than visible radiation from a lamp [46,47], with a pollutant degradation of up to 95% after 80 min of irradiation. However, as confirmed by the packed bed photoreactor experiments, the solar light was not enough to degrade the ciprofloxacin, with a value lower than 40% after more than 5.5 h of irradiation at the studied operating conditions. This fact is indicative that most of the contaminant content would remain in the wastewater, affecting the environment in which it would be in contact.
According to these results, it is possible to claim the need for catalytic materials that accelerate the pollutant degradation processes to decrease the effect of these emergent contaminants on the environment. Moreover, it is necessary to implement an accessible and valuable packed bed reactor system that allows studying possible photocatalytic materials at operating conditions similar to industrial needs and operations.
2.3. Photocatalytic Activity
Based on references [18], it is expected that the use of TiO2 as a photocatalyst will significantly increase pollutant degradation at laboratory and pilot plant scales. At the same operating conditions as the photolysis experiments, the unsupported (TiO2 in a powder suspension in wastewater) and supported TiO2 (configured in a packed bed photoreactor) were tested. Figure 4 shows the pollutant degradation in the batch reactor (under UV and visible radiation) and using the catalytic material in a packed bed configuration (under solar radiation).
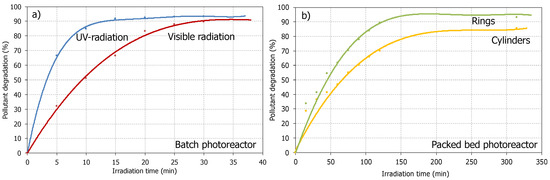
Figure 4.
Pollutant degradation by photocatalysis: (a) Batch photoreactor (using several radiation sources: UV or Visible lamps); (b) Packed bed photoreactor (TiO2 supported on Raschig cylinders or TiO2 supported on rings).
As mentioned in the photolysis study, the photocatalytic activity in the batch reactor strongly depended on the type of radiation. In this way, pollutant degradation under UV radiation was much faster than under visible radiation. This behavior was expected considering the nature of UV radiation [47] and its role in the activation of the semiconductor material used as a photocatalyst. Thus, after 15 min (25 min for visible radiation), the pollutant content in wastewater decreased to up to 90%, which is a significant degradation rate.
On the other hand, the experiments developed using the packed bed photoreactor configuration seem to need more contact to degrade the pollutant at the same percentage as a laboratory scale. It makes sense considering the unit’s configuration, which is more complex and with higher volumes of wastewater to process. Nevertheless, the obtained pollutant degradation rates were always similar, with values up to 95% with TiO2 supported in a packed bed. The most striking observation to emerge from the data analysis was the faster degradation of the pollutant in the glass rings than in the cylinders. This finding suggests that the packed bed configured with rings offers better light distribution conditions into the bed than the packed bed with cylinders [48], increasing the light available to activate more photocatalytic material, the contact area among substances and providing better conditions for the photocatalytic pollutant degradation in a pilot plant scale.
To distinguish between adsorption, photolysis, and photocatalytic reaction phenomena, Figure 5 shows the pollutant adsorption in the photocatalytic surface and the emergent pollutant degradation by photolysis and photocatalysis.
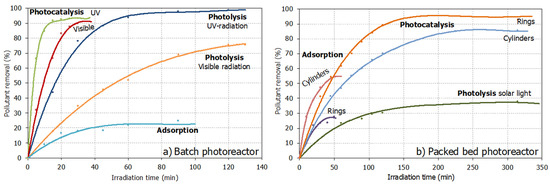
Figure 5.
Emerging pollutant removal in wastewater: (a) Batch photoreactor; (b) Packed bed photoreactor. (Operating conditions described in Table 1).
At batch scale experiments, the presence of TiO2 significantly promotes emergent contaminant degradation in wastewater, decreasing the ciprofloxacin content in wastewater at low irradiation times. This result matches those of references studying the photocatalytic effect of TiO2 for some other emerging pollutants degradation [13,14,15,16,17,18]. The kind of radiation (UV or visible) plays the most critical role in photocatalytic kinetics [47], increasing the pollutant degradation rate when UV radiation is used, as could be expected for TiO2 acting as a photocatalyst. Comparing the photocatalytic and photolytic phenomena, it is also clear that the presence of TiO2 significantly increases the degradation ratio, which confirms the photocatalytic activity.
