Abstract
Inulin is a renewable and cheap carbon source used in microbial fermentations. Bacillus licheniformis 24 is known as an excellent 2,3-butanediol (2,3-BD) producer from fructose; therefore, the cloning and expression of a robust heterologous inulinase could enhance its 2,3-BD production from inulin. The inu gene of Lacticaseibacillus paracasei DSM 23505 encoding fructan-β-fructosidase (EC 3.2.1.80) was chosen for the purpose. PCR fragments containing the complete inu (3.6 kb) and its truncated variant inu-tr (2.2 kb, lacking Big3 cell wall attachment domains) were cloned into Escherichia coli StellarTM and B. licheniformis 24. The high quality of the recombinant constructs was confirmed by restriction analysis, PCR, sequencing, and phenotypic tests. The results showed that the inulinase activity of B. licheniformis cells harboring the full-length inu variant (T26) was eightfold higher compared to the wild type, retaining cell wall attachment in the B. licheniformis host. In contrast, the truncated variant inu-tr (T14) showed mostly extracellular but weak activity, thus suggesting that the Big3 domains are also important for the enzyme’s function. During flask-batch fermentation of 100 g/L raw chicory flour (containing 90% inulin), T26 produced acetoin and 2,3-BD from inulin. Contrariwise, T14 and the wild type formed products only from the mono- and disaccharides naturally found in the chicory flour. In the fermenter, from 200 g/L of raw chicory flour, the recombinant T26 degraded approximately 140 g/L of the inulin. However, the final concentrations of the produced 2,3-BD and acetoin were 18.5 g/L and 8.2 g/L, respectively, because of the accumulation of unconverted sucrose. To conclude, further strain improvement is necessary to make the process efficient for obtaining 2,3-BD from inulin by simultaneous saccharification and fermentation (SSF).
1. Introduction
According to a recent forecast, the global market for 2,3-butanediol (2,3-BD) will grow to USD 300 million by 2030 [1]. This incredibly high demand is due to the application of 2,3-BD in several industries, namely, food (as a flavoring ingredient), chemical (as a platform reagent in polymer synthesis), cosmetics and pharmaceuticals, and also as antifreeze and additive in oils and fuels [2,3,4,5,6]. The perspectives on the production of bio-based chemicals in the forthcoming 2022–2050 period include an increase in the demand for carbon for chemicals from 450 million metric tons (MMt) per annum in 2021 to 1000 MMt per annum in 2050 [7]. A new concept in biotechnology known as “funneling” envisages the development of microbial fermentations to convert heterogeneous raw materials into a single product. Therefore, the current trend in microbial production of 2,3-BD should be directed towards the use of a cheap and renewable substrate such as plant biomass [8].
Inulin is a polysaccharide composed of fructose units linked by β (2→1) bonds, with a single glucose moiety at the reducing end. It is a natural reserve polysaccharide in plants of the Asteraceae family such as Jerusalem artichoke, chicory, dahlia, and globe artichoke [9]. Among them, chicory is the most widely used crop because it can be harvested three times a year, it is not pretentious to soils, and contains up to 98% inulin in its fibers [10]. Chicory inulin is an abundant, renewable, cheap, and inedible microbial substrate and was used for 2,3-BD production by Klebsiella pneumoniae, Paenibacillus polymyxa, the engineered Bacillus sp. BRC1, and Bacillus licheniformis ATCC 14580 [11,12,13,14].
As a 2,3-BD producer, B. licheniformis is known for having the highest yield compared to other bacilli, its advantageous GRAS (generally regarded as safe) status [15], and the ability to convert various carbohydrates such as glucose, fructose, cellobiose, starch, and glycerol, as well as the lignocellulosic sugars galactose, mannose, and xylose [16,17,18]. Another advantage of B. licheniformis is its extremely high tolerance to 2,3-BD, withstanding titers of 14% (w/v), and the maintenance of predominant 2,3-BD synthesis (instead of acetoin), even at a high aeration regime, due to its aerophilic nature [19]. However, the genetic modification of B. licheniformis strains has so far been focused primarily on eliminating the expression (knockout) of specific 2,3-BD dehydrogenases (budC, gdh, acoR) in order to improve the stereoisomeric profile of the resulting 2,3-BD from glucose as a substrate [20,21]. In contrast, there is no evidence of any literature in which genetically improving B. licheniformis strains was attempted in order to expand their substrate spectrum by the heterologous expression of glycoside hydrolases.
B. licheniformis 24 was isolated recently as a promising 2,3-BD producer from plant biomass. The strain displayed a slight inulinase activity during batch cultivation in a medium containing 50 g/L inulin [22]. It was also a highly efficient 2,3-BD producer from fructose [23]; therefore, B. licheniformis 24 could be a very useful inulin-based 2,3-BD producer after its inulinase activity enhancement. Thus, the aim of the present study was the genetic improvement of B. licheniformis 24 by cloning and expression of the inu gene encoding a powerful inulinase enzyme of Lacticaseibacillus paracasei DSM 23505 [24,25]. The major novelties of the work consist in cloning a heterologous inulinase gene in B. licheniformis and the successful expression of a Lactobacillus glycoside hydrolase in a Bacillus host. The potential of the resulting recombinants to convert crude, insoluble inulin is described below.
2. Results
2.1. Selection of Inulinase: The Inu Gene of Lc. paracasei DSM 23505
In order to complement the existing inulinase activity of B. licheniformis 24, the inu gene of Lc. paracasei DSM 23505 (GenBank KP663715.1) was selected. It consists of 3645 bp and encodes a protein of the glycosyl hydrolase family GH32 (Figure 1).

