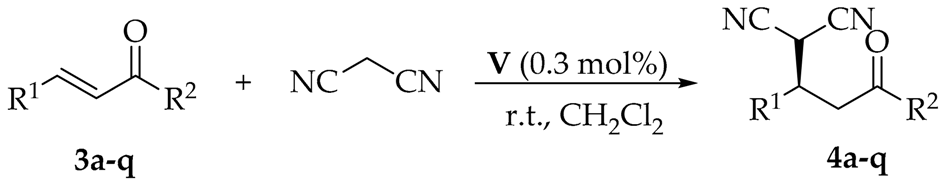Abstract
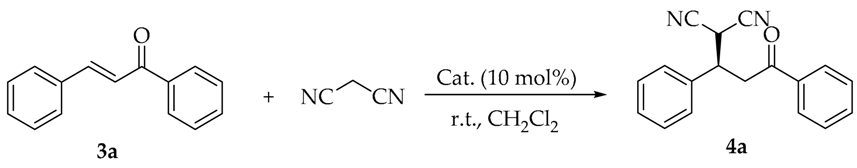
A rosin-derived bifunctional squaramide catalyzed asymmetric Michael addition of malononitrile with chalcones was discovered. This protocol provides a methodology for the facile synthesis of chiral γ-cyano carbonyl compounds in high yields and enantioselectivities (up to 99% yield and 90% ee) with a lower catalyst loading (0.3 mol%). The predominant R-configured adducts were obtained by this organocatalystic reaction, according to the experimental findings.
1. Introduction
The Michael reaction of carboanion nucleophiles to activated olefins represents a powerful type of the most remarkable transformations for the new carbon–carbon bond formation in modern organic synthesis, and has been immensely exploited over the past few decades [1,2,3,4,5,6,7]. Among the versatile nucleophiles, the employment of malononitrile for asymmetric Michael addition has received extensive attention since its nitrile group could be efficiently converted to valuable functionalities [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. To date, a few research groups have devoted their efforts to the catalytic asymmetric Michael reaction of malononitrile onto chalcones and their analogues, by either metal-catalytic [19,20] or organocatalytic [21,22,23,24,25,26,27] methods. Despite those gratifying advances, it should be reminded that most of the ligands and organocatalysts utilized in this transformation are commonly cinchona alkaloid-type. Therefore, to seek an efficient catalytic system with a novel organocatalyst is still a challenging and interesting task.
Rosin-derived bifunctional thiourea organocatalysts, originated from the abundantly available natural rosin, were revealed to be highly efficient for some catalytic asymmetric reactions, including Aza-Henry [28], Mannich reaction [29,30], Aldol reaction [31,32], Michael addition [33,34,35,36], and Friedel-Crafts alkylation [37]. The thiourea moiety of those organocatalysts is usually introduced at position C-4 of rosin skeleton, while the tertiary amine moiety is either 1,2-diaminocyclohexane 1 or cinchona alkaloid 2 (Figure 1). In addition to thiourea, squaramide is also a good hydrogen-bonding donor and has been successfully applied to facilitate various asymmetric transformations [38,39,40,41,42,43]. Recently, we have developed several bifunctional squaramide catalysts at position C-4 or C-7 of rosin scaffold, which exhibited excellent enantioselectivities in asymmetric catalytic 1,3-dipolar cycloaddition reactions [44] and Michael/cyclization cascade reactions [45]. However, to the best of our knowledge, rosin-derived chiral squaramides have not been applied for the enantioselective Michael reaction of malononitrile and chalcones. As our ongoing interest in organocatalysis of rosin-derived catalysts, we herein reported the results from the asymmetric Michael addition of malononitrile with chalcones catalyzed by rosin-derived bifunctional squaramide organocatalysts.
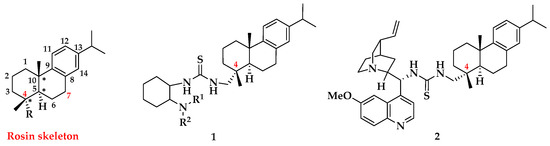
Figure 1.
Representative thiourea organocatalysts based on rosin skeleton.
2. Results and Discussion
2.1. Screening of the Catalysts for the Asymmetirc Michael Addtion
Initial investigation started with testing several rosin-derived bifunctional catalysts in a model reaction of malononitrile to trans-chalcone 3a with 10 mol% catalyst loading in CH2Cl2 at room temperature (Figure 2 and Table 1). The Michael reaction proceeded smoothly by thiourea catalyst I, affording the product 4a with 88% yield and 76% ee (entry 1). Unexpectedly, thiourea catalyst II exhibited inferior catalytic activity merely due to the opposite configuration at the position of C-7 of rosin skeleton, and enantioselectivity decreased sharply with the reversed absolute configuration (entry 2, 31% yield, and 26% ee). Those results displayed that the configuration at the position of C-7 plays a crucial role in the control of reactivity and enantioselectivity. Thus, we would like to affirm S-configuration at C-7 as the optimal for the choice of catalysts.
