1. Introduction
The solid oxide fuel cell (SOFC) is an attractive device for direct conversion of chemical energy of fuel to electricity with additional heat production [
1]. Due to its high working temperature, a SOFC can operate on both pure hydrogen and synthesis gas (syngas) [
2] obtained from hydrocarbon fuels (methane, liquified petroleum gas (LPG), liquid hydrocarbons) by steam conversion [
3], partial oxidation (PO) [
4], dry reforming [
5,
6], or autothermal reforming [
7]. In the case of a SOFC stack with power of several kilowatts or more, the heat released during the reaction can be efficiently utilized to conduct endothermal steam reforming of the initial fuel. This increases the efficiency of the entire system (reformer + SOFC system). For small power SOFCs, usually less than 1–2 kW, the heat generated is not always enough to simultaneously maintain the required stack temperature and for endothermic steam reforming. In this case, it is preferable to use an exothermic partial oxidation process:
However, the overall efficiency of the power plant is significantly reduced. In addition, when using LPG (which can be compactly stored in a liquid state in cylinders at low pressure) during partial oxidation, the conversion temperature usually exceeds 900 °C, which leads to accelerated degradation of the catalyst [
8].
One of the options for increasing the efficiency of fuel conversion is a process that combines the weakly exothermic process of LPG pre-reforming at low temperatures and partial oxidation of the resulting methane–hydrogen mixture, the temperature of which is significantly lower than the temperature of LPG partial oxidation:
In addition, the pre-reforming process requires much smaller amounts of water to be supplied than for the classic steam reforming process [
9].
In this work we considered the possibility of combining pre-reforming at a low steam to carbon ratio and subsequent partial oxidation to maximize the energy efficiency of syngas production from LPG and carried out proof-of-concept experiments.
2. Results and Discussion
Propane, the main component of LPG, was chosen as a model compound. In our previous work the Ni-Cr
2O
3-Al
2O
3 catalyst was shown to be effective in propane pre-reforming [
10]. As an example,
Figure 1 shows the temperature dependences of the outlet concentrations of propane, methane, carbon dioxide, and hydrogen during propane pre-reforming under 1 bar pressure, with a H
2O:C molar ratio of 1.0 and gas hourly space velocity (GHSV) of 12,000 h
−1. It is seen that the propane conversion and CH
4, H
2 and CO
2 concentrations increased with the increase of temperature, reaching equilibrium values at 380 °C.
Methane-rich gas (MRG) after pre-reforming typically contains 40–70 vol.% CH4, 10–30 vol.% H2O, 5–15 vol.% CO2, 5–20 vol.% H2, and 0.1–2 vol.% CO. The obtained methane-rich gas can be converted to syngas via the addition of the required quantity of oxygen or air. As the resulting mixture will contain water steam together with oxygen, the process will be near autothermal, which is especially convenient for compact systems.
It was previously demonstrated [
8,
11] that Rh/Ce
0.75Zr
0.25O
2-δ–ƞ-Al
2O
3/FeCrAl catalyst (further Rh-block) was highly efficient in the autothermal reforming of diesel fuel, exhibiting high catalytic activity, stability, and a low tendency to carbonization. In this work, its properties in the partial oxidation of methane-rich gas are studied.
Figure 2 shows the dependences of the outlet concentrations of H
2, N
2, CO, CO
2, and CH
4 and the temperatures at the inlet (T
in) and outlet (T
out) centerline points of the catalytic block as a function of the flow rate of the reaction mixture (GHSV) for the PO of MRG over the Rh-block at 730 °C. It is seen that, for all flows, the distribution of products was close to equilibrium at 700 °C. The temperature at the outlet of the block varied from 693 to 730 °C, i.e., was close to 700 °C, which explains the closeness of the product distribution to equilibrium. However slight increase of CH
4 outlet concentration, accompanied with the decrease of H
2 and CO level, was observed at GHSVs 40,000–60,000 h
−1, indicating the limitation of the overall reaction rate.
The temperature at the catalyst inlet exceeded the reactor wall temperature (730 °C) and the outlet temperature, which was associated with the exothermic reaction of complete oxidation of methane and hydrogen. The outlet temperature, on the contrary, did not exceed the temperature of the walls of the reactor, which was due to the endothermic reactions of steam and dry reforming of methane. In this case, with an increase in the flow rate, T
in decreased and T
out increased. This is apparently associated with an increase in the heat release rate on the catalyst and with the stretching of the zone in which complete oxidation of methane and hydrogen occurs, with an increase of GHSV, which is caused by external diffusion limitation of the rate of total oxidation [
12].
