Energy policies in the US and in the EU during the last decades have been focused on enhanced oil and gas recovery, including the so-called tertiary extraction or enhanced oil recovery (EOR), on one hand, and the development and implementation of renewable energy vectors, on the other, including biofuels as bioethanol (mainly in US and Brazil) and biodiesel (mainly in the EU). EU objectives for 2020 suppose an increase in renewable energy up to 20% of total primary energy, and a share of biofuels in transportation up to 10%. Indeed, the EU adopted the Strategic Energy Technology Plan (SET-Plan) (COM(2007)723 final to progressively implement low-carbon energy technologies as a way to meet the proposed goals for this year 2020 energy and the concomitant climate change goals. To this end, certain European Industrial Initiatives have been developed to promote the development of critical energy technologies, such as wind, solar, electricity grids, bioenergy vectors, carbon capture devices and facilities, as well as storage and fission centrals and facilities for nuclear energy [1]. In the 2007–2017 decade, global investments in renewable energies exceeded 280 billion US$, which, in the case of EU means almost 50 billion US$, not meeting the 2020 goal for renewables [2]. Indeed, to meet the net-zero scenario for GHG by 2050, a share of these energy sources in final primary energy consumption should exceed 50% [3]. Up to now, wind and solar energy are paving the way.
Crude glycerol is the main by-product from biodiesel processes based on homogeneous acid and basic catalysis, the main technology used nowadays. However more pure glycerol is, and will be obtained, by heterogeneous catalysis, including mixed oxides, basic catalysts and enzymes (purity up to 98.5% without costly downstream purification) [4]. Glycerol is a well-known polyol with a plethora of applications: (1) in the food industry (sweetener, thickener and ingredient): 11% in 2014; (2) in the basic organic chemistry industry (as a building block): 32%; (3) in pharmaceutical and cosmetic industries (additive, skin and hair care, and more): 34%; and (4) as an ingredient and reagent in explosives: 2%, hydraulic fluids: 11%, and plasticizers in tobacco: 6%,among other products [5]. This polyol is obtained from triglycerides by saponification and transesterification and, when needed, from propylene via epichlorohydrin or glycidol. The advent of biodiesel as a promising biofuel in the last few decades has involved an uprising of the production of glycerol, without a concomitant increase of the purification capacity or the development of new applications for this compound. Therefore, crude glycerol prices have decreased enormously, and its production, only in the EU, has increased from 1.16 to 2 Mtons from 2015 to 2017. Worldwide, this chemical is expected to reach >5 Mtons by 2026 [5]. Thus, its application as a platform chemical to obtain acrylic acid, propylene glycol, epichlorohydrin, and methanol has consumed almost 25% of total glycerol in 2015. Thus, its future as a platform chemical is hardly deniable and thermal, catalytic, enzymatic and microbial processes are being developed [6].
Organic synthetic processes based on thermal and catalytic routes are being developed from crude (with a market prices of about 300–500 US$ per ton) and from pure glycerol (including UPS glycerol), whose prices are around 2000–3000 US$ per ton [5]. Reactions include oxidation, telomerisation, ketalyzation, alkylation, esterification, transcarbonation, and reforming, among others, to render acrylic acid, acrolein, glycerol carbonates, solketal, and other ketals and hemiketals (some of which are renewable substitutes for ETBE and MTBE), polyglycerols and hydrogen, synthesis gas and biomethanol, to name a few [5,6,7,8,9]. In particular, methanol is one of the main platform chemicals in the petrochemical industry, so its production out of several biomass-based resources, including glycerol, is subject to intense research nowadays [10]. Diols, such as 1,2 propanediol, are important commodities for the food, cosmetic, chemical and pharma industries, while 1-propanol is an important chemical and solvent that can be also obtained from glycerol [11]. Finally, organic carbonates, including glycerol carbonate, are considered green solvents and organic intermediates, a way to chemically activate CO2 after its capture [12,13].
Biotechnological routes driven by enzymatic biocatalysts (mainly, lipases such as the lipase B of Candida antarctica (CALB) or the lipase of Rhizomucor miehei) are being employed in the pharma, drug and cosmetic industries [14,15]. In particular, the high regiospecificity for the extreme positions of glycerides of the R. miehei lipase permits for the production of active ingredients from glycerol useful in the food and cosmetic industries, such as 1,3-dicaprin, that can be subsequently acylated with palmitic acid (or other long fatty acids) with the lipase from Burkholderia cepacia to render ingredients for functional foods that reduce fat accumulation and promote energy production [15]. Moreover, lipases, such as CALB, can be employed to produce prodrugs that reduce secondary effects due to the original drugs, such as ibuprofen and other profens [16]. Figure 1 shows a general overview of several thermal, catalytic and biocatalytic processes and products from crude and pure glycerol.
