Abstract
Ethyl esters of omega-3 fatty acids are active pharmaceutical ingredients used for the reduction in triglycerides in the treatment of hyperlipidemia. Herein, an ultrasonic packed-bed bioreactor was developed for continuous production of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) ethyl esters from DHA+EPA concentrate and ethyl acetate (EA) using an immobilized lipase, Novozym® 435, as a biocatalyst. A three-level–two-factor central composite design combined with a response surface methodology (RSM) was employed to evaluate the packed-bed bioreactor with or without ultrasonication on the conversion of DHA + EPA ethyl ester. The highest conversion of 99% was achieved with ultrasonication at the condition of 1 mL min−1 flow rate and 100 mM DHA + EPA concentration. Our results also showed that the ultrasonic packed-bed bioreactor has a higher external mass transfer coefficient and a lower external substrate concentration on the surface of the immobilized enzyme. The effect of ultrasound was also demonstrated by a kinetic model in the batch reaction that the specificity constant (V′max/K2) in the ultrasonic bath was 8.9 times higher than that of the shaking bath, indicating the ultrasonication increased the affinity between enzymes and substrates and, therefore, increasing reaction rate. An experiment performed under the highest conversion conditions showed that the enzyme in the bioreactor remained stable at least for 5 days and maintained a 98% conversion.
1. Introduction
At present, the global annual production of fish oil is around 1,000,000 tons, and the global market of ω-3 polyunsaturated fatty acids was valued at USD 2.49 billion in 2019 [1]. As people’s consumption levels improve, the fish oil market continues to grow, and the amount of fish oil used in health care products is also rapidly expanding. DHA and EPA are well-known active ingredients in fish oil. Fish oils vary in DHA and EPA levels, depending on the source [2], so their value varies greatly. As nutritional supplements, the total content of DHA and EPA is generally greater than 30%. As a pharmaceutical grade, the total DHA and EPA content must be over 60% [3]. However, the DHA and EPA content in fish oil is typically below 30%. Several methods have been used for concentrating ω-3 PUFA to meet the specifications for health-promoting food or medicine, including urea complexation [4], molecular distillation [5], supercritical fluid method [6], reverse phase HPLC [7], solvent fractionation [8], and enzyme method [9].
The concentrated or purified fish oil products rich in DHA and EPA can be divided into three types according to their chemical structure: (1) glyceride; (2) fatty acid ethyl ester; (3) free fatty acid (FFA). The glyceride type is commonly sold as a health-promoting food. For pharmaceutical grade products, high purity is required and usually present as ethyl esters sold on the market, such as Lovaza® and Vascepa® [10,11]. To increase the content of DHA and EPA in the fish oil, the concentrated FFA with a high amount of DHA and EPA can be used as raw material to synthesize into the form of glyceride or ester through lipase catalyzed esterification, i.e., the transesterification of phophatidylcholine with PUFA in hexane [12], acidolysis of ethyl acetate (EA) with DHA+ EPA concentrate in hexane [13], esterification of tuna–FFA with various alcohol [14,15], esterification of sardine–FFA with glycerol [16].
Lipase-catalyzed esterification was performed in the packed-bed bioreactor [17,18]. The packed-bed bioreactor consists of simple equipment, a pump and a column packed with immobilized lipase. The reagents are pumped into a long tubular column to react directly with the immobilized lipase, resulting in high reaction rates and shortened reaction times to achieve a higher conversion [19,20]. Due to the advantages of high efficiency, lower energy consumption, minimum reaction volume and convenient operation, the packed-bed bioreactor is often used in the industry to maximize the efficiency for continuous operation of enzyme reaction [21]. In addition, ultrasound improves the mass transfer rate [22] and delivers activation energy to trigger reactions, which is a useful tool to accelerate enzyme reactions in different systems [23]. Ultrasonic-assisted enzymatic reactions have been widely investigated and reported [24,25]. Ultrasonication accelerated lipase-catalyzed acetylation of resveratrol and their potential kinetic response models were widely reported [26]. Lipase-catalyzed transesterification and esterification reactions were reported that could proceed with a two-substrate reaction model, ping-pong bi–bi [27] and order bi–bi mechanism [13,28]. In contrast, there are limited data available on the packed-bed bioreactors for continuous synthesis of DHA and EPA ethyl esters. In particular, DHA and EPA are long carbon chain FFA; when the enzyme acts on the substrate, it should be in a specific position to synthesize the DHA and EPA ethyl esters, making the reaction longer.
A solvent-free system means that the reactant also plays the role of solvent in the reaction. Esters synthesized using solvent-free systems have several advantages, such as high substrate concentrations, high conversion and less reaction time [29]. The ethyl acetate (EA) has been used in solvent-free system for the lipase-catalyzed synthesis of many esters. Lipase-catalyzed transesterification of geraniol to geranyl acetate at a mole ratio of 1:7 of geraniol to ethyl acetate, obtaining 83% conversion [30]. A high substrate concentration of 2-phenethyl alcohol (493.4 mM) was reacted with EA in a solvent-free system to produce 2-phenylethyl acetate, and a conversion of 97.64% was achieved at reaction time of 3.07 h [31]. In a previous study, we used lipase-catalyzed transesterification of EA with DHA and EPA concentrate in n-hexane to obtain in DHA and EPA ethyl ester [13]. Therefore, a solvent-free system employing the reactant EA as the solvent for the transesterification reaction to synthesize DHA and EPA ethyl esters was explored. It should accelerate the reaction to the direction in which the product is synthesized.
