Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
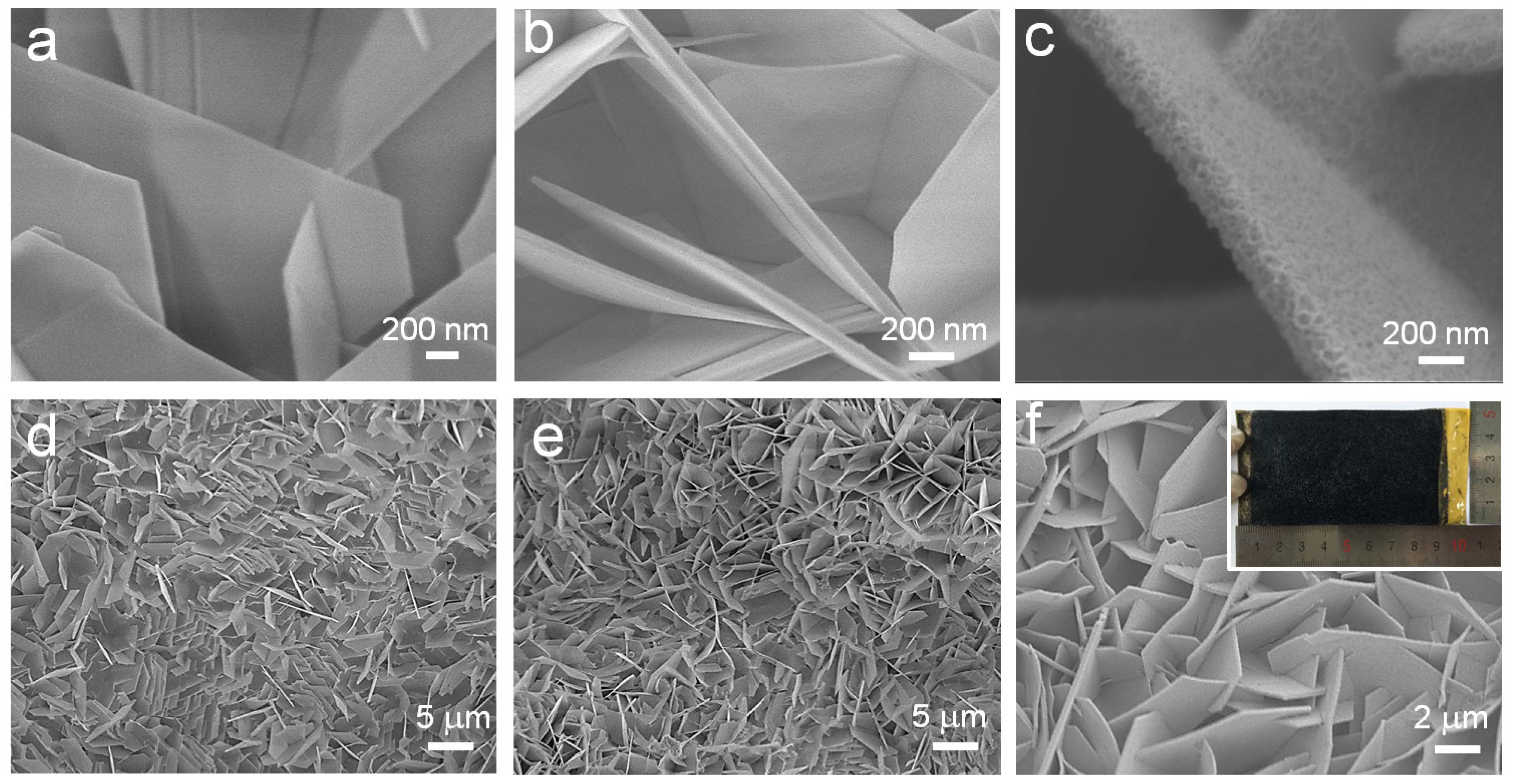
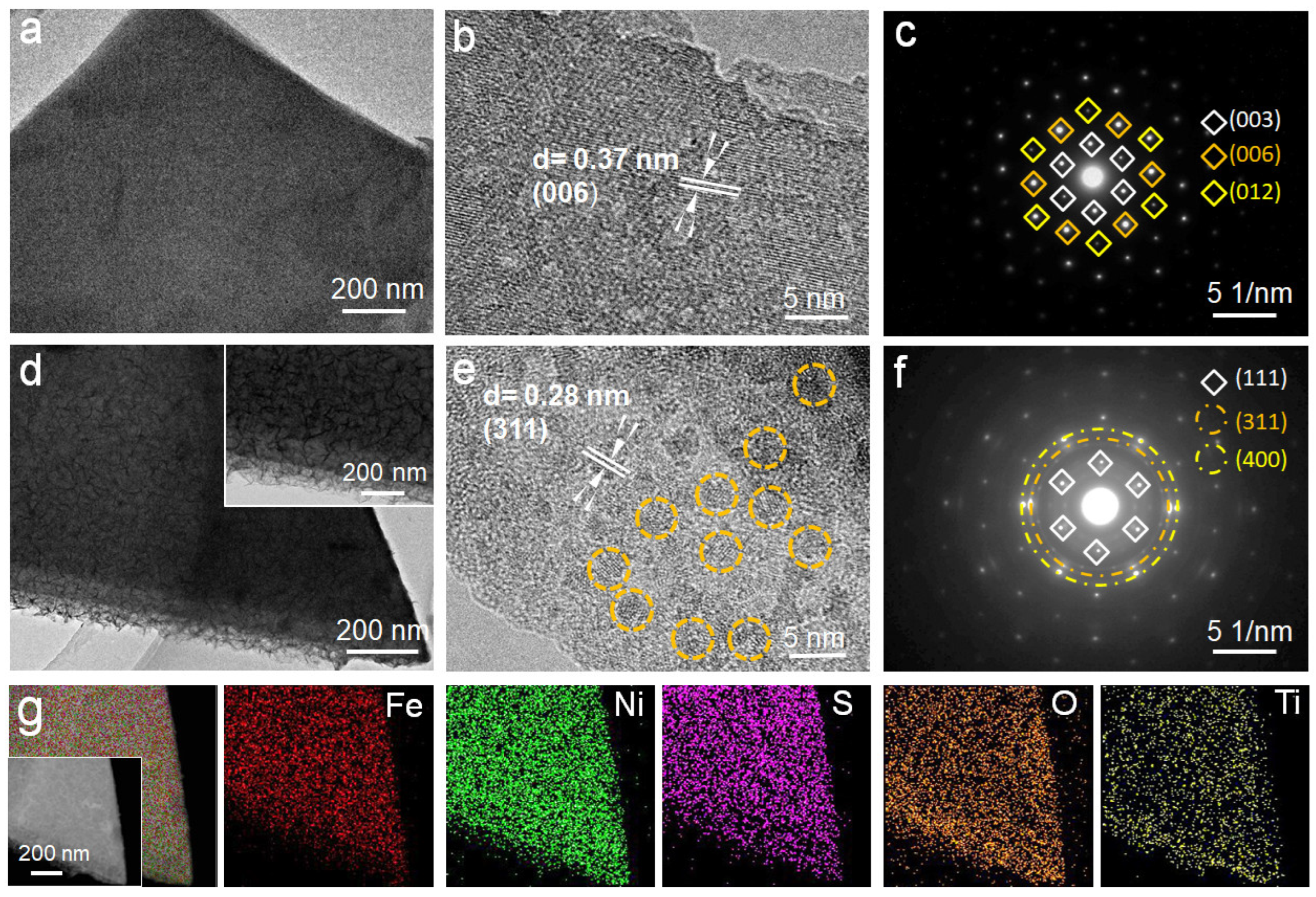
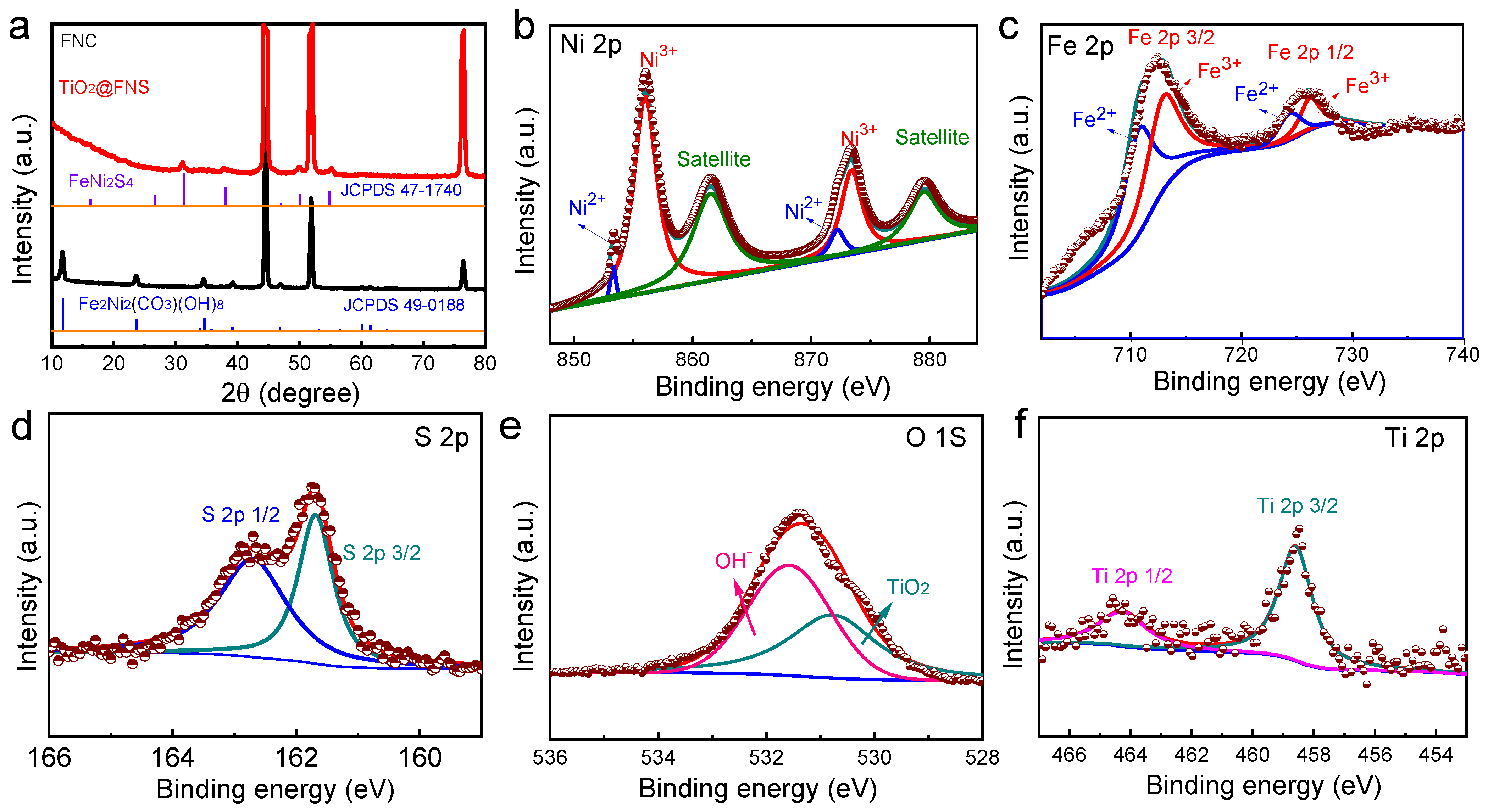
2.1. Morphology and Structure Analyses
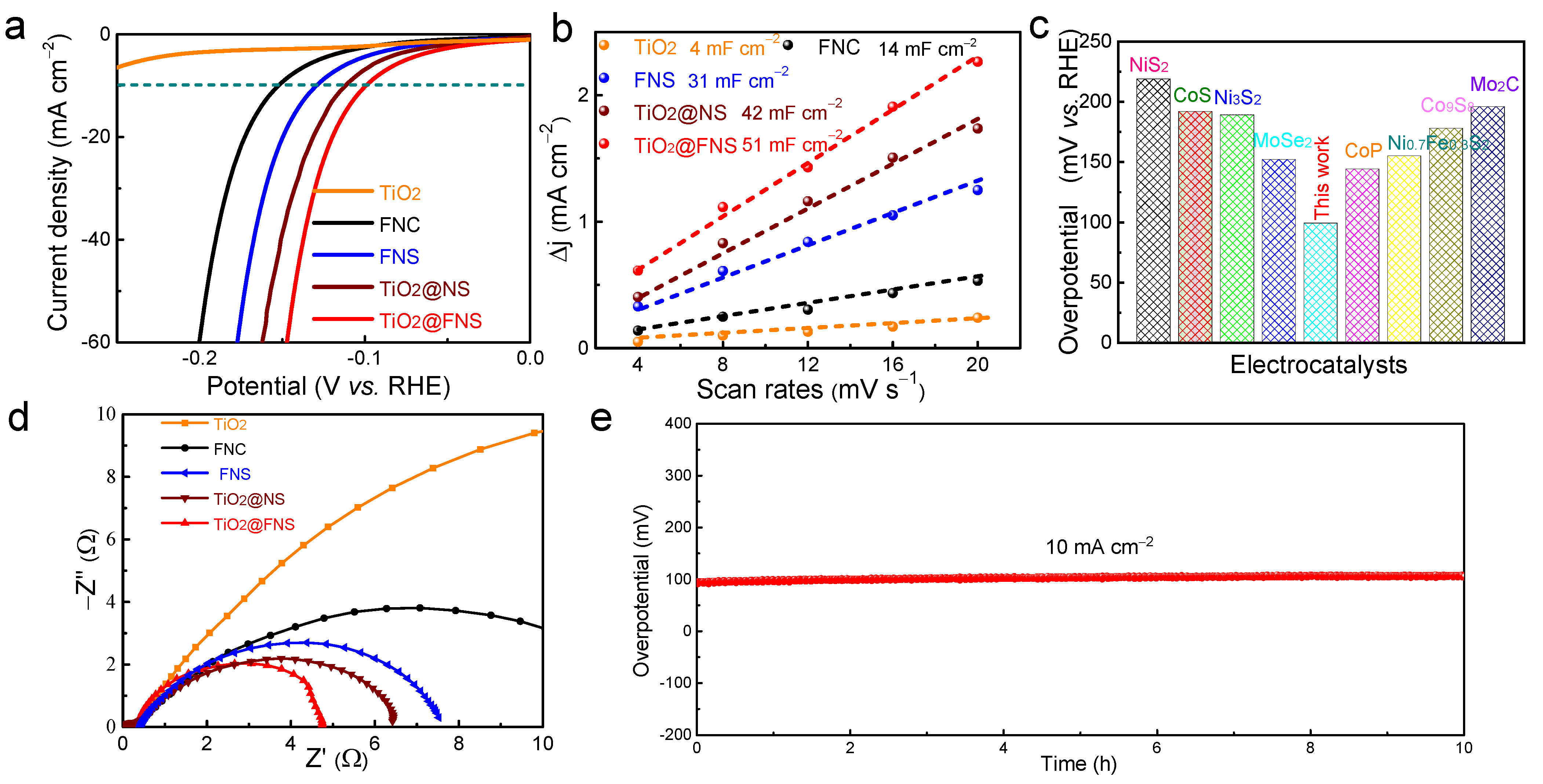
2.2. Electrochemical Performance Analyses
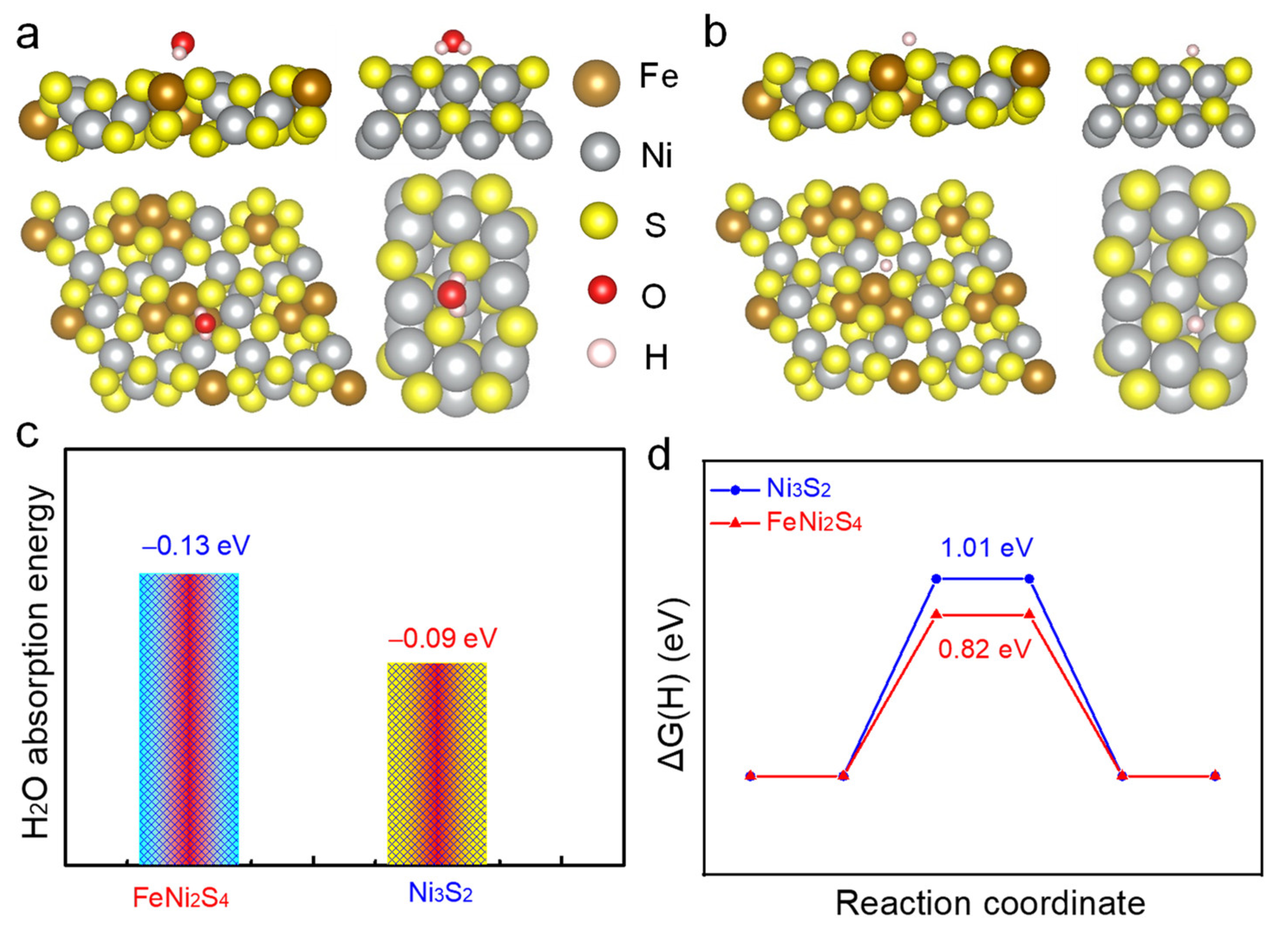
2.3. DFT Calculation
3. Materials and Methods
3.1. Synthesis of TiO2@FNS Arrays on Ni Foam
3.2. Characterization
3.3. Electrochemical Measurements
3.4. Computational Methods and Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Wang, Z.; Liu, M.; Wang, E.; Wang, B.; Xu, L.; Jiang, K.; Fan, S.; Sun, Y.; Li, J.; et al. Ultrafast self-heating synthesis of robust heterogeneous nanocarbides for high current density hydrogen evolution reaction. Nat. Commun. 2022, 13, 3338. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.; Zhou, J. Facet-tunable coral-like Mo2C catalyst for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2023, 451, 138977. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Che, S.; Ta, N.; Yang, F.; Yang, Y.; Li, Y. I Interfacial electronic engineering of NiSe–anchored Ni–N–C composite electrocatalyst for efficient hydrogen evolution. Catalysts 2022, 12, 1525. [Google Scholar] [CrossRef]
