Simple Summary
Uveal melanoma is the most common primary cancer of the eye in adults, usually beginning in the choroid. It is a rare but aggressive disease with nonspecific symptoms and many cases are discovered incidentally during eye examinations. Although effective local therapies, such as radiotherapy, surgery, or enucleation, can control the primary tumor, the risk of metastases remains high, affecting up to 70% of patients. The liver is the most frequent location of distant spreading, and once metastases occur, the prognosis is poor, with survival often less than one year. Liver-directed treatments, particularly surgery, may prolong survival, but systemic therapies are generally limited in efficacy. Recently, tebentafusp, a bispecific protein, has shown a survival benefit in selected patients, representing an important step forward. This article summarizes the current knowledge of UM, including its epidemiology, genetic background, clinical features, treatment options, and perspectives for future therapy.
Abstract
Background/Objectives: Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, most commonly arising in the choroid. Its development is associated with phenotypic characteristics, ultraviolet radiation, and germline or somatic genetic alterations. Despite progress in diagnostics and local therapies, UM remains characterized by high metastatic risk and poor overall prognosis. This review aimed to summarize current knowledge on epidemiology, clinical features, genetic background, prognostic factors, and therapeutic approaches in metastatic UM. Methods: A structured literature review was conducted to evaluate epidemiological trends, genetic alterations, prognostic markers, clinical presentation, and therapeutic strategies. The results of different systemic treatments were analyzed, with special attention to liver-directed interventions and emerging systemic therapies. Results: The incidence of UM in Europe increases with latitude, ranging from two per million in the southern regions to more than eight per million in the North. The median age at diagnosis is 62 years, and most cases are detected incidentally during ophthalmological examinations due to nonspecific symptoms. Some genetic alterations serve as important prognostic indicators. Local treatment consists of globe-preserving procedures, including radiation therapy, surgery, laser therapy, or enucleation, with failure rates between 6.15% and 20.8%. Up to 70% of patients develop distant metastases, predominantly in the liver. Metastatic UM (mUM) carries a poor prognosis, with overall survival ranging from 3 to 30 months. Liver-directed therapies, particularly surgical resection, provide the most favorable outcomes. Systemic therapies demonstrate limited efficacy; however, tebentafusp has shown an overall survival benefit in HLA-A*02:01 (human leukocyte antigen A*02:01)-positive patients. Conclusions: UM is a rare but aggressive malignancy with limited treatment options once metastatic. Liver-directed strategies remain the mainstay of management, while novel systemic approaches, including tebentafusp, represent promising advances. Further research is required to improve survival and expand therapeutic opportunities.
Keywords:
uveal melanoma; metastases; liver-directed therapy; immunotherapy; tebentafusp; anti-PD1; anti-CTLA4 1. Introduction
Uveal melanoma (UM) is a rare disease, but it is the most common ocular malignancy in adults [1]. UM arises from melanocytes in the choroid (90%), ciliary body (6%), or iris (4%). This malignancy, which develops from ocular melanocytes, significantly differs from skin melanomas in terms of biology, clinical features, pathogenesis, molecular abnormalities, and clinical course, including the pattern of metastases [2]. The risk factors include light eye color, fair skin, ocular and oculodermal melanocytosis, cutaneous, iris, or choroidal nevi, sunlight exposure to ultraviolet radiation, and mutations in BRCA1 and BAP1 [3]. Additionally, mutations in the GNA11, GNAQ, SF3B1, and EIF1AX genes are often detected in UM. Moreover, mutations in the promoter of BRAF, NRAS, and TERT genes have been described [3,4]. Congenital or infantile UM are rare, and this malignancy mainly develops in adults [5], and most often presents between 50 and 70 years of age, with a median age of 62 at diagnosis. UM is usually unilateral. The incidence worldwide is 1–9/100,000, increases with latitude, and ranges from 2/106 in Spain, to 4–6/106 in Central Europe, to above 8/106 in Denmark and Norway [6]. In about half of the patients, this disease is asymptomatic [7]. The signs and symptoms of UM are nonspecific and include myodesopsia (seeing flying flies), photopsia, high intraocular pressure, or even loss of vision. UMs are most often diagnosed incidentally during ophthalmological examinations. Mutations in TETR, BAP1, SF3B1, and EIF1AX are of prognostic significance [8]. Management of localized UM includes either eye-preserving treatment strategies or enucleation. Eye-preserving approaches encompass surgical interventions, radiation therapy, and laser-based modalities. The average treatment failure in all radiation therapies is 6.15%, 18.6% in surgical therapies, and 20.8% in laser therapies [9]. Kaplan–Meier analyses of metastasis-free survival in UM demonstrate age-dependent differences. At 3, 5, 10, and 20 years, the estimated rates are 2%, 9%, 9%, and 20% in younger patients; 6%, 12%, 23%, and 34% among middle-aged adults; and increase to 11%, 19%, 28%, and 39% in elderly individuals [10]. Distant metastases develop in up to 70% of patients. The risk of metastases primarily depends on the stage of the primary tumor and its molecular profile [10,11,12,13,14]. The most frequent sites of metastasis are shown on Figure 1. Metastatic UM (mUM) is a disease with a poor prognosis. The expected overall survival (OS) in the metastatic setting ranges from 3 to 12 months. Based on systematic reviews, meta-analyses, and retrospective studies, the best outcomes are observed in patients treated with a liver-directed approach, in particular, complete resection [15,16,17,18]. Systemic therapies, which are of limited efficacy, may be considered if liver-directed therapies are not feasible. Tebentafusp, a bispecific protein, has been approved for the treatment of patients with mUM with HLA-A*02:01 (human leukocyte antigen A*02:01)-positive disease, based on improved OS [19]. The study aimed to review current and prospective approaches in metastatic ocular melanoma.
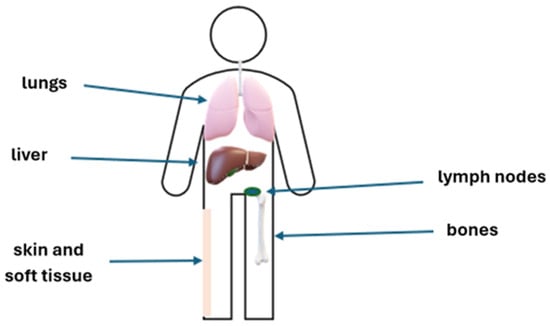
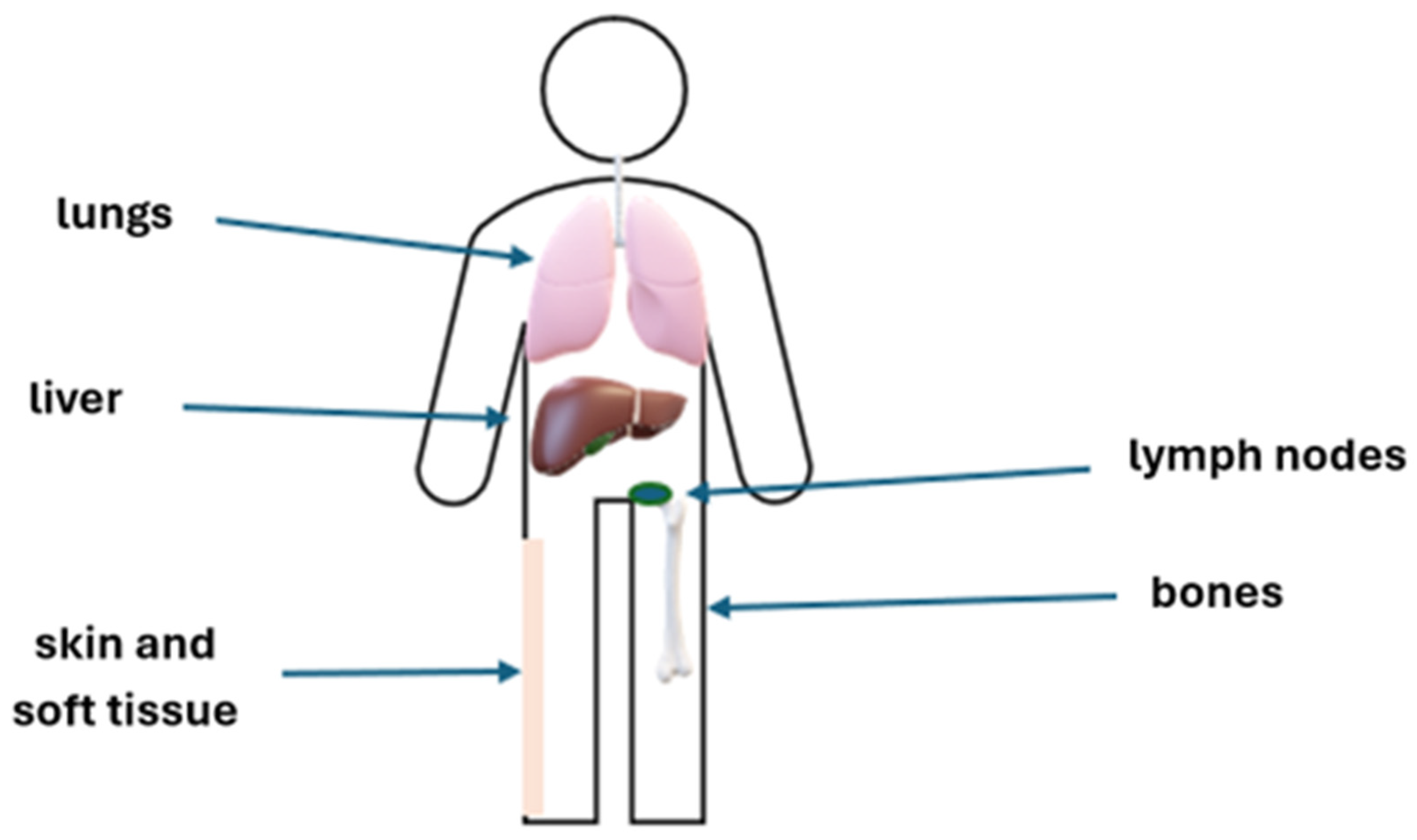
Figure 1.
The most frequent sites of UM metastases.
2. Risk Stratification and Surveillance of Extraocular Disease in UM
At the time of diagnosis, approximately 97% of patients with UM are limited to the eye, and proper treatment prevents local recurrence in about 87% of these patients. Only 3% of patients are diagnosed in the metastatic stage at the time of presentation. Patients who have undergone treatment for primary tumors should undergo ophthalmological examinations every 3–6 months for the first 2–5 years (duration of follow-up every 3–6 months differs between regions and countries), and every 6–12 months thereafter to detect potential local recurrence or treatment complications [20,21,22,23]. Due to the risk of dissemination, the patients should also be properly monitored to detect and treat any potential metastases. Up to 70% of patients after treatment of localized UM will develop distant metastases within 20 years. The main risk factors are primary tumor size and genetic characteristics. The liver is the most frequent site of metastases, but these may also develop in other organs, such as the lungs, bones, skin, soft tissue, and lymph nodes. The recommended follow-up is based on the assessment of the risk of metastases, which is mostly associated with the stage of ocular disease (stage T assessed based on AJCC, American Joint Committee on Cancer) and molecular abnormalities (Table 1). If liver metastases are suspected, liver MRI (magnetic resonance imaging) with contrast is recommended. Liver function tests can also be performed, but their value is limited due to their low sensitivity [24,25]. Additional imaging tests, including CT (computed tomography) with contrast and PET (positron emission tomography), should be performed if necessary; however, neither CT with contrast nor PET is sensitive enough to replace liver MRI. There is no need to perform a brain MRI unless the patient has signs and/or symptoms of brain metastases, but it may be considered after 2 years of metastatic disease [26]. For extrahepatic metastases, the recommended imaging modalities are CT with contrast and PET-CT. In patients with metastatic disease or a high risk of metastases, assessment of HLA-A*02:01 is recommended for potential treatment with tebentafusp [27,28]. Additional lab tests, such as liver function, renal function, hematology, and LDH (lactate dehydrogenase), should also be performed. Patients with elevated LDH usually have more advanced disease and a worse prognosis [29]. The assessment of ctDNA at the metastatic setting diagnosis and throughout therapy to monitor disease activity is also recommended [30].

