Abstract
The utilization of charcoal from woody biomass is an efficient way to reduce CO2 emissions from the metallurgical industry. The main aim of this work is to study the charcoal production process from torrefied biomass. For this purpose, torrefaction (3 °C min−1, 250 °C, 30 min) and carbonization (3 °C min−1, 750 °C, 30 min) experiments of eucalyptus wood were carried out in a 3.5 L tank reactor. In the carbonization experiments, a thermo-catalytic treatment of the vaporized phase was also performed, with the objective of producing less condensates and H2-rich gases. The results show that the torrefaction pre-treatment does not affect the chemical properties of charcoal but significantly improves the performance of the carbonization process, where more than 50 wt% of charcoal is obtained. In addition, the thermal and thermo-catalytic treatment of the vaporized phase during the carbonization of torrefied biomass yields better results than in the case of fresh biomass. When torrefied biomass is used as raw material and the reforming catalyst is employed to treat the vapors and gases, a proportion of 71 vol% of H2 in the gases is achieved, together with very low quantities of condensates (8.0 wt%). This allows designing a carbonization process in which, in addition to charcoal, pure H2 can also be produced.
1. Introduction
The metallurgical industry is a very energy-demanding sector and generates approximately one-third of the industrial CO2 emissions into the atmosphere. As a result, different strategies have been implemented in last few years in order to reduce the energy and environmental impact [1]. Among them, the production of charcoal from wood is one of the most promising options for a few reasons. On the one hand, it can be carried out using lignocellulosic waste generated in agricultural and industrial activities or by using wood coming from energy crops [2,3]. On the other hand, carbonization is a simple process from a technological point of view. It consists in the slow heating of the material in the absence of oxygen to temperatures >700 °C; in other words, a slow pyrolysis process focused on maximizing the solid yield. It must be mentioned that wood charcoal cannot replace fossil coal in all applications, much less replace coke. However, it can be used in specific applications, e.g., metallurgical rotary kilns, or blended with coal/coke in certain percentages. This already means important savings in terms of CO2 emissions due to the climate-neutral nature of wood charcoal [2,4,5]. For these reasons, several charcoal production facilities can be found worldwide at an industrial or semi-industrial scale [3,6,7]. However, one problem is not yet solved concerning charcoal production, which in turn prevents the development of this technology in countries with restrictive environmental legislation: The emission of hazardous gases into the atmosphere [8] and/or the generation, as a by-product, of tars and other condensates. Tar and condensates are composed of many organic substances, e.g., linear and cyclic hydrocarbons, phenol, and its derivatives, mono and polyaromatic hydrocarbons (PAH). Therefore, they hardly present any industrial application and eventually constitute a hazardous waste in the process [9,10].
The elimination or minimization of tars and other condensed products is one of the most critical and studied aspects in biomass gasification [11,12,13,14]. However, this issue is not reported so often in carbonization processes. In two studies recently published by Rodrigues and Braghini, they analyzed the technology of 172 patents on charcoal production kilns. They found that only four of those patents included gas purification or treatment systems [15,16]. For this reason, the authors of this work have dedicated several years to study the removal of tars and the minimization of condensed products in biomass carbonization by using thermo-catalytic treatments in vapor phase contact [17,18,19]. The objective is to valorize the vaporized fraction in the form of hydrogen-rich gases, which is a way to obtain another product of wide industrial utilization [18,19]. Concerning hydrogen production, the main reactions expected to occur in the thermo-catalytic treatment of gases and vapors coming from biomass carbonization are the steam and dry reforming of hydrocarbons or “bio-oil like” oxygenated compounds (Equations (1) and (2)) and water-gas shift reaction (Equation (3)). On the other hand, some secondary reactions such as coking through Boudouard reaction (Equation (4)) or water–gas reaction (Equation (5)) and methanation (Equation (6)) are also likely to occur:
CnHmOk + (n − k)H2O ↔ nCO + n + ((m/2) − k)H2
CnHm + nCO2 ↔ 2nCO + m/2H2
CO + H2O ↔ CO2 + H2
2CO ↔ CO2 + C
CO + H2 ↔ C + H2O
CO2 + 4H2 ↔ CH4 + 2H2O
Taking into account that carbonization is a cracking process in which many compounds are formed by random scission of the chemical bonds present in biomass, the chemical system that arrives to the catalytic bed is very complex. This limits the performance of the catalyst, even working at high temperatures, which is probably due to the poisoning and deactivation of the catalyst [20,21]. Consequently, it is difficult to obtain a gaseous fraction yielding more than 50% by volume of H2. This is the minimum concentration that allows separating H2 in a profitable way through pressure swing adsorption (PSA), which is the most used industrial process of H2 separation nowadays [22]. Thus, the present work aims to study a new approach, consisting of the pre-treatment of biomass by torrefaction before the carbonization process. Torrefaction is a low-temperature thermal treatment (200–300 °C) in which the moisture and the most volatile substances present in the biomass are volatilized. As a result, torrefied biomass is obtained, which is a solid with a calorific value higher than raw biomass (energetically denser) and also biologically more stable due to the absence of moisture [23,24,25]. Consequently, torrefaction is successfully applied as a pre-treatment of the biomass to be used as fuel, since, in addition to being a high calorific product, it can also be stored for long periods without biological deterioration [26,27]. The loss of water and volatile substances (acetic acid, phenol derivatives, furfural, etc.) during torrefaction can be beneficial for the later treatment of the vaporized products generated during carbonization. That is, previously torrefied biomass will release a lower quantity of vapors and gases during carbonization than fresh biomass. Consequently, the amount of this type of substance passing through the catalytic bed will be lower, which can be beneficial for the activity and performance of the catalyst. This statement is the hypothesis of this work, which will compare the products obtained in the carbonization of torrefied biomass with those obtained in the carbonization of fresh biomass, with special emphasis on the performance of a reforming catalyst in the removal of condensed products and the production of hydrogen. The authors are not aware of any research concerning the production of charcoal from torrefied biomass to date, and much less about the influence of this fact on the performance and activity of a catalyst used to upgrade the gases and vapors from carbonization. Therefore, the results of this research will be innovative and may represent a considerable advance in the industrialization of charcoal production.
