Abstract
Nanofibers are considered versatile materials with remarkable potential in tissue engineering and regeneration. In addition to their extracellular matrix-mimicking properties, nanofibers can be functionalized with specific moieties (e.g., antimicrobial nanoparticles, ceramics, bioactive proteins, etc.) to improve their overall performance. A novel approach in this regard is the use of enzymes immobilized onto nanofibers to impart biocatalytic activity. These nanofibers are capable of carrying out the catalysis of various biological processes that are essential in the healing process of tissue. In this review, we emphasize the use of biocatalytic nanofibers in various tissue regeneration applications. Biocatalytic nanofibers can be used for wound edge or scar matrix digestion, which reduces the hindrance for cell migration and proliferation, hence displaying applications in fast tissue repair, e.g., spinal cord injury. These nanofibers have potential applications in bone regeneration, mediating osteogenic differentiation, biomineralization, and matrix formation through direct enzyme activity. Moreover, enzymes can be used to undertake efficient crosslinking and fabrication of nanofibers with better physicochemical properties and tissue regeneration potential.
1. Introduction
Nanofibers can be of synthetic or natural origin, which are preferably fabricated through a technique termed electrospinning. Electrospinning affords us 1D nanomaterials in the form of micro and nanofibers. Nanofibers have attained a premier place in the field of tissue engineering and are the main focus of current regenerative medicine owing to their remarkable extracellular matrix-mimicking functional properties [1,2,3]. Nanofibers can be functionalized with additional moieties to impart specific properties in addition to their inherent tissue regenerative properties [4,5,6]. Nanofibers have been studied and successfully evaluated for different uses because of their textured microstructure, which imparts high surface area. The unique structural features and the increased surface area to volume ratio, controllable pore size, biomimetic morphology, and the presence of interconnected pores present in nanofibers suggest efficient delivery of bioactive compounds through nanofiber.
Nanofibers support enzyme immobilization, which can improve enzymes’ performance by increasing surface area, mass transfer resistance, loading efficiency, and the possibility of recycling the enzymes after catalytic performances. The primary emphasis after fabrication of nanofibers so far, however, has been mainly on improving their mechanical properties, cellular adhesion, and biocompatibility. Nevertheless, several studies have used bioactive proteins in nanofibers as moieties for triggering signaling pathways for regulating tissue regeneration [7,8,9]. A unique approach for nanofiber modification is that of enzyme immobilization [10]. As we know, enzymes are versatile biological entities that carry out the catalysis of various reactions to regulate the rate of processes in living organisms, and hence enzymes play a vital role in the optimal working of cellular systems. In tissue repair and regeneration, many enzymes operate to catalyze important reactions for maintaining tissue homeostasis, e.g., collagenases, transglutaminases, metalloproteinases, trypsin, serine proteases, alkyl phosphatase, etc. [11,12,13,14,15]. Inspired by the properties of these natural biocatalysts (i.e., enzymes), several studies have reported enzyme-immobilized nanofibers for improved tissue regeneration applications. These studies show that enzyme-immobilized biocatalytic nanofibers offer a novel approach for the repair and reconstruction of tissues. These nanofibers work by undertaking the catalysis of processes that are essential for the regeneration of tissue, e.g., polymerization of extracellular matrix (ECM) components, increasing gaseous diffusion to assist artificial respiratory devices, etc. Alternatively, these biocatalytic nanofibers help to eradicate certain obstacles that hinder the process of tissue repair or healing, e.g., digestion of components (such as collagen) of a scar to increase its porosity for unrestrained cellular proliferation. Other strategies involve the use of enzymes to facilitate better crosslinking in nanofibers for improved properties and performance. This review provides a comprehensive account of numerous strategies explored for improving the utilization of biocatalytic nanofibers in tissue engineering. In this perspective, here we discuss the applications of biocatalytic nanofibers in wound healing, ECM polymerization, artificial tissue fabrication, bone regeneration, etc.
It is noteworthy to mention that a number of techniques are used to immobilize enzymes on nanofibers utilizing the principles of encapsulation, adsorption, and covalent bonding between the polymers and the enzymes [16,17]. However, encapsulation involves the entrapment of enzymes into the nanofiber network, wherein the enzymes are mixed with the polymer solution, which is later used in nanofiber fabrication. The encapsulation technique increases the enzyme’s stability and ensures that no enzyme leakage occurs from the nanofibers. On the other hand, adsorption involves binding of the enzyme through the formation of weak forces, e.g., electrostatic attraction, hydrophobic and/or Van der Waal’s force. A robust immobilization technique is one through which the formation of covalent bonds between the nanofiber and the enzyme can occur. This covalent bonding-mediated immobilization can occur through the functional groups present on the nanofibers that can interact with enzymes to form bonds, hence arresting the enzyme in the polymer [18]. Physical binding, i.e., adsorption, generally produces weak bonds between the nanofiber and the enzyme, resulting in reversible bond formation. Such type of binding is highly sensitive to process conditions such as pH, temperature, etc. On the other hand, covalent linkage gives rise to highly robust and durable enzyme-immobilized nanofibers but, at the same time, is an expensive method. [19]. We provide in the following sections a comprehensive account of strategies adopted using enzyme-immobilized nanofibers for tissue engineering applications.
2. ECM Digestion
The ECM in the musculoskeletal system’s fibrous connective tissue comprises of aligned collagen fiber bundles that function as microstructural reinforcement to impart mechanical strength to the whole system [20,21,22]. The ECM imparts tissue resistance against external stresses along the fiber length and provides the strength to withstand the external load. During an injury to such tissues, the ECM–collagen microstructure gets disrupted, and hence the mechanical strength and load-bearing capacity of the tissue is altered. During the course of self-healing at the injury site, the tissue develops a scar mass that consists of a disordered high-density collagen fiber structure [22]. The application of a tissue-engineered scaffold at that site is limited because of the already deposited dense ECM of the scar. This is because the naturally existing high-density ECM bears less porosity, which hinders cell proliferation, adhesion, and migration. Hence, it acts as the barrier for endogenous tissue repair, resulting in inadequate healing. In this regard, researchers have suggested the use of the collagen digestion approach as an effective strategy for managing the efficient working of a nanofibrous implant. In this course, several studies have shown effective wound healing while applying digestion of wound edges using matrix-digesting enzymes that are immobilized on nanofibers. Examples of such enzymes that many researchers use as biocatalysts for tissue repair are collagenase, trypsin, hyaluronidase, etc. [23,24,25].
An excellent illustration of the application of this strategy to tissue repair is the one demonstrated by Qu et al. for restoration after knee meniscal injury [26]. It is well known that tearing in joint menisci leads to the destruction of collagen microstructure and the development of dense collagen scar that often results in degenerative osteoarthritis [27,28]. The primary treatment for meniscal injuries is meniscectomy or resection of the affected meniscal area through the surgery. However, the recovery rate during such a procedure is relatively low, and the meniscectomy imparts only temporary pain relief. Several patients need a second surgery, resulting in increased morbidity rates. Furthermore, the surgical procedures may damage articular cartilage and meniscal fibrocartilage, requiring total arthroplasty in some cases [29,30].
