Preparation and Electrocatalysis Application of Pure Metallic Aerogel: A Review
Abstract
1. Introduction
2. Preparation Methods of Aerogels
2.1. Two-Step Process
2.2. One-Step Process
3. Drying Process
3.1. Freezing-Drying Method
3.2. Supercritical Drying Method
4. Preparation of Alloy Aerogel
5. Preparation of Aerogel with Special Structures
5.1. Core–Shell Structure
5.2. Hollow Structure
5.3. Other Structures
6. Application of Aerogels in Electrocatalysis
6.1. Cathodic Reduction Reaction
6.1.1. Oxygen Reduction Reaction (ORR)
6.1.2. H2O2 Reduction Reaction (H2O2RR)
6.1.3. CO2 Reduction Reaction (CO2RR)
6.2. Anodic Oxidation Reaction
6.2.1. Methanol/Ethanol Oxidation Reaction (MOR/EOR)
6.2.2. Formate Oxidation Reactions (FOR)
7. Mechanism
7.1. Oriented Attachment Mechanism
7.2. Movement Mechanism
8. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Graham, T. XXXV.—On the properties of silicic acid and other analogous colloidal substances. J. Chem. Soc. 1864, 17, 318–327. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nat. Cell Biol. 1931, 127, 741. [Google Scholar] [CrossRef]
- Alwin, S.; Shajan, X.S. Aerogels: Promising nanostructured materials for energy conversion and storage applications. Mater. Renew. Sustain. Energy 2020, 9, 1–27. [Google Scholar] [CrossRef]
- Teichner, S.; Nicolaon, G.; Vicarini, M.; Gardes, G. Inorganic oxide aerogels. Adv. Colloid Interface Sci. 1976, 5, 245–273. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; White, R.J.; Titirici, M.-M.; Antonietti, M. Borax-Mediated Formation of Carbon Aerogels from Glucose. Adv. Funct. Mater. 2012, 22, 3254–3260. [Google Scholar] [CrossRef]
- Mohanan, J.L.; Brock, S.L. A new addition to the aerogel community: Unsupported CdS aerogels with tunable optical properties. J. Non-Cryst. Solids 2004, 350, 1–8. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chou, J.-C. Preparation and characterization of the titanium dioxide thin films used for pH electrode and procaine drug sensor by sol–gel method. Mater. Chem. Phys. 2009, 114, 542–548. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; Del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering Ordered and Nonordered Porous Noble Metal Nanostructures: Synthesis, Assembly, and Their Applications in Electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef]
- Bigall, N.C.; Herrmann, A.; Vogel, M.; Rose, M.; Simon, P.; Carrillo-Cabrera, W.; Dorfs, D.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Hydrogels and Aerogels from Noble Metal Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 9731–9734. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.; Geiger, D.; Borchardt, L.; Simon, F.; Kaskel, S.; Gaponik, N.; Eychmüller, A. High-Performance Electrocatalysis on Palladium Aerogels. Angew. Chem. Int. Ed. 2012, 51, 5743–5747. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Nie, X.; Yang, W.; Wang, Y.; Yu, R.; Shui, J. Multifunctional Organic–Inorganic Hybrid Aerogel for Self-Cleaning, Heat-Insulating, and Highly Efficient Microwave Absorbing Material. Adv. Funct. Mater. 2019, 29, 1807624. [Google Scholar] [CrossRef]
