Abstract
In this work, calcium oxide (CaO) extracted from eggshell impregnated with magnetite (Fe3O4) is prepared successfully and it had been applied on transesterification of palm oil off-grade. Prior experiment, the eggshells material are powdered and calcined at 900 °C then impregnated with Fe3O4 and recalcined. The obtained Fe3O4/CaO catalyst is characterized using X-ray diffraction and Braunaeur–Emmet–Teller (BET) surface area. The influence of various parameters including recalcined time and temperature are investigated. The prepared catalyst is tested for transesterification of palm oil off-grade to produce biodiesel in which the optimal conditions of a methanol/palm oil off-grade molar ratio of 10:1, the catalyst weight of 6%, the reaction temperature of 70 °C, and the reaction time of 2 h. The transesterification product was analyzed using GC-MS, which showed the biodiesel yield of 90% at the recalcined temperature of 600 °C and reaction time of 2 h. It has been noted that the catalyst activity is achieved when the moderate recalcination temperature is applied and the disordered structure of the catalyst is maintained. This study also confirms that CaO impregnated with Fe3O4 could be a solid catalyst for the biodiesel synthesis through transesterification reaction of palm oil off-grade.
1. Introduction
Due to global warming and other environmental concern today, many countries are trying to reduce carbon dioxide emissions in atmosphere by reducing the fossil fuel as the energy resources. There is one of the most effective ways to replace the fossil fuels with implementations of renewable energy [1]. Usually, different countries have different types of renewable energy available. Concerning this issue, several countries have successfully implemented some renewables from bioenergy, including biogas, biodiesel, and bioethanol [2,3,4,5]. On the other sides, some countries have also successfully generated the electrical power from renewable energy resources, such as geothermal, waves, hydro, wind, and solar energy [6,7,8,9,10]. However, there are some weak points resulted while the renewable energy is used, such as it is unstable, only available for certain periods, and therefore, its required the energy storage devices such as battery. However, the energy storage capacity of batteries is very limited, and for this reason some scientists are attempting to discover another type of energy storage materials that can store significant amount of energy [11,12,13].
Calcium is one of the most abundant metals on earth. It can be mostly found as an ion in seawater [14], as well as geothermal hot springs [15,16,17,18]. It can be also found as an oxide in limestone and fossilized remnant sites [14]. However, in order to obtain the calcium from these resources is required the natural resource exploitation that is often given negative impact to environment. Considering the environmental safety, an alternative way has been used to produce calcium source, namely it produce from some renewable resources which are available abundantly in nature, such as egg shell material wastes, because the egg shell well known as a natural material contained lots of calcium oxide [19]. As fact, the eggshell waste has been an abundant wastes in environment either come from egg consumption or the food industries. The waste materials are interesting to use as eco-friendly material to produce CaO [20].
Nowdays, the alkali catalysts including CaO is popular used in biodiesel synthesis through the transesterification process by using unedible oils, such as Jatropha curcas oil [21]. However, on practical application while the CaO was used directly as a catalyst in the transesterification process, the oxygen ions in the CaO surface will form a hydrogen bond with methanol and glycerin, resulting in an increasing of the glycerin viscosity and formation of the suspension of CaO; thus, the CaO and glycerin are difficult to separate from the product [22]. To overcome this problem, the CaO should be impregnated with any catalyst supports or other metal oxides [22,23]. The utilization of solid base catalysts (CaO) which are supported by metal oxides has gained attention from other research. There are some published articles which concern the modification of CaO catalyst, including those concerning KF/CaO-Fe3O4 [24], CaO/Al/Fe3O4 [25], CaO.ZnO [23], CaO/CoFe2O4 [26], K2O/CaO-ZnO [27], MgFe2O4-CaO [28], and Fe3O4/CaO [29]. These studies were focused on the investigation of appropriate heterogeneous solid base catalysts are able to be separated from the product. Furthermore, the catalysts showed activity and good catalytic properties, and also showed the highest biodiesel yields [25]. Additionally, the utilization of a catalyst enables an increase in the biodiesel yield from commercial edible sunflower oil.
Considering some advantages of Fe3O4/CaO catalysts such as their inexpensive supports, the fact that they are easy to find, and the catalyst preparation method, these catalysts have been subjected to study by many researchers. Niju et al. [30] have reported the modification of waste eggshells as CaO catalysts to biodiesel production from waste frying oil. The catalysts were prepared using various processes, such as having a calcination temperature of 900 °C, and were prepared through a calcination-hydration-dehydration process. Regarding the results, the surface area of the obtained catalysts was around 8.6401 m2/g and the alkalinity was about 12.2 < H ≤ 15.0, even though the catalysts were synthesized using calcination, showing a surface area and alkalinity of 3.73 m2/g and 9.8 < H ≤ 12.2, respectively.
