Simple Summary
Patients with breast cancer are all confronted with different needs and preferences concerning their treatment and care. Understanding what matters to breast cancer patients can help tailor decisions. This scoping review aims to provide an overview of published studies identifying breast cancer patient preferences. The review shows how the diversity within and between the preference studies complicates a robust and standardized implementation of preferences in decision-making processes. The article suggests how stakeholders can create valuable impact for and with breast cancer patients, for example, by linking preference studies to clear objectives and implementation strategies, and by involving patients throughout the whole process.
Abstract
Background: Breast cancer is one of the most common cancers worldwide, with both the disease and the treatments affecting patients’ quality of life and overall survival. Patient preference studies can help to identify what matters most to patients. This study aims to provide an overview of published patient preference studies in breast cancer, focusing on (i) the design of the study, (ii) preference outcomes including preference heterogeneity, (iii) recruitment strategies, and (iv) patient involvement. Methods: For this scoping review, a search strategy was created for five databases (PubMed, Embase, Scopus, Web of Science Core Collection, and CINAHL), based on the concepts ‘breast cancer’ and ‘patient preference’. The articles were screened double-blind in two phases, after which, the relevant data was extracted and analyzed. Results: A total of 31 articles were selected based on the eligibility criteria. The studies were published over a wide time range (1995–2024), and across different countries. Different methodologies were used, and different attributes were included, mainly related to adverse events of treatments and survival elements. Survival elements were almost always considered important when included. Preference heterogeneity was assessed in 24 of the included studies. The involvement of patients in these studies varied; in two studies, patients were involved in an advisory board; in five studies, patients provided feedback on the preference study conduct via interviews or focus group discussions; and in twelve studies, pilot tests were mentioned. Conclusions: The studies show diverse methods, outcomes, and patient involvement strategies. This may complicate the implementation of preferences in decision-making, suggesting preference studies with clear objectives and implementation strategies to create impact for and with patients.
1. Introduction
Breast cancer is the most common cancer among women worldwide and remains a leading cause of mortality in this population [1]. Both the disease and its treatments profoundly affect patients’ overall survival as well as their quality of life due to a wide range of symptoms and side effects, necessitating a multidisciplinary approach to care. To fully understand the needs and experiences of patients, information on patient experiences, alongside clinical trial evidence on physical parameters, is necessary.
One type of patient experience data is data on patient preferences, demonstrating what matters most to patients in terms of their experiences with treatments or disease characteristics, and which trade-offs (e.g., between treatment benefits and risks) patients are willing to make. Such data result from ‘patient preference studies’ (PPS) [2].
Methods used in patient preference studies can be categorized as qualitative (preference exploration) and quantitative (preference elicitation) [2]. Qualitative methods aim to explore patients’ perspectives in depth; examples are interviews and focus group discussions. Quantitative methods aim to collect quantifiable data and can be grouped into four categories: (i) discrete choice-based methods, (ii) ranking methods, (iii) indifference techniques, and (iv) rating methods [2,3]. In the paper of Soekhai et al. [3], an overview of the different methods in these four categories can be found.
The different methods offer distinct advantages for capturing the complexities of patient preferences and to guide and support patient decision-making.
Besides the importance of choosing the adequate method to answer the specific research questions of the patient preference study, other important elements (such as the heterogeneity in the responses of patients on preferences in view of their demographic backgrounds, or particular recruitment strategies to include patients in the study) should be considered.
To study patient heterogeneity, different techniques can be used, e.g., sub-group analyses, mixed logit models, and latent class analysis [2,4,5]. To determine preferences from heterogenous samples of breast cancer, patients’ effective recruitment strategies are crucial. In the literature review of Wang et al. [6], effective recruitment strategies for adolescent and young adult (AYA) cancer survivors were identified, showing that for this target group, internet and social networking were the strategies mainly used, and with a higher participation rate compared to other more conventional methods (such as recruitment via hospitals).
This scoping review aims to provide a comprehensive overview of the design and outcomes of patient preference studies conducted among breast cancer patients concerning their disease, treatment, and care, and to (i) provide insights on the elements assessed in the preference studies, (ii) investigate if variations in patient preferences (preference heterogeneity) are considered, (iii) analyze recruitment strategies, and (iv) identify patient involvement and communication strategies.
2. Methods
The methodology of the scoping review was predefined and documented in a registered protocol at the Open Science Framework (OSF) database (registration DOI: 10.17605/OSF.IO/M2H4K). The review was compliant with the PRISMA-ScR guidelines for scoping reviews (Supplementary Materials).
2.1. Search Strategy
Five databases were systematically searched: PubMed, Embase, Web of Science, CINAHL, and Scopus. The search string was developed by the research team and verified and further refined with a biomedical information specialist. The search strategy was based on two key concepts: ‘Breast cancer’ and ‘Patient preference’. Relevant synonyms and related terms were included, combined with Boolean operators to ensure an optimal search strategy. The detailed search strategies can be found in Table S1. The search was conducted on 9 October 2024. The articles were extracted from all databases, added and manually deduplicated in the Rayyan Software [7].
2.2. Study Selection
2.2.1. Inclusion and Exclusion Criteria
Inclusion criteria were (i) studies involving breast cancer patients or breast cancer survivors (population), (ii) qualitative and/or quantitative patient preference studies (study design) eliciting patient preferences concerning disease, treatments, and care (concept), and (iii) publication in English. Exclusion criteria were (i) studies not involving breast cancer patients or involving a broader population, e.g., a mixed population of breast cancer patients and healthcare professionals, (ii) all other study designs than patient preference studies (e.g., reviews, retrospective data analysis, and clinical trials), (iii) studies with another aim than the elicitation of patient preferences, (iv) papers where the full text was not available, preprints, conference abstracts, conference proceedings, and book chapters, and (v) publications that were not in English. A detailed overview of the inclusion and exclusion criteria can be found in Table S2.
