Comparison of Catalytic Properties of Vanadium Centers Introduced into BEA Zeolite and Present on (010) V2O5 Surface–DFT Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. The Substitution of Si for V–OH
2.2. The Substitution of Si for V=O
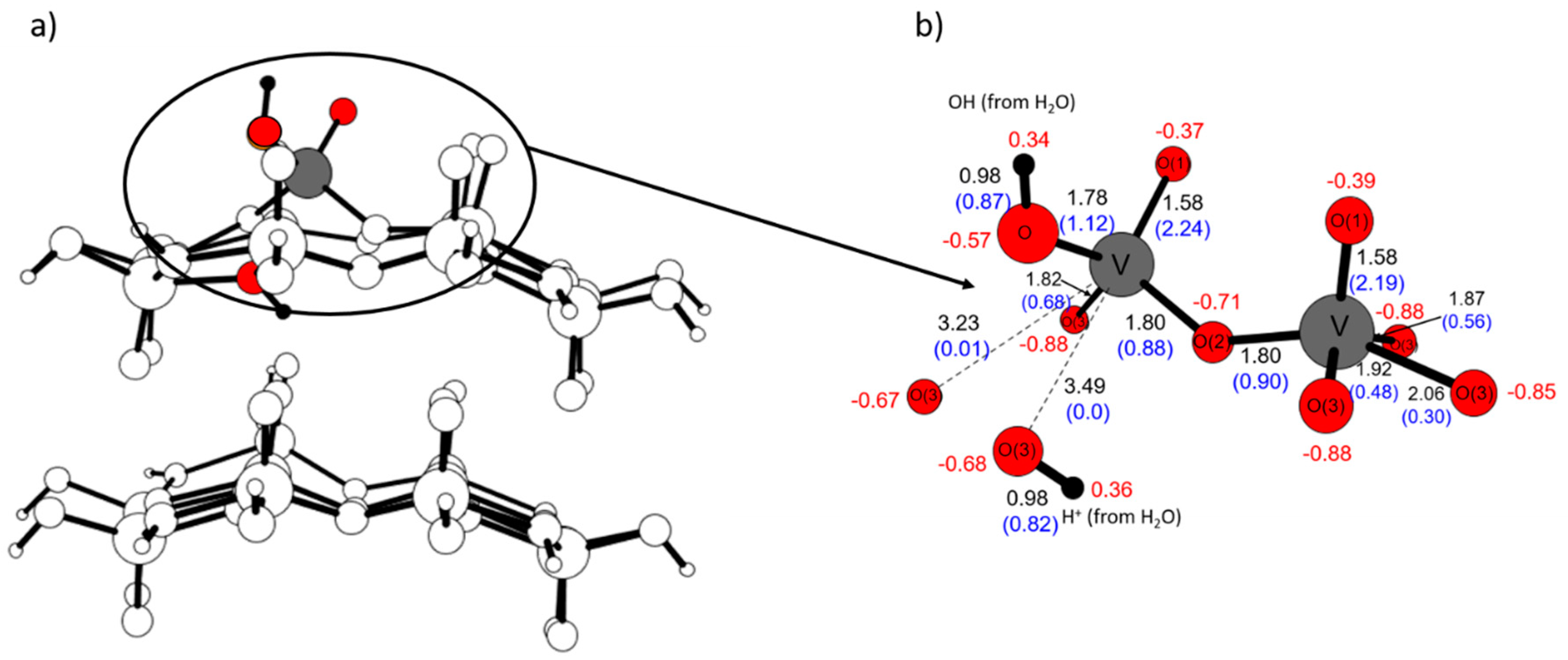
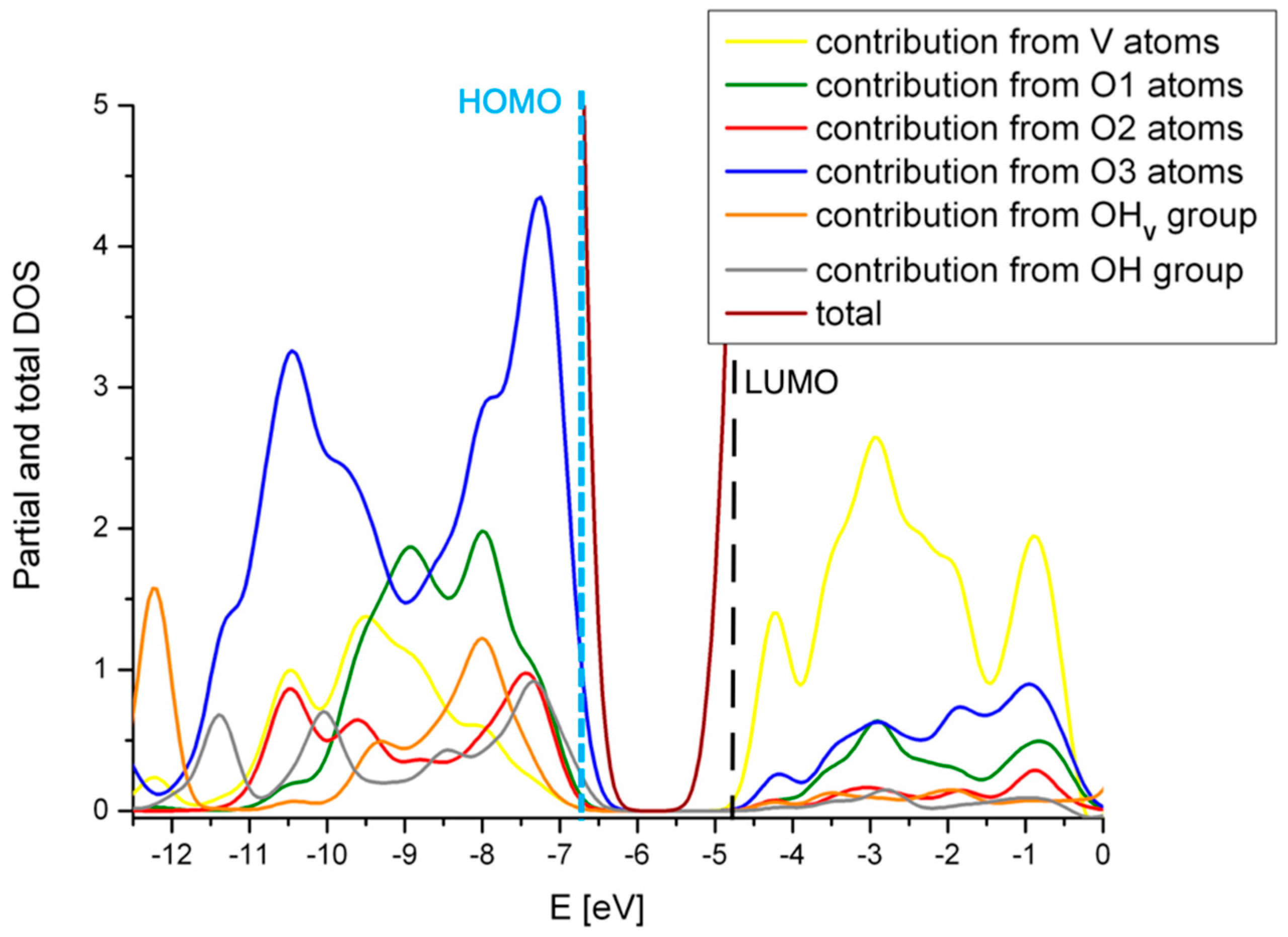
2.3. The Substitution of Si for V(=O)(–OH)
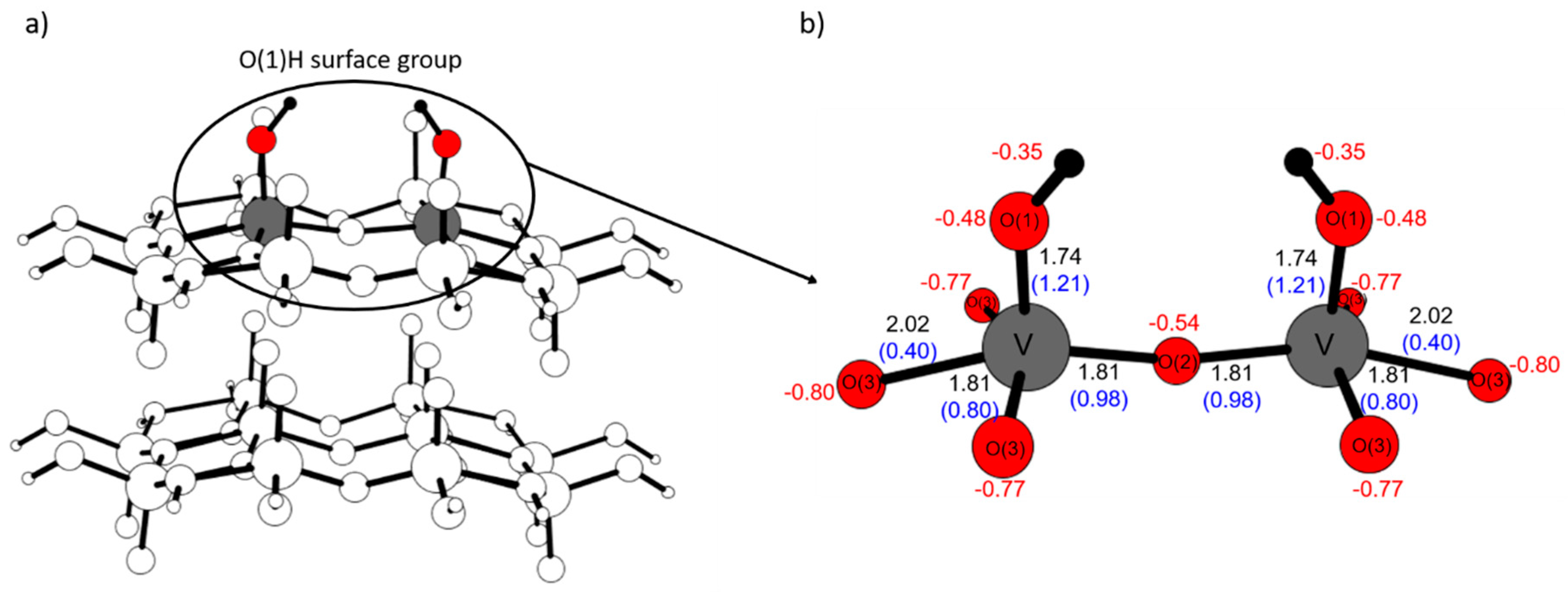
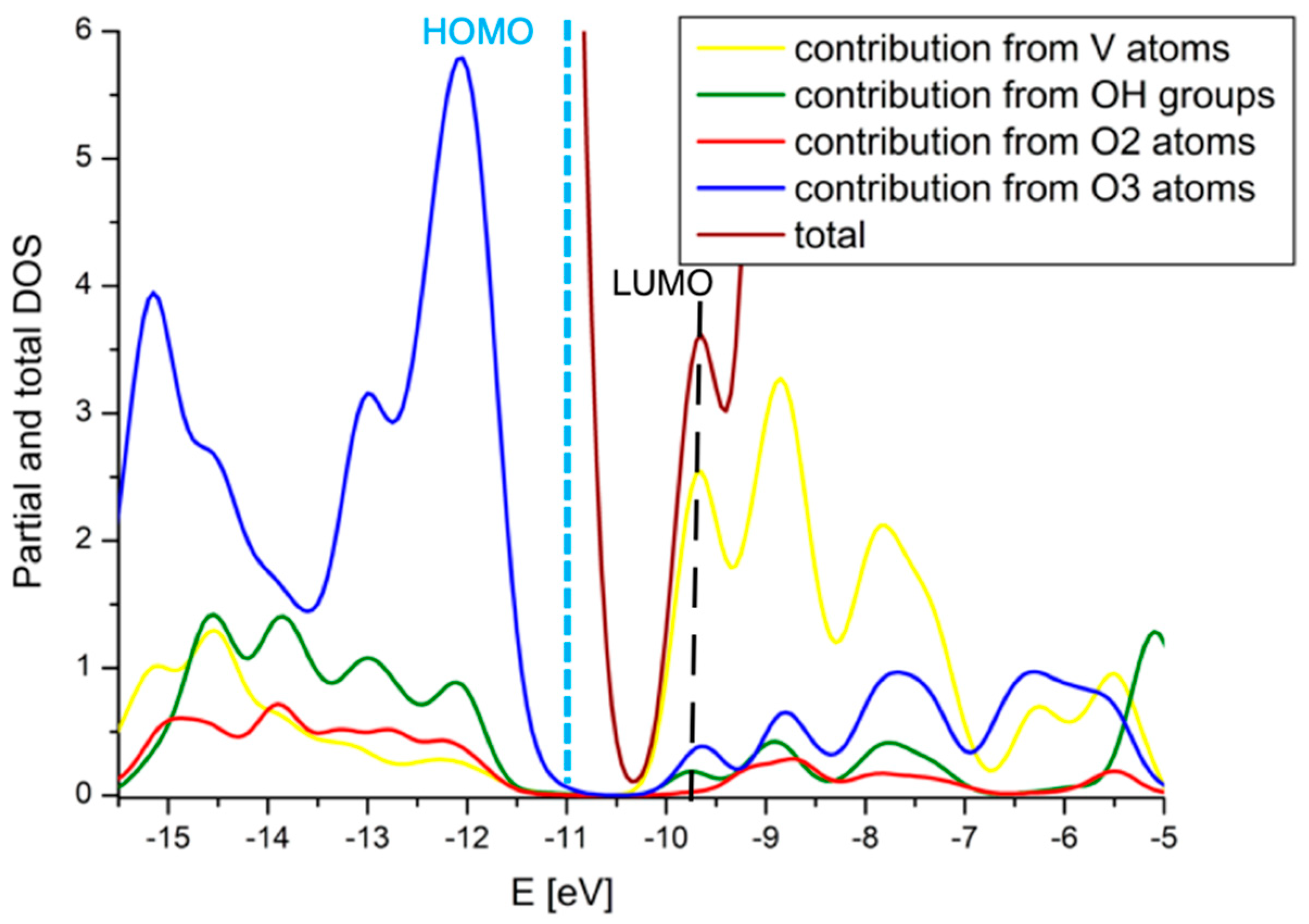
2.4. Comparison with V2O5
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornils, B.; Herrmann, W.A.; Wong, C.-H.; Zanthoff, H.-W. Catalysis from A to Z. A Concise Encyclopedia; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 1995, 24, 307–313. [Google Scholar] [CrossRef]
- Grasselli, R.K. Fundamental Principles of Selective Heterogeneous Oxidation Catalysis. Top. Catal. 2002, 21, 79–88. [Google Scholar] [CrossRef]
- Knotek, P.; Čapek, L.; Bulánek, R.; Adam, J. Vanadium supported on hexagonal mesoporous silica: Active and stable catalysts in the oxidative dehydrogenation of alkanes. Top. Catal. 2007, 45, 51–55. [Google Scholar] [CrossRef]
- Han, Z.-F.; CXue, X.-L.; Wu, H.-M.; Lang, W.-Z.; Guo, Y.-J. Preparation and catalytic properties of mesoporous nV-MCM-41 for propane oxidative dehydrogenation in the presence of CO2. Chin. J. Catal. 2018, 39, 1099–1109. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Loridant, S.; Launay, H.; Pigamo, A.; Dubois, J.L.; Millet, J.M.M. Study of new catalysts based on vanadium oxide supported on mesoporous silica for the partial oxidation of methane to formaldehyde: Catalytic properties and reaction mechanism. J. Catal. 2006, 237, 38–48. [Google Scholar] [CrossRef]
- Wallis, P.; Wohlrab, S.; Kelevaru, V.N.; Frank, M.; Martin, A. Impact of support pore structure and morphology on catalyst performance of VOx/SBA-15 for selective methane oxidation. Catal. Today 2016, 278, 120–126. [Google Scholar] [CrossRef]
- Baran, R.; Millot, Y.; Onfroy, T.; Averseng, F.; Krafft, J.-M.; Dzwigaj, S. Influence of the preparation procedure on the nature and environment of vanadium in VSiBEA zeolite: XRD, DR UV–vis, NMR, EPR and TPR studies. Microporous Mesoporous Mater. 2012, 161, 179–186. [Google Scholar] [CrossRef]
- El-Roz, M.; Lakiss, L.; Telegeiev, I.; Lebedev, O.I.; Bazin, P.; Vicente, A.; Fernandez, C.; Valtchev, V. High-Visible-Light Photoactivity of Plasma-Promoted Vanadium Clusters on Nanozeolites for Partial Photooxidation of Methanol. ACS Appl. Mater. Interfaces 2017, 9, 17846–17855. [Google Scholar] [CrossRef]
