Photocatalytic Degradation of Pharmaceutical Amisulpride Using g-C3N4 Catalyst and UV-A Irradiation
Abstract
1. Introduction
2. Results and Discussion
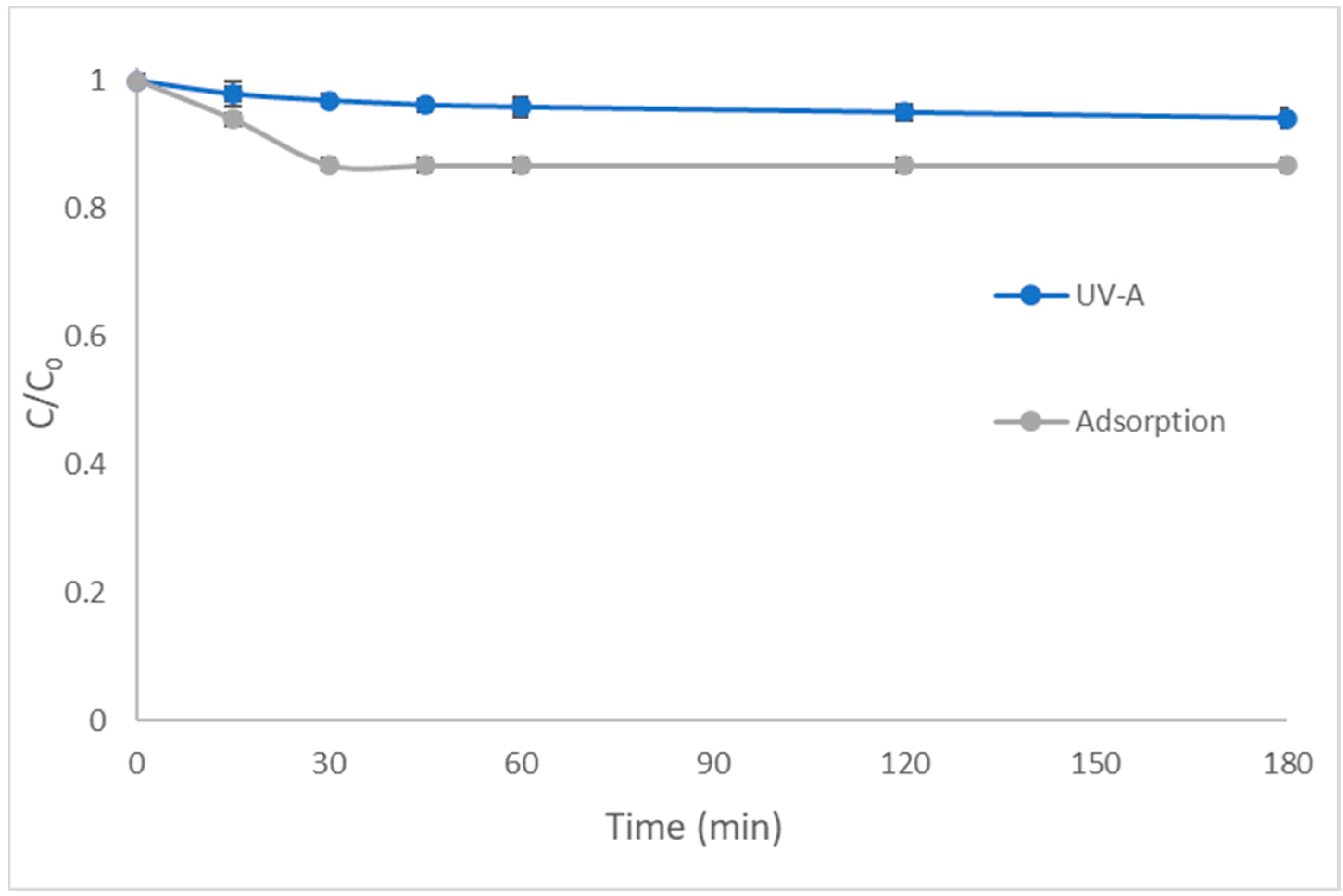
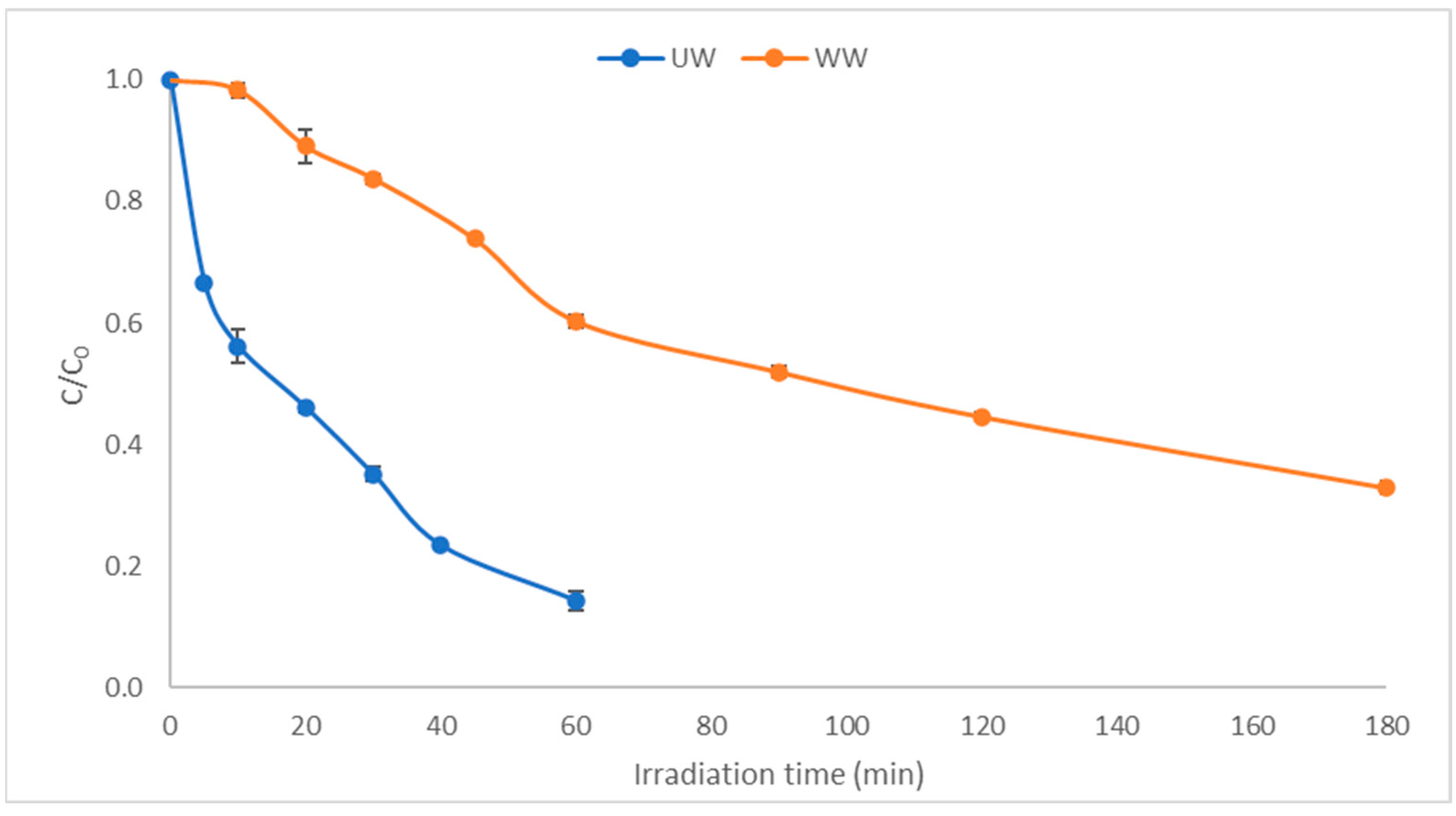
2.1. Photocatalytic Degradation Kinetics
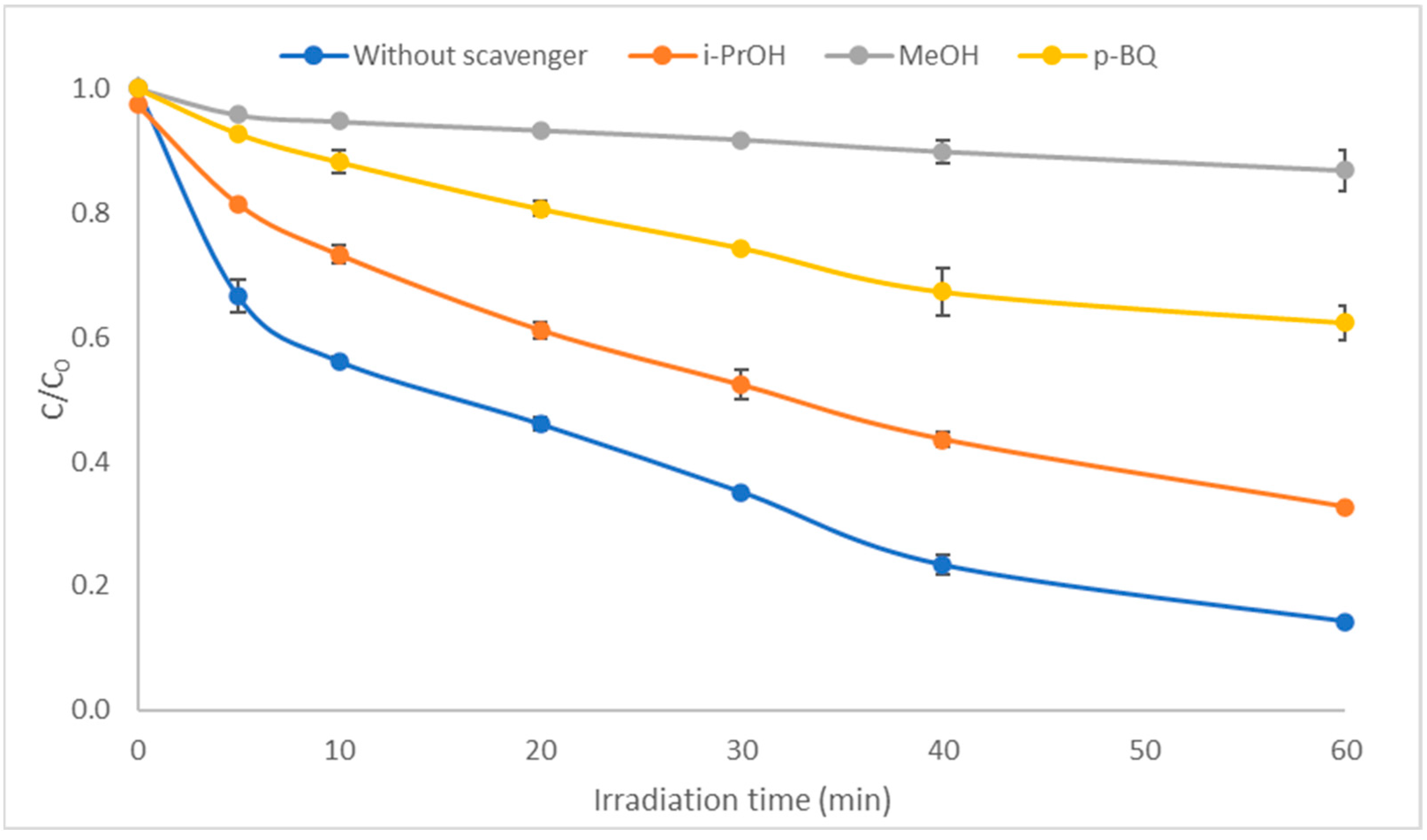
2.2. Role of Reactive Species to the Degradation Mechanism
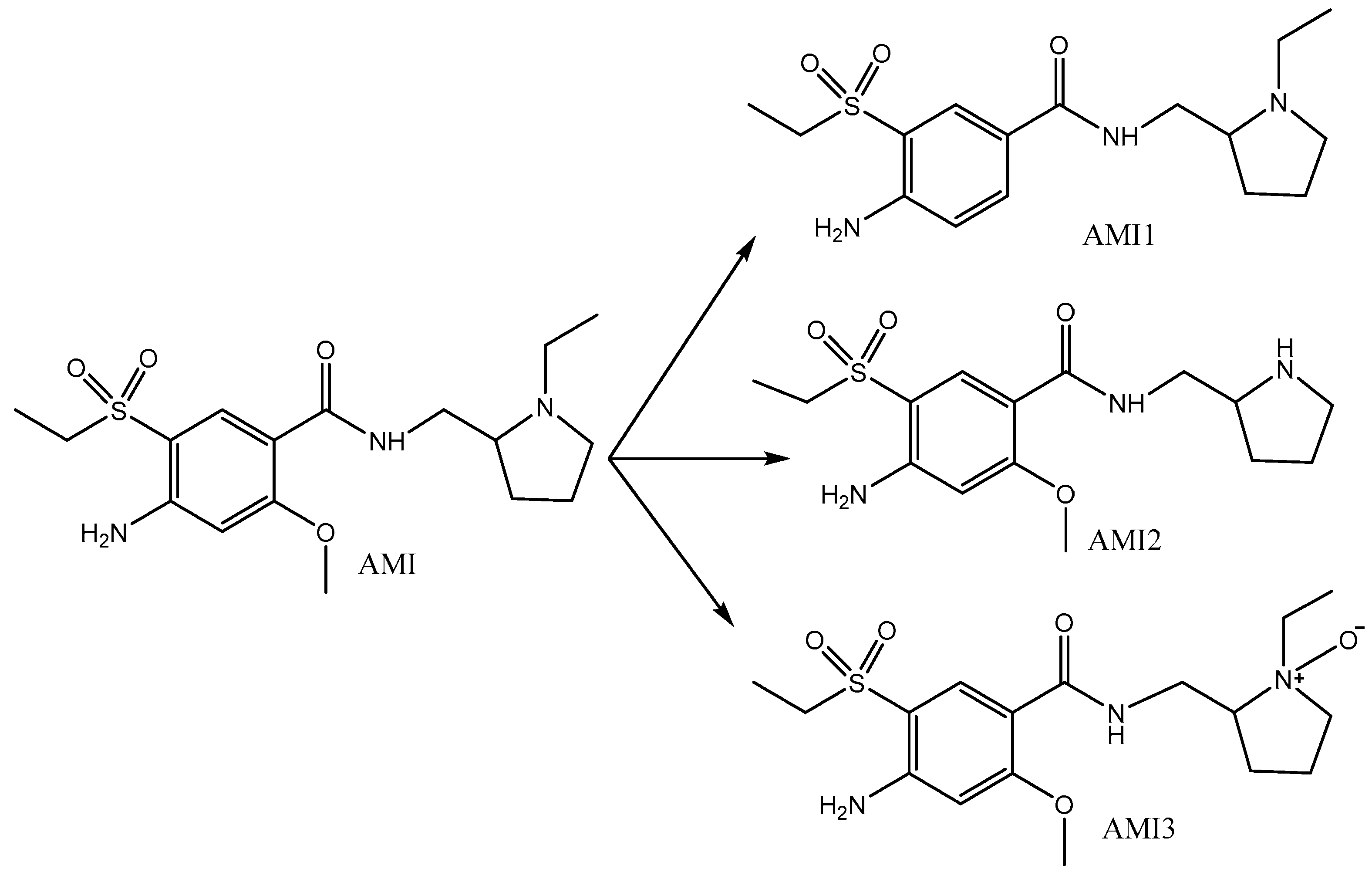
2.3. Photocatalytic Degradation Mechanism
2.4. Ecotoxicity Evolution
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Photocatalytic Experiments
3.3. Scavenging Experiments
3.4. Analytical Methods
3.5. UHPLC/MS Analysis
3.6. Algal Biotest
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calisto, V.; Esteves, V.I. Psychiatric Pharmaceuticals in the Environment. Chemosphere 2009, 77, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium Dioxide Photocatalysis for Pharmaceutical Wastewater Treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Rapti, I.; Kosma, C.; Albanis, T.; Konstantinou, I. Solar Photocatalytic Degradation of Inherent Pharmaceutical Residues in Real Hospital WWTP Effluents Using Titanium Dioxide on a CPC Pilot Scale Reactor. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Gros, M.; Williams, M.; Llorca, M.; Rodriguez-Mozaz, S.; Barceló, D.; Kookana, R.S. Photolysis of the Antidepressants Amisulpride and Desipramine in Wastewaters: Identification of Transformation Products Formed and Their Fate. Sci. Total Environ. 2015, 530-531, 434–444. [Google Scholar] [CrossRef]