2.2.2. Screening Process
The screening of the articles was conducted double-blind in two phases. Prior to the screening phase, a pilot step assessing a limited number of articles on eligibility was performed by independent researchers (AB, FS, and FV; double-blind with CV) to ensure alignment and refine the inclusion and exclusion criteria where needed. In the first screening phase, researchers AB, FS, and FV each reviewed one third of the articles, while researcher CV screened all the articles on title and abstract. Disagreements were discussed and resolved among the research team (AB, FS, FV, and CV). Then, in the second screening phase on the full text, researchers FS and FV each assessed half of the articles, and researcher CV assessed all articles. Again, all disagreements were resolved by consensus among the researchers (FS, FV and CV), and the selection of the studies for data extraction was finalized.
2.3. Data Extraction and Analysis
Data extraction was performed using a predefined extraction table template. A pilot phase was performed prior to the actual data extraction, to ensure that all relevant elements were extracted and interpreted in the same way among the research team. During the subsequent consensus meeting, the extraction table template was refined and finalized (Table S3). The data extracted was related to (i) general characteristics of the publication, (ii) characteristics of the patient preferences study, (iii) patient heterogeneity, and (iv) patient involvement, recruitment, and communication strategies. Researchers FS and FV extracted the data from half of the articles; researcher CV extracted the data from all of the articles independently to ensure consistency. The extracted raw data was consolidated in an Excel spreadsheet, categorized based on predefined themes, and summarized for further analysis. Descriptive analysis of the extracted data was performed by FS, FV, and CV.
3. Results
3.1. Study Inclusion
A total of 19.017 articles were identified through the database search, from which 10.511 duplicates were removed. A total of 8.506 unique articles were screened on title and abstract in the first phase. A total of 8.261 articles were excluded based on the eligibility criteria. Eight reports were not retrieved, resulting in 237 articles eligible for full-text screening in a second screening phase. Thirty-one articles met the inclusion criteria and were analyzed in the review, as shown in the flow diagram (Figure 1).
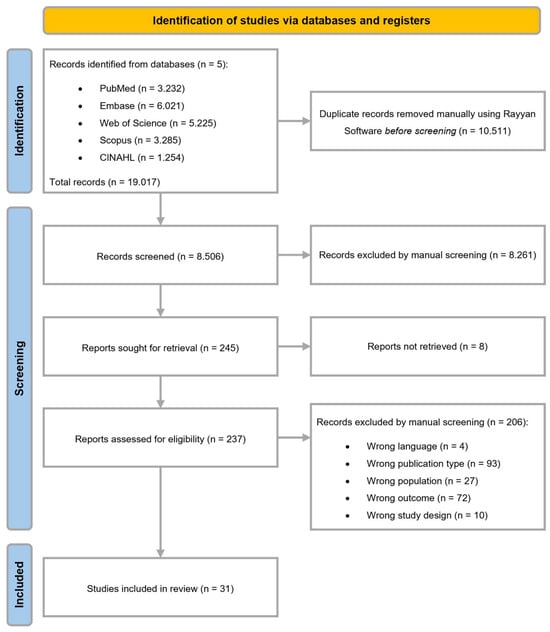
Figure 1.
Flow diagram.
3.2. Study Characteristics
The study characteristics of the 31 studies are summarized in (Table S4), including general characteristics of the publication (year, first author, country of the first author, journal, and funding) and general characteristics of the patient preference study (objectives, PPS methods, ranking/grading elements). For most of the studies, the country of the first author was the USA (n = 11). The year of publication ranged from 1995 to 2024.
Patient Characteristics
As stated in the inclusion criteria, both studies involving breast cancer survivors and/or breast cancer patients were included. In Table 1, the types of breast cancer of the participants in the studies are summarized. In some studies, patients across different breast cancer stages and with different subtypes were included.

Table 1.
Type of breast cancer of the participants in the study.
An overview of the study population characteristics can be found in Table S5, including the inclusion and exclusion criteria, breast cancer types of the study population, and information on the age and country/ethnicity of the included participants.
3.3. Patient Preference Studies
3.3.1. Steps Prior to the Patient Preference Studies
Prior to the actual assessment of what patients prefer or value most in view of their treatment or disease characteristics, most of the studies (n = 21) (Table 2) included literature reviews to identify studies on breast cancer disease or treatment characteristics to be used in the preference study as starting points [8,10,11,13,14,15,16,17,18,19,20,22,23,24,25,27,28,29,36,37,38]. Two studies mentioned reviewing breast cancer web forums to verify if the selected side effects were influencing patients’ daily life [11,16].

Table 2.
Methods and ranking/grading elements of the patient preference studies.
Moreover, in seven studies, clinicians or experts in the field were involved in the selection or validation steps [8,10,16,17,19,25,27]. In two studies, an advisory board, consisting of clinicians, breast cancer patients, and former health policy makers, finalized the characteristics included in the preference studies [13,14].
Also, in some studies (n = 7), interviews or focus group discussions with patients and/or clinical experts were conducted, to provide feedback, to verify, and to refine elements of the study designs [15,18,20,22,24,28,33]. The study of Chou et al. [19] mentioned that non-medical people reviewed the descriptions for each health state used in the study.
Twelve studies mentioned conducting a pilot study [11,13,14,15,21,23,24,26,27,33,37,38]; in nine cases, patients were involved in this step [11,14,15,23,24,26,33,37,38]. In two studies, the pretest of the questionnaire was performed with the general population [13,27], to ensure that patients hard to recruit would participate in the actual study instead of the pilot phase [13].
