Abstract
An integrated cleaner biocatalyst process was performed for biodiesel production from crude palm oil (CPO) and refined palm oil (RPO). It was evaluated on process efficiency in terms of high purity of biodiesel as well as by-products without purification, less wastewater, less time consuming, and a simple downstream process. A first saponification step was carried out in both f CPO and RPO, a high purity of glycerol (86.25% and 87.5%) was achieved, respectively, while free fatty acids (FFASs) in soap were obtained after hexane extraction. High yields of FFASs were obtained from both CPO and RPO (98.83% and 90.94%). Subsequently, the FFAs were esterified to biodiesel by a biocatalyst of immobilized lipase. The highest biodiesel yields achieved were of 92.14% and 92.58% (CPO and RPO). Remarkably, biodiesel yields obtained from CPO and RPO achieved satisfactory values and the biocatalyst used could be reused for more than 16–17 cycles.
1. Introduction
Currently, biodiesel, an alternative biofuel is being considered to replace fossil diesel [1,2]. It can be used as pure 100% biodiesel (B100) or blended with petroleum-based diesel due to its several advantages; biodegradability, excellent lubricity, non-toxicity, cleaner combustion, high colorific value, and production of less engine exhaust gas emissions (CO, CO2, and SOx) than petroleum-based diesel [3,4,5,6]. Typically, biodiesel can be produced from several oil crops, such as soybean oil, jatropha oil, sunflower oil, coconut oil, palm oil etc. Among these, refined palm oil (RPO) has the lowest price, the highest yield per hectare in Thailand and is widely used as a raw material for biodiesel production in South East Asia [7]. Naturally, there are two kinds of oils obtained from the palm tree; palm oil (PO) and palm kernel oil (PKO). Some physical and chemical methods are required to purify these oils by fractional distillation, leading to different forms of oil, including crude palm oil (CPO), semi-refined palm oil (SRPO), and refined palm oil (RPO). The current palm oil refinery process in Thailand is explained by Dujjanutat et al. [8]. In addition, CPO is also of significant interest to researchers for use as a feedstock for biodiesel production being a non-edible oil with low production cost compared to other plant oils [9,10]. Therefore, both CPO and RPO were chosen to be feedstocks with their good potential for biodiesel production. Traditionally, biodiesel is mostly produced via transesterification of triglycerides (TG) and short chain alcohols, catalyzed by homogeneous alkaline catalysts including NaOH, KOH, CH3CH2ONa, etc. The process shows many advantages including high reaction rate, high catalytic efficiency, and low amount of catalyst loading [11,12]. However, the major drawbacks are soap formation, difficult separation, and high impurity biodiesel as well as glycerol. In contrast, the presence of FFAs does not have any effect on the esterification reaction if catalyzed by homogenous acid catalysts such as H2SO4, HClO4, HNO3, etc. In addition, several problems have been encountered for example; long reaction time, low conversion rate, and low biodiesel yield [13,14].
Biodiesel purification is typically carried out in a number of ways, namely by washing with water, hot deionized water, pre-washing, organic solvents, and acidified water [15,16,17,18]. These processes are mainly hindered by the wastewater generation. The impurities commonly found in biodiesel can be removed by adsorbents such as magnesium silicate, calcium magnesium silicate, etc. In addition, ion-exchange resin can also be used to obtain pure biodiesel without glycerol contamination. However, the resin reusability is low, presenting a challenge [19]. Impurities including FFAs, inorganic salts, and free ions found in the glycerol are still a major problem for further applications, for example using as feedstocks in the food, pharmaceutical, and cosmetics industries, which require quite high purity glycerol (96–99 wt%.). In addition, glycerol also has beneficial applications for bio-hydrogen, monoacylglycerol (MAG), diacylglycerol (DAG), triacylglycerol (TAG), and other value-added chemicals [20,21,22]. Recently, it was reported that several methods can be used to purify glycerol such as neutralization, ion exchange/activated carbon adsorption, vacuum distillation, and membrane distillation [23]. In a previous study, Dhabhai et al. reported that the highest purity of glycerol (97.5%) was obtained via serial physicochemical treatment, membrane filtration, and activated carbon treatment [24]. However, saponification of triglycerides (TG) is also reported as being the simplest process to produce high purity glycerol (83–84%) without subsequent additional purification [25]. In addition, López et al. [26] applied a saponification reaction to obtain FFAs from microalgae lipids, these were further used as a substrate for biodiesel production via an esterification reaction catalyzed by lipase Novozyme 435 from Candida antarctica. They found that high purity of biodiesel (83 wt %) was obtained within four hours and the biocatalyst could be reused for more than six cycles.