Referring to previous works as reported above [24,25], waste palm oil has been successfully used as a feedstock for the transesterification reaction to produce biodiesel in which the yields of around 95% with the catalyst prepared via calcination-hydration-dehydration, and 80% with the prepared catalyst which was synthesized using calcination. The experiment was designed with a catalyst weight of 5%wt. CaO and a molar ratio of methanol to oil of 12:1; the reaction temperature was 65 °C and the reaction time was 1 h. The research proved that the highest surface area and alkalinity of the catalyst influenced the catalyst activity and leading to increase the biodiesel yield. Hence, based on some scientific reasons as explained above, this research is focused on the preparation of a Fe3O4/CaO catalyst based on eggshell waste and the study of its catalytic activity for transesterification of palm oil off-grade.
2. Results and Discussion
2.1. Extraction of Palm Oil Off-Grade
The raw material of crude palm oil off-grade was steamed to mash the mesocarp of fruit and deactivate the lipase enzyme in order to prevent increases in free fatty acid (FFA) content from the oil. The fruit was extracted using a spindle hydraulic press which produced palm oil off-grade of around 17%, or 170 g of oil for 1 kg of palm fruit off-grade. The crude palm oil off-grade was characterized with regard to density, viscosity, water content, and FFA content. The properties of the crude palm oil off-grade which resulted from this experiment are shown in Table 1.

Table 1.
Properties of crude palm oil off-grade. Legend: FFA, free fatty acid.
As it shown in Table 1, the palm oil off-grade had high water and FFA contents. The highest water content in the feed stock oil had been a negative impact on the oil quality because it is improving the FFA content through hydrolysis process. Due to the fact that the CPO off-grade used as a sample in this experiment contained high FFA (6.9%), the esterification process was carried out as a prior experiment in order to reduce the FFA value, and the obtained CPO off-grade FFA value become around 1.26%.
2.1.1. Catalyst Characterization
XRD analysis was used in this experiment. The purpose of X-ray diffraction analysis is to identify the structure, crystallinity, and formation of a metal oxide [31] in the material, in this case CaO and Fe3O4 in the catalyst. Figure 1 showed the XRD patterns of obtained Fe3O4/CaO catalysts prepared at different dehydration times of 2, 3, and 4 h, respectively and the reaction temperature of 500 °C.
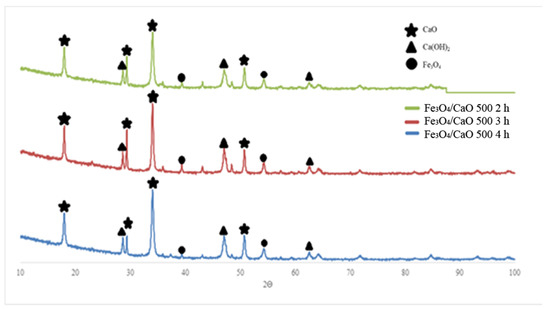
Figure 1.
XRD pattern of obtained Fe3O4/CaO catalyst at a dehydration temperature of 500 °C with various dehydration times of 2 h, 3 h, and 4 h, respectively.
Based on a matching process between the XRD pattern obtained in this experiment and the standard diffractogram of a calcium compound which exists in the JCPDS system, the obtained catalyst was found to be composed of CaO (34.044°, 50.73°, 62.56°, and 71.75°), Ca(OH)2 (18.06°, 34.10°, and 64.29°), and Fe3O4 (39.36°, 54.33°, 62.61°, and 71.743°), whereas the highest intensity of a CaO particle was detected at 2θ = 32.19° with an intensity value of 957.4 and then at 2θ = 37.35° and 53.85°, as it matched with the JCPDS file number 37-1497. It can be seen clearly that in natural calcined eggshell a mixture of CaO and Ca(OH)2 phases are present; however, CaO is the main phase. CaO intensity decreases because CaO has reacted with H2O in the impregnation process to form Ca(OH)2 [32], resulting in a reaction as in the following equation, Equation (1), i.e.,
CaO + H2O → Ca(OH)2 + H2O
Based on the X-ray diffractogram shown in Figure 1, it can be seen that all the prepared catalysts (Fe3O4/CaO) show similar peaks that indicate their being of the same phase of crystal. However, considering that the peak intensity of obtained Fe3O4/CaO increased slightly while the dehydration time increased, it could be assumed dehydration has a time-driven positive impact on the active site of the catalyst surface, as has been reported previously by Mutreja, et al. [33].