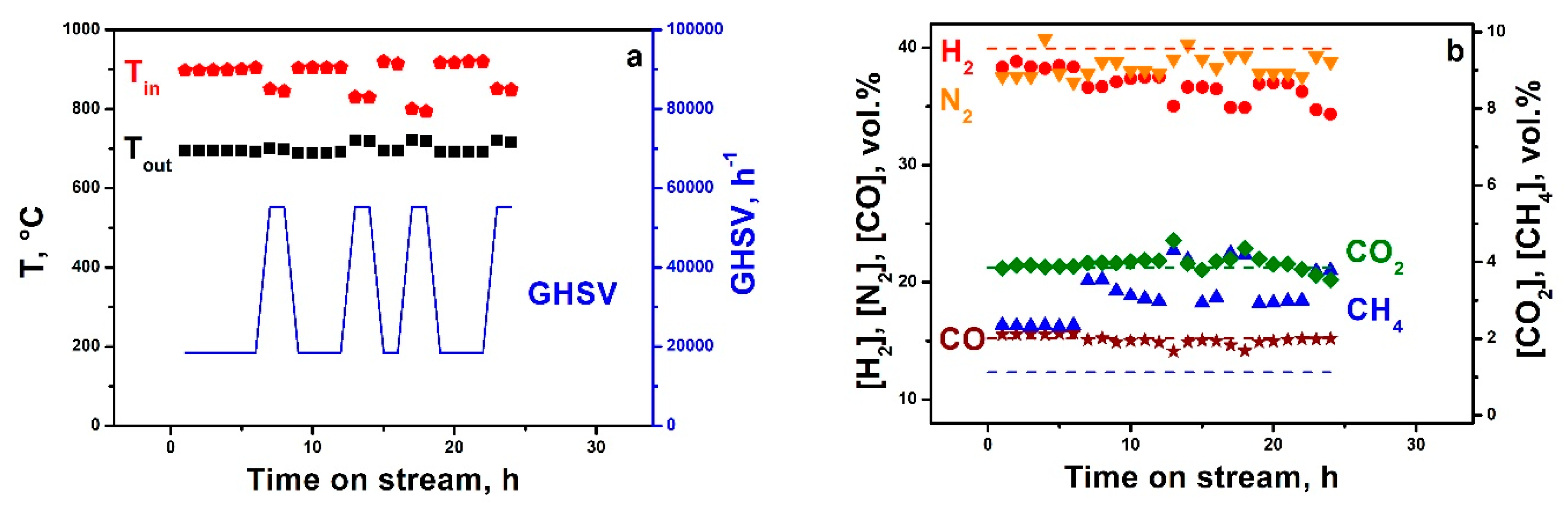
The stability of catalytic properties was studied during 25 h on stream. The test was performed at a reactor wall temperature of 730 °C and GHSV of 20,000 h
−1 and the following reaction mixture (vol.%): 25.6 CH
4, 13.4 O
2, 10.5 H
2O, and 50.5 N
2. The GHSV was periodically raised to 55,000 h
−1 (
Figure 3a) to check the catalyst activity in the conditions at which the reaction was under kinetic control (product distribution differs from the equilibrium). The properties of the catalyst were constant during 25 h on stream (
Figure 3b). The weight of the block did not change after the experiment; no damage (detachments) of the catalytic coating was detected. No carbon deposits were detected by temperature-programmed oxidation. Thus, the Rh-block showed stable operation under PO MRG conditions and provided a syngas (H
2 + CO) productivity of 28 m
3·L
cat−1·h
−1 (STP).