- Zhou, Q.; Bian, Q.; Liao, L.; Yu, F.; Li, D.; Tang, D.; Zhou, H. In situ electrochemical dehydrogenation of ultrathin Co(OH)2 nanosheets for enhanced hydrogen evolution. Chin. Chem. Lett. 2023, 34, 107248. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Wang, L.; Su, B.-J.; Wu, K.-H.; Juang, J.-Y.; Hou, F.; Yin, L.; Dou, S.; Liu, J.; et al. Carbon-Shielded Single-Atom Alloy Material Family for Multi-Functional Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2205654. [Google Scholar] [CrossRef]
- Karmodak, N.; Andreussi, O. Catalytic Activity and Stability of Two-Dimensional Materials for the Hydrogen Evolution Reaction. ACS Energy Lett. 2020, 5, 885–891. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, M.; Liu, X.; Ma, J.; Wang, L.; Li, C.; Wang, W. Co(OH)2 nanoflowers decorated α-NiMoO4 nanowires as a bifunctional electrocatalyst for efficient overall water splitting. Catalysts 2022, 12, 1417; [Google Scholar] [CrossRef]
- Li, C.; Jang, H.; Kim, M.G.; Hou, L.; Liu, X.; Cho, J. Ru-incorporated oxygen-vacancy-enriched MoO2 electrocatalysts for hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 307, 121204. [Google Scholar] [CrossRef]
- Ye, J.; Zang, Y.; Wang, Q.; Zhang, Y.; Sun, D.; Zhang, L.; Wang, G.; Zheng, X.; Zhu, J. Nitrogen doped FeS2 nanoparticles for efficient and stable hydrogen evolution reaction. J. Energy Chem. 2020, 56, 283–289. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, Y.; Zhou, H.; Li, D.; Qi, Y.; Zhang, Y.; Sun, Y.; Zhou, Q.; Yu, F. Edge-oriented N-Doped WS2 Nanoparticles on Porous Co3N Nanosheets for Efficient Alkaline Hydrogen Evolution and Nitrogenous Nucleophile Electrooxidation. Small 2022, 18, 2203171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Y.; He, R.; Yu, F.; Sun, J.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. One-step synthesis of self-supported porous NiSe2/Ni hybrid foam: An efficient 3D electrode for hydrogen evolution reaction. Nano Energy 2016, 20, 29–36. [Google Scholar] [CrossRef]
- Deng, S.; Yang, F.; Zhang, Q.; Zhong, Y.; Zeng, Y.; Lin, S.; Wang, X.; Lu, X.; Wang, C.-Z.; Gu, L.; et al. Phase Modulation of (1T-2H)-MoSe2/TiC-C Shell/Core Arrays via Nitrogen Doping for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, 1802223. [Google Scholar] [CrossRef]
- Zang, Y.; Yang, B.; Li, A.; Liao, C.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tuning Interfacial Active Sites over Porous Mo2N-Supported Cobalt Sulfides for Efficient Hydrogen Evolution Reactions in Acid and Alkaline Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 41573–41583. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Xue, S.; Zhou, S.; Qu, K.; Li, Y.; Cai, W. Pt/Mo2C heteronanosheets for superior hydrogen evolution reaction. J. Energy Chem. 2020, 47, 317–323. [Google Scholar] [CrossRef]