As a comparison, Figure 2 shows an XRD pattern of the Fe3O4/CaO catalyst which was synthesized at different temperatures of 600 °C and 700 °C, respectively, with each having a dehydration time of 3 h.
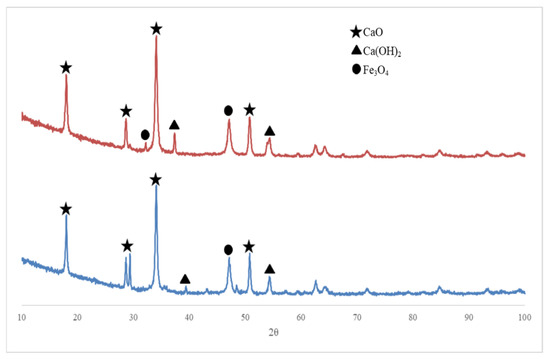
Figure 2.
XRD pattern of prepared Fe3O4/CaO catalyst with the same dehydration time (3 h) at different dehydration temperatures of 600 °C and 700 °C, respectively.
The XRD pattern shows a strong intensity of 2θ at 39.36°, 54.33°, 62.61°, and 71.743°, which indicates the existing Fe3O4 phase in the sample. The XRD pattern in Figure 2 also appears to show the CaO phase in the prepared sample, indicated by the specific peak of CaO at 2θ values of 32.22°, 37.37°, 42.40°, and 53.89°. This experiment also confirmed the existence of Ca(OH)2, with its specific values at 17.55° and 43.93°, which was possibly produced by the reaction process between calcium oxide and some trace amount of air in the atmosphere. Concerning the basic strength of the CaO catalyst, Kouzu et al. [34] have reported that the highest order of base strength was CaO followed by Ca(OH)2, with the lowest being CaCO3. Thus, the presence of Ca(OH)2 compounds on the surface of CaO solids results in a decrease of base strength on the catalyst.
Based on the XRD diffractogram obtained from three catalyst samples synthesized in this experiment it can be seen that the formation of CaO crystalline increased with increasing temperature and dehydration time due to high temperature and a longer recalcination time causing Ca(OH)2 to decompose into CaO, which indicates that the results of this experiment are consistent with the previous work as reported by Wong et al. [31].
2.1.2. Hammett Indicator Titration
Hammett indicator experiments were conducted to determine the H range of basic sites in each catalyst. The basic strength of the catalyst was taken to be higher than the weakest indicator that underwent a color change and lower than the strongest indicator that underwent no color change [35]. Among the catalysts tested, Fe3O4/CaO recalcined at 600 °C for 3 h had the strongest basic strength; the results obtained are presented in Table 2. According to Ho et al. (2014) [36], a strongly developed base catalyst of H_ > 9.3 is considered a relatively strong base catalyst for the transesterification process, although its basic strength is lower than that of pure CaO (15 < H_ < 18.4).

Table 2.
The base strength of the Fe3O4/CaO catalyst.
2.1.3. Braunaeur–Emmet–Teller (BET)
The surface area of a solid catalyst has a direct impact on its catalytic activity [37,38]. Hydration and dehydration (recalcination) treatments play a vital role for improving the surface area of a catalyst [39]. As it shown in Table 3, the surface area of the CaO from eggshell calcined at 850 °C was found to be 1.96 m2/g [29]. However, the surface area of CaO obtained from calcination-hydration and dehydration (recalcination) treatment of eggshell was determined to be 15.67–265.37 m2/g. These results are in accordance with the findings of [39]. The surface area of the CaO obtained from the calcination-hydration and dehydration (recalcination) treatment was twice that of CaO obtained from the calcination of calcium carbonate [39].

Table 3.
BET surface area of the obtained Fe3O4/CaO catalyst.
Based on the BET results shown in Table 3, the large catalyst surface area at the dehydration temperature of 600 °C was 265.371 m2/g. This is due to an increase in the temperature of calcination, after which the catalyst gradually turns into a stable crystal and has increased surface area [23]. The closing of pores from magnetite by CaO crystals causes a decrease in the surface area of the catalyst [40]. Hence, the magnitude of the catalyst surface area obtained due to the possibility of CaO is not evenly distributed across the pores and magnetite surfaces in the impregnation process.
