Simple Summary
The aim of this clinical study was to compare the accuracy of an automated oral cancer screening platform in individuals with different modes of tobacco use. The reason for this study is that the tobacco-related changes in the soft tissues of the mouth vary depending on the type of tobacco that is being used. The findings from this research indicate that screening accuracy is greatly affected by the type of tobacco use practiced. Thus, in the future, this variable needs to be included in oral cancer screening approaches to ensure more accurate detection of oral cancer risk, resulting in earlier referral and better treatment outcomes.
Abstract
Background/Objectives: Effective screening for oral cancer (OC) remains challenging. Inaccuracies contribute to delayed diagnosis and poor outcomes. Tobacco-related changes in oral mucosa may compromise the accuracy of oral screening approaches, and, in emerging “smart” screening modalities, they may overshadow the influence of other predictive variables. The objective of this study was to evaluate the screening accuracy of an imaging- and risk factor-based OC screening platform in individuals practicing different types of tobacco usage. Methods: 318 subjects who had previously screened positive for increased OC risk were recruited and sorted into “tobacco smoker”, “tobacco vaper”, “tobacco chewer”, “hookah user”, “multiple tobacco usage”, or “tobacco non-user” groups. Next, demographic information, risk factors, outcome of clinical examination, as well as AFI and pWLI were recorded using a prototype OC screening platform. The OC risk assessment outcome from the OC screening platform was compared to that from an oral medicine specialist. Results: The screening platform demonstrated high sensitivity in tobacco chewers and hookah users, and it also exceeded 90% in smokers, vapers, and multi-product users. In tobacco non-users, 80% screening sensitivity was recorded. Screening specificity was considerably better in tobacco non-users than in the tobacco-user groups, and low in tobacco chewers (33.3%), vapers (55.6%), and smokers (62.5%). Across all groups, agreement between the screening platform outcome and specialist evaluation exceeded 80%. Significant differences in probe accuracy were noted between tobacco non-users and users (p < 0.05), except for tobacco vapers. Conclusions: These findings highlight the need to consider the effects of type of tobacco use on the OC screening approach, and to integrate these variables into imaging-and risk-factor-based algorithms for OC screening.
1. Introduction
Oral cancer (OC) remains a significant global health issue, with an estimated 377,713 new cases and 177,757 deaths reported worldwide every year [1]. In the United States alone, approximately 54,000 new cases and 11,000 deaths are attributed to OC annually [2]. OC exhibits notably higher mortality and morbidity than many major cancers, surpassing other common cancers such as cervical, laryngeal, Hodgkin’s lymphoma, and many others [3]. The five-year survival rate for OC in the U.S. is approximately 62%, with the patient’s prognosis heavily influenced by the disease stage at diagnosis [3]. The majority of cases are identified at advanced stages, when metastasis has occurred, complicating treatment and leading to poor outcomes with high morbidity and mortality [3,4,5,6,7,8].
The reason for the predominantly late diagnosis of most oral squamous cell carcinomas (OSCCs) is attributed to the subtle and asymptomatic nature of oral potentially malignant (OPML) and early-stage lesions, which often mimic benign conditions. These similarities in presentation hinder early detection and are considered a primary cause of referral delay [4,5,9]. Barriers to effective risk screening are particularly pronounced in tobacco users, in whom other tobacco use-related mucosal changes often mask clinical manifestations of OPMLs or OCs.
OPMLs carry a heightened risk of malignant transformation in tobacco users compared to non-users [10,11,12,13,14]. Consequently, tobacco use is recognized as one of the most significant etiological risk factors for OC, with 75–90% of individuals diagnosed with OC having a history of tobacco consumption [3,15,16,17,18,19,20]. Studies indicate that tobacco smokers are five times more likely to develop OC than nonsmokers, with smokeless tobacco also increasing OC risk, particularly at the sites of tobacco placement in the mouth [5,21,22,23,24]. Different forms of tobacco use—including smoking, chewing, vaping, and hookah smoking—are associated with varying usage patterns and health risks [25,26,27,28,29,30,31,32,33,34,35,36,37]. Moreover, mucosal manifestations vary considerably between different types of tobacco use, yet existing screening tools are not tailored to these differences [25,26,27,28,29,30,31,32,33,34,35,36,37]. Tobacco smoking remains the most common form of consumption, followed by vaping, chewing, and hookah smoking [38]. The primary dysplastic oral lesions linked to tobacco use include leukoplakia, erythroplakia, and oral submucous fibrosis [27,28,29,30,31,32,33,34,35,39]. OSCC accounts for over 90% of oral cancer cases and is often preceded by OPMLs, which encompass a variety of mucosal and submucosal abnormalities, with a malignant transformation rate up to 10% per year [5,39,40,41,42,43].
Low socioeconomic status (SES) is strongly associated with increased tobacco use, placing this demographic at heightened risk for OC and OPMLs. Indeed, individuals with a low SES carry the highest risk of OC in the United States [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. Minority groups and those living in remote areas also face elevated OC risks due to higher tobacco use, low health literacy, and limited access to healthcare [44,58,61,62,63]. Minority disparities in five-year survival rates are evident, with survival rates over five years measuring 66% and 71%, respectively, in white men and women, compared to 56% and 64% in black men and women [64]. On a global scale, low- and middle-income countries (LMICs), particularly in Southeast Asia and India, face significant OC burdens due to high areca and betel-nut-based tobacco consumption, which often begins at a young age [61,62,65,66,67,68,69]. India alone accounts for approximately one-quarter of global OC cases, with a five-year survival rate of only 50%, compared to 68% in developed countries [3,68,70]. Healthcare facilities in LMICs are predominantly urban-centered, leaving low- and middle-income populations, who typically reside in rural areas, at a significant disadvantage due to limited access to screening, diagnosis, and care [62,65].
Clinical oral examination (COE), the current standard for OC screening, has limited accuracy due to the similar presentation of many common non-cancerous conditions [71,72,73]. Its findings are often subjective and heavily reliant on the clinician’s experience and expertise [39,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. Various adjunctive screening tools exist; however, their effectiveness remains ambiguous [11,88,89,90,91,92,93], and they are not currently recommended [39]. A mobile health (mHealth) program has shown promise in assisting community healthcare workers (CHWs) in India with screening effectiveness and implementation in remote populations [94]. This technology-driven platform incorporates a questionnaire, image-capturing technology, and rapid data transmission to specialists, enabling frontline, non-specialist clinicians to screen individuals in low-resource and remote settings [94].
