Abstract
In this study, three CuFe-MOFs were successfully synthesized by a solvothermal process by changing the ratio of solvents, salts, or temperature. These MOFs named CuFe(BDC-NH2)R, CuFe(BDC-NH2)S, and CuFe(BDC-NH2)D showed rod-shaped, spindle-like, and diamond-like structures, respectively. The CuFe(BDC-NH2)D and CuFe(BDC-NH2)S were found to exhibit an improved PMS activation for Rhodamine B removal attaining levels around 92%. Their effective removal capability was investigated as a function of the pH, catalyst dosage, and the effect of the application of UV radiation. The best degradation system was photo-assisted activation of PMS when CuFe(BDC-NH2)D and CuFe(BDC-NH2)S were used. Under these conditions, the degradation of a mixture of antibiotic and anti-inflammatory drugs (sulfamethoxazole and antipyrine) was evaluated with the results revealing the total degradation of both drugs after 1 h. A higher antibacterial activity was attained with the system CuFe(BDC-NH2)R/PMS due to the high copper content with respect to the others.
1. Introduction
In recent years, sulfate radicals-based advanced oxidation processes (SR-AOPs) or peroxymonosulfate (PMS) activation using catalysts have drawn notable attention in wastewater treatment due to their high efficiency and cost-effectiveness. Such a process does not require external energy and offers several operational advantages, including its application at a wide range of pHs [1,2]. There are several pathways by which the O–O bond of PMS may be disrupted, including heating, ultrasound, light, and transition metals. Among them, transition metal-based material activation using Co, Cu, Fe, or Mn seems to be the most usual method [3,4,5,6].
Several studies have demonstrated the high degradation capability of sulfate radicals generated in SR-AOPs when used to remove organic contaminants such as dyes, phenolic and pharmaceuticals compounds, pesticides, and pathogens [7,8]. However, to improve its application several drawbacks must be overcome. Among these factors is the difficulty of recovering and recycling the catalyst in a homogeneous process, which is one reason why heterogeneous catalysts are preferred for PMS activation. The main advantages of these heterogeneous catalysts are as follows: (i) reusability and easiness for the separation from the reaction system; (ii) minimum release of metal catalyst that could be considered as a secondary pollutant; (iii) ability to operate in extreme conditions; (iv) higher effectiveness at a low economical cost [9,10,11]. Among the heterogeneous catalysts, metal–organic frameworks (MOFs) have drawn great interest because of their high surface area, enormous framework flexibility, and huge structural variety, which facilitate the development of tailored properties materials, as well as tunable pore sizes and catalytic activities [12,13]. By combining metallic ions with organic linkers, MOFs are synthesized producing crystalline porous structures with periodic structures coordinated with inorganic metal nodes and organic bridge ligands. They are capable of serving as heterogeneous catalysts as they possess exposed metal sites in functional pores. Thus, their application in this field has received considerable attention in recent years [14,15].
According to the literature, many MOFs exhibit excellent catalytic properties for PMS activation due to the abundance of oxygen vacancies (as active sites), synergistic mechanisms, and high efficiency [16]. Considering that MOFs are composed of both organic and inorganic components, there is a greater degree of freedom when selecting the starting materials. The versatility of MOFs is attributed to their ability to interact with metal ions and organic ligands in a variety of ways. Furthermore, a variety of conditions can be used to achieve the desired reaction. The presence of two or more metals in a matter can improve their catalytic activity. Mixed metals usually have high stability and low leaching compared to single metals [17].
In this context, recent research has been conducted on the design and synthesis of bimetallic–organic frameworks that take advantage of the synergistic effects of metals. As compared to single-metal MOFs, these MOFs are likely to provide higher activity, stability, and surface area [18]. Bimetallic CuCo-MOFs were synthesized, and it was determined that the introduction of another metal ion into MOFs could significantly enhance the catalytic performance [19,20]. A bimetallic MOF containing Fe and Co showed improved catalytic activity as compared to a monometallic MOF containing a single metal. However, it was also reported that the process could be strongly affected by the shape of the FeCo MOF nanocrystals [21]. Thus, MOFs such as MIL-88B-Fe and MIL-101-Fe, with identical chemical compositions but different exposed facets or shapes, exhibited different activity, MIL-88B-Fe being around five times higher than that of MIL-101-Fe [22]. Similarly, Liao et al. [23] determined that the morphology of MIL-88A-Fe greatly affects its catalytic performance. By a solvent-mediated method, they synthesized several shape-controlled MIL-88A-Fe nanocrystals with different oriented facets and confirmed that the nucleation rate of the MOF defines the morphology or particle size. They synthesized three MIL-88A-Fe with different morphologies such as rod-shaped (100–300 nm length), spindle-like, and diamond-like structures showing different catalytic behaviors.
The catalytic performance is significantly influenced by the composition, structure, and properties of MOFs. For this reason, in this study, different methods were evaluated to synthesize bimetallic CuFe-MOFs and to determine their influence on the synthesis conditions (temperature and solvent compositions) on the catalytic performance for the activation of PMS. Thus, three CuFe-MOFs were prepared the by solvothermal process: two of them using water-free solvents and different salts at 150 °C, CuFe(BDC-NH2)S and CuFe(BDC-NH2)D, respectively, and the other one in an aqueous solvent at 90 °C, CuFe(BDC-NH2)R. The catalytic performance of these bimetallic MOFs was assessed for the removal of dyes, drugs, and pathogens.
2. Results
2.1. Preliminary Catalytic Performance
Based on the literature, Cu-MOF is becoming a promising alternative transition metal-based heterogeneous PMS activator [24]. However, the use of bimetallic MOFs could provide synergistic effects to increase their catalytic activity. Thus, a comparative study among the Cu(BDC-NH2)R and CuFe(BDC-NH2)R catalysts, synthesized in the presence of water as described in Section 3, was performed to determine the possible synergistic effects between metals.
The catalytic activity of these two MOFs towards PMS was evaluated by determining the removal rates of Rhodamine B. These tests were conducted operating at natural pH with 0.25 g/L of catalysts and 1 mM of PMS and compared with PMS alone. Around 16% of Rhodamine B was removed in 60 min by direct oxidation of PMS. However, this level was improved in the presence of Cu(BDC-NH2)R and CuFe(BDC-NH2)R, attaining removal levels of Rhodamine B around 20% and 45%, respectively. These results demonstrated the superiority of bimetallic CuFe(BDC-NH2)R over Cu(BDC-NH2)R, which is in accordance with other studies that had reported the synergistic effect in bimetallic catalysts for PMS activation [25]. Based on these results, other synthesis procedures of bimetallic CuFe(BDC-NH2) were undertaken to determine their effect on the degradation of Rhodamine B.
The adsorption capabilities of the three different CuFe-MOFs, (CuFe(BDC-NH2)R, CuFe(BDC-NH2)S, and CuFe(BDC-NH2)D) for dye removal were evaluated before catalytic oxidation. As shown in Figure 1, in the presence of catalysts alone, Rhodamine B was rapidly removed within the first five minutes with an adsorption/desorption equilibrium being reached after 60 min. It was observed that CuFe(BDC-NH2)D exhibited the greatest efficiency in the adsorption of Rhodamine B. There was rapid adsorption of Rhodamine B, with nearly 50% of Rhodamine B being adsorbed within 60 min. Then, to determine the ability of these bimetallic-MOFs to activate PMS, Rhodamine B was degraded in the presence of PMS. The performance of all Rhodamine B degradation assays was significantly improved when catalysts and PMS were present simultaneously. Thus, CuFe(BDC-NH2)S and CuFe(BDC-NH2)D achieved the highest removal efficiency with removal percentages of Rhodamine B higher than 90%. For an in-depth understanding of the degradation process, Rhodamine B degradation data were assessed by using three kinetic models (zero-order, pseudo-first-order, and pseudo-second-order). According to the results, Rhodamine B degradation follows a pseudo-first-order kinetic. It is also clear that CuFe(BDC-NH2)S and CuFe(BDC-NH2)D have elevated k values (0.0650 and 0.0637 min−1, respectively), seven times higher than that of CuFe(BDC-NH2)R (0.0091 min−1). Consequently, the MOF synthesized in an aqueous media at 90 °C showed different catalytic activities (Table 1).
