The Asymmetric Petasis Borono-Mannich Reaction: Insights on the Last 15 Years
Abstract
1. Introduction
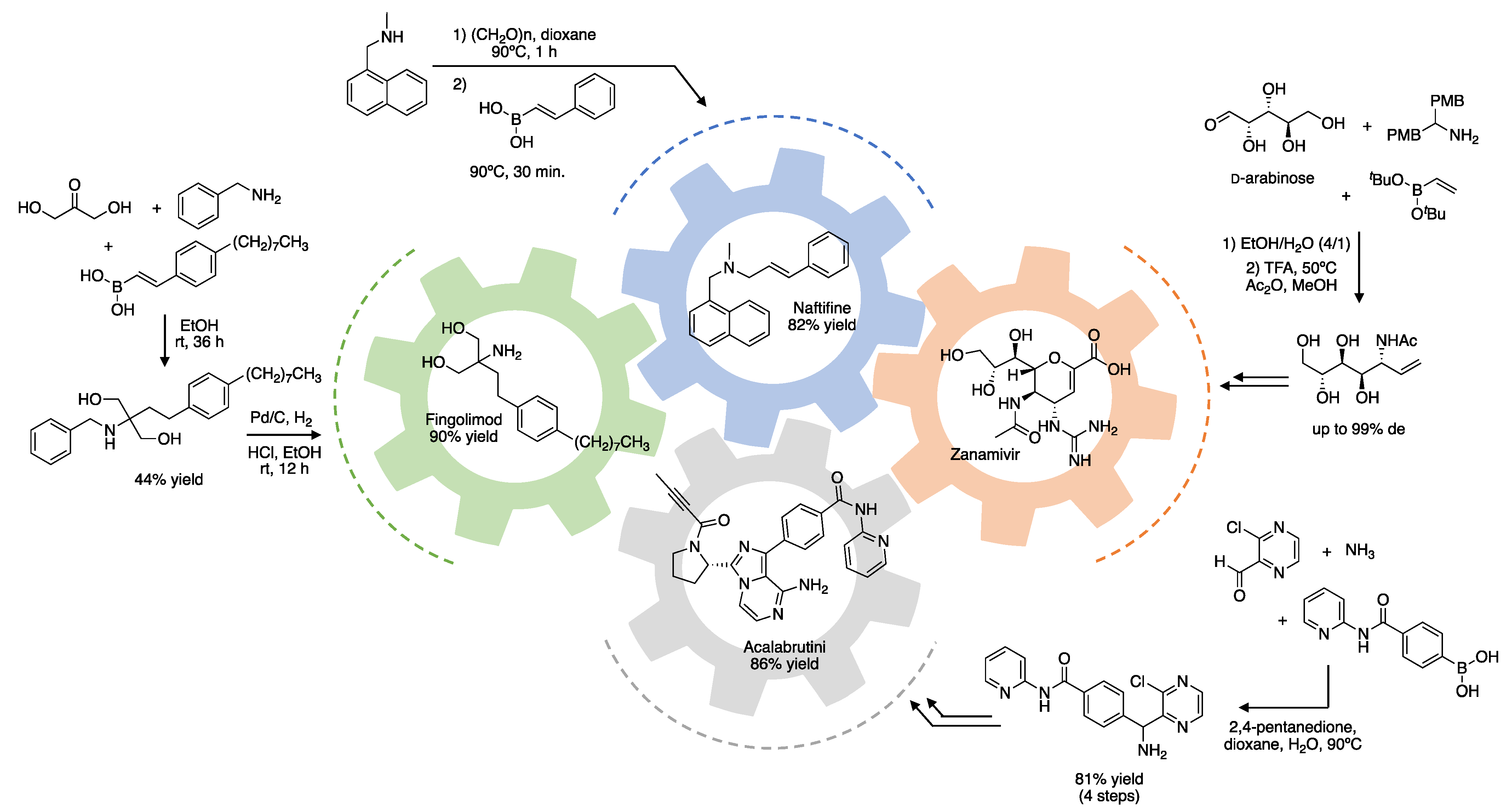
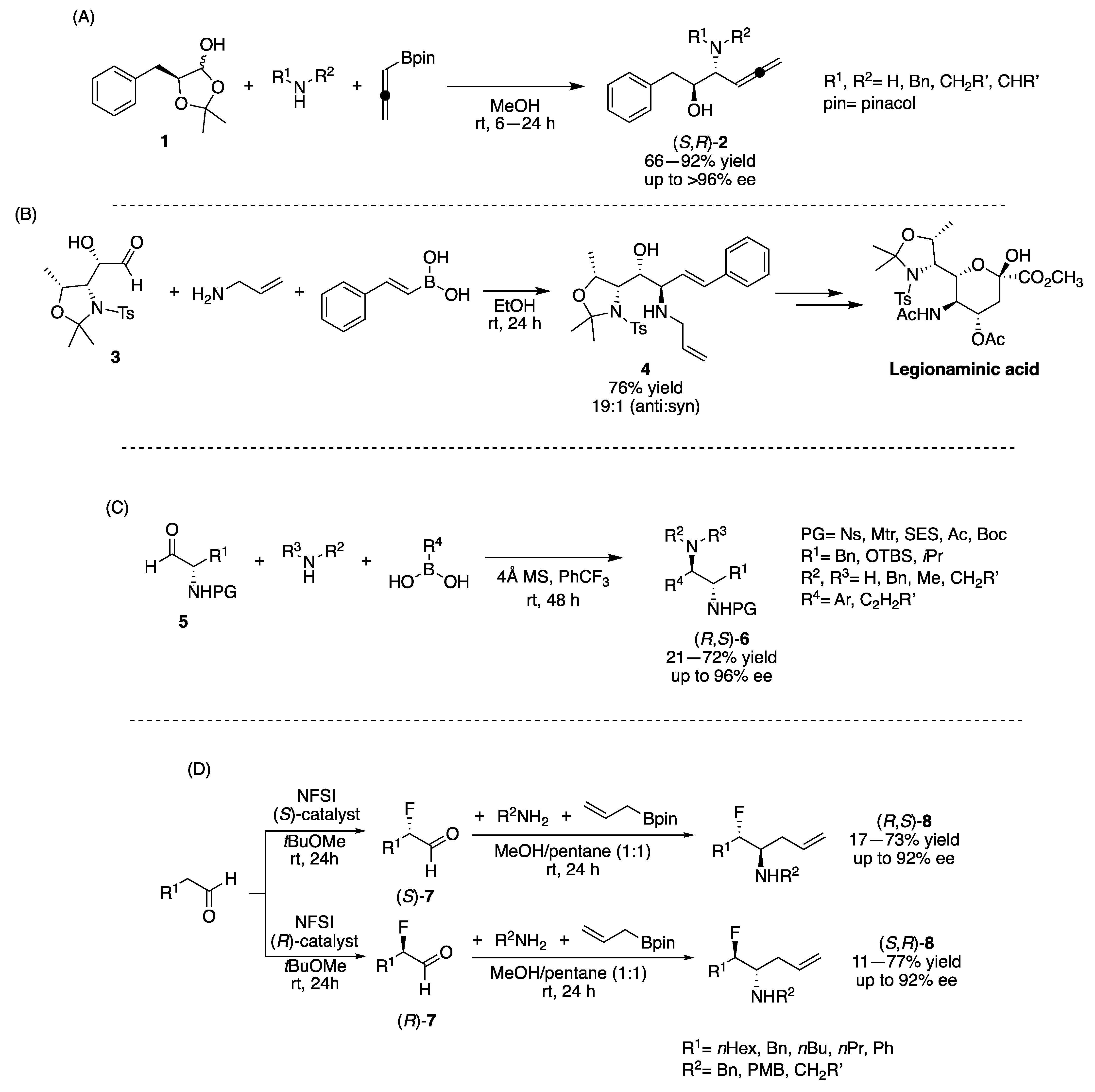
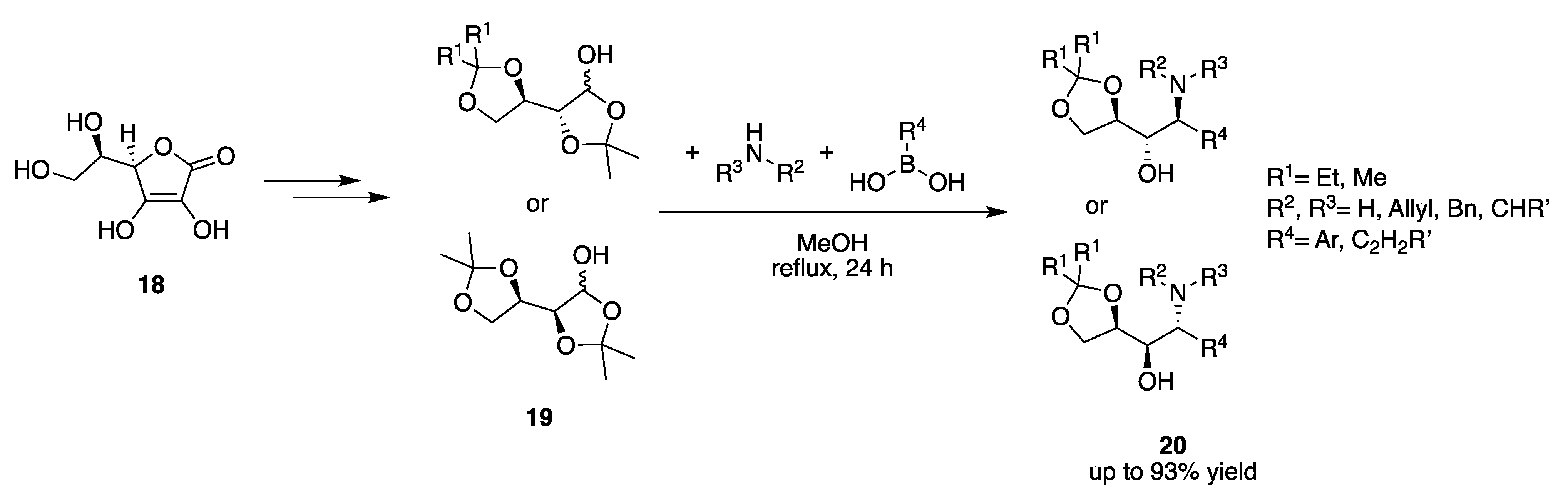
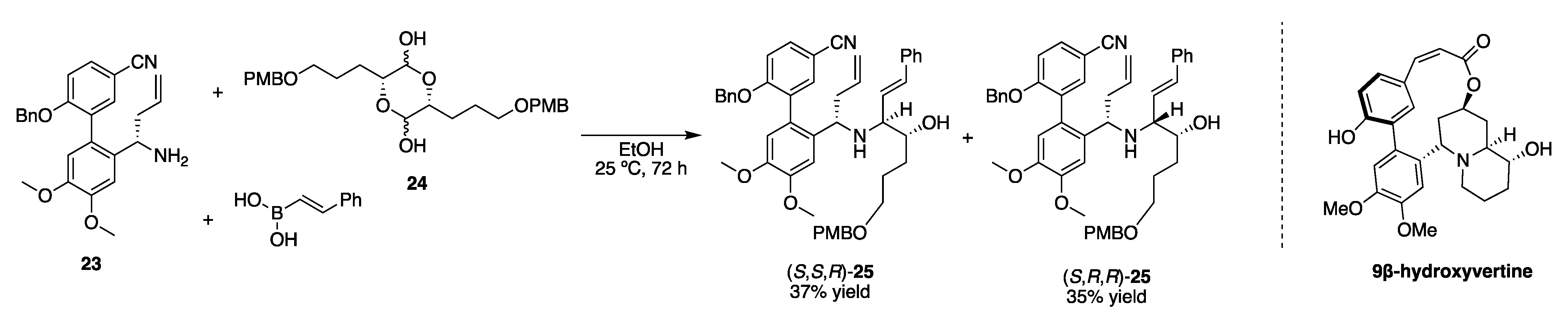
2. Asymmetric Induction by Reaction Components (Chiral Pool)
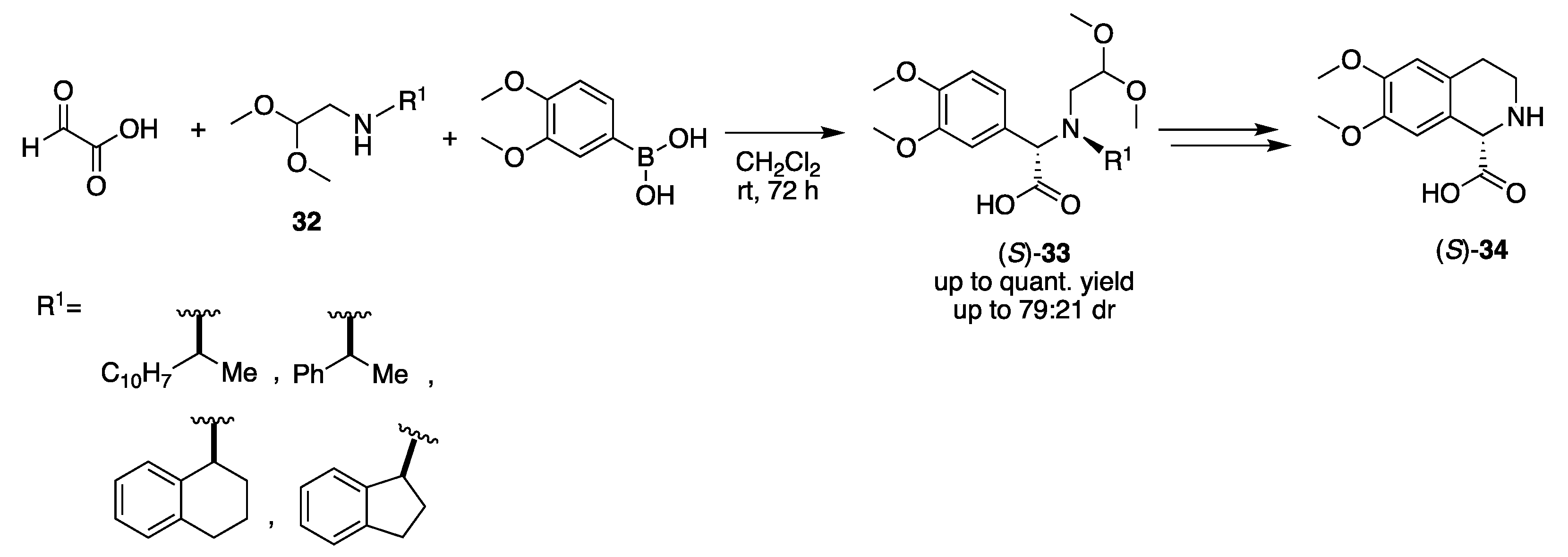
2.1. Carbonyl Component
2.2. Amine Component
2.3. Boronic Acid Component
3. Organocatalysts versus Transition-Metal Catalysts
3.1. Organocatalysts
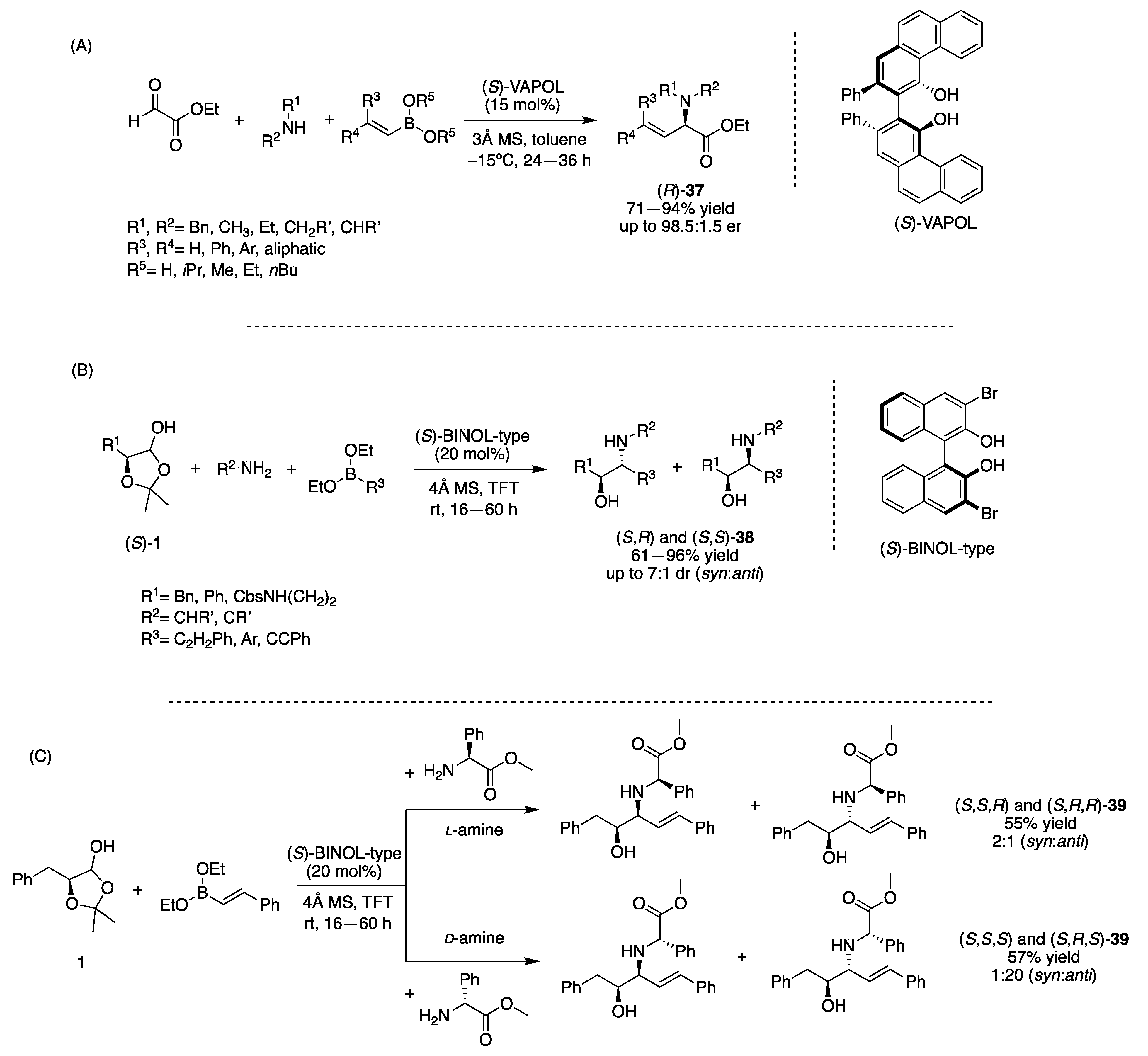
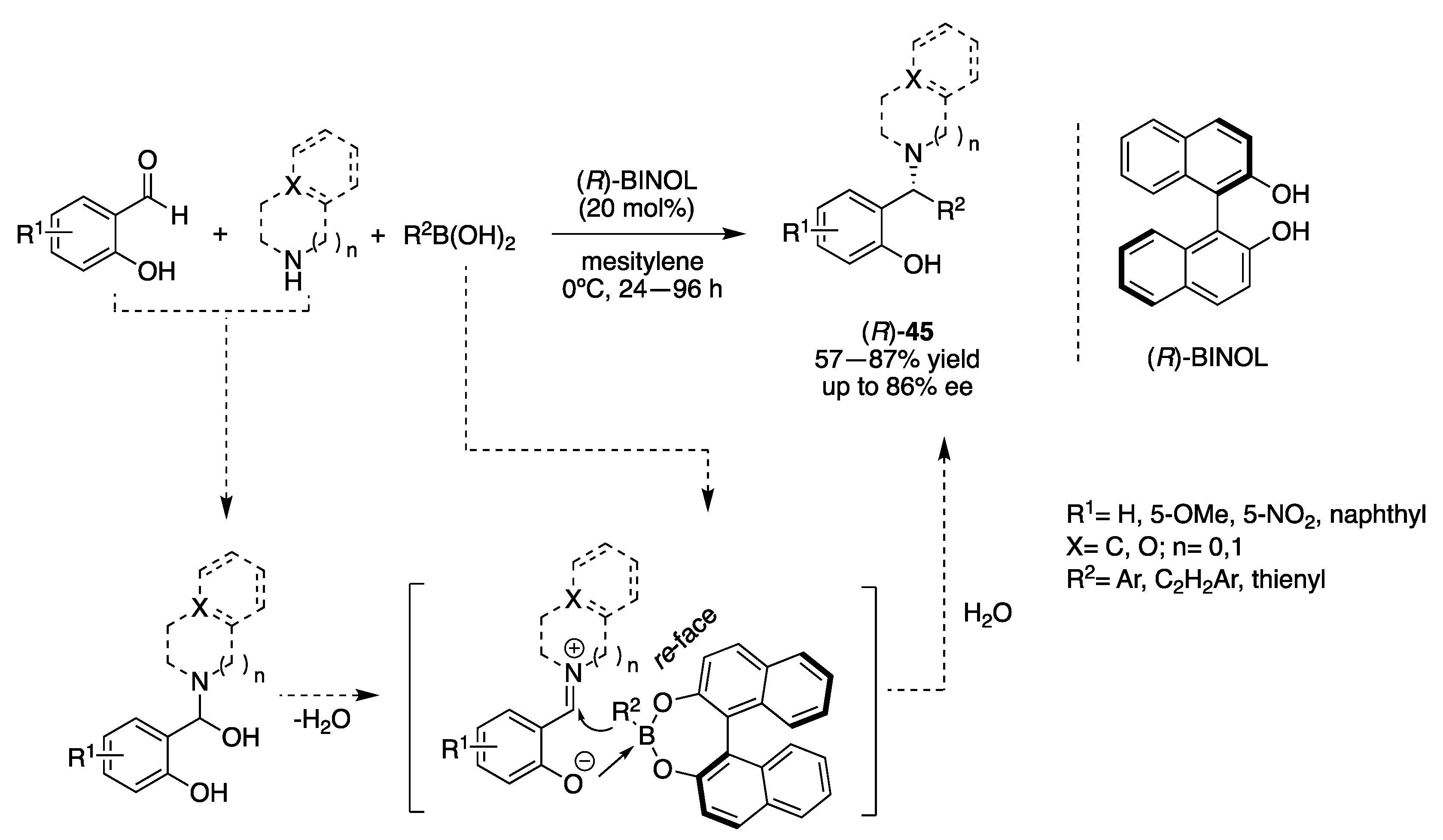
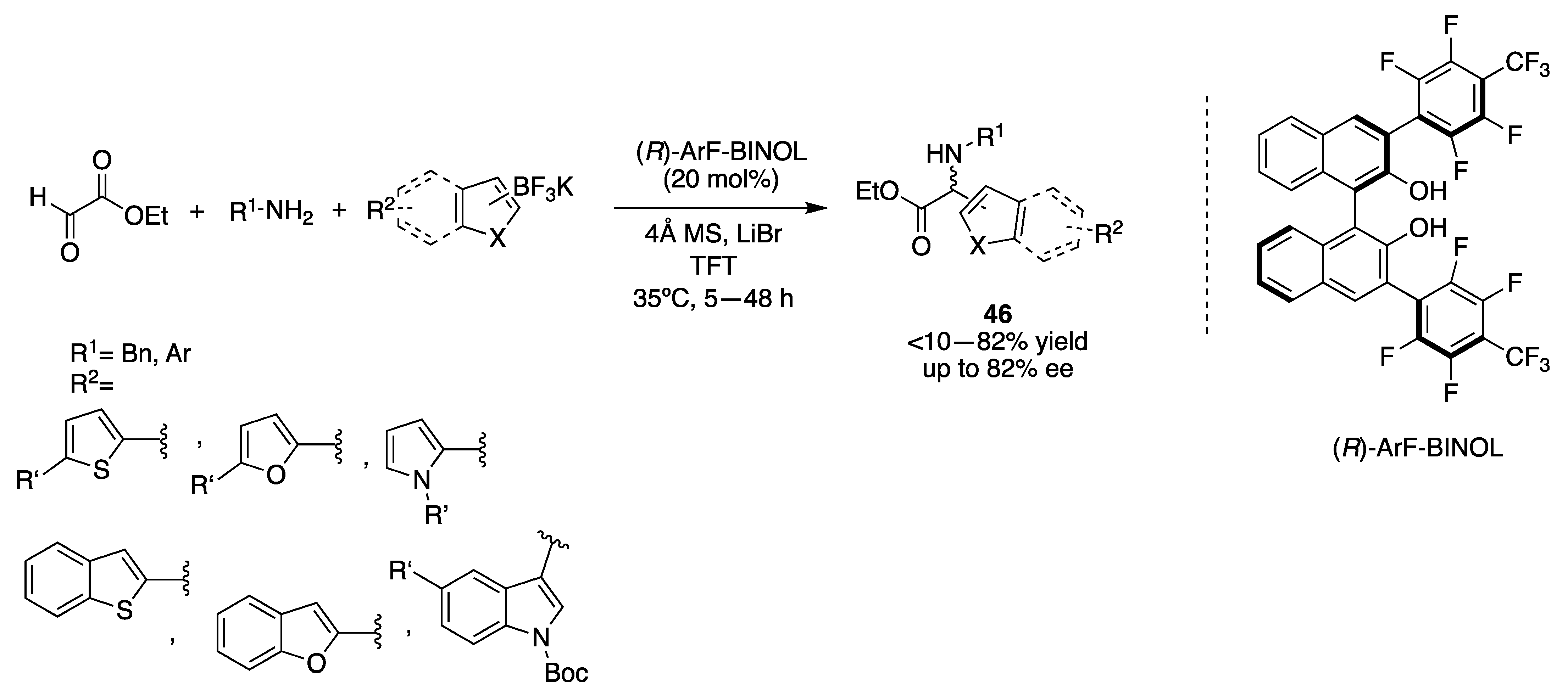
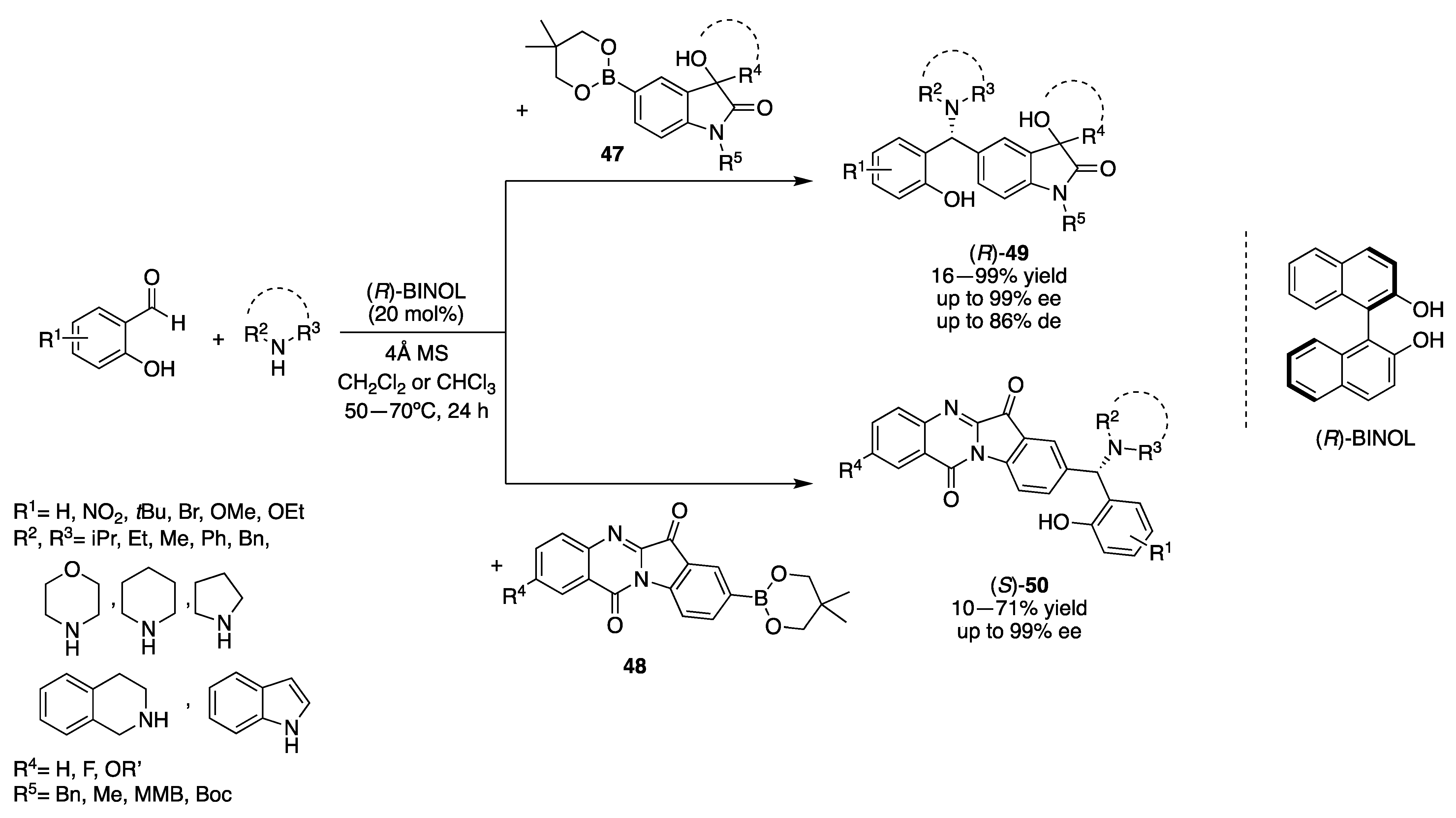
3.1.1. Chiral Diol-Based Organocatalysts
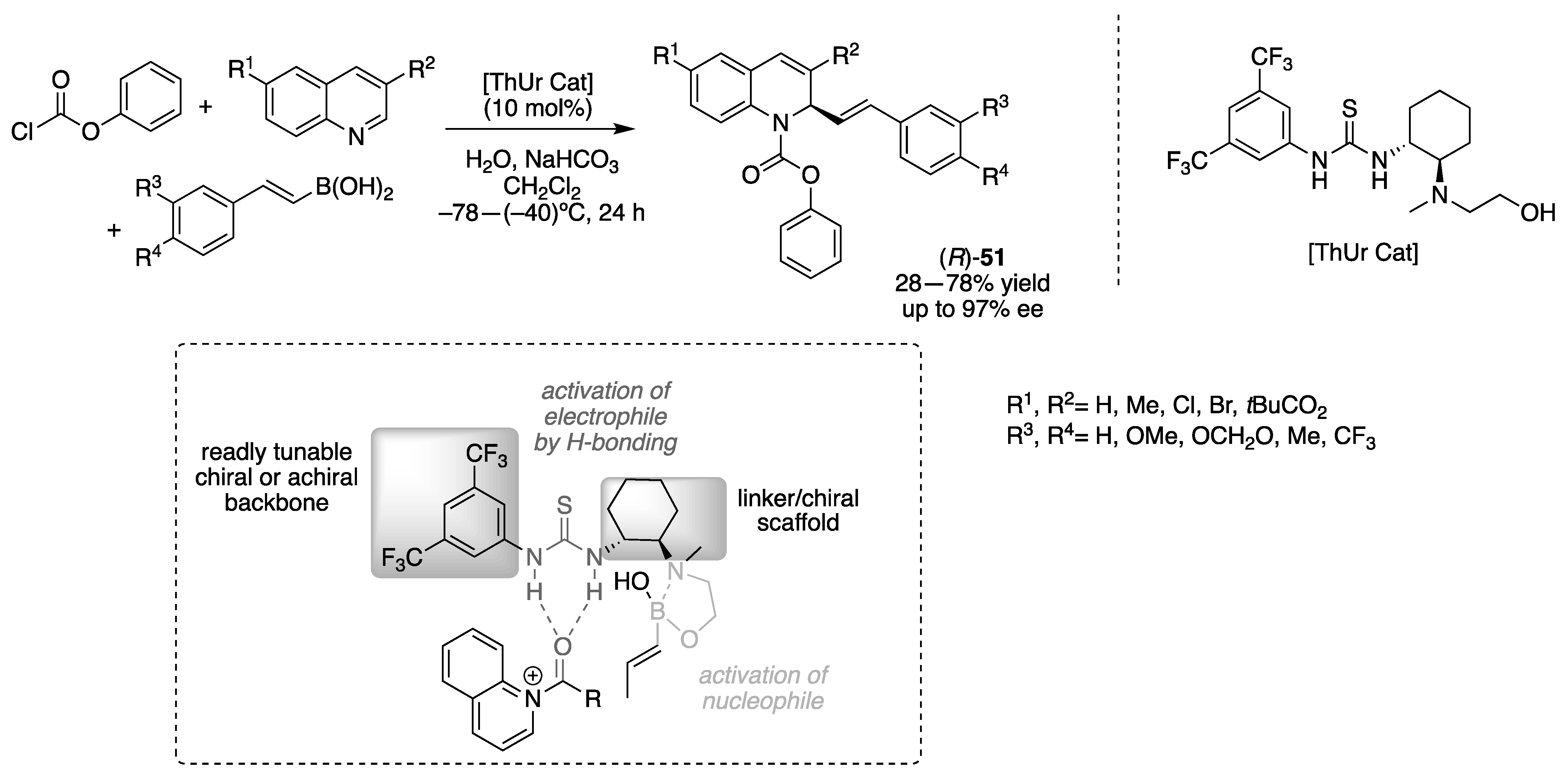
3.1.2. Chiral Thiourea-Based Organocatalysts
3.1.3. Miscellaneous
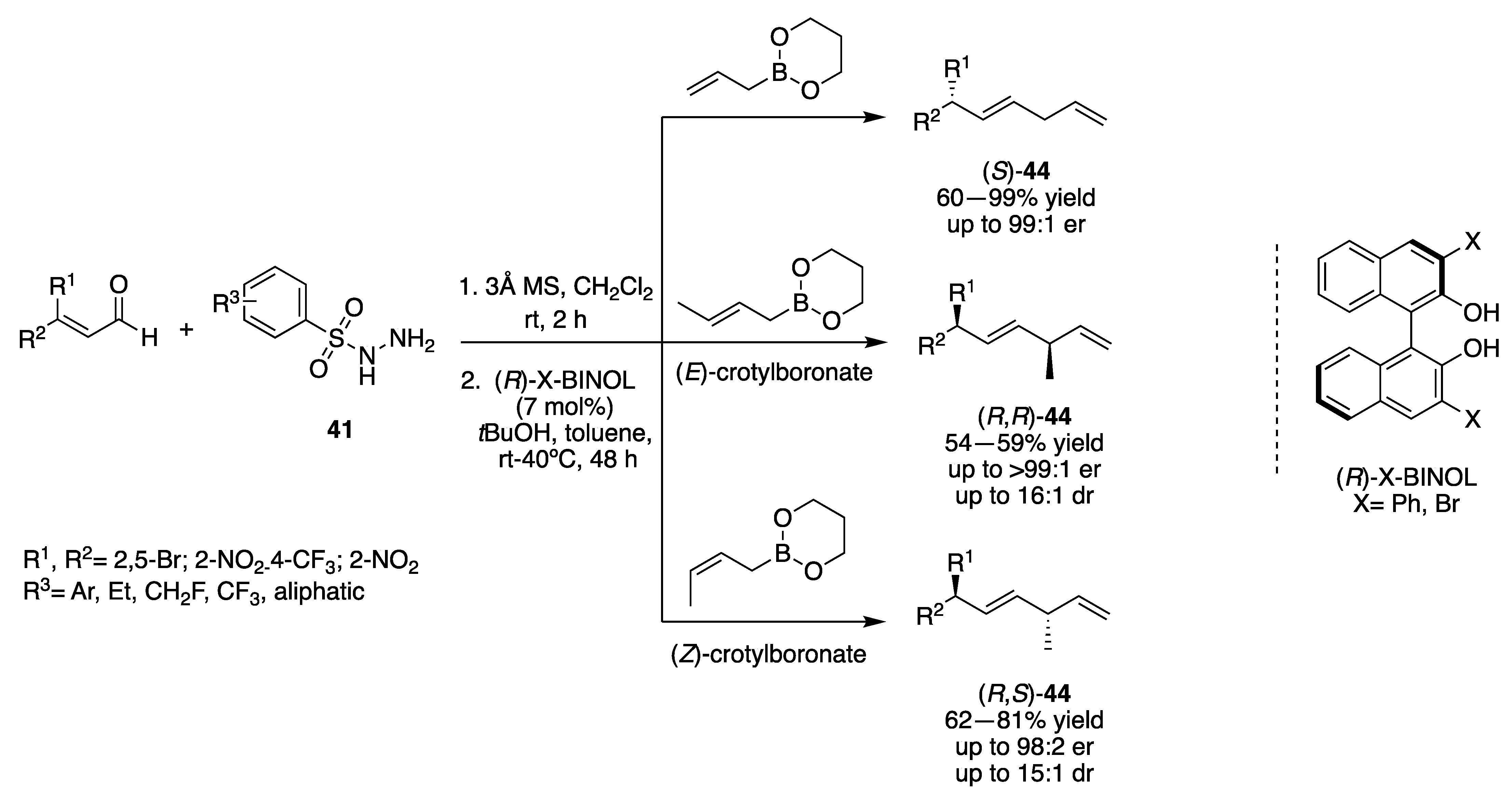
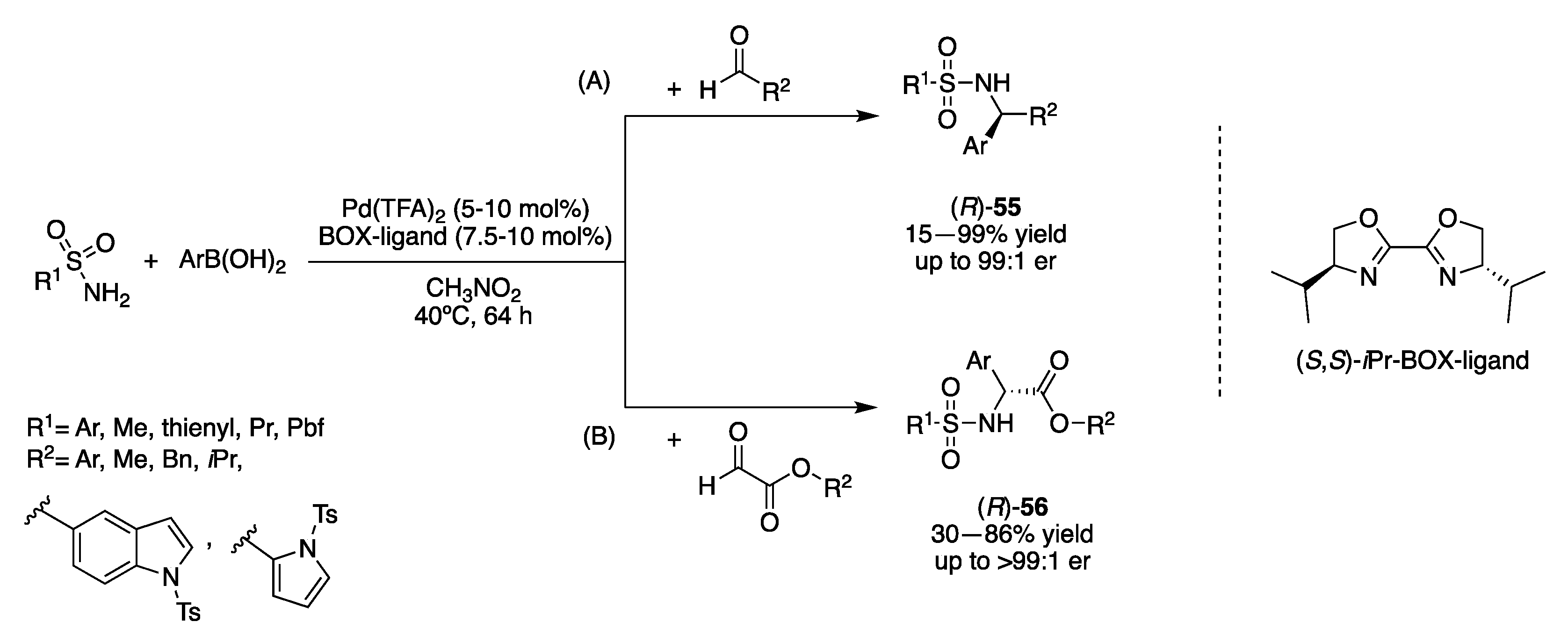
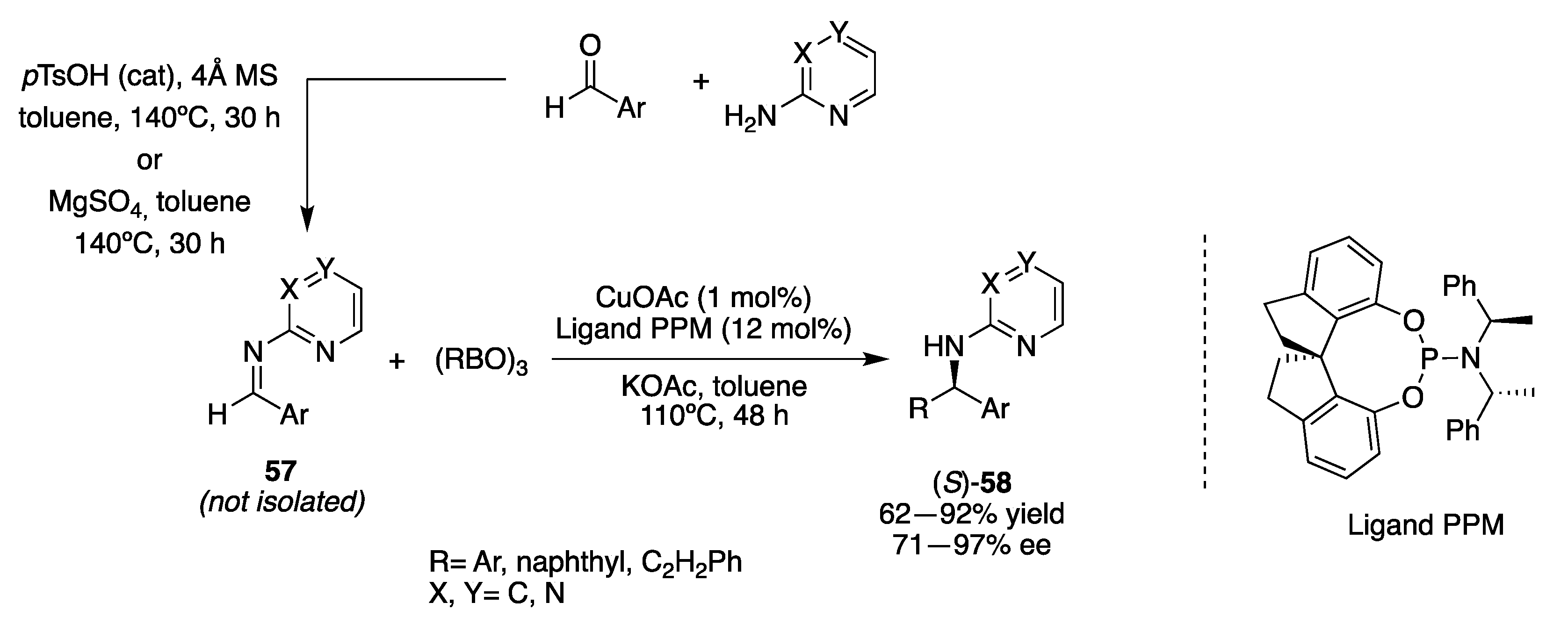
3.2. Transition-Metal Catalysts
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alegre-Requena, J.V.; Marqués-López, E.; Raquel, P.; Herrera, R.P. Multicomponent Strategies. In Multicomponent Reactions: Concepts and Applications for Design and Synthesis; Herrera, R.P., Marqués-López, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- John, S.E.; Gulatia, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Hafiza Amna Younus, H.A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K.M. Multicomponent reactions (MCR) in medicinal chemistry: A patent review (2010–2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Cores, Á.; Clerigué, J.; Orocio-Rodríguez, E.; Menéndez, J.C. Multicomponent Reactions for the Synthesis of Active Pharmaceutical Ingredients. Pharmaceuticals 2022, 15, 1009. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Petasis, N.A.; Akritopoulou, I. The boronic acid mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1992, 34, 583–586. [Google Scholar] [CrossRef]
- Sugiyama, S.; Arai, S.; Kiriyama, M.; Ishii, K. A Convenient Synthesis of Immunosuppressive Agent FTY720 Using the Petasis Reaction. Chem. Pharm. Bull. 2005, 53, 100–102. [Google Scholar] [CrossRef]
- Zhangyong, H.; Zeming, C.; Kui, Y.; Jin, W. Novel Method for Synthesis of Anti-Influenza Medicament Zanamivir. CN104744415(A). 1 July 2015. Available online: https://patents.google.com/patent/CN104744415A/en (accessed on 31 March 2023).
