Abstract
Photocatalysis is an effective technology for preventing the spread of pandemic-scale viruses. This review paper presents an overview of the recent progress in the development of an efficient visible light-sensitive photocatalyst, i.e., a copper oxide nanoclusters grafted titanium dioxide (CuxO/TiO2). The antiviral CuxO/TiO2 photocatalyst is functionalised by a different mechanism in addition to the photocatalytic oxidation process. The CuxO nanocluster consists of the valence states of Cu(I) and Cu(II); herein, the Cu(I) species denaturalizes the protein of the virus, thereby resulting in significant antiviral properties even under dark conditions. Moreover, the Cu(II) species in the CuxO nanocluster serves as an electron acceptor through photo-induced interfacial charge transfer, which leads to the formation of an anti-virus Cu(I) species and holes with strong oxidation power in the valence band of TiO2 under visible-light irradiation. The antiviral function of the CuxO/TiO2 photocatalyst is maintained under indoor conditions, where light illumination is enabled during the day but not during the night; this is because the remaining active Cu(I) species works under dark conditions. The CuxO/TiO2 photocatalyst can thus be used to reduce the risk of virus infection by acting as an antiviral coating material.
1. Introduction
Human beings have suffered from numerous kinds of pandemic viruses, such as SARS [1], Ebola virus [2], H1N2/2009 influenza [3], and COVID-19 (SARS-CoV-2) [4]. These viruses spread through direct person-to-person contact and/or indirect contact via virus-containing airborne droplets or contaminated surfaces of objects such as floors, handrails, touch panel/buttons, or furniture [5]. Therefore, antiviral chemicals and/or materials are useful for protecting against the spread of pandemic-scale viruses. For example, alcohol [6], hydrogen peroxide [7], and hypochlorous acid [8] have been widely used to disinfect various objects against bacteria or viruses. These chemicals deactivate viruses by denaturising their proteins [9]. However, the antiviral effect of these chemicals is not sustainable over the long term because of their evaporation and/or dissipation. Conversely, solid-state antiviral metal compounds could be useful because of their robustness and feasibility for use as coating materials. Although the biocidal properties of copper and silver have been reported previously [10], their antiviral effects are insufficient and do not last over the long term. Once their surfaces become contaminated by organic molecules, contact between the active metal and the viruses is inhibited.
Among various antiviral materials, the titanium dioxide (TiO2)-based photocatalysts are promising [11,12,13,14], because their antiviral effect is functioned under ultraviolet (UV) light irradiation [15,16]. Photogenerated holes in the valence band of TiO2 exhibit strong oxidation power for decomposing organic molecules [17,18,19]; thus, virus components such as surface proteins are oxidized under UV irradiation, resulting in virus disinfection [12]. Furthermore, a TiO2 photocatalyst film has a self-cleaning function by the strong oxidation power of holes [20] and its super-hydrophilic function [21,22,23,24,25], which helps the film retain its clean surface under UV light. Thus, surface contaminants are removed to expose antiviral active sites. However, TiO2 can only be activated by UV light, which is hardly contained in normal room light. Because viral infections mainly occur in indoor environments, it is necessary to use a visible light-sensitive antiviral photocatalyst. It is also noted that lighting is usually turned off during the night; thus, the sustained antiviral properties of photocatalysts under dark conditions are also important for their practical use.
Recently, we developed an efficient visible light-sensitive photocatalyst based on Cu(II) oxide nanoclusters grafted onto TiO2 [Cu(II)/TiO2] by using the concept of interfacial charge transfer (IFCT) [26,27,28,29,30,31,32]. Although the Cu(II)/TiO2 photocatalyst exhibited efficient photocatalytic oxidation activity and antiviral properties under visible light irradiation, its antiviral activity under dark conditions was limited. To improve the antiviral activity in the dark, we further developed CuxO (1 < x < 2) nanoclusters, which consisted of Cu(I) and Cu(II) species, and grafted them onto the TiO2 surface (denoted as CuxO/TiO2) [33]. While the Cu(II) species in CuxO nanoclusters is indispensable for the photocatalysis process, the Cu(I) species plays a crucial role in denaturing virus proteins, thereby causing their disinfection under dark conditions [33,34,35].