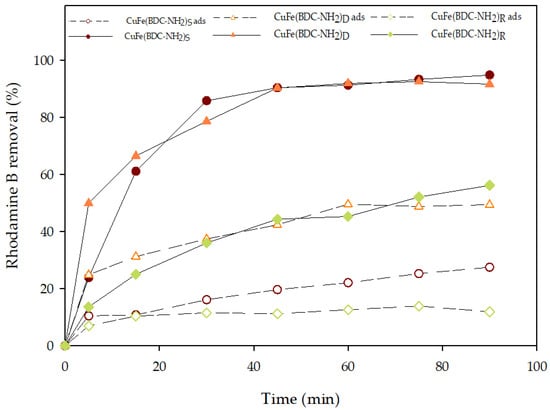
Figure 1.
Profiles of Rhodamine B by PMS activation and adsorption using the different synthesized CuFe-MOFs. Adsorption and PMS assays were performed at natural pH, with a catalyst concentration of 0.25 g/L and a PMS concentration of 1 mM.

Table 1.
Pseudo-first-order kinetic parameters and Rhodamine B removal after 60 min.
In these heterogeneous catalytic systems, the reaction between the metal ions Cu and Fe and oxidant PMS occurs in both solutions and catalyst surface. The CuFe-MOF can easily adsorb the pollutants and PMS molecules, and subsequently, with reactions taking place at the catalyst surface generate sulfate and hydroxyl radicals (Equations (1)–(7)).
≡Fe2+ + HSO5− → ≡Fe3+ + SO4•− + OH−
≡Fe3+ + HSO5− → ≡Fe2+ + SO5•− + H+
≡Cu2+ + HSO5− → ≡Cu+ + SO5•− + H+
≡Cu+ + HSO5− → ≡Cu2+ + SO4•− + OH−
≡Cu+ + ≡Fe3+ → ≡Cu2+ + ≡Fe2+
SO4•− + H2O → H+ + SO42− + •OH
SO4•− or •OH + Pollutant → Degradation products
These reactions explain the synergistic effect observed between Cu2+ and Fe3+, as the generated Cu+ could undergo disproportionation readily, then the electron transfer from Cu+ to Fe3+ produces Fe2+ which is thermodynamically more stable, increasing the generation of sulfate radical from PMS.
In order to determine the contribution of sulfate and hydroxyl radicals, preliminary experiments were performed with excessive masking agents. As is known, methanol has a distinct masking effect on both radicals, but tertbutyl-alcohol only acts on hydroxyl radical [26]. The results indicated the inhibition of dye removal after their addition. However, the inhibiting effect of methanol was stronger than tertbutyl-alcohol. This fact demonstrated that the sulfate radical plays a major role compared to the hydroxyl radical.
2.2. Characterization
The SEM images (Figure 2) depicted the surface structure of the prepared bimetallic CuFe-MOFs, pointing to different types of morphology. CuFe(BDC-NH2)D showed a diamond-like structure (Figure 2c) that is similar to that observed in other MOFs, which suggested that the amine-functionalized organic ligand would be conducive to forming a regular structure [27]. However, for CuFe(BDC-NH2)S, two types of morphology, octahedron and spindle-shaped structures, were detected (Figure 2b). If water was used in the synthesis procedure, a rod-like morphology was observed (Figure 2a) [28]. In addition, the presence of Cu and Fe elements was corroborated by EDS analysis. It was detected that the amount of Cu in the CuFe(BDC-NH2)S and CuFe(BDC-NH2)D samples was lower than the amount used in their preparation. As was concluded by Khosravi et al. [29], this fact could be due to the observed tendency of carboxylate groups to form strong bonds with iron. However, in the presence of water, the elemental mapping proved the existence of a similar percentage of Fe and Cu elements. In addition, the temperature in the synthesis of CuFe(BDC-NH2)S and CuFe(BDC-NH2)D was 150 °C which increased the crystal growth rate and size of the MOF [30].
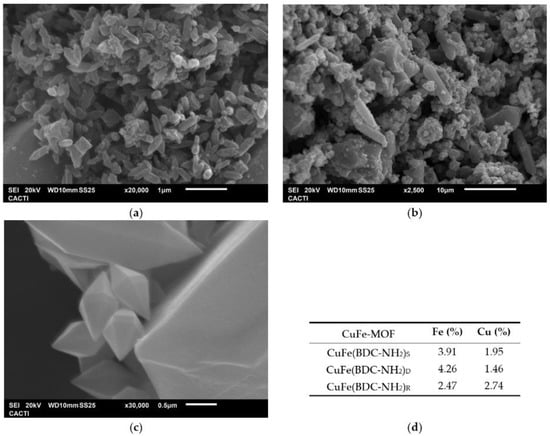
Figure 2.
SEM images of different CuFe-MOFs: (a) CuFe(BDC-NH2)R; (b) CuFe(BDC-NH2)S; and (c) CuFe(BDC-NH2)D. (d) Percentage of Fe and Cu from the EDS analysis.
After analyzing the morphology of the three synthesized MOFs, FTIR and XRD techniques were used to determine the crystalline structure and detect the functional groups of the CuFe-MOFs which could be responsible for the different behavior in the reaction patterns.
First, XRD analysis was carried out to determine whether the MOFs had different crystal lattices. First, it was possible to confirm the differences between CuFe-MOFs due to the Fe/Cu ratio. These differences between the three CuFe-MOFs were investigated by analyzing Fe-MOF-NH2 and Cu-MOF-NH2 in the literature. In Figure 3a, the peaks of the three synthesized CuFe-MOFs are shown. Since CuFe(BDC-NH2)R has a Fe/Cu = 1 ratio, it makes the identification of the copper and iron peaks easier. As shown in Figure 3a, the peaks found in CuFe(BDC-NH2)R are intense reflections in 2θ = 9.2°, 10.3°, 11.8°, and 16.6° and weak reflections in 2θ = 13.2°, 18.1°, 18.5°, 20.8°, 24.7°, and 33.7°. From the work of Zhong et al. [31], who synthesized a Cu-MOF, and Abdel-Azim et al. [32] a Cu-MOF-NH2, it has been possible to associate the peaks with the 2θ values of 11.8°, 18.1°, and 24.7°. In addition, a small peak at 2θ = 33.7° was noted. Considering the Fe/Cu ratios of each CuFe-MOFs, it is logical that the intensity and appearance of these peaks are higher in CuFe(BDC-NH2)R than in CuFe(BDC-NH2)D. In fact, in CuFe(BDC-NH2)D, the 33.7° peak was not detected and the 24.7° peak had a low intensity. This fact may be due to the low copper content (1.46% Cu) compared to CuFe(BDC-NH2)S (1.95% Cu) and CuFe(BDC-NH2)R (2.74% Cu). However, although CuFe(BDC-NH2)S has a slight increase in copper content, compared to CuFe(BDC-NH2)D, all the aforementioned copper peaks were detected except the 2θ = 11.8°.
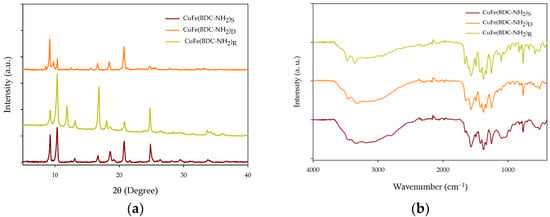
Figure 3.
CuFe-MOF characterization: (a) XRD patterns of different CuFe-MOFs and (b) FTIR spectra of different CuFe-MOFs.
As for the peaks corresponding to iron, they were associated, by difference, with the peaks of 2θ = 9.2°, 10.3°, 13.2°, 16.6°, 18.6°, and 20.8°. These peaks have also been found in other works such as that of Zango et al. [33], who synthetized MIL-88(Fe), NH2-MIL-88(Fe), and mixed-MIL-88(Fe). It should be noted that a peak with a value of 2θ = 9.8° has only been detected in CuFe(BDC-NH2)D and CuFe(BDC-NH2)S. This can be related to crystal formation as both have been synthesized at the same temperature, but with different metal salts.
Figure 3b shows the FTIR analysis of these CuFe-MOFs in the range of 4000 to 400 cm−1. From these FTIR spectra, the bonds that ensure MOF formation and that it is bimetallic are highlighted. Based on the results, it has been possible to confirm in the three CuFe-MOFs the 516 cm−1 bond, which is associated with the tension in the Fe–O bond [34], and the 1066 cm−1 bond, which corresponds to the stretching in the C–O–Cu bond. Additionally, an intense band at 1570 cm−1 can be seen in all three CuFe-MOFs and this corresponds to the asymmetric tension of COO−, a group of the NH2-BDC ligand. However, as a doublet appears at 1425 cm−1 and another at 1377 cm−1, it seems that the symmetric tension mode of this group has unfolded. This may be due to the ligand acting as a bridge, allowing binding to two metals. In addition, it was observed that at 3500 cm−1 and 3200 cm−1, N–H peaks are visible due to amine group stretching [33]. In summary, all the peaks discussed above indicate that the CuFe-MOFs were synthesized successfully.