- Flick, A.C.; Leverett, C.A.; Ding, H.X.; McInturff, E.; Fink, S.J.; Helal, C.J.; O’Donnell, C.J. Synthetic Approaches to the New Drugs Approved During 2017. J. Med. Chem. 2019, 62, 7340–7382. [Google Scholar] [CrossRef]
- Candeias, N.R.; Montalbano, F.; Cal, P.M.S.D.; Gois, P.M.P. Boronic Acids and Esters in the Petasis-Borono Mannich Multicomponent Reaction. Chem. Rev. 2010, 110, 6169–6193. [Google Scholar] [CrossRef]
- Souza, R.Y.; Bataglion, G.A.; Ferreira, D.A.C.; Gatto, C.C.; Eberlin, M.N.; Neto, B.A.D. Insights on the Petasis Borono–Mannich multicomponent reaction mechanism. RSC Adv. 2015, 5, 76337–76341. [Google Scholar] [CrossRef]
- Filho, J.F.A.; Lemos, B.C.; de Souza, A.S.; Pinheiro, S.; Greco, S.J. Multicomponent Mannich reactions: General aspects, methodologies and applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.H. Applications of Asymmetric Petasis Reaction in the Synthesis of Chiral Amines. Acta Chim. Sin. 2021, 79, 1345–1359. [Google Scholar] [CrossRef]
- De Graaff, C.; Ruijter, E.; Orru, R.V.A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 2012, 41, 3969–4009. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.M.; Jeong, M.; Jo, J.; Heo, Y.M.; Han, Y.T.; Yun, H. Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis. Molecules 2016, 21, 951. [Google Scholar] [CrossRef] [PubMed]
- Rouf, A.; Taneja, S.C. Synthesis of Single-enantiomer Bioactive Molecules: A Brief Overview. Chirality 2014, 26, 63–78. [Google Scholar] [CrossRef]
- Thaima, T.; Pyne, S.G. Regioselective and Diastereoselective Borono-Mannich Reactions with Pinacol Allenylboronate. Org. Lett. 2015, 17, 778–781. [Google Scholar] [CrossRef]
- Matthies, S.; Stallforth, P.; Seeberger, P.H. Total Synthesis of Legionaminic Acid as Basis for Serological Studies. J. Am. Chem. Soc. 2015, 137, 2848–2851. [Google Scholar] [CrossRef]
- Norsikian, S.; Beretta, M.; Cannillo, A.; Martin, A.; Retailleau, P.; Beau, J.M. Synthesis of enantioenriched 1,2-trans-diamines using the borono-Mannich reaction with N-protected α-amino aldehydes. Chem. Commun. 2015, 51, 9991–9994. [Google Scholar] [CrossRef]
