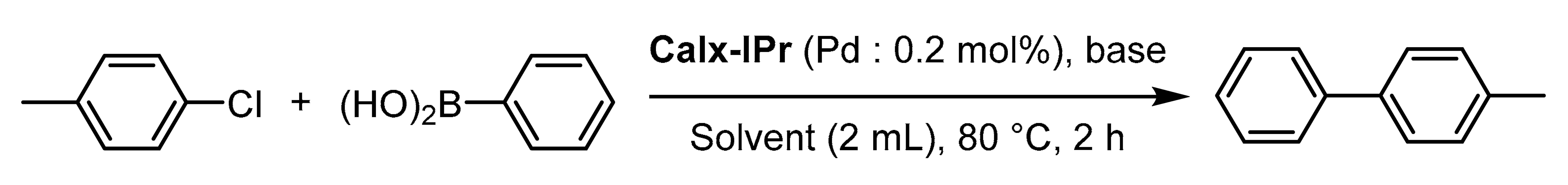Abstract
We report here the synthesis and characterization of a new calix[8]arene-supported PEPPSI-IPr Pd polymetallic complex. This complex, showing greater steric hindrance around the Pd centers compared with previous calix[8]arene-based catalysts, demonstrated high reactivity and selectivity for the Suzuki–Miyaura coupling of aryl chlorides under mild conditions. Along with this good performance, the new catalyst showed low Pd leaching into the final Suzuki–Miyaura coupling products, indicative of a heterogeneous-type reactivity. This rare combination of good reactivity towards challenging substrates and low metal leaching offers great promise at both academic and industrial levels.
1. Introduction
Transition metal catalysis has emerged as one of the most powerful synthetic tools over the last century [1]. Thanks to these advances, a broad scope of organic transformations can now be performed with relative ease. Among them, metal-catalyzed C-C bond formation reactions are of central importance, and palladium chemistry offers highly efficient catalysts in this regard. Since the discovery of the first example of homogeneous palladium catalysis in the 1950s, namely the Hoechst–Wacker process for the production of aldehydes from alkenes, palladium-based catalysis has had a major impact on industrial applications and has opened a wealth of opportunities for new reactions [2,3,4,5]. A dozen years after the discovery of the Hoechst–Wacker process, palladium species were found to catalyze C-C cross-coupling reactions between haloarenes and alkenes, organozinc compounds or aryl boronic acids, ultimately leading to the 2010 Nobel Prize in Chemistry for Heck, Negishi and Suzuki and the reactions bearing their names [6,7,8]. In particular, the Suzuki–Miyaura reaction has been applied extensively throughout organic chemistry for the synthesis of pharmaceutical compounds [9,10,11,12,13,14] and in materials science [15,16]. Recently, the modification of biological species such as proteins by palladium catalysis has attracted much attention for biological chemistry applications [17,18]. Despite the well-documented efficiency of palladium catalysts, these species still suffer from drawbacks, especially regarding industrial applications. Within this context, the efficiency of the catalyst (i.e., the catalyst loading applied in a given transformation) is not the only criterion to be taken into account. Indeed, metal leaching (from catalyst decomposition and/or activation processes [19], or from the intrinsic solubility of the catalyst used) is an undesired phenomenon that is frequently observed. This leaching often results in unacceptable levels of Pd-contamination in the final products, an issue that is critical for the pharmaceutical industry, where toxicity concerns are of paramount importance. The amount of Pd in active ingredients is thus strictly regulated, and in some cases must remain below 1 ppm [20]. To avoid contamination in the final products by the leaching of palladium and to enable greener chemical processes, heterogenous catalysis appears to be one of the most effective solutions [21]. As an alternative to classical catalyst supports such as polymers or inorganic materials [22,23], a calix[8]arene has recently been employed as a support for a palladium catalyst for Suzuki–Miyaura cross-coupling reactions, allowing both high activity and low metal leaching to be achieved simultaneously. In a published study, calix[8]arenes were functionalized by imidazole-derived ligands, and these were subsequently used for the anchoring of Pd(II) centers (Cat1 and Cat2; Figure 1) [24,25]. A nanoformulation of Cat1 (Figure 1) was also developed to enhance the reactivity of this catalyst in Suzuki–Miyaura cross-coupling reactions in water and under physiological conditions [26]. A few other studies have described calix[8]arene-supported catalysts [27,28,29]. Alongside this, some reports also demonstrate the use of calix[4]arene as catalysts in Suzuki–Miyaura cross-coupling reactions [30,31]. The active centers of the previously-reported first-generation catalysts Cat1 and Cat2 were based on palladium bound to mono(aryl)-N-heterocyclic carbene (NHC) units. It has been shown that greater steric crowding around the active center considerably increases the activity and the stability of the corresponding catalyst [32,33,34]. This effect is due to both favorable weak interactions between the ligands around the palladium and the incoming substrate, and promotion of the reductive elimination step (see Supplementary Materials). We thus targeted a related complex bearing bis(aryl)-NHC units. In this work, we describe our efforts to tackle the synthesis of sterically hindered imidazolium ligands, their grafting onto calixarenic supports, and the use of this platform as a Pd ligand for challenging Suzuki–Miyaura transformations.

Figure 1.
Previously described calix[8]arene-based catalysts Cat1 and Cat2 and new catalyst Calx-IPr.
