Abstract
The enzymatic acetylation in the organic solvents of a number of the important bioactive cardiac glycosides was investigated. With the bufanolide proscillaridin A and the cardenolide lanatoside C, acylation, as expected, occurred at the secondary 4′-OH of the rhamnopyranosyl unit of the former (by the action of Novozym 435 lipase) and the primary 6′′′′-OH of the terminal glucopyranosyl unit of the latter (best results obtained by the action of the lipase PS). Only lipase PS was found to be able to acylate the cardenolides digitoxin and digoxin at the 4‴-OH of their terminal digitoxose unit. The corresponding monoacetyl derivatives, both of which are commercialized drugs, could be isolated with good yields. The investigation of the Novozym 435-catalyzed acetylation of free D-digitoxose provided a possible explanation for the inability of this lipase to acylate digitoxin and digoxin.
1. Introduction
Among pharmacologically active natural products, cardiotonic glycosides (Figure 1) are of prominent interest due to their peculiar and diverse biological activities. Historically, they have been investigated for their ability to improve cardiac contractility in the treatment of congestive heart failure [1,2,3,4,5]. A particular focus was dedicated to their inhibitory activity against Na+/K+-ATPase, a cell-membrane-located enzyme that plays a key role in the active transport of monovalent cations across membranes [6,7,8]. More recently, the biological profile of this family of molecules has become attractive since the discovery of their ability to act as modulators of neuroinflammation and as anticancer agents [9,10,11,12].
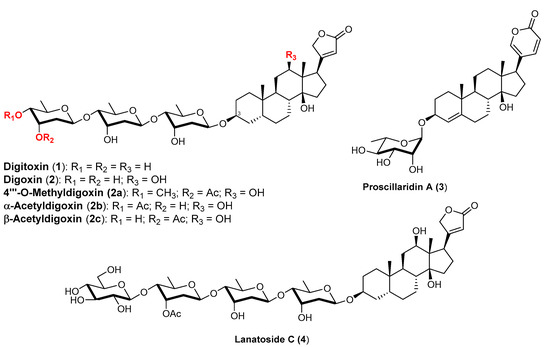
Figure 1.
Chemical structures of the cardiac glycosides 1, 2, 3, and 4 and their methylated (2a) and acetylated derivatives (2b—trade name: Novodigal and 2c—trade names: Dioxanin, Lanatilin, and Sandolanid; both commercially available oral drugs).
Cardiac glycosides are isolated from many plant sources belonging to different families and are structurally characterized by the presence of a steroidal genine having a cis A/B junction and carrying a lactone ring at the 17β-position and a sugar chain at position 3β. When the lactone ring is a butanolide, they are called cardenolides, while if an α-pyrone ring is present, they are called bufadienolides. The saccharide chain can contain up to four monosaccharides belonging to the D- and L-series, joined either through 1–4 or 1–6 glycosidic linkages [13].
The prototypes of cardiotonic glycosides (Figure 1) are digitoxin (1), isolated from Digitalis purpurea and made up of the genine digitoxigenine and a trisaccharide containing three molecules of the deoxysugar D-digitoxose, and digoxin (2), isolated from Digitalis lanata, made up of the same trisaccharide and the genine digixigenine. They are both used routinely for congestive heart failure and in the treatment of cardiac arrhythmias, in particular atrial fibrillation, under strictly controlled dosage because the therapeutic dose is very close to the toxic dose.
The highly valuable pharmacological activity of these compounds depends on the aglycone moiety, but it is significantly modulated by the nature of the sugar chain. Therefore, with the aim of improving and modifying the therapeutic action and bioavailability compared to the parent compounds, extensive research has been conducted, mainly by pharmaceutical companies, to modify the sugar chain, as shown, i.e., by a long list of patents. For example, with digoxin, worthy of mention is the chemical methylation to give the 4‴-O-methylderivative (2a) or acetylation to furnish the 3‴- and 4‴-O-acetylderivatives (2b) and (2c), respectively, used as drugs with improved oral absorption for the treatment of heart failure.
The exploitation of biocatalytic approaches has also been reported. For instance, in the early 1980s, Satoh and colleagues described the use of β-galactosidases for the one-step selective glycosylation of the genine digitoxigenine [14,15]. More recently and more efficiently, the glycosylation of the same genine has been obtained via sequential glycosylation using a steroid glycosyltransferase UGT74AN3 from Catharanthus roseus and a cyclodextrin glycosyltransferase from Bacillus licheniformis [16].
More recently, organocatalytic protocols have been proposed for the selective acylation of structurally complex and fragile natural products [17], specifically polyols and carbohydrates [18,19]. For instance, Kawabata and colleagues reported the regioselective acylation of digitoxin (1) with different esters at its 4′′′′-OH with a monoacylation yield of up to 98% [20].
The esterification of OH’s group under the catalysis of hydrolytic enzymes has proved to be a mild and selective methodology for the preparation of the specific derivatives of saccharides. We contributed to the development of this methodology by preparing the selected acyl derivatives of a large number of naturally occurring glycosides, ranging from flavonoid glycosides [21,22,23], colchicoside and thiocolchcicosode [24], ginsenosides [25], stevioside [26], and others.
Cardiac glycosides appeared as attractive substrates to extend the application of this methodology to the substrates of pharmaceutical value and to highlight the importance of natural catalysts for their mild and selective derivatization. Here, we report the results obtained, in terms of product characterization and regioselectivity determination, in the enzymatic acetylation of digitoxin and digoxin (1 and 2) and of proscillaridin A and lanatoside C (3 and 4), other examples of this important family of bioactive natural products.
2. Results and Discussion
Our investigation started with Proscillaridin A (3), a cardiac glicosyde belonging to the bufenolide family that can be isolated from plants of the genus Scilla, such as Urginea (or Drimia) Maritima [27,28,29,30], and that, besides its cardiotonic effects, possesses promising anticancer activity [31,32]. The sugar moiety of this compound, one of the most potent cardiac glycosides [33], is the simple monosaccharide L-rhamnose. From previous investigations [34,35], it was known that the lipase B from Candida antarctica (and specifically its immobilized form Novozym 435) is able to regioselectively acylate the hydroxyl group at position C-4 of a terminal α-rhamopyranoside unit. In addition, this was also the case with compound 3, once dissolved in vinyl acetate, acting both as solvent and acylating agent, and stirred at 45 °C for 24 h in the presence of this immobilized lipase. (Reaction conditions and enzyme dosage (5 mg/mL) were based on our previous investigations.) [34,35]. The less polar product formed was purified by flash chromatography on silica gel (ca 50% isolated yield) and characterized as 4′-O-acetylproscillaridin A (3a, Scheme 1). Monoacetylation was confirmed by the expected value of molecular mass (HR-ESI-MS, m/z: 595.28834 [M + Na]+) and the regioselectivity indicated by the diagnostic downfield shift of 4′-H (a triplet, now at 4.94 ppm) and the downfield shift of C-4′ (at 76.8 ppm from 73.3 ppm in the parent 3) accompanied by the upfield shift of the adjacent C-3′ and C-5′.