In this study, a packed-bed bioreactor was developed for the continuous synthesis of DHA and EPA ethyl esters using a solvent-free system. For understanding the relationship between conversion, flow rate and substrate concentration, we applied the central composite design and response surface methodology (RSM). More specifically, the mass transfer effects on the performance of the packed bed bioreactor with ultrasonication were also studied. Besides, we developed a kinetic model to evaluate ultrasonication’s effects on the lipase-catalyzed synthesis of DHA and EPA ethyl esters. Finally, the stability of the packed-bed bioreactor was evaluated under long-term operation.
2. Results and Discussion
2.1. Effect of Ultrasonication on DHA and EPA Ethyl Ester Synthesized Using Lipase Packed-Bed Bioreactor
Figure 1 shows a diagram of the apparatus. The column packing with immobilized lipase was placed in a temperature-controlled ultrasonic bath. The DHA + EPA concentrate was well mixed with EA in a feeding flask. The experimental design is based on the three-level–two-factor central composite design as shown in Table 1. Initially, the lipase-catalyzed synthesis of DHA + EPA ethyl ester was carried out at a reaction temperature of 60 °C with a flow rate of 1 to 5 mL min−1, without ultrasonication or with ultrasonication. Zanwar and Pangarkar have reported that the mass transfer coefficient was increased with acoustic power [32]. Therefore, the ultrasonic bath was set at 100% output power. The substrate mixture was fed through the column using a reciprocating piston pump. The HPLC analysis of reactants and products eluting from the packed-bed bioreactor is shown in Figure 2a,b, respectively. After lipase catalysis, the DHA + EPA concentrate was mostly converted into DHA + EPA ethyl ester, as shown in Table 1. The highest conversion was 98.84% in treatment 1 (DHA + EPA 100 mM, flow rate 1 mL min−1), and the lowest conversion was 86.61% in treatment 10 (DHA + EPA 500 mM, flow rate 5 mL min−1) for the packed-bed bioreactor. In the contrast, the ultrasonic packed-bed bioreactor had the highest conversion of 99.21% in treatment 1 and the lowest conversion of 93.03% in treatment 10. As a result, the ultrasonication-improved synthetic conversion was obtained for all treatments in Table 1. These results showed that ultrasonication can effectively enhance the synthesis of DHA + EPA ethyl ester in the packed-bed bioreactor and can obtain a higher conversion when it operates at higher substrate concentration and higher flow rates.
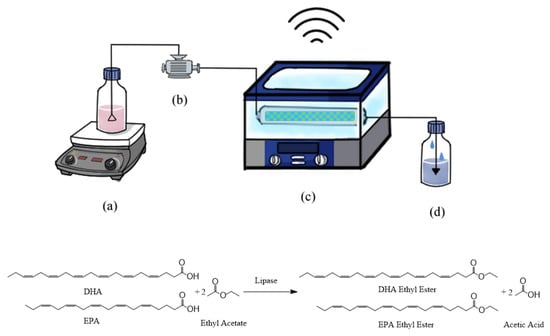
Figure 1.
Diagram of the ultrasonic packed-bed bioreactor: (a) DHA + EPA concentrate in the EA; (b) pump; (c) temperature-controlled ultrasonic bath; (d) product.

Table 1.
Central composite design and experimental conversion of DHA + EPA ethyl ester from the packed-bed bioreactor and ultrasonic packed-bed bioreactor.
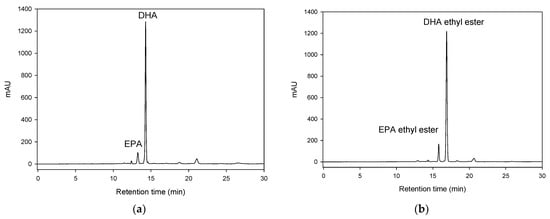
Figure 2.
HPLC analysis of (a) the reaction mixture and (b) product eluting from the lipase packed-bed bioreactor.
The second-order polynomial Equations (1) and (2) were obtained from the Design-Expert software by fitting the data in Table 1.
where Y1 is the conversion of DHA + EPA ethyl ester synthesized using a packed-bed bioreactor; Y2 is the conversion of DHA + EPA ethyl ester synthesized using an ultrasonic packed-bed bioreactor; x1 is the concentration of DHA + EPA (100–500 mM), x2 is the flow rate (1–5 mL min−1).
Y1 (%) = 97.985136 + 0.088092x1 + 0.008523x2 − 0.074570x1x1 − 0.003473x2x1 − 0.000020509x2x2
Y2 (%) = 100.683977 + 0.172551x1 − 0.015220x2 − 0.108444x1x1 − 0.001154x2x1 + 0.000019055x2x2
According to the results of the analysis of variance (ANOVA) data in Table 2, the polynomial model can adequately describe the actual relationship between response and significant variables, as indicated by a significant total model for p < 0.05, and a satisfactory coefficient of determination (R2 = 0.99). Moreover, the ANOVA results indicate that the linear term and interaction term had a significant influence (p < 0.05) on responses for both models. The quadratic terms had less influence (p > 0.05), except for the quadratic terms of Equation (2) (p < 0.05).

Table 2.
Analysis of variance (ANOVA) analysis for continuous lipase-catalyzed synthesis of DHA + EPA ethyl ester by packed-bed bioreactor and ultrasonic packed-bed bioreactor.