Figure 1.
(a) 3D model of inulinase (EC 3.2.1.80) of Lc. paracasei DSM 23505; the amino acids of the catalytic triad, two aspartates (D) and glutamate (E), are shown in purple. The model was made by homology in SWISS-MODEL Workspace, using QMEAN assessment of the model’s quality [26]. (b) The amino acid sequence of the enzyme. The signal peptide is shown in green; the polypeptide of 731 aa belonging to GH32 Glyco Hydro family is shown in blue; Big3 domains responsible for the cell wall anchoring are grey; the amino acids of the catalytic triad are highlighted yellow; and the conserved regions are underlined.
The enzyme contains a putative secretion sequence of 38 amino acids, a β-fructosidase catalytic domain, a C-terminal domain, and four Big3 domains responsible for the anchoring to the cell wall. Alignment of the amino acid sequences of GH32 family enzymes revealed three highly conserved motifs (underlined in Figure 1b), each containing an amino acid from a catalytic triad composed of two aspartates (D) and one glutamate (E) [24].
2.2. Cloning of the Inu Gene in pBE-S and pMA5 E. coli/Bacillus spp. Shuttle Vectors
The gene inu was cloned into two different shuttle vectors and in two different length variants. Maps of the constructs used to transform E. coli and B. licheniformis are shown in Figure 2.
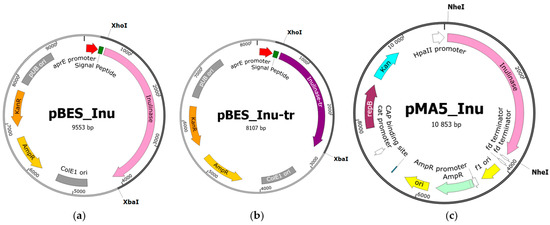
Figure 2.
Physical maps of the recombinant constructs containing inu gene. (a) pBES_Inu; (b) pBES_Inu-tr; (c) pMA5_Inu. The plasmid constructs maps were conducted with SnapGene software version 6.2.1 (GSL Biotech LLC, San Diego, CA, USA).
The obtained constructs were digested with the relevant restriction enzymes, and the proper sequence of the inu gene was confirmed by PCR and sequencing (Figure 3).
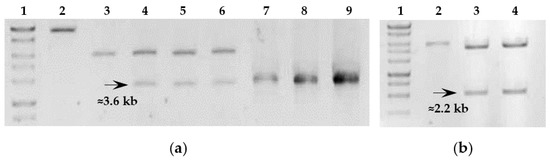
Figure 3.
Analysis of the recombinant constructs isolated from E. coli STELLARTM clones based on pBE-S vector. (a) Line 2: linearized construct pBES_Inu; Line 3: linear pBE-S vector; Lines 4, 5, and 6: plasmid DNA of pBES_Inu clones digested with XbaI and XhoI; Lines 7, 8, and 9: PCR amplification of the insert of 3.6 kb. (b) Line 2: linearized pBE-S vector; Lines 3 and 4: plasmid DNA of pBES_Inu-tr clones digested with XbaI and XhoI. Perfect Plus 1 kb DNA Ladder (EURx, Gdansk, Poland) was used as a molecular weight marker with fragments of the following sizes: 10.0, 8.0, 6.0, 5.0, 4.0, 3.0, 2.5, 2.0, 1.5 kb.
The plasmids isolated from E. coli clones containing pMA5_Inu were analyzed by BamHI restriction digest to determine the orientation of the cloned inu gene (Figure 4). Fragments of 3446 bp and 7404 bp indicated the correct orientation of the gene, while 10 602 bp and 251 bp fragments indicated the opposite orientation. The restriction analysis of 12 clones revealed that half of them had the correct orientation.
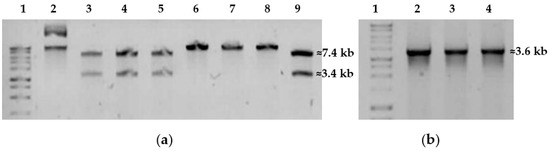
Figure 4.
Analysis of the recombinant constructs isolated from E. coli STELLARTM clones based on pMA5 vector. (a) Line 2: pMA5_Inu, undigested control; Lines 3, 4, 5, and 9: pMA5_Inu digested by BamHI confirming the proper inu gene orientation; (b) Lines 2, 3, and 4: PCR amplification of the insert of 3.6 kb with template plasmid DNA isolated from E. coli clones containing pMA-5_Inu. Perfect Plus 1 kb DNA Ladder (EURx, Gdansk, Poland) was used as a molecular weight marker with fragments of the following sizes: 10.0, 8.0, 6.0, 5.0, 4.0, 3.0, 2.5, 2.0, 1.5 kb.
All recombinant constructs were introduced into B. licheniformis 24 via electroporation. A summary of the results obtained from analysis of the transformants is presented in Table 1. B. licheniformis 24 clones were selected by their resistance to kanamycin and increased hydrolase activity (Figure 5), which was analyzed by subculturing the colonies on LB-agar plates containing 1% raw, insoluble inulin.

Table 1.
Introducing recombinant constructs containing inu gene variants into B. licheniformis 24.
