Abstract
Development of new tools to improve the efficiency of iron minerals in promoting Fenton oxidation for environmental remediation is a highly promising field. Here, we examine for the first time the role of ascorbic acid (AA) in improving the magnetite (Fe3O4)-mediated Fenton oxidation to remove pentachlorophenol (PCP) in a historically contaminated soil. Experiments were performed in batch and flow-through conditions. In batch slurry experiments, the combination of Fe3O4/AA/H2O2 removed up to 95% of PCP as compared to the 43% removal by Fe3O4/H2O2. Dissolved Fe(II) measurements and Mössbauer spectroscopy highlight the role of AA in increasing the Fe(II) generation. Therefore, its presence enabled the Fe3O4 to maintain its structural Fe(II) content even after the oxidation reaction. Despite kinetic limitations in water-saturated columns, use of Fe3O4/AA/H2O2 removed about 70% of PCP contrary to the 20% PCP removal with Fe3O4/H2O2. This oxidation performance was affected by an injection flow rate or column residence time of AA and H2O2 in columns. Thus, the presence of AA significantly improved the ability of magnetite in promoting the Fenton reaction. Owing to the crucial role of AA in the Fe(II)/Fe(III) redox cycling, a mixed-valent character of magnetite makes it a potential catalyst for Fenton oxidation of organic pollutants.
1. Introduction
Fenton oxidation is among the most efficient advanced oxidation processes (AOPs) to remediate contaminated water and soil [1,2]. Traditional Fenton oxidation generates hydroxyl radicals (HO•) by the reaction of hydrogen peroxide (H2O2) and dissolved Fe(II) [3]. This process, however, requires an acidic pH for optimum oxidation efficiency [1]. Use of traditional Fenton oxidation has been associated with different drawbacks such as a narrow pH range, hazardous effects of acidification, elevated treatment cost, and formation of Fe(III) sludge in large amounts [4]. Therefore, development of suitable alternatives is gaining interest to extend the pH range but without compromising the oxidation efficiency.
In this regard, Fenton-like oxidation (heterogeneous Fenton oxidation) has gained widespread recognition in recent decades [4,5]. Instead of soluble Fe(II), this process relies on Fe minerals/solids whereas structural iron (Fe) in minerals allows the chemical oxidation to take place at a wide range of pH [6,7]. There exist many iron minerals in the environment characterized by different Fe valence states and chemical compositions [8]. Among their characteristics, the contents of structural Fe(II) were found of crucial significance to dictate the catalytic ability of a mineral [6]. Owing to the presence of structural Fe(II), magnetite (Fe(II) Fe(III)2 O4, simply denoted as Fe3O4, was found more reactive to trigger Fenton-like oxidation than ferric minerals (ferrihydrite, goethite, hematite, etc.) [5,6]. Moreover, its magnetic nature enables quick separation from the reaction medium by applying a magnetic field, which is highly advantageous in seeking the reuse of such materials for repeated treatment cycles [9]. Therefore, its use as a catalyst has been widely explored to treat a variety of organic pollutants, such as pharmaceuticals, dyes, hydrocarbons, and pesticides [6,10,11].
Despite the strong stability of magnetite, oxidation of structural Fe(II) can limit its efficiency in subsequent oxidation cycles [10], which is challenging to address for the development of efficient catalytic materials. In this context, recent studies reported that ascorbic acid (AA), also termed as vitamin C, can be used to improve the ability of iron-based minerals in Fenton oxidation [12,13,14]. Ascorbic acid is an environment-friendly reducing and chelating agent [15]. It has been found that use of AA improved the reactivity of iron minerals in aqueous solution [12,13,14] or in an aquifer sediment (goethite and hematite mainly) [16]. However, application of the AA-mediated heterogeneous oxidation process has never been evaluated in the soil matrix, which is the major objective of this study. For this, the present study was designed to assess the efficiency of AA-mediated magnetite to improve Fenton-like oxidation of pentachlorophenol (PCP) in a historically contaminated soil. Soil experiments were performed under batch and flow through conditions (saturated soil columns). Compared to the batch system, soil columns are better representative of the field conditions and, thus, provide better insights for real field applications of remediation strategies [17,18]. Based on our knowledge, this study is the first report of soil application of AA-mediated magnetite Fenton oxidation. PCP has been used as a wood preservative and pesticide for more than 100 years and its extensive use has resulted in its ubiquitous contamination in water and soil [19]. It is listed among the persistent organic pollutants under the Stockholm Convention. Considering its persistence, toxicity, and carcinogenic effects, PCP is also categorized as a priority pollutant by the U.S. Environmental Protection Agency (USEPA) and listed as an environmental carcinogen by the International Association for Research on Cancer (IARC) [20]. Deterioration of groundwater quality, which is the major drinking water resource in Europe, due to soil pollution, is now a serious threat. Therefore, developing strategies and tools to mitigate soil pollution and protect water resources include major challenges.
