Abstract
To achieve low-carbon and sustainable development it is imperative to explore water treatment technologies in a carbon-neutral model. Because of its advantages of high efficiency, low consumption, and no secondary pollution, electrocatalytic oxidation technology has attracted increasing attention in tackling the challenges of organic wastewater treatment. The performance of an electrocatalytic oxidation system depends mainly on the properties of electrodes materials. Compared with the instability of graphite electrodes, the high expenditure of noble metal electrodes and boron-doped diamond electrodes, and the hidden dangers of titanium-based metal oxide electrodes, a titanium sub-oxide material has been characterized as an ideal choice of anode material due to its unique crystal and electronic structure, including high conductivity, decent catalytic activity, intense physical and chemical stability, corrosion resistance, low cost, and long service life, etc. This paper systematically reviews the electrode preparation technology of Magnéli phase titanium sub-oxide and its research progress in the electrochemical advanced oxidation treatment of organic wastewater in recent years, with technical difficulties highlighted. Future research directions are further proposed in process optimization, material modification, and application expansion. It is worth noting that Magnéli phase titanium sub-oxides have played very important roles in organic degradation. There is no doubt that titanium sub-oxides will become indispensable materials in the future.
1. Electrocatalytic Oxidation Technologies
The rapid development of modern industries in various fields directly brings about growing pollution and ever-increasing refractory and toxic substances in the different liquid effluent. Traditional wastewater treatment methods have difficulty meeting the environmental requirements for such organic wastewater [1]. Advanced oxidation technology has developed rapidly in recent years as a practical approach to constructing a carbon-neutral treatment mode and realizing the green development of wastewater treatment technologies. Electrocatalytic oxidation technology is a promising way of removing organic pollutants. It is a green chemical technology that adopts electrodes with favorable catalytic performance to generate hydroxyl radicals (or other radicals and groups) with strong oxidation ability and react with the organic pollutants in the solution to decompose them into H2O and CO2 [2,3]. Compared with traditional water purification technologies, it has drawn significant interest thanks to its advantages, such as strong oxidation capacity, with no chemical agents added, and clean and mild conditions [4,5,6].
1.1. Technique Principle
As shown in Figure 1, the principle of electrocatalytic oxidation technology can be divided into direct oxidation and indirect oxidation [7,8]. Direct oxidation refers to the process in which the pollutant is adsorbed directly on the anode surface and oxidized by losing its electron. It can be oxidized to substances easily for less toxic biochemical treatment, or can even be directly and deeply oxidized to H2O and CO2 to achieve the purpose of removing organic pollutants from wastewater. Kirk et al. [9] studied the anodic oxidation process of aniline and believed that aniline was oxidized through an electron transfer reaction, which was a typical direct anodic oxidation process.
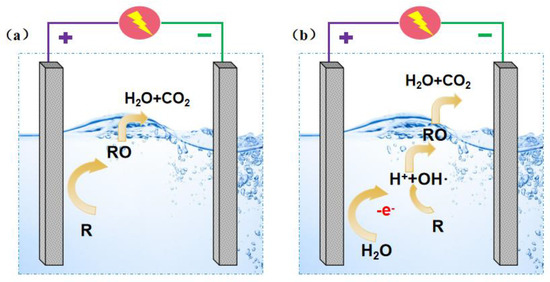
Figure 1.
Schematic diagram of direct oxidation (a) and indirect oxidation (b) of electrocatalytic oxidation technology.
Indirect oxidation refers to strong oxidizing free radicals from an anode reaction (• OH [10], O2 •, • HO2, etc.), or products from an intermediate reaction (such as a chlorine-active substance) that are generated in the electrode surface or spread to the aqueous solution for pollutants oxidation, ultimately achieving the degradation of organic pollutants. Taking hydroxyl radicals as an example, the reaction processes are as follows:
H2O → ·OH + H+ + e−
R + 2·OH → RO + H2O
In most actual electrocatalytic oxidation processes, there is no definite sharp boundary between direct oxidation and indirect oxidation. However, the above two processes are usually both included [11]. Li et al. [8] studied removing azo dyes from water by oxidation using different kinds of electrodes. They found that the oxidation process was mainly determined by direct oxidation for some active electrodes (Ru and IrO2). In contrast, both direct oxidation and indirect oxidation of active radicals influenced the oxidation process of inactive electrodes (BDD and PbO2).
1.2. Anode
The electrocatalytic oxidation system is a composite system, mainly including an anode, cathode, power supply, electrolytic cell, and other supporting equipment [12]. Electrode material is the key point of electrocatalytic technology because any research on electrocatalytic technology must be based on a certain electrode material with superior performance [13,14,15]. The electrode not only maintains the conductive process (i.e., conductivity) but also activates the reactants (i.e., catalytic activity), increases the electron transfer rate, and performs the promotion and selection functions for electrochemical reactions. In addition, to meet the requirements of practical use, electrode materials are also supposed to have satisfactory properties in stability and economic performance.
The active substances with oxidizing ability produced by the anode play an essential role in the removing of organic pollutants. At the same time, side reactions (such as oxygen evolution reactions) can also occur at the anode, which will burden the reaction energy consumption of the system. Therefore, selecting electrode materials (especially anode materials) in the electrocatalytic oxidation system is crucial. The electrode materials used in academic research and engineering practice mainly include graphite electrodes (graphite electrode and BDD electrode), noble metal electrodes (Pt electrode), and titanium-based metal oxide electrodes.
Graphite electrode refers to the electrode composed of carbon, which is usually used for dye decolorization studies due to its high adsorption and larger specific surface area [16,17,18]. Its strong adsorption can also incur adverse effects for the spread of the intermediate, causing the accumulation of the products on the electrode surface and covering the active site to impair the electrode, resulting in a loss of catalytic activity, which extensively limited its widespread application. Common noble metal electrodes are Pt, Ru, Au, etc. [19,20]. This electrode has good corrosion resistance and high oxygen evolution potential, while the high expenditure restrains its use in the industrial field of electrocatalytic oxidation. The BDD electrode is considered competent in electrocatalytic oxidation to organic matters with superior performance, which has good electrical conductivity, high oxygen evolution potential, strong corrosion resistance, and good stability [21]. However, its preparation process is relatively complex with high costs. The electrode surface can form more thin-polymer film in the degradation process, leading to electrode passivation, which cannot be ideally used in practical engineering [22,23]. Titanium-based metal oxide electrode uses a titanium sheet as the substrate and is covered with one or more metal oxide coatings, also known as a dimension stable anode (DSA). Common metal oxide coatings on DSA electrodes mainly include Sb-SnO2 [24,25,26,27], IrO2 [28], RuO2 [29], and PbO2 [30,31,32,33,34]. At present, there are many investigations on the doping modification methods of the Ti/PbO2 electrode, mainly including the doping ion [35,36,37], introducing the intermediate layer [38], doping nanoparticles [39,40] and regulating the micro-morphology of electrode materials [41,42]. Compared with the instability of graphite electrodes and the high price of noble metal electrodes and boron-doped diamond electrodes, titanium-based metal oxide electrodes have attracted great attention and are widely applied in practice because of their relatively low-price and simple preparation as well as easy functionalization and modification [43].
Nonetheless, there are still many obstacles when using titanium-based metal oxide electrodes in the practical application of the electrocatalytic oxidation system, mainly focusing on the following four aspects, namely safety, catalytic activity, economy, and stability:
- (1)
- Potential safety hazards: Due to intrinsic properties, traditional titanium-based metal oxide electrodes (including the PbO2 electrode, Sb-SnO2 electrode, and Ir/Ru/Ta series electrode) have an issue of surface metal element dissolution in reactions, which will last during the entire life cycle of the electrode, resulting in a significant risk of secondary pollution [44]. In addition, there is a significant scaling phenomenon on the cathode surface in the engineering practice of electrocatalytic oxidation technology. Once the scale layer thickens and results in the connection between anode and cathode, the anode surface layer will be penetrated and corroded, seriously impairing the safe and stable operation of the system [45,46];
- (2)
- Inadequate catalytic capacity: The yield of the hydroxyl radical on the surface of the Sb-SnO2 electrode and Ir/Ru/Ta electrode is not sufficient. Although the hydroxyl radical yield of the PbO2 electrode is higher than that of the former two, it is mainly in the adsorption state [47] and incompetent for effective degradation, bringing its oxidation capacity even lower than that of the Sb-SnO2 electrode [48,49];
- (3)
- Poor economy: Although it is relatively cheap compared with the noble metal electrode and BDD electrode, the titanium-based metal oxide electrode still requires the use of noble metal salt to prepare a brush coating solution in the preparation process, resulting in high cost and vulnerability to fluctuations in noble metal market price [50,51];
- (4)
- Stability to be improved: The above-mentioned PbO2 electrode, Sb-SnO2 electrode, and Ir/Ru/Ta series electrode all have a surface-active element dissolution, surface oxide layer loss, and titanium base passivation in the use process (especially with F− ion or high concentration Cl− ion), which leads to the irreversible inactivation of the electrode [52,53]. Thus, the stability needs further improvement.
The above discussion demonstrates many problems in the practical application of the anode materials currently used. Hence, it is urgent to develop new electrode materials to meet the requirements of electrocatalytic oxidation technology.
1.3. Magnéli Titanium Sub-Oxides Material
Titanium is a metal material with abundant storage (only second to iron) and excellent biosafety performance. However, it was used as the substrate material for electrodes because of the easy formation of an insulating oxide layer on its surface [54]. However, when the valence state of titanium is lower than Ti3+, its properties will be completely different from those of traditional titanium oxides [55,56], which have attracted widespread attention since its discovery.
1.3.1. Crystal Structure of Titanium Sub-Oxides Material
Magnéli titanium sub-oxide is a general term for a series of non-stoichiometric titanium oxides, whose generic formula is TinO2n−1 (4 ≤ n ≤ 10) [57], including a series of titanium oxides, such as Ti4O7, Ti5O9, and Ti6O11.
