Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems
Abstract
1. Introduction
1.1. Activation of PMS and PS
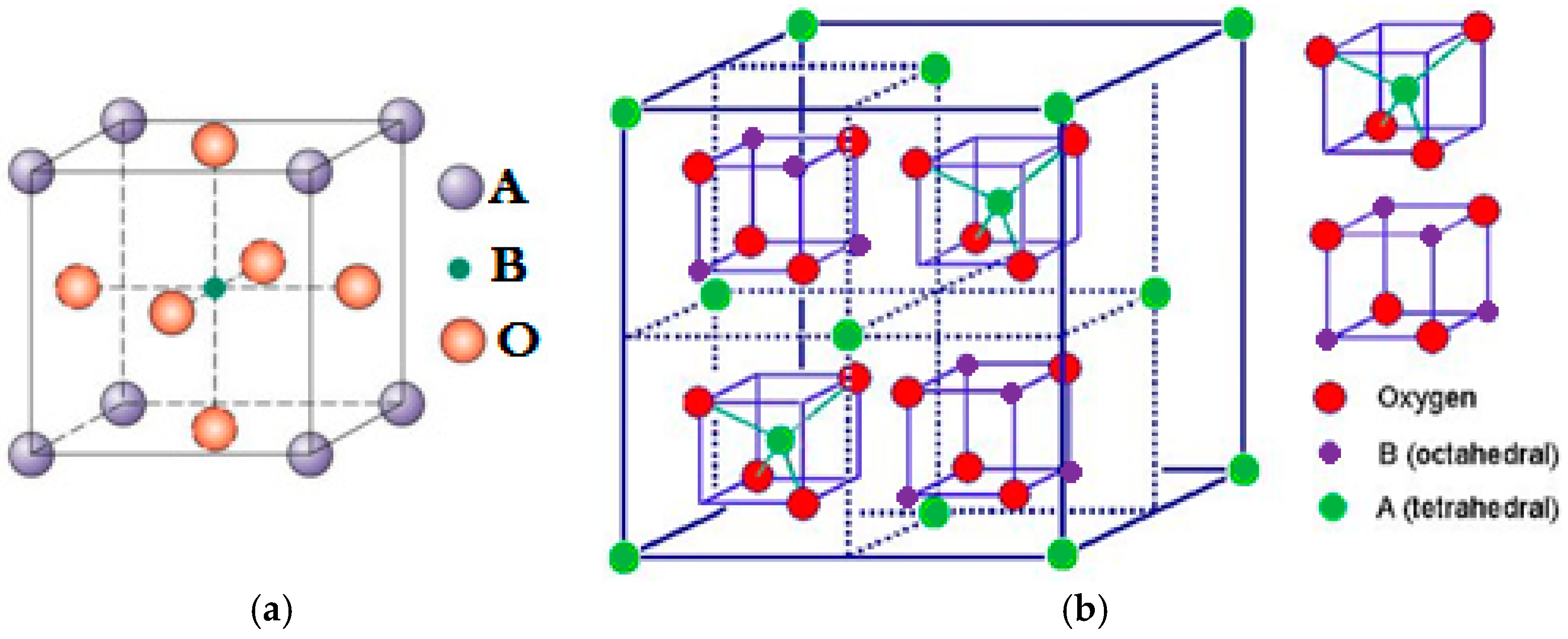
1.2. Perovskites and Spinel Catalysts
2. Perovskite Catalysts for the Activation of PMS, PS and Degradation of Organic Pollutants
2.1. Simple Perovskite Catalysts
| Concentration | Treatment Efficiency | |||||||
|---|---|---|---|---|---|---|---|---|
| Catalyst | Synthesis Method | Pollutant | Catalyst (g L−1) | Pollutant (mg L−1) | PMS (mM) | % Degradation (Time) | TOC Removal | Reference |
| SrCoO3 | citric acid sol-gel | phenol | 0.2 | 20 | 0.1 | 100% (3 h) | - | [53] |
| LaCoO3 | phenol | 0.2 | 20 | 0.1 | 95% (3 h) | - | ||
| BaCoO3 | phenol | 0.2 | 20 | 0.1 | 84% (3 h) | - | ||
| CeCoO3 | phenol | 0.2 | 20 | 0.1 | 80% (3 h) | - | ||
| LaCoO3 | precipitation method | PBSA | 0.22 | 5 | 5 | 100% (4.5 min) | - | [54] |
| LaCoO3-CTAB | modified precipitation method | 0.22 | 5 | 5 | 100% (3.5 min) | - | ||
| LaCoO3-SiO2 | single-stage hydrothermal | 0.5 | 5 | 5 | 100% (30 min) | - | ||
| LaCoO3-500 | sol-gel | CBZ | 0.05 | 5 | 0.5 | 100% (30 min) | - | [55] |
| LaCoO3-600 | CBZ | 0.05 | 5 | 0.5 | 100% (30 min) | - | ||
| LaCoO3-700 | CBZ | 0.05 | 5 | 0.5 | 98% (30 min) | - | ||
| LaCoO3 | citric acid sol-gel | metazachlor | 0.5 | 1 | 0.1 | 100% (<1 min) | - | [56] |
| LaCoO3 | citric acid sol-gel | tembotrione | 0.5 | 1 | 0.1 | 100% (<1 min) | - | [56] |
| LaCoO3 | citric acid sol-gel | tritosulfuron | 0.5 | 1 | 0.1 | 90% (>90 min) | - | |
| LaCoO3 | citric acid sol-gel | ethofumesate | 0.5 | 1 | 0.1 | 100% (<1 min) | - | |
| LaFeO3-500 | sol-gel | DCF | 0.1 | 7.5 | 0.5 | 100% (30 min) | - | [57] |
| LaFeO3-600 | sol-gel | DCF | 0.1 | 7.5 | 0.5 | 80% (30 min) | - | |
| LaFeO3-700 | sol-gel | DCF | 0.1 | 7.5 | 0.5 | 60% (30 min) | - | |
| LaFeO3-900 | sol-gel | DCF | 0.1 | 7.5 | 0.5 | 20% (30 min) | - | |
| LaFeO3 | sol-gel | DCF | 0.6 | 7.5 | 0.3 | 100% (60 min) | 50% (120 min) | [58] |
| LaMnO3-3 bar-600 | post synthesis | RhB | 0.2 | 20 | 4.9 | 100% (45 min) | - | [59] |
| LaMnO3-5 bar-600 | post synthesis | RhB | 0.2 | 20 | 4.9 | 100% (30 min) | - | |
| LaMnO3-8 bar-600 | post synthesis | RhB | 0.2 | 20 | 4.9 | 100% (45 min) | - | |
| LaMnO3-5 bar-300 | post synthesis | RhB | 0.2 | 20 | 4.9 | 100% (45 min) | - | |
| LaMnO3-5 bar-900 | post synthesis | RhB | 0.2 | 20 | 4.9 | 100% (60 min) | - | |
| La1.15MnO3+δ | complexing sol-gel | RhB | 0.2 | 20 | 1.3 | 100% (40 min) | - | [60] |
| LaMnO3+δ | complexing sol-gel | RhB | 0.2 | 20 | 1.3 | 86% (40 min) | - | |
| LaMnO3+δ | novel post synthesis | RhB | 0.2 | - | - | 100% (30 min) | ||
| LaCoO3/ZrO2 | Piccini (LaCoO3) hydrothermal (ZrO2) | RhB | 0.1 | 10 | 0.16 | 100% (60 min) | - | [61] |
| 23% wt LaCoO3/Al2O3 | citrate sol-gel | ATZ | 0.1 | 20 | 0.16 | 100% (30 min) | 30.8% (30 min) | [62] |
| LaFeO3/CeO2 | citric sol-gel | AO7 | 0.1 | 20 | 0.5 | 59% (120 min) | 28.4% | [63] |
| LaFeO3/SiO2 | citric sol-gel | AO7 | 0.1 | 20 | 0.5 | 52,3% (120 min) | 26.3% | |
| LaFeO3/TiO2 | citric sol-gel | AO7 | 0.1 | 20 | 0.5 | 32.2% (120 min) | 8.1% | |
| LaFeO3/Al2O3 | citric sol-gel | AO7 | 0.1 | 20 | 0.5 | 86.2% (120 min) | 56.5% | |
| LaFeO3 | citric sol-gel | AO7 | 0.1 | 20 | 0.5 | 70.8% (120 min) | 40.7% | |
| LaFeO3 | glycine combustion | RhB | 0.1 | 10 | 0.16 | 70% (60 min) | - | [64] |
| LaCuO3 | glycine combustion | RhB | 0.1 | 10 | 0.16 | 90% (60 min) | - | |
| LaNiO3 | glycine combustion | RhB | 0.1 | 10 | 0.16 | 100% (60 min) | - | [64] |
| LaCoO3 | glycine combustion | RhB | 0.1 | 10 | 0.16 | 100% (60 min) | - | |
| LaFeO3 | sol-gel | OFX | 0.2 | 10 | 0.8 | 15% (30 min) | - | [65] |
| LaMnO3 | sol-gel method | OFX | 0.2 | 10 | 0.8 | 90% (30 min) | - | |
| LaNiO3 | sol-gel method | OFX | 0.2 | 10 | 0.8 | 93% (30 min) | - | |
| PrBaCo2O5+δ | combined EDTA-citric acid | phenol | 0.1 | 20 | 3.25 | 100% (15 min) | 39.3% at pH 2 | [66] |
| SrCo1−xTixO3−δ (SCTx, x = 0.1, 0.2, 0.4, and 0.6) | combined EDTA-citric acid | phenol | 0.1 | 20 | 3.25 | 100% (15 min) (SCT0.2) | 82% (5h) (SCT0.2) | [67] |
| SrCo0.6Ti0.4O3−δ@CoOOH | post-synthesis hydrothermal treatment method | phenol | 0.06 | 20 | 3.25 | 100% (20 min) | - | [68] |
| LaCo0.6Cu0.4O3 | citric acid sol-gel method | phenol | 0.1 | 20 | 0.32 | 99% (12 min) | 30% (60 min) | [69] |
| LaCo1-xMnxO3+δ (LCM, x= 0, 0.3, 0.5, 0.7) | combined EDTA-citric acid | phenol | 0.1 | 20 | 3.25 | 100% (20 min) | - | [70] |