Currently, enzymatic esterification of feedstock rich in FFA content is becoming an attractive approach. The key point is minimal pretreatment of low-quality feedstock in an upstream process with a lipase biocatalyst (EC.3.1.1) which shows high potential to promote acidic oil esterification [27]. Furthermore, the enzymatic process presents advantages such as a mild reaction temperature, non-corrosive reaction, high biodiesel purity, simple downstream process, no wastewater from washing steps, and reusable catalyst (if the enzyme is suitably immobilized). Thus, lipase could be applied as a biocatalyst during an esterification reaction to produce biodiesel. There are some doubts which might arise in the use of lipase as a biocatalyst to produce biodiesel such as the high cost, difficulties in downstream recovery, and low alcohol resistance. These problems can be solved by using immobilized lipase on a matrix support. Enzyme entrapment is a simple and cost-effective method which is widely used in industrial applications [28]. During entrapment, the enzyme either reacts with the supporting material or other enzyme molecules through encapsulation within a network of insoluble polymer, such as alginate silica aerogel and celite support sol gel. This is achieved by adding enzyme to a monomer solution before forming a gel, thus trapping the enzyme molecules. After immobilization, the enzyme can move freely within a solid support which allows only substrate and product to pass though and retains the enzyme inside the polymeric network [29,30]. Therefore, this technique was used throughout this study. Alginate is widely used as a supporting material for enzyme immobilization by using the entrapment technique because of the desirable properties; the mild conditions required, high porosity, easy to control porosity and the retention of enzymatic activity. However, fast degradation of the alginate matrix could occur under conditions of high temperature, low pH, and presence of organic solvents [31,32,33]. In addition, a non-toxic synthetic polymer of polyvinyl alcohol (PVA) provides good properties; high strength and a high ability to stabilize and preserve protein activity. Previously, Martinja et al. [34] reported biodiesel production from palm oil mill effluent using immobilized Candida rugosa lipase in PVA-alginate-sulfate beads. Despite low biocatalyst loading (2 g), the esterification reaction was complete within five hours. Accordingly, lipase immobilization on PVA-alginate beads is appropriate to use as biocatalyst for biodiesel production from FFAs in acid oil.
Thus, in this work, an alternative new cleaner biodiesel production process using CPO and RPO as feedstocks was developed and evaluated in terms of wastewater reduction, an easy separation process, a simple purification of biodiesel and glycerol. Saponification of CPO/RPO was first carried out to obtain soap and glycerol. CPO/RPO glycerol was then discharged from the reaction medium. Subsequently, the soap was extracted by hexane to generate FFAs for use as a substrate in subsequent steps. Second, enzymatic esterification of FFAs from CPO/RPO for biodiesel production was optimized by varying the reaction temperature, the MEOH to FFAs ratio, and the agitation rate. Furthermore, the reusability of biocatalyst was also investigated under the optimal condition. The characterization of the fatty acid methyl esters (FAMEs) obtained was also determined.
2. Results
2.1. Fatty Acid Composition of CPO/RPO
FFA content in both CPO and RPO was determined and the results are presented in Table 1. It was revealed that the CPO was mainly composed of oleic acid, palmitoleic acid, stearic acid, and palmitic acid, respectively. Meanwhile, in the case of RPO, the main fatty acids present were oleic acid and palmitoleic acid.

Table 1.
Fatty acid composition of crude palm oil (CPO) and refined palm oil (RPO).
2.2. Yield of Fatty Acid and Glycerol Content
High fatty acids yields of 98.83% (RPO) and 90.94% (CPO) were achieved after the extraction process modified from López et al. [26]. Furthermore, high glycerol content from CPO (86.25%) and RPO (87.50%) was also obtained without an additional purification step (See Table 2). It should be noted that the glycerol content obtained in this work was higher than in previous studies in which the reaction was stopped by adding organic solvent [19] or acid pretreatment [35]. Furthermore, the glycerol content obtained from the saponification reaction of CPO/RPO was also lower than in some previous works, who reported various methods such as vacuum distillation [36], sludge-derived activated carbon [37], including a sequential extraction of organic solvents together with discoloration by activated coal [38] and moreover, serial purification steps; physico-chemical treatment, membrane filtration, and activated charcoal adsorption [24], physicochemical and membrane pretreatments [39], ion exchange resins [40]. Other methods used were neutralization, vacuum distillation, adsorption onto activated carbon and adsorption onto a metal filter [41], and a hydro-esterification process [42].

Table 2.
Comparative glycerol content obtained from different processing routes.
2.3. Effect of Temperature on Esterification Reaction
Both CPO and RPO biodiesel yields illustrate a significant increase from 30 °C to 40 °C (See Figure 1). A higher reaction temperature could shift the equilibrium to the right-hand side, leading to higher conversion rate because the fast mobility of the reactants led to a greater number of collisions between the reactants than occurs at lower reaction temperatures. This is in agreement with a previous study [43]. On the other hand, biodiesel yields were reduced as reaction temperatures became higher than 40 °C, due to enzyme denaturation at higher temperatures. Similar results were explained by Jambulingam et al. 2019 who studied the effect of temperature on the esterase activity of immobilized lipase and observed that the maximum esterase activity occurred at a temperature of 45 °C. In this study the highest biodiesel yields from CPO (79.67%) and RPO (74.23%) and the lowest FFA content from CPO (2.21 mg KOH/g oil) and RPO (0.20 mg KOH/g oil) were obtained at 40 °C. Therefore, the temperature at 40 °C was further used in subsequent experiments. Similar results were also reported by Tan et al. [44]. Esterification of FFAs in hydrolyzed palm oil using Candida sp. 99–125 lipase as biocatalyst (15 wt%. enzyme loading) led to the highest biodiesel yield (95%) at 40 °C at a total reaction time of 30 h. Previously, biodiesel production from FFAs of CPO via the esterification reaction in the presence of isooctane catalyzed by a commercial enzyme, Novozyme 435, was reported by Talukder et al. [45], with the maximum biodiesel yield (98%) obtained at 40 °C.