In the present work, oxidation experiments were performed in a contaminated soil sampled from a wood treatment facility (Waikato Region, New Zealand). PCP degradation was studied at different experimental conditions (reaction time, magnetite dose, and oxidant dose). In column experiments, both solid and effluent analyses were performed to assess the PCP removal efficiency under flow-through conditions. Different flow rates or column residence times were tested in order to evaluate the impact of chemical non-equilibrium on the PCP removal. Mössbauer spectroscopy was then used to assess any changes in magnetite composition and its structural Fe(II) content (stoichiometry). A present feasibility study notably demonstrates the substantial capability of the combined system (Fe3O4/AA/H2O2) to remove PCP in a historically contaminated soil.
2. Results and Discussion
Experiments were performed to evaluate the efficiency of H2O2/magnetite (Fenton oxidation) in the presence of AA in a historically PCP contaminated soil. The investigated soil is a “loamy sand” with pH 7.2, 1.5% soil organic matter, 0.3 wt% carbonate contents (CaCO3), and high iron contents (9.8 g kg−1). The value of PCP in soil was 6 mg kg−1, which exceeds the safe limit for agricultural soils. Therefore, tested soil is categorized as a soil with serious threats in New Zealand and in Europe. Properties of the tested soil are summarized in Table S1. The following sections describe the chemical oxidation efficiency in the tested soil under batch and column conditions.
2.1. PCP Remediation Efficiency in Soil Slurry Experiments
The degradation of PCP in contaminated soil was first evaluated by using different doses of Fe3O4 (5 or 25 g kg−1 soil w/w), AA (5 and 125 mM), and H2O2 (200 and 500 mM) in different oxidation systems including single (Fe3O4 or H2O2), binary (Fe3O4/AA or Fe3O4/H2O2), and ternary systems (Fe3O4/AA/H2O2) (Table 1). Obtained results (Figure 1) indicate that application of Fe3O4 alone did not cause any PCP removal. Similarly, low PCP removal (< 6%) was observed when H2O2 was applied alone (even at a higher oxidant dose) due to its inability to perform without a catalytic agent. Use of Fe3O4/AA also resulted in slight removal of PCP (7%). However, application of Fe3O4 with H2O2 removed 43% of PCP due to the considerable ability of magnetite to improve the Fenton-like oxidation. It should be noted that negligible PCP degradation by Fe3O4/H2O2 has been noted in the same soil by Rybnikova et al. [19] (under similar experimental conditions), which could be linked to the lower reagent dose (0.2–4 mM H2O2 and 5 mg Fe3O4 g−1 soil). In the present study, higher oxidant doses (200–500 mM) were chosen in the soil to compensate the non-target use of oxidizing species by soil organic matter and/or other soil constituents [4,21,22].

Table 1.
A summary of the experiments performed in real contaminated soil (batch and column) and aqueous solution.
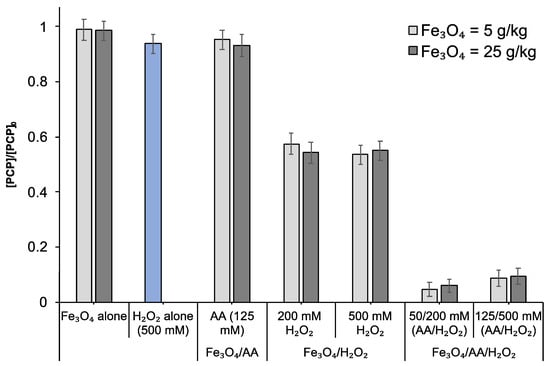
Figure 1.
Degradation of PCP in contaminated soil (PCP = 6 mg Kg−1) evaluated in the presence of two different amounts of magnetite- Fe3O4 (5 or 25 g kg−1 soil w/w) by using single (Fe3O4 alone, H2O2 alone), binary (Fe3O4/AA and Fe3O4/ H2O2), and ternary treatments (Fe3O4/AA/H2O2). Experiments were performed in batch conditions at a liquid-to-solid ratio (L/S) of 1 (total solution volume = 2 mL) without pH adjustment (initial pH = 7.2) for 24 h. [PCP] represents the PCP concentration after oxidation (t = 24 h) and [PCP]0 is its concentration at t = 0 (before treatment) determined by HPLC.
Maximum PCP removal (95%) was attained when AA was introduced in the reaction medium (Fe3O4/AA/H2O2). This highlights the indispensable role of AA in enhancing the Fenton-like oxidation of PCP in the contaminated soil. Increasing the dose of magnetite (from 5 to 25 g kg−1 soil w/w) exhibited a negligible impact on the removal efficiency. It has been reported by that the addition of incremental Fe3O4 or H2O2 did not further enhance the removal of contaminants in aqueous solution [13]. As reported in Figure 1, PCP degradation slightly decreased (from 95% to 91%) with an increase in the dose of H2O2 or AA/H2O2. This can be correlated with the parasite reactions (i.e., scavenging effects, self-destruction of H2O2), which generally happen when a high amount of oxidant is introduced in the soil [23]. H2O2 can scavenge the HO• radicals through the reaction 1 decreasing the PCP removal when it is used at a high dose [24]. Similarly, the generated reactive oxygen species (ROS) could be consumed by AA (reaction 2), thereby, limiting the treatment efficiency [25].