The response surface plots of the packed-bed bioreactor show the effect of the DHA + EPA concentration and flow rate on the conversion as shown in Figure 3a. The conversion was only ~87% when the flow rate was 5 mL min−1 and the DHA + EPA concentration was 500 mM. However, the conversion was ~98% when the flow rate was decreased to 1 mL min−1 and the DHA + EPA concentration was decreased to 100 mM. In contrast, the conversions of DHA + EPA ethyl ester for the ultrasonic packed-bed bioreactor (Figure 3b) were ~93% at a flow rate of 5 mL min−1 and DHA + EPA concentration of 500 mM and ~99% at a flow rate of 1 mL min−1 and DHA + EPA concentration of 100 mM, the conversion was higher than that of the packed-bed bioreactor at the same flow rate and DHA + EPA concentration. Therefore, ultrasonication can significantly improve the efficiency for the synthesis of DHA/EPA ethyl ester, allowing the packed-bed bioreactor to operate at higher concentrations and flow rates. Stavarache et al. used base-catalyzed synthesis of biodiesel with low-frequency ultrasonic waves (28 and 40 kHz); the time required for ultrasonic synthesis was shorter [33]. Ji et al. studied soybean oil transesterification using alkali as a catalyst to produce biodiesel with ultrasonication; the highest yield of 100% can be obtained within the reaction time of 10–30 min [34]. Liu et al. compared the effect of lipase-catalyzed hydrolysis of soy oil in a shaking bath and in an ultrasonic bath. The overall hydrolysis reaction rate in the ultrasonic bath was above 2-fold than that in the shaking bath [35]. Yu et al. conducted the transesterification of soybean oil and methanol with Novozym® 435 to synthesize biodiesel and discussed the effect of vibration speed and ultrasonication. The authors found that enzymes work at a vibration speed of 50 rpm with ultrasonication; a yield of 96% can be obtained in 4 h [36]. Patchimpet et al. reported that ultrasonic irradiation coupled with stirring enhanced the transesterification with the highest yield of 97.59% [37]. Our experimental results may be shown in the same way as the results of the above literature. It is deduced that ultrasonication can assist the enzymatic reaction, which increases the reaction rate and decreases the reaction time.
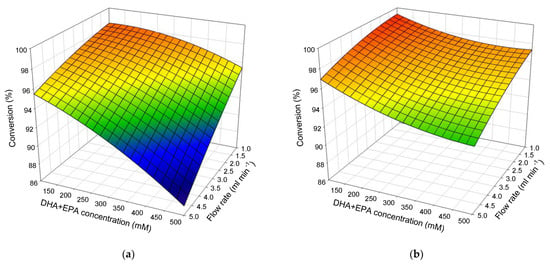
Figure 3.
Response surface plots show the mutual effects of DHA + EPA concentration and flow rate on the conversion for (a) packed-bed bioreactor and (b) ultrasonic packed-bed bioreactor.
2.2. Mass Transfer Kinetic Model of an Ultrasonic Packed-Bed Reactor
It is possible to determine a mass transfer limited model of a packed-bed bioreactor based on the mass balance of DHA + EPA in the column and the mass transfer rate of DHA + EPA from the bulk fluid to the surface of the immobilized enzyme. The mass transfer limited model is as follows:
where V is the substrate flow rate (mL min−1), Ax is the cross-sectional area of column (cm2), dS/dZ is the concentration gradient along the column length (mM cm−1), Z is the column length (cm), km is the external mass transfer coefficient (cm min−1), am is area of mass transfer (cm2 cm−3), S is DHA + EPA concentration in the bulk liquid (mM), and Si is DHA + EPA concentration at the external surface of the immobilized enzyme (mM) under mass transfer condition.
Equation (3) uses the boundary conditions as Z = 0; S = S0; Z = h; S = Si to obtain Equation (4).
The km can be obtained by plotting ln(S0 − Si/S − Si) versus amAxZ/V. The experimental data obtained from response surface plots were then used to plot the graphs for the mass transfer limited model. The value of Si was obtained by fitting the data to Equation (4), which gives a high value of R2. Figure 4 is an example to show a plot ln(S0 − Si/S − Si) versus amAxZ/V for the mass transfer limited condition at a DHA + EPA concentration of 500 mM. In this way, the best fit of Si value gives the highest value of R2 for packed-bed bioreactor and ultrasonic packed-bed bioreactor listed in Table 3. In both bioreactors, km decreased and Si increased with increasing substrate concentration. Similar results have been reported by Todero et al. in the case of the enzymatic synthesis of isoamyl butyrate under optimal experimental conditions [38]. In addition, our results also showed that the ultrasonic packed-bed bioreactor had a higher external mass transfer coefficient km and a lower Si as compared to the packed-bed bioreactor. At this stage, the mass transfer model experiments confirmed that ultrasonication could indeed assist the action of enzymes, which was reflected in the increase in the reaction rate and the higher conversion.
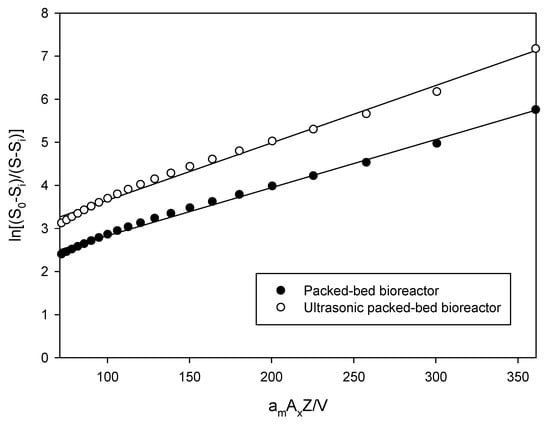
Figure 4.
The mass transfer limited model of ● packed-bed bioreactor and ○ ultrasonic packed-bed bioreactor operated at a DHA + EPA concentration of 500 mM and different flow rates.

Table 3.
km and Si obtained from Equation (5) for packed-bed bioreactor and ultrasonic packed-bed bioreactor operated at a different substrate concentration.