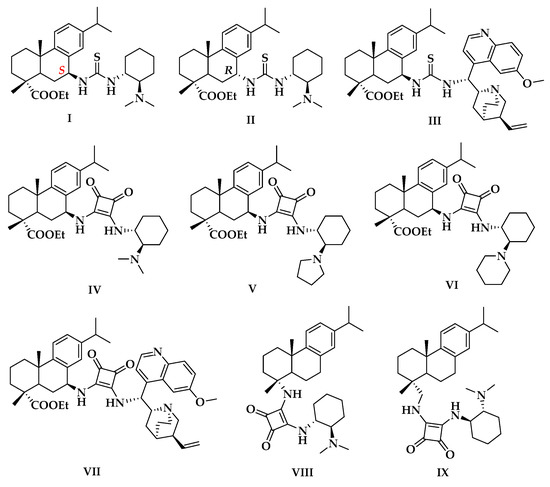
Figure 2.
Rosin-derived bifunctional catalysts for this study.

Table 1.
Effect of catalysts on the model reaction 1.
When thiourea catalyst III was used, both reactivity and enantioselectivity dropped deeply (entry 3 vs. entry 1). This phenomenon may be due to steric effects around the hydrogen-bonding donor of the catalyst derived from more sterically bulky quinine instead of 1,2-diaminocyclohexane. By contrast, squaramide catalysts IV–VI were better catalysts, which could promote this reaction steadily in very good to excellent yields (85%–>99%) and high ees (80–90%) (entries 4–6). In terms of reactivity and enantioselectivity, catalyst V gave the best results (entry 5, >99% yield, and 90% ee). Notably, like the sterically hindered thiourea catalyst III, squaramide catalyst VII resulted in the same poor outcome of this reaction, but with the reversed absolute configuration (entry 7, 20% yield, and 23% ee). Moreover, squaramide catalysts VIII and IX were also surveyed, whose squaramide moiety were introduced at C-4 of rosin skeleton. They could promote the reaction smoothly as well, however, enantioselectivities of the desired product were moderate (entries 8 and 9).
2.2. Optimization of the Reaction Conditions
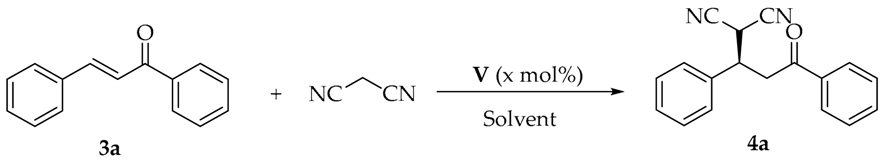
Having identified the optimal squaramide catalyst V for this Michael reaction, we looked forward to subsequent screening of other reaction conditions (Table 2). Optimization studies show that the solvent had a significant influence on the reactivity and enantioselectivity of this transformation (entries 1–7). It was observed that dicloromethane and chloroform were the best solvents. Considering the toxicity of chloroform, dicloromethane was used as the best solvent for further studies. Also, different temperatures were investigated for this reaction, when the reaction was conducted under 0 °C or −20 °C, enantioselectivities would not greatly improve, while the reactivities dramatically declined (entries 8 and 9). Gradually reducing catalyst loading did not make any obvious difference from the enantioselectivities, whereas the yield of the reaction became worse (entries 10–14). To our delight, the catalytic activity could be notably enhanced when the reaction was carried out in 0.5 mL CH2Cl2 with 0.3 mol% catalyst loading (entry 15). After taking many factors into consideration, including reactivity, enantioselectivity, catalyst loading, and solvent volume, the asymmetric Michael reaction of malononitrile to trans-chalcone 3a could achieve high yield and ee value (87% yield and 90% ee, entry 15) in the presence of squaramide catalyst V with a lower catalyst loading (0.3 mol%) in CH2Cl2 (0.5 mL) at room temperature compared with the present literature data [21,22,23,24,25,26,27], where only chiral quinine-derived squaramide organocatalyst prepared for the same model reaction by Du [25] could show high activity and enatioselectivity (82% yield and 89% ee) under 0.5 mol% catalyst loading.

Table 2.
Optimization of the reaction conditions 1.