H2O2 + HO• = HOO• + H2O
AA + HO• = AA(oxidized)
PCP + HO• = PCP(oxidized)
In addition to these reactions (1 and 2), the ROS could also be scavenged by soil constituents, such as soil organic matter and minerals [4,21,22]. These scavenging reactions compete with PCP oxidation (reaction 3) by hydroxyl radicals and, thus, could decrease its removal.
2.2. Combined Treatments in Batch Slurry Experiments
Preliminary investigations were conducted in PCP contaminated aqueous solutions to evaluate the PCP removal using single or combined systems, i.e., single (AA, H2O2, or Fe3O4), binary (Fe3O4/AA, Fe3O4/H2O2, or AA/H2O2), and ternary systems (Fe3O4/AA/H2O2) (Figure 2). Experiments in aqueous solution could offer a better understanding of the operation of this novel activation treatment in a simple setup by avoiding the complexities associated with the complex soil medium [2].
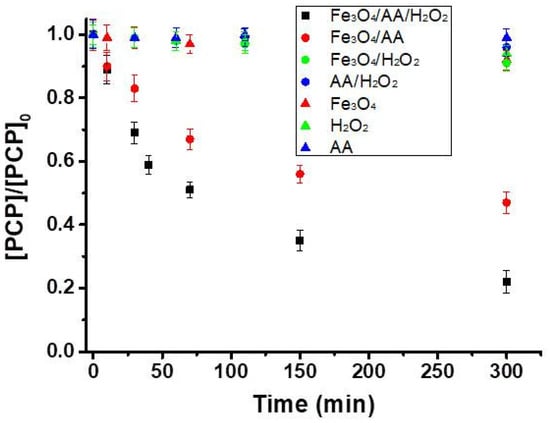
Figure 2.
PCP removal in the single, binary, and ternary systems with 20 mg L−1 of PCP using 0.5 g L−1 of magnetite, 4 mM of ascorbic acid, and 4 mM of H2O2 at pH 7.2 (total solution volume = 100 mL). [PCP] represents the PCP concentration after oxidation at a specified oxidation time, and [PCP]0 is its concentration at t = 0 (before treatment) determined by HPLC.
One of the most important effects of AA is likely to be the reduction of Fe (III) at the surface of magnetite into Fe (II). Small degradation (<10% PCP removal) was observed in the binary systems except for Fe3O4/AA, which removed 67% of PCP (Figure 2). A plausible explanation for higher efficiency of Fe3O4/AA as compared to the other binary systems is the activation of dissolved O2 and the formation of superoxide radicals, which can either degrade the target pollutant or form hydroxyl radicals in acidic conditions (reactions 4–7) [12,26]. In addition to the formation of Fe(II)-ascorbate complexes that contribute toward higher oxidation efficiency, ascorbic acid decreased the solution pH from 7.2 to 3.8. This decrease in pH might promote the mineral dissolution and formation of HO• radicals in the Fe3O4/AA system (reactions 4–7) [13]. Therefore, all these processes can contribute toward the removal of PCP in the Fe3O4/AA system.
Fe(II) + xAA = Fe(II)(AA)x
Fe(II)(AA)x + O2 = Fe(III)(AA)x + O2•−
Fe(II)(AA)x + O2•− + 2H+ = H2O2 + Fe(III)(AA)x
Fe(II)(AA)x + H2O2 = Fe(III)(AA)x + HO• + HO−
In the ternary system (Fe3O4/AA/H2O2), the main reactions include (i) the generation of Fe(II) species through ascorbate-mediated reduction of Fe(III) (reaction 8), (ii) and the generation of reactive oxygen species (HO•) via the H2O2 decomposition with the produced iron (II) species (reaction 9) [13,25]. Therefore, more HO• generation can be expected in the Fe3O4/AA/H2O2 system compared to the single and binary systems (Figure 2), thus, leading to better efficiency in PCP removal. These reactions (8-9) provide the simplified mechanisms of catalytic oxidation of ascorbic acid.
2Fe(III) + AA = 2Fe(II) + 2H+ + AA(oxidized)
Fe(II) + H2O2 = Fe(III) + HO• + OH−
As mentioned in the previous section, the PCP oxidation occurs concomitantly with that of AA, whereas hydroxyl radicals can react with all co-present compounds (reactions 1-3). Therefore, to optimize the degradation of PCP, the effect of the molar ratio of AA/H2O2 on PCP removal efficiency was evaluated (Figure 3). The use of H2O2/AA in stoichiometric molar ratios of 2:2 and 4:4 resulted in removal extents of 66% and 78%, respectively. Application of higher doses (4:4) was more efficient to activate the Fenton reaction due to increased generation of dissolved Fe(II) by AA, and subsequent activation of H2O2 to produce hydroxyl radicals (reactions 8 and 9). An increase of AA as compared to the H2O2 (H2O2/AA ratio of 2:4) dramatically decreased the PCP degradation to 45%, which can be associated with the scavenging properties of AA toward the formed HO• radicals (reaction 2). Similarly, an increase in concentration of H2O2 (H2O2/AA ratio 8:4) decreased the PCP degradation to 62%, which can be attributed to the scavenging effect through reaction of H2O2 with HO• radicals [24] (reaction 1). The percentage of the hydroxyl radical (HO•) reacting with each compound can be estimated by using the bimolecular rate constants (k′) and concentration of each species [13,27] (Table 2). Given its higher reactivity and concentration used here, 99% of the produced HO• would react with AA (for H2O2/AA ratio of 4:4). Though only 0.7% of HO• would react with PCP, 78% of PCP removal was achieved after 300 min, suggesting that the production rate of HO• could be very important under the experimental conditions of this study.