- Liu, W.; Geng, P.; Li, S.; Liu, W.; Fan, D.; Lu, H.; Lu, Z.; Liu, Y. Tuning electronic configuration of WP2 nanosheet arrays via nickel doping for high-efficiency hydrogen evolution reaction. J. Energy Chem. 2020, 55, 17–24. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Morozan, A.; Johnson, H.; Roiron, C.; Genay, G.; Aldakov, D.; Ghedjatti, A.; Nguyen, C.T.; Tran, P.D.; Kinge, S.; Artero, V. Nonprecious Bimetallic Iron–Molybdenum Sulfide Electrocatalysts for the Hydrogen Evolution Reaction in Proton Exchange Membrane Electrolyzers. ACS Catal. 2020, 10, 14336–14348. [Google Scholar] [CrossRef]
- Yang, H.; Driess, M.; Menezes, P.W. Self-Supported Electrocatalysts for Practical Water Electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Zhao, Y.; Mavrokefalos, C.K.; Zhang, P.; Erni, R.; Li, J.; Triana, C.A.; Patzke, G.R. Self-Templating Strategies for Transition Metal Sulfide Nanoboxes as Robust Bifunctional Electrocatalysts. Chem. Mater. 2020, 32, 1371–1383. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Gao, Y.; Li, N.; Fu, Y.; Ge, L.; Song, W.; Liu, J.; Ma, T. In Situ Electronic Redistribution Tuning of NiCo2S4 Nanosheets for Enhanced Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2109731. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Thorat, G.M.; Chung, W.-J.; Gil Seo, J. Hierarchical free-standing networks of MnCo2S4 as efficient Electrocatalyst for oxygen evolution reaction. J. Ind. Eng. Chem. 2018, 71, 452–459. [Google Scholar] [CrossRef]