- Konstas, P.S.; Kosma, C.; Konstantinou, I.; Albanis, T. Photocatalytic Treatment of Pharmaceuticals in Real Hospital Wastewaters for Effluent Quality Amelioration. Water 2019, 11, 2165. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and Spatial Distribution of 158 Pharmaceuticals, Drugs of Abuse and Related Metabolites in Offshore Seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Bletsou, A.A.; Damalas, D.E.; Aalizadeh, R.; Alygizakis, N.A.; Singer, H.P.; Hollender, J.; Thomaidis, N.S. Wide-Scope Target Screening of >2000 Emerging Contaminants in Wastewater Samples with UPLC-Q-ToF-HRMS/MS and Smart Evaluation of Its Performance through the Validation of 195 Selected Representative Analytes. J. Hazard. Mater. 2020, 387, 121712. [Google Scholar] [CrossRef]
- Cappelli, F.; Longoni, O.; Rigato, J.; Rusconi, M.; Sala, A.; Fochi, I.; Palumbo, M.T.; Polesello, S.; Roscioli, C.; Salerno, F.; et al. Suspect Screening of Wastewaters to Trace Anti-COVID-19 Drugs: Potential Adverse Effects on Aquatic Environment. Sci. Total Environ. 2022, 824, 153756. [Google Scholar] [CrossRef]
- Bollmann, A.F.; Seitz, W.; Prasse, C.; Lucke, T.; Schulz, W.; Ternes, T. Occurrence and fate of amisulpride, sulpiride, and lamotrigine in municipal wastewater treatment plants with biological treatment and ozonation. J. Hazard. Mater. 2016, 320, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, A.; Tzamaria, A.; Dormousoglou, M.; Skourti, A.; Vlastos, D.; Papadaki, M.; Antonopoulou, M. The overall assessment of simultaneous photocatalytic degradation of Cimetidine and Amisulpride by using chemical and genotoxicological approaches. Sci. Total Environ. 