Simple Summary
A blood culture result indicating a hidden tumor is a crucial scenario that highlights the need to reconsider the clinical significance of some infections traditionally viewed as isolated events. Recent research demonstrates that certain infections may serve as early indicators of occult malignancies. Specific infection patterns, such as unexplained liver abscesses, persistent pneumonia, or bloodstream infections with gut bacteria, frequently precede cancer diagnosis by several months. These events are not random. Tumors can compromise immune function, disrupt tissue barriers, and alter the microbiome, thereby facilitating bacterial infections. Recognizing these infection patterns challenges conventional clinical approaches and prompts the critical question of whether cancer should be sought when infections lack an obvious cause. Identifying this association may enable earlier cancer detection, reduce the incidence of advanced-stage diagnoses, and improve patient survival.
Abstract
Emerging evidence suggests some bacterial infections may be early signs of hidden cancers rather than random events. Yet this link remains under-recognized in practice, representing an often-missed diagnostic opportunity. Large registry studies show that certain infections are linked to a sharply increased short-term risk of cancer detection, with most of the excess diagnoses clustering in the first 6 months after the index episode. Key associations include the following: (i) anaerobic or gut-derived bacteremia with Bacteroides, Clostridium, Fusobacterium, or pks+ Escherichia coli before colorectal neoplasm; (ii) Streptococcus gallolyticus/bovis bacteremia and colorectal neoplasm; (iii) cryptogenic Klebsiella pneumoniae liver abscess and pancreaticobiliary or colorectal cancer; (iv) non-resolving pneumonia and segmental collapse before lung cancer. Overall, short-term cancer detection risks range from about 3% after unselected Gram-negative bacteremia to ~8% or higher after cryptogenic liver abscess—similar to accepted thresholds that justify targeted cancer work-up. Even hidden tumors disrupt immunity, compromise barriers, and create conditions that favor microbial invasion. This review synthesizes evidence for the “sentinel infection phenotype”; outlines pathogen-specific associations, including their possible pathogenetic mechanisms; and proposes a practical diagnostic framework. Recognizing these infection signatures may enable earlier cancer detection and better outcomes.
1. Introduction
A 68-year-old male patient presented with Escherichia coli bacteremia. No identifiable infectious focus was found, and inflammatory markers normalized rapidly with antibiotic therapy, leading to a diagnosis of “transient gut translocation”. Six weeks later, he was re-admitted with Enterobacter spp. bacteremia. This time computed tomography and colonoscopy identified a stage II sigmoid adenocarcinoma. Although such scenarios are routinely encountered in hospitals globally, the oncologic significance is often initially overlooked, highlighting a critical knowledge gap—when should unexplained Gram-negative bacteremia prompt targeted evaluation for occult malignancy?
Global cancer incidence is increasing, with 19.3 million new cases reported in 2020 and projections of up to 30 million annually by 2040 [1]. For most solid tumors, delayed diagnosis remains the main barrier to better outcomes. Only a minority of colorectal (CRC) and lung cancers are found through screening—up to 70% are diagnosed at stage III–IV, with nearly half of lung cancers already metastatic [2,3,4].
The link between chronic inflammation and cancer is not a modern concept. In early Chinese medical texts, Galen’s humoral pathology and Virchow’s “chronic irritation theory”, persistent inflammation was linked with “mass-forming disease”. Modern infectious diseases re-exposed this link in a clinically actionable way. In 1951, McCoy and Mason described an association of enterococcal endocarditis with CRC [5], raising the concept that CRC can serve as a portal of entry for gut bacteria, which was later confirmed by Klein et al. in 1977 leading to current recommendations for colonoscopic evaluation in patients with Streptococcus bovis bacteremia or endocarditis [6].
Since then, multiple studies have shown that certain infections are followed by sharply increased short-term cancer detection. For example, overall cancer incidence during the first 6 months after Gram-negative bacteremia is about threefold higher than expected, after pneumonia 2.5-fold higher, and in patients with pyogenic liver abscess (PLA) the risk of subsequently diagnosed cancer is increased by fourfold, with an especially high excess risk in the first few months after the infection episode [7,8,9,10,11,12,13]. This highlights that infection is not generating cancer—it can be the first visible sign of a malignancy that has been present silently for years.
Despite substantial evidence, this association is not consistently recognized in clinical practice. The segmentation of contemporary medicine is often the reason why no single specialty oversees the entire diagnostic process from the initial infectious event to the eventual identification of occult malignancy. Consequently, the oncologic significance of certain infections is frequently overlooked or recognized too late. Furthermore, very few reports synthesize epidemiologic data, mechanistic immunobiology, and diagnostic implications into a coherent clinically applicable framework.
This review uniquely integrates epidemiologic evidence, immunobiology, and bedside algorithms to demonstrate that specific infections may serve as early indicators of occult malignancy. It examines the biological mechanisms through which tumors predispose individuals to infection and proposes a practical, pathogen-specific diagnostic framework termed the “sentinel infection phenotype”. In defined clinical contexts, infections should be viewed not only as isolated events but also as potential indicators of underlying malignancy.
2. Infections Forecasting Cancer: What the Numbers Reveal
Recent studies show that certain bacterial infections often occur shortly before the diagnosis of solid cancers and that this timing pattern is replicable across different populations. Multiple epidemiological studies showed that the excess cancer risk following infection is highest in the first months, begins to decline by about 6 months, and continues to attenuate thereafter [8,13,14]. In general populations, the absolute risk of being diagnosed with cancer in the first six months after a bacterial infection is usually around 1–3%, with standardized incidence ratios (SIRs) of 2 to 4 for any cancer type during this period [13,15]. Higher detection rates are seen in certain clinical subgroups, particularly older adults with unexplained bacteremia and smokers who present with lobar pneumonia [10,16]. This temporal pattern supports a reverse-causation model, in which the infection is often the first clinical manifestation of an already existing but previously undiagnosed tumor, rather than a distant causal factor in cancer development. Some of this early excess in cancer diagnoses is likely driven by greater healthcare contact and more intensive diagnostic testing around the index infection; however, the magnitude and consistent, organ-specific clustering of risk across cohorts suggests that detection bias alone is unlikely to fully explain these associations.