- Norsikian, S.; Beretta, M.; Cannillo, A.; Auvray, M.; Martin, A.; Retailleau, P.; Iorga, B.I.; Beau, J.M. Stereoselective Synthesis of 1,2-trans-Diamines Using the Three-Component Borono-Mannich Condensation—Reaction Scope and Mechanistic Insights. Eur. J. Org. Chem. 2017, 2017, 1940–1951. [Google Scholar] [CrossRef]
- Chevis, P.J.; Wangngae, S.; Thaima, T.; Carroll, A.W.; Willis, A.C.; Pattarawarapan, M.; Pyne, S.G. Highly diastereoselective synthesis of enantioenriched anti-α-allyl-β-fluoroamines. Chem. Commun. 2019, 55, 6050–6053. [Google Scholar] [CrossRef] [PubMed]
- Moosophon, P.; Baird, M.C.; Kanokmedhakul, S.; Pyne, S.G. Total Synthesis of Calystegine B4. Eur. J. Org. Chem. 2010, 2010, 3337–3344. [Google Scholar] [CrossRef]
- Bouillon, M.E.; Pyne, S.G. Diastereoselective concise syntheses of the polyhydroxylated alkaloids DMDP and DAB. Tetrahedron Lett. 2014, 55, 475–478. [Google Scholar] [CrossRef]
- Bharathkumar, H.K.; Krishna, J.S.; Surender, B.; Batchu, V.R. Approaches to the Total Synthesis of Conduramines: A Review. SynOpen 2022, 6, 238–257. [Google Scholar] [CrossRef]
- Ghosal, P.; Shaw, A.K. A Chiron Approach to Aminocytitols by Petasis-Borono-Mannich Reaction: Formal Synthesis of (+)-Conduramine E and (−)-Conduramine E. J. Org. Chem. 2012, 77, 7627–7632. [Google Scholar] [CrossRef] [PubMed]
- Norsikian, S.; Soule, J.F.; Cannillo, A.; Guillot, R.; Dau, M.; Beau, J.M. Remarkable Stereoselectivity in Intramolecular Borono-Mannich Reactions: Synthesis of Conduramines. Org. Lett. 2012, 14, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Mandai, H.; Yamada, H.; Shimowaki, K.; Mitsudo, K.; Suga, S. An Efficient Petasis Boronic–Mannich Reaction of Chiral Lactol Derivatives Prepared from D-Araboascorbic Acid. Synthesis 2014, 46, 2672–2681. [Google Scholar] [CrossRef]
- Cannillo, A.; Norsikian, S.; Dau, M.; Retailleau, P.; Iorga, B.I.; Beau, J.M. From Enantiopure Hydroxyaldehydes to Complex Heterocyclic Scaffolds: Development of Domino Petasis/Diels–Alder and Cross-Metathesis/Michael Addition Reactions. Chem. Eur. J. 2014, 20, 12133–12143. [Google Scholar] [CrossRef]
- Thaima, T.; Willis, A.C.; Pyne, S.G. Progress toward the total synthesis of 9β-hydroxyvertine: Construction of an advanced quinolizidine intermediate. Tetrahedron 2019, 75, 130476. [Google Scholar] [CrossRef]
- Carroll, A.W.; Savaspun, K.; Willis, A.C.; Hoshino, M.; Kato, A.; Pyne, S.G. Total Synthesis of Natural Hyacinthacine C5 and Six Related Hyacinthacine C5 Epimers. J. Org. Chem. 2018, 83, 5558–5576. [Google Scholar] [CrossRef] [PubMed]