2022, 838, 156140. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of Multiclass Pharmaceutical Active Compounds (PhACs) in Hospital WWTP Influent and Effluent Samples by UHPLC-Orbitrap MS: Temporal Variation, Removals and Environmental Risk Assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef] [PubMed]
- Parry, E.; Young, T.M. Comparing Targeted and Non-Targeted High-Resolution Mass Spectrometric Approaches for Assessing Advanced Oxidation Reactor Performance. Water Res. 2016, 104, 72–81. [Google Scholar] [CrossRef]
- Rapti, I.; Bairamis, F.; Konstantinou, I. g-C3N4/MoS2 Heterojunction for Photocatalytic Removal of Phenol and Cr(VI). Photochem 2021, 1, 358–370. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I. WO3 Fibers/g-C3N4 z-Scheme Heterostructure Photocatalysts for Simultaneous Oxidation/Reduction of Phenol/Cr (Vi) in Aquatic Media. Catalysts 2021, 11, 792. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sampaio, M.J.; Ribeiro, A.R.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Metal-Free g-C3N4 Photocatalysis of Organic Micropollutants in Urban Wastewater under Visible Light. Appl. Catal. B Environ. 2019, 248, 184–192. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic Carbon Nitride (g-C3N4) Nanocomposites: A New and Exciting Generation of Visible Light Driven Photocatalysts for Environmental Pollution Remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Antonopoulou, Μ.; Hela, D.; Konstantinou, I. Photocatalytic degradation kinetics, mechanism and ecotoxicity assessment of tramadol metabolites in aqueous TiO2 suspensions. Sci. Total Environ. 2016, 545-546, 476–485. [Google Scholar] [CrossRef]