The crystal structure of Magnéli titanium sub-oxides can be regarded as a network structure composed of a two-dimensional chain of titanium dioxide with multiple titanium atoms at the center and oxygen atoms at the apex in an octahedral structure [58]. For example, as shown in Figure 2a, the Ti4O7 crystal is composed of three regular octahedral TiO2 layers and one TiO layer due to the vacancy of an oxygen atom in every four layers and the apparent shear plane of the crystal structure. The TiO layer forms a shared edge perpendicular to the surface due to the vacancy of oxygen atoms, resulting in titanium atoms closer together to form a conductive band. In contrast, a TiO2 layer on both sides of the TiO layer can wrap the conductive band of titanium sub-oxides, which determines the strong corrosion resistance of Ti4O7.
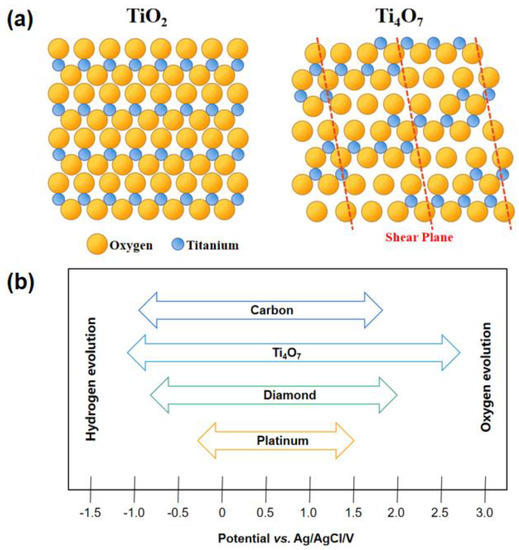
Figure 2.
Comparison of crystal structures of TiO2 and Ti4O7 (a); hydrogen and oxygen evolution potentials of some electrode materials (1.0 M H2SO4) (b) [58].
1.3.2. Physicochemical Properties of Titanium Sub-Oxides Material
The unique lattice structure makes titanium sub-oxide materials possess unique physical and electrochemical properties, such as high conductivity, superior chemical stability, and a wide electrochemical stability potential window [59]. The conductivity of titanium sub-oxides is in the same order of magnitude as carbon rods. As shown in Table 1, the conductivity of Ti4O7 material is the highest, which can reach 1500 S·cm−1, about twice that of graphite (727 S·cm−1).

Table 1.
Electrical conductivity of each phase of titanium sub-oxides at 298 K [58].
Recent research shows that Ti4O7 is exceptionally stable in a strong corrosive solution and an alkali environment. As shown in Table 2, the mass loss of Ti4O7 in the fluorine-containing acidic electrolyte is only 0.29% after 350 h. In a high concentration HF/HNO3 electrolyte, the mass loss is only 12.7%, far better than that of metal Ti. Some studies have also stated that the expected half-life of Ti4O7 is 50 years in 1.0 M H2SO4 at room temperature [60].

Table 2.
Comparison of corrosion resistance between Ti4O7 and Ti [58].
In addition, Figure 2b illustrates that the difference between the oxygen evolution potential and hydrogen evolution potential of Ti4O7 is nearly 4 V in a 1.0 m H2SO4 solution, which exhibits a promising application prospect in the field of electrocatalytic oxidation [61].
Furthermore, the titanium sub-oxide material can be considered a low-cost material, and the properties of the titanium-based material make it intrinsic for electrode safety. Therefore, titanium sub-oxide is suitable as electrode material in electrocatalytic degradation, with superior properties meeting the requirements for selecting electrodes in the electrocatalytic oxidation system [62,63,64].
As a kind of conductive material, titanium sub-oxide material has the advantages of high conductivity, decent catalytic activity, strong physical and chemical stability, high corrosion resistance, low cost, and long service life, etc., which is considered a kind of catalytic electrode with great development potential. At present, many domestic and foreign studies have used titanium sub-oxide material to treat various types of refractory organic wastewater, highlighting the unique advantages of titanium sub-oxides in the field of electrocatalytic oxidation.
According to the classification of different electrode forms, a great deal of work was summarized on the preparation and application of titanium sub-oxide electrodes. Then, the existing technical problems and challenges were also put forward, with further prospects for the future development direction.
2. Preparation Methods of Titanium Sub-Oxides Electrode
At present, there are three types of titanium sub-oxide materials used in the electrocatalytic oxidation wastewater systems, including a coated electrode, integrated electrodes, and composite electrodes. Different kinds of electrodes have their own advantages and disadvantages. Many researchers have made many contributions in optimizing the preparation process and improving the performance of electrodes, which is conducive to broadening the application prospect of titanium sub-oxides electrodes.
2.1. Preparation of Titanium Sub-Oxides Powder
As the primary raw material for electrode preparation, titanium sub-oxides powder has been used in many processes. Current synthesis methods of titanium sub-oxides powder focus on reducing of titanium dioxide at a high temperature between 600 and 1000 °C [65,66,67]. In addition, there are also studies on the preparation of titanium sub-oxides powder using metallic titanium, organic titanium, inorganic titanium, and other raw materials as precursors [68,69,70]. Briefly, titanium dioxide is used as a prevalent raw material due to its abundant reserves and relatively low cost [71]. Table 3 lists the principles and process comparisons of the three methods of carbothermal reduction, hydrogen reduction, and metallothermic reduction using titanium dioxide as the precursor.

Table 3.
Synthesis methods of titanium sub-oxides powder with TiO2 as precursor.
2.2. Titanium Sub-Oxides-Coated Electrode
Titanium sub-oxides-coated electrode refers to the preparation of titanium sub-oxides powder by reduction and other methods, which is then coated or deposited on the anode substrate, which is adopted as a catalytic electrode. The preparation methods of coating electrodes mainly include the coating methods, magnetron sputtering methods, the electrodeposition method, and the sol–gel method, etc. As shown in Figure 3, the performance of the electrodes prepared vary depending on the preparation processes.
2.2.1. Coating Method
The coating method includes coating, spraying, and powder curing technology, among which plasma spraying is a new kind of multipurpose spraying method with relative maturity and precision. Thanks to its characteristics of ultra-high temperature, fast jet particles, compact coating density, high bond strength, inert gas as the working gas, and no easy oxidation of spraying material, etc., it is widely used in the preparation of titanium sub-oxides electrodes. Teng et al. [76] coated titanium sub-oxides powder on titanium mesh and titanium plate by plasma spraying technology, which successfully prepared a Ti/Ti4O7 electrode. The preparation process and characterizations are shown in Figure 3a,b. Ti4O7 powder was approximately spherical and uniformly and compactly covered on a Ti matrix, providing a large surface area and more active sites for the electrochemical reaction. The electrode had high conductivity and low oxygen evolution potential, presenting fair oxidation activity to sulfadiazine. Soliu et al. [77] also prepared a Ti/Ti4O7 electrode by plasma spraying technology, which was employed for the electrocatalytic degradation of amoxicillin. In the plasma spraying system, 20–60 μm of Ti4O7 particles were sprayed on the surface of the pretreated titanium plate under the conditions of a 700 A current, argon, and with hydrogen-mixed gas (19.6% H2) as the carrier at 2 rpm.
Al, carbon cloth, and other conductive substrates are also alternatives for the matrix and the coating on a titanium base. Han et al. [78] successfully coated Ti4O7 ceramic particles on an Al substrate by plasma spraying. The system was operated under the condition of a 10 V spray voltage in a nitrogen atmosphere, and the accelerated life of the prepared electrode was 69.6% longer than that of the commonly used titanium-based SnO2 electrode. The current efficiency and energy consumption were increased by 5.59% and 16.2%, respectively, with electrochemical performance greatly improved. Geng et al. [79] impregnated a layer of TiO2 on a porous Al2O3 matrix by the coating reduction method. The as-prepared electrode was aged at room temperature for 2 h, dried at 100 °C for 1 h, and sintered in air at 800 °C, which was all repeated two to eight times, and it was finally reduced in the H2 atmosphere at 1050 °C to obtain the Ti4O7 layer. The conductivity of the prepared electrode is more than 200 S cm−1, with the particle size of the coating in the 200–300 nm range and the average pore size being 350 nm, exhibiting superior electrochemical properties.
This technology pretreated the substrate and coated the anode surface with a dense and uniform Ti4O7 particle coating, which has the advantages of simple operation, low equipment maintenance cost, and flexible regulation performance. However, there are still technical problems, such as the weak adhesion between the substrate and the coating, which impairs the stability of the electrode.
2.2.2. Magnetron Sputtering Method
Magnetron sputtering is a physical vapor deposition method with the advantages of easy control, a large coating area, and strong adhesion. In Ar + O2 plasma, Wong et al. [80] deposited a series of TiOx(0 < x ≤ 2) films with different O/Ti ratios on silicon substrates by reactive magnetron sputtering and discussed the effects of the oxygen flow rate and substrate temperature on the structural properties, mechanical properties, and electrical properties of the films, respectively. The results showed that the titanium, Ti(-O) solid solution, TiO, Ti2O3, Magnéli, rutile, and anatase TiO2 phases appeared successively with the increase of the oxygen flow rate. The conductivity decreased with the rise of oxygen content but increased with the elevation of the deposition temperature. The hardness of the films changed in the order of TiO > Ti2O3 > Ti4O7. Titanium sub-oxides films with high performance can be obtained at the deposition temperature of 500 °C. These thin films possessed properties in terms of a small crystal diameter, the proper crystal structure of defects and grain boundaries, and good electrocatalytic oxidation performance.
Li et al. [81] also explored the magnetron sputtering method to deposit titanium sub-oxide on MGO-ZrO2 plasma electrolytic oxidation (PEO) coating at different lining temperatures. XRD results shown in Figure 3e demonstrate that the relative content of titanium sub-oxide in the coating is relatively high at a higher temperature, while the content is almost zero at room temperature. Therefore, the temperature of the lining greatly influences the composition of the coating.
This technology is easy to control, with a stable prepared electrode morphology and a strong coating adhesion. In contrast, the composition is relatively impure, and is greatly affected by various preparation factors.
