Abstract
Lipase Amano A from Aspergillus niger (AA-ANL) is among the most commonly applied enzymes in biocatalysis processes, making it a significant scientific subject in the pharmaceutical and medical disciplines. In this study, we investigated the lipolytic activity of AA-ANL immobilized onto polyacrylic support IB-150A in 23 oils of natural origin containing various amounts of polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs). The created systems were expressed as an ‘ESS catalytic triangle’. A distinct ‘jump’ (up to 2400%) of lipolytic activity of immobilized AA-ANL compared to free lipase and hyperactivation in mostly tested substrates was observed. There was a ‘cutoff limit’ in a quantitative mutual ratio of ω-PUFAs/MUFAs, for which there was an increase or decrease in the activity of the immobilized AA-ANL. In addition, we observed the beneficial effect of immobilization using three polyacrylic supports (IB-150A, IB-D152, and IB-EC1) characterized by different intramolecular interactions. The developed substrate systems demonstrated considerable hyperactivation of immobilized AA-ANL. Moreover, a ‘lipolytic jump’ in the full range of tested temperature and pH was also observed. The considerable activity of AA-ANL-IB-150A after four reuse cycles was demonstrated. On the other hand, we observed an essential decrease in stability of immobilized lipase after 168 h of storage in a climate chamber. The tested kinetic profile of immobilized AA-ANL confirmed the decreased affinity to the substrate relative to lipase in the free form.
1. Introduction
Biocatalysis has become an increasingly important scientific field in recent years. Given the multidisciplinary interconnections between biotechnology, pharmacology, and biochemistry, the use of enzymes in synthesis reactions and analysis is emerging as an interesting alternative to chemical catalysts. Catalytic reactions are characterized by low cost, mild conditions, and environmental friendliness. Therefore, they are included in the so-called ‘green chemistry’ domain. A wide range of enzymes with specific properties is applied in biocatalysis. As catalysts, lipases (EC 3.1.1.3) in particular deserve the attention of researchers. These enzymes, which are a significant part of the hydrolase group, show activity such as hydrolysis of triacylglycerols to fatty acids, di- and monoglycerols, and glycerol (Figure 1) [,,,,,]. The action occurs in the water–oil interface. Furthermore, they are characterized by substrate specificity and stereoselectivity toward the ester bond [,,]. An interesting phenomenon is so-called ‘interfacial activation’, an exposition of the lipase active center by the movement of the characteristic ‘lid’ [,,,]. The consequence is a change in lipase conformation from closed to open form, resulting in increased enzymatic activity. The interfacial activation is specific for most lipases (e.g., Aspergillus niger, Burkholderia cepacia, Candida rugosa, Thermomyces lanuginosus, and Rhizomucor miehei). However, its occurrence has not been observed in some cases (e.g., lipase B from Candida antarctica, Pseudomonas aeruginosa, and Burkholderia glumae) []. However, the characteristic features of this enzyme group have been commonly applied in medicine, pharmacy, biotechnology, and cosmetology [,,,,,,]. Lipases catalyze a wide range of reactions, e.g., esterification, transesterification, and interesterification, as well as acid-, amino-, and alcoholysis [,].
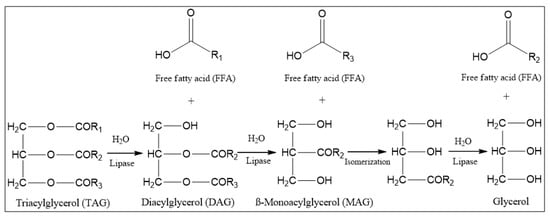
Figure 1.
Hydrolysis of triacylglycerols catalyzed by lipase.
Amano lipase A from Aspergillus niger (AA-ANL) is a commercially available lipase of Aspergillus sp. origin. It is the most commonly applied enzyme in numerous catalytic reactions [,,,,]. According to the literature, AA-ANL is characterized by positional specificity toward 1- and 3-glycerol groups and substrate specificity to the medium-length carbon chains. The optimal temperature range of this lipase is 40–50 °C, with an optimal pH of 4–7. Lipase from Aspergillus niger (ANL) exhibits high levels of activity, stability, and specificity [,,]. Therefore, it has been used in the pharmaceutical, biotechnological, chemical, food, cosmetics, and agricultural industries [,]. Apart from commonly applied commercial preparations, ANL is often acquired in laboratories, e.g., by fermentation or genetic recombination [,,,]. Owing to its properties, AA-ANL has proven its usefulness in catalyzing abundant reactions in both free [,,,,,,,] and immobilized forms. Qiao et al. [] exploited AA-ANL in the native (free) form to hydrolyze triglycerides from soybean oil to obtain biodiesel by efficient and environmentally-friendly means. On the other hand, Yildiz et al. [] performed enzymatic hydrolysis of 1,3-ketoacetates catalyzed by AA-ANL to obtain chiral β-hydroxy ketones, which are used as building blocks in the synthesis of macrolide antibiotics and antitumor drugs. In another work, Kuboki et al. [] applied AA-ANL to deacetylation to obtain regioselective cytidine. AA-ANL has also been tested as a catalyst in Henry reactions (nitroaldol reactions) to afford chiral β-nitroalcohols, which are used as intermediates in the synthesis of antibiotics, fungicides, and insecticides. The studies mentioned above [,,] demonstrate the wide applicability of AA-ANL in catalyzing reactions of pharmaceutical significance. Given currently dominant research trends, improvement of the catalytic parameters of lipase (activity, stability, and specificity) is required. Immobilization is one of the most popular processes associated with the modification of enzymatic properties [,,,,]. The interaction between lipase and immobilization material, so-called ‘support’, occurs by various mechanisms, as shown in Figure 2.
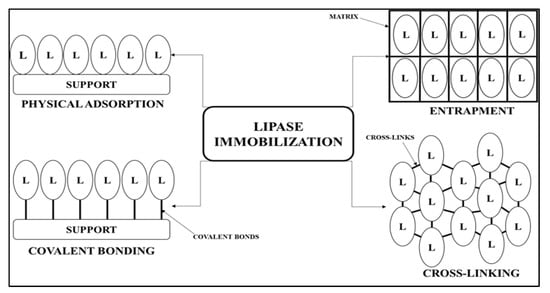
Figure 2.
The simplified division of immobilization techniques. “L” indicates a lipase molecule.
Covalent binding should be included in the above-mentioned immobilization methods, among others, due to the remarkably durable connection of lipase support [,,]. This interaction is stronger than physical adsorption. Therefore, the risk of ‘leakage’ of lipase from the formed complex is significantly decreased. Compared with crosslinking and entrapment methods, covalent binding is characterized by lower rigidity, which results in increased lipase activity. Furthermore, a sustainable immobilization technique should provide the optimal rotation in enzyme molecular structure towards open conformation exposition. However, in the case of the functional group of the support, containing the so-called ‘short spacer arm’, this interaction can cause attraction of the substrate too close to the enzyme active center, resulting in their blockade and, ultimately, limited availability, which determines decreased enzyme activity. On the other hand, the stability of the formed bond allows for the reuse of immobilized lipase in subsequent reaction cycles, owing to the strong interaction, preventing enzyme loss. An additional advantage of the above-mentioned technique is low reaction cost compared with crosslinking and entrapment. Moreover, immobilization does not require extreme conditions or aggressive chemicals (disturbing or destroying protein lipase structure), which is considered environmentally friendly. Therefore, this method was commonly applied in the immobilization of many lipases, such as lipase B from Candida antarctica (CAL-B), lipase from Candida methylica (CML), Thermomyces lanuginosus (TLL), Rhizomucor miehei (RML), or Amano lipase PS from Burkholderia cepacia (APS-BCL) [,,,,,,,]. In the case of supports, the widely tested materials in covalent binding are of polyacrylic origin. These include, among others, commercially available Immobead 150 (IB-150). As materials with various functional groups (IB-150P: polar; IB-150A: apolar), they are used for immobilization of various lipases [,,,,], as well as enzymes from other groups, such as β-galactosidases, pullulanases, dehydrogenases, or laccases [,,,]. IB-150 was investigated for immobilization of β-galactosidase from Aspergillus oryzae for the modification of support functional groups. Regarding AA-ANL, immobilization has been performed with several methods. However, the process of applying the Immobead polyacrylic supports for AA-ANL has not been reported in the literature to date. Zdarta et al. [] immobilized AA-ANL onto the surface of silica Stöber to improve the immobilization and testing of lipase kinetic parameters. In other studies conducted by the same authors, lipase AA-ANL was immobilized onto sponges from Luffa cylindrica in an attempt to improve the thermal and chemical stability of the catalyst []. As mentioned above, the application of AA-ANL immobilized onto polymeric support IB-150A has not been studied. Therefore, it was suggested that studies concerning the above issues could be helpful in the modulation and development of a ‘catalytic library’ composed of a wide range of designed enzymatic models and support materials. To create such a system, and optimal substrate must be selected. The enzymatic activity of lipase is expressed by its lipolytic activity. The application of triglycerides containing fatty acids is determined by the length of the carbon chain appropriate for lipase specificity, e.g., CAL-B prefers short-chain fatty acids. In contrast, lipase APS-BCL shows high activity toward carbon chains, regardless of their length [,,]. In turn, AA-ANL prefers substrates containing medium-length carbon chains []. The wide spectrum of substrates used for lipase-catalyzed hydrolysis includes fats with unsaturated fatty acids from both vegetables and animals, such as polyunsaturated fatty acids (PUFAs) ω3 (with three double bonds), ω6 (with two double bonds), and monounsaturated fatty acids (MUFAs) ω9 (with one double bond) of natural origin (not synthetic). α-linolenic (ALA), eicosapentaenoic (EPA), and docosahexaenoic (DHA) are among the most important ω3 PUFAs. They are an important component of a healthy human diet, especially EPA and DHA, the supplementation of which decreases cardiovascular disease risk. Additionally, they exert a slight antidepressive effect and support lipogenesis, which is a promising obesity treatment [,,,,,]. According to recent reports, ω3 PUFA supplementation, especially DHA, can accelerate the regeneration of the organism after COVID-19 infection [,]. For the above-mentioned reasons, increasing interest in including ω3 PUFAs in the diet has been observed [,,,]. Moreover, the formulation of cod liver oil, registered as an OTC drug, was recently introduced into the pharmaceutical market. Until recently, only preparations registered as dietary supplements or food for special medical purposes were available [,]. Regarding ω6 PUFAs, the main compound from this group is linoleic acid (LA), which, in its coupled form (CLA), despite transconfiguration, shows many health-promoting effects, such as decreasing cholesterol oxidation and increasing β-oxidation in skeletal muscles [,]. Another advantage of LA is the possibility of its endogenous production in human organisms compared with EPA and DHA. On the other hand, excessive dietary consumption of LA can cause a fatty liver [] Oleic acid (OA) is the main representative of ω9 MUFAs. Like LA, OA is an important energetic reservoir for the organism and exhibits antisclerotic activity. However, excess OA can be harmful to the metabolism. Therefore, a PUFA-rich diet should be properly balanced. Hence, unsaturated fatty acids are applied in developed multicomponent (MC-UFA) systems. As suggested by the authors of previous works [,], the ω6/ω3 PUFA supplementation system seems to be the most beneficial for the human organism. In contrast, the proportion of ω6 to ω3 PUFAs should not be high due to the previously mentioned risk of obesity associated with fatty liver []. With respect to dietary supplements containing PUFAs and MUFAs, MC-UFAs contain both and occur in vegetable oils, which are, together with fish oil, the main sources of PUFAs in the human diet. The ratios of ω-PUFAs and ω-MUFAs in fish and vegetable oils are been shown in Table 1.