- Grajewska, A.; Rozwadowska, M.D.; Gzella, A. Synthesis and stereochemistry assignment of (3R,5R)- and (3S,5R)-4-benzyl-3-(3,4-dimethoxyphenyl)-5-phenyl-1,4-oxazin-2-ones. J. Mol. Struct. 2019, 1194, 204–210. [Google Scholar] [CrossRef]
- Churches, Q.I.; Johnson, J.K.; Fifer, N.L.; Hutton, C.A. Anomalies in the Stereoselectivity of the Petasis Reaction Using Styrenyl Boronic Acids. Aust. J. Chem. 2011, 64, 62–67. [Google Scholar] [CrossRef]
- Yang, X.; Cao, Z.H.; Zhou, Y.; Cheng, F.; Lin, Z.W.; Ou, Z.; Yuan, Y.; Huang, Y.Y. Petasis-Type gem-Difluoroallylation Reactions Assisted by the Neighboring Hydroxyl Group in Amines. Org. Lett. 2018, 20, 2585–2589. [Google Scholar] [CrossRef]
- Lin, Z.W.; Zhou, Y.; Zhao, Z.N.; Zhao, Y.; Liu, J.; Huang, Y.Y. 1,2-Amino alcohol-dependent Petasis allylboration for racemic and chiral homoallylamines. Org. Chem. Front. 2019, 6, 751–755. [Google Scholar] [CrossRef]
- Xie, Y.W.; Zhao, Z.N.; Lin, Z.W.; Wang, Y.H.; Liu, Y.Q.; Huang, Y.Y. Asymmetric Petasis reaction for the synthesis of chiral α- and β-butadienyl amines. Chem. Commun. 2021, 57, 2364–2367. [Google Scholar] [CrossRef]
- Churches, Q.I.; White, J.M.; Hutton, C.A. Synthesis of β,γ-Dihydroxyhomotyrosines by a Tandem Petasis–Asymmetric Dihydroxylation Approach. Org. Lett. 2011, 13, 2900–2903. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.H. Lewis Acid Promoted Highly Diastereoselective Petasis Borono-Mannich Reaction: Efficient Synthesis of Optically Active β,γ-Unsaturated α-Amino Acids. Org. Lett. 2012, 14, 2062–2065. [Google Scholar] [CrossRef]
- Bułyszko, I.; Chrzanowska, M.; Grajewska, A.; Rozwadowska, M.D. Synthesis of (+)-6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline-1-carboxylic Acid, a Diastereoselective Approach. Eur. J. Org. Chem. 2015, 2015, 383–388. [Google Scholar] [CrossRef]
- Hellou, N.; Mace, A.; Martin, C.; Dorcet, V.; Roisnel, T.; Jean, M.; Vanthuyne, N.; Berree, F.; Carboni, B.; Crassous, J. Synthesis of Carbo[6]helicene Derivatives Grafted with Amino or Aminoester Substituents from Enantiopure [6]Helicenyl Boronates. J. Org. Chem. 2018, 83, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.G.; Cardoso, M.F.D.C.; Forezi, L.S.M. Organocatalysis: A Brief Overview on Its Evolution and Applications. Catalysts 2018, 8, 605. [Google Scholar] [CrossRef]