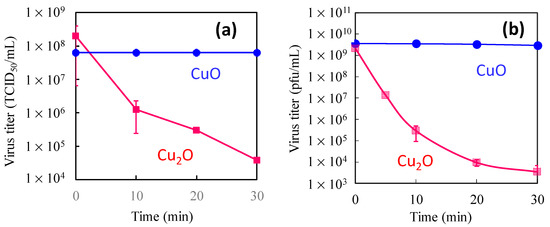
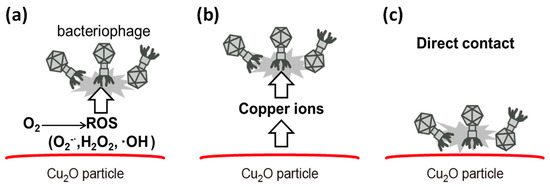
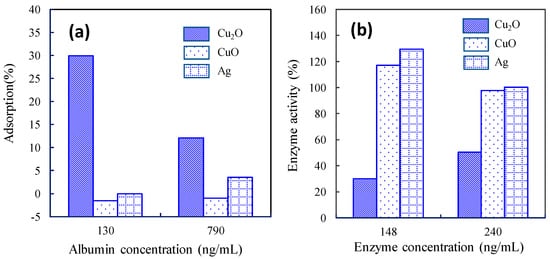
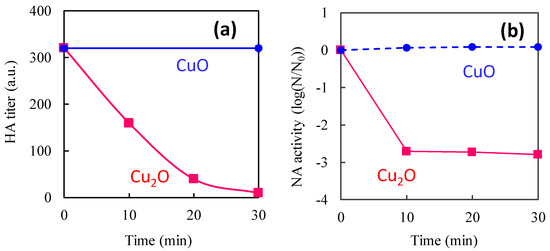
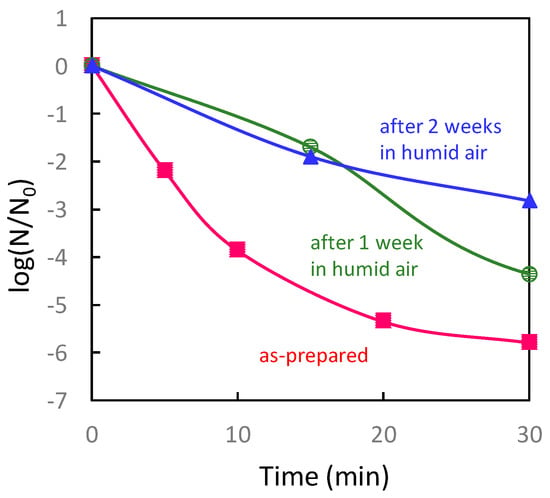
This review paper explains the role of the Cu(I) and Cu(II) species on TiO2 in terms of efficient antiviral activity. We first introduce the antiviral properties of pristine copper oxides (CuO and Cu2O) under dark conditions in the next section on the basis of our previous reports [34,35] and discuss the role of the Cu(I) species in Cu2O in terms of its antiviral properties. We then show the disadvantage of Cu2O for practical use because its surface can easily be oxidized into the inactive Cu(II) state in ambient humid air. Subsequently, we introduce our recent studies regarding Cu(II)/TiO2 as a visible light-sensitive photocatalyst [26,27,32], and CuxO/TiO2 as a visible light-sensitive as well as an efficient antiviral catalyst even under dark conditions [33]. The characterization, photocatalytic working principle, and sustained antiviral mechanism of these materials have been presented in this paper. We also show the results of the antiviral tests using a pseudo splash-containing bacteriophage Qβ on CuxO/TiO2-coated sheet fabric. This review paper comprehensively introduces the practical advantage of using CuxO/TiO2 as an antiviral coating material to protect against the spread of pandemic-scale viruses.
3. Visible Light-Sensitive Cu(II)/TiO2 Photocatalyst
The previous section suggests that maintaining the Cu(I) species is critical for sustaining antiviral properties over the long term. The main goal of this paper is to introduce the combination of a TiO2 photocatalyst with CuxO nanoclusters containing Cu(I) and Cu(II) species to achieve sustained antiviral properties. Before providing a detailed explanation of the CuxO/TiO2 system, we describe the role of the Cu(II) species attached to the TiO2 photocatalyst.
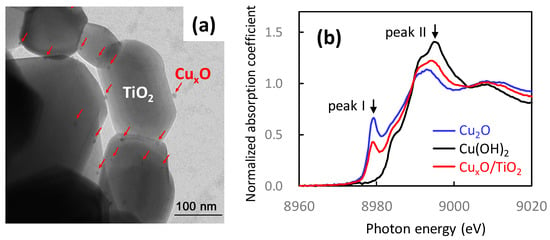
We previously reported Cu(II) nanoclusters grafted onto TiO2 [Cu(II)/TiO2] as an efficient visible light-sensitive photocatalyst for the oxidation of organic molecules [26,27]. Cu(II) nanoclusters could be grafted onto TiO2 (rutile, MT-150A, TAYCA Corporation) by wet chemical impregnation method using copper chloride dissolved aqueous media (0.1 wt % versus TiO2) as reported in our previous studies [26,27]. Figure 6a shows a transmission electron microscope (TEM) image of Cu(II)/TiO2, where Cu(II) clusters a few nanometres in size were grafted onto the TiO2 surface. Although the size of the Cu(II) nanocluster was too small to detect its X-ray diffraction, a previous study determined the local chemical structure of the Cu(II) nanoclusters by X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) [27]. Figure 6b shows the XANES spectra of Cu(II)/TiO2 and commercial reference powders. The spectrum of Cu(II)/TiO2 resembles that of Cu(OH)2, indicating that the valence number of the nanoclusters is in the 2+ state and that the Cu(II) species are likely to be in the five-coordinate square pyramidal form [53,54,55]. Figure 6c shows the EXAFS results of Cu(II)/TiO2 and commercial powder references of Cu(OH)2 and CuO. In contrast to the XANES results, the local chemical environment of the Cu(II) nanoclusters resembles that of CuO. The EXAFS data were carefully analysed using the REX2000 (Rigaku Corporation) and the FEFF program [56], and a one-coordinate Cu–O bond length (2.1–2.2 Å) was observed in Cu(OH)2 and Cu(II)/TiO2. Thus, the grafted Cu(II) nanoclusters are in the five-coordinate environment, which is consistent with the XANES results. In addition, one four-coordinate Cu–Cu and three types of two-coordinate Cu–Cu were observed, and the Cu–Cu bond lengths were similar to those in CuO, and so it can be considered that the grafted Cu(II) nanoclusters resemble the chemical environment of Cu(II) in CuO. That is, the local structure of the Cu(II) nanoclusters is distorted CuO, wherein the apical oxygen approaches Cu(II), forming a five-coordinate square pyramid attached to the TiO2 surface [27].