2.2. Catalytic Study of Fe3O4/CaO
2.2.1. Biodiesel Yield
To understand how the temperature and dehydration time influence the activity of the Fe3O4/CaO catalyst, the transesterification process was designed in this experiment with the experimental conditions are consisted of 10:1 for the methanol oil molar ratio, the catalyst weight of 1% (wt.), the reaction temperature of 70 °C, and the reaction time of 2 h. Figure 3 showed the yield of biodiesel resulted in this experiment.
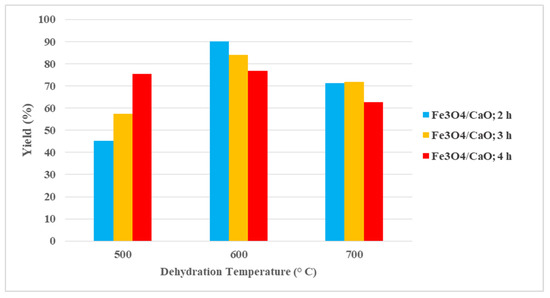
Figure 3.
The yield of biodiesel produced from palm oil off-grade using Fe3O4/CaO catalyst prepared at various dehydration temperatures and reaction times.
As it has been mentioned, the chicken eggshell has been modified via calcination-hydration-dehydration followed by impregnation process with magnetite (Fe3O4) through the wet impregnation method. Specifically, the catalysts (Fe3O4/CaO) were treated at different dehydration temperatures of 500, 600, and 700 °C, respectively, and dehydration times of 2, 3, and 4 h, respectively. The optimal yield of biodiesel obtained was around 90% using the catalyst Fe3O4/CaO. This result is quite different from that of previous work, as reported by Niju et al. [30], in which a result of 95% was obtained by using CaO at a dehydration temperature of 600 °C and a reaction time of 3 h. The different result of the biodiesel yield is due to the CaCO3 compound still existing in the prepared catalyst, so that the amount of CaO as the main substance of the prepared catalyst was only 74% wt. This result indicates that a temperature and calcination time of 900 °C and 2.5 h, respectively, was inconvenient to convert CaCO3 to CaO. However, in the case of the prepared catalyst modified with magnetite, the yield of biodiesel produced was high. Hence, it can be noted that increasing dehydration temperature and dehydration time affected the catalytic activity, which was referred to by the obtained biodiesel. It can be assumed that the catalytic activity increased due to the homogenous distribution of magnetite substances on the outer surface of the CaO particles, which produced a larger surface area of the obtained catalyst (Fe3O4/CaO). On the other hand, it can be observed that the increase in dehydration temperature produced a stable crystal with a high specific area [25]. A comparison study was performed in which different transesterification processes of palm oil off-grade were carried out by using CaO only, Fe3O4 only, and without catalyst, respectively. The summary of the transesterification processes is shown in Figure 4. It can be seen that in the case of the CaO catalyst only, the biodiesel yield was 62%, and the biodiesel yield was 33% for the Fe3O4 catalyst.
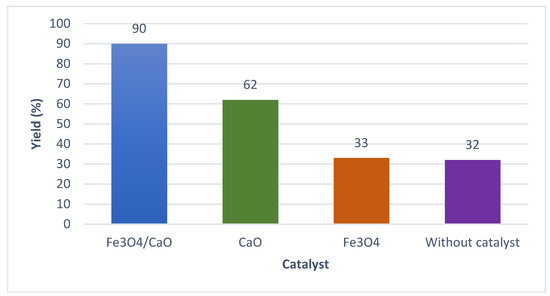
Figure 4.
The biodiesel yield produced from palm oil off-grade catalyzed by Fe3O4/CaO, CaO, Fe3O4, and without catalyst.
2.2.2. Biodiesel Characterization
In order to evaluate the quality of the transesterification product, the obtained biodiesel was characterized and compared with the Indonesian biodiesel quality standard (SNI 7182:2015). Characterization parameters, which are including density, kinematic viscosity, flash point, and acidic number, are shown in Table 4.

Table 4.
Characteristics of obtained biodiesel compared to Indonesian quality standard (SNI).
Myristic, palmitic, linoleic, oleic, and stearic acids are the major compounds of carboxylic acid contained in coconut and palm oils [41,42,43]. These compounds are converted to methyl ester through a transesterification process with alcohol assisted by a catalyst substance [44]. The converted percentage is used to justify a catalyst’s ability. The transesterification product was analyzed using GC-MS, which indicated that methyl ester was the main compound in the obtained biodiesel, which are specific retention times of 35.39 and 37.42 min, respectively, as reported in Figure 5.