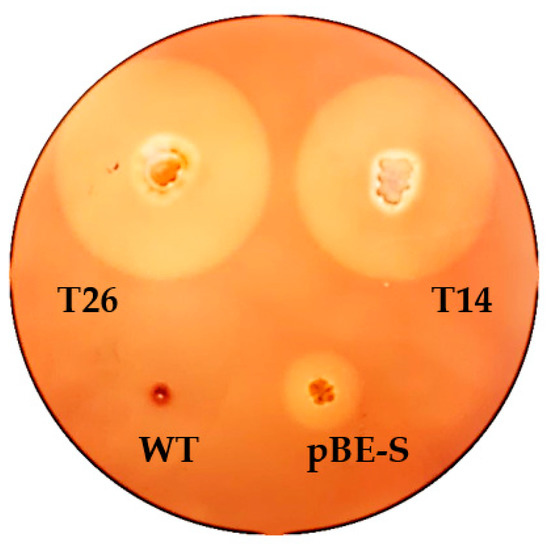
Figure 5.
Comparison of the inulin hydrolysis zones obtained by selected B. licheniformis transformants on LB agar supplemented with 1% HD inulin and 50 μg/mL kanamycin. Designations: T26, B. licheniformis containing pBES_Inu construct; T14, B. licheniformis with pBES_Inu-tr; pBE-S, B. licheniformis transformed with the “empty” vector; WT, wild type B. licheniformis 24 (does not grow in media with kanamycin).
The largest halos (2.5–2.7 cm) were formed by B. licheniformis clones containing pBES_Inu. Of them, clone 26 (T26, Figure 5) was selected for further fermentations of inulin. Clone 14 (T14), containing pBES_Inu-tr (with a halo of 2.2–2.3 cm), looked just as promising and was chosen for further batch processes. Of the total 164 analyzed clones obtained with pMA5_Inu, none were found to have a hydrolysis zone significantly larger than that of the wild type.
2.3. Production of 2,3-BD by B. licheniformis T14 and T26 during Flask-Batch Processes
The ability of B. licheniformis recombinant clones to produce 2,3-BD from raw, insoluble inulin was studied in the course of flask-batch fermentations (Figure 6).
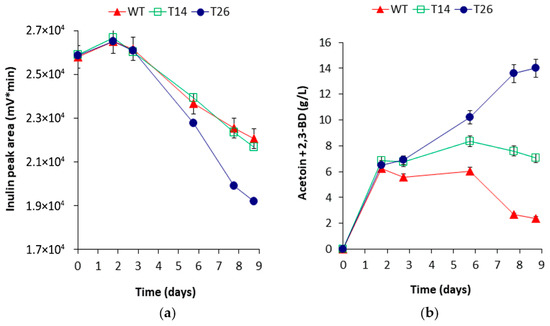
Figure 6.
Batch fermentation of 100 g/L insoluble inulin by B. licheniformis recombinant clones T26, T14, and the host B. licheniformis 24 (WT) in optimized medium and process parameters. (a) Time course of inulin hydrolysis (estimated as peak area of oligosaccharides with degree of polymerization DP3–DP9). (b) Time course of acetoin+2,3-BD production.
Raw chicory flour with an initial concentration of 100 g/L, containing 90% insoluble inulin with DP8-DP13, and a 10% mix of fructose, sucrose, and glucose, was used as a substrate. Inulin was most efficiently consumed by T26 cells, while T14 and WT showed a similar profile of slower degradation (Figure 6a). By the 40 h mark, T26, T14, and WT completely utilized the substrate-introduced sugars producing about 6 g/L acetoin + 2,3-BD. Thereafter, WT and T14 failed to form more of the products investigated. In contrast, T26 produced approximately 8 g/L more 2,3-BD and acetoin, reaching a final concentration of 14 g/L (Figure 6b). Evaluation of the inulinase activity of the recombinant clones showed a significant increase over WT. The inulinase activity shown by T26 was more than eight times higher (mean 8.7 U/mL) compared to WT (ranging from 0.5 to 1.5 U/mL) and more than three times higher than that of T14 (2.7 U/mL). Examination of the localization of the heterologous enzyme by assaying cells and cell-free supernatants showed predominantly cell-bound inulinase activity of T26 and predominantly extracellular activity of T14 and WT.
2.4. Production of 2,3-BD by B. licheniformis T26 from Inulin during the Fermenter-Batch Process
The batch cultivation in the fermenter was performed from 200 g/L raw chicory flour using process parameters previously optimized for B. licheniformis 24-based 2,3-BD production from glucose and fructose [19,23]. Thus, the inulin fermentation was carried out at pH 6.23, a temperature of 37.8 °C, and aeration of 3.68 vvm.
In this process, the initial sugars (fructose, glucose, and sucrose) were completely used up within the first 16 h, yielding 7.22 g/L 2,3-BD and 3.96 g/L acetoin. The biomass grew exponentially until the 72nd hour, reaching 8.8 × 107 CFU/mL, and completely plateauing after the 96th hour (1.2 × 108 CFU/mL). No degradation of inulin was observed during the first 48 h. As a result, between the 16th and 48th hour, some amount of the 2,3-BD was converted to acetoin. When inulin degradation began, the concentration of 2,3-BD rose again. Nevertheless, a sharp accumulation of sucrose was observed in the broth after the 66th hour. Thus, after 160 h of fermentation, although approximately 140 g/L inulin was degraded, 53 g/L sucrose and only 18.5 g/L 2,3-BD were accumulated. The amounts of by-products were relatively low: acetoin (8.2 g/L), acetic acid (6.5 g/L), lactic acid (1.27 g/L), and glycerol (0.33 g/L) (Figure 7).