Table 1.
The quantitative ratios of ω-PUFA and ω-MUFA acids in fish and vegetable oils. The data were established based on information provided by Oleofarm (Poland) and Sigma-Aldrich (Steinheim, Germany).
Preliminary studies and literature data show that the ratio of ω-PUFA content can influence lipase lipolytic activity in the lipolysis process. Therefore, applying fish and vegetable oils with various amounts of unsaturated fatty acids could be helpful in the development of optimal catalytic systems. An interesting model is the so-called ‘catalytic triangle’, which is composed of the enzyme, support, and substrate (ESS) related to each other by intramolecular interactions []. In this study, we aimed to investigate the effect of the broad spectrum of substrate systems with varying content of ω-PUFAs and ω-MUFAs on the lipolytic activity of AA-ANL in free form and immobilized onto polyacrylic support IB-150A. We tested the influence of six oil mixtures on the enzymatic activity of immobilized AA-ANL. In addition, the effect of temperature, pH, and three supports on the selected substrates was investigated. Moreover, the influence of reusing immobilization complex AA-ANL-IB-150A on lipase activity was evaluated. We also verified the stability of the enzyme following storage in a climate chamber. Kinetic parameters were determined to establish the profile of the lipase affinity for the substrate in light of the lipolytic activity of immobilized AA-ANL corresponding to various amounts of substrate.
2. Results and Discussion
2.1. Effect of Substrates on the Lipolytic Activity of Immobilized AA-ANL
In the present study, we evaluated the lipolytic activity of AA-ANL immobilized onto polyacrylic support IB-150A (UI) and in its free form in an amount corresponding to the amount of lipase immobilized onto the support (UB), as determined by the Bradford method (LAB) [,]. We screened 22 vegetable oils and fish oil. Lipase loading (LL), immobilization yield (Iy), and immobilization efficiency (Ie) were calculated based on the obtained results. The data are juxtaposed in Table 2. The amount of lipase immobilized onto polyacrylic support IB-150A was in the range of 4.0 ± 0.3–6.5 ± 0.1 mg, with an immobilization yield of 40 ± 3.0–65 ± 1.0%, whereas (LL) was in the range of 80.0 ± 6.0–130.0 ± 2.0 mg on 1.0 g of support. According to the lipase amount corresponding to the amount immobilized onto the support, AA-ANL showed the highest activity in olive (11.33 ± 0.25 U), grape seed (6.83 ± 0.35 U), and rice (5.33 ± 0.51 U) oils. In contrast, the tested lipase was the least active in sesame, fish, argan, corn, and hazelnut oils (0.50 ± 0.10 U). In the case of AA-ANL immobilized onto IB-150A beads, the most activity was observed in milk thistle, linseed (9.17 ± 0.25 and 9.17 ± 0.10 U, respectively), and grapeseed (8.67 ± 0.17 U) oils. The results were compared with literature data [,]. We studied the lipolytic activity of the immobilization complex of AA-ANL-IB-150A toward a significantly wide spectrum of substrates for the first time. Dulęba et al. [] investigated the lipolytic activity of free APS-BCL (initial amount: 10.0 mg) in 13 vegetable oils. High levels of enzymatic activity were achieved in the 27.5–44.3 U range. The highest activity of APS-BCL was shown in black cumin, hemp, safflower, and grapeseed oils, and the lowest levels of activity were observed in peanut and walnut oils. On the other hand, Siódmiak et al. [] studied the lipolytic activity of free CAL-B in olive oil (2.69 U). Our studies showed significantly lower (more than tenfold) enzymatic activity of AA-ANL relative to APS-BCL, which was simultaneously higher than that of CAL-B. The results confirmed that AA-ANL prefers fatty acids with a medium-length carbon chain. However, compared with APS-BCL, AA-ANL presents a lower affinity to the long carbon chains of fatty acids. Furthermore, AA-ANL is characterized by higher levels of activity than CAL-B, which favors the short carbon chains of fatty acids. The suggested conclusions concern lipase in its native form. The discussed relations altered in the case of immobilized AA-ANL. Considering the Ie parameters, immobilized AA-ANL, apart from a few cases, presents significant hyperactivation (≥100%) compared to the free lipase in an amount corresponding to the amount immobilized onto the support. In turn, with respect to the immobilization efficiency parameter, immobilized AA-ANL was the most active in pumpkin seed (2400.00%), fish (1433.33%), and corn (1266.67%) oils. A comparison of the obtained results with literature data [] suggests that immobilization significantly improved the lipolytic activity of AA-ANL. Lipase APS-BCL hyperactivation occurred in four oils (peanut, pumpkin seed, walnut, and corn oils), as expressed by activity recovery, whereas hyperactivation expressed by activity retention was only demonstrated for peanut oil. The hyperactivation value of immobilized APS-BCL slightly exceeded 100%. However, it should be noted that APS-BCL showed high levels of activity in its free form. According to the enzymatic activity value (U), we hypothesized that immobilization decreased the lipolytic activity of APS-BCL. The results achieved in this study indicate a beneficial effect of immobilization on the lipolytic activity of AA-ANL. Immobilization of lipase onto a polyacrylic support was based on covalent binding of distinct groups contained in the support (oxirane group), with a strong nucleophilic group expressed in the lipase molecule (probably the amine group from the catalytic triad in the active enzyme center). In the case of APS-BCL, the so-called short ‘spacer arm’ formed by this bond is localized too close to the lipase active center. Therefore, the availability of lipase to the substrate was hindered []. Alagoz et al. [] studied the effect of the ‘spacer arm’ on the activity of pectinase immobilized onto two types of support: Florisil (magnesium silicate) and nanosilicate. The results of the study showed that immobilization using a 3-glioxypropyltriethyloxisilicate (3-GTPMS) ‘spacer arm’ caused enzyme inactivation. The authors explained the effect of the significant rigidity of the immobilization complex, which contributed to the blockage of the pectinase active center. On the other hand, the ‘spacer arm’ of the glutaraldehyde had a positive effect on lipase retention. Glutaraldehyde has also been applied as a ‘spacer arm’ in the immobilization of lipase from Thermomyces lanuginosus on a multicomponent support system (metalorganic structure hydroxyapatite-glycyrrhizin-lithium) []. According to our research, regarding AA-ANL, the short ‘spacer arm’ seems not to block substrate access to the enzyme active site. The immobilization onto the support and stiffening of the complex promotes the substrate, ipso facto increasing lipase reactivity []. However, the steric hindrance in these complexes may impede the availability of the substrate to the enzyme active site [,,,]. The extremal increase in lipolytic activity is dependent on the type of substrate used. Each oil contains a certain amount of unsaturated fatty acids. All used substrates have ω6 PUFA, such as LA. Additionally, blackberry, rapeseed, walnut, hemp, borage, linseed, and fish oils contain ω3 PUFAs, such as ALA (fish oil also contains EPA and DHA). In turn, blackberry, linseed, and borage oils do not contain ω9 MUFAs acids, such as OA. The main representatives of the unsaturated fatty acids group differ in terms of the number of double bonds and chain rotation (so-called “kinks”), which could influence lipase lipolytic activity [,,,,]. The effect of the ratio of ω6/ω9 PUFAs/MUFAs on APS-BCL activity has been described in previous works [,]. Authors assumed that in oils characterized by a ratio of ω6/ω9 of more than 2.3 (cut-off limit = 2.3) [], lipase lipolytic activity is higher than in oils with a ratio below this value. This dependence was observed for both (free and immobilized) lipase forms. In the case of our studies, the cutoff limit (the ω6/ω9 PUFA/MUFA ratio) for AA-ANL lipase was 3.44, above which value it showed higher activity than in the case of other oils. With respect to the ratio of ω3/ω9 PUFAs/MUFAs, as the value declined, the AA-ANL activity decreased. However, comparison was only possible between oils containing ω3 PUFAs; hence, the comparative spectrum was relatively limited. Taking into account the results of prior research [,], the effect of the ω6/ω9 ratio on AA-ANL activity was confirmed, although without linear dependence. Notwithstanding, the investigation showed the extremely beneficial effect of immobilization by the covalent binding method on AA-ANL lipolytic activity. The obtained catalytic parameters are undoubtedly helpful for the development of substrate systems to obtain modified optimal models. Such a system could be applied to in vitro tests with the aim of fat digestion, especially of fats unfavorable to human health. As mentioned in the Introduction, a diet containing unsaturated fatty acids should be properly balanced, as an excess of ω6 PUFAs can be harmful to the organism []. Our results showed the high catalytic specificity of AA-ANL to ω6 PUFAs, which can be useful in the future to create a therapeutic system supporting the release of fat excess from the body. However, further studies on wider substrate and support spectra should be conducted to confirm the beneficial lipase activity in the developed substrate systems; we attempted to do so, which will be described in a subsequent report.