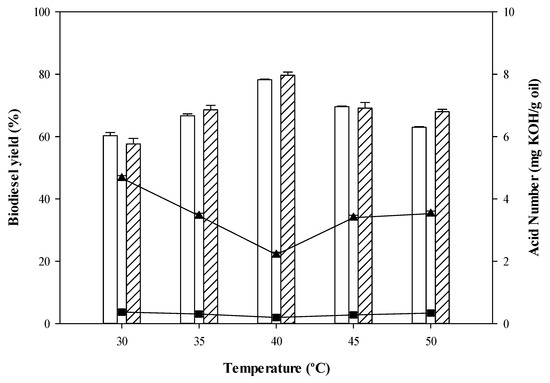
Figure 1.
Biodiesel yield (%) as function of temperature (°C) and acid number (mg KOH/g oil) (CPO biodiesel yield RPO biodiesel yield ▲ CPO acid number ■ RPO acid number.
2.4. Effect of MEOH to FFA Ratio on Esterification Reaction
The MEOH to FFA ratio was investigated. It was found that biodiesel yields of CPO/RPO continually increased between ratios of 1:1 and 4:1 as shown in Figure 2. Based on stoichiometry, the reaction required one mole of both FFAs and methanol generated FAMEs. As esterification is a reversible reaction, a higher MEOH to FFAs ratio more than 1:1 could drive esterification forward. However, a reduction of biodiesel yield was observed when the ratio used exceeded 4:1. The maximum biodiesel yields of FFAs from CPO (87.36%) and RPO (89.84%) together with the minimum FFAs content of CPO (1.39 mg KOH/g oil) and RPO (0.09 mg KOH/g oil) were reached at a ratio of 4:1. Thus, the ratio of methanol to fatty acid of 4:1 was deemed to be the best ratio for biodiesel production from CPO/RPO. Likewise, in earlier literature [44] which studied biodiesel production from FFAs in hydrolyzed palm oil via esterification catalyzed by Candida sp.99-125 lipase (15 wt%. enzyme loading). They also found that the highest biodiesel yield (90%) was also obtained when the ratio of MEOH to FFA ratio was at 3:1 with methanol being added twice at different stages of the biodiesel production process. Furthermore, Kareem et al. [46] also reported that the optimal MEOH to FFA ratio for enzymatic biodiesel production from palm oil and palm kernel oil using 5 wt%. enzyme loading was 3:1. Maximum biodiesel yields from palm oil (95%) and palm kernel oil (82.5%) were observed at this molar ratio.
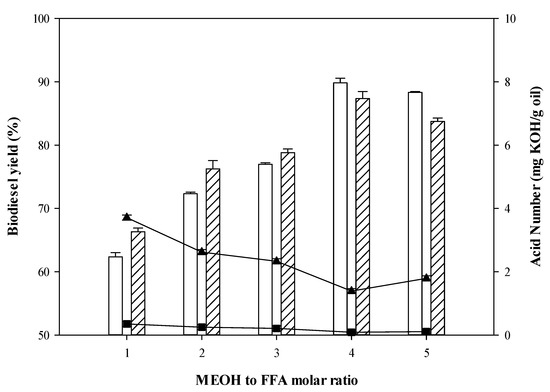
Figure 2.
Biodiesel yield (%) as function of MeOH to FFA molar ratio and acid number (mg KOH/g oil) (CPO biodiesel yield RPO biodiesel yield ▲ CPO acid number ■ RPO acid number).
2.5. Effect of Agitation Speed on Esterification Reaction
Third, the influence of agitation speed on biodiesel production was investigated. Both CPO and RPO biodiesel yields presented an upward trend as agitation speed increased from 100 rpm to 250 rpm (See Figure 3). Seemingly, a high agitation speed can promote high efficiency mass transfer of the liquid phases (CPO/RPO and methanol) and also maintain homogeneity leading to effective conversion of reactants in high biodiesel yields [47]. The biodiesel yield was remarkably reduced at agitation speed of 300 rpm, perhaps due to insufficient interaction time between the reactants [43]. The highest biodiesel yields of CPO (92.14%) and RPO (95.28%) and the lowest FFAs content of CPO (0.87 mg KOH/g oil)/RPO (0.25 mg KOH/g oil) were achieved at 250 rpm. Therefore, an agitation speed of 250 rpm was chosen as a suitable agitation speed for esterification of FFAs from CPO/RPO. Similarly, the influence of agitation speed on enzymatic esterification of FFAs in hydrolyzed CPO in the presence of isooctane was previously investigated by Talukder et al. [45]. Their results also revealed that an agitation speed of 250 rpm was the best condition for biodiesel production.