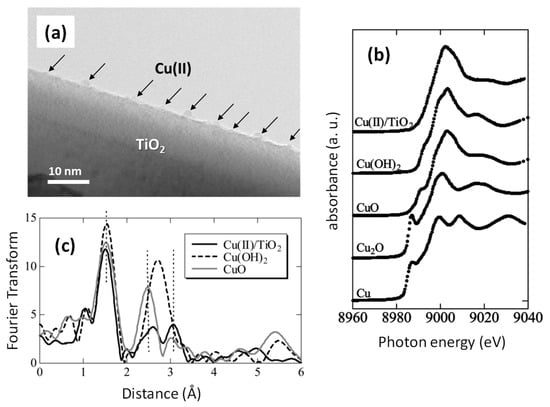
Figure 6.
(a) TEM image, (b) XANES analyses, and (c) Fourier transforms of EXAFS for Cu(II)/TiO2 [27]. Commercial powder of Cu, Cu2O, CuO, and Cu(OH)2 (Wako Ltd.) were used as references.
Figure 7a shows the UV-vis absorption spectra of pristine TiO2 and Cu(II)/TiO2. The pristine TiO2 exhibited strong UV light absorption shorter than 400 nm owing to its bandgap excitation. Meanwhile, Cu(II)/TiO2 exhibited additional visible-light absorption around 400–480 nm and over 650 nm. The former absorption is owing to the inter facial charge transfer (IFCT) excitation from the valence band of TiO2 to the Cu(II) nanocluster [26,27], whereas the latter originates in the d–d transition in the Cu(II) species [57]. The IFCT process is theoretically feasible between a semiconductor and ligand under photon irradiation [58], and visible-light absorption through IFCT was experimentally observed in previous studies [59,60,61]. The IFCT transition was also observed in the iron oxide-based Fe(III) nanocluster-grafted TiO2 [31,62].
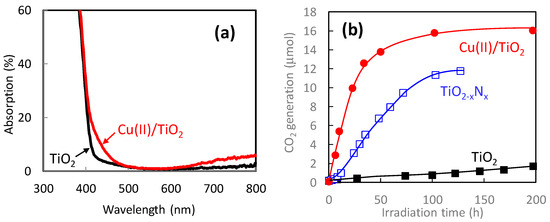
Figure 7.
(a) Optical absorption spectra of TiO2 (black line) and Cu(II)/TiO2 (red line). Amount of Cu(II) was 0.1 wt% versus TiO2 particles. (b) Photocatalytic oxidation activities of 2-propanol under visible-light irradiation for bare TiO2 (black), TiO2-xNx (blue), and Cu(II)/TiO2 (red). Visible-light irradiation was conducted using a xenon lamp passed through optical filters to set the wavelength at 400–530 nm with an illuminance of 1 mW/cm2.
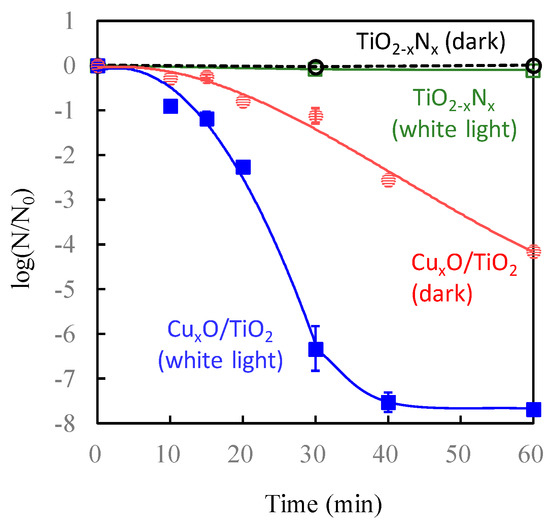
Figure 7b shows the photocatalytic oxidation activities of gaseous 2-propanol to carbon dioxide (CO2) under visible-light irradiation. As control groups, we also evaluated the photocatalytic activities of bare TiO2 and nitrogen-doped TiO2 (TiO2-xNx). The TiO2-xNx photocatalyst, which is recognized as an efficient visible-light photocatalyst [63], was prepared by a wet chemical method using titanium tetrachloride and ammonia, similar to a previous report [64]. The activity of pristine TiO2 was limited because of the lack of its visible-light absorption. In the case of TiO2-xNx, CO2 molecules were generated by the oxidation of 2-propanol; however, its activity was worse than that of Cu(II)/TiO2 because of the lower oxidation power of the holes excited in the nitrogen orbital [65,66,67]. It is noted that the Cu(II)/TiO2 photocatalyst decomposed 2-propanol with an initial amount of 5 μmol, producing approximately 15 μmol of CO2, showing that complete decomposition was achieved under visible-light irradiation. The quantum efficiency of the Cu(II)/TiO2 system reached over 80% by the optimization of the fabrication process [29], and thus it was significantly superior to that of TiO2-xNx [65,66].