2.3. Continuous Synthesis of DHA + EPA Ethyl Ester by Packed-Bed Bioreactor with Long-Term Operation
In the batch reactor, the by-product, acetic acid, is produced continuously and accumulates in the reactor. It has been reported that the high acid concentration may cause enzyme inhibition [39,40]. Unlike batch reactors, by-products continually flow out of the packed-bed bioreactor, and there is no accumulation of by-products in the reactor. Therefore, the reusability and productivity of enzymes are better when used in continuous production than that in batch production [41]. In this way, the experiments in this section were to test the long-term stability of the packed-bed bioreactor for the continuous synthesis of DHA + EPA ethyl ester. Because the long-term operation required a large amount of DHA + EPA concentrate, the long-term operation was carried out at a low flow rate and low DHA + EPA concentration. In the packed-bed bioreactor or ultrasonic packed-bed bioreactor, the bioreactors were in continuous operation for 5 days at a DHA + EPA concentration of 100 mM, a flow rate of 1 mL min−1 and a temperature of 60 °C. The results are shown in Figure 5. After continuous operation for 5 days, the conversion remained at 98% without a downtrend. The reason for no difference in conversion between ultrasonic packed-bed and packed-bed bioreactors is that the experiments were operated at a low flow rate and low DHA + EPA concentration. The conversion is proportional to the residence time of the substrate in the packed-bed bioreactor and is inversely proportional to the substrate concentration. A lower flow rate indicated that the substrate had sufficient residence time to fully convert to product, so the benefit of ultrasonication cannot be seen. However, operating under conditions of high flow rate (5 mL min−1) and high substrate concentration (500 mM), as shown in Figure 3a,b, due to insufficient residence time, the conversion of the packed-bed bioreactor was 86.61%, while the conversion of the packed-bed bioreactor was 93.03%. Under the conditions of high substrate concentration and high flow rate, the ultrasound showed a significant positive impact on reaction conversion. Zenevicz et al. [42] found that the enzymatic production of ethyl esters in continuous mode coupled with an ultrasound bath increased conversion from 87% to 95%, which is consistent with our results. Furthermore, the long-term operation showed the packed-bed bioreactor was very stable. This result confirmed Novozym® 435 is suitable for the packed-bed bioreactor for continuous production of DHA + EPA ethyl ester. The packed-bed bioreactor is a continuous operation system, which is superior to batch production in terms of enzyme utilization and production cost and has good feasibility for future application in production capacity or industrial mass production. It should be kept in mind that mass production required the operating conditions of flow rate and substrate concentration as high as possible. Our results confirmed that the use of ultrasound improved the conversion effectively when production of DHA + EPA ethyl ester operated at a higher flow rate or substrate concentration. In addition, extending the length of the column to increase the residence time of the substrate, or connecting the columns in series, can also achieve the effect of improving the conversion [43,44].
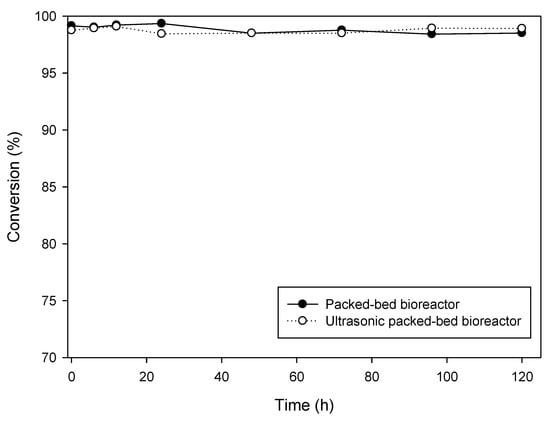
Figure 5.
Long-term operation of ● packed-bed bioreactor and ○ ultrasonic packed-bed bioreactor for continuous synthesis of DHA + EPA ethyl ester.
2.4. Recovery of DHA/EPA Ethyl Ester
An amount of 100 mL of the product produced by the ultrasonic packed-bed bioreactor using a DHA + EPA concentration of 100 mM after 5 days of operation as described in Section 2.3 was collected for recovery of DHA + EPA ethyl ester. The vacuum rotary evaporator was used to remove ethyl acetate and acetic acid at 80 °C; a 3.15 g of DHA + EPA ethyl ester was obtained with a yield of 96%, and a conversion of 98%. Next, the ultrasonic packed-bed bioreactor was operated at 60 °C with increasing DHA + EPA concentration to 500 mm and flow rate to 5 mL min−1. A 15.9 g of DHA + EPA ethyl ester was obtained, with a yield of 97% and a conversion of 93%. It can be seen from the recovery results that when the conversion is high enough, the purified product can be easily obtained.
2.5. Evaluate the Ultrasonication Effect by the Kinetic Model in the Batch Reaction
In the packed-bed bioreactor, the substrate was reacted in the presence of excess enzymes. In order to further examine the effect of ultrasonication, we performed enzyme kinetic studies in the batch reaction with trace amount of enzyme. The lipase-catalyzed synthesis of ester is a two-substrates reaction. The kinetic mechanism of the dual substrate reaction is more complicated as compared with one substrate reaction. Generally, three mechanisms can be used to describe the multi-substrate enzyme-catalyzed reaction: the ping-pong Bi–Bi mechanism, the ordered mechanism and the random order mechanism. In the first mechanism, the first substrate is combined with the enzyme to form a substituted enzyme intermediate and release the first product. As the second substrate interacts with the substituted enzyme intermediate, the second product is produced, and the native enzyme is regenerated. The latter two mechanisms are before the release of the product; the enzyme must be fully combined with the substrate to react, and then the product will appear. Our previous studies have shown that lipase-catalyzed transesterification can be represented by an ordered mechanism [28]. The kinetic model is as follows:
where v is the initial reaction rate, Vmax is the maximum initial reaction rate, [A] is the initial concentration of DHA + EPA, [B] is the initial concentration of EA, and KmA and KmB are the Michaelis constants for DHA + EPA and EA, respectively. KdA is the dissociation constant of the DHA + EPA–lipase complex.