- Wong, J.C.; Kaymak, H.; Tingaut, P.; Brunner, S.; Koebel, M.M. Mechanical and thermal properties of nanofibrillated cellulose reinforced silica aerogel composites. Microporous Mesoporous Mater. 2015, 217, 150–158. [Google Scholar] [CrossRef]
- Tang, Y.; Yeo, K.L.; Chen, Y.; Yap, L.W.; Xiong, W.; Cheng, W. Ultralow-density copper nanowire aerogel monoliths with tunable mechanical and electrical properties. J. Mater. Chem. A 2013, 1, 6723–6726. [Google Scholar] [CrossRef]
- Du, R.; Fan, X.; Jin, X.; Hübner, R.; Hu, Y.; Eychmüller, A. Emerging Noble Metal Aerogels: State of the Art and a Look Forward. Matter 2019, 1, 39–56. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.-K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels—Synthesis, Characterization, and Application as Electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef]
- Wittstock, A.; Wichmann, A.; Bäumer, M. Nanoporous Gold as a Platform for a Building Block Catalyst. ACS Catal. 2012, 2, 2199–2215. [Google Scholar] [CrossRef]
- Asao, N.; Ishikawa, Y.; Hatakeyama, N.; Menggenbateer; Yamamoto, Y.; Chen, M.; Zhang, W.; Inoue, A. Nanostructured Materials as Catalysts: Nanoporous-Gold-Catalyzed Oxidation of Organosilanes with Water. Angew. Chem. Int. Ed. 2010, 49, 10093–10095. [Google Scholar] [CrossRef]
- Tappan, B.C.; Steiner, S.A.; Luther, E.P. Nanoporous Metal Foams. Angew. Chem. Int. Ed. 2010, 49, 4544–4565. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J. Mater. Res. 2017, 32, 4153–4165. [Google Scholar] [CrossRef]
- Cai, B.; Sayevich, V.; Gaponik, N.; Eychmüller, A. Emerging Hierarchical Aerogels: Self-Assembly of Metal and Semiconductor Nanocrystals. Adv. Mater. 2018, 30, e1707518. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zhang, P.; XiaHou, Y.; Song, Y.; Bi, C.; Zhan, J.; Du, W.; Huang, L.; Möhwald, H.; Xia, H. Regulating Surface Facets of Metallic Aerogel Electrocatalysts by Size-Dependent Localized Ostwald Ripening. ACS Appl. Mater. Interfaces 2018, 10, 23081–23093. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Freytag, A.; Deutsch, J.; Wendt, N.; Behrens, P.; Köckritz, A.; Bigall, N.C. Porous Aerogels from Shape-Controlled Metal Nanoparticles Directly from Nonpolar Colloidal Solution. Chem. Mater. 2017, 29, 9208–9217. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, F.; Wang, J.; Guo, L.; Jin, T.; Liu, H. Lamellar platinum–rhodium aerogels with superior electrocatalytic performance for both hydrogen oxidation and evolution reaction in alkaline environment. J. Power Sources 2019, 435. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, J.Z.; Chang, S.L.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241. [Google Scholar] [CrossRef]