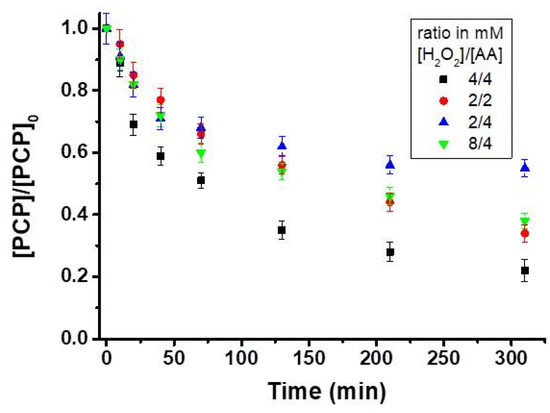
Figure 3.
Effect of different [H2O2]/ascorbic acid (AA) ratio on the degradation of PCP (20 mg L−1) in the presence of 0.5 g L−1 of magnetite at pH 7.2 (total solution volume = 100 mL). [PCP] represents the PCP concentration after oxidation at a specified oxidation time and [PCP]0 is its concentration at t = 0 (before treatment) determined by HPLC.

Table 2.
Reactivity of hydroxyl radicals with species present in the aqueous Fe3O4/AA/H2O2 system.
The degradation of PCP in contaminated soil is the most efficient system (Fe3O4/AA/H2O2) and Fe3O4 alone (as the control), as shown in Figure 4. Clearly, the Fe3O4/AA/H2O2 was highly efficient, which was able to degrade about 87% of the PCP in 5 h that reached to 93% in 24 h. This can be linked to the exhaustion of the AA and H2O2 as observed elsewhere [13]. It should be noted that the presence of AA can significantly increase (174 times) the H2O2 decomposition rate in the Fe3O4/AA/H2O2 system as compared to the Fe3O4/H2O2 system [13].
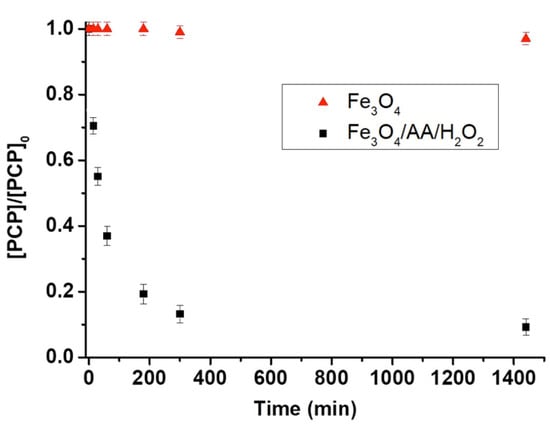
Figure 4.
PCP degradation in contaminated soil using Fe3O4 alone and Fe3O3/AA/H2O2. [PCP] represents the PCP concentration at a specified oxidation time and [PCP]0 is its concentration at t = 0 (before treatment) determined by HPLC. Experimental conditions are: Fe3O3 = 5 g kg−1 soil w/w, AA = 50 mM, H2O2 = 200 mM, liquid-to-solid ratio (L/S) = 1, total solution volume = 2 mL, and initial pH = 7.2.
In contrast with the high performance observed in aqueous solution, Fe3O4/AA removed only 7% of PCP in the contaminated soil. It is, however, difficult to compare the findings in aqueous solution and the soil matrix as the latter is characterized by lower pollutant availability and the presence of many non-target constituents as potential scavengers [4]. The interaction of AA with other soil constituents in addition to the magnetite might have limited the remediation potential of Fe3O4/AA as compared to the aqueous solution. Moreover, Fe3O4/H2O2 removed less than 10% of PCP in aqueous solution which can be correlated to the lower amount of the oxidant (4 mM H2O2 in aqueous solution against 200 mM in soil). The ternary system (Fe3O4/AA/H2O2) was shown to be the most efficient system to remove up to 78% of the PCP, thereby, highlighting the role of AA in improving the oxidation efficiency.