2. Results
Herein, eight 2,6-diisopropylphenyl NHC-palladium complexes are supported on benzyloxycalix[8]arene, the full complex being denoted Calx-IPr, and its catalytic activity is explored. Towards this goal, we performed the synthesis of an alkyne-functionalized IPr as described by Organ et al. (see Scheme 1) [35]. The corresponding copper(I) complex would act as both a catalyst for its covalent grafting onto the easily-accessible calix[8]octaazide A [28] and an efficient precursor for obtaining the corresponding Pd precursor. Therefore, and according to Scheme 1, the addition of 2,6-diisopropylaniline to oxazolium 1 [36] gave imidazolium 3 in 70% yield. The imidazolium salt 3 was subjected to anion exchange (BF4 to Cl) with a DOWEX resin, providing imidazolium 4 in good yield, followed by complexation to copper and desilylation of the alkyne to give the NHC-copper complex 5 in 58% yield. The combination of calix[8]octaazide A with complex 5 provided calix[8]arene complex 6 by Huisgen cycloaddition in 84% yield. Finally, the target complex Calx-IPr was obtained by transmetalation with the palladium precursor Cl2Pd(3-Cl-Py)2 in 77% yield (Scheme 1) [35,37].

Scheme 1.
Synthetic route to Calx-IPr.
The complex Calx-IPr was first analyzed by 1H NMR spectroscopy in DMSO-d6 at 300 K. However, the signals were found to be significantly broader than those of complex 6, suggesting reduced flexibility of Calx-IPr due to the steric hindrance of the NHC-Pd unit (Figure 2).

Figure 2.
1H NMR spectrum of complex 6 at 300 K in CDCl3 (Top) and Calx-IPr at 300 K in DMSO-d6 (Bottom).
In order to obtain better resolved 1H NMR data for Calx-IPr, variable-temperature experiments were performed, leading to sharpening of all the signals, and identification of a characteristic symmetric hydroquinone signal at 6.55 ppm (Figure 3), indicating the full metalation of all calixarenic arms.

Figure 3.
Stack plot of 1H NMR spectra of Calx-IPr obtained at 370 K (Top) and 300 K (Bottom).
Unfortunately, all attempts to obtain single crystals of Calx-IPr suitable for X-ray analysis were unsuccessful. Moreover, it was not possible to obtain useful mass spectral analyses, regardless of the technique used (ESI, MALDI). In order to confirm the structure of Calx-IPr, we resorted to X-ray photoelectron spectroscopy analysis, which showed the presence of palladium atoms with two main unsymmetrical peaks at 337.98 and 343.28 eV, separated by 5.3 eV (Figure 4). The observed binding energy is characteristic of Pd(II) species. Moreover, the absence of shoulders at lower binding energies rules out the presence of metallic palladium impurities.

Figure 4.
X-ray photoelectron spectrum of Calx-IPr at the Pd 3d core level.
Semiquantitative elemental analysis of Calx-IPr powder was also performed by X-ray photoelectron spectroscopy (Table 1), providing results close to those expected (calculated for the formula C416H472Cl24N48O16Pd).

Table 1.
Elemental and X-ray photoelectron spectroscopy analysis of complex Calx-IPr.
After confirmation of the structure of the new catalyst Calx-IPr, an evaluation of its activity in the Suzuki–Miyaura cross-coupling between an aryl chloride and an aryl boronic acid was performed using the model reaction of 4-chlorotoluene with phenylboronic acid. Solvent optimization was first performed, using a 0.2 mol% loading of Calx-IPr (relative to Pd). With K3PO4 as a base, and conventional solvents for the reaction in which the catalyst is soluble (THF, DMF, toluene, etc.), the conversion was found to be very low (1–4%). In marked contrast, a 94% conversion was obtained with a high selectivity when EtOH was used. As the catalyst is not soluble in EtOH, the reaction likely proceeds via heterogeneous catalysis. It should be noted that this type of reactivity was also observed with Cat1 and Cat2 [24,25]. Other bases were also tested, but potassium phosphate was found to give the best results, followed by potassium carbonate and potassium hydroxide (Table 2) for achieving high conversion in a short reaction time.

Table 2.
Optimization of solvents and bases in Suzuki–Miyaura cross-coupling reactions catalyzed by Calx-IPr.
Then, an adjustment of the catalytic loading was performed to determine the optimum reactivity of Calx-IPr. By using 1 or 0.5 mol% [Pd], complete conversion was obtained with high selectivity. At 0.2 and 0.1 mol% [Pd], 95 and 80% conversion was observed, respectively. This activity is significantly higher than Cat1 and Cat2 [24], for which only 50 and 38% conversion (respectively) was obtained with 2 mol% [Pd] at 80 °C for this benchmark reaction. Finally, the temperature was decreased to 50 °C, giving 70% of conversion after 2 h using 0.5 mol% [Pd] of Calx-IPr. In addition, using the same catalyst loading at 25 °C led to 15% conversion after 2 h, but 98% conversion was noted after 20 h (Table 3). Overall, these results indicate that the new catalyst Calx-IPr is significantly more active for the Suzuki–Miyaura cross-coupling of aryl chlorides than related catalysts Cat1 and Cat2. This new catalyst is efficient under mild conditions and with low catalytic loadings, highlighting the benefit of using sterically hindered ligands.