Beyond this short-term diagnostic window, a second pattern is apparent: several chronic colonization or infection states (for example chronic Salmonella Typhi carriage, recurrent urinary tract infections, or persistent Chlamydia pneumoniae seropositivity) have been associated with a modest but sustained increase in long-term cancer risk, probably mediated through chronic inflammation, genotoxic effects, and pro-oncogenic changes in the local microbiome. The strength of evidence for these infection–cancer links is heterogeneous, ranging from well-established associations supported by large population-based cohorts (e.g., Streptococcus gallolyticus and colorectal cancer) to emerging or largely anecdotal signals. Importantly, most of the evidence summarized in this section comes from retrospective observational studies, which are inherently vulnerable to confounding, detection bias and surveillance bias; these methodological limitations are discussed in more detail in Section 7.
2.1. Gastrointestinal Cancers (Colorectal and Gastric)
CRC shows the strongest and most clinically relevant association with infection-related warning signs (Table 1). National Danish registry data show that among more than 11,000 patients with Gram-negative bacteremia, about 3% are diagnosed with cancer within six months, with gastrointestinal cancers occurring 3- to 13-times more often during this period [13]. A recent population-based study from Queensland further confirmed this pattern, demonstrating elevated short-term CRC detection following bloodstream infection, particularly after anaerobic and Gram-negative episodes [14]. Pathogen-specific cohort analyses confirm that bacteremia with Fusobacterium nucleatum, Bacteroides and Clostridium septicum is associated with a markedly increased short-term risk of CRC (with adjusted risks that are several-fold higher than for other bacteremias and exceeding ten-fold for C. septicum) [9,17,18,19]. In patients with Enterococcus endocarditis, colorectal neoplasia was found in over half of cases, similar to Streptococcus gallolyticus, supporting the role of routine colonoscopy as part of diagnostic evaluation [20,21,22]. A large Danish study recently confirmed that patients with Enterococcus faecalis bloodstream infection have a significantly higher short-term risk of CRC, with a 3.7-fold increased hazard compared to matched population controls [23]. Patients with PLA, especially caused by Klebsiella pneumoniae, are also at higher risk of having an underlying CRC, and colonoscopy in those patients should be considered as well [11,24,25]. Among these, the associations of S. gallolyticus and C. septicum with CRC can be considered high-certainty sentinel links, whereas the signals involving F. nucleatum, Bacteroides spp. and Enterococcus spp. are strong but still emerging and will benefit from further prospective validation.
In addition to these sentinel infections, some chronic infection states may increase the long-term risk of cancer development. For example, Helicobacter pylori is a well-established long-latency carcinogen. Prospective studies and meta-analyses show that H. pylori infection increases gastric cancer risk by two- to threefold, with the risk rising more than sixfold when the infection is acquired early in life [26]. In regions with high prevalence, population-level eradication programs have led to measurable declines in gastric cancer incidence, further supporting a causal relationship [27].
2.2. Pancreaticobiliary Cancers
A Taiwanese study described that more than 2% of patients with PLA presented with the disease as the initial manifestation of underlying hepatocellular carcinoma (HCC) [28]. Similar patterns have been described for biliary tract cancers presenting with cholangitis or liver abscess due to tumor-related ductal obstruction.
Beyond these sentinel presentations, chronic colonization states and dysbiosis appear to influence long-term pancreaticobiliary cancer risk. Chronic Salmonella Typhi carriage in typhoid-endemic areas is linked to a four- to fivefold higher risk of gallbladder cancer, especially in individuals who also have gallstones [29]. Pancreatic cancer shows an association with oral dysbiosis. In a case–control study, higher pancreatic cancer risk was observed in individuals with increased oral levels of Porphyromonas gingivalis (OR 1.60) and Aggregatibacter actinomycetemcomitans (OR 2.20) [30].
2.3. Lung Cancer and Pulmonary Infection Phenotypes
Pulmonary infections can serve as early warning signs of hidden lung cancer. Danish national data show a 1.4% risk of being diagnosed with lung cancer within six months after hospitalization for pneumonia, with SIR around 2.5 and an eightfold excess among patients who eventually develop lung cancer [12]. In high-risk groups, especially heavy smokers hospitalized with pneumonia, the twelve-month lung cancer risk can reach 8%, and up to 24% when pneumonia affects the upper lobe [16]. This pattern is consistent with post-obstructive infection caused by tumor-related airway obstruction [16].
In parallel, chronic Chlamydia pneumoniae infection contribute to long-term lung cancer risk, with meta-analyses showing overall OR of 1.5 and subgroup risks up to 2.3 in IgA-positive individuals, likely driven by chronic inflammation [31].
2.4. Genitourinary Cancers
Strong links between urinary tract infections (UTIs) and bladder cancer are seen in large population studies. In a Taiwanese cohort of more than 70,000 UTI patients, the adjusted hazard ratios (aHR) for urinary tract cancers ranged from 4.5 to 4.7, with bladder cancer being the most common and the highest risk observed in people with repeated, unexplained infections [32]. In the Nijmegen bladder cancer study recurrent UTIs were linked to a higher risk of bladder cancer, with the strongest association seen in people who regularly experience episodes of cystitis (OR 6.6 in men and 2.7 in women), with strong association in men with more than ten antibiotic-treated UTIs (OR 6.0) [33].