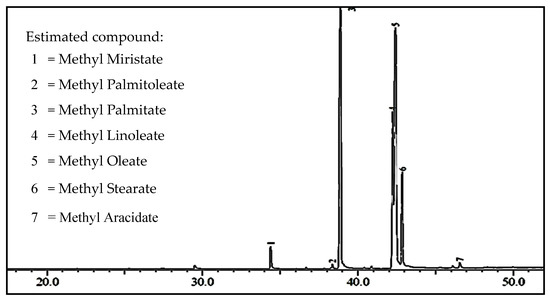
Figure 5.
The chromatogram of transesterification products resulted from palm oil off-grade using Figure catalyst.
Based on the results shown in Figure 5 above, it can be concluded that methyl palmitate was the main methyl ester compound in the biodiesel produced in this experiment, with a percentage of 35%, while the minor compound was methyl stearate (9.58 wt.%). On the other hand, the yield of biodiesel was 90%, which indicated the produced biodiesel still contained some impurities such as catalyst residue, water, methanol, and glycerol.
2.3. Separation of Fe3O4/CaO Catalyst
In order to separate the catalyst (Fe3O4/CaO) from the other substances in the final product of the transesterification experiment, the catalyst was separated physically through a decantation process and then filtrated using filter paper. With regard to a previous work, the introduction of magnetite onto the CaO surface results in magnetic properties of the produced catalyst (Fe3O4/CaO) [29]. According to Kazeminezhad and Mosivand (2014) [45], iron (III) oxide is known as hematite (α-Fe2O3) or maghemite (γ-Fe2O3), and has the same structure as magnetic compounds. Conversely, the magnetite compound (Fe3O4) is converted to hematite (Fe2O3) through the calcination process at a high temperature of around 800–900 °C. Introduction of the magnetite (Fe3O4) on CaO material resulting the Fe3O4/CaO catalyst had been positive impact on the separation process of the Fe3O4/CaO catalyst from transesterification products of palm oil off grade. Figure 6 shows the obtained biodiesel contained and free of the catalyst after the transesterification process.
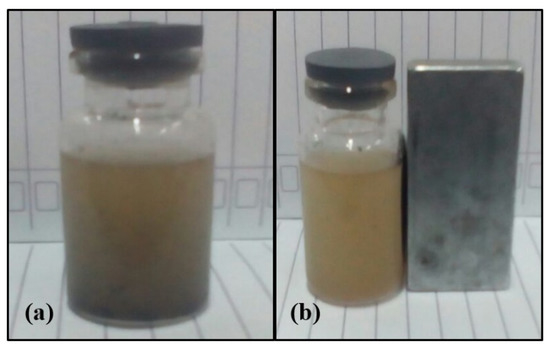
Figure 6.
The obtained biodiesel produced from palm oil off-grade: (a) still contained the Fe3O4/CaO catalyst; (b) after being separated from the Fe3O4/CaO catalyst.
3. Experimental Section
3.1. Materials
Waste chicken eggshell and palm oil off-grade used as raw materials in this experiment were collected from a palm oil production in Riau province, Indonesia. Magnetite (Fe3O4) was collected from iron trellis waste in Indonesia. Some of the chemicals used, including methanol, sulfuric acid (H2SO4), ethanol, potassium oxide (KOH), and oxalic acid, were purchased from Aldrich products.
3.2. Equipment
The equipment used for this experiment was a three-neck flask with a capacity of 500 mL as a batch reactor for a transesterification reaction, which was equipped with a heating mantel, condenser, thermometer, and magnetic stirrer. A strainer with a hole size of around 100 and 200 mesh, an oven, a furnace, and analytic weights were used to prepare the catalysts. A spindle press was used to collect palm oil off-grade. GC-MS, XRD, and BET characterization equipment were used to characterize the biodiesel products and catalysts.
3.3. Raw Material Preparation
The crude palm oil off-grade was extracted using an artisanal method in which the first step was to wash the fruit to remove the impurities of the palm fruit. Afterward, the palm fruit was steamed for 120 min and then extracted using a spindle hydraulic press. Furthermore, the extracted oil was transferred into a separate funnel so that the oil and water could be separated completely. The upper layer, which was the obtained palm oil, was evaluated to determine its FFA and water contents.