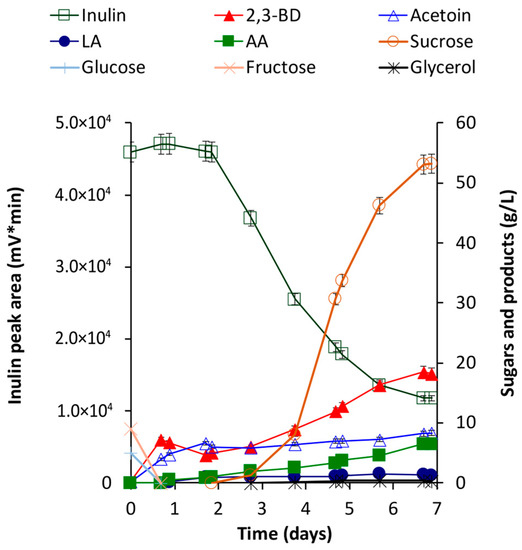
Figure 7.
Batch fermentation of 200 g/L raw chicory flour by B. licheniformis T26. Designations: LA, lactic acid; AA, acetic acid. Mean values from three processes are presented.
3. Discussion
Bacillus spp. are efficient and reliable producers of 2,3-BD not only from glucose but from various substrates, especially inexpensive cellulosic and non-cellulosic ones [27]. The raw materials containing sucrose (sugar cane and beet), starch, molasses, and inulin have been most widespread in microbial processes for 2,3-BD synthesis [3,6]. On the other hand, B. licheniformis 24 is capable of producing extremely high amounts of 2,3-BD from fructose [23]. During a 72-h fed-batch process, from a total of 370 g/L fructose, the strain gained 156.1 g/L 2,3-BD, as well as having appeared to be a promising fructose polymer-based 2,3-BD producer. However, the use of natural substrates in SSF processes is always problematic because, as a rule, the resulting yields and productivity are low. For example, in the case of molasses, the natural strain B. subtilis CICC10025 [28], the engineered B. subtilis 1A1 [29], and the isolate B. subtilis DL01 [30] yielded between 35 and 65 g/L 2,3-BD, which may seem high for the point of view of bacterial yields, but it remains a yield that is hardly sufficient for downstream industrial processes.
It is notable that alongside molasses, the strain of B. amyloliquefaciens TUL 308 required glucose feeding for better results [31]. Similarly, B. subtilis strain TUL 322 needed four additions of glucose to yield 75 g/L 2,3-BD [32]. For better results, microbial fermentation for 2,3-BD and acetoin synthesis requires preliminary hydrolysis of the substrate. For example, the high productivity of B. licheniformis NCIMB 8059 from starch (0.58 g/L/h) was achieved by treatment of the corn flour with α-amylase and amyloglucosidase [33]. Similarly, B. subtilis producers of acetoin needed preliminary hydrolysis of the substrates okara flour or sugarcane bagasse [34,35].
Many authors rank inulin as the most attractive substrate for microbial production of 2,3-BD [3,4,36,37,38]. This opinion is based on the development of several very efficient processes engaging Klebsiella pneumoniae (belonging to the Risk 1 bacterial group). Notably, Sun et al. [11] obtained 84 g/L 2,3-BD in SSF fed-batch fermentation by K. pneumoniae CICC 10011 from Jerusalem artichoke tubers. Further, Li et al. [10] obtained 80.5 g/L target products (2,3-BD plus acetoin) by the same strain from Jerusalem artichoke stalk hydrolysate, while Dai et al. obtained 80.4 g/L 2,3-BD from Jerusalem artichoke tuber extract by the K. pneumoniae H3 strain, gaining a yield of 0.426 g/g inulin [39].
In contrast, impressive reports of inulin conversion into 2,3-BD by nonpathogenic strains are quite rare. One successful example was the SSF developed by Gao et al. [12] where, though the use of the Paenibacillus polymyxa ZJ-9 strain, 36.92 g/L 2,3-BD was yielded from Jerusalem artichoke tubers extract. However, from commercial inulin, ZJ-9 produced 6.6 g/L 2,3-BD only. A higher concentration, 103 g/L 2,3-BD, was obtained by Li et al. by B. licheniformis ATCC 14580 [14]. Although the authors claimed that this process was SSF, the fermentation of sugars actually occurred after several external additions (30 U/mL each) of purified inulinase enzyme. A real SSF, involving the inulin of the Jerusalem artichoke tuber, was conducted by Park et al. [13]. By introducing the gene, sacC, responsible for inulin hydrolysis in Bacillus sp. strain BRC1, the enzyme activity of the recombinant was increased twofold, and its 2,3-BD production increased from 3.98 to 8.10 g/L.
The observation of the genome of B. licheniformis type strain ATCC 14580T reveals that it contains several genes for glycoside-hydrolase enzymes related to inulin hydrolysis (sacA, sacB, sacC, lev, and fru). Some of these enzymes are characterized, such as sucrose-6-phosphate hydrolase (sucrase, β-fuctofuranosidase) [40], levansucrase [41,42], and levanase [22]. Since these enzymes act as hydrolases displaying cross-specificity to similar substrates, i.e., both levanases and sucrases have minor inulinase activity, a slight natural inulinase activity of B. lichenifromis strains is to be expected.