Recurrent UTIs were also linked with prostate cancer in several cohorts. In an Italian case–control study cystitis was linked to a higher risk of prostate cancer (OR 1.76), with a markedly stronger association when infection occurred within one year before diagnosis (OR 7.6) [34]. In a nationwide Swedish cohort of over 600,000 adults aged 50 years and older, acute cystitis was also shown to precede urogenital cancers, with the highest risks observed within three months after infection and the reported SIR for prostate cancer of 7.1 [35]. Similarly, in Taiwanese study men with lower UTIs had a significantly higher risk of developing prostate cancer (aHR of 1.46 to 1.72) and the association was particularly strong among patients with recurrent infections, whose prostate cancer risk increased more than ninefold [36].
A meta-analysis including 47 studies found that men with a history of sexually transmitted infections had a 49% higher risk of prostate cancer (SIR 1.5), with a particularly consistent association for gonorrhea (SIR 1.20) [37]. In another meta-analysis Chlamydia trachomatis infection has been associated with an increased risk of cervical cancer (OR 2.2), and co-infection with human papillomavirus further amplifies this risk [38].
2.5. Other Pathogens and Malignancies
Frequent community-acquired infections such as bronchitis, sinusitis and pneumonia have been associated with a higher risk of subsequent multiple myeloma diagnosis [39]. Staphylococcus aureus bacteremia showed a higher incidence of malignancy during the first year, with the highest risks observed for cervical cancer, hematologic malignancy, sarcoma, liver and pancreatic carcinoma, and urinary tract carcinoma [8].
A variety of other bacterial pathogens have been reported preceding cancer detection; however, data are limited, and associations were largely anecdotal, based mainly on case reports and small series, and should be interpreted with caution. These include organisms such as the Streptococcus anginosus group, Aeromonas spp., Clostridium perfringens, Actinomyces spp., and Listeria monocytogenes [40,41,42,43,44,45], as further described.

Table 1.
Pathogen-specific infection phenotypes and their association with subsequent cancer detection: a summary of selected published studies.
Table 1.
Pathogen-specific infection phenotypes and their association with subsequent cancer detection: a summary of selected published studies.
| First Author, Year | Infection Phenotype | Study Design | Time Window | Effect Size/Absolute Risk |
|---|---|---|---|---|
| Colorectal and other gastrointestinal cancers | ||||
| Østergaard, 2025 [23] | Enterococcus faecalis bacteremia | Danish nationwide registries | First 6 months | CRC 0.45%, CRN 2.3%; CRC HR 3.7; CRN HR 4.6 |
| Ursi, 2021 [22] | Enterococcus spp. and S. gallolyticus IE | Single-center cohort | At time of IE work-up | In patients with colonoscopy: 71–83% intestinal disease; 13–15% CRC |
| Abe, 2024 [19] | Anaerobic bacteremia | Single-center cohort | Within 1 year | OR 3.44 for any GI cancer |
| Justesen, 2022 [46] | Anaerobic bacteremia | Population-based cohort | Within 1 year | C. septicum HR 76; Bacteroides HR 5.95; GPAC ~11; Fusobacterium ~8.5 |
| Kwong, 2018 [17] | Anaerobic bacteremia | Population-based cohort | Within 1 year | Pathogen-specific HRs: B. fragilis ~3.8; S. gallolyticus ~5.7; F. nucleatum ~6.9; C. septicum ~17 |
| Tsai, 2016 [21] | Streptococcus bovis bacteremia | Single-center cohort | No defined time window | CRC detected in 30.7% of patients with S. bovis bacteremia |
| Laupland, 2023 [14] | Community-onset bacteremia | Population-based surveillance cohort | Within 1 year | Overall IRR 16; RR C. septicum 25, B. ovatus 11.8, C. paraputrificum 11.4, S. infantarius 10.6, G. morbillorum 6.5 |
| Gaab, 2023 [18] | Colibactin-producing pks+ Escherichia coli | Systematic review and meta-analysis | No defined time window | Overall OR 2.3; Western countries OR 2.3; tissue-based studies OR 2.2 |
| Huang, 2012 [24] | Pyogenic liver abscess | Retrospective cohort | Highest risk within first 2 years | CRC 2.3%; SIR 4.00; Klebsiella spp. PLA SIR 5.8; aHR 2.7 |
| Suzuki, 2023 [25] | Pyogenic liver abscess | Retrospective cohort | Highest risk within 3 years after PLA | CRC 1.9% vs. 0.8%; time-dependent HRs: 3.6 (0.5 years), 2.5 (1 yr), 1.7 (2 yr), 1.4 (3 yr) |
| Hepatobiliary cancers (liver and gallbladder) | ||||
| Lin, 2011 [28] | Pyogenic liver abscess | Nationwide retrospective cohort | HCC diagnosed within 60 days of PLA | HCC in 2.1%; in liver cirrhosis (OR 5.1), HBV (OR 3.8), HCV (OR 3.5) |
| Koshiol, 2016 [29] | Chronic Salmonella Typhi carriage | Case–control studies/meta-analysis | No defined time window | High Vi antibody titer OR ≈ 4.0; meta-analysis summary RR 4.6–5.0 for S. Typhi and gallbladder cancer |
| Lung cancer | ||||
| Zhan, 2011 [31] | C. pneumoniae infection | Meta-analysis | No defined time window | Overall OR 1.5; prospective OR 1.2; retrospective OR 2.2; IgA ≥ 64 OR 2.4 |
| Shepshelovich, 2016 [16] | Pneumonia in smokers | Retrospective cohort | 1-year follow-up | 1-year lung cancer 8.1% (≈24% after upper-lobe pneumonia 76% located in same lobe) |
| Urinary tract cancers | ||||
| Sun, 2013 [32] | Urinary tract infection | Nationwide cohort | Risk highest in first 4 years | Any UTI HR 4.7; upper UTI HR 4.3 for renal pelvis/ureter cancer; lower UTI HR 5.7 for bladder cancer. |