Table 1.
Follow-up after primary uveal melanoma treatment—summary of recommendations [20,21,22,23].
3. Treatment of Metastatic Uveal Melanoma
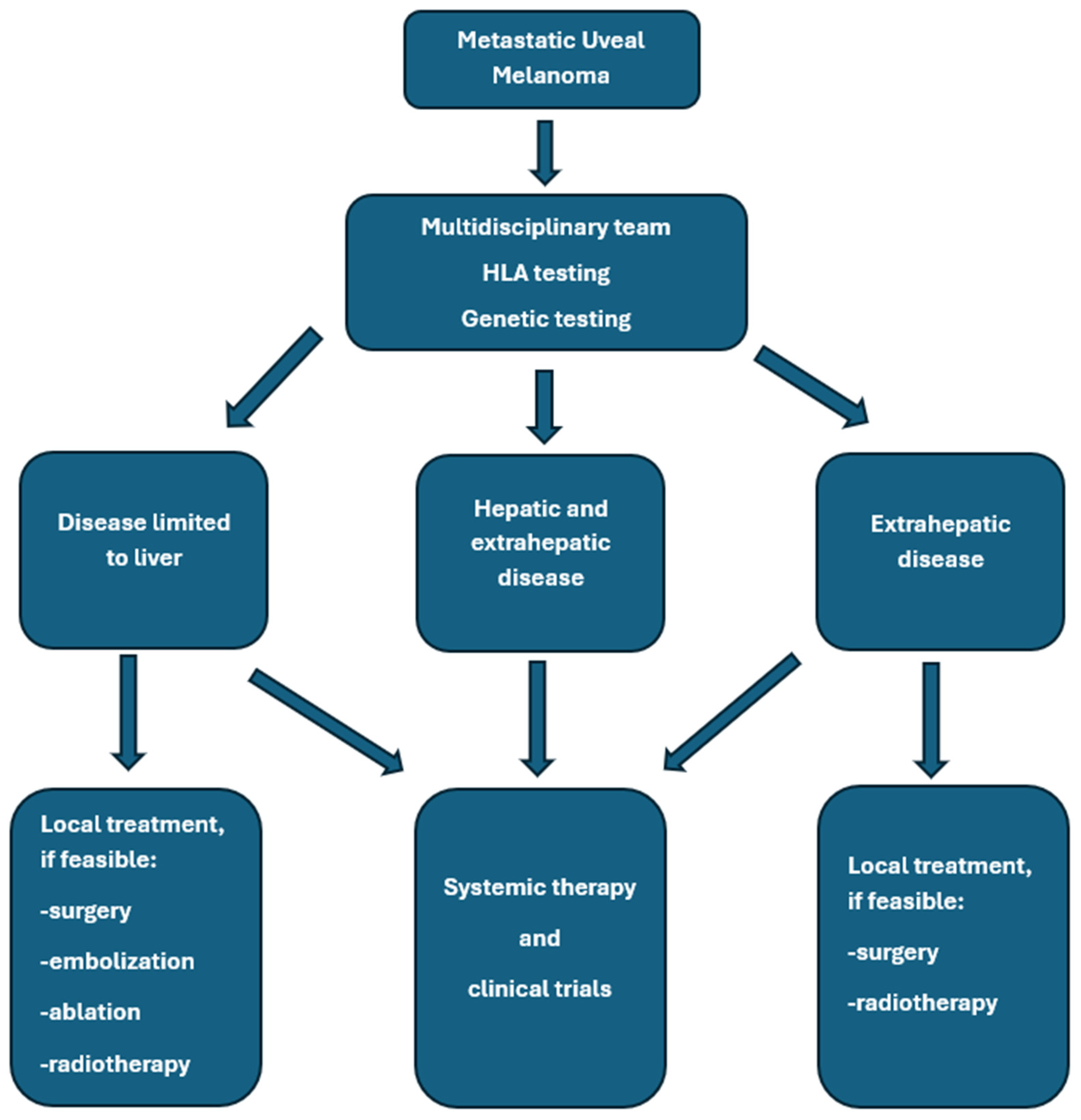
Treatment of mUM requires a multimodal approach, which may include local and systemic therapies. Liver-directed therapies remain an essential component in the management of hepatic metastases of UM and can provide effective local control, symptomatic relief, and potential survival benefits in carefully selected patients [20,31,32]. Systemic treatment options are limited in efficacy [31]. Khoja et al. published the results of a meta-analysis based on data from 965 patients with mUM who participated in 29 prospective studies. The median progression-free survival (mPFS) was 3.3 months in the entire group of patients; the PFS was 5.2 months in the liver-directed therapy group, 2.8 months in patients treated with immunotherapy, kinase inhibitors, or anti-angiogenic drugs, and 2.6 months in the chemotherapy group. The median OS (median OS) was 10.2 months in all enrolled patients and reached 14.6 months in the liver-directed therapy-treated group, and, respectively, 11.0, 9.2, 9.1, and 8.9 months for the groups treated with anti-angiogenic drugs, chemotherapy, kinase inhibitors, and immunotherapy. Univariate analysis revealed that shorter PFS was associated with liver metastases greater than 3 cm, elevated lactate dehydrogenase, and elevated alkaline phosphatase [33]. Personalized treatment planning based on tumor burden and location, disease course, hepatic function, patient performance status, comorbidities, and their treatment, HLA-A*02:01 genotype testing, is essential for the proper treatment of patients with mUM. A valuable option remains participation in clinical trials if available [32]. In general, surgery provides superior local control and OS improvement compared to systemic therapy alone. Hepatic metastasectomy or partial hepatectomy remains a valuable option for patients with limited disease. Only selected patients with liver metastases from UM are good candidates for complete metastasis resection, mainly due to the spread of multifocal malignancy. This treatment should be used in patients with an expected long survival time, without extra-hepatic metastases, and with potentially radically resected lesions (R0). Usually, two liver segments are removed. Retrospective analyses suggest that complete resection may improve OS, especially if complete resection is feasible. In different studies, the complete resection rates ranged from 27% to 88% [34,35,36]. For example, Mariani et al. reported 30% R0 resections in a group of 255 patients treated at a single center in France [37]. In this study, mOS in all patients treated with surgery, irrespective of the completeness of resection, was 14 months compared to 8 months in patients who did not undergo surgery, and was 27, 17, and 11 months after complete surgical resection (R0), R1 resection, and R2 resection, respectively. Three percent of patients died within 30 days after surgery due to liver insufficiency, hemorrhagic events, and subphrenic abscess. Based on a systematic review of 793 patients, OS ranged from 10 to 35 months in patients treated with surgery [38]. The general summary of treatment options is shown in Figure 2 and in Table 2.
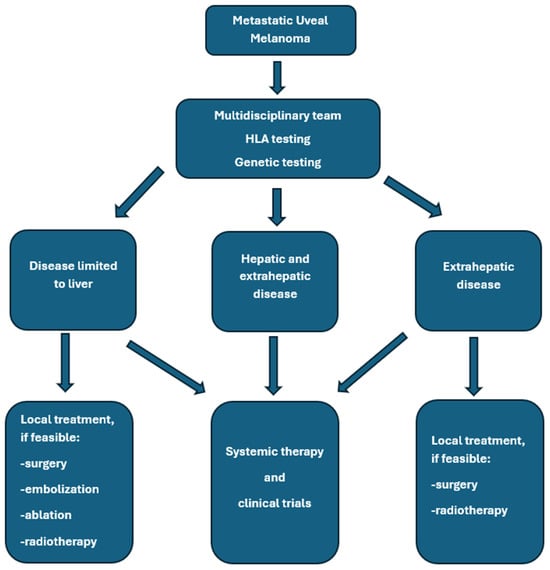
Figure 2.
General rules of metastatic uveal melanoma management.

Table 2.
Treatment options in metastatic uveal melanoma.
4. Systemic Treatment in Metastatic Uveal Melanoma
Metastatic uveal melanoma (mUM) is characterized by poor prognosis and limited systemic treatment options [55,56]. Multiple clinical trials have not shown any benefit from the use of the tested therapeutic options. The first approved systemic therapy with OS benefit in patients with HLAA*02:01-positive nonresectable mUM is tebentafusp. New molecules are being developed in clinical studies, and participation in these trials is strongly recommended unless local therapies are more suitable [56,57].
In view of the historically limited efficacy and unfavorable toxicity profile of conventional cytotoxic chemotherapy, contemporary clinical practice guidelines and recent systematic reviews increasingly emphasize a transition toward precision-based approaches, particularly targeted agents and immuno-oncologic therapies [21,28,58,59]. Nevertheless, systemic chemotherapy can retain clinical utility in highly selected patient populations, most notably in settings where approved targeted or immune-based strategies are unavailable, contraindicated, or accessible exclusively within clinical trial frameworks. Consequently, chemotherapy is mainly relegated to a palliative role. In contrast, current standards of care prioritize enrollment in well-designed clinical trials and the implementation of evidence-based targeted or immunotherapeutic regimens when appropriate. Crucially, comprehensive multidisciplinary evaluation at specialized referral centers remains indispensable to optimizing individualized treatment strategies and ensuring equitable access to emerging therapeutic modalities within this rapidly evolving landscape [20,21,58,59,60].
4.1. Chemotherapy
Systemic chemotherapy generally shows minimal efficacy in mUM. Several chemotherapy regimens have been evaluated in prospective trials in this patient population, but it remains unclear whether monotherapy improves survival. Single-agent treatments, including dacarbazine, fotemustine, and temozolomide, as well as combinations such as treosulfan plus gemcitabine, have demonstrated low ORR, with mPFS typically under 4 months, and mOS around 10 months [60,61,62]. Standard first-line triplet chemotherapy with cisplatin, vinblastine, and dacarbazine yielded an ORR of 20%, mOS of 13 months, and PFS of 5.5 months [63]. In a phase 2 trial, treosulfan combined with gemcitabine achieved an ORR of 28.6%, mOS of approximately 14 months, PFS of about 6.5 months, and 1-year survival of 80% [64]. Across four prospective clinical trials evaluating combination chemotherapy protocols, median progression-free survival ranged from 2.5 to 6.7 months, while median overall survival varied between 7.5 and 14.2 months [60,61,62]. Additionally, clinical trial data indicate therapeutic activity of the BOLD regimen—comprising dacarbazine, vincristine, bleomycin, and lomustine—when administered in combination with interferon [65]. Other studies confirm that objective responses are rare, and survival benefits are marginal, underscoring the substantial chemoresistance of UM [66,67].
4.2. Immunotherapy
Immune checkpoint inhibitors (ICIs), targeting PD-1 (nivolumab, pembrolizumab) and CTLA-4 (ipilimumab), have been evaluated in multiple clinical trials and real-world studies; however, their efficacy is generally lower than in cutaneous melanoma [56,58,68]. Meta-analyses and clinical trials have shown that the treatment results are not superior to conventional chemotherapy. Single-agent anti-PD-1 therapy in mUM showed low ORR (3–5%), with mPFS of 2–3 months and mOS of 7–10 months [69,70]. Ipilimumab monotherapy yields similar ORR (0–10%) and OS (6–10 months), with grade ≥ 3 immune-related adverse events in up to 36% of patients [70,71,72]. Combination therapy with ipilimumab plus nivolumab increases ORR to 11–17%, mPFS to 3–4 months, mOS 12.7–18 months, but grade ≥ 3 toxicity rises to 40–54%, predominantly colitis, hepatitis, and endocrinopathies [36,55,73]. A combination of anti-PD1 and anti-LAG3 monoclonal antibodies has been assessed in a phase 2 study (NCT04552223, n = 27). The safety profile was consistent with previous data, but the ORR was low (7.7%) [74]. Nevertheless, combination nivolumab of with relatlimab as salvage treatment has been reported as successful in selected cases [75].