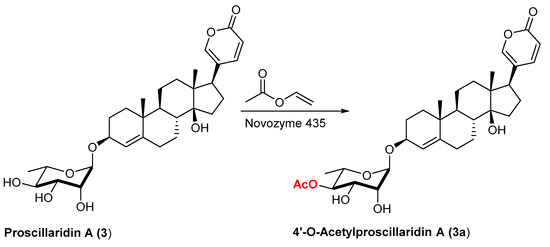
Scheme 1.
Enzymatic acetylation of proscillaridin A catalyzed by Novozym 435.
The enzymatic acylation of the structurally more complex lanatoside C (4) was investigated next. Lanatoside C is a clinically used cardiac glycoside composed of the aglycon digoxigenin and a tetrasaccharide composed of three digitoxose units and a glucopyranoside at its non-reducing end [36]. Currently, medical research is devoted to the characterization of the oncological potential of the administration of this drug as an apoptosis inducer and/or as a partner in combinatorial therapies with other anticancer compounds [37,38,39,40]. With this compound too, our previous experience with aglycones carrying oligosaccharides [41,42] suggested that, possibly, enzymatic esterification could be effective on the terminal glucose moiety, with a preference for the primary OH. In this case too, the reaction conditions and enzymatic dosages were based on protocols proposed by us that highlighted the use of a mixed-solvent system, made of t-amyl alcohol and pyridine, as optimal to guarantee substrate solubility and efficient biotransformations [41,42].
In a preliminary screening, compound 4, dissolved in a 3:1 mixture of t-amyl alcohol/pyridine containing an excess of vinyl acetate, was subjected to the action of different lipases and the protease subtilisin, following the reaction by TLC and HPLC (see Supplementary Materials). In detail, the lipases investigated were: Candida antarctica lipase B, lipozyme IM20, lipase from porcine pancreas, lipase from Humicola lanuginosa, lipase PS from Pseudomonas cepacea, lipase from Candida rugosa, lipase AK, lipase AG, and lipase from Chromobacterium viscosum. The best results in terms of substrate acetylation (conversion of the starting material quantified by HPLC—ca 80%) and apparent regioselectivity (only one spot detected in TLC) were obtained with lipase PS supported on celite (0.3% w/w).
The reaction was scaled up, and a single product was isolated in 90% yield. Monoacylation was confirmed by its mass spectrum, and NMR analysis confirmed the expected acylation of the primary hydroxyl of the glucose moiety (4a, Figure 2). Specifically, the two dd due to the diastereotopic proton, H-6a′′′′ (J1 = 11.8 Hz, J2 = 2.1 Hz) and H-6b′′′′ (J1 = 11.8 Hz, J2 = 5.5 Hz), were downfield shifted at 4.36 and 4.16 ppm, respectively. Similarly, the signal due to the C-6′′′′ carbon was downfield shifted to 65.52 ppm.
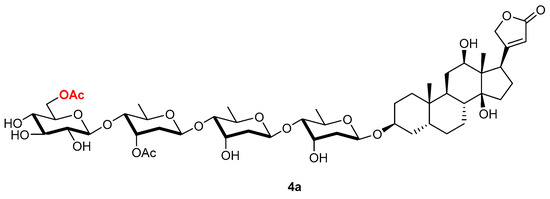
Figure 2.
Acetylation product obtained by reacting lanatoside C (4) in the presence of vinyl acetate and lipase PS.
The other lipases were either inactive or, according to TLC analysis, formed the same product, albeit with a much lower yield. The protease subtilisin behaved differently: substrate acetylation was almost quantitative (as judged by TLC), but two additional less polar products could be detected. Based on our previous experience with glucosylated derivatives, we could hypothesize that the two additional products could be a C-3′′′′-acetylated monoester and a C-3′′′′- and C-6′′′′-acetylated diester [43,44]. However, this reaction was not further investigated.
The results obtained in the enzymatic acylation of lanatoside C compare well with those obtained by Kawataba with their organocatalyst, as with their proposed protocols, mixtures of monoacylated derivatives mainly at C-3′′′′ OH and C-4′′′′ OH were always obtained, albeit in good yields [45].
The next step was to consider as substrates digitoxin (1) and its cognate digoxin (2), bufadienolide-based cardiac glycosides, whose acetylated derivatives are the drugs Novodigal (2b) and Dioxanin (2c) (Scheme 2). These two molecules differ only slightly in their aglycon moiety (the former contains a 12β-OH) and possess the same glycosidic structure, a trisaccharide formed by three units of D-digitoxose (5). As previously discussed [46], several examples of the enzymatic esterification of carbohydrates and natural glycosides have been reported in the literature, but, in the specific case of these compounds, to the best of our knowledge, there have been no previous examples of the enzymatic acylation of digitoxose or of its derivative.
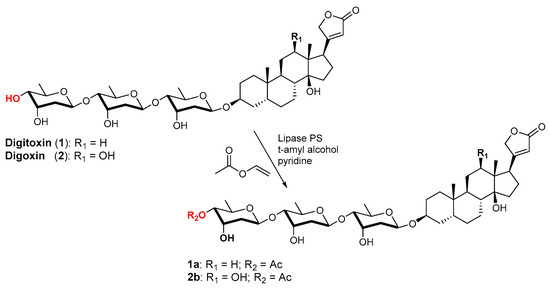
Scheme 2.
Acetylation products obtained by reacting digitoxin (1) and digoxin (2) in the presence of lipase PS and vinyl acetate in a 3 to 1 mixture of t-amyl alcohol and pyridine.
Since digitoxin (1) is a poly-glycosylated digoxigenin natural compound such as lanatoside C (4), its acetylation was conducted exploiting the same reaction conditions described for 4. Specifically, 1 was thus dissolved in a 3:1 mixture of t-amyl alcohol and pyridine containing vinyl acetate as an acyl donor and submitted to the action of a panel of lipases and of protease subtilisin. Only two lipases showed the formation of less polar products in TLC (see supporting information). With lipase “AK” and lipase from Chromobacterium viscosum, substrate acetylation was poor, while lipase PS showed the transformation of the starting material to a single acylation product that could be isolated after chromatography (46% i.y.). The HR-MS-ESI spectrum of this product showed a peak at m/z: 829.43310 [M + Na]+, indicating that the isolated product was a monoacetylated digitoxin. A careful inspection of the NMR spectra (1D and 2D) in comparison with those of 1 allowed the determination of the position of acetylation. In the 1H-NMR spectrum, the signals due to the genine (aglycone) moiety were unaffected, whereas a considerable perturbation was observed in the region of the hydroxymethyne protons. A clean dd appeared downfield at 4.44 ppm, connected via a trans-diaxial coupling to a dq at 4.05 ppm and via an ax-eq coupling to an apparent q at 4.19 ppm. From these data, the downfield oxymethyne proton can be attributed only to the H-4‴ and the other protons to the H-5‴ and H-3″, respectively, thus establishing that the reaction product was a monoacetylated derivative of the terminal digitoxose unit and that it was 4‴-O-acetyldigitoxin (1a).