3.4. Preparation of Fe3O4/CaO Base Catalysts
In the first step, the magnetite samples were grained to produce a particle size of around 75 μm and 150 μm, respectively, by using a metal mesh separation. The magnetite was washed using deionized water to remove the sand and other impurities and then subsequently dried in the oven at 105 °C. Additionally, the CaO was prepared from the waste chicken eggshell; the waste chicken eggshell was washed with distilled water to remove impurities such as sand from the surface and subsequently treated in an oven at 105 °C for 24 h for removal of adhering water. The dried eggshells were ground and strained to sizes of 75 and 150 μm, respectively. Afterward, the fine eggshells were calcined in a furnace at 900 °C for 2.5 h to generate CaO. The obtained CaO and magnetite were weighed based on a weight ratio of CaO and magnetite of around 60:40 (wt.). Typically, in order to prepare 30 wt.% CaO loaded catalysts, 18 g of calcined eggshell powder was dissolved in 250 mL of water in a glass and stirred using a magnetic stirrer on a hotplate until a temperature of around 70 °C was reached. A hydration process was used to form an aqueous solution of Ca(OH)2; this solution was subsequently added to 12 g of magnetite and mixed for 4 h at 700 rpm until an homogeneous solution was obtained. A slurry was formed from this process and dried in an oven at 105 °C for 24 h to remove the excess water (H2O). Furthermore, the dried mass was dehydrated using calcination in the furnace at varying temperatures of 500 °C, 600 °C, and 700 °C, with time variations of 2, 3, and 4 h, to change the hydroxide to oxide particles and to produce a magnetic base catalyst containing CaO particles produced from chicken eggshells.
3.5. Catalytic Study
The transesterification process in this experiment was started by placing 60 g of extracted oil into a three-neck flask and heating to reach 60 °C. Methanol (with a mole ratio of methanol:oil = 12:1) and 1% (wt.) of H2SO4 were added. The reaction lasted for 1 h, with the speed of the stirrer kept at 400 rpm throughout. The mixture of the H2SO4 catalyst and the residual methanol was separated using a separating funnel. The lower layer was checked for FFA content [46] and then the transesterification reaction stage was commenced. The lower layers of esterification product separation were fed into 50 g of transesterification reactor and heated to 70 °C; methanol was added with a mole ratio of methanol:oil = 10:1, Fe3O4/CaO catalyst of 1% wt.% oil was used, and the stirring rate was kept at 400 rpm for 2 h. After the reaction was completed, the catalyst was separated and the transesterification products were allowed to settle overnight for the clear separation of biodiesel and glycerol.
3.6. Catalyst Characterization
There were some characterization methods, including X-ray diffraction (Shimadzu XRD 600 X-ray Diffractometer, 30 kV, 30 mA) and BET surface area, which were used in this experiment. A prepared sample was filled through gold sputtering on the surface of Fe3O4/CaO. The pictures were gained through scanning electron microscope pictures gained at 15 kV with 10,000 times enlargement. For these calcium compounds, basic properties were determined by the indicator method. After the catalyst was dispersed in a toluene solution of the indicator, the color change of the indicator was observed. The strength of the basic site was expressed by an acidity function (H_). The indicators used were bromothymol blue (pKBH = 7.2), phenolphthalein (pKBH = 9.3), indigo carmine (pKa = 12.2), and 2,4-dinitroaniline (pKBH = 15.0).
4. Conclusions
The present study reveals that the calcination-hydration-dehydration treatment is a sufficient method to increase the activity of a solid catalyst prepared from eggshells waste to produce biodiesel through transesterification reaction from palm oil off-grade substance. The optimal conditions of the transesterification experiment were found to include a reaction time of 2 h, the reaction temperature of 70 °C, the methanol to oil molar ratio of 10:1, and the catalyst loading of 1 % wt. The results showed that the methyl ester was the main compound in the obtained biodiesel, with the yield of 62% was obtained when using the CaO catalyst and a yield of 90% obtained when Fe3O4/CaO was applied as a catalyst instead of CaO. This experiment also found that the calcination temperature of the catalyst was an important parameter during the catalyst treatment; while high calcination temperature was required in order to gain mechanical strength to prevent the leaching process on their application.