Equation (5) can be arranged into the following equation by combining the parameters:
where , let V′max = VmaxK1, obtain Equation (7) that is similar to Michaelis–Menten equation.
Therefore, the kinetic model of the reaction is only related to the concentration of DHA + EPA. This particular kinetic model can be used to analyze lipase-catalyzed reactions in solvent-free systems, such as esterification of formic acid [45], synthesis of amyl levulinate [46], synthesis of monoglyceryl phenolic acids [47], synthesis of ethyl valerate [48], synthesis of geranyl acetate [49]. Since the solvent concentration is saturated and much greater than the substrate concentration, the solvent concentration can be regarded as a constant. Thus, the kinetic parameters can be obtained by using the Lineweaver–Burk plot method [50].
Lipase-catalyzed synthesis of DHA + EPA ethyl esters was performed using different DHA + EPA concentrations (50–400 mM) in EA. The reactions were carried out in a shaking bath at 150 rpm or in an ultrasonic bath at 37 Hz, respectively. According to Equation (7), plot the reciprocal initial reaction rate (1/v) versus the reciprocal substrate concentrations (1/[A]) (i.e., Lineweaver–Burk plot) is shown in Figure 6. An acceptable value of the determination coefficient (R2 = 0.98) confirmed the fitness of the kinetic Equation (7). The Lineweaver–Burk plot showed both curves were linear and no upward curve at high substrate concentrations (lower 1/[A]), indicating that the substrate inhibition did not occur in this solvent-free system at high concentrations of DHA + EPA. Moreover, the kinetic parameters can be obtained from the slope and intercept in Figure 6. The values of the apparent Michaelis constant (K2), apparent maximum initial reaction rate (V′max) and specificity constant (V′max/K2) were 960.02 mM, 11.76 mM min−1 and 0.012 min−1, respectively, for shaking bath, and 715.98 mM, 76.92 mM min−1 and 0.107 min−1 for ultrasonic bath. The V′max of the lipase-catalyzed synthesis of DHA/EPA ethyl ester in the ultrasonic bath increased about 6.54 times, while the K2 decreased. K2 represents the affinity of enzymes and substrates; the smaller K2 means the greater affinity of the enzyme and the substrate. The ultrasonic bath showed lower K2 indicating that the ultrasonication increased the substrate affinity toward immobilized lipase. V′max/K2 can be used to express the specificity constant, which reflects both affinity and catalytic ability [28,51,52]. The ultrasonic bath showed 8.9 times higher V′max/K2 value than that of the shaking bath, indicating that ultrasonication highly enhanced the efficiency of lipase-catalyzed transesterification. The higher reaction rate increased by ultrasonication can shorten the reaction time. Lipase-catalyzed synthesis of cetyl oleate with ultrasonication has been reported to reduce reaction time by about 75% [53]. Ultrasonication-assisted lipase-catalyzed synthesis of cinnamyl acetate also found that the reaction time for the maximum conversion was reduced from 60 min to 20 min [54].
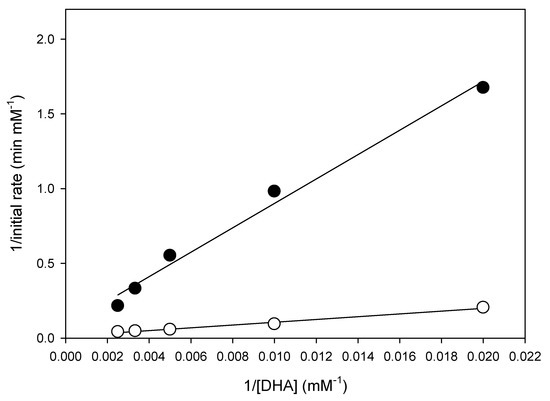
Figure 6.
Lineweaver–Burk plot of reciprocal initial reaction rate versus reciprocal concentrations of DHA + EPA concentrate for lipase-catalyzed transesterification in ● shaking bath and ○ ultrasonic bath.
3. Materials and Methods
3.1. Materials
Immobilized lipase (Novozym® 435, 10,000 PLU U/g; propyl laurate units) was purchased from Novo Nordisk Bioindustrials Inc. (Copenhagen, Denmark). As described previously [8,13], DHA + EPA concentrate with an average molecular weight of 312 was produced from cobia liver oil by fractionating fatty acids salts using acetone. There was 54.4% DHA, 16.8% EPA, 7.0% docosadienoic acid, 14.2% oleic acid, 0.8% linoleic acid, 0.5% linolenic acid, 3.1% palmitoleic acid, 2.7% palmitic acid and 0.5% myristic acid in DHA + EPA concentrate. Ethyl acetate (99.8%) was produced by Merck (Darmstadt, Germany). cis-4,7,10,13,16,19-Docosahexaenoic acid and cis-5,8,11,14,17-eicosapentaenoic acid used for analysis were purchased from Acros (Fair Lawn, NJ, USA) and TCI Co., LTD. (Tokyo, Japan), respectively. All chemicals and reagents used in this study were of analytical grade, unless otherwise noted.
3.2. Continuous Synthesis of DHA and EPA Ethyl Ester in Packed-Bed Bioreactor
The apparatus of the packed-bed bioreactor is shown in Figure 2. The packed-bed bioreactor was implemented in a stainless-steel column at 60 °C. The stainless-steel column was 25 cm in length with an inner diameter of 0.25 cm and packed with 1.3 g of Novozym® 435. The upper and lower end of the column was layered with a 2 μm filter. The flow rates were controlled by a Hitachi Pump (L-2130, Hitachi, Tokyo, Japan). The ultrasonic packed-bed bioreactor was carried out in the ultrasonic bath and operated at 37 kHz with 100% output. A feeding flask containing various concentrations of DHA + EPA (100–500 mM) in EA was thoroughly mixed before reaction. The reaction mixture in the feeding flask was pumped continuously into the column under designed flow rates (1–5 mL min−1). The product was collected at the end of the column. After each experiment, ethyl acetate was used to flush the column at a flow rate of 1 mL min−1 for 30 min.