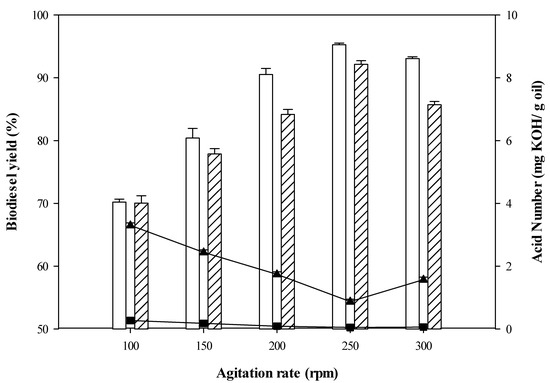
Figure 3.
Biodiesel yield (%) as function of agitation rate (rpm) and acid number (mg KOH/g oil) (CPO biodiesel yield RPO biodiesel yield ▲ CPO acid number ■ RPO acid number).
2.6. Reusability and Stability of Immobilized Lipase
The reusability and stability of immobilized alginate-PVA lipase beads in esterification of FFAs obtained from CPO/RPO were carried out under the optimal conditions (40 °C, 4:1 MEOH to FFA ratio and 250 rpm agitation speed for 20 cycles. Remarkably, the immobilized lipase beads gave high biodiesel yields of CPO (92.14%) and RPO (95.28%). It should be noted that the biocatalyst provided high catalytic efficiency as well as high reusability for CPO (16 cycles) and RPO (17 cycles) as shown in Figure 4. Lower catalytic efficiency of biocatalyst was observed after eighteen cycles onwards because lipase lost its stability in methanol. Furthermore, enzyme leakage from the immobilized beads could occur with a high cycle number [48]. Both the CPO and RPO biodiesel yields obtained were in agreement with other works [49,50,51,52,53]. However, considered in terms of reusability and stability, immobilized alginate-PVA lipase R. oryzae illustrated higher stability than other biocatalysts when comparing to previous works (See Table 3). Furthermore, the reusability of biocatalyst obtained agreed with other previous works. The immobilized alginate-PVA lipase bead could be reused for over 10–15 cycles with high catalytic efficiency for biodiesel production [14,32]. Therefore, it can be stated that an alternative cleaner process for biodiesel production from CPO/RPO could also enhance catalytic efficiency and stability of immobilized lipase.
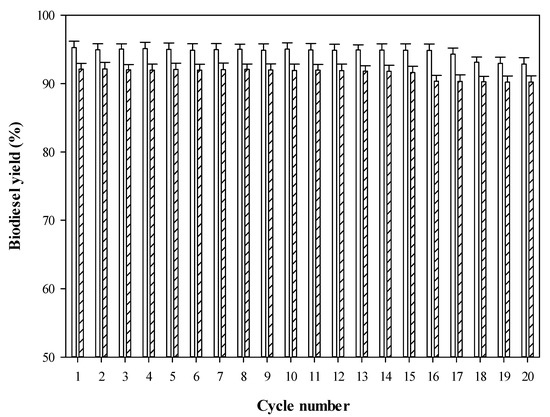
Figure 4.
Biodiesel yield (%) as a function of cycle number for testing reusability and stability of immobilized lipase during esterification reaction (CPO biodiesel yield RPO biodiesel yield).

Table 3.
Comparative biodiesel yield obtained from CPO and RPO via different processes.
2.7. Composition of Biodiesel
The composition of CPO/RPO biodiesel was analyzed by the GC-MS method. For CPO, the biodiesel obtained mainly consisted of methyl esters of oleic acid (45.02%), palmitoleic acid (24.54%), stearic acid (10.49%), and palmitic acid (9.56%), respectively. Meanwhile, RPO biodiesel was found to contain mostly oleic and palmitoleic acid methyl ester at 76.27% and 12.50% (See Figure 5). The proportion of monounsaturated fatty acid was higher than that of saturated fatty acid found in both CPO/RPO biodiesel, for example palmitoleic acid (C16:1) and oleic acid (C18:1). This improved the biodiesel quality in terms of low viscosity, good oxidative stability, and good cold flow properties [2]. In addition, the reduction of solidification problems at low temperature could also be addressed [54]. However, the saturated fatty acid or palmitic acid (C16:0) and stearic acid (C18:0) assisted in obtaining a high cetane number and led to the formation of less nitrogen oxides (NOx) [54,55,56].
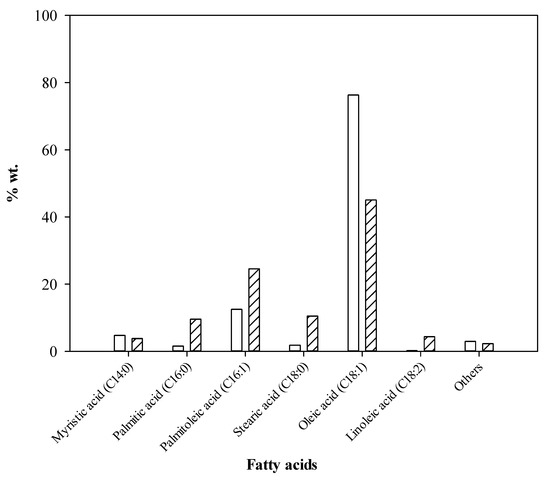
Figure 5.