- Smoliło, M.; Samson, K.; Zhou, T.; Duraczyńska, D.; Ruggiero-Mikołajczyk, M.; Drzewiecka-Matuszek, A.; Rutkowska-Zbik, D. Oxidative Dehydrogenation of Propane over Vanadium-Containing Faujasite Zeolite. Molecules 2020, 25, 1961. [Google Scholar]
- Lie Bøyesen, K.; Meneau, F.; Mathisen, K. A combined in situ XAS/Raman and WAXS study on nanoparticulate V2O5 in zeolites ZSM-5 and Y. Phase Transit. 2011, 84, 675–686. [Google Scholar] [CrossRef]
- Chalupka, K.; Thomas, C.; Millot, Y.; Averseng, F.; Dzwigaj, S. Mononuclear pseudo-tetrahedral V species of VSiBEA zeolite as the active sites of the selective oxidative dehydrogenation of propane. J. Catal. 2013, 305, 46–55. [Google Scholar] [CrossRef]
- Held, A.; Kowalska-Kuś, J.; Millot, Y.; Averseng, F.; Calers, C.; Valentin, L.; Dzwigaj, S. Influence of the Preparation Procedure of Vanadium-Containing SiBEA Zeolites on Their Catalytic Activity in Propene Epoxidation. J. Phys. Chem. C 2018, 122, 18570–18582. [Google Scholar] [CrossRef]
- Tranca, D.C.; Keil, F.J.; Tranca, I.; Calatayud, M.; Dzwigaj, S.; Trejda, M.; Tielens, F. Methanol Oxidation to Formaldehyde on VSiBEA Zeolite: A Combined DFT/vdW/Transition Path Sampling and Experimental Study. J. Phys. Chem. C 2015, 119, 13619–13631. [Google Scholar] [CrossRef]
- Baran, R.; Onfroy, T.; Grzybek, T.; Dzwigaj, T. Influence of the nature and environment of vanadium in VSiBEA zeolite on selective catalytic reduction of NO with ammonia. Appl. Catal. B Environ. 2013, 136–137, 186–192. [Google Scholar] [CrossRef]
- Tielens, F. Exploring the reactivity of intraframework vanadium, niobium and tantalum sites in zeolitic materials using the molecular electrostatic potential. J. Mol. Struct. THEOCHEM 2009, 903, 23–27. [Google Scholar] [CrossRef]
- Tielens, F.; Calatayud, M.; Dzwigaj, S.; Che, M. What do vanadium framework sites look like in redox model silicate zeolites? Microporous Mesoporous Mater. 2009, 119, 137–143. [Google Scholar] [CrossRef]
- Tielens, F.; Trejda, M.; Ziolek, M.; Dzwigaj, S. Nature of vanadium species in V substituted zeolites: A combined experimental and theoretical study. Catal. Today 2008, 139, 221–226. [Google Scholar] [CrossRef]
- Tielens, F.; Dzwigaj, S. Probing acid–base sites in vanadium redox zeolites by DFT calculation and compared with FTIR results. Catal. Today 2010, 152, 66–69. [Google Scholar] [CrossRef]