The mechanism of the photocatalytic reaction by Cu(II)/TiO2 was previously investigated by various spectroscopic analyses. For example, Nosaka et al. examined the in situ electron spin resonance (ESR) of Cu(II)/TiO2 under visible-light irradiation [68]. Cu(II) species involve unpaired electrons, thus exhibiting an ESR signal, whereas Cu(I) is ESR-inactive. Furthermore, the photogenerated electrons and holes in TiO2 can be detected by ESR. When the Cu(II)/TiO2 sample was irradiated by visible light under vacuum conditions, the ESR signal of the Cu(II) species decreased and that of photogenerated holes in the valence band of TiO2 appeared. These results strongly suggest that the electron transition occurs from the valence band of TiO2 to the Cu(II) species through their interface under visible-light irradiation to generate Cu(I) species and holes in TiO2. The signal of the photogenerated holes decreased by the introduction of gaseous 2-propanol into the ESR chamber, whereas that of Cu(II) recovered by exposure to oxygen [68]. These results also indicate that the photogenerated holes oxidize 2-propanol, whereas excited electrons in the copper ion species react with oxygen molecules. Formation of Cu(I) species on TiO2 under light irradiation was also reported in the other previous literature [69]. The redox potential of Cu(II)/Cu(I) is approximately 0.16 V [versus a normal hydrogen electrode (NHE)] [26,27], which is more negative than that of the multi-electron reduction reaction of oxygen molecules to hydrogen peroxide (0.68 V vs. NHE) [70,71,72]. Therefore, excited electrons in the Cu(I) species react with oxygen molecules through a multi-electron reduction process under an oxygen-abundant atmosphere. A similar electron transition trend was seen in the XANES results [27]. Furthermore, Osako et al. visualized the reduction and oxidation sites in a Cu(II)/TiO2 system by using an ultrathin CuO film with a well-defined pattern coated onto a TiO2 single crystal prepared by pulsed laser deposition and photolithography [73]. Using an atomic force microscope (AFM), the authors observed the formation of metal Ag particles on the film resulting from the photoreduction of Ag+ ions, and Ag particles were selectively deposited on the edge of a CuO film under visible-light irradiation [74]. These results also suggest that the IFCT transition occurs by visible light and that the Cu(II) species acts as reduction sites. The concept of an IFCT transition for the development of visible light-sensitive photocatalysts has been extended to semiconductor systems other than TiO2, such as ZnO [75,76], SrTiO3 [77,78], SnO2 [79], Nb3O8- [80], Ag3PO4, Bi2O3 [81], BiOCl [82], BiVO4 [83], and Ag-based compounds [84]. The concept of an IFCT transition was also adopted for impurity-doped TiO2, such as Ti(III) self-doped TiO2 [28], Nb(IV)-doped TiO2 [85], and W(IV) and Ga(III)-codoped TiO2 [86].
Figure 8 shows the antiviral bacteriophage Qβ activity of TiO2-xNx and Cu(II)/TiO2 under white-light irradiation and dark conditions. Among these samples, the antiviral activity of Cu(II)/TiO2 under white-light irradiation was the most significant. Even though TiO2-xNx exhibited photocatalytic oxidation activity for 2-propanol [Figure 7b], its antiviral activity was negligible, attributed to its limited oxidation power [65,66,67]. In contrast, the number of bacteriophage Qβ on contact with Cu(II)/TiO2 under white-light irradiation decreased more than two orders of magnitude after 60 min of exposure. The antiviral properties of Cu(II)/TiO2 under dark conditions, however, were limited because the Cu(II) species was not as effective for the disinfection of viruses, as described in the previous section.
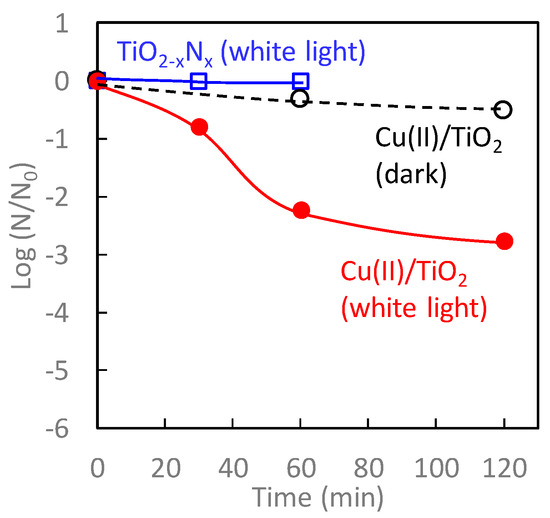
Figure 8.
Antiviral bacteriophage Qβ for Cu(II)/TiO2 under dark (black), TiO2-xNx under white-light irradiation (blue), and Cu(II)/TiO2 under white-light irradiation (red). Light irradiation was conducted using a commercial 10 W cylindrical white fluorescent lightbulb (FL-10, Mitsubishi) with a UV cut-off film shorter than 400 nm at an illuminance of 800 lux, which was measured by photometer (Topcon IM-5).
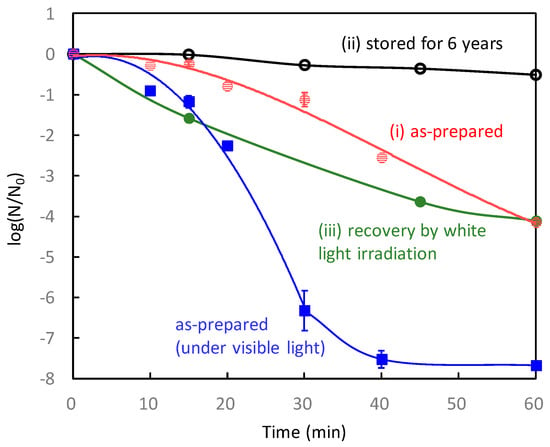
Through the IFCT transition in Cu(II)/TiO2, the Cu(I) species are created, in addition to the generation of holes in the valence band of TiO2. The produced Cu(I) species are effective for protein denaturation, and the holes, which have strong oxidation power, causing protein decomposition, and leading to virus disinfection. The contribution of the Cu(I) species generated by an IFCT transition to the antiviral properties was suggested by a previously reported “pre-irradiation” experiment [32]. Figure 9 shows the antiviral activities of Cu(II)/TiO2 under dark conditions without/with pre-irradiation. As a pre-irradiation treatment, the Cu(II)/TiO2 sample was placed under a white fluorescence lightbulb passed through a UV cut-off film below 400 nm before the evaluation of the antiviral effect. After the pre-irradiation treatment, the Cu(II)/TiO2 film was subjected to antiviral activity testing using bacteriophage Qβ under dark conditions. As shown in Figure 9, the pre-irradiation treatment improved the antiviral activity of Cu(II)/TiO2. This result suggests that pre-irradiation produced the Cu(I) species through the IFCT process, and some of them reacted with oxygen molecules in air, but the others remained even in the dark for a while, causing an antiviral effect. The previous study also showed that pre-irradiation with UV light improved the antiviral activity of Cu(II)/TiO2 [32], indicating that the excited electrons in the conduction band of TiO2 would also be injected into Cu(II) nanoclusters to form Cu(I) species.