| Vermeulen, 2015 [33] | Recurrent cystitis | Case–control study | No defined time window | Recurrent cystitis: OR 6.6 in men, OR 2.7 in women. Frequent recurrence (>10 episodes) in men: OR ~6 |
| Fan, 2017 [36] | Lower urinary tract infection | Nationwide population-based cohort | Follow-up for up to 14 yrs | Prostate cancer higher in cystitis aHR 1.5 and urethritis aHR 1.7 vs. no UTI; >5 LUTI visits/yr → aHR 9.3 |
| Zhu, 2016 [38] | Chlamydia trachomatis infection | Systematic review and meta-analysis | No defined time window | Overall OR ≈ 2.2 for cervical cancer; HPV + C. trachomatis OR ≈ 4.0 |
| Any cancer detection—multiple-site signal after severe infection | ||||
| Søgaard, 2020 [15] | Community-acquired Escherichia coli bacteremia (age ≥ 50) | Population-based cohort | Strongest association within 1 year | 1-year cancer incidence 3.0% (GI/hepatobiliary 1.9%, urinary 1.0%) SIR < 1 yr: GI/hepatobiliary 5.4; CRC 4.4; pancreas 7.2; kidney 10.5 SIR ≥ 1 yr: CRC 1.4; pancreas 2.3; overall 1.3 |
| Søgaard, 2017 [13] | First-time Gram-negative bacteremia | Nationwide cohort | 0–6 and 6–12 months after bacteremia | Overall SIR ≈ 1.4. Any cancer SIR ≈ 3.3 in first 6 months; particularly high SIRs (>4–10) for GI and GU cancers |
| Thomsen, 2013 [20] | Infective endocarditis | Retrospective cohort | Very high risk 0–3 months; persistent 3 months–5 yrs; modest > 5 yrs | Overall SIR 1.6: 0–3 months SIR 8; 3 months–5 yrs SIR 1.5; >5 yrs SIR 1.2 Site-specific SIR < 3 months: colon ~12, liver ~46, hematologic ~24 |
| Gotland, 2020 [8] | Staphylococcus aureus bacteremia | Nationwide matched cohort study | Within first year | 1-year IRR 1.65; highest site-specific IRRs: cervical 37.8, myeloma 6.3, leukemia 4.7, sarcoma 4.7, liver 3.6, pancreas 2.8, urinary 2.6. |
| McShane, 2014 [39] | Common community-acquired infections | Population-based case–control study | >13 months prior to diagnosis | ORs 1.1–1.4 for significant infections (strongest: pneumonia OR 1.3; sinusitis OR 1.15; bronchitis OR 1.14) Associations persist up to >72 months |
Effect sizes are reported as the hazard ratio (HR), adjusted hazard ratio (aHR), odds ratio (OR), relative risk (RR), incidence rate ratio (IRR), or standardized incidence ratio (SIR) as provided by each study. Time windows refer to the period between infection episode and cancer diagnosis. Abbreviations: CRC—colorectal cancer; CRN—colorectal neoplasia; GI—gastrointestinal; GPAC—Gram-positive anaerobic cocci.
3. Infection-Cancer Nexus: A Pathogen Map
The infection–cancer signal is not random. Across studies, distinct microbial niches map to specific malignancies. First, gut-associated anaerobes consistently cluster with colorectal neoplasia: Streptococcus gallolyticus, Clostridium septicum, Fusobacterium nucleatum, enterotoxigenic Bacteroides fragilis, and pks+ Escherichia coli are all enriched at or within colorectal tumors and can adhere to or invade dysplastic epithelium while expressing virulence factors (e.g., colibactin, FadA adhesin, B. fragilis toxin) that induce DNA double-strand breaks, activate β-catenin/NF-κB/IL-17–STAT3 signaling, and drive epithelial proliferation and inflammation [14,17,18,47,48]. Emerging data also implicate Enterococcus faecalis bacteremia and Streptococcus anginosus group infections, which frequently arise from mucosal barrier breach, as a possible signal of colorectal neoplasia [14,17,19,22].
Second, hepatobiliary pathogens appear to be linked to pancreaticobiliary cancers. Klebsiella pneumoniae in cryptogenic PLA repeatedly points to occult colon or biliary tract neoplasm, while chronic Salmonella Typhi carriage remains one of the strongest region-specific associations with gallbladder cancer [24,25,29]. Aeromonas spp. bacteremia is likewise enriched in patients with hepatobiliary malignancy, although evidence is limited to smaller clinical studies [41].
Third, respiratory pathogens and airway dysbiosis may be associated with lung cancer. While pneumonia in smokers (especially of the upper-lobe) can unmask obstructive lung tumors, Chlamydia pneumoniae seropositivity—particularly elevated or persistent IgA titers—has been associated with a modest increase in lung cancer risk, especially in smokers [16,49]. Beyond classical infections, bronchoalveolar lavage and lung-tissue microbiome studies show that lung cancer samples are disproportionately populated by oral commensal/anaerobic genera—including Veillonella, Prevotella, Fusobacterium, Porphyromonas and related taxa—consistent with chronic microaspiration of the oropharyngeal microbiota [50,51,52].
In addition to some well-described pathogen–cancer relations, several organisms show weak or anecdotal associations that may reflect mucosal disruption, immune compromise, or incidental detection. Staphylococcus aureus bacteremia is followed by a short-term diagnostic spike in several malignancies [8]. Clostridium perfringens sepsis, pelvic Actinomyces infection, and invasive Listeria monocytogenes disease have each been described in patients later diagnosed with gastrointestinal malignancy [40,41,42,43,44,45]. However, Listeria infections more commonly occur in individuals with previously diagnosed malignancy [53], whereas Actinomyces infections typically mimic malignant disease rather than precede it [54,55]. A comprehensive organism–cancer–mechanism map is shown in Figure 1.