Recent advances in artificial intelligence (AI) present promising opportunities to enhance OC screening accuracy, particularly through combined autofluorescence imaging (AFI) and polarized white light imaging (pWLI) [92,95,96,97,98,99]. When integrated with AI, these optical imaging modalities create “smart”, cost-effective screening platforms that can easily be used by non-specialists to enhance early detection and, ultimately, improve oral cancer outcomes [97,98,99,100,101,102,103].
Our overall objective is to improve OC outcomes by developing an effective and accurate smartphone-based screening platform for non-specialist use in low-resource communities. This platform aims to assess OC risk levels by integrating multimodal intraoral imaging, clinical signs and symptoms, and relevant risk factors. The specific objective of this study was to evaluate the impact of various forms of tobacco use on the accuracy of a prototype imaging- and risk factor-based OC screening platform.
2. Materials and Methods
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of California Irvine (protocol number 2002-2805) for studies involving humans. Written informed consent was obtained from all subjects involved in the study, all of whom completed the study in full compliance with the approved protocol.
2.1. Subjects
A total of 318 participants who had screened positive for increased OC risk at Concorde College of Dental Hygiene in Garden Grove, CA, West Coast University Dental Hygiene Clinic in Anaheim, CA, and the University of California, Irvine’s Clinics were recruited and classified into ‘tobacco smoker’, ‘tobacco vaper’, ‘tobacco chewer’, ‘hookah user’, ‘multiple tobacco usage ’, or ‘tobacco non-user’ groups. Subjects self-categorized their tobacco user status. Participants in each tobacco use category had engaged in their reported tobacco use habits for at least 1 year prior to their participation in this study. Subject demographics are shown in Table 1.

Table 1.
Subject demographics.
2.2. Oral Cancer Screening Platform
A prototype oral health screening platform whose technology and algorithm were previously developed by our group was used in this study (Figure 1). It was connected wirelessly to a Dell laptop computer (Dell, Round Rock, TX, USA) on which the prototype software and user interface had been installed. The platform was used by the same experienced dental hygienist throughout this study to collect and record all the data and images that were subsequently analyzed by the screening algorithm.

Figure 1.
Handheld prototype intra-oral scanner system designed and built by our team: (a) scanner device with extended reach to improve intraoral access, (b) flexible tip permitting imaging access to all intra-oral sites, including base of tongue and tonsillar region.
Demographic information, OC risk factors, as well as polarized white light (pWLI) and autofluorescence (AFI) images from the prototype intra-oral scanner were recorded using the platform’s App. These data were then automatically uploaded to a secure, cloud-based, HIPAA-compliant site, where a prototype algorithm processed them and registered an OC risk categorization for each intra-oral site. The algorithmic triage output had 3 categories: ‘no increased risk’, ‘moderate risk’, or ‘high’ risk, and these were displayed on the linked laptop computer.
2.3. Protocol
Patients who had previously screened positive for OPMLs were provided full details about this study, provided with the opportunity to ask questions of the study team, and then they were invited to participate in the study. Individuals were told that non-participation would not affect their treatment in any way. Those who opted into the study provided written informed consent according to the approved IRB protocol. Data collection included 3 separate steps. First, the dental hygienist completed a standard oral cancer screening process according to her daily clinical practice and as taught at dental hygiene schools. This includes a clinical exam and risk factor assessment. These findings were recorded in the patient’s file. The same experienced, pre-standardized hygienist completed all examinations and all data collection throughout the study. She had previously obtained 94% accuracy for clinical recognition of lesions using a biopsy-benchmarked database of 200 images of oral lesions. The second step of the protocol encompassed data collection using the screening platform. First, the hygienist completed a de novo standard oral cancer risk habit assessment and recorded the results directly on the platform App. Next, she acquired pWLI and AFI images of any potentially suspicious lesions, and these, too, were recorded on the platform App. The automated HIPAA-compliant system then uploaded all the recorded data to our proprietary, secure website, where a previously developed algorithm processed the data and provided triage output. In the third step of the data collection process, one oral medicine specialist who was not aware of the previous screening outcomes from the hygienist and the automated algorithm performed a full standard of care OC screening. The specialist subsequently recorded this screening outcome in conventional patient records as either “no increased risk”, “moderate risk”, or “high risk” for each study participant. The same specialist performed all screening in all participants according to the standard of care, combining clinical examination with risk factors and patient history. This specialist screening outcome was used as the gold standard to subsequently evaluate the accuracy of the screening algorithm in each patient and for each lesion. Finally, the standard of care specialist screening outcome was communicated to all study participants, and they underwent referral in accordance with that outcome and in accordance with the standard of care (Figure 2).
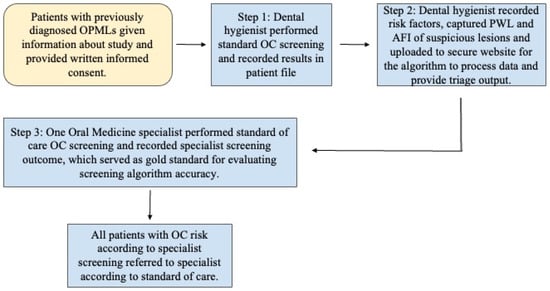
Figure 2.
Flow diagram of study protocol.
2.4. Statistical Analysis
Data were analyzed using standard SPSS 19 software (IBM®, Armonk, NY, USA). Sensitivity, specificity, specialist agreement, false positive rate, false negative rate, and positive and negative predictive values were estimated from the observed results. Standard errors (SE) and 95% confidence intervals were calculated for all the rates. A level of p < 0.05 was used to achieve statistical significance.
3. Results
1099 AFI and pWLI image pairs were analyzed by the prototype algorithm in combination with the matching risk factor data. The algorithm processed these datasets and then indicated the screening outcome on the laptop screen: either no increased risk, moderate risk, or high risk.