Comparison of fatty acid methyl esters (FAME) found in CPO/RPO biodiesel as a function of weight (%) (CPO FAME, RPO FAME).
3. Materials and Methods
3.1. Feedstocks and Chemicals
Different grades of palm oil; crude palm oil (CPO) containing acid value of about 11.02 and saponification value (211.25) together with refined palm oil (RPO) were used throughout this study. The CPO sample was kindly provided by Esarn Palm Oil (ESP) Co., Ltd. Sakon Nakorn province, North-East Thailand. Prior to use, samples of CPO were pretreated by heating at 80 °C and filtering with a cotton sheet. Meanwhile, samples of RPO with acid value 0.92 and saponification value 217.06 were collected from a local supermarket near KhonKaen University, KhonKaen, Thailand. All chemical substances used such as calcium chloride (CaCl2), potassium hydroxide (KOH), methanol, hydrochloric acid (HCl), and polyvinyl alcohol were analytical grade. The solid form of alginate was food grade. Only an enzyme lipase powder of Rhizopus oryzae was purchased from Sigma Co., Ltd., Bangkok, central area of Thailand.
3.2. Separation of Free Fatty Acid and Glycerol
To obtain FFAs, 100 mL of CPO or RPO was separately added to a 250 mL-Erlenmeyer flask and then hexane was added to carry out the extraction. The method used was modified from a previous work [26]. The flow diagram of the process is shown in Figure 6. First, CPO and RPO were saponified by 5 M potassium hydroxide (KOH) solution. The reaction mixture consisted of 100 mL of either CPO or RPO and 5 M KOH. The saponification reaction was performed at 60 °C, 250 rpm, for about 30 min. After that, it was left at room temperature. The by-product of glycerol was separated from the reaction medium, with the glycerol bottom layer settling out from the top soap layer. Subsequently, water (30% v/v) was added and 37% HCl was used to adjust the pH down to 1. Second, FFAs were extracted with 45 mL hexane with shaking at 250 rpm for 30 min. Then, both the hydro-alcoholic and hexanic phases were separated using a separating funnel. Finally, hexane was eliminated by evaporation and the FFA solution was washed in triplicate before being used as a substrate in further experiments. The FFA solution was analyzed as described in Section 3.3. The glycerol content was determined using the iodometric titration method [57].
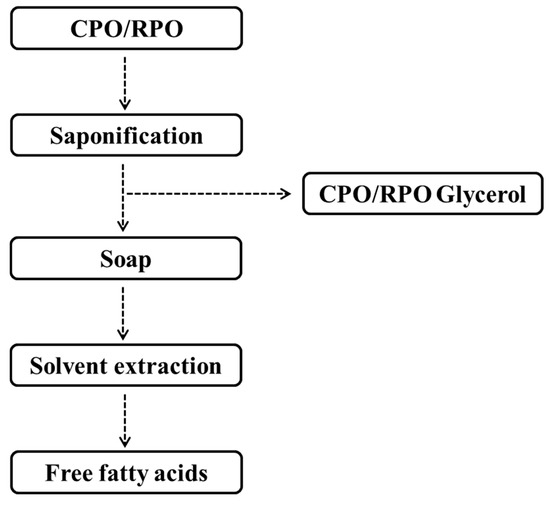
Figure 6.
Flow diagram of free fatty acids (FFAs) extracted from crude palm oil (CPO) and refined palm oil (RPO).
3.3. Determination of Recovery FFAs
The FFA content was determined in terms of acid number. First, 5 g of oil sample was dissolved in 50 mL of alcohol–ether solution. Then, it was titrated with KOH (0.1 M) using phenolphthalein as an indicator. The end point was indicated by a stable pink color appearance. Acid number (mg KOH/g oil) was calculated as shown in Equation (1). The amount of FFAs obtained from CPO/RPO was determined as increasing acid number. The percentage of recovery FFAs was calculated using Equation (2).
where V = volume of KOH consumed (ml); N = normality of KOH; W = sample weight (g)
where AN1 = acid number of oil sample at initial time; AN2 = acid number of oil sample after extraction process
3.4. Preparation of Immobilized Alginate and PVA Lipase
The immobilized lipase beads were prepared by mixing 5% (w/v) lipase solution in pH 7.0 phosphate buffer (10 mL) and 90 mL of matrix solution of polyvinyl alcohol (PVA) and alginate (ratio of 1:1). Then, it was dropped into 0.1M CaCl2 solution onto a magnetic stirrer. The immobilized beads were incubated in CaCl2 at 4 °C for 24 h and the obtained beads were washed in fresh water for 30 min. Finally, the biocatalyst of immobilized alginate-PVA beads was then kept at 4 °C for biodiesel production.