- Wojtaszek, A.; Ziolek, M.; Dzwigaj, S.; Tielens, F. Comparison of competition between T=O and T–OH groups in vanadium, niobium, tantalum BEA zeolite and SOD based zeolites. Chem. Phys. Lett. 2011, 514, 70–73. [Google Scholar] [CrossRef]
- Witko, M.; Tokarz-Sobieraj, R. Surface oxygen in catalysts based on transition metal oxides—What can we learn from cluster DFT calculations? Catal. Today 2004, 91–92, 171–176. [Google Scholar] [CrossRef]
- Kerber, T.; Sierka, M.; Sauer, J. Application of semiempirical long-range dispersion corrections to periodic systems in density functional theory. J. Comput. Chem. 2008, 29, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Dobrota, A.S.; Rafailović, L.D.; Mentus, S.V.; Pašti, I.A.; Johansson, B.; Skorodumova, N.V. Structural and electronic properties of V2O5 and their tuning by doping with 3d elements—Modelling using the DFT+U method and dispersion correction. Phys. Chem. Chem. Phys. 2018, 20, 13934–13943. [Google Scholar] [CrossRef] [PubMed]
- Ganduglia-Pirovano, M.V.; Sauer, J. Stability of reduced V2O5 (001) surfaces. Phys. Rev. B 2004, 70, 045422. [Google Scholar] [CrossRef]
- Helali, Z.; Jedidi, A.; Syzgantseva, O.A.; Calatayud, M.; Minot, C. Scaling reducibility of metal oxides. Theor. Chem. Acc. 2017, 136, 100. [Google Scholar] [CrossRef]
- Słoczyński, J.; Grabowski, R.; Kozłowska, A.; Tokarz-Sobieraj, R.; Witko, M. Interaction of oxygen with the surface of vanadia catalysts. J. Mol. Catal. A Chem. 2007, 277, 27–34. [Google Scholar] [CrossRef]
- Goclon, J.; Grybos, R.; Witko, M.; Hafner, J. Relative stability of low-index V2O5 surfaces: A density functional investigation. J. Physics: Condens. Matter 2009, 21, 095008. [Google Scholar]
- Oshio, T.; Sakai, Y.; Ehara, S. Scanning tunneling microscopy/spectroscopy study of V2O5 surface with oxygen vacancies. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1994, 12, 2055–2059. [Google Scholar] [CrossRef]
- Smith, R.L.; Rohrer, G.S.; Lee, K.S.; Seo, D.-K.; Whangbo, M.-H. A scanning probe microscopy study of the (001) surfaces of V2O5 and V6O13. Surf. Sci. 1996, 367, 87–95. [Google Scholar] [CrossRef]
- Goschke, R.A.; Vey, K.; Maier, M.; Walter, U.; Goering, E.; Klemm, M.; Horn, S. Tip induced changes of atomic scale images of the vanadiumpentoxide surface. Surf. Sci. 1996, 348, 305–310. [Google Scholar] [CrossRef]
- Structure Commission of the International Zeolite Association. Zeolite Structures Database. Available online: http://www.iza-structure.org/databases/ (accessed on 21 November 2017).