- Aukland, M.H.; List, B. Organocatalysis emerging as a technology. Pure Appl. Chem. 2021, 93, 1371–1381. [Google Scholar] [CrossRef]
- Han, B.; He, X.H.; Liu, Y.Q.; He, G.; Peng, C.; Li, J.L. Asymmetric organocatalysis: An enabling technology for medicinal chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Schaus, S.E. Asymmetric Petasis Reactions Catalyzed by Chiral Biphenols. J. Am. Chem. Soc. 2008, 130, 6922–6923. [Google Scholar] [CrossRef] [PubMed]
- Muncipinto, G.; Moquist, P.N.; Schreiber, S.L.; Schaus, S.E. Catalytic Diastereoselective Petasis Reactions. Angew. Chem. Int. Ed. 2011, 50, 8172–8175. [Google Scholar] [CrossRef]
- Jiang, Y.; Diagne, A.B.; Thomson, R.J.; Schaus, S.E. Enantioselective Synthesis of Allenes by Catalytic Traceless Petasis Reactions. J. Am. Chem. Soc. 2017, 139, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Schaus, S.E. Asymmetric Petasis Borono-Mannich Allylation Reactions Catalyzed by Chiral Biphenols. Angew. Chem. Int. Ed. 2017, 56, 1544–1548. [Google Scholar] [CrossRef]
- Jiang, Y.; Thomson, R.J.; Schaus, S.E. Asymmetric Traceless Petasis Borono-Mannich Reactions of Enals: Reductive Transposition of Allylic Diazenes. Angew. Chem. Int. Ed. 2017, 56, 16631–16635. [Google Scholar] [CrossRef]
- Mundal, D.A.; Lutz, K.E.; Thomson, R.J. A Direct Synthesis of Allenes by a Traceless Petasis Reaction. J. Am. Chem. Soc. 2012, 134, 5782–5785. [Google Scholar] [CrossRef]
- Sikandar, S.; Zahoor, A.F.; Ghaffar, A.; Anjum, M.N.; Noreen, R.; Irfan, A.; Munir, B.; Kotwica-Mojzych, K.; Mojzych, M. Unveiling the Chemistry and Synthetic Potential of Catalytic Cycloaddition Reaction of Allenes: A Review. Molecules 2023, 28, 704. [Google Scholar] [CrossRef]
- Han, W.Y.; Zuo, J.; Zhang, X.M.; Yuan, W.C. Enantioselective Petasis reaction among salicylaldehydes, amines, and organoboronic acids catalyzed by BINOL. Tetrahedron 2013, 69, 537–541. [Google Scholar] [CrossRef]
- Shi, X.L.; Kiesman, W.F.; Levina, A.; Xin, Z.L. Catalytic Asymmetric Petasis Reactions of Vinylboronates. J. Org. Chem. 2013, 78, 9415–9423. [Google Scholar] [CrossRef]