Table 2.
AA-ANL lipolytic activity in free form and immobilized onto IB-150A support in 22 vegetable oils and fish oil.
2.2. The Effect of the Support on AA-ANL Lipolytic Activity
We evaluated the effect of three polymeric supports (Immobead: IB-150A, IB-D152, and IB-EC1) on the enzymatic activity of immobilized AA-ANL. The determination was performed on a free lipase sample in an amount corresponding to the amount immobilized on the support. The results, along with each support characteristic feature, are juxtaposed in Table 3. The experiment was carried out using peanut oil as the reaction substrate. According to analysis of the various amounts of lipase immobilized onto the support, the largest amounts of lipase were immobilized onto polyacrylic IB-EC1 (5.0 ± 0.1 mg, Iy = 50.0 ± 1.0), and the smallest amounts were immobilized onto polyacrylic IB-D152 (1.3 ± 0.1 mg, Iy = 13.0 ± 1.0). Taking UI as the Ie value, increased lipolytic activity of AA-ANL immobilized onto IB-D152 support was observed (UI = 2.33 ± 0.10, Ie = 233%), compared with AA-ANL immobilized onto other supports. Siódmiak et al. [] investigated the lipolytic activity of CAL-B immobilized onto 12 polymeric supports (Immobead) with olive oil as the reaction substrate. The results indicated the highest activity retention (Aret) of CAL-B immobilized onto IB-D152 support (Aret = 178.0%). The authors stated that high lipase activity immobilized onto this support may have been caused by electrostatic interactions of the polarized carboxylic group as the characteristic functional group of the support. It should be mentioned that the reaction medium was slightly alkaline (pH 7.4). Therefore, substrate binding and increasing concentration were promoted near the enzyme active center. This phenomenon may have been additionally facilitated by the strong bond between the polarized carboxylic group and the nucleophile (probably amine group) occurring in the active site of the enzyme. This binding (probably peptide), due to its considerable rigidity (conditioned by partially double-bond character) [], could guarantee the high durability of the complex, i.e., resistant to ‘enzyme leakage’, with simultaneous blockage of the associated rotation. This phenomenon could allow for optimal location adjustment of lipase localization for high substrate concentrations. Because the immobilization type is characterized by a small amount of immobilized protein, the risk of steric hindrance is decreased. This means that the lipase molecules can hydrolyze more of the substrate. Comparing the activity results of AA-ANL onto IB-D152 support with that of AA-ANL onto IB-EC1 support, it seems that a larger amount of the immobilized lipase did not correlate with increased activity. However, the immobilization technique of lipase onto IB-EC1 was based on adsorption interactions characterized by strong hydrogen bonds []. Despite this, lipase molecules after adsorption were significantly less ordered [] than in the case of covalent bonding, as well as less stable, which made them more susceptible to enzyme leakage. The adsorption of larger lipase molecules on the support surface [] could increase steric hindrance, as expressed by decreased activity. Nevertheless, in all immobilization complexes, AA-ANL showed hyperactivation without the loss of activity compared to the free form—in the case of AA-ANL-IB-150A and AA-ANL-IB-D152 complexes, the activity increased. On the other hand, in the AA-ANL-IB-EC1 complex, the activity did not change. The obtained results confirm the beneficial effect of immobilization on AA-ANL lipolytic activity in each support. However, the study of lipase for a broader spectrum of supports and substrates is required to investigate, in more detail, the effects of immobilization on AA-ANL activity. Therefore, as mentioned in the previous subsection, this subject will be the aim of further studies.

Table 3.
Lipolytic activity of AA-ANL immobilized onto three polyacrylic supports, as well as the characteristic features of each support (data achieved from ChiralVision).
2.3. The Effect of Temperature on the Lipolytic Activity of the Immobilized AA-ANL
We investigated the effect of temperature on the lipolytic activity of AA-ANL in free form and immobilized onto polyacrylic support IB-150A with peanut oil as the reaction substrate. The results are listed in Table 4. Two dependencies were observed: a change in the activity of free and immobilized AA-ANL (Figure 3) and the effect of immobilization on lipase activity, depending on thermal conditions (Figure 4). The study showed the highest enzymatic activity of immobilized AA-ANL at 45 °C, whereas free AA-ANL was the most active at temperatures of 45 °C and 65 °C. An interesting phenomenon (for both lipase forms) is a significant decrease in activity in the temperature range of 45–55 °C and an increase in activity in the range of 55–65 °C. Siódmiak et al. [] studied the effect of temperature on the lipolytic activity of CAL-B immobilized onto polyacrylic support IB-D152. The maximum activity of immobilized CAL-B was in the range of 25–55 °C, and that of free CAL-B was 55 °C. At temperatures above 55 °C, the activity decreased. On the other hand, Sun et al. [] demonstrated the thermal optimum of immobilized CAL-B to be 30 °C, above which a significant activity decrease was noted. Thermal stability studies (temperature range of 30–65 °C) on immobilized lipase from Aspergillus niger were performed by Dos Santos et al. [] The results indicated a slight increase in ANL activity after immobilization and an activity maximum at 55 °C. Our studies demonstrated an essential improvement of activity of immobilized AA-ANL compared with the free form. However, the increase in activity of AA-ANL-IB-150A did not correlate with activity maximum (U). As mentioned above, AA-ANL showed the highest activity at 45 °C (2.67 ± 0.17 U), whereas the maximum increase in activity compared to the free form occurred at 25 °C (Ie = 800.00%). Despite a significant improvement in the catalytic parameters of AA-ANL after immobilization, low enzymatic activity compared to other lipases, such as APS-BCL [,], should be noted. However, modified thermal conditions did not seem to abolish the beneficial effects of immobilization on AA-ANL activity. The covalent interactions between IB-150A support and the active lipase center probably stabilized AA-ANL against unfavorable conditions. Therefore, further studies with the application of a broader spectrum of substrates, especially with higher AA-ANL activity, are suggested to further thermally optimize AA-ANL. Given the increase in AA-ANL activity in the temperature range of 55–65 °C, we propose extending the temperature range to reaching the point at which lipase activity decreases (also by denaturation).

Table 4.
Effect of temperature on the lipolytic activity of AA-ANL immobilized onto polyacrylic support IB-150A.
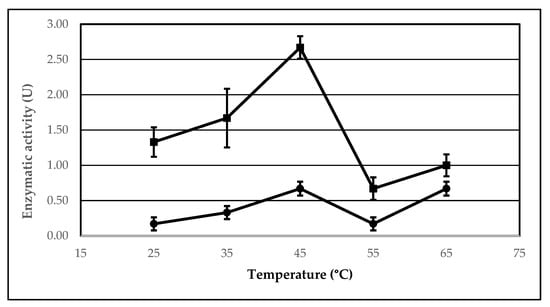
Figure 3.
Effect of temperature on the activity of AA-ANL in free form (circles) and immobilized onto IB-150A support (squares). Reaction mixture: free AA-ANL (5.0 ± 0.2 mg) or AA-ANL immobilized on IB-150A beads, phosphate buffer (100 mM, pH 7.4), and an emulsion containing equal volumes of peanut oil and gum arabic suspension. The mixture was incubated for 30 min. The error bars represent the standard deviations of the mean.
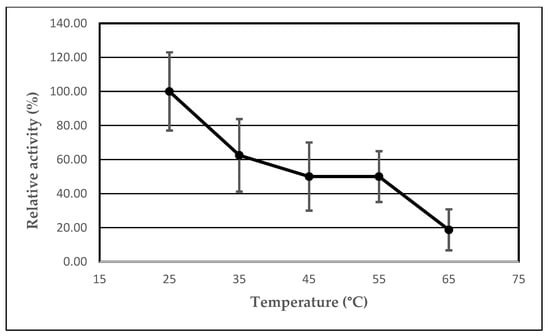
Figure 4.
Effect of immobilization on AA-ANL activity under various thermal conditions. Reaction mixture: free AA-ANL (5.0 ± 0.2 mg) or AA-ANL immobilized on IB-150A beads, phosphate buffer (100 mM, pH 7.4), and an emulsion containing equal volumes of peanut oil and gum arabic suspension. The mixture was incubated for 30 min. The error bars represent the standard deviations of the mean.