Author Contributions
Conceptualization, Z.H., B.B., D.Y., M.R. and R.I.; data curation, E.S., W.F. and M.M.; formal analysis, R.I.; funding acquisition, T.M.I.M.; investigation, Z.H., B.B., D.Y., W.F. and M.R.; methodology, Z.H.; project administration, R.I.; resources, T.M.I.M.; software, G.M.I. and T.M.I.M.; supervision, R.I. and T.M.I.M.; validation, E.S., D.Y., M.M., M.R. and T.M.I.M.; visualization, E.S. and B.B.; writing—original draft, Z.H.; writing—review and editing, W.F., G.M.I., M.M. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universitas Riau through the “Penelitian Energi dan Energi Terbarukan” scheme, grant number 287/UN.19.5.1.3/PP/2018 and Universitas Syiah Kuala through “Penelitian Dasar” scheme, grant number: 215/SP2H/LT/DPRM/2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Kusumo, F.; Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Siswantoro, J.; Mahlia, T.M.I. Optimization of transesterification process for Ceiba pentandra oil: A comparative study between kernel-based extreme learning machine and artificial neural networks. Energy 2017, 134, 24–34. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Chong, W.T.; Mahlia, T.M.I.; Sebayang, A.H.; Berawi, M.A.; Nur, H. Second generation bioethanol potential from selected Malaysia’s biodiversity biomasses: A review. Waste Manag. 2016, 47, 46–61. [Google Scholar] [CrossRef]
- Silitonga, A.; Mahlia, T.M.; Ong, H.C.; Riayatsyah, T.M.; Kusumo, F.; Ibrahim, H.; Dharma, S.; Gumilang, D. A comparative study of biodiesel production methods for Reutealis trisperma biodiesel. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 2006–2014. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Saiful, S.; Alatas, M.; Subhan, S.; Lala, A.; Muslem, M.; Suhendra, R.; Idroes, G.M.; Marwan, M.; et al. Geochemistry Exploration and Geothermometry Application in the North Zone of Seulawah Agam, Aceh Besar District, Indonesia. Energies 2019, 12, 4442. [Google Scholar] [CrossRef]
- Schloer, S.; Bredmose, H.; Bingham, H.B. The influence of fully nonlinear wave forces on aero-hydro-elastic calculations of monopile wind turbines. Mar. Struct. 2016, 50, 162–188. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Techno-economic analysis of an optimized photovoltaic and diesel generator hybrid power system for remote houses in a tropical climate. Energy Convers. Manag. 2013, 69, 163–173. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.M. Phase change materials (PCM) for solar energy usages and storage: an overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Preparation and properties of highly conductive palmitic acid/graphene oxide composites as thermal energy storage materials. Energy 2013, 58, 628–634. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Lala, A.; Suhendra, R.; Idroes, G.M. Marwan Geochemistry of Sulphate spring in the Ie Jue geothermal areas at Aceh Besar district, Indonesia. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 12 September 2019; Volume 523, p. 012012. [Google Scholar]
- Idroes, R.; Yusuf, M.; Alatas, M.; Lala, A.; Suhendra, R.; Idroes, G.M. Geochemistry of hot springs in the Ie Seu’um hydrothermal areas at Aceh Besar district, Indonesia. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 12 September 2018; Volume 334, p. 12002. [Google Scholar]
- Idroes, R.; Yusuf, M.; Alatas, M.; Lala, A.; Suhendra, R.; Idroes, G.M.; Riza, M. Geochemistry of warm springs in the Ie Brôuk hydrothermal areas at Aceh Besar district. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 12 September 2019; Volume 523, p. 12010. [Google Scholar]
- Nuraskin, C.; Idroes, R.; Soraya, C. Djufri Identification of secondary metabolite of laban leaf extract (Vitex pinnata l) from geothermal areas and non-geothermal of agam mountains in Aceh Besar, Aceh province, Indonesia. Rasayan J. Chem. 2020, 13, 18–23. [Google Scholar]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Santos, A.F.; Arim, A.L.; Lopes, D.V.; Gando-Ferreira, L.M.; Quina, M.J. Recovery of phosphate from aqueous solutions using calcined eggshell as an eco-friendly adsorbent. J. Environ. Manag. 2019, 238, 451–459. [Google Scholar] [CrossRef]
- Yaakob, Z.; Sukarman, I.S.B.; Narayanan, B.; Abdullah, S.R.S.; Ismail, M. Utilization of palm empty fruit bunch for the production of biodiesel from Jatropha curcas oil. Bioresour. Technol. 2012, 104, 695–700. [Google Scholar] [CrossRef]
- Liu, C.; Lv, P.; Yuan, Z.; Yan, F.; Luo, W. The nanometer magnetic solid base catalyst for production of biodiesel. Renew. Energy 2010, 35, 1531–1536. [Google Scholar] [CrossRef]