When the reaction was carried out on digoxin (2), using lipase PS as a biocatalyst and the same conditions described for the acetylation of 1, the reaction product (51% i.y.) was also recognized as the now expected 4‴-O-acetyl derivative 2b—the structure of the drug Novodigal—by HR-MS-ESI and NMR spectra, with signals analogous to those of 1a.
The identification of the signals due to the carbohydrate moieties of 1a and 2b (as well as of their parents 1 and 2) was also supported by the investigation of the spectrum of the sugar digitoxose (5, Figure 3).
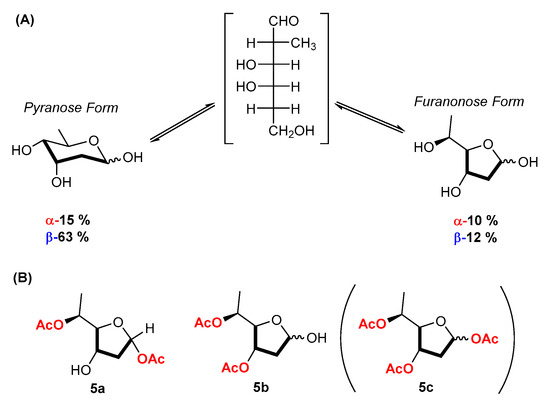
Figure 3.
(A) Equilibrium of D-digitoxose α- and β-pyranose and furanose forms in solution; (B) isolated products from the Novozym-435-mediated acylation of digitoxose.
The structure of crystalline 5 is configured as a β-pyranose form, as evidenced by its 1H-NMR spectrum recorded immediately after dissolution in CD3OD. (The signals are reported in the Experimental section, and the spectra are in the Supplementary Materials). After equilibration in solution, 5 mutarotated via the open aldehyde (not detected) to afford a mixture of four different forms. The β-pyranose form was still predominant (ca 63%) accompanied by the anomeric α-pyranose form (ca 15%), which displayed H-1 at 5.11 ppm as a brd (J = 3.2 Hz). The two other forms were identified as the β-digitoxyfuranose form (ca 12%) with H-1 as a triplet at 5.52 ppm (J = 4.4 Hz) and the anomeric α-furanose form (ca 10%) with H-1 as a dd at 5.46 ppm (J1 = 5.6 Hz, J2 = 2.1 Hz). Similar results had been reported by dissolving digitoxose in deuterated DMSO [47].
As we were quite surprised by the inability of Novozym 435 to acylate the two cardiac glycosides 1 and 2, we decided to investigate the outcome of the same reaction (solvent t-amyl alcohol/pyridine 3:1, acylating agent vinyl acetate) using the free sugar digitoxose as the substrate. Indeed, this compound was converted into a mixture of at least three less polar products. Two of them could be isolated in sufficient amounts for their characterization and were identified as two diacetates in furanose form. Specifically, the isolated less polar spot in TLC (Rf 0.63) proved to be a single diacetylated compound and gave a clean 1H-NMR spectrum characterized by the presence of an anomeric proton at 6.27 ppm as a dd (J = 5.5 and 1.0 Hz) and of a qd (J = 6.5 and 4.1 Hz) at 4.96 ppm due to H-5. From H-1 via H-2 at 2.40 and 2.05, H-3 was detected at 4.34 as a ddd (J = 7.5, 3.2, and 2.1 Hz) and H-4 at 4.06 ppm as a dd (J = 3.8 and 3.5 Hz). These data are in agreement with an α-furanose form and suggest, for this product, the structure of 1,5-diacetil-α-D-digitoxofuranose (5a). The more polar spot (Rf 0.54) was due to an inseparable mixture of two diacetylated products as emerged from the presence in the 1H-NMR spectrum of two anomeric protons at 5.56 ppm (t, J = 4.9 Hz) and at 5.54 ppm (J1 = 5.4 Hz and J2 = 1.1 Hz). The first anomeric proton was correlated to the downfield signal at 5.32 ppm, a ddd due to H-3 (J1 = 6.9, J2 = 4.3, and J3 = 3.2 Hz), to the qt due to H-4 at 3.86 ppm (J1 = 6.6 and J2 = 2.9 Hz), and this in turn to H-5 in the region 4.95–5.05 ppm. The second anomeric proton at 5.54 ppm was correlated to H-3 at 5.21 ppm (ddd, J1 = 7.8, J2 = 3.6, and J3 = 2.0 Hz), and then to H-4 and H-5 in the region 4.95–5.05 ppm. To this compound, the structure of the epimeric mixture of 3,5-diacetate (5b) could be assigned. The third compound could not be isolated, but, being significantly less polar than 5a and 5b, it might likely have been the triacetate 5c (Figure 3). The lack of products in the pyranose configuration might explain why Novozym 435 was not able to acylate digitoxin (1) and digoxin (2).
3. Materials and Methods
3.1. General Information
The 1H and 13C-NMR spectra were recorded in CD3OD (unless otherwise stated) with a Bruker Avance AC400 instrument (400.13 MHz and 100.1 MHz, respectively). Chemical shifts are given on a δ (ppm) scale, and coupling constants J are given in Hz.
HR-ESI-MS were measured with a Fourier Transfer Ion Cyclotronic Resonance (FT-ICR) ApexTM II instrument (Bruker Daltonics, Billerica, MA, USA) equipped with a 4.7 Tesla cryo-magnet (Magnex). Compounds were dissolved in CH3OH/H2O.
Thin-layer chromatography (TLC) was performed on silica plates (Merck 60 F254). Substrates and products were visualized by plate treatment with the molibdate reagent ((NH4)6MoO24·4H2O, 42 g; Ce(SO4)2, 2 g; H2SO4, concentrated 62 mL; made up to 1 L with deionized water). Purifications were performed by flash chromatography on silica gel (Merck 60, 230—400 mesh).
Candida antarctica lipase B was from Novozyme (Novozym 435); lipozyme IM20, lipase PS from Pseudomonas capacea, lipase AK, lipase AG, and lipase CE5 from Humicola lanuginosa were from Amano; the protease subtilisin and the lipases from porcine pancreas, Candida rugosa, and Chromobacterium viscosum were from Sigma Aldrich (St. Louis, MO, USA).
Lipase PS was immobilized by mixing the crude enzyme powder (3 g, ca 30 mg of biocatalyst) with 10 g of celite (Hyflo Super Cell). A total of 10 mL of PB (0.1 M, pH 7.0) was added, and the obtained solid-liquid combination was energetically mixed and then dried in vacuo for 24 h.