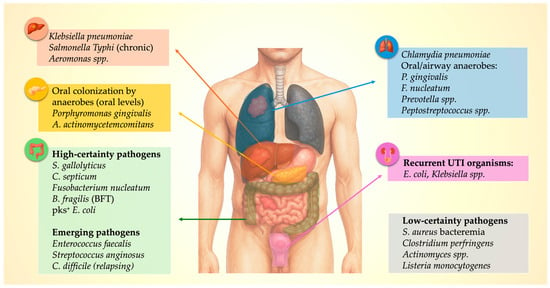
Figure 1.
Pathogen–Organ–Cancer Map: Microbial clusters linked to specific sentinel malignancies.
4. Mechanistic Insights: How Tumors Enable Infection
Even clinically or radiologically invisible cancers can act like silent saboteurs of the immune system, possibly leaving the host vulnerable to infections. Mechanistically, early tumors induce (i) systemic immunosuppression, (ii) local ecological changes in the tumor microenvironment (TME) that enable microbial persistence, (iii) gut and mucosal dysbiosis with enrichment of pro-oncogenic and invasive taxa, creating (iv) chronic inflammatory feedback loops that might accelerate malignant evolution while progressively degrading host defense (Figure 2).
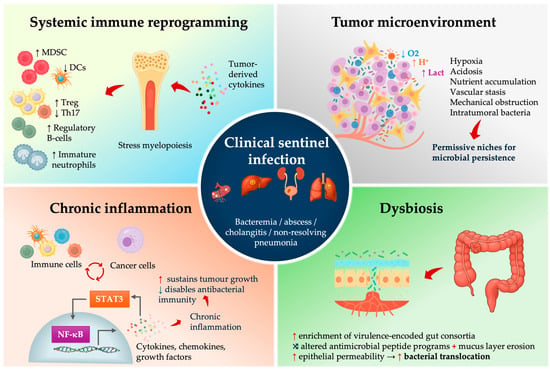
Figure 2.
Mechanistic drivers linking occult malignancy to clinical sentinel infection.
4.1. Early Systemic Immune Reprogramming in Cancer—Relevance for Infection Biology
Clinically relevant immunosuppression is not confined to late-stage malignancy. Transcriptomic profiling consistently shows that even early or pre-malignant solid tumors, such as colorectal advanced adenomas, induce systemic immune reprogramming—innate immune activation followed by T-cell suppression—a feature that is detectable in circulating leukocytes, possibly facilitating early, pre-symptomatic cancer detection [56,57,58,59]. Tumor-derived cytokines and growth factors (e.g., IL-6, GM-CSF, G-CSF, IL-1β, and VEGF) skew hematopoiesis toward stress myelopoiesis and expansion of immature neutrophil and monocyte populations that acquire myeloid-derived suppressor cell (MDSC) phenotypes. The MDSCs, together with exhausted T cells, blunt T-cell effector function and contribute to systemic T-cell dysfunction (Figure 2) [60,61,62,63].
In parallel, tumor-derived TGF-β, IL-10 and CCL22 recruit and expand regulatory T cells (Tregs), further attenuating Th1/Th17-type responses [64,65,66]. Peripheral blood transcriptomic studies in patients with advanced adenomas or early CRC demonstrate stage-specific systemic immune remodeling, with inflammatory and innate immune networks already altered before clinically overt cancer [57,58]. The Th17/IL-17 axis is crucial for maintaining intestinal epithelial barrier integrity and coordinating neutrophil recruitment to control gut bacteria, so its disruption weakens mucosal containment of opportunistic intestinal bacteria and supports the view of cancer-associated immune dysregulation as a systemic, rather than purely local, immune disorder [67,68]. Tumor-derived cytokines and growth factors (VEGF, G-CSF) further impair conventional type 1 dendritic cell (cDC1) development and function resulting in an immune landscape that is inflamed yet functionally paralyzed [69,70].
These systemic signatures can precede clinical cancer diagnosis by months to years. Circulating hematopoietic stem and progenitor cell compartments show consistent myeloid-biased expansion across several solid cancers [71,72]. Immunotranscriptomic assays can already detect systemic innate immune activation in patients with advanced colorectal adenomas, with emerging T-cell–suppressive signatures that become more pronounced as lesions progress to overt CRC [57,73].
For infectious-disease biology, the consequence is an IL-6/STAT3-inflamed, Th17/IL-17-deficient, DC-poor, and MDSC-expanded immune state, which is permissive for mucosal barrier failure and suboptimal neutrophil antimicrobial function. Minor translocation events from the gut or biliary tree can present as bacteremia, PLA biliary sepsis, or difficult-to-clear pneumonia, illustrating how early-stage cancer functions as a systemic immune disorder that specifically disrupts antibacterial defense.
4.2. The TME as a Microbial Incubator
The physical and metabolic state of solid tumors creates permissive microbial niches. Solid tumors are hypoxic and acidic, with abnormal vasculature and disrupted epithelial and endothelial barriers that impair immune cell access and clearance of microbes. These changes alter epithelial antimicrobial programs and tight junction integrity, so barrier function and mucosal containment deteriorate [74,75,76]. Mechanical obstruction of the colon lumen, bile duct, or bronchus produces stasis, bacterial overgrowth, and ascending or post-obstructive infection [77,78]. Intratumoral microbiome studies show that bacteria are not just surface contaminants; they localize intracellularly within tumor and immune cells and form tumor-type–specific communities [79,80]. The same hypoxic, leaky, barrier-disrupted niches that permit this colonization likely also lower the threshold for progression from subclinical colonization to overt infection.