- Herrmann, A.-K.; Formanek, P.; Borchardt, L.; Klose, M.; Giebeler, L.; Eckert, J.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Multimetallic Aerogels by Template-Free Self-Assembly of Au, Ag, Pt, and Pd Nanoparticles. Chem. Mater. 2013, 26, 1074–1083. [Google Scholar] [CrossRef]
- Du, R.; Jin, W.; Hübner, R.; Zhou, L.; Hu, Y.; Eychmüller, A. Engineering Multimetallic Aerogels for pH-Universal HER and ORR Electrocatalysis. Adv. Energy Mater. 2020, 10, 1903857. [Google Scholar] [CrossRef]
- Liu, W.; Rodriguez, P.; Borchardt, L.; Foelske, A.; Yuan, J.; Herrmann, A.-K.; Geiger, D.; Zheng, Z.; Kaskel, S.; Gaponik, N.; et al. Bimetallic Aerogels: High-Performance Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 9849–9852. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Luo, X.; Jiao, L.; Wei, X.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Ternary PtRuCu aerogels for enhanced methanol electrooxidation. Nanoscale 2019, 11, 10575–10580. [Google Scholar] [CrossRef] [PubMed]
- Prabhudev, S.; Bugnet, M.; Zhu, G.-Z.; Bock, C.; Botton, G.A. Surface Segregation of Fe in Pt-Fe Alloy Nanoparticles: Its Precedence and Effect on the Ordered-Phase Evolution during Thermal Annealing. ChemCatChem 2015, 7, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Tymoczko, A.; Kamp, M.; Prymak, O.; Rehbock, C.; Jakobi, J.; Schürmann, U.; Kienle, L.; Barcikowski, S. How the crystal structure and phase segregation of Au–Fe alloy nanoparticles are ruled by the molar fraction and size. Nanoscale 2018, 10, 16434–16437. [Google Scholar] [CrossRef] [PubMed]
- Kühn, L.; Herrmann, A.-K.; Rutkowski, B.; Oezaslan, M.; Nachtegaal, M.; Klose, M.; Giebeler, L.; Gaponik, N.; Eckert, J.; Schmidt, T.J.; et al. Alloying Behavior of Self-Assembled Noble Metal Nanoparticles. Chem.-Eur. J. 2016, 22, 13446–13450. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Du, D.; Su, D.; Engelhard, M.H.; Yan, X. Core–shell PdPb@Pd aerogels with multiply-twinned intermetallic nanostructures: Facile synthesis with accelerated gelation kinetics and their enhanced electrocatalytic properties. J. Mater. Chem. A 2018, 6, 7517–7521. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Li, Y.; Xia, H.; Engelhard, M.H.; Fu, S.; Du, D.; Lin, Y. A Facile Method for Synthesizing Dendritic Core–Shell Structured Ternary Metallic Aerogels and Their Enhanced Electrochemical Performances. Chem. Mater. 2016, 28, 7928–7934. [Google Scholar] [CrossRef]
- Cai, B.; Hübner, R.; Sasaki, K.; Zhang, Y.; Su, D.; Ziegler, C.; Vukmirovic, M.B.; Rellinghaus, B.; Adzic, R.R.; Eychmüller, A. Core-Shell Structuring of Pure Metallic Aerogels towards Highly Efficient Platinum Utilization for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2018, 57, 2963–2966. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Tian, M.; Su, D.; Fu, M.; Engelhard, M.H.; Chowdhury, I.; Feng, S.; Du, D.; Lin, Y. Ultrafine Pd ensembles anchored-Au2Cu aerogels boost ethanol electrooxidation. Nano Energy 2018, 53, 206–212. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, W.; Zhong, H.; Zhu, C.; Tian, H.; Li, J.; Xu, M.; Su, D.; Li, X.; Liu, D.; et al. Highly Dispersed Platinum Atoms on the Surface of AuCu Metallic Aerogels for Enabling H2O2 Production. ACS Appl. Energy Mater. 2019, 2, 7722–7727. [Google Scholar] [CrossRef]