3.2. Synthesis
4′-O-acetyl-proscillaridin A (3a). Proscillaridin A (3, 90 mg) was dissolved in 10 mL of vinyl acetate and treated with 50 mg of Novozym 435. The suspension was stirred (220 rpm) at 45 °C for 24 h, then filtered, solvent evaporated, and the residue purified by flash column chromatography on silica gel using the eluent CHCl3—MeOH (25:2) to obtain 46 mg of pure 3a.
Rf (CHCl3-MeOH-H2O, 10:2:0.25): 3a, 0.34; proscillaridin A, 0.14. 1H-NMR (CDCl3): selected data, δ: 8.01 (1H, dd, J1 = 9.7 Hz, J2 = 2.5 Hz, H-22); 7.45 (1H, bs, H-21); 6.30 (1H, d, J = 9.7 Hz, H-23); 5.36 (1H, bs, H-4); 4.94 (1H, t, J = 9.4 Hz, H-4′); 4.91 (1H, bs, H-1′); 4.15 (1H, m, H-3); 3.81 (3H, m, H-2′, H-3′, and H-5′); 2.57 (H-17); 2.10 (3H, s, CH3CO); 1.15 (3H, d, J = 5.9 Hz, CH3-6′); 1.09 (3H, s, CH3-19); 0.76 (3H, s, CH3-18). 13C-NMR (CDCl3): δ: 173.49 (CH3CO); 151.43 (C-21); 150.25 (C-22); 149.78 (C-5); 125.91 (C-20); 122.403 (C-4); 116.30 (C-23); 101.712 (C-1′); 86.66; 76.76 (C-4′); 76.19 (C-3); 73.79, 71.44, and 68.78 (C-2′, C-3′, and C-5′); 53.19 (C-17); 52.44; 44.41; 42.65; 39.59; 37.43; 34.44; 34.20; 30.96; 30.63; 29.04, 23.37; 21.87 (CH3CO); 20.46 (CH3-19); 18.65 (CH3-6′); 18.12 (CH3-18). HR-ESI-MS, m/z: 595.28834 [M + Na]+, calc for C32H44O9Na 595.28775.
6′′′′-O-Acetyl-lanatoside C (4a). Lanatoside C (4, 90 mg) was dissolved in a 1:3 mixture (8 mL) of pyridine and tert-amyl alcohol (t-AmOH) and reacted with 2 mL of vinyl acetate and 1 g of lipase PS on celite at 45 °C (220 rpm) for 96 h. After filtration and solvent evaporation, the residue was purified via flash column chromatography on silica gel using the eluent CHCl3-MeOH 15:1 to obtain 81 mg of pure 4a as an amorphous powder.
Rf (CHCl3-MeO-H2O, 20:2:0.25): 4a, 0.21; lanatoside C, 0.11. 1H-NMR; selected data, δ: 5.93 (1H, s, H-22); 5.84 (1H, q, J = 3.0 Hz, H-3‴); 5.00 (1H, dd, J1 = 18.6 Hz, J2 = 1.7 Hz, H-21a); 4.92 (1H, dd, J1 = 18.6 Hz, J2 = 1.8 Hz, H-21b); 4.94, 4.92, and 4.89 (1H each, d each, J = 7.8 Hz, J = 8.2 Hz, and J = 7.4 Hz, H-1′, H-1″, and H-1‴); 4.38 (1H, d, J = 7.8 Hz, H-1′′′′); 4.36 (1H, dd, J1 = 11.8 Hz, J2 = 2.1 Hz, H-6a′′′′); 4.28 and 4.26 (1H each, q each, J = 2.9 Hz, and J = 3.0 Hz, H-3′, and H-3″); 4.16 (1H, dd, J1 = 11.8 Hz, J2 = 5.5 Hz, H-6b′′′′); 4.03 (1H, br m, H-3); 3.97 (1H, dq, J1 = 9.6 Hz, J2 = 6.2 Hz, H-5‴); 3.85 and 3.81 (1H each, dq each, J1 = 9.6 Hz and J2 = 6.2 Hz each, H-5′, and H-5”); 3.45 (1H, ddd, J1 = 9.4 Hz, J2 = 5.5 Hz, J3 = 2.1 Hz, H-5′′′′); 3.41 (1H, dd, J1 = 9.5 Hz, J2 = 3.2 Hz, H-4‴); 3.37 (1H, t, J = 9.2 Hz, H-4′′′′); 3.29 (1H, t, J = 8.9 Hz, H-3′′′′); 3.29, and 3.24 (1H each, dd each, J1 = 9.6 Hz, J2 = 2.9 Hz and J1 = 9.5 Hz, J2 = 2.9 Hz, H-4′, and H-4″); 3.17 (1H, t, J = 8.2 Hz, H-2′′′′); 2.09 and 2.06 (3H each, s each, 2 CH3CO); 1.35 (3H, d, J = 6.2 Hz, CH3-6‴); 1.22 and 1.21 (3H each, d each, J = 6.2 Hz each, CH3-6′, and CH3-6″); 0.97 (3H, s, CH3-19); 0.80 (3H, s, CH3-18). 13C-NMR; δ: 179.34 and 178.16 (C-20 and C-23); 173.78 and 172.67 (2 CH3CO); 118.62 (C-22); 106.46 (C-1′′′′); 101.35, 101.32, and 97.92 (C-1′, C-1″, and C-1‴); 87.69 (C-14); 84.72 and 84.42 (C-4′ and C-4″); 82.22; 78.84 (C-4′′′′); 76.64 (C-4‴); 76.34 (C-21); 76.05 (C-2′′′′ and C-5′′′′); 75.39 (C-3); 72.49 and 72.37 (C-3‴ and C-3′′′′); 71.55 (C-5‴); 70.40 (C-5′ and C-5″); 69.30 and 69.12 (C-3′ and C-3″); 65.52 (C-6′′′′); 58.22; 48.05 (C-17); 43.21; 39.80 and 39.38 (C-2′ and C-2″); 37.80; 37.10; 34.63; 34.51; 32.34 (C-16); 32.01 (C-11); 31.80; 29.29; 28.70; 28.40; 25.10 (C-19); 23.63; 21.93 and 21.66 (2 CH3CO); 19.51 (C-6‴); 19.35 (C-6′ and C-6″); 10.76 (C-18). HR-ESI-MS m/z: 1049.49123 [M + Na]+, calculated for C51H78O21 Na 1049.49278.
4‴-O-Acetyl-digitoxin (1a). Digitoxin (1, 100 mg) was dissolved in a 1:3 mixture (8 mL) of pyridine and t-AmOH and reacted with 2 mL of vinyl acetate and 1 g of lipase PS at 45 °C (220 rpm) for 96 h. After filtration and solvent evaporation, the residue was purified via flash column chromatography on silica gel using the eluent CHCl3-MeOH 15:1 to obtain 48 mg of pure 1a as an amorphous powder.