The basic idea behind employing a biocatalytic approach for such tissue repair is to decrease the density of meniscal scar to reduce the impedance in the healing process and allow easy cell migration, proliferation, and matrix deposition. Qu et al. fabricated collagenase-incorporated nanofibers for the repair of knee meniscal injury, employing the biocatalysis of collagen degradation in the dense matrix of wound scar. The nanofiber scaffolds were fabricated by electrospinning using 8% w/v polyethylene solution (in 1:1 ethanol/water) or 40–50% w/v polyethylene oxide/poly(ε-caprolactone) solution containing 1.25% w/v collagenase for 10–15 min onto glass coverslips. The in vitro release studies indicated that the nanofibers released the collagenase enzyme in a controllable manner. The scaffolds were evaluated in vitro for meniscal repair in juvenile and adult bovine menisci, as indicated in Figure 1. The scaffolds were placed in the meniscus defects, which significantly reduced the matrix integrity, increased the porosity, and reduced the levels of proteoglycans and collagen at the wound edge. This resulted in an increase in the cell infiltration and integration of fibrils, leading to the closure of more than 90% of the wound within four weeks. The annulus-core boundary of the meniscus displayed relatively higher cell density.
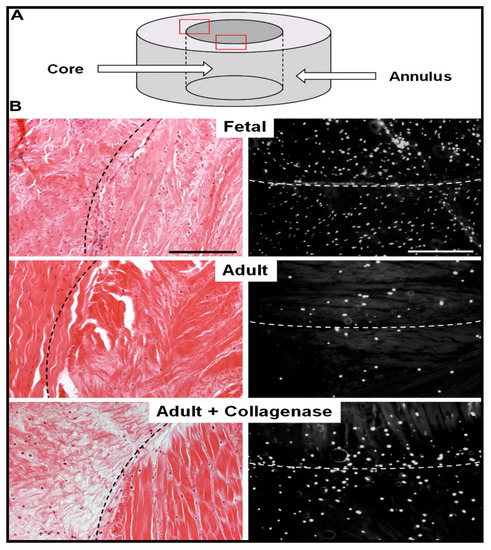
Figure 1.
Reduction in the adult bovine meniscal matrix density and cellularity increase through collagenase-immobilized nanofiber treatment. (A) Schematic showing meniscal annulus and core. (B) Histological analysis of repair constructs using hematoxylin and eosin staining (left) and 4′,6-diamidino-2-phenylindole (right) staining. Reproduced from [26]. Copyright (2013) Elsevier Ltd.
Feini et al. adopted the same approach for the fabrication of biocatalytic nanofibers that incorporated a chemotactic agent in addition to the immobilized collagenase [31]. These researchers hypothesized that in addition to reducing the stiffness and density of native ECM, it was essential to guide the adult repairing cells to migrate to the wound site. Therefore, a chemotactic agent (i.e., platelet-derived growth factor-AB) was used to encapsulate in the nanofibers. These nanofibers were fabricated by electrospinning using 15% hyaluronic acid, 35% poly(ethylene oxide), and 50% poly(ε-caprolactone). Various nanofibrous scaffolds of different compositions that were evaluated are shown in Figure 2.
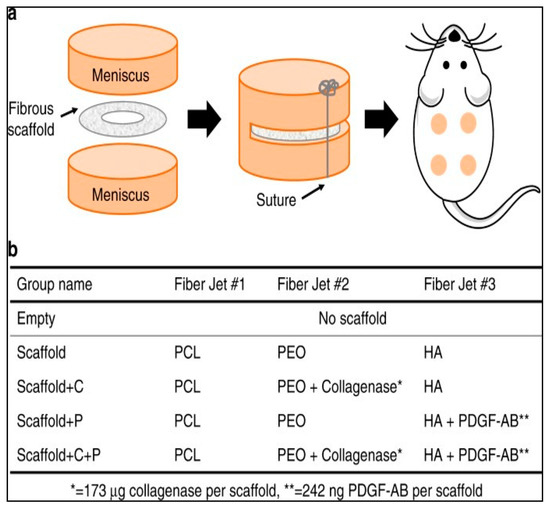
Figure 2.
Nanofibrous scaffolds used for in vivo evaluation of collagenase-immobilized nanofibers. (a) Animal model for tissue repair evaluation: scaffolds placed subcutaneously in rats; (b) composition of various scaffolds used by Feini et al. in their study. PCL: poly(ε-caprolactone); PEO: poly(ethylene oxide); HA: hyaluronic acid; PDGF-AB: platelet-derived growth factor-AB. Reproduced from [31]. Copyright (2017), Feini Qu et al.
The nanofibers were able to successfully release the collagenase with an initial 20% showing a burst release and the remaining 80% released in a controlled manner over a period of 5 h in a phosphate buffer saline. On the other hand, 67% of platelet-derived growth factor-AB was released over a period of 12 days. The nanofibers were evaluated for their tissue repairing application in meniscal defects. The results showed increased cellularity at the wound site and integration with the adjoining tissues. The nanofibers were assessed for their meniscal repair potential in vivo in athymic rats by inserting the nanofibrous scaffolds subcutaneously. This revealed the role of the dense extracellular matrix in hindering the healing process of the wound. The degradation of the wound matrix collagen increased significantly with the use of the novel collagenase scaffold, while allowing for the formation of its own cellular matrix. This was evident from the appearance of interconnected thin collagen fibers that were found to bridge the scaffold with the wound tissue within four weeks (Figure 3).
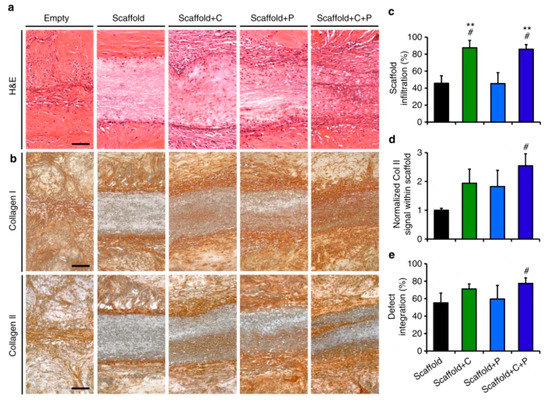
Figure 3.
Effect of collagenase-immobilized, platelet-derived growth factor-AB nanofibrous scaffolds on tissue integration, matrix formation, and cellularity. (a) Histological images of hematoxylin and eosin-stained wound after scaffold fixation. (b) Histological images of the wound after immunostaining showing collagen I and II; (c–e). The cell infiltration, normalized collagen II signal within the scaffold and % defect integration (% integrated tissue at the wound site) for various scaffolds are also presented. # = p < 0.05 vs. Scaffold, ** = p < 0.05 vs. Scaffold + P. These figures are reproduced from [31]. Copyright (2017), Feini Qu et al.
In these results, the cell proliferation and new collagen formation were more evident in the case of collagenase-immobilized scaffolds. An important aspect of these novel scaffolds is that the collagen degradation takes place only up to 300 µm from the wound edge. The study further revealed endogenous cell-mediated wound closure by recruitment was possible through the chemotactic property of the scaffolds.