- Kesica, Z.; Lukic, I.; Zdujic, M.; Liu, H.; Skala, D. Mechanochemically Synthesized CaO ZnO Catalyst For Biodiesel Production. Procedia Eng. 2012, 42, 1169–1178. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Zhang, Y.; Li, S.; Tian, S.; Wang, B. Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process. Technol. 2012, 95, 84–89. [Google Scholar] [CrossRef]
- Zhang, P.; Han, Q.; Fan, M.; Jiang, P. Magnetic solid base catalyst CaO/CoFe2O4 for biodiesel production: Influence of basicity and wettability of the catalyst in catalytic performance. Appl. Surf. Sci. 2014, 317, 1125–1130. [Google Scholar] [CrossRef]
- Istadi, I.; Prasetyo, S.A.; Nugroho, T.S. Characterization of K2O/CaO-ZnO Catalyst for Transesterification of Soybean Oil to Biodiesel. Procedia Environ. Sci. 2015, 23, 394–399. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@CaO. Fuel 2016, 164, 314–321. [Google Scholar] [CrossRef]
- Hafiz, F.; Helwani, Z.; Saputra, E. Sintesis Katalis Basa Padat Nanomagnetik CaO/Serbuk Besi untuk Reaksi Transesterifikasi Minyak Sawit Off Grade menjadi Biodiesel. Ph.D. Thesis, Riau University, Riau, Indonesia, 2017. [Google Scholar]
- Niju, S.; Meera, K.M.; Begum, S.; Anantharaman, N. Modification of egg shell and its application in biodiesel production. J. Saudi Chem. Soc. 2014, 18, 702–706. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I.; Tee, H.S. Biodiesel production via transesterification of palm oil by using CaO–CeO2 mixed oxide catalysts. Fuel 2015, 162, 288–293. [Google Scholar] [CrossRef]
- Umdu, E. Methyl Ester Production From Vegetable Oils on Heterogeneous Basic Catalysts; İzmir Institute of Technology: Urla, Turkey, 2008. [Google Scholar]
- Mutreja, V.; Singh, S.; Ali, A. Potassium impregnated nanocrystalline mixed oxides of La and Mg as heterogeneous catalysts for transesterification. Renew. Energy 2014, 62, 226–233. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Watkins, R.S.; Lee, A.F.; Wilson, K. Li–CaO catalysed tri-glyceride transesterification for biodiesel applications. Green Chem. 2004, 6, 335–340. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S.; Tan, S.H. Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energy Convers. Manag. 2014, 88, 1167–1178. [Google Scholar] [CrossRef]
- Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Md Sani, N.D.; Safitri, E.; Yook Heng, L.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Nanocrystalline K–CaO for the transesterification of a variety of feedstocks: Structure, kinetics and catalytic properties. Biomass Bioenergy 2012, 46, 459–468. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Improving transesterification acitvity of CaO with hydration technique. Bioresour. Technol. 2010, 101, 3784–3786. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Development and characterisation of novel heterogeneous palm oil mill boiler ash-based catalysts for biodiesel production. Bioresour. Technol. 2012, 125, 158–164. [Google Scholar] [CrossRef]
- Earlia, N.; Rahmad, R.; Amin, M.; Prakoeswa, C.; Khairan, K.; Idroes, R. The potential effect of fatty acids from Pliek U on epidermal fatty acid binding protein: Chromatography and bioinformatic studies. Sains Malaysiana 2019, 48, 1019–1024. [Google Scholar] [CrossRef]
- Rahmad, R.; Earlia, N.; Nabila, C.; Inayati, I.; Amin, M.; Prakoeswa, C.R.S.; Khairan, K.; Idroes, R. Antibacterial cream formulation of ethanolic Pliek U extracts and ethanolic residue hexane Pliek U extracts against Staphylococcus aureus. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012011. [Google Scholar] [CrossRef]
- Earlia, N.; Suhendra, R.; Amin, M.; Prakoeswa, C.R.S.; Idroes, R. GC/MS Analysis of Fatty Acids on Pliek U Oil and Its Pharmacological Study by Molecular Docking to Filaggrin as Drug Candidate in Atopic Dermatitis Treatment. Sci. World J. 2019, 2019, 8605743. [Google Scholar] [CrossRef]
- Hasanah, U.; Md Sani, N.D.; Yook Heng, L.; Idroes, R.; Safitri, E. Construction of a Hydrogel Pectin-Based Triglyceride Optical Biosensor with Immobilized Lipase Enzymes. Biosensors 2019, 9, 135. [Google Scholar] [CrossRef]
- Iraj, K.; Mosivand, S. Phase Transition of Electrooxidized Fe3O4 to gamma and alpha-Fe2O3 Nanoparticles Using Sintering Treatment. Acta Phys. Pol. Ser. A 2014, 125, 1210–1214. [Google Scholar]
- Hindryawati, N.; Maniam, G.P.; Karim, M.R.; Chong, K.F. Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst. Eng. Sci. Technol. Int. J. 2014, 17, 95–103. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).