- Cai, B.; Wen, D.; Liu, W.; Herrmann, A.-K.; Benad, A.; Eychmüller, A. Function-Led Design of Aerogels: Self-Assembly of Alloyed PdNi Hollow Nanospheres for Efficient Electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 13101–13105. [Google Scholar] [CrossRef]
- Liu, W.; Haubold, D.; Rutkowski, B.; Oschatz, M.; Hübner, R.; Werheid, M.; Ziegler, C.; Sonntag, L.; Liu, S.; Zheng, Z.; et al. Self-Supporting Hierarchical Porous PtAg Alloy Nanotubular Aerogels as Highly Active and Durable Electrocatalysts. Chem. Mater. 2016, 28, 6477–6483. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Zhong, H.; Su, D.; Li, N.; Engelhard, M.H.; Xia, H.; Zhang, Q.; Feng, S.; Beckman, S.P.; et al. Nanovoid Incorporated IrxCu Metallic Aerogels for Oxygen Evolution Reaction Catalysis. ACS Energy Lett. 2018, 3, 2038–2044. [Google Scholar] [CrossRef]
- Ranmohotti, K.G.S.; Gao, X.; Arachchige, I.U. Salt-Mediated Self-Assembly of Metal Nanoshells into Monolithic Aerogel Frameworks. Chem. Mater. 2013, 25, 3528–3534. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Hammond, C.; Brett, G.L.; Kesavan, L.; White, S.; Miedziak, P.; Tiruvalam, R.; Jenkins, R.L.; Carley, A.F.; et al. Facile removal of stabilizer-ligands from supported gold nanoparticles. Nat. Chem. 2011, 3, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Nguyen, T.M.T.; Le, H.V.; Nguyen, D.N.; Truong, Q.D.; Tran, P.D. Gold nanoparticles as an outstanding catalyst for the hydrogen evolution reaction. Chem. Commun. 2018, 54, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-R.; Xu, B. Surprisingly strong effect of stabilizer on the properties of Au nanoparticles and Pt^Au nanostructures in electrocatalysis. Nanoscale 2010, 2, 2798–2804. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-R.; Bai, J.; Jiang, J.-X.; Lee, J.; Chen, Y. Polyethyleneimine functionalized platinum superstructures: Enhancing hydrogen evolution performance by morphological and interfacial control. Chem. Sci. 2017, 8, 8411–8418. [Google Scholar] [CrossRef]
- Shan, J.; Zheng, Y.; Shi, B.; Davey, K.; Qiao, S. Regulating Electrocatalysts via Surface and Interface Engineering for Acidic Water Electrooxidation. ACS Energy Lett. 2019, 4, 2719–2730. [Google Scholar] [CrossRef]