| La0.4Sr0.6MnO3-δ | EDTA-citric acid sol-gel method | phenol | 0.2 | 20 | 6.5 | 100% (90 min) | 70.1% (5 h) | [71] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | sol-gel | phenol | 0.1 | 20 | 6.5 | 100% (30 min) | - | [72] |
| La2CoMnO6−δ | evaporation-induced self-assembly | ATZ | 0.1 | 2 | 1 | 97% (30 min) | - | [73] |
| LaFe0.8Cu0.2O3−δ | citrate sol-gel | ATZ | 0.5 | 5 | 0.5 | 100% (60 min) | 52% (120 min) | [74] |
| Ag-La0.8Ca0.2Fe0.95O3−δ/Al2O3 | sol-gel | MB | 0.3 | 20 | 0.5 | 100% (45 min) | - | [75] |
| Ag-La0.8Ca0.2Fe0.94O3−δ | sol-gel (LCF) phase inversion/sintering (Ag-LCF) | MB | - | 10 | 0.6 | 90% (75 min) | - | [76] |
| La0.8Ca0.2Fe0.94O3−δ | sol-gel method | MB | 1 | 10 | 0.4 | 84% (45 min) | - | [77] |
| Ag-La0.8Ca0.2Fe0.94O3−δ | silver doping of La0.8Ca0.2Fe0.94O3-δ | MB | 1 | 10 | 0.4 | 90% (45 min) | - | |
| LaAl0.8Cu0.2O3 | sol-gel | TAP | 0.25 | 5 | 0.8 | 97.4% (30 min) | 71.4% (30 min) | [78] |
| LaAl0.8Cu0.2O3 | solvothermal | DCF | 0.25 | 5 | 0.8 | 99.3% (30 min) | 52.2% (30 min) | |
| solvothermal | IBF | 0.25 | 5 | 0.8 | 93.8% (30 min) | 59.2% (30 min) | ||
| solvothermal | PR | 0.25 | 5 | 0.8 | 97.7% (30 min) | 42.3% (30 min) | ||
| La0.8Sr0.2CoO3-δ | Combustion method | SMX | 0.5 | 0.5 | 0.4 | 100% (45 min) | - | [79] |
2.1.1. Co-Based Perovskites
2.1.2. Fe-Based Perovskites
2.1.3. LaMnO3 Catalyst
2.1.4. Various LaMO3 Perovskite Catalysts
2.2. Supported Simple Perovskite Catalysts
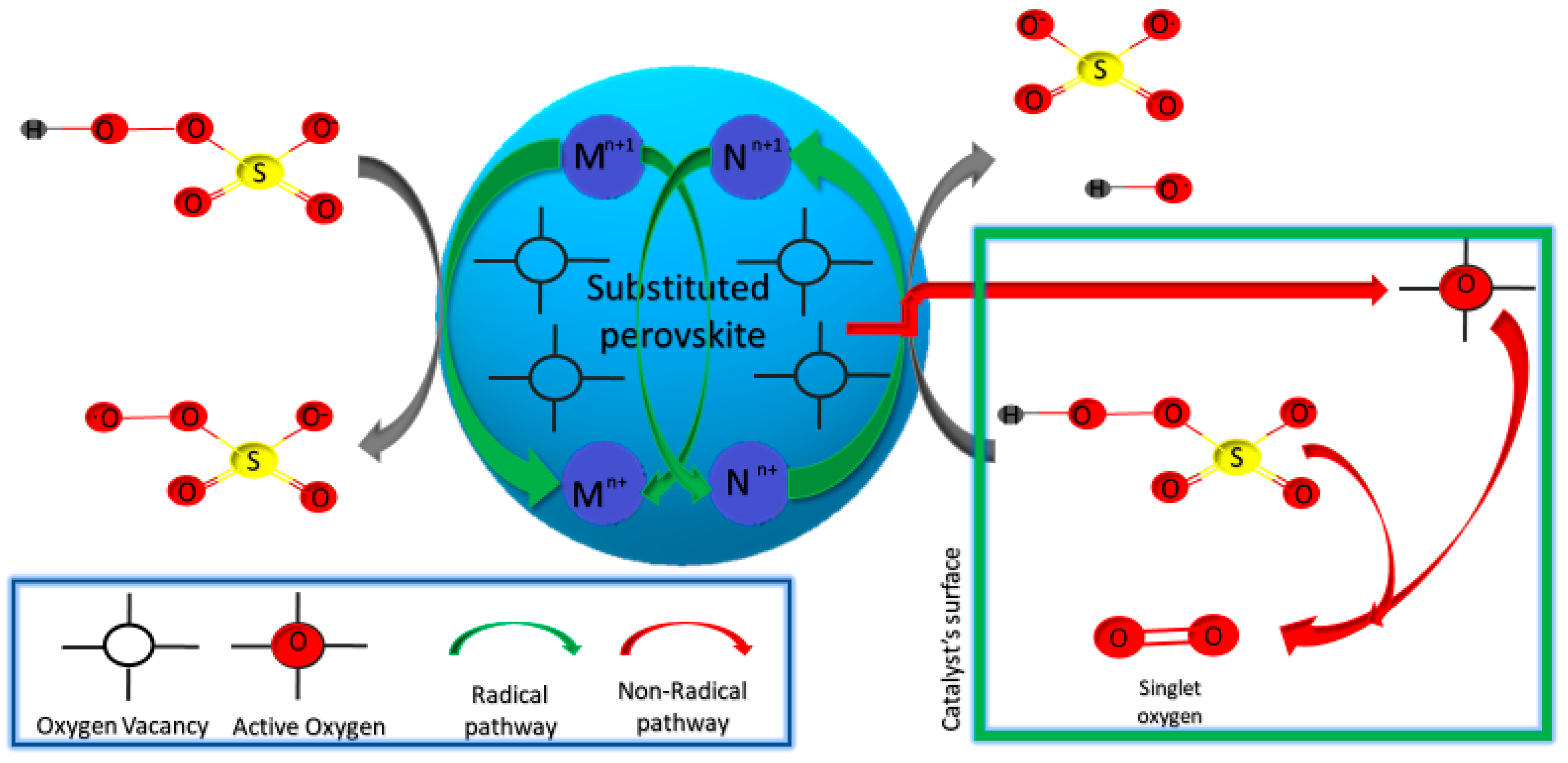
2.3. Simple Substituted Perovskites
2.3.1. Substituted in Position B Perovskites
2.3.2. Substituted in Position A Perovskite Catalysts
2.3.3. Doubly Substituted in Position A,B Perovskites
2.4. Double Perovskite Catalysts
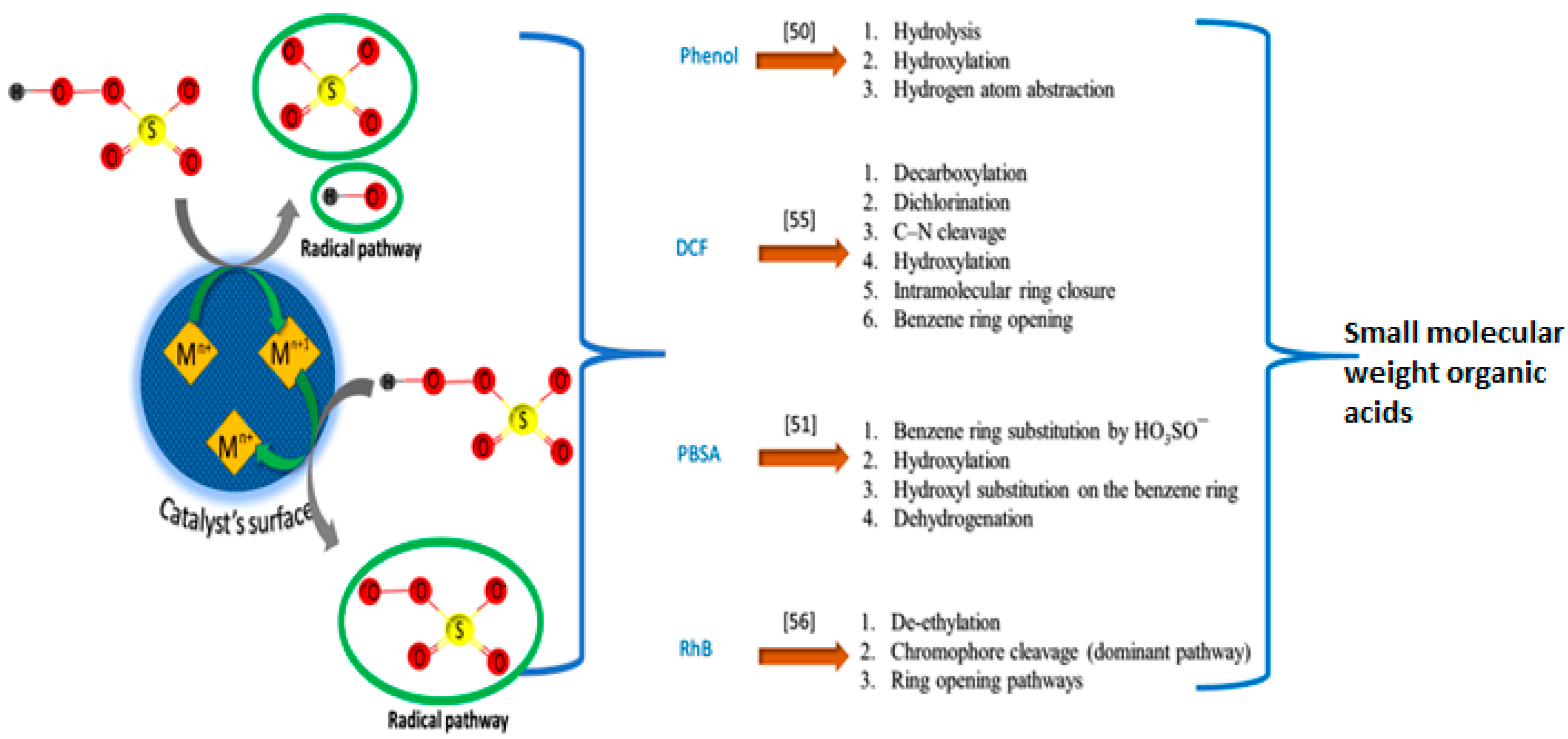
2.5. Metal Leaching and Secondary Pollution
3. Spinel Catalysts for the Activation of PMS, PS and Degradation of Organic Pollutants
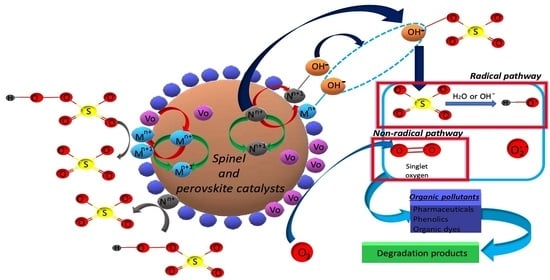
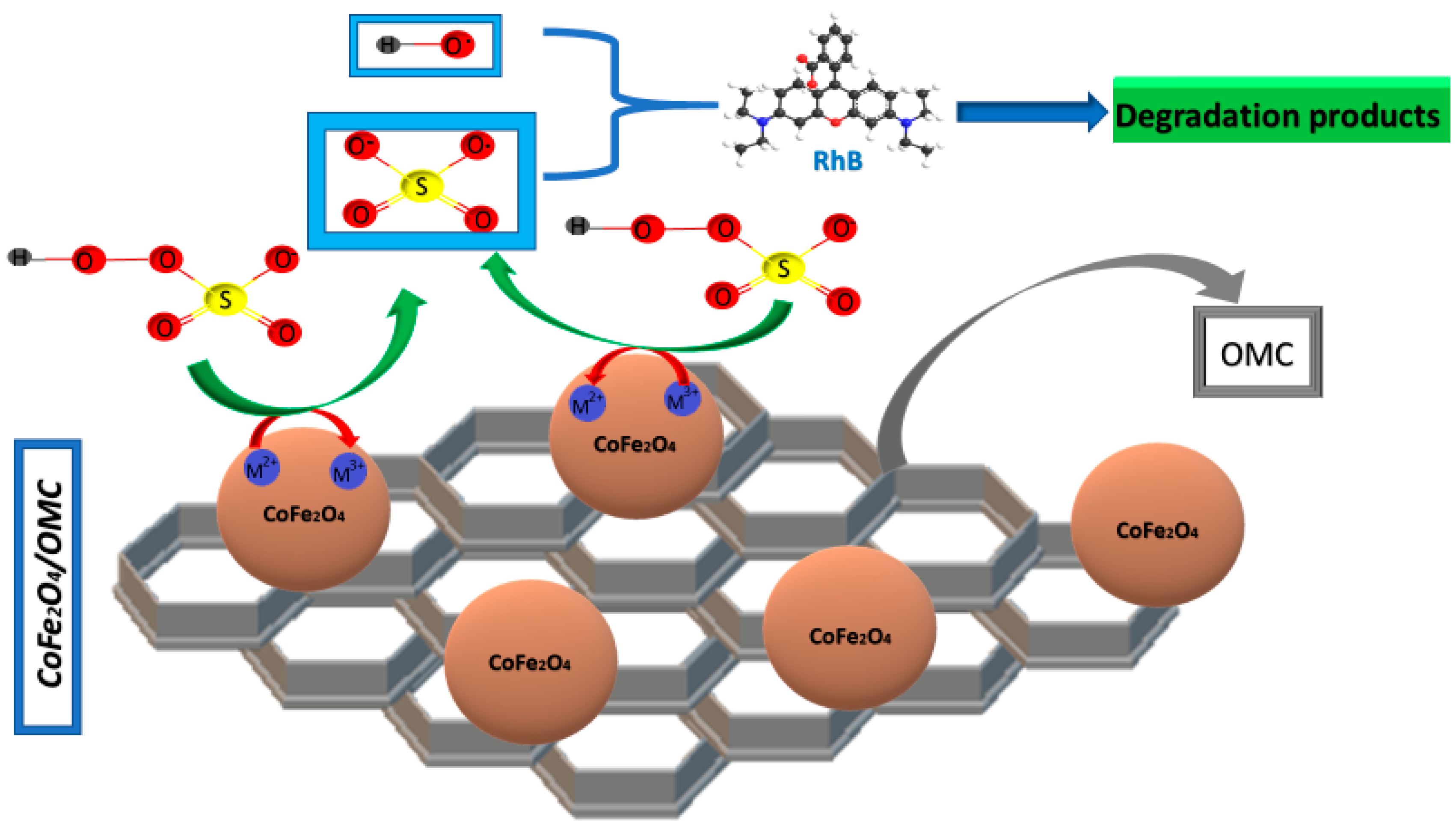
3.1. Cobalt-Ferrite (CoxFe3−xO4) Spinels
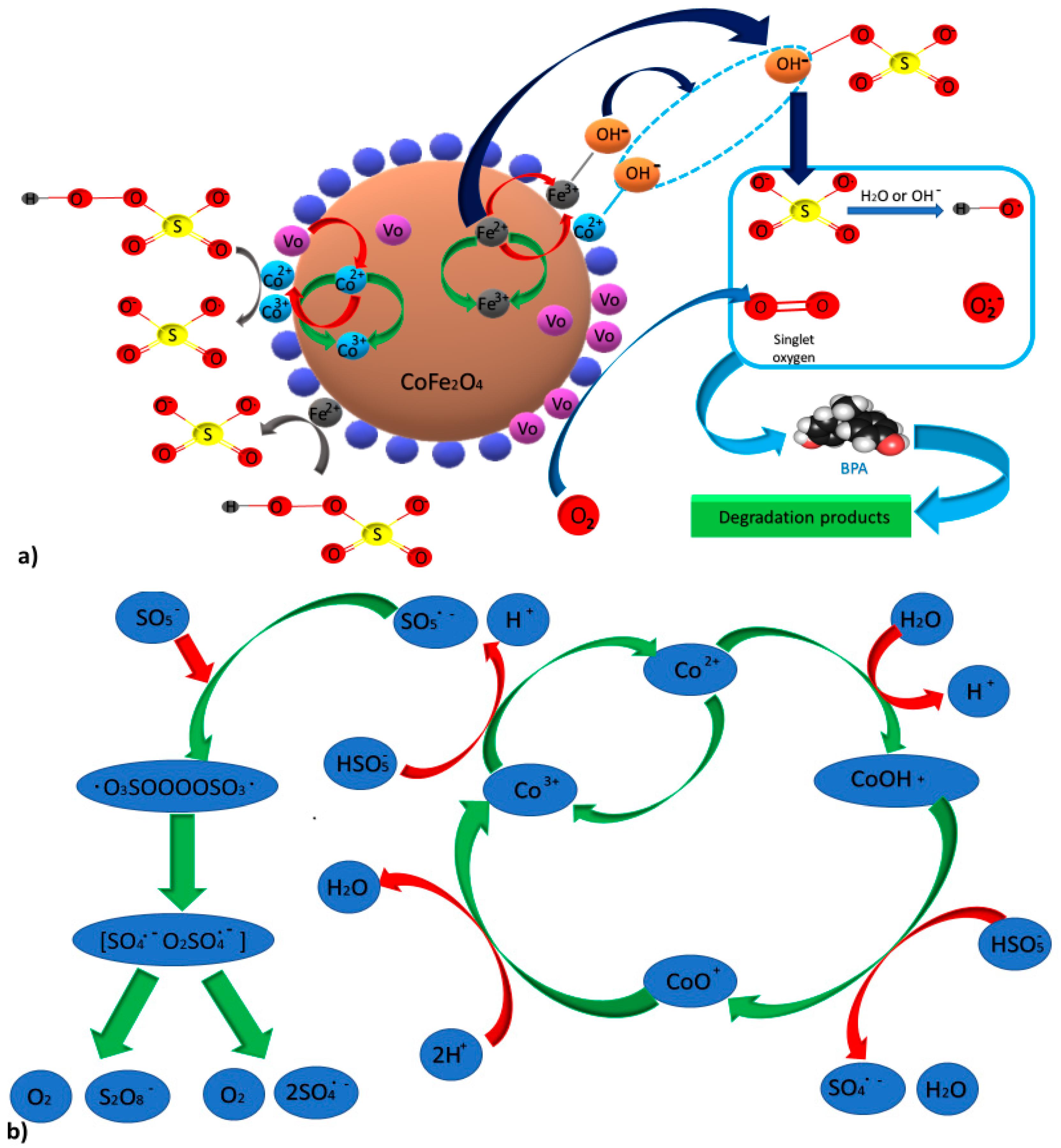
3.2. Cobalt-Manganese (CoxMn2-xO4) Spinels
3.3. Copper Cobaltite (CuCo2O4) Spinels
3.4. Zinc Ferrite and Cobaltite (ZnFe2O4, ZnCo2O4) Spinels
3.5. Nickel Ferrite (NiFe2O4) Spinel
4. Current Trends and Future Research Needs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Machulek, A.; Oliveira, S.C.; Osugi, M.E.; Ferreira, V.S.; Quina, F.H.; Dantas, R.F.; Oliveira, S.L.; Casagrande, G.A.; Anaissi, F.J.; Silva, V.O.; et al. Application of Different Advanced Oxidation Processes for the Degradation of Organic Pollutants. In Organic Pollutants-Monitoring, Risk and Treatment; InTech: London, UK, 2013; Volume 6, pp. 141–166. [Google Scholar] [CrossRef]
- Kilic, M.Y.; Abdelraheem, W.H.; He, X.; Kestioglu, K.; Dionysiou, D.D. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). J. Hazard. Mater. 2019, 367, 734–742. [Google Scholar] [CrossRef]
- Ferkous, H.; Merouani, S.; Hamdaoui, O.; Pétrier, C. Persulfate-enhanced sonochemical degradation of naphthol blue black in water: Evidence of sulfate radical formation. Ultrason. Sonochem. 2017, 34, 580–587. [Google Scholar] [CrossRef]
- Sun, Z.; Li, S.; Ding, H.; Zhu, Y.; Wang, X.; Liu, H.; Zhang, Q.; Zhao, C. Electrochemical/Fe3+/peroxymonosulfate system for the degradation of Acid Orange 7 adsorbed on activated carbon fiber cathode. Chemosphere 2020, 241, 125125. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Teng, J.; Zhang, J.; You, S. Visible-light-driven photocatalytic activation of peroxymonosulfate by Cu2(OH)PO4 for effective decontamination. Chemosphere 2018, 201, 197–205. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Mahdi Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Zhang, B.T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate radical and its application in decontamination technologies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1756–1800. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbk, H.; Siegrist, R.L.; Bjerg, P.L. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
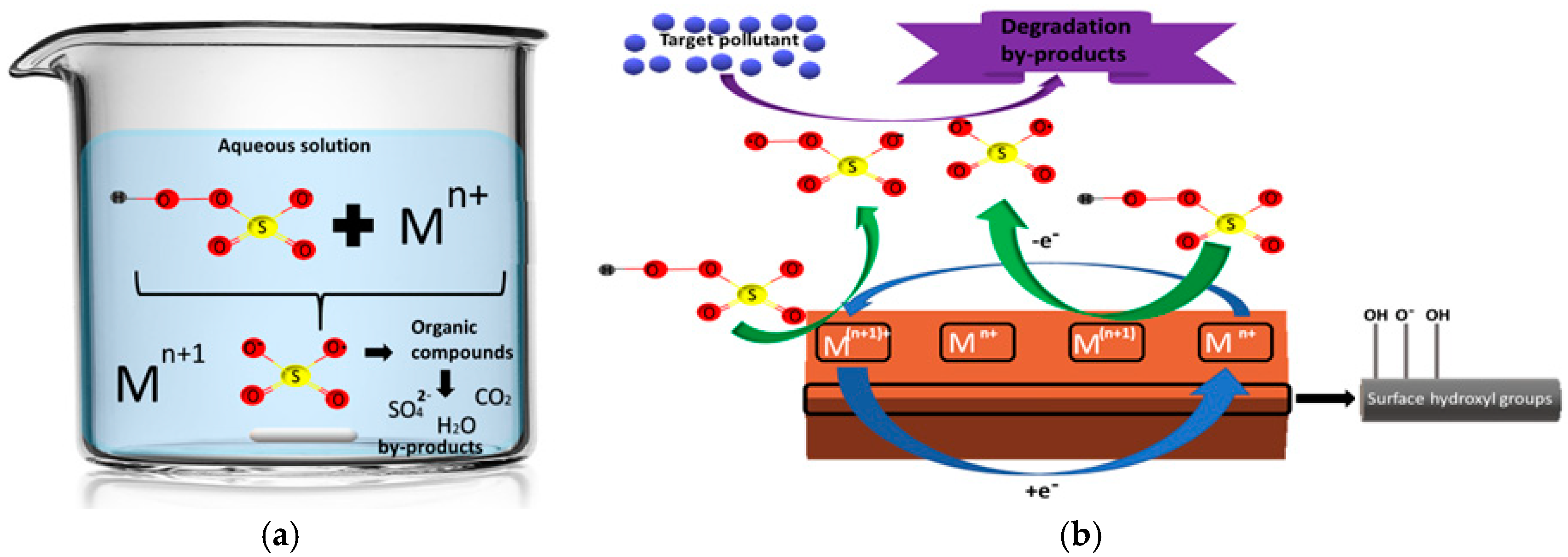