Table 3.
Optimization of catalytic loading and temperature in Suzuki–Miyaura cross-coupling reactions catalyzed by Calx-IPr.
A kinetic study of the reaction using Calx-IPr was undertaken under heterogeneous conditions in order to compare its reactivity with the performances of Cat1; Cat2 [24]; and homogeneous, commercially available catalyst PEPPSI-IPr (Figure 1). We used a 0.5 mol% catalytic loading with hexadecane as the internal standard (Figure 5). The homogeneous catalyst PEPPSI-IPr led to complete conversion after 5 min, while the heterogeneous catalyst Calx-IPr required five additional minutes to reach the same result. Although lower, the reactivity of Calx-IPr is thus comparable to the reference catalyst PEPPSI-IPr. In contrast, Cat1 and Cat2 provided very low conversions even after extended reaction times (<10%). The addition of aromatic substituents to the imidazolium groups of the calix[8]arene increases its steric hindrance, which appears to have a major beneficial effect on its catalytic activity.

Figure 5.
Conversion/time plots for various catalysts. 4-chlorotoluene (1 equiv., 1 M), phenylboronic acid (1.5 equiv.), K3PO4 (2 equiv.), [Pd] = 0.5 mol%, 80 °C, reaction performed under argon atmosphere. Yield determined by GC (Gas chromatography) analyses using hexadecane as internal standard.
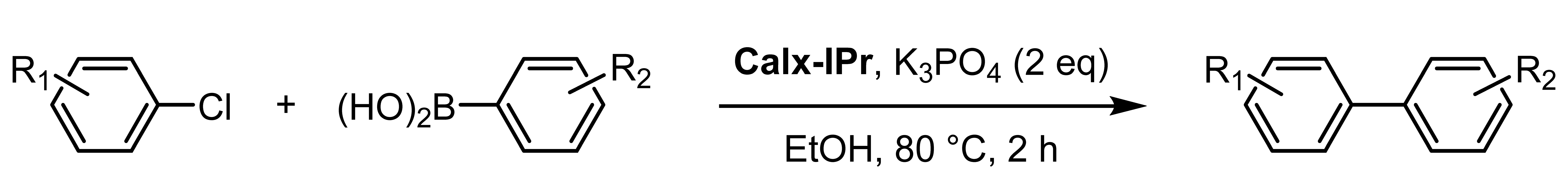
Using these optimized conditions, catalyst Calx-IPr showed high reactivity towards a broad range of substrates (Table 4). Cross-coupling of electron-rich 4-chloroanisole with phenyl boronic acid provided 92% yield (2 h of reaction with 0.5 mol% [Pd] of Calx-IPr at 80 °C) after purification by chromatography. Interestingly, the reaction with 2-chloroanisole needed only 0.2 mol% for complete conversion of the starting material to the biphenyl product in 93% yield (entries 1–2). The electron-rich 2-aminophenylboronic acid was coupled with 3-chloroanisole in 90% yield in the presence of 1 mol% of [Pd] of Calx-IPr at 80 °C (entry 3). A test of the reactivity of 2-chlorobenzonitrile using phenylboronic acid gave a 67% yield using 1 mol% [Pd] of Calx-IPr, while a second test replacing phenyl boronic acid by p-tolylboronic acid allowed the preparation of 4′-methyl-[1,1′-biphenyl]-2-carbonitrile (a precursor to Valsartan, a drug used for the treatment of cardiovascular disease) [38] in 56% isolated yield with 1 mol% [Pd] of Calx-IPr (entries 4–5). Concerning heterocycles, both 2- and 3-chloropyridine were tested in the cross-coupling process with phenylboronic acid. This resulted in complete conversion of 2- and 3-chloropyridine with 0.5 mol% [Pd] of Calx-IPr at 80 °C, and the corresponding products were isolated with yields of 98 and 89%, respectively (entries 6–7). Changing the boronic acid partner to 4-formylphenylboronic acid in the presence of 2-chloropyridine gave a 68% yield with 1 mol% [Pd] of Calx-IPr at 80 °C (entry 8). It is worth noting that this allows access to the corresponding pyridinyl benzaldehyde coupling product, a precursor used in the synthesis of the HIV protease inhibitor Atazanavir [39]. Moreover, this result was significantly better than previously tested Cat1, which provided a yield of only 51% for the transformation of 2-bromopyridine with a higher catalytic loading and at a higher temperature [25].

Table 4.
Scope of the Suzuki cross-coupling reaction catalyzed by Calx-IPr.
Finally, cross-coupling of other relevant heterocycles such as 2-chlorothiophene and 2-chloroquinoline with phenylboronic acid derivatives gave full conversion in the presence of 0.5 mol% [Pd] of Calx-IPr at 80 °C, providing yields of 87 and 90%, respectively (entries 9–10). It is worth mentioning here that complete selectivity towards the coupling products was observed, without any dehalogenation.
In order to obtain a more accurate evaluation of the synthetic utility of Calx-IPr, we performed a detailed analysis of the amount of Pd contamination in the final products resulting from catalyst leaching. ICP-MS analyses of the crude filtrates were performed for two representative examples of Suzuki–Miyaura cross-coupling reactions (Table 5).

Table 5.
ICP-MS analysis of two Suzuki–Miyaura coupling products.