In our previous work, dedicated to the characterization of new Bacillus spp. isolates from nature, B. licheniformis 24 was found to possess some natural inulinase activity [22]. Thus, attempting to enhance this activity, in this study, the inu gene of Lp. paracasei DSM 23505 [24] was chosen for cloning. The inu gene encodes an exoinulinase (3.2.1.80) with high substrate specificity to inulin. A preliminary investigation in the Km and Vmax of the original enzyme of Lc. paracasei showed that Km for substrate HD inulin is 0.33, while for sucrose it is 55.37 [25]. In the presence of the preferred substrate inulin (to which the enzyme has a higher affinity), sucrose hydrolysis is hindered. The accumulation of high amounts of sucrose suggests that the heterologous enzyme was the major acting inulin-degrading hydrolase in B. licheniformis recombinant T26. With the cell wall anchoring being retained in T26, this suggests that the enzyme was efficiently produced and transported through the cell membrane. The recombinant T26 possessed a higher (eightfold) increase of the inulinase activity compared to the wild type, being capable of degrading high amounts of inulin (140 g/L). While T26 was able to produce 18.5 g/L 2,3-BD, which is quite an impressive amount for a genetically modified Bacillus spp., the yield was still low (0.1 g/g). Although various tools for the genetic improvement of B. licheniformis have been developed [43,44,45,46], further research is needed to establish successful cloning systems suitable for this microorganism. For example, shuttle vectors, which have been developed for cloning into B. subtilis, cannot be directly applied. If the two shuttle vectors used in this study are to be compared, pBE-S, which contains the aprE promoter, was found to be more suitable for inu gene expression. Contrastingly, the hpaII promoter of pMA-5 vector was not effective in B. licheniformis, confirming the observation of Li et al. [47].
4. Materials and Methods
4.1. Bacterial Strains, Media, and Cultivation Conditions
E. coli STELLARTM competent cells with genotypes F-, endA1, supE44, thi-1, recA1, relA1, gyrA96, phoA, Φ80d lacZΔ M15, Δ(lacZYA-argF) U169, Δ(mrr-hsdRMS-mcrBC), ΔmcrA, λ- were purchased from Clontech Laboratories Inc. (Takara Bio Company, Mountain View, CA, USA).
Lc. paracasei strain B41 has been isolated from the Bulgarian traditional beverage Boza [48] and was deposited in the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ) under registration number DSM 23505.
B. licheniformis 24 was isolated from a soil sample [22] and stored in the microbial collection of the Institute of Microbiology, Bulgarian Academy of Sciences.
E. coli and B. licheniformis strains were cultivated in LB broth, supplemented with 1.5% agar (when needed) (Alfa Aesar GmbH and Co. KG; Karlsruhe, Germany), and ampicillin or kanamycin with final concentrations of 50 μg/mL (AppliChem GmbH, Darmstadt, Germany). Lc. paracasei strain B41 was grown in MRS broth. The strains were stored in slanted agar tubes at 4 °C, or frozen at −80 °C, and supplemented with glycerol (20% v/v).
The flask-batch cultivation of B. licheniformis clones was carried out in 500 mL flasks containing 100 mL medium with the following content (g/L): yeast extract, 5; tryptone, 5; (NH4)2SO4, 3; KH2PO4, 3.5; K2HPO4, 2.75; MgSO4, 0.2; ammonium acetate, 1.5; CoCl2 × 6H2O, 0.09; salt solution, 3 mL per liter, containing (g/L): FeSO4, 0.4; H3BO3, 0.8; CuSO4 × 5H2O, 0.04; NaMoO4 × 2H2O, 0.04; MnCl2 × 4H2O, 5.0; ZnSO4 × 7H2O, 0.1; Co(NO3)2 × 6H2O, 0.08; CaCl2 × 2H2O, 1.0; and Biotin, 0.01 [49]. As a substrate, raw chicory flour was used, which contains 90% insoluble inulin and up to 10% sugars (Sensus B.V., Roosendaal, The Netherlands). The flasks were incubated on a rotary shaker at 37 °C and 140 rpm.
Batch processes with pH and aeration control were performed in a 1 L stirred bioreactor (Biostat® A Plus, Sartorius Stedim Biotech, Gottingen, Germany), additionally equipped with bumpers, air pump, and rotameter in an attempt to ensure higher levels of oxygen supply. The pH, temperature, and aeration rate were maintained at their optimal values of 6.23 (by addition of 6M NaOH or 5M HCl), 37.8 °C, and 3.68 vvm [19].
Two E. coli/B. subtilis shuttle vectors were used. The pBE-S (PaprE, aprE SP, Kanr, Ampr) vector was purchased from Clontech Laboratories Inc. (Takara Bio Company, Mountain View, CA, USA). The pMA5 (PHpaII, Ampr, Kanr) vector was purchased from Nova Lifetech Pte Ltd. (Hong Kong, China).
4.2. Molecular cloning of Inulinase Genes into pBE-S and pMA5 Vectors
The complete inulinase gene (inu, 3645 bp) from Lc. paracasei B41 was obtained by digestion with XbaI and XhoI of a pJET2.1/blunt recombinant construct previously obtained by Petrova et al. [24]. Separately, the inu gene was amplified from the total DNA of Lc. paracasei B41 with primers designed to contain NheI restriction sites. The truncated version (without the Big3 domains) of the same inulinase gene (inu-tr, 2913 bp) was amplified from the total DNA of Lc. paracasei B41 with primers designed to contain restriction sites for XbaI and XhoI. PCR amplification was performed in QB-96 Satellite Gradient Thermal Cycler (LKB Vertriebs GmbH, Vienna, Austria) with primers specially designed to contain XbaI, XhoI, and NheI restriction sites. The primers used, as well as the optimal annealing temperatures, are listed in Table 2.

Table 2.
Primers that were designed to amplify the inulinase gene of Lc. paracasei DSM 23505 with NCBI GenBank accession number KP663715. The introduced sites for endonuclease enzymes are underlined. The introduced “stop” codons are shown in bold and italics.