2.4. The Effect of pH on the Lipolytic Activity of Immobilized AA-ANL
In this experiment, we investigated the effect of pH on the lipolytic activity of AA-ANL in its free form and immobilized on IB-150A polyacrylic support with peanut oil as the reaction substrate. The results are presented in Table 5. Analogous to the temperature studies, the following two dependencies were observed: a change in activity of AA-ANL in free and immobilized form (Figure 5) and the effect of immobilization on lipase activity, depending on the pH conditions (Figure 6). According to analysis of the received data, the maximum activity of immobilized AA-ANL was obtained at pH 7 (3.00 ± 0.51 U), whereas in the case of the free form, maximum activity occurred at pH 4–5, 9 (0.33 ± 0.10 U, 0.33 ± 0.17 U, and 0.33 ± 0.10 U, respectively). With respect to the profile of the free lipase, native AA-ANL demonstrated low activity in the whole range of tested pH values. Moreover, the values remained stable, and the increase occurred with the alkalization of the reaction medium. In the case of immobilized AA-ANL, the activity increased in the pH range of 4–7 and decreased at pH 8, with slight increase at pH 9. Compared with studies performed by other authors [,], the activity improvement with alkalization of the medium can be perceived as surprising, as authors had previously noted an activity decrease under these conditions. Our research confirmed the optimal pH for immobilized AA-ANL []. Similarly to the temperature studies, the pH experiment demonstrated the beneficial effect of immobilization on AA-ANL activity in the whole range of tested conditions. The most hyperactivation was noted at pH 7 (Ie = 1800%). However, a decrease in this value was observed with the alkalization of the medium. The literature data [] show that lipases immobilized onto Immobead support demonstrate a consistent profile of catalytic parameters, as in the free form. Our results confirmed that immobilization of AA-ANL is not entirely responsible for increased activity with the neutralization of the medium, and free AA-ANL activity is maintained at a stable level. On the basis of the obtained results, it can be concluded that the immobilized AA-ANL seems more readable for the interpretation of lipase behavior under varying pH conditions because the free AA-ANL showed low activity, practically independent of the tested conditions. However, analogous to temperature studies, testing AA-ANL activity under more extreme pH conditions with a synchronously extended range of the substrate spectrum could help to optimize AA-ANL properties under various medium conditions.

Table 5.
Effect of pH on the lipolytic activity of AA-ANL immobilized onto polyacrylic IB-150-A support.
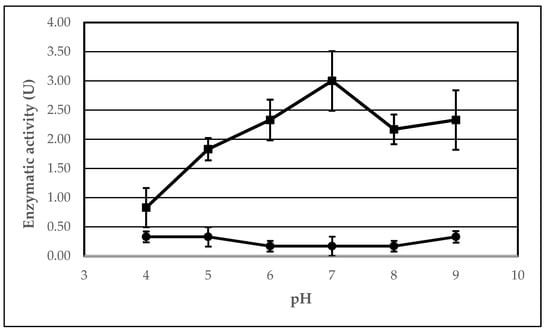
Figure 5.
Effect of pH on AA-ANL activity in free form (circles) and immobilized onto IB-150A (squares). Reaction mixture: free AA-ANL (5.0 ± 0.2 mg) or AA-ANL immobilized onto IB-150A beads, phosphate buffer (100 mM, pH in the range of 4–9), and an emulsion containing equal volumes of peanut oil and gum arabic suspension. The mixture was incubated for 30 min. The error bars represent the standard deviations of the mean.
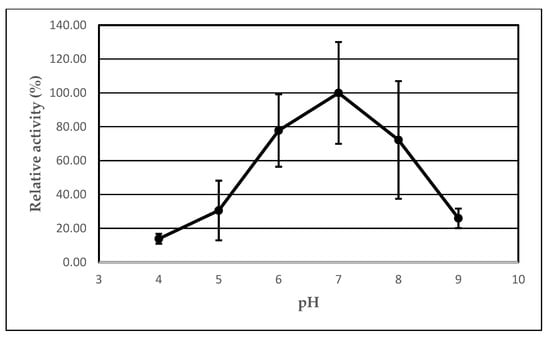
Figure 6.
Effect of immobilization on AA-ANL activity under various pH conditions. Reaction mixture: free AA-ANL (5.0 ± 0.2 mg) or AA-ANL immobilized on IB-150A beads, phosphate buffer (100 mM, pH in the range of 4–9), and an emulsion containing equal volumes of peanut oil and gum arabic suspension. The mixture was incubated for 30 min). The error bars represent the standard deviations of the mean.
2.5. The Effect of Substrate Mixtures on the Lipolytic Activity of Immobilized AA-ANL
We investigated, for the first time, the activity of free and immobilized AA-ANL in a substrate mixture containing two oils (in equal volumes). The results are shown in Table 6. The mixtures were tested in five forms: (a) mixture of two oils with medium lipolytic activity of immobilized AA-ANL, (b) and (c) mixture of one oil with medium and one oil with low lipolytic activity of immobilized AA-ANL, (d) mixture of two oils with the highest lipolytic activity of immobilized AA-ANL, (e) mixture of two oils with the lowest lipolytic activity of immobilized AA-ANL, (f) mixture of one oil with the highest and one oil with the lowest lipolytic activity of immobilized AA-ANL. Oils were selected based on the results obtained in Section 2.1, according to Ie parameters. The hyperactivation of immobilized lipase was observed in all mixtures, except a mixture of avocado and sunflower oils. High levels of hyperactivation (1800% and 1600%) were observed in the formulation containing oils from (a). Despite the essential activity values, they were lower than the lipase activity obtained with a single oil. Therefore, the additive effect of ω-PUFAs/ω-MUFAs, expressed in tested oils, should be excluded. However, AA-ANL-IB-150A in the rapeseed and olive oil mixture showed significantly higher hyperactivation than in the one-substrate mixture. In turn, immobilized AA-ANL showed lower activity in sunflower and avocado oil composition than in single oils. With respect to the activity of immobilized AA-ANL in formulations composed of oil, in which the highest activity of the catalytic system was obtained, and oil, in which the lowest activity of the catalytic system was obtained, the phenomenon of averaging the enzymatic activity was not observed. The dominant influence of the substrate towards which the lipase showed the highest and the medium activity was observed. In addition, the various activities of two mixtures containing oil with medium and low lipolytic activity of AA-ANL were. In terms of divergent dependencies, the additive or inhibiting effect was conditioned by the use of substrates and with ω-PUFAs/ω-MUFAs. Mixtures were selected on the basis of various activity ratios. As this was a novel research method (there is a lack of studies on lipase lipolytic activity in various substrates in one sample in the literature to date), it was not possible to compare our results to references. Moreover, the suggestions and interpretations presented herein are of a of pioneering nature and shed new light on the importance of the given dependencies. Therefore, it is necessary to develop this research in the future by using a wider spectrum of substrate mixtures, along with other lipases and supports, which will be the goal of future experiments.

Table 6.
Lipolytic activity of free and immobilized AA-ANL in substrate mixtures. The oils in which (Section 2.1) AA-ANL-IB-150A showed the highest lipolytic activity are indicated by + +, the oils with medium lipolytic activity are indicated by +, and the oils with the low AA-ANL-IB-150A activity are indicated by −.
2.6. Reuse of Immobilized AA-ANL
We determined the activity of AA-ANL immobilized onto polyacrylic support IB-150A after four reuse cycles. The results are shown in Table 7. A minimal decrease was observed in immobilized AA-ANL activity in the subsequent reaction cycles (13% Arel in four cycles). This phenomenon could be caused by leakage of protein from the support, as well as denaturation, which is probably related to the interaction of lipase molecules interacted with the support via physical adsorption. As it mentioned in the Introduction, this action is less pronounced than the covalent binding between the lipase and support. The remaining lipase molecules were immobilized on the beads by interactions guaranteeing the activity of the AA-ANL-IB-150A complex, which prevented the protein from being washed out. Therefore, separating supports from the primary reaction mixture by filtration through the polyamide layer enables the efficient separation of solid and fat emulsions. However, a slight loss of carrier occurred (caused by the high viscosity of the emulsion and getting stuck in the pores of the polyamide layer), which may have also contributed to the reduced AA-ANL activity after subsequent cycles. Dos Santos et al. [] tested the effect of support reuse on the lipolytic activity of ANL, i.e., so-called ‘process stability’. In this case, ANL was increased due to residual fermentation of mangaba fruits and immobilization by covalent interactions on silicone matrix sol-gel. Lipase immobilized onto supports was reused for seven cycles. The lipase activity was maintained at a high level for four cycles (10% decrease in Arel). After five cycles, a significant decrease to 50% of activity was observed. The authors separated the immobilized lipase from the substrate (olive oil) by filtration under a vacuum. Then, the immobilized complex was washed with n-hexane to remove of the residual substrate and product. The technique of separating Immobead carriers (containing lipase) by filtration with a polyamide layer for reuse presented herein is a novel method for determining lipolytic activity of lipase by acid-base titration. However, this method undoubtedly requires optimization, especially an increased number of reuse cycles, which will be the subject of future studies.

Table 7.
Lipolytic activity of immobilized AA-ANL after support reuse (one, two, three, and four cycles). The activity parameter of free AA-ANL is shown as the initial value (one cycle).