Screening Accuracy vs. Specialist (Gold Standard)
Table 2 summarizes a comparison of the screening algorithm performance vs. the screening outcome from the oral medicine specialist in tobacco non-users and tobacco smokers. Briefly, the algorithm achieved a higher sensitivity but a lower specificity and a slightly higher agreement with specialist screening outcome in tobacco smokers vs. non-smokers. Its output performance achieved 90% sensitivity, 62.5% specificity, and 84.2% agreement with the specialist screening outcome in tobacco smokers, and 80% sensitivity, 100% specificity, and 82.4% agreement with the specialist screening outcome in tobacco non-users. The remaining performance measures are summarized in Table 2.

Table 2.
Accuracy of screening algorithm in non-tobacco users and tobacco smokers.
Table 3 provides a comparison of the screening performance of the algorithm vs. the oral medicine specialist in tobacco vapers. The algorithm achieved 93.3% sensitivity, 55.6% specificity, and 84.6% agreement with the specialist screening outcome in tobacco vapers. A more detailed breakdown of the results is shown in Table 3.

Table 3.
Accuracy of screening algorithm in tobacco vapers.
Table 4 details screening algorithm performance in tobacco chewers. The algorithm achieved a very high level of sensitivity (100%) and a good level of agreement with the specialist screening outcome (81.8%) but a remarkably low level of specificity in its triage guidance (33.3%). A comprehensive summary of the statistical analysis is presented in Table 4.

Table 4.
Accuracy of screening algorithm in tobacco chewers.
Table 5 provides an overview of the screening algorithm performance in tobacco hookah smokers. While the algorithm achieved 100% sensitivity and 86.7% agreement with the specialist screening outcome in this population, its specificity was only moderate (71.4%). A more extensive analysis of the data is shown in Table 5.

Table 5.
Accuracy of screening algorithm in tobacco hookah smoker.
Table 6 presents an overview of the performance of the screening algorithm in multi-tobacco users. In this group, too, the algorithm achieved a good sensitivity of 95.2% and reasonable agreement (81.5%) agreement with the specialist screening outcome. However, the specificity of the algorithm was poor (33.3% specificity). A full overview of the performance measures is presented in Table 6.

Table 6.
Accuracy of screening algorithm in tobacco multi-users.
A summary of the overall performance of the screening algorithm in all tobacco users combined is summarized in Table 7. Briefly, the algorithm achieved 94.3% sensitivity, 52.8% specificity, and 83.7% agreement with the specialist screening outcome.

Table 7.
Accuracy of screening algorithm in all tobacco users.
Table 8 provides a summary of the screening platform performance in all the tobacco use groups included in this study. Agreement between the specialist screening outcome and the probe screening output varied considerably between the different risk groups.

Table 8.
Accuracy of screening algorithm in all groups.
The algorithm achieved moderate levels of agreement with the gold standard in all tobacco use categories, with a high level of risk except for tobacco non-users, where agreement with specialist screening outcome was only 66.7%. In individuals with a gold standard screening outcome of “moderately increased risk”, agreement between the specialist and the algorithm ranged from 66.7 to 84.0%, with a mean value of 80.2%. Algorithm performance was poorest in tobacco chewers. Finally, in individuals with a gold standard screening outcome of “no increased risk”, agreement between the algorithmic and the specialist screening outcome ranged from 66.7 to 100%, with the algorithm performing best in tobacco non-users and vapers, while it performed most poorly in tobacco chewers and multi-users.
Screening accuracy by the algorithm differed significantly between tobacco non-users and each tobacco use group (p < 0.05), except for the vaping group, where screening accuracy did not differ significantly from that observed in the tobacco non-user group.
4. Discussion
The goal of this study was to deepen our understanding of the effect of different tobacco-use types on the performance of an AI-generated imaging- and risk-factor-based screening algorithm for OC risk. Our previous research had compared the screening accuracy of a “smart” OC screening platform in tobacco non-users vs. tobacco users [99]. However, it did not examine the influence of different types of tobacco consumption, such as vaping, chewing, and hookah smoking on automated screening outcomes. Because daily usage habits and clinical oral manifestations are changing [104], and can differ considerably between the various types of tobacco consumption, it seemed reasonable to assume that these differences might affect the variables that are collected by the screening platform, the weighting of these variables within the algorithm architecture, and hence also the accuracy of the screening algorithm output. Multiple studies have indeed confirmed that the accuracy of oral cancer screening by conventional methods is affected by tobacco use [105]. Another study reported that the chronic inflammatory, white, and/or red lesions as well as keratotic changes that can be related to tobacco use can closely resemble potentially malignant disease and can therefore increase false positives [106].
Findings from the current study revealed that the type of tobacco use does indeed influence greatly the screening accuracy of the “smart” OC screening platform. While agreement with the specialist screening outcome remained consistently above 80% across all groups, and the automated system tested in this study manifested overall high screening sensitivity, specificity was low in chewers, vapers, and smokers relative to the much higher specificity observed in tobacco non-users. These outcomes are supported by reports from other studies that optically based screening modalities are especially susceptible to the confounding effects of tobacco-related mucosal changes [105]. The risk of poor screening accuracy is further compounded when such devices use only one wavelength of autofluorescence to assess OC risk, without incorporating risk factors and habits and/or other optical parameters or multiple wavelengths of light [106].
To the best of our knowledge, there exists little information to date regarding the specific effects of different types of tobacco products on screening accuracy, nor has the role of variables such as frequency and duration of tobacco usage been elucidated. While our previous studies have investigated extensively the design and technology as well as the merits and disadvantages of various algorithmic approaches [107,108,109,110,111,112], the results of this study suggest that further refinement of the algorithm—particularly in calibrating the “weight” or “impact” of each tobacco-related variable within the algorithm architecture—should be considered. Moreover, expanded training data—to take into account the frequency of tobacco use, and to capture a broader spectrum of mucosal presentations that may co-exist with tobacco-related lesions—should be included in the next stages of algorithm refinement.
In this study, on-site specialist screening served as the gold standard. This approach was adopted in order to provide ready access to a wide range of tobacco users in whom biopsy would not be considered appropriate according to the standard of care. However, given the potentially negative effect of tobacco use on conventional screening outcomes, additional studies that use biopsy results as the gold standard are needed to provide a more accurate and differentiated determination of the screening platform’s performance.