The differences observed in the chemical and morphological characterization could explain the different behavior of the synthesized CuFe-MOF in the previous assays of Section 2.1. Based on the obtained results, the following experiments were performed using the CuFe-MOFs by the three synthesis methods. The main objective was to determine the best PMS activator for their application in the degradation of pollutants such as dyes, drugs, or pathogens.
2.3. Rhodamine B Removal
2.3.1. Effect of pH
In PMS activation by CuFe-MOFs, different reaction conditions may lead to different activation pathways and result in the formation of different reactive oxidants. Consequently, acidic pH levels support the dominance of sulfate radicals, whereas alkaline pH levels shift the reactions towards more production of hydroxyl radicals. This is due to the substantial presence of hydroxide ions under alkaline conditions [35,36]. For this reason, in this study, the behavior of the degradation process was evaluated at pH 3, 9, and natural. As shown in Figure 4, for each CuFe-MOF similar profiles were obtained operating at the selected pHs.
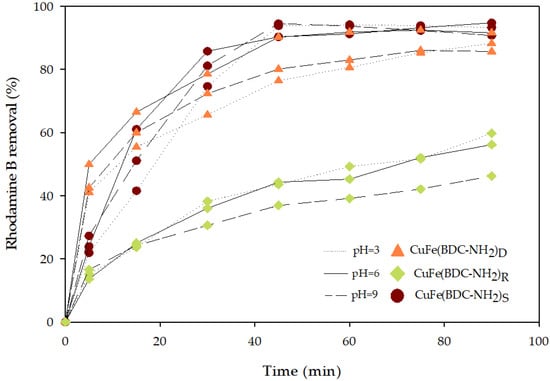
Figure 4.
Profiles of Rhodamine B removal by PMS activation using the three synthesized CuFe-MOFs operating at different pHs.
These results describing the dominant role of sulfate radicals are in accordance with previous studies [37,38] where it was observed that the value of fenuron pesticide or dye removal decreased when the initial pH was increased. The slight differences in Rhodamine B removal could be due to the evolution of the system of CuFe-MOF/PMS in this pH range in which the final pH of the system achieved a similar value of around pH 3. This fact could be explained due to the oxidation of sulfate radicals and their transformation to hydroxyl radicals (Equation (6)), contributing to the production of hydrogen ions and reducing the pH to acid values in which the production of sulfate radicals is boosted [27,39]. As was determined by Li et al. [38], by the CuFe-MOF/PMS system, acidic conditions are preferred for the removal of methyl blue as under strongly alkaline conditions it was detected that the ferrous iron ions form iron sludge with lost catalytic performance.
Based on the attained results and that reported in the literature, it was confirmed that at acid and natural pH ranges, the generated sulfate radicals are similar. Thus, the operation at natural pH was selected as it is then not required to alter the pH of the water, reducing the cost of the process.
2.3.2. Effect of Catalyst Dosage
In this study, different catalyst dosages (0.125–0.5 g/L) were studied to determine their influence on Rhodamine B removal (Table 1). According to the results, Rhodamine B removal rates increased substantially when the catalyst dosages of the three synthesized CuFe-MOFs were increased. The best results were attained using 0.5 g/L of CuFe(BDC-NH2)D and with dye removal ranging from 52% for 0.125 g/L to 94% for 0.5 g/L after 60 min. The kinetic parameters of the degradation were calculated, and the pseudo-first-order model fitted well to the Rhodamine B removal with all R2 values exceeding 0.95 (Table 1). It can be also observed from the table that as catalyst dosage increased from 0.125 to 0.5 g/L, the rate constant for Rhodamine B removal also increased.
Based on these results, the removal of Rhodamine B in this system showed a strong dependency on catalyst dosage as this increases the number of active sites for oxidizing PMS and also accelerates the generation of reactive oxygen species. However, it is important to highlight that a significant effect was detected when the catalyst (CuFe(BDC-NH2)D or CuFe(BDC-NH2)R) concentration increased from 0.125 to 0.25 g/L, with a slight increase when the dosage reached 0.5 g/L or decrease in the case of CuFe(BDC-NH2)S. Accordingly, in terms of economics and removal effects, it was decided to operate the next experiments at 0.25 g/L because the low concentration dosage enabled a reduction of process costs. These results are in accordance with previous studies reported in the literature [40]. When MOFs with small catalyst particles are used as catalysts, it may be advantageous to increase their surface area. It is expected that the effectiveness of the whole system will be greatly increased if more surface-active sites are provided for PMS activation, which is a typical heterogeneous reaction process. Nevertheless, if the quantity of active sites exceeds the required amount, the opposite effect could be detected [40].
2.3.3. Effect of PMS Concentration
The oxidant concentration is another factor that influences the behavior of the system and that needs to be assessed. In Figure 5, the Rhodamine B degradation profiles show that the removal efficiency of the dye improved with the increase of PMS concentration until a value of 1 mM was attained. Even though the PMS alone can reach a maximum dye degradation value of 24.52% at 1 mM after 90 min, the removal rate is lower than the ones obtained in the presence of CuFe-MOFs (Figure 5a). This degradation level was increased when CuFe(BDC-NH2)R was added, doubling their removal levels (Figure 5b). Near total Rhodamine B removal was achieved when CuFe(BDC-NH2)S/PMS (1 mM) and CuFe(BDC-NH2)D/PMS (1 mM) systems were evaluated (Figure 5c,d). However, in the presence of CuFe(BDC-NH2)S and CuFe(BDC-NH2)D, Rhodamine B removal at 0.5 mM and 1 mM differed only by 11–13%. For this reason, the use of higher concentrations is not recommended as excessive PMS concentration could produce free radical self-quenching instead of promoting Rhodamine B degradation [41]. Similarly, Li et al. [42,43] in the perfluorooctane sulfonate degradation determined that a continued increase in PMS concentration brought low removal efficiency and low rate kinetic constants, which was mainly ascribed to the scavenging reaction between PMS and the generated radicals (Equations (8) and (9)) [44]. Despite the fact that increasing the PMS concentration enhances Rhodamine B degradation efficiency, 1 mM was thought to be a suitable concentration and was used in the following assays.
SO4•− + HSO5− → SO5•− + HSO42−
•OH + HSO5− → SO5•− + H2O
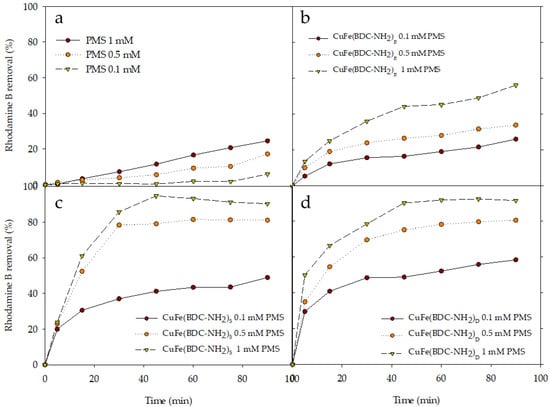
Figure 5.
Effect of PMS concentration on profiles of Rhodamine B by different CuFe-MOFs/PMS systems: (a) without CuFe-MOF, only PMS; (b) CuFe(BDC-NH2)R; (c) CuFe(BDC-NH2)S, and (d) CuFe(BDC-NH2)D with PMS concentrations.
2.3.4. Rhodamine B Removal by Photo-Assisted Activation of PMS over CuFe-MOFs
As aforementioned, PMS can be activated and decomposed to sulfate radicals by using different methods such as UV light according to Equation (10). According to the literature, processes with two or more activators for the decomposition of PMS molecules and the generation of sulfate radicals are more effective than ones with one activator [1,45,46].
HSO5− + hυ → SO4•− + •OH
Thus, Hassani et al. [47] determined the synergistic role of photocatalytic activation of PMS by UV-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation. The coupling catalyst and UV-LED increased the Bisphenol A degradation rate via a sum of mechanisms for the generation of oxidative agents, mainly, SO4•− and •OH radicals.