Another target for extracellular digestion as a fast tissue repair facilitation strategy is the chondroitin sulfate proteoglycan digestion. The formation of a glial scar is considered as one of the major factors restricting the nerve regeneration process during the healing of a spinal cord injury. Therefore, a glial scar remains a chief therapeutic target for the treatment of spinal cord injury and efforts are being put forward to devise methods for eradicating glial scar to facilitate adequate reconstruction. Because the significant component of such a scar is chondroitin sulfate proteoglycans, several researchers have used a biocatalytic approach to eradicate these glial scars associated with spinal cord injury by using the enzyme chondroitinase as the biocatalyst. There is not much literature available demonstrating this application of the biocatalytic nanofibers; however, Liu et al. have applied this approach to the treatment of spinal cord injury [32]. Presently, intrathecal injection of chondroitinase ABC (lasting for weeks) is the central treatment approach for spinal cord injury, which is quite invasive, apart from chondroitinase ABC being thermal sensitive and susceptible to degradation in the host. Additionally, the use of intrathecal chondroitinase ABC injection is restricted because it overflows beyond the injection site, reducing the amount reaching the actual injury site. Liu et al. fabricated collagen scaffolds containing neurotrophin-3 and chondroitinase ABC for the repair of spinal cord injury. These nanofibers were able to release both neurotrophin-3 and chondroitinase ABC in a sustained manner, thus avoiding the rapid clearance from the site of action, which generally happens with conventional drugs. A similar combination for spinal cord injury treatment has already been attempted by other researchers who demonstrated an enhanced locomotor function and sensory axon growth in rats [33]. However, hydrogels are generally isotropic and weak. Furthermore, hydrogels exhibit poor mechanical and morphological properties, which are pivotal for cell alignment, migration, and proliferation. On the other hand, nanofibers fulfill all these requirements and provide an excellent alternative as a scaffold for neurotrophin-3 and chondroitin ABC delivery for neuronal repair in the treatment of spinal cord injury. Nanofibers’ excellent topographical properties enable their use in spinal cord injury due to their ability to increase cells, as demonstrated by Chew et al. using Schwann cells [34]. Further, when incorporating neurotrophic factors in the topographically favorable nanofibers, an increase in nerve regeneration was observed [35]. As demonstrated by Liu et al., nanofibers have been used in the treatment of acute spinal cord injury in rats [36].
The nanofibers fabricated by Liu et al. provided sustained release of both neurotrophin-3 and chondroitin ABC, minimizing the chances of losing therapeutic agents. These researchers used rat tail extracted collagen scaffolds for immobilization of neurotrophin-3 and chondroitin ABC. The immobilization was achieved by crosslinking through microbial transglutaminase crosslinking. In addition to this, heparin was added to increase the protective effect on chondroitin ABC. This was evident because the chondroitin ABC showed 32% bioactivity after 32 days compared with the 1.9% action after 22 days.
3. Extracellular Matrix Protein Polymerization
Quite contrary to the previous approach (ECM digestion) for tissue repair is ECM polymerization, which is justifiable in its own space. The ECM is considered the primary component of tissue and focuses on current material science research for tissue engineering applications. The main reason for nanofibers’ success in wound healing and tissue engineering is their ECM-mimicking factor [6,37]. The ECM consists of several fibrous proteins and glycosaminoglycans (such as fibronectin, elastin, hyaluronic acid, collagen, etc.) embedded in the fluid matrix that act as reinforcement and impart structural integrity to the tissues (Figure 4) [38].
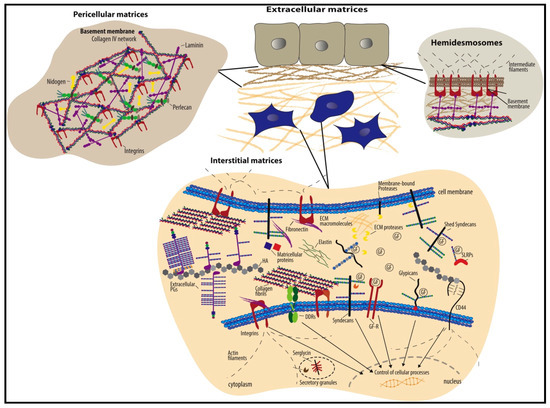
Figure 4.
Schematic representation of the ECM structure consisting of two major types, viz. pericellular matrix and interstitial matrix. The figure shows some of the significant components of the ECM. It is reproduced with permission from [38], Copyright (2015), Elsevier B.V.
Hence, focusing on ECM proteins’ production is another strategy for the repair and regeneration of degenerated or injured tissues. One of the prime groups of enzymes is transglutaminases that mediate extracellular matrix proteins’ polymerization through glutamine–lysine interaction (Figure 5). These enzymes carry out transamidation and catalyze the formation of isopeptide crosslinks between proteins and/or amino acids. Hence, these facilitate the polymerization of proteins, such as fibronectin, collagen, osteopontin, osteonectin, fibrinogen, etc., which are essential constituents of the ECM. Transglutaminases show protective action in some pathological conditions, such as osteoarthritis, hepatic injury, kidney disease, and wound healing. In osteoarthritis, transglutaminases have been shown to exhibit enhanced activity, thus regulating bone mass formation and preventing bone resorption and osteoclastogenesis. Transglutaminases demonstrate bone regeneration properties by interacting and binding to various substrates and by mediating their accumulation in the bones. A well-known substrate of transglutaminase is fibronectin, which is essential for cell proliferation and ECM deposition. Fibronectin deposition and accumulation in osteoblasts in the bones has been shown to be dependent on transglutaminases. Another mechanism by which transglutaminases are known to induce ECM polymerization is ECM–cell adhesion through surface-related high-affinity fibronectin crosslinking. This has been attributed to the observation that inhibition of transglutaminases makes the cells more liable to detach from the surface.
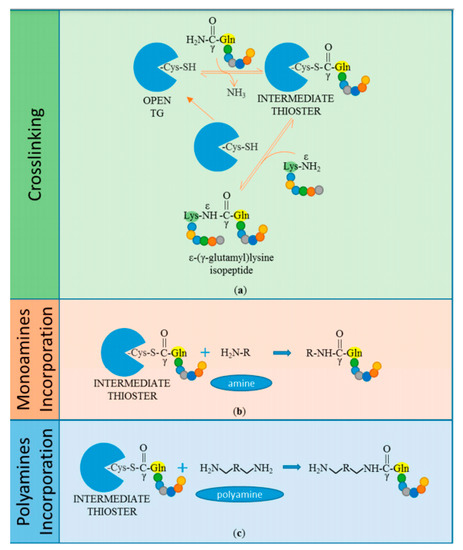
Figure 5.
Transglutaminases undertaking the catalysis of transamidation of substrates. (a) The cysteine-thiol moiety of the transglutaminase enzyme binds to the γ-carboxamide of glutamine residue on peptide substrate, resulting in a thioesterbon formation d, additionally releasing ammonia. When an acyl intermediate product comes in contact with a nucleophilic substrate, it results in a crosslink bond which is an ε-(γ-glutamyl)lysine isopeptide bond. Similarly, the enzyme can catalyze the acyl transfer to (b) monoamines and (c) polyamines. Reproduced with permission from [47]. Copyright (2018) multidisciplinary digital publishing institute.
Further, the fact that externalization of transglutaminases occurs only at the basal surface of endothelial cells compared with the apical surface also shows the involvement of transglutaminases in cell adhesion [39]. The transglutaminase enzyme activity is regulated by Factor XIIIA, which converts plasma fibronectin into the ECM, as shown by Cui et al. Transglutaminase also carries out vascular lesion healing through induction of platelet–fibrin–endothelium stability and TGF-β activation [40,41]. Transglutaminase enzymes are also involved in the crosslinking of proteins constituting dermo–epidermal junction at the tissue interface. This has been revealed by findings of stable skin grafts corresponding to the presence of transamidation of reinforcing fibrils and ECM of the skin. The ECM protein transamidation catalyzed by transglutaminases results in stabilized protein complexes that are impervious to digestion by metalloproteinases [42]. Nardacci et al. have demonstrated the protective role of transglutaminase II in hepatic injury. It was found that transglutaminase’s low activity in mice was responsible for the delayed healing or lack of necrotic tissue clearance of carbon tetrachloride-induced liver injury. An increase in transglutaminase expression up to 3–4 times was observed in hepatitis C virus-infected patients [43]. These enzymes induce the matrix production during fibrotic scarring through post-translational protein modification through their catalytic triad (Cys/His/Asp). Hence this enzyme seems to be involved mainly in the natural healing process and can be pivotal in inducing tissue repair through ECM polymerization when used as nanofiber dressings.