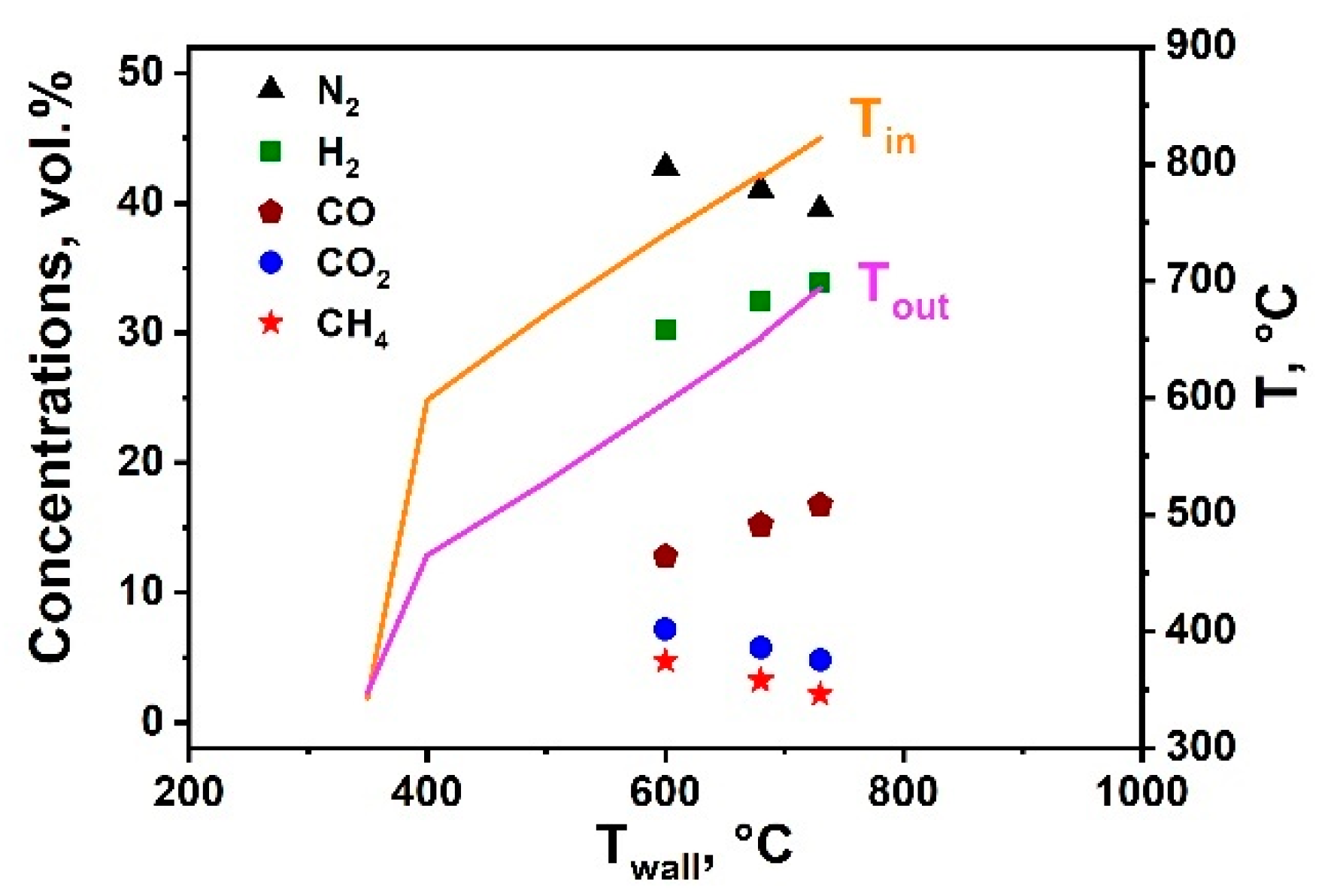
We also studied the light-on curve for the Rh-block in PO MRG, as it is important to determine the minimal outlet temperature after pre-reforming at which the PO could be launched.
Figure 4 shows the dependence of the temperatures at the inlet (T
in) and outlet (T
out) centerline points of the catalytic block and the outlet concentrations of H
2, N
2, CO, CO
2, and CH
4 as a function of the reactor wall temperature. It is seen that at the initial temperature of 350 °C, T
in and T
out were similar to T
wall, indicating the absence of a PO reaction. A T
wall increase to 400 °C induced a sharp rise of T
in and T
out to 598 and 465 °C, respectively. Thus, the temperature of 400 °C could be considered as a light-on one. Further increases of T
wall provided a gradual increase of T
in, T
out, and progress of the reaction. Product distribution at 600–730 °C was close to equilibrium. Rapid product composition changes at 400–600 °C were difficult to register by gas chromatography. Transient switch on/off regimes will be the subject of further studies.