- Haeffner, F.; Pickel, T.C.; Hou, A.; Walker, D.G.; Kiesman, W.F.; Shi, X. The Chelate Effect Rationalizes Observed Rate Acceleration and Enantioselectivity in BINOL-Catalyzed Petasis Reactions. Chem. Eur. J. 2023, 29, e202203331. [Google Scholar] [CrossRef]
- Tong, M.N.; Bai, X.; Meng, X.; Wang, J.F.; Wang, T.; Zhu, X.Y.; Mao, B. Enantioselective synthesis of α-amino esters through Petasis borono-Mannich multicomponent reaction of potassium trifluoroborate salts. J. Chem. Res. 2019, 43, 557–564. [Google Scholar] [CrossRef]
- Marques, C.S.; López, Ó.; Leitzbach, L.; Fernández-Bolaños, J.G.; Stark, H.; Burke, A.J. Survey of New, Small-Molecule Isatin-Based Oxindole Hybrids as Multi-Targeted Drugs for the Treatment of Alzheimer’s Disease. Synthesis 2022, 54, 4304–4319. [Google Scholar] [CrossRef]
- Busto, N.; Leitão-Castro, J.; García-Sosa, A.T.; Cadete, F.; Marques, C.S.; Freitas, R.; Burke, A.J. N-1,2,3-Triazole–isatin derivatives: Anti- proliferation effects and target identification in solid tumour cell lines. RSC Med. Chem. 2022, 13, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; González-Bakker, A.; Padrón, J.M.; Burke, A.J. Easy access to Ugi-derived isatin-peptoids and their potential as small-molecule anticancer agents. New J. Chem. 2023, 47, 743–750. [Google Scholar] [CrossRef]
- Brandão, P.; López, Ó.; Leitzbach, L.; Stark, H.; Fernández-Bolaños, J.G.; Burke, A.J.; Pineiro, M. Ugi Reaction Synthesis of Oxindole-Lactam Hybrids as Selective Butyrylcholinesterase Inhibitors. ACS Med. Chem. Lett. 2021, 12, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Brandão, P.; Puerta, A.; Padrón, J.M.; Kuznetsov, M.L.; Burke, A.J.; Pineiro, M. Ugi Adducts of Isatin as Promising Antiproliferative Agents with Druglike Properties. Asian J. Org. Chem. 2021, 10, 3434–3455. [Google Scholar] [CrossRef]
- Marques, C.S.; McArdle, P.; Erxleben, A.; Burke, A.J. Accessing New 5-α-(3,3-Disubstituted Oxindole)-Benzylamine Derivatives from Isatin: Stereoselective Organocatalytic Three Component Petasis Reaction. Eur. J. Org. Chem. 2020, 2020, 3622–3634. [Google Scholar] [CrossRef]
- Brandao, P.; Marques, C.S.; Pinto, E.; Pineiro, M.; Burke, A.J. Petasis adducts of tryptanthrin–synthesis, biological activity evaluation and druglikeness assessment. New J. Chem. 2021, 45, 14633–14649. [Google Scholar] [CrossRef]
- Brandão, P.; Pineiro, M.; Burke, A.J. Tryptanthrin and Its Derivatives in Drug Discovery: Synthetic Insights. Synthesis 2022, 54, 4235–4245. [Google Scholar] [CrossRef]