Retrospective analyses confirmed the limited activity of immunotherapy in mUM. To compare the efficacy of chemotherapy used before 2017 and immunotherapy used after 2017, Magomedova et al. analyzed data from 124 patients treated with chemotherapy and 144 treated with immunotherapy. The chemotherapy regimens used the most frequently were cyclophosphamide plus vincristine plus dacarbazine (n = 39) and paclitaxel plus carboplatin (n = 18). In the immunotherapy group, patients were treated with anti-PD-1 (n = 78), ipilimumab plus nivolumab (n = 48), ipilimumab (n = 10), and tebentafusp (n = 8). In the immunotherapy group, 26 patients received additional liver-directed therapies. Grade 3 and 4 treatment-related AEs were observed in 28.2% and in 30.6% of patients in the chemotherapy group and immunotherapy group, respectively. The survival was prolonged with immunotherapy in comparison to chemotherapy, mPFS was 4.1 months vs. 1.8 months and mOS 21.7 months vs. 5.6 months. There were no responses in the chemotherapy group while 11.8% of patients treated with immunotherapy achieved response (4-CR, 13-PR) [76].
4.3. Tebentafusp
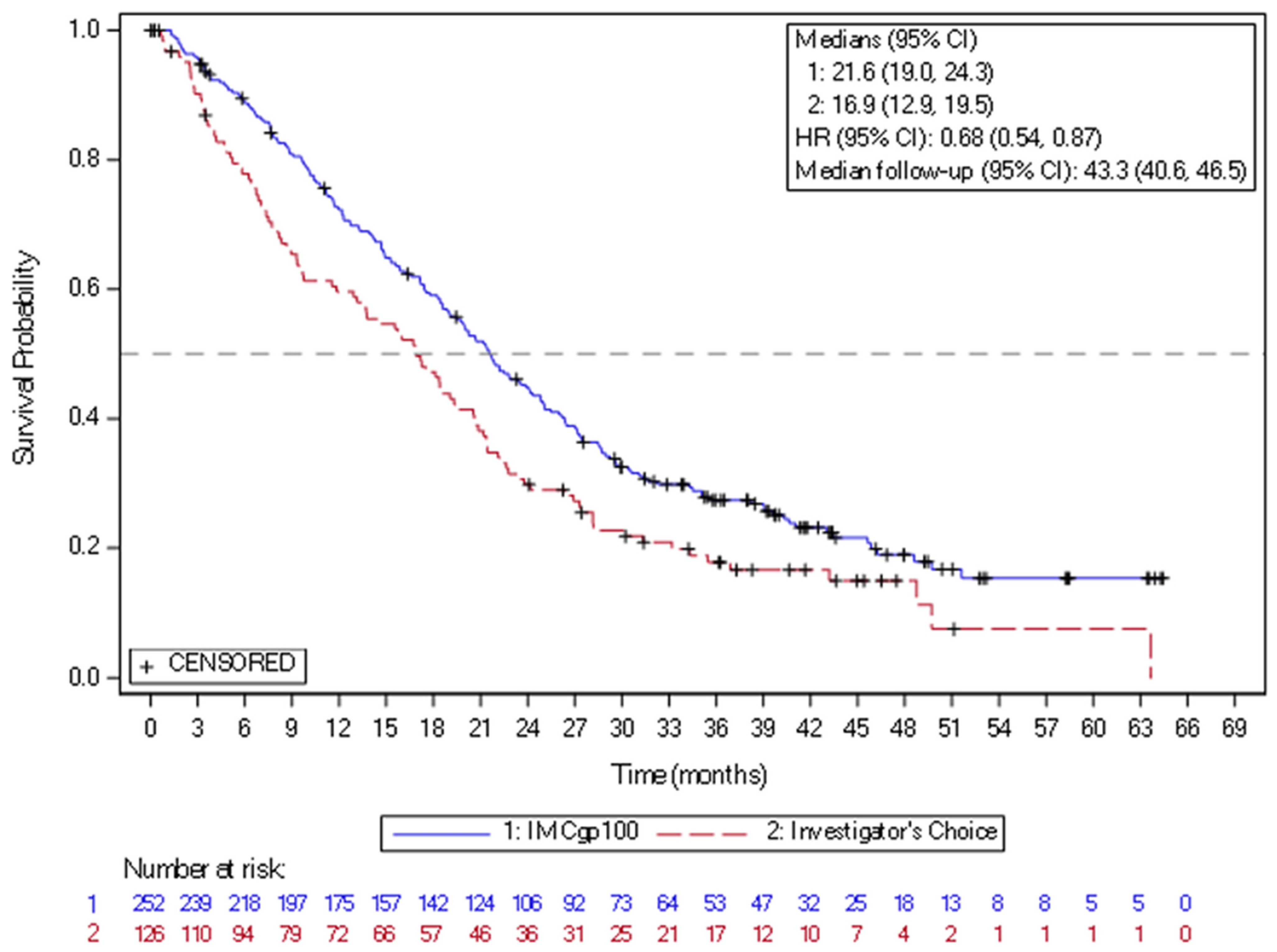
In 2022, the new drug, tebentafusp, a bispecific gp100 peptide-HLA-directed CD3 T-cell engager, had been approved by FDA and EMA, based on the positive efficacy results in mUM. Tebentafusp is a bispecific fusion protein composed of a T-cell receptor (TCR)-targeting moiety linked to an anti-CD3 antibody fragment that serves as the effector component. The TCR domain exhibits high-affinity binding to a gp100 peptide presented by HLA-A*02:01 on the surface of UM cells, while the effector domain engages the CD3 receptor in polyclonal T cells. This drug demonstrated a reproducible OS benefit in prospective trials and real-world series. In the randomized phase 3 IMCgp100-202 study (NCT03070392) (n = 378), the patients with previously untreated HLA-A02:01-positive mUM were randomized to tebentafusp arm or control group treated based on investigator choice with pembrolizumab, ipilimumab, or dacarbazine. The mOS was 21.6 months in the tebentafusp group and 16.9 months in the control group (HR 0.68) (Figure 3). The PFS rate at 6 months was 31% vs. 19, despite modest ORR around 9%. The most frequently observed treatment-related adverse events of any grade in the tebentafusp group were rash (83%), pyrexia (76%), pruritus (70%), and hypotension (38%). Grade 3 or 4 treatment-related adverse events occurred in 109 patients (44%) in the tebentafusp group compared with 19 patients (17%) in the control group. Extended follow-up confirmed a durable survival benefit, with a 3-year OS of 27% vs. 18%. No new safety signals were observed [77]. Tebentafusp is indicated as monotherapy for the treatment of human leukocyte antigen (HLA)-A*02:01-positive adult patients with unresectable or mUM. This medication is given weekly, with the dose increased for the first three infusions and close patient follow-up lasting at least 16 h after the first three infusions, mostly due to the risk of cytokine release syndrome. Based on the data from two clinical trials with tebentafusp in mUM (IMCgp100-102 and IMCgp100-202, n = 378), the most frequently reported AEs in patients receiving tebentafusp included cytokine release syndrome (88%), rash (85%), pyrexia (79%), pruritus (72%), fatigue (66%), nausea (56%), chills (55%), abdominal pain (49%), edema (49%), hypo- or hyperpigmentation (48%), hypotension (43%), dry skin (35%), vomiting (34%), and headache (32%). Four percent of patients required treatment discontinuation due to AEs [19,27].
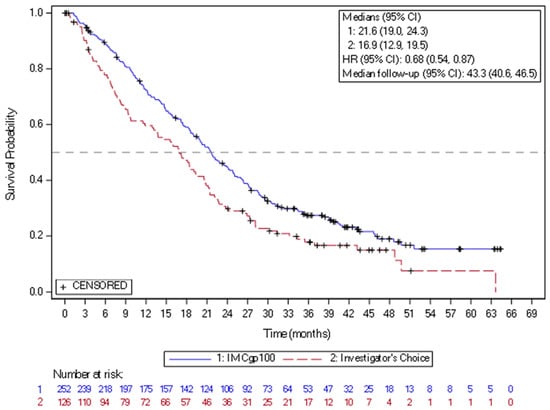
Figure 3.
Kaplan–Meier curves of overall survival in the study of IMCgp100-202 (3-year follow-up analysis) in the intention-to-treat population. CI = confidence interval; HR = hazard ratio; IMCgp100 = tebentafusp; ITT = Intent-to-treat.
Real-world evidence confirmed the efficacy of tebentafusp in mUM. A multicenter real-world cohort study including centers in Belgium, France, and Poland confirmed these findings, showing tebentafusp to be effective and safe in a broad patient population (n = 175), with high-risk features such as elevated LDH and extrahepatic metastases correlating with shorter OS. mPFS was 4 months (95% CI 2.7–5.3), and mOS was 20 months (95% CI 15.3–24.6) with 1-year OS rate of 63.6% [78]. In the Department of Soft Tissue/Bone Sarcoma and Melanoma Maria Sklodowska-Curie National Research Institute of Oncology in Poland, over 550 UM patients at different stages have been treated and followed. Among them, 132 patients were treated with chemotherapy, a few with immunotherapy, and 50 with tebentafusp. The most frequently used chemotherapy regimens were CVD (63 patients) and BOLD (47 patients). mOS in patients treated with chemotherapy containing dacarbazine was 9.0 (7.2–11.4) months [n = 98] vs. 13.4 months (n = 32) in patients treated with tebentafusp. Notably, approximately 50% of patients treated with tebentafusp continued treatment beyond disease progression. Tebentafusp safety profile was consistent with that previously reported [79].
Based on the reported analyses, early reductions in ctDNA after tebentafusp treatment may be associated with improved OS in patients with metastatic uveal melanoma, which was shown in the clinical trial. Patients with early ctDNA reduction had prolonged mOS compared to patients with lower reduction; the mOS was 21.2 months vs. 12.9 months. Although retrospective and early-phase analyses suggest this correlation, prospective validation is still required. In addition, patients with detectable ctDNA before starting treatment with tebentafusp had significantly lower mOS (12.9 months vs. 40.5 months) and mPFS (2.5 months vs. 10.8 months) [30]. In summary, tebentafusp consistently provides a clinically meaningful OS benefit across randomized and observational datasets, regardless of RECIST (Response Evaluation Criteria in Solid Tumors) responses or conventional PFS.
J.M. Piulats et al. compared OS on tebentafusp or pembrolizumab in the IMCgp100-202 study with nivolumab plus ipilimumab in the GEM1402 study in previously untreated patients with mUM using the inverse probability of treatment weighting based on the propensity score (IPTW) [80]. The IPTW-adjusted analysis demonstrated that tebentafusp significantly favored OS (HR 0.52; 1-year OS was 73% for tebentafusp vs. 50% for nivolumab plus ipilimumab). Sensitivity analyses confirmed the superior OS for tebentafusp. The findings support the conclusion that tebentafusp provides a similar OS benefit compared with nivolumab plus ipilimumab.
4.4. Targeted Therapy
Metastatic UM is characterized by distinct genetic alterations compared to cutaneous melanoma, notably activating mutations in GNAQ and GNA11, and secondary alterations in BAP1, SF3B1, and EIF1AX [81]. These molecular insights have guided the development of targeted therapies, including MEK inhibitors, PKC inhibitors, and other pathway-directed agents. In most studies with targeted therapy, the ORR was up to 10% [82,83]. UM often express KIT, but the c-KIT mutations are of low frequency and imatinib showed limited efficacy in clinical trials. In a phase 2 study with 25 patients with mUM enrolled and treated with imatinib, mPFS was 2.8 months and mOS was 6.9 months [84].
Selumetinib, an MEK1/2 inhibitor, was evaluated in a randomized phase 2 trial against chemotherapy (temozolomide or dacarbazine) in patients with mUM (NCT01143402). A modest improvement in PFS, mPFS of 15.9 vs. 7 weeks and ORR of 14% vs. 0% was reported, and there was no improvement in OS, mOS, which was 11.8 vs. 9.1 months [85]. Generally, the targeted therapy in biomarker-unselected patients is of limited benefit in mUM [86].