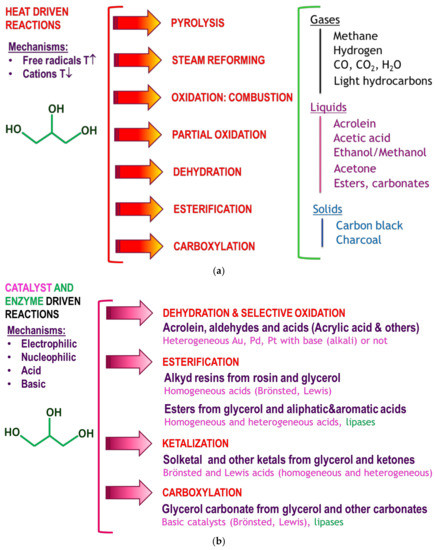
Figure 1.
The glycerol biorefinery: several products obtained by thermal, catalytic and enzymatic routes.
Microbial transformations are envisaged as one of the main strategies to obtain several new products from crude glycerol: diols, ketones, multifunctional compounds (as hydroxyaldehydes and hydroxyacids) and diacids. Diols such as 2,3-butanediol and 1,3-propanediol can be obtained from glycerol using oxidative and reductive pathways of biosafety 1 microorganisms (for example, Raoultella terricola); they are prospective biomonomers but, in the case of 2,3 butanediol, it can be also a precursor of biobutadiene [17,18]. Succinic, itaconic and malic acid are very promising monomers to obtain polyamides and other bioplastics by condensation reactions, and they can be produced from glycerol using fungi of geni Rhizopus, Aspergillus, and Ustilago, among others [19,20,21]. One of the most successful biomonomers to date is D- and L-lactic acid, the precursors of several types of D,L-PLA; these enantiomers can be obtained up to 50 g/L or higher titers using genetically modified strains of Escherichia coli, Saccharomyces cerevisiae and Pichia pastoris [22,23]. The catalytic routes from glycerol to racemic lactic acid, one of the main acidifiers and preservers used in the food industry, are also being developed using oxidative processes with bifunctional catalysts with Lewis acid and redox sites and also hydrothermal reactions driven by CuO/ZrO2 [24]. As for ketones, dihydroxyacetone can not only be obtained as one of the main byproducts on the route to biomethanol from glycerol [10], but is also very selectively produced by several bacteria such as Gluconobacter oxidans out of crude and pure glycerol. Its production using resting cells allows for the separation of microbial biomass production and the ketone production, optimizing the process conditions of the production step in what refers to oxygen mass transfer and hydrodynamic stress of the cells [25].
Although the production of chemicals and food, pharma and cosmetic ingredients are important due to the medium to high-added value of the target products, the production of energy vectors, such as hydrogen out of crude glycerol, either alone or mixed with manure, food residues, and other organic wastes, is a trending topic as one of the most sustainable strategies to obtain profit from residues, avoiding costly purification operations. To this end, optimization of anaerobic processes to hydrogen from crude glycerol is a must [26]. The following Figure 2 compiles several products and microbial processes that are being developed and implemented using crude glycerol as feedstock.
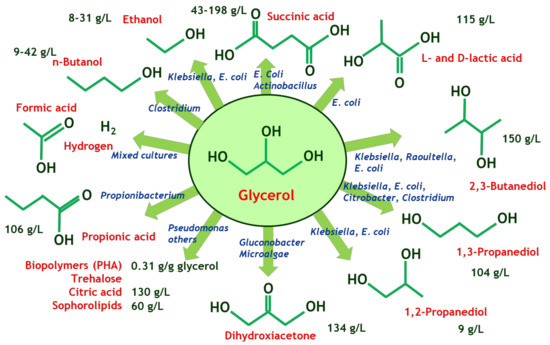
Figure 2.
The glycerol biorefinery: microbial processes to several bioproducts.
This Special Issue on new processes to upgrade pure and crude glycerol from biodiesel compiles a general review on the valorization of crude glycerol [4], a review focused on the production of one of the most relevant products from glycerol: glycerol carbonate [12], two research articles focused on catalytic processes to methanol and byproducts (acetaldehyde and DHA) [10], and 1,2-propanediol and 1- propanol [11], two research papers on enzymatic processes to functional glycerides [15] and ibuprofen prodrugs [16], and three research contributions focused on the production of succinic acid [19], DHA [25] and hydrogen [26]. All these contributions show a wide perspective of the ample and promising set of processes and products that are being developed and implemented for the valorization and upgrading of pure and crude glycerol, creating new opportunities for the biodiesel sector and, in general, for the chemical and related industries.
Funding
Our activities in research on pure and crude glycerol valorization were funded by MICINN through projects VALOGLI (CTQ 2010-15460) and PUBB (EUI20082008-03600) and by MMA under project MMA-PR21/06-0.39/2006/3-11.2.
Acknowledgments
The guest editor would like to thank the interest and the commitment of the authors who have contributed to this special issue and to all the editorial team of Catalysts for their kind support. He is especially grateful to Adela Liao for her assistance during this marvelous and rewarding experience.
Conflicts of Interest
The author declares no conflict of interest.