2.3. The Scope of the Asymmetric Michael Reaction
After the optimal reaction conditions were established, the scope of the asymmetric Michael reaction of malononitrile with various trans-chalcones 3a–o and the analogues 3p–q were investigated. The results were summarized in Table 3. The electronic property of different substituents on the aromatic ring (R1) of trans-chalcones 3b–h had no significant effect on the enantioselectivities. In detail, the trans-chalcones bearing electron-donating groups (Me and OMe, entries 2–3) or electron-withdrawing groups (-F, Cl, and Br, entries 4–6) at the 4-position of the aromatic ring led to high enantioselectivities with 85–90% ee. However, the radius of the halogen noticeably impacted the reactivities of chalcones. Furthermore, the stereoselectivities and reactivities of this reaction rely on the position of the aryl substituents. The substrates bearing the chloro at 3-position (entry 7), methoxy group at 3-position (entry 8), and 2-position (entry 9) of the aromatic ring were all converted to the corresponding products, but the enantioselectivities and yields markedly decreased due to the position and steric hindrance. When trans-chalcones 3j–n with different electronic substituents on the aromatic ring (R2) were used in this reaction (entries 10–14), the same results were observed as the substituents (R1) in 4-position of the aromatic ring. Substrate 3o with 4-Me substituent on both R1 and R2 phenyl reacted with malononitrile smoothly to afford the corresponding adduct with good yield and high enantioselectivity (70% yield and 86% ee, entry 15). Other chalcone analogues, such as 2-enoylpyridine 3p and 1-naphthlaldehyde derivative 3q, were also tested, both giving low results (entries 16–17). The pyridine nitrogen of substrate 3p might involve the hydrogen bonding of the squaramide catalyst V in the H-bond framework to change the steric environment of the catalytic system, which made the activity of catalyst fade out, leading to lower reactivity and enantioselectivity [26]. Poor results of substrate 3q may be due to steric hindrance, similar to substrate 3i.

Table 3.
Scope of the asymmetric Michael reaction 1.
2.4. Plausible Transition-State Model of the Asymmetric Michael Reaction
Based on literature reports [25,26,27,43] and the predominant R-configured product 4a, the feasible activation model for the asymmetric Michael reaction was proposed via cooperative catalysis of the squaramide functionality and the tertiary amino group of the rosin-derived squaramide V (Figure 3). The squaramide moiety activated trans-chalcone 3a through bidentate hydrogen bonds. Simultaneously, α-proton of malononitrile was captured by the basic tertiary nitrogen to form an active carbanion. Subsequent addition of the carbanion to the Si-face of 3a led to the desired adduct as major stereoisomer, which is in agreement with the observed experimental results.
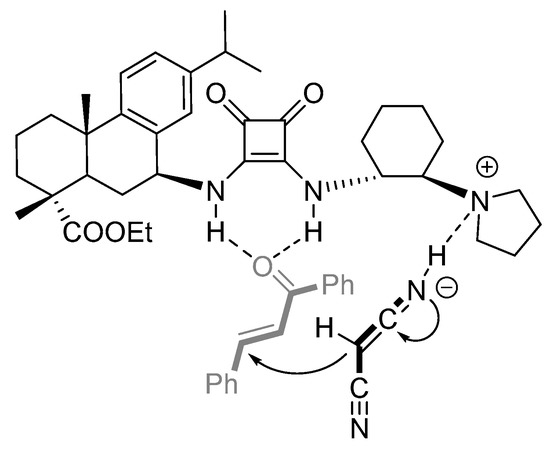
Figure 3.
Plausible transition-state model.
3. Experimental Section
3.1. General Information
Unless noted otherwise, all commercial reagents were purchased from chemical reagent suppliers (Alfa Aesar Chemical Co. Ltd., Shanghai, China; Sigma-Aldrich Chemical Company, Darmstadt, Germany; and Aladdin Chemical Co. Ltd., Shanghai, China) and used as received, without further purification. Isolation of the crude products was accomplished by flash chromatography on silica gel (200–300 mesh, Qingdao Sea Chemical Reagent Co. Ltd., Qingdao, Shandong, China). Thin layer chromatography (TLC) analysis was carried out using EM separations percolated TLC sheets (silica gel GF254, 0.2 mm, Qingdao Sea Chemical Reagent Co. Ltd., Qingdao, Shandong, China) under UV light. 1H NMR spectra were obtained with a Bruker Avance III spectrometer (400 MHz, Switzerland). Chemical shifts were published as parts per million (ppm) in δ units internally, with tetramethylsilane (TMS, δ = 0.00 ppm) as the referenced standard. The enantiomeric excesses (ee) were determined by HPLC analyses using a Shimadzu 10A instrument (Japan) with Daicel Chiralcel OD-H or AD-H column (0.46 cm diameter × 25 cm length) in comparison with racemic samples and n-hexane/i-PrOH as the eluent. Known adducts 4b, 4e, and 4j were assigned as R-configuration by HPLC comparisons with the reported data [23], respectively, and the absolute configurations of other products were confirmed by analogy with compounds 4b, 4e, and 4j. All the rosin-derived chiral squaramide organocatalysts I–IX were synthesized according to the literature procedures [25,28,29,46]. All 1H NMR and HPLC spectra of compounds 4a-q could be found in Supplementary Materials.
3.2. Typical Procedure for the Michael Addition
Catalyst V (7.1 mg, 0.012 mmol) was dissolved into dicloromethane to prepare the solution of catalyst V (20.0 mL, 0.6 mmol/L). To the above solution (0.5 mL, containing catalyst 0.0003 mmol, 0.3 mol%) was added malononitrile (8 mg, 0.12 mmol), chalcones 3 (0.1 mmol) subsequently. The resulting mixture was then stirred at room temperature for 36 h. The corresponding adducts 4 were isolated through flash silica gel chromatography (eluent, ethyl acetate/petroleum ether).