Promising results were observed in a phase 2 trial using molecularly targeted therapy, using crizotinib in combination with the protein kinase C (PKC) inhibitor darovasertinib, which targets GNAQ/GNA11 mutations, in patients with mUM (NCT03947385). In this study, 30% of patients achieved a response to treatment, and another 60% achieved stable disease [87,88].
4.5. Other Systemic Treatments for Metastatic Uveal Melanoma
Antibody–drug conjugates, which are widely used in some malignancies, have been tested in early-phase clinical trials in patients with melanoma, including uveal melanoma. Glembatumumab vedotin, a fully human IgG2 monoclonal antibody directed against the glycoprotein NMB coupled by a peptide linker to monomethyl auristatin E, was evaluated in the phase 2 clinical study (NCT02363283) in patients with mUM or locally recurrent UM. The ORR was 6%, and DCR 57%, with mPFS of 3.1 months and OS 11.9 months [89]. In a phase 1 study of the anti-endothelin B receptor antibody–drug conjugate DEDN6526A in patients with metastatic or unresectable cutaneous, mucosal, or UM (NCT01522664), DEDN6526A at the selected dose showed an acceptable profile and preliminary antitumor activity in all melanomas [90].
Treatment of mUM with adoptive transfer of tumor-infiltrating lymphocytes has also been assessed in clinical trials. In the single-center phase 2 study (NCT01814046), 21 patients with UM were enrolled. Autologous TILs cultures were generated after metastasectomy, and patients received lymphodepleting conditioning chemotherapy before infusion of autologous TILs and high-dose interleukin-2. Eight patients responded to treatment. The AEs were mostly related to lymphodepleting chemotherapy [91].
The use of oncolytic viral therapy has been assessed in clinical trials in patients with mUM. RP2, a selective, replication-competent HSV-1 engineered to express GM-CSF, a modified GALV-GP glycoprotein, and an anti-CTLA-4 antibody-like molecule, has been evaluated in a phase 1 study (NCT04336241) in patients with mUM. In total, 17 patients received treatment either in monotherapy (n = 3) or in combination with nivolumab (n = 14). The ORR was 29.4%, and DCR 58.8%. The most common AEs were pyrexia, chills, fatigue, hypotension, and pruritus [92]. The data about cancer vaccine use in mUM are limited. Bol et al. reported responses induced with dendritic cell vaccination in four (29%) of 14 patients, with mOS of 19.2 months and no severe treatment-related toxicities [93].
In the phase 1a/1b study (NCT03686124), 16 adult HLA-A*02:01+ patients with UM were treated with IMA203, a PRAME-directed TCR T-cell therapy engineered to recognize intracellular PRAME-derived peptides presented by HLA. Following leukapheresis and the manufacture of IMA203, the patients underwent lymphodepletion and IMA203 infusion followed by low-dose IL-2. The median of previous lines of therapy was two (62.5% prior tebentafusp), 75% patients had the largest metastatic lesion measuring 3.1–8.0 cm in diameter, and 56% had elevated LDH. ORR was 67%; tumor shrinkage was observed in all participants. Median DOR was 11.0 months, and mPFS and mOS were 8.5 months and 16.2 months, respectively. Tolerability was favorable [94,95].
Recent studies show benefit from a precision oncology approach based on molecular testing and tumor mutational burden testing [86,96].
5. Local Therapies in Metastatic Uveal Melanoma
The results of local treatment have been confirmed in many prospective analyses and in a systematic review published in 2025 [16,97]. Surgery, described previously in Section 3, is a valuable option in eligible patients. Radiofrequency ablation (RFA), microwave ablation, and cryoablation are minimally invasive options for small lesions (<3 cm). Local control rates exceed 70–80% for appropriately sized tumors, and procedure-related morbidity is generally low [52,97,98]. Various techniques have been developed for the localized delivery of pharmaceutical therapies to the liver, allowing the administration of higher doses than would be tolerable systemically due to toxicity constraints. These techniques include embolization-based therapies, percutaneous hepatic perfusion (PHP), and isolated hepatic perfusion (IHP). Transarterial therapies, including transarterial chemoembolization (TACE) and transarterial radioembolization (TARE, e.g., Yttrium-90) are also valuable options in patients with UM with liver metastases.
5.1. Therapies Based on Liver Metastases-Directed Embolization
Isolated hepatic perfusion—IHP—and percutaneous hepatic perfusion—PHP—enable perfusion of the liver with high-dose chemotherapy while minimizing systemic exposure. Isolated hepatic perfusion (IHP) is a more prolonged and complex procedure than PHP and is used infrequently. It is an open surgical procedure and requires vascular isolation of the liver to allow the delivery of high doses of heated chemotherapy, for example, melphalan or cisplatin, directly to the organ through an arterial catheter. It requires a few days of hospitalization and can cause significant complications, including transient hepatotoxicity and portal vein thrombosis [18,42].
Prospective studies of PHP with melphalan report hepatic response rates of 30–40% and mOS up to 16 months [99,100,101,102]. A phase III randomized trial of hepatic intra-arterial fotemustine vs. IV fotemustine (ClinicalTrials.gov NCT00110123) showed no OS benefit (14.6 vs. 13.8 months) but improved PFS (4.5 vs. 3.5 months) and RR (10.5% vs. 2.4%) [103]. Nevertheless, based on the FOCUS phase 3 trial (NCT02678572), PHP with melphalan had been approved by the FDA for UM with liver metastases. In this study, patients were treated with PHP with melphalan or the investigator’s choice of TACE, pembrolizumab, ipilimumab, or dacarbazine. Patients treated with PHP with melphalan had an ORR (objective response rate) of 35.2% (n = 91) compared to 12.5% in the comparator group (n = 42), with a median duration of response of 14 months, and mOS of 20.5 months compared to 14.1 months in the control group. Adverse events were observed in 42.6% of patients in the group receiving liver-directed treatment with melphalan, but these were mostly transient. No death had been reported in the treatment group [104]. PHP is associated with hematologic toxicity, primarily thrombocytopenia and neutropenia, which are usually reversible [17].
In the SCANDIUM phase 3 clinical trial (NCT01785316), 87 patients with UM with previously untreated isolated liver metastases were randomized in a 1:1 ratio to the group treated with melphalan IHP (n = 41) or to the control group treated according to the choice of the investigator (chemotherapy, immunotherapy, or other localized interventions) (n = 44). The mOS was 21.7 in the IHP group vs. 17.6 months in the control group (HR: 0.64; 95% CI: 0.37–1.10) and mPFS was 7.4 months in the IHP group vs. 3.3 months in the control group (HR = 0.21, 95% CI, 0.12 to 0.36) [100,105].
5.2. Transarterial Therapies Directed to Liver Metastases
Transarterial chemoembolization (TACE) is a liver-directed catheter-based therapy that combines hepatic-arterial chemotherapy with embolization. TACE results in response rates of 20–35% and an mOS of around 10–14 months [16]. In a first TACE clinical trial using cisplatin (±carboplatin) followed by polyvinyl alcohol embolization, partial responses were reported in 57% of patients with median time to progression of 8.5 months and median survival 11.5 months after first TACE (17 vs. 11 months when liver tumor burden was <25% vs. >25%) [51]. Subsequently, in a larger series, chemoembolization yielded a partial response rate of 11%, along with mPFS of 3.8 months, and mOS 6.7 of months [106]. At the same time, in an Asian cohort treated with cisplatin (and gelatin sponge), ORR was 21%, with mOS reaching 23 months, and 1-, 2-, and 5-year survival rates of 72.4%, 39.4%, and 0%, respectively. In these patients, liver enzyme elevation was reported in 100% of them, with grade ≥ 3 AST/ALT elevations of 34.5% and 51.7%, respectively [48]. A small prospective first-line study using irinotecan drug-eluting beads reported an ORR of 80%, with all patients alive at ~10–16 months of follow-up [47]. TACE is often repeated and may be used alone or alongside systemic therapy. The efficacy of tebentafusp + TACE has not been reported yet, but transarterial immunoembolization with concurrent checkpoint blockade was reported to induce an ORR of 17%, mPFS of 4.9 months, and mOS of 35 months, at the price of immune-related hepatitis during such combination therapy [107].
Transarterial radioembolization—TARE, also termed selective internal radiation therapy (SIRT)—delivers intra-arterial Yttrium-90 (90Y) microspheres (made of resin or glass) via catheter into the hepatic artery to locally irradiate uveal melanoma metastases while sparing normal liver parenchyma. TARE is typically performed as a lobar or segmental procedure after angiographic mapping. It is used either as a standalone liver metastases-directed therapy or with systemic therapy [108]. TARE uses beta radiation. It was demonstrated to induce disease control rates of 60–70% and mOS of 12–15 months in several retrospective series [98]. In the prospective phase II radiology trial, reported ORR was 18%, with hepatic mPFS of 5.5 months, and mOS of 19.1 months resulting from 1-year OS as high as 70.4% [109]. When used in a first-line treatment, SIRT induced partial responses in 30% of cases and disease control of 80%, with mPFS of 8.1 months and mOS of 18.7 months [45].
A systematic review of SIRT for uveal melanoma liver metastases reported median OS of 12.3 months and hepatic PFS of 5.4 months across included studies [46]. German multicenter registry analysis confirmed longer OS with liver-directed therapy plus immune checkpoint blockade vs. immune checkpoint blockade only (20.1 vs. 13.8 months) and a higher ORR for multidisciplinary treatment compared to immune checkpoint blockade (16.7% vs. 3.8%) [110]. Moreover, sequential SIRT and nivolumab with ipilimumab was associated with grade 3–4 immune-related adverse events in 44.4% and an mOS from metastatic diagnosis of 49.6 months [111]. At this point in time, tebentafusp–TARE combinations are being prospectively evaluated (e.g., NCT06627244), but efficacy results are not yet available.
For both TACE and TARE, post-embolization syndrome, transient elevations in liver enzymes, and fatigue are common [47,50,112].
5.3. Liver-Directed Radiotherapy
Stereotactic body radiation therapy (SBRT) may be a valuable option in case other liver-directed therapies are not feasible and for patients with extrahepatic metastases. SBRT provides high-dose, precisely targeted radiation to hepatic lesions while sparing surrounding liver tissue. EBRT, though less precise, can be used in palliative settings to reduce tumor burden and alleviate symptoms (Table 2) [20,66,68].
5.4. Local Treatment of Extrahepatic Disease
Isolated extrahepatic metastases are rare in UM. Extrahepatic metastases in UM are most commonly located in the lung, bone, and central nervous system, often in the context of multiorgan metastatic disease. In a multicenter cohort of 1845 metastatic UM patients, symptomatic brain metastases were identified in 6.3%, and median survival after brain metastases diagnosis was 7.6 months [113]. The data about local treatment of extrahepatic metastases is limited and this treatment is not considered the standard of care. In highly selected cases with isolated or oligometastatic disease, absence of hepatic metastases, long disease-free intervals, surgery, and radiotherapy including radiosurgery may be considered based on a multidisciplinary team (MDT) decision. Management is typically individualized per other solid-tumor oligometastatic practices including surgical metastasectomy for technically resectable solitary lesions, and stereotactic or conventionally fractionated radiotherapy for CNS, bone or spine metastases, and selected thoracic metastases. Systemic therapy remains essential because extrahepatic lesions frequently coexist with occult micrometastatic liver disease [113,114,115].
6. Liver-Directed Therapy in Combination with Systemic Treatment
Integration of liver-directed therapies with systemic approaches, including ICI and molecularly targeted agents, is an area of growing clinical and translational interest. Emerging evidence indicates that achieving local tumor control may potentiate systemic immune activation, thereby augmenting the therapeutic efficacy of immunotherapy, particularly in patients with liver-dominant disease [98,101,116].
The evolving therapeutic strategy for mUM with predominant hepatic involvement focuses on the integration of liver-directed locoregional modalities with systemic immunotherapies. Retrospective data from a single-center cohort (n = 45) indicate that the combination of ICI and liver-directed therapies (e.g., surgical resection or transarterial approaches) significantly prolongs OS—mOS of 22.5 months vs. 11.4 months with systemic therapy alone [117]. Furthermore, a larger retrospective multicenter analysis reveals that patients receiving liver-directed therapy as first-line treatment demonstrated a median melanoma-specific survival of 28 months vs. 10 months for systemic therapy alone, with further survival gains observed when followed by ICI [118].