Inspired by these previous studies, we evaluated the PMS activation under low-intensity light irradiation in the presence of the synthesized CuFe-MOFs [48,49]. In Figure 6 the effect of UV irradiation on Rhodamine B degradation was examined using PMS activated by the different evaluated CuFe-MOFs. UV irradiation alone and in combination with PMS did not appear to be effective in dye removal during the testing period. The main bottleneck in relation to UV activation of PMS is low utilization efficiency due to the poor UV penetration and rapid disruption by pollutants and impurities contained in the water matrix since the UV irradiation is in accordance with the transmittance of the treated effluent. Therefore, the UV light activation of PMS systems might not be suitable for effluents with low transmittance [17]. Similarly, the systems UV/CuFe-MOFs showed similar behavior to the adsorption process (Figure 1).
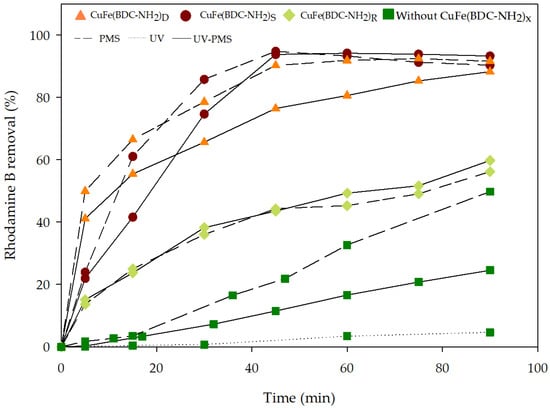
Figure 6.
Comparison among several processes such as UV/PMS, PMS, UV alone separately and in combination with the three synthesized CuFe-MOFs in the Rhodamine B removal. As a reference, the working conditions for the experiment were natural pH, 0.25 g/L catalyst dose, and 1 mM PMS concentration.
Nevertheless, when the UV was added to the PMS/CuFe-MOFs system, Rhodamine B degradation kinetics were significantly faster than without UV irradiation. A complete degradation of the dye was achieved in the presence of CuFe(BDC-NH2)S or CuFe(BDC-NH2)D after 45 min. The corresponding rate constants for the systems with CuFe(BDC-NH2)S or CuFe(BDC-NH2)D were 0.0941 and 0.191 min−1, respectively. These values were much higher than the rate constant of the test with CuFe(BDC-NH2)R (0.03 min−1). The increase in the kinetics of the reaction is the result of UV radiation facilitating the generation of sulfate radicals and other species and by the effect of the UV irradiation exciting the electron/holes pairs of the CuFe-MOF [50,51].
Similar results were reported by Karim et al. [52] who reported that the introduction of UV irradiation for catalyst-based PMS/PS activation accelerated the transfer of e− and enhanced the production of radicals in the system. The reactive species generated under photocatalytic irradiations and enhanced charge transfer assisted in the activation of PMS to generate SO4•− in the solution. Thus, the HSO5− and dissolved oxygen would obtain an e− to generate several radicals and hydrogen peroxide, such as described in Equations (11)–(13), with the following generation of reactive oxidation species that increase the dye degradation rate. In addition, the UV light could accelerate the degradation rate via the indirect formation of free radicals by the regeneration of the metal ions Cu and Fe [51,53,54].
O2 + eˉ → •O2ˉ
HSO5ˉ + eˉ → SO4•ˉ + OH−
2•O2ˉ + 2H+ → H2O2 + 1O2
In order to evaluate the cost/commercial value of a product, its recovery and reuse is an important factor to consider. Based on the previously obtained results, the reusability of both CuFe-MOFs (CuFe(BDC-NH2)S or CuFe(BDC-NH2)D) for PMS activation is crucial for their application for long-term continuous degradation processes. Thus, the UV/[CuFe(BDC-NH2)S or CuFe(BDC-NH2)D]/PMS systems were evaluated in seven successive cycles. It was determined that only a slight decrease in the removal efficiency was observed after six cycles, similar to other studies using MOFs as activators for sulfate radical generation [55]. Both CuFe-MOFs showed higher catalytic activity after each run, suggesting high stability. Thus, the proposed system presented good behavior and usability for the removal of the pollutants with low iron and cooper leaching (lower than 5%). This behavior was comparable to that reported in the literature in which the mixed metals usually have high stability and low leaching compared to single metals [17].
According to previous studies, the enhancement in catalytic activity of both CuFe-MOFs across successive runs could be explained by the action of the oxidant on the heterogeneous catalyst, generating more defects that increase the contact surface [55]. In addition, the literature reported that bi-metallic, and multi-metallic catalysts have higher stability in comparison with mono-metallic catalysts due to the strong interactions between the metallic species [56]. Thus, the obtained results confirm the applicability of these CuFe-MOFs and open the door of opportunity to use these systems in the continuous treatment of polluted effluents in the future.
2.4. Drug Removal
As expected, the synthesized CuFe-MOFs showed remarkable catalytic activity to induce the oxidation process with sulfate radical generated from PMS to efficiently remove Rhodamine B. This study might open an avenue to study the multiple applications of these MOFs in the degradation of other pollutants such as drugs and pathogens. Nowadays, drugs such as antibiotics have been extensively used in human healthcare and livestock production. However, they are poorly metabolized in animals and human bodies. A great number of consumed drugs are eliminated through excretion and eventually released into our water environment [57]. Their residues left in the water can constitute a potential risk for the ecological environment through inducing antibiotic-resistant bacteria, antibiotic resistance, and toxicity effects on living organisms [58,59]. Due to the large amounts of drugs that have been detected in aquatic ecosystems, their degradation is an urgent task that must be performed by an effective technique [60].
Antibiotic and analgesic drugs such as sulfamethoxazole (SMX) or antipyrine (ANT) are widely used currently in human medicine and detected worldwide in effluents near discharge points of plants for wastewater treatment. It has been revealed that these organic compounds cannot be eliminated by conventional treatments [61]. For this reason, in this study, a mixture of SMX and ANT were selected as target pollutants in the developed treatment systems. The application of these CuFe-MOFs in different degradation systems operating in all cases at natural pH, MOF concentration of 0.25 g/L, and PMS concentration of 1 mM was assessed (Figure 7). Although the typical drug concentrations in the wastewater range from 0.1 to 200 µg/L, in these tests, an initial concentration of 10 mg/L of each drug was used to perform the kinetic studies by conventional analytical techniques with adequate reliability.
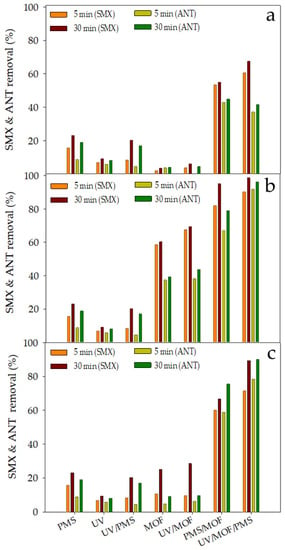
Figure 7.
Concentration of SMX and ANT with different systems using CuFe-MOFs: (a) CuFe(BDC-NH2)R; (b) CuFe(BDC-NH2)D; (c) CuFe(BDC-NH2)S at different times.
As can be observed from Figure 7, similar results were obtained showing the same tendency that was found for Rhodamine B as target pollutant. UV/PMS system can degrade SMX and ANT, reaching 20% of pollutant removal after 30 min. It seems that a low number of radicals are generated from PMS under light irradiation at the beginning of the reaction. In the case of UV/CuFe-MOF, it was highlighted that CuFe(BDC-NH2)D exhibited a very fast initial degradation of SMX and ANT. This fact could be due to adsorption, the predominant mechanism that, regrettably, stops after the first minutes of treatment. The high SMX and ANT degradation levels of the systems UV/[CuFe(BDC-NH2)S or CuFe(BDC-NH2)D]/PMS could be attributed to the redox cycles of both metallic species that are made available for the activation of PMS to generate the radicals mentioned above to oxidize SMX and ANT under UV [62].
Near complete degradation was obtained for SMX and ANT after 90 min by the photo-assisted system followed by CuFe-MOF/PMS with lower levels for the control systems. In these experiments, the carboxylic acids were detected at 30, 60, and 90 min to prove the generation of degradation products. It was highlighted by the formation of several compounds such as oxalic, succinic, formic, malonic, and acetic acid. The obtained results of short-chain carboxylic acids and the generation of intermediates that correspond to peaks detected by measurement on high-performance liquid chromatography (HPLC), not identified or quantified, are indicative of the generation of reaction products [63]. In Figure 8, the intermediates detected with the systems UV/[CuFe(BDC-NH2)R, CuFe(BDC-NH2)S, CuFe(BDC-NH2)D]/PMS are presented as HPLC area.