(R)-2-(3-oxo-1,3-diphenylpropyl)malononitrile (4a) [19,25]: 99% yield; white solid; 90% ee, determined by HPLC (Daicel Chiralcel OD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 23.8 min (major), 38.6 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 7.3 Hz, 2H), 7.65–7.60 (m, 1H), 7.52–7.42 (m, 7H), 4.65 (d, J = 5.1 Hz, 1H), 3.96 (dt, J = 8.1, 5.2 Hz, 1H), 3.71–3.66 (m, 2H).
(R)-2-(3-oxo-3-phenyl-1-p-tolylpropyl)malononitrile (4b) [19,23,25]: 97% yield; colorless oil; 86% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 10.8 min (major), 15.6 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.98–7.93 (m, 2H), 7.64–7.58 (m, 1H), 7.48 (dd, J = 10.6, 4.8 Hz, 2H), 7.33 (d, J = 8.1 Hz, 2H), 7.24–7.21 (m, 2H), 4.62–4.56 (m, 1H), 3.92 (dt, J = 8.2, 5.4 Hz, 1H), 3.67–3.52 (m, 2H), 2.35 (s, 3H).
(R)-2-(1-(4-methoxyphenyl)-3-oxo-3-phenylpropyl)malononitrile (4c) [19,25]: 92% yield; colorless oil; 86% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 14.2 min (major), 23.2 min (minor). 1H NMR (400 MHz, CDCl3): δ 8.02–7.93 (m, 2H), 7.66–7.59 (m, 1H), 7.50 (dd, J = 10.6, 4.8 Hz, 2H), 7.40–7.34 (m, 2H), 6.99–6.91 (m, 2H), 4.61 (d, J = 5.0 Hz, 1H), 3.92 (dt, J = 8.5, 5.2 Hz, 1H), 3.82 (s, 3H), 3.67–3.63 (m, 2H).
(R)-2-(1-(4-fluorophenyl)-3-oxo-3-phenylpropyl)malononitrile (4d) [19,25]: 94% yield; white solid; 90% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 10.4 min (major), 16.4 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.97 (dd, J = 8.3, 1.1 Hz, 2H), 7.66–7.60 (m, 1H), 7.50 (t, J = 7.7 Hz, 2H), 7.47–7.42 (m, 2H), 7.15–7.09 (m, 2H), 4.62 (d, J = 5.1 Hz, 1H), 4.01–3.93 (m, 1H), 3.72–3.60 (m, 2H).
(R)-2-(1-(4-chlorophenyl)-3-oxo-3-phenylpropyl)malononitrile (4e) [19,23,25]: 72% yield; white solid; 85% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 11.4 min (major), 18.7 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.96 (dd, J = 8.3, 1.2 Hz, 2H), 7.64 (s, 1H), 7.51 (t, J = 7.7 Hz, 2H), 7.41 (d, J = 1.5 Hz, 4H), 4.63 (d, J = 5.1 Hz, 1H), 3.95 (d, J = 8.4 Hz, 1H), 3.68–3.63 (m, 2H).
(R)-2-(1-(4-bromophenyl)-3-oxo-3-phenylpropyl)malononitrile (4f) [25]: 45% yield; white solid; 85% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 11.6 min (major), 18.8 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.96 (d, J = 7.9 Hz, 2H), 7.64 (t, J = 7.2 Hz, 1H), 7.57 (d, J = 8.2 Hz, 2H), 7.50 (t, J = 7.6 Hz, 2H), 7.34 (d, J = 8.2 Hz, 2H), 4.62 (d, J = 5.0 Hz, 1H), 3.93 (dd, J = 8.3, 5.2 Hz, 1H), 3.67–3.59 (m, 2H).
(R)-2-(1-(3-chlorophenyl)-3-oxo-3-phenylpropyl)malononitrile (4g) [19]: 72% yield; white solid; 90% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 9.8 min (major), 12.2 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.95 (dd, J = 8.3, 1.1 Hz, 2H), 7.60 (d, J = 7.4 Hz, 1H), 7.50–7.43 (m, 3H), 7.38–7.33 (m, 3H), 4.61 (d, J = 5.2 Hz, 1H), 3.98–3.90 (m, 1H), 3.66–3.62 (m, 2H).
(R)-2-(1-(3-methoxyphenyl)-3-oxo-3-phenylpropyl)malononitrile (4h) [19]: 65% yield; white solid; 85% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 90/10, 25 °C, 1.0 mL min−1, 254 nm): tR = 16.9 min (major), 19.5 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.98–7.93 (m, 2H), 7.63–7.58 (m, 1H), 7.48 (dd, J = 10.7, 4.8 Hz, 2H), 7.33 (t, J = 8.0 Hz, 1H), 7.03–6.89 (m, 3H), 4.62 (d, J = 5.2 Hz, 1H), 3.95–3.89 (m, 1H), 3.82 (s, 3H), 3.66–3.64 (m, 2H).