Overall, the findings of this study highlight the multifaceted impact of tobacco use on oral mucosal status, with each form of consumption presenting distinct screening challenges. The results underscore the importance of further refining the AI-based screening algorithm to ensure that it provides the most accurate screening outcomes possible as an important step in the pathway to improving early oral cancer detection and treatment outcomes.
5. Conclusions
This study highlights the need to consider the type of tobacco use during OC screening, as this variable considerably affects screening accuracy when an imaging and risk factor-based OC screening algorithm is used.
Author Contributions
Conceptualization, P.W.-S., S.Y. and C.W.; methodology, P.W.-S., A.A., C.W., S.Y., A.D., M.H. and J.B.; software, P.W.-S., S.Y. and A.A.; validation, T.T., S.Y., T.T. and P.W.-S.; formal analysis, T.T. and S.Y.; investigation, E.K., A.A., T.T., C.W. and S.Y.; resources, A.D., M.H., J.B. and P.W.-S.; data curation, E.K., A.A., C.W. and S.Y.; writing—original draft preparation, A.A., E.K. and P.W.-S.; writing—review and editing, E.K., T.T. and P.W.-S.; visualization, E.K., T.T. and P.W.-S.; supervision, P.W.-S. and C.W.; project administration, S.Y. and P.W.-S.; funding acquisition, P.W.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Arnold and Mabel Beckman Foundation, TRDRP T31IR1825, NIH UL1 TR0001414, NIH UH3EBO22623, NIH RO1DE030682, and the University of California Irvine Undergraduate Research Opportunities Program.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of California Irvine (protocol code 2002-2805; date of approval: 14 July 2024).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Acknowledgments
This study was supported by the Arnold and Mabel Beckman Foundation, TRDRP T31IR1825, NIH UL1 TR0001414, NIH UH3EBO22623, NIH RO1DE030682, and the University of California Irvine Undergraduate Research Opportunities Program. The OC screening device uses an AI algorithm. During the preparation of this manuscript, the authors used ChatGPT 5.2 for the purposes of formatting and spell check only. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 22 February 2025).
- National Institute of Dental and Craniofacial Research. Oral Cancer. Available online: https://www.nidcr.nih.gov/research/data-statistics/oral-cancer (accessed on 22 February 2025).
- Oral Cancer Foundation. Oral Cancer Facts. Available online: https://oralcancerfoundation.org/facts/ (accessed on 22 February 2025).
- González-Moles, M.Á.; Aguilar-Ruiz, M.; Ramos-García, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Day, T.A. Oral Cancer and Precancerous Lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Vernham, G.A.; Crowther, J.A. Head and Neck Carcinoma—Stage at Presentation. Clin. Otolaryngol. 1994, 19, 120–124. [Google Scholar] [CrossRef]
- McGurk, M.; Chan, C.; Jones, J.; O’Regan, E.; Sherriff, M. Delay in Diagnosis and Its Effect on Outcome in Head and Neck Cancer. Br. J. Oral Maxillofac. Surg. 2005, 43, 281–284. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Smith, W.P. Analysis of Survival Rates Following Primary Surgery of 178 Consecutive Patients with Oral Cancer in a Large District General Hospital. J. Maxillofac. Oral Surg. 2017, 16, 158–163. [Google Scholar] [CrossRef]
- Chhabra, N.; Chhabra, S.; Sapra, N. Diagnostic Modalities for Squamous Cell Carcinoma: An Extensive Review of Literature-Considering Toluidine Blue as a Useful Adjunct. J. Maxillofac. Oral Surg. 2015, 14, 188–200. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, J.; Wang, J.; Huang, R. Tobacco and Oral Squamous Cell Carcinoma: A Review of Carcinogenic Pathways. Tob. Induc. Dis. 2019, 17, 29. [Google Scholar] [CrossRef]
- Kaur, J.; Srivastava, R.; Borse, V. Recent Advances in Point-of-Care Diagnostics for Oral Cancer. Biosens. Bioelectron. 2021, 178, 112995. [Google Scholar] [CrossRef]
- Khariwala, S.S.; Ma, B.; Ruszczak, C.; Carmella, S.G.; Lindgren, B.; Hatsukami, D.K.; Hecht, S.S.; Stepanov, I. High Level of Tobacco Carcinogen–Derived DNA Damage in Oral Cells Is an Independent Predictor of Oral/Head and Neck Cancer Risk in Smokers. Cancer Prev. Res. 2017, 10, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Johnson, N.W. Systematic Review and Meta-Analysis of Association of Smokeless Tobacco and of Betel Quid without Tobacco with Incidence of Oral Cancer in South Asia and the Pacific. PLoS ONE 2014, 9, e113385. [Google Scholar] [CrossRef] [PubMed]
- Gajendra, S.; McIntosh, S.; Ghosh, S. Effects of Tobacco Product Use on Oral Health and the Role of Oral Healthcare Providers in Cessation: A Narrative Review. Tob. Induc. Dis. 2023, 21, 1–16. [Google Scholar] [CrossRef]
- Mashberg, A.; Boffetta, P.; Winkelman, R.; Garfinkel, L. Tobacco Smoking, Alcohol Drinking, and Cancer of the Oral Cavity and Oropharynx among U.S. Veterans. Cancer 1993, 72, 1369–1375. [Google Scholar] [CrossRef]
- Varshney, P.K.; Agrawal, N.; Bariar, L.M. Tobacco and Alcohol Consumption in Relation to Oral Cancer. Indian J. Otolaryngol. Head Neck Surg. 2003, 55, 25–28. [Google Scholar] [CrossRef]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and Drinking in Relation to Oral and Pharyngeal Cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar] [PubMed]
- Morse, D.E.; Psoter, W.J.; Cleveland, D.; Cohen, D.; Mohit-Tabatabai, M.; Kosis, D.L.; Eisenberg, E. Smoking and Drinking in Relation to Oral Cancer and Oral Epithelial Dysplasia. Cancer Causes Control 2007, 18, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Lewin, F.; Norell, S.E.; Johansson, H.; Gustavsson, P.; Wennerberg, J.; Biӧrklund, A.; Rutqvist, L.E. Smoking Tobacco, Oral Snuff, and Alcohol in the Etiology of Squamous Cell Carcinoma of the Head and Neck. Cancer 1998, 82, 1367–1375. [Google Scholar] [CrossRef]