As mentioned in the bibliography, titanium oxide is a photocatalyst that is used in powder form, with the consequent problems that this can cause when separating the photocatalyst from the treated wastewater effluent. Therefore, a solution to this separation problem is the use of supported TiO2.
The selection of support for the photocatalytic active material must meet a series of requirements and properties. It must favor a strong physical-chemical interaction with TiO2 without negatively affecting the chemical properties of the photocatalyst. It must be structured to favor its separation from the water to be treated. It must have a suitable geometry so that the configuration of the reactor facilitates the mass transfer processes. It should be transparent, ideally, to radiation. It should have a high surface area and a high adsorption capacity for the pollutant to be treated and it must be chemically inert.
For this reason, the aim of this work was to test the photocatalytic material immobilized on support as an adequate configuration for a packed bed photoreactor with continuous flow operation, avoiding the subsequent filtrations necessary to separate the photocatalyst from the effluent if a suspended photocatalyst is used. In addition, the use of catalytic material in a packed bed would avoid deterioration problems in the photocatalyst particles.
When the photocatalytic active material, TiO2, is supported on glass structures, the photocatalytic activity was also significant under solar radiation using the packed bed photoreactor. At this scale, the photocatalytic activity is much more noticeable than adsorption or photolysis phenomena, with a photocatalysis activity of up to 95% by using the rings after 300 min of solar irradiation.
The experiments developed using the packed bed photoreactor configuration seem to need more contact to degrade the pollutant at the same percentage as the batch photoreactor. It makes sense considering the unit’s configuration, which is more complex and with higher volumes of wastewater to process. Furthermore, it is well known that TiO2 requires UV radiation to overcome the band gap, and in solar radiation, only a small portion is UV [43,45,49,50]. Under these findings, it is possible to claim that the used pilot plant schema was valid for establishing a suitable photocatalytic system for wastewater treatment [49].
3. Materials and Methods
3.1. Feedstocks and Photocatalytic Material
The feedstock consisted of synthetic wastewater (pH in the neutral range) containing 50 mg/L of ciprofloxacin (Alter—500 mg film-coated tablets) as a model molecule of emerging contaminants (considered as an endocrine disrupter and microbial growing inhibitory), compounds significantly discharged and accumulated in domestic wastewater and that have been shown to pass through conventional wastewater treatments without being degraded. The studied concentration is even higher than the usual found in real wastewater effluents [51]. The photocatalyst consisted of TiO2 (Degussa P25) powder form (SBET 50 m2/g and mean particle size 21 nm). This photocatalytic material has been widely described in the bibliography [23,43,44,45] (including XRD, XPS, and Hg-porosimetry), so here it is only described the impregnation process into glass Raschig cylinders and rings and pilot plant operation.
The first step of impregnation consisted of increasing the superficial area and rugosity of the glass structures serving as catalytic support. For this purpose, the material acting as support was dipped in acetic acid 96% for 72 h. After washing with distilled water, the supports were dried at 105 °C. Later, they were dipped in a suspension of TiO2 in water/acetone (20 g of TiO2, 200 mL of water, and 4 mL of acetone) and dried for 30 min at 350 °C. This part of the process was repeated two times to obtain a thicker TiO2 layer on the surface of the supports [52]. Figure 6 shows rings and Raschig cylinders after TiO2 impregnation.
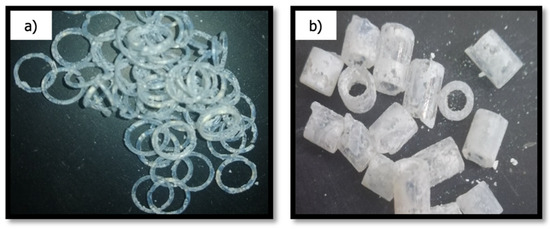
Figure 6.