2.3. Dissolved and Structural Fe(II) Analyses
Further experiments were performed to evaluate whether the higher efficiency of Fe3O4/AA/H2O2 can be correlated with the reductive dissolution of Fe(III). For this, the production of dissolved Fe(II) was monitored against the reaction time, and AA concentration (Figure 5). Dissolved Fe(II) concentration with 4 mM AA in solution was noted to be 1.1 µM after 10 min, which increased up to 2 µM after 2 h (Figure 5). In other words, Fe(II) is released in solution by reductive dissolution of magnetite that leads to the formation of Fe(II)-ascorbate complexes [12,14,16]. As reported previously [13], surface complexation of AA with reactive sites of iron oxide (Fe(III)-AA) is followed by electron transfer, thus, generating Fe(II) species and oxidized AA molecules. These Fe(II) species are the integral component to promote the formation of the HO• radical in the presence of H2O2. Therefore, the catalytic cycle of iron, which is essential in the Fenton reaction is improved, thereby, enhancing the oxidation efficiency of Fe3O4/AA/H2O2. Mössbauer spectroscopy analysis also supports this (Figure 6, Table 3) as magnetite retained its Fe(II) contents after the oxidation without AA. The Mössbauer spectra (Figure 6) are very characteristic of magnetite, with two sextets easily identifiable and corresponding to a tetrahedral (MagT) Fe(III) and an octahedral (MagO) Fe(II)-Fe(III) [6]. Using the integrated areas of the magnetite sextets (i.e., relative abundance of MagT and MagO), it is possible to estimate the stoichiometry (i.e., Fe(II)/Fe(III) ratio) based on the following formula [28]:
Fe(II)/Fe(III)Mag = (1/2 MagO)/((1/2 MagO)+MagT)
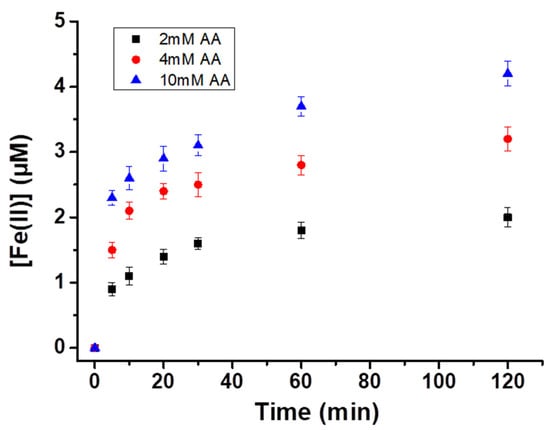
Figure 5.
Evolution of Fe(II) concentration in the solution against time using various concentrations of ascorbic acid (2, 4, and 10 mM) in the presence of 0.5 g L−1 magnetite and 20 mg L−1 of PCP (total solution volume = 100 mL).
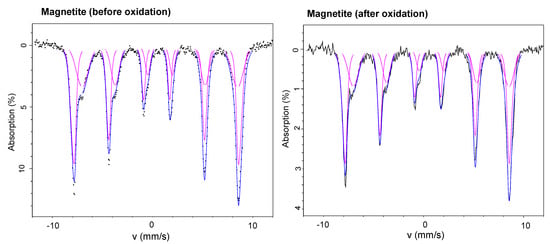
Figure 6.
Mössbauer spectra of magnetite samples (before and after their use in chemical oxidation experiments) collected at 140 K.

Table 3.
Fitting results of Mössbauer spectroscopy. CS—center shift. e—quadrupole shift. H—hyperfine field. stdev(H)—standard deviation of hyperfine field. R.A.—Relative abundance. χ2—goodness of fit. Fe(II)/Fe(III)—iron II to iron III ratio.
Table 3 shows the Fe(II)/Fe(III) ratios determined through Mössbauer spectroscopy, which remained almost the same before and after oxidation in the presence of AA. Thus, the presence of AA allowed the magnetite to retain its structure (Figure 6) and structural Fe(II) (Table 3) even after oxidation. These results highlight the contribution of AA in increasing the transfer of Fe(II) in solution (Figure 5), which improves the ability of magnetite in heterogeneous Fenton oxidation. It should be noted that oxidation of magnetite could reduce its structural Fe(II) and its ultimate oxidation ability for pollutant removal. For example, a decline in degradation efficiency in sequential experiments has been deduced to the conversion of structural Fe(II) to Fe(III) on the surface of magnetite [29]. Thus, our study indicates that the presence of AA helps magnetite to maintain its structural Fe(II) contents for a prolonged reaction time.
2.4. PCP Remediation in Dynamic Column Experiments
Though an improved contact between the oxidant, pollutant, and soil matrix is the prominent feature of batch experiments, such conditions are not prevalent in field conditions. Therefore, batch experiments do not truly represent the real field conditions. To get closer to the field settings, we performed experiments under flow through conditions in soil columns. For this, experiments were performed by using different treatments where reagents (in different amounts) were injected at varying flow rates (25, 100, 500, and 1000 µL min−1) to evaluate the impact of residence time on the remediation efficiency (Figure 7). Blank experiments, performed by flushing deionized water, did not indicate any PCP degradation or loss in the column effluent (not shown). Similar to the soil experiments in batch, only <7% of PCP removal was observed by using Fe3O4/AA. With Fe3O4/H2O2, PCP removal extent was less than 20%. However, PCP removal extent reached ~70% when Fe3O4/(AA/H2O2: 5/10 mM) was injected at a flow rate of 25 µL min−1. Increasing the dose of AA/H2O2 from 5/10 mM to 10/10 mM slightly improved the PCP degradation (from 63% to 70%). As expected from a relatively lower oxidant, pollutant, and soil matrix contact in the column, oxidation efficiency decreased in the column as compared to the batch experiments. It should be noted that PCP degradation in these column experiments have been assessed on soil after the completion of the experiment and no release of PCP was observed in column effluents.