Only a small amount of Pd was observed in the final products after a simple filtration over a paper filter, followed by trituration in diethyl ether.
Our Calx-IPr catalyst combines a reactivity that is comparable to those of the reference homogeneous catalysts (at the same catalytic rate), with the low leaching of heterogenous catalysts. Thus, it represents a significant improvement over the first generation of calix[8]arene-based Pd catalysts.
3. Materials and Methods
All reactions were carried out under argon atmosphere, and all glassware was flame-dried before use. PEPPSI-IPr was purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France). Ethanol (EtOH), THF, DMF and toluene were purchased from ACROS Organics (Geel, Belgium) and Alfa Aesar (Kandel, Germany). Potassium phosphate (K3PO4), cesium carbonate (Cs2CO3), potassium carbonate (K2CO3), and potassium hydroxide (KOH) were purchased from ACROS Organics. 3-chloropyridine was purchased from Sigma-Aldrich. All commercially available reagents were used as received. Complexes Cat1, Cat2 [24,25] and Cl2Pd(3-ClPy)2 [40] were prepared according to previously reported procedures. 1H NMR and 13C NMR spectra were recorded on either a Bruker DPX 250, Bruker 300 MHz, Bruker Avance 360 MHz, Bruker 400 (400 MHz) or Bruker DRX 400 (400 MHz) instrument, and data were reported in ppm with the solvent signal as reference. Gas chromatography (GC) analyses were performed on a GC 2010 Plus Shimadzu gas chromatograph with a Phenomenex column ZB-5 MS (15 m × 0.25 mm × 0.25 µm), and GC-MS analyses were achieved on a DSQ apparatus (Thermo Scientific, Orsay, France). HRMS analyses were performed with a MicroTOFq (Orsay, France) (quadrupole coupled with TOF analyzer). ICP-MS analyses were performed by IRAMIS (CEA-Saclay, Saclay, France).
Synthesis of 1:
The preparation of N-(2,6-diisopropylphenyl)-N-(2-oxoethyl)formamide (51 mmol) was performed following a procedure described by Fürstner et al. [36]. This compound was dissolved in acetic anhydride (1 mL/mmol), and HBF4 (7.7 mL, 48% w/w in water, 1.15 equiv.) was added at 0 °C, and then warmed to room temperature. The mixture was stirred overnight before Et2O (130 mL) was added to induce precipitation of the salt. The solid was collected by filtration and washed with Et2O (2 × 30 mL). Recrystallization from MeCN/Et2O (1:1) gave pure 1-(2,6-diisopropylphenyl)-3-acetoxyoxazolinium tetrafluoroborate as a white solid, which was collected by filtration and dried in air (9.1 g, 47%). The product was found to decompose slowly at ambient temperature; however, it could be stored at −10 °C for prolonged periods without significant decomposition.
1H NMR (300 MHz, CDCl3): δ 8.93 (dd, J = 1.9 Hz and 1.2 Hz, 1H), 7.70–7.54 (m, 1H), 7.44 (d, J = 7.8 Hz, 2H), 7.37 (dd, J = 7.0 Hz and 3.1 Hz, 1H), 4.69 (ddd, J = 14.4 Hz, 7.0 Hz and 1.9 Hz, 1H), 4.27 (ddd, J = 14.4 Hz, 3.1 Hz and 1.2 Hz, 1H), 2.85 (dhept, J = 20.4 Hz and 6.8 Hz, 2H), 2.24 (s, 3H), 1.36–1.20 (m, 12H). 13C NMR (90 MHz, CDCl3): δ 168.8, 167.8, 146.5, 146.2, 133.2, 127.1, 126.1, 126.0, 100.6, 58.4, 29.1, 28.9, 24.4, 24.3, 24.1, 23.8, 20.4. 19F NMR (235 MHz, CDCl3): −145.91. 11B NMR (96 MHz, CDCl3): 4.13.
Synthesis of 3:
The synthesis of 3 was performed following a procedure described by Organ et al. [35]. In air, a round-bottomed flask was charged with 2,6-diisopropyl-4-[2-(trimethylsilyl)ethynyl]-aniline (2.3 g, 8.5 mmol) and a mixture of CH2Cl2 and toluene (70 mL, 1:1). 1-(2,6-diisopropylphenyl)-3-acetoxyoxazolinium tetrafluoroborate (1) (2.6 g, 7.0 mmol) was added as a single portion, and the solution was stirred at rt overnight. The solvent was removed in vacuum, and the resultant oil was filtered through a plug of SiO2 using CH2Cl2 as eluent. The filtrate was concentrated and then triturated with pentane to give a red/brown solid, which was collected by filtration. The solid was placed in a flame-dried round-bottomed flask under argon, CH2Cl2 (20 mL) was added and the solution was cooled to 0 °C. The addition of pyridine (2.8 mL, 35.0 mmol, 5 equiv.) was followed by the dropwise addition of SOCl2 (1.0 mL, 14.0 mmol, 2 equiv.). The mixture was stirred at rt for 1 h. The volatiles were removed under reduced pressure, and the residue was taken up in CH2Cl2 and filtered through a plug of SiO2 using a 1:1 mixture of EtOAc and CH2Cl2 as eluent. The solvent was evaporated from the filtrate and the brown oil placed in an ultrasound bath with pentane to produce the tetrafluoroborate salt as an off-white solid, which was collected by filtration (2.8 g, 70%).