2.7. Storage Stability of the Immobilized AA-ANL
We investigated the effect of complex AA-ANL-IB-150A storage on lipase stability. The study was performed under standard storage conditions (24 h at room temperature) and in a KBF P240 climate chamber (168 h, 40 °C). The obtained results (Table 8) demonstrated a significant (nearly ninefold) decrease in lipolytic activity of immobilized lipase after storage in a climate chamber, which suggests that the central factor conditioning the drastic activity loss could be the storage time. After 168 h of AA-ANL storage in a climate chamber, the enzyme leakage probably occurred due to the disengagement of covalent binding between lipase and support or protein denaturation. Therefore, despite the high rigidity, this connection was characterized by high lability during long storage, which was reflected by the essential activity decrease. Da Silva et al. [] immobilized ANL on Celite diatomaceous earth by physical adsorption. The effect of storage on lipase activity was also studied. As in our study, the lipase was kept at temperature of 40 °C for five days. The investigation demonstrated a decrease in residual activity of 27%. In our research, the comparison concerned another catalytic parameter (Ie). Notwithstanding, the activity decrease was much higher. Therefore, applying immobilization complex AA-ANL-IB-150A is not beneficial in reaction requiring a long duration. Nevertheless, the exact stability profile of immobilized AA-ANL must be determined, which will be studied in future experiments.

Table 8.
Lipolytic activity of immobilized ANL stored under the conditions recommended by ChiralVision (24 h, room temperature) and in a climate chamber (168 h).
2.8. Kinetic Studies
The kinetic studies of the reaction were performed with the aim of evaluating the affinity of immobilized AA-ANL to the substrate. We calculated the following kinetic parameters based on Lineweaver–Burk curves: Km, Vmax, and kcat. In addition, the correlation coefficient (R2) was determined. The results are shown in Table 9. With respect to the Km parameter, an extremal decrease was observed in the affinity of the immobilized lipase to the substrate (immobilized AA-ANL (Km = 1827.95 ± 0.17 mg/mL) compared with free AA-ANL (Km = 29.20 ± 0.08 mg/mL)). That is, an essential effect of immobilization on lipase affinity to the hydrophobic substrate has been observed. It should be mentioned that the previous sections noted a significant increase in the lipolytic activity of the immobilized lipase compared to the free sample. Hence, the linear relationship between lipase activity and its affinity to the substrate has not been shown. Dos Santos et al. [] studied the kinetic parameters of ANL obtained by fermentation in free form and immobilized onto a sol-gel matrix. The obtained results of immobilized lipase (Km = 115 ± 4 mM) juxtaposed those of with free lipase (Km = 77 ± 2 mM) indicate lower affinity of ANL to the substrate, which was confirmed in our study. The authors suggested that lipase immobilization by covalent binding could change the lipase conformation and hinder substrate availability. The catalytic parameters resulting from the evaluation of lipolytic activity were determined by titration. However, olive oil was used as the substrate for the reaction. In our study, peanut oil was used as the substrate. On the other hand, Zubiolo et al. [], performing a similar experiment investigating catalytic parameters of free and immobilized ANL (encapsulation in a sol-gel matrix), achieved a change in enzyme affinity to the substrate that affected limiting in mass transfer. Substrate solubility could also influence the behavior of encapsulated lipase []. In our study, covalent interactions between lipase and the support seemed to form a rigid complex that prevented protein leakage due to the rotation limitation of other lipase molecules relative to one another. Therefore, the aggregation risk was limited. However, the catalytic parameters were mainly dependent on the curve course, which differed for each screening. Hence, we propose the development of kinetic studies of AA-ANL based on a wider support spectrum (the effect of intramolecular interactions between enzymes and support on lipase affinity to the substrate). In future studies, other kinetic mechanisms will be investigating considering new factors influencing lipase properties.

Table 9.
Kinetic parameters of immobilized AA-ANL compared with lipase in free form. Data are presented as a means ± standard deviations of three analyses (n = 3).
3. Materials and Methods
3.1. Materials
Amano lipase A from Aspergillus niger, 2-propanol (IPA), Bradford reagent, fish oil, and TrizmaBase were received from Sigma Aldrich (Steinheim, Germany). Acetone, methanol, disodium hydrogen phosphate, sodium dihydrogen phosphate, concentrated hydrochloric acid, citric acid, and arabic gum were purchased from POCH (Gliwice, Poland). IB-150A, IB-D152, and IB-EC1 supports were purchased from ChiralVision (Leiden, The Netherlands). Vegetable oils (peanut, blackberry, rapeseed, pumpkin seed, walnut, sesame, avocado, rice, corn, black cumin, hemp, safflower, grape seed, hazelnut, evening primrose, argan, milk thistle, borage, linseed, sunflower, apricot kernel, and olive) were purchased from Oleofarm (Wroclaw, Poland). The water used in the study was purified by Milli-Q Water Purification System equipment (Millipore, Bedford, MA, USA). Lipolytic activity was investigated using a Seven Multi pH meter (Mettler Toledo, Schwerzenbach, Switzerland), a UV-Vis U-1800 spectrophotometer (Hitachi, Tokyo, Japan), and a Unimax 1010 incubator (Heidolph, Germany). The stability of immobilized AA-ANL was determined using a KBF P240 climate chamber (Tuttingen, Germany).
3.2. Immobilization of AA-ANL onto Polyacrylic Supports IB-150A, IB-D152, and IB-EC1
The immobilization process was performed based on the procedure recommended by ChiralVision and literature data [], with slight modifications. A total of 50.0 mg of IB-150A support was placed in Eppendorf vials (2.0 mL). Then, beads were washed with 0.3 mL of 2-propanol for 15 min, and the solvent was removed by washing the sample with purified water and filtration with fluted filters. The separated supports were then dried. Then, they were transferred to Eppendorf vials (2.0 mL) containing a precisely mixed suspension of AA-ANL in phosphate buffer pH 7.0 (10.0 mg/mL). The samples were mixed with a spatula for 5 min in an ice bath and stored in a fridge (4.0 °C) overnight. The procedure was repeated in the case of polyacrylic supports IB-D152 and IB-EC1.
3.3. Determination of the Amount of Immobilized AA-ANL
The amount of protein immobilized on the support was determined by a modified Bradford method [,]. The study was performed using the UV-Vis spectrophotometric method (λ = 595.0 nm), measuring the absorbance of the free lipase remaining in the suspension after the immobilization process (concentration range: 1.0–10.0 mg/mL). The measurement was conducted twice. The amount of lipase immobilized onto the support was calculated with a calibration curve equation (R2 = 0.998). The result was the two-sample mean. The lipase loading (LL) was determined based on the obtained data. Then, the supports were transferred to a filter for 24 h until they were completely dry.
3.4. Determination of the Lipolytic Activity of Immobilized AA-ANL
The lipolytic activity of immobilized AA-ANL relative to free form was determined by titrimetric method, as described in previous studies and literature data [,,,,]. The study was performed using 22 vegetable oils and fish oil. The reaction mixture (emulsion) containing the immobilized lipase was composed of supports with lipase, 3.0 mL phosphate buffer (pH 7.4), 2.5 mL of the appropriate oil (substrate), and 2.5 mL of the water suspension containing arabic gum. The reaction mixture was composed of lipase in free form in the amount of native AA-ANL corresponding to the amount embedded onto the support during immobilization (calculated using the Bradford method). An additional sample containing 10 mg AA-ANL (free sample assuming 100% effective immobilization) was added to each substrate. The mixtures were incubated at 37 °C for 30 min with rotation at 600 rpm. Then, the reaction was stopped by adding a 10.0 mL methanol and acetone mixture (volume ratio 1:1). Titration was conducted using 0.05 M NaOH standard solution at temperature, with phenolphthalein as the indicator. A change in mixture color to orange, together breaking of the emulsion, was considered the end point of titration. The activity of free lipase in the amount corresponding with the amount immobilized onto the support (UB) and the activity of the lipase on the support (UI) were determined based on obtained results. In addition, the ratio of immobilized lipase activity to free lipase activity, i.e., the immobilization efficiency (Ie) was calculated with the following equation:
Immobilization yield (Iy) was calculated according to the following formula []:
where Iy = immobilization yield; LAB = amount of lipase, defined as the difference between the initial amount of lipase and the amount remaining in the supernatant after immobilization onto 50 mg of IB-150A (calculated by Bradford’s method); and LA10 = initial amount of lipase (10 mg).
3.5. Effect of the Support on Lipolytic Activity of Immobilized AA-ANL
The experiment was conducted using methods similar to those described in Section 3.2, Section 3.3 and Section 3.4. Apart from the immobilization of lipase onto a covalent polyacrylic support, the process was also carried out on polyacrylic supports IB-D152 (cationic) and IB-EC1 (non-ionic). Peanut oil was applied as the reaction substrate. Based on obtained results, we calculated the immobilization yield (Iy), activity of free lipase in the amount corresponding with the amount immobilized onto the support (UB), the activity of the lipase on the support (UI), and immobilization efficiency (Ie).
3.6. Effect of Temperature and pH on the Lipolytic Activity of Immobilized AA-ANL
We determined the effect of temperature and pH on AA-ANL lipolytic activity in a similar manner as in Section 3.4. Among the tested oils, peanut oil was chosen for condition optimization. In the case of the temperature study, the mixture was incubated in the temperature range of 25–65 °C for 30 min. The samples were titrated at room temperature. For the pH study, lipase samples in free form (lipase weight) and in immobilized form were incubated by the appropriate buffer (3 mL) in the range of pH 4.0–9.0 (citric buffer 100 mM in the pH range of 4.0–6.0, phosphate buffer 100 mM in the pH range of 7.0–8.0, and tris-based buffer for pH 9.0). The following parameters were determined based on the achieved results,: activity of free lipase in the amount corresponding with the amount immobilized onto the support (UB), the activity of the lipase on the support (UI), immobilization efficiency (Ie), and the relative activity (Arel), i.e., the ratio of the enzymatic activity of tested sample to the sample with maximal activity.