(R)-2-(1-(2-methoxyphenyl)-3-oxo-3-phenylpropyl)malononitrile (4i) [19,25]: 52% yield; white solid; 35% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 90/10, 25 °C, 1.0 mL min−1, 254 nm): tR = 11.7 min (major), 13.2 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.97–7.92 (m, 2H), 7.58 (d, J = 7.4 Hz, 1H), 7.47 (t, J = 7.7 Hz, 2H), 7.35–7.29 (m, 2H), 6.99–6.91 (m, 2H), 4.66 (d, J = 6.6 Hz, 1H), 4.44 (d, J = 6.8 Hz, 1H), 3.88 (s, 3H), 3.73–3.64 (m, 2H).
(R)-2-(3-oxo-1-phenyl-3-p-tolylpropyl)malononitrile (4j) [47]: 64% yield; colorless oil; 88% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 12.3 min (major), 18.5 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 8.2 Hz, 2H), 7.46–7.38 (m, 5H), 7.28 (d, J = 8.0 Hz, 2H), 4.65 (d, J = 5.1 Hz, 1H), 3.97–3.88 (m, 1H), 3.66–3.61 (m, 2H), 2.42 (s, 3H).
(R)-2-(3-(4-methoxyphenyl)-3-oxo-1-phenylpropyl)malononitrile (4k) [25]: 76% yield; colorless oil; 80% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 19.9 min (major), 31.5 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.95 (d, J = 9.0 Hz, 2H), 7.44 (d, J = 5.8 Hz, 5H), 6.95 (d, J = 8.9 Hz, 2H), 4.69 (d, J = 5.0 Hz, 1H), 3.97–3.91 (m, 1H), 3.88 (s, 3H), 3.65–3.59 (m, 2H).
(R)-2-(3-(4-fluorophenyl)-3-oxo-1-phenylpropyl)malononitrile (4l) [25]: 99% yield; colorless oil; 80% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 11.5 min (major), 13.6 min (minor). 1H NMR (400 MHz, CDCl3): δ 8.04–7.96 (m, 2H), 7.47–7.38 (m, 5H), 7.20–7.13 (m, 2H), 4.62 (d, J = 5.2 Hz, 1H), 3.99–3.92 (m, 1H), 3.67–3.63 (m, 2H).
(R)-2-(3-(4-chlorophenyl)-3-oxo-1-phenylpropyl)malononitrile (4m) [25]: 57% yield; white solid; 80% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 12.9 min (major), 15.5 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.92–7.87 (m, 2H), 7.48–7.44 (m, 2H), 7.44–7.39 (m, 5H), 4.60 (d, J = 5.2 Hz, 1H), 3.98–3.91 (m, 1H), 3.65–3.62 (m, 2H).
(R)-2-(3-(4-bromophenyl)-3-oxo-1-phenylpropyl)malononitrile (4n) [19,25]: 41% yield; white solid; 79% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 80/20, 25 °C, 0.8 mL min−1, 254 nm): tR = 14.3 min (major), 17.2 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.80 (d, J = 8.4 Hz, 2H), 7.61 (d, J = 8.4 Hz, 2H), 7.41 (s, 5H), 4.58 (d, J = 5.2 Hz, 1H), 3.97–3.89 (m, 1H), 3.63–3.60 (m, 2H).
(R)-2-(3-oxo-1,3-di-p-tolylpropyl)malononitrile (4o) [25]: 70% yield; white solid; 86% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 90/10, 25 °C, 1.0 mL min−1, 254 nm): tR = 14.6 min (major), 22.3 min (minor). 1H NMR (400 MHz, CDCl3): δ 7.84 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 8.1 Hz, 2H), 7.26 (d, J = 8.0 Hz, 2H), 7.20 (d, J = 7.9 Hz, 2H), 4.59 (d, J = 5.1 Hz, 1H), 3.89 (d, J = 8.3 Hz, 1H), 3.62–3.58 (m, 2H), 2.40 (s, 3H), 2.34 (s, 3H).
(R)-2-(3-oxo-1-phenyl-3-(pyridin-2-yl)propyl)malononitrile (4p) [26]: 55% yield; white solid; 22% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 90/10, 25 °C, 1.0 mL min−1, 254 nm): tR = 17.9 min (major), 20.8 min (minor). 1H NMR (400 MHz, CDCl3): δ 8.69 (ddd, J = 4.7, 1.6, 0.9 Hz, 1H), 8.03–7.99 (m, 1H), 7.85 (td, J = 7.7, 1.7 Hz, 1H), 7.52 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.48–7.45 (m, 2H), 7.44–7.37 (m, 3H), 4.52 (d, J = 5.1 Hz, 1H), 4.08 (dd, J = 17.5, 4.7 Hz, 1H), 3.97–3.87 (m, 2H).