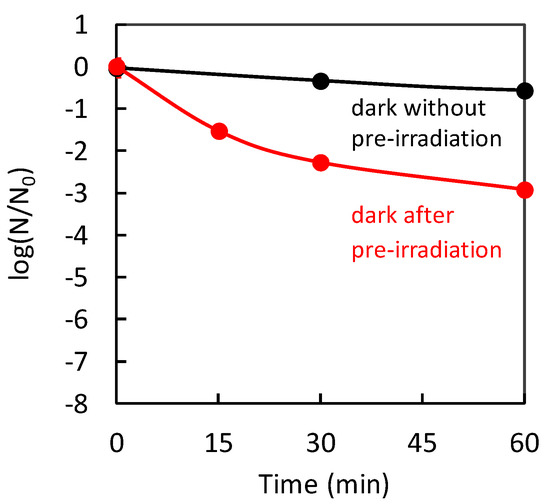
Figure 9.
Inactivation of bacteriophage Qβ by Cu(II)/TiO2 under dark conditions without pre-irradiation (black) and after pre-irradiation treatment (red) [32]. The pre-irradiation treatment was conducted using a white fluorescence lightbulb passed through a UV cut-off film below 400 nm.
5. Viruses Droplet Splash Test of CuxO/TiO2 Photocatalyst
Considering the practical application of the CuxO/TiO2 photocatalyst, we conducted antiviral tests on the CuxO/TiO2-coated sheet fabrics using the pseudo splash-containing bacteriophage Qβ. Figure 16 shows a photograph of the experimental setup for the antiviral splash test. An atomizer generated an aerosol that contained 6 × 107 pfu/h of bacteriophage Qβ, and the particle size of the aerosol was approximately 0.3 μm. The virus aerosol from the atomizer attached to the photocatalyst sheets on a desk of 1 m high from the floor under white fluorescence light at an illuminance of 1000 lux. After 4 h, the number of bacteriophages was counted using the same procedure with the previous studies [33,34,35]. Bacteriophages on a control sheet without CuxO/TiO2 coating were also sampled at 1 h and 2 h.

Figure 16.
Procedure for evaluating an antiviral CuxO/TiO2-coated fabric sheet using pseudo splash-containing bacteriophage Qβ. Room volume was (4 m × 3 m × 2 m) and the ventilation frequency was 1.8 time/h. White light was irradiated by fluorescent lightbulbs at an illuminance of 1000 lux. Bacteriophage Qβ containing 6 × 107 pfu/h was sprayed for 4 h by an atomizer (ATM-226, KANOMAX JAPAN INC.) to attach it on the sheet surfaces.
Figure 17 shows the changes in the number of bacteriophages on the photocatalyst sheet and control sheet. It is noteworthy that the number of bacteriophages on the CuxO/TiO2 sheet was negligible, indicating its strong antiviral function against the virus attached to the surface. A CuxO/TiO2-coated material can thus potentially disinfect viruses on any surface derived from droplets and aerosol to protect against viral disease spread by contact infection.
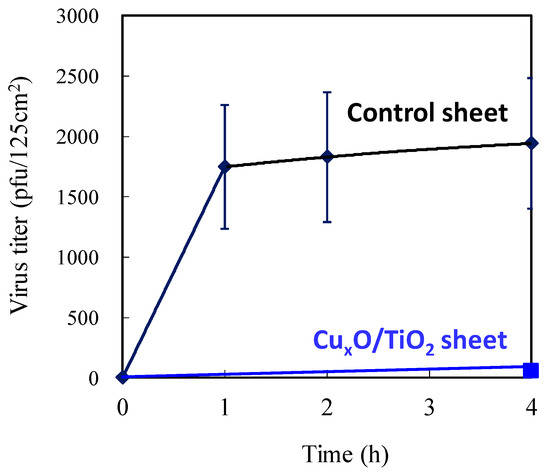
Figure 17.
Antiviral properties of the CuxO/TiO2-coated sheet and the control sheet without the photocatalyst using splash-containing bacteriophage Qβ. In the case of CuxO/TiO2 to avoid the overestimation of its antiviral property, the number of experiments was set to 1 time (after 4h) in order to exclude the influence of air flow due to human’s entering into the room for measurement.
6. Conclusions
This review paper introduces the recent progress in the development of CuxO/TiO2 as an efficient visible light-sensitive photocatalyst for antiviral applications. The CuxO nanocluster consists of the valence states of Cu(I) and Cu(II). Cu(I) species in CuxO nanoclusters can denature viral proteins, resulting in significant antiviral properties even under dark conditions. Unfortunately, the Cu(I) species in CuxO are easily oxidized to inactive Cu(II) in ambient air. However, the combination of CuxO with the TiO2 photocatalyst maintained its antiviral function by visible-light irradiation. In the CuxO/TiO2 photocatalyst, electron transition occurs by visible-light irradiation through the IFCT process; this results in the generation of antiviral Cu(I) species and holes in the valence band of TiO2, which are effective in disinfecting viruses. Once the Cu(I) species in CuxO turn into Cu(II) by self-oxidation, antiviral active Cu(I) species can be regenerated by visible light like a white fluorescence bulb. Therefore, the antiviral function of CuxO/TiO2 can be maintained, even under indoor conditions, where light illumination is turned on during the day and off during the night. It is also noted that the CuxO/TiO2 composite samples have been commercialized (NAKA CORPORATION, Tokyo Japan). We expect the CuxO/TiO2 material to be applied to various antiviral industrial items in indoor circumstances, such as hospitals, airports, metro stations, and schools, as coating materials for air filters, respiratory face masks, and antifungal fabrics to prevent the COVID-19 spread. Furthermore, the present concept contributes to the design of various antiviral materials, such as bimetallic catalysts [88,89,90].