- Fan, X.; Zerebecki, S.; Du, R.; Hübner, R.; Marzum, G.; Jiang, G.; Hu, Y.; Barcikowki, S.; Reichenberger, S.; Eychmüller, A. Promoting the Electrocatalytic Performance of Noble Metal Aerogels by Ligand-Directed Modulation. Angew. Chem. Int. Ed. 2020, 59, 5706–5711. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Duan, W.; Xing, L.; XiaHou, Y.; Du, W.; Xia, H. Fabrication of Au aerogels with {110}-rich facets by size-dependent surface reconstruction for enzyme-free glucose detection. J. Mater. Chem. B 2019, 7, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Brown, E.; Su, Q.; Li, J.; Wang, J.; Xu, C.; Zhou, C.; Lin, D. 3D Printing Hierarchical Silver Nanowire Aerogel with Highly Compressive Resilience and Tensile Elongation through Tunable Poisson’s Ratio. Small 2017, 13, 1701756. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, F.; Jin, Y.; Johnston, R.L. Gold-Copper Aerogels with Intriguing Surface Electronic Modulation as Highly Active and Stable Electrocatalysts for Oxygen Reduction and Borohydride Oxidation. ChemSusChem 2018, 11, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-H.; Yu, S.-H. Engineering Interface and Surface of Noble Metal Nanoparticle Nanotubes toward Enhanced Catalytic Activity for Fuel Cell Applications. Acc. Chem. Res. 2013, 46, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, F.; Jin, Y.; Guo, L.; Gong, X.; Wang, X.; Johnston, R.L. In situ high-potential-driven surface restructuring of ternary AgPd–Ptdilute aerogels with record-high performance improvement for formate oxidation electrocatalysis. Nanoscale 2019, 11, 14174–14185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Wang, J.; Yang, S.; Liu, T.; Tao, K.; Chang, H. A silver wire aerogel promotes hydrogen peroxide reduction for fuel cells and electrochemical sensors. J. Mater. Chem. A 2019, 7, 11497–11505. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly Efficient Electroreduction of CO2 to Methanol on Palladium-Copper Bimetallic Aerogels. Angew. Chem. Int. Ed. 2018, 57, 14149–14153. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, Y.; Huang, F.; Zhang, H.; Tao, K.; Zhang, R.; Shen, Q.; Chang, H. A Facile Microfluidic Hydrogen Peroxide Fuel Cell with High Performance: Electrode Interface and Power-Generation Properties. ACS Appl. Energy Mater. 2018, 1, 5328–5335. [Google Scholar] [CrossRef]
- Cui, M.; Qian, Q.; He, Z.; Ma, J.; Kang, X.; Hu, J.; Liu, Z.; Han, B. Synthesizing Ag Nanoparticles of Small Size on a Hierarchical Porosity Support for the Carboxylative Cyclization of Propargyl Alcohols with CO2under Ambient Conditions. Chem. A Eur. J. 2015, 21, 15924–15928. [Google Scholar] [CrossRef]
- Lee, J.-S.; Anselmi-Tamburini, U.; Munir, Z.A.; Kim, S. Direct Evidence of Electron Accumulation in the Grain Boundary of Yttria-Doped Nanocrystalline Zirconia Ceramics. Electrochem. Solid-State Lett. 2006, 9, J34. [Google Scholar] [CrossRef]
- Zhou, Y.; Sarwat, S.G.; Jung, G.S.; Buehler, M.J.; Bhaskaran, H.; Warner, J.H. Grain Boundaries as Electrical Conduction Channels in Polycrystalline Monolayer WS2. ACS Appl. Mater. Interfaces 2019, 11, 10189–10197. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, L.; Zhao, Q.; Cheng, D.; Deng, W.; Liu, B.; Zhang, G.; Dong, H.; Yuan, X.; Zhao, Z.; et al. Concentrating and activating carbon dioxide over AuCu aerogel grain boundaries. J. Chem. Phys. 2020, 152, 204703. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A. Anode Catalysts and Cathode Catalysts of Direct Methanol Fuel Cells. Prog. Chem. 2015, 27, 1147–1157. [Google Scholar]