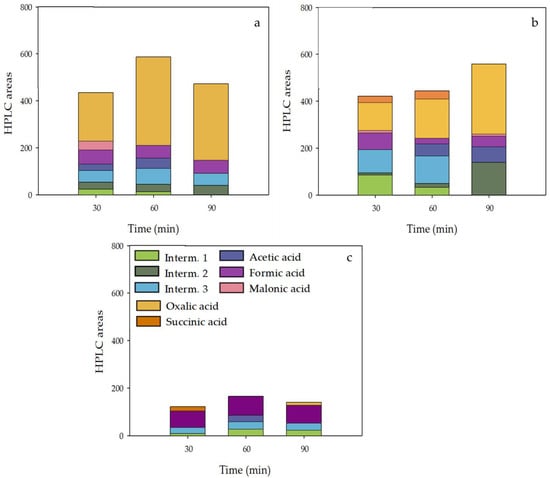
Figure 8.
Bar graphs with the identified carboxylic acids and several detected intermediates in terms of HPLC area in the degradation of SMX and ANT using UV/PMS with different CuFe-MOFs: (a) CuFe(BDC-NH2)R; (b) CuFe(BDC-NH2)S; (c) CuFe(BDC-NH2)D—in this last figure the common legends of all detected intermediates are shown.
As shown in Figure 8c, the amount of compounds measured during the treatment of SMX and ANT in the system UV/CuFe(BDC-NH2)D/PMS is lower than the other catalysts. This fact is indicative of the higher degradation action of this system that aligns with the results shown in Figure 7. Furthermore, formic acid, the shortest carboxylic acid, constitutes a high percentage of the carboxylic acids. However, the other systems showed a lower degradation rate, and they are rather resistant to degradation with higher intermediates that are accumulated in the solution (Figure 8a,b).
2.5. Antibacterial Capability
Copper-containing compounds display high efficiency and extensive spectrum activity against pathogens through mechanisms such as reactive oxygen species production and penetration of bacterial cell walls [64]. The antibacterial capability of the system of synthesized CuFe-MOFs and PMS was determined by the colony-forming unit assay as described in Section 3. In this study, Escherichia coli (E. coli) was selected as the model bacteria based on previous studies of our group [8,65]. Due to PMS alone at a concentration of 1 mM removing colonies, its concentration was reduced to 0.1 mM. Figure 9 depicted a comparative antibacterial activity of PMS without activator and in the presence of the different PMS activator CuFe-MOFs by the number of colonies at 5, 15, 30, and 60 min. In this case, the best results were obtained when CuFe(BDC-NH2)R was used and the increased incubation time significantly reduced the number of colony-forming units (CFU) reaching total disinfection after 1 h. These results suggested that CuFe(BDC-NH2)R showed potential as antibacterial material with respect to the others. In the presence of CuFe(BDC-NH2)S and CuFe(BDC-NH2)D the CFU reduction was lower than 50% after 1 h. This fact could be explained by the composition of each CuFe-MOF (Table 1) where CuFe(BDC-NH2)R presented the highest percentage of Cu. Other studies have proved that Cu is a proficient disruptor of the bacterial cell envelope and the killing effect increased when the Cu concentration was increased [66,67]. Thus, a double benefit of the use of CuFe(BDC-NH2)R was detected, with the capacity to generate sulfate radicals and with a structure that contributes to bacterial disinfection strategy.
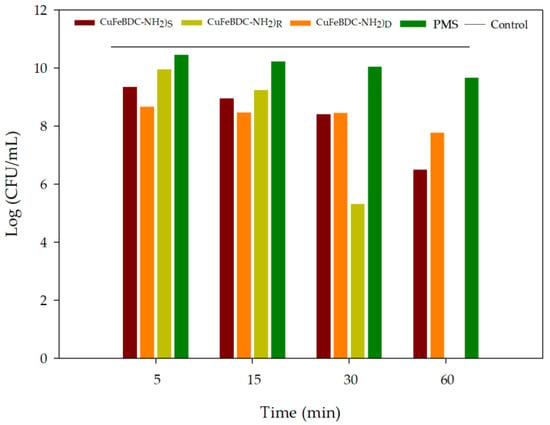
Figure 9.
Comparative antibacterial activity of different CuFe-MOFs/PMS systems: CuFe(BDC-NH2)R; CuFe(BDC-NH2)S; CuFe(BDC-NH2)D. Experimental conditions: concentration of PMS (0.1 mM), catalyst dosage (0.25 g/L), dark and room temperature.
3. Materials and Methods
3.1. Chemicals and Microorganisms
The reagents used for the synthesis of the three bimetallics were dimethylformamide (DMF), 2-aminoterephthalic acid (NH2BDC), ethanol, iron (II) sulfate heptahydrate (FeSO4·7H2O), copper (II) acetate (Cu(CH3COO)2⋅H2O), copper (II) chloride hexahydrate (CuCl2·6H2O), and iron (III) chloride hexahydrate (FeCl3·6H2O). All the chemicals mentioned were purchased from Sigma-Aldrich (Madrid, Spain).
As for the reagents utilized for the antibacterial test with E. coli culture, meat peptone broth (MPB) culture medium was prepared, including 10 g/L of peptone, 5 g/L of meat extract, and 5 g/L of sodium chloride. MPB culture medium was used as liquid and solid medium. For the microorganism, the strain E. coli CECT 102 was used, provided by the Spanish Type Culture Collection.
Several model pollutants were used to determine degradation ability. The dye Rhodamine B, ANT, and SMX, all reagents, and the PMS (2KHSO5·KHSO4·K2SO4) were supplied also by Sigma-Aldrich.
All experimental solutions were prepared using ultrapure deionized water.
3.2. Synthesis of the Bimetallic CuFe(BDC-NH2)x
The catalysts were prepared through a solvothermal method and subsequent thermal treatment. Considering the slight differences among these catalysts, the following synthesis procedure is provided here:
- -
- CuFe(BDC-NH2)R: The procedure of this synthesis was adapted, with slight modifications, from the study of Fu et al. [68]. In this case, 0.724 g of NH2BDC was added to 32 mL of DMF. When NH2BDC was completely dissolved, 0.4 g of Cu(CH3COO)2⋅H2O and 0.556 g of FeSO4·7H2O, were added simultaneously with 4 mL of ethanol and 4 mL of ultrapure water. The mixture was dissolved completely after 30 min and transferred to a 100-mL Teflon-lined autoclaved reactor, which was kept in an oven at 90 °C for 24 h. The obtained solid was washed with ethanol and dried overnight at 80 °C. The monometallic Cu(BDC-NH2)R was synthesized analogously using only 0.4 g of Cu(CH3COO)2⋅H2O as precursor.
- -
- CuFe(BDC-NH2)D: For this catalyst, the synthesis was carried out as described in the work of Khosravi et al. [29]. Briefly, 0.362 g of NH2BDC was mixed in 14 mL of DMF for 15 min. Meanwhile, 0.341 g CuCl2·6H2O and 0.541 g FeCl3⋅6H2O were stirred in 14 mL of DMF. Subsequently, both dilutions were mixed, and 2 mL of ethanol added and shaken vigorously for 30 min, followed by 20 min in ultrasound. Once finished, it was transferred to a 100-mL Teflon-lined autoclaved reactor, which was kept in an oven at 150 °C for 24 h. Subsequently, the obtained solid was washed and filtered with DMF and ethanol and dried overnight at 80 °C.
- -
- CuFe(BDC-NH2)S: The previous procedure was followed, the only difference being the salts used which were 0.4 g of Cu(CH3COO)2⋅H2O and 0.556 g of FeSO4·7H2O.
3.3. Culture Conditions
According to Fdez-Sanromán et al. [8], similar steps were taken to activate and disinfect E. coli CECT 102. In summary, the E. coli CECT 102 inoculum was transferred into a 250 mL Erlenmeyer flask, with 50 mL of MPB medium. Then, it was grown for 20 h at 180 rpm and 37 °C under darkness. For all disinfection experiments, this culture was used as an inoculum (1% v/v inoculum). The culture was incubated until the stationary phase (approx. 20 h) and then centrifuged at 8000 rpm for 15 min (Sigma Laboratory Centrifuges, 3K18, Osterode am Harz, Germany). As a result of this procedure, a minimum concentration of 1010 CFU per mL was assured. Subsequently, it was resuspended in 5 mL of sterile saline solution at 0.9% w/w. For the E. coli experiments, all materials and solutions mentioned were sterilized in an autoclave Presoclave II (J.P. Selecta®, Barcelona, Spain). Each cycle lasted 20 min at 121 °C and 1 bar pressure.