Author Contributions
Conceptualization, K.H.; antiviral investigation, K.S.; photocatalysis investigation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, K.H.; project leader, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by New Energy and Industrial Technology Development Organisation (NEDO) in Japan, project name: Project to Create Photocatalyst Industry for Recycling-Oriented Society. This research was also funded by JSPS Kakenhi, grant No. 18H02055.
Acknowledgments
We appreciate the project members of NEDO, including H. Irie at Yamanashi University, Japan, M. Minoshima at Osaka University, Japan, Y. Kuroda at Showa Denko K.K., Japan, H. Yu at Wuhan University of Technology, China, X. Qiu and M. Liu at Central South University, China, and other collaborators for their great help in the development of the present photocatalyst. Nitrogen-doped TiO2 (TiO2-xNx) was provided by Showa Denko K.K. (HP-N08).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Délicat, A.; Paweska, J.T.; Gonzalez, J.P.J.; Swanepoel, R. Fruit bats as reservoirs of Ebola virus. Nature 2005, 438, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishna, D.; Poon, L.L.M.; Zhu, H.C.; Ma, S.K.; Li, O.T.W.; Cheung, C.L.; Smith, G.J.D.; Peiris, J.S.M.; Guan, Y. Reassortment of pandemic h1n1/2009 influenza a virus in swine. Science 2010, 328, 1529. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Brankston, G.; Gitterman, L.; Hirji, Z.; Lemieux, C.; Gardam, M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007, 7, 257–265. [Google Scholar] [CrossRef]
- Kampf, G.; Grotheer, D.; Steinmann, J. Efficacy of three ethanol-based hand rubs against feline calicivirus, a surrogate virus for norovirus. J. Hosp. Infect. 2005, 60, 144–149. [Google Scholar] [CrossRef]
- Tuladhar, E.; Terpstra, P.; Koopmans, M.; Duizer, E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J. Hosp. Infect. 2012, 80, 110–115. [Google Scholar] [CrossRef]
- Barclay, L.; Park, G.W.; Vega, E.; Hall, A.; Parashar, U.; Vinjé, J.; Lopman, B. Infection control for norovirus. Clin. Microbiol. Infect. 2014, 20, 731–740. [Google Scholar] [CrossRef]
- Anson, M. Protein denaturation and the properties of protein groups. In Advances in Protein Chemistry; Anson, M.L., Edsall, J.T., Eds.; Academic Press: Cambridge, MA, USA, 1945; Volume 2, pp. 361–386. [Google Scholar]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Control. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and detoxification effects of TiO2 thin film Photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal activity of copper-deposited TiO2 thin film under weak UV light illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Process. Polym. 2007, 4, S397–S401. [Google Scholar] [CrossRef]
- Ishiguro, H.; Nakano, R.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, A.M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of bacteriophages by TiO2-coated glass plates under low-intensity, long-wavelength UV irradiation. Photochem. Photobiol. Sci. 2011, 10, 1825–1829. [Google Scholar] [CrossRef]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, A.M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Paz, Y.; Luo, Z.; Rabenberg, L.; Heller, A. Photooxidative self-cleaning transparent titanium dioxide films on glass. J. Mater. Res. 1995, 10, 2842–2848. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Photogeneration of highly amphiphilic tio2 surfaces. Adv. Mater. 1998, 10, 135–138. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Hashimoto, K.; Watanabe, T. A highly hydrophilic thin film under 1 μW/cm2 UV illumination. Adv. Mater. 2000, 12, 1923–1927. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and photoinduced hydrophilicity of various metal oxide thin films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Miyauchi, M.; Tokudome, H. Highly hydrophilic conversion on oriented TiO2 thin films synthesized by a facile spin-coating method. Appl. Phys. Lett. 2007, 91, 43111. [Google Scholar] [CrossRef]
- Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) ions onto TiO2 and WO3 photocatalysts. Chem. Phys. Lett. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Cu(II) oxide amorphous nanoclusters grafted Ti3+self-doped TiO2: An efficient visible light photocatalyst. Chem. Mater. 2011, 23, 5282–5286. [Google Scholar] [CrossRef]
- Liu, M.; Inde, R.; Nishikawa, M.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced photoactivity with nanocluster-grafted titanium dioxide photocatalysts. ACS Nano 2014, 8, 7229–7238. [Google Scholar] [CrossRef]
- Miyauchi, M.; Liu, Z.; Zhao, Z.-G.; Anandan, S.; Tokudome, H. Visible-light-driven superhydrophilicity by interfacial charge transfer between metal ions and metal oxide nanostructures. Langmuir 2010, 26, 796–801. [Google Scholar] [CrossRef]
- Miyauchi, M.; Irie, H.; Liu, M.; Qiu, X.; Yu, H.; Sunada, K.; Hashimoto, K. Visible-light-sensitive photocatalysts: Nanocluster-grafted titanium dioxide for indoor environmental remediation. J. Phys. Chem. Lett. 2016, 7, 75–84. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-light sensitive Cu(II)–TiO2 with sustained anti-viral activity for efficient indoor environmental remediation. J. Mater. Chem. A 2015, 3, 17312–17319. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, A.M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Minoshima, A.M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Minoshima, A.M.; Lü, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef]