- Calza, P.; Hadjicostas, C.; Sakkas, V.A.; Sarro, M.; Minero, C.; Medana, C.; Albanis, T.A. Photocatalytic transformation of the antipsychotic drug risperidone in aqueous media on reduced graphene oxide—TiO2 composites. Appl. Catal. B Environ. 2016, 183, 96–106. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Ma, W.; Zhao, J.; Hidaka, H.; Serpone, N. Effect of Transition Metal Ions on the TiO2-Assisted Photodegradation of Dyes under Visible Irradiation: A Probe for the Interfacial Electron Transfer Process and Reaction Mechanism. J. Phys. Chem. B 2002, 106, 318–324. [Google Scholar] [CrossRef]
- Palominos, C.R.; Freer, J.; Mondaca, M.A.; Mansilla, H. Evidence for hole participation during the photocatalytic oxidation of the antibiotic flumequine. J. Photochem. Photobiol. Chem. 2008, 193, 139–145. [Google Scholar] [CrossRef]
- Antonopoulou, Μ.; Konstantinou, I. Photocatalytic degradation and mineralization of tramadol pharmaceutical in aqueous TiO2 suspensions: Evaluation of kinetics, mechanisms and ecotoxicity. Appl. Catal. Gen. C 2016, 515, 136–143. [Google Scholar] [CrossRef]
- Hazime, R.; Ferronato, C.; Fine, L.; Salvador, A.; Jaber, F.; Chovelon, J.-M. Photocatalytic degradation of imazalil in an aqueous suspension of TiO2 and influence of alcohols on the degradation. Appl. Catal. B Environ. 2012, 126, 90–99. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Cat. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I.; Petrakis, D.; Vaimakis, T. Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts 2019, 9, 880. [Google Scholar] [CrossRef]