- Li, L.; Dai, Y.; Xu, Q.; Zhang, B.; Zhang, F.; You, Y.; Ma, D.; Li, S.-S.; Zhang, Y.-X. Interlayer expanded nickel-iron layered double hydroxide by intercalation with sodium dodecyl sulfate for enhanced oxygen evolution reaction. J. Alloy. Compd. 2021, 882, 160752. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Srinivas, K.; Wang, M.; Liu, D.; Chen, X.; Wang, B.; Wang, S.; Chen, Y. Heterogeneous FeNi2S4/Ni3S4 nanoparticles embedded CNT networks for efficient and stable water oxidation. Journal Alloys Compounds 2022, 914, 165327. [Google Scholar] [CrossRef]
- Ren, L.; Wang, C.; Li, W.; Dong, R.; Sun, H.; Liu, N.; Geng, B. Heterostructural NiFe-LDH@Ni3S2 nanosheet arrays as an efficient electrocatalyst for overall water splitting. Electrochim. Acta 2019, 318, 42–50. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.-J.; Zhu, X.-J.; Lu, S.; Long, L.-L.; Chen, J.-J. Nanostructured metallic FeNi2S4 with reconstruction to generate FeNi-based oxide as a highly-efficient oxygen evolution electrocatalyst. Nano Energy 2020, 81, 105619. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Yuan, M.; Hao, H.; San, X.; Lv, Z.; Xu, L.; Wei, B. Operando capturing of surface self-reconstruction of Ni3S2/FeNi2S4 hybrid nanosheet array for overall water splitting. Chem. Eng. J. 2022, 427, 131944. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Zhu, Z.; Sun, J.; He, R.; Bao, J.; Chen, S.; Ren, Z. Three-Dimensional Nanoporous Iron Nitride Film as an Efficient Electrocatalyst for Water Oxidation. ACS Catal. 2017, 7, 2052–2057. [Google Scholar] [CrossRef]
- Liu, C.; Jia, D.; Hao, Q.; Zheng, X.; Li, Y.; Tang, C.; Liu, H.; Zhang, J.; Zheng, X. P-Doped Iron–Nickel Sulfide Nanosheet Arrays for Highly Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 27667–27676. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Guan, P.-Y.; Li, Q.-Y.; Zhang, M.; Zang, S.-Q. MOF-Derived Flower-like MoS2@TiO2 Nanohybrids with Enhanced Activity for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 26794–26800. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zavabeti, A.; Wang, B.; Ren, R.; Yang, C.; Liu, Z.; Wang, Y. Nickel Phosphides Electrodeposited on TiO2 Nanotube Arrays as Electrocatalysts for Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 4542–4551. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, K.; Xie, D.; Zhang, Y.; Zhang, Y.; Wang, Y.; Wu, J.; Wang, X.; Fan, H.J.; Xia, X.; et al. High-Index-Faceted Ni3S2 Branch Arrays as Bifunctional Electrocatalysts for Efficient Water Splitting. Nano-Micro Lett. 2019, 11, 12. [Google Scholar] [CrossRef]