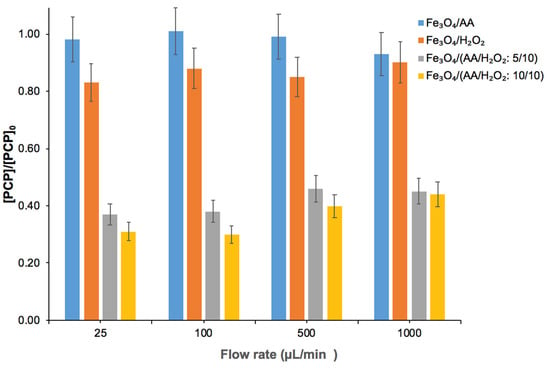
Figure 7.
Degradation of PCP in saturated soil columns by using Fe3O4/AA, Fe3O4/H2O2, and Fe3O4/(AA/H2O2: 5/10 or 10/10 mM) at different flow rates (25, 100, 500, and 1000 µL min−1). For the first two treatments, 10 mM of both AA and H2O2 were used. For all columns, 5 g of PCP-contaminated soil (initial pH 7.2) pre-mixed with magnetite (5 g kg−1 soil w/w) was used. [PCP] represents the PCP concentration after oxidation and [PCP]0 is its concentration at t = 0 (before treatment) determined by HPLC.
Obtained PCP degradation was different at different flow rates, suggesting the impact of oxidant residence time on the treatment efficiency. Degradation extent of PCP was almost similar (70%) at lower flow rates (25 and 100 µL min−1). However, the oxidation efficiency of Fe3O4/AA/H2O2 decreased from 70% to 56% when the flow rate was increased from 100 to 1000 µL min−1. A decrease in PCP degradation extent with an increase in flow rate suggests the absence of local equilibrium in these columns. Given the oxidation rate constant of PCP in the Fe3O4/AA/H2O2 system (5.78 × 10−3 min−1 and 16.29 × 10−2 min−1 in aqueous solution and soil, respectively), the Damköhler number, which is based on the ratio of hydrodynamic residence time to characteristic time for a chemical reaction in the column, is relatively low. This suggests that the PCP oxidation reaction can be kinetically limited under flow-through conditions. Column experiments at a lower flow rate exhibited higher PCP degradation due to higher oxidant residence time leading to a prolonged reaction between the oxidant and the pollutant [23,30].
3. Conclusions
In this work, we have notably demonstrated the strong ability of ascorbic acid to improve the efficiency of magnetite-mediated Fenton oxidation to remove PCP from contaminated soil and aqueous solution. The presence of AA increased the PCP removal from 43% (Fe3O4/H2O2) to 95% (Fe3O4/AA/H2O2) in a soil slurry batch. High efficiency of Fe3O4/AA/H2O2 has also been evident in water-saturated soil columns despite a small decrease in oxidation efficiency (from 95% to 70%) due to the kinetic limitations. Degradation extent was higher at lower flow rates in columns, due to the higher residence time of reactants in magnetite-amended soil columns. Improved treatment efficiency in the presence of AA has been correlated to its role in reducing Fe(III) to Fe(II) as the amount of soluble Fe(II) increased with a growing amount of AA. Mössbauer spectroscopy showed that magnetite retained its structural Fe(II) contents and crystal structure for an improved efficiency as a sustainable process over a longer period of reaction time. Thus, use of AA could enhance the Fe(II) generation, thus, improving its treatment ability to remove persistent organic pollutants in contaminated soil. Strong efficiency of Fe3O4/AA/H2O2 in historically contaminated soil under dynamic column conditions contributes to a better understanding of this process for field experiments. Further investigations are certainly needed in order to optimize the operational parameters for high-scale applications, and then develop an effective technology for soil remediation.
4. Materials and Methods
4.1. Materials
Magnetite (Fe3O4, 95%, <5 μm), hydrogen peroxide (H2O2, 30 wt%), pentachlorophenol (PCP, 97%), and L-ascorbic acid (AA, 99%) were obtained from the Sigma-Aldrich (Steinheim am Albuch, Baden-Württemberg, Germany). Throughout the study, solutions were prepared by using deionized water produced by Milli-Q system from Millipore (Darmstadt, Germany).
The historically PCP-contaminated soil was obtained from a timber mill located in Waipa district of Waikato Region, New Zealand and has been characterized by a French Institute for Agro-environmental Health (ISAE, France). Properties of the tested soil are described in Table S1 and Section 2.2. The soil was dried, ground, and sieved through a 1-mm mesh for chemical oxidation experiments.
4.2. Oxidation Experiments
A summary of the different experiments performed in this study is presented in Table 1. All experiments were performed in triplicates to calculate the standard deviation represented as the error bars in the figures.
First, experiments were performed in real soil samples under batch and column conditions. For batch experiments, 2 g of soil was homogenized with a suitable amount of magnetite, which is followed by the addition of required amounts of ascorbic acid and H2O2 (detailed in Table 1). Experiments were performed at liquid-to-solid ratios (L/S) of 1 chosen on the basis of our previous study [30]. Impact of reagent doses (AA, magnetite, and H2O2) and reaction time on the removal of PCP was evaluated (Table 1).