The polymerization of proteins, e.g., collagen using transglutaminases, has been already demonstrated by many researchers and has stirred great interest in its use for the fabrication of nanofibrous scaffolds. However, these generally require a covalent crosslinking agent to display stable integrity since it is prone to physicochemical changes and degradation by collagenases. Chemical crosslinking agents, such as glutaraldehyde, have been primarily used to improve these properties; however, these agents always present the risk of toxicity. For this reason, several researchers have used biocatalytic methods to insert crosslink bonds between the polymer molecules. An excellent example is that of collagen, which is a component of the extracellular matrix essential for maintaining the structural integrity and mechanical properties of connective tissues and thus has been extensively used as a scaffolding material. Transglutaminases have been shown to efficiently induce crosslink bonds between collagen fibrils and collagen and ECM proteins such as fibronectin [44,45]. Transglutaminase mediates the crosslinking of collagen molecules through chemical interaction between the glutamine on one fiber with the lysine on the other fibers, thus providing a strong reinforcement to hold the 3D scaffold structure. Orban et al. have used transglutaminase for biocatalytic crosslinking of collagen for scaffold fabrication for tissue engineering application [46]. The amount of crosslinking was revealed by amine analysis (assessing the content of amine groups) of the nanofibers since the crosslinking occurs by transamidation. The amine analysis showed a decrease in the number of amine groups as the concentration of transglutaminase was increased, indicating intensification in the degree of crosslinking. The resulting scaffolds possessed excellent mechanical properties, as depicted by the increased transition temperature by transglutaminase-aided nanofiber fabrication through differential scanning calorimetry results. In addition to providing for an excellent option for macromolecular crosslinking, transglutaminase use also reduced the cytotoxic effect of the nanofibers, as demonstrated in bone marrow stromal cell culturing.
4. Artificial Tissue Fabrication
Nowadays, fibrous scaffolds are considered a promising tool for the fabrication of artificial tissues and organs, especially the lungs. Moreover, lung transplantation, mechanical ventilators, and respiratory assist devices have been vital options for treating chronic obstructive pulmonary disease and/or chronic irreversible pulmonary failure. Current devices used for the purpose are intravenacaval devices, such as intravenous membrane oxygenators and intravascular oxygenators. These devices consist of hollow fiber membranes that mediate gaseous exchange at the blood/gas interface. Although these devices have found a premier place in addressing respiratory failure, they have been reported to mediate inefficient gaseous exchange and removal of CO2, attributed to their inadequate diffusion characteristic due to small membrane contact area with the blood. This is because large blood contact surfaces of these devices present biocompatibility and thrombotic response issues. In contrast to increasing the respiratory devices’ contact surface, many researchers have sought to increase the diffusional efficiency of these devices instead, using mechanical methods, e.g., increasing the blood flow through agitation near the artificial respiratory device [48,49,50,51,52]. Biologically, our red blood cells and the lung capillary surfaces contain carbonic anhydrase that carries out the catalytic conversion of bicarbonates to CO2 in the course of maintaining a sufficient CO2 concentration gradient across the blood–lung interface. Inspired by the natural red blood cell functionality, scientists have developed new biocatalytic devices harboring carbonic anhydrase on their surfaces that works similarly to the red blood cells and lung capillary surfaces present in the human body. These biocatalytic fibrous scaffolds are a novel approach that the researchers are seeking for a solution to the aforementioned problem. In this regard, Kaar et al. have devised novel bioactive fiber-based membranes to demonstrate their potential in eradicating the diffusional limitation of hollow fiber membrane-based respiratory devices and artificial lungs. These devices use hollow fiber membranes containing surface-immobilized carbonic anhydrase for the purpose. Carbonic anhydrase is an enzyme that in blood mediates the conversion of bicarbonates to CO2. The novel bioactive device comprising immobilized carbonic anhydrase has demonstrated more significant potential in improving the hollow fiber membrane-facilitated diffusion and exchange of CO2. The device improves the CO2 concentration and increases its diffusion, thus providing a versatile option for addressing the otherwise diffusional inadequacy of the artificial lungs. The enzyme was covalently immobilized onto the hollow fiber membrane surface after plasma modification of the fibers. Before the enzyme immobilization, the membranes were modified by introducing surface-active hydroxyl groups through plasma treatment with a plasma discharge of 25–100 W for 30 to 180 s. Additional activation of the membranes was achieved by the treatment of cyanogen bromide, followed by treatment with carbonic anhydrase and then the samples were incubated for 3 h. The performance of the fiber membranes was evaluated by assessment of CO2 exchange using a model respiratory device. The evaluation was done by measuring the CO2 removal rate of carbonic anhydrase-immobilized membranes from a buffer containing sodium bicarbonate. Thus, this study provides a demonstration of how carbonic anhydrase-immobilized fiber membranes can increase the CO2 removal efficiency and thus improve the performance of artificial lungs and respiratory devices. Figure 6 shows the working of carbonic anhydrase-assisted CO2 diffusion using enzyme-immobilized respiratory devices.
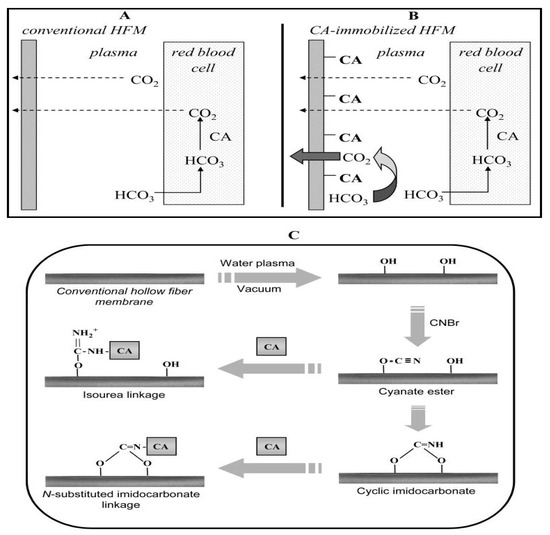
Figure 6.
CO2 diffusion through (A) a conventional and (B) a carbonic anhydrase-immobilized hollow fiber membrane. (C) Steps for the immobilization of carbonic anhydrase on hollow fiber membranes. The membranes are initially plasma modified, resulting in the deposition of –OH groups on the substrate, followed by activation with cyanogen bromide, which results in the introduction of cyanate ester and cyclic imidocarbonate groups. Carbonic anhydrase is then subsequently immobilized through N-substituted imidcarbonate or isourea bond formation. Reproduced with permission from [53].