- Li, J.; Zou, S.; Liu, X.; Lu, Y.; Dong, D. Electronic Modulation of CoP by Ce Doping as Highly Efficient Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 10009–10016. [Google Scholar] [CrossRef]
- Tian, T.; Huang, L.; Ai, L.; Jiang, J. Surface anion-rich NiS2 hollow microspheres derived from metal–organic frameworks as a robust electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 20985–20992. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, M.; Li, H.; Sun, G.; Ma, S. Controllable synthesis of ultrathin Co9S8 nanosheets as a highly efficient electrocatalyst for overall water splitting. Electrochim. Acta 2018, 281, 198–207. [Google Scholar] [CrossRef]
- Li, L.; Sun, C.; Shang, B.; Li, Q.; Lei, J.; Li, N.; Pan, F. Tailoring the facets of Ni3S2 as a bifunctional electrocatalyst for high-performance overall water-splitting. J. Mater. Chem. A 2019, 7, 18003–18011. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Li, G.-D.; Wu, Y.; Gao, R.; Zou, X. Vertically grown CoS nanosheets on carbon cloth as efficient hydrogen evolution electrocatalysts. Int. J. Hydrogen Energy 2017, 42, 9914–9921. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Gao, T.; Yao, T.; Zhang, X.; Han, J.; Wang, X.; Zhang, Z.; Xu, P.; Zhang, P.; et al. Synergistic Phase and Disorder Engineering in 1T-MoSe2 Nanosheets for Enhanced Hydrogen-Evolution Reaction. Adv. Mater. 2017, 29, 1700311. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Chen, H.; Han, X.; Wu, Y.; He, J.; Han, C.; Yang, G.; Shan, Y. Strongly coupled Mo2C and Ni nanoparticles with in-situ formed interfaces encapsulated by porous carbon nanofibers for efficient hydrogen evolution reaction under alkaline conditions. J. Colloid Interface Sci. 2019, 558, 100–105. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, G.; Luo, W. Ternary nickel–iron sulfide microflowers as a robust electrocatalyst for bifunctional water splitting. J. Mater. Chem. A 2017, 5, 15838–15844. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Liu, C.; Zhang, Y.; Ji, Y.; Mei, B.; Yao, Z.; Lin, S. Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction. Catalysts 2023, 13, 174. https://doi.org/10.3390/catal13010174
Deng S, Liu C, Zhang Y, Ji Y, Mei B, Yao Z, Lin S. Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction. Catalysts. 2023; 13(1):174. https://doi.org/10.3390/catal13010174
Chicago/Turabian StyleDeng, Shengjue, Changsheng Liu, Yan Zhang, Yingxi Ji, Bingbao Mei, Zhendong Yao, and Shiwei Lin. 2023. "Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction" Catalysts 13, no. 1: 174. https://doi.org/10.3390/catal13010174
APA StyleDeng, S., Liu, C., Zhang, Y., Ji, Y., Mei, B., Yao, Z., & Lin, S. (2023). Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction. Catalysts, 13(1), 174. https://doi.org/10.3390/catal13010174