PCR reactions consisted of a 15 ng DNA template, 0.4 µM primers, Premix Ex Taq Hot Start Version (Clontech Laboratories, Inc., A Takara Bio Company, Mountain View, CA, USA), and sterile water to 25 µL final volume. Between initial denaturation for 3 min and 30 s at 98 °C and final elongation for 5 min at 72 °C, the following temperature profile was used for 38 cycles: 10 s denaturation at 98 °C, 45 s annealing at either 57 °C or 60 °C, and 2.5 min elongation at 72 °C.
Both genes (inu and inu-tr) were cloned into the pBE-S vector digested with XhoI and XbaI. The inu gene was also inserted into the pMA5 shuttle vector digested with NheI.
The recombinant constructs were transformed in E. coli STELLARTM competent cells. Sufficient amounts of plasmid DNA from the E. coli clones were obtained with Plasmid Miniprep DNA Purification Kit (EURx®, Gdansk, Poland). Plasmids and DNA fragments were visualized using gel electrophoresis on 1–1.5% agarose (AlfaAesar, Kandel, Germany), in TAE buffer (40 mM Tris-base, 20 mM acetic acid, 1 mM EDTA), and stained with SimplySafeTM (EURx®, Gdansk, Poland).
4.3. Transformation and Clone Selection
Transformation of E. coli was carried out following Protocol PT5055-2 of STELLARTM competent cells manufacturer’s instructions.
Transformation of B. licheniformis 24 with recombinant constructs was performed via electroporation following a modified version of the high-osmolarity protocol by Xue et al. [50]. An overnight culture in standard LB media (1% tryptone, 0.5% yeast extract, 0.5% NaCl) was diluted 16 times in LB media with 0.5 M sorbitol in 500 mL Erlenmeyer flask and grown until OD600 of 0.9. The flask was chilled on ice for 10 min and the bacteria were washed four times with an ice-cold electroporation medium (0.5 M sorbitol, 0.5 M mannitol, 10% glycerol). After the final centrifugation (3350 g/10 min/4 °C), the competent cells were resuspended in a 625 µL electroporation medium. Aliquots of 60 µL were used for electroporation in ice-cold GenePulser cuvettes with 0.1 cm electrode gap on MicroPulser electroporator (BioRad Laboratories, Hercules, CA, USA). A pulse of 2.1 kV was applied for 4–5 ms, and 1 mL recovery medium (0.5 M sorbitol and 0.38 M mannitol in LB) was added as quickly as possible. The culture was transferred into 15 mL glass tubes, incubated for 3 h, spread on Petri dishes with LB-agar, and left overnight at 37 °C. Competent cells were stored at –70 °C and reused several times with a minimal loss of electroporation efficiency.
Clone selection was performed on grid dishes with agar medium supplement with 1% inulin. Iodine staining and water destaining were used to visualize the zones of hydrolysis after 48 h of cultivation.
4.4. Inulinase Activity Assay
The inulinase activity of intact cells twice washed with water and cell-free culture supernatants was investigated. The samples were suitably diluted in phosphor citrate buffer (0.16 M Na2HPO4, 0.02 M citrate, pH 5.0, containing 1% inulin) and incubated at 50 °C for 60 min. The amount of reducing sugars was estimated with a DNS reagent (2.18% 3,5-dinitorsalycilic acid in 0.4 M NaOH with 30% (w/v) Rochelle salt). The absorbance at 540 nm was measured on a Helios Omega UV–VIS spectrophotometer (Thermo Scientific, Waltham, MA, USA) with a separate control for each reaction, and a fructose standard was used to estimate the increased amount of reducing sugars. One unit of enzyme activity was defined as the amount of the enzyme that releases 1 μmol fructose per minute.
4.5. Analytical Methods
Sugars and fermentative products were determined by HPLC analysis using YL Instrument 9300 HPLC System (YL Instrument Co., Ltd., Anyang, Republic of Korea). They were separated by HPLC column Aminex HPX-87H at 65 °C with mobile phase 5 mM H2SO4 at a flow rate 0.6 mL/min (BioRad Laboratories, Hercules, CA, USA) as sugars and 2,3-BD were detected by RI detector (YL 9170 RI Detector), while acetoin, lactic, and acetic acids were detected by UV detector (YL 9120 UV/Vis Detector) at wavelengths of 190 and 210 nm. Glucose, fructose, sucrose, and oligo-sugars were additionally analyzed by BioRad column HPX-87C at 85 °C with mobile phase water with a flow rate of 0.6 mL/min.
5. Conclusions
The microbial production of 2,3-BD by B. licheniformis 24 from raw chicory flour was investigated. The approach to enhance the weak natural inulinase activity of the strain was successfully applied, as this study is the first to report heterologous inulinase production by B. licheniformis and the successful transfer of an inulinase gene of Lactobacillus origin into a Bacillus host. Some of the recombinants showed inulinase activity many times higher compared to the wild type. The findings of this study indicate that despite the successful cloning and expression of the heterologous inu gene, the resulting 2,3-BD is hardly sufficient for an efficient process. The hydrolysis of insoluble inulin is slow, and the resulting sugars are not converted to 2,3-BD efficiently enough, due to the accumulation of sucrose. During a batch process in a fermenter, 18.5 g/L 2,3-BD and 8.2 g/L acetoin were obtained from a starting amount of 200 g/L raw chicory flour. Thus, the engineered B. licheniformis has some potential as a microbial cell factory to produce 2,3-BD during the simultaneous hydrolysis and fermentation of inulin, but the recombinants need further improvement, and the process needs intensification to be industrially applicable.