Structures impregnated with TiO2 to configure the packed bed photoreactor: (a) Rings; (b) Raschig cylinders.
3.2. Experimental Setup
The photocatalytic experiments were carried out on both: bench-top and pilot plant scales. The bench-top experiments aimed to evaluate the suitability of TiO2 to decompose ciprofloxacin as a model for an emerging contaminant in wastewater. The bench-top unit consisted of a stirred batch photoreactor of 500 mL (1000 rpm) with a thermometer for manual temperature control and an air inlet to optimize oxidative conditions. The light source consisted of a 150 W medium pressure Hg vapor lamp (Heraeus TQ-150, 150 W—Hanau, Germany) for UV-radiation experiments and a 150 W Xe-lamp (Hamamatsu L2274, Ammersee, Germany) for simulating solar radiation (visible range). Figure 7 shows a schematic diagram of the unit used in these experiments.
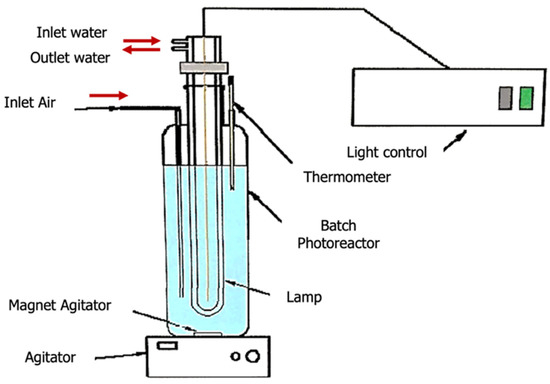
Figure 7.
Schematic diagram of the experimental unit used for photocatalytic reaction study at bench-top scale.
The experiments on the pilot plant photoreactor aimed to evaluate the suitability of a packed bed photoreactor configuration to study the photocatalytic behavior of supported photocatalytic material under a recirculation regime. This unit consisted of a packed bed quartz reactor (18 cm length and 1 cm outlet diameter) filled with glass Raschig cylinders or glass rings coated with photo-catalytically active material, TiO2. The photoreactor unit was placed on the reflecting surface with an inclination of 45°. The polluted water was pumped from a stirred deposit with an oxygen supply and covert by foil to protect it from solar radiation. The photocatalytic system structure has the capacity of up to six quartz-packed bed photoreactors, so it is possible to increase the capacity of the wastewater decontamination treatment system, or duplicate experiments if required. In this pilot plant, the light source consisted of solar radiation towards the photoreactors and indirect reflection to activate all the photocatalytic particles inside the bed. Figure 8 shows a schematic diagram and some photographs of the photoreactor unit.
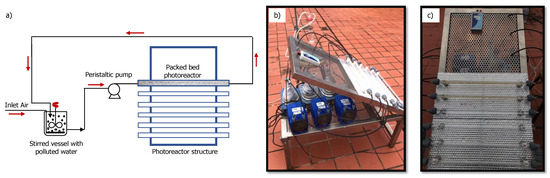
Figure 8.
Packed bed photoreactor: (a) schematic diagram; (b) photo of packed bed photoreactor system—side detail; (c) photo of packed bed photoreactor system—front detail.
3.3. Photocatalytic Tests
Photocatalytic experiments were performed in order to evaluate the activity of the TiO2 supported on glass structures, specifically for the degradation of emergent contaminants in wastewater. The first point consisted of evaluating the adsorption properties of the photocatalytic material, capturing the pollutant on its surface. Later, the photolysis activity has to be evaluated without the intervention of photocatalysts. The last experimental step evaluates the photocatalytic activity under an artificial light source and solar radiation.