3.4. Experimental Set-Up
3.4.1. Pollutant Degradation
The catalytic performance was evaluated by the decrease of Rhodamine B concentration and further confirmed using a mixture of two pharmaceuticals, ANT and SMX. In both cases, the catalytic activity was evaluated using several AOPs such as Fenton-like, photocatalysis and photo-PMS. In all of them, the heterogeneous degradation experiments were performed in a 0.1 L individual cylindrical cell with an operating volume of 0.05 L and containing Rhodamine B solution (10 mg/L) or the pharmaceutical mixture solution (10 mg/L of each drug).
Concerning the Rhodamine B assays, the experiment was developed under magnetic stirring at 300 rpm at a determined initial pH (natural, 3, and 9). This pH was adjusted by adding 1 M sodium hydroxide or sulfuric acid (H2SO4). The PMS concentration values were from 1 to 0.1 mM and the catalyst dosage was from 0.125 to 0.5 g/L. The assays involving light irradiation, such as photolysis, photocatalysis ,and photo-PMS, were developed using as a light source a UV-A LED lamp (30 W) operating at 365 nm. Sample aliquots of 1 mL were withdrawn at predetermined time intervals and the residual Rhodamine B concentration was measured with a UV-Vis spectrophotometer (Thermo Fisher Genesys M-150, Waltham, MA, USA) at 554 nm. The removal of ANT and SMX was performed by using different AOPs at the best conditions of initial pH, PMS concentration, and catalyst dosage, which were obtained from Rhodamine B experiments. To analyze the sample by HPLC, aliquots of 1 mL were withdrawn at predetermined times and filtered through 0.22 μm PTFE filters. All the results are the average of duplicated assays, and the standard deviations were below 5%.
3.4.2. Disinfection Experiment
As mentioned previously, the disinfection process was followed by Fdez-Sanromán et al. [8]. As a first step, 0.5 mL of E. coli CECT 102 inoculum was transferred to a 250 mL Erlenmeyer flask, containing 50 mL of MPB medium This was incubated for 20 h at 180 rpm, at 37 °C, in the dark. In order to obtain a concentrated culture of E. coli, that was incubated until the stationary phase (approx. 20 h), it was centrifuged at 8000 rpm for 15 min (Sigma Laboratory Centrifuges, 3K18, Osterode am Harz, Germany).
After that, E. coli CECT 102 inactivation experiments were carried out to determine the concentration of the three catalysts (0.25 g/L), CuFe(BDC-NH2)R, CuFe(BDC-NH2)S, and CuFe(BDC-NH2)D for a concentration of PMS (0.1 mM). The disinfection experiment was accomplished by adding 1 mL of concentrated E. coli culture to 99 mL of synthetic water [8] and a mixture of catalyst and PMS. During the experiment, the mixture was kept in an incubator at 80 rpm, 25 °C, and in the dark and samples were taken at 5, 15, 30, and 60 min to follow the disinfection process. At those times, the bacteria concentration was assessed by the standard plate counting method through a serial 10-fold dilution.
3.5. Analytical Methods
3.5.1. Determination of ANT and SMX
The concentrations of ANT and SMX were monitored by HPLC using an Agilent instrument equipped (Agilent 1260, Santa Clara, CA, USA) with a diode array detector at two wavelengths: 242 nm for ANT and 263 nm for SMX. ZORBAX Eclipse XDB-C8 column (Agilent, Santa Clara, CA, USA) (dimensions of 4.6 × 150 mm; 5 μm) was used to carry out the chromatographic separation. The eluent was a mixture consisting of acetonitrile and 1.5% acetic acid aqueous solution (10/90%). The mobile phase flow rate was at 1 mL/min and the injection volume was 10 µL.
For carboxylic acids measurement, the same analytical equipment was employed. The chromatographic separation was carried out at room temperature on a Rezex ROA-Organic Acid H+ column, supplied by Phenomenex (Torrance, CA, USA). The mobile phase used was H2SO4 (0.05 M) at a flow rate of 0.5 mL/min. The carboxylic acids were detected at 210 nm with an injection volume of 20 µL.
3.5.2. Disinfection Efficiency
The disinfection process efficiency was measured by the logarithmic of the CFU/mL. Through a serial 10-fold dilution, the bacteria’s concentrations were assessed using the standard plate counting method. After dilutions were performed in buffered peptone water (15 g/L), aliquots for each dilution were spread onto MPB plates and incubated at 37 °C for 24 h. Afterwards, colonies were counted. For the mean counts (of triplicate samples) in CFU/mL, the coefficient of variation was always less than 15%.
3.5.3. Characterization of CuFe-MOFs
Characterization of the catalyst’s surface was accomplished by scanning electron microscopy and energy dispersive spectrometry (SEM/EDS) using a JEOL JSM6010LA with EDS Oxford AZtecOne SEM (C.A.C.T.I., University of Vigo, Vigo, Spain). For the crystallographic analysis of CuFe-MOFs, the X-ray diffraction (XRD) was made on a Siemens D5000 diffractometer (C.A.C.T.I., University of Vigo, Vigo, Spain). Fourier transform infrared spectroscopy (FTIR) analyses, Nicolet 6700, Thermo Fisher Scientific Inc. (C.A.C.T.I., University of Vigo, Vigo, Spain) were used to analyze the CuFe-MOF bonds and functional groups.
4. Conclusions
In this study, three different bimetallic CuFe-MOFs were assessed and applied for PMS activation. Initially, these heterogeneous bimetallic MOFs were investigated for the removal of Rhodamine B and it was confirmed that the bimetallic catalyst improved the performance of the Cu-MOF. The effects of variables such as pH, catalyst, and PMS dosage were ascertained, and the best conditions were determined for each CuFe-MOF. Their behavior was affected by the Fe/Cu ratio which was found through characterization. It was detected that the MOF with the higher Fe/Cu ratio had better results in the degradation of Rhodamine B and drugs (SMX and ANT). However, for disinfection, the best results were obtained when the Cu content of MOF was increased. In addition, it was proved that the combination system PMS/CuFeMOF/UV is the best system as the radiation improved the generation of radicals. As a result, Rhodamine B and two drugs, namely SMX and ANT, were degraded more efficiently.
The high stability over several cycles and the wide range of applications of these CuFe-MOFs proved the feasibility of the proposed system for application in the environmental field. Thus, these results confirm the applicability of CuFe-MOFs and open the door of opportunity to use these systems for the continuous treatment of polluted effluents in the future.
Author Contributions
Conceptualization, A.S. and A.F.-S.; methodology, A.S. and E.R.; formal analysis, A.F.-S. and B.L.-F.; investigation, A.F.-S. and B.L.-F.; resources, M.P. and A.S.; data curation, A.F.-S. and B.L.-F.; writing—original draft preparation, A.F.-S. and B.L.-F.; writing—review and editing, A.F.-S. and A.S.; visualization, A.F.-S., B.L.-F. and A.S.; supervision, A.S. and E.R.; project administration, A.S.; funding acquisition, M.P. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through the join 2019–2020 Biodiversa & Water JPI joint call for research proposals, under the BiodivRestore ERA-Net COFUND programme with the Project PCI2022-132941 funded by MCIN/AEI/10.13039/501100011033 and PID2020-113667GBI00, funded by MCIN/AEI/10.13039/501100011033 and Xunta de Galicia, and the European Regional Development Fund (ED431C 2021-43).
Data Availability Statement
Not applicable.