2.2.3. Electrodeposition Method
Ertekin et al. [82] successfully prepared titanium sub-oxide thin-film electrodes by the electrochemical deposition of titanium peroxide solution on indium tin oxide-coated glass with acetonitrile and hydrogen peroxide as supporting electrolytes. This study also confirmed the feasibility of the electrochemical deposition of crystalline TinO2n−1 film, a preparation method with an electrochemical application prospect. SEM characterization in Figure 3c shows that the surface of the film electrode was porous and granular. Figure 3d XRD results confirm that different potentials and temperatures greatly influence the crystallinity of the film. At different electrochemical deposition potentials and temperatures, TinO2n−1 was Ti3O5, Ti4O7, and Ti5O9, among which there was a low content of the Ti4O7 phase with the best performance.
This method is simple to operate with a relatively low cost. However, much the same as magnetron sputtering, the biggest problem of this technology is that the coating composition is not easy to control, which will have a significant influence on the performance of the electrode.
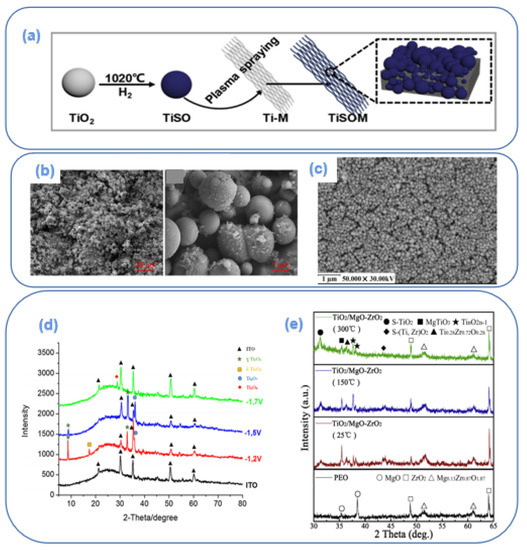
Figure 3.
Processes of plasma spraying technology [76] (a); SEM images of coating electrode [76] (b); SEM images of electrodeposition electrode [82] (c); XRD pattern of electrodeposition electrode [82] (d); XRD pattern of magnetron sputtering electrode [81] (e).
2.2.4. Sol–Gel Method
The sol–gel method can reduce the temperature required for titanium sub-oxide electrode preparation. Sasmita et al. [60] used synthetic titanium dioxide powder dispersed in an aqueous solution of polyacrylic acid as a raw material, with the addition of monomer (MAM) and a crosslinking agent (MBAM) to the slurry in a ratio of 4:1. Next, 0.01 wt% APS was added to the mix for 6 h. The slurry was injected into a Teflon mold by adding 10 mL of TEMED to the slurry for catalytic polymerization. Polymerization took place within 5 min, and the pellets were cast. After drying at 50 °C, gel-cast electrodes were prepared by sintering for 6 h in a tubular furnace at 1050 °C.
Perica Paunovic et al. [83] used the Magnéli phase instead of a carbon-based carrier material as a catalyst carrier for the hydrogen/oxygen evolution electrocatalyst. Magnéli phase raw materials were pulverized by a ball mill at 200 rpm of acceleration in a dry ball mill for 4, 8, 12, 16, and 20 h, respectively. The sol–gel method deposited the Co (10 wt%) metal phase on the Magnéli matrix. With the extension of mechanical treatment time, the size of the Magnéli phase decreased and the catalytic activity of the corresponding electrocatalyst increased. However, due to the low surface area of the Magnéli phase, its activity is not as good as that of corresponding electrocatalysts deposited on other support materials, such as multi-walled carbon nanotubes.
This method is flexible because of the low cost required for preparation. However, the catalytic activity of the electrode could be affected to a certain extent due to the doping of complex coagulant aids.
2.3. Titanium Sub-Oxides-Integrated Electrode
The methods of preparing integrated electrodes mainly include compression reduction, powder sintering, and membrane preparation by hydrothermal reduction, etc. The preparation diagrams of different processes are shown in Figure 4.
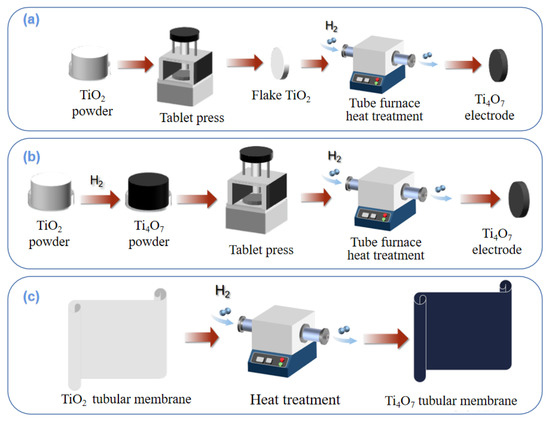
Figure 4.
Preparation processes of compression reduction method (a); powder sintering method (b); membrane preparation by hydrothermal reduction method (c).
2.3.1. Compression Reduction Method
As shown in Figure 4a, TiO2 powder is first pressed, molded, and then calcined in a reducing atmosphere to produce a titanium sub-oxides-integrated electrode. Regonini et al. [84] mixed titanium dioxide particles with polyethylene glycol as binder under pressure, sintered them into spheres, and then reduced them into titanium sub-oxide electrodes under the protection of Ar gas at 1300 °C. As shown in SEM images of Figure 5a, compared with titanium dioxide, the prepared electrode had conspicuous furrows to provide more active sites but did not form porous particles. The XRD results in Figure 5b also reveal different kinds of titanium sub-oxide phases such as Ti4O7, Ti5O9, and Ti6O11 in the integrated electrode due to the reduction after pressing. Difficulty in controlling the reduction degree and an insufficient internal reduction degree resulted in the composite structure of different layers. The TiO2 layer remaining in the inner layer generated the internal barrier layer capacitor (IBLC) effect, significantly weakening the electrode performance.
2.3.2. Powder Sintering Method
The powder sintering method can effectively avoid the disadvantages of the compression reduction method. The technical route is shown in Figure 4b, where titanium sub-oxides powder is prepared before compression sintering. Lin et al. [85] first synthesized Ti4O7 nanopowder by reducing titanium dioxide nanopowder at 950 °C in a H2 atmosphere. Then, the prefabricated Ti4O7 nanopowder was mixed with polyacrylamide/polyvinyl alcohol binder to form slurry, and spray dried to small ceramic particles (40–80 mesh, water volume 5%). After the ceramic particles were loaded into the mold and fully vibrated, the prefabricated ceramic parts were pressed for 5 min by a 60 MPa static press. The macroporous Ti4O7 ceramic-integrated electrode was successfully prepared by drying the ceramic preform and sintering it for 11 h in a vacuum at 1350 °C. The average pore size of the electrode surface was 2.6 μm, and the porosity was 21.6%. The electrode had excellent electrochemical performance, including ultra-high oxygen evolution potential, decent electrochemical stability, high electroactive surface, and weak adsorption performance.
In addition, Lin et al. [86] studied a new spark plasma sintering (SPS) process, in which a pulse current was directly applied between powder particles to heat and sintering. Firstly, 2g performed Ti4O7 powder was put into a graphite mold and vacuumed to less than 1 Pa. Start sintered at 1100 °C for 40 min, then rapid cooling in the vacuum to below 60 °C with maintaining sintering pressure is 5 MPa. Finally, the Ti4O7-integrated electrode was prepared. The characteristics of this process can be evenly heated with a high heating rate, low sintering temperature, and time, which could avoid the electrode surface cracks to improve the stability of the electrode.
Sasmita et al. [60] also successfully adopted the powder sintering method to successfully produce a titanium sub-oxides-integrated electrode. They mixed Ti4O7 powder and paraffin oil, pressed and formed under the action of a hydraulic press, and sintered at 1050 °C for 6 h in a H2 atmosphere. As shown in Figure 5c,d, the electrode surface is porous and granular, and the component is still Ti4O7.
The binder selection varies in different studies, but the principle is that the selected binder will volatilize entirely as far as possible in the operation process of high-temperature reduction. To sum up, a pure, stable, and uniformly integrated titanium sub-oxides electrode can be prepared by the powder sintering method; however, its application is limited to the shape and size. The cost is relatively high because the integrated titanium sub-oxides electrode with a large volume is not accessible to press and sinter.
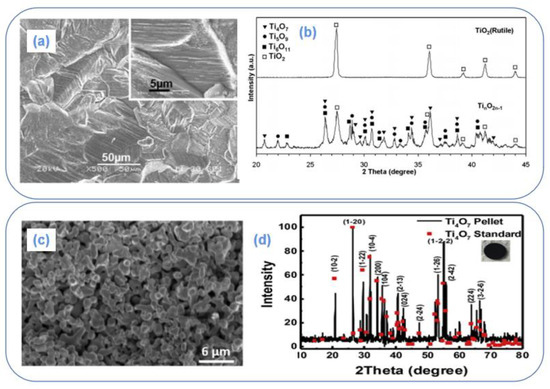
Figure 5.
XRD pattern of compression reduction method [84] (a); SEM image of compression reduction method [84] (b); XRD pattern of powder sintering method [60] (c); SEM figure of powder sintering electrode [60] (d).
2.3.3. Membrane Preparation by Hydrothermal Reduction Method
A tubular titanium dioxide membrane can also be used to synthesize a titanium sub-oxides electrode, where titanium dioxide will be reduced to Ti4O7 after hydrothermal treatment. The specific path is shown in Figure 4c. Guo et al. [85] used a tubular titanium dioxide ultrafiltration membrane and reduced it to Ti4O7 by a hydrothermal method in a tubular furnace at 1050 °C in a H2 atmosphere. With different treatment times, the main composition of the membrane also changed, and the phase change from Ti6O11 to Ti4O7 occurred from 30 h to 50 h. Based on this, Liang et al. [86] designed a set of tubular electrode assembly reactors using titanium oxide thin film, in which stainless steel tubes (SSP) were used as cathodes, and tubular titanium oxide film was placed in the center of the tubes as anodes, making full use of advantages such as the good stability and low internal resistance of thin-film electrodes. The optimal conditions for the catalytic degradation of methylene blue are as follows: the removal rate was close to 100% when the current density was 9 mA·cm−2 for 90 min.