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Banja, M.; Motola, V. Renewable energy policy framework and bioenergy contribution in the European Union–An overview from National Renewable Energy Action Plans and Progress Reports. Renew. Sustain. Energy Rev. 2015, 51, 969–985. [Google Scholar] [CrossRef]
- Bórawski, P.; Bełdycka-Bórawska, A.; Szymańska, E.J.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of renewable energy sources market and biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- BP plc. The BP Energy Outlook. 2020. Available online: https://www.bp.com/en/global/corporate/energy-economics/energy-outlook.html (accessed on 31 December 2020).
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Chong, C.C.; Aqsha, A.; Ayoub, M.; Sajid, M.; Abdullah, A.Z.; Yusup, S.; Abdullah, B. A review over the role of catalysts for selective short-chain polyglycerol production from biodiesel derived waste glycerol. Environ. Technol. Innov. 2020, 19, 100859. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Basir, N.I.; Abdullah, A.Z. A review on one-pot synthesis of acrylic acid from glycerol on bi-functional catalysts. J. Ind. Eng. Chem. 2020, 93, 216–227. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. A review on glycerol valorization to acrolein over solid acid catalysts. J. Taiwan Inst. Chem. Eng. 2016, 67, 29–44. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L.; Lam, M.K.; Chen, W.H. Organic carbonate production utilizing crude glycerol derived as by-product of biodiesel production: A review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef]
- Mitran, G.; Neațu, F.; Neațu, Ș.; Trandafir, M.M.; Florea, M. VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol. Catalysts 2020, 10, 728. [Google Scholar] [CrossRef]
- Gatti, M.N.; Cerioni, J.L.; Pompeo, F.; Santori, G.F.; Nichio, N.N. High Yield to 1-Propanol from Crude Glycerol Using Two Reaction Steps with Ni Catalysts. Catalysts 2020, 10, 615. [Google Scholar] [CrossRef]
- Ji, Y. Recent development of heterogeneous catalysis in the transesterification of glycerol to glycerol carbonate. Catalysts 2019, 9, 581. [Google Scholar] [CrossRef]
- Esteban, J.; Fuente, E.; González-Miquel, M.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Sustainable joint solventless coproduction of glycerol carbonate and ethylene glycol via thermal transesterification of glycerol. RSC Adv. 2014, 4, 53206–53215. [Google Scholar] [CrossRef]
- Pradima, J.; Kulkarni, M.R. Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resour. Effic. Technol. 2017, 3, 394–405. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Valorization of glycerol through the enzymatic synthesis of acylglycerides with high nutritional value. Catalysts 2020, 10, 116. [Google Scholar] [CrossRef]
- Ravelo, M.; Wojtusik, M.; Ladero, M.; García-Ochoa, F. Synthesis of ibuprofen monoglyceride in solventless medium with novozym® 435: Kinetic analysis. Catalysts 2020, 10, 76. [Google Scholar] [CrossRef]
- Ripoll, V.; Rodríguez, A.; Ladero, M.; Santos, V.E. High 2, 3-butanediol production from glycerol by Raoultella terrigena CECT 4519. Bioprocess Biosyst. Eng. 2020, 43, 685–692. [Google Scholar] [CrossRef]
- Rodriguez, A.; Wojtusik, M.; Masca, F.; Santos, V.E.; Garcia-Ochoa, F. Kinetic modeling of 1, 3-propanediol production from raw glycerol by Shimwellia blattae: Influence of the initial substrate concentration. Biochem. Eng. J. 2017, 117, 57–65. [Google Scholar] [CrossRef]
- Kuenz, A.; Hoffmann, L.; Goy, K.; Bromann, S.; Prüße, U. High-level production of succinic acid from crude glycerol by a wild type organism. Catalysts 2020, 10, 470. [Google Scholar] [CrossRef]
- Kuenz, A.; Krull, S. Biotechnological production of itaconic acid—things you have to know. Appl. Microbial. Biotechnol. 2018, 102, 3901–3914. [Google Scholar] [CrossRef]
- Iyyappan, J.; Bharathiraja, B.; Baskar, G.; Kamalanaban, E. Process optimization and kinetic analysis of malic acid production from crude glycerol using Aspergillus niger. Bioresour. Technol. 2019, 281, 18–25. [Google Scholar] [CrossRef]
- Mazumdar, S.; Blankschien, M.D.; Clomburg, J.M.; Gonzalez, R. Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb. Cell Factories 2013, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kwon, E.Y.; Kim, Y.H.; Hahn, J.S. Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2737–2748. [Google Scholar] [CrossRef]
- Razali, N.; Abdullah, A.Z. Production of lactic acid from glycerol via chemical conversion using solid catalyst: A review. Appl. Catal. A Gen. 2017, 543, 234–246. [Google Scholar] [CrossRef]
- De la Morena, S.; Wojtusik, M.; Santos, V.E.; Garcia-Ochoa, F. Kinetic modeling of dihydroxyacetone production from glycerol by gluconobacter oxydans ATCC 621 resting cells: Effect of fluid dynamics conditions. Catalysts 2020, 10, 101. [Google Scholar] [CrossRef]
- FA, A.A.; Longoria, A.; AU, J.; AS, S.; LA, P.; Sebastian, P.J. Optimization of hydrogen yield from the anaerobic digestion of crude glycerol and swine manure. Catalysts 2019, 9, 316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).