- Yin, P.; Zhou, M.; Chen, J.; Tan, C.; Liu, G.; Ma, Q.; Yun, Q.; Zhang, X.; Cheng, H.; Lu, Q.; et al. Synthesis of Palladium-Based Crystalline@Amorphous Core–Shell Nanoplates for Highly Efficient Ethanol Oxidation. Adv. Mater. 2020, 32, e2000482. [Google Scholar] [CrossRef] [PubMed]
- El Sawy, E.N.; Brueckner, T.M.; Pickup, P.G. Electrochemical Oxidation of Methanol and Ethanol at Rh@Pt and Ru@Pt Catalysts. J. Electrochem. Soc. 2020, 167, 106507. [Google Scholar] [CrossRef]
- Song, P.; Cui, X.; Shao, Q.; Feng, Y.; Zhu, X.; Huang, X. Networked Pt–Sn nanowires as efficient catalysts for alcohol electrooxidation. J. Mater. Chem. A 2017, 5, 24626–24630. [Google Scholar] [CrossRef]
- Dubale, A.A.; Zheng, Y.; Wang, H.; Hübner, R.; Li, Y.; Yang, J.; Zhang, J.; Sethi, N.K.; He, L.; Zheng, Z.; et al. High-Performance Bismuth-Doped Nickel Aerogel Electrocatalyst for the Methanol Oxidation Reaction. Angew. Chem. Int. Ed. 2020, 59, 13891–13899. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, F.; Guo, L.; Jin, T.; Liu, H.; Wang, X.; Gong, X.; Liu, Y. Nanoalloying effects on the catalytic activity of the formate oxidation reaction over AgPd and AgCuPd aerogels. J. Mater. Chem. A 2019, 7, 16122–16135. [Google Scholar] [CrossRef]
- Li, H.-H.; Fu, Q.-Q.; Xu, L.; Ma, S.-Y.; Zheng, Y.-R.; Liu, X.-J.; Yu, S.-H. Highly crystalline PtCu nanotubes with three dimensional molecular accessible and restructured surface for efficient catalysis. Energy Environ. Sci. 2017, 10, 1751–1756. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; Van Der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef]
- Shin, H.J.; Ryoo, R.; Liu, Z.; Terasaki, O. Template Synthesis of Asymmetrically Mesostructured Platinum Networks. J. Am. Chem. Soc. 2001, 123, 1246–1247. [Google Scholar] [CrossRef]
- Wang, D.; Kou, R.; Gil, M.P.; Jakobson, H.P.; Tang, J.; Yu, D.; Lu, Y. Templated Synthesis, Characterization, and Sensing Application of Macroscopic Platinum Nanowire Network Electrodes. J. Nanosci. Nanotechnol. 2005, 5, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, H.; Kou, R.; Gil, M.P.; Xiao, S.; Golub, V.; Yang, Z.; Brinker, C.J.; Lu, Y. A General Route to Macroscopic Hierarchical 3D Nanowire Networks. Angew. Chem. Int. Ed. 2004, 43, 6169–6173. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Garcia, R.M.; Dorin, R.M.; Wang, H.; Qiu, Y.; Coker, E.N.; Steen, W.A.; Miller, A.J.E.; Shelnutt, J.A. Synthesis of Platinum Nanowire Networks Using a Soft Template. Nano Lett. 2007, 7, 3650–3655. [Google Scholar] [CrossRef] [PubMed]
- Ramanath, G.; D’Arcy-Gall, J.; Maddanimath, T.; Ellis, A.V.; Ganesan, P.G.; Goswami, R.; Kumar, A.; Vijayamohanan, K. Templateless Room-Temperature Assembly of Nanowire Networks from Nanoparticles. Langmuir 2004, 20, 5583–5587. [Google Scholar] [CrossRef] [PubMed]