2. Results and Discussion
2.1. Torrefaction
Table 1 shows the yields obtained in the torrefaction experiments, as well as the composition of generated gases and liquids. The table also presents the higher heating value (HHV) of the gases. As it can be seen, the main product of the torrefaction process of woody biomass is precisely the torrefied solid fraction, ranging 53.1 wt%. Secondly, a significant amount of liquid fraction is obtained (38.0 wt%), which is mainly composed of water and acetic acid. Finally, a gas fraction composed by CO2, CO, and small quantities of hydrocarbons is also obtained. As it can be seen, the torrefaction process leads to the loss of almost the half of the initial mass, which is consistent with other research works concerning the torrefaction of eucalyptus wood at the same or similar operating conditions [23]. Taking into account that the torrefaction process takes place at 200 °C, such a mass loss mainly corresponds to the evaporation of water and light extractives, and the thermal decomposition of hemicellulose, which starts right in the 150–200 °C range. Additionally, there is also some breakdown of cellulose and lignin, although their main decomposition takes place at higher temperatures [23,26,28]. The composition of the liquid and gases shown in Table 1 agrees with that obtained by other authors in the torrefaction of woody biomass at 250 °C [29,30,31]. On the one hand, water and acetic acid have been reported as the dominant condensable products of torrefaction, the former coming from the evaporation and dehydration of hydroxyl groups, the latter derived from acetoxy and methoxy groups mainly present in hemicellulose [26]. Due to the high content of acetic acid, torrefaction condensate has been reported to be a good co-feedstock for anaerobic digestion processes [32]. Apart from water and acetic acid, some other elements were detected at trace levels, mainly derivatives of dimethoxy-phenol and furfural. On the other hand, concerning gas composition, the predominant formation of CO and CO2 in the torrefaction of woody biomass is also a very well-known fact, and it is attributed to the decarbonylation and decarboxylation of hemicellulose and, to a smaller extent, cellulose [33].

Table 1.
Torrefaction yields and composition of gases and liquids.
2.2. Carbonization Yields and Gas Composition
Table 2 shows the yields obtained in the carbonization experiments, together with the composition of liquids, the composition of gases, and their higher heating value (HHV). The chemical properties of the obtained solid products will be discussed in the next section. As commented in the introduction, the authors normally produce charcoal with the simultaneous treatment of the vapors and gases that come from the carbonization process, with the aim of obtaining high-quality gases and minimizing as much as possible the production of tars and condensed liquids. In this sense, the results presented in the first column of Table 2 are those obtained in a carbonization process at 750 °C, where the gases and vapors are thermally treated at 900 °C in contact with a charcoal bed. As can be seen, the solid yield is 26 wt%, which matches with typical solid yields obtained in woody biomass carbonization processes at those temperatures [34]. In addition to the solid product, 31 wt% of condensed liquids and 43 wt% of gases are also obtained. On the one hand, it should be noted that there is no generation of tars in the process and that the condensed liquids are composed mainly of water, with small amounts of phenol. Some other compounds were also found at trace levels, mainly methyl-phenol isomers and pyridine in the experiments without catalyst and 3-methyl-phenol and 2,6-dimethoxy-phenol in the catalytic experiments. On the other hand, a great quantity of gases is obtained with a significant percentage of H2 and, in general, with an attractive composition to be used as fuel or for certain chemical syntheses, mainly dimethyl-ether (DME) [35]. The results obtained for the liquid and gas phases are better than the usual ones in carbonization processes. In a previous study on charcoal production from woody biomass carried out by the authors without gas and vapor treatment, 17 wt% of tars and a total of 45.4 wt% of condensed products were reported, leaving gas production at 33.4 wt% with an H2 content of 11.5 vol% [10]. This better performance of liquids and gases can be attributed to cracking reactions that are enhanced at 900 °C but also to the presence of the charcoal bed in the tubular reactor, since charcoal has been reported to act as a tar remover and acid gases adsorbent (including CO2) in the pyrolysis and gasification of biomass [36,37,38].

Table 2.
Carbonization yields, liquid composition, and gas composition.
The second column of Table 2 shows the results of the same carbonization process but in this case, apart from the charcoal bed, the reforming catalyst Katalco 57-4Q is used in the second reactor at the same temperature. The use of this reforming catalyst is intended to convert the organic substances that pass through the tubular reactor (phenol, methane, and other hydrocarbons) into H2, given the high amount of steam in the reaction media (see the water quantity of liquids in column 1). At the same time, dry reforming reactions with CO2 could also be promoted. Additionally, taking into account that the support of the employed catalyst contains both acid and base properties (see Table 3, a dual behavior related to this nature is somehow expected. On the one hand, the efficient catalytic activity related to acid catalysts (cracking, aromatization, deoxygenation [34]) and, on the other hand, that related to CaO (the elimination of sugar-like compounds [35]). It must be said that although acidity may be a negative property for classical reforming reactions, it is a desired property in the particular chemical environment of this application. In this system, cracking reactions are of great importance, as can be seen in the non-catalyzed tests. This is why reforming catalysts with an important acidic function are required.