Rf (CHCl3-MeOH-H2O, 20:2:0.25): 1a, 0.18; digitoxin, 0.06. 1H-NMR; selected data, δ: 5.91 (1H, brs, H-22); 5.05 (1H, dd, J1 = 18.4 Hz, J2 = 1.2 Hz, H-21a); 5.00 (1H, dd, J1 = 9.6 Hz, J2 = 1.6 Hz, H-1‴); 4.94 and 4.92 (1H each, brd each, J = 9.8 Hz, and J = 9.5 Hz; H-1′ and H-1″); 4.93 (1H, dd, J1 = 18.6 Hz, J2 = 1.6 Hz, H-21b); 4.44 (1H, dd, J1 = 10.0 Hz, J2 = 2.8 Hz, H-4‴); 4.27 (2H, m, H-3′, and H-3″); 4.19 (1H, q, J = 3.0 Hz, H-3‴); 4.05 (1H, dq, J1 = 9.9 Hz, J2 = 6.4 Hz, H-5‴); 4.03 (1H, brm, H-3); 3.86 and 3.81 (1H each, dq each, J1 = 9.7 Hz, J2 = 6.4 Hz each, H-5′, and H-5″); 3.28 and 3.24 (1H each, dd each, J1 = 9.6 Hz, J2 = 3.2 Hz, and J1 = 9.6 Hz, J2 = 2.8 Hz, H-4′, and H-4″); 2.85 (1H, brdd, J1 = 8.2 Hz, J2 = 5.6 Hz, H-17); 2.10 (3H, s, CH3CO); 1.24 and 1.22 (3H each, d each, J = 6.4 Hz each, H-6′, and H-6″); 1.18 (3H, d, J = 6.4 Hz, H-6‴); 0.96 (3H, s, CH3-19); 0.90 (3H, s, CH3-18). 13C-NMR, δ: 177.04 and 175.15 (C-20 and C-23); 170.66 (CH3CO) 116.38 (C-22); 99.30 (C-1‴); 99.11 and 95.51 (C-1′ and C-1″); 85.04 (C-14); 82.39 and 82.22 (C-4′ and C-4″); 75.21 (C-4‴); 73.94 (C-21); 73.10 (C-3); 68.07 (C-5′ and C-5″); 67.07 (C-3′ and C-3″); 66.99 (C-5‴); 64.91 (C-3‴); 50.75 (C-17); 49.67; 41.31; 39.58 (C-2‴); 37.82 and 37.52 (C-2′ and C-2″); 37.16; 36.57; 35.48; 34.93; 32.00; 29.98; 29.62; 26.66; 26.47; 26.11; 22.87 (C-19); 21.17; 20.96; 19.49 (CH3CO); 17.04 (C-6′ and C-6″); 16.89 (C-6‴); 14.99 (C-18). HR-ESI-MS m/z: 829.43310 [M + Na]+, calculated for C43H66O14Na 829.43448.
4‴-O-Acetyldigoxin (2b). To a solution of 100 mg of digoxin (2) prepared in a 1:3 mixture (8 mL) of pyridine and t-AmOH, 2 mL of vinyl acetate and 1 g of lipase PS were added. The reaction mixture was held at 45 °C (220 rpm) for 96 h, then filtered, and solvents evaporated. The purification of the residue via flash column chromatography on silica gel using the eluent CHCl3-MeOH 23:2 yielded 52 mg of pure 2b as an amorphous powder.
Rf (CHCl3-MeOH-H2O, 20:2:0.25): 2b, 0.20; digoxin 0.09. 1H-NMR; selected data, δ: 5.93 (1H, brs, H-22); 5.00 (1H, dd, J1 = 18.6 Hz, J2 = 1.6 Hz, H-21a); 4.98, 4.96, and 4.92 (1H each, brd each, J = 9.2 Hz, J = 9.3 Hz, and J = 9.0 Hz; H-1′, H-1″, and H-1‴); 4.94/(1H, dd, J1 = 18.6 Hz, J2 = 1.6 Hz, H-21b); 4.44 (1H, dd, J1 = 9.9 Hz, J2 = 2.8 Hz, H-4‴); 4.27 and 4.24 (1H each, dd, each; J1 = 9.9 Hz, J2 = 2.8; Hz, H-3′, and H-3″); 4.20 (1H, q, J = 3.1 Hz, H-3‴); 4.05 (1H, dq, J1 = 9.9 Hz, J2 = 6.4 Hz, H-5‴); 4.03 (1H, brm, H-3); 3.84 and 3.81 (1H each, dq each, J1 = 9.7 Hz, J2 = 6.4 Hz each, H-5′, and H-5″); 3.40 (1H, dd, J1 = 11.9 Hz, J2 = 4.1 Hz, H-12); 3.34 (1H, m, H-17); 3.28 and 3.24 (1H each, dd each, J1 = 9.6 Hz, J2 = 3.2 Hz, and J1 = 9.6 Hz, J2 = 2.8 Hz; H-4′ and H-4″); 2.10 (3H, s, CH3CO); 1.24 and 1.22 (3H each, d each, J = 6.8 Hz and J = 6.7 Hz; H-6′ and H-6″); 1.18 (3H, d, J = 6.2 Hz, H-6‴); 0.97 (3H, s, CH3-19); 0.81 (3H, s, CH3-18). 13C-NMR; δ: 179.35 and 178.19 (C-20 and C-23); 172.98 (CH3CO); 118.62 (C-22); 101.56 (C-1‴); 101.37 and 97.91 (C-1′ and C-1″); 87.69 (C-14); 84.72 and 84.55 (C-4′ and C-4″); 77.56 (C-4‴); 76.64 (C-12); 76.34 (C-21); 75.40 (C-3); 70.41 (C-5′ and C-5″); 69.44 and 69.31 (C-3′ and C-3″); 69.25 (C-5‴); 67.26 (C-3‴); 48.04 (C-17); 43.21; 40.18 (C-2‴); 39.79 and 39.44 (C-2′ and C-2″); 38.90; 38.01; 37.11; 34.62; 34.49; 32.34 (C-16); 32.00 (C-11); 31.79; 29.28; 28.69; 28.39; 25.08 (C-19); 23.68; 21.76 (CH3CO); 19.35 (C-6′ and C-6″); 19.18 (C-6‴); 10.74 (C-18). HR-ESI-MS m/z: 845.42899 [M + Na]+, calculated for C43H66O15Na 845.42939.
- 1H-NMR of D-digitoxose (5).
(a) Immediately after dissolution in CD3OD (β-pyranose conformation): δ: 5.08 (1H, dd, J1 = 9.7 Hz, J2 = 2.0 Hz, H-1); 4.02 (1H, app q, J = 3.1 Hz, H-3); 3.80 (1H, dd, J1 = 9.5 Hz, J2 = 6.2 Hz, H-5); 3.16 (1H, dd, J1 = 9.5 Hz, J2 = 3.1 Hz, H-4); 2.03 (1H, ddd, J1 = 13.6 Hz, J2 = 2.5 Hz, J3 = 2.1 Hz, H-2eq); 1.64 (1H, ddd, J1 = 13.7 Hz, J2 = 9.8 Hz, J3 = 2.8 Hz, H-2ax); 1.25 (3H, d, J = 6.3, CH3-6).