(R)-2-(1-(naphthalen-1-yl)-3-oxo-3-phenylpropyl)malononitrile (4q) [25]: 50% yield; white solid; 39% ee, determined by HPLC (Daicel Chiralcel AD-H, n-hexane/i-PrOH = 90/10, 25 °C, 1.0 mL min−1, 254 nm): tR = 15.4 min (major), 17.4 min (minor). 1H NMR (400 MHz, CDCl3): δ 8.13 (d, J = 8.5 Hz, 1H), 8.00–7.96 (m, 2H), 7.91 (dd, J = 18.2, 8.0 Hz, 2H), 7.70 (d, J = 7.1 Hz, 1H), 7.66–7.56 (m, 3H), 7.50 (dt, J = 10.1, 7.8 Hz, 3H), 5.03 (d, J = 6.7 Hz, 1H), 4.70 (d, J = 5.3 Hz, 1H), 3.86–3.83 (m, 2H).
4. Conclusions
In summary, we have developed an effective asymmetric Michael addition of malononitrile onto various trans-chalcones and their analogues catalyzed by chiral squaramide derived from commercially available rosin under mild conditions with a low catalyst loading (0.3 mol%), affording optical products (up to 90% ee). Further studies on bifunctional rosin-derived chiral squaramide organocatalysts for other enantioselective reactions are currently underway in our laboratory.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/1/14/s1, containing NMR spectra of compounds 4a–q, and HPLC spectra of racemic and chiral products 4a–q.
Author Contributions
Conceptualization, N.L. and Z.-W.Z.; methodology, N.L., Q.-X.W., and L.-H.J.; validation, Q.-X.W. and L.-H.J.; formal analysis, Q.-X.W., L.-H.J., and Y.-Q.D.; investigation, N.L., Q.-X.W., and L.-H.J.; resources, N.L. and Q.C.; data curation, Q.-X.W. and L.-H.J.; writing—original draft preparation, Z.-W.Z.; writing—review and editing, N.L.; visualization, Q.C.; supervision, N.L.; project administration, N.L.; funding acquisition, N.L., Z.-W.Z., and Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 21861009; Guangxi Natural Science Foundation, grant number 2018GXNSFAA281317 and 2018GXNSFBA138032; Guangxi University of Chinese Medicine Research Foundation for introduced Ph.D., grant number XB0170027; Innovation Project of Guangxi Graduate Education, grant number YCSW2019175; Guangxi University of Chinese Medicine First-class Discipline Construction of Chinese Medicine.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ballini, R.; Bosica, G.; Fiorini, D.; Palmieri, A.; Petrini, M. Conjugate additions of nitroalkanes to electron-poor alkenes: Recent results. Chem. Rev. 2005, 105, 933–971. [Google Scholar] [CrossRef] [PubMed]
- Almasi, D.; Alonso, D.A.; Nájera, C. Organocatalytic asymmetric conjugate additions. Tetrahedron Asymmetry 2007, 18, 299–365. [Google Scholar] [CrossRef]
- Tsogoeva, S.B. Recent advances in asymmetric organocatalytic 1, 4-conjugate additions. Eur. J. Org. Chem. 2007, 11, 1701–1716. [Google Scholar] [CrossRef]
- Csaky, A.G.; Herran, G.D.L.; Murcia, M.C. Conjugate addition reactions of carbon nucleophiles to electron-deficient dienes. Chem. Soc. Rev. 2010, 39, 4080–4102. [Google Scholar] [CrossRef] [PubMed]
- Roca-Lopez, D.; Sadaba, D.; Delso, I.; Herrera, R.P.; Tejero, T.; Merino, P. Asymmetric organocatalytic synthesis of γ-nitrocarbonyl compounds through Michael and Domino reactions. Tetrahedron Asymmetry 2010, 21, 2561–2601. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W. Recent advances in organocatalytic asymmetric Michael reactions. Catal. Sci. Technol. 2012, 2, 42–53. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, X.; Feng, X. Recent advances in metal-catalyzed asymmetric 1, 4-conjugate addition (ACA) of nonorganometallic nucleophiles. Chem. Rev. 2018, 118, 7586–7656. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Enantioselective Michael additions to α, β-unsaturated imides catalyzed by a salen−Al complex. J. Am. Chem. Soc. 2003, 125, 11204–11205. [Google Scholar] [CrossRef]
- Taylor, M.S.; Zalatan, D.N.; Lerchner, A.M.; Jacobsen, E.N. Highly enantioselective conjugate additions to α, β-unsaturated ketones catalyzed by a (salen) Al complex. J. Am. Chem. Soc. 2005, 127, 1313–1317. [Google Scholar] [CrossRef]
- Hoash, Y.; Okino, T.; Takemoto, Y. Enantioselective Michael addition to α, β-unsaturated imides catalyzed by a bifunctional organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 4032–4035. [Google Scholar] [CrossRef]
- Inokuma, T.; Hoashi, Y.; Takemoto, Y. Thiourea-catalyzed asymmetric Michael addition of activated methylene compounds to α, β-unsaturated imides: Dual activation of imide by intra-and intermolecular hydrogen bonding. J. Am. Chem. Soc. 2006, 128, 9413–9419. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.W.; Huang, X.; Fan, L.P.; Xu, D.C.; Li, X.S.; Su, H.; Wen, Y.H. Efficient method for the synthesis of optically active 2-amino-2-chromene derivatives via one-pot tandem reactions. Adv. Synth. Catal. 2009, 351, 3077–3082. [Google Scholar] [CrossRef]