For the bench-top scale experiments, the photocatalytic activity (including adsorption and photolysis) was evaluated using the stirred bath photoreactor with 250 mL of the synthetic wastewater (50 mg/L of ciprofloxacin) and 0.2 g of TiO2 (Degussa P25) as a suspension in water. The reaction time changed depending on the aim of the experiment stage (adsorption, photolysis, or photocatalysis). For the pilot plant scale experiments, the synthetic wastewater was continuously pumped into the packed bed photoreactors with a flow rate of 0.005 L/s. Photocatalytic experiments were performed simultaneously with both TiO2-coated structures in two separated packed bed photoreactor systems, using filling the photocatalytic packed bed reactor with glass cylinders and glass rings (in the range of 16–22 g). All the experiments were performed at ambient temperature and Solar radiation was measured and monitored during pilot plant scale experiments. Table 1 and Table 2 gather the experiments performed and operating conditions details.

Table 1.
Description of tests and operating conditions.

Table 2.
Solar radiation during Packed-bed experiments (CMP-21 pyranometer, Kipp and Zonen, Holland).
The solar radiation-packed bed experiments were carried out under variable atmospheric weather to study real operating conditions when developing the photocatalytic treatment under solar radiation and with insolation and variable cloudiness.
During each experimental run, wastewater aliquots were sampled every 15 min (5 min in case of 0.5 h time duration experiments) to monitor the pollutant degradation. Then, the sample was centrifugated to remove solids particles in some cases and analyzed by using a UV-Visible spectrophotometer (Varian—Cary 50 UV-Vis Spectrophotometer, Agilent Technologies, Santa Clara, CA, USA). The instrument was calibrated at different ciprofloxacin concentrations in the range of 1–20 mg/L to determine the concentration based on the absorbance. The absorption spectrum of the emergent contaminant was performed in a range between 200 and 400 nm, resulting in the maximum absorption peak of ciprofloxacin corresponding to a wavelength value of 275 nm [53,54].
4. Conclusions
This work studied the upscaling of photocatalysis as a suitable process for wastewater treatment to remove emerging pollutants and non-degradable contaminants by conventional wastewater treatment processes. For this purpose, unsupported and supported TiO2 as photocatalyst were tested, at laboratory and pilot plant scale, in the photodegradation of ciprofloxacin as a model molecule of an emerging pollutant in domestic wastewaters, using visible, UV radiation and solar light.
From the photolysis, adsorption, and photocatalytic studies carried out for both stirred batch photoreactor and packed bed photoreactor, it can be affirmed that the effective elimination of emerging contaminants as ciprofloxacin is possible using solar radiation in a packed bed photoreactor. The efficacy of TiO2 Degussa P25 as a photocatalyst for emergent pollutants degradation and mineralization is confirmed since contaminant degradation values very close to 100% in relatively short periods have been obtained in all the photocatalytic reactions.
After the studies were carried out, it can be affirmed that both supports are good options since they allow the adequate immobilization of the photocatalytic material on its surface using a simple and effective immobilization method. On the other hand, it should be noted that glass rings achieve higher degradation values in the same operating conditions as cylinders. This point may be due to the fact that the packed bed constituted by rings shows a greater contact surface between the photocatalyst and the wastewater, which leads to better yields of the photodegradation of the pollutant. In addition, it constitutes a better disposition to allow the passage of light into the interior of the photocatalytic bed, achieving a better activation of the photocatalytic active material with sunlight.
The suitability of TiO2 as a photocatalyst to decompose emerging pollutants as ciprofloxacin was confirmed in experiments developed in the batch photoreactor under Visible and UV radiation, with degradation rates up to 90% after 30 min of irradiation and low adsorption values (less than 30%). In this way, at the packed bed photoreactor configuration, the supported TiO2 photocatalysts showed significant ratios of pollutant degradation under solar radiation, up to 95%, with glass rings acting as support for probed photocatalytic material. The glass support seems to be useful favoring the operating variables that set limits to the photoactivity of the material when direct solar light is used in a continuous flow reactor configuration. Our results enabled us to validate our scaling process from the laboratory scale (bench top) to a pilot plant scale used for decomposing emerging pollutants in wastewater, such as ciprofloxacin. Simulating industrial conditions for treating high volumes of wastewater offers an actual scene for the wastewater decontamination process, especially with emergent pollutants, a critical factor in the challenging target of UE and World commitments for a better environment.