- Xu, T.; Wang, D.; Dong, L.; Shen, H.; Lu, W.; Chen, W. Graphitic carbon nitride co-modified by zinc phthalocyanine and graphene quantum dots for the efficient photocatalytic degradation of refractory contaminants. Appl. Catal. B Environ. 2019, 244, 96–106. [Google Scholar] [CrossRef]
- Skibiński, R. Identification of photodegradation product of amisulpride by ultra-high-pressure liquid chromatography–DAD/ESI-quadrupole time-of-flight-mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 904–910. [Google Scholar] [CrossRef]
- Gomes, J.F.; Lopes, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Study of the influence of the matrix characteristics over the photocatalytic ozonation of parabens using Ag-TiO2. Sci. Total Environ. 2019, 646, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.C.; Rossi, A.F.; Quinta-Ferreira, R.M. Fenton’s oxidation process for phenolic wastewater remediation and biodegradability enhancement. J. Hazard. Mater. 2010, 180, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Konstas, P.-S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts 2018, 8, 554. [Google Scholar] [CrossRef]
- Baxendale, J.H.; Bridge, N.K. The photoreduction of some ferric compounds in aqueous solution. J. Phys. Chem. 1955, 59, 783–788. [Google Scholar] [CrossRef]
- Calvert, J.; Pitts, J.N. Liquid–Phase Chemical Actinometry Using Potassium Ferrioxalate. Photochemistry; John Wiley: New York, NY, USA, 1966; pp. 783–786. [Google Scholar]
- Organization for the Economic Cooperation and Development. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar] [CrossRef]