3.3. Experimental Design
In this study, the three-level-two-factor central composite design was employed, and the experiments were carried out in a packed-bed reactor or ultrasonic packed-bed bioreactor at 60 °C. The experimental variables were DHA + EPA concentration (100–500 mM), mixture flow rate (1–5 mL min−1), as shown in Table 1. The experimental data (Table 1) were analyzed by Design-Expert software 8.0 (StatEase Inc., Minneapolis, MN, USA) to fit the following second-order polynomial equation:
where Y is the response (conversion of DHA+EPA ethyl ester; %), β0 is the constant term; β1 and β2 are coefficients of the linear effects, β11 and β22 are coefficients of quadratic effects and β12 is coefficients of interaction effects; X1 and X2 are the uncoded independent variables.
3.4. Measure Initial Rate of DHA and EPA Ethyl Ester Synthesized in the Batch Reaction
Various amounts of DHA + EPA concentrate were added with EA to a total volume of 3 mL to make the solution concentration of 50–400 mM. Then, 20 mg immobilized lipase Novozym® 435 was added to 3 mL of the reaction mixture. The reactions were performed at 60 °C for 5 min and 20 min with or without ultrasonication, respectively. The ultrasonic bath (Elmasonic P 70 H, Elma, Siegen, Germany) was operated at 37 kHz with 100% output power. A liquid sample was withdrawn from the reaction mixture to determine the production of DHA + EPA ethyl ester using HPLC after the reaction. Initial reaction rates are expressed as produced DHA + EPA ethyl ester mM per min (mM min−1). The V′max and K2, were obtained from the Lineweaver–Burk plot.
3.5. Analytical Methods
Inertsil ODS-3 column (5 µM, 250 mm × 4.6 mm) and HPLC system, consisting of a Hitachi L-2130 HPLC pump and a Hitachi L-2420 UV/VIS detector (Hitachi, Tokyo, Japan), were used to analyze the samples. Deionized water and methanol containing 0.1% acetic acid were used for eluting the sample. From 80% to 100% methanol, gradient elution was carried out for 10 min, followed by 20 min at 100% methanol. Flow rate and wavelength were both set to 1.0 mL min−1 and 303 nm, respectively. The conversion (%) was calculated from the peak areas of the substrate (DHA + EPA) and product (DHA + EPA ethyl ester).
4. Conclusions
Continuous synthesis of DHA + EPA ethyl ester using a lipase packed-bed bioreactor was successfully studied. The benefits of ultrasonication in increasing the reaction rate were successfully demonstrated in both kinetic and mass transfer models. A three-level–two-factor central composite design and RSM were employed for the experimental design and data analysis. A model for the DHA + EPA ethyl ester synthesis was established. With ultrasonication, the highest conversion of 99% was obtained with ultrasonication to be a flow rate of 1 mL min−1, and substrate concentration of 100 mM. The mass transfer rates could be increased by ultrasonication. Therefore, when the substrate concentration increased to 500 mM and the flow rate increased to 5 mL min−1, the conversion rate still is maintained at 93%. The ultrasonic packed-bed bioreactor used for continuous synthesis of DHA + EPA ethyl ester was quite stable under long-term operation, and no reduction in conversion was observed. The high conversion makes the product easy to recover. Therefore, an ultrasonic packed-bed bioreactor combined with a solvent-free system has the potential to be utilized in industrial applications for the continuous production of DHA + EPA ethyl ester. In addition, we also successfully developed an apparent Michaelis–Menten equation that can be used to compare the efficiency of lipase-catalyzed reactions under different reactor operations.
Author Contributions
Conceptualization, C.-H.K. and C.-J.S.; methodology, C.-H.K. and Y.-C.L.; formal analysis, M.-L.T. and Y.-H.T.; investigation, C.-H.K.; resources, H.-M.D.W. and C.H.; data curation, C.-H.K.; writing—original draft preparation, C.-H.K. and C.-Y.H.; writing—review and editing, C.-H.K., C.-Y.H. and C.-J.S.; supervision, C.-D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by research funding grants provided by the Ministry of Science and Technology of Taiwan, MOST 104-2218-E-022-001-MY2 and MOST 110-2221-E-992-009-.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest in this research.