(b) The equilibrated mixture of four different forms of β-D-digitoxose after 2–3 days of dissolution in CD3OD: β-D-digitoxopyranose (62.9%): δ 5.09 (1H, dd, J1 = 9.6 Hz, J2 = 2.0 Hz, H-1); 4.02 (1H, app q, J = 2.8 Hz, H-3); 3.81 (1H, dd, J1 = 9.6 Hz, J2 = 6.2 Hz, H-5); 3.16 (1H, dd, J1 = 9.6 Hz, J2 = 3.2 Hz, H-4); 2.03 (1H, ddd, J1 = 13.6 Hz, J2 = 3.6 Hz, J3 = 2.4 Hz., H-2eq); 1.64 (1H, ddd, J1 = 13.6 Hz, J2 = 10.0 Hz, J3 = 2.8 Hz, H-2ax); 1.25 (3H, d, J = 6.2, CH3-6). α-D-digitoxopyranose (15.2%): δ 5.11 (1H, brd, J = 3.2 Hz, H-1); 4.10 (1H, dq, J1 = 9.6 Hz, J2 = 6.4 Hz, H-5); 3.80–3.85 (1H, m, H-3); 3.18 (1H, dd, J1 = 9.6 Hz, J2 = 3.2 Hz, H-4); 2.05–2.08 (1H, m, H-2ax); 1.86 (1H, ddd, J1 = 13.9 Hz, J2 = 3.6 Hz, J3 = 3.2 H-2eq); 1.27 (3H, d, J = 6.2, CH3-6). β-D-digitoxofuranose (13.0%): δ 5.52 (1H, t, J = 4.4 Hz, H-1); 4.48 (1H, td, J1 = 5.6 Hz, J2 = 3.6 Hz, H-3); 3.80 (1H, m, H-5); 3.64 (1H, dd, J1 = 7.6 Hz, J2 = 4.8 Hz, H-4); 2.08–2.05 (2H, m, H-2eq, and H-2ax); 1.21 (3H, d, J = 6.4, CH3-6). α-D-digitoxofuranose (8.4%): δ 5.46 (1H, dd, J1 = 5.6 Hz, J2 = 2.1 Hz, H-1); 4.30 (1H, td, J1 = 6.8 Hz, J2 = 3.2 Hz, H-3); 3.89 (1H, dd, J1 = 4.8 Hz, J2 = 3.6 Hz, H-4); 3.75 (1H, qd, J1 = 6.6 Hz, J2 = 3.9 Hz, H-5); 2.23 (1H, ddd, J1 = 13.6 Hz, J2 = 7.2 Hz, J3 = 5.2 Hz, H-2ax); 1.85 (1H, ddd, J1 = 13.5 Hz, J2 = 3.6 Hz, J3 = 3.2 Hz, H-2 eq); 1.24 (3H, d, J = 6.4, CH3-6).
Acetylation of D-digitoxose (5). To a solution of 70 mg of D-digitoxose (5) prepared in a 1:3 mixture (8 mL) of pyridine and tert-amyl alcohol (t-AmOH), 2 mL of vinyl acetate and 100 mg of Novozym 435 were added. The reaction mixture was held at 45 °C (220 rpm) for 7 h, then filtered, and solvents evaporated. Three new spots could be detected by TLC (eluent AcOEt–MeOH–H2O, 7:1:0.5) with Rf 0.71 (5c), 0.63 (5b), and 0.54 (5a) (Rf 5, 0.37). Samples of almost pure 5a (40 mg, as an inseparable mixture of anomeric epimers) and 5b (5 mg) could be isolated by flash column chromatography on silica gel using the eluent AcOEt-MeOH 25: 1.
Selected 1H-NMR signals (CD3OD) are reported below for the isolated compounds:
5a (1,5-diacetyl-digitoxose, α-furanose conformation), δ 6.27 (1H, dd, J1 = 5.5 Hz, J2 = 1.0 Hz, H-1);
- 5b (3,5-diacetyl-digitoxose)
α-furanose conformation: δ 5.54 (1H, dd, J1 = 5.4 Hz, J2 = 1.1 Hz, H-1); 5.21 (1H, ddd, J1 = 7.8 Hz, J2 = 3.6 Hz, J3 = 2.0 Hz, H-3); 5.00 (2H, m, H-5, and H-4); 2.15 (1H, m, H-2ax); 1.92 (1H, ddd, J1 = 14.5 Hz, J2 = 1.9 Hz, J3 = 1.2, H-2eq); 2.05 and 2.03 (3H each, s, CH3CO), 1.29 (3H, d, J = 6.5 Hz, CH3-6);
β-Furanose conformation: δ 5.56 (1H, t, J = 5.56 Hz, H-1); 5.32 (1H, ddd, J1 = 6.9 Hz, J2 = 4.2 Hz, J3 = 3.2 Hz, H-3); 5.00 (1H, m, H-5); 3.86 (1H, dd, J1 = 6.6 Hz, J2 = 2.9 Hz, H-4); 2.37 (1H, ddd, J1 = 14.4 Hz, J2 = 7.8 Hz, J3 = 5.3, H-2ax); 2.15 (1H, m, H- H-2eq); 2.08 and 2.06 (3H each, s, CH3CO), 1.25 (3H, d, J = 6.5 Hz, CH3-6).
4. Conclusions
In light of the increasing interest in cardiac glycosides as potential immuno- and cancer-chemotherapeutics, this work has been focused on a selective, enzymatic entry to the mono-acetylated derivatives of selected cardiotonics isolated from Digitalis sp. These simple compounds are, in fact, known to possess improved pharmacokinetic profiles compared to their respective parental molecules. Accordingly, we took inspiration from the efficient regioselective esterification protocols developed by us and others in the late 1980s and early 1990s for the regioselective enzymatic acylation of polyhydroxylated natural compounds.
The acetylation of compounds carrying terminal L-rhamnopyranosyl or D-glupyranosyl units was achieved by following previous literature reports on natural glycosides carrying the same sugar moieties and allowing the easy and selective preparation of mono-acetylated derivatives of lanatoside C and proscillaridin A. Conversely, the regioselective enzymatic acetylation of derivatives carrying a terminal D-digitoxose unit was achieved for the first time, identifying lipase PS as the catalyst of choice. This novel biocatalytic entry allowed the regioselective preparation of the drug Novodigal, a mono-acetylated derivative of digoxin.
These preliminary findings could be useful for the preparative-scale synthesis of new bioactive derivatives of these glycosides, either acetylated or carrying other acyl moieties on their oligosaccharidic units.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13050819/s1: Copies of the NMR and mass spectra of the starting and the obtained compounds as well as HPLC and TLC screening from lanatoside C and digitoxin acetylation, respectively.