1H NMR (360 MHz, CDCl3): δ 8.99 (t, J = 1.6 Hz, 1H), 7.67 (d, J = 1.6 Hz, 2H), 7.52 (t, J = 7.8 Hz, 1H), 7.37 (s, 2H), 7.33–7.24 (m, 2H), 2.32 (dhept, J = 13.2 Hz and 7.0 Hz, 4H), 1.20 (d, J = 6.8 Hz, 12H), 1.12 (t, J = 6.4 Hz, 12H), 0.30 (s, 9H). 13C NMR (90 MHz, CDCl3): δ 145.3, 145.0, 138.1, 132.1, 129.9, 129.8, 128.3, 127.2, 126.5, 126.3, 124.7, 103.9, 96.9, 29.1, 24.2, 24.0, 23.9, 23.7, 0.0. 19F NMR (235 MHz, CDCl3): −151.91. 11B NMR (96 MHz, CDCl3): −1.30. HRMS [ESI(+)]: m/z [M-BF4]+ calculated for [C32H45N2Si]+: 485.3346, found: 485.3327.
Synthesis of 4:
The synthesis of 4 was performed following a procedure described by Organ et al. [35]. A fritted funnel was packed with Dowex® 1X4 chloride form, 200–400 mesh ion exchange resin by suspending the resin in MeOH and packing by vacuum filtration. Water was removed by passing MeOH (50 mL) followed by acetone (50 mL) through the column. A mixture of 15% toluene/5% MeOH/80% CH2Cl2 was passed through the resin (100 mL) before filtering a solution of 1-(2,6-diisopropylphenyl)-3-(2,6-diisopropylphenyl-4-((trimethylsilyl)ethynyl)phenyl)imidazolium tetrafluoroborate (3) (1.0 g, 1.75 mmol) in 15% toluene/5% MeOH/80% CH2Cl2 (0.014 M, 125 mL) through the ion-exchange column. The filtrate was evaporated to dryness and triturated with pentane to give an off-white solid, which was collected by filtration (846 mg, 93%).
1H NMR (360 MHz, CDCl3): δ 10.66 (t, J = 1.6 Hz, 1H), 8.13 (t, J = 1.8 Hz, 1H), 8.00 (t, J = 1.7 Hz, 1H), 7.52 (t, J = 7.8 Hz, 1H), 7.38 (s, 2H), 7.30 (d, J = 7.8 Hz, 2H), 2.43–2.29 (m, 4H), 1.26–1.12 (m, 24H), 0.31 (s, 9H). 13C NMR (90 MHz, CDCl3): δ 145.2, 144.9, 139.6, 132.0, 130.0, 129.9, 128.2, 127.0, 126.6, 126.6, 124.6, 103.9, 96.8, 29.1, 29.0, 24.7, 24.5, 23.7, 23.5, −0.1. HRMS [ESI(+)]: m/z [M-Cl]+ calculated for [C32H45N2Si]+: 485.3346, found: 485.3327.
Synthesis of 5:
The synthesis of 5 was performed following a procedure described by Organ et al. [35]. Under argon, a two-necked round-bottomed flask equipped with a condenser was charged with Cu2O (161 mg, 1.13 mmol, 0.75 equiv.) and 1-(2,6-diisopropylphenyl)-3-(2,6-diisopropylphenyl-4-((trimethylsilyl) ethynyl)phenyl)imidazolium chloride (4) (784 mg, 1.50 mmol). Toluene (11 mL) was added, and the suspension was stirred at reflux for 48 h. After cooling to rt, the solvent was removed in vacuum, and the remaining solid was taken up in CH2Cl2 and filtered through a SiO2 plug with CH2Cl2 as eluent. The filtrate was evaporated, and then THF (3.5 mL) was added, followed by TBAF (1 M in THF, 1.50 mL, 1.50 mmol), and the mixture was stirred at rt for 16 h. The solvent was removed under reduced pressure and the residue passed through a plug of SiO2 using CH2Cl2 as eluent. The filtrate was concentrated to ca. 0.6 mL, hexanes (7 mL) added and the CH2Cl2 slowly removed under reduced pressure to induce precipitation of complex 5 as an off-white solid, which was collected by filtration (432 mg, 56%).
1H NMR (360 MHz, CDCl3): δ 7.49 (t, J = 7.8 Hz, 1H), 7.41 (s, 2H), 7.29 (d, J = 7.8 Hz, 2H), 7.14 (d, J = 1.8 Hz, 1H), 7.11 (d, J = 1.8 Hz, 1H), 3.17 (s, 1H), 2.62–2.46 (m, 4H), 1.29 (dd, J = 6.9 Hz and 1.3 Hz, 12H), 1.22 (dd, J = 6.9 Hz and 1.6 Hz, 12H). 13C NMR (90 MHz, CDCl3): δ 180.8, 146.1, 145.6, 134.9, 134.4, 130.8, 128.3, 124.7, 124.4, 123.5, 123.1, 83.2, 78.4, 28.9, 28.9, 25.0, 24.7, 24.0, 23.9. HRMS [ESI(+)]: m/z [M-Cl]+ calculated for [C29H36CuN2]+: 475.2149, found: 475.2168.