Table 3.
Main properties of the catalyst used in the work (Katalco 57-4Q).
As expected, the presence of the catalyst does not affect the quantity of the solid obtained in the tank reactor, which is very similar to the previous experiment. However, a variation is observed in the yield of condensed liquids and gases. Specifically, a slight increase in the yield of gases to the detriment of the yield of liquids was obtained, which indicates that the catalyst is able to convert to some extent the liquid phase hydrocarbons into gaseous compounds. This catalyst showed similar performance, in yields, in a previous work carried out by the authors concerning the pyrolysis of woody biomass in an auger reactor [18]. With regard to the composition of the liquids and gases, it can be observed on the one hand, a decrease of the quantity of phenol in the condensed liquids, as well as that of methane and the rest of light hydrocarbons in the gas fraction. On the other hand, the amount of hydrogen produced in the gases is double than obtained in the experiment without a catalyst, and an increase of CO is also noticed. Therefore, it seems clear that the catalyst promotes the reforming reactions. Although it appears that more water has been produced in the liquids in the catalyzed experiment compared to the previous test (98.6 vs. 95.6 area%), it is worth mentioning that the total production of liquids is lower in the catalyzed one, so the total amount of water collected is lower, explaining the steam-reforming route. There is no evident decrease in CO2 between the two experiments, so, at first sight, it does not seem that dry reforming has been produced to a great extent. However, it must be said that the decomposition of phenol into CO2 could hide the consumption of CO2 in dry reforming reactions, so, definitely, it cannot be clarified whether the dry-reforming pathway has taken place in the process or not. In any case, with the use of the catalyst, it is possible to produce gases with more than 40% H2 in volume and more than 70% of H2 + CO with a CO:H2 ratio around 0.65. This makes the gas very interesting from the point of view of its industrial use, for example in the production of alcohols such as ethanol, where the maximum conversion of CO and maximum selectivity to ethanol occurs when CO:H2 ratios are in the 0.5–1 range [39].
In spite of these good results and in view of the amount of steam in the media, it seems that there is still more potential for reforming organic compounds (phenol, methane, other hydrocarbons) and that the catalyst is not capable of converting them into H2 and CO. It must be kept in mind that the scission of multiple C-C bonds takes place during carbonization, where highly reactive carbon radicals are formed, increasing the possibility of carbon formation [40]. Besides, Ni-based catalysts usually present high carbon deposition. The authors have previous experience improving the production of H2 and CO from biomass carbonization vapors by increasing the catalyst load and/or increasing the amount of Ni in the catalyst [18]. However, it is also necessary to take into account the cost that the use of large quantities of catalyst can have on the process, especially if they are expensive. For this reason, torrefaction is proposed in this manuscript as a pre-treatment of the biomass before the carbonization process to see if the catalyst performs better in contact with a lower quantity of gases and vapors.
The carbonization of the torrefied biomass is presented in columns 3 and 4 of Table 2. Column 3 corresponds to the experiment without a catalyst, while the catalyzed experiment is shown in column 4 (in the two experiments, the gases and vapors passed through the treatment reactor at 900 °C in the presence of the charcoal bed, as in the previous ones). In these experiments, it was not possible to analyze the liquids because no liquids were found in the condensers. The liquid yield in this case corresponds to liquids that condensed in pipelines and reactors. Consequently, their composition is missing in the table. Firstly, it can be seen that the charcoal yield is greater than 50 wt%, which is twice the yield obtained in the carbonization of fresh biomass. However, this should not be misleading, since it is the yield of a biomass that has already lost approximately half of its weight during torrefaction. In global terms, the same amount of charcoal is achieved. Nevertheless, it is important to note that the effect of the biomass pre-treatment is decisive in the production of condensed liquids, which drops to very low values (8.0 wt%), and at the same time, the gas yield is maintained in similar values to those obtained in the carbonization of fresh biomass (41.6 vs. 43.0 wt%). In this case, if the overall mass balances are taken into account, the total production of liquids in the torrefaction process (38.0 wt%, Table 1) and their subsequent carbonization (8.0 wt%, Table 2, column 3) is higher than that obtained from the direct carbonization of fresh biomass (31.0 wt%, Table 2, column 1). That is, taking into account the solid yield in torrefaction, the total value of liquids produced would be around 42 wt%. If a similar calculation is made for gases, less gases are produced globally in the torrefaction + carbonization treatment (31 wt%) than in direct carbonization (43 wt%). A priori, this could seem to be a bad result, but it should be noted that the objective of this work is to evaluate the potential of charcoal production from torrefied biomass, providing a new market option for this product, in addition to combustion. In this sense, producing 8.0 wt% of liquids is much better than producing 31.0 wt%. With regard to gases, comparing columns 1 and 3, it can be seen that the gases almost doubled the H2 percentage (quite close to 50% in volume in column 3), and the percentage of CO2, CH4, and the other gaseous compounds decreased or even disappeared. This can be an indication of a simpler vapor phase being produced in the tank reactor with the torrefied biomass. Therefore, the pre-treatment of the biomass by torrefaction substantially improves the results obtained concerning the liquid and gas phases of the process.
