Switching Competitors Reduces Win-Stay but Not Lose-Shift Behaviour: The Role of Outcome-Action Association Strength on Reinforcement Learning
Abstract
1. Introduction
2. Method
2.1. Participants
2.2. Stimuli and Apparatus
2.3. Design
2.4. Procedure
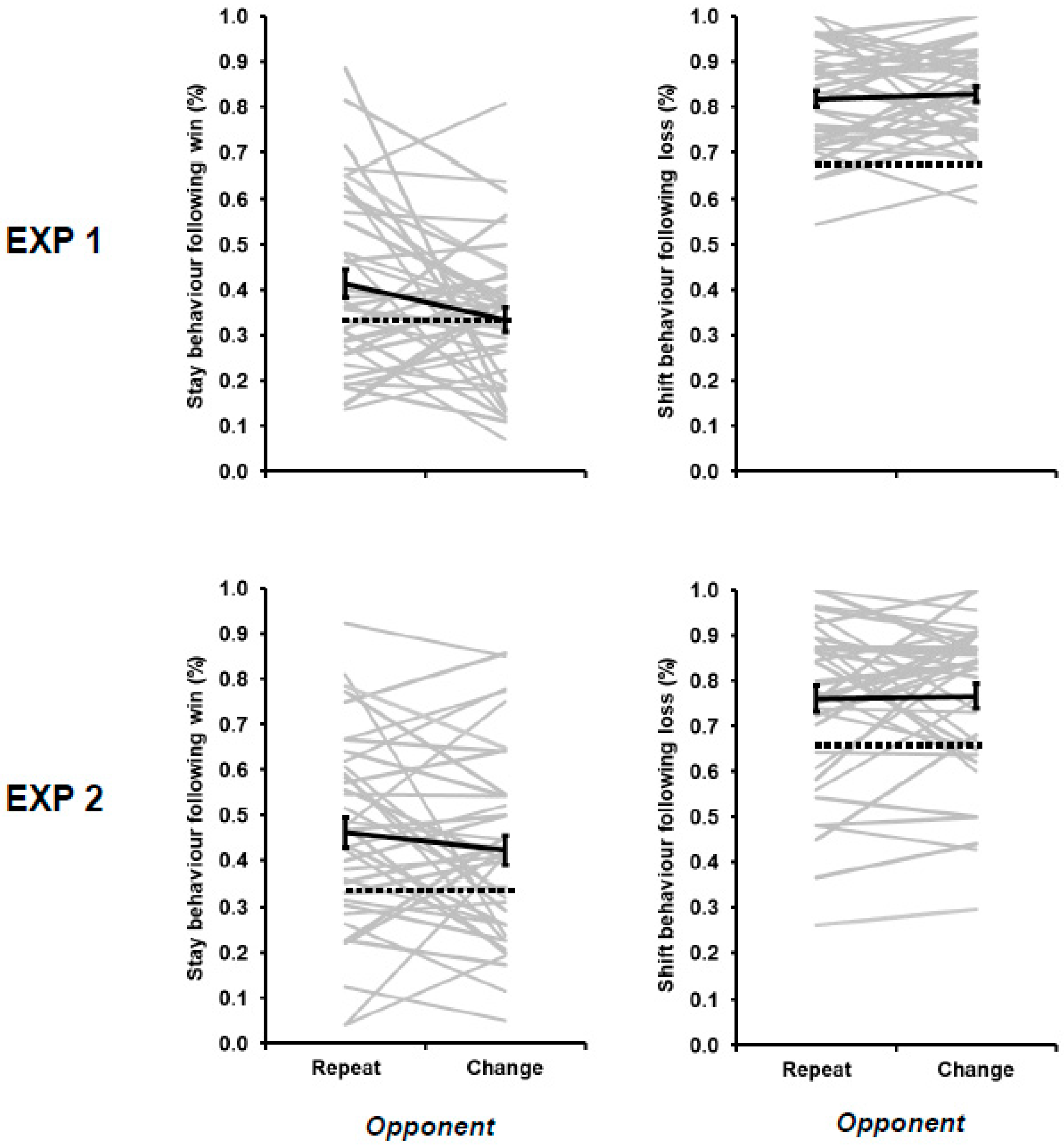
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miltner, W.H.R.; Brown, C.H.; Coles, M.G.H. Event related brain potentials following incorrect feedback in a time estimation task: Evidence for a generic neural system for error detection. J. Cogn. Neurosci. 1997, 9, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Lee, D. Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron 2011, 70, 731–741. [Google Scholar] [CrossRef]
- Baek, K.; Kim, Y.-T.; Kim, M.; Choi, Y.; Lee, M.; Lee, K.; Hahn, S.; Jeong, J. Response randomization of one- and two-person Rock-Paper-Scissors games in individuals with schizophrenia. Psychiatry Res. 2013, 207, 158–163. [Google Scholar] [CrossRef]
- Bi, Z.; Zhou, H.-J. Optimal cooperation-trap strategies for the iterated rock-paper-scissors game. PLoS ONE 2014, 9, e111278. [Google Scholar] [CrossRef]
- Loertscher, S. Rock-Scissors-Paper and evolutionarily stable strategies. Econ. Lett. 2013, 118, 473–474. [Google Scholar] [CrossRef]
- Griessinger, T.; Coricelli, G. The neuroeconomics of strategic interaction. Curr. Opin. Behav. Sci. 2015, 3, 73–79. [Google Scholar] [CrossRef]
- Scheibehenne, B.; Wilke, A.; Todd, P.M. Expectations of clumpy resources influence predictions of sequential events. Evol. Hum. Behav. 2011, 32, 326–333. [Google Scholar] [CrossRef]
- Dyson, B.J. Behavioural isomorphism, cognitive economy and recursive thought in non-transitive game strategy. Games 2019, 10, 32. [Google Scholar] [CrossRef]
- Thorndike, E.L. Animal Intelligence; Macmillan Company: New York, NY, USA, 1911. [Google Scholar]
- Kahneman, D.; Tversky, A. Prospect theory: An analysis of decision under risk. Econometrica 1979, 47, 263–291. [Google Scholar] [CrossRef]
- Bolles, R.C. Species-specific defense reactions and avoidance learning. Psychol. Rev. 1970, 77, 32–48. [Google Scholar] [CrossRef]
- West, R.L.; Lebiere, C.; Bothell, D.J. Cognitive architectures, game playing and human evolution. In Cognition and Multi-Agent Interaction: From Cognitive Modeling to Social Smulation; Sun, R., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 102–123. [Google Scholar]
- Gruber, A.J.; Thapa, R. The memory trace supporting lose-shift responding decays rapidly after reward omission and is distinct from other learning mechanisms in rats. ENeuro 2016, 3, 6. [Google Scholar] [CrossRef]
- Kubanek, J.; Snyder, L.H.; Abrams, R.A. Reward and punishment act as distinct factors in guiding behavior. Cognition 2015, 139, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.B.; Ariely, D. The enduring impact of transient emotions on decision making. Organ. Behav. Hum. Decis. Process. 2009, 109, 1–8. [Google Scholar] [CrossRef]