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Grübel, K. Simple spectrophotometric determination of monopersulfate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B. Metalloporphyrins as Versatile Catalysts for Oxidation Reactions and Oxidative DNA Cleavage. Chem. Rev. 1992, 92, 1411–1456. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Behrman, E.J.; Dean, D.H. Sodium peroxydisulfate is a stable and cheap substitute for ammonium peroxydisulfate (persulfate) in polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 723, 325–326. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Costa, D.; Romero, A.; Santos, A. Oxidation of Orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere 2014, 101, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Saputra, E.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Heterogeneous catalytic oxidation of aqueous phenol on red mud-supported cobalt catalysts. Ind. Eng. Chem. Res. 2012, 51, 15351–15359. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Peng, W.; Fang, Z.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/B-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2019, 356, 904–914. [Google Scholar] [CrossRef]
- Xie, Y.; Li, P.; Zeng, Y.; Li, X.; Xiao, Y.; Wang, Y.; Zhang, Y. Thermally treated fungal manganese oxides for bisphenol A degradation using sulfate radicals. Chem. Eng. J. 2018, 335, 728–736. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Shen, T.; Wang, Q.; Wang, X.; Xu, P.; Zheng, Q.; Zhang, G. The application of microwaves in sulfate radical-based advanced oxidation processes for environmental remediation: A review. Sci. Total Environ. 2020, 722, 137831. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Yang, Y.; Jiang, C.; Bian, C.; Liu, C.; Zhang, M.; Cai, T. Degradation of atrazine and structurally related s-triazine herbicides in soils by ferrous-activated persulfate: Kinetics, mechanisms and soil-types effects. Chem. Eng. J. 2018, 351, 523–531. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Waldemer, R.H.; Tratnyek, P.G.; Johnson, R.L.; Nurmi, J.T. Oxidation of chlorinated ethenes by heat-activated persulfate: Kinetics and products. Environ. Sci. Technol. 2007, 41, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.; Gimeno, O.; Borralho, T.; Beltrán, F. Influence of oxygen and free radicals promoters on the UV-254nm photolysis of diclofenac. Chem. Eng. J. 2010, 163, 35–40. [Google Scholar] [CrossRef]
- Su, S.; Guo, W.; Yi, C.; Leng, Y.; Ma, Z. Degradation of amoxicillin in aqueous solution using sulphate radicals under ultrasound irradiation. Ultrason. Sonochem. 2012, 19, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, Y.; Wang, P.; Li, H.; Dong, W. Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ. Sci. Pollut. Res. 2013, 20, 4947–4953. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Huang, A.; Zhao, X.; Zhang, X.; Tang, H. A heterogeneous Co3O4-Bi2O3 composite catalyst for oxidative degradation of organic pollutants in the presence of peroxymonosulfate. Catal. Sci. Technol. 2012, 2, 1977–1984. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D. Iron-cobalt mixed oxide nanocatalysts: Heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications. Appl. Catal. B Environ. 2009, 88, 462–469. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-Based Solar Cells: Materials, Methods, and Future Perspectives. J. Nanomater. 2018. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharm. J. 2019, 27, 817–829. [Google Scholar] [CrossRef]
- Hsu, K.C.; Fang, T.H.; Hsiao, Y.J.; Wu, P.C. Response and characteristics of TiO2/perovskite heterojunctions for CO gas sensors. J. Alloys Compd. 2019, 794, 576–584. [Google Scholar] [CrossRef]
- Cai, B.; Zhao, M.; Wang, Y.; Zhou, Y.; Cai, H.; Ye, Z.; Huang, J. A perovskite-type KNbO3 nanoneedles based biosensor for direct electrochemistry of hydrogen peroxide. Ceram. Int. 2014, 40, 8111–8116. [Google Scholar] [CrossRef]
- Chandrasekhar, P.S.; Dubey, A.; Qiao, Q. High efficiency perovskite solar cells using nitrogen-doped graphene/ZnO nanorod composite as an electron transport layer. Sol. Energy 2020, 197, 78–83. [Google Scholar] [CrossRef]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrogen Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Yazdanbakhsh, M. Fabrication of two perovskite-type oxide nanoparticles as the new adsorbents in efficient removal of a pesticide from aqueous solutions: Kinetic, thermodynamic, and adsorption studies. Microp. Mesop. Mater. 2013, 176, 86–94. [Google Scholar] [CrossRef]
- Kumar Sekhar, P.; Mukundan, R.; Brosha, E.; Garzon, F. Effect of perovskite electrode composition on mixed potential sensor response. Sens. Actuators B 2013, 183, 20–24. [Google Scholar] [CrossRef]