Author Contributions
Conceptualization, S.R. and B.D.; methodology and experiment, S.R. and L.R.; formal analysis, B.D.; data curation, B.D. and L.R.; writing—original draft preparation, B.D.; writing—review and editing, I.B. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AcOEt = ethyl acetate; t-AmOH = tert-amyl alcohol; MeOH = methanol; i.y. = isolated yield; brd = broad; s = singlet; d = doubled; t = triplet; ax = axial; eq = equatorial; PB = phosphate buffer.
References
- Albrecht, H.P. Cardiac Glycosides in Naturally Occurring Glycosides; John Wiley & Sons, Inc.: Chichester, UK, 1999. [Google Scholar]
- Gaignault, J.C.; Bidet, D. Hétérosides Cardiotoniques. Fitoterapia 1988, 59, 259–315. [Google Scholar]
- Melero, C.P.; Medarde, M.; San Feliciano, A. A Short Review on Cardiotonic Steroids and Their Aminoguanidine Analogues. Molecules 2000, 5, 51–81. [Google Scholar] [CrossRef]
- Patel, S. Plant-Derived Cardiac Glycosides: Role in Heart Ailments and Cancer Management. Biomed. Pharmacother. 2016, 84, 1036–1041. [Google Scholar] [CrossRef]
- Ren, J.; Gao, X.; Guo, X.; Wang, N.; Wang, X. Research Progress in Pharmacological Activities and Applications of Cardiotonic Steroids. Front. Pharmacol. 2022, 13, 902459. [Google Scholar] [CrossRef] [PubMed]
- Krstić, D.; Krinulović, K.; Spasojević-Tišma, V.; Joksić, G.; Momić, T.; Vasić, V. Effects of Digoxin and Gitoxin on the Enzymatic Activity and Kinetic Parameters of Na+/K+-ATPase. J. Enzyme Inhib. Med. Chem. 2004, 19, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, F.; Mahmmoud, Y.A. Interaction between Cardiotonic Steroids and Na,K-ATPase. Effects of PH and Ouabain-Induced Changes in Enzyme Conformation. Biochemistry 2009, 48, 10056–10065. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.N.; Tverskoi, A.M.; Sidorenko, S.V.; Smolyaninova, L.V.; Lopina, O.D.; Dulin, N.O.; Klimanova, E.A. Na,K-ATPase as a Target for Endogenous Cardiotonic Steroids: What’s the Evidence? Genes Dis. 2021, 8, 259–271. [Google Scholar] [CrossRef]
- Shah, K.; Chhabra, S.; Singh Chauhan, N. Chemistry and Anticancer Activity of Cardiac Glycosides: A Review. Chem. Biol. Drug. Des. 2022, 100, 364–375. [Google Scholar] [CrossRef]
- Orellana, A.M.; Kinoshita, P.F.; Leite, J.A.; Kawamoto, E.M.; Scavone, C. Cardiotonic Steroids as Modulators of Neuroinflammation. Front. Endocrinol. 2016, 7, 10. [Google Scholar] [CrossRef]
- Schneider, N.; Cerella, C.; Simões, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Kren, V.; Martinkova, L. Glycosides in Medicine: “The Role of Glycosidic Residue in Biological Activity”. Curr. Med. Chem. 2001, 8, 1303–1328. [Google Scholar] [CrossRef]
- Ooi, Y.; Toshihiro, H.; Mituso, N.; Satoh, T. Enzymic Formation of β-Alkyl Glycosides by β-Galactosidase from Aspergillus Oryzae and Its Application to the Synthesis of Chemically Unstable Cardiac Glycosides. Chem. Pharm. Bull. 1984, 35, 1808–1814. [Google Scholar]
- Ooi, Y.; Hashimoto, T.; Mitsuo, N.; Satoh, T. Enzymic Synthesis of Chemically Unstable Cardiac Glycosides by β-Galactosidase from Aspergillus Oryzae. Tetrahedron Lett. 1984, 25, 2241–2244. [Google Scholar] [CrossRef]
- Huang, W.; Wen, C.; Zhou, Z.-R.; Fu, Z.-H.; Katz, A.; Plotnikov, A.; Karlish, S.J.D.; Jiang, R.-W. An Efficient One-Pot Enzymatic Synthesis of Cardiac Glycosides with Varied Sugar Chain Lengths. Adv. Synth. Cat. 2019, 361, 3114–3119. [Google Scholar] [CrossRef]
- Shugrue, C.R.; Miller, S.J. Applications of Nonenzymatic Catalysts to the Alteration of Natural Products. Chem. Rev. 2017, 117, 11894–11951. [Google Scholar] [CrossRef]
- Sun, X.; Lee, H.; Lee, S.; Tan, K.L. Catalyst Recognition of Cis-1,2-Diols Enables Site-Selective Functionalization of Complex Molecules. Nat. Chem. 2013, 5, 790–795. [Google Scholar] [CrossRef]
- Ueda, Y.; Kawabata, T. Organocatalytic Site-Selective Acylation of Carbohydrates and Polyol Compounds. Top. Curr. Chem. 2016, 372, 203–232. [Google Scholar]
- Yoshida, K.; Furuta, T.; Kawabara, T. Perfectly Regioselective Acylation of a Cardiac Glycoside, Digitoxin, via Catalytic Amplification of the Intrinsic Reactivity | Elsevier Enhanced Reader. Tetrahedron Lett. 2010, 51, 4830–4832. [Google Scholar] [CrossRef]
- Danieli, B.; De Bellis, P.; Carrea, G.; Riva, S. Enzyme-Mediated Regioselective Acylations of Flavonoid Disaccharide Monoglycosides. Helv. Chim. Acta 1990, 73, 1837–1844. [Google Scholar] [CrossRef]
- Danieli, B.; Bertario, A.; Carrea, G.; Redigolo, B.; Secundo, F.; Riva, S. Chemo-Enzymatic Synthesis of 6″-O-(3-Arylprop-2-Enoyl) Derivatives of the Flavonol Glucoside Isoquercitrin. Helv. Chim. Acta 1993, 76, 2981–2991. [Google Scholar] [CrossRef]
- Riva, S.; Danieli, B.; Luisetti, M. A Two-Step Efficient Chemoenzymatic Synthesis of Flavonoid Glycoside Malonates. J. Nat. Prod. 1996, 59, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Danieli, B.; De Bellis, P.; Carrea, G.; Riva, S. Regioselective Enzyme-Mediated Acylation of Colchicoside and Thiocolchicoside. Gazz. Chim. Ital. 1991, 121, 123–125. [Google Scholar]
- Danieli, B.; Luisetti, M.; Riva, S.; Bertinotti, A.; Ragg, E.; Scaglioni, L.; Bombardelli, E. Regioselective Enzyme-Mediated Acylation of Polyhydroxy Natural Compounds. A Remarkable, Highly Efficient Preparation of 6′-Acetyl and 6′-O-Carboxyacetyl Ginsenoside Rg1. J. Org. Chem. 1995, 60, 3637–3642. [Google Scholar] [CrossRef]