- Migues, A.N.; Muskat, A.; Auerbach, S.M.; Sherman, W.; Vaitheeswaran, S. On the Rational Design of Zeolite Clusters. ACS Catal. 2015, 5, 2859–2865. [Google Scholar] [CrossRef]
- Migues, A.N.; Sun, Q.; Vaitheeswaran, S.; Sherman, W.; Auerbach, S.M. On the Rational Design of Zeolite Clusters for Converging Reaction Barriers: Quantum Study of Aldol Kinetics Confined in HZSM-5. J. Phys. Chem. C 2018, 122, 23230–23241. [Google Scholar] [CrossRef]
- Fermann, J.T.; Moniz, T.; Kiowski, O.; McIntire, T.J.; Auerbach, S.M.; Vreven, T.; Frisch, M.J. Modeling Proton Transfer in Zeolites: Convergence Behavior of Embedded and Constrained Cluster Calculations. J. Chem. Theory Comput. 2005, 1, 1232–1239. [Google Scholar] [CrossRef]
- de Vries, A.H.; Sherwood, P.; Collins, S.J.; Rigby, A.M.; Rigutto, M.; Kramer, G.J. Zeolite Structure and Reactivity by Combined Quantum-Chemical−Classical Calculations. J. Phys. Chem. B 1999, 103, 6133–6141. [Google Scholar] [CrossRef]
- Sierka, M.; Sauer, J. Finding transition structures in extended systems: A strategy based on a combined quantum mechanics–empirical valence bond approach. J. Chem. Phys. 2000, 112, 6983–6996. [Google Scholar] [CrossRef]
- Sherwood, P.; De Vries, A.H.; Collins, S.J.; Greatbanks, S.P.; Burton, N.A.; Vincent, M.A.; Hillier, I.H. Computer simulation of zeolite structure and reactivity using embedded cluster methods. Faraday Discuss. 1997, 106, 79–92. [Google Scholar] [CrossRef]