- Maddanimath, T.; Kumar, A.; D’Arcy-Gall, J.; Ganesan, P.G.; Vijayamohanan, K.; Ramanath, G. Wet-chemical templateless assembly of metal nanowires from nanoparticles. Chem. Commun. 2005, 11, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wan, L.; Jiang, Q.; Zhang, H.; Tang, X.; Qin, X.; Shao, Z.; Wei, Z.-D. Wavy PtCu alloy nanowire networks with abundant surface defects enhanced oxygen reduction reaction. Nano Res. 2019, 12, 2766–2773. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, Y.; Wang, S.; Zhou, G.; Zhao, N.; Wong, C.-P. A highly stretchable and conductive composite based on an emulsion-templated silver nanowire aerogel. J. Mater. Chem. A 2020, 8, 1724–1730. [Google Scholar] [CrossRef]
- Yang, D.; Yan, Z.; Li, B.; Higgins, D.C.; Wang, J.; Lv, H.; Chen, Z.; Zhang, C. Highly active and durable Pt–Co nanowire networks catalyst for the oxygen reduction reaction in PEMFCs. Int. J. Hydrog. Energy 2016, 41, 18592–18601. [Google Scholar] [CrossRef]
- Feng, Y.; Bu, L.; Guo, S.; Guo, J.; Huang, X. 3D Platinum-Lead Nanowire Networks as Highly Efficient Ethylene Glycol Oxidation Electrocatalysts. Small 2016, 12, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, L.-X.; Wang, A.-J.; Yuan, J.; Shen, L.; Feng, J.-J. Hydrogen bubbles template-directed synthesis of self-supported AuPt nanowire networks for improved ethanol oxidation and oxygen reduction reactions. Int. J. Hydrog. Energy 2016, 41, 8871–8880. [Google Scholar] [CrossRef]
- Ying, J.; Jiang, G.; Cano, Z.P.; Ma, Z.; Chen, Z. Spontaneous weaving: 3D porous PtCu networks with ultrathin jagged nanowires for highly efficient oxygen reduction reaction. Appl. Catal. B Environ. 2018, 236, 359–367. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, W.; Bellabarba, R.; Tooze, R. One-step synthesis and shape-control of CuPd nanowire networks. Nanoscale 2014, 6, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Guo, Y.; Wang, E. Facile fabrication of PdRuPt nanowire networks with tunable compositions as efficient methanol electrooxidation catalysts. Nano Res. 2018, 11, 4348–4355. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D. Controlling the Growth of Charged-Nanoparticle Chains through Interparticle Electrostatic Repulsion. Angew. Chem. Int. Ed. 2008, 47, 3984–3987. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Hu, Y.; Hübner, R.; Joswig, J.-O.; Fan, X.; Schneider, K.; Eychmüller, A. Specific ion effects directed noble metal aerogels: Versatile manipulation for electrocatalysis and beyond. Sci. Adv. 2019, 5, eaaw4590. [Google Scholar] [CrossRef]
- Du, R.; Wang, J.; Wang, Y.; Hübner, R.; Fan, X.; Senkovska, I.; Hu, Y.; Kaskel, S.; Eychmüller, A. Unveiling reductant chemistry in fabricating noble metal aerogels for superior oxygen evolution and ethanol oxidation. Nat. Commun. 2020, 11, 1590. [Google Scholar] [CrossRef]