3.7. Effect of Substrate Mixtures on the Lipolytic Activity of Immobilized AA-ANL
Based on the results of the immobilization efficiency calculated in Section 3.4, the six substrate systems composed of two oils were developed: (a) oil mixture in which AA-ANL demonstrated medium lipolytic activity (milk thistle oil + sesame oil), (b) and (c) oil mixture in which AA-ANL demonstrated medium and low lipolytic activity (sunflower oil + avocado oil, milk thistle oil + sunflower oil), (d) oil mixture in which AA-ANL demonstrated the highest lipolytic activity (pumpkin seed oil + fish oil), (e) oil mixture in which AA-ANL demonstrated the lowest lipolytic activity (olive oil + rapeseed oil), (f) oil mixture in which AA-ANL demonstrated the highest and the lowest lipolytic activity (pumpkin seed oil + olive oil). The investigation was performed similarly as in Section 3.4 (incubation at 37 °C, lipase suspended in buffer (pH 7.4)) with modifications with respect to preparation of the reaction mixture; the emulsion was composed of 2.5 mL water suspension with arabic gum and 1.25 mL of each oil. Taking into account the obtained results, as in Section 3.4, the following parameters were calculated: the activity of free lipase in the amount corresponding with the amount immobilized onto the support (UB), the activity of the lipase on the support (UI), and immobilization efficiency (Ie).
3.8. Reusability of Immobilized AA-ANL
We investigated the reusability of supports with immobilized lipase according to the procedure described in Section 3.4 by introducing a novel reuse method. After incubation, the supports were separated from the reaction mixture by filtration with a polyamide layer to a conical flask with a volume of 50 mL. The fat emulsion, without supports, was inactivated by adding 10.0 mL of a methanol and acetone mixture (volume ratio 1:1). A volume of 3.0 mL of phosphate buffer (pH 7.4) and 5.0 mL of fat emulsion were added to the flask with lipase immobilized on the supports, and the sample was incubated for 30 min at 37 °C with rotation at 600 rpm. The procedure was repeated twice for a total of four reuse cycles. The immobilized lipase was inactivated in the mixture after four cycles. The lipolytic activity of inactivated mixtures was determined by titration according to the technique described in Section 3.4. Based on the achieved results, the following parameters were calculated: activity of free lipase in the amount corresponding with the amount immobilized onto the support (UB), the activity of the lipase immobilized on the support (UI), immobilization efficiency (Ie), and the relative activity (Arel), i.e., the ratio of enzymatic activity of the tested sample to the sample with maximal activity.
3.9. Storage Stability of Immobilized AA-ANL
The effect of the storage of complex AA-ANL-IB-150A determined similarly as in Section 3.4. Lipase was immobilized on supports stored in a KBF P240 climate chamber for 7 days (168 h) at a temperature of 40 °C and 75% rH and compared to a sampled immobilized using the standard procedure recommended by ChiralVision (24 h at room temperature). The free lipase was not stored in the climate chamber. Based on achieved results, we calculated the activity of free lipase in the amount corresponding with the amount immobilized onto the support (UB), the activity of the lipase on the support (UI), and immobilization efficiency (Ie).
3.10. Kinetic Studies
The kinetic studies of AA-ANL in free from and immobilized onto polyacrylic support IB-150A were conducted according to the procedure described in Section 3.4 and literature data [,,,,]. Peanut oil was used as the substrate in the concentration range of 100–800 mg/mL. Based on obtained results, the kinetic Michaelis-Menten constant (Km), the maximal velocity of the reaction (Vmax), and turnover number (kcat) according to the Lineweaver-Burk curve were calculated using the following equations:
where V is the initial velocity, Vmax is the maximal velocity, Km is the Michaelis-Menten constant, and [S] is the initial substrate concentration.
where [ET] is the total enzyme amount in the reaction mixture.
4. Conclusions
In this work, we described a study of the lipolytic activity of AA-ANL immobilized onto polyacrylic support IB-150A in 22 vegetable oils and fish oil. We tested the effects of three supports, six substrate mixtures, temperature, and pH on the lipolytic activity of immobilized AA-ANL. In most substrates, a significant ‘jump’ of enzymatic activity of immobilized AA-ANL was observed compared to lipase in free form, the activity of which was very low. High levels of hyperactivation parameters were demonstrated (the highest Ie = 2400% in pumpkin seed oil). A mutual quantitative effect of ratios of ω3, ω6-PUFAs, and ω9 MUFAs on AA-ANL activity was suggested. A ‘cutoff limit’ of ≥3.44 in the ω6/ω9 PUFAs/MUFAs ratio was proposed, above which increased activity of AA-ANL was observed; when the ratio of ω3/ω9 PUFAs/MUFAs declined, the AA-ANL activity decreased. The beneficial effect of polyacrylic supports on AA-ANL activity (highest activity of complex AA-ANL-IB-D152 — Ie = 233%) was confirmed. The hyperactivation of AA-ANL was demonstrated in the oil mixture. A ‘lipolytic jump’ of immobilized AA-ANL was observed in the whole range of tested temperature and pH values (maximal activity at pH 7). On the other hand, high levels of lipase activity were observed under extremal thermal (65 °C) and medium (pH 9.0) conditions. In terms of reuse of the immobilized lipase, considerable activity of this complex was observed after four cycles, indicating the positive effect of covalent interactions between enzymes and the support. However, due to the drastic loss of lipolytic activity, low stability of immobilization complex AA-ANL-IB-150A was observed after storage for 168 h in a climate chamber. The calculated kinetic parameters confirmed a low affinity of immobilized AA-ANL to the substrate. The obtained results could be used to develop enzymatic systems, so-called ‘catalytic triangles of lipase-support-substrate’, with the aim of obtaining an optimal model for application in in vitro studies. The investigated wide substrate spectrum, i.e., oils with varying content of PUFAs/MUFAs, enables selection of appropriate oil systems, which positively influence digestive enzymes and can be used in obesity treatment and to balance the fat contained in the diet with the aim of achieving optimal homeostasis. Activation of lipase toward PUFAs/MUFAs can promote ‘healthy fats’ with multidirectional activity, with beneficial effects not only on lipid metabolism but also on other systems in the human body.
Author Contributions
Conceptualization, T.S., J.D. and M.P.M.; methodology, T.S., J.D., D.W.-Ś. and M.P.M.; formal analysis, T.S. and J.D.; investigation, T.S., J.D. and N.K.; writing—original draft preparation, T.S. and J.D.; writing—review and editing, T.S., J.D., N.K. and D.W.-Ś.; visualization, T.S. and J.D.; project administration, T.S., J.D., D.W.-Ś. and M.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland, grant DEC-2013/09/N/NZ7/03557.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tacias-Pascacio, V.G.; Ortiz, C.; Rueda, N.; Berenguer-Murcia, A.; Acosta, N.; Aranaz, I.; Civera, C.; Fernandez-Lafuente, R.; Alcantara, A.R. Dextran Aldehyde in Biocatalysis: More Than a Mere Immobilization System. Catalysts 2019, 9, 622. [Google Scholar] [CrossRef]
- Alcalde, M.; Ferrer, M.; Plou, F.J.; Ballesteros, A. Environmental biocatalysis: From remediation with enzymes to novel green processes. Trends Biotechnol. 2006, 24, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Soumanou, M.M.; Bornscheuer, U.T.; Menge, U.; Schmid, R.D. Synthesis of structured triglycerides from peanut oil with immobilized lipase. J. Am. Oil Chem. 1997, 74, 427–433. [Google Scholar] [CrossRef]
- Busch, S.; Horlacher, P.; Both, S.; Westfechtel, A.; Schorken, U. Green synthesis routes toward triglycerides of conjugated linoleic acid. Eur. J. Lipid Sci. Technol 2011, 113, 92–99. [Google Scholar] [CrossRef]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R,S)-ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Marszałł, M.P.; Siódmiak, T. Immobilization of Candida rugosa lipase onto magnetic beads for kinetic resolution of (R,S)- ibuprofen. Catal. Commun. 2012, 24, 80–84. [Google Scholar] [CrossRef]
- Graffner-Nordberg, M.; Sjodin, K.; Tunek, A.; Hallberg, A. Synthesis and enzymatic hydrolysis of esters, constituting simple models of soft drugs. Chem. Pharm. Bull. 1998, 46, 591–601. [Google Scholar] [CrossRef]