Another example of fabricating biocatalysis-assisted artificial tissue is that of artificial blood vessels facilitating the in situ release of nitric oxide for better cardiovascular functioning and vascular tissue regeneration. Nitric oxide released by endothelial cells plays a vital role in maintaining the homeostasis of the cardiovascular system. It regulates blood pressure through vasodilation and prevents vascular smooth muscle cell proliferation and platelet aggregation and adhesion to the vascular wall. Insufficiency of nitric oxide in the blood is an underlying cause of various cardiovascular diseases, such as hypertension, stroke, arterial thrombotic disorders, atherosclerosis, coronary heart disease, and heart failure. This is in agreement with the fact that most of these pathological conditions are accompanied by vascular endothelium dysfunction, which is the prime source of nitric oxide in the blood. Drugs are known as nitric oxide donors and many other nitric oxide release pathway modulators are being frequently used in the treatment of cardiovascular diseases. These therapeutic agents work by increasing the concentration of nitric oxide in the blood and restoring the nitric oxide-mediated cardiac functions. In addition to these drugs, vascular bypass surgery using artificial vessels has a primary place in cardiovascular disease treatment. Artificial blood vessels are a new interest of researchers for replacing blood vessels having defunct endothelium, which has proven quite beneficial. However, such procedures still do not provide adequate support for long-term relief from cardiac problems and a lot of research is dedicated to making these vessels more clinically useful. Most of the research’s prime focus has been on rendering these artificial vessels more and more biocompatible, improving cell attachment, and increasing cell proliferation, rather than incorporating any bioactive components for additional applicability. In some cases, nitric oxide donors have been used in artificial blood vessel scaffolds to compensate for the nitric oxide deficiency [54,55,56,57,58,59,60,61]. However, these drug-loaded scaffolds’ most significant limitation is that nitric oxide supply persists only until the nitric oxide donor reservoirs last. A novel strategy, known as enzyme prodrug therapy, has been used for addressing this problem by fabricating biocatalytic fibrous vascular grafts immobilizing galactosidase enzyme. The advantage of such a treatment is that it allows for precise dose control and duration of action. Wang et al. fabricated artificial blood vessels (tubular grafts) by electrospinning (under 11 kV voltage) a mixture of poly(ε-caprolactone) and azide group terminated poly(ε-caprolactone) [62]. The azide terminated poly(ε-caprolactone) enabled the introduction of azide groups on the tubular grafts’ surface, which mediated surface functionalization with alkynyl–biotin and avidin. Through the biotin–avidin complex, the galactosidase enzyme immobilization was then carried out on the tubular graft surfaces. The artificial tubular graft enables the in situ decomposition of nitric oxide prodrug, injected intravenously after the in vivo implantation, releasing nitric oxide at the site of action. The in vitro catalytic activity of these biocatalytic grafts was evident during testing with diazeniumdiolates, which underwent hydrolysis in the presence of biocatalytic grafts in PBS, spontaneously releasing nitric oxide. These grafts were able to display significant catalytic activity even after subcutaneous implantation for up to 30 days. Further, the real-time release of nitric oxide was assessed in actual blood flow conditions in rats using arteriovenous shunt assay. Using diaminofluoresceins as a probe, the nitic oxide was detected in the circulation after 1 h of the tubular graft fixation and nitric oxide prodrug injection, verifying the galactosidase catalyzed in situ nitric oxide release. It is important to mention that avidinavidin’s presence did not interfere with the nitric oxide release, which was evident from the fact that avidin-functionalized grafts not immobilized with galactosidase could not catalyze the release of nitric oxide. Finally, the in vivo evaluation was carried out by implantation of the tubular grafts as a replacement for abdominal artery in rats. The in vivo results revealed the formation of the new and significantly thick tissue on the vascular tubular grafts and speedy endothelialization after one month. Further, the regenerated endothelium was able to respond to the adrenaline and acetylcholine for vasoconstriction and vasodilation, respectively, unlike the control group.
5. Bone Regeneration
Alkaline phosphatase is an enzyme that undertakes dephosphorylation of many compounds and plays a role in bone development in our body other than regulating various other physiological functions, e.g., in the liver, kidney, bile duct, etc. A schematic representation of the dephosphorylation process is depicted in Figure 7.
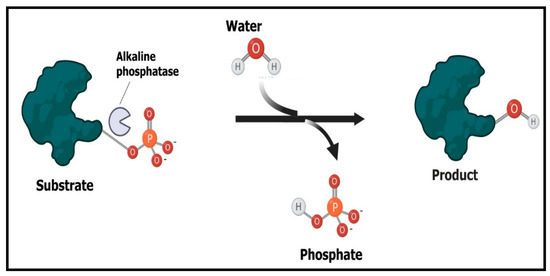
Figure 7.
Mechanism of working of alkaline phosphatase enzyme. The enzyme catalyzes the dephosphorylation process through the cleavage of phosphate bond in phosphoric acid monoesters to give rise to phosphate and alcohol.
The alkaline phosphatase promotes bone development through bone mineralization by hydrolyzing inorganic pyrophosphate, a natural inhibitor of hydroxyapatite formation and mineralization. Thus, the increased levels of inorganic phosphate are consumed for the construction of hydroxyapatite, a naturally existing mineral form of calcium apatite and a vital element of bone and teeth. Researchers have utilized the above-discussed functionality of alkaline phosphatase as a biocatalytic strategy for bone regeneration using nanofibrous scaffolds. Immobilizing alkaline phosphatase onto nanofibrous scaffolds has been demonstrated as being effective in the revival of new bone tissue. Moreover, alkaline phosphate is believed to undertake the deposition of acellular cementum in periodontal sites. This has evidence from studies in alkaline phosphate knockout mice, where the absence of alkaline phosphate significantly reduced the cementum deposition [63]. These researchers also observed the enhanced mineralization on alkaline phosphate-immobilized collagen membranes compared with non-functionalized membranes. Osathanon et al. have developed nanofibrous fibrin scaffolds using the polymer poly(methyl methacrylate) immobilized with alkaline phosphate with defined pore size and interconnection [64]. Prior to enzyme immobilization, the –COOH groups on the nanofibrous scaffolds were activated by 1-ethyl-3-(dimethylaminopropyl)carbodiimide hydrochloride (EDC). The nanofibrous scaffolds containing immobilized alkaline phosphate showed excellent results during the in vitro proliferation and differentiation of calvarial cells. The novel biocatalytic nanofibrous scaffolds were effective in filling the mouse calvarial defects and inducing bone formation as well. The main effect of these novel biocatalytic scaffolds was an increase in the concentration of phosphate locally, which promoted the formation of osteoblasts vis-à-vis being biodegradable. Culturing calvarial cells on the alkaline phosphate-immobilized nanofibrous scaffolds resulted in enhanced expression of alkaline phosphate mRNA and higher phosphate concentration, suggesting increased degradation of β-glycerophosphate. Furthermore, the mineral deposition on the scaffolds also increased when the scaffolds immobilized with alkaline phosphate were incubated in β-glycerophosphate-containing medium.
Oortgiesen et al. demonstrated the bone and periodontal regeneration potential of alkaline phosphatase-immobilized membranes using Bio-Gide® and Bio-Oss® for periodontal regeneration [65]. The enzyme immobilization was achieved through an electrospray deposition. Obtained from bovine intestine, the alkaline phosphate-immobilized membranes increased the in vitro mineralization within 30 min and in vivo bone formation in Wistar rats. Furthermore, the micro-CT and histopathological results indicated immobilization of enzymes in these fibrous membranes could lead to guided tissue and bone regeneration.