3.5. Biodiesel Production
Biodiesel production via esterification reaction of FFAs from CPO/RPO was carried out in a 250 mL-Erlenmeyer flask with a working volume of 50 mL of FFA solution mixed with methanol together with 2% w/v biocatalyst. After incubation, the immobilized beads were separated from reaction mixture by filtration to stop the reaction. The final reaction mixture was further analyzed for biodiesel yield as described in Section 3.3. Experiments were carried out to optimize variables; temperatures (30–50 °C), ratios of MEOH to FFAs (1:1–5:1), and 100–300 rpm agitation rate. All sample reactions were carried out in triplicate to give an understanding of reproducibility and results obtained are all shown as a mean value ± standard deviation. The acid numbers in oil samples were analyzed using the standard method of biodiesel (ASTM D 6751). The percentage of biodiesel yield was determined as a reduction of acid number in oil samples at the initial time and after the reaction time as shown in Equation (3). Biodiesel or fatty acid methyl esters (FAMEs) were analyzed in both CPO/RPO biodiesel in accordance with European Standard method (EN 14103:2003) by Gas Chromatography–Mass Spectroscopy (GC-MS) as described in our previous studies [58].
where AN1 = acid number of oil sample at initial time; AN2 = acid number of oil sample after the reaction
4. Conclusions
Chemical fatty acid extraction was successful in chemical fatty acid extraction coupling with an enzymatic esterification to clearly improve biodiesel production from CPO and RPO. Under the optimal conditions, high biodiesel yields of 92.14% (CPO) and 95.28% (RPO) were achieved. Furthermore, the immobilized biocatalyst used, possesses high reusability and stability up to 16–17 cycles. Moreover, high glycerol content was also obtained without any additional purification processes. This new route for cleaner biodiesel production presents an alternative approach for biodiesel production from both refined and unrefined vegetable oils with several advantages; low cost production, low water demand, and together with a simple downstream process.
Author Contributions
This article was performed as collaborative research between P.K. and J.W. as well as P.M and P.W. who are assistant researchers. For P.K.; the work included: conceptualization, methodology, resources, formal analysis, data curation, visualization, and writing—original draft preparation, P.M., P.W. planned and performed the experiments and prepared the software. Meanwhile, J.W., carried out the validation and investigation. In addition, P.K. performed the main supervision, project administration, funding acquisition, review, and corrections. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by Royal Golden Jubilee (RGJ) PhD Programme (Contract no. PHD/0018/2557) and Newton Fund Institutional Links (IL) 2019/2020.
Acknowledgments
All authors would like to sincerely and gratefully acknowledge all sponsors: The Royal Golden Jubilee (RGJ) Ph.D. Programme (Contract no. PHD/0018/2557), Bangkok, Thailand and Newton Fund for Institutional Links (IL 2019/2020), the British Council, London, UK for for mainly funding research support, collaborative research, travel bursa-ry and PhD researcher exchange. In addition, the Centre for Alternative Energy Research and Development (AERD), Faculty of Engineering, Khon Kaen University, Khon Kaen, Thailand and are also thanked for the matching fund and travel bursary.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melikoglu, M.; Cinel, A.M. Food waste-water-energy nexus: Scrutinising sustainability of biodiesel production from sunflower oil consumption wastes in Turkey till 2030. Environ. Technol. Innov. 2020, 17, 100628. [Google Scholar] [CrossRef]
- Navaneeth, P.V.; Suraj, C.K.; Mehta, P.S.; Anand, K. Predicting the effect of biodiesel composition on the performance and emission of a compression ignition engine using a phenomenological model. Fuel 2021, 293, 120453. [Google Scholar] [CrossRef]
- Jambulingam, R.; Shalma, M.; Shankar, V. Biodiesel production using lipase immobilised functionalized magnetic nanocatalyst from oleaginous fungal lipid. J. Clean. Prod. 2019, 215, 245–258. [Google Scholar] [CrossRef]
- Wongsawaeng, D.; Ngaosuwan, K.; Kiatkittipong, W.; Laosuttiwong, T.; Chanthon, N.; Kanjanapaparkul, K.; Assabumrungrat, S. Simple and effective technology for sustainable biodiesel production using high-power household fruit blender. J. Clean. Prod. 2019, 237, 117842. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Arul Mozhi Selvan, V. Experimental investigation on the effects of micro eggshell and nano-eggshell catalysts on biodiesel optimization from waste chicken fat. Bioresour. Technol. Rep. 2021, 14, 100658. [Google Scholar] [CrossRef]
- Vinoth Arul Raj, J.; Praveen Kumar, R.; Vijayakumar, B.; Gnansounou, E.; Bharathiraja, B. Modelling and process optimization for biodiesel production from Nannochloropsis salina using artificial neural network. Bioresour. Technol. 2021, 329, 124872. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Kaewkannetra, P. Production of bio-hydrogenated kerosene by catalytic hydrocracking from refined bleached deodorised palm/ palm kernel oils. Renew. Energy 2020, 147, 464–472. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. Optimization of bio-hydrogenated kerosene from refined palm oil by catalytic hydrocracking. Energies 2019, 12, 3196. [Google Scholar] [CrossRef]
- Boons, F.; Mendoza, A. Constructing sustainable palm oil: How actors define sustainability. J. Clean. Prod. 2010, 18, 1686–1695. [Google Scholar] [CrossRef]
- Ayoola, A.A.; Hymore, F.K.; Omonhinmin, C.A.; Babalola, P.O.; Fayomi, O.S.I.; Olawole, O.C.; Olawepo, A.V.; Babalola, A. Response surface methodology and artificial neural network analysis of crude palm kernel oil biodiesel production. Chem. Data Collect. 2020, 28, 100478. [Google Scholar] [CrossRef]
- B10 Biodiesel Implementation in Malaysia—We Speak with MPOB’s Biodiesel Researcher, Dr Harrison Lau. Available online: https://paultan.org/2017/03/06/b10-biodiesel-implementation-in-malaysia-we-speak-with-mpobs-biodiesel-researcher-dr-harrison-lau/#comments (accessed on 14 June 2021).