Finally, the results obtained in the carbonization of torrefied biomass using the reforming catalyst for the treatment of gases and vapors are presented in the fourth column of Table 2. It can be observed that the catalyst does not exert any appreciable effect on the process yields, which remain very similar to those obtained in the experiment without the catalyst. However, a significant variation in the composition of the gases can be seen, where there is an increase of more than double in the H2 content (wt%), together whit a very important reduction of CO and an increase of almost double in CO2 and CH4 contents. Comparing the results of columns 2 and 4, it can be observed that the catalytic effect in the gaseous products is very different. It is important to remark the change in two critical parameters in the tubular reactor of the experiment shown in column 4 with regard to that of column 2. The first one is the amount of condensed liquids. In the carbonization of torrefied biomass, there is a far less amount of liquid phase, which implies much less water content in the tubular reactor, influencing the reaction mechanism. The second one is that this liquid amount decrease also implies a decrease in the space velocity inside the tubular reactor. This may allow slow reactions to also take place, or even for secondary reactions to occur that were not observed in the results of column 2. Taking into consideration the change in reaction conditions explained before, the justification for the gaseous composition observed in column 4 could be the following. Ni-based catalysts are known to have a good cleavage of C–C and C-O bonding, which is favorable for the water–gas shift reaction (WGS), decreasing the CO content and increasing CO2 [38]. Hydrogen is generated from the reforming of organic compounds, as it occurred in column 2, generating also CO. The enormous decrease in CO amount can be explained by the WGS reaction, by which a lot of CO2 and H2 would have been produced, decreasing also the quantity of water. Due to the smaller space velocity and higher residence time compared to the experiment of column 2, the methanation side reaction may have taken place, as it has been previously reported by other authors [41]. The increase of CH4 can be explained through methanation. In methanation, water is again produced, compensating for the water consumed in WGS and ending in a similar amount of water in the experiments of columns 3 and 4 (assuming that the liquid products are essentially water, as is expected).
In short, when torrefaction is applied as pre-treatment, the hydrogen production shows an increase of 116% (in weight), while in the case of the fresh biomass, the increase is 88% (in weight). Therefore, the presence of the catalyst generates gases with an H2 percentage of more than 70% in volume, which is an important milestone because above 50%, the option of separating the H2 from a mixture of gases by pressure swing adsorption (PSA) can be economically viable. This means producing pure H2 and, therefore, accessing the specific markets and applications of this valuable chemical compound.
2.3. Evolution of Solids during Torrefaction and Carbonization
Table 4 shows the proximate and ultimate analyses of the solid products involved in this investigation: fresh biomass, torrefied biomass, carbonized biomass, and torrefied and carbonized biomass. As it can be seen, the fresh biomass shows typical characterization results concerning woody biomass: moisture around 10 wt%, volatiles/fixed carbon ratio ≈ 70/15, low ash content, and elemental composition based on carbon and oxygen [17,18,34]. When the fresh biomass is torrefied, the resultant solid is an almost dry product (1 wt% moisture) that contains a lower quantity of volatile matter (58.3 wt%), and in consequence, a higher content of fixed carbon (38.4 wt%). This chemical transformation improves the fuel properties of the biomass, whose HHV increases up to 23.1 MJ kg−1. The aforementioned chemical changes are common to torrefaction processes and have been reported by other authors before [32,42,43]. Regarding the ultimate analysis, a significant increase in carbon is produced at the expense of a decrease in oxygen and some hydrogen content. These results are similar to those obtained by other authors before [36], and they are attributed to the loss of water and the decomposition of hemicellulose, which is a polymer with high O/C content [23]. Additionally, taking into account that the bond energy of the C–O and C–H linkages is lower than that of the C–C, a preferential process of deoxygenation, and to a lesser extent of dehydrogenation, is expected when cellulose and lignin starts decomposition during torrefaction. Regarding the charcoals, the carbonized solid shown in column 3 of Table 4 is a “low-volatiles charcoal” mainly composed of fixed carbon, as expected after the carbonization process at 750 °C. The chemical properties of this charcoal concerning moisture, volatiles, ash, and sulfur make it a suitable product as a substitute for metallurgical coke, petroleum coke, or anthracite in metallurgical rotary kilns, as shown in a previous paper by the authors [44]. At last, the charcoal obtained from torrefied biomass is shown in column 4. It can be observed that this charcoal has chemical properties very similar to those of the charcoal obtained directly from fresh biomass, so it can be concluded that the pre-treatment of the biomass through torrefaction has no significant effect on the chemical properties of the charcoal obtained.

Table 4.
Characterization of the solid samples.
Figure 1 shows the aforementioned four solid products in the Van Krevelen diagram, where the H/C atomic ratio of a solid fuel is plotted against its O/C atomic ratio. It can be clearly seen in Figure 1 that the torrefaction process generates an important loss in atomic oxygen and atomic hydrogen in the torrefied solid. This is a very well documented fact in the literature concerning torrefaction and is directly related to the release of oxygenated compounds such as CO2, CO, H2O, and acetic acid, coming from the loss of moisture and the decomposition of hemicellulose [26,31,33,42,45,46]. The reduction in H/C and O/C ratios caused by torrefaction makes the fresh biomass move toward the H/C and O/C ratios of the carbonized products, as it is evident from Figure 1. In fact, the atomic ratios of the torrefied biomass obtained in this work (0.91, 0.37) are in the range of peat and lignites [29,47,48]. Regarding the charcoals, although their small differences in the elemental analysis make them not coincide in the same point, both are placed in the region of the anthracites, corroborating the theory that they can be used as substitutes of this coal in some of its applications.