Author Contributions
Conceptualization and methodology, M.E.B. and P.E.; formal analysis, H.d.P.C. and P.E.; investigation, M.E.B., M.G. and P.E.; resources, M.E.B. and P.E.; data curation, M.G., H.d.P.C. and P.E.; writing—original draft preparation, M.E.B. and H.d.P.C.; writing—review and editing, M.E.B., and H.d.P.C.; supervision, M.E.B. and P.E.; funding acquisition, M.E.B. and P.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
State Program for R&D&I Oriented to the Challenges of Society PDC2021-121131-C22. Call for grants for the requalification of the Spanish university system 2021–2023 (B.O.C. 140, of 9 July 2021), Order UNI/551/2021, of 26 May, of the Ministry of Universities. Financing by the European Union-Next Generation EU Funds. The authors would like to thank Ignacio Melián Cabrera for his collaboration and review of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschor-reck, J. Emerging Pollutants in the EU: 10 Years of NORMAN in Support of Environmental Policies and Regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Imparato, C.; Bifulco, A.; Silvestri, B.; Vitiello, G. Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials. Catalysts 2022, 12, 289. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.; Ritsema, C.J. Emeging Pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of Fluo-roquinolone Antibiotics to Aquatic Organisms. Environ. Toxicol. Chem. 2005, 24, 423–430. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 155. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Z.; Zhang, H.; Hyldgaard Klausen, L.; Moonhee, R.; Kang, S. Emerging high-ammonia-nitrogen wastewater remediation by biological treatment and photocatalysis techniques. Sci. Total Environ. 2023, 875, 162603. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Dwivedi, S.K.; Gupta, D.K. Constructed wetland, an eco-technology for wastewater treatment: A review on types of wastewaters treated and components of the technology (macrophyte, biolfilm and substrate). J. Environ. Manag. 2021, 283, 111986. [Google Scholar] [CrossRef]
- Hou, T.; Du, H.; Yang, Z.; Tian, Z.; Shen, S.; Shi, Y.; Yang, W.; Zhang, L. Flocculation of different types of combined contaminants of antibiotics and heavy metals by thermo-responsive flocculants with carious architectures. Sep. Purif. Technol. 2019, 223, 123–132. [Google Scholar] [CrossRef]
- Nickless, E. Resourcing Future Generations: A Contribution by the Earth Science Community. Nat. Resour. Res. 2018, 27, 143–158. [Google Scholar] [CrossRef]
- Nakagawa, Y. Taking a Future Generation’s Perspective as a Facilitator of Insight Problem-Solving: Sustainable Water Supply Management. Sustainability 2020, 12, 1000. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- González-Burciaga, L.A.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. Challenges of TiO2 heterogeneous photocatalysis on cystostatic compounds degradation: State of the art. Environ. Sci. Pollut. Res. 2022, 29, 42251–42274. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Ali, H.M.; Roghabadi, F.A.; Ahmadi, V. Solid-supported photocatalysts for wastewater treatment: Supports contribution in the photocatalysis process. Sol. Energy 2023, 255, 99–125. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.; Hai, F. A critical review on advanced oxidation processes for the removal of trace organic contaminants: A voyage from individual to integrated processes. Chemosphere 2020, 260, 127460. [Google Scholar] [CrossRef] [PubMed]
- Son, B.T.; Long, N.V.; Hang, N.T.N. Fly ash-, foundry sand-, clay-, and pumice-based metal oxide nanocomposites as green catalysts. RSC Adv. 2021, 11, 30805. [Google Scholar] [CrossRef]