- Sharma, M.; Pathak, M.; Kapoor, P.N. The sol-gel method: Pathway to ultrapure and homogeneous mixed metal oxide nanoparticles. Asian J. Chem. 2018, 30, 1405–1412. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef]
- Kirankumar, V.S.; Sumathi, S. A review on photodegradation of organic pollutants using spinel oxides. Mater. Today Chem. 2020, 18, 100355. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, S.B.; Zhao, F.; Safaei, Z.; Srivastava, V.; Lakshmi Ramasamy, D.; Iftekhar, S.; Kalliola, S.; Sillanpää, M. Degradation and mineralization of phenol in aqueous medium by heterogeneous monopersulfate activation on nanostructured cobalt based-perovskite catalysts ACoO3 (A = La, Ba, Sr and Ce): Characterization, kinetics and mechanism study. Appl. Catal. B Environ. 2017, 215, 60–73. [Google Scholar] [CrossRef]
- Pang, X.; Guo, Y.; Zhang, Y.; Xu, B.; Qi, F. LaCoO3 perovskite oxide activation of peroxymonosulfate for aqueous 2-phenyl-5-sulfobenzimidazole degradation: Effect of synthetic method and the reaction mechanism. Chem. Eng. J. 2016, 304, 897–907. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, X.; Zhang, Y.; Yao, Q.; Qian, Y.; Chu, H.; Chen, J. Carbamazepine degradation by heterogeneous activation of peroxymonosulfate with lanthanum cobaltite perovskite: Performance, mechanism and toxicity. J. Environ. Sci. 2020, 91, 10–21. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Gimeno, O. Removal of aqueous metazachlor, tembotrione, tritosulfuron and ethofumesate by heterogeneous monopersulfate decomposition on lanthanum-cobalt perovskites. Appl. Catal. B Environ. 2017, 200, 83–92. [Google Scholar] [CrossRef]
- Rao, Y.; Han, F.; Chen, Q.; Wang, D.; Xue, D.; Wang, H.; Pu, S. Efficient degradation of diclofenac by LaFeO3-Catalyzed peroxymonosulfate oxidation—kinetics and toxicity assessment. Chemosphere 2019, 218, 299–307. [Google Scholar] [CrossRef]
- Rao, Y.F.; Zhang, Y.; Han, F.; Guo, H.; Huang, Y.; Li, R.; Qi, F.; Ma, J. Heterogeneous activation of peroxymonosulfate by LaFeO3 for diclofenac degradation: DFT-assisted mechanistic study and degradation pathways. Chem. Eng. J. 2018, 352, 601–611. [Google Scholar] [CrossRef]
- Miao, J.; Li, J.; Dai, J.; Guan, D.; Zhou, C.; Zhou, W.; Duan, X.; Wang, S.; Shao, Z. Postsynthesis Oxygen Nonstoichiometric Regulation: A New Strategy for Performance Enhancement of Perovskites in Advanced Oxidation. Ind. Eng. Chem. Res. 2020, 59, 99–109. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ma, X.; Miao, J.; Ran, R.; Zhou, W.; Shao, Z. Nonstoichiometric perovskite for enhanced catalytic oxidation through excess A-site cation. Chem. Eng. Sci. 2020, 219, 115596. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, Y.-C.; Lin, T.-Y.; Yang, H. Lanthanum Cobaltite Perovskite supported on Zirconia as an Efficient Hetero-geneous Catalyst for Activating Oxone in Water. J. Colloid Interface Sci. 2017, 497, 325–332. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by LaCoO3/Al2O3 catalyzed peroxymonosulfate: Performance, degradation intermediates and mechanism. Chem. Eng. J. 2019, 372, 796–808. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Yang, C.; Du, C.; Teng, Q.; Ma, Y.; Zhang, D.; Nie, L.; Zhong, Y. Enhanced activation of peroxymonosulfte by LaFeO3 perovskite supported on Al2O3 for degradation of organic pollutants. Chemosphere 2019, 237, 124478. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Chen, Y.C.; Lin, Y.F. LaMO3 perovskites (M = Co, Cu, Fe and Ni) as heterogeneous catalysts for activating peroxymonosulfate in water. Chem. Eng. Sci. 2017, 160, 96–105. [Google Scholar] [CrossRef]
- Gao, P.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Wang, Y. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration. Chem. Eng. J. 2019, 359, 828–839. [Google Scholar] [CrossRef]
- Su, C.; Duan, X.; Miao, J.; Zhong, Y.; Zhou, W.; Wang, S.; Shao, Z. Mixed Conducting Perovskite Materials as Superior Catalysts for Fast Aqueous-Phase Advanced Oxidation: A Mechanistic Study. ACS Catal. 2017, 7, 388–397. [Google Scholar] [CrossRef]
- Miao, J.; Sunarso, J.; Su, C.; Zhou, W.; Wang, S.; Shao, Z. SrCo1-xTixO3-δ perovskites as excellent catalysts for fast degradation of water contaminants in neutral and alkaline solutions. Sci. Rep. 2017, 7, 44215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miao, J.; Duan, X.; Guan, D. Post-synthesis growth of CoOOH nanostructure on SrCo0.6Ti0.4O3−δ perovskite surface for enhanced degradation of aqueous organic contaminants. ACS Sustain. Chem. Eng. 2018, 6, 15737–15748. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef]