Large registry studies show that bacteremias caused by specific gut taxa are strongly associated with a concurrent or subsequent diagnosis of CRC [9,17,46]. In pancreaticobiliary cancer, acute or recurrent cholangitis frequently reflects malignant biliary obstruction with bile stasis from tumor-induced strictures and may be the first clinical manifestation of an otherwise occult cancer. In lung cancer, ‘non-resolving’ or post-obstructive pneumonia that fails to clear despite appropriate antibiotics is a classic presentation of endobronchial tumor, where airway obstruction and mucus plugging create hypoxic niches that favor local overgrowth and secondary infection [78]. Together, these represent canonical infection phenotypes of cancer-driven ecological failure: colorectal → anaerobe-linked bacteremia; pancreaticobiliary → ascending cholangitis from malignant obstruction; lung → post-obstructive, non-resolving pneumonia.
4.3. Dysbiosis, Microbial Virulence Programs, and Translocation Risk
Dysbiosis in colorectal neoplasia is reproducible across cohorts and geography, and the organisms enriched in this state may not be entirely passive. Pks+ E. coli produces colibactin, generating DNA double-strand breaks; its specific mutational signature has now been detected directly in human CRC genomes and is enriched in early-onset CRC [81,82]. Enterotoxigenic Bacteroides fragilis secretes the metalloproteases, cleaves E-cadherin, activates β-catenin and NF-κB signaling, drives epithelial IL-6/STAT3 activation and Th17/IL-17-dependent inflammation and can promote tumorigenesis in experimental models [83,84]. Fusobacterium nucleatum binds tumor glycans, inhibits NK and T cell cytotoxicity, activates a TLR4–MYD88–microRNA–autophagy axis [85,86]. In other words, these bacteria do not merely exploit established tumors; they might actively help shape tumor evolution and the immune microenvironment. Importantly, dysbiosis is not unique to CRN: distinct, cancer-type–specific microbial communities have been described in pancreatic ductal adenocarcinoma, cholangiocarcinoma, and lung cancer, including intracellular tumor- and immune-cell–associated bacteria that differ by tumor type and correlate with immune programs, prognosis, and therapy response [80].
Dysbiosis also increases the risk of bacterial translocation. Dysbiotic mucosa shows reduced antimicrobial peptide expression, increased permeability and thinning of the mucus layer [87]. Consequently, the gut-derived organisms that are normally contained (anaerobes, Enterobacterales, even some streptococcal groups) are more likely to cross the epithelial barrier. Multiple studies have shown that patients with adenomas and early CRC have a higher prevalence of potentially pathogenic enteric strains, including pathotype-carrying E. coli and virulence-encoded Bacteroides [88]. This helps explain why a host with occult CRC can develop “spontaneous” anaerobic bacteremia or cryptogenic liver abscess—the mucosal barrier is not intact, and the microbial ecology is shifted toward invasive phenotypes.
4.4. Chronic Inflammation as Integrator and Amplifier
Chronic inflammation is the central mechanism that links these processes. NF-κB and IL-6/STAT3 form an inflammatory positive-feedback loop that promotes tumor survival and proliferation, while suppressing antitumor immunity and compromising microbicidal defenses at mucosal barriers [89,90]. Dysbiosis-associated ligands (e.g., LPS, flagellin) activate TLRs to drive NF-κB and IL-6/STAT3 signaling, thereby amplifying tumor fitness, blunting antitumor immunity, altering antimicrobial-peptide programs, and increasing epithelial permeability [91,92]. The net effect suggests that infection can be viewed as an expected clinical phenotype of the developing tumor’s inflammatory architecture.
Taken together, these domains act in series and in parallel; occult tumor induces systemic immune dysfunction; dysbiosis and barrier failure emerge downstream; the TME becomes a microbial incubator; chronic inflammation maintains the malignant state. The clinical infection is simply the visible tip of a multi-year remodeling process driven by tumor evolution. Infection is therefore not always separate from cancer—it can represent one component of the malignant phenotype and, in some patients, the first clinically visible sign of malignancy (Figure 3).
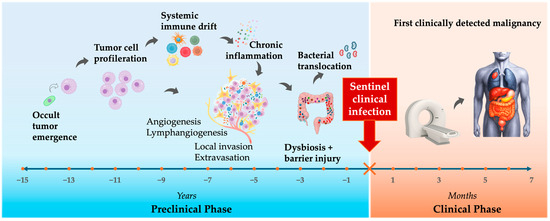
Figure 3.
The Sentinel Infection Window: timeline from tumor evolution to infection to cancer detection.
5. Clinical Red Flags: Infection Signatures Suggesting Malignancy
For physicians, the key question is not only “what is the source?” but also “why did this infection occur in this host, in this way, at this time?” Large nationwide cohort studies of pneumonia and bloodstream infection show that when an underlying cancer is uncovered after such “sentinel infections”, the excess cancer diagnoses are front-loaded, with cancer incidence peaking in the first 3–6 months after the index episode and then declining thereafter [12,13,15].
Gram-negative bacteremia without an obvious anatomic source is one of the most reproducible infectious “red flags” for occult cancer. In a Danish nationwide cohort of 11,753 adults with Gram-negative bacteremia, the absolute risk of being diagnosed with cancer within 6 months was about 3%, and cancer incidence in that 6-month window was more than threefold higher than expected; over longer follow-up, the overall cancer risk remained about 40% higher than in the general population [13]. Recurrent Gram-negative bacteremia—especially with shifting gut organisms (for example E. coli followed by Klebsiella and later an anaerobe) in a patient without clear immunodeficiency or a fixed focus—can be interpreted as a pattern suggestive of tumor-driven ecological failure, although this specific recurrence pattern has not yet been systematically quantified in large cohorts.