- Alkhubaizi, Q.; Khalaf, M.M.; Dashti, H.; Sharma, P.N. Oral Cancer Screening among Smokers and Nonsmokers. J. Int. Soc. Prev. Community Dent. 2018, 8, 553. [Google Scholar] [CrossRef]
- Brown, R.L.; Suh, J.M.; Scarborough, J.E.; Wilkins, S.A.; Smith, R.R. Snuff Dippers’ Intraoral Cancer: Clinical Characteristics and Response to Therapy. Cancer 1965, 18, 2–13. [Google Scholar] [CrossRef]
- Winn, D.M.; Blot, W.J.; Shy, C.M.; Pickle, L.W.; Toledo, A.; Fraumeni, J.F. Snuff Dipping and Oral Cancer among Women in the Southern United States. N. Engl. J. Med. 1981, 304, 745–749. [Google Scholar] [CrossRef]
- Johnson, N. Tobacco Use and Oral Cancer: A Global Perspective. J. Dent. Educ. 2001, 65, 328–339. [Google Scholar] [CrossRef]
- Noor, A.E.; Ramanarayana, B. Relationship of Smokeless Tobacco Uses in the Perspective of Oral Cancer: A Global Burden. Oral Oncol. Rep. 2024, 10, 100516. [Google Scholar] [CrossRef]
- Taybos, G.; Crews, K.; AAOM Web Writing Group. Oral Changes Associated with Tobacco Use. The American Academy of Oral Medicine 2008. Available online: https://www.aaom.com/index.php?option=com_content&view=article&id=136%3Aoral-changes-associated-with-tobacco-use&catid=22%3Apatient-condition-information&Itemid=120 (accessed on 24 February 2025).
- Taybos, G. Oral Changes Associated with Tobacco Use. Am. J. Med. Sci. 2003, 326, 179–182. [Google Scholar] [CrossRef]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines; International Agency for Research on Cancer: Lyon, France, 2007; ISBN 9789283212898. [Google Scholar]
- Kusiak, A.; Maj, A.; Cichońska, D.; Kochańska, B.; Cydejko, A.; Świetlik, D. The Analysis of the Frequency of Leukoplakia in Reference of Tobacco Smoking among Northern Polish Population. Int. J. Environ. Res. Public Health 2020, 17, 6919. [Google Scholar] [CrossRef]
- Cullen, J.W.; Blot, W.; Henningfield, J.; Boyd, G.; Mecklenburg, R.; Massey, M.M. Health Consequences of Using Smokeless Tobacco: Summary of the Advisory Committee’s Report to the Surgeon General. Public Health Rep. 1986, 101, 355–373. [Google Scholar] [PubMed]
- Office of the Surgeon General. The Health Consequences of Smoking; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2004; ISBN 0160515762. [Google Scholar]
- Grajewski, S.; Groneberg, D. Leukoplakia and Erythroplakia—Two Orale Precursor Lesions. Laryngo-Rhino-Otol. 2009, 88, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sinha, A.; Kumar, S.; Iqbal, H. Oral Submucous Fibrosis: Current Concepts on Aetiology and Management—A Review. J. Indian Acad. Oral Med. Radiol. 2018, 30, 407–411. [Google Scholar] [CrossRef]
- Ekanayaka, R.P.; Tilakaratne, W.M. Oral Submucous Fibrosis: Review on Mechanisms of Malignant Transformation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 192–199. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020, 27, 1862–1880. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; Van Der Waal, I. Nomenclature and Classification of Potentially Malignant Disorders of the Oral Mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Gallagher, K.P.; Vargas, P.A.; Santos-Silva, A.R. The Use of E-Cigarettes as a Risk Factor for Oral Potentially Malignant Disorders and Oral Cancer: A Rapid Review of Clinical Evidence. Med. Oral Patol. Oral Cir. Bucal 2024, 29, 18–26. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Tobacco Use Supplement Current Population Survey; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://cancercontrol.cancer.gov/sites/default/files/2020-06/2018-19-tus-factsheet.pdf (accessed on 22 February 2025).
- Lingen, M.W.; Abt, E.; Agrawal, N.; Chaturvedi, A.K.; Cohen, E.; D’Souza, G.; Gurenlian, J.; Kalmar, J.R.; Kerr, A.R.; Lambert, P.M.; et al. Evidence-Based Clinical Practice Guideline for the Evaluation of Potentially Malignant Disorders in the Oral Cavity. J. Am. Dent. Assoc. 2017, 148, 712–727.e10. [Google Scholar] [CrossRef]
- Villa, A.; Woo, S.B. Leukoplakia—A Diagnostic and Management Algorithm. J. Oral Maxillofac. Surg. 2017, 75, 723–734. [Google Scholar] [CrossRef]
- Qi, M.; Li, L.; Tang, X.; Lu, Y.; Wang, M.; Yang, J.; Zhang, M. Nicotine Promotes the Development of Oral Leukoplakia via Regulating Peroxiredoxin 1 and Its Binding Proteins. Braz. J. Med. Biol. Res. 2021, 54, e10931. [Google Scholar] [CrossRef] [PubMed]
- Chaouachi, K. Hookah Epidemic. Br. Dent. J. 2009, 207, 192–193. [Google Scholar] [CrossRef]
- Elmahdi, F.M.; Alsebaee, R.O.; Ballaji, M.M.; Alharbi, A.M.; Alhejaili, M.E.; Altamimi, H.S.; Abu Altaher, M.J.; Ballaji, M.B.; Aljohani, A.S.; Alahmdi, A.A.; et al. Cytological Changes and Immunocytochemistry Expression of P53 in Oral Mucosa among Waterpipe Users in the Kingdom of Saudi Arabia. Cureus 2022, 14, e31190. [Google Scholar] [CrossRef]
- Hegde, S.; Ajila, V.; Zhu, W.; Zeng, C. Artificial Intelligence in Early Diagnosis and Prevention of Oral Cancer. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100133. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.I.; Petticrew, M.; Marlborough, H.; Berthiller, J.; Hashibe, M.; Macpherson, L.M.D. Socioeconomic Inequalities and Oral Cancer Risk: A Systematic Review and Meta-Analysis of Case-Control Studies. Int. J. Cancer 2008, 122, 2811–2819. [Google Scholar] [CrossRef]