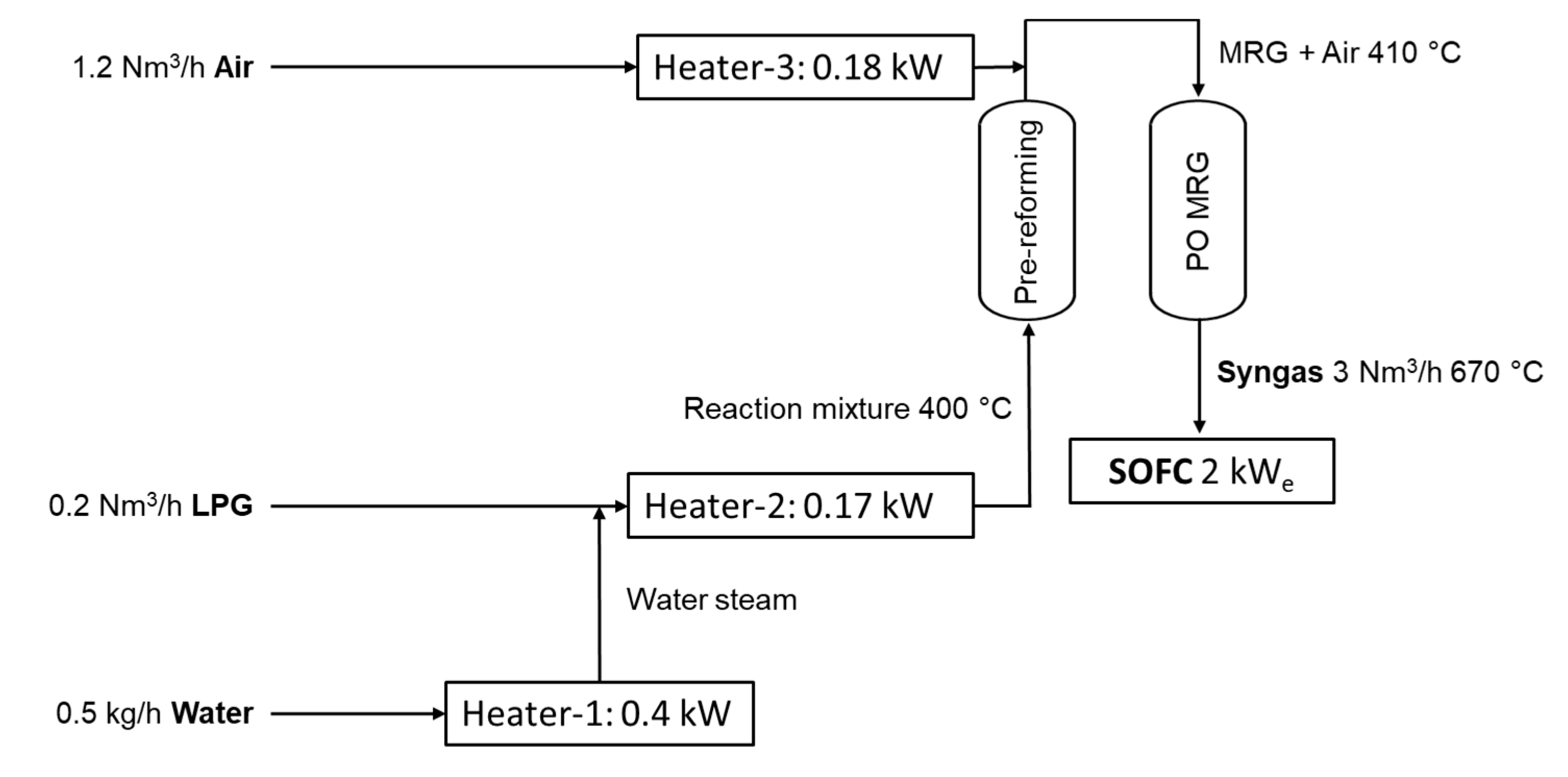
Coupling pre-reforming and partial oxidation of the following syngas production scheme could be supposed (
Figure 5): after mixing and heating the mixture of LPG and water steam (H
2O:C = 1, molar) was supplied to the adiabatic pre-reformer at 400 °C; the outlet temperature of methane-rich gas (MRG) was 410 °C; heated air was mixed with MRG and supplied to the adiabatic partial oxidation reactor at 410 °C; the resulted syngas with temperature of 670 °C was supplied directly to SOFC. The obtained syngas contained (vol.%) 1.1 CH
4, 36.4 H
2, 13.1 CO, 10.7 H
2O, 7.1 CO
2, 0.4 Ar, and 31.2 N
2 and could be successfully used for SOFC feeding. For 2 kW
e SOFC only 0.2 Nm
3/h LPG (80 mol.% C
3H
8 and 20 mol.% C
4H
10) was required due to the high energy density of LPG. Furthermore, only 0.75 kW of heat power was required for water evaporation and gas superheating. Instead of the conventional steam reforming process, the heat in this scheme had low- and medium-potential: the maximal temperature had to be reached at 410 °C. Thus, the process needs could be satisfied by SOFC heat output (ca. 0.5 kW for 2 kW
e SOFC) and anode gases afterburning (ca. 0.4 kW).