- Huang, X.; Li, P.; Li, X.-S.; Xu, D.-C.; Xie, J.-W. The organocatalytic two-step synthesis of diversely functionalized tricyclic tetrazoles. Org. Biomol. Chem. 2010, 8, 4527–4529. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-P.; Lou, C.-L.; Wang, J.-J.; Chen, C.-X.; Yan, M. Organocatalytic conjugate addition of malononitrile to conformationally restricted dienones. J. Org. Chem. 2011, 76, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Wang, B.; Zhang, J.-M.; Yan, M. Asymmetric organocatalytic double-conjugate addition of malononitrile to dienones: Efficient synthesis of optically active cyclohexanones. Org. Lett. 2011, 13, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, W.; Du, D.-M. Efficient organocatalytic asymmetric synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives. Tetrahedron Asymmetry 2012, 23, 339–344. [Google Scholar] [CrossRef]
- Arai, T.; Oka, I.; Morihata, T.; Awata, A.; Masu, H. A neutral, chiral, bis (imidazolidine)-derived NCN-type palladium pincer complex with catalytic activity. Chem. Eur. J. 2013, 19, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.R.; Gayen, P.; Panda, S.; Ghorai, P. Enantioselective, organocatalytic, dissymmetric 1, 4- and 1, 2-addition of malononitrile to a keto-bisenone followed by an oxa-Michael addition cascade. Org. Lett. 2019, 21, 5793–5797. [Google Scholar] [CrossRef]
- Shi, J.; Wang, M.; He, L.; Zheng, K.; Liu, X.; Lin, L.; Feng, X. Enantioselective Michael addition of malononitrile to chalcones catalyzed by a simple quinine–Al (OiPr)3 complex: A simple method for the synthesis of a chiral 4H-pyran derivative. Chem. Commun. 2009, 31, 4711–4713. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Xing, Z.; Tang, N.; Zhu, J.; Deng, J. The asymmetric addition of malononitrile to α, β-unsaturated ketones catalyzed by RuCl2 [(R, R)-DPEN] (PPh3)2 as the precatalyst. Tetrahedron Lett. 2014, 55, 3868–3872. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Zu, L.; Jiang, W.; Xie, H.; Duan, W.; Wang, W. Organocatalytic enantioselective conjugate additions to enones. J. Am. Chem. Soc. 2006, 128, 12652–12653. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cun, L.; Lian, C.; Zhong, L.; Chen, Y.; Liao, J.; Zhu, J.; Deng, J. Highly enantioselective Michael addition of malononitrile to α, β-unsaturated ketones. Org. Biomol. Chem. 2008, 6, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Perfetto, A.; Lattanzi, A. Back to natural cinchona alkaloids: Highly enantioselective Michael addition of malononitrile to enones. Adv. Synth. Catal. 2009, 351, 3067–3071. [Google Scholar] [CrossRef]
- Russo, A.; Capobianco, A.; Perfetto, A.; Lattanzi, A.; Peluso, A. Enantioselective conjugate addition of malononitrile to chalcones promoted by α, α-L-diaryl prolinols: Noncovalent versus covalent catalysis? Eur. J. Org. Chem. 2011, 10, 1922–1931. [Google Scholar] [CrossRef]
- Yang, W.; Jia, Y.; Du, D.-M. Squaramide-catalyzed enantioselective Michael addition of malononitrile to chalcones. Org. Biomol. Chem. 2012, 10, 332–338. [Google Scholar] [CrossRef]
- Molleti, N.; Rana, N.K.; Singh, V.K. Highly enantioselective conjugate addition of malononitrile to 2-enoylpyridines with bifunctional organocatalyst. Org. Lett. 2012, 14, 4322–4325. [Google Scholar] [CrossRef]
- Yan, L.; Wang, H.; Xiong, F.; Tao, Y.; Wu, Y.; Chen, F. Chloramphenicol base chemistry. Part 11: Chloramphenicol base-derived thiourea-catalyzed enantioselective Michael addition of malononitrile to α, β-unsaturated ketones. Tetrahedron Asymmetry 2017, 28, 921–929. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Wu, L.; Zhang, G.; Liu, X.; Zhang, H.; Fu, D.; Wang, R. Doubly stereocontrolled asymmetric aza-Henry reaction with in situ generation of N-Boc-imines catalyzed by novel rosin-derived amine thiourea catalysts. Adv. Synth. Catal. 2009, 351, 2096–2100. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Liu, X.; Zhang, G.; Lai, L.; Wu, L.; Zhang, J.; Wang, R. Enantio and diastereoselective asymmetric addition of 1, 3-dicarbonyl compounds to nitroalkenes in a doubly stereocontrolled manner catalyzed by bifunctional rosin-derived amine thiourea catalysts. J. Org. Chem. 2009, 74, 5562–5567. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Chan, A.S.C.; Wang, R. Highly enantioselective synthesis of γ-nitro heteroaromatic ketones in a doubly stereocontrolled manner catalyzed by bifunctional thiourea catalysts based on dehydroabietic amine: A doubly stereocontrolled approach to pyrrolidine carboxylic acids. Org. Lett. 2009, 11, 153–156. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, D.; Zhang, G.; Cao, Y.; Liu, L.; Song, J.; Wang, R. Highly diastereo-and enantioselective Mannich reaction of lactones with N-Boc-aldimines catalyzed by bifunctional rosin-derived amine thiourea catalysts. Chem. Commun. 2010, 46, 4294–4296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, G.; Fu, D.; Cao, Y.; Shen, F.; Wang, R. Direct organocatalytic asymmetric Aldol reaction of α-isothiocyanato imides to α-ketoesters under low ligand loading: A doubly stereocontrolled approach to cyclic thiocarbamates bearing chiral quaternary stereocenters. Org. Lett. 2010, 12, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, Y.; Wang, Y.; Liu, L.; Shen, F.; Wang, R. A unique approach to the concise synthesis of highly optically active spirooxazolines and the discovery of a more potent oxindole-type phytoalexin analogue. J. Am. Chem. Soc. 2010, 132, 15328–15333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Jiang, X.; Yan, W.; Wang, R. Highly enantioslective synthesis of multisubstituted polyfunctional dihydropyrrole via an organocatalytic tandem Michael/cyclization sequence. Org. Lett. 2011, 13, 3806–3809. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, X.; Liu, L.; Shen, F.; Zhang, F.; Wang, R. Enantioselective Michael/cyclization reaction sequence: Scaffold-inspired synthesis of spirooxindoles with multiple stereocenters. Angew. Chem. Int. Ed. 2011, 50, 9124–9127. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, L.; Xing, Y.; Wang, L.; Wang, S.; Chen, Z.; Wang, R. Highly enantioselective Friedel–Crafts alkylation reaction catalyzed by rosin-derived tertiary amine–thiourea: Synthesis of modified chromanes with anticancer potency. Chem. Commun. 2012, 48, 446–448. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, X.; Li, X.; Hou, C.; Jiang, Y.; Hou, K.; Wang, R.; Li, Y. Highly enantioselective synthesis of N-protected β-amino malonates catalyzed by magnetically separable heterogeneous rosin-derived amino thiourea catalysts: A stereocontrolled approach to β-amino acids. ChemCatChem 2013, 5, 2187–2190. [Google Scholar] [CrossRef]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef]
- Alemán, J.; Parra, A.; Jiang, H.; Jørgensen, K.A. Squaramides: Bridging from molecular recognition to bifunctional organocatalysis. Chem. Eur. J. 2011, 17, 6890–6899. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional amine-squaramides: Powerful hydrogen-bonding organocatalysts for asymmetric domino/cascade reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Held, F.E.; Tsogoeva, S.B. Asymmetric cycloaddition reactions catalyzed by bifunctional thiourea and squaramide organocatalysts: Recent advances. Catal. Sci. Technol. 2016, 6, 645–667. [Google Scholar] [CrossRef]
- Zhao, B.-L.; Li, J.-H.; Du, D.-M. Squaramide-catalyzed asymmetric reactions. Chem. Rec. 2017, 17, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Mika, L.T.; Csámpai, A.; Holczbauer, T.; Kardosa, G.; Soós, T. Mechanistic investigations of a bifunctional squaramide organocatalyst in asymmetric Michael reaction and observation of stereoselective retro-Michael reaction. RSC Adv. 2015, 5, 95079–95086. [Google Scholar] [CrossRef]
- Huang, W.-J.; Chen, Q.; Lin, N.; Long, X.-W.; Pan, W.-G.; Xiong, Y.-S.; Weng, J.; Lu, G. Asymmetric synthesis of trifluoromethylsubstituted 3, 3′-pyrrolidinyl-dispirooxindoles through organocatalytic 1, 3-dipolar cycloaddition reactions. Org. Chem. Front. 2017, 4, 472–482. [Google Scholar] [CrossRef]
- Lin, N.; Long, X.-W.; Chen, Q.; Zhu, W.; Wang, B.; Chen, K.; Jiang, C.; Weng, J.; Lu, G. Highly efficient construction of chiral dispirocyclic oxindole/thiobutyrolactam/chromanone complexes through Michael/cyclization cascade reactions with a rosin-based squaramide catalyst. Tetrahedron 2018, 74, 3734–3741. [Google Scholar] [CrossRef]
- Jiang, L.; Long, X.; Huang, W.; Lin, N.; Jiang, C.; Lu, G. Synthesis and characterization of chiral ethyl 7-amino-dehydroabietate. Fine Chem. 2014, 31, 807–811. [Google Scholar]
- Yue, L.; Du, W.; Liu, Y.-K.; Chen, Y.-C. Organocatalytic asymmetric direct Michael addition of aromatic ketones to alkylidenemalononitriles. Tetrahedron Lett. 2008, 49, 3881–3884. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).