Besides, Mitsuhiro et al. [87] further studied the preparation of Ti4O7 film by the gradual oxidation of titanium foil. Ti4O7 films with a thickness of 3 μm were successfully prepared on the surface of titanium foil by annealing in air and heating to 1173 K at low-oxygen partial pressure. The surface of the electrode was characterized by four layers of several hundred nanometer-sized equiaxed particles. The thin film has excellent electrocatalytic oxidation performance and can absorb light in visible and near-infrared regions, making it have specific application prospects in photocatalysis.
2.4. Titanium Sub-Oxides Composite Electrode
The composite electrode is also a recent research hotspot for electrode performance improvement. It can be mainly divided into titanium sub-oxides modified by other kinds of electrodes and titanium sub-oxides doped with other metals.
2.4.1. Titanium Oxide-Doped Composite Electrode
By doping Magnéli phase Ti4O7, the electrocatalytic activity and the stability and conductivity of specific electrode materials can be increased to some extent. Zhang et al. [88] prepared a Ru@Pt-type core–shell catalyst containing Ti4O7 (Ru@Pt/Ti4O7) by microwave pyrolysis. In this method, the mixture of the ruthenium precursor and TiO2 was heat-treated in a H2 atmosphere, Ru nuclei were obtained on the Ti4O7 carrier, and platinum shells were generated by microwave radiation. The characterization results showed that the catalytic material had a core–shell structure of Ru core and Pt shell, which significantly improved PT-Ru’s durability. Zhao et al. [89] synthesized a MoS2/Ti4O7 composite HER electrocatalyst by the hydrothermal method. Under the condition of 0.5 M H2SO4, the hydrogen evolution activity of the MoS2/Ti4O7 composite catalyst was significantly improved. The results presented that, with the addition of conductive carrier Ti4O7, the HER activity of MoS2 could be significantly enhanced by the formation of an interface SeO-Ti bond, thus improving the electrochemically active surface area fast charge transferability.
Wang et al. [90] compared the morphology, structure, and electrochemical properties of Ti4O7-modified LiNi0.8Co0.15Al0.05O2 (NCA) with the original NCA. Compared with the original one, the Ti4O7 modified NCA electrode exhibited better cycling performance and specific capacity thanks to its improved stability and conductivity. At the current density of 2000 mA·g−1, the electrode with 0.5 wt% Ti4O7 had a capacity of 159 mA·g−1, higher than the original electrode with a capacity of 94 mA·g−1. After 50 cycles, the capacity retention rate increased to 88% at 40 mA·g−1. Guo et al. [91] studied the morphology, crystal structure, electrochemical properties, electrocatalytic oxidation performance, and stability of a PbO2 electrode modified with different doses of Ti4O7 by the electrochemical deposition method. The results showed that Ti4O7 modification could significantly improve the surface morphology of the electrode, improve the current response of the electrode, and reduce the impedance of the electrode. Under 1.0g·L−1 as the best deposition amount, the decolorization rate of dye wastewater can reach 99%, and the TOC removal rate can reach 65%, in 180 min. The accelerated lifetime of the electrode was 175 h, 1.65 times longer than that of the PbO2 electrode (105.5 h). The above studies indicate that the composite electrode doped with Ti4O7 has a good application prospect.
2.4.2. Metals-Doped Composite Titanium Sub-Oxides Electrode
In addition, there are many types of research on the modification of titanium sub-oxides electrodes doped with other metals. For example, Linet et al. [86] doped a Ce3+ electrode preparation by the powder sintering method. Compared with the electrode before doping, the increased oxygen evolution potential and the reduced internal resistance of the charge transfer of the prepared electrode could be obtained, and the degradation effect of perfluorooctane sulfonic acid (PFOS) was further improved because the doping of Ce3+ resulted in more active sites on the electrode surface, which improved the rapid transfer of charge. Huang et al. [92] studied the effects of loaded crystalline and amorphous Pd on titanium sub-oxides electrodes prepared by plasma spraying technology. The results showed that the performance of electrodes doped with Pd was greatly improved. The effect of amorphous Pd was more obvious due to the disordered atomic arrangement of amorphous metals and the unsaturated coordination of amorphous metals. The amorphous metal formed a stronger interaction with the substrate, and this electron-metal interaction support (EMSI) enhanced electron transfer, which ultimately promoted the oxidation kinetics of the electrode. The above study demonstrated that doping Pt, Ce, Pd, and other active substances positively affects the performance of the titanium sub-oxides electrode, which means it is of further research interest.
3. Application of Titanium Sub-Oxides Electrode in Electrocatalytic Oxidation Wastewater
Compared with other commercial electrodes, including commercial graphite, stainless steel, and dimensional stability anode (DSA) electrodes, the electrochemical oxidation rate of the Ti4O7 anode was reported to be higher than that of other electrodes. Table 4 lists the performance comparison of several common electrodes with the Ti4O7 anode. The Ti4O7 anode has superior electrocatalytic activity, high oxygen evolution overpotential, and stable material properties that make it suitable for the electrochemical treatment of many pollutants, such as drugs, dyes, and phenolic organic matter (Table 5 lists the effects of titanium sub-oxide anode treatment on different organic matters).

Table 4.
Performance comparison of several common electrodes with Ti4O7 anode.

Table 5.
Titanium sub-oxides as electrode electro-oxidation to degrade organic matter.
3.1. Antibiotic Wastewater
Antibiotic pollutants have attracted attention due to high COD concentration, difficulty in biodegradation, and strong pollution. Titanium sub-oxide materials can play a strong role in the degradation of antibiotic pollutants.
Teng et al. [76] successfully prepared Ti/Ti4O7 electrodes using plasma spraying technology. They studied the electrochemical catalytic oxidation reaction of sulfadiazine (SDZ), a typical antibiotic, using the electrode as an electrode anode. The results showed that under the degradation conditions of 0.05 mol L−1 sodium sulfate, pH 6.33, and a current density of 10 mA·cm−2, the SDZ removal rate reached almost 100% after 60 min. It is demonstrated that the Ti/Ti4O7 anode had a high oxygen evolution potential and long-term stability in the treatment of actual pharmaceutical wastewater. Moreover, the network structure of the Ti/Ti4O7 anode can obtain a large electrochemically active surface area and improve the mass transfer between the electrolyte solution and anode.
Soliu et al. [77] also used plasma spraying technology to prepare Ti4O7 electrodes and compared it with several common commercial electrodes to study the electrochemical catalytic oxidation reaction of amoxicillin. The results revealed that, under low current intensity, the mineralization efficiency of the DSA and Pt anode was low. The TOC removal rate of the DSA and Pt anode was 36% and 41%, respectively. In comparison, the TOC removal rate of the Ti4O7 anode can reach 69%.
Wang et al. [102] evaluated the performance and complete pathway of tetracycline (TC) electrochemical oxidation on a Ti/Ti4O7 anode prepared by plasma spraying. The results showed that the electrochemical oxidation of TC on the Ti/Ti4O7 anode followed pseudo-first-order kinetics, and the TC removal efficiency can reach 95.8% in 40 min. The anode also had high stability, and the TC removal efficiency remained above 95% after five times of repeated use.
3.2. Dye Wastewater
Dye wastewater is also a significant field of electrocatalytic oxidation wastewater treatment technology. Wang et al. [98] successfully prepared titanium sub-oxides electrode material by spark plasma sintering technology and conducted electrocatalytic oxidation research on azo dye methyl orange (MO). The results showed that with the increase of the current density and the decrease of the initial concentration of MO, the removal rates of MO and COD presented an upward trend, and the complete removal of MO was achieved in a relatively short time. When the current density is 10 mA·cm−2 and the initial dye concentration is 100 mg·L−1, the COD removal rate can reach 91.7%. The degradation pathways of MO on the Ti4O7 electrode can be divided into two types: (1) the active substance first attacked the azo bond and the large conjugated system formed by benzene ring; (2) the active substance first attacked the azo bond and the C−N bond on the benzene ring. As the degradation reaction goes on, the final products are H2O and CO2.
Wang Yu [99] compared the carbon cloth and the titanium sub-oxides electrochemical oxidation device on the degradation of methylene blue decolorizing effects, and the results demonstrated that the reaction rate of the titanium sub-oxides anode was 3.7 times that of carbon cloth and the titanium sub-oxides electrode of methylene blue mineralization rate can reach 83.5%, but carbon cloth was only 42.6%. Therefore, the degradation of methylene blue by titanium sub-oxides was mainly mineralization. The experimental results showed that when the pH of the electrolyte is 3.01, the current density is 1.13 mA·cm−2, and the concentration is less than 100 mg·L−1; therefore, the electrochemical oxidation device of titanium sub-oxides can be operated with a better degradation rate and mineralization rate, higher Coulomb efficiency, and a lower reaction energy consumption.
3.3. Wastewater Containing Phenols
The refractory organic wastewater containing phenols has the characteristics of comprehensive sources, brutal treatment, and substantial destruction. At the same time, the titanium sub-oxides electrode can also achieve the effective degradation of phenolic pollutants. For example, Tan Yang [101] studied the electrochemical oxidation process of 4-chlorophenol wastewater by anodizing the titanium sub-oxides film material. The results showed that the target substance diffused faster at the titanium sub-oxides film electrode/solution interface compared with the BDD film electrode. When the initial concentration of 4-chlorophenol was 20 mg·L−1, the electrolyte solution, membrane flux, and current density were 0.04 mol·L−1 sodium sulfate solution, 0.023 mL·cm−2·s−1, and 5 mA·cm−2, respectively, and the removal rate of 4-chlorophenol can reach 100%. After 150 min, both COD and chromaticity can reach the discharge standard with low energy consumption.
In addition, Ping Geng [62] synthesized a pure Magnéli Ti4O7 nanotube array (NTA) by reducing lithium titanate with hydrogen at 850 °C. Compared with Ti4O7 particles and Ti4O7 NTA, the chemical oxygen demand (COD) removal rate of phenol increased by 20%. The degradation coefficient, COD removal rate, and current efficiency of pure Ti4O7 NTA were higher than those of boron-doped diamond and other Magnéli phase nanotube arrays.