Synthesis of 6:
The synthesis of 6 was performed following a procedure described by Organ et al. [35,37]. Under argon, a vial was charged with 1-(2,6-diisopropylphenyl)-3-(2,6-diisopropylphenyl-4-ethynylphenyl) imidazol-2-ylidene]copper(I) chloride (5) (894 mg, 1.74 mmol) and sealed with a septum. Distilled MeCN (20 mL) and benzyloxycalix[8]arene azide (A) (360 mg, 0.145 mmol) was added, and the mixture was stirred for 36 h at 35 °C. After cooling to rt, the solvent was removed in vacuo. The residue was then extracted into CH2Cl2 and precipitated by the addition of Et2O to give pure 6 as an off-white solid after filtration (804 mg, 84%).
1H NMR (360 MHz, CDCl3): δ 8.14 (bs, 8H), 7.75 (s, 16H), 7.46 (t, J = 7.8 Hz, 8H), 7.34–7.22 (m, 24H), 7.22–6.96 (m, 56H), 6.52 (bs, 16H), 4.63 (bs, 16H), 4.26 (bs, 16H), 3.95 (bs, 8H), 3.63 (bs, 16H), 2.60–2.48 (m, 32H), 2.02 (bs, 16H), 1.64 (bs, 16H), 1.39–1.05 (m, 192H). 13C NMR (90 MHz, CDCl3): δ 180.6, 154.9, 149.1, 146.9, 146.4, 145.7, 137.0, 135.0, 134.5, 134.1, 133.1, 130.6, 128.5, 127.9, 127.8, 124.3, 123.5, 123.4, 121.6, 121.2, 73.0, 70.0, 50.3, 28.9, 28.8, 27.6, 27.3, 25.0, 24.8, 24.0, 23.9. HRMS [ESI(+)]: m/z [M-3Cl]3+ calculated for [C376H440Cl5Cu8N40O16]3+: 2153.5914, found: 2153.5867.
Synthesis of Calx-IPr:
The synthesis of Calx-IPr was performed following a procedure described by Organ et al. [35,37]. Under argon, a two-necked round-bottomed flask equipped with a condenser was charged with solid 6 (733 mg, 0.112 mmol) followed by Cl2Pd(3-ClPy)2 (451 mg, 1.12 mmol, 10 equiv.) and toluene (50 mL). The suspension was then stirred at 110 °C for 48 h. After cooling to rt, the mixture was condensed under reduced pressure, and was then centrifuged (10,000 rpm for 20 min) and filtered through a PTFE 0.2 µm filter. The filtrate was precipitated by the addition of Et2O to give Calx-IPr as an off-white solid (700 mg, 77%).
1H NMR at 370 K (400 MHz, DMSO-d6): δ 8.53 (bs, 8H), 7.87 (d, J = 7.9 Hz, 8H), 7.78 (bs, 16H), 7.57 (bs, 16H), 7.51 (t, J = 7.7 Hz, 8H), 7.35 (d, J = 7.7 Hz, 16H), 7.06 (bs, 48H), 6.56 (bs, 16H), 4.68 (bs, 16H), 4.37 (bs, 16H), 3.97 (bs, 16H), 3.71 (bs, 16H), 3.19–3.05 (m, 32H), 2.01 (bs, 16H), 1.68 (bs, 16H), 1.38 (t, J = 7.1 Hz, 96H), 1.21 (t, J = 7.6 Hz, 16H), 1.07 (d, J = 6.8 Hz, 96H). 13C NMR at 370 K (100 MHz, DMSO-d6): δ 153.5, 150.0, 148.1, 147.6, 146.4, 145.7, 145.2, 141.3, 136.1, 134.2, 133.7, 131.5, 129.1, 127.2, 126.5, 126.4, 125.7, 125.5, 122.8, 119.9, 114.3, 71.9, 68.9, 48.8, 27.4, 27.3, 26.0, 25.7, 24.9, 24.8, 23.2, 23.1, 22.6, 22.5, 22.1, 22.0.
General procedure for Suzuki–Miyaura cross-coupling reactions:
Under argon, a Schlenk tube was charged with the arylhalide derivative (1 mmol), the boronic acid (1.5 mmol), K3PO4 (2 mmol) and the Calx-IPr (x mol % of Pd). The mixture was dried under vacuum for 10 min, and dry EtOH (2 mL) was added under argon. The Schlenk tube was flushed with three vacuum/argon cycles. The mixture was stirred at 80 °C for two hours. After cooling at room temperature, the heterogeneous mixture was filtered through a DicaliteTM pad, rinsed with EtOAc and concentrated under reduced pressure. The crude reaction was purified by column chromatography on silica gel to obtain the pure products.
General procedure for kinetic studies
Under argon, a Schlenk tube was charged with 4-chlorotoluene (3 mmol), the boronic acid (5 mmol), K3PO4 (6 mmol) and the catalyst (0.5 mol% of Pd). The mixture was dried under vacuum for 10 min. Dry EtOH (6 mL) and 3 mmol of hexadecane as the internal standard were added under argon. The Schlenk tube was flushed with three vacuum/argon cycles. The mixture was stirred at 80 °C for two hours, and samples were taken under argon using a syringe at regular time intervals. The conversion monitoring was carried out by GC.