In prospective early-phase studies, sequential application of selective internal radiation therapy (SIRT) using Yttrium-90 followed by combination ICI (ipilimumab + nivolumab) yielded overall response rates and survival outcomes that support a synergistic interaction, despite the need for dose modifications due to toxicity [111]. These findings are echoed in additional retrospective investigations, which affirm a more favorable mOS when systemic ICI is combined with liver-directed interventions compared to either modality alone [118].
In the SCANDIUM II phase 1b study (NCT01785316), IHP plus systemic treatment with nivolumab and ipilimumab was compared with immunotherapy alone. The number of patients enrolled was small (N = 18). However, based on preliminary results, it appears that the combination of IHP with immunotherapy may be feasible in patients with liver-dominant metastatic UM [119].
In the CHOPIN phase 2 study (NCT04283890) (n = 79), the addition of ipilimumab and nivolumab to PHP in mUM patients with hepatic-only or hepatic-dominant disease significantly improved PFS (12.8 vs. 8.3 months), OS (23.1 vs. 19.6 months), and ORR (76.3% vs. 39.5%) with a manageable safety profile [43,120].
7. Drugs in Development
Multiple agents are currently in development, targeting key oncogenic pathways and the immune microenvironment to improve outcomes. The current pipeline includes antibody-based therapies, adoptive cell transfers, combination regimens of targeted agents, and novel immunomodulatory strategies to improve efficacy and overcome intrinsic resistance mechanisms [32]. Recent advances in genomic, transcriptomic, and broader multi-omic profiling have provided a more comprehensive understanding of tumor biology, facilitating biomarker-driven patient stratification and the identification of actionable molecular targets [83]. Integrating these multidimensional datasets with clinical outcomes has allowed the design of rational combination therapies and predictive frameworks to anticipate the response to treatment. Continued translational efforts connecting molecular insights with therapeutic development are essential to advance personalized interventions. In addition to strategies for advanced disease, ongoing studies also focus on interventions to reduce local recurrence and metastatic spread in high-risk patients (Table 3).

Table 3.
Summary of clinical trials investigating systemic treatments for metastatic uveal melanoma (based on https://clinicaltrials.gov, access date 20 August 2025).
8. Discussion
This article provides a comprehensive overview of the current standards of care for UM, highlighting significant advances in diagnostics and therapy while also pointing out persistent challenges, particularly in the context of metastatic disease. Unlike cutaneous melanoma, UM is characterized by a unique biology and a predilection for liver metastases, which is a key factor influencing prognosis.
The prognosis for metastatic UM (mUM) remains poor, with an mOS ranging from 3 to 30 months, which is mostly due to the limited efficacy of systemic therapies. Considering this, liver-directed therapies, such as surgical resection, embolization techniques (TACE, TARE), ablative and other methods like PHP and IHP, and SBRT, have become the cornerstone of treatment in patients with disease limited to the liver and eligible for local treatment. These therapies, particularly complete resection, have been shown to significantly improve median survival compared to systemic treatment. Notably, data from the phase 3 FOCUS trial confirms that PHP with melphalan provides better ORR and OS compared to conventional therapies. The isolated extrahepatic disease is rare and is associated with better prognosis, and despite data about local treatment being limited, the local therapies, including surgery and radiotherapy, may be considered in selected cases based on the MDT decision.
The efficacy of conventional chemotherapy in mUM is generally limited. Similarly, ICI therapy shows limited activity compared to cutaneous melanoma, although “real-world” data suggest a small benefit, especially in combination with liver-directed therapies. A breakthrough is the approval of tebentafusp, the first systemic agent to demonstrate a significant advantage in OS in the IMCgp100-202 study, which has been confirmed in daily practice. Importantly, early reduction in ctDNA after tebentafusp treatment may be an early indicator of a better prognosis, even in cases of radiologically assessed disease progression, suggesting the need for further research on biomarkers.
The emerging opportunities, including systemic treatment for HLA-A*02:01-negative patients, a combination of local treatment with systemic therapies leading to further survival improvement, and development of new drugs targeting known molecular abnormalities based on multi-omic profiling studies, may change the treatment paradigm in the near future. A key factor in optimizing treatment is an individualized approach to the patient, considering the molecular profile of the tumor (e.g., BAP1, SF3B1, EIF1AX, PRAME mutations) and HLA-A*02:01 status, as well as participation in clinical trials. The proper management should be personalized and include MDT discussion, especially in candidates for local treatment, local treatment combined with systemic therapy, and patients with extrahepatic disease only.
9. Conclusions
UM is a rare but aggressive cancer with a high risk of metastases, particularly to the liver, despite great opportunities for primary tumor treatment. In spite of the limited efficacy of systemic therapies, there is some progress observed in the treatment of patients with mUM. Liver-directed strategies remain the cornerstone of therapy, offering the most favorable outcomes for liver metastases. New systemic approaches, such as tebentafusp, represent a promising advance, providing significant OS benefit for a selected group of patients. Future research should focus on further understanding the genetic and molecular underpinnings of UM, as well as on identifying new systemic therapies, including drug combinations that can overcome chemoresistance and induce lasting immune responses. The implementation of individualized treatment plans in specialized centers within MDT, supported by precise molecular diagnostics, availability of local and systemic treatments, and access to clinical trials, is crucial for improving treatment outcomes and the prognosis for patients with mUM.
Author Contributions
Conceptualization—P.R. (Piotr Rutkowski); methodology—P.R. (Paweł Rogala) and A.M.C.; software—P.R. (Paweł Rogala) and A.M.C.; resources—P.R. (Paweł Rogala) and A.M.C.; writing—original draft preparation—all authors; writing—review and editing—all authors; reviewer response and text correction—P.R. (Paweł Rogala) and A.M.C.; visualization—P.R. (Paweł Rogala) and A.M.C.; supervision—P.R. (Piotr Rutkowski); project administration—P.R. (Paweł Rogala) and A.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created or analyzed in this study.
Conflicts of Interest
P.R. (Paweł Rogala) Medison Pharma—honoraria for lectures; M.D.-Ś., Medison Pharma- honoraria for lectures and writing, Incyte Sarl—employee; A.D. Medison Pharma—funding the participation in the conference; P.R. (Piotr Rutkowski) Medison Pharma—Consulting fees, payment or honoraria for lectures, presentations, or educational events; other authors declared no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AJCC | American Joint Committee on Cancer |
| BAP1 | BRCA1-associated protein 1 |
| COMS | Collaborative Ocular Melanoma Study |
| CT | computed tomography |
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen 4 |
| EIF1AX | Initiation Factor 1A X-linked |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GNA11 | Guanosine Nucleotide-Binding Protein Alpha-11 |
| GNAQ | Guanosine Nucleotide-Binding Protein Alpha-Q Gene |
| gp100 | Glycoprotein 100 |
| HDAC | Histone deacetylase |
| HLA | Human Leukocyte Antigen |
| HR | Hazard Ratio |
| ICI | Immune Checkpoint Inhibitor |
| LDH | lactate dehydrogenase |
| LITT | Laser-induced interstitial thermotherapy |
| MAPK | Mitogen-activated protein kinase |
| MEK | Mitogen-Activated Extracellular Signal-Regulated Kinase |
| mOS | Median overall survival |
| mPFS | Median progression-free survival |
| MRI | Magnetic Resonance Imaging |
| MWA | Microwave Ablation |
| mUM | metastatic Uveal Melanoma |
| NCCN | National Comprehensive Cancer Network |
| ORR | objective response rate |
| OS | overall survival |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PET-CT | Positron Emission Tomography/Computed Tomography |
| PHP | percutaneous hepatic perfusion |
| PKC | Protein Kinase C |
| QoL | quality of life |
| RFA | Radiofrequency ablation |
| SF3B1 | Splicing Factor 3B Subunit 1 |
| SIRT | Selective Internal Radiotherapy |
| TACE | Transarterial Chemoembolization |
| TARE | transarterial radioembolization |
| TCR | T-cell Receptor |
| TMB | Tumor Mutational Burden |
| UM | Uveal Melanoma |
| VEGF | Vascular Endothelial Growth Factor |
References
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Silva-Rodriguez, P.; Fernandez-Diaz, D.; Bande, M.; Pardo, M.; Loidi, L.; Blanco-Teijeiro, M.J. GNAQ and GNA11 Genes: A Comprehensive Review on Oncogenesis, Prognosis and Therapeutic Opportunities in Uveal Melanoma. Cancers 2022, 14, 3066. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Wdowiak, K.; Dolar-Szczasny, J.; Rejdak, R.; Drab, A.; Maciocha, A. A Brief Overview of Uveal Melanoma Treatment Methods with a Focus on the Latest Advances. J. Clin. Med. 2025, 14, 4058. [Google Scholar] [CrossRef]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58, 10. [Google Scholar] [CrossRef]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef]
- Damato, E.M.; Damato, B.E. Detection and time to treatment of uveal melanoma in the United Kingdom: An evaluation of 2,384 patients. Ophthalmology 2012, 119, 1582–1589. [Google Scholar] [CrossRef]