3.3.2. Patient Preference Methods
Patient preferences were obtained through various methods (Table 2). In the study of Silva et al. [17], preferences were explored via interviews and ranked by importance in an online survey. In three studies, hypothetical scenarios of treatments were presented to patients and discussed during an interview [30,35,37].
The method used in the other studies could be classified in one of the elicitation categories as outlined in the PREFER recommendations [2]. The study methods used are distributed as followed: (i) twelve studies using discrete choice-based methods (discrete choice experiment [13,14,15,18,20,23,26,27,38] and adaptive conjoint analysis [16,24,28]); (ii) six studies using ranking methods (choice-based conjoint [12,21,22,25,29] and Q-sort task [36]); (iii) eight studies using indifference methods (standard gamble [10,11], threshold task [8], time trade-off [9,19], and trade-off technique [31,32,33]); and (iv) three studies using rating methods (visual analog scale [10,19] and analytical hierarchy process [34]).
3.3.3. Ranking or Grading Elements
In all the studies, patients had to rank or grade certain (treatment) elements and/or hypothetical (treatment) scenarios. An overview of the ranking/grading elements can be found in Table 2.
Such elements presented in these studies are based on characteristics of breast cancer therapies (for instance, nausea and fatigue due to chemotherapy [11,16], or joint and muscle pain and libido decrease due to endocrine therapies [28]). In five studies, the elements were related to endocrine therapies [10,28,32,35,36]; one study presented elements related to radiotherapy [31], and another one to targeted therapy [23]. In seven studies, the elements were based on chemotherapy [8,9,11,16,21,29,33].
In the study of Omori et al. [26], the attributes were selected based on findings from the MONARCH 2 trial [39], with elements related to the treatment categories targeted agents and endocrine therapy. Also, in the study of Nazari et al. [27], elements were identified starting from endocrine and targeted therapies. Different hypothetical scenarios, consisting of chemotherapy and endocrine therapy, were used in the study of McQuellon et al. [30].
The elements in three of the preference studies were not formulated based on treatments. In one study, characteristics of an axillary dissection were used as starting point for the elements in the preference study [37]. In another study, the preferences of centrale venous devices are examined [38]. The third study based the elements on the treatment aspects currently considered in value frameworks [20].
The elements evaluated in the studies can be classified as (i) adverse events(related to the treatments) (n = 23) [8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,34], (ii) effectiveness/efficacy (n = 4) [21,22,28,34], (iii) life expectancies and survival (n = 14) [8,13,14,17,18,20,23,24,26,27,32,33,35,37], (iv) quality of life (n = 7) [12,13,14,17,22,24,38], (v) administration (n = 7) [8,16,21,22,26,27,28], (vi) breast-cancer-related disease stages (n = 5) [10,19,29,31,37], (vii) costs (n = 8) [12,13,14,18,20,25,27,38], and (viii) other (n = 6) [8,12,15,20,37,38] (Table S6).
Adverse events that appeared in several studies were as follows: diarrhea (n = 10) [11,15,16,19,22,23,24,25,26,34], nausea/vomiting (n = 9) [11,15,16,19,22,23,24,25,34], fatigue (n = 8) [11,15,16,19,22,24,25,34], and alopecia (n = 6) [11,16,22,24,25,34]. The study of Omori et al. [26] included three attributes in the DCE on diarrhea: (i) frequency of stools, (ii) incidence of diarrhea, and (iii) duration of diarrhea.
The attribute that appeared most in the category ‘life expectancies and survival’ was progression-free survival (n = 6) [14,17,18,23,26,27]. Seven studies included elements related to ‘quality of life’ [12,13,14,17,22,24,38].
3.4. Preference Outcomes
An overview of (i) the (statistical) methods to obtain preferences and (ii) some of the preference results can be found in Table S7. If elements of life expectancies and survival were included, these elements were in most studies evaluated as important [8,14,17,20,24,26,27,32,33,35,37]. In the studies with elements on quality of life, it seemed that in some studies, these elements influence patients’ decisions and were ranked as important [12,13,14,17,24]. The preference outcomes are very dependent on the elements that were evaluated in the study and the design of the study.
In 24 of the included studies [8,9,10,11,12,13,14,15,19,20,21,23,24,26,27,28,29,30,31,32,33,35,37,38], preference heterogeneity was assessed. Statistical analyses were performed to assess heterogeneity, and the heterogeneity results are also summarized in Table S7.
3.5. Patient Recruitment and Patient Involvement
The studies included in this review aim to identify which elements in their treatment or disease matter (most) to patients, and therefore recruiting patients willing to participate is needed. Different recruitment strategies were described. Moreover, the involvement of patients is crucial in patient preference studies. Table 3 provides an overview of patient recruitment strategies and patient involvement in the selected studies. Patient involvement is divided into two parts: (1) patients as participants, including communication to patients, and (2) patients as co-researchers and their involvement throughout the study (from the study design and pilot studies until the interpretation of the results).

Table 3.
Patient recruitment and patient involvement.
3.5.1. Patient Recruitment
Recruitment Strategies
The recruitment strategy used most was via hospitals and cancer centers (n = 13) [9,10,11,15,16,18,19,27,28,32,33,36,38]. One of the studies also mentioned community pharmacies [28].
In some of the studies, eligible participants were recruited via personal contact by a researcher or physician (n = 5) [15,24,30,34,37]. In two studies, information was sent to patients, followed by a phone call if patients were open to participate [35,37]. Also, in the study of McQuellon et al. [30], eligible patients were called by the study coordinator, for the explanation and invitation to participate.
Another recruitment strategy frequently mentioned was through online channels (n = 10) [12,15,17,21,22,23,24,29,34,36]. One of the studies used internet advertisements [34].