Author Contributions
Conceptualization, K.P. and P.P.; methodology, K.P. and P.P.; investigation, A.A., L.T. and K.P.; writing—original draft preparation, P.P. and K.P., software, visualization, E.G.; writing—review and editing, K.P. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bulgarian Ministry of Education and Science under the National Research Programme “Young scientists and postdoctoral students-2” approved by DCM 206/07.04.2022.
Data Availability Statement
Data supporting the reported results can be provided on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.-J.; Koutinas, A.; Venus, J. Volumetric oxygen transfer coefficient as fermentation control parameter to manipulate the production of either acetoin or D(−)2,3-butanediol using bakery waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state of the art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- Song, D.; Cho, S.-Y.; Vu, T.-T.; Duong, H.-P.-Y.; Kim, E. Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone: Modeling, Numerical Analysis and Validation Using Pilot-Scale Reactor Data. Catalysts 2021, 11, 999. [Google Scholar] [CrossRef]
- Petrov, K.; Petrova, P. Current Advances in Microbial Production of Acetoin and 2,3-Butanediol by Bacillus spp. Fermentation 2021, 7, 307. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef] [PubMed]
- Ewing, T.A.; Nouse, N.; van Lint, M.; van Haveren, J.; Hugenholtz, J.; van Es, D.S. Fermentation for the production of biobased chemicals in a circular economy: A perspective for the period 2022–2050. Green Chem. 2022, 24, 6373–6405. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic bio-refinery approach for microbial 2,3-Butanediol production. Bioresour. Technol. 2020, 302, 122873. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods 2022, 11, 3032. [Google Scholar] [CrossRef]
- Li, D.; Dai, J.Y.; Xiu, Z.L. A novel strategy for integrated utilization of Jerusalem artichoke stalk and tuber for production of 2,3-butanediol by Klebsiella pneumoniae. Bioresour. Technol. 2010, 101, 8342–8347. [Google Scholar] [CrossRef]
- Sun, L.H.; Wang, X.D.; Dai, J.Y.; Xiu, Z.L. Microbial production of 2,3-butanediol from Jerusalem artichoke tubers by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2009, 82, 847–852. [Google Scholar] [CrossRef]
- Gao, J.; Xu, H.; Li, Q.-j.; Feng, X.-h.; Li, S. Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R,R-2,3-butanediol. Bioresour. Technol. 2010, 101, 7087–7093. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Oh, B.-R.; Kang, I.Y.; Heo, S.-Y.; Seo, J.-W.; Park, S.-M.; Hong, W.-K.; Kim, C.H. Enhancement of 2,3-butanediol production from Jerusalem artichoke tuber extract by a recombinant Bacillus sp. strain BRC1 with increased inulinase activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Chen, C.; Li, K.; Wang, Y.; Gao, C.; Ma, C.Q.; Xu, P. Efficient simultaneous saccharification and fermentation of inulin to 2,3-butanediol by thermophilic Bacillus licheniformis ATCC 14580. Appl. Environ. Microbiol. 2014, 80, 6458–6464. [Google Scholar] [CrossRef] [PubMed]
- Lü, C.; Ge, Y.; Cao, M.; Guo, X.; Liu, P.; Gao, C.; Xu, P.; Ma, C. Metabolic Engineering of Bacillus licheniformis for Production of Acetoin. Front. Bioeng. Biotechnol. 2020, 8, 125. [Google Scholar] [CrossRef]
- Jurchescu, I.M.; Hamann, J.; Zhou, X.; Ortmann, T.; Kuenz, A.; Prusse, U.; Lang, S. Enhanced 2,3-butanediol production in fed batch cultures of free and immobilized Bacillus licheniformis DSM 8785. Appl. Microbiol. Biotechnol. 2013, 97, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- Kallbach, M.; Horn, S.; Kuenz, A.; Prusse, U. Screening of novel bacteria for the 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Li, K.; Wang, Y.; Gao, C.; Han, B.; Ma, C.; Xu, P. A newly isolated Bacillus licheniformis strain thermophilically produces 2,3-butanediol, a platform and fuel bio-chemical. Biotechnol. Biofuels 2013, 6, 123. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Ganchev, D.; Petrova, P.; Petrov, K. Highly efficient 2,3-butanediol production by Bacillus licheniformis via complex optimization of nutritional and technological parameters. Fermentation 2021, 7, 118. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, J.; Li, L.; Wen, Z.; Nomura, C.T.; Wu, S.; Chen, S. Engineering Bacillus licheniformis for the production of me-so-2,3-butanediol. Biotechnol. Biofuels 2016, 9, 117. [Google Scholar] [CrossRef]
- Qi, G.; Kang, Y.; Li, L.; Xiao, A.; Zhang, S.; Wen, Z.; Xu, D.; Chen, S. Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnol. Biofuels 2014, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petlichka, S.; Petrov, K. New Bacillus spp. with potential for 2,3-butanediol production from biomass. J. Biosci. Bioeng. 2020, 130, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Tsigoriyna, L.; Petrov, K. Production of 2,3-butanediol from fructose by Bacillus licheniformis 24. Acta Microbiol. Bulg. 2021, 37, 183–187. Available online: https://actamicrobio.bg/archive/issue-4-2021/amb-4-2021-article-2.pdf (accessed on 18 March 2023).