- Song, Y.; Peng, Y.; Long, N.V.; Huang, Z.; Yang, Y. Multifunctional self-assembly 3D Ag/g-C3N4/RGO aerogel as highly efficient adsorbent and photocatalyst for R6G removal from wastewater. Appl. Surf. Sci. 2021, 542, 148584. [Google Scholar] [CrossRef]
- Bui, T.S.; Bansal, P.; Lee, B.; Lee, K.; Mahvelati-Shamsabadi, T.; Soltani, T. Facile fabrication of novel Ba-doped g-C3N4 photocatalyst with remarkably enhanced photocatalytic activity towards tetracycline elimination under visible-light irradiation. Appl. Surf. Sci. 2020, 506, 144184. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Biogenic synthesis of a Ag-graphene nanocomposite with efficient photocatalytic defradation, electrical conductivity and photoelectrochemical performance. New J. Chem. 2015, 39, 8121–8129. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis, 1st ed.; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 19–40. [Google Scholar] [CrossRef]
- Borges, M.E.; Sierra, M.; Méndez-Ramos, J.; Acosta-Mora, P.; Ruiz-Morales, J.C.; Esparza, P. Solar degradation of contaminants in water: TiO2 solar photocatalysis assisted by up-conversion luminescent materials. Sol. Energy Mater. Sol. Cells 2016, 155, 194–201. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Kobylański, M.P.; Zaleska-Medynska, A. Fundamentals of metal oxide-based photocatalysis. In Metal Oxide-Based Photocatalysis, 1st ed.; Zaleska-Medynska, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 3–50. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts 2021, 11, 1118. [Google Scholar] [CrossRef]
- Vatika, S.; Khosla, A.; Singh, P.; Nguyen, V.H.; Van Le, Q.; Selvasembian, R.; Hussain, C.M.; Thakur, S.; Raizada, P. Current Perspective in Metal Oxide Based Photocatalysts for Virus Disinfection: A Review. J. Environ. Manag. 2022, 308, 114617. [Google Scholar] [CrossRef]
- Prakruthi, K.; Ujwal, M.P.; Yashas, S.R.; Mahesh, B.; Swamy, N.K.; Shivaraju, H.P. Recent advances in photocatalytic remediation of emerging organic pollutants using semiconducting metal oxides: An overview. Environ. Sci. Pollut. Res. 2022, 29, 4930–4957. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; El Aatik, A.; Aliste, M.; Navarro, G.; Fenoll, J.; Navarro, S. Removal of Contaminants of Emmerging Convern from a Wastewater Effluent by Solar-Driven Heterogeneous Photocatalysis: A Case Study of Pharmaceuticals. Water Air Soil. Pollut. 2023, 234, 55. [Google Scholar] [CrossRef]
- do Nascimento, J.L.A.; Chantelle, L.; dos Santos, I.M.G.; Menezes de Oliveira, A.L.; Alves, M.C.F. The Influence of Synthesis Methods and Experimental Conditions on the Photocatalytic Properties of SnO2: A Review. Catalysts 2022, 12, 428. [Google Scholar] [CrossRef]
- Ekta, S.; Thakur, V.; Sangar, S.; Singh, K. Recent Progress on Heterostructures of Photocatalysts for Environmental Remediation. Mater. Today Proc. 2020, 32, 584–593. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M. Titanium dioxide nanostructures as efficient photocatalyst: Progress, challenges and perspective. Int. J. Energy Res. 2021, 45, 3569–3589. [Google Scholar] [CrossRef]
- Sraw, A.; Kaur, T.; Pandey, Y.; Sobti, A.; Wanchoo, R.K.; Toor, A.P. Fixed bed recirculation type photocatalytic reactor with TiO2 immobilized clay beads for the degradaton of pesticide polluted water. J. Environ. Chem. Eng. 2018, 6, 7035–7043. [Google Scholar] [CrossRef]
- Nguyen-Dinh, M.; Bui, T.; Bansal, P.; Jourshabani, M.; Lee, B. Photocatalytic and photo-electrochemical behavior of novel SnO2-modified-g-C3N4 for complete elimination of tetracycline under visible-light irradiation: Slurry and fixed-bed approach. Sep. Purif. Technol. 2021, 267, 118607. [Google Scholar] [CrossRef]
- Muñoz-Flores, P.; Poon, P.S.; Sepulveda, C.; Ania, C.O.; Matos, J. Photocatalytic Performance of Carbon-Containing CuMo-Based Catalysts under Sunlight Illumination. Catalysts 2022, 12, 46. [Google Scholar] [CrossRef]