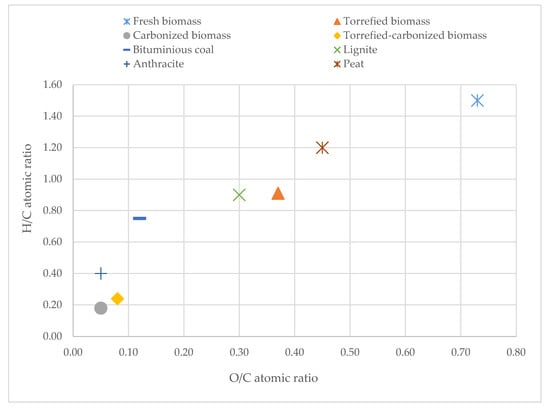
Figure 1.
Van Krevelen diagram for fresh biomass, torrefied biomass, carbonized biomass, and torrefied-carbonized biomass.
3. Materials and Methods
3.1. Materials
Eucalyptus wood coming from chopped pre-crushed trees was used as a biomass sample for the experiments. This biomass came from an energetic crop and in consequence, it was a very clean and homogeneous sample; on a dry basis, it consisted mainly of cellulose (37.6 wt%, mainly glucanes) and lignin (28.6 wt%), followed by hemicellulose (13.3 wt%, xylanes and arabinanes), extractives (8.1 wt%) and ashes (2.4 wt%) [19]. The proximate analysis, ultimate analysis, and HHV of the eucalyptus sample are presented in Table 4, together with those analyses of the solid samples obtained after the thermal treatments shown in this paper.
The catalyst is a non-alkalized nickel oxide catalyst (NiO 16 wt% = Ni 12.6 wt%) on a calcium aluminate support (CaO/Al2O3). It is a commercial catalyst normally used for the steam reforming of natural gas, other light hydrocarbons, and hydrogen-rich industrial streams. This catalyst has been also used by the authors in the thermo-catalytic treatment of vapors and gases coming from the pyrolysis of different types of waste [18,19,49]. Its main properties are summarized in Table 3. These properties have been all determined in the laboratories of the authors by using the following techniques: N2 sorption/desorption for surface area and pore analysis (Autosorb-1C, Quantachrome, USA); Inductively coupled plasma with atomic emission system, ICP/AES (Perkin-Elmer Optima 2000-DV, USA) for chemical analysis; CO chemisorption for active metal surface and metal dispersion (Autosorb-1C, Quantachrome, USA); temperature-programmed reduction, TPR (AutoChem II, Micromeritics, USA) for reduction temperature; XRD for crystallinity (X-ray Powder Diffraction, Philips X’Pert PRO), and temperature-programmed desorption of ammonia, NH3-TPD (Autosorb-1C, Quantachrome, USA) for acidity. A thorough discussion on such properties and the methodology employed has been already published elsewhere [18,19]. In a few words, it can be said that the catalyst has quite a low surface area with big pores, which will prevent pore blocking due to coke deposition. The Ni content of the catalyst and the small crystal size indicates that the nickel is well distributed in the surface, which will discourage the sintering of Ni particles and suppress further carbon deposition.
3.2. Experimental Procedure
The torrefaction experiments were performed in a non-stirred 3.5 L stainless steel tank reactor, where 100 g of wood pieces smaller than 10 mm were heated at a 3 °C min−1 to 250 °C and maintained there for 30 min. Such temperature was selected to prevent the eucalyptus wood from starting carbonization [50]. The gases and vapors generated in the reactor were driven by a 1 L min−1 nitrogen flow to a vapors condensation and gas–liquid separation unit. Once the gas–liquid separation took place, the liquids remained in the condensers, and the non-condensed gases were collected to be analyzed afterwards.
The carbonization experiments were performed in the same tank reactor, but in this case, they were followed by a second tubular reactor where the pyrolysis vapors and gases were upgraded by means of thermal and thermo-catalytic treatment, with the aim of diminishing tars and improving the composition of the gas phase. In such experiments, the tank reactor was heated at a heating rate of 3 °C min−1 to 750 °C for 30 min. In this case, the 1 L min−1 nitrogen flow was also used during the whole experiment. The vapors and gases leaving the tank reactor passed through the tubular reactor, which was loaded with a charcoal bed and the catalyst (when necessary) and was maintained at 900 °C. The charcoal bed was used due to the catalytic activity given to char in the removal of tars [51,52]. This ex situ treatment of gases and vapors allows controlling the temperature of both reactors and reduces the effect of coking and prevents metal contamination of the catalyst, expanding its life. The catalyst was impregnated into a monolithic cordierite ceramic carrier (400 cpsi corning) through the incipient wetness impregnation (IWI) method [53]. The steps to place the catalytic monolith inside the tubular reactor, together with the advantages of using this type of catalyst placement, have been previously described [17]. Prior to the reaction, the catalyst was reduced in situ at 800 °C during 4 h with a 10 vol% H2 − 10 vol% N2 - 80 vol% Ar mixture. The flowsheet of the lab-scale installation employed for the experiments is shown in Figure 2. Solid, liquid, and gas yields were determined as follows. Solid yield was calculated as the mass of torrefied/carbonized solid found in the tank reactor after the experiment. Liquid yield was determined by the weight difference in the condensers and pipelines of the installation, but only the liquids in the condensers were analyzed (the liquids in pipelines cannot be recovered). Consequently, it was not possible to analyze the liquid fraction produced in the carbonization of the torrefied biomass samples despite constituting 8 wt% in yield (everything condensed in pipelines). At least, gas yield was calculated by difference to 100. Both the torrefaction and the carbonization experiments were performed twice or more until a standard deviation smaller than 3 wt% in pyrolysis yields was obtained.