4. Materials and Methods
Propane pre-reforming was studied using NIAP-07-05 industrial catalyst (wt.%): 42 NiO, 12 Cr
2O
3, 46 Al
2O
3, and 4 graphite (NIAP Ltd., Novomoskovsk, Russia). This catalyst demonstrated high activity in the low-temperature steam conversion of light hydrocarbons in methane excess [
9,
10].
The experiments on propane pre-reforming were carried out in a fixed-bed U-shaped quartz reactor (internal diameter 8 mm, catalyst bed volume 1.7 cm3). Experimental conditions were as follows: 1 bar pressure, temperature of 240–400 °C, GHSV of 12,000 h−1. Before the experiment, 2 g of the catalyst (fraction 0.5–1 mm) were reduced at 420 °C in a flow of 10 mL/min H2 and 100 mL/min Ar. The composition of the initial reaction mixture was 25 vol.% C3H8 and 75 vol.% H2O, which corresponded to an H2O/C molar ratio of 1. The temperature of the catalyst was measured using a K-type thermocouple placed in the center of the catalyst bed. The composition of the gas phase components were determined using a Chromos GC-1000 chromatograph (CHROMOS Engineering company, Moscow, Russia). The concentration of carbon monoxide was negligible (below 0.1 vol.%), and therefore CO was not taken into account when processing the results. The carbon imbalance in all the experiments did not exceed ±1 rel.%.
The composite Rh/Ce
0.75Zr
0.25O
2-δ–ƞ-Al
2O
3/FeCrAl (Rh-block) was prepared according to [
8]. The cylindric Rh-block was 60 mm long and 18 mm in diameter. The weight of the Rh-block was 13.7 g, Total weight of the Rh/ Ce
0.75Zr
0.25O
2-δ–ƞ-Al
2O
3 coating was 1.58 g.
Partial oxidation of methane-rich gas over the Rh/Ce0.75Zr0.25O2-δ–ƞ-Al2O3/FeCrAl catalyst was studied in a stainless steel flow reactor under atmospheric pressure. The catalyst was preliminarily reduced in a flow of 5 vol.% H2/N2 at 600 °C for 1 h. Afterwards, the reaction mixture was fed into the reactor. The volumetric flow rate of the mixture was varied in the range 10,000–55,000 h−1. The temperature at the inlet and outlet of the block was measured with thermocouples along the central axis of the block. The composition of the gas phase components of the reaction mixture was determined using a Chromos GC-1000 (CHROMOS Engineering company, Moscow, Russia) gas chromatograph equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID) with a methanator. Before analysis, water was removed from the reformate using a moisture trap. Concentrations of H2 and N2 separated in a CaA column with an Ar carrier gas were determined with TCD. The CO, CO2, and CH4 were separated in a Chromosorb 106 column and quantified using FID. The “blank” experiment without a catalyst showed that at the used volumetric flow rates, the reaction on the walls of the reactor was not significant and could be ignored when analyzing the results.
Equilibrium concentrations were calculated using the HSC Chemistry 7.1 software package (HSC Chemistry, version 7.1, Outotec (company), 2011) under the assumption that the equilibrium mixture contains only gaseous substances.