- Mahy, J.G.; Tsaffo Mbognou, M.H.; Léonard, C.; Fagel, N.; Woumfo, E.D.; Lambert, S.D. Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water. Catalysts 2022, 12, 148. [Google Scholar] [CrossRef]
- Conte, F.; Pellegatta, V.; Di Michele, A.; Ramis, G.; Rossetti, I. Photocatalytic Reduction of Nitrates and Combined Photodegradation with Ammonium. Catalysts 2022, 12, 321. [Google Scholar] [CrossRef]
- Murzin, P.D.; Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of the Heterovalent Doping of TiO2 with Sc3+ and Nb5+ on the Defect Distribution and Photocatalytic Activity. Catalysts 2022, 12, 484. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, B.; Chen, Q.; Bai, Z.; Cao, Y.; Davaa, B. Synthesis, Structure, and Photocatalytic Activity of TiO2-Montmorillonite Composites. Catalysts 2022, 12, 486. [Google Scholar] [CrossRef]
- Zaruma-Arias, P.E.; Núñez-Núñez, C.M.; González-Burciaga, L.A.; Proal-Nájera, J.B. Solar Heterogenous Photocatalytic Degradation of Methylthionine Chloride on a Flat Plate Reactor: Effect of pH and H2O2 Addition. Catalysts 2022, 12, 132. [Google Scholar] [CrossRef]
- Borges, M.E.; García, D.M.; Hernández, T.; Ruiz-Morales, J.C.; Esparza, P. Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration. Catalysts 2015, 5, 77–87. [Google Scholar] [CrossRef]
- Borges, M.E.; Sierra, M.; Cuevas, E.; García, R.D.; Esparza, P. Photocatalysis with solar energy: Sunlight-responsive photocatalyst based on TiO2 loaded on a natural material for wastewater treatment. Sol. Energy 2016, 135, 527–535. [Google Scholar] [CrossRef]
- Borges, M.E.; Sierra, M.; Esparza, P. Solar photocatalysis at semi-pilot scale: Wastewater decontamination in a packed-bed photocatalytic reactor system with a visible-solar-light-driven photocatalyst. Clean Technol. Environ. Policy 2017, 19, 1239–1245. [Google Scholar] [CrossRef]
- Kalikeri, S.; Shetty Kodialbail, V. Solar light-driven photocatalysis using mixed-phase bismuth ferrite (BiFeO3/Bi25FeO40) nanoparticles for remediation of dye-contaminated water: Kinetics and comparison with artificial UV and visible light-mediated photocatalysis. Environ. Sci. Pollut. Res. 2018, 25, 13881–13893. [Google Scholar] [CrossRef] [PubMed]
- Iervolino, G.M.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. (Z) 2020, 378, 7. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zeng, F.; Jeong, T.; Wu, G.; Guan, Q. CFD Modeling of UV/H2O2 Process in Internal Airlift Circulating Photoreactor. Water 2020, 12, 3237. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D. Main parameters influencing the design of photocatalytic reactors for wastewater treatment: A mini review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar] [CrossRef]
- Enesca, A. The Influence of Photocatalytic Reactors Design and Operating Parameters on the Wastewater Organic Pollutants Removal—A Mini-Review. Catalysts 2021, 11, 556. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process Saf. Environ. Prot. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Thompson, W.A.; Perier, C.; Maroto-Valer, M.M. Systematic study of sol-gel parameters on TiO2 coating for CO2 photoreduction. Appl. Catal. B Environ. 2018, 238, 136–146. [Google Scholar] [CrossRef]
- Mostafa, S.; El-Sadek, M.; Awad Alla, E. Spectrophotometric determination of ciprofloxacin, enrofloxacin and pefloxacin thoright change transfer complex formation. J. Pharm. Biomed. Anal. 2002, 27, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.; Waheed, N. Simple UV spectrophotometric assay of ciprofloxacin. Mintage J. Pharm. Med. Sci. 2014, 3, 10–13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).