Wang et al. [101] prepared three anodes (Ti/Ti4O7, Ti/Ti4O7-PbO2-Ce, and Ti/Ti4O7 nanotube array (NTA)), which were used to treat a p-nitrophenol (PNP) solution by electrocatalytic oxidation. After 30 min of treatment, the removal rate of PNP by Ti/Ti4O7 NTA and Ti/Ti4O7 was 89–92%, 10–60% higher than that of Ti/Pt, Ti/RuO2-IrO2, and Ti/ IrO2-Ta2O5, and equivalent to BDD (95%). In addition, Ti/Ti4O7 NTA and Ti/Ti4O7 have a decent mineralization effect on the PNP solution.
Maria et al. [103] successfully prepared Ti4O7-porous electrodes with a continuous layered structure. When the liquid was recirculated through the layered structure of the electrode, the performance was significantly improved. After 6 h of p-bendasone treatment, the removal rate was 85% and the mineralization rate was 57%, which was much better than the commercial electrode currently developed. Ti4O7 is a potential anode material for electrochemical oxidation due to its high efficiency and low energy consumption.
3.4. Treatment of Mixed Pollutants
The titanium sub-oxides electrode has sound degradation effects on single pollutants and is suitable for treating wastewater containing mixed contaminants.
Wang et al. [104] studied the feasibility and effectiveness of a titanium sub-oxides anode in simultaneously removing bacterial pathogens, antibiotics, and antibiotic resistance genes in water by using an integrated electrode prepared by high-temperature sintering. The results showed that the degradation rates of tetracycline (TC) and sulfamedimethazine (SDM) in the same treatment system reached more than 95% after 3 h of treatment. Further studies found that with the extension of treatment time the activity of bacterial pathogens was basically zero after 15 min, and the resistance genes were effectively removed. This was because the EO process produces a large amount of •OH, which can effectively attack pathogens, resulting in cell damage and reduction of cytoplasm content. This study confirmed the practical electrochemical degradation ability to mix medical wastewater in a titanium sub-oxides anode system.
Dan et al. [105] used the titanium sub-oxides-coated electrode prepared by plasma spraying technology as an anode and compared it with a Ti/RuO2-IrO2 anode to conduct the electrochemical treatment of coking wastewater (CW). The degradation results presented that the titanium sub-oxides electrode had apparent advantages in the COD removal rate, TOC removal rate, current efficiency, and energy consumption. The COD removal rate of CW was 78.7% and the TOC removal rate of CW was 50.3%, which were higher than that of the Ti/RuO2-IrO2 anode. In addition, the removal effects of the two electrodes for different kinds of PAHs in mixed wastewater were compared, where the titanium sub-oxides electrode had better performance for most organic compounds.
Pei et al. [61] employed a titanium sub-oxides membrane electrode to treat printing and dyeing industrial wastewater, which proved that the membrane electrode prepared by a high-temperature reduction method was mainly composed of Ti4O7 and a small amount of Ti5O9, with good conductivity, and high oxygen precipitation potential and electrochemical stability. When the current density was 8 mA·cm−2 and the membrane flux was 2.31 × 10−3 mL·cm−2·s−1, electrolysis for 1.5 h can effectively treat the actual printing and dyeing industrial wastewater. The COD removal rate was up to 96.07%, the current efficiency up to 24.22%, and the energy consumption was reduced by 32.99% compared with that without the membrane flux. It has important research value and development potential in small-scale decentralized industrial wastewater treatment.
3.5. Coupling Technology of Titanium Sub-Oxides Anodic Electrocatalytic Oxidation System
The titanium sub-oxides anode electrocatalytic oxidation system still cannot achieve the best effect for treating complex practical wastewater, which has the disadvantages of high energy consumption and low electronic utilization rate [106,107]. Therefore, the coupling application of electrocatalytic oxidation and other technologies has a promising prospect for development. The coupling technologies mainly focus on photoelectron-Fenton, electro-Fenton, and ultrasonic enhancement.
Han et al. [108] used a TF/Ti4O7 electrode prepared by loading titanium sub-oxides powder on the surface of titanium foam (TF) material as the anode and carbon with nitrogen-modified nickel foam (NF/CN) as the cathode (the experimental device is shown in Figure 6b). When the current density was 10 mA·cm−2 and the initial pH was 2, the removal rate of sulfamethazine (SMR) was 48.09% after the electrocatalytic reaction took 60 min. When the electro-Fenton anodic oxidation system constructed with NF/CN-TF/Ti4O7 degraded SMR again, the removal rate of 8 h could reach 99.48%, which was far better than that of the electrocatalytic system. The results showed that the electro-Fenton mechanism was the primary way to degrade pollutants in the electrochemical system, with a contributing rate of 71.60%. The coupling of the electro-Fenton and anodic oxidation system resulted in significant growth in the production of hydroxyl radicals, thus improving the degradation capacity of the whole system.
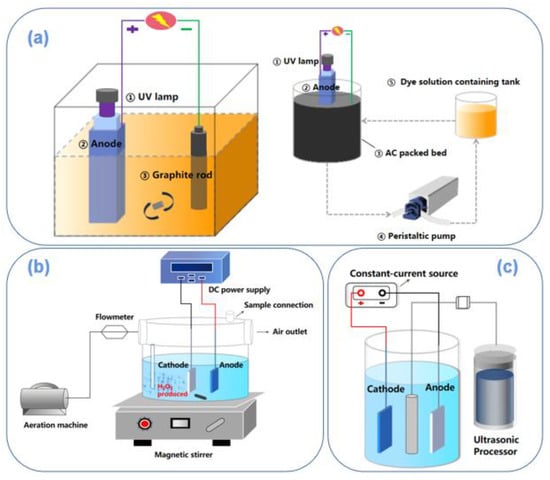
Figure 6.
Photoelectro-Fenton coupling device [108] (a); electro-Fenton coupling device [107] (b); ultrasonic strengthening coupling device (c).
Zwane et al. [109] also used electro-Fenton (EF)-, anodic oxidation (AO)-, and electro-Fenton-combined anodic oxidation (EF/AO) to degrade tetracycline on the titanium sub-oxides (Ti4O7) layer (anode) deposited on carbon felt (cathode)/titanium substrate. The EF/AO coupling system had the highest pollutant removal efficiency compared with EF and AO. When the initial concentration of tetracycline was 20 ppm and 50 ppm, the removal rate of total organic carbon was 69 ± 1% and 68 ± 1%, respectively. The reason was that the EF/AO process with Ti4O7 as anode and CF as a cathode can generate hydroxyl radicals on the surface of the inactive Ti4O7 electrode and the electro-Fenton process solution on the cathode carbon blanket, which could reduce the energy consumption and improve the removal efficiency of tetracycline. It was observed from HPLC data that the optimal conditions for 20 ppm and 50 ppm tetracycline concentrations were 120 mA and 210 mA, with complete removal of tetracycline after 30 min.
Estrada et al. [110] studied an embedded activated carbon-filled bed cathode and a TiO2/Ti4O7 photoelectric anode (the experimental device is shown in Figure 6a). The experimental results of the photocatalysis (PC), electrooxidation (EO), and photoelectrochemical (PEC) of the methyl orange (Mo) model dye solution showed that TiO2/Ti4O7 composite film combined the advantages of the two materials so that anatase TiO2 had better photocatalytic performance. The partially reduced Ti4O7 had higher conductivity, thus promoting the effective decolorization of the dye solution, and the decolorization rate was close to 70% within 60 min. Based on simulating calculations of (1) photoelectric chemicals-induced discoloration on the surface of the anode (electric catalytic oxidation and light contribution), (2) the adsorption and desorption process of dye molecules on the surface of activated carbon particles and pores, and the (3) Fenton-induced decolorization of the dye on the surface of the cathode existing in the system, it was concluded that the surface of the anode photoelectric catalytic contribution was up to 54%. Technical coupling [111] can improve the yield and utilization rate of hydroxyl radicals in the system. The Fenton process can also avoid electron quenching in the degradation process and reduce the energy consumption. In addition, it was proposed that further optimization of the area/volume ratio of the membrane to the reactor can improve the removal efficiency of target pollutants.
Yang et al. [112] used a Ti4O7-plate electrode as an anode prepared by plasma spraying, through coupling ultrasonic strengthening, and the electrical catalytic degradation of chloramphenicol (CAP) wastewater was conducted (experimental research device, as shown in Figure 6c). The results confirmed that under the condition of the ultrasonic, targets in the titanium sub-oxides film electrode/solution interface diffused faster, improving the efficiency of mass transfer. When the initial concentration of chloramphenicol was 20 mg·L−1, the electrolyte solution, ultrasonic power, current density, and pH were 0.05 mol·L−1 sodium sulfate solution, 150 W, 20 mA·cm−2, and 5, respectively, and the removal rate of chloramphenicol could reach 100%. The degradation mechanism was direct electrochemical oxidation induced by •OH and the indirect electrooxidation of Cl−/ClO− intermediates.
4. Summary and Outlook
Titanium sub-oxide materials are considered a candidate for the electrode with great potential in electrocatalytic oxidation due to their excellent electrochemical performance. At present, some achievements have been made in the research of titanium sub-oxides in electrode preparation and wastewater treatment. A variety of stable electrode preparation technology routes were developed to be applied in the treatment process of wastewater with various contaminants, further coupling with other technologies to enhance the treatment efficiency. However, there are still great challenges in the research process, where the current preparation process is still cumbersome and inefficient, the conditions for scaling-up production are not available, the stability of the electrode needs to be improved, and the degradation effect of the complex water system is still to be studied. Thus, the future development direction of titanium sub-oxide materials can be focused on the following aspects:
- (1)
- Optimization of preparation process: The current relatively stable preparation route still has the disadvantages of high energy consumption requirements, high temperature, and low efficiency, in which high-temperature calcination is prone to agglomeration. Therefore, it is necessary to further optimize the preparation route at high temperatures to prepare relatively pure titanium sub-oxides electrodes with high activity. In addition, the medium- and low-temperature synthesis should be further explored to avoid the disadvantages resulting from high temperatures and reduce the preparation costs;
- (2)
- Modification of electrode materials: Studies show that the performance of electrodes can be significantly promoted by doping with highly active metal elements, and its degradation mechanisms need further elucidation. By identifying the mechanisms, the titanium sub-oxide electrode can be further modified and optimized to retain its excellent electrochemical performance. The original defects are to be made up by doping and other technical means to develop high-efficiency and low-consumption electrode products;
- (3)
- Application expansion: The performance of titanium sub-oxides is not limited to electrocatalysis. Combining an electrocatalytic oxidation system and other technical means can better develop the hidden performance of the electrode to achieve twice the result with half the effort. In addition, the effective construction of an electrocatalytic oxidation system can also make the electrode material fulfill the maximum function;
- (4)
- Principles exploration: Titanium sub-oxides are a kind of material with excellent stability. Therefore, it is of great importance to explore the deactivation mechanism of electrodes for prolonging electrode life. In the electrocatalytic oxidation system, the effects of different impurity ions on the electrode and the synergistic degradation principle of complex pollutants demand to be further studied.