Preparation of samples for ICP-MS analyses [24,25]:
After a Suzuki–Miyaura reaction, the mixture was filtered on a Whatman 5 filter; the ethanol solution was then evaporated under reduced pressure. Water was added (10 mL), and the residue was extracted with Et2O (3 × 10 mL). The organic layer was finally dried over MgSO4 and concentrated under reduced pressure. The solid was heated under high vacuum at 200 °C for 1 h, and the residue was mineralized in nitric acid (69%) at 140 °C for 2 h.
4. Conclusions
We disclose here a new p-(benzyloxy)calix[8]arene-supported catalyst, which exhibits increased steric crowding around the metallic centers. This new catalyst shows a considerably higher reactivity than previously described ones of the same family, with low levels of Pd leaching into the final products. Interestingly, the reactivity of this new calixarene-supported catalyst is comparable with those of reference PEPPSI-IPr catalysts. This compound thus holds great promise for future developments in the field of catalysis, such as C-C and C-X cross-coupling reactions and C-H activation reactions.
Supplementary Materials
Copies of NMR spectra of the compounds are available in the online supplementary materials. The following are available online at https://www.mdpi.com/2073-4344/10/9/1081/s1.
Author Contributions
V.H., I.A. wrote the paper, V.H., I.A., E.S. and C.M. conceived and designed the experiments; A.L., J.B., S.A.F., E.A. and I.A. performed the experiments. D.D. performed XPS (X-ray photoelectron) analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The CNRS, the Ministère de l’Enseignement Supérieur et de la Recherche (for a Ph.D. grant, A.L.) and the SATT Paris-Saclay are acknowledged for financial support (NOVECAL project, I.A., C.M.). The authors also wish to thank the French National Research Agency (IdEx Paris-Saclay, ANR-IdEx-0039RA14, grant to C.M.; the Charmmmat ANR-11-LABX-0039, grant to I.A.; and the ANRT CIFRE, grant to S.A.F.) for their support.
Conflicts of Interest
V.H., C.M. and E.S. are cofounders of Novecal.
References
- Beller, M.; Bolm, C. Transition Metals for Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; Volume 1, pp. I–XIX. [Google Scholar]
- Hafner, W.; Jira, R.; Sedlmeier, J.; Smidt, J. Über die Reaktionen von Olefinen mit wassrigen Lösungen von Palladiumsalzen. Chem. Ber. 1962, 95, 1575–1581. [Google Scholar] [CrossRef]
- Smidt, J.; Hafner, W.; Jira, R.; Sieber, R.; Sedlmeier, J.; Sabel, A. The Oxidation of Olefins with Palladium Chloride Catalysts. Angew. Chem. Int. Ed. 1962, 1, 80–88. [Google Scholar] [CrossRef]
- Jira, R. Acetaldehyde from Ethylene. Angew. Chem. Int. Ed. 2009, 48, 9034–9037. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.E.; Benhamou, L.; Sheppard, T.D. Palladium(II)-Catalysed Oxidation of Alkenes. Synthesis 2015, 47, 3079–3117. [Google Scholar]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Gildner, P.G.; Colacot, T.J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics 2015, 34, 5497–5508. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef]
- Busacca, A.C.; Fan-drick, R.D.; Song, J.J.; Senanayake, C.H. Applications of Transition Metal Catalysis in Drug Discovery and Development; John Wiley & Sons: New York, NY, USA, 2012; pp. 1–24. [Google Scholar]
- Hayler, D.; Leahy, K.D.; Simmons, E.M. A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38, 36–46. [Google Scholar] [CrossRef]
- Diner, C.; Organ, M.G. What Industrial Chemists Want—Are Academics Giving It to Them? Organometallics 2019, 38, 66–75. [Google Scholar] [CrossRef]
- Borths, C.J.; Walker, S.D. Accelerating Pharmaceutical Development via Metal-Mediated Bond Formation. Isr. J. Chem. 2020, 60, 1–12. [Google Scholar] [CrossRef]
- Cheng, F.; Adronov, A. Suzuki Coupling Reactions for the Surface Functionalization of Single-Walled Carbon Nanotubes. Chem. Mater. 2006, 18, 5389–5391. [Google Scholar] [CrossRef]
- Fei, Z.; Soo Kim, J.; Smith, J.; Buchaca Domingo, E.; Anthopoulos, T.D.; Stingelin, N.; Watkins, S.E.; Kim, J.S.; Heeney, M. A low band gap co-polymer of dithienogermole and 2,1,3-benzothiadiazole by Suzuki polycondensation and its application in transistor and photovoltaic cells. J. Mater. Chem. 2011, 21, 16257–16263. [Google Scholar] [CrossRef]
- Jabara, M.; Kumar Maity, S.; Brik, A. Palladium in the Chemical Synthesis and Modification of Proteins. Angew. Chem. Int. Ed. 2017, 56, 10644–10655. [Google Scholar] [CrossRef]
- Isengger, P.G.; Davis, G.B. Concepts of Catalysis in Site-Selective Protein Modifications. J. Am. Chem. Soc. 2019, 141, 8005–8013. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Beletskaya, I.P. Toward the Ideal Catalyst: From Atomic Centers to a “Cocktail” of Catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- ICH Guideline Q3D (R1) on Elemental Impurities. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-32.pdf (accessed on 28 March 2019).