- van Poppelen, N.M.; de Bruyn, D.P.; Bicer, T.; Verdijk, R.; Naus, N.; Mensink, H.; Paridaens, D.; de Klein, A.; Brosens, E.; Kili, E. Genetics of Ocular Melanoma: Insights into Genetics, Inheritance and Testing. Int. J. Mol. Sci. 2020, 22, 336. [Google Scholar] [CrossRef]
- Chang, M.Y.; McCannel, T.A. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br. J. Ophthalmol. 2013, 97, 804–811. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef]
- The AJCC Ophthalmic Oncology Task Force. International Validation of the American Joint Committee on Cancer’s 7th Edition Classification of Uveal Melanoma. JAMA Ophthalmol. 2015, 133, 376–383. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013, 120, 2066–2071. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer Classification of Uveal Melanoma (Anatomic Stage) Predicts Prognosis in 7,731 Patients: The 2013 Zimmerman Lecture. Ophthalmology 2015, 122, 1180–1186. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Rivoire, M.; Kodjikian, L.; Baldo, S.; Kaemmerlen, P.; Negrier, S.; Grange, J.D. Treatment of liver metastases from uveal melanoma. Ann. Surg. Oncol. 2005, 12, 422–428. [Google Scholar] [CrossRef]
- Ini, C.; Foti, P.V.; Farina, R.; Tiralongo, F.; Castiglione, D.G.; Cannarozzo, M.; Spatola, C.; David, E.; Palmucci, S.; Russo, A.; et al. Percutaneous Locoregional Therapies for the Treatment of Liver Metastases from Uveal Melanoma: A Systematic Review. Technol. Cancer Res. Treat. 2025, 24, 15330338251343144. [Google Scholar] [CrossRef]
- Bethlehem, M.S.; Katsarelias, D.; Olofsson Bagge, R. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers 2021, 13, 4726. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Eggermont, A.M.; O’Day, S.; Zager, J.S. Metastatic melanoma to the liver: A contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer 2014, 120, 781–789. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Rutkowski, P.; Romanowska-Dixon, B.; Markiewicz, A.; Zieniewicz, K.; Kozak, K.; Rogala, P.; Świtaj, T.; Dudzisz-Sledź, M. Diagnostic and therapeutic management of patients with ocular melanomas—Recommendations of the Polish Society of Oncology. Nowotw. J. Oncol. 2022, 72, 342–352. [Google Scholar] [CrossRef]
- Rutkowski, P.; Rogala, P.; Wysocki, P.J.; Kozak, K.; Nasierowska-Guttmejer, A.; Jeziorski, A.; Wysocki, W.M.; Kalinka, E.; Świtaj, T.; Kamińska-Winciorek, G.; et al. Postępowanie diagnostyczno-terapeutyczne u chorych na czerniaki oka—Zalecenia ekspertów 2025. Onkol. Prakt. Klin.—Eduk. 2025, 11, 243–256. [Google Scholar]
- Rao, P.K.; Barker, C.; Coit, D.G.; Joseph, R.W.; Materin, M.; Rengan, R.; Sosman, J.; Thompson, J.A.; Albertini, M.R.; Boland, G.; et al. NCCN Guidelines Insights: Uveal Melanoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2020, 18, 120–131. [Google Scholar] [CrossRef]
- Rodrigues, M.; Servois, V.; Mariani, P.; Pracht, M.; Dutriaux, C.; Grillet, F.; Ryckwaert, T.; Ducoulombier, A.; Neidhardt, E.M.; Piperno-Neumann, S. Management of metastatic uveal melanoma: French expert consensus guidelines. Bull. Cancer 2025, 112, 1334–1341. [Google Scholar] [CrossRef]
- Kaiserman, I.; Amer, R.; Pe’er, J. Liver function tests in metastatic uveal melanoma. Am. J. Ophthalmol. 2004, 137, 236–243. [Google Scholar] [CrossRef]
- Hendler, K.; Pe’er, J.; Kaiserman, I.; Baruch, R.; Kalickman, I.; Barak, V.; Frenkel, S. Trends in liver function tests: A comparison with serum tumor markers in metastatic uveal melanoma (part 2). Anticancer. Res. 2011, 31, 351–357. [Google Scholar]
- Jaarsma-Coes, M.G.; Klaassen, L.; Marinkovic, M.; Luyten, G.P.M.; Vu, T.H.K.; Ferreira, T.A.; Beenakker, J.M. Magnetic Resonance Imaging in the Clinical Care for Uveal Melanoma Patients-A Systematic Review from an Ophthalmic Perspective. Cancers 2023, 15, 2995. [Google Scholar] [CrossRef]
- Montazeri, K.; Pattanayak, V.; Sullivan, R.J. Tebentafusp in the Treatment of Metastatic Uveal Melanoma: Patient Selection and Special Considerations. Drug Des. Dev. Ther. 2023, 17, 333–339. [Google Scholar] [CrossRef]
- Remiszewski, P.; Siedlecki, E.; Wąż, J.; Filipek, K.; Pisklak, A.; Gaik, W.; Dudzisz-Śledź, M.; Rutkowski, P.; Czarnecka, A.M. Tebentafusp (IMCgp100) in the treatment of uveal melanoma—From preclinical evidence to clinical practice. Nowotw. J. Oncol. 2025, 75, 78–96. [Google Scholar] [CrossRef]
- Mariani, P.; Dureau, S.; Savignoni, A.; Rouic, L.L.-L.; Levy-Gabriel, C.; Piperno-Neumann, S.; Rodrigues, M.J.; Desjardins, L.; Cassoux, N.; Servois, V. Development of a Prognostic Nomogram for Liver Metastasis of Uveal Melanoma Patients Selected by Liver MRI. Cancers 2019, 11, 863. [Google Scholar]
- de Bruyn, D.P.; van Poppelen, N.M.; Brands, T.; van den Boom, S.C.; Eikenboom, E.; Wagner, A.; van Veghel-Plandsoen, M.M.; Geeven, G.; Beverloo, B.; van Rij, C.M.; et al. Evaluation of Circulating Tumor DNA as a Liquid Biomarker in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2024, 65, 11. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Foteinou, D.; Theocharopoulos, C.; Martinos, A.; Petsiou, D.P.; Anastasopoulou, A.; Gogas, H. State-of-the-art in Metastatic Uveal Melanoma Treatment: A 2025 Update. Curr. Oncol. Rep. 2025, 27, 803–821. [Google Scholar] [CrossRef]
- Seedor, R.S.; Aplin, A.E.; Bertolotto, C.; Carvajal, R.D.; Deacon, N.; Doble, K.; Hamid, O.; Haq, R.; Kadosh, M.; Khan, S.; et al. Meeting Report from the 2023 Cure Ocular Melanoma (CURE OM) Global Science Meeting, Philadelphia, PA, November 2023. Pigment. Cell Melanoma Res. 2025, 38, e13205. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.P.; Piperno-Neumann, S.; et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 2019, 30, 1370–1380. [Google Scholar] [CrossRef]
- Hand, F.; Doherty, S.; Gullo, G.; Geoghegan, J.; Crown, J.; Hoti, E. Metastatic uveal melanoma: A valid indication for liver resection. J. Balk. Union Oncol. 2020, 25, 1161–1165. [Google Scholar]
- Maspero, M.; Pezzoli, I.; Di Guardo, L.; Angi, M.; Lo Dico, S.; Sposito, C.; Battiston, C.; Mazzaferro, V. Intention-to-Treat Analysis of Hepatic Resection for Liver Metastases from Uveal Melanoma: A Single-Center Experience. Ann. Surg. Oncol. 2025, 32, 4989–4996. [Google Scholar] [CrossRef]
- Aoyama, T.; Mastrangelo, M.J.; Berd, D.; Nathan, F.E.; Shields, C.L.; Shields, J.A.; Rosato, E.L.; Rosato, F.E.; Sato, T. Protracted survival after resection of metastatic uveal melanoma. Cancer 2000, 89, 1561–1568. [Google Scholar] [CrossRef]
- Mariani, P.; Piperno-Neumann, S.; Servois, V.; Berry, M.G.; Dorval, T.; Plancher, C.; Couturier, J.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Desjardins, L.; et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur. J. Surg. Oncol. 2009, 35, 1192–1197. [Google Scholar] [CrossRef]
- Rowcroft, A.; Loveday, B.; Thomson, B.; Banting, S.; Knowles, B. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB 2020, 22, 497–505. [Google Scholar] [CrossRef]
- Yu, I.S.; Wee, K.; Williamson, L.; Titmuss, E.; An, J.; Naderi-Azad, S.; Metcalf, C.; Yip, S.; Horst, B.; Jones, S.J.M.; et al. Exceptional response to combination ipilimumab and nivolumab in metastatic uveal melanoma: Insights from genomic analysis. Melanoma Res. 2022, 32, 278–285. [Google Scholar] [CrossRef]
- Salaun, H.; de Koning, L.; Saint-Ghislain, M.; Servois, V.; Ramtohul, T.; Garcia, A.; Matet, A.; Cassoux, N.; Mariani, P.; Piperno-Neumann, S.; et al. Nivolumab plus ipilimumab in metastatic uveal melanoma: A real-life, retrospective cohort of 47 patients. Oncoimmunology 2022, 11, 2116845. [Google Scholar] [CrossRef]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P.; et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results from a Single-Arm Phase II Study. J. Clin. Oncol. 2021, 39, 599–607. [Google Scholar] [CrossRef]
- Tong, T.M.L.; Fiocco, M.; van Duijn-de Vreugd, J.J.; Lutjeboer, J.; Speetjens, F.M.; Tijl, F.G.J.; Sitsen, M.E.; Zoethout, R.W.M.; Martini, C.H.; Vahrmeijer, A.L.; et al. Quality of Life Analysis of Patients Treated with Percutaneous Hepatic Perfusion for Uveal Melanoma Liver Metastases. Cardiovasc. Interv. Radiol. 2024, 47, 741–750. [Google Scholar] [CrossRef]
- Tong, T.M.L.; Burgmans, M.C.; Speetjens, F.M.; van Erkel, A.R.; van der Meer, R.W.; van Rijswijk, C.S.P.; Jonker-Bos, M.A.; Roozen, C.F.M.; Sporrel-Blokland, M.; Lutjeboer, J.; et al. Combining Melphalan Percutaneous Hepatic Perfusion with Ipilimumab Plus Nivolumab in Advanced Uveal Melanoma: First Safety and Efficacy Data from the Phase Ib Part of the Chopin Trial. Cardiovasc. Interv. Radiol. 2023, 46, 350–359. [Google Scholar] [CrossRef]
- Tulokas, S.; Maenpaa, H.; Peltola, E.; Kivela, T.; Vihinen, P.; Virta, A.; Makela, S.; Kallio, R.; Hernberg, M. Selective internal radiation therapy (SIRT) as treatment for hepatic metastases of uveal melanoma: A Finnish nation-wide retrospective experience. Acta Oncol. 2018, 57, 1373–1380. [Google Scholar] [CrossRef]
- Peuker, C.-A.A.; Bucourt, M.D.; Gebauer, B.; Amthauer, H.; Erxleben, C.; Eucker, J.; Keller, U.; Leyvraz, S.; Joussen, A.M.; Keilholz, U.; et al. First interim analysis of the SirTac trial: A randomized phase II study of SIRT and DSM-TACE in patients with liver metastases from uveal melanoma. J. Clin. Oncol. 2022, 40, 9511. [Google Scholar] [CrossRef]
- Alexander, H.; Wen, D.; Chu, M.; Han, C.; Hadden, P.; Thomas, R.; Bartlett, A. Selective internal radiation therapy for hepatic metastases of uveal melanoma: A systematic review. Br. J. Radiol. 2022, 95, 20210200. [Google Scholar] [CrossRef]
- Venturini, M.; Pilla, L.; Agostini, G.; Cappio, S.; Losio, C.; Orsi, M.; Ratti, F.; Aldrighetti, L.; De Cobelli, F.; Del Maschio, A. Transarterial chemoembolization with drug-eluting beads preloaded with irinotecan as a first-line approach in uveal melanoma liver metastases: Tumor response and predictive value of diffusion-weighted MR imaging in five patients. J. Vasc. Interv. Radiol. 2012, 23, 937–941. [Google Scholar] [CrossRef]
- Shibayama, Y.; Namikawa, K.; Sone, M.; Takahashi, A.; Tsutsumida, A.; Sugawara, S.; Arai, Y.; Aihara, Y.; Suzuki, S.; Nakayama, J.; et al. Efficacy and toxicity of transarterial chemoembolization therapy using cisplatin and gelatin sponge in patients with liver metastases from uveal melanoma in an Asian population. Int. J. Clin. Oncol. 2017, 22, 577–584. [Google Scholar] [CrossRef]