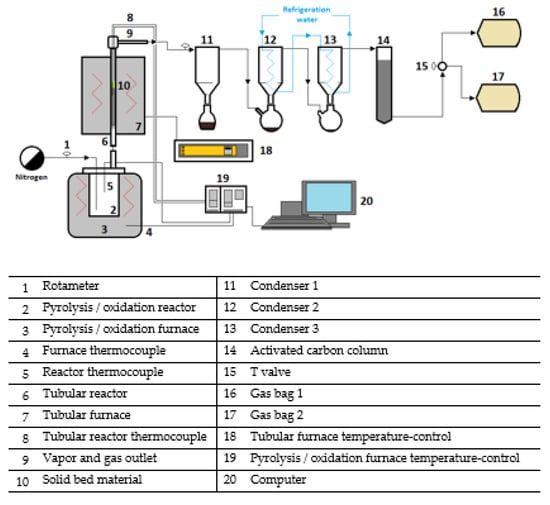
Figure 2.
Flowsheet of the lab-scale plant used for the torrefaction and carbonization experiments.
A conceptual scheme of the experiments carried out is presented in Figure 3.
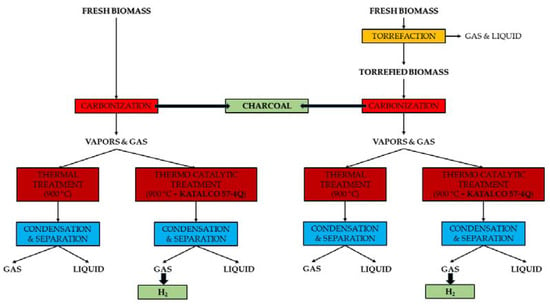
Figure 3.
Conceptual scheme of the experiments.
3.3. Analytical Techniques
The initial feedstock (fresh biomass) and all the products from torrefaction and carbonization were thoroughly characterized. The biomass and thermally treated solid samples were crushed to <2 mm particle size in order to have an appropriate homogeneous sample available for characterization. The proximate analysis of these samples was determined by thermogravimetry with a LECO TGA 500 thermobalance, according to D3173 (moisture) and D3174 (ash) ASTM standards. Their ultimate analysis was determined with a Euro Elemental Analyzer (CHN-S-O) made by EuroVector, and the HHV of the samples was obtained with a LECO AC-500 calorimeter.
The composition of the liquid products was analyzed using gas chromatography coupled to a mass spectroscopy detector (GC/MS, Agilent 6890 and Agilent 5973N). Tetrahydrofuran (THF), supplied by Panreac (99.9% purity), was used as solvent, and the sample-to-solvent ratio was 1/10. The column employed in the GC was the Agilent 123-3262, with a program that consisted of an injection at 250 °C (split 75:1), 2.3 mL min−1 of He as carrier gas, and a final oven temperature of 240 °C. This column and method have demonstrated high-quality results in the determination of the composition of aqueous phases in the previous research works of the authors [9,17,19].
The compositional analysis of the gases was carried out with an AGILENT 7890A gas chromatograph coupled with thermal conductivity and flame ionization detectors (GC-TCD/FID). For the simultaneous separation and determination of gas fraction, the chromatograph was equipped with two columns (HP-Molesieve and HP-Plot Q) interconnected with each other and with the detectors through a system of valves. The injection was carried out at 150 °C, and the analyses were performed using He as the carrier gas (5 mL min−1). The oven program started at 40 °C and finished at 200 °C. TCD and FID detectors’ temperature was fixed at 250 and 300 °C, respectively. The calibration of the GC was carried out with a “refinery” standard prepared by Air Liquide, which consisted of N2, H2, CO, CO2, H2S, CH4, C2H6, C2H4, C3H8, C3H6, C4H10, C4H8, C5H12, C5H10, C6H14, and C6H12. The calibration curve was established for each component by modifying the split ratio of the injection. Finally, the HHV of the gas fraction was calculated based on the composition and HHV of the individual components of the gases.
4. Conclusions
The torrefaction of woody biomass generates more than 50 wt% of an improved solid product, without moisture, with less volatility, higher carbon content, and higher heating value than the fresh biomass. At the same time, a liquid product consisting of water and acetic acid and a gas stream of CO2 and CO are generated.
Producing charcoal from torrefied biomass is beneficial from the standpoint of carbonization performance: more than 50 wt% of charcoal is obtained from torrefied biomass compared to 26 wt% when fresh biomass is carbonized.
In addition, producing charcoal from torrefied biomass is also beneficial for the thermal treatment of the vaporized phase. At 900 °C, the production of condensates is very low (8 wt% vs. 31 wt% when fresh biomass is carbonized) and a gas fraction with almost 50 vol% of H2 is generated (28 vol% with fresh biomass).
Regarding the chemical properties of charcoal (proximate and ultimate analysis), there is no significant difference between producing charcoal from fresh biomass or from torrefied biomass.
On the other hand, the performance of the reforming catalyst improves significantly when treating the vaporized phase of the torrefied biomass in comparison to that of the fresh biomass. The hydrogen production shows an increase of 116% (in weight), while in the case of the fresh biomass, the increase is 88% (in weight). Consequently, the amount of H2 in the gaseous stream reaches 71 vol%, which allows this gaseous stream to be used as a source of pure H2.