In some studies, the (online) distribution of the survey was facilitated by a third party; (i) patient support organizations (n = 7) [12,17,21,24,28,29,36] and (ii) (market research) companies (n = 5) [13,14,20,23,29].
In the study of DaCosta DiBonaventura et al. [22], invitations were sent to panelists of cancer-specific online panels. In two studies, invitations were sent via email after identification from a panel [25,26].
In three studies, a combination of different recruitment channels was applied [15,24,34].
Some studies (n = 6) also described the identification of eligible patients: via the hospital pharmacy [10], via a database [35,37], by the treating physician [30], or via a (web) panel [25,26]. In two of the studies, the recruitment strategy was not specified [8,31].
3.5.2. Patient Involvement
Involvement in Study Design
In 14 of the studies [11,13,14,15,18,20,22,23,24,26,28,33,37,38], certain roles of patients as co-researchers were identified. There are two categories in how patients were involved in the studies:
- (a)
- Patient providing advice and input
In two studies, patients are part of an advisory board in the study [13,14]. In one study, patient advocacy groups provided input on the study protocol [23]. Moreover, qualitative interviews and focus groups were conducted in five studies to provide insights and verify attributes [18,20,22,24,28].
- (b)
- Pretest/pilot test/think-aloud interviews
In nine studies, the study was pretested with patients [11,14,15,23,24,26,33,37,38].
3.6. Evaluation and Future Preference Research
The strengths, limitations, and opportunities for future research described in the papers are summarized in (Table S8). Some of the elements are explained more in detail below.
Different limitations on preference research in breast cancer could be identified. The first category of limitations is related to the sample in the preference studies. Several studies reported that the sample in their preference studies was small [8,10,15,17,18,20,27], homogeneous [20,37], and/or not representative [9,12,17,21,28,32,37,38]. Some studies highlighted that due to these sample limitations, it was not possible to generalize the results [20,23,25,26,33,35].
Moreover, selection biases [8,16,36] were mentioned due to the choices in, for example, recruitment, as part of convenience sampling [10,19,22]. In one of the studies [10], only outpatients were recruited, which may create a selection bias towards patients with better health and mobility.
Studies recruiting via an online setting [23] mentioned (i) bias towards patients that have access to technology, are technology savvy, and have better health [11,14,26], and (ii) that it may lead to illogical responses [11].
A second category of limitations relates to the design of preference studies. In the study of Tan et al. [10], a starting point bias was mentioned, because of the design where 50% perfect health was given as starting point, creating a narrower range of preferences.
Concerning the choice of attributes and the descriptions in the preference studies, some considerations were highlighted: (i) the higher the number of attributes, the greater the burden on respondents [20]; (ii) in the case of a limited number of attributes, preferences might be different if other attributes would be included [12,15,18,22,23,25,26,38]; (iii) some attributes may be difficult to understand [15,38]; (iv) descriptions might not be representative for a real-life situation [11,16]; and (v) attributes could not be deselected [24].
Moreover, the limitations related to hypothetical scenarios often make it impossible for patients to know which choices would be made in actual therapeutic decisions [18,20,23,29].
In some studies, patients themselves reported the data [12,20,22,23], which can lead to recall biases and self-presentation effects [22].
Only few studies (n = 9) clearly highlighted strengths of their study, both on the design [8,9,10,14,15,26,28,34,36] and on the sample [26,36].
4. Discussion
4.1. Points to Highlight from the Included Breast Cancer Preference Studies
4.1.1. Various Preference Designs and Outcomes
The findings of this scoping review highlight the wide variety in which patient preference studies among breast cancer patients are conducted and the preference results are obtained. The 31 included studies are published during a wide time range (1995–2024), across a diverse range of countries.
The methods mostly used in the included studies (n = 12) were discrete choice-based methods (DCEs and adaptive conjoint analysis). As highlighted in previous literature reviews, DCEs are widely used in health economics, and this number continues to grow [40,41,42]. This is in line with the increasing recognition of the importance of obtaining and integrating patient preferences in decision-making as seen by various stakeholders [43,44].
Various ranking and grading elements were identified across the selected studies; most of them were based on adverse events of treatments (n = 23) and survival elements (n = 14). The descriptions of the survival elements were distinct. In one of the studies, it was mentioned that including both progression-free survival and overall survival as elements in a preference study was confusing for patients. In some studies, the effectiveness (n = 4) of the treatments was added as attribute to evaluate the overall survival. If a survival element was added, it was valued in almost all cases as (the most) important. Quality of life elements were only included in seven of the studies. As has been mentioned in the scoping review on patient preference in metastatic breast cancer by Bland et al. [45], often the survival elements and superior treatment benefits are concluded to be the most important to patients. Quality of life elements are not as often included in the studies, so it is not possible to assess the value.
4.1.2. Selection and Formulation of Attributes
Besides the selection of attributes, the importance of including clearly formulated and understandable attributes for patients in a preference study is crucial. As reported as one of the limitations by Liu et al. [38], there might be difficulties in understanding the chosen attributes. In the study of Chou et al. [19], the health states were drafted without input from patients.
The qualitative (preference exploration) phase in patient preference studies can serve as a starting point to determine which elements are important for patients, and based on these insights, attributes can be formulated. However, as highlighted in the study of Clark et al. [40], conducting patient preferences studies is on the rise, but a decline is observed in the qualitative phases of a preference study that is aimed to inform attribute selection.
Moreover, the study of Reinisch et al. [24] mentioned, as one of the limitations, the fact that attributes could not be deselected. This again shows the relevance and importance of defining meaningful attributes, and highlights the crucial previous steps of a patient preference study where literature is screened and patients are consulted via interviews or focus groups.