- Petrova, P.; Velikova, P.; Popova, L.; Petrov, K. Direct conversion of chicory flour into L(+)-lactic acid by the highly effective inulinase producer Lactobacillus paracasei DSM 23505. Bioresour. Technol. 2015, 186, 329–333. [Google Scholar] [CrossRef]
- Velikova, P.; Petrov, K.; Petrova, P. The cell wall anchored β-fructosidases of Lactobacillus paracasei: Overproduction, purification, and gene expression control. Proc. Biochem. 2017, 52, 53–62. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Ge, Y.; Li, K.; Li, L.; Gao, C.; Zhang, L.; Ma, C.; Xu, P. Contracted but effective production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem. 2016, 18, 4693–4703. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Liu, P.H.; Qin, J.Y.; Xu, P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl. Microbiol. Biotechnol. 2007, 74, 61–68. [Google Scholar] [CrossRef]
- Deshmukh, A.N.; Nipanikar-Gokhale, P.; Jain, R. Engineering of Bacillus subtilis for the Production of 2,3-Butanediol from Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 179, 321–331. [Google Scholar] [CrossRef]
- Dai, J.Y.; Cheng, L.; He, Q.F.; Xiu, Z.L. High acetoin production by a newly isolated marine Bacillus subtilis strain with low requirement of oxygen supply. Proc. Biochem. 2015, 50, 1730–1734. [Google Scholar] [CrossRef]
- Sikora, B.; Kubik, C.; Kalinowska, H.; Gromek, E.; Białkowska, A.; Jędrzejczak-Krzepkowska, M.; Schüett, F.; Turkiewicz, M. Application of byproducts from food processing for production of 2,3-butanediol using Bacillus amyloliquefaciens TUL 308. Prep. Biochem. Biotechnol. 2016, 46, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, A. Strategies for efficient and economical 2,3-butanediol production: New trends in this field. World J. Microbiol. Biotechnol. 2016, 32, 200. [Google Scholar] [CrossRef] [PubMed]
- Perego, P.; Converti, A.; del Borghi, M. Effects of temperature, inoculum size and starch hydrolyzate concentration on butanediol production by Bacillus licheniformis. Bioresour. Technol. 2003, 89, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, P.; Guo, G.; Liu, Z.; Zhong, L.; Guo, L.; Chen, C.; Hao, N.; Ouyang, P. Production of acetoin and its derivative tetramethylpyrazine from okara hydrolysate with Bacillus subtilis. AMB Express 2023, 13, 25. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Ren, K.; Han, R.; Lu, R.; Bao, T.; Pan, X.; Yang, T.; Xu, M.; Rao, Z. Acetoin production from lignocellulosic biomass hydrolysates with a modular metabolic engineering system in Bacillus subtilis. Biotechnol. Biofuels 2022, 15, 87. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; da Silva, S.P.; Converti, A.; Porto, T.S. Production, Biochemical Characterization, and Kinetic/Thermodynamic Study of Inulinase from Aspergillus terreus URM4658. Molecules 2022, 27, 6418. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Piłat, B.; Starowicz, M.; Jeliński, T.; Szmatowicz, B. High-Quality Gluten-Free Sponge Cakes without Sucrose: Inulin-Type Fructans as Sugar Alternatives. Foods 2020, 9, 1735. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Zhu, G.; Song, W.; Yi, C. Cleaner production and downstream processing of bio-based 2,3-butanediol: A review. J. Clean. Prod. 2022, 343, 131033. [Google Scholar] [CrossRef]
- Dai, J.Y.; Guan, W.T.; Xiu, Z.L. Bioconversion of inulin to 2,3-butanediol by a newly isolated Klebsiella pneumoniae producing inulinase. Proc. Biochem. 2020, 98, 247–253. [Google Scholar] [CrossRef]
- Mera, A.; de Lima, M.Z.T.; Bernardes, A.; Garcia, W.; Muniz, J.R.C. Low-resolution structure, oligomerization and its role on the enzymatic activity of a sucrose-6-phosphate hydrolase from Bacillus licheniformis. Amino Acids 2019, 51, 599–610. [Google Scholar] [CrossRef]
- Klaewkla, M.; Pichyangkura, R.; Chunsrivirot, S. Computational Design of Oligosaccharide-Producing Levansucrase from Bacillus licheniformis RN-01 to Increase Its Stability at High Temperature. J. Phys. Chem. B 2021, 125, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, T.T.; Tran, T.P.H.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.-L. Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source. Polymers 2021, 13, 1959. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wang, H.; He, P.; Chengjun Zhu, C.; Wang, Q.; Wei, X.; Nomura, C.T.; Chen, S. A novel strategy to improve protein secretion via overexpression of the SppA signal peptide peptidase in Bacillus licheniformis. Microb. Cell Fact. 2017, 16, 70. [Google Scholar] [CrossRef]
- Shen, P.; Niu, D.; Liu, X.; Tian, K.; Permaul, K.; Singh, S.; Mchunu, N.P.; Wang, Z. High-efficiency chromosomal integrative amplification strategy for overexpressing α-amylase in Bacillus licheniformis. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac009. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Y.; Zhao, Y.; Shen, W.; Chen, X. Systematic engineering of transport and transcription to boost alkaline α-amylase production in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2020, 104, 2973–2985. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. Engineering of Bacillus Promoters Based on Interacting Motifs between UP Elements and RNA Polymerase (RNAP) α-Subunit. Int. J. Mol. Sci. 2022, 23, 13480. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Direct starch conversion into L-(+)-lactic acid by a novel amylolytic strain of Lactobacillus paracasei B41. Starch 2012, 64, 10–17. [Google Scholar] [CrossRef]
- Arsov, A.; Petrov, K.; Petrova, P. Enhanced activity by genetic complementarity: Heterologous secretion of clostridial cellulases by Bacillus licheniformis and Bacillus velezensis. Molecules 2021, 26, 5625. [Google Scholar] [CrossRef]
- Xue, G.-P.; Johnson, J.S.; Dalrymple, B.P. High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J. Microbiol. Methods 1999, 34, 183–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).