6. Enzyme Mimetic Nanofibers
In addition to the above-mentioned strategies involving enzyme immobilization onto the micro-nanofibers that can be adopted to catalyze specific processes to improve their functionality, nanofibers can be instead fabricated from materials (e.g., peptides) to impart biocatalytic action. Such nanofibers are fabricated from artificial structures resembling enzymes that mimic the catalytic activity of enzymes without actually immobilizing the enzyme. A prime example of an enzyme mimicked by nanofibers is that of alkaline phosphatase. In an enzyme mimetic nanofiber, the most crucial consideration is that of the active sites of essential enzymes. Therefore, precisely knowing the mechanism by which alkaline phosphatase works, is essential. The biocatalytic-mimicking properties of these peptide nanofibers is exhibited through its imidazole-containing histidine moieties, which catalyze substrate hydrolysis through a simple chemical reaction. These moieties catalyze the deprotonation of water, thus generating a nucleophile with high affinity with the phosphate monoester, yielding phosphate ions that initiate hydroxyapatite formation. The construction of hydroxyapatite, an essential component of the bone matrix, is an indication of the successful biocatalytic activity of these enzyme mimetic nanofibers. However, it is hypothesized that the peptide nanofibers show higher catalytic activities when imidazole/histidine is present in high density on the nanofibers. Gulseren et al. fabricated this type of peptide-based nanofiber for osteogenic differentiation, mimicking the biocatalytic activity of the enzyme alkaline phosphatase [66]. Figure 8 shows the TEM images of these nanofibers and the peptide amphiphiles structures used for nanofiber fabrication with and without imidazole rings.
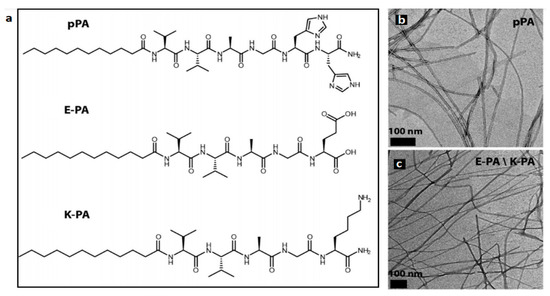
Figure 8.
Peptide amphiphile molecules. (a) Chemical structure and (b,c) TEM images of peptide amphiphile fibers. pPA: Lauryl-VVAGH-Am, E-PA: Lauryl-VVAGE, K-PA: Lauryl-VVAGK-Am. Reproduced from [67]. Copyright (2015), American Chemical Society.
The biocatalytic peptide nanofibers developed by these researchers had three distinct properties. For instance, they possessed an extracellular matrix mimicking the fibrous structure and maintained high catalytic sites with alkaline phosphatase-like activity and biomineralization-inducing functions. The nanofibrous structures (diameter 5–10 nm) fabricated from peptide amphiphile, Lauryl-VVAGH-Am, possessed catalytic sites composed of imidazole-containing histidine residues at the boundary. The peptide nanofibers’ catalytic activity was evaluated by determining the conversion of p-nitrophenyl phosphate to p-nitrophenol, which is mediated by histidine. The peptide nanofibers exhibited significantly high catalytic activity, as depicted by the high value of the Michaelis−Menten constant (kcat = 1.83 × 10−5 s−1). The nanofibers also displayed a significant deposition of calcium phosphate crystals compared with the free imidazole and imidazole-free nanofibers, as determined by the energy-dispersive X-ray analysis. An essential aspect of the biomimetic peptide nanofibers was that the nanofibers were able to impart time-dependent regulation of biocatalytic activity. This implies that as the biomineralization progresses, it results in the formation of calcium phosphate crystals that occupy and hence block the access of the substrate on the active sites on the nanofibers. In this regard, Figure 9 shows the SEM and optical microscopy images of these calcium deposited nanofibers after culturing them for 6 days in osteogenic medium.
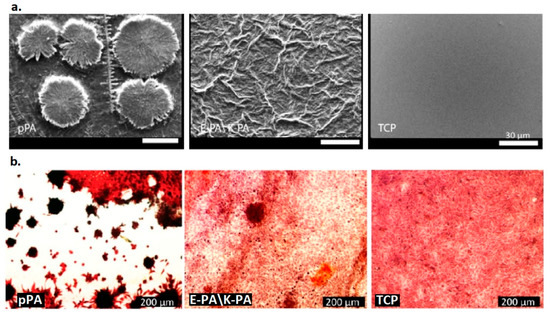
Figure 9.
Calcium phosphate deposition on samples. (a) SEM images of calcium phosphate deposited on peptide amphiphile nanofibers and (b) optical microscope images of samples (Alizarin Red stained) at day 6 in osteogenic medium. pPA: Lauryl-VVAGH-Am, E-PA\K-PA: Lauryl-VVAGE/Lauryl-VVAGK-Am, TCP: uncoated tissue culture plates. Reproduced from [67] Copyright (2015), American Chemical Society.
The nanofibers were further able to initiate osteogenic differentiation of Saos-2 cells through directly regulating the mineralization process, unlike many other extracellular matrix-mimicking nanofibrous scaffolds that induce signaling pathways for cellular differentiation. The cells grown on the alkaline phosphate-mimicking nanofibers could carry out the formation of bone-like nodules within 3–6 days. This was in contrast to the cells grown on culture plates and nanofibers devoid of histidine sites, where significantly less calcium deposition was observed. An extraordinary property of the novel peptide nanofibers was that osteogenic differentiation and bone regeneration required only the presence of an organic phosphate without many osteogenic supplements being used in the osteogenic medium, unlike many other types of nanofibers. This resulted in the formation of bone nodules depicting the strong osteogenic and biomineralization potential of the novel alkaline phosphate-mimicking peptide nanofibers. In addition to these observations, various osteogenesis-related transcription factors were also observed to increase, including Runt-related transcription factor-2, collagen type I osteopontin, and bone sialoprotein. The study was further extended for evaluating the osteogenic potential of the nanofibers in mesenchymal stem cells, which also revealed promising results.
7. Limitations of Enzyme-Based Nanofiber and Future Perspective
Although the enzyme-immobilized nanofibers have tremendous advantages that can be exploited for diverse applications, due to certain limitations they can still not be realized for clinical or large-scale use. The first issue with the enzyme-based nanofibers is their bulk production, owing to the difficulty in the fabrication of nanofibers at a large scale. Secondly, the nanofibers’ enzyme-loading efficiency is also generally low, which means a high cost on large-scale production. Further, the immobilization of the enzymes on nanofibers may change the behavior and enzyme activity, rendering the enzymes less stable. The enzymes’ attachment to the nanofiber surface may lead to conformational changes in the enzyme, resulting in alterations in the enzyme kinetic parameters [67]. This can lead to the inactivation of the enzymes, e.g., by aggregate formation, destruction of bonds, or formation of bonds between wrong side chains. Another limitation of the enzyme-immobilized nanofibers is that the enzyme-based nanofibers may also require complicated cofactors for their functioning, which makes this approach cumbersome and costlier [68].