- Centi, G.; Trifiro, F. Catalytic behavior of V-containing zeolites in the transformation of propane in the presence of oxygen. Appl. Catal. A-Gen. 1996, 143, 3–16. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Hieu, N.V.; Lichtman, D. Bandgap radiation induced photodesorption from V2O5 powder and vanadium oxide surfaces. J. Vac. Sci. Technol. 1981, 18, 49–53. [Google Scholar] [CrossRef]
- Cogan, S.F.; Nguyen, N.M.; Perrotti, S.J.; Rauh, R.D. Optical properties of electrochromic vanadium pentoxide. J. Appl. Phys. 1989, 66, 1333–1337. [Google Scholar] [CrossRef]
- Moshfegh, A.Z.; Ignatiev, A. Formation and characterization of thin film vanadium oxides: Auger electron spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, scanning electron microscopy, and optical reflectance studies. Thin Solid Films 1991, 198, 251–268. [Google Scholar] [CrossRef]
- Hermann, K.; Witko, M.; Druzinic, R.; Tokarz, R. Hydrogen assisted oxygen desorption from the V2O5(010) surface. Top. Catal. 2000, 11, 67–75. [Google Scholar] [CrossRef]
- Hejduk, P.; Szaleniec, M.; Witko, M. Molecular and dissociative adsorption of water at low-index V2O5 surfaces: DFT studies using cluster surface models. J. Mol. Catal. A 2010, 325, 98–104. [Google Scholar] [CrossRef]
- TURBOMOLE V6.3 adoUoKa; Forschungszentrum Karlsruhe GmbH; TURBOMOLE GmbH, saf. 2011. Available online: http://www.turbomole.com (accessed on 7 February 2011).