As a general conclusion, it can be said that the production of charcoal from torrefied biomass is much more efficient than that from fresh biomass in terms of solid and liquid production, performance of the catalyst, and gas quality. In this sense, future techno-economic studies are welcome in order to evaluate the sustainability of charcoal production from torrefied woody biomass. If sustainability is reached, charcoal production could be also a potential market for torrefied biomass, which, in addition to energy use, could stimulate the industrial implementation of torrefaction.
Author Contributions
Conceptualization, A.L.-U. and I.d.M. Methodology, A.L.-U. and A.A. Investigation, A.A., J.S. and E.A. Resources, I.d.M. and B.M.C. Writing—original draft preparation, A.L.-U. and A.A. Writing—review and editing, E.A. Supervision, A.L.-U. and I.d.M. Project administration, I.d.M. Funding acquisition, I.d.M. and B.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basque Government through the “SAIOTEK” program, grant number SAI13/190 (S-PE13UN126), by University of the Basque Country (UPV/EHU), Spanish Ministry of Economy and Innovation and European Union through the European Regional Development Fund (FEDER) (Projects: ENE2017-82250-R). The work was also funded by the financing provided to the “Sustainable Process Engineering” research group for the 2016-2021 period (reference: GIC 15/13, IT993-16).
Acknowledgments
The authors want thank the Central Analysis Service of the University of the Basque Country (UPV/EHU) for the ultimate analysis of the solid samples. Additionally, the authors are very grateful to Maria Felisa Laresgoiti for her technical advice in the laboratory.
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- Abdul Quader, M.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking–Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Mansor, A.M.; Theo, W.L.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Potential commercialisation of biocoke production in Malaysia—A best evidence review. Renew. Sustain. Energy Rev. 2018, 90, 636–649. [Google Scholar] [CrossRef]
- Dufourny, A.; Van De Steene, L.; Humbert, G.; Guibal, D.; Martin, L.; Blin, J. Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. J. Anal. Appl. Pyrolysis 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. Bioreducer use in Finnish blast furnace ironmaking–Analysis of CO2 emission reduction potential and mitigation cost. Appl. Energy 2014, 124, 82–93. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Pereira, E.G.; Martins, M.A.; Pecenka, R.; Carneiro, A.d.C.O. Pyrolysis gases burners: Sustainability for integrated production of charcoal, heat and electricity. Renew. Sustain. Energy Rev. 2017, 75, 592–600. [Google Scholar] [CrossRef]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Solar, J.; Requies, J.; De Marco, I.; Cambra, J.F. Upgrading of pyrolysis vapours from biomass carbonization. J. Anal. Appl. Pyrolysis 2013, 103, 293–299. [Google Scholar] [CrossRef]
- Min, Z.; Asadullah, M.; Yimsiri, P.; Zhang, S.; Wu, H.; Li, C.Z. Catalytic reforming of tar during gasification. Part I. Steam reforming of biomass tar using ilmenite as a catalyst. Fuel 2011, 90, 1847–1854. [Google Scholar] [CrossRef]
- Nordgreen, T.; Nemanova, V.; Engvall, K.; Sjöström, K. Iron-based materials as tar depletion catalysts in biomass gasification: Dependency on oxygen potential. Fuel 2012, 95, 71–78. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L.; Zhao, X.Y.; Cao, J.P. Biomass thermochemical conversion: A review on tar elimination from biomass catalytic gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- Rodrigues, T.; Braghini Junior, A. Technological prospecting in the production of charcoal: A patent study. Renew. Sustain. Energy Rev. 2019, 111, 170–183. [Google Scholar] [CrossRef]
- Rodrigues, T.; Braghini Junior, A. Charcoal: A discussion on carbonization kilns. J. Anal. Appl. Pyrolysis 2019, 143. [Google Scholar] [CrossRef]
- Adrados, A.; De Marco, I.; Lopez-Urionabarrenechea, A.; Solar, J.; Caballero, B. Avoiding tar formation in biocoke production from waste biomass. Biomass Bioenergy 2015, 74, 172–179. [Google Scholar] [CrossRef]
- Solar, J.; Caballero, B.; De Marco, I.; López-Urionabarrenechea, A.; Gastelu, N. Optimization of Charcoal Production Process from Woody Biomass Waste: Effect of Ni-Containing Catalysts on Pyrolysis Vapors. Catalysts 2018, 8, 191. [Google Scholar] [CrossRef]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Acha, E.; Solar, J.; Caballero, B.M.; de Marco, I. Hydrogen rich reducing gases generation in the production of charcoal from woody biomass carbonization. Energy Convers. Manag. 2017, 148, 352–359. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: A review. Renew. Sustain. Energy Rev. 2020, 119. [Google Scholar] [CrossRef]
- Ochoa, A.; Arregi, A.; Amutio, M.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Castaño, P. Coking and sintering progress of a Ni supported catalyst in the steam reforming of biomass pyrolysis volatiles. Appl. Catal. B Environ. 2018, 233, 289–300. [Google Scholar] [CrossRef]