| Matrix | k × 10−2 (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| UW | 3.04 | 22.80 | 0.9420 |
| WW | 0.7 | 99.02 | 0.9809 |
| Scavenger | k × 10−2 (min−1) | R2 | Δk (%) |
|---|---|---|---|
| Without Scavenger | 3.04 | 0.9420 | - |
| i-PrOH | 1.76 | 0.9800 | 42.1 |
| MeOH | 0.21 | 0.9292 | 93.1 |
| p-BQ | 0.80 | 0.9720 | 73.7 |
| TPs | [M + H]+ | Molecular Formula |
|---|---|---|
| AMI | 370.30 | C17H27N3O4S |
| AMI1 | 340.37 | C16H25N3O3S |
| AMI2 | 342.39 242.05 196.01 | C15H23N3O4S |
| AMI3 | 386.26 196.01 | C17H27N3O5S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulou, M.; Papadaki, M.; Rapti, I.; Konstantinou, I. Photocatalytic Degradation of Pharmaceutical Amisulpride Using g-C3N4 Catalyst and UV-A Irradiation. Catalysts 2023, 13, 226. https://doi.org/10.3390/catal13020226
Antonopoulou M, Papadaki M, Rapti I, Konstantinou I. Photocatalytic Degradation of Pharmaceutical Amisulpride Using g-C3N4 Catalyst and UV-A Irradiation. Catalysts. 2023; 13(2):226. https://doi.org/10.3390/catal13020226
Chicago/Turabian StyleAntonopoulou, Maria, Maria Papadaki, Ilaeira Rapti, and Ioannis Konstantinou. 2023. "Photocatalytic Degradation of Pharmaceutical Amisulpride Using g-C3N4 Catalyst and UV-A Irradiation" Catalysts 13, no. 2: 226. https://doi.org/10.3390/catal13020226
APA StyleAntonopoulou, M., Papadaki, M., Rapti, I., & Konstantinou, I. (2023). Photocatalytic Degradation of Pharmaceutical Amisulpride Using g-C3N4 Catalyst and UV-A Irradiation. Catalysts, 13(2), 226. https://doi.org/10.3390/catal13020226