- Deka, P.; Borah, B.J.; Saikia, H.; Bharali, P. Cu-based nanoparticles as emerging environmental catalysts. Chem. Rec. 2019, 19, 462–473. [Google Scholar] [CrossRef]
- Scotti, N.; Monticelli, D.; Zaccheria, F. Dispersed copper oxide: A multifaceted tool in catalysis. Inorganica Chim. Acta 2012, 380, 194–200. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Pang, H.; Gao, F.; Lu, Q. Morphology effect on antibacterial activity of cuprous oxide. Chem. Commun. 2009, 9, 1076–1078. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Tornkvist, C.; Liedberg, B. Photoelectron and infrared reflection absorption spectroscopy of benzotriazole adsorbed on copper and cuprous oxide surfaces. Appl. Surf. Sci. 1989, 37, 306–326. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. ChemInform abstract: Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Glover, R.D.; Miller, J.M.; Hutchison, J.E. Generation of metal nanoparticles from silver and copper objects: Nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano 2011, 5, 8950–8957. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Aramini, J.M.; Ma, L.C.; Krug, R.M.; Arnold, E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010, 17, 530–538. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004, 78, 12665–12667. [Google Scholar] [CrossRef]
- Donald, H.B.; Isaacs, A. Counts of influenza virus particles. J. Gen. Microbiol. 1954, 10, 457–464. [Google Scholar] [CrossRef]
- Buxton, R.C.; Edwards, B.; Juo, R.R.; Voyta, J.C.; Tisdale, M.; Bethell, R.C. Development of a sensitive chemiluminescent neuraminidase assay for the determination of influenza virus susceptibility to zanamivir. Anal. Biochem. 2000, 280, 291–300. [Google Scholar] [CrossRef]
- Gattinoni, C.; Michaelides, A. Atomistic details of oxide surfaces and surface oxidation: The example of copper and its oxides. Surf. Sci. Rep. 2015, 70, 424–447. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of polycrystalline copper thin films at ambient conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Nian, J.N.; Chen, S.A.; Tsai, C.C.; Teng, H. Structural feature and catalytic performance of cu species distributed over TiO2 nanotubes. J. Phys. Chem. B 2006, 110, 25817–25824. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, T.L.; Wang, H.P.; Lu, Y.M.; Hsiao, M.C. In situ XANES studies of CuO/TiO2 thin films during photocatalytic degradation of CHCl3. Radiat. Phys. Chem. 2006, 75, 2054–2057. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kubota, T.; Gotoh, H.; Ohto, Y.; Aritani, H.; Tanaka, T.; Yoshida, S. XAFS study of zirconia-supported copper catalysts for the NO–CO reaction: Deactivation, rejuvenation and stabilization of Cu species. J. Chem. Soc. Faraday Trans. 1998, 94, 3743–3752. [Google Scholar] [CrossRef]
- Stern, E.A.; Newville, M.; Ravel, B.; Yacoby, Y.; Haskel, D. The UWXAFS analysis package: Philosophy and details. Phys. B Condens. Matter 1995, 208, 117–120. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Hush, N. Homogeneous and heterogeneous optical and thermal electron transfer. Electrochim. Acta 1968, 13, 1005–1023. [Google Scholar] [CrossRef]
- Creutz, C.; Brunschwig, B.S.; Sutin, N. Interfacial charge-transfer absorption: Semiclassical treatment. J. Phys. Chem. B 2005, 109, 10251–10260. [Google Scholar] [CrossRef]
- Creutz, C.; Brunschwig, B.S.; Sutin, N. Interfacial charge-transfer absorption: 3. Application to semiconductor−molecule assemblies. J. Phys. Chem. B 2006, 110, 25181–25190. [Google Scholar] [CrossRef]
- Nakamura, R.; Okamoto, A.; Osawa, H.; Irie, H.; Hashimoto, K. Design of all-inorganic molecular-based photocatalysts sensitive to visible light: Ti(iv)−o−ce(iii) bimetallic assemblies on mesoporous silica. J. Am. Chem. Soc. 2007, 129, 9596–9597. [Google Scholar] [CrossRef]
- Yu, H.; Irie, H.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; Miyauchi, M.; Hashimoto, K. An efficient visible-light-sensitive fe(iii)-grafted tio2 photocatalyst. J. Phys. Chem. C 2010, 114, 16481–16487. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Kisch, H. Photocatalytic and photoelectrochemical properties of nitrogen-doped titanium dioxide. ChemPhysChem 2003, 4, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of tio2-xnx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ikezawa, A.; Tobimatsu, H.; Irie, H.; Hashimoto, K. Zeta potential and photocatalytic activity of nitrogen doped TiO2 thin films. Phys. Chem. Chem. Phys. 2004, 6, 865–870. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for visible light responses in anodic photocurrents at N-doped TiO2 film electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Nosaka, Y.; Takahashi, S.; Sakamoto, H.; Nosaka, A.Y. Reaction mechanism of cu(ii)-grafted visible-light responsive TiO2 and WO3 photocatalysts studied by means of ESR spectroscopy and chemiluminescence photometry. J. Phys. Chem. C 2011, 115, 21283–21290. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Scott, J.A.; Ng, Y.H.; Jiang, Y.; Amal, R. Exploring Cu oxidation state on TiO2 and its transformation during photocatalytic hydrogen evolution. Appl. Catal. A Gen. 2016, 521, 190–201. [Google Scholar] [CrossRef]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Ab initio molecular dynamics simulations of the oxygen reduction reaction on a pt(111) surface in the presence of hydrated hydronium (H3O)+(H2O)2: Direct or series pathway? J. Phys. Chem. B 2005, 109, 14896–14907. [Google Scholar] [CrossRef]