- Lerner, J.S.; Li, Y.; Veldesolo, P.; Kassam, K.S. Emotion and decision making. Annu. Rev. Psychol. 2015, 66, 799–823. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.T. Emotion and rationality: A critical review and interpretation of empirical evidence. Rev. Gen. Psychol. 2007, 11, 155–178. [Google Scholar] [CrossRef]
- Sanfey, A.G.; Rilling, J.K.; Aronson, J.A.; Nystrom, L.E.; Cohen, J.D. The neural basis of economic decision-making in the ultimatum game. Science 2003, 300, 1755–1758. [Google Scholar] [CrossRef]
- Dixon, M.J.; MacLaren, V.; Jarick, M.; Fugelsang, J.A.; Harrigan, K.A. The frustrating effects of just missing the jackpot: Slot machine near-misses trigger large skin conductance responses, but no post-reinforcement pauses. J. Gambl. Stud. 2013, 29, 661–674. [Google Scholar] [CrossRef]
- Dixon, M.R.; Schreiber, J.E. Near-miss effects on response latencies and win estimations of slot machine players. Psychol. Rec. 2004, 54, 335–348. [Google Scholar] [CrossRef]
- Dyson, B.J.; Sundvall, J.; Forder, L.; Douglas, S. Failure generates impulsivity only when outcomes cannot be controlled. J. Exp. Psychol. Hum. Percept. Perform. 2018, 44, 1483–1487. [Google Scholar] [CrossRef]
- Verbruggen, F.; Chambers, C.D.; Lawrence, N.S.; McLaren, I.P.L. Winning and losing: Effects on impulsive action. J. Exp. Psychol. Hum. Percept. Perform 2017, 43, 147–168. [Google Scholar] [CrossRef]
- Williams, P.; Heathcote, A.; Nesbitt, K.; Eidels, A. Post-error recklessness and the hot hand. Judgm. Decis. Mak. 2016, 11, 174–184. [Google Scholar]
- Zheng, Y.; Li, Q.; Zhang, Y.; Li, Q.; Shen, H.; Gao, Q.; Zhou, S. Reward processing in gain versus loss context: An ERP study. Psychophys 2017, 54, 1040–1053. [Google Scholar] [CrossRef]
- Dyson, B.J.; Steward, B.A.; Meneghetti, T.; Forder, L. Behavioural and neural limits in competitive decision making: The roles of outcome, opponency and observation. Biol. Psychol. 2020, 149, 107778. [Google Scholar] [CrossRef] [PubMed]
- Dyson, B.J.; Wilbiks, J.M.P.; Sandhu, R.; Papanicolaou, G.; Lintag, J. Negative outcomes evoke cyclic irrational decisions in Rock, Paper, Scissors. Sci. Rep. 2016, 6, 20479. [Google Scholar] [CrossRef] [PubMed]
- Dyson, B.J.; Musgrave, C.; Rowe, C.; Sandhur, R. Behavioural and neural interactions between objective and subjective performance in a Matching Pennies game. Int. J. Psychophysiol. 2020, 147, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Nevo, I.; Erev, I. On surprise, change, and the effects of recent outcomes. Front. Psychol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Forder, L.; Dyson, B.J. Behavioural and neural modulation of win-stay but not lose-shift strategies as a function of outcome value in Rock, Paper, Scissors. Sci. Rep. 2016, 6, 33809. [Google Scholar] [CrossRef]