- Zhao, C.; Yang, L.; Xing, S.; Luo, W.; Wang, Z.; Lv, P. Biodiesel production by a highly effective renewable catalyst from pyrolytic rice husk. J. Clean. Prod. 2018, 199, 772–780. [Google Scholar] [CrossRef]
- de Haro, J.C.; del Prado Garrido, M.; Pérez, Á.; Carmona, M.; Rodríguez, J.F. Full conversion of oleic acid to estolides esters, biodiesel and choline carboxylates in three easy steps. J. Clean. Prod. 2018, 184, 579–585. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Optimization of biodiesel synthesis from nonedible oil using immobilized bio-support catalysts in jacketed packed bed bioreactor by response surface methodology. J. Clean. Prod. 2020, 244, 118700. [Google Scholar] [CrossRef]
- Stojković, I.J.; Stamenković, O.S.; Povrenović, D.S.; Veljković, V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Berrios, M.; Skelton, R.L. Comparison of purification methods for biodiesel. Chem. Eng. J. 2008, 144, 459–465. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Hassan, M.A.; Taufiq-Yap, Y.H.; Shirai, Y.; Hasan, M.Y.; Zakaria, M.R. Waterless purification using oil palm biomass-derived bioadsorbent improved the quality of biodiesel from waste cooking oil. J. Clean. Prod. 2017, 165, 262–272. [Google Scholar] [CrossRef]
- Bashir, M.A.; Thiri, M.; Yang, X.; Yang, Y.; Safdar, A.M. Purification of biodiesel via pre-washing of transesterified waste oil to produce less contaminated wastewater. J. Clean. Prod. 2018, 180, 466–471. [Google Scholar] [CrossRef]
- Vávra, A.; Hájek, M.; Skopal, F. Acceleration and simplification of separation by addition of inorganic acid in biodiesel production. J. Clean. Prod. 2018, 192, 390–395. [Google Scholar] [CrossRef]
- Gargalo, C.L.; Cheali, P.; Posada, J.A.; Carvalho, A.; Gernaey, K.V.; Sin, G. Assessing the environmental sustainability of early stage design for bioprocesses under uncertainties: An analysis of glycerol bioconversion. J. Clean. Prod. 2016, 139, 1245–1260. [Google Scholar] [CrossRef]
- Tu, Q.; Lu, M.; Knothe, G. Glycerolysis with crude glycerin as an alternative pretreatment for biodiesel production from grease trap waste: Parametric study and energy analysis. J. Clean. Prod. 2017, 162, 504–511. [Google Scholar] [CrossRef]
- Haron, R.; Mat, R.; Tuan Abdullah, T.A.; Rahman, R.A. Overview on utilization of biodiesel by-product for biohydrogen production. J. Clean. Prod. 2018, 172, 314–324. [Google Scholar] [CrossRef]
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Dhabhai, R.; Ahmadifeijani, E.; Dalai, A.K.; Reaney, M. Purification of crude glycerol using a sequential physico-chemical treatment, membrane filtration, and activated charcoal adsorption. Sep. Purif. Technol. 2016, 168, 101–106. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 133, 1181–1189. [Google Scholar] [CrossRef]
- López, B.C.; Cerdán, L.E.; Medina, A.R.; López, E.N.; Valverde, L.M.; Peña, E.H.; Moreno, P.A.; Grima, E.M. Production of biodiesel from vegetable oil and microalgae by fatty acid extraction and enzymatic esterification. J. Biosci. Bioeng. 2015, 119, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.B.; Agerbaek, M.A.; Nielsen, P.M.; Rancke-Madsen, A.; Woodley, J.M. Esterification using a liquid lipase to remove residual free fatty acids in biodiesel. Process. Biochem. 2020, 97, 213–221. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Pilipenko, N.; Gonçalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef] [PubMed]
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energy 2020, 146, 901–906. [Google Scholar] [CrossRef]
- Muanruksa, P.; Dujjanutat, P.; Kaewkannetra, P. Entrapping immobilisation of lipase on biocomposite hydrogels toward for biodiesel production from waste frying acid oil. Catalysts 2020, 10, 834. [Google Scholar] [CrossRef]
- Matinja, A.I.; Mohd Zain, N.A.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA-alginate-sulfate beads. Renew. Energy 2019, 135, 1178–1185. [Google Scholar] [CrossRef]
- Asad-ur-Rehman; Saman Wijesekara, R.G.; Nomura, N.; Sato, S.; Matsumura, M. Pre-treatment and utilization of raw glycerol from sunflower oil biodiesel for growth and 1,3-propanediol production by Clostridium butyricum. J. Chem. Technol. Biotechnol. 2008, 83, 1072–1080. [Google Scholar] [CrossRef]
- Yong, K.; Ooi, T.L.; Dzulkefly, K.; Wan Yunus, W.M.Z.; Hazimah, A. Refining of Crude Glycerine Recovered from Glycerol Residue by Simple Vacuum Distillation. J. Oil Palm Res. 2001, 13, 39–44. [Google Scholar]