- Luberti, M.; Friedrich, D.; Brandani, S.; Ahn, H. Design of a H2 PSA for cogeneration of ultrapure hydrogen and power at an advanced integrated gasification combined cycle with pre-combustion capture. Adsorption 2014, 20, 511–524. [Google Scholar] [CrossRef]
- Da Silva, C.M.S.; de Carneiro, A.C.O.; Vital, B.R.; Figueiró, C.G.; de Fialho, L.F.; de Magalhães, M.A.; Carvalho, A.G.; Cândido, W.L. Biomass torrefaction for energy purposes–Definitions and an overview of challenges and opportunities in Brazil. Renew. Sustain. Energy Rev. 2018, 82, 2426–2432. [Google Scholar] [CrossRef]
- Shang, L.; Ahrenfeldt, J.; Holm, J.K.; Bach, L.S.; Stelte, W.; Henriksen, U.B. Kinetic model for torrefaction of wood chips in a pilot-scale continuous reactor. J. Anal. Appl. Pyrolysis 2014, 108, 109–116. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. A review on torrefied biomass pellets as a sustainable alternative to coal in power generation. Renew. Sustain. Energy Rev. 2014, 40, 153–160. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Basu, P. Pyrolysis and Torrefaction. In Biomass Gasification and Pyrolysis; Elsevier: Amsterdam, The Netherlands, 2010; pp. 65–96. ISBN 978-0-12-374988-8. [Google Scholar]
- Granados, D.A.; Chejne, F.; Basu, P. A two dimensional model for torrefaction of large biomass particles. J. Anal. Appl. Pyrolysis 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Gul, S.; Ramzan, N.; Hanif, M.A.; Bano, S. Kinetic, volatile release modeling and optimization of torrefaction. J. Anal. Appl. Pyrolysis 2017, 128, 44–53. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Solar, J.; Hernandez, A.; Lopez-Urionabarrenechea, A.; de Marco, I.; Adrados, A.; Caballero, B.M.; Gastelu, N. From woody biomass waste to biocoke: Influence of the proportion of different tree components. Eur. J. Wood Wood Prod. 2017, 75, 485–497. [Google Scholar] [CrossRef]
- Peinado, C.; Liuzzi, D.; Retuerto, M.; Boon, J.; Peña, M.A.; Rojas, S. Study of catalyst bed composition for the direct synthesis of dimethyl ether from CO2-rich syngas. Chem. Eng. J. Adv. 2020, 100039. [Google Scholar] [CrossRef]
- Shen, Y. Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification. Renew. Sustain. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. A review on common adsorbents for acid gases removal: Focus on biochar. Renew. Sustain. Energy Rev. 2018, 81, 1705–1720. [Google Scholar] [CrossRef]
- De Miranda, J.C.C.; Ponce, G.H.S.F.; Arellano-Garcia, H.; Maciel Filho, R.; Wolf Maciel, M.R. Process design and evaluation of syngas-to-ethanol conversion plants. J. Clean. Prod. 2020, 269, 122078. [Google Scholar] [CrossRef]
- Haynes, D.J.; Shekhawat, D. Chapter 6—Oxidative Steam Reforming. In Full Cells: Technologies for Fuel Processing; Shekhawat, D., Spivey, J.J., Berry, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 129–190. ISBN 978-0-444-53563-4. [Google Scholar]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Hydrogen production via acetic acid steam reforming: A critical review on catalysts. Renew. Sustain. Energy Rev. 2017, 79, 1091–1098. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Keipi, T.; Tolvanen, H.; Kokko, L.; Raiko, R. The effect of torrefaction on the chlorine content and heating value of eight woody biomass samples. Biomass Bioenergy 2014, 66, 232–239. [Google Scholar] [CrossRef]
- Adrados, A.; De Marco, I.; López-Urionabarrenechea, A.; Solar, J.; Caballero, B.M.; Gastelu, N. Biomass Pyrolysis Solids as Reducing Agents: Comparison with Commercial Reducing Agents. Materials 2015, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Xu, F.; Sun, R.C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Basu, P.; Sadhukhan, A.K.; Gupta, P.; Rao, S.; Dhungana, A.; Acharya, B. An experimental and theoretical investigation on torrefaction of a large wet wood particle. Bioresour. Technol. 2014, 159, 215–222. [Google Scholar] [CrossRef]
- Doassans-Carrère, N.; Muller, S.; Mitzkat, M. REVE—A new industrial technology for biomass torrefaction: Pilot studies. Fuel Process. Technol. 2014, 126, 155–162. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment—Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Gastelu, N.; Lopez-Urionabarrenechea, A.; Solar, J.; Acha, E.; Caballero, B.M.; López, F.A.; de Marco, I. Thermo-Catalytic Treatment of Vapors in the Recycling Process of Carbon Fiber-Poly (Benzoxazine) Composite Waste by Pyrolysis. Catalysts 2018, 8, 523. [Google Scholar] [CrossRef]
- Fan, Y.; Tippayawong, N.; Wei, G.; Huang, Z.; Zhao, K.; Jiang, L.; Zheng, A.; Zhao, Z.; Li, H. Minimizing tar formation whilst enhancing syngas production by integrating biomass torrefaction pretreatment with chemical looping gasification. Appl. Energy 2020, 260, 114315. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Zeng, X.; Ueki, Y.; Yoshiie, R.; Naruse, I.; Wang, F.; Han, Z.; Xu, G. Recent progress in tar removal by char and the applications: A comprehensive analysis. Carbon Resour. Convers. 2020, 3, 1–18. [Google Scholar] [CrossRef]
- Delmon, B.; Haber, J.; Block, J.H. Manual of methods and procedures for catalyst characterization (technical report). Pure Appl. Chem. 1995, 67, 1257–1306. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).