- Miao, J.; Sunarso, J.; Duan, X.; Zhou, W.; Wang, S.; Shao, Z. Nanostructured Co-Mn containing perovskites for degradation of pollutants: Insight into the activity and stability. J. Hazard. Mater. 2018, 349, 177–185. [Google Scholar] [CrossRef]
- Miao, J.; Duan, X.; Li, J.; Dai, J.; Liu, B.; Wang, S.; Zhou, W. Enhanced performance of LaFeO3 perovskite for peroxymonosulfate activation through strontium doping towards 2,4-D degradation. Chem. Eng. J. 2019, 355, 721–730. [Google Scholar] [CrossRef]
- Duan, X.; Su, C.; Miao, J.; Zhong, Y.; Shao, Z.; Wang, S.; Sun, H. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals. Appl. Catal. B Environ. 2018, 220, 626–634. [Google Scholar] [CrossRef]
- Luo, X.; Bai, L.; Xing, J.; Zhu, X.; Xu, D.; Xie, B.; Gan, Z.; Li, G.; Liang, H. Ordered Mesoporous Cobalt Containing Perovskite as a High-Performance Heterogeneous Catalyst in Activation of Peroxymonosulfate. ACS Appl. Mater. Interfaces 2019, 11, 35720–35728. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cheng, C.; Zhu, J.; Wang, L.; Gao, S.; Xia, X. Enhanced degradation of atrazine by nanoscale LaFe1-xCuxO3-δ perovskite activated peroxymonosulfate: Performance and mechanism. Sci. Total Environ. 2019, 673, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yao, Z.; Ye, H.; Zhang, C.; Liang, P.; Sun, H.; Wang, S.; Liu, S. Efficient removal of organic pollutants by ceramic hollow fibre supported composite catalyst. Sustain. Mater. Technol. 2019, 20, e00108. [Google Scholar] [CrossRef]
- Ma, T.; Liu, L.; Meng, B.; Gao, J.; Wang, S.; Liu, S. Heterogeneous activation of peroxymonosulfate via a Ag-La0.8Ca0.2Fe0.94O3−Δ perovskite hollow fibre membrane reactor for dye degradation. Sep. Purif. Technol. 2019, 211, 298–302. [Google Scholar] [CrossRef]
- Chu, Y.; Tan, X.; Shen, Z.; Liu, P.; Han, N.; Kang, J.; Duan, X.; Wang, S.; Liu, L.; Liu, S. Efficient removal of organic and bacterial pollutants by Ag-La0.8Ca0.2Fe0.94O3-Δ perovskite via catalytic peroxymonosulfate activation. J. Hazard. Mater. 2018, 356, 53–60. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, Z.; Wang, Z.; Zhang, H.; Qiu, Y.; Yin, D.; Zhao, G. 3D hollow sphere-like Cu-incorporated LaAlO3 perovskites for peroxymonosulfate activation: Coaction of electron transfer and oxygen defect. Chem. Eng. J. 2020, 385, 123935. [Google Scholar] [CrossRef]
- Gkika, C.; Petala, A.; Frontistis, Z.; Bampos, G.; Hela, D.; Konstantinou, I.; Mantzavinos, D. Heterogeneous activation of persulfate by lanthanum strontium cobaltite for sulfamethoxazole degradation. Catal. Today 2020. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Martínez, F.; Melero, J.A.; Milieni, A. Catalytic wet peroxide oxidation of phenolic solutions over a LaTi1-xCuxO3 perovskite catalyst. Appl. Catal. B Environ. 2004, 47, 281–294. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Qiao, X.; Wang, D.; Cai, X. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using Acid Orange 7 as a model compound. Appl. Catal. B Environ. 2008, 80, 116–121. [Google Scholar] [CrossRef]
- Zuo, Z.; Cai, Z.; Katsumura, Y.; Chitose, N.; Muroya, Y. Reinvestigation of the acid-base equilibrium of the (bi)carbonate radical and ph dependence of its reactivity with inorganic reactants. Radiat. Phys. Chem. 1999, 55, 15–23. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Siddiqi, W.A. Perovskite-type catalytic materials for water treatment. In Hybrid Perovskite Composite Materials Design to Applications; Khan, I., Khan, A., Khan, M.M.A., Khan, S., Verpoort, F., Umar, A., Eds.; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, H.; Croué, J.P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Degradation of carbamazepine by radiation-induced activation of peroxymonosulfate. Chem. Eng. J. 2018, 336, 595–601. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Dionysiou, D.D. Nanocrystalline cobalt oxide immobilized on titanium dioxide nanoparticles for the heterogeneous activation of peroxymonosulfate. Appl. Catal. B Environ. 2007, 74, 170–178. [Google Scholar] [CrossRef]
- Yamaguchi, T. Application of ZrO2 as a catalyst and a catalyst support. Catal. Today 1994, 20, 199–217. [Google Scholar] [CrossRef]