- Stanisławska, K.; Stadnik, H.; Nawrocki, M.; Ramlau-Piątek, K.; Juszkat, R.; Drews, M. Long-term survival in a patient with unresectable liver metastases from uveal melanoma treated with transarterial chemoembolization with irinotecan eluting beads—Case report and review of literature. Contemp. Oncol. 2017, 21, 244–248. [Google Scholar] [CrossRef]
- Schuster, R.; Lindner, M.; Wacker, F.; Krössin, M.; Bechrakis, N.; Foerster, M.H.; Thiel, E.; Keilholz, U.; Schmittel, A. Transarterial chemoembolization of liver metastases from uveal melanoma after failure of systemic therapy: Toxicity and outcome. Melanoma Res. 2010, 20, 191–196. [Google Scholar] [CrossRef]
- Huppert, P.E.; Fierlbeck, G.; Pereira, P.; Schanz, S.; Duda, S.H.; Wietholtz, H.; Rozeik, C.; Claussen, C.D. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur. J. Radiol. 2010, 74, e38–e44. [Google Scholar] [CrossRef]
- Mariani, P.; Almubarak, M.M.; Kollen, M.; Wagner, M.; Plancher, C.; Audollent, R.; Piperno-Neumann, S.; Cassoux, N.; Servois, V. Radiofrequency ablation and surgical resection of liver metastases from uveal melanoma. Eur. J. Surg. Oncol. 2016, 42, 706–712. [Google Scholar] [CrossRef]
- Servois, V.; Bouhadiba, T.; Dureau, S.; Da Costa, C.; Almubarak, M.M.; Foucher, R.; Savignoni, A.; Cassoux, N.; Pierron, G.; Mariani, P. Iterative treatment with surgery and radiofrequency ablation of uveal melanoma liver metastasis: Retrospective analysis of a series of very long-term survivors. Eur. J. Surg. Oncol. 2019, 45, 1717–1722. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Prevoo, W.; Meier, M.A.J.; Sikorska, K.; Van, T.M.; van de Wiel, B.A.; van der Wal, J.E.; Mallo, H.A.; Grijpink-Ongering, L.G.; Broeks, A.; et al. Phase Ib/II trial testing combined radiofrequency ablation and ipilimumab in uveal melanoma (SECIRA-UM). Melanoma Res. 2020, 30, 252–260. [Google Scholar] [CrossRef]
- Polaczek, M.M.; Koseła-Paterczyk, H. Treatment of metastatic uveal melanoma. Nowotw. J. Oncol. 2022, 72, 378–383. [Google Scholar] [CrossRef]
- Seth, R.; Agarwala, S.S.; Messersmith, H.; Alluri, K.C.; Ascierto, P.A.; Atkins, M.B.; Bollin, K.; Chacon, M.; Davis, N.; Faries, M.B.; et al. Systemic Therapy for Melanoma: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4794–4820. [Google Scholar] [CrossRef]
- Kulbay, M.; Marcotte, E.; Remtulla, R.; Lau, T.H.A.; Paez-Escamilla, M.; Wu, K.Y.; Burnier, M.N. Uveal Melanoma: Comprehensive Review of Its Pathophysiology, Diagnosis, Treatment, and Future Perspectives. Biomedicines 2024, 12, 1758. [Google Scholar]
- Synoradzki, K.J.; Paduszyńska, N.; Solnik, M.; Toro, M.D.; Bilmin, K.; Bylina, E.; Rutkowski, P.; Yousef, Y.A.; Bucolo, C.; Zweifel, S.A.; et al. From Molecular Biology to Novel Immunotherapies and Nanomedicine in Uveal Melanoma. Curr. Oncol. 2024, 31, 778–800. [Google Scholar] [CrossRef]
- Carter, T.J.; Broadfoot, J.; Coupland, S.E.; Damato, B.; Evans, H.; Fenwick, S.; Khoja, L.; Krausa, T.; Lewis, R.; Nathan, P.; et al. Uveal Melanoma UK national guidelines: 2023 update. Eur. J. Cancer 2025, 228, 115687. [Google Scholar] [CrossRef]
- Schmittel, A.; Schmidt-Hieber, M.; Martus, P.; Bechrakis, N.E.; Schuster, R.; Siehl, J.M.; Foerster, M.H.; Thiel, E.; Keilholz, U. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann. Oncol. 2006, 17, 1826–1829. [Google Scholar] [CrossRef]
- Schmittel, A.; Schuster, R.; Bechrakis, N.E.; Siehl, J.M.; Foerster, M.H.; Thiel, E.; Keilholz, U. A two-cohort phase II clinical trial of gemcitabine plus treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005, 15, 447–451. [Google Scholar] [CrossRef]
- Corrie, P.G.; Shaw, J.; Spanswick, V.J.; Sehmbi, R.; Jonson, A.; Mayer, A.; Bulusu, R.; Hartley, J.A.; Cree, I.A. Phase I trial combining gemcitabine and treosulfan in advanced cutaneous and uveal melanoma patients. Br. J. Cancer 2005, 92, 1997–2003. [Google Scholar] [CrossRef]
- Schinzari, G.; Rossi, E.; Cassano, A.; Dadduzio, V.; Quirino, M.; Pagliara, M.; Blasi, M.A.; Barone, C. Cisplatin, dacarbazine and vinblastine as first line chemotherapy for liver metastatic uveal melanoma in the era of immunotherapy: A single institution phase II study. Melanoma Res. 2017, 27, 591–595. [Google Scholar] [CrossRef]
- Pföhler, C.; Cree, I.A.; Ugurel, S.; Kuwert, C.; Haass, N.; Neuber, K.; Hengge, U.; Corrie, P.G.; Zutt, M.; Tilgen, W.; et al. Treosulfan and gemcitabine in metastatic uveal melanoma patients: Results of a multicenter feasibility study. Anti-Cancer Drugs 2003, 14, 337–340. [Google Scholar]
- Nathan, F.E.; Berd, D.; Sato, T.; Shield, J.A.; Shields, C.L.; De Potter, P.; Mastrangelo, M.J. BOLD+interferon in the treatment of metastatic uveal melanoma: First report of active systemic therapy. J. Exp. Clin. Cancer Res. 1997, 16, 201–208. [Google Scholar]
- Rodriguez-Vidal, C.; Fernandez-Diaz, D.; Fernandez-Marta, B.; Lago-Baameiro, N.; Pardo, M.; Silva, P.; Paniagua, L.; Blanco-Teijeiro, M.J.; Piñeiro, A.; Bande, M. Treatment of Metastatic Uveal Melanoma: Systematic Review. Cancers 2020, 12, 2557. [Google Scholar]
- Buder, K.; Gesierich, A.; Gelbrich, G.; Goebeler, M. Systemic treatment of metastatic uveal melanoma: Review of literature and future perspectives. Cancer Med. 2013, 2, 674–686. [Google Scholar] [CrossRef]
- Magomedova, Z.; Nazarova, V.; Orlova, K.V.; Romanov, D.; Demidov, L. 156P Stereotactic radiotherapy and immunotherapy for metastatic uveal melanoma: A retrospective observational study. ESMO Open 2025, 10, 104470. [Google Scholar] [CrossRef]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef]
- Grigoruta, M.; Kong, X.; Qin, Y. Advances and Challenges in Immunotherapy for Metastatic Uveal Melanoma: Clinical Strategies and Emerging Targets. J. Clin. Med. 2025, 14, 5137. [Google Scholar]
- Heppt, M.V.; Steeb, T.; Schlager, J.G.; Rosumeck, S.; Dressler, C.; Ruzicka, T.; Nast, A.; Berking, C. Immune checkpoint blockade for unresectable or metastatic uveal melanoma: A systematic review. Cancer Treat. Rev. 2017, 60, 44–52. [Google Scholar] [CrossRef]
- Luke, J.J.; Callahan, M.K.; Postow, M.A.; Romano, E.; Ramaiya, N.; Bluth, M.; Giobbie-Hurder, A.; Lawrence, D.P.; Ibrahim, N.; Ott, P.A.; et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013, 119, 3687–3695. [Google Scholar] [CrossRef]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kämpgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naïve patients with metastatic uveal melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef]
- Lutzky, J.; Hernandez-Aya, L.; Feun, L.; Correa, Z.; King, J.; Estevez, C.; Decatur, C.; Dollar, J.J.; Reis, I.; Harbour, J.W. 1126P A phase II study of nivolumab/relatlimab in metastatic uveal melanoma. Ann. Oncol. 2024, 35, S741. [Google Scholar] [CrossRef]
- Mynam, R.S.; Menon, N.; Birbrair, A.; Ma, V.T. Successful use of nivolumab and relatlimab as salvage therapy for metastatic uveal melanoma. Melanoma Res. 2025, 35, 352–356. [Google Scholar] [CrossRef]
- Magomedova, Z.R.; Nazarova, V.V.; Orlova, K.V.; Kharkevich, G.; Samoylenko, I.; Petenko, N.N.; Baryshnikov, K.A.; Akhmetianova, A.; Demidov, L.V. Real-world impact of immunotherapy in metastatic uveal melanoma: A retrospective single-center study. J. Clin. Oncol. 2025, 43, e21594. [Google Scholar] [CrossRef]
- Hassel, J.C.; Piperno-Neumann, S.; Rutkowski, P.; Baurain, J.F.; Schlaak, M.; Butler, M.O.; Sullivan, R.J.; Dummer, R.; Kirkwood, J.M.; Orloff, M.; et al. Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2023, 389, 2256–2266. [Google Scholar] [CrossRef]
- van der Kooij, M.K.; Kapiteijn, E.; Rutkowski, P.; Baurain, J.-F.; de Koning, L.; Speetjens, F.M.; Bartoszek, P.; Rogala, P.; Cornélis, F.; Dudzisz- Śledź, M.; et al. Real-life data on tebentafusp in metastatic uveal melanoma patients from four EURACAN Expert Centres. Eur. J. Cancer 2025, 227, 115634. [Google Scholar] [CrossRef]
- Rutkowski, P.; Dudzisz-Śledź, M.; Urbonas, V.; Rogala, P.; Simetic, L.; Putnik, K.; Teterycz, P. Advances in the therapeutic management of metastatic uveal melanoma including real-world experience with tebentafusp. Oncol. Clin. Pract. 2025, 21, 317–324. [Google Scholar] [CrossRef]
- Piulats, J.M.; Watkins, C.; Costa-García, M.; Del Carpio, L.; Piperno-Neumann, S.; Rutkowski, P.; Hassel, J.C.; Espinosa, E.; de la Cruz-Merino, L.; Ochsenreither, S.; et al. Overall survival from tebentafusp versus nivolumab plus ipilimumab in first-line metastatic uveal melanoma: A propensity score-weighted analysis. Ann. Oncol. 2024, 35, 317–326. [Google Scholar] [CrossRef]
- Gentien, D.; Saberi-Ansari, E.; Servant, N.; Jolly, A.; de la Grange, P.; Nemati, F.; Liot, G.; Saule, S.; Teissandier, A.; Bourc’his, D.; et al. Multi-omics comparison of malignant and normal uveal melanocytes reveals molecular features of uveal melanoma. Cell Rep. 2023, 42, 113132. [Google Scholar] [CrossRef]
- Croce, M.; Ferrini, S.; Pfeffer, U.; Gangemi, R. Targeted Therapy of Uveal Melanoma: Recent Failures and New Perspectives. Cancers 2019, 11, 846. [Google Scholar] [CrossRef]
- Hanratty, K.; Finegan, G.; Rochfort, K.D.; Kennedy, S. Current Treatment of Uveal Melanoma. Cancers 2025, 17, 1403. [Google Scholar]
- Nathan, P.D.; Marshall, E.; Smith, C.T.; Bickerstaff, M.; Escriu, C.; Marples, M.; Damato, B.; Kalirai, H.; Coupland, S. A Cancer Research UK two-stage multicenter phase II study of imatinib in the treatment of patients with c-kit positive metastatic uveal melanoma (ITEM). J. Clin. Oncol. 2012, 30, 8523. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA 2014, 311, 2397–2405. [Google Scholar] [CrossRef]
- Leyvraz, S.; Konietschke, F.; Peuker, C.; Schutte, M.; Kessler, T.; Ochsenreither, S.; Ditzhaus, M.; Sprunken, E.D.; Dorpholz, G.; Lamping, M.; et al. Biomarker-driven therapies for metastatic uveal melanoma: A prospective precision oncology feasibility study. Eur. J. Cancer 2022, 169, 146–155. [Google Scholar] [CrossRef]
- McKean, M.; Chmielowski, B.; Butler, M.O.; Carvajal, R.; Rodon, J.; Carlino, M.; Kim, K.B.; Wise-draper, T.; Khan, S.; Salama, A.K.S.; et al. 1081O ctDNA reduction and clinical efficacy of the darovasertib + crizotinib (daro + crizo) combination in metastatic uveal melanoma (MUM). Ann. Oncol. 2023, 34, S651. [Google Scholar] [CrossRef]