References
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Sankar, T.V.; Anandan, R.; Chakraborty, K.; Paul, B.N.; Sarma, D.; Syama Dayal, J.; Venkateshwarlu, G.; et al. DHA and EPA Content and Fatty Acid Profile of 39 Food Fishes from India. BioMed Res. Int. 2016, 2016, 4027437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innes, J.K.; Calder, P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovale-Rosabal, G.; Rodríguez, A.; Contreras, E.; Ortiz-Viedma, J.; Muñoz, M.; Trigo, M.; Aubourg, S.P.; Espinosa, A. Concentration of EPA and DHA from refined salmon oil by optimizing the urea–fatty acid adduction reaction conditions using response surface methodology. Molecules 2019, 24, 1642. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Wu, F.; Yue, L.; Du, Q.; Tian, L.; Wang, Z. Combination of urea complexation and molecular distillation to purify DHA and EPA from sardine oil ethyl esters. J. Am. Oil Chem. Soc. 2014, 91, 687–695. [Google Scholar] [CrossRef]
- Montañés, F.; Tallon, S. Supercritical fluid chromatography as a technique to fractionate high-valued compounds from lipids. Separations 2018, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Oh, C.-E.; Kim, G.-J.; Park, S.-J.; Choi, S.; Park, M.-J.; Lee, O.-M.; Seo, J.-W.; Son, H.-J. Purification of high purity docosahexaenoic acid from Schizochytrium sp. SH103 using preparative-scale HPLC. Appl. Biol. Chem. 2020, 63, 1–8. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Chen, J.-W.; Wang, H.-M.D.; Shieh, C.-J. Concentration of Docosahexaenoic and Eicosapentaenoic Acid from Cobia Liver Oil by Acetone Fractionation of Fatty Acid Salts. Appl. Biochem. Biotechnol. 2020, 192, 517–529. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Zhang, S.; Zhang, W.; Barrow, C.J. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012, 131, 639–644. [Google Scholar] [CrossRef]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and bioactive components from fish for dyslipidemia and cardiovascular risk reduction. Mar. Drugs 2016, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The anticancer drug discovery potential of marine invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, A.; Gładkowski, W.; Grudniewska, A. Lipase-catalyzed transesterification of egg-yolk phophatidylcholine with concentrate of n-3 polyunsaturated fatty acids from cod liver oil. Molecules 2017, 22, 1771. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Huang, C.-Y.; Lee, C.-L.; Kuo, W.-C.; Hsieh, S.-L.; Shieh, C.-J. Synthesis of DHA/EPA ethyl esters via lipase-catalyzed acidolysis using Novozym® 435: A kinetic study. Catalysts 2020, 10, 565. [Google Scholar] [CrossRef]
- Shimada, Y.; Sugihara, A.; Nakano, H.; Kuramoto, T.; Nagao, T.; Gemba, M.; Tominaga, Y. Purification of docosahexaenoic acid by selective esterification of fatty acids from tuna oil with Rhizopus delemar lipase. J. Am. Oil Chem. Soc. 1997, 74, 97–101. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Baba, T.; Ooguri, T.; Moriyama, S.; Terai, T.; Tominaga, Y. Ethyl esterification of docosahexaenoic acid in an organic solvent-free system with immobilized Candida antarctica lipase. J. Biosci. Bioeng. 2001, 92, 19–23. [Google Scholar] [CrossRef]
- Bispo, P.; Batista, I.; Bernardino, R.J.; Bandarra, N.M. Preparation of triacylglycerols rich in omega-3 fatty acids from sardine oil using a Rhizomucor miehei lipase: Focus in the EPA/DHA Ratio. Appl. Biochem. Biotechnol. 2014, 172, 1866–1881. [Google Scholar] [CrossRef]
- Chen, H.C.; Kuo, C.H.; Twu, Y.K.; Chen, J.H.; Chang, C.M.J.; Liu, Y.C.; Shieh, C.J. A continuous ultrasound-assisted packed-bed bioreactor for the lipase-catalyzed synthesis of caffeic acid phenethyl ester. J. Chem. Technol. Biotechnol. 2011, 86, 1289–1294. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Shieh, C.-J. Biocatalytic Process Optimization. Catalysts 2020, 10, 1303. [Google Scholar] [CrossRef]
- Huang, S.-M.; Huang, H.-Y.; Chen, Y.-M.; Kuo, C.-H.; Shieh, C.-J. Continuous production of 2-phenylethyl acetate in a solvent-free system using a packed-bed reactor with Novozym® 435. Catalysts 2020, 10, 714. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, L.; Dong, Z.; Shen, J.; Du, L.; Luo, X. Enzymatic synthesis of thioesters from thiols and vinyl esters in a continuous-flow microreactor. Catalysts 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Lindeque, R.M.; Woodley, J.M. Reactor selection for effective continuous biocatalytic production of pharmaceuticals. Catalysts 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.-R.; Tsai, M.-F.; Shieh, C.-J.; Arakawa, O.; Dong, C.-D.; Huang, C.-Y.; Kuo, C.-H. Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage. Foods 2021, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Kuo, C.-H.; Chen, B.-Y.; Li, Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Murillo, G.; He, Y.; Yan, Y.; Sun, J.; Bartocci, P.; Ali, S.S.; Fantozzi, F. Scaled-up biodiesel synthesis from Chinese Tallow Kernel oil catalyzed by Burkholderia cepacia lipase through ultrasonic assisted technology: A non-edible and alternative source of bio energy. Ultrason. Sonochem. 2019, 58, 104658. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; Garcia-Galan, C.; Schein, M.F.; Silva, A.M.; Barbosa, O.; Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules 2014, 19, 9562–9576. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Hsiao, F.-W.; Chen, J.-H.; Hsieh, C.-W.; Liu, Y.-C.; Shieh, C.-J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4′-acetoxyresveratrol. Ultrason. Sonochem. 2013, 20, 546–552. [Google Scholar] [CrossRef]