Acknowledgments
Antía Fdez-Sanromán thanks Ministerio de Ciencia e Innovación (PRE2021-098540) for her predoctoral fellowships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arellano, M.; Sanromán, M.A.; Pazos, M. Electro-Assisted Activation of Peroxymonosulfate by Iron-Based Minerals for the Degradation of 1-Butyl-1-Methylpyrrolidinium Chloride. Sep. Purif. Technol. 2019, 208, 34–41. [Google Scholar] [CrossRef]
- Nguyen, A.Q.K.; Ahn, Y.Y.; Shin, G.; Cho, Y.; Lim, J.; Kim, K.; Kim, J. Degradation of Organic Compounds through Both Radical and Nonradical Activation of Peroxymonosulfate Using CoWO4 Catalysts. Appl. Catal. B Environ. 2023, 324, 122266. [Google Scholar] [CrossRef]
- Bouzayani, B.; Rosales, E.; Pazos, M.; Elaoud, S.C.; Sanromán, M.A. Homogeneous and Heterogeneous Peroxymonosulfate Activation by Transition Metals for the Degradation of Industrial Leather Dye. J. Clean. Prod. 2019, 228, 222–230. [Google Scholar] [CrossRef]
- Hammad, M.; Angel, S.; Al-Kamal, A.K.; Asghar, A.; Amin, A.S.; Kräenbring, M.A.; Wiedemann, H.T.A.; Vinayakumar, V.; Ali, M.Y.; Fortugno, P.; et al. Synthesis of novel LaCoO3/graphene catalysts as highly efficient peroxymonosulfate activator for the degradation of organic pollutants. Chem. Eng. J. 2023, 454, 139900. [Google Scholar] [CrossRef]
- Dung, N.T.; Thuy, B.M.; Son, L.T.; Ngan, L.V.; Thao, V.D.; Takahashi, M.; Maenosono, S.; Thu, T.V. Mechanistic Insights into Efficient Peroxymonosulfate Activation by NiCo Layered Double Hydroxides. Environ. Res. 2023, 217, 114488. [Google Scholar] [CrossRef]
- Ni, T.; Yang, Z.; Zhang, H.; Zhou, L.; Guo, W.; Pan, L.; Yang, Z.; Chang, K.; Ge, C.; Liu, D. Peroxymonosulfate activation by Co3O4/SnO2 for efficient degradation of ofloxacin under visible light. J. Colloid Interface Sci. 2022, 615, 650–662. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Sanroman, A. Peroxymonosulphate Activation by Basolite® F-300 for Escherichia coli Disinfection and Antipyrine Degradation. Int. J. Environ. Res. Public Health 2022, 19, 6852. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Curiel, S.; Pazos, M.; Sanromán, A. Sustainable regeneration of a honeycomb carbon aerogel used as a high-capacity adsorbent for Fluoxetine removal. J. Mol. Liq. 2022, 357, 119079. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Joseph, J.; Iftekhar, S.; Srivastava, V.; Fallah, Z.; Zare, E.N.; Sillanpää, M. Iron-based metal-organic framework: Synthesis, structure and current technologies for water reclamation with deep insight into framework integrity. Chemosphere 2021, 284, 131171. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanroman, A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Appl. Sci. 2022, 12, 8240. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling Orientation of Metal-Organic Frameworks (MOFs): The Quest to Enhance MOF Performance. Coord. Chem. Rev. 2023, 481, 215043. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, G.; Yi, J.; Cheng, M.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Zhou, C.; Xue, W.; et al. Progress and Challenges of Metal-Organic Frameworks-Based Materials for SR-AOPs Applications in Water Treatment. Chemosphere 2021, 263, 127672. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Scaria, J.; Ghanbari, F.; Nidheesh, P.V. Sulfate Radicals-Based Advanced Oxidation Processes for the Degradation of Pharmaceuticals and Personal Care Products: A Review on Relevant Activation Mechanisms, Performance, and Perspectives. Environ. Res. 2023, 217, 114789. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qiu, X.; Jin, P.; Dzakpasu, M.; Wang, X.C.; Zhang, Q.; Zhang, L.; Yang, L.; Ding, D.; Wang, W.; et al. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Lu, S.; Su, L.; Wang, C.; Huang, J.; Zhou, J.; Tang, J.; Huang, M. Nano-porous bimetallic CuCo-MOF-74 with coordinatively unsaturated metal sites for peroxymonosulfate activation to eliminate organic pollutants: Performance and mechanism. Chemosphere 2021, 273, 129643. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Chen, J.; Wang, C.; Huang, J.; Du, J.; Xu, S.; Tang, J.; Zhao, H.; Huang, M. Heterogeneous activation of peroxymonosulfate by bimetallic MOFs for efficient degradation of phenanthrene: Synthesis, performance, kinetics, and mechanisms. Sep. Purif. Technol. 2021, 259, 118217. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Liu, B.; Zhao, C.; Kuang, Z.; Hu, R.; Liu, B.; Ao, Z.; Wang, J. Shape-Controlled Synthesis of Metal-Organic Frameworks with Adjustable Fenton-Like Catalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 38051–38056. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Liao, X.; Wang, F.; Wang, F.; Cai, Y.; Yao, Y.; Teng, B.T.; Hao, Q.; Shuxiang, L. Synthesis of (100) surface oriented MIL-88A-Fe with rod-like structure and its enhanced fenton-like performance for phenol removal. Appl. Catal. B Environ. 2019, 259, 118064. [Google Scholar] [CrossRef]
- Bai, Y.; Nie, G.; He, Y.; Li, C.; Wang, X.; Ye, L. Cu-MOF for effectively organic pollutants degradation and E. coli inactivation via catalytic activation of peroxymonosulfate. J. Taiwan Inst. Chem. Eng. 2022, 132, 104154. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Li, B.; Zhang, C.; Zhang, M.; Huang, D.; Qin, L.; Yi, H.; Liu, X.; Huang, F.; et al. Role of Radical and Non-Radical Pathway in Activating Persulfate for Degradation of p-Nitrophenol by Sulfur-Doped Ordered Mesoporous Carbon. Chem. Eng. J. 2020, 384, 123304. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhang, Y.; Li, R.; Meng, W.; Song, Z.; Qi, F.; Xu, B.; Chu, W.; Yuan, D.; et al. Enhancement of Fe@porous Carbon to Be an Efficient Mediator for Peroxymonosulfate Activation for Oxidation of Organic Contaminants: Incorporation NH2-Group into Structure of Its MOF Precursor. Chem. Eng. J. 2018, 354, 835–848. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F. Hybridization of MOFs and COFs: A New Strategy for Construction of MOF@COF Core–Shell Hybrid Materials. Adv. Mater. 2018, 30, 1705454. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Gholinejad, M.; Sansano, J.M.; Luque, R. Bimetallic Fe–Cu Metal Organic Frameworks for Room Temperature Catalysis. Appl. Organomet. Chem. 2022, 36, e6749. [Google Scholar] [CrossRef]
- Usman, K.A.S.; Maina, J.W.; Seyedin, S.; Conato, M.T.; Payawan, L.M.; Dumée, L.F.; Razal, J.M. Downsizing Metal–Organic Frameworks by Bottom-up and Top-down Methods. NPG Asia Mater. 2020, 12, 58. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, S.; Dong, A.; Sui, Z.; Feng, L.; Chen, Q. Cu-MOF/Au–Pd Composite Catalyst: Preparation and Catalytic Performance Evaluation. J. Mater. Sci. 2020, 55, 10388–10398. [Google Scholar] [CrossRef]
- Abdel-Azim, S.; Aman, D.; Van Steen, E.; El Salam, H.A. Visible-Light Responsive Cu–MOF–NH2 for Highly Efficient Aerobic Photocatalytic Oxidation of Benzyl Alcohol. Kinet. Catal. 2021, 62, S9–S20. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Hanif Abu Bakar, N.H.; Fathihah Abdullah, N.A.; Basheer, C.; Saad, B. Removal of Anthracene in Water by MIL-88(Fe), NH2-MIL-88(Fe), and Mixed-MIL-88(Fe) Metal-Organic Frameworks. RSC Adv. 2019, 9, 41490–41501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Ding, Z.; Wan, D.; Nie, X.; Zhong, C. A Functionalized Magnetic Graphene-Based MOFs Platform as the Heterogeneous Mimic Enzyme Sensor for Glucose Detection. Catal. Lett. 2022, 152, 2375–2385. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of PH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Ding, Y.; Fu, L.; Peng, X.; Lei, M.; Wang, C.; Jiang, J. Copper Catalysts for Radical and Nonradical Persulfate Based Advanced Oxidation Processes: Certainties and Uncertainties. Chem. Eng. J. 2022, 427, 131776. [Google Scholar] [CrossRef]