- Kustov, A.L.; Tkachenko, O.P.; Kustov, L.M.; Romanovsky, B.V. Lanthanum cobaltite perovskite supported onto mesoporous zirconium dioxide: Nature of active sites of VOC oxidation. Environ. Int. 2011, 37, 1053–1056. [Google Scholar] [CrossRef]
- Taran, O.P.; Ayusheev, A.B.; Ogorodnikova, O.L.; Prosvirin, I.P.; Isupova, L.A.; Parmon, V.N. Perovskite-like catalysts LaBO3 (B = Cu, Fe, Mn, Co, Ni) for wet peroxide oxidation of phenol. Appl. Catal. B Environ. 2016, 180, 86–93. [Google Scholar] [CrossRef]
- Guo, W.; Su, S.; Yi, C.; Ma, Z. Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system. Environ. Prog. Sustain. Energy 2013, 32, 193–197. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W.; Xu, B. Modeling the heterogeneous peroxymonosulfate/Co-MCM41 process for the degradation of caffeine and the study of influence of cobalt sources. Chem. Eng. J. 2014, 235, 1–378. [Google Scholar] [CrossRef]
- Dai, D.; Yang, Z.; Yao, Y.; Chen, L.; Jia, G.; Luo, L. Highly efficient removal of organic contaminants based on peroxymonosulfate activation by iron phthalocyanine: Mechanism and the bicarbonate ion enhancement effect. Catal. Sci. Technol. 2017, 7, 934–942. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Wu, G.; Wei, F.; Li, X.; Chen, H.; Wang, S. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water. J. Hazard. Mater. 2015, 296, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.T.; Wang, R.; Webster, R.D.; Hu, X. Urea-assisted one-step synthesis of cobalt ferrite impregnated ceramic membrane for sulfamethoxazole degradation via peroxymonosulfate activation. Chem. Eng. J. 2018, 343, 737–747. [Google Scholar] [CrossRef]
- Gao, D.; Junaid, M.; Lin, F.; Zhang, S.; Xu, N. Degradation of sulphachloropyridazine sodium in column reactor packed with CoFe2O4−loaded quartz sand via peroxymonosulfate activation: Insights into the amorphous phase, efficiency, and mechanism. Chem. Eng. J. 2020, 390, 124549. [Google Scholar] [CrossRef]
- Chen, L.; Ding, D.; Liu, C.; Cai, H.; Qu, Y.; Yang, S.; Gao, Y.; Cai, T. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration. Chem. Eng. J. 2018, 334, 273–284. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.; Feng, Y.; Zhou, Y.; Wen, L.; Shih, K. Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites. Chem. Eng. J. 2020, 383, 123056. [Google Scholar] [CrossRef]
- Lashkaryani, E.B.; Kakavandi, B.; Kalantary, R.R.; Jafari, A.J.; Gholami, M. Activation of peroxymonosulfate into amoxicillin degradation using cobalt ferrite nanoparticles anchored on graphene (CoFe2O4 @Gr). Toxin Rev. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Xu, L.J.; Chu, W.; Gan, L. Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chem. Eng. J. 2015, 263, 435–443. [Google Scholar] [CrossRef]
- Pi, Y.; Gao, H.; Cao, Y.; Cao, R.; Wang, Y.; Sun, J. Cobalt ferrite supported on carbon nitride matrix prepared using waste battery materials as a peroxymonosulfate activator for the degradation of levofloxacin hydrochloride. Chem. Eng. J. 2020, 379, 122377. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Xu, S.; Fu, H.; Li, Z.; Li, K.; Sheng, K.; Du, J.; Lu, X.; Li, X.; et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chem. Eng. J. 2020, 387, 124094. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Y.J.; Lu, Y.A.; Ma, X.Y.; Feng, S.F.; Gao, N.; Li, J. Synthesis of magnetic CoFe2O4/ordered mesoporous carbon nanocomposites and application in Fenton-like oxidation of rhodamine B. Environ. Sci. Pollut. Res. 2017, 24, 14396–14408. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Yang, M.T.; Lin, J.T.; Du, Y. Cobalt ferrite nanoparticles supported on electrospun carbon fiber as a magnetic heterogeneous catalyst for activating peroxymonosulfate. Chemosphere 2018, 208, 502–511. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Niu, P.; Li, C.; Jia, C.; Wang, D.; Liu, S. Facile synthesis of CoFe2O4 magnetic nanomaterial by natural cellulose template and catalytic performance in heterogeneous activation of peroxymonosulfate. J. Sol-Gel Sci. Technol. 2020, 93, 419–427. [Google Scholar] [CrossRef]
- Shao, S.; Qian, L.; Zhan, X.; Wang, M.; Lu, K.; Peng, J.; Miao, D.; Gao, S. Transformation and toxicity evolution of amlodipine mediated by cobalt ferrite activated peroxymonosulfate: Effect of oxidant concentration. Chem. Eng. J. 2020, 382, 123005. [Google Scholar] [CrossRef]