- Cohen, M.X.; Elger, C.E.; Ranganath, C. Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage 2007, 35, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Moser, J.S.; Holroyd, C.B.; Simons, R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 2006, 71, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Engelstein, G. Achievement Relocked: Loss Aversion and Game Design; MIT Press: London, UK, 2020. [Google Scholar]
- Laakasuo, M.; Palomäki, J.; Salmela, J.M. Emotional and social factors influence poker decision making accuracy. J. Gambl. Stud. 2015, 31, 933–947. [Google Scholar] [CrossRef]
- Mitzenmacher, M.; Upfal, E. Probability and Computing: Randomized Algorithms and Probabilistic Analysis; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Barreda-Tarrazona, I.; Jaramillo-Gutierrez, A.; Pavan, M.; Sabater-Grande, G. Individual Characteristics vs. Experience: An Experimental Study on Cooperation in Prisoner’s Dilemma. Front. Psychol. 2017, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, M.; Pancani, L.; Cappelletti, E.R.; Greco, A.; Monzani, D.; Steca, P. The hidden side of the Ultimatum Game: The role of motivations and mind-reading in a two-level one-shot Ultimatum Game. J. Cogn. Psychol. 2015, 27, 898–907. [Google Scholar] [CrossRef]
- Coleman, A.M. Cooperation, psychological game theory, and limitation of rationality in social interaction. Behav. Brain Sci. 2003, 26, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.N.; Rosenthal, R.W. Testing the minimax hypothesis: A re-examination of O’Neill’s game experiment. Econometrica 1990, 58, 1065–1081. [Google Scholar] [CrossRef]
- Garnefski, N.; Kraaij, V. Cognitive emotion regulation questionnaire–development of a short 18-item version (CERQ-short). Personal. Individ. Differ. 2006, 41, 1045–1053. [Google Scholar] [CrossRef]
- O’Neill, B. Nonmetric test of the minimax theory of two-person zerosum games. Proc. Natl. Acad. Sci. USA 1987, 84, 2106–2109. [Google Scholar] [CrossRef]
- O’Neill, B. Comments on Brown and Rosenthal’s re-examination. Econometric 1991, 59, 503–507. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Farrar, Straus and Giroux: New York, NY, USA, 2011. [Google Scholar]
- Sloman, S.A. The empirical case for two systems of reasoning. Psychol. Bull. 1996, 119, 3–22. [Google Scholar] [CrossRef]
- Weibel, D.; Wissmath, B.; Habegger, S.; Steiner, Y.; Groner, R. Playing online games against computer- vs. human-controlled opponents: Effects on presence, flow, and enjoyment. Comput. Hum. Behav. 2008, 24, 2274–2291. [Google Scholar] [CrossRef]
- West, R.L.; Lebiere, C. Simple games as dynamic, coupled systems: Randomness and other emergent properties. J. Cogn. Syst. Res. 2001, 1, 221–239. [Google Scholar] [CrossRef]
- Sundvall, J.; Dyson, B.J. Breaking the bonds of reinforcement: Effects of trial outcome, rule consistency and rule complexity against exploitable and unexploitable opponents. submitted.