In practice, infection phenotypes tend to track with the anatomic location of the underlying malignancy. Anaerobic bacteremia, pks+ E. coli, cryptogenic PLA and even Fusobacterium brain abscess cluster with colorectal neoplasia. Recurrent cholangitis without stones or strictures often precedes pancreaticobiliary malignancy, and non-resolving pneumonia is a recognized presenting mode of lung cancer in bronchoscopy series. Recurrent culture-proven UTI in older adults without stones or instrumentation enriches for urothelial carcinoma, and Clostridium septicum bacteremia or gas gangrene is a classical signal for occult CRC or hematologic malignancy. C. difficile infection (CDI) has also been linked to subsequent CRC diagnosis, and in older adults with recurrent/non-resolving CDI—particularly without recent antibiotics—CRC evaluation could be reasonable [93]. The principal infection–malignancy “red flag” patterns are summarized in Table 2 and illustrated as clinical vignettes in Figure 4.

Table 2.
Recommended immediate organ-directed evaluation after sentinel infection.
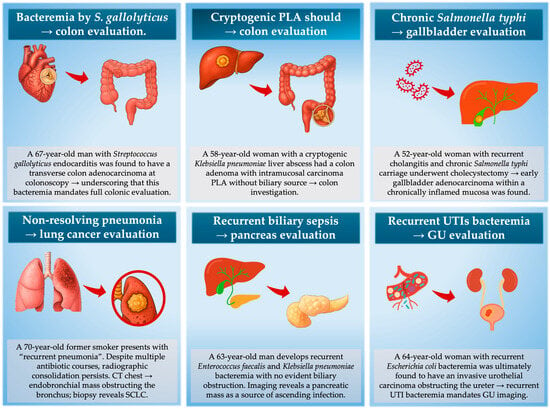
Figure 4.
Representative clinical vignettes demonstrating “sentinel infection phenotypes” that should trigger targeted malignancy evaluation. In these scenarios, the infection is not the cause of cancer but the first visible consequence of tumor-driven immune dysfunction, mucosal barrier breakdown, or anatomical obstruction. Acting on these infection signatures enables cancer detection during a narrow diagnostic window.
Laboratory and imaging features increase the probability of cancer: unexplained normocytic anemia, persistent low-grade CRP elevation after microbiologic clearance, cholestatic liver enzymes without biliary explanation, or incidental lymphadenopathy/hepatic hypodensities on CT performed for infection. The major diagnostic error is negative reassurance. A colonoscopy 3–5 years earlier does not exclude CRC. A rapid decrease in CRP after initiation of antibiotic treatment does not exclude malignancy (cancer-associated infections are usually microbiologically responsive). A non-diagnostic CT at the index admission does not exclude cancer (sub-radiologic mucosal or ductal infiltration can drive obstruction, stasis, and overgrowth).
In summary, atypical, recurrent, polymicrobial, or anatomically incongruent infections in adults without conventional risk factors should prompt consideration of both infectious and underlying malignant etiologies.
6. From Clue to Diagnosis—“Don’t Miss the Window”
Collectively, these epidemiologic and mechanistic data provide actionable guidance for clinical reasoning. The central principle is that “red-flag infections” which are unusual in presentation, anatomically incongruent, or recurrent without a clear explanation should lower the threshold for targeted cancer evaluation in the anatomically relevant organ system. Therefore, the diagnostic aim after a sentinel infection is not long-term risk prediction, but identification of an already existing malignancy.
Diagnostic evaluation should be parallel and phenotype-directed. Specific organisms and syndromes map to specific anatomic cancer domains, and that mapping is sufficiently reproducible for diagnostic actions to be protocolized. Table 2 summarizes a clinically rational, phenotype-driven diagnostic strategy for the most validated sentinel infections. Simplified, colon phenotypes require colonoscopy, pancreaticobiliary phenotypes require MRCP or pancreas-protocol MRI, lung phenotypes require bronchoscopy—and in high-risk cases, a single cross-sectional CT chest–abdomen–pelvis or FDG-PET/CT should be performed early after the index infection [94,95].
Crucially, the clinical efficiency of this strategy is not theoretical. When recalculated and expressed as estimated number-needed-to-test (NNT, Table 3) to detect one occult malignancy, the magnitudes are comparable to, or higher than, the thresholds used in accepted screening programs. In unselected adults with Gram-negative bacteremia, about 3% are diagnosed with a new cancer within 6 months (NNT ≈ 33) [13]. In bacteremia caused by colorectal-associated anaerobes and in cryptogenic PLA, colorectal neoplasia is found in roughly 3–8% of patients overall (NNT ≈ 12–30) in population-based cohorts and meta-analyses, while East Asian colonoscopy series report colonic neoplasia (cancer or advanced adenoma) in about 15–25% of cryptogenic abscess cases (NNT ≈ 4–7) [9,11,46,96,97]. In Streptococcus gallolyticus bacteremia, colorectal neoplasia is detected at colonoscopy in roughly 40–70% of patients (NNT ≈ 2–3), making colon evaluation essentially mandatory [7,98,99]. In non-resolving pneumonia, bronchogenic carcinoma accounts for up to 30% of etiologies overall (NNT ≈ 3–4) [100]. These magnitudes are not negligible; they are similar to accepted screening triggers and importantly, they are obtained without any new screening tool just simply by better interpretation of infections we already see.

Table 3.
Infection phenotypes that should trigger targeted cancer evaluation.