- Güneri, P.; Çankaya, H.; Yavuzer, A.; Güneri, E.A.; Erişen, L.; Özkul, D.; El, S.N.; Karakaya, S.; Arican, A.; Boyacioğlu, H. Primary Oral Cancer in a Turkish Population Sample: Association with Sociodemographic Features, Smoking, Alcohol, Diet and Dentition. Oral Oncol. 2005, 41, 1005–1012. [Google Scholar] [CrossRef]
- Rejineveld, S.A. The Impact of Individual and Area Characteristics on Urban Socioeconomic Differences in Health and Smoking. Int. J. Epidemiol. 1998, 27, 33–40. [Google Scholar] [CrossRef]
- Barbeau, E.M.; Krieger, N.; Soobader, M.-J. Working Class Matters: Socioeconomic Disadvantage, Race/Ethnicity, Gender, and Smoking in NHIS 2000. Am. J. Public Health 2004, 94, 269–278. [Google Scholar] [CrossRef]
- Sharma, A.; Lewis, S.; Szatkowski, L. Insights into Social Disparities in Smoking Prevalence Using Mosaic, a Novel Measure of Socioeconomic Status: An Analysis Using a Large Primary Care Dataset. BMC Public Health 2010, 10, 755. [Google Scholar] [CrossRef]
- Hiscock, R.; Bauld, L.; Amos, A.; Fidler, J.A.; Munafò, M. Socioeconomic Status and Smoking: A Review. Ann. N. Y. Acad. Sci. 2012, 1248, 107–123. [Google Scholar] [CrossRef]
- Hosseinpoor, A.R.; Parker, L.A.; Tursan d’Espaignet, E.; Chatterji, S. Social Determinants of Smoking in Low- and Middle-Income Countries: Results from the World Health Survey. PLoS ONE 2011, 6, e20331. [Google Scholar] [CrossRef]
- Branstetter, S.; Lengerich, E.; Dignan, M.; Muscat, J. Knowledge and Perceptions of Tobacco-Related Media in Rural Appalachia. Rural Remote Health 2015, 15, 3136. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Machiorlatti, M.; Krebs, N.M.; Muscat, J.E. Socioeconomic Differences in Nicotine Exposure and Dependence in Adult Daily Smokers. BMC Public Health 2019, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.; Jerrett, M.; Eyles, J. Heterogeneity in the Determinants of Health and Illness: The Example of Socioeconomic Status and Smoking. Soc. Sci. Med. 2000, 51, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Peretti-Watel, P.; Seror, V.; Constance, J.; Beck, F. Poverty as a Smoking Trap. Int. J. Drug Policy 2009, 20, 230–236. [Google Scholar] [CrossRef]
- Clare, P.; Bradford, D.; Courtney, R.J.; Martire, K.; Mattick, R.P. The Relationship between Socioeconomic Status and “Hardcore” Smoking over Time—Greater Accumulation of Hardened Smokers in Low-SES than High-SES Smokers. Tob. Control 2014, 23, e133–e138. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Significant Oral Cancer Risk Associated with Low Socioeconomic Status. Evid.-Based Dent. 2009, 10, 4–5. [Google Scholar] [CrossRef]
- Jamal, A.; Agaku, I.T.; O’Connor, E.; King, B.A.; Kenemer, J.B.; Neff, L. Current Cigarette Smoking among Adults—United States, 2005–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 1108. [Google Scholar]
- Silverman, S.; Bhargava, K.; Mani, N.J.; Smith, L.W.; Malaowalla, A.M. Malignant Transformation and Natural History of Oral Leukoplakia in 57,518 Industrial Workers of Gujarat, India. Cancer 1976, 38, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral Potentially Malignant Disorders: Risk of Progression to Malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef]
- Kar, A.; Wreesmann, V.B.; Shwetha, V.; Thakur, S.; Rao, V.U.S.; Arakeri, G.; Brennan, P.A. Improvement of Oral Cancer Screening Quality and Reach: The Promise of Artificial Intelligence. J. Oral Pathol. Med. 2020, 49, 727–730. [Google Scholar] [CrossRef]
- Barik, D.; Thorat, A. Issues of Unequal Access to Public Health in India. Front. Public Health 2015, 3, 245. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, A.M.; Slack-Smith, L.M.; Parsons, R.; Threlfall, T. Oral Cavity Squamous Cell Carcinoma—Characteristics and Survival in Aboriginal and Non-Aboriginal Western Australians. Open Dent. J. 2014, 8, 168–174. [Google Scholar]
- Shiboski, C.H.; Schmidt, B.L.; Jordan, R.C.K. Racial Disparity in Stage at Diagnosis and Survival among Adults with Oral Cancer in the US. Community Dent. Oral Epidemiol. 2007, 35, 233–240. [Google Scholar] [CrossRef]
- Chhabra, A.; Hussain, S.; Rashid, S. Recent Trends of Tobacco Use in India. J. Public Health 2019, 29, 27–36. [Google Scholar] [CrossRef]
- Laprise, C.; Shahul, H.P.; Madathil, S.A.; Thekkepurakkal, A.S.; Castonguay, G.; Varghese, I.; Shiraz, S.; Allison, P.; Schlecht, N.F.; Rousseau, M.-C.; et al. Periodontal Diseases and Risk of Oral Cancer in Southern India: Results from the HeNCe Life Study. Int. J. Cancer 2016, 139, 1512–1519. [Google Scholar] [CrossRef]
- Akashanand; Zahiruddin, Q.S.; Jena, D.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R.; Rustagi, S.; Gaidhane, A.M.; et al. Burden of Oral Cancer and Associated Risk Factors at National and State Levels: A Systematic Analysis from the Global Burden of Disease in India, 1990–2021. Oral Oncol. 2024, 159, 107063. [Google Scholar] [CrossRef]
- Thavarool, S.B.; Muttath, G.; Nayanar, S.; Duraisamy, K.; Bhat, P.; Shringarpure, K.; Nayak, P.; Tripathy, J.P.; Thaddeus, A.; Philip, S.; et al. Improved Survival among Oral Cancer Patients: Findings from a Retrospective Study at a Tertiary Care Cancer Centre in Rural Kerala, India. World J. Surg. Oncol. 2019, 17, 15. [Google Scholar] [CrossRef]
- Jena, D.; Swain, P.K.; Gandhi, N.C.; Guthi, V.R.; Ramasamy, A.; TY, S.S.; P, P.; Valli Sreepada, S.S.; Quispevicuna, C.; Beig, M.A.; et al. Cancer Burden and Trends across India (1990–2021): Insights from the Global Burden of Disease Study. Evidence 2024, 2. [Google Scholar] [CrossRef]
- Cancer Research UK. Survival for Mouth and Oropharyngeal Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/mouth-cancer/survival (accessed on 23 February 2025).