A significant health risk associated with nanofibers is that these require organic solvents for their fabrication, which can prove toxic to humans. Further, the nanofibers act as excellent substrates for the growth of microbes, resulting in the formation of biofilms facilitating the development of diverse microorganisms on nanofiber surfaces. If not appropriately addressed using antimicrobial agents, this can spoil the scaffolds or implants, exaggerate the pathological condition, and finally reduce the enzyme activity, thus worsening the tissue repair further. In the future, research focusing on novel strategies employing enzyme-immobilized biocatalytic nanofibers for diverse tissue engineering applications is required. A thorough understanding of enzyme functioning in various organs can be used for the development of a vast number of biocatalytic nanofibers exhibiting multiple applications. Suitable approaches (e.g., using whole cells instead of isolated enzymes) can be devoted to overcoming these limitations, thus rendering these biocatalytic nanofibers fully functional for improved tissue engineering. Therefore, biocatalytic nanofibers can find applications as wound dressings, artificial tissues, bone and tissue regenerators, etc. Moreover, enzyme-based nanofibers can be used to improve the functioning of tissues such as the cardiovascular system, liver, and kidney.
8. Conclusions
From the above discussion, it follows that enzyme-immobilized nanofibers have potential implications in tissue engineering, including wound healing, tissue repair, tissue regeneration, cell differentiation, artificial tissue fabrication, etc. However, not much research in this field has been undertaken to exploit enzyme-immobilized nanofiber applications in tissue engineering. The available literature depicts a considerable prospect for this novel approach, one which requires prompt scientific attention. Such a strategy can help in the fabrication of nanofibers with better properties, improving nanofiber performance and the fabrication of artificial tissues. These biocatalytic nanofibers have application in tissue regeneration after surgery, bone regeneration after a fracture, as support for patients with lung disease, and in wound healing.
Author Contributions
Conceptualization, T.U.W. and F.A.S.; Methodology, T.U.W.; Software, T.U.W., A.H.R. and R.S.K.; Validation, M.P., B.P., and F.A.S.; Formal analysis, M.A.B. and F.A.S.; Investigation, T.U.W.; Resources, F.A.S.; writing—original draft preparation, T.U.W.; Writing—review and editing, T.U.W. and F.A.S.; Visualization, A.H.R. and R.S.K.; Supervision, F.A.S.; project administration, F.A.S.; funding acquisition, F.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
ICMR File No: 45/13/2020-Nan/BMS, SERB File No: CRG/2020/000113 and NRF-Korea File No: 2018M3C1B5052283.
Data Availability Statement
No applicable.
Acknowledgments
The authors are thankful to the Indian Council of Medical Research (ICMR) research grants 45/13/2020-Nan/BMS, New Delhi, and the Science and Engineering Research Board (SERB) research grants (CRG/2020/000113) for supporting this work. This work was also supported by the Traditional Culture Convergence Research Program through the National Research Foundation of Korea (NRF), grant funded by the Ministry of Science, CT & Future Planning (2018M3C1B5052283).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric Nanofibers in Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef]
- Liao, S.; Li, B.; Ma, Z.; Wei, H.; Chan, C.; Ramakrishna, S. Biomimetic electrospun nanofibers for tissue regeneration. Biomed. Mater. 2006, 1, R45–R53. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.-I.; Ko, S.W.; Kim, H.-J.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar] [CrossRef]
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Sridhar, S.; Ramakrishna, S. Functionalized hybrid nanofibers to mimic native ECM for tissue engineering applications. Appl. Surf. Sci. 2014, 322, 162–168. [Google Scholar] [CrossRef]
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.-H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kim, J.; Kim, S.E.; Song, S.-J.; Hong, S.W.; Oh, J.-W.; Lee, J.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regen. Biomater. 2017, 4, 159–166. [Google Scholar] [CrossRef]
- Junka, R.; Valmikinathan, C.M.; Kalyon, D.M.; Yu, X. Laminin Functionalized Biomimetic Nanofibers for Nerve Tissue Engineering. J. Biomater. Tissue Eng. 2013, 3, 494–502. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Sun, Y.; Weber, K.T. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J. Mol. Cell. Cardiol. 1996, 28, 851–858. [Google Scholar] [CrossRef]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Jiang, F.; Peshavariya, H.M.; Dusting, G.J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: Potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 2009, 122, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Mignatti, P.; Rifkin, D.B.; Welgus, H.G.; Parks, W.C. Proteinases and tissue remodeling. In The Molecular and Cellular Biology of Wound Repair; Clark., R.A.F., Ed.; Springer: Boston, MA, USA, 1988; pp. 427–474. [Google Scholar] [CrossRef]
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)—A wound response enzyme. Front. Biosci. 2006, 2006. 11, 867–882. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, A.M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Sirisha, V.; Jain, A.; Jain, A. Enzyme immobilization. Adv. Food Nutr. Res. 2016, 79, 179–211. [Google Scholar]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 2011. 44, 318–331. [Google Scholar] [CrossRef]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Subramanian, A.; Schilling, T.F. Tendon development and musculoskeletal assembly: Emerging roles for the extracellular matrix. Development 2015, 142, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Bravenboer, J.V.D.B.; Der Maur, C.D.I.; Bos, P.K.; Feenstra, L.; Verhaar, J.A.N.; Weinans, H.; Van Osch, G.J.V.M. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res. 2004, 6, R469. [Google Scholar] [CrossRef]
- Obradovic, B.; Martin, I.; Padera, R.F.; Treppo, S.; Freed, L.E.; Vunjak-Navakovic, G. Integration of engineered cartilage. J. Orthop. Res. 2001, 19, 1089–1097. [Google Scholar] [CrossRef]
- Janssen, L.M.; Der Maur, C.D.I.; Bos, P.K.; Hardillo, J.A.; Van Osch, G.J.V.M. Short-Duration Enzymatic Treatment Promotes Integration of a Cartilage Graft in a Defect. Ann. Otol. Rhinol. Laryngol. 2006, 115, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Lin, J.-M.G.; Esterhai, J.L.; Fisher, M.B.; Mauck, R.L. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013, 9, 6393–6402. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Cole, B.J.; Freedman, K.B. Augmentation of meniscus repair. Oper. Tech. Sports Med. 2003, 11, 127–133. [Google Scholar] [CrossRef]
- Aagaard, H.; Verdonk, R. Function of the normal meniscus and consequences of meniscal resection. Scand. J. Med. Sci. Sports 2007, 9, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Englund, M. Meniscal tear—A feature of osteoarthritis. Acta Orthop. Scand. 2004, 75 (Suppl. 312), 1–45. [Google Scholar]