- Matter, F.; Luna, A.L.; Niederberger, M. From colloidal dispersions to aerogels: How to master nanoparticle gelation. Nano Today 2020, 30, 100827. [Google Scholar] [CrossRef]
- Fan, X.; Cai, B.; Du, R.; Hübner, R.; Georgi, M.; Jiang, G.; Li, L.; Khoshkhoo, M.S.; Sun, H.; Eychmüller, A. Ligand-Exchange-Mediated Fabrication of Gold Aerogels Containing Different Au(I) Content with Peroxidase-like Behavior. Chem. Mater. 2019, 31, 10094–10099. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Sherman, Z.M.; Swan, J.W.; Doyle, P.S. Colloidal Gelation through Thermally Triggered Surfactant Displacement. Langmuir 2019, 35, 9464–9473. [Google Scholar] [CrossRef]
- Gao, X.; Esteves, R.J.; Luong, T.T.H.; Jaini, R.; Arachchige, I.U. Oxidation-Induced Self-Assembly of Ag Nanoshells into Transparent and Opaque Ag Hydrogels and Aerogels. J. Am. Chem. Soc. 2014, 136, 7993–8002. [Google Scholar] [CrossRef]
- Han, X.; Goebl, J.; Lu, Z.; Yin, Y. Role of Salt in the Spontaneous Assembly of Charged Gold Nanoparticles in Ethanol. Langmuir 2011, 27, 5282–5289. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Jin, X.; Hübner, R.; Fan, X.; Hu, Y.; Eychmüller, A. Engineering Self-Supported Noble Metal Foams Toward Electrocatalysis and Beyond. Adv. Energy Mater. 2019, 10, 1901945. [Google Scholar] [CrossRef]

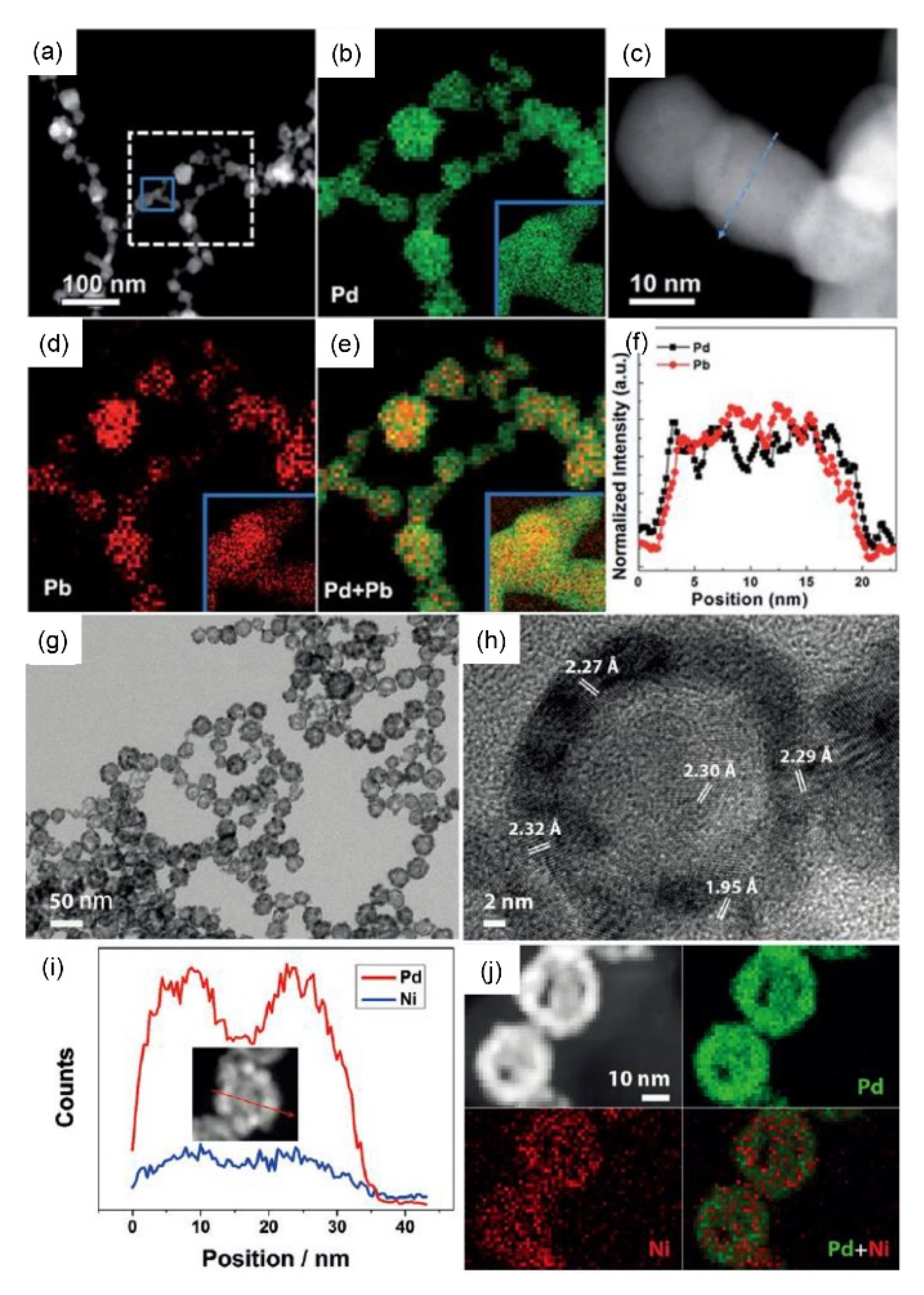
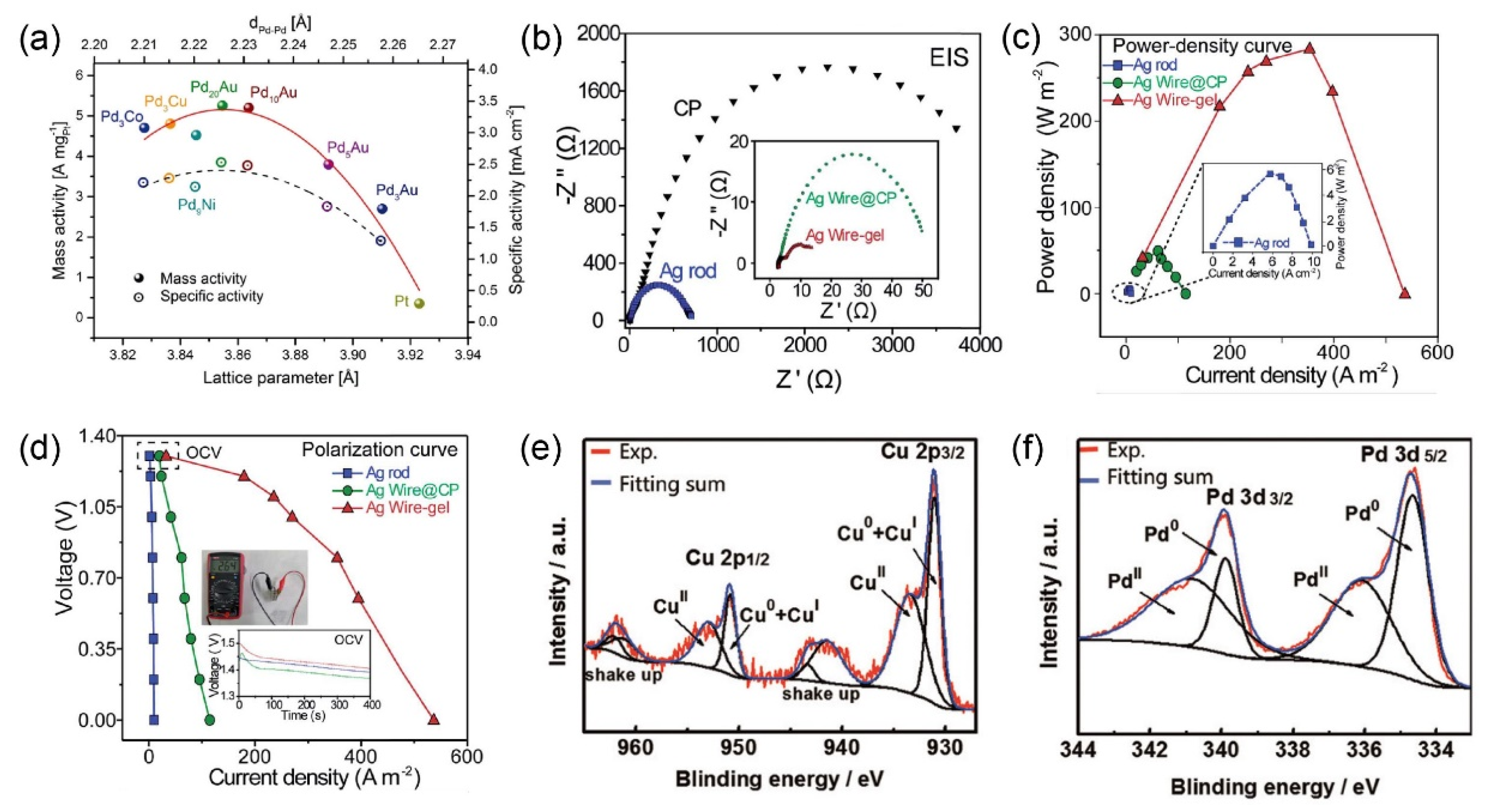
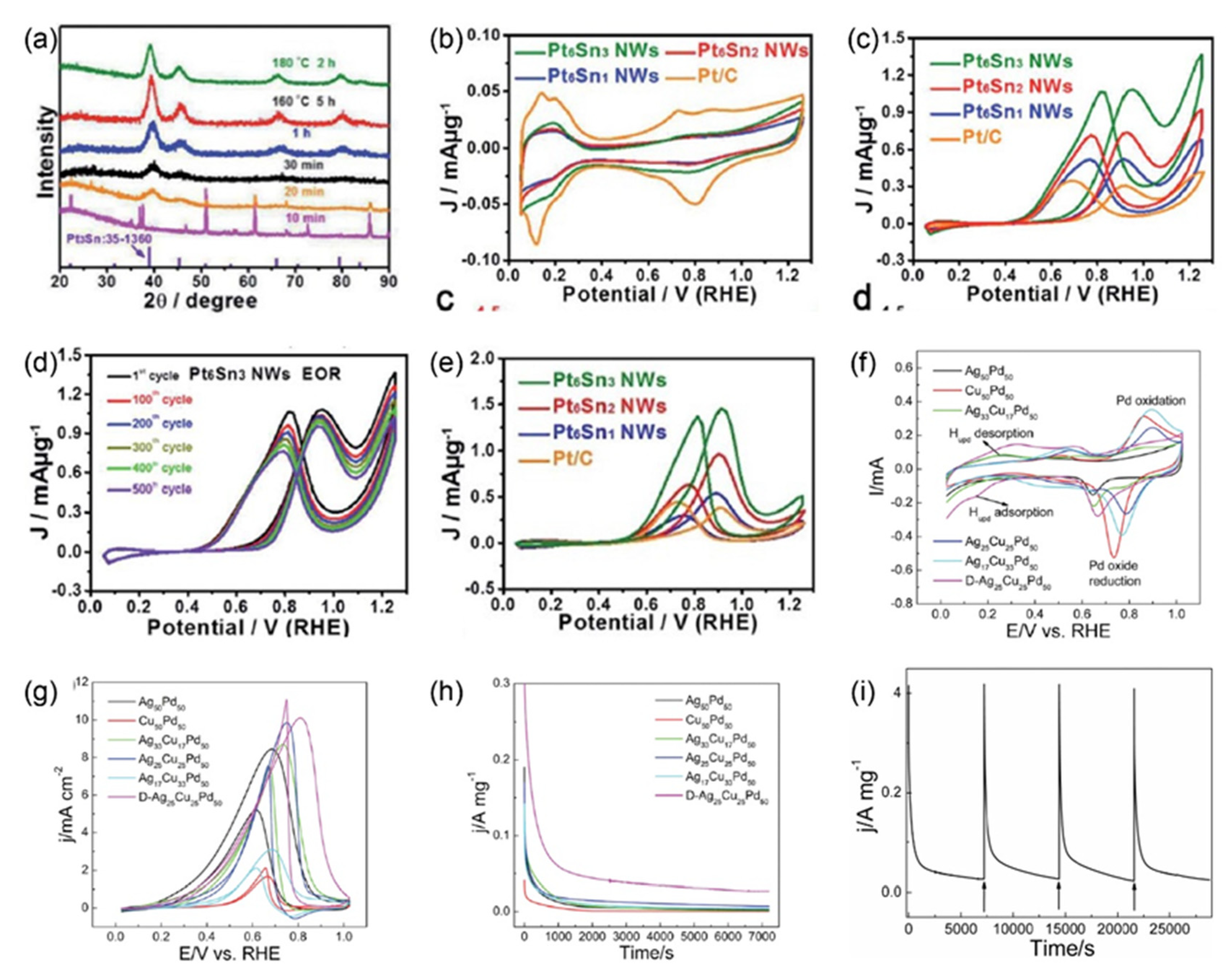
| Materials | Electrolytes | Mass Activities | Specific Activities | ECSAs | Reactions | Ref |
|---|---|---|---|---|---|---|
| Au@Pt3Pd aerogel | 0.1 M HClO4 | 0.812 A mgPt+Pd−1 | 24.3 mA/cm2 | 58.7 m2/gPt+Pd | ORR | 36 |
| PdAu@Pt aerogel | 0.1 M HClO4 | 0.75 A mgnoblemetal−1 | 2.53 mA/cm2 | 192.3 m2/gPt | ORR | 37 |
| Au2Cu@Pd aerogel | 1.0 M KOH + 1.0 M ethanol | 22.1 A mgPd−1 | 89.2 m2/gPd | EOR | 38 | |
| PdNi hollow aerogels | 1.0 M NaOH +1.0 M ethanol | 3.63 A mgPd−1 | 6.54 mA/cm2 | 45.2 m2/gPd | EOR | 41 |
| PVP-modified Au-Pd aerogel | 1.0 M KOH + 1.0 M ethanol | 6.75 A mgPd−1 | 47.7 m2/g | EOR | 49 | |
| PtAg hollow nanotubular aerogel | 0.1 M HClO4 + 0.5 M HCOOH | 237 mA mgmetal−1 | 19.4 m2/gmetal | FOR | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhao, Y. Preparation and Electrocatalysis Application of Pure Metallic Aerogel: A Review. Catalysts 2020, 10, 1376. https://doi.org/10.3390/catal10121376
Zhang R, Zhao Y. Preparation and Electrocatalysis Application of Pure Metallic Aerogel: A Review. Catalysts. 2020; 10(12):1376. https://doi.org/10.3390/catal10121376
Chicago/Turabian StyleZhang, Ran, and Yan Zhao. 2020. "Preparation and Electrocatalysis Application of Pure Metallic Aerogel: A Review" Catalysts 10, no. 12: 1376. https://doi.org/10.3390/catal10121376
APA StyleZhang, R., & Zhao, Y. (2020). Preparation and Electrocatalysis Application of Pure Metallic Aerogel: A Review. Catalysts, 10(12), 1376. https://doi.org/10.3390/catal10121376