- Colombo, G.; Riva, S.; Danieli, B. Remote Control of Enzyme Selectivity: The Case of Stevioside and Steviolbioside. Tetrahedron 2004, 60, 741–746. [Google Scholar] [CrossRef]
- Kedra, M.; Kedrowa, S. Clinical evaluation of Proscillaridin A, a glycoside of Scilla maritima. Pol. Tyg. Lek. 1968, 23, 714–716. [Google Scholar]
- Pagliara, R. Clinical use of a new cardioactive glycoside: Proscillaridin A. Case studies. Minerva Med. 1967, 58, 4296–4301. [Google Scholar]
- Krenn, L.; Kopp, B.; Deim, A.; Robien, W.; Kubelka, W. Zum Bufadienolidmuster der roten “Meerzwiebel”. Planta Med. 1994, 60, 63–69. [Google Scholar] [CrossRef]
- Kopp, B.; Krenn, L.; Draxler, M.; Hoyer, A.; Terkola, R.; Vallaster, P.; Robien, W. Bufadienolides from Urginea Maritima from Egypt. Phytochemistry 1996, 42, 513–522. [Google Scholar] [CrossRef]
- Da Costa, E.M.; Armaos, G.; McInnes, G.; Beaudry, A.; Moquin-Beaudry, G.; Bertrand-Lehouillier, V.; Caron, M.; Richer, C.; St-Onge, P.; Johnson, J.R.; et al. Heart Failure Drug Proscillaridin A Targets MYC Overexpressing Leukemia through Global Loss of Lysine Acetylation. J. Exp Clin. Cancer Res. 2019, 38, 251. [Google Scholar] [CrossRef]
- Li, R.-Z.; Fan, X.-X.; Duan, F.-G.; Jiang, Z.-B.; Pan, H.-D.; Luo, L.-X.; Zhou, Y.-L.; Li, Y.; Yao, Y.-J.; Yao, X.-J.; et al. Proscillaridin A Induces Apoptosis and Suppresses Non-Small-Cell Lung Cancer Tumor Growth via Calcium-Induced DR4 Upregulation. Cell Death Dis. 2018, 9, 696. [Google Scholar] [CrossRef]
- Mori, J.; Nagai, S.-I.; Sakakibara, J.; Takeya, K.; Hotta, Y.; Ando, H. Studies on Cardiac Ingredients of Plants. III. Structural Confirmation and Biological Activity of Reduced Proscillaridins. Chem. Pharm. Bull. 1987, 35, 1839–1846. [Google Scholar] [CrossRef]
- Danieli, B.; Luisetti, M.; Sampognaro, G.; Carrea, G.; Riva, S. Regioselective Acylation of Polyhydroxylated Natural Compounds Catalyzed by Candida Antarctica Lipase B (Novozym 435) in Organic Solvents. J. Mol. Catal. B Enzym. 1997, 3, 193–201. [Google Scholar] [CrossRef]
- Monti, D.; Candido, A.; Cruz Silva, M.M.; Křen, V.; Riva, S.; Danieli, B. Biocatalyzed Generation of Molecular Diversity: Selective Modification of the Saponin Asiaticoside. Adv. Synth. Catal. 2005, 347, 1168–1174. [Google Scholar] [CrossRef]
- Hammarström, L.; Smith, C.I.E.; Persson, U. Functional Characterization of Lanatoside-C-Responsive Cells. Scand. J. Immunol. 1978, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.-W.; Chen, T.-H.; Haung, H.-L.; Chang, Y.-W.; HangFu, W.-C.; Lee, Y.-C.; Teng, C.-M.; Pan, S.-L. Lanatoside C, a Cardiac Glycoside, Acts through Protein Kinase Cδ to Cause Apoptosis of Human Hepatocellular Carcinoma Cells. Sci. Rep. 2017, 7, 46134. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.P.; Tsai, Y.-L.; Lee, A.S. Suppression of ER-Stress Induction of GRP78 as an Anti-Neoplastic Mechanism of the Cardiac Glycoside Lanatoside C in Pancreatic Cancer. Neoplasia 2021, 23, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Vinod, N.; Kim, J.H.; Choi, S.; Lim, I. Combination of I-131-Trastuzumab and Lanatoside C Enhanced Therapeutic Efficacy in HER2 Positive Tumor Model. Sci. Rep. 2021, 11, 12871. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Shao, J.; Jiang, C.; Zhao, Y.; Li, Y.; Ke, H.; Zhang, R.; Zhu, J.; Yu, M. Lanatoside C Inhibits Human Cervical Cancer Cell Proliferation and Induces Cell Apoptosis by a Reduction of the JAK2/STAT6/SOCS2 Signaling Pathway. Oncol. Lett. 2021, 22, 740. [Google Scholar] [CrossRef]
- Danieli, B.; Luisetti, M.; Steurer, S.; Michelitsch, A.; Likussar, W.; Riva, S.; Reiner, J.; Schubert-Zsilavecz, M. Application of Lipase-Catalyzed Regioselective Esterification in the Preparation of Digitonin Derivatives. J. Nat. Prod. 1999, 62, 670–673. [Google Scholar] [CrossRef]
- Gebhardt, S.; Bihler, S.; Schubert-Zsilavecz, M.; Riva, S.; Monti, D.; Falcone, L.; Danieli, B. Biocatalytic Generation of Molecular Diversity: Modification of Ginsenoside Rb1 by β-1,4-Galactosyltransferase and Candida Antarctica Lipase, Part 4. Helv. Chim. Acta 2002, 85, 1943–1959. [Google Scholar] [CrossRef]
- Danieli, B.; De Bellis, P.; Carrea, G.; Riva, S. Enzyme-Mediated Acylation of Flavonoid Monoglycosides. Heterocycles 1989, 29, 2061. [Google Scholar] [CrossRef]
- Riva, S.; Carrea, G.; Ottolina, G.; Secundo, F.; Danieli, B.; De Bellis, P. Enzymatic Regioselective Acylation of Polyhydroxylated Natural Compounds in Organic Solvents. Ann. N. Y. Acad. Sci. 1990, 613, 712–716. [Google Scholar] [CrossRef]
- Ueda, Y.; Mishiro, K.; Yoshida, K.; Furuta, T.; Kawabata, T. Regioselective Diversification of a Cardiac Glycoside, Lanatoside C, by Organocatalysis. J. Org. Chem. 2012, 77, 7850–7857. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Enzymatic Modification of the Sugar Moieties of Natural Glycosides. J. Mol. Catal. B Enzym. 2002, 19–20, 43–54. [Google Scholar] [CrossRef]
- Coxon, B. Two-Dimensional Proton Chemical Shift Correlated NMR Spectroscopy of Digitoxose1. J. Carbohydr. Chem. 1984, 3, 525–543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).