- Onoja, E.; Chandren, S.; Razak, F.I.A.; Wahab, R.A. Enzymatic synthesis of butyl butyrate by Candida rugosa lipase supported on magnetized-nanosilica from oil palm leaves: Process Optimization, Kinetic and Thermodynamic Study. J. Taiwan Inst. Chem. Eng. 2018, 91, 105–118. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, G.-J.; Chen, C.-I.; Liu, Y.-C.; Shieh, C.-J. Kinetics and optimization of lipase-catalyzed synthesis of rose fragrance 2-phenylethyl acetate through transesterification. Process Biochem. 2014, 49, 437–444. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-free esterifications mediated by immobilized lipases: A Review from Thermodynamic and Kinetic Perspectives. Catal Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. Synthesis of geranyl acetate by transesterification of geraniol with ethyl acetate over Candida antarctica lipase as catalyst in solvent-free system. Flavour Fragr. J. 2019, 34, 288–293. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liu, T.-A.; Chen, J.-H.; Chang, C.-M.J.; Shieh, C.-J. Response surface methodology and artificial neural network optimized synthesis of enzymatic 2-phenylethyl acetate in a solvent-free system. Biocatal. Agric. Biotechnol. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Zanwar, S.; Pangarkar, V. Solid-liquid mass transfer in packed beds: Enhancement Due to Ultrasound. Chem. Eng. Commun. 1988, 68, 133–142. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrason. Sonochem. 2005, 12, 367–372. [Google Scholar] [CrossRef]
- Ji, J.; Wang, J.; Li, Y.; Yu, Y.; Xu, Z. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, e411–e414. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Q.; Shan, L.; Liu, Y.; Shen, W.; Wang, X. The effect of ultrasound on lipase-catalyzed hydrolysis of soy oil in solvent-free system. Ultrason. Sonochem. 2008, 15, 402–407. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Patchimpet, J.; Zhang, Y.; Simpson, B.K.; Rui, X.; Sangkharak, K.; Eiad-ua, A.; Klomklao, S. Ultrasonic enhancement of lipase-catalyzed transesterification for biodiesel production from used cooking oil. Biomass Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Todero, L.M.; Bassi, J.J.; Lage, F.A.; Corradini, M.C.C.; Barboza, J.; Hirata, D.B.; Mendes, A.A. Enzymatic synthesis of isoamyl butyrate catalyzed by immobilized lipase on poly-methacrylate particles: Optimization, Reusability and Mass Transfer Studies. Bioprocess Biosyst. Eng. 2015, 38, 1601–1613. [Google Scholar] [CrossRef]
- Cavallaro, V.; Tonetto, G.; Ferreira, M.L. Optimization of the enzymatic synthesis of pentyl oleate with lipase immobilized onto novel structured support. Fermentation 2019, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Claon, P.A.; Akoh, C.C. Effect of reaction parameters on SP435 lipase-catalyzed synthesis of citronellyl acetate in organic solvent. Enzym. Microb. Technol. 1994, 16, 835–838. [Google Scholar] [CrossRef]
- Kuo, C.; Chen, C.; Chiang, B. Process characteristics of hydrolysis of chitosan in a continuous enzymatic membrane reactor. J. Food Sci. 2004, 69, 332–337. [Google Scholar] [CrossRef]
- Zenevicz, M.C.P.; Jacques, A.; Silva, M.J.A.; Furigo, A.; Oliveira, V.; de Oliveira, D. Study of a reactor model for enzymatic reactions in continuous mode coupled to an ultrasound bath for esters production. Bioprocess. Biosyst. Eng. 2018, 41, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Continuous biodiesel conversion via enzymatic transesterification catalyzed by immobilized Burkholderia lipase in a packed-bed bioreactor. Appl. Energy 2016, 168, 340–350. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zhao, C.; Ding, Y.; Xu, P. Biodiesel production in packed-bed reactors using lipase–nanoparticle biocomposite. Bioresour. Technol. 2011, 102, 6352–6355. [Google Scholar] [CrossRef]
- Aljawish, A.; Heuson, E.; Bigan, M.; Froidevaux, R. Lipase catalyzed esterification of formic acid in solvent and solvent-free systems. Biocatal. Agric. Biotechnol. 2019, 20, 101221. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Green synthesis of amyl levulinate using lipase in the solvent free system: Optimization, Mechanism and Thermodynamics Studies. Catal. Today 2021, 375, 120–131. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Shi, J.; Zheng, M.; Xiang, X.; Huang, F.; Xiao, J. Ultrasound irradiation promoted enzymatic alcoholysis for synthesis of monoglyceryl phenolic acids in a solvent-free system. Ultrason. Sonochem. 2018, 41, 120–126. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Hazer, B.; Altıntaş, B.; Arıca, M.Y. Covalent immobilization of lipase onto amine functionalized polypropylene membrane and its application in green apple flavor (ethyl valerate) synthesis. Process Biochem. 2011, 46, 372–378. [Google Scholar] [CrossRef]
- Mahapatra, P.; Kumari, A.; Kumar, G.V.; Banerjee, R.; Nag, A. Kinetics of solvent-free geranyl acetate synthesis by Rhizopus oligosporus NRRL 5905 lipase immobilized on to cross-linked silica. Biocatal. Biotransform. 2009, 27, 124–130. [Google Scholar] [CrossRef]
- Rodriguez, J.-M.G.; Hux, N.P.; Philips, S.J.; Towns, M.H. Michaelis–Menten graphs, Lineweaver–Burk plots, and reaction schemes: Investigating Introductory Biochemistry Students’ Conceptions of Representations in Enzyme Kinetics. J. Chem. Educ. 2019, 96, 1833–1845. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Shieh, C.-J.; Huang, S.-M.; Wang, H.-M.D.; Huang, C.-Y. The effect of extrusion puffing on the physicochemical properties of brown rice used for saccharification and Chinese rice wine fermentation. Food Hydrocoll. 2019, 94, 363–370. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Z.; Tian, Y.; Fan, H.; Huang, S.; Lu, Y.; Jin, Z. Highly efficient regioselective decanoylation of hyperoside using nanobiocatalyst of Fe3O4@ PDA-thermomyces lanuginosus lipase: Insights of Kinetics and Stability Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Jadhav, S.V.; Rathod, V.K. Lipase catalysed synthesis of cetyl oleate using ultrasound: Optimisation and Kinetic Studies. Ultrason. Sonochem. 2015, 27, 522–529. [Google Scholar] [CrossRef]
- Tomke, P.D.; Rathod, V.K. Ultrasound assisted lipase catalyzed synthesis of cinnamyl acetate via transesterification reaction in a solvent free medium. Ultrason. Sonochem. 2015, 27, 241–246. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).