For the column study, experiments were performed under water-saturated conditions. For this, reagent solutions are injected from the bottom of the column in an upward direction. Choosing saturated conditions eliminate any preferential pathways by uniform distribution of the reagents in the soil column for better treatment [19]. Briefly, glass columns were packed with 5 g of PCP-contaminated soil pre-mixed with magnetite (5 g kg−1 soil w/w). These soil columns were subjected to oxidation by injecting inflow solutions in the upward direction to maintain the saturated flow conditions. Using soils pre-mixed with magnetite, column experiments were performed with AA alone (10 mM), H2O2 alone (10 mM), and AA+ H2O2 (5:10 and 10:10 ratio) at different flow rates (0.025, 0.1, 0.5, and 1 mL min−1), as summarized in Table 1. For this, required amounts of AA and/or H2O2 were simultaneously pumped from different containers into the column at the same flow rate. After oxidation, soil in the column bed was analyzed for PCP. Column effluents were also collected to detect any released PCP.
Further experiments to study the AA-activated Fe3O4 system for the oxidation of PCP were performed in aqueous solution. For a typical experiment in aqueous solution, batch studies were performed in 100 mL of 20 mg L−1 of PCP solution at circumneutral pH (pH = 7.2 as in soil) for 5 h. Magnetite (0.5 g L−1) was first added to the PCP containing solution, which is followed by the addition of suitable amounts of ascorbic acid. Then after 10 min of stirring, hydrogen peroxide (H2O2) was added to the reaction mixture to trigger the Fenton reaction.
Formation of soluble Fe(II) in the Fe3O4/AA system was determined using the 1,10-phenantroline complexation method [31]. Furthermore, 2 mL of sample was introduced in a tube containing 10 mL of acidified 1,10-phenentroline (0.08% w/v at pH = 2). After 5 min, the Fe(II)-phenanthroline complex with the maximum absorbance at 510 nm is analyzed by UV-visible spectrophotometry where the intensity is proportional to the iron(II) concentration.
4.3. Extraction and Analysis of PCP
The PCP was extracted from soil slurries, as reported elsewhere [19]. For this, in an ultrasonic bath, water/methanol solution (50/50, v/v) was blended with soil slurries (L/S ratio: 2/1) for 1 h. This was followed by 10 min of centrifugation at 4000 rpm. The obtained supernatant solution (2 mL) was, then, filtered (0.2 μm filter) for further analyses. NaOH (1 M) solution was used to adjust the pH at 7.2 ± 0.2 before extraction (if needed). Rybnikova et al. [19] reported that >95% of the PCP can be extracted at a pH of 7–7.5. For experiments in aqueous solution, 2 mL of sample are taken at a fixed reaction time, filtered by using 0.2 µm of polytetrafluoroethylene (PTFE) microfilter, and quenched in 200 µL of methanol, which is followed by PCP analyses.
The PCP concentrations are quantified by using HPLC [30], which was performed on a Alliance Waters 2487 device (San Diego, CA, USA) equipped with an XBridge C18 column fixed at 30 °C and ultraviolet (UV) detector at 254 nm. A mixture of acetonitrile/ultra-pure water (ACN/UPW), acidified with formic acid 0.1 vol%, was used as a mobile phase. The isocratic mode ACN/UPW 70:30 was realized for 9 min with an injection volume of 50 µL at 1 mL min−1 flow rate.
4.4. Mössbauer Characterization
Magnetite samples before and after chemical oxidation were characterized by the Mössbauer spectroscopy (Burlington, MA, USA). For this, dried powders were loaded into Plexiglas holders (1 cm²). Samples were then transferred to the instrument and loaded inside a closed-cycle exchange gas cryostat (Janis cryogenics Co., Woburn, MA, USA). Measurements were collected at 140 K with a constant acceleration drive system (WissEL, Wissenschaftliche Elektronik GmbH, Starnberg, Germany) in a transmission mode with a 57Co/Rh source and calibrated against a 7-µm thick α-57Fe foil measured at room temperature. All spectra were analysed using Recoil (University of Ottawa, Ontario, Canada) by applying an extended Voight Based Fitting (xVBF) routine [32]. The half width at half maximum (HWHM) was fixed to a value of 0.13 mm/s for all samples.
Supplementary Materials
The following is available online at https://www.mdpi.com/2073-4344/11/3/331/s1. Table S1: Physico-chemical characteristics of the PCP-contaminated soil.