- Bourouina, A.; Meille, V.; de Bellefon, C. About Solid Phase vs. Liquid Phase in Suzuki-Miyaura Reaction. Catalysts 2019, 9, 60. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings -Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Das, P.; Linert, W. Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki-Miyaura reaction. Coord. Chem. Rev. 2016, 311, 1–23. [Google Scholar] [CrossRef]
- Abdellah, I.; Kasongo, P.; Labattut, A.; Guillot, R.; Schulz, E.; Martini, C.; Huc, V. Benzyloxycalix[8]arene: A new valuable support for NHC palladium complexes in C–C Suzuki–Miyaura couplings. Dalton Trans. 2018, 47, 13843–13848. [Google Scholar] [CrossRef] [PubMed]
- Labattut, A.; Abi Fayssal, S.; Buendia, J.; Abdellah, I.; Huc, V.; Martini, C.; Schulz, E. Calixarene-supported Pd-NHC complexes as efficient catalysts for scalable Suzuki-Miyaura cross-couplings. React. Chem. Eng. 2020, 5, 1509–1514. [Google Scholar] [CrossRef]
- Peramo, A.; Abdellah, I.; Pecnard, S.; Mougin, J.; Martini, C.; Couvreur, P.; Huc, V.; Desmaële, D. A Self-Assembling NHC-Pd-Loaded Calixarene as a Potent Catalyst for the Suzuki-Miyaura Cross-Coupling Reaction in Water. Molecules 2020, 25, 1459. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Percástegui, E.; Hernándeza, D.J.; Castillo, I. Calix[8]arene nanoreactor for Cu(I)-catalysed C–S coupling. Chem. Commun. 2016, 52, 3111–3114. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, I.; Martini, C.; Dos Santos, A.; Dragoe, D.; Guérineau, V.; Huc, V.; Schulz, E. Calix[8]arene as New Platform for Cobalt-Salen Complexes Immobilization and Use in Hydrolytic Kinetic Resolution of Epoxides. ChemCatChem 2018, 10, 4761–4767. [Google Scholar] [CrossRef]
- Reyes-Mata, C.A.; Castillo, I. Calix[8]arene-based Ni(II) complexes for electrocatalytic CO2 reduction. Inorg. Chim. Acta 2020, 507, 119607. [Google Scholar] [CrossRef]
- Frank, M.; Maas, G.; Schatz, J. Calix[4]arene-Supported N-Heterocyclic Carbene Ligands as Catalysts for Suzuki Cross-Coupling Reactions of Chlorotoluene. Eur. J. Org. Chem. 2004, 2004, 607–613. [Google Scholar] [CrossRef]
- Brenner, E.; Matt, D.; Henrion, M.; Teci, M.; Toupet, L. Calix[4]arenes with one and two N-linked imidazolium units as precursors of N-heterocyclic carbene complexes. Coordination chemistry and use in Suzuki-Miyaura cross-coupling. Dalton Trans. 2011, 40, 9889–9898. [Google Scholar] [CrossRef]
- Szilvási, T.; Veszprémi, T. Internal Catalytic Effect of Bulky NHC Ligands in Suzuki—Miyaura Cross-Coupling Reaction. ACS Catal. 2013, 3, 1984–1991. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Nasielski, J.; Hadei, N.; Achonduh, G.; Kantchev, E.A.B.; O’Brien, C.J.; Lough, A.; Organ, M.G. Structure–Activity Relationship Analysis of Pd–PEPPSI Complexes in Cross-Couplings: A Close Inspection of the Catalytic Cycle and the Precatalyst Activation Model. Chem. Eur. J. 2010, 16, 10844–10853. [Google Scholar] [CrossRef] [PubMed]
- Price, G.A.; Bogdan, A.R.; Aguirre, A.L.; Iwai, T.; Djuric, S.W.; Organ, M.G. Continuous flow Negishi cross-couplings employing silica-supported Pd-PEPPSI–IPr precatalyst. Catal. Sci. Technol. 2016, 6, 4733–4742. [Google Scholar] [CrossRef]
- Fürstner, A.; Alcarazo, M.; César, V.; Lehmann, C.W. Convenient, scalable and flexible method for the preparation of imidazolium salts with previously inaccessible substitution patterns. Chem. Commun. 2006, 20, 2176–2178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Price, G.A.; Hassan, A.; Chandrasoma, N.; Bogdan, A.R.; Djuric, S.W.; Organ, M.G. Pd-PEPPSI-IPent-SiO2: A Supported Catalyst for Challenging Negishi Coupling Reactions in Flow. Angew. Chem. Int. Ed. 2017, 56, 1–5. [Google Scholar] [CrossRef]
- Beutler, U.; Boehm, M.; Fuenfschilling, P.C.; Heinz, T.; Mutz, J.P.; Onken, U.; Mueller, M.; Zaugg, W. A High-Throughput Process for Valsartan. Org. Process Res. Dev. 2007, 11, 892–898. [Google Scholar] [CrossRef]
- Fan, X.; Song, Y.-L.; Long, Y.-Q. An Efficient and Practical Synthesis of the HIV Protease Inhibitor Atazanavir via a Highly Diastereoselective Reduction Approach. Org. Process Res. Dev. 2008, 12, 69–75. [Google Scholar] [CrossRef]
- Krogul, A.; Skupinska, J.; Litwinienko, G. Catalytic activity of PdCl2 complexes with pyridines in nitrobenzene carbonylation. J. Mol. Catal. A Chem. 2011, 337, 9–16. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).