- Lee, D.; McGreevy, B.P.; Barraclough, D.J. Learning decision making in monkeys during a rock-paper-scissors game. Cogn. Brain Res. 2005, 25, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Budescu, D.V.; Rapoport, A. Subjective randomization in one- and two-person games. J. Behav. Decis. Mak. 1992, 7, 261–278. [Google Scholar] [CrossRef]
- Pulford, B.D.; Colman, A.M.; Loomes, G. Incentive magnitude effects in experimental games: Bigger is not necessarily better. Games 2018, 9, 4. [Google Scholar] [CrossRef]
- Yechiam, E.; Hochman, G. Losses as modulators of attention: Review and analysis of the unique effects of losses over gains. Psychol. Bull. 2013, 139, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jin, J.; Meng, L.; Shen, Q. The dark side of monetary incentive: How does extrinsic reward crowd out intrinsic motivation. Neuroreport 2014, 25, 194–198. [Google Scholar] [CrossRef]
- Rapoport, A.; Budescu, D.V. Generation of random series in two-person strictly competitive games. J. Exp. Psychol. Gen. 1992, 121, 352–363. [Google Scholar] [CrossRef]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J. Personal. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Anderson, J.R. The Adaptive Character of Thought; Erlbaum: Hillsdale, NJ, USA, 1990. [Google Scholar]
- Hillstrom, A. Repetition effects in visual search. Percept. Psychophys. 2000, 62, 800–817. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Hajcak, G.; Larsen, J.T. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Res. 2006, 1105, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.V.; Moller, J.; Rodriguez-Fornells, A.; Munte, T.F. Brain potentials related to self-generated and external information used for performance monitoring. Clin. Neurophysiol. 2005, 116, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ivan, V.E.; Banks, P.J.; Goodfellow, K.; Gruber, A.J. Lose-shift responding in humans is promoted by increased cognitive load. Front. Integr. Neurosci. 2018, 12, 9. [Google Scholar] [CrossRef] [PubMed]
| 1 | Such predictions also appear in [38], p. 1081 in their suggestion that with opponent ‘rotation,’ participants “ought to feel freer to condition on their own past choices as an aid in achieving any desired move frequencies.” |
| 2 | For one participant, only 287 trials were recorded resulting in 143 pairs (286 trials) being used. For a second participant, only 285 trials were recorded resulting in 142 pairs (284 trials) being used. |
| 3 | The decrease in win–stay behavior during opponent change relative to opponent repeat trials was replicated when the 2 opponents playing with an item bias were removed from the overall averages for Experiment 1 (43.55% versus 38.15%; F[1,78] = 5.693, MSE = 0.020, p = 0.019, ƞp2 = 0.068) |
| 4 | These differences in win–stay and lose–shift flexibility cannot be attributed to the variable experience of positive and negative outcomes in the current study. At a group level, the proportion of wins (33.51%) was equivalent to the proportion of losses (33.12%; t[79] = 0.814, p = 0.418). At an individual level, the degree of win–stay behavior was not correlated with individually experienced (r = 0.106, p = 0.350), nor was lose–shift behavior correlated with individually experienced lose rates (r = −0.131, p = 0.247). |
| 5 | It is interesting to note that when comparing unexploitable with exploitable computer opponents, participants report an increased sense of co-presence for an automated program that played randomly [46]. |
| 6 | More broadly, the mental representation of zero-sum games only with respect to the relationship between items can lead to identical behavior with more traditional representations that preserve both items and outcomes. For example, reference [8] discusses the behavioral isomorphism between zero-sum game strategy rules expressed in terms of the opponent’s previous response only versus the participant’s previous response and outcome. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srihaput, V.; Craplewe, K.; Dyson, B.J. Switching Competitors Reduces Win-Stay but Not Lose-Shift Behaviour: The Role of Outcome-Action Association Strength on Reinforcement Learning. Games 2020, 11, 25. https://doi.org/10.3390/g11030025
Srihaput V, Craplewe K, Dyson BJ. Switching Competitors Reduces Win-Stay but Not Lose-Shift Behaviour: The Role of Outcome-Action Association Strength on Reinforcement Learning. Games. 2020; 11(3):25. https://doi.org/10.3390/g11030025
Chicago/Turabian StyleSrihaput, Vincent, Kaylee Craplewe, and Benjamin James Dyson. 2020. "Switching Competitors Reduces Win-Stay but Not Lose-Shift Behaviour: The Role of Outcome-Action Association Strength on Reinforcement Learning" Games 11, no. 3: 25. https://doi.org/10.3390/g11030025
APA StyleSrihaput, V., Craplewe, K., & Dyson, B. J. (2020). Switching Competitors Reduces Win-Stay but Not Lose-Shift Behaviour: The Role of Outcome-Action Association Strength on Reinforcement Learning. Games, 11(3), 25. https://doi.org/10.3390/g11030025