- Hunsom, M.; Autthanit, C. Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH. Chem. Eng. J. 2013, 229, 334–343. [Google Scholar] [CrossRef]
- Contreras-Andrade, I.; Avella-Moreno, E.; Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A.; Sodré, J.R. Purification of glycerol from biodiesel production by sequential extraction monitored by 1H NMR. Fuel Process. Technol. 2015, 132, 99–104. [Google Scholar] [CrossRef]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Lopes, A.P.; Souza, P.R.; Bonafé, E.G.; Visentainer, J.V.; Martins, A.F.; Canesin, E.A. Purified glycerol is produced from the frying oil transesterification by combining a pre-purification strategy performed with condensed tannin polymer derivative followed by ionic exchange. Fuel Process. Technol. 2019, 187, 73–83. [Google Scholar] [CrossRef]
- Pitt, F.D.; Domingos, A.M.; Barros, A.A.C. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Mowla, O.; Kennedy, E.; Stockenhuber, M. Mass transfer and kinetic study on BEA zeolite-catalysed oil hydroesterification. Renew. Energy 2019, 135, 417–425. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Tan, T.; Nie, K.; Wang, F. Production of biodiesel by immobilized Candida sp. lipase at high water content. Appl. Biochem. Biotechnol. 2006, 128, 109–116. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Wu, J.C.; Fen, N.M.; Melissa, Y.L.S. Two-step lipase catalysis for production of biodiesel. Biochem. Eng. J. 2010, 49, 207–212. [Google Scholar] [CrossRef]
- Kareem, S.O.; Falokun, E.I.; Balogun, S.A.; Akinloye, O.A.; Omeike, S.O. Enzymatic biodiesel production from palm oil and palm kernel oil using free lipase. Egypt. J. Pet. 2017, 26, 635–642. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Ibrahim, S.M.; Yakout, S.M.; El-Zaidy, M.E.; Abdeltawab, A.A. Synthesis of Na+ trapped bentonite/zeolite-P composite as a novel catalyst for effective production of biodiesel from palm oil; Effect of ultrasonic irradiation and mechanism. Energy Convers. Manag. 2019, 196, 739–750. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Jun-Yee, L.; Chan, E.S.; Ravindra, P. Production of biodiesel from palm oil using liquid core lipase encapsulated in κ-carrageenan. Fuel 2010, 89, 2272–2277. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; Alnashef, I.M. A new processing route for cleaner production of biodiesel fuel using a choline chloride based deep eutectic solvent. J. Clean. Prod. 2014, 65, 246–251. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Qing, K.G. Biodiesel production from acidic crude palm oil using perchloric acid. Energy Procedia 2014, 61, 2745–2749. [Google Scholar] [CrossRef]
- Raita, M.; Arnthong, J.; Champreda, V.; Laosiripojana, N. Modification of magnetic nanoparticle lipase designs for biodiesel production from palm oil. Fuel Process. Technol. 2015, 134, 189–197. [Google Scholar] [CrossRef]
- Nongbe, M.C.; Ekou, T.; Ekou, L.; Yao, K.B.; Le Grognec, E.; Felpin, F.X. Biodiesel production from palm oil using sulfonated graphene catalyst. Renew. Energy 2017, 106, 135–141. [Google Scholar] [CrossRef]
- Galeano, J.D.; Mitchell, D.A.; Krieger, N. Biodiesel production by solvent-free ethanolysis of palm oil catalyzed by fermented solids containing lipases of Burkholderia contaminans. Biochem. Eng. J. 2017, 127, 77–86. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Ruhul, M.A.; Abedin, M.J.; Rahman, S.M.A.; Masjuki, B.H.H.; Alabdulkarem, A.; Kalam, M.A.; Shancita, I. Impact of fatty acid composition and physicochemical properties of Jatropha and Alexandrian laurel biodiesel blends: An analysis of performance and emission characteristics. J. Clean. Prod. 2016, 133, 1181–1189. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; Bento, H.B.S.; Izário Filho, H.J.; de Castro, H.F. Approaches to convert Mucor circinelloides lipid into biodiesel by enzymatic synthesis assisted by microwave irradiations. Renew. Energy 2018, 125, 747–754. [Google Scholar] [CrossRef]
- Hájek, M.; Skopal, F.; Vávra, A.; Kocík, J. Transesterification of rapeseed oil by butanol and separation of butyl ester. J. Clean. Prod. 2017, 155, 28–33. [Google Scholar] [CrossRef]
- Muanruksa, P.; Winterburn, J.; Kaewkannetra, P. A novel process for biodiesel production from sludge palm oil. MethodsX 2019, 6, 2838–2844. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).