- Mustain, W.E.; Prakash, J. A model for the electroreduction of molecular oxygen. J. Electrochem. Soc. 2007, 154, A668–A676. [Google Scholar] [CrossRef]
- Osako, K.; Matsuzaki, K.; Hosono, H.; Yin, G.; Atarashi, D.; Sakai, E.; Susaki, T.; Miyauchi, M. Examination of interfacial charge transfer in photocatalysis using patterned CuO thin film deposited on TiO2. APL Mater. 2015, 3, 104409. [Google Scholar] [CrossRef]
- Osako, K.; Matsuzaki, K.; Susaki, T.; Ueda, S.; Yin, G.; Yamaguchi, A.; Hosono, H.; Miyauchi, M. Direct Observation of interfacial charge transfer between rutile tio2 and ultrathin cuox film by visible-light illumination and its application for efficient photocatalysis. ChemCatChem 2018, 10, 3666–3670. [Google Scholar] [CrossRef]
- Anandan, S.; Ohashi, N.; Miyauchi, M. Zno-based visible-light photocatalyst: Band-gap engineering and multi-electron reduction by co-catalyst. Appl. Catal. B 2010, 100, 502–509. [Google Scholar] [CrossRef]
- Anandan, S.; Miyauchi, M. Ce-doped ZnO (CexZn1−xO) becomes an efficient visible-light-sensitive photocatalyst by co-catalyst (Cu2+) grafting. Phys. Chem. Chem. Phys. 2011, 13, 14937–14945. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Miyauchi, M.; Yu, H.; Irie, H.; Hashimoto, K. Visible-light-driven cu(ii)−(sr1−ynay)(ti1−xmox)o3 photocatalysts based on conduction band control and surface ion modification. J. Am. Chem. Soc. 2010, 132, 15259–15267. [Google Scholar] [CrossRef]
- Nosaka, Y.; Takahashi, S.; Mitani, Y.; Qiu, X.; Miyauchi, M. Reaction mechanism of visible-light responsive Cu(II)-grafted Mo-doped SrTiO3 photocatalyst studied by means of ESR spectroscopy and chemiluminescence photometry. Appl. Catal. B Environ. 2012, 111–112, 636–640. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Zhang, Y.; Xu, S.; Kong, F.; Luo, Y.; Tian, Y.; Teng, X.; Li, G. Surface Fe3+-decorated pristine SnO2 nanoparticles with enhanced ·OH radical generation performance. Catal. Commun. 2012, 24, 96–99. [Google Scholar] [CrossRef]
- Yin, G.; Nishikawa, M.; Nosaka, Y.; Srinivasan, N.; Atarashi, D.; Sakai, E.; Miyauchi, M. Photocatalytic carbon dioxide reduction by copper oxide nanocluster-grafted niobate nanosheets. ACS Nano 2015, 9, 2111–2119. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Huang, C.; Liu, M.; Qiu, X. Enhanced photocatalytic activity of Bi2O3 under visible light irradiation by Cu(II) clusters modification. Appl. Catal. B Environ. 2013, 142–143, 598–603. [Google Scholar] [CrossRef]
- Huang, C.; Hu, J.; Cong, S.; Zhao, Z.; Qiu, X. Hierarchical BiOCl microflowers with improved visible-light-driven photocatalytic activity by Fe(III) modification. Appl. Catal. B Environ. 2015, 174, 105–112. [Google Scholar] [CrossRef]
- Yang, Y.; Yamaguchi, A.; Jiang, H.; Van Der Kooy, A.; Okunaka, S.; Hosogai, M.; Tokudome, H.; Miyauchi, M. Green light active photocatalyst for complete oxidation of organic molecules. Chem. Commun. 2020, 56, 9210–9213. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, Y.; Wu, P.; Wang, X.; Yu, H.; Yu, J. Cu(II) as a general cocatalyst for improved visible-light photocatalytic performance of photosensitive ag-based compounds. J. Phys. Chem. C 2014, 118, 8891–8898. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Hashimoto, K.; Miyauchi, M. Cu(ii) nanocluster-grafted, Nb-doped TiO2 as an efficient visible-light-sensitive photocatalyst based on energy-level matching between surface and bulk states. J. Mater. Chem. A 2014, 2, 13571–13579. [Google Scholar] [CrossRef]
- Yu, H.; Irie, H.; Hashimoto, K. Conduction band energy level control of titanium dioxide: Toward an efficient visible-light-sensitive photocatalyst. J. Am. Chem. Soc. 2010, 132, 6898–6899. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravorty, D. Optical absorption by nanoparticles of Cu2O. EPL Europhys. Lett. 2000, 52, 468–473. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Ma, T.; Li, W.; Zhang, J.; Zhang, M. Mechanistic understanding of Cu-based bimetallic catalysts. Front. Chem. Sci. Eng. 2020, 14, 689–748. [Google Scholar] [CrossRef]
- Spanu, D.; Recchia, S.; Mohajernia, S.; Tomanec, O.; Kment, S.; Zbořil, R.; Schmuki, P.; Altomare, M. Templated dewetting–Alloying of NiCu bilayers on TiO2 nanotubes enables efficient noble-metal-free photocatalytic H2 evolution. ACS Catal. 2018, 8, 5298–5305. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Ryl, J.; Nowaczyk, G.; Zielińska-Jurek, A. Morphology, photocatalytic and antimicrobial properties of TiO2 modified with mono- and bimetallic copper, platinum and silver nanoparticles. Nanomaterials 2019, 9, 1129. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).