- Walsh, T.; Warnakulasuriya, S.; Lingen, M.W.; Kerr, A.R.; Ogden, G.R.; Glenny, A.-M.; Macey, R. Clinical Assessment for the Detection of Oral Cavity Cancer and Potentially Malignant Disorders in Apparently Healthy Adults. Cochrane Database Syst. Rev. 2021, 12, CD010173. [Google Scholar]
- Epstein, J.B.; Güneri, P.; Boyacioglu, H.; Abt, E. The Limitations of the Clinical Oral Examination in Detecting Dysplastic Oral Lesions and Oral Squamous Cell Carcinoma. J. Am. Dent. Assoc. 2012, 143, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Essat, M.; Cooper, K.; Bessey, A.; Clowes, M.; Chilcott, J.B.; Hunter, K.D. Diagnostic Accuracy of Conventional Oral Examination for Detecting Oral Cavity Cancer and Potentially Malignant Disorders in Patients with Clinically Evident Oral Lesions: Systematic Review and Meta–Analysis. Head Neck 2022, 44, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B. Effect of Screening on Oral Cancer Mortality in Kerala, India: A Cluster-Randomised Controlled Trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Thara, S.; Muwonge, R.; Thomas, G.; Anju, G.; Mathew, B. Long Term Effect of Visual Screening on Oral Cancer Incidence and Mortality in a Randomized Trial in Kerala, India. Oral Oncol. 2013, 49, 314–321. [Google Scholar] [CrossRef]
- Chuang, S.-L.; Su, W.; Hsiu, T.; Ming, A.; Wang, C.-P.; Ching, J.; Yueh, S.; Lee, Y.-C.; Chiu, H.-M.; Chang, D.-C.; et al. Population-Based Screening Program for Reducing Oral Cancer Mortality in 2,334,299 Taiwanese Cigarette Smokers And/or Betel Quid Chewers. Cancer 2017, 123, 1597–1609. [Google Scholar] [CrossRef]
- Ford, P.J.; Farah, C.S. Early Detection and Diagnosis of Oral Cancer: Strategies for Improvement. J. Cancer Policy 2013, 1, e2–e7. [Google Scholar] [CrossRef]
- Speer, K. Workforce Strategies to Improve Access to Oral Health Care. National Conference of State Legislatures. 2023. Available online: https://www.ncsl.org/health/workforce-strategies-to-improve-access-to-oral-health-care (accessed on 23 February 2025).
- Shuman, A.G.; Entezami, P.; Chernin, A.S.; Wallace, N.E.; Taylor, J.M.G.; Hogikyan, N.D. Demographics and Efficacy of Head and Neck Cancer Screening. Otolaryngol.–Head Neck Surg. 2010, 143, 353–360. [Google Scholar] [CrossRef]
- Garrote, L.F.; Sankaranarayanan, R.; Lence Anta, J.J.; Salvá, A.R.; Parkin, D.M. An Evaluation of the Oral Cancer Control Program in Cuba. Epidemiology 1995, 6, 428–431. [Google Scholar] [CrossRef]
- Santana, J.; Delgado, L.; Miranda, J.; Sanchez, M. Oral Cancer Case Finding Program (OCCFP). Oral Oncol. 1997, 33, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. The World Oral Health Report 2003: Continuous Improvement of Oral Health in the 21st Century—The Approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Northridge, M.E.; Kumar, A.; Kaur, R. Disparities in Access to Oral Health Care. Annu. Rev. Public Health 2020, 41, 513–535. [Google Scholar] [CrossRef]
- Nicotera, G.; Di Stasio, S.M.; Angelillo, I.F. Knowledge and Behaviors of Primary Care Physicians on Oral Cancer in Italy. Oral Oncol. 2004, 40, 490–495. [Google Scholar] [CrossRef]
- Shepperd, J.A.; Howell, J.L.; Logan, H. A Survey of Barriers to Screening for Oral Cancer among Rural Black Americans. Psycho-Oncol. 2013, 23, 276–282. [Google Scholar] [CrossRef]
- Edwards, P.; Subramanian, S.; Hoover, S.; Ramesh, C.; Ramadas, K. Financial Barriers to Oral Cancer Treatment in India. J. Cancer Policy 2016, 7, 28–31. [Google Scholar] [CrossRef]
- Panzarella, V.; Pizzo, G.; Calvino, F.; Domenico, C.; Colella, G.; Campisi, G. Diagnostic Delay in Oral Squamous Cell Carcinoma: The Role of Cognitive and Psychological Variables. Int. J. Oral Sci. 2014, 6, 39–45. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 002203452110147. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of Tissue Autofluorescence Imaging (Velscope) in the Visualization of Oral Mucosal Lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef]
- Macey, R.; Walsh, T.; Brocklehurst, P.; Kerr, A.R.; Liu, J.L.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriya, S.; Scully, C. Diagnostic Tests for Oral Cancer and Potentially Malignant Disorders in Patients Presenting with Clinically Evident Lesions. Cochrane Database Syst. Rev. 2015, 7, CD010276. [Google Scholar] [CrossRef]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Evaluation of an Autofluorescence Based Imaging System (VELscopeTM) in the Detection of Oral Potentially Malignant Disorders and Benign Keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef]
- Jayaprakash, V.; Sullivan, M.; Merzianu, M.; Rigual, N.R.; Loree, T.R.; Popat, S.R.; Moysich, K.B.; Ramananda, S.; Johnson, T.; Marshall, J.R.; et al. Autofluorescence-Guided Surveillance for Oral Cancer. Cancer Prev. Res. 2009, 2, 966–974. [Google Scholar] [CrossRef]
- Tanriver, G.; Soluk Tekkesin, M.; Ergen, O. Automated Detection and Classification of Oral Lesions Using Deep Learning to Detect Oral Potentially Malignant Disorders. Cancers 2021, 13, 2766. [Google Scholar] [CrossRef]
- Birur, N.P.; Gurushanth, K.; Patrick, S.; Sunny, S.; Raghavan, S.; Gurudath, S.; Hegde, U.; Tiwari, V.; Jain, V.; Imran, M.; et al. Role of Community Health Worker in a Mobile Health Program for Early Detection of Oral Cancer. Indian J. Cancer 2019, 56, 107. [Google Scholar] [CrossRef]
- Lane, P.M.; Gilhuly, T.; Whitehead, P.; Zeng, H.; Poh, C.F.; Ng, S.; Williams, P.M.; Zhang, L.; Rosin, M.P.; MacAulay, C.E. Simple Device for the Direct Visualization of Oral-Cavity Tissue Fluorescence. J. Biomed. Opt. 2006, 11, 024006. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, I.; Williams, M.; El-Naggar, A.; Richards-Kortum, R.; Gillenwater, A. Understanding the Biological Basis of Autofluorescence Imaging for Oral Cancer Detection: High-Resolution Fluorescence Microscopy in Viable Tissue. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, K.; Tofangchiha, M. Oral Cancer Screening by Artificial Intelligence-Oriented Interpretation of Optical Coherence Tomography Images. Radiol. Res. Pract. 2022, 2022, 1614838. [Google Scholar] [CrossRef] [PubMed]
- Roblyer, D.; Kurachi, C.; Stepanek, V.M.; Schwarz, R.A.; Williams, M.; El-Naggar, A.K.; Lee, J.; Gillenwater, A.M.; Richards-Kortum, R. Comparison of Multispectral Wide-Field Optical Imaging Modalities to Maximize Image Contrast for Objective Discrimination of Oral Neoplasia. J. Biomed. Opt. 2010, 15, 066017. [Google Scholar] [CrossRef]
- Yang, S.M.; Song, B.; Wink, C.; Abouakl, M.; Takesh, T.; Hurlbutt, M.; Dinica, D.; Davis, A.; Liang, R.; Wilder-Smith, P. Performance of Automated Oral Cancer Screening Algorithm in Tobacco Users vs. Tobacco non-users. Appl. Sci. 2023, 13, 3370. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ingole, N.; Roblyer, D.; Stepanek, V.; Richards-Kortum, R.; Gillenwater, A.; Shastri, S.; Chaturvedi, P. Evaluation of a Low-Cost, Portable Imaging System for Early Detection of Oral Cancer. Head Neck Oncol. 2010, 2, 10. [Google Scholar] [CrossRef]
- Ilhan, B.; Lin, K.; Guneri, P.; Wilder-Smith, P. Improving Oral Cancer Outcomes with Imaging and Artificial Intelligence. J. Dent. Res. 2020, 99, 241–248. [Google Scholar] [CrossRef]
- Cicciù, M.; Herford, A.S.; Cervino, G.; Troiano, G.; Lauritano, F.; Laino, L. Tissue Fluorescence Imaging (VELscope) for Quick Non-Invasive Diagnosis in Oral Pathology. J. Craniofacial Surg. 2017, 28, e112–e115. [Google Scholar] [CrossRef]
- Jeyaraj, P.R.; Samuel Nadar, E.R. Computer-Assisted Medical Image Classification for Early Diagnosis of Oral Cancer Employing Deep Learning Algorithm. J. Cancer Res. Clin. Oncol. 2019, 145, 829–837. [Google Scholar] [CrossRef]
- Cooper, M.; Pacek, L.R.; Guy, M.C.; Barrington-Trimis, J.L.; Simon, P.; Stanton, C.; Kong, G. Hookah Use among US Youth: A Systematic Review of the Literature from 2009 to 2017. Nicotine Tob. Res. 2018, 21, 1590–1599. [Google Scholar] [CrossRef]
- Olson, C.M.; Burda, B.U.; Beil, T.; Whitlock, E.P. Screening for Oral Cancer: A Targeted Evidence Update for the U.S. Preventive Services Task Force; Evidence Syntheses, No. 102; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013; p. 4. [Google Scholar]
- McKinney, R.; Buhler, C.; Brizuela, M. Smokeless Tobacco Oral Pathology. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Song, B.; Sunny, S.; Li, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Gurudath, S.; Raghavan, S.; Imchen, T.; et al. Mobile-based oral cancer classification for point-of-care screening. J. Biomed. Opt. 2021, 26, 065003. [Google Scholar] [CrossRef]
- Figueroa, K.C.; Song, B.; Sunny, S.; Li, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Gurudath, S.; Raghavan, S.; et al. Interpretable deep learning approach for oral cancer classification using guided attention inference network. J. Biomed. Opt. 2022, 27, 015001. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, S.; Sunny, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Peterson, T.; Gurudath, S.; Raghavan, S.; et al. Exploring uncertainty measures in convolutional neural network for semantic segmentation of oral cancer images. J. Biomed. Opt. 2022, 27, 115001. [Google Scholar] [CrossRef]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Gurushanth, K.; Wooten, K.; Gupta, V.; Platek, M.E.; et al. Small form factor, flexible, dual-modality handheld probe for smartphone-based, point-of-care oral and oropharyngeal cancer screening. J. Biomed. Opt. 2019, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Sunny, S.; Uthoff, R.D.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Anbarani, A.; Wilder-Smith, P.; Kuriakose, M.A.; et al. Automatic classification of dual-modalilty, smartphone-based oral dysplasia and malignancy images using deep learning. Biomed. Opt. Express 2018, 9, 5318–5329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.