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary Basis Sets to Approximate Coulomb Potentials. Chem. Phys. Lett. 1995, 240, 283–289. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO[Single Bond]MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lever, A.B.P. Electronic structure and spectra of ruthenium diimine complexes by density functional theory and INDO/S. Comparison of the two methods. J. Organomet. Chem. 2001, 635, 187–196. [Google Scholar] [CrossRef]
- Gorelsky, S.I. AOMix: Program for Molecular Orbital Analysis. version X.X. 2015. Available online: http://www.sg-chem.net/ (accessed on 21 May 2013).
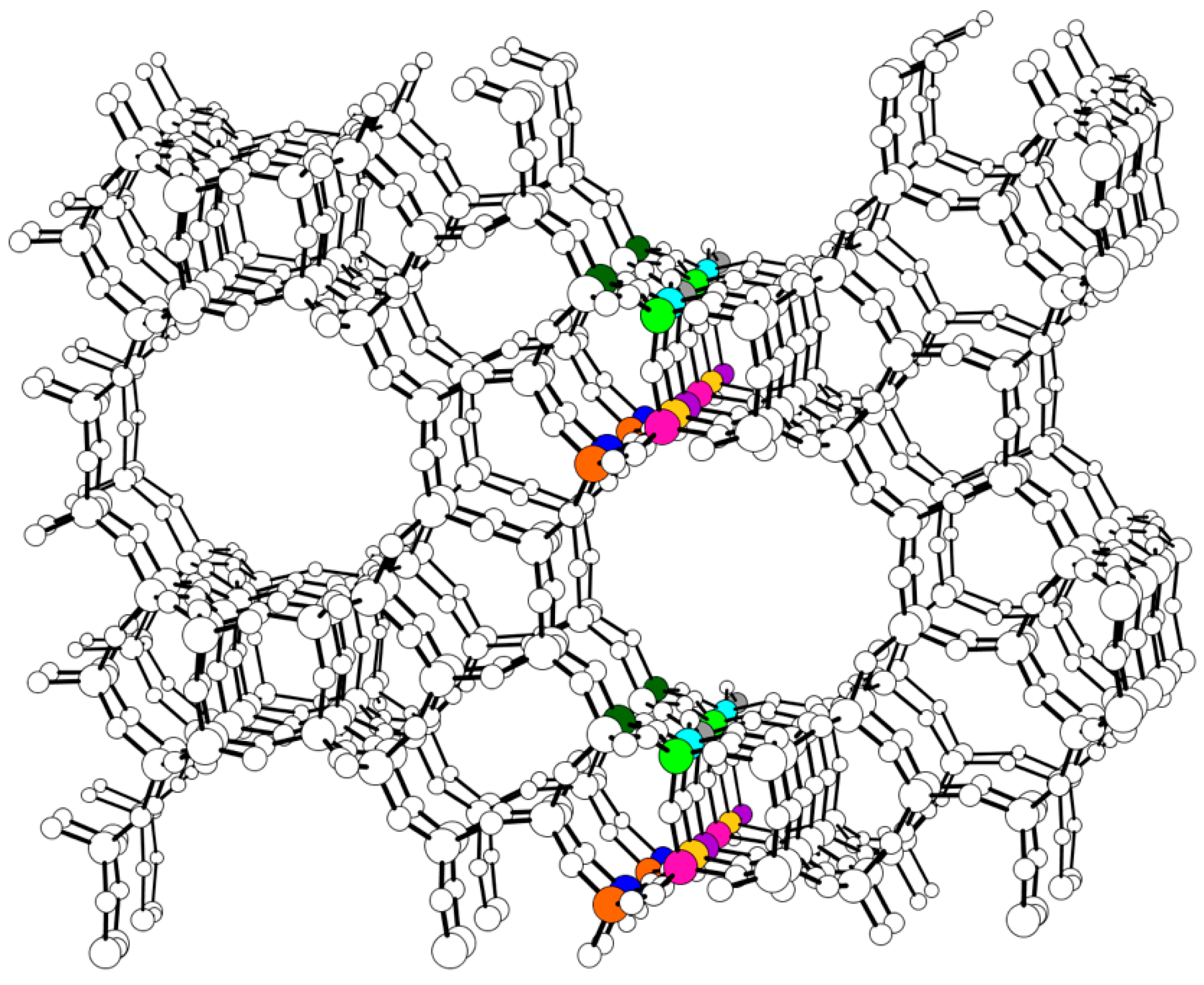
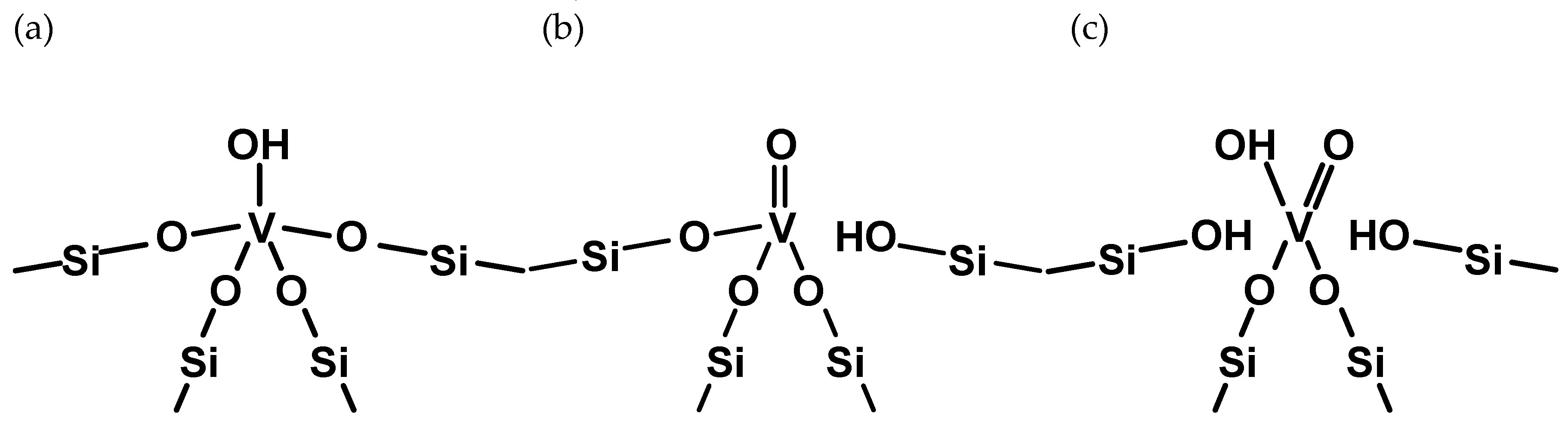
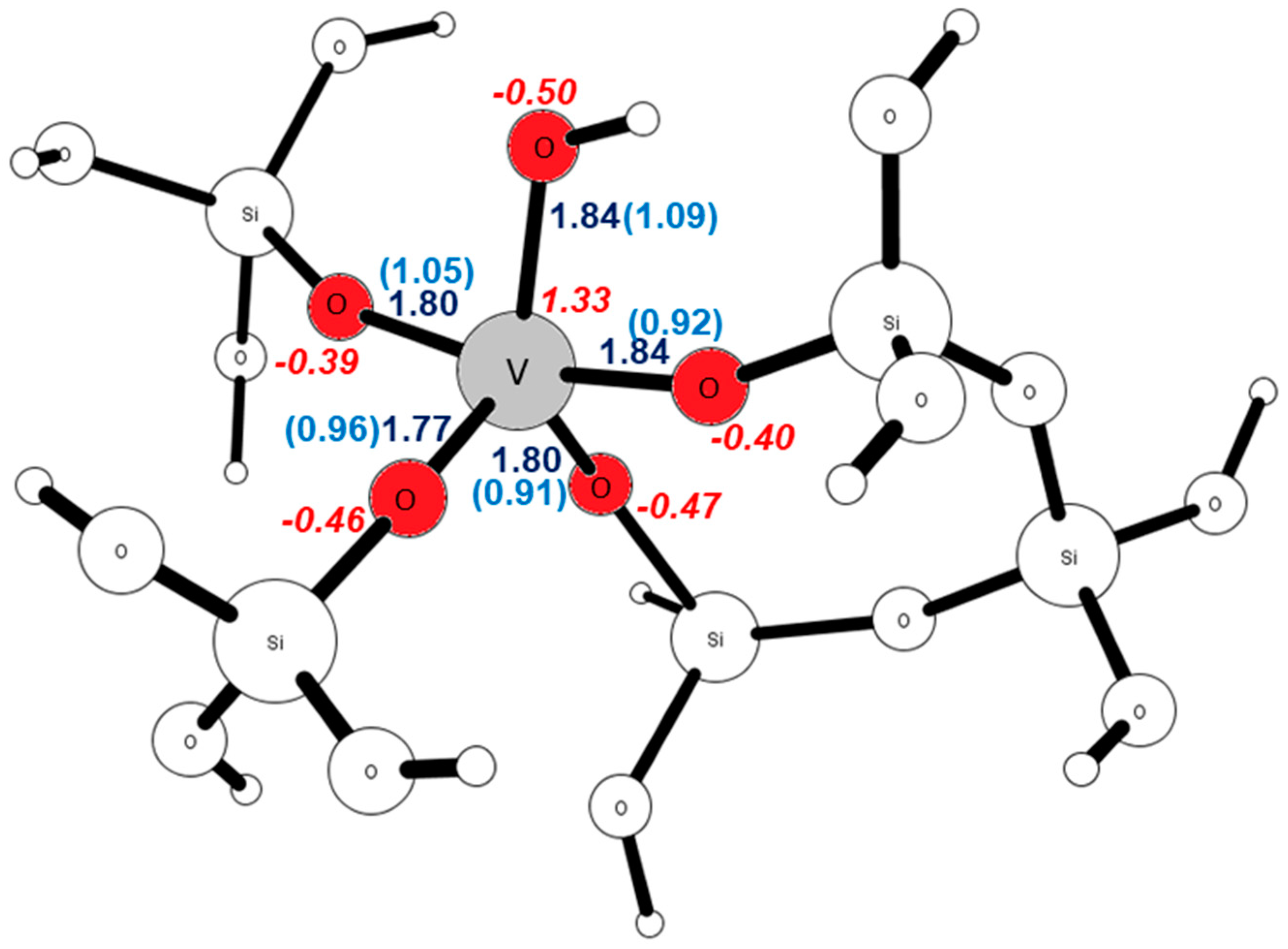
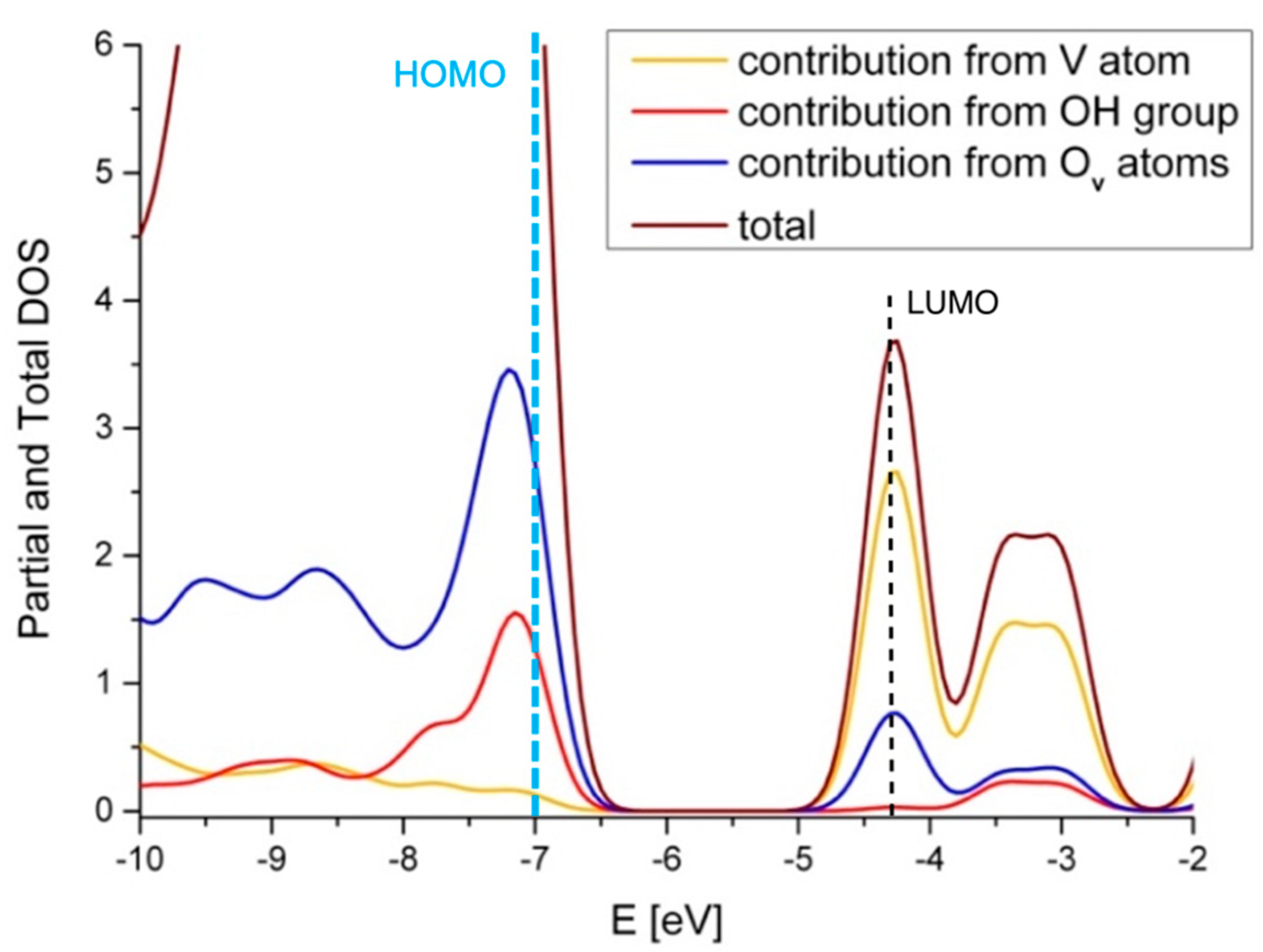
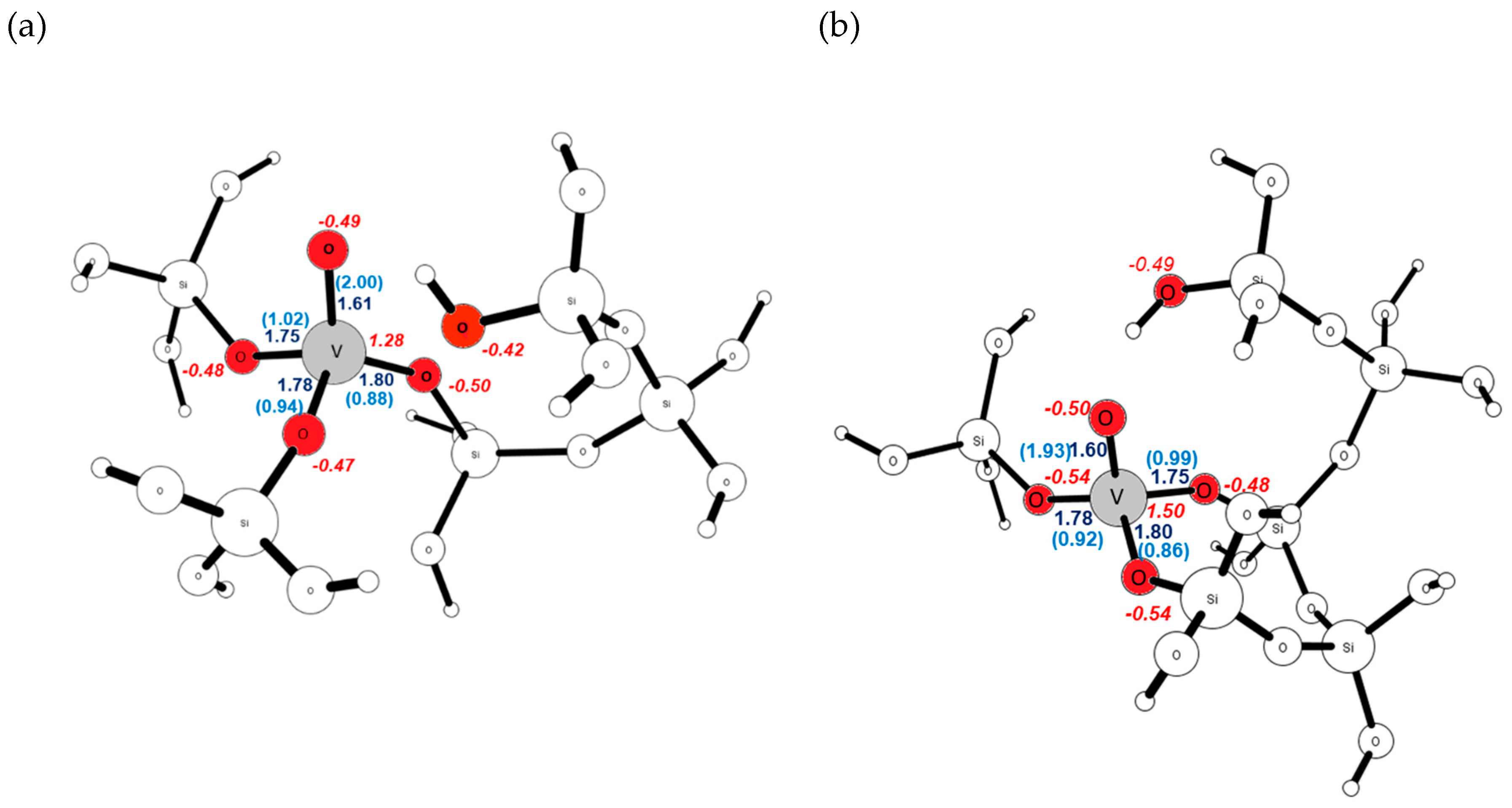
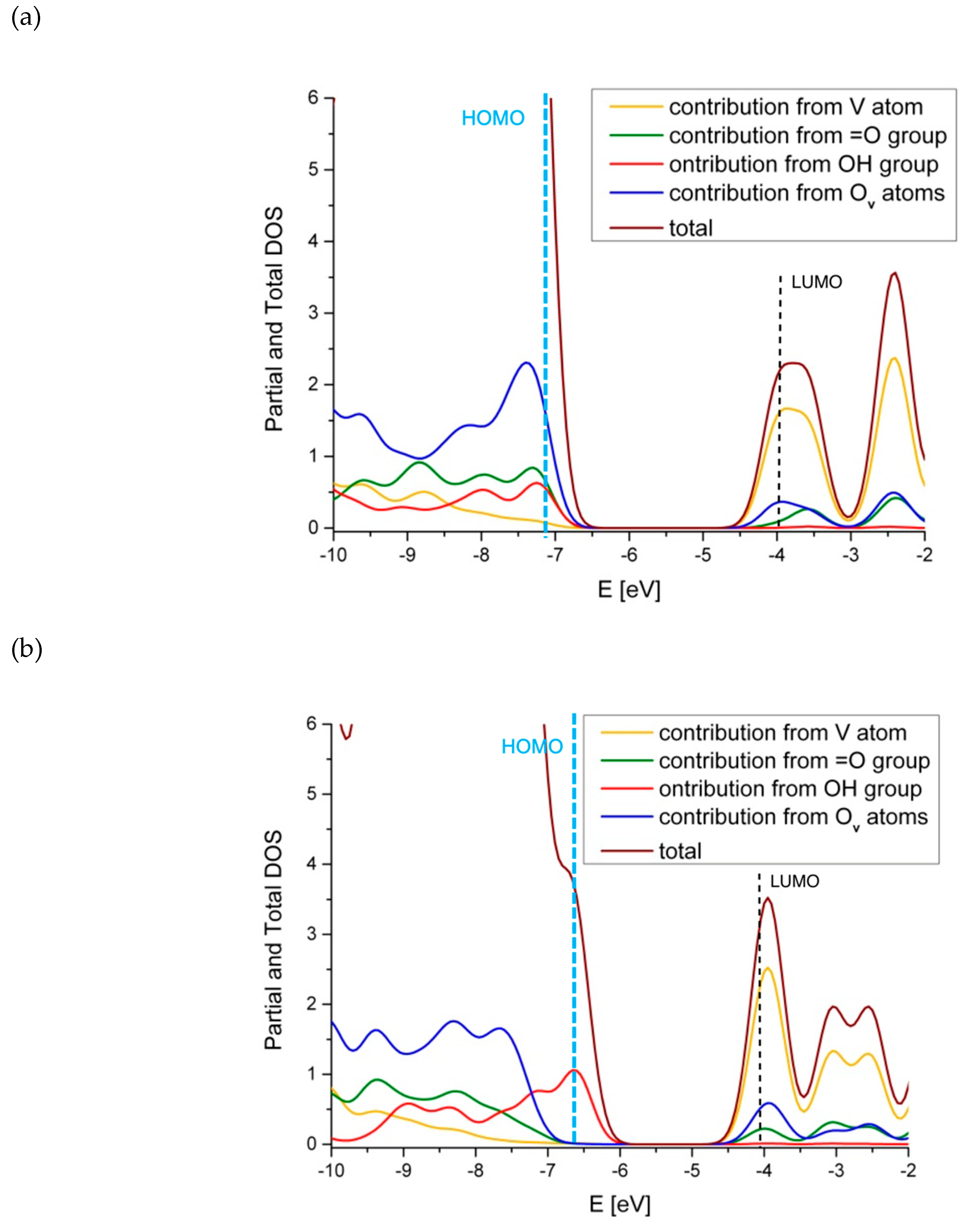
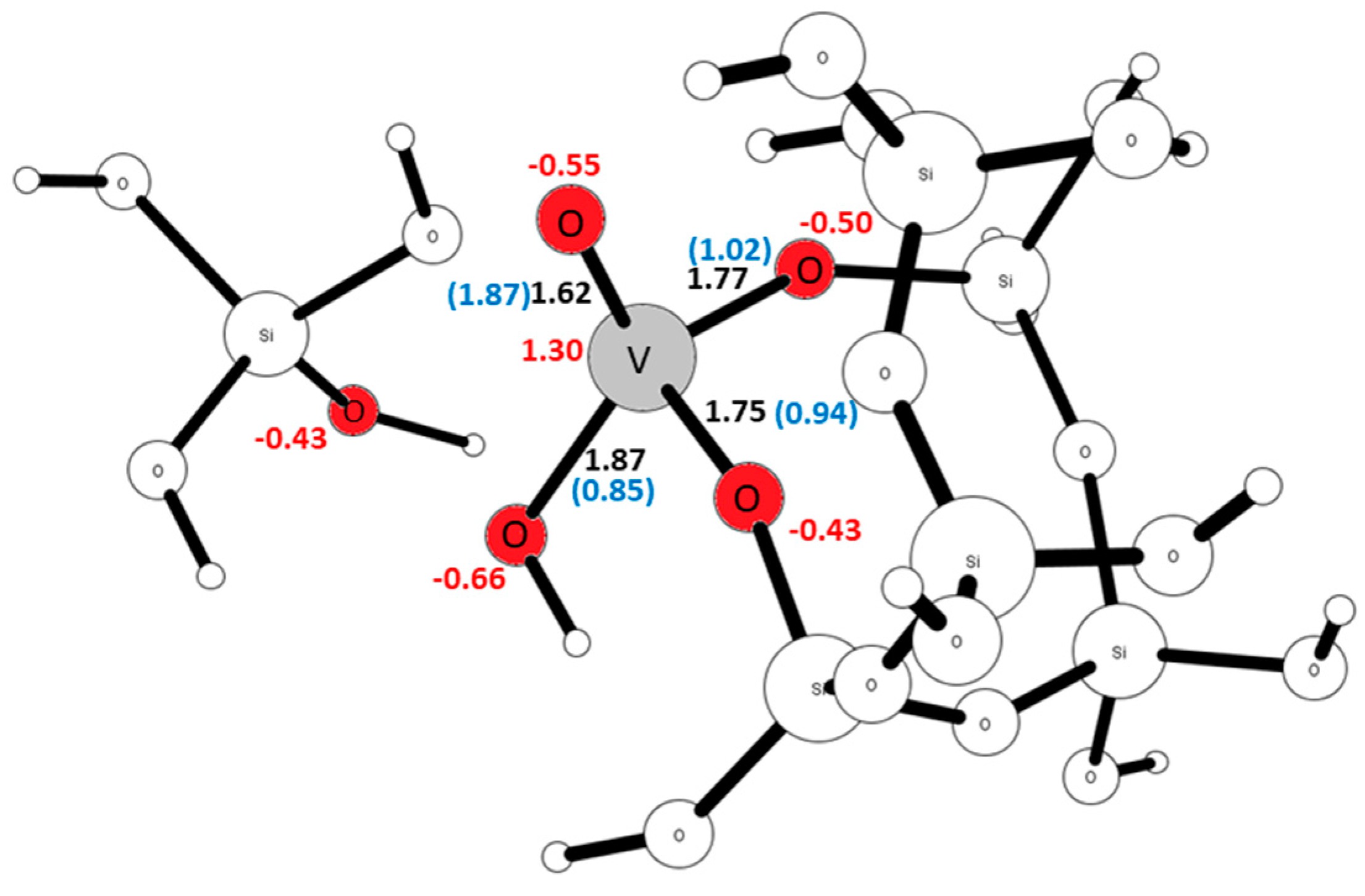
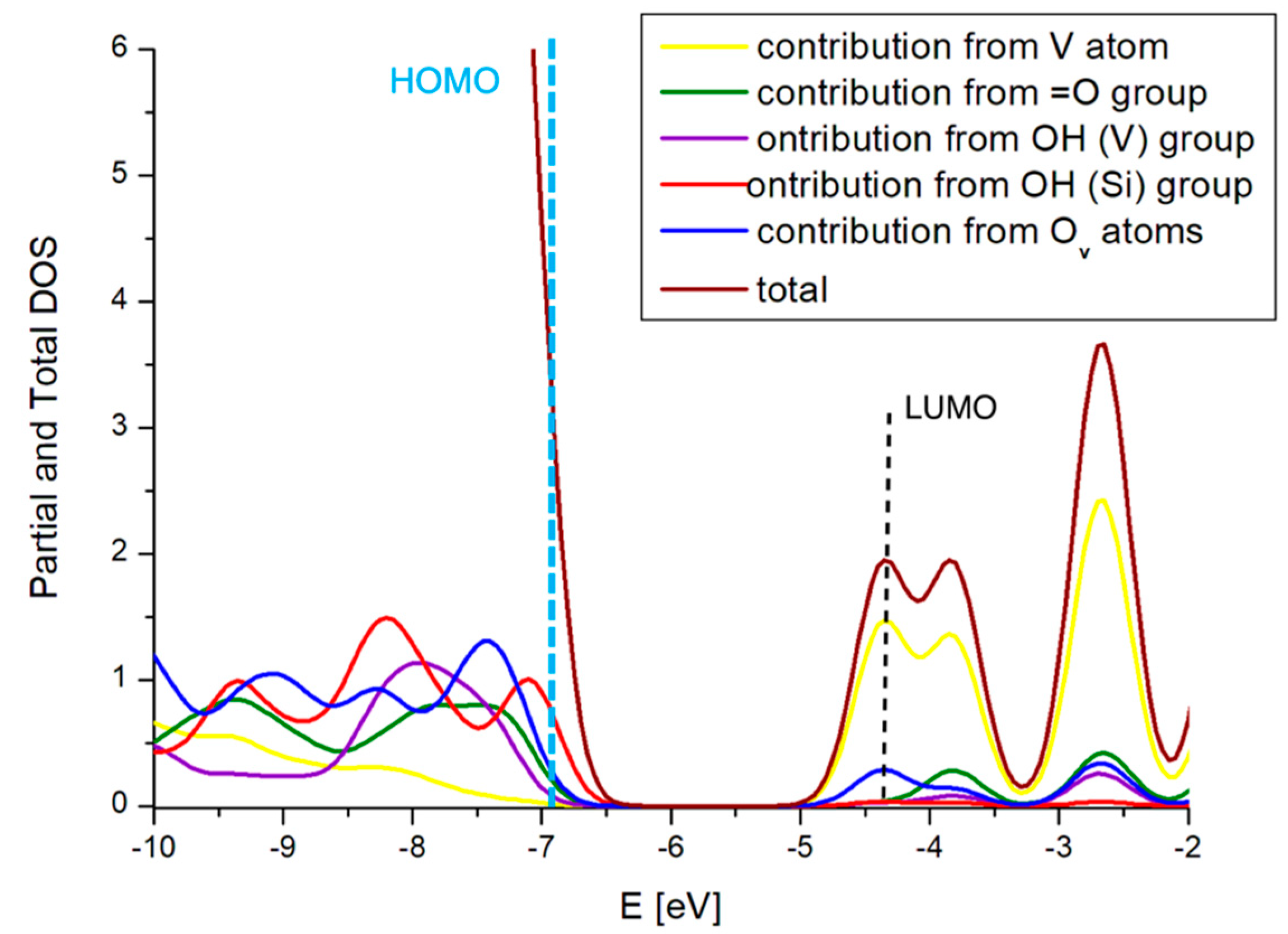


| Site | ΔE V–OH [eV] | ΔE V=O [eV] | ΔE V(=O)(–OH) [eV] |
|---|---|---|---|
| T1 | 0.23 | −0.69 | 1.43 |
| T2 | −2.16 | −2.24 | −1.51 |
| T3 | −1.69 | −2.21 | −2.19 |
| T4 | −0.42 | −1.14 | −1.29 |
| T5 | −0.41 | −0.85 | −1.09 |
| T6 | 0.10 | 0.00 | −0.40 |
| T7 | −0.48 | −1.12 | −0.14 |
| T8 | 0.06 | −1.42 | −1.38 |
| T9 | −1.19 | −1.79 | −1.41 |
| Site | R(V–OH) [Å] | B.O. | qOOH | qOH | qV |
|---|---|---|---|---|---|
| T1 | 1.85 | 1.12 | −0.48 | −0.16 | 1.25 |
| T2 | 1.84 | 1.09 | −0.50 | −0.17 | 1.33 |
| T3 | 1.82 | 1.12 | −0.49 | −0.16 | 1.37 |
| T4 | 1.83 | 1.09 | −0.50 | −0.17 | 1.39 |
| T5 | 1.83 | 1.09 | −0.49 | −0.16 | 1.38 |
| T6 | 1.84 | 1.14 | −0.48 | −0.16 | 1.31 |
| T7 | 1.84 | 1.09 | −0.50 | −0.17 | 1.39 |