- Jia, S.Q.; Qin, W.L.; Wang, P.F.; Yan, H.L. Organocatalytic atroposelective construction of axially chiral nonsymmetric biaryltriols and their applications in asymmetric synthesis and heavy metal ion detection. Org. Chem. Front. 2022, 9, 923–928. [Google Scholar] [CrossRef]
- Parvin, T.; Yadav, R.; Choudhury, L.H. Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs). Org. Biomol. Chem. 2020, 18, 5513–5532. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, C.J. Recent advances in asymmetric organocatalysis mediated by bifunctional amine–thioureas bearing multiple hydrogen-bonding donors. Chem. Commun. 2015, 51, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Serdyuk, O.V.; Heckel, C.M.; Tsogoeva, S.B. Bifunctional Primary Amine-Thioureas in Asymmetric Organocatalysis. Org. Biomol. Chem. 2013, 11, 7051–7071. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H.; Takemoto, Y. Discovery and Application of Asymmetric Reaction by Multi-Functional Thioureas. Bull. Chem. Soc. Jpn. 2008, 81, 785–795. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Miyabe, H.; Takemoto, Y. Catalytic Enantioselective Petasis-Type Reaction of Quinolines Catalyzed by a Newly Designed Thiourea Catalyst. J. Am. Chem. Soc. 2007, 129, 6686–6687. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, T.; Suzuki, Y.; Sakaeda, T.; Takemoto, Y. Synthesis of Optically Active N-Aryl Amino Acid Derivatives through the Asymmetric Petasis Reaction Catalyzed by a Novel Hydroxy–Thiourea Catalyst. Chem. Asian J. 2011, 6, 2902–2906. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, Y.; Inokuma, T. Chapter 29: Bifunctional Thiourea Catalysts. In Asymmetric Synthesis II: More Methods and Applications, 1st ed.; Christmann, M., Bräse, S., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2012; pp. 233–237. [Google Scholar]
- Takemoto, Y. Development of Chiral Thiourea Catalysts and Its Application to Asymmetric Catalytic Reactions. Chem. Pharm. Bull. 2010, 58, 593–601. [Google Scholar] [CrossRef]