- Song, X.; Qi, X.Y.; Hao, B.; Qu, Y.B. Studies of substrate specificities of lipases from different sources. Eur. J. Lipid Sci. Technol. 2008, 110, 1095–1101. [Google Scholar] [CrossRef]
- Peter, F.; Preda, G. Characterisation of pancreatic lipase substrate specificity in organic reaction media by a kinetic method. J. Mol. Catal. B Enzym. 2002, 19, 467–472. [Google Scholar] [CrossRef]
- Sanchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia lipase: A versatile catalyst in synthesis reactions. Biotechnol. Bioeng. 2018, 115, 6–24. [Google Scholar] [CrossRef]
- Stauch, B.; Fisher, S.J.; Cianci, M. Open and closed states of Candida antarctica lipase B: Protonation and the mechanism of interfacial activation. J. Lipid Res. 2015, 56, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseing, M.C.F.; Rowan, A.E.; Blank, K.G. Interfacial Activation of Candida antarctica Lipase B: Combined Evidence from Experiment and Simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F.; Carrea, G.; Tarabiono, C.; Gatti-Lafranconi, P.; Brocca, S.; Lotti, M.; Jaeger, K.E.; Puls, M.; Eggert, T. The lid is a structural and functional determinant of lipase activity and selectivity. J. Mol. Catal. B Enzym. 2006, 39, 166–170. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Bezerra, C.S.; Lemos, C.; de Sousa, M.; Goncalves, L.R.B. Enzyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J. Appl. Polym. Sci. 2015, 132, 42125. [Google Scholar] [CrossRef]
- Siódmiak, T.; Mangelings, D.; Vander Heyden, Y.; Ziegler-Borowska, M.; Marszałł, M.P. High Enantioselective Novozym 435-Catalyzed Esterification of (R,S)-Flurbiprofen Monitored with a Chiral Stationary Phase. Appl. Biochem. Biotechnol. 2015, 175, 2769–2785. [Google Scholar] [CrossRef] [PubMed]
- Jose, C.; Toledo, M.V.; Briand, L.E. Enzymatic kinetic resolution of racemic ibuprofen: Past, present and future. Crit. Rev. Biotechnol. 2016, 36, 891–903. [Google Scholar] [CrossRef]
- Kourist, R.; de Maria, P.D.; Miyamoto, K. Biocatalytic strategies for the asymmetric synthesis of profens—recent trends and developments. Green Chem. 2011, 13, 2607–2618. [Google Scholar] [CrossRef]
- Carvalho, A.; Fonseca, T.D.; de Mattos, M.C.; de Oliveira, M.D.F.; de Lemos, T.L.G.; Molinari, F.; Romano, D.; Serra, I. Recent Advances in Lipase-Mediated Preparation of Pharmaceuticals and Their Intermediates. Int. J. Mol. Sci. 2015, 16, 29682–29716. [Google Scholar] [CrossRef]
- Rios, N.S.; Neto, D.M.A.; dos Santos, J.C.S.; Fechine, P.B.A.; Fernandez-Lafuente, R.; Goncalves, L.R.B. Comparison of the immobilization of lipase from Pseudomonas fluorescens on divinylsulfone or p-benzoquinone activated support. Int. J. Biol. Macromol. 2019, 134, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Sałek, K.; Kołodziejczak-Radzimska, A.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Norman, M.; Klapiszewski, Ł.; Bartczak, P.; Kaczorek, E.; Jesionowski, T. Immobilization of Amano Lipase A onto Stober silica surface: Process characterization and kinetic studies. Open Chem. 2015, 13, 138–148. [Google Scholar] [CrossRef]
- Le, Z.G.; Guo, L.T.; Jiang, G.F.; Yang, X.B.; Liu, H.Q. Henry reaction catalyzed by Lipase A from Aspergillus niger. Green Chem. Lett. Rev. 2013, 6, 277–281. [Google Scholar] [CrossRef]
- Yildiz, T. An Enzymatic and Environmentally Friendly Route for the Synthesis of Chiral beta-Hydroxy Ketones. ChemistrySelect 2019, 4, 7927–7931. [Google Scholar] [CrossRef]
- Yildiz, T.; Yasa, H.; Hasdemir, B.; Yusufoglu, A.S. Different bio/Lewis acid-catalyzed stereoselective aldol reactions in various mediums. Monatsh. Chem. 2017, 148, 1445–1452. [Google Scholar] [CrossRef]
- Dunne, A.; Palomo, J.M. Efficient and green approach for the complete deprotection of O-acetylated biomolecules. RSC Adv. 2016, 6, 88974–88978. [Google Scholar] [CrossRef]
- Xu, L.; Ke, C.X.; Huang, Y.; Yan, Y.J. Immobilized Aspergillus niger Lipase with SiO2 Nanoparticles in Sol-Gel Materials. Catalysts 2016, 6, 149. [Google Scholar] [CrossRef]
- Feng, K.L.; Huang, Z.C.; Peng, B.; Dai, W.J.; Li, Y.Q.; Zhu, X.A.; Chen, Y.J.; Tong, X.; Lan, Y.Q.; Cao, Y. Immobilization of Aspergillus niger lipase onto a novel macroporous acrylic resin: Stable and recyclable biocatalysis for deacidification of high-acid soy sauce residue oil. Bioresour. Technol. 2020, 298, 122553. [Google Scholar] [CrossRef]
- Contesini, F.J.; Calzado, F.; Madeira, J.V.; Rubio, M.V.; Zubieta, M.P.; de Melo, R.R.; Goncalves, T.A. Aspergillus Lipases: Biotechnological and Industrial Application. Fungal Metab. 2017, 639–666. [Google Scholar] [CrossRef]
- Silva, W.S.D.; Lapis, A.A.M.; Suarez, P.A.Z.; Neto, B.A.D. Enzyme-mediated epoxidation of methyl oleate supported by imidazolium-based ionic liquids. J. Mol. Catal. B Enzym. 2011, 68, 98–103. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Contesini, F.J.; Ikegaki, M. Enzymatic resolution of (R,S)-ibuprofen and (R,S)-ketoprofen by microbial lipases from native and commercial sources. Braz. J. Microbiol. 2006, 37, 329–337. [Google Scholar] [CrossRef]
- El-Ghonemy, D.H.; Ali, T.H.; Hassanein, N.M.; Abdellah, E.M.; Fadel, M.; Awad, G.E.A.; Abdou, D.A.M. Thermo-alkali-stable lipase from a novel Aspergillus niger: Statistical optimization, enzyme purification, immobilization and its application in biodiesel production. Prep. Biochem. Biotechnol. 2021, 51, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.Z.; Tian, K.M.; Zhang, X.; Lu, F.P.; Singh, S.; Prior, B.; Wang, Z.X. Synthesis of flavor esters by a novel lipase from Aspergillus niger in a soybean-solvent system. 3 Biotech 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Pera, L.M.; Romero, C.M.; Baigori, M.D.; Castro, G.R. Catalytic properties of lipase extracts from Aspergillus niger. Food Technol. Biotechnol. 2006, 44, 247–252. [Google Scholar]
- Qiao, H.Z.; Zhang, F.; Guan, W.T.; Zuo, J.J.; Feng, D.Y. Optimisation of combi-lipases from Aspergillus niger for the synergistic and efficient hydrolysis of soybean oil. J. Anim. Sci. 2017, 88, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, A.; Ishihara, T.; Kobayashi, E.; Ohta, H.; Ishii, T.; Inoue, A.; Mitsuda, S.; Miyazaki, T.; Kajihara, Y.; Sugai, T. Synthesis of regioselectively protected forms of cytidine based on enzyme-catalyzed deacetylation as the key step. Biosci. Biotechnol. Biochem. 2000, 64, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Silano, V.; Baviera, J.M.B.; Bolognesi, C.; Bruschweiler, B.J.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mortensen, A.; et al. Enzyme, Safety evaluation of the food enzyme triacylglycerol lipase from Aspergillus niger (strain LFS). Efsa J. 2019, 17, e05630. [Google Scholar] [PubMed]
- Carvalho, P.D.; Contesini, F.J.; Bizaco, R.; Calafatti, S.A.; Macedo, G.A. Optimization of enantioselective resolution of racemic ibuprofen by native lipase from Aspergillus niger. J. Ind. Microbiol. Biotechnol. 2006, 33, 713–718. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part II: Carrier Materials. ChemBioEng Rev. 2019, 6, 167–194. [Google Scholar] [CrossRef]
- Mokhtar, N.F.; Rahman, R.A.; Noor, N.D.M.; Shariff, F.M.; Ali, M.S.M. The Immobilization of Lipases on Porous Support by Adsorption and Hydrophobic Interaction Method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Poppe, J.K.; Costa, A.P.O.; Brasil, M.C.; Rodrigues, R.C.; Ayub, M.A.Z. Multipoint covalent immobilization of lipases on aldehyde-activated support: Characterization and application in transesterification reaction. J. Mol. Catal. B Enzym. 2013, 94, 57–62. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef]
- Siódmiak, T.; Haraldsson, G.G.; Dulęba, J.; Ziegler-Borowska, M.; Siódmiak, J.; Marszałł, M.P. Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica. Catalysts 2020, 10, 876. [Google Scholar] [CrossRef]
- Alagoz, D.; Celik, A.; Yildirim, D.; Tukel, S.S.; Binay, B. Covalent immobilization of Candida methylica formate dehydrogenase on short spacer arm aldehyde group containing supports. J. Mol. Catal. B Enzym. 2016, 130, 40–47. [Google Scholar] [CrossRef]
- Matte, C.R.; Bussamara, R.; Dupont, J.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Immobilization of Thermomyces lanuginosus Lipase by Different Techniques on Immobead 150 Support: Characterization and Applications. Appl. Biochem. Biotechnol. 2014, 172, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Yousefi, M.; Nikseresht, A.; Omidi, H. Covalent binding and in-situ immobilization of lipases on a flexible nanoporous material. Process Biochem. 2021, 102, 92–101. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Hayyan, A.; Hayyan, M.; Rashid, S.N.; Nor, M.R.M.; Zulkifli, M.Y.; Alias, Y.; Mirghani, M.E.S. Shedding Light on Lipase Stability in Natural Deep Eutectic Solvents. Chem. Biochem. Eng. Q. 2018, 32, 359–370. [Google Scholar] [CrossRef]