| T8 | 1.81 | 0.94 | −0.46 | −0.13 | 1.31 |
| T9 | 1.82 | 1.22 | −0.44 | −0.12 | 1.27 |
| Site | R(V–O) [Å] | B.O. | qO | qV |
|---|---|---|---|---|
| T1 | 1.60 | 2.09 | −0.46 | 1.23 |
| T2 | 1.61 | 2.00 | −0.49 | 1.28 |
| T3 | 1.60 | 1.93 | −0.50 | 1.50 |
| T4 | 1.60 | 1.83 | −0.54 | 1.58 |
| T5 | 1.61 | 1.87 | −0.54 | 1.49 |
| T6 | 1.59 | 1.91 | −0.50 | 1.53 |
| T7 | 1.61 | 1.89 | −0.52 | 1.49 |
| T8 | 1.60 | 1.91 | −0.51 | 1.48 |
| T9 | 1.60 | 1.91 | −0.51 | 1.60 |
| Site | R(V=O) [Å] | B.O. V=O | R(V–OH) [Å] | B.O. V–OH | qOO | qOOH | qOH | qV |
|---|---|---|---|---|---|---|---|---|
| T1 | 1.62 | 2.18 | 1.89 | 1.03 | −0.34 | −0.58 | −0.27 | 0.76 |
| T2 | 162 | 2.17 | 1.83 | 1.03 | −0.51 | −0.65 | −0.33 | 1.06 |
| T3 | 1.62 | 1.87 | 1.87 | 0.85 | −0.55 | −0.66 | −0.35 | 1.30 |
| T4 | 1.62 | 1.84 | 1.85 | 0.90 | −0.57 | −0.64 | −0.32 | 1.30 |
| T5 | 1.61 | 1.99 | 1.89 | 0.82 | −0.49 | −0.70 | −0.38 | 1.24 |
| T6 | 1.58 | 2.17 | 1.80 | 1.03 | −0.41 | −0.61 | −0.26 | 1.40 |
| T7 | 1.62 | 1.90 | 1.86 | 0.82 | −0.54 | −0.69 | −0.38 | 1.24 |
| T8 | 1.60 | 1.98 | 1.78 | 1.14 | −0.51 | −0.54 | −0.21 | 1.33 |
| T9 | 1.62 | 1.78 | 1.76 | 1.19 | −0.61 | −0.54 | −0.20 | 1.40 |
| Parameter | Site on (010) V2O5 | Site in V-BEA |
|---|---|---|
| V(–OH) group | ||
| R (V–OH) | 1.74 | 1.84 a |
| B.O. (V–OH) | 1.21 | 1.09 a |
| qO | −0.48 | −0.50 a |
| qOH | −0.13 | −0.17 a |
| qV | 1.70 | 1.33 a |
| V(=O) group | ||
| R (V=O) | 1.58 | 1.60 a, 1.60 b |
| B.O. (V=O) | 2.19 | 2.00 a, 1.93 b |