Author Contributions
S.G.: conceptualization, investigation, writing—original draft; Z.X.: writing-reviewing and editing, investigation; W.H.: investigation; D.Y.: writing—reviewing and editing; X.W.: investigation; H.X.: conceptualization, writing—reviewing and editing, resources, funding acquisition; X.X. and Z.L.: resources, investigation; W.Y.: supervision, reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [the Natural Science Basic Research Plan in the Shaanxi Province of China] grant number [2021JM-012], [the Welfare Technology Research Plan of Zhejiang Province] grant number [Program No. LZY21E080003]. and [the Fundamental Research Funds for the Central Universities] grant number [Program No. xjh012020037].
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Panizza, M.; Michaud, P.-A.; Iniesta, J.; Comninellis, C.; Cerisola, G. Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim. Acta 2001, 46, 3573–3578. [Google Scholar]
- Lei, J.; Xu, Z.; Xu, H.; Qiao, D.; Liao, Z.; Yan, W.; Wang, Y. Pulsed electrochemical oxidation of acid Red G and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773. [Google Scholar]
- Zhang, Y.; Zhang, C.; Shao, D.; Xu, H.; Rao, Y.; Tan, G.; Yan, W. Magnetically assembled electrodes based on Pt, RuO2-IrO2-TiO2 and Sb-SnO2 for electrochemical oxidation of wastewater featured by fluctuant Cl− concentration. J. Hazard. Mater. 2022, 421, 126803. [Google Scholar] [CrossRef]
- Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination 2006, 190, 201–211. [Google Scholar] [CrossRef]
- Dargahi, A.; Shokoohi, R.; Asgari, G.; Ansari, A.; Nematollahi, D.; Samarghandi, M.R. Moving-bed biofilm reactor combined with three-dimensional electrochemical pretreatment (MBBR-3DE) for 2,4-D herbicide treatment: Application for real wastewater, improvement of biodegradability. RSC Adv. 2021, 11, 9608–9620. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Li, A.; Weng, J.; Yan, X.; Li, H.; Shi, H.; Wu, X. Electrochemical oxidation of acid orange 74 using Ru, IrO2, PbO2, and boron doped diamond anodes: Direct and indirect oxidation. J. Electroanal. Chem. 2021, 898, 115622. [Google Scholar] [CrossRef]
- Kirk, D.W.; Sharifian, H.; Foulkes, F.R. Anodic oxidation of aniline for waste water treatment. J. Appl. Electrochem. 1985, 15, 285–292. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhou, L.; Zhu, H.; Wan, F.; Wang, Y.; Zhang, D. Performance of nitrogen-doped graphene aerogel particle electrodes for electro-catalytic oxidation of simulated Bisphenol A wastewaters. J. Hazard. Mater. 2017, 332, 70–78. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Xu, H.; Qiao, D.; Xu, Z.; Guo, H.; Chen, S.; Xu, X.; Gao, X.; Yan, W. Application of electro-catalytic oxidation technology in organic wastewater treatment. Ind. Water Treat. 2021, 41, 1–9. (In Chinese) [Google Scholar]
- Liu, Y.; Liu, H. Comparative studies on the electrocatalytic properties of modified PbO2 anodes. Electrochim. Acta 2008, 53, 5077–5083. [Google Scholar] [CrossRef]
- Patel, P.S.; Bandre, N.; Saraf, A.; Ruparelia, J.P. Electro-catalytic Materials (Electrode Materials) in Electrochemical Wastewater Treatment. Procedia Eng. 2013, 51, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Ignasi, S. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 8336–8367. [Google Scholar]
- Ihara, I.; Umetsu, K.; Kanamura, K.; Watanabe, T. Electrochemical oxidation of the effluent from anaerobic digestion of dairy manure. Bioresour. Technol. 2006, 97, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.; Yang, B.; Deng, S.; Huang, J.; Wang, B.; Yu, G. Electrochemical Anodic Materials Used for Degradation of Organic Pollutants. Prog. Chem. 2012, 24, 628–636. (In Chinese) [Google Scholar]
- Dargahi, A.; Hasani, K.; Mokhtari, S.A.; Vosoughi, M.; Moradi, M.; Vaziri, Y. Highly effective degradation of 2,4-Dichlorophenoxyacetic acid herbicide in a three-dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J. Environ. Chem. Eng. 2021, 9, 105889. [Google Scholar] [CrossRef]
- Salazar-Banda, G.R.; Santos, G.d.O.S.; Duarte Gonzaga, I.M.; Dória, A.R.; Barrios Eguiluz, K.I. Developments in electrode materials for wastewater treatment. Curr. Opin. Electrochem. 2021, 26, 100663. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Anodic oxidation of phenol for waste water treatment. J. Appl. Electrochem. 1991, 21, 703–708. [Google Scholar] [CrossRef]
- Bai, H.; He, P.; Pan, J.; Chen, J.; Chen, Y.; Dong, F.; Li, H. Boron-doped diamond electrode: Preparation, characterization and application for electrocatalytic degradation of m-dinitrobenzene. J. Colloid Interface Sci. 2017, 497, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Zhao, G.; Li, P.; Nong, F.; Li, M.; Gao, J.; Li, D. Construction and High Performance of a Novel Modified Boron-Doped Diamond Film Electrode Endowed with Superior Electrocatalysis. J. Phys. Chem. C 2010, 114, 5906–5913. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, C.; Yu, G.; Zhuo, Q.; Deng, S.; Wu, J.; Zhang, H. Highly efficient electrochemical degradation of perfluorooctanoic acid (PFOA) by F-doped Ti/SnO2 electrode. J. Hazard. Mater. 2015, 299, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Salman, N.M.; Guo, H.; Xu, Z.; Xu, H.; Yan, W.; Liao, Z.; Wang, Y. A 2.5D Electrode System Constructed of Magnetic Sb-SnO2 Particles and a PbO2 Electrode and Its Electrocatalysis Application on Acid Red G Degradation. Catalysts 2019, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhu, K.; Xu, H.; Yan, W. Electrochemical oxidation of rhodamine B by PbO2/Sb-SnO2/TiO2 nanotube arrays electrode. Chin. J. Catal. 2019, 40, 917–927. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Sun, Y.; Jiang, S.; Shi, L.; Bi, Q.; Xue, J. Preparation of a novel Ni/Sb co-doped Ti/SnO2 electrode with carbon nanotubes as growth template by electrodeposition in a deep eutectic solvent. J. Electroanal. Chem. 2022, 911, 116225. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Dimou, A.; Mantzavinos, D.; Katsaounis, A. Electrochemical oxidation of model compounds and olive mill wastewater over DSA electrodes: 1. The case of Ti/IrO2 anode. J. Hazard. Mater. 2009, 167, 268–274. [Google Scholar] [CrossRef]
- Qu, J.P.; Zhang, X.G.; Wang, Y.G.; Xie, C.X. Electrochemical reduction of CO2 on RuO2/TiO2 nanotubes composite modified Pt electrode. Electrochim. Acta 2005, 50, 3576–3580. [Google Scholar] [CrossRef]
- Samet, Y.; Elaoud, S.C.; Ammar, S.; Abdelhedi, R. Electrochemical degradation of 4-chloroguaiacol for wastewater treatment using PbO2 anodes. J. Hazard. Mater. 2006, 138, 614–619. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Z.; Qiao, D.; Wan, D.; Xu, H.; Yan, W.; Jin, X. Fabrication and characterization of porous titanium-based PbO2 electrode through the pulse electrodeposition method: Deposition condition optimization by orthogonal experiment. Chemosphere 2020, 261, 128157. [Google Scholar]
- Zhang, X.; Shao, D.; Lyu, W.; Xu, H.; Yang, L.; Zhang, Y.; Wang, Z.; Liu, P.; Yan, W.; Tan, G. Design of magnetically assembled electrode (MAE) with Ti/PbO2 and heterogeneous auxiliary electrodes (AEs): The functionality of AEs for efficient electrochemical oxidation. Chem. Eng. J. 2020, 395, 125145. [Google Scholar] [CrossRef]
- Shao, D.; Wang, Z.; Zhang, C.; Li, W.; Xu, H.; Tan, G.; Yan, W. Embedding wasted hairs in Ti/PbO2 anode for efficient and sustainable electrochemical oxidation of organic wastewater. Chin. Chem. Lett. 2022, 33, 1288–1292. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Ansari, A.; Dargahi, A.; Shabanloo, A.; Nematollahi, D.; Khazaei, M.; Nasab, H.Z.; Vaziri, Y. Enhanced electrocatalytic degradation of bisphenol A by graphite/beta-PbO2 anode in a three-dimensional electrochemical reactor. J. Environ. Chem. Eng. 2021, 9, 106072. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Jiang, C.; Li, J.; Yu, G.; Deng, S.; Lu, S.; Zhang, P.; Zhu, C.; Zhuo, Q. Electrochemical mineralization of perfluorooctane sulfonate by novel F and Sb co-doped Ti/SnO2 electrode containing Sn-Sb interlayer. Chem. Eng. J. 2017, 316, 296–304. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Liu, H.; Niu, J. Highly efficient and stable Zr-doped nanocrystalline PbO2 electrode for mineralization of perfluorooctanoic acid in a sequential treatment system. Sci. Total Environ. 2017, 579, 1600–1607. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Fan, X.; Zhang, Z.; Dou, R.; Wen, S.; Chen, Y.; Chen, Y.; Hu, Y. Preparation and Characterization of a Pd modified Ti/SnO2-Sb anode and its electrochemical degradation of Ni-EDTA. Electrochim. Acta 2017, 231, 354–362. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Cao, X.; Wang, D.; Wei, J. Facile preparation of a Ti/α-PbO2/β-PbO2 electrode for the electrochemical degradation of 2-chlorophenol. Chin. J. Catal. 2015, 36, 975–981. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.-H.; Hu, Z.-T.; He, C.; Lim, T.-T. High performance duplex-structured SnO2-Sb-CNT composite anode for bisphenol A removal. Sep. Purif. Technol. 2017, 179, 25–35. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, H.; Niu, J.; Zhou, Y.; Wang, C.; Wang, Y. Hydroxyl multi-walled carbon nanotube-modified nanocrystalline PbO2 anode for removal of pyridine from wastewater. J. Hazard. Mater. 2017, 327, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liang, G.; Yin, M. A promising electrode material modified by Nb-doped TiO2 nanotubes for electrochemical degradation of AR 73. Chemosphere 2017, 173, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jin, T.; Hu, Z.; Zhou, L.; Zhou, M. TiO2-NTs/SnO2-Sb anode for efficient electrocatalytic degradation of organic pollutants: Effect of TiO2-NTs architecture. Sep. Purif. Technol. 2013, 102, 180–186. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, Y.; Lyu, W.; Zhang, X.; Tan, G.; Xu, H.; Yan, W. A modular functionalized anode for efficient electrochemical oxidation of wastewater: Inseparable synergy between OER anode and its magnetic auxiliary electrodes. J. Hazard. Mater. 2020, 390, 122174. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, W.; Yang, H. Surface Analysis of Ti/Sb-SnO2/PbO2 Electrode after Long Time Electrolysis. Rare Met. Mater. Eng. 2015, 44, 2637–2641. [Google Scholar]
- Guo, Y.; Xu, Z.; Guo, S.; Chen, S.; Xu, H.; Xu, X.; Gao, X.; Yan, W. Selection of anode materials and optimization of operating parameters for electrochemical water descaling. Sep. Purif. Technol. 2021, 261, 118304. [Google Scholar] [CrossRef]
- Xu, H.; Xu, Z.; Guo, Y.; Guo, S.; Xu, X.; Gao, X.; Wang, L.; Yan, W. Research and application progress of electrochemical water quality stabilization technology for recirculating cooling water in China: A short review. J. Water Process Eng. 2020, 37, 101433. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Z.; Wang, D.; Chen, S.; Qiao, D.; Wan, D.; Xu, H.; Yan, W.; Jin, X. Evaluation of diclofenac degradation effect in “active” and “non-active” anodes: A new consideration about mineralization inclination. Chemosphere 2022, 286, 131580. [Google Scholar] [CrossRef]
- Xing, X.; Ni, J.; Zhu, X.; Jiang, Y.; Xia, J. Maximization of current efficiency for organic pollutants oxidation at BDD, Ti/SnO2-Sb/PbO2, and Ti/SnO2-Sb anodes. Chemosphere 2018, 205, 361–368. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Chen, J.; Niu, J. Electrochemical degradation of sunscreen agent benzophenone-3 and its metabolite by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, mechanism, toxicity and energy consumption. Sci. Total Environ. 2019, 688, 75–82. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P.; Singh, N. Electro-oxidation of amoxicillin trihydrate in continuous reactor by Ti/RuO2 anode. Sci. Total Environ. 2019, 677, 84–97. [Google Scholar] [CrossRef]
- Duan, P.; Hu, X.; Ji, Z.; Yang, X.; Sun, Z. Enhanced oxidation potential of Ti/SnO2-Cu electrode for electrochemical degradation of low-concentration ceftazidime in aqueous solution: Performance and degradation pathway. Chemosphere 2018, 212, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Feng, J.; Fan, S.; Zhou, W.; Dai, Q. Fabrication of a multi-layer CNT-PbO2 anode for the degradation of isoniazid: Kinetics and mechanism. Chemosphere 2021, 263, 128069. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, H.; Feng, J.; Wang, D.; Liao, Z.; Wang, Y.; Wei, Y. Electrochemical Oxidation Combined with Adsorption: A Novel Route for Low Concentration Organic Wastewater Treatment. Int. J. Electrochem. Sci. 2019, 14, 8110–8120. [Google Scholar]
- Kong, D.; Lue, W.; Feng, Y.; Bi, S. Advances and Some Problems in Electrocatalysis of DSA Electrodes. Prog. Chem. 2009, 21, 1107–1117. (In Chinese) [Google Scholar]
- Tang, C.; Zhou, D.; Zhang, Q. Synthesis and characterization of Magnéli phases: Reduction of TiO2 in a decomposed NH3 atmosphere. Mater. Lett. 2012, 79, 42–44. [Google Scholar] [CrossRef]
- Geng, P.; Chen, G. Antifouling ceramic membrane electrode modified by Magnéli Ti4O7 for electro-microfiltration of humic acid. Sep. Purif. Technol. 2017, 185, 61–71. [Google Scholar] [CrossRef]
- Smith, J.R.; Walsh, F.C.; Clarke, R.L. Reviews in applied electrochemistry. Number 50—Electrodes based on Magnéli phase titanium oxides: The properties and applications of Ebonex (R) materials. J. Appl. Electrochem. 1998, 28, 1021–1033. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex (R) electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Pei, S.; Teng, J.; Ren, N.; You, S. Low-Temperature Removal of Refractory Organic Pollutants by Electrochemical Oxidation: Role of Interfacial Joule Heating Effect. Environ. Sci. Technol. 2020, 54, 4573–4582. [Google Scholar] [CrossRef]
- Nayak, S.; Chaplin, B.P. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes. Electrochim. Acta 2018, 263, 299–310. [Google Scholar] [CrossRef]
- Pei, S.; Zhu, L.; Zhang, Z.; Teng, J.; Liu, X.; You, S. Electrochemical properties of titanium sub-oxide membrane electrode and application for electro-oxidation treatment of dyeing wastewater. Acta Sci. Circumstantiae 2020, 40, 3658–3665. (In Chinese) [Google Scholar]
- Geng, P.; Su, J.; Miles, C.; Comninellis, C.; Chen, G. Highly-Ordered Magnéli Ti4O7 Nanotube Arrays as Effective Anodic Material for Electro-oxidation. Electrochim. Acta 2015, 153, 316–324. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Shen, C.; Zhang, C.; Ren, N.; You, S. Characterization of the Interfacial Joule Heating Effect in the Electrochemical Advanced Oxidation Process. Environ. Sci. Technol. 2019, 53, 4406–4415. [Google Scholar] [CrossRef]
- Xu, B.; Sohn, H.Y.; Mohassab, Y.; Lan, Y. Structures, preparation and applications of titanium suboxides. RSC Adv. 2016, 6, 79706–79722. [Google Scholar] [CrossRef]
- Malik, H.; Sarkar, S.; Mohanty, S.; Carlson, K. Modelling and synthesis of Magnéli Phases in ordered titanium oxide nanotubes with preserved morphology. Sci. Rep. 2020, 10, 8050. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ye, J.; Zhu, R. Fabrication and characterisation of Magnéli phase Ti4O7 nanoparticles. Micro Nano Lett. 2013, 8, 251–253. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, D.; Sohn, H.Y.; Mohassab, Y.; Yang, B.; Lan, Y.; Yang, J. Flash synthesis of Magnéli phase (TinO2n−1) nanoparticles by thermal plasma treatment of H2TiO3. Ceram. Int. 2018, 44, 3929–3936. [Google Scholar] [CrossRef]
- Han, W.-Q.; Wang, X.-L. Carbon-coated Magnéli-phase TinO2n-1 nanobelts as anodes for Li-ion batteries and hybrid electrochemical cells. Appl. Phys. Lett. 2010, 97, 243104. [Google Scholar] [CrossRef] [Green Version]
- Conze, S.; Veremchuk, I.; Reibold, M.; Matthey, B.; Michaelis, A.; Grin, Y.; Kinski, I. Magnéli phases Ti4O7 and Ti8O15 and their carbon nanocomposites via the thermal decomposition-precursor route. J. Solid State Chem. 2015, 229, 235–242. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Qiu, W. Synthesis, microstructural characterization, and electrochemical performance of novel rod-like Ti4O7 powders. J. Alloy. Compd. 2017, 704, 18–25. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of carbon-coated Magnéli phases TinO2n−1 and their photocatalytic activity under visible light. Appl. Catal. B-Environ. 2009, 88, 160–164. [Google Scholar] [CrossRef]
- You, S.; Liu, B.; Gao, Y.; Wang, Y.; Tang, C.Y.; Huang, Y.; Ren, N. Monolithic Porous Magnéli-phase Ti4O7 for Electro-oxidation Treatment of Industrial Wastewater. Electrochim. Acta 2016, 214, 326–335. [Google Scholar] [CrossRef]
- Gusev, A.; Avvakumov, E.; Medvedev, A.; Masliy, A. Ceramic electrodes based on Magnéli phases of titanium oxides. Sci. Sinter. 2007, 39, 51–57. [Google Scholar] [CrossRef]
- Hauf, C.; Kniep, R.; Pfaff, G. Preparation of various titanium suboxide powders by reduction of TiO2 with silicon. J. Mater. Sci. 1999, 34, 1287–1292. [Google Scholar] [CrossRef]
- Teng, J.; Liu, G.; Liang, J.; You, S. Electrochemical oxidation of sulfadiazine with titanium suboxide mesh anode. Electrochim. Acta 2019, 331, 135441. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Esmilaire, R.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: A case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res. 2016, 106, 171–182. [Google Scholar] [CrossRef]