Also, advisory boards consisting of multiple stakeholders could help to create and formulate adequate attributes. In only two of the included studies, an advisory board was involved in the study.
4.1.3. Patient Recruitment Strategies
Almost all of the studies provided some insights on their recruitment strategy. However, often it remained vague and somewhat unclear. The main recruitment channel was via hospitals and cancer centers. As has been highlighted by Tomiwa et al. [46], different digital tools can help to target a more representative sample and to enhance the diversity of the patients participating. An evolution towards digital channels was observed, as well as the inclusion of patient organizations and market research companies.
Yet, all these recruitment strategies innately bring with them the risk of selection bias. To overcome the biases and move towards a better representation of (breast cancer) patients, hybrid recruitment strategies are suggested.
4.1.4. Opportunities for Future Research
The main opportunities mentioned in the studies relate to the way information could be used in decision-making. Moreover, the importance of patient data was mentioned several times; however, clear implementation strategies and actions for the inclusion of preference data in decision-making are still lacking.
4.2. Suggestions for Patient Preference Studies in Breast Cancer
Different guidelines and recommendations are currently available on the conduct of patient preference studies. The PREFER recommendations provide guidance on how, when, and why to assess and use patient preferences [2]. Also, the ISPOR task force provides a roadmap to increase the usefulness and the impact of patient preferences in decision-making [47].
Below, some suggestions for patient preference studies among breast cancer patients are outlined.
- (1)
- Standardization of methods and designs making findings comparable
A variety of preference designs and outcomes were identified, making it hard to know what matters most and how findings can be compared among studies. Furthermore, all the studies were conducted within a certain context, year, country, and type of breast cancer.
Suggestions would be (i) making choices in methods and design with a clear strategy and aim of implementation in specific decision-making processes (decisions for clinical trial designs, regulatory decision-making, HTA decision-making, and decisions in clinical practice), and (ii) consulting upfront the decision-makers involved to enhance the value of the data and impact on decisions.
- (2)
- Selection of the attributes to be included
Many of the studies added a survival element that was often highly valued by patients. This should not be a surprise, given the fact that many women with breast cancer want to fight and survive, and want to receive treatments that prolong their life expectancies as much as possible, especially in the earlier phase of breast cancer.
Suggestions would be: (i) focusing on elements beyond survival when conducting a patient preference study, (ii) looking at elements that patients might accept but that make them suffer during treatments and impact patients’ quality of life, and (iii) treating data on patients’ needs and patient perspectives as needed and crucial next to clinical trial data to optimize decisions (a suggestion for decision-makers).
- (3)
- Representative sample: for the chosen target or for the potential population
Different breast cancer stages and subtypes were at hand in different studies. However, in specific studies, the sample was often not representative for the eligible population of the study, so it is not possible to establish generalizability of results.
Suggestions would be (i) clearly defining which sample is needed based on the research questions, (ii) creating a hybrid recruitment strategy (combining traditional and digital channels), (iii) focusing the recruitment strategy on how to include more diverse and underrepresented patient groups, and (iv) considering appropriate compensation that might also enhance the recruitment of underrepresented groups.
- (4)
- Preferences from experiences vs. hypothetical preferences.
Preference designs are created with the idea to determine what patients would choose, and which tradeoffs patients would be willing to make in hypothetical scenarios. When targeting breast cancer patients, some patients might have already experienced the side effects mentioned, and others might not.
Suggestions would be (i) asking patients to indicate their experiences with the attributes in the study, and (ii) considering targeting a broader population. Given the hypothetical scenarios, it might be considered to target all potential breast cancer patients.
- (5)
- The concept of preference heterogeneity
It seems that different options related to treatment elements exist among patients, which may be influenced by the demographic or treatment or be disease-related (early vs. metastatic) or other characteristics of patients (preference heterogeneity). Such preference heterogeneity is assessed via a diverse range of ways:
- (i)
- In subgroup analyses, subgroups are defined based on patients’ characteristics starting from the sample. Associations between preferences and patients’ characteristics are tested.
- (ii)
- In latent class analyses, patients are segmented based on attribute importance patterns. Patients’ characteristics correlated with the class assignment can be determined (i.e., hidden elements that determine preferences).
Suggestions would be (i) to define upfront which heterogeneity to determine, (ii) to see if the results (for example, hidden preferences) will be able to inform the decision-making processes, and (iii) discuss upfront with decision-makers involved how the data will be treated.
- (6)
- Information and communication with the included patients
Attention should be given to the patients participating in the study. The information that was given to patients prior to participating in the preference study was often not clearly reported and remained vague. Moreover, in none of the studies were patients informed about the results after the study. In the interview study of van Overbeeke et al. [48], the value of involving patients in all steps was underscored.
Suggestions would be (i) emphasizing the objectives of the study to patients, (ii) providing information via explanatory videos and interactive briefings, and (iii) systematically informing patients of the study results to improve transparency between the patients and researchers.
- (7)
- Patient involvement as co-researchers
Involving patients from the start of the preference studies improves the acceptability, comprehensibility, and relevance of the study.
Suggestions would be (i) setting up an advisory board with all the different stakeholders involved (including patients), (ii) asking for feedback and input from patients on protocols and study designs, (iii) focusing on the formulation of attributes and how patients interpret and answer the questions, and (iv) pilot testing the study with patients.
4.3. Strengths and Limitations of the Scoping Review
Both the strengths and limitations for this scoping review could be highlighted. The study offers several strengths concerning design. Firstly, the research team collaborated with Research Library 2Bergen Desiré Collen, who were providing support throughout the process, helping with the search strategies, and providing articles that could not be retrieved. Secondly, five databases were used in the scoping review, allowing us to obtain as many relevant articles as possible.
Moreover, throughout all steps of the research, a double-blind approach was applied and consensus meetings to resolve disagreements were organized, aiming to reduce the risks of including or excluding articles and misinterpretations of the data.
Finally, the research teams’ choice to exclusively focus on the patients, and hence only include patient preference studies that exclusively deal with breast cancer patients or breast cancer survivors, allowed for the exploration of patients’ own preferences, choices, and experiences, leading to more authentic and patient-centered insights. This can also be seen as a limitation, since the perspectives of other actors was not analyzed, such as healthcare professionals who obviously may play a significant role in treatment decision-making.
Some other limitations should be acknowledged. Firstly, not all articles were available in full text, which may have limited the depth and completeness of the literature review. Moreover, only English-language articles were included, which may introduce a language or cultural bias, as important studies in other languages have been excluded. Furthermore, even though a double-blind approach was performed over the whole process, other researchers might still have made other decisions concerning the inclusion or exclusion of the papers and the data extracted. Also, in terms of the interpretation and reporting of the data, the researchers made choices regarding what to report and how to structure and categorize the findings of the included studies.
5. Conclusions
This scoping review provides an overview of different preference studies among breast cancer patients. The findings show diversity, both in terms of the methods and designs used, as well as the preference outcomes. Moreover, differences in preferences among patients were observed. Given this diversity within and between the different studies, drawing general conclusions remains challenging. This could also potentially slow down the clear and standardized implementation of patient preference studies in decisions. The proposed suggestions for patient preference studies in breast cancer aim to provide ideas for studies targeting elements with an implementation plan in mind, to create impact with the valuable insights patients provide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers18010134/s1, Table S1: search string; Table S2: inclusion_exclusion; Table S3: extraction table template; Table S4: Study descriptives; Table S5: Population; Table S6: Classification ranking_grading elements; Table S7: preference outcomes; Table S8: Evaluation and future research. Reference [49] is cited in the supplementary materials.
Funding
This research received no external funding. This research is partly supported by the internal KU Leuven funding C3/22/052.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data is available upon request.
Acknowledgments
The authors want to thank the support provided by Chayenne Van Meel, biomedical reference librarian at the KU Leuven Libraries—2Bergen—Learning Centre Désiré Collen (Leuven, Belgium), for the assistance in conducting the literature review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global Patterns and Trends in Breast Cancer Incidence and Mortality across 185 Countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- The PREFER Consortium. Recommendations—Why, When and How to Assess and Use Patient Preferences in Medical Product Decision-Making; Zenodo: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Soekhai, V.; Whichello, C.; Levitan, B.; Veldwijk, J.; Pinto, C.A.; Donkers, B.; Huys, I.; van Overbeeke, E.; Juhaeri, J.; de Bekker-Grob, E.W. Methods for Exploring and Eliciting Patient Preferences in the Medical Product Lifecycle: A Literature Review. Drug Discov. Today 2019, 24, 1324–1331. [Google Scholar] [CrossRef]
- Cheung, K.L.; Mayer, S.; Simon, J.; de Vries, H.; Evers, S.M.A.A.; Kremer, I.E.H.; Hiligsmann, M. Comparison of Statistical Analysis Methods for Object Case Best–Worst Scaling. J. Med. Econ. 2019, 22, 509–515. [Google Scholar] [CrossRef]
- Vass, C.; Boeri, M.; Karim, S.; Marshall, D.; Craig, B.; Ho, K.A.; Mott, D.; Ngorsuraches, S.; Badawy, S.M.; Mühlbacher, A.; et al. Accounting for Preference Heterogeneity in Discrete-Choice Experiments: An ISPOR Special Interest Group Report. Value Health 2022, 25, 685–694. [Google Scholar] [CrossRef]
- Wang, R.R.; Schweitzer, J.B.; Hernandez, S.; Molina, S.C.; Keegan, T.H.M. Strategies for Recruitment and Retention of Adolescent and Young Adult Cancer Patients in Research Studies. J. Clin. Transl. Sci. 2023, 7, e240. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Srikanthan, A.; Amir, E.; Gupta, A.; Baxter, N.; Kennedy, E.D. Assisting with Decision-Making: How Standardized Information Impacts Breast Cancer Patient Decisions Regarding Fertility Trade-Offs and Chemotherapy. J. Adolesc. Young Adult Oncol. 2019, 8, 660–667. [Google Scholar] [CrossRef]
- Simes, R.J.; Coates, A.S. Patient Preferences for Adjuvant Chemotherapy of Early Breast Cancer: How Much Benefit Is Needed? J. Natl. Cancer Inst. Monogr. 2001, 2001, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Aung, M.M.; Ngai, M.I.; Xie, F.; Ko, Y. Assessment of Preference for Hormonal Treatment-Related Health States among Patients with Breast Cancer. Value Health Reg. Issues 2014, 3, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, I.; Bouganim, N.; Beusterien, K.; Grinspan, J.; Vandermeer, L.; Gertler, S.; Dent, S.F.; Song, X.; Segal, R.; Mazzarello, S.; et al. Preference Weights for Chemotherapy Side Effects from the Perspective of Women with Breast Cancer. Breast Cancer Res. Treat. 2013, 142, 101–107. [Google Scholar] [CrossRef]
- Williams, C.P.; Gallagher, K.D.; Deehr, K.; Aswani, M.S.; Azuero, A.; Daniel, C.L.; Ford, E.W.; Ingram, S.A.; Balch, A.J.; Rocque, G.B. Quantifying Treatment Preferences and Their Association with Financial Toxicity in Women with Breast Cancer. Cancer 2021, 127, 449–457. [Google Scholar] [CrossRef]
- Stamuli, E.; Corry, S.; Foss, P. Patient Preferences Do Matter: A Discrete Choice Experiment Conducted with Breast Cancer Patients in Six European Countries, with Latent Class Analysis. Int. J. Technol. Assess. Health Care 2023, 39, e21. [Google Scholar] [CrossRef]
- Stamuli, E.; Corry, S.; Ross, D.; Konstantopoulou, T. Patient Preferences for Breast Cancer Treatments: A Discrete Choice Experiment in France, Ireland, Poland and Spain. Future Oncol. 2022, 18, 1115–1132. [Google Scholar] [CrossRef]
- Bullen, A.; Ryan, M.; Ennis, H.; Gray, E.; Loría-Rebolledo, L.E.; McIntyre, M.; Hall, P. Trade-Offs between Overall Survival and Side Effects in the Treatment of Metastatic Breast Cancer: Eliciting Preferences of Patients with Primary and Metastatic Breast Cancer Using a Discrete Choice Experiment. BMJ Open 2024, 14, e076798. [Google Scholar] [CrossRef]
- Beusterien, K.; Grinspan, J.; Kuchuk, I.; Mazzarello, S.; Dent, S.; Gertler, S.; Bouganim, N.; Vandermeer, L.; Clemons, M. Use of Conjoint Analysis to Assess Breast Cancer Patient Preferences for Chemotherapy Side Effects. Oncologist 2014, 19, 127–134. [Google Scholar] [CrossRef]
- Silva, A.S.; França, A.C.W.; Padilla, M.P.; Macedo, L.S.; Magliano, C.A.d.S.; Santos, M.d.S. Brazilian Breast Cancer Patient-Reported Outcomes: What Really Matters for These Women. Front. Med. Technol. 2022, 4, 809222. [Google Scholar] [CrossRef]
- Ngorsuraches, S.; Thongkeaw, K. Patients’ Preferences and Willingness-to-Pay for Postmenopausal Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer Treatments after Failure of Standard Treatments. Springerplus 2015, 4, 674. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Chiang, S.C.; Ko, Y. Health State Utilities for Metastatic Breast Cancer in Taiwan. Breast 2020, 51, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hollin, I.L.; González, J.M.; Buelt, L.; Ciarametaro, M.; Dubois, R.W. Do Patient Preferences Align with Value Frameworks? A Discrete-Choice Experiment of Patients with Breast Cancer. MDM Policy Pract. 2020, 5, 2381468320928012. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; White, C.B.; Railey, E.; Sledge, G.W. Examining and Predicting Drug Preferences of Patients with Metastatic Breast Cancer: Using Conjoint Analysis to Examine Attributes of Paclitaxel and Capecitabine. Breast Cancer Res. Treat. 2014, 145, 83–89. [Google Scholar]
- DiBonaventura, M.D.; Copher, R.; Basurto, E.; Faria, C.; Lorenzo, R. Patient Preferences and Treatment Adherence Among Women Diagnosed with Metastatic Breast Cancer. Am. Health Drug Benefits 2014, 7, 386. [Google Scholar]
- Mansfield, C.; Botha, W.; Vondeling, G.T.; Klein, K.; Wang, K.; Singh, J.; Hackshaw, M.D. Patient Preferences for Features of HER2-Targeted Treatment of Advanced or Metastatic Breast Cancer: A Discrete-Choice Experiment Study. Breast Cancer 2023, 30, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, M.; Marschner, N.; Otto, T.; Korfel, A.; Stoffregen, C.; Wöckel, A. Patient Preferences: Results of a German Adaptive Choice-Based Conjoint Analysis (Market Research Study Sponsored by Eli Lilly and Company) in Patients on Palliative Treatment for Advanced Breast Cancer. Breast Care 2021, 16, 491–499. [Google Scholar] [CrossRef]
- Lalla, D.; Carlton, R.; Santos, E.; Bramley, T.; D’Souza, A. Willingness to Pay to Avoid Metastatic Breast Cancer Treatment Side Effects: Results from a Conjoint Analysis. Springerplus 2014, 3, 350. [Google Scholar] [CrossRef]
- Omori, Y.; Enatsu, S.; Cai, Z.; Ishiguro, H. Patients’ Preferences for Postmenopausal Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer Treatments in Japan. Breast Cancer 2019, 26, 652–662. [Google Scholar] [CrossRef]
- Nazari, A.; Lopez-Valcarcel, B.G.; Najafi, S. Preferences of Patients With HR+ & HER2- Breast Cancer Regarding Hormonal and Targeted Therapies in the First Line of Their Metastatic Stage: A Discrete Choice Experiment. Value Health Reg. Issues 2021, 25, 7–14. [Google Scholar] [CrossRef]
- Wouters, H.; Maatman, G.A.; Van Dijk, L.; Bouvy, M.L.; Vree, R.; Van Geffen, E.C.G.; Nortier, J.W.; Stiggelbout, A.M. Trade-Offpreferences Regarding Adjuvant Endocrine Therapy among Women with Estrogen Receptor-Positive Breast Cancer. Ann. Oncol. 2013, 24, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Kassem, N.; Shen, F.; Jiang, G.; Smith, M.L.; Railey, E.; Howell, J.; White, C.B.; Schneider, B.P. Discerning the Clinical Relevance of Biomarkers in Early Stage Breast Cancer. Breast Cancer Res. Treat. 2017, 164, 89–97. [Google Scholar] [CrossRef]
- McQuellon, R.P.; Muss, H.B.; Hoffman, S.L.; Russell, G.; Craven, B.; Yellen, S.B. Patient Preferences for Treatment of Metastatic Breast Cancer: A Study of Women with Early-Stage Breast Cancer. J. Clin. Oncol. 1995, 13, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Spaich, S.; Krickeberg, S.; Hetjens, S.; Wenz, F.; Gerhardt, A.; Sütterlin, M. Patient Preferences Regarding Intraoperative versus External Beam Radiotherapy for Early Breast Cancer and the Impact of Socio-Demographic Factors. Arch. Gynecol. Obstet. 2019, 299, 1121–1130. [Google Scholar] [CrossRef]
- Duric, V.M.; Fallowfield, L.J.; Saunders, C.; Houghton, J.; Coates, A.S.; Stockler, M.R. Patients’ Preferences for Adjuvant Endocrine Therapy in Early Breast Cancer: What Makes It Worthwhile? Br. J. Cancer 2005, 93, 1319–1323. [Google Scholar] [CrossRef]
- Duric, V.M.; Stockler, M.R.; Heritier, S.; Boyle, F.; Beith, J.; Sullivan, A.; Wilcken, N.; Coates, A.S.; Simes, R.J. Patients’ Preferences for Adjuvant Chemotherapy in Early Breast Cancer: What Makes AC and CMF Worthwhile Now? Ann. Oncol. 2005, 16, 1786–1794. [Google Scholar] [CrossRef]
- Thill, M.; Pisa, G.; Isbary, G. Targets for Neoadjuvant Therapy–The Preferences of Patients with Early Breast Cancer. Geburtshilfe Frauenheilkd 2016, 76, 551–556. [Google Scholar] [CrossRef]
- Thewes, B.; Meiser, B.; Duric, V.M.; Stockler, M.R.; Taylor, A.; Stuart-Harris, R.; Links, M.; Wilcken, N.; McLachlan, S.A.; Phillips, K.A.; et al. What Survival Benefits Do Premenopausal Patients with Early Breast Cancer Need to Make Endocrine Therapy Worthwhile? Lancet Oncol. 2005, 6, 581–588. [Google Scholar] [CrossRef]
- Wouters, H.; van Geffen, E.C.G.; Baas-Thijssen, M.C.; Krol-Warmerdam, E.M.; Stiggelbout, A.M.; Belitser, S.; Bouvy, M.L.; van Dijk, L. Disentangling Breast Cancer Patients’ Perceptions and Experiences with Regard to Endocrine Therapy: Nature and Relevance for Non-Adherence. Breast 2013, 22, 661–666. [Google Scholar] [CrossRef]
- Galper, S.R.; Lee, S.J.; Tao, M.L.; Troyan, S.; Kaelin, C.M.; Harris, J.R.; Weeks, J.C. Patient Preferences for Axillary Dissection in the Management of Early-Stage Breast Cancer. J. Natl. Cancer Inst. 2000, 92, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiang, Y.; Gu, Y.; Chen, N.; Fu, P.; Wei, Y.; Zhao, P.; Li, Y.; Du, C.; Mu, W.; et al. Patient Preferences and Willingness to Pay for Central Venous Access Devices in Breast Cancer: A Multicenter Discrete Choice Experiment. Int. J. Nurs. Stud. 2024, 152, 104695. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2-Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Clark, M.D.; Determann, D.; Petrou, S.; Moro, D.; de Bekker-Grob, E.W. Discrete Choice Experiments in Health Economics: A Review of the Literature. Pharmacoeconomics 2014, 32, 883–902. [Google Scholar] [CrossRef]
- Soekhai, V.; de Bekker-Grob, E.W.; Ellis, A.R.; Vass, C.M. Discrete Choice Experiments in Health Economics: Past, Present and Future. Pharmacoeconomics 2019, 37, 201–226. [Google Scholar] [CrossRef]
- Nouwens, S.P.H.; Marceta, S.M.; Bui, M.; van Dijk, D.M.A.H.; Groothuis-Oudshoorn, C.G.M.; Veldwijk, J.; van Til, J.A.; de Bekker-Grob, E.W. The Evolving Landscape of Discrete Choice Experiments in Health Economics: A Systematic Review. PharmacoEconomics 2025, 43, 879–936. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.J.; Edwards, A.; Kinnersley, P.; Grol, R. Shared Decision Making and the Concept of Equipoise: The Competences of Involving Patients in Healthcare Choices. Br. J. General. Pract. 2000, 50, 892. [Google Scholar]
- Mühlbacher, A.C.; Juhnke, C.; Beyer, A.R.; Garner, S. Patient-Focused Benefit-Risk Analysis to Inform Regulatory Decisions: The European Union Perspective. Value Health 2016, 19, 734–740. [Google Scholar] [CrossRef]
- Bland, K.A.; Mustafa, R.; McTaggart-Cowan, H. Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review. Cancers 2023, 15, 4331. [Google Scholar] [CrossRef]
- Tomiwa, T.; Wong, E.; Miller, H.N.; Ogungbe, O.; Byiringiro, S.; Plante, T.; Himmelfarb, C.R. Leveraging Digital Tools to Enhance Diversity and Inclusion in Clinical Trial Recruitment. Front. Public Health 2024, 12, 1483367. [Google Scholar]
- Bridges, J.F.P.; de Bekker-Grob, E.W.; Hauber, B.; Heidenreich, S.; Janssen, E.; Bast, A.; Hanmer, J.; Danyliv, A.; Low, E.; Bouvy, J.C.; et al. A Roadmap for Increasing the Usefulness and Impact of Patient-Preference Studies in Decision Making in Health: A Good Practices Report of an ISPOR Task Force. Value Health 2023, 26, 153–162. [Google Scholar] [CrossRef]
- van Overbeeke, E.; Vanbinst, I.; Jimenez-Moreno, A.C.; Huys, I. Patient Centricity in Patient Preference Studies: The Patient Perspective. Front. Med. 2020, 7, 93. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).