- Sharma, A.; Bhandari, K.; Jain, A.; Chaurasia, S.P.; Dalai, A.K. Lipase catalyzed esterification of Docosahexaenoic acid (DHA) with immobilized Pseudomonas cepacia and Thermomyces lanuginosus. Indian J. Chem. Technol. 2021, 28, 139–149. [Google Scholar]
- Diwan, B.; Gupta, P. Synthesis of MCFA and PUFA rich oils by enzymatic structuring of flax oil with single cell oils. LWT 2020, 133, 109928. [Google Scholar] [CrossRef]
- Hebda, P.; Wiśniowska, L.; Szafrański, P.W.; Cegła, M. Multigram-scale enzymatic kinetic resolution of trans-2-azidocyclohexyl acetate and chiral reversed-phase HPLC analysis of trans-2-azidocyclohexanol. Chirality 2022, 34, 428–437. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.L.; Assuncao, J.C.D.; Pascoal, C.V.P.; Bezerra, M.L.S.; Silva, A.C.S.; de Souza, B.V.; Rodrigues, F.E.A.; Ricardo, N.; Arruda, T. Waste of Nile Tilapia (Oreochromis niloticus) to Biodiesel Production by Enzymatic Catalysis.Optimization Using Factorial Experimental Design. Ind. Eng. Chem. Res. 2021, 60, 3554–3560. [Google Scholar] [CrossRef]
- Sanchez, D.A.; Alnoch, R.C.; Tonetto, G.M.; Krieger, N.; Ferreira, M.L. Immobilization and bioimprinting strategies to enhance the performance in organic medium of the metagenomic lipase LipC12. J. Biotechnol. 2021, 342, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Kahar, U.M.; Chan, K.G.; Sani, M.H.; Noh, N.I.M.; Goh, K.M. Effects of single and co-immobilization on the product specificity of type I pullulanase from Anoxybacillus sp SK3–4. Int. J. Biol. Macromol. 2017, 104, 322–332. [Google Scholar] [CrossRef]
- Gonzalez-Coronel, L.A.; Cobas, M.; Rostro-Alanis, M.D.; Parra-Saldivar, R.; Hernandez-Luna, C.; Pazos, M.; Sanroman, M.A. Immobilization of laccase of Pycnoporus sanguineus CS43. New Biotechnol. 2017, 39, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Mobayed, F.H.; Rafael, R.D.; Rodrigues, R.C.; Sperotto, R.A.; Volpato, G.; de Souza, C.F.V. Modification of Immobead 150 Support for Protein Immobilization: Effects on the Properties of Immobilized Aspergillus oryzae beta-Galactosidase. Biotechnol. Prog. 2018, 34, 934–943. [Google Scholar] [CrossRef]
- Binay, B.; Alagoz, D.; Yildirim, D.; Celik, A.; Tukel, S.S. Highly stable and reusable immobilized formate dehydrogenases: Promising biocatalysts for in situ regeneration of NADH. Beilstein J. Org. Chem. 2016, 12, 271–277. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T. Luffa cylindrica Sponges as a Thermally and Chemically Stable Support for Aspergillus niger Lipase. Biotechnol. Prog. 2016, 32, 657–665. [Google Scholar] [CrossRef]
- Dulęba, J.; Siódmiak, T.; Marszałł, M.P. Amano Lipase PS from Burkholderia cepacia—Evaluation of the Effect of Substrates and Reaction Media on the Catalytic Activity. Curr. Org. Chem. 2020, 24, 798–807. [Google Scholar] [CrossRef]
- Dulęba, J.; Siódmiak, T.; Marszałł, M.P. The influence of substrate systems on the enantioselective and lipolytic activity of immobilized Amano PS from Burkholderia cepacia lipase (APS-BCL). Process. Biochem. 2022, 130, 126–137. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.O.; Soomro, H.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3,-6 and-9 fatty acids in animals. Anim. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 137–141. [Google Scholar] [CrossRef]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E.; Mayangell, L.B.; Richardson, A.J.; Lake, J.; et al. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar] [CrossRef]
- Curioni, C.C.; Alves, N.N.R.; Zago, L. Omega-3 supplementation in the treatment of overweight and obese children and adolescents: A systematic review. J. Funct. Foods 2019, 52, 340–347. [Google Scholar] [CrossRef]
- Das, U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020, 51, 282–286. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Jin, W.H.; Cheng, X.Y.; Dong, Z.; Chang, M.; Wang, X.S. Enzymatic enrichment of n-3 polyunsaturated fatty acid glycerides by selective hydrolysis. Food Chem. 2021, 346, 128743. [Google Scholar] [CrossRef]
- Osadnik, K.; Jaworska, J. Analysis of ω-3 fatty acid content of polish fish oil drug and dietary supplements. Acta Pol. Pharm. 2016, 73, 875–883. [Google Scholar]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and improving the extraction of omega-3 from fish oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD011094. [Google Scholar] [PubMed]
- Halfen, S.; Jacometo, C.B.; Mattei, P.; Fenstenseifer, S.R.; Pfeifer, L.F.M.; Del Pino, F.A.B.; Santos, M.A.Z.; de Pereira, C.M.P.; Schmitt, E.; Correa, M.N. Diets Rich in Polyunsaturated Fatty Acids With Different Omega-6/Omega-3 Ratio Decrease Liver Content of Saturated Fatty Acids Across Generations of Wistar Rats. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.F.; Ge, J.; Liu, Z. Enhanced Activity of Immobilized or Chemically Modified Enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alagoz, D.; Tukel, S.S.; Yildirim, D. Immobilization of pectinase on silica-based supports: Impacts of particle size and spacer arm on the activity. Int. J. Biol. Macromol. 2016, 87, 426–432. [Google Scholar] [CrossRef]
- Ameri, A.; Asadi, F.; Shakibaie, M.; Forootanfar, H.; Ranjbar, M. Hydroxyapatite/Glycyrrhizin/Lithium-Based Metal-Organic Framework (HA/GL/Li-MOF) Nanocomposite as Support for Immobilization of Thermomyces lanuginosus Lipase. Appl. Biochem. Biotechnol. 2022, 194, 2108–2134. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisan, J.M.; Fernandez-Lafuente, R. Self-assembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Gocen, T.; Bayari, S.H.; Guven, M.H. Effects of chemical structures of omega-6 fatty acids on the molecular parameters and quantum chemical descriptors. J. Mol. Struct. 2018, 1174, 142–150. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Candida antarctica lipase A effectively concentrates DHA from fish and thraustochytrid oils. Food Chem. 2017, 229, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Smith, C.E.; Lai, C.Q.; Irvin, M.R.; Parnell, L.D.; Lee, Y.C.; Pham, L.D.; Aslibekyan, S.; Claas, S.A.; Tsai, M.Y.; et al. The effects of omega-3 polyunsaturated fatty acids and genetic variants on methylation levels of the interleukin-6 gene promoter. Mol. Nutr. Food Res. 2016, 60, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef]
- Holt, A.P.; Bocharova, V.; Cheng, S.W.; Kisliuk, A.M.; White, B.T.; Saito, T.; Uhrig, D.; Mahalik, J.P.; Kumar, R.; Imel, A.E.; et al. Controlling Interfacial Dynamics: Covalent Bonding versus Physical Adsorption in Polymer Nanocomposites. ACS Nano 2016, 10, 6843–6852. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.N.; Jiang, Y.J.; Zhou, L.Y.; Gao, J. Immobilization of Candida antarctica lipase B by adsorption in organic medium. New Biotechnol. 2010, 27, 53–58. [Google Scholar] [CrossRef]
- dos Santos, E.A.L.; Lima, A.S.; Soares, C.M.F.; Santana, L. Lipase from Aspergillus niger obtained from mangaba residue fermentation: Biochemical characterization of free and immobilized enzymes on a sol-gel matrix. Acta Sci. Technol. 2017, 39, 1–8. [Google Scholar] [CrossRef][Green Version]
- Muley, A.B.; Awasthi, S.; Bhalerao, P.P.; Jadhav, N.L.; Singhal, R.S. Preparation of cross-linked enzyme aggregates of lipase from Aspergillus niger: Process optimization, characterization, stability, and application for epoxidation of lemongrass oil. Bioprocess. Biosyst. Eng. 2021, 44, 1383–1404. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.C.F.; Contesini, F.J.; Carvalho, P.D. Characterization and Catalytic Activity of Free and Immobilized Lipase from Aspergillus niger: A Comparative Study. J. Braz. Chem. Soc. 2008, 19, 1468–1474. [Google Scholar] [CrossRef]
- Zubiolo, C.; Santos, R.C.A.; Carvalho, N.B.; Soares, C.M.F.; Lima, A.S.; Santana, L. Encapsulation in a sol-gel matrix of lipase from Aspergillus niger obtained by bioconversion of a novel agricultural residue. Bioprocess. Biosyst. Eng. 2014, 37, 1781–1788. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Immobilization of lipase on carboxylic acid-modified silica nanoparticles for olive oil glycerolysis. Bioprocess. Biosyst. Eng. 2018, 41, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Bai, Y.X.; Li, Y.F.; Lin, L.; Cui, Y.J.; Xia, C.G. Characterization of Candida rugosa lipase immobilized onto magnetic microspheres with hydrophilicity. Process. Biochem. 2008, 43, 1179–1185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).