- Qu, F.; Holloway, J.L.; Esterhai, J.L.; Burdick, J.A.; Mauck, R.L. Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat. Commun. 2017, 8, 1780. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Chan, B.P.; Chew, S.Y. Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2012, 100, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 3340–3345. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 2008, 29, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Houle, J.D.; Xu, J.; Chan, B.P.; Chew, S.Y. Nanofibrous Collagen Nerve Conduits for Spinal Cord Repair. Tissue Eng. Part A 2012, 18, 1057–1066. [Google Scholar] [CrossRef]
- Srouji, S.; Kizhner, T.; Suss-Tobi, E.; Livne, E.; Zussman, E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J. Mater. Sci. Mater. Med. 2007, 19, 1249–1255. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Aeschlimann, D.; Thomazy, V. Protein Crosslinking in Assembly and Remodelling of Extracellular Matrices: The Role of Transglutaminases. Connect. Tissue Res. 2000, 41, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Nara, K.; Rifkin, D.B. Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J. Cell Biol. 1993, 121, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Nunes, I.; Gleizes, P.-E.; Metz, C.N.; Rifkin, D.B. Latent Transforming Growth Factor-β Binding Protein Domains Involved in Activation and Transglutaminase-dependent Cross-Linking of Latent Transforming Growth Factor-β. J. Cell Biol. 1997, 136, 1151–1163. [Google Scholar] [CrossRef]
- Folk, J.; Finlayson, J. The ɛ-(γ-glutamyl) lysine crosslink and the catalytic role of transglutaminases. Adv. Protein Chem. 1977, 31, 1–133. [Google Scholar] [PubMed]
- Nardacci, R.; Iacono, O.L.; Ciccosanti, F.; Falasca, L.; Addesso, M.; Amendola, A.; Antonucci, G.; Craxi, A.; Fimia, G.M.; Iadevaia, V.; et al. Transglutaminase Type II Plays a Protective Role in Hepatic Injury. Am. J. Pathol. 2003, 162, 1293–1303. [Google Scholar] [CrossRef]
- Kleman, J.-P.; Aeschlimann, D.; Paulsson, M.; van der Rest, M. Transglutaminase-catalyzed crosslinking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry 1995, 34, 13768–13775. [Google Scholar] [CrossRef] [PubMed]
- Mosher, D.F.; Schad, P.E. Cross-linking of fibronectin to collagen by blood coagulation Factor XIIIa. J. Clin. Investig. 1979, 64, 781–787. [Google Scholar] [CrossRef]
- Orban, J.M.; Wilson, L.B.; Kofroth, J.A.; El-Kurdi, M.S.; Maul, T.M.; Vorp, D.A. Crosslinking of collagen gels by transglutaminase. J. Biomed. Mater. Res. 2004, 68, 756–762. [Google Scholar] [CrossRef]
- Savoca, M.P.; Tonoli, E.; Atobatele, A.G.; Verderio, E.A.M. Biocatalysis by Transglutaminases: A Review of Biotechnological Applications. Micromachines 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Hattler, B.G.; Federspiel, W.J. Gas exchange in the venous system: Support for the failing lung. In The Artificial Lung; Landes Bioscience: Georgetown, TX, USA, 2002; pp. 133–174. [Google Scholar]
- Wu, Z.J.; Gartner, M.; Litwak, K.N.; Griffith, B.P. Progress toward an ambulatory pump-lung. J. Thorac. Cardiovasc. Surg. 2005, 130, 973–978. [Google Scholar] [CrossRef][Green Version]
- Federspiel, W.J.; Svitek, R. Lung, Artificial: Current Research and Future Directions. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G., Bowlin, G., Eds.; Marcel Dekker: New York, NY, USA, 2008; pp. 1673–1682. [Google Scholar] [CrossRef]
- Krantz, W.B.; Bilodeau, R.R.; Voorhees, M.E.; Elgas, R.J. Use of axial membrane vibrations to enhance mass transfer in a hollow tube oxygenator. J. Membr. Sci. 1997, 124, 283–299. [Google Scholar] [CrossRef]
- Svitek, R.G.; Frankowski, B.J.; Federspiel, W.J. Evaluation of a Pumping Assist Lung That Uses a Rotating Fiber Bundle. ASAIO J. 2005, 51, 773–780. [Google Scholar] [CrossRef][Green Version]
- Kaar, J.L.; Oh, H.-I.; Russell, A.J.; Federspiel, W.J. Towards improved artificial lungs through biocatalysis. Biomaterials 2007, 28, 3131–3139. [Google Scholar] [CrossRef]
- Jen, M.C.; Serrano, M.C.; Van Lith, R.; Ameer, G.A. Polymer-Based Nitric Oxide Therapies: Recent Insights for Biomedical Applications. Adv. Funct. Mater. 2012, 22, 239–260. [Google Scholar] [CrossRef]
- Batchelor, M.M.; Reoma, S.L.; Fleser, P.S.; Nuthakki, V.K.; Callahan, R.E.; Shanley, C.J.; Politis, J.K.; Elmore, J.; Merz, S.I.; Meyerhoff, M.E. More Lipophilic Dialkyldiamine-Based Diazeniumdiolates: Synthesis, Characterization, and Application in Preparing Thromboresistant Nitric Oxide Release Polymeric Coatings. J. Med. Chem. 2003, 46, 5153–5161. [Google Scholar] [CrossRef]
- Fleser, P.S.; Nuthakki, V.K.; Malinzak, L.E.; Callahan, R.E.; Seymour, M.L.; Reynolds, M.M.; Merz, S.I.; Meyerhoff, M.E.; Bendick, P.J.; Zelenock, G.B.; et al. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J. Vasc. Surg. 2004, 40, 803–811. [Google Scholar] [CrossRef]
- Annich, G.M.; Meinhardt, J.P.; Mowery, K.A.; Ashton, B.A.; Merz, S.I.; Hirschl, R.B.; Meyerhoff, M.E.; Bartlett, R.H. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit. Care Med. 2000, 28, 915–920. [Google Scholar] [CrossRef]
- Kushwaha, M.; Anderson, J.M.; Bosworth, C.A.; Andukuri, A.; Minor, W.P.; Lancaster, J.R.; Anderson, P.G.; Brott, B.C.; Jun, H.-W. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials 2010, 31, 1502–1508. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Delivering nitric oxide with nanoparticles. J. Control. Release 2015, 205, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Taite, L.J.; West, J.L. Sustained Delivery of Nitric Oxide from Poly(ethylene glycol) Hydrogels Enhances Endothelialization in a Rat Carotid Balloon Injury Model. Cardiovasc. Eng. Technol. 2011, 2, 113–123. [Google Scholar] [CrossRef]
- Reynolds, M.M.; Saavedra, J.E.; Showalter, B.M.; Valdez, C.A.; Shanklin, A.P.; Oh, B.K.; Keefer, L.K.; Meyerhoff, M.E. Tailored synthesis of nitric oxide-releasing polyurethanes using O2-protected diazeniumdiolated chain extenders. J. Mater. Chem. 2010, 20, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y.; Qin, K.; Wu, Y.; Tian, Y.; Wang, J.; Zhang, J.; Hou, J.; Cui, Y.; Wang, K.; et al. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Control. Release 2015, 210, 179–188. [Google Scholar] [CrossRef]
- Beertsen, W.; Vandenbos, T.; Everts, V. Root Development in Mice Lacking Functional Tissue Non-specific Alkaline Phosphatase Gene: Inhibition of Acellular Cementum Formation. J. Dent. Res. 1999, 78, 1221–1229. [Google Scholar] [CrossRef]
- Osathanon, T.; Giachelli, C.M.; Somerman, M.J. Immobilization of alkaline phosphatase on microporous nanofibrous fibrin scaffolds for bone tissue engineering. Biomaterials 2009, 30, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Oortgiesen, D.A.W.; Plachokova, A.S.; Geenen, C.; Meijer, G.J.; Walboomers, X.F.; Beucken, J.J.J.P.V.D.; Jansen, J.A. Alkaline phosphatase immobilization onto Bio-Gide® and Bio-Oss® for periodontal and bone regeneration. J. Clin. Periodontol. 2012, 39, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Gulseren, G.; Yasa, I.C.; Ustahuseyin, O.; Tekin, E.D.; Tekinay, A.B.; Guler, M.O. Alkaline Phosphatase-Mimicking Peptide Nanofibers for Osteogenic Differentiation. Biomacromolecules 2015, 16, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.S.; Bailey, J.E. Structure-function relationships in immobilized chymotrypsin catalysis. Biotechnol. Bioeng. 1983, 25, 1027–1047. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S.; Riebel-Bommarius, B.R. Biocatalysis: Fundamentals and Applications; John Wiley & Sons: Weinheim, Germany, 2004. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).