- Hayat, W.; Liu, Z.H.; Wan, Y.P.; Zhang, Y. The Analysis of Efficiency of Activated Peroxymonosulfate for Fenuron Degradation in Water. Environ. Technol. Innov. 2022, 26, 102352. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Li, N.; Rao, T.; Zhou, Z.; Zhou, Q.; Wang, C.; Xu, S.; Tang, J. Synthesis of Bimetallic FeCu-MOF and Its Performance as Catalyst of Peroxymonosulfate for Degradation of Methylene Blue. Materials 2022, 15, 7252. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Xing, L.; Li, J.; Xu, M.; Pan, G.; Li, J. Superoxide Radical Mediated Persulfate Activation by Nitrogen Doped Bimetallic MOF (FeCo/N-MOF) for Efficient Tetracycline Degradation. Sep. Purif. Technol. 2022, 282, 120124. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Y.; Zhang, T.C.; Liang, Y.; Wang, Q. Degradation of Methylene Blue with Magnetic Co-Doped Fe3O4@FeOOH Nanocomposites as Heterogeneous Catalysts of Peroxymonosulfate. RSC Adv. 2019, 9, 17664–17673. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Guo, H.; Liu, Y. Heterogeneous Activation of Peroxymonosulfate for Bisphenol AF Degradation with BiOI0.5Cl0.5. RSC Adv. 2019, 9, 14060–14071. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jin, Y.T.; Yan, J.F.; Liu, Z.; Feng, N.X.; Han, W.; Huang, L.W.; Li, Q.K.; Yeung, K.L.; Zhou, S.Q.; et al. Exploration of Perfluorooctane Sulfonate Degradation Properties and Mechanism via Electron-Transfer Dominated Radical Process. Water Res. 2022, 215, 118259. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jin, Y.T.; Cao, D.Y.; Yang, L.L.; Yan, J.F.; Zhang, Z.X.; Liu, Z.; Huang, L.W.; Zhou, S.Q.; Cheng, J.L.; et al. Efficient Decomposition of Perfluorooctane Sulfonate by Electrochemical Activation of Peroxymonosulfate in Aqueous Solution: Efficacy, Reaction Mechanism, Active Sites, and Application Potential. Water Res. 2022, 221, 118778. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Lin, C.; Zhang, H.; Zhou, Z.; Fan, G.; Ma, J. Cobalt Ferrite Nanoparticles Supported on Drinking Water Treatment Residuals: An Efficient Magnetic Heterogeneous Catalyst to Activate Peroxymonosulfate for the Degradation of Atrazine. Chem. Eng. J. 2019, 367, 208–218. [Google Scholar] [CrossRef]
- Noorisepehr, M.; Ghadirinejad, K.; Kakavandi, B.; Ramazanpour Esfahani, A.; Asadi, A. Photo-Assisted Catalytic Degradation of Acetaminophen Using Peroxymonosulfate Decomposed by Magnetic Carbon Heterojunction Catalyst. Chemosphere 2019, 232, 140–151. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, C.P. Mineralization of Aniline in Aqueous Solution by Electrochemical Activation of Persulfate. Chemosphere 2015, 125, 175–181. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.Y.A.; Ghanbari, F. Insights into the Synergistic Role of Photocatalytic Activation of Peroxymonosulfate by UVA-LED Irradiation over CoFe2O4-RGO Nanocomposite towards Effective Bisphenol A Degradation: Performance, Mineralization, and Activation Mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Shen, Y.; Martín de Vidales, M.J.; Espíndola, J.C.; Gómez-Herrero, A.; Dos santos-García, A.J. Paracetamol Degradation by Photo-Assisted Activation of Peroxymonosulfate over ZnxNi1−xFe2O4@BiOBr Heterojunctions. J. Environ. Chem. Eng. 2021, 9, 106797. [Google Scholar] [CrossRef]
- Lin, K.A.; Chang, H. Zeolitic Imidazole Framework-67 (ZIF-67) as a Heterogeneous Catalyst to Activate Peroxymonosulfate for Degradation of Rhodamine B in Water. J. Taiwan Inst. Chem. Eng. 2015, 53, 40–45. [Google Scholar] [CrossRef]
- Bandala, E.R.; Peláez, M.A.; Dionysiou, D.D.; Gelover, S.; Garcia, J.; Macías, D. Degradation of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Using Cobalt-Peroxymonosulfate in Fenton-like Process. J. Photochem. Photobiol. A Chem. 2007, 186, 357–363. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Jiang, F.; Liu, P.; Zhu, M. Photo-Assisted Peroxymonosulfate Activation via 2D/2D Heterostructure of Ti3C2/g-C3N4 for Degradation of Diclofenac. Chemosphere 2020, 258, 127339. [Google Scholar] [CrossRef]
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P.V. Nanostructured Modified Layered Double Hydroxides (LDHs)-Based Catalysts: A Review on Synthesis, Characterization, and Applications in Water Remediation by Advanced Oxidation Processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Wang, J.; Li, W. Fe@C Carbonized Resin for Peroxymonosulfate Activation and Bisphenol S Degradation. Environ. Pollut. 2019, 252, 1042–1050. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A Review of the Innovations in Metal- and Carbon-Based Catalysts Explored for Heterogeneous Peroxymonosulfate (PMS) Activation, with Focus on Radical vs. Non-Radical Degradation Pathways of Organic Contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Khalil, Z.A.; Baalbaki, A.; Bejjani, A.; Ghauch, A. MIL88-A as a Mediator for the Degradation of Sulfamethoxazole in PS Systems: Implication of Solar Irradiation for Process Improvement. Environ. Sci. Adv. 2022, 1, 797–813. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D. Iron-Cobalt Mixed Oxide Nanocatalysts: Heterogeneous Peroxymonosulfate Activation, Cobalt Leaching, and Ferromagnetic Properties for Environmental Applications. Appl. Catal. B Environ. 2009, 88, 462–469. [Google Scholar] [CrossRef]
- Huang, F.; An, Z.; Moran, M.J.; Liu, F. Recognition of Typical Antibiotic Residues in Environmental Media Related to Groundwater in China (2009–2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The Effect of Bioelectrochemical Systems on Antibiotics Removal and Antibiotic Resistance Genes: A Review. Chem. Eng. J. 2019, 358, 1421–1437. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Gao, R.; Zhao, Q.; Yang, Z.; Gao, X.; Wang, Z.; Zhang, F.; Wu, W. Enhanced Visible Light Photoelectrocatalytic Degradation of Tetracycline Hydrochloride by I and P Co-Doped TiO2 Photoelectrode. J. Hazard. Mater. 2021, 406, 124309. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.J.; Kronka, M.S.; Fortunato, G.V.; Lanza, M.R.V. Recent Advances in Electrochemical Water Technologies for the Treatment of Antibiotics: A Short Review. Curr. Opin. Electrochem. 2021, 26, 100674. [Google Scholar] [CrossRef]
- Peralta-Hernández, J.M.; Brillas, E. A Critical Review over the Removal of Paracetamol (Acetaminophen) from Synthetic Waters and Real Wastewaters by Direct, Hybrid Catalytic, and Sequential Ozonation Processes. Chemosphere 2023, 313, 137411. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Z.; Deng, L. Enhanced Heterogeneous Degradation of Sulfamethoxazole via Peroxymonosulfate Activation with Novel Magnetic MnFe2O4/GCNS Nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126531. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Moratalla, A.; Pazos, M.; Sanroman, A.; Sáez, C.; Rodrigo, M.A. Exploring the Pressurized Heterogeneous Electro-Fenton Process and Modelling the System. Chem. Eng. J. 2022, 431, 133280. [Google Scholar] [CrossRef]
- Yu, Y.-P.; Pan, M.-M.; Jiang, M.; Yu, X.; Xu, L. Facile Synthesis of Self-Assembled Three-Dimensional Flower-like Cu-MOF and Its Pyrolytic Derivative Cu-N-C450 for Diverse Applications. J. Environ. Chem. Eng. 2023, 11, 109400. [Google Scholar] [CrossRef]
- Blanco-Canella, P.; Lama, G.; Sanromán, M.A.; Pazos, M. Disinfection through Advance Oxidation Processes: Optimization and Application on Real Wastewater Matrices. Toxics 2022, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S.J. Role of Copper in Reducing Hospital Environment. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef]
- Chakraborty, D.; Musib, D.; Saha, R.; Das, A.; Raza, M.K.; Ramu, V.; Chongdar, S.; Sarkar, K.; Bhaumik, A. Highly Stable Tetradentate Phosphonate-Based Green Fl Uorescent Cu-MOF for Anticancer Therapy and Antibacterial Activity. Mater. Today Chem. 2022, 24, 100882. [Google Scholar] [CrossRef]
- Fu, A.; Liu, Z.; Sun, Z. Cu/Fe Oxide Integrated on Graphite Felt for Degradation of Sulfamethoxazole in the Heterogeneous Electro-Fenton Process under near-Neutral Conditions. Chemosphere 2022, 297, 134257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).