Author Contributions
Conceptualization, M.U., O.M., and K.H. Methodology, M.U. and O.M. Investigation, M.U. and O.M. Writing—original draft preparation, M.U. and O.M. Writing—review and editing, M.U., O.M., S.H., and K.H. Supervision, K.H. and S.H. Funding acquisition, S.H. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the French Agency for Environment and Energy Management (ADEME) and the Humboldt Foundation of Germany.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
We thank N. Singhal (University Auckland, New Zealand) for providing the PCP-contaminated soil, J. Sorwat (University Tübingen) for Mössbauer characterization and P. Martin (University Tübingen) for assistance in HPLC analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Usman, M.; Ho, Y.-S. A bibliometric study of the Fenton oxidation for soil and water remediation. J. Environ. Manag. 2020, 270, 110886. [Google Scholar] [CrossRef]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef]
- Usman, M.; Hanna, K.; Haderlein, S. Fenton Oxidation to Remediate PAHs in Contaminated Soils: A Critical Review of Major Limitations and Counter-Strategies. Sci. Total Environ. 2016, 569–570, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.; Hanna, K.; Chiron, S. Fenton-Like Oxidation of 2,4,6-Trinitrotoluene Using Different Iron Minerals. Sci. Total Environ. 2007, 385, 242–251. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Hanna, K.; Faure, P. Remediation of oil-contaminated harbor sediments by chemical oxidation. Sci. Total Environ. 2018, 634, 1100–1107. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Wei, X.; Wu, H.; Sun, F. Magnetite/Fe-Al-montmorillonite as a Fenton catalyst with efficient degradation of phenol. J. Colloid Interface Sci. 2017, 504, 611–619. [Google Scholar] [CrossRef]
- Munoz, M.; Domínguez, P.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Naturally-Occurring Iron Minerals as Inexpensive Catalysts for CWPO. Appl. Catal. B 2017, 203, 166–173. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic acid/Fe@Fe2O3: A highly efficient combined Fenton reagent to remove organic contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xie, G.; He, D.; Zhang, L. Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling. Appl. Catal. B Environ. 2020, 267C, 118383. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Jia, F.; Song, F.; Zhao, J.; Zhang, L. Ascorbate-Promoted Surface Iron Cycle for Efficient Heterogeneous Fenton Alachlor Degradation with Hematite Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 8751–8758. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, H.; Wang, J.; Ai, J. Rapid and continuous oxidation of organic contaminants with ascorbic acid and a modified ferric/persulfate system. Chem. Eng. J. 2015, 270, 73–79. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Li, M.; Zhang, Y.; Yuan, S.; Ai, Z.; Zhao, J.; Zhang, L. Fenton oxidation of organic contaminants with aquifer sediment activated by ascorbic acid. Chem. Eng. J. 2018, 348, 255–262. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Usman, M.; Faure, P.; Lorgeoux, C.; Ruby, C.; Hanna, K. Treatment of hydrocarbon contamination under flow through conditions by using magnetite catalyzed chemical oxidation. Env. Sci. Pollut. Res. 2013, 20, 22–30. [Google Scholar] [CrossRef]
- Rybnikova, V.; Singhal, N.; Hanna, K. Remediation of an aged PCP-contaminated soil by chemical oxidation under flow-through conditions. Chem. Eng. J. 2017, 314, 202–211. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Pentachlorophenol and Some Related Compounds. Some Ind. Chem. 1994, 60, 389–433. [Google Scholar]
- Usman, M.; Tascone, O.; Faure, P.; Hanna, K. Chemical oxidation of hexachlorocyclohexanes (HCHs) in contaminated soils. Sci. Total Environ. 2014, 476–477, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Faure, P.; Ruby, C.; Hanna, K. Remediation of PAH-contaminated soils by magnetite catalyzed Fenton-like oxidation. Appl. Catal. B 2012, 117–118, 10–17. [Google Scholar] [CrossRef]
- Usman, M.; Tascone, O.; Rybnikova, V.; Faure, P.; Hanna, K. Application of chemical oxidation to remediate HCH-contaminated soil under batch and flow through conditions. Env. Sci. Pollut. Res. 2017, 24, 14748–14757. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Hou, Y.; Zhang, E.; Tu, S.; Xiong, S. Ascorbic acid induced activation of persulfate for pentachlorophenol degradation. Chemosphere 2019, 229, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Liu, H.; Ma, J. Ascorbic acid/Fe0 composites as an effective persulfate activator for improving the degradation of rhodamine B. RSC Adv. 2018, 8, 12791–12798. [Google Scholar] [CrossRef]
- Hanna, K.; Chiron, S.; Oturan, M.A. Coupling enhanced water solubilization with cyclodextrin to indirect electrochemical treatment for pentachlorophenol contaminated soil remediation. Water Res. 2005, 39, 2763–2773. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of nanoparticulate magnetite stoichiometry by Mössbauer spectroscopy, acidic dissolution, and powder X-ray diffraction: A critical review. Am. Mineral. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Xue, X. Ultrasound Enhanced Heterogeneous Activation of Peroxydisulfate by Magnetite Catalyst for the Degradation of Tetracycline in Water. Sep. Purif. Technol. 2012, 84, 147–152. [Google Scholar] [CrossRef]
- Monfort, O.; Usman, M.; Hanna, K. Ferrate (VI) oxidation of pentachlorophenol in water and soil. Chemosphere 2020, 253, 126550. [Google Scholar] [CrossRef]
- Bertinetti, S.; Hanna, K.; Minella, M.; Minero, C.; Vione, D. Fenton-type processes triggered by titanomagnetite for the degradation of phenol as model pollutant. Desalin. Water Treat. 2019, 151, 117–127. [Google Scholar] [CrossRef]
- Rancourt, D.G.; Ping, J.Y. Voigt-based methods for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B 1991, 58, 85–97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).