A core principle is to balance the benefit of early cancer detection against the risks/costs of over-testing. Most infections do not mean cancer—so the question is not “should we test everyone?” but “who is at sufficiently elevated post-infection risk to justify targeted evaluation?”. Several datasets help narrow this—the excess cancer risk begins to decline after 6 months [12,13]. Therefore, if an initial evaluation after a sentinel infection is negative and the patient remains clinically well for ~12 months, further extensive testing is generally not warranted. Conversely, unusually severe or anatomically unlike infections in younger adults (where background cancer risk is lower) generate a substantially higher SIRs than the same infections in multi-morbid elderly patients, because the event is more biologically aberrant. Clinicians should therefore individualize the threshold for initiating a malignancy evaluation based on age, baseline risk, infection severity, and feasibility of screening tests. These relationships are illustrated through representative clinical vignettes in Figure 4, which highlight when specific infectious presentations warrant targeted cancer evaluation.
Importantly, acting within this window may improve outcomes. Several series report that cancers detected following sentinel infection are more frequently operable. For example, even though a high short-term risk of CRC has been demonstrated in anaerobic bacteremia and detailed comparative data on stage distribution versus usual symptomatic presentation are still limited, CRCs identified after evaluation of cryptogenic PLA are typically nonmetastatic, supporting opportunistic colon investigation in this setting [11,46,97]. In other words, the biology that makes infection an early phenotypic manifestation of cancer also creates a clinical opportunity—if we act on the signal, we may shift diagnosis toward a stage range where cure and long-term control are still possible.
7. Future Directions—From Sentinel Infections to Early Cancer Detection
Despite robust associations between selected bacterial infections and subsequent cancer detection, several methodological and interpretive limitations must be recognized. Most studies are observational, often retrospective, and vulnerable to confounding (e.g., smoking and alcohol use as shared drivers of both infection and cancer), detection bias (more imaging after severe infection), and selection bias (hospital-based cohorts enriched for high-risk phenotypes). In particular, patients hospitalized with severe infection undergo more intensive investigations (e.g., imaging, endoscopy), so greater diagnostic intensity likely contributes to the short-term “spikes” in cancer diagnoses after infection. The specificity of individual pathogens is also not absolute—although anaerobic bacteremia strongly points to CRC, similar organisms can also be isolated in advanced diverticular disease or postoperative states. In addition, geography matters; Northern European registries quantify generalizable baseline risks (e.g., ~3% six-month cancer detection after Gram-negative sepsis) [13,15], whereas several East Asian cohorts report larger effect sizes for specific infection–cancer phenotypes. For instance, a Taiwanese population-based study found that prior UTI was associated with a 4–5-fold increased risk of subsequent urinary tract cancers [32]. Some classic infection–cancer links, such as chronic Salmonella Typhi carriage and gallbladder carcinoma, are concentrated in typhoid-endemic regions, with meta-analyses and regional studies from South Asia and China showing markedly elevated risks [29,108]. Thus, sentinel infection phenotypes should not be interpreted as stand-alone diagnostic tools, but as context-dependent triggers to lower the threshold for targeted evaluation.
Prospective clinical studies are crucial to advancing the translational momentum in recognizing bacterial infections as early indicators of underlying malignancies. Current efforts include the establishment of prospective cohorts that embed structured cancer evaluation pathways following sentinel infection events, aiming to rigorously quantify the diagnostic yield and clinical utility of infection-based early cancer detection strategies. In parallel, translational research is increasingly focusing on integrating microbial genomic data, host immune profiling, and circulating tumor DNA analyses to develop multi-modal biomarker signatures that can enhance diagnostic precision and specificity. Emerging diagnostics leveraging artificial intelligence to analyze pathogen characteristics—such as species type, recurrence patterns, and infection site incongruence—within comprehensive cancer risk models show promising potential for personalized early detection frameworks. Notably, microbiome-cancer associations are already being explored in cancers such as pancreatic [109], head, neck, and oral malignancies [110], although larger, more robust studies are needed to strengthen their clinical applicability. These efforts underscore a dynamic and growing field that bridges infection biology with oncology, increasing chances for earlier cancer detection and improved patient outcomes.
8. Conclusions
Growing epidemiologic evidence, reinforced by modern insights into tumor immunobiology, barrier integrity, and microbiome disruption, shows that certain severe or anatomically unexpected infections can represent early clinical manifestations of occult malignancy. When recognized as sentinel events, these infections provide a practical opportunity for earlier cancer detection, often at stages when curative treatment remains achievable. Although current data are largely observational, the consistency of these associations and the feasibility of focused, organ-specific evaluation support broader clinical application and prospective validation. Emerging approaches, including microbiome and immune profiling and AI-enabled risk stratification, may further enhance recognition of infection-linked cancer signals and expand timely diagnostic opportunities.
Author Contributions
Conceptualization, L.M., B.G. and N.P.; methodology, N.P.; formal analysis, N.P. and L.M.; investigation, B.G.; data curation, L.M. and B.G.; writing—original draft preparation, L.M.; writing—review and editing, B.G. and N.P.; visualization, N.P.; supervision, N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Icons representing organs and cells used in figure preparation were created with the assistance of Microsoft Copilot (accessed November 2025). The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aHR | Adjusted hazard ratio |
| CAP | Community-acquired pneumonia |
| CRC | Colorectal cancer |
| CRN | Colorectal neoplasia |
| CT | Computed tomography |
| CXCL | C-X-C motif chemokine ligand |
| FUO | Fever of unknown origin |
| GBC | Gallbladder cancer |
| GI | Gastrointestinal |
| GPAC | Gram-positive anaerobic cocci |
| GNR | Gram-negative rod |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HR | Hazard ratio |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| IRR | Incidence rate ratio |
| IV | Intravenous |
| LUTI | Lower urinary tract infection |
| MDSC | Myeloid-derived suppressor cells |
| MRCP | Magnetic resonance cholangiopancreatography |
| MRI | Magnetic resonance imaging |
| NNT | Number needed to test |
| OR | Odds ratio |
| PLA | Pyogenic liver abscess |
| ROS | Reactive oxygen species |
| RR | Relative risk |
| SARI | Severe acute respiratory infection |
| SIR | Standardized incