Simple Summary
Vascular endothelial growth factor (VEGF)-A is known to play a crucial role in the tumor microenvironment. This study investigated the relationship between circulating total VEGF-A (tVEGF-A) and its isoforms with the therapeutic effects of anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) antibody monotherapy in patients with non-small-cell lung cancer (NSCLC). Higher levels of tVEGF-A were associated with shorter progression-free survival (PFS) in anti-PD-1/PD-L1 antibody monotherapy only when measured in serum, not in plasma. Notably, higher levels of serum VEGF121, an isoform of VEGF-A, were significantly associated with not only shorter PFS but also a lower objective response rate. Serum VEGF121 levels could serve as a useful biomarker for predicting anti-PD-1/PD-L1 antibody monotherapy efficacy in patients with NSCLC.
Abstract
Background/Objectives: Vascular endothelial growth factor (VEGF)-A promotes an immunosuppressive tumor microenvironment, potentially affecting the efficacy of anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) antibody therapy. VEGF121 and VEGF165, VEGF-A isoforms, promote and inhibit tumor growth, respectively. Additionally, VEGF-A levels differ depending on whether they are measured in serum or plasma. However, whether the serum or plasma levels of total VEGF-A (tVEGF-A) or its isoforms are the most suitable for predicting anti-PD-1/PD-L1 antibody therapy efficacy remains unclear. Methods: Eighty-six patients with non-small-cell lung cancer (NSCLC) who were treated with anti-PD-1/PD-L1 antibody monotherapy between December 2015 and December 2023 were retrospectively enrolled. The association between the serum and plasma levels of tVEGF-A and its isoforms (VEGF121 and VEGF165) and treatment outcomes was analyzed. Results: The median progression-free survival (PFS) was 2.9 months, and the objective response rate (ORR) was 23.3%. PFS was significantly shorter in patients with higher tVEGF-A serum levels (≥484.2 pg/mL) than in those without (median PFS 2.1 vs. 3.7 months, p = 0.004). In contrast, plasma tVEGF-A levels could not be used to stratify PFS. Therefore, the serum levels of VEGF-A isoforms were measured. Patients with higher VEGF121 serum levels (≥523.5 pg/mL) showed both significantly shorter PFS (median PFS 2.3 vs. 3.3 months, p = 0.022) and a lower ORR (9.7% vs. 30.9%, p = 0.033) than those without. Multivariate Cox and logistic regression analyses showed that higher levels of serum VEGF121 were significantly associated with shorter PFS and a lower ORR. Conclusions: Serum VEGF121 levels may be useful in predicting anti-PD-1/PD-L1 antibody monotherapy efficacy.
1. Introduction
Non-small-cell lung cancer (NSCLC) has a poor prognosis compared to many other cancers [1]. The prognosis of advanced NSCLC has dramatically improved with the advent of nivolumab [2,3], pembrolizumab [4,5] (anti-programmed cell death 1 [PD-1] antibodies), and atezolizumab [6] (anti-programmed cell death ligand 1 [PD-L1] antibodies). Currently, the PD-L1 tumor proportion score (TPS) is a predictor of the response to anti-PD-1/PD-L1 antibody therapy and is used in clinical practice [7]. However, even in patients with advanced NSCLC harboring high PD-L1 expression, the response rate for anti-PD-1/PD-L1 antibody monotherapy as a first-line treatment is only 38.3–44.8% [4,6], and the accuracy of predictions based on PD-L1 expression is limited. Therefore, there is an urgent need to identify new biomarkers other than PD-L1 that can predict the efficacy of anti-PD-1/PD-L1 antibody therapy.
Vascular endothelial growth factor (VEGF)-A is a homodimer protein of 40–45 kDa that is secreted by various cells, including tumor cells, immune cells, and platelets [8,9,10,11]. VEGF-A binds to vascular endothelial growth factor receptor (VEGFR) and neuropilin (NRP) [12]. VEGF-A expression is regulated by hypoxia-inducible factor-1α and is induced under hypoxic conditions [13]. Secreted VEGF-A is involved in angiogenesis, tumor growth, and tumor metastasis [13,14]. VEGF-A is highly expressed in lung cancer tissues, and its overexpression is a poor prognostic factor [15]. Furthermore, VEGF-A increases the presence and function of myeloid-derived suppressor cells, regulatory T cells, and tumor-associated macrophages, which suppress anticancer immunity and inhibit cytotoxic T lymphocytes and dendritic cells [16]. Hence, VEGF-A promotes the development of an immunosuppressive tumor microenvironment, which may affect the therapeutic efficacy of anti-PD-1/PD-L1 antibodies.
The VEGF-A gene is located on chromosome 6p21.1 and consists of eight exons separated by seven introns [17]. The alternative splicing of VEGF-A mRNA from exons 5 to 8 produces different VEGF-A isoforms, such as VEGF121, VEGF165, VEGF189, and VEGF206 [18,19,20]. Of these, VEGF121 and VEGF165 are primarily secreted by tumor cells [21]. In a cancer mouse model with the overexpression of VEGF121 or VEGF165, VEGF121 promotes tumor growth, whereas VEGF165 suppresses it [22]. However, no studies have examined the association between the efficacy of anti-PD-1/PD-L1 antibody monotherapy and the levels of VEGF-A isoforms in the blood.
VEGF-A can be measured in both serum and plasma; however, because serum VEGF-A levels include VEGF-A pooled in the platelets, serum VEGF-A levels have been reported to be approximately 2–7 times higher than plasma levels [23,24]. Additionally, conflicting reports have demonstrated a relationship between the efficacy of anti-PD-1 antibody therapy in NSCLC and circulatory VEGF-A levels [25,26]. Shibaki et al. revealed that higher levels of VEGF-A in serum were associated with shorter survival [25], although Tiako et al. showed that there was no significant association between VEGF-A levels in plasma and efficacy in patients with NSCLC [26]. These data suggest that the usefulness of VEGF-A as a blood marker for predicting the efficacy of anti-PD-1/PD-L1 antibody therapy in patients with NSCLC depends on the sample type, such as serum or plasma.
Therefore, we investigated whether the association between the efficacy of anti-PD-1/PD-L1 antibody monotherapy and the circulatory levels of total VEGF-A (tVEGF-A) was dependent on sample types, such as serum and plasma, and compared the predictive value of tVEGF-A and its major isoforms, VEGF121 and VEGF165, for the efficacy of anti-PD-1/PD-L1 antibody monotherapy.
2. Materials and Methods
2.1. Study Population and Design
This study screened 137 patients with NSCLC treated with anti-PD-1/PD-L1 antibody monotherapy (nivolumab, pembrolizumab, or atezolizumab) at the Department of Respiratory Medicine, Hiroshima University Hospital, between December 2015 and December 2023 (Figure 1). Forty-two patients without serum and plasma samples were excluded. Because the administration of bevacizumab and ramucirumab has been reported to cause fluctuations in circulatory VEGF-A [27,28,29], eight patients with a history of bevacizumab or ramucirumab before anti-PD-1/PD-L1 antibody administration were also excluded. Moreover, one patient who developed radiation pneumonitis immediately before the initiation of anti-PD-1/PD-L1 antibody monotherapy was excluded because VEGF-A levels may fluctuate owing to the development of pneumonitis [30]. Ultimately, 86 patients with serum and plasma samples were included in this study. This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Hiroshima University Hospital (E2004-0326-23, approved 7 August 2024). Written informed consent was obtained from all the participants.
Figure 1.
Flowchart of patient enrollment. This study included patients with non-small-cell lung cancer (NSCLC) treated with anti-programmed cell death 1(PD-1)/programmed cell death ligand 1(PD-L1) antibody monotherapy (nivolumab, pembrolizumab, or atezolizumab) at the Department of Respiratory Medicine, Hiroshima University Hospital, between December 2015 and December 2023, for whom serum and plasma samples were stored. After excluding eight patients who had a history of bevacizumab or ramucirumab prior to anti-PD-1/PD-L1 antibody administration and one patient who developed radiation pneumonitis just prior to the initiation of anti-PD-1/PD-L1 antibody monotherapy, 86 patients were finally included in the study. NSCLC, non-small-cell lung cancer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
2.2. Evaluations of the Objective Response Rate and Progression-Free Survival
Complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and not evaluable (NE) were determined based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [31]. The objective response rate (ORR) was defined as the proportion of patients who achieved CR or PR. Progression-free survival (PFS) was defined as the time from the start of each treatment until progression or death from any cause. Patients who failed to follow-up were censored on the date of their last known survival.
2.3. Measurement of tVEGF-A and Its Isoforms
Serum and plasma samples were collected prior to anti-PD-1/PD-L1 antibody administration and stored at −80 °C. Serum and plasma tVEGF-A levels were determined using an ELISA system developed by Shino-Test Corporation. Polystyrene microtiter plates were coated and incubated with 100 μL of anti-human VEGF-A polyclonal antibody (R&D Biosystems, Minneapolis, MN, USA) in PBS overnight at 4 °C. The plates were washed three times with PBS containing 0.05% Tween 20, and the remaining binding sites in the wells were blocked by incubating the plates for 2 h with 400 μL/well of PBS containing 0.5% casein. After the plates were washed, 100 μL of each dilution of the calibrator and samples (1:1 dilution in 0.2 mol/L Tris pH 8.5 and 0.15 mol/L sodium chloride containing 1% casein) was added to the wells. The plates were then incubated for 15 h at 25 °C. The plates were washed again and were incubated with 100 μL/well of peroxidase-conjugated anti-human VEGF-A monoclonal antibody (R&D Biosystems, Minneapolis, MN) for 2 h at 25 °C. After another washing step, chromogenic substrate 3,3′,5,5′-tetra-methylbenzidine (Dojindo Laboratories, Kumamoto, Japan) was added to each well. The reaction was terminated with sulfuric acid, and the absorbance at 450 nm was read using a microplate reader (Model 680, Bio-Rad, Irvine, CA, USA). VEGF121 and VEGF165 levels were measured using ELISA kits (Shino-Test, Kanagawa, Japan) [19].
2.4. Statistical Analysis
Values are expressed as a median (interquartile range [IQR]) unless stated otherwise. Differences among the groups were examined using the Fisher’s exact, Wilcoxon signed-rank, and Mann–Whitney U tests. Spearman’s rank correlation coefficient was calculated to evaluate the association between the levels of tVEGF-A and its isoforms. A receiver operating characteristic (ROC) curve analysis was performed to identify the optimal cut-off levels of tVEGF-A and its isoforms for predicting the objective response (CR or PR) to anti-PD-1/PD-L1 antibody monotherapy. The optimal cut-off level was determined by maximizing the sum of sensitivity plus specificity − 1. PFS was evaluated using a Kaplan–Meier analysis and the log-rank test. Median PFS intervals with a corresponding 95% confidence interval (CI) were calculated. Univariate and multivariate Cox proportional hazard models and logistic regression analyses were used to identify the independent predictors of PFS and the objective response for anti-PD-1/PD-L1 antibody monotherapy, respectively. Statistical significance was set at p < 0.05. All data analyses were performed using JMP statistical software version 17.0.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient Characteristics
The baseline characteristics of the patients are shown in Table 1. Of the 86 patients, the median age was 73 years (67–77), 60 (69.8%) were male, and 14 (16.3%) were NSCLC positive for driver oncogenes. PD-L1 TPS was ≥50% in 41 (47.7%), 1–49% in 18 (20.9%), <1% in 8 (9.3%), and unknown in 19 (22.1%). Anti-PD-1/PD-L1 antibody monotherapy was administered to 54 patients (62.8%) as a second- or later-line treatment.

Table 1.
Baseline characteristics.
The median observation period was 10.6 months (4.6–30.6). At the data cut-off in July 2024, progression or death from any cause was observed in 78 patients (90.7%). The treatment responses to anti-PD-1/PD-L1 antibody monotherapy in 86 patients were classified as CR in 4 (4.7%), PR in 16 (18.6%), SD in 17 (19.8%), PD in 36 (41.9%), and NE in 13 (15.1%). The ORR and median PFS of anti-PD-1/PD-L1 antibody monotherapy were 23.3% and 2.9 months (95% CI: 2.1–3.4), respectively.
3.2. Prediction of the Therapeutic Effect of Anti-PD-1/PD-L1 Antibody Monotherapy by Serum and Plasma tVEGF-A
The serum and plasma levels of tVEGF-A were measured. Serum tVEGF-A levels were significantly higher than plasma levels (452.9 pg/mL [252.3–704.7] vs. 49.4 pg/mL [0.0–131.6], p < 0.001) (Figure 2a, Supplementary Table S1). Serum and plasma tVEGF-A levels were positively correlated (ρ = 0.502, p < 0.001) (Supplementary Figure S1a). The ROC curve analysis revealed that the optimal cut-off levels for predicting the objective response to anti-PD-1/PD-L1 antibody monotherapy were 484.2 pg/mL for serum tVEGF-A (area under the curve [AUC] = 0.54 [95% CI: 0.40–0.68], specificity = 48.5%, sensitivity = 70.0%) and 137.1 pg/mL for plasma tVEGF-A (AUC = 0.54 [95% CI: 0.39–0.68], specificity = 81.8%, sensitivity = 35.0%) (Supplementary Figure S2a,b). There was no significant difference in the ORR between the groups stratified by serum and plasma tVEGF-A cut-off levels (Figure 3a,b). Conversely, the Kaplan–Meier analysis showed that PFS was significantly shorter in patients with higher levels of serum tVEGF-A than in those with lower levels (median PFS 2.1 months [95% CI: 1.2–3.3] vs. 3.7 months [95% CI: 2.1–5.4], p = 0.004), but there was no significant difference in PFS between patients stratified by the cut-off levels of plasma tVEGF-A (median PFS 2.3 months [95% CI: 0.7–4.0] vs. 2.9 months [95% CI: 2.1–4.4], p = 0.611) (Figure 4a,b). The univariate Cox proportional hazards model revealed that serum tVEGF-A levels, a history of chronic obstructive pulmonary disease (COPD), an immune checkpoint inhibitor (ICI) treatment line, and the ICI agent were significant predictors of PFS (Table 2). Furthermore, the multivariate Cox proportional hazards model (model 1) revealed that serum tVEGF-A levels (≥484.2 pg/mL) were independent predictors of shorter PFS when adjusted for a history of COPD, ICI treatment line, and ICI agent (Table 2).
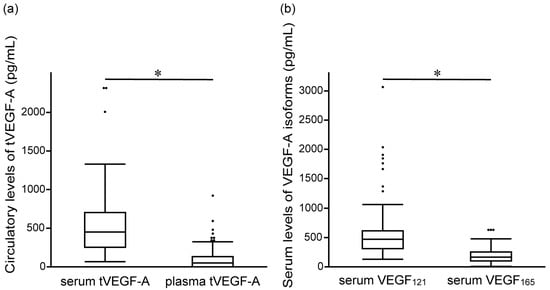
Figure 2.
Comparison of baseline levels of (a) serum and plasma tVEGF-A, and (b) serum VEGF121 and VEGF165 before the initiation of anti-programmed cell death 1/programmed cell death ligand 1 antibody monotherapy. The serum levels of tVEGF-A are significantly higher than the plasma levels of tVEGF-A (452.9 pg/mL [interquartile range (IQR), 252.3–704.7] vs. 49.4 pg/mL [IQR, 0.0–131.6], p < 0.001) (a). The serum levels of VEGF121 are significantly higher than the serum levels of VEGF165 (466.4 pg/mL [IQR, 309.3–611.9] vs. 169.4 pg/mL [IQR, 98.8–251.8], p < 0.001) (b). The boxes represent the 25th to 75th percentiles; the solid lines within the boxes show the median values; the whiskers represent the 10th and 90th percentiles; the dots represent outliers. IQR, interquartile range; tVEGF, total vascular endothelial growth factor. * p < 0.001, using the Wilcoxon signed-rank test.
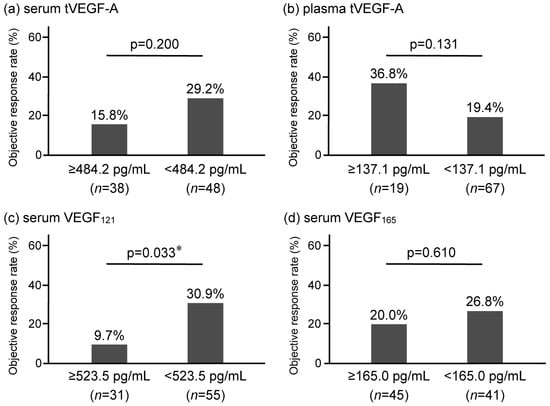
Figure 3.
Comparison of the objective response rate (ORR) of anti-programmed cell death 1/programmed cell death ligand 1 antibody monotherapy in non-small-cell lung cancer stratified by baseline levels of (a) serum tVEGF-A, (b) plasma tVEGF-A, (c) serum VEGF121, and (d) serum VEGF165. The ORR is not significantly different for serum tVEGF-A (a) and plasma tVEGF-A (b). In contrast, the ORR is significantly lower in the high serum VEGF121 group (9.7% vs. 30.9, p = 0.033) (c) but not significantly different in serum VEGF165 (20.0% vs. 26.8%, p = 0.610) (d). ORR, objective response rate; tVEGF, total vascular endothelial growth factor. * p < 0.05, using the Fisher’s exact test.
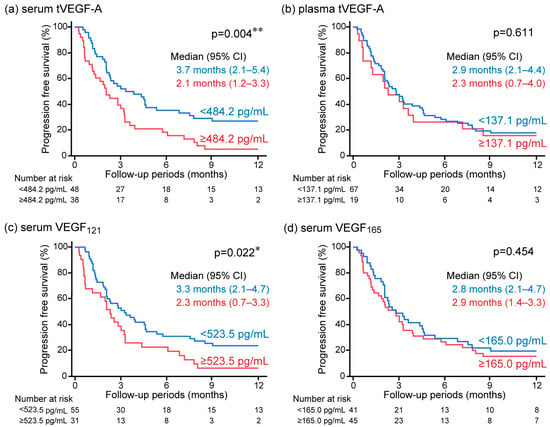
Figure 4.
Kaplan–Meier analysis for progression-free survival (PFS) in anti-programmed cell death 1/programmed cell death ligand 1 antibody monotherapy in non-small-cell lung cancer stratified by baseline (a) serum tVEGF-A, (b) plasma tVEGF-A, (c) serum VEGF121, and (d) serum VEGF165. Patients with higher levels of serum tVEGF-A showed significantly shorter PFS than those with lower levels (a), but no significant difference in PFS was observed when stratified by plasma tVEGF-A (b). Also, patients with higher levels of serum VEGF121 showed significantly shorter PFS than those with lower levels (c), but there was no significant difference in PFS when evaluated by serum VEGF165 (d). CI, confidence interval; PFS, progression-free survival; tVEGF, total vascular endothelial growth factor. * p < 0.05 and ** p < 0.01 using the log-rank test.

Table 2.
Univariate and multivariate Cox proportional hazards model for predicting progression-free survival in patients with non-small-cell lung cancer treated with anti-programmed cell death 1(PD-1)/programmed cell death ligand 1(PD-L1) antibody monotherapy.
3.3. Prediction of the Therapeutic Effect of Anti-PD-1/PD-L1 Antibody Monotherapy by Serum VEGF-A Isoforms
This study additionally measured VEGF121 and VEGF165 levels using serum samples, as only the serum levels of tVEGF-A, not the plasma levels, were used to stratify PFS. The serum levels of VEGF121 were significantly higher than the serum levels of VEGF165 (466.4 pg/mL [309.3–611.9] vs. 169.4 pg/mL [98.8–251.8], p < 0.001) (Figure 2b, Supplementary Table S1). The serum levels of VEGF121 and VEGF165 were positively correlated with the serum levels of tVEGF-A (ρ = 0.607, p < 0.001 and ρ = 0.865, p < 0.001, respectively) (Supplementary Figure S1b,c). The ROC curve analysis revealed that the optimal cut-off levels for predicting the objective response to anti-PD-1/PD-L1 antibody monotherapy were 523.5 pg/mL for serum VEGF121 (AUC = 0.61 [95% CI: 0.47–0.73], specificity = 42.4%, sensitivity = 85.0%) and 165.0 pg/mL for serum VEGF165 (AUC = 0.50 [95% CI: 0.36–0.65], specificity = 54.6%, sensitivity = 55.0%) (Supplementary Figure S2c,d).
The ORR was significantly lower in patients with higher levels of serum VEGF121 (≥523.5 pg/mL) than in those without (9.7% vs. 30.9%, p = 0.033), although there was no significant difference in the ORR between patients with and without VEGF165 serum levels higher than 165.0 pg/mL (20.0% vs. 26.8%, p = 0.610) (Figure 3c,d). Univariate and multivariate logistic regression analyses revealed that, among circulatory tVEGF-A and its isoforms, only higher levels of serum VEGF121 were independently and significantly associated with a failure to achieve the objective response (Table 3).

Table 3.
Univariate and multivariate logistic regression analyses for predicting the objective response in patients with non-small-cell lung cancer treated with anti-programmed cell death 1(PD-1)/programmed cell death ligand 1(PD-L1) antibody monotherapy.
The Kaplan–Meier analysis also showed a significantly shorter PFS in the group with higher levels of serum VEGF121 than in the group without (median PFS 2.3 months [95% CI: 0.7–3.3] vs. 3.3 months [95% CI: 2.1–4.7] months, p = 0.022), but not in the group with and without higher levels of serum VEGF165 (median PFS 2.9 months [95% CI: 1.4–3.3] vs. 2.8 months [95% CI: 2.1–4.7] months, p = 0.454) (Figure 4c,d). The univariate Cox proportional hazards model revealed that serum VEGF121 levels were significant predictors of PFS (Table 2). A positive correlation was observed between the serum levels of tVEGF-A and VEGF121; therefore, the association between PFS and serum VEGF121 was analyzed in a multivariate Cox proportional hazards model not including serum tVEGF-A. The multivariate Cox proportional hazards model (model 2) revealed that higher levels of serum VEGF121 were an independent predictor of shorter PFS when adjusted for a history of COPD, ICI treatment line, and ICI agent (Table 2).
4. Discussion
In this study, serum and plasma tVEGF-A levels were examined to predict the efficacy of anti-PD-1/PD-L1 antibody monotherapy in patients with NSCLC. Higher levels of tVEGF-A in serum, but not in plasma, were significantly associated with a shorter PFS. Furthermore, among the serum levels of tVEGF-A and its isoforms, higher levels of serum VEGF121 were useful for predicting a lower ORR and shorter PFS in patients treated with anti-PD-1/PD-L1 antibody monotherapy.
This study demonstrated that serum samples were suitable for measuring tVEGF-A levels to stratify the PFS of patients receiving anti-PD-1/PD-L1 antibody monotherapy. Consistent with our results, high serum tVEGF-A levels have been reported to be associated with shorter PFS in patients with NSCLC who are elderly or have poor PS [25]; however, these associations have not been shown in other studies using plasma samples [26]. This discrepancy in the association between the efficacy of anti-PD-1/PD-L1 antibodies and serum or plasma tVEGF-A levels is potentially caused by platelet-derived VEGF-A in the serum. First, in circulation, most VEGF-A is pooled in the alpha granules of platelets, and VEGF-A is released from platelets, particularly when platelets are activated by several factors, including blood coagulation [32,33]. When serum samples are obtained, VEGF-A is released from platelets owing to blood coagulation in the serum collection tubes [33]. Accordingly, serum VEGF-A levels have been reported to be approximately two to seven times higher than plasma VEGF-A levels [23,24]. It has also been shown that the VEGF-A content of platelets increases with tumor progression, and much of the serum VEGF-A in patients with cancer is thought to be derived from VEGF-A pooled in platelets [10,11]. Secondly, VEGF-A released from activated platelets by tumor cells plays a role in promoting tumor progression and metastasis [34,35]. Additionally, VEGF-A promotes the development of an immunosuppressive tumor microenvironment associated with resistance to anti-PD-1/PD-L1 antibody therapy [16]. These data suggest that the measurement of VEGF-A levels pooled in platelets is needed to predict the efficacy of anti-PD-1/PD-L1 antibody therapy, and therefore, serum tVEGF-A could be a predictive biomarker for efficacy by reflecting the amount of VEGF-A in platelets in this study.
This study also showed that higher levels of serum VEGF121 were significantly and independently associated with a lower ORR and shorter PFS in patients treated with anti-PD-1/PD-L1 antibody monotherapy, although high levels of serum tVEGF-A were associated with shorter PFS but not the ORR. Additionally, VEGF165 serum levels could not be used to stratify PFS or the ORR. VEGF121 promotes tumor angiogenesis and increases vascular permeability around the tumor [22,36,37,38]. Moreover, VEGF121 promotes lymphangiogenesis in the sentinel lymph nodes of NSCLC cells [39]. Furthermore, in various types of cancer, including lung cancer, high expression of VEGF121 evaluated using tumor samples has been shown to be associated with poor prognosis [40,41,42]. In contrast, VEGF165 inhibits tumor growth by normalizing tumor blood vessels and reducing the hypoxic state of the tumors. VEGF165 has an NRP-binding domain and can bind to NRP1, although VEGF121 cannot accelerate NRP1 signaling [43]. The interaction between VEGF165 and NRP1 recruits NRP1-expressing monocytes (NEMs) to newly formed blood vessels [43]. NEMs produce molecules that contribute to the stabilization of tumor blood vessels (such as transforming growth factor-β, platelet-derived growth factor-B, and stromal cell-derived factor-1) and chemokines with anti-tumor activity (C-C motif chemokine ligand [CCL]2, CCL4, CCL5, C-X-C motif chemokine ligand [CXCL]9, CXCL10, etc.), thereby inhibiting tumor growth [44]. Therefore, the use of VEGF121, which is involved in tumor growth more specifically than tVEGF-A, including VEGF165, could stratify both the PFS and ORR of anti-PD-1/PD-L1 antibody treatment.
This study has several limitations. First, this was a retrospective study with a limited sample size. Second, the study included patients who received anti-PD-1/PD-L1 antibody monotherapy from the second-line treatment onwards. Currently, ICI alone or in combination with chemotherapy is administered as the first-line therapy for the treatment of advanced-stage NSCLC in most cases. To further investigate the usefulness of VEGF121, it is necessary to confirm the results of this study in a prospective cohort of patients with NSCLC who received anti-PD-1/PD-L1 antibody therapy as the first-line treatment. Additionally, to overcome ICI resistance in patients with higher levels of serum VEGF121, our future perspective is that the efficacy of the combination therapy with molecular targeted therapies for VEGF-A or VEGFR needs to be evaluated. Third, the AUC value of serum VEGF121 was 0.61, which is not sufficiently high. To enhance its predictive accuracy, a combination with other predictive markers may be necessary. Fourth, VEGF-A levels may have fluctuated due to the influence of the fasting state and time of day [45,46], as the conditions for obtaining blood samples were not unified with considering these factors in this study.
5. Conclusions
In patients with NSCLC who received anti-PD-1/PD-L1 antibody monotherapy, high serum tVEGF-A levels, but not plasma levels, were significantly associated with a shorter PFS. Furthermore, as the focus was on the VEGF-A isoforms VEGF121 and VEGF165, only VEGF121 could be used as a predictive marker for the efficacy of anti-PD-1/PD-L1 antibody monotherapy, and high serum VEGF121 levels were associated with a low ORR and shorter PFS. Therefore, VEGF-A levels, particularly VEGF121, measured by serum levels, could be a predictive biomarker for the efficacy of anti-PD-1/PD-L1 antibody monotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17040572/s1. Figure S1: Association between circulatory levels of tVEGF-A and VEGF-A isoforms; Figure S2: Receiver operating characteristic (ROC) curve analysis for predicting the complete response or partial response to anti-programmed cell death 1/programmed cell death ligand 1 antibody monotherapy; Table S1: Baseline levels of circulatory tVEGF-A and VEGF-A isoforms before the initiation of anti-programmed cell death 1/programmed cell death ligand 1 antibody monotherapy.
Author Contributions
Conceptualization, K.Y.; methodology, K.Y.; validation, T.H. and K.Y.; formal analysis, T.H. and K.Y.; investigation, T.H.; resources, T.H., K.Y., K.F., K.S., S.S., Y.H., T.M., T.N., H.I., H.H., S.Y. and N.H.; data curation, T.H.; writing—original draft preparation, T.H.; writing—review and editing, K.Y., K.F., K.S., S.S., Y.H., T.M., T.N., H.I., H.H., S.Y. and N.H.; visualization, T.H.; supervision, H.I., H.H., and N.H.; project administration, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hiroshima University Hospital (protocol code E2004-0326-23, approved 7 August 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank Editage (https://www.editage.jp/, accessed on 9 January 2025) for the English language editing. Measurements of the levels of tVEGF-A, VEGF121, and VEGF165 were performed by Shino-Test Corporation.
Conflicts of Interest
Kakuhiro Yamaguchi reports personal fees from Chugai Pharmaceutical and Ono Pharmaceutical and research funding from Shino-Test Corporation. Takeshi Masuda reports personal fees from Daiichi-Sankyo, Taiho Pharmaceutical, Boehringer Ingelheim, Kyowa Kirin, Eli Lilly, Ono Pharmaceutical, Otsuka Pharmaceutical, Chugai Pharmaceutical, and AstraZeneca. Noboru Hattori reports personal fees from AstraZeneca, Boehringer Ingelheim, and Chugai Pharmaceutical and research funding from Ono Pharmaceutical, Chugai Pharmaceutical, and Shino-Test Corporation. All other authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | area under the curve |
| CCL | C-C motif chemokine ligand |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| CR | complete response |
| CXCL | C-X-C motif chemokine ligand |
| ELISA | enzyme-linked immunosorbent assay |
| ICI | immune checkpoint inhibitor |
| IQR | interquartile range |
| NSCLC | non-small-cell lung cancer |
| NE | not evaluable |
| NRP | neuropilin |
| ORR | objective response rate |
| PD-1 | programmed cell death 1 |
| PD-L1 | programmed cell death ligand 1 |
| PFS | progression-free survival |
| PR | partial response |
| PD | progressive disease |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| ROC | receiver operating characteristic |
| SD | stable disease |
| tVEGF | total vascular endothelial growth factor |
| TPS | tumor proportion score |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Lantuejoul, S.; Damotte, D.; Hofman, V.; Adam, J. Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J. Thorac. Dis. 2019, 11, S89–S101. [Google Scholar] [CrossRef]
- Nagy, J.A.; Dvorak, A.M.; Dvorak, H.F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2007, 2, 251–275. [Google Scholar] [CrossRef]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef]
- Salgado, R.; Benoy, I.; Bogers, J.; Weytjens, R.; Vermeulen, P.; Dirix, L.; Van Marck, E. Platelets and vascular endothelial growth factor (VEGF): A morphological and functional study. Angiogenesis 2001, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- George, M.L.; Eccles, S.A.; Tutton, M.G.; Abulafi, A.M.; Swift, R.I. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: Clinical evidence of platelet scavenging? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 3147–3152. [Google Scholar]
- Silva-Hucha, S.; Pastor, A.M.; Morcuende, S. Neuroprotective Effect of Vascular Endothelial Growth Factor on Motoneurons of the Oculomotor System. Int. J. Mol. Sci. 2021, 22, 814. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, D.; Han, Y.S.; Baek, M.J. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann. Surg. Treat. Res. 2015, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, J.; Lv, X.J.; Wang, Q.; Qiu, L.X.; Lin, X.Q.; Yu, L.K.; Song, Y. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: A systematic review with meta-analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2009, 4, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Al Kawas, H.; Saaid, I.; Jank, P.; Westhoff, C.C.; Denkert, C.; Pross, T.; Weiler, K.B.S.; Karsten, M.M. How VEGF-A and its splice variants affect breast cancer development—Clinical implications. Cell. Oncol. 2022, 45, 227–239. [Google Scholar] [CrossRef] [PubMed]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-Angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef]
- Yamakuchi, M.; Okawa, M.; Takenouchi, K.; Bibek, A.; Yamada, S.; Inoue, K.; Higurashi, K.; Tabaru, A.; Tanoue, K.; Oyama, Y.; et al. VEGF-A165 is the predominant VEGF-A isoform in platelets, while VEGF-A121 is abundant in serum and plasma from healthy individuals. PLoS ONE 2023, 18, e0284131. [Google Scholar] [CrossRef]
- Ferrara, N. Binding to the extracellular matrix and proteolytic processing: Two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 2010, 21, 687–690. [Google Scholar] [CrossRef]
- Cheung, N.; Wong, M.P.; Yuen, S.T.; Leung, S.Y.; Chung, L.P. Tissue-specific expression pattern of vascular endothelial growth factor isoforms in the malignant transformation of lung and colon. Hum. Pathol. 1998, 29, 910–914. [Google Scholar] [CrossRef]
- Kazemi, M.; Carrer, A.; Moimas, S.; Zandonà, L.; Bussani, R.; Casagranda, B.; Palmisano, S.; Prelazzi, P.; Giacca, M.; Zentilin, L.; et al. VEGF121 and VEGF165 differentially promote vessel maturation and tumor growth in mice and humans. Cancer Gene Ther. 2016, 23, 125–132. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Arimura, K.; Matsumuro, K.; Otsuka, R.; Watanabe, O.; Jonosono, M.; Maruyama, Y.; Maruyama, I.; Osame, M. Highly concentrated vascular endothelial growth factor in platelets in Crow-Fukase syndrome. Muscle Nerve 2000, 23, 1051–1056. [Google Scholar] [CrossRef]
- Cappelletto, E.; Fasiolo, L.T.; Salizzato, V.; Piccin, L.; Fabozzi, A.; Contato, A.; Bianco, P.D.; Pasello, G.; Chiarion-Sileni, V.; Gion, M.; et al. Cytokine and soluble programmed death-ligand 1 levels in serum and plasma of cancer patients treated with immunotherapy: Preanalytical and analytical considerations. Int. J. Biol. Markers 2024, 39, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Shibaki, R.; Murakami, S.; Shinno, Y.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Kanda, S.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; et al. Predictive value of serum VEGF levels for elderly patients or for patients with poor performance status receiving anti-PD-1 antibody therapy for advanced non-small-cell lung cancer. Cancer Immunol. Immunother. 2020, 69, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Tiako Meyo, M.; Jouinot, A.; Giroux-Leprieur, E.; Fabre, E.; Wislez, M.; Alifano, M.; Leroy, K.; Boudou-Rouquette, P.; Tlemsani, C.; Khoudour, N.; et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers 2020, 12, 473. [Google Scholar] [CrossRef]
- Ninomiya, H.; Ozeki, M.; Matsuzawa, Y.; Nozawa, A.; Yasue, S.; Kubota, K.; Endo, S.; Asano, T.; Taguchi, K.; Ohe, N.; et al. A pediatric case of anaplastic astrocytoma with a gliomatosis cerebri; the growth pattern and changes in serum VEGF-121 levels after bevacizumab treatment. J. Clin. Neurosci. 2020, 81, 431–433. [Google Scholar] [CrossRef]
- Segerström, L.; Fuchs, D.; Bäckman, U.; Holmquist, K.; Christofferson, R.; Azarbayjani, F. The anti-VEGF antibody bevacizumab potently reduces the growth rate of high-risk neuroblastoma xenografts. Pediatr. Res. 2006, 60, 576–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spratlin, J.L.; Cohen, R.B.; Eadens, M.; Gore, L.; Camidge, D.R.; Diab, S.; Leong, S.; O’Bryant, C.; Chow, L.Q.; Serkova, N.J.; et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Flower, V.A.; Pauling, J.D.; Millar, A.B. VEGF (Vascular Endothelial Growth Factor) and Fibrotic Lung Disease. Int. J. Mol. Sci. 2018, 19, 1269. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Wartiovaara, U.; Salven, P.; Mikkola, H.; Lassila, R.; Kaukonen, J.; Joukov, V.; Orpana, A.; Ristimäki, A.; Heikinheimo, M.; Joensuu, H.; et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb. Haemost. 1998, 80, 171–175. [Google Scholar] [CrossRef]
- Banks, R.E.; Forbes, M.A.; Kinsey, S.E.; Stanley, A.; Ingham, E.; Walters, C.; Selby, P.J. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br. J. Cancer 1998, 77, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Amirkhosravi, A.; Amaya, M.; Siddiqui, F.; Biggerstaff, J.P.; Meyer, T.V.; Francis, J.L. Blockade of GpIIb/IIIa inhibits the release of vascular endothelial growth factor (VEGF) from tumor cell-activated platelets and experimental metastasis. Platelets 1999, 10, 285–292. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Tian, Y.; Li, Z.; Liu, Z.; Zhu, S. The critical role of platelet in cancer progression and metastasis. Eur. J. Med. Res. 2023, 28, 385. [Google Scholar] [CrossRef] [PubMed]
- Küsters, B.; de Waal, R.M.; Wesseling, P.; Verrijp, K.; Maass, C.; Heerschap, A.; Barentsz, J.O.; Sweep, F.; Ruiter, D.J.; Leenders, W.P. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res. 2003, 63, 5408–5413. [Google Scholar]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Manenti, L.; Riccardi, E.; Marchini, S.; Naumova, E.; Floriani, I.; Garofalo, A.; Dossi, R.; Marrazzo, E.; Ribatti, D.; Scanziani, E.; et al. Circulating plasma vascular endothelial growth factor in mice bearing human ovarian carcinoma xenograft correlates with tumor progression and response to therapy. Mol. Cancer Ther. 2005, 4, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Minamiya, Y.; Ito, M.; Saito, H.; Ogawa, J. VEGF121 promotes lymphangiogenesis in the sentinel lymph nodes of non-small cell lung carcinoma patients. Lung Cancer 2008, 59, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Watanabe, Y.; Murakami, S.; Oda, M.; Hayashi, Y.; Nonomura, A.; Endo, Y.; Sasaki, T. Vascular endothelial growth factor and lymph node metastasis in primary lung cancer. Br. J. Cancer 1997, 76, 1041–1045. [Google Scholar] [CrossRef][Green Version]
- Ljungberg, B.; Jacobsen, J.; Häggström-Rudolfssson, S.; Rasmuson, T.; Lindh, G.; Grankvist, K. Tumour vascular endothelial growth factor (VEGF) mRNA in relation to serum VEGF protein levels and tumour progression in human renal cell carcinoma. Urol. Res. 2003, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Broséus, J.; Mourah, S.; Ramstein, G.; Bernard, S.; Mounier, N.; Cuccuini, W.; Gaulard, P.; Gisselbrecht, C.; Brière, J.; Houlgatte, R.; et al. VEGF(121), is predictor for survival in activated B-cell-like diffuse large B-cell lymphoma and is related to an immune response gene signature conserved in cancers. Oncotarget 2017, 8, 90808–90824. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Pattarini, L.; Zentilin, L.; Moimas, S.; Carrer, A.; Sinigaglia, M.; Arsic, N.; Tafuro, S.; Sinagra, G.; Giacca, M. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J. Clin. Investig. 2008, 118, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Carrer, A.; Moimas, S.; Zacchigna, S.; Pattarini, L.; Zentilin, L.; Ruozi, G.; Mano, M.; Sinigaglia, M.; Maione, F.; Serini, G.; et al. Neuropilin-1 identifies a subset of bone marrow Gr1- monocytes that can induce tumor vessel normalization and inhibit tumor growth. Cancer Res. 2012, 72, 6371–6381. [Google Scholar] [CrossRef]
- Galeati, G.; Spinaci, M.; Govoni, N.; Zannoni, A.; Fantinati, P.; Seren, E.; Tamanini, C. Stimulatory effects of fasting on vascular endothelial growth factor (VEGF) production by growing pig ovarian follicles. Reproduction 2003, 126, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Engelmann, K.; Appelt, D.; Sandner, D.; Weigmann, I.; Ganz, X.; Pistrosch, F.; Köhler, C.; Gasparic, A.; Birkenfeld, A.L. Intra-individual variability and circadian rhythm of vascular endothelial growth factors in subjects with normal glucose tolerance and type 2 diabetes. PLoS ONE 2017, 12, e0184234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).