- Han, W.Y.; Wu, Z.J.; Zhang, X.M.; Yuan, W.C. Enantioselective Organocatalytic Three- Component Petasis Reaction among Salicylaldehydes, Amines, and Organoboronic Acids. Org. Lett. 2012, 14, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Selander, N.; Kipke, A.; Sebelius, S.; Szabo, K.J. Petasis Borono-Mannich Reaction and Allylation of Carbonyl Compounds via Transient Allyl Boronates Generated by Palladium-Catalyzed Substitution of Allyl Alcohols. An Efficient One-Pot Route to Stereodefined r-Amino Acids and Homoallyl Alcohols. J. Am. Chem. Soc. 2007, 129, 13723–13731. [Google Scholar] [CrossRef]
- Beisel, T.; Manolikakes, G. Palladium-Catalyzed Enantioselective Three-Component Synthesis of α-Substituted Amines. Org. Lett. 2015, 17, 3162–3165. [Google Scholar] [CrossRef] [PubMed]
- Beisel, T.; Diehl, A.M.; Manolikakes, G. Palladium-Catalyzed Enantioselective Three-Component Synthesis of α-Arylglycines. Org. Lett. 2016, 18, 4116–4119. [Google Scholar] [CrossRef] [PubMed]
- Beisel, T.; Manolikakes, G. A Lewis Acid Palladium(II)-Catalyzed Three-Component Synthesis of α-Substituted Amides. Org. Lett. 2013, 15, 6046–6049. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Hendrix, J.A. Asymmetric Synthesis of Arylglycines. Chem. Rev. 1992, 92, 889–917. [Google Scholar] [CrossRef]
- Diehl, A.M.; Manolikakes, G. Palladium-Catalyzed Decarboxylative Three-Component Synthesis of α-Arylglycines: Replacing Boronic with Carboxylic Acids in the Petasis Reaction. ChemCatChem 2020, 12, 3463–3466. [Google Scholar] [CrossRef]
- Wu, C.L.; Qin, X.R.; Moeljadi, A.M.P.; Hirao, H.; Zhou, J.S. Copper-Catalyzed Asymmetric Arylation of N-Heteroaryl Aldimines: Elementary Step of a 1,4-Insertion. Angew. Chem. Int. Ed. 2019, 58, 2705–2709. [Google Scholar] [CrossRef] [PubMed]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, C.; Brandão, P. The Asymmetric Petasis Borono-Mannich Reaction: Insights on the Last 15 Years. Catalysts 2023, 13, 1022. https://doi.org/10.3390/catal13061022
Marques C, Brandão P. The Asymmetric Petasis Borono-Mannich Reaction: Insights on the Last 15 Years. Catalysts. 2023; 13(6):1022. https://doi.org/10.3390/catal13061022
Chicago/Turabian StyleMarques, Carolina, and Pedro Brandão. 2023. "The Asymmetric Petasis Borono-Mannich Reaction: Insights on the Last 15 Years" Catalysts 13, no. 6: 1022. https://doi.org/10.3390/catal13061022
APA StyleMarques, C., & Brandão, P. (2023). The Asymmetric Petasis Borono-Mannich Reaction: Insights on the Last 15 Years. Catalysts, 13(6), 1022. https://doi.org/10.3390/catal13061022