- Han, Z.; Xu, Y.; Zhou, S.; Zhu, P. Preparation and electrochemical properties of Al-based composite coating electrode with Ti4O7 ceramic interlayer for electrowinning of nonferrous metals. Electrochimica Acta 2019, 325, 134940. [Google Scholar] [CrossRef]
- Geng, P.; Chen, G. Magnéli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property. J. Membr. Sci. 2016, 498, 302–314. [Google Scholar] [CrossRef]
- Wong, M.-S.; Lin, Y.-J.; Pylnev, M.; Kang, W.-Z. Processing, structure and properties of reactively sputtered films of titanium dioxide and suboxides. Thin Solid Films 2019, 688, 137351. [Google Scholar] [CrossRef]
- Li, H.; Lu, S.; Li, Y.; Qin, W.; Wu, X. Tunable thermo-optical performance promoted by temperature selective sputtering of titanium oxide on MgO-ZrO2 coating. J. Alloy. Compd. 2017, 709, 104–111. [Google Scholar] [CrossRef]
- Ertekin, Z.; Tamer, K.; Pekmez, U. Cathodic electrochemical deposition of Magnéli phases TinO2n-1 thin films at different temperatures in acetonitrile solution. Electrochim. Acta 2015, 163, 77–81. [Google Scholar] [CrossRef]
- Paunović, P.; Popovski, O.; Fidančevska, E.; Ranguelov, B.; Gogovska, D.S.; Dimitrov, A.T.; Jordanov, S.H. Co-Magnéli phases electrocatalysts for hydrogen/oxygen evolution. Int. J. Hydrogen Energy 2010, 35, 10073–10080. [Google Scholar] [CrossRef]
- Regonini, D.; Adamaki, V.; Bowen, C.R.; Pennock, S.R.; Taylor, J.; Dent, A.C.E. AC electrical properties of TiO2 and Magnéli phases, TinO2n−1. Solid State Ion. 2012, 229, 38–44. [Google Scholar] [CrossRef]
- Guo, L.; Jing, Y.; Chaplin, B.P. Development and Characterization of Ultrafiltration TiO2 Magnéli Phase Reactive Electrochemical Membranes. Environ. Sci. Technol. 2016, 50, 1428–1436. [Google Scholar] [CrossRef]
- Liang, J.; You, S.; Yuan, Y.; Yuan, Y. A tubular electrode assembly reactor for enhanced electrochemical wastewater treatment with a Magnéli-phase titanium suboxide (M-TiSO) anode and in situ utilization. RSC Adv. 2021, 11, 24976–24984. [Google Scholar] [CrossRef]
- Matsuda, M.; Yamada, Y.; Himeno, Y.; Shida, K.; Mitsuhara, M.; Matsuda, M. Magnéli Ti4O7 thin film produced by stepwise oxidation of titanium metal foil. Scr. Mater. 2021, 198, 113829. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, J.; Zhang, J.; Nan, F.; Gauquelin, N.; Botton, G.A.; He, P.; Bashyam, R.; Knights, S. Ti4O7 supported Ru@Pt core-shell catalyst for CO-tolerance in PEM fuel cell hydrogen oxidation reaction. Appl. Energy 2013, 103, 507–513. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Wu, S.; Xu, F.; Du, J.; Li, J.; Li, K.; Ren, J.; Zhao, Y. Strong interfacial interaction significantly improving hydrogen evolution reaction performances of MoS2/Ti4O7 composite catalysts. Electrochim. Acta 2020, 337, 135850. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Zhang, K.; Li, Y.; Xu, B.; Liang, F.; Dai, Y.; Yao, Y. Enhancing the rate performance of high-capacity LiNi(0.8)Co(0.15)Al(0.05)O(2) cathode materials by using Ti4O7 as a conductive additive. J. Energy Storage 2020, 28, 101182. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Z.; Qiao, D.; Wang, L.; Xu, H.; Yan, W. Fabrication and characterization of titanium-based lead dioxide electrode by electrochemical deposition with Ti4O7 particles. Water Environ. Res. 2021, 93, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, K.; Niu, J.; Chu, C.; Weon, S.; Zhu, Q.; Lu, J.; Stavitski, E.; Kim, J.-H. Amorphous Pd-Loaded Ti4O7 Electrode for Direct Anodic Destruction of Perfluorooctanoic Acid. Environ. Sci. Technol. 2020, 54, 10954–10963. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Feng, Y.; Liu, J. Comparison of electrocatalytic performance of different anodes with cyclic voltammetry and Tafel curves. Chin. J. Catal. 2007, 28, 646–650. (In Chinese) [Google Scholar]
- Zhang, Y.; Zhang, R.; Ma, J.; Liu, L. Advanced Electrochemical Oxidation Process with BDD Electrodes for Organic Wastewater Treatment. China Water Wastewater 2006, 22, 15–18. (In Chinese) [Google Scholar]
- Wei, Z.; Kang, X.; Xu, S.; Zhou, X.; Jia, B.; Feng, Q. Electrochemical oxidation of Rhodamine B with cerium and sodium dodecyl benzene sulfonate co-modified Ti/PbO2 electrodes: Preparation, characterization, optimization, application. Chin. J. Chem. Eng. 2021, 32, 191–202. [Google Scholar] [CrossRef]
- Qiao, D.; Xu, Z.; Guo, H.; Wang, X.; Wan, D.; Li, X.; Xu, H.; Yan, W. Non-traditional power supply mode: Investigation of electrodeposition towards a better understanding of PbO2 electrode for electrochemical wastewater treatment. Mater. Chem. Phys. 2022, 284, 126066. [Google Scholar] [CrossRef]
- Han, J. Degradation of Sulfamerazine by NF/CNTF/Ti4O7 Electrochemical System and its Mechanism. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2019. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Lin, Z.; Yang, X. Electrochemical oxidation of methyl orange by a Magnéli phase Ti4O7 anode. Chemosphere 2019, 241, 125084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Anodic Oxidation for Degradation of Dyeing Wastewater in Eleochemical Systems with Titanium Sub-Oxide Anode. Harbin Inst. Technol. 2016. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201701&filename=1016913685.nh&uniplatform=NZKPT&v=dl01S0dpi5oBa4sLKxvjDs9DEAi3BmuNaVpj_rsz9T2dfFtl53U0KdpUdR80Ru5s (accessed on 8 May 2022).
- Wang, J. Preparation of Titanium Sub-Oxide Electrode and Research on Degradation Efficiency of Phenol Wastewater by Three-dimensional Electrode System. Harbin Inst. Technol. 2020. [Google Scholar] [CrossRef]
- Tan, Y. Magnéli-phase Ti4O7 Conductive Membrane for Effective Electrochemical Degradation of 4-Chlorophenol Wastewater. Harbin Inst. Technol. 2018. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201901&filename=1018894521.nh&uniplatform=NZKPT&v=NX9XUuHDAefm1QlL7hWOo6L9Dfcwokx8EQsfUQ5NPnf6Wqe-A6luvnTUcpYkTcg4 (accessed on 8 May 2022).
- Wang, J.; Zhi, D.; Zhou, H.; He, X.; Zhang, D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Zhang, F.; Wang, Y.; Zhang, X.; Wang, J.; He, X. Comparison of Ti/Ti4O7, Ti/Ti4O7-PbO2-Ce, and Ti/Ti4O7 nanotube array anodes for electro-oxidation of p-nitrophenol and real wastewater. Sep. Purif. Technol. 2021, 266, 118600. [Google Scholar] [CrossRef]
- Barni, M.F.S.; Doumic, L.I.; Procaccini, R.A.; Ayude, M.A.; Romeo, H.E. Layered platforms of Ti4O7 as flow-through anodes for intensifying the electro-oxidation of bentazon. J. Environ. Manag. 2020, 263, 110403. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, H.; Habteselassie, M.Y.; Deng, X.; Teng, Y.; Wang, Y.; Huang, Q. Simultaneous removal of multidrug-resistant Salmonella enterica serotype typhimurium, antibiotics and antibiotic resistance genes from water by electrooxidation on a Magnéli phase Ti4O7 anode. Chem. Eng. J. 2021, 407, 127134. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, J.; Wang, J.; Luo, L.; Zhou, Y.; Zhou, Y. Electrochemical treatments of coking wastewater and coal gasification wastewater with Ti/Ti4O7 and Ti/RuO2-IrO2 anodes. J. Environ. Manag. 2020, 265, 110571. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, Z.; Guo, S.; Liu, J.; Xu, H.; Xu, X.; Gao, X.; Yan, W. Practical optimization of scale removal in circulating cooling water: Electrochemical descaling-filtration crystallization coupled system. Sep. Purif. Technol. 2022, 284, 120268. [Google Scholar]
- Liang, S.; Lin, H.; Yan, X.; Huang, Q. Electro-oxidation of tetracycline by a Magnéli phase Ti4O7 porous anode: Kinetics, products, and toxicity. Chem. Eng. J. 2018, 332, 628–636. [Google Scholar] [CrossRef]
- Zwane, B.N.; Orimolade, B.O.; Koiki, B.A.; Mabuba, N.; Gomri, C.; Petit, E.; Bonniol, V.; Lesage, G.; Rivallin, M.; Cretin, M.; et al. Combined Electro-Fenton and Anodic Oxidation Processes at a Sub-Stoichiometric Titanium Oxide (Ti4O7) Ceramic Electrode for the Degradation of Tetracycline in Water. Water 2021, 13, 2772. [Google Scholar] [CrossRef]
- Becerril-Estrada, V.; Robles, I.; Martinez-Sanchez, C.; Godinez, L.A. Study of TiO2/Ti4O7 photo-anodes inserted in an activated carbon packed bed cathode: Towards the development of 3D-type photo-electro-Fenton reactors for water treatment. Electrochim. Acta 2020, 340, 135972–135981. [Google Scholar] [CrossRef]
- Safajou, H.; Khojasteh, H.; Salavati-Niasari, M.; Mortazavi-Derazkola, S. Enhanced photocatalytic degradation of dyes over graphene/Pd/TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles. J. Colloid Interface Sci. 2017, 498, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. Ultrasound Enhanced Electrochemical Oxidation of Chloramphenicol Wastewater with Titanium Sub-Oxide Anode. Harbin Inst. Technol. 2019. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).