- Song, Q.; Feng, Y.; Wang, Z.; Liu, G.; Lv, W. Degradation of triphenyl phosphate (TPhP) by CoFe2O4-activated peroxymonosulfate oxidation process: Kinetics, pathways, and mechanisms. Sci. Total Environ. 2019, 681, 331–338. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Zhang, H.; Zhou, Z.; Fan, G.; Ma, J. Cobalt ferrite nanoparticles supported on drinking water treatment residuals: An efficient magnetic heterogeneous catalyst to activate peroxymonosulfate for the degradation of atrazine. Chem. Eng. J. 2019, 367, 208–218. [Google Scholar] [CrossRef]
- Oh, D.; Lee, C.S.; Kang, Y.G.; Chang, Y.S. Hydroxylamine-assisted peroxymonosulfate activation using cobalt ferrite for sulfamethoxazole degradation. Chem. Eng. J. 2020, 386, 123751. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Hu, X. Synergistic coupling Co3Fe7 alloy and CoFe2O4 spinel for highly efficient removal of 2,4-dichlorophenol by activating peroxymonosulfate. Chemosphere 2020, 242, 125244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhao, J.; Shen, N.; Ma, T.; Su, Y.; Ren, H. Efficient degradation of 2,4-dichlorophenol in aqueous solution by peroxymonosulfate activated with magnetic spinel FeCo2O4 nanoparticles. Chemosphere 2018, 197, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.; Abdelraheem, W.H.; Han, C.; Nadagouda, M.N.; Sygellou, L.; Arfanis, M.K.; Falaras, P.; Sharma, V.K.; Dionysiou, D.D. Cobalt ferrite nanoparticles with controlled composition-peroxymonosulfate mediated degradation of 2-phenylbenzimidazole-5-sulfonic acid. Appl. Catal. B Environ. 2018, 221, 266–279. [Google Scholar] [CrossRef]
- Lin, C.; Shi, D.; Wu, Z.; Zhang, L.; Zhai, Z.; Fang, Y.; Sun, P.; Han, R.; Wu, J.; Liu, H. CoMn2O4 catalyst prepared using the sol-gel method for the activation of peroxymonosulfate and degradation of UV filter 2-phenylbenzimidazole-5-sulfonic acid (PBSA). Nanomaterials 2019, 9, 774. [Google Scholar] [CrossRef]
- Li, C.X.; Chen, C.B.; Lu, J.Y.; Cui, S.; Li, J.; Liu, H.Q.; Li, W.W.; Zhang, F. Metal organic framework-derived CoMn2O4 catalyst for heterogeneous activation of peroxymonosulfate and sulfanilamide degradation. Chem. Eng. J. 2018, 337, 101–109. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L.; Lan, Y.; Li, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Liu, Y.; Zou, B.; Xiao, J.; Ma, J. Degradation of organic pollutants by NiFe2O4/peroxymonosulfate: Efficiency, influential factors and catalytic mechanism. RSC Adv. 2016, 6, 11040–11048. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Wu, D.; Zhou, Z.; Deng, Y.; Zhang, T.; Shih, K. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Optimization of the catalytic activity of a ZnCo2O4 catalyst in peroxymonosulfate activation for bisphenol A removal using response surface methodology. Chemosphere 2018, 212, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Lyu, L.; Hu, C. Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl. Catal. B Environ. 2020, 51, 11288–11296. [Google Scholar] [CrossRef]
- Tan, Y.; Li, C.; Sun, Z.; Bian, R.; Dong, X.; Zhang, X.; Zheng, S. Natural diatomite mediated spherically monodispersed CoFe2O4 nanoparticles for efficient catalytic oxidation of bisphenol A through activating peroxymonosulfate. Chem. Eng. J. 2020, 51, 12611–12618. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Y.; Zhang, Q.; Hong, J.; Wang, J.; She, Y. A novel magnetic heterogeneous catalyst oxygen-defective CoFe2O4−x for activating peroxymonosulfate. Appl. Surf. Sci. 2019, 480, 717–726. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef]
- Chen, C.B.; Zhang, F.; Li, C.X.; Lu, J.Y.; Cui, S.; Liu, H.Q.; Li, W.W. A magnetic CoFe2O4-CNS nanocomposite as an efficient, recyclable catalyst for peroxymonosulfate activation and pollutant degradation. RSC Adv. 2017, 7, 55020–55025. [Google Scholar] [CrossRef]
- Dung, N.T.; Thu, T.V.; Van Nguyen, T.; Thuy, B.M.; Hatsukano, M.; Higashimine, K.; Maenosono, S.; Zhong, Z. Catalytic activation of peroxymonosulfate with manganese cobaltite nanoparticles for the degradation of organic dyes. RSC Adv. 2020, 10, 3775–3788. [Google Scholar] [CrossRef]



| SR-AOPs | Photo-Fenton | |
|---|---|---|
| Radical species | (SO4•−) | (•OH) |
| Oxidation potential (V) | 2.5–3.1 | 2.8 |
| Selectivity | Reacts selectively | Reacts non selectively |
| Main reaction mechanism | Electron transfer | Electron transfer/Hydrogen atom abstraction |
| Half-life | 30–40 μs | 20 ns |
| pH range | 2–8 | 2–4 |
| Properties | PMS | PS | References |
|---|---|---|---|