- Cao, L.; Chen, S.; Sun, R.; Ashby, C.R., Jr.; Wei, L.; Huang, Z.; Chen, Z.S. Darovasertib, a novel treatment for metastatic uveal melanoma. Front. Pharmacol. 2023, 14, 1232787. [Google Scholar] [CrossRef]
- Hasanov, M.; Rioth, M.J.; Kendra, K.; Hernandez-Aya, L.; Joseph, R.W.; Williamson, S.; Chandra, S.; Shirai, K.; Turner, C.D.; Lewis, K.; et al. A Phase II Study of Glembatumumab Vedotin for Metastatic Uveal Melanoma. Cancers 2020, 12, 2270. [Google Scholar] [CrossRef]
- Sandhu, S.; McNeil, C.M.; LoRusso, P.; Patel, M.R.; Kabbarah, O.; Li, C.; Sanabria, S.; Flanagan, W.M.; Yeh, R.F.; Brunstein, F.; et al. Phase I study of the anti-endothelin B receptor antibody-drug conjugate DEDN6526A in patients with metastatic or unresectable cutaneous, mucosal, or uveal melanoma. Investig. New Drugs 2020, 38, 844–854. [Google Scholar] [CrossRef]
- Chandran, S.S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- Sacco, J.J.; Harrington, K.J.; Olsson-Brown, A.; Chan, T.Y.; Nenclares, P.; Leslie, I.; Bommareddy, P.; Kalbasi, A.; Xie, B.; Mishal, M.; et al. Safety, efficacy, and biomarker results from an open-label, multicenter, phase 1 study of RP2 alone or combined with nivolumab in a cohort of patients with uveal melanoma. J. Clin. Oncol. 2024, 42, 9511. [Google Scholar] [CrossRef]
- Bol, K.F.; Mensink, H.W.; Aarntzen, E.H.; Schreibelt, G.; Keunen, J.E.; Coulie, P.G.; de Klein, A.; Punt, C.J.; Paridaens, D.; Figdor, C.G.; et al. Long overall survival after dendritic cell vaccination in metastatic uveal melanoma patients. Am. J. Ophthalmol. 2014, 158, 939–947. [Google Scholar] [CrossRef]
- Wermke, M.; Araujo, D.M.; Chatterjee, M.; Tsimberidou, A.M.; Holderried, T.A.W.; Jazaeri, A.A.; Reshef, R.; Bokemeyer, C.; Alsdorf, W.; Wetzko, K.; et al. Autologous T cell therapy for PRAME(+) advanced solid tumors in HLA-A*02(+) patients: A phase 1 trial. Nat. Med. 2025, 31, 2365–2374, Correction in Nat. Med. 2025, 31, 2453. https://doi.org/10.1038/s41591-025-03731-6. [Google Scholar] [CrossRef]
- Patel, S.P.; Tsimberidou, A.M.; Luke, J.J.; Alsdorf, W.; Busse, A.; DeVane, S.C.; Hengler, S.; Hilf, N.; Kursunel, M.A.; Mayer-Mokler, A.; et al. 1600O Efficacy and safety of IMA203, a PRAME-directed T-cell receptor (TCR) T-cell therapy, in patients with previously treated advanced or metastatic uveal melanoma from a phase I trial. Ann. Oncol. 2025, 36, S946. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; Garcia-Honduvilla, N.; Coca, S.; Alvarez-Mon, M.; Bujan, J.; Teus, M.A. Update on uveal melanoma: Translational research from biology to clinical practice (Review). Int. J. Oncol. 2020, 57, 1262–1279. [Google Scholar] [CrossRef]
- Caplan, M.M.; Chen, L.N.; Krishnasamy, V.; Sperling, D.; Weintraub, J.; DeStephano, D.; Pan, S.M.; McDonnell, D.; Marr, B.P.; Carvajal, R.D.; et al. Survival outcomes associated with liver-directed therapies in patients with metastatic uveal melanoma. J. Clin. Oncol. 2023, 41, 9590. [Google Scholar] [CrossRef]
- Huibers, A.; Wong, A.; Burgmans, M.; Ny, L.; Stalhammar, G.; Kapiteijn, E.; Zager, J.S.; Olofsson Bagge, R. Management of liver metastases from uveal melanoma. Br. J. Surg. 2025, 112, znaf130. [Google Scholar] [CrossRef]
- Meijer, T.S.; Burgmans, M.C.; de Leede, E.M.; de Geus-Oei, L.F.; Boekestijn, B.; Handgraaf, H.J.M.; Hilling, D.E.; Lutjeboer, J.; Vuijk, J.; Martini, C.H.; et al. Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Ocular Melanoma Metastases Confined to the Liver: A Prospective Phase II Study. Ann. Surg. Oncol. 2021, 28, 1130–1141. [Google Scholar] [CrossRef]
- Bagge, R.O.; Nelson, A.; Shafazand, A.; All-Eriksson, C.; Cahlin, C.; Elander, N.; Helgadottir, H.; Kiilgaard, J.F.; Kinhult, S.; Ljuslinder, I.; et al. Isolated Hepatic Perfusion with Melphalan for Patients with Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial). J. Clin. Oncol. 2023, 41, 3042–3050. [Google Scholar] [CrossRef]
- Sajan, A.; Fordyce, S.; Sideris, A.; Liou, C.; Toor, Z.; Filtes, J.; Krishnasamy, V.; Ahmad, N.; Reis, S.; Brejt, S.; et al. Minimally Invasive Treatment Options for Hepatic Uveal Melanoma Metastases. Diagnostics 2023, 13, 1836. [Google Scholar]
- Dewald, C.L.A.; Warnke, M.-M.; Brüning, R.; Schneider, M.A.; Wohlmuth, P.; Hinrichs, J.B.; Saborowski, A.; Vogel, A.; Wacker, F.K. Percutaneous Hepatic Perfusion (PHP) with Melphalan in Liver-Dominant Metastatic Uveal Melanoma: The German Experience. Cancers 2022, 14, 118. [Google Scholar]
- Leyvraz, S.; Piperno-Neumann, S.; Suciu, S.; Baurain, J.F.; Zdzienicki, M.; Testori, A.; Marshall, E.; Scheulen, M.; Jouary, T.; Negrier, S.; et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): A multicentric randomized trial. Ann. Oncol. 2014, 25, 742–746. [Google Scholar] [CrossRef]
- Zager, J.S.; Orloff, M.M.; Ferrucci, P.F.; Glazer, E.S.; Ejaz, A.; Richtig, E.; Ochsenreither, S.; Lowe, M.C.; Reddy, S.A.; Beasley, G.; et al. FOCUS phase 3 trial results: Percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases (PHP-OCM-301/301A). J. Clin. Oncol. 2022, 40, 9510. [Google Scholar] [CrossRef]
- Olofsson Bagge, R.; Nelson, A.; Shafazand, A.; All-Eriksson, C.; Cahlin, C.; Elander, N.; Gustavsson, A.; Helgadottir, H.; Kiilgaard, J.F.; Kinhult, S.; et al. Survival and Quality of Life After Isolated Hepatic Perfusion with Melphalan as a Treatment for Uveal Melanoma Liver Metastases: Final Results from the Phase III Randomized Controlled Trial SCANDIUM. Ann. Surg. 2025, 282, 100–107. [Google Scholar] [CrossRef]
- Gupta, S.; Bedikian, A.Y.; Ahrar, J.; Ensor, J.; Ahrar, K.; Madoff, D.C.; Wallace, M.J.; Murthy, R.; Tam, A.; Hwu, P. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: Response, survival, and prognostic factors. Am. J. Clin. Oncol. 2010, 33, 474–480. [Google Scholar] [CrossRef]
- Miller, N.J.; Kwan, S.W.; Leary, J.B.; Hippe, D.S.; McCamy, W.; Veatch, J.R.; Hall, E.T.; Monsky, W.L.; Bhatia, S. Concurrent treatment with transarterial immunoembolization of hepatic metastases and systemic immune checkpoint inhibitors to overcome immune evasion in patients with metastatic uveal melanoma. Cancer Immunol. Immunother. 2025, 74, 270. [Google Scholar] [CrossRef]
- Gonsalves, C.F.; Eschelman, D.J.; Adamo, R.D.; Anne, P.R.; Orloff, M.M.; Terai, M.; Hage, A.N.; Yi, M.; Chervoneva, I.; Sato, T. A Prospective Phase II Trial of Radioembolization for Treatment of Uveal Melanoma Hepatic Metastasis. Radiology 2019, 293, 223–231. [Google Scholar] [CrossRef]
- Gonsalves, C.F.; Eschelman, D.J.; Adamo, R.D.; Anne, P.R.; Orloff, M.M.; Sato, T. Radioembolization for treatment of uveal melanoma hepatic metastasis: Results of a phase II, single institution, prospective trial. J. Clin. Oncol. 2018, 36, 9535. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Petzold, A.; Wessely, A.; Dippel, E.; Eckstein, M.; Gesierich, A.; Gutzmer, R.; Hassel, J.C.; Knorr, H.; Kreuzberg, N.; et al. Liver-directed treatment is associated with improved survival and increased response to immune checkpoint blockade in metastatic uveal melanoma: Results from a retrospective multicenter trial. Front. Med. 2023, 17, 878–888. [Google Scholar] [CrossRef]
- Aedo-Lopez, V.; Gérard, C.L.; Boughdad, S.; Gautron Moura, B.; Berthod, G.; Digklia, A.; Homicsko, K.; Schaefer, N.; Duran, R.; Cuendet, M.A.; et al. Safety and Efficacy of Ipilimumab plus Nivolumab and Sequential Selective Internal Radiation Therapy in Hepatic and Extrahepatic Metastatic Uveal Melanoma. Cancers 2022, 14, 1162. [Google Scholar] [CrossRef]
- Buijs, M.; Vossen, J.A.; Hong, K.; Georgiades, C.S.; Geschwind, J.-F.H.; Kamel, I.R. Chemoembolization of Hepatic Metastases from Ocular Melanoma: Assessment of Response with Contrast-Enhanced and Diffusion-Weighted MRI. Am. J. Roentgenol. 2008, 191, 285–289. [Google Scholar] [CrossRef]
- Wei, A.Z.; Uriel, M.; Porcu, A.; Manos, M.P.; Mercurio, A.C.; Caplan, M.M.; Hulse, L.; Seedor, R.S.; Holovatska, M.; Francis, J.; et al. Characterizing metastatic uveal melanoma patients who develop symptomatic brain metastases. Front. Oncol. 2022, 12, 961517. [Google Scholar] [CrossRef]
- Comanici, M.; Robertus, J.L.; Rice, A.; Nathan, P.; Anikin, V. Surgical management of pulmonary metastases secondary to uveal melanoma: A case series. Melanoma Res. 2026, 36, 70–74. [Google Scholar] [CrossRef]
- Sniegocki, M.; Smuczynski, W.; Wozniak-Dabrowska, K.; Nowacka, A. Brain metastasis from ocular malignant melanoma: A case report of a brain secondary lesion occurring 5 years after the primary lesion treatment. Postep. Dermatol. Alergol. 2019, 36, 371–373. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Zhou, Y.-W.; Wei, G.-X.; Luo, Y.-Q.; Qiu, M. Locoregional therapies combined with immune checkpoint inhibitors for liver metastases. Cancer Cell Int. 2024, 24, 302. [Google Scholar] [CrossRef]
- Blomen, C.L.; Kött, J.; Hartung, T.I.; Torster, L.K.; Gebhardt, C. Combination of Immune Checkpoint Inhibitors and Liver-Specific Therapies in Liver-Metastatic Uveal Melanoma: Can We Thus Overcome Its High Resistance? Cancers 2021, 13, 6390. [Google Scholar] [CrossRef]
- Wiens, L.; Grözinger, G.; Dittmann, H.; Thiel, K.; Leiter, U.; Amaral, T.; Nanz, L.; Flatz, L.; Forschner, A. Melanoma-specific survival of patients with uveal melanoma and liver metastases diagnosed between 2005 and 2021. Ther. Adv. Med. Oncol. 2024, 16, 17588359241273020. [Google Scholar] [CrossRef]
- Olofsson Bagge, R.; Nelson, A.; Shafazand, A.; Cahlin, C.; Carneiro, A.; Helgadottir, H.; Levin, M.; Rizell, M.; Ullenhag, G.; Wirén, S.; et al. A phase Ib randomized multicenter trial of isolated hepatic perfusion in combination with ipilimumab and nivolumab for uveal melanoma metastases (SCANDIUM II trial). ESMO Open 2024, 9, 103623. [Google Scholar] [CrossRef]
- Tong, T.M.L.; van der Kooij, M.K.; Speetjens, F.M.; van Erkel, A.R.; van der Meer, R.W.; Lutjeboer, J.; van Persijn van Meerten, E.L.; Martini, C.H.; Zoethout, R.W.M.; Tijl, F.G.J.; et al. Combining Hepatic Percutaneous Perfusion with Ipilimumab plus Nivolumab in advanced uveal melanoma (CHOPIN): Study protocol for a phase Ib/randomized phase II trial. Trials 2022, 23, 137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.