| qO | −0.38 | −0.49 a, −0.50 b |
| qV | 1.70 | 1.28 a, 1.50 b |
| V(=O)(–OH) group | ||
| R (V–OH) | 1.78 | 1.83 b |
| R (V=O) | 1.58 | 1.62 b |
| B.O. (V–OH) | 1.12 | 0.85 b |
| B.O. (V=O) | 2.24 | 1.87 b |
| qOOH | −0.57 | −0.66 b |
| qOH | −0.23 | −0.35 b |
| qOO | −0.37 | −0.55 b |
| qV | 1.41 | 1.30 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzewiecka-Matuszek, A.; Tokarz-Sobieraj, R.; Witko, M.; Rutkowska-Zbik, D. Comparison of Catalytic Properties of Vanadium Centers Introduced into BEA Zeolite and Present on (010) V2O5 Surface–DFT Studies. Catalysts 2020, 10, 1080. https://doi.org/10.3390/catal10091080
Drzewiecka-Matuszek A, Tokarz-Sobieraj R, Witko M, Rutkowska-Zbik D. Comparison of Catalytic Properties of Vanadium Centers Introduced into BEA Zeolite and Present on (010) V2O5 Surface–DFT Studies. Catalysts. 2020; 10(9):1080. https://doi.org/10.3390/catal10091080
Chicago/Turabian StyleDrzewiecka-Matuszek, Agnieszka, Renata Tokarz-Sobieraj, Małgorzata Witko, and Dorota Rutkowska-Zbik. 2020. "Comparison of Catalytic Properties of Vanadium Centers Introduced into BEA Zeolite and Present on (010) V2O5 Surface–DFT Studies" Catalysts 10, no. 9: 1080. https://doi.org/10.3390/catal10091080
APA StyleDrzewiecka-Matuszek, A., Tokarz-Sobieraj, R., Witko, M., & Rutkowska-Zbik, D. (2020). Comparison of Catalytic Properties of Vanadium Centers Introduced into BEA Zeolite and Present on (010) V2O5 Surface–DFT Studies. Catalysts, 10(9), 1080. https://doi.org/10.3390/catal10091080