| Formula | HSO5− | S2O82− | |
| Structure | 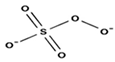 | 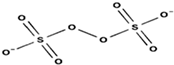 | |
| Molecular weight (g/mol) | 113.07 (614.738 as Oxone®) | 192.12 (238.03 as sodium-PS) | |
| Solubility in H2O at 25 °C | >250 1 | 730 2 | [3] |
| Oxidation potential (V) | 1.8 | 2.01 | |
| O-O bond dissociation energy (kJ/mol) | 377 | 92 | [3] |
| O-O bond length (Å) | 1.460 | 1.493 | [20] |
| Preferred activation method | Activation via electron transfer (metal-catalysts and nanocarbons) | Activation via energy transfer (thermolysis, photolysis, etc.) | [21] |
| Concentration | Treatment Efficiency | |||||||
|---|---|---|---|---|---|---|---|---|
| Catalyst | Synthesis Method | Target Pollutant | Catalyst (g L−1) | Pollutant (mg L−1) | PMS (mM) | Degradation | TOC Removal | Reference |
| CoFe2O4/Al2O3 | Combustion | SMX | 0.15 | 10 | 0.66 | 90% (90 min) | - | [98] |
| CoFe2O4-QS | Combustion | SCP | 5 | 20 | 0.5 | 90% (30 min) | 6.1% (30 min) | [99] |
| CoFe2O4-GO | Hydrothermal treatment—ultrasound | NOR | 0.3 | 4.8 | 0.5 | 100% (20 min) | 64.1% (60 min) | [100] |
| CoFe2O4-rGO | Hydrothermal treatment | OFX | 0.1 | 14 | 1 | 100% (60 min) | - | [101] |
| CFZ | 0.1 | 18 | 0.1 | 100% (60 min) | - | |||
| CoFe2O4/Gr | Hydrothermal treatment | AMX | 0.5 | 20 | 3 | 99.3% (60 min) | 61.1% (60 min) | [102] |
| Hydrothermal treatment | DMP | 0.5 | 9.7 | 2 | 100% (30 min) | 24% (60 min) | [103] | |
| CoFe2O4/TNTs | Hydrothermal treatment—combustion | RhB | 0.02 | 100 | 26 | 97% (60 min) | 50% (60 min) | [51] |
| Phenol | 0.02 | 20 | 20 | 97.2% (60 min) | 81.3% (60 min) | |||
| CoFe2O4/CN | Hydrothermal treatment with melamine | LVF | 0.15 | 10 | 0.5 | 89.4% (60 min) | 30% (40 min) | [104] |
| CoFe2O4/HPC | Pyrolysis | BPA | 0.05 | 10 | 3.3 | 86% (8 min) | - | [105] |
| CoFe2O4/OMC | Hydrothermal treatment | RhB | 0.05 | 100 | 1.5 | 92.7% (60 min) | - | [106] |
| CoFe2O4/OMC | Hydrothermal treatment | RhB | 0.05 | 100 | 1.5 | 92.7% (60 min) | - | [106] |
| CoFe2O4/CCNF | Hydrothermal treatment | DMP | 0.5 | 9.7 | 1.5 | 100% (30 min) | - | [107] |
| CF@CNF | Electrospinning techniques | Amaranth dye | 0.05 | 10 | 1.3 | 100% (120 min) | - | [108] |
| CoFe2O4 | Hydrothermal treatment | ATZ | 0.4 | 10 | 0.8 | 99% (30 min) | 27% (30 min) | [109] |
| CoFe2O4 | Sol-Gel process | AO7 | 0.5 | 20 | 0.8 | 96% (40 min) | - | [110] |
| AML | 0.04 | 4 | 10 | 92.5% (30 min) | 70% (120 min) | [111] | ||
| CoFe2O4 | Sol-Gel process | TPhP | 0.25 | 3.3 | 0.2 | 80% (30 min) | <7% (180 min) | [112] |
| CoFe2O4/WTRs | co-precipitation combustion | ATZ | 0.03 | 2 | 0.25 | 98.2% (20 min) | - | [113] |
| CoFe2O4/HA | co-precipitation | SMX | 0.1 0.1 mM HA | 20 | 1 | 96% SMX (120 min) | - | [114] |
| Co3O7-CoFe2O4 | Pyrolysis | 2,4-DCP | 0.05 | 50 | 8.2 | 93.8% 2,4-DCP (30 min) | 55.7% (30 min) | [115] |
| FeCo2O4 | Sol-Gel | 2,4-DCP | 0.06 | 100 | 4 | 95.8% (90 min) | 44.7% (90 min) | [116] |
| CoxFe3−xO4 (x = 0.1, 0.5, 0.7 and 1) | Combustion | PBSA | 0.17 | 11 | 0.1 | 70% (240 min) | 32% (240 min) | [117] |
| CoMn2O4 | Sol-Gel | PBSA | 0.05 | 5 | 1.6 | 93.7% (60 min) | 30% (30 min) | [118] |
| CoMn2O4/MD | Solvothermal | SA | 0.05 | 10 | 0.66 | 100% (30 min) | - | [119] |
| CoxMn2−xO4 (8:1, 4:1, 2:1, 1:2, 1:4 and 1:8) | Precipitation | TCS | 0.02 | 10 | 0.33 | 96.4% (30 min) | - | [50] |
| CuCo2O4 | Combustion | SMZ | 0.1 | 50 | 6.5 | 98% (30 min) | - | [120] |
| NiFe2O4 | Combustion | BA | 0.1 | 1.2 | 1 | 82.5% (60 min) | - | [121] |
| CuCo2O4 | Solvothermal | SMZ | 0.01 | 5 | 0.13 | 87.5% (20 min) | - | [122] |
| ZnCo2O4 | Microwave-assisted | BPA | 0.2 | 20 | 0.26 | 99.2% (5 min) | 70% (30 min) | [123] |
| ZnFeCoO4 | Sol-gel and combustion | BPA | 0.2 | 10 | 1 | 100% (4 min) | - | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems. Catalysts 2020, 10, 1299. https://doi.org/10.3390/catal10111299
Manos D, Miserli K, Konstantinou I. Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems. Catalysts. 2020; 10(11):1299. https://doi.org/10.3390/catal10111299
Chicago/Turabian StyleManos, Donatos, Kleopatra Miserli, and Ioannis Konstantinou. 2020. "Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems" Catalysts 10, no. 11: 1299. https://doi.org/10.3390/catal10111299
APA StyleManos, D., Miserli, K., & Konstantinou, I. (2020). Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems. Catalysts, 10(11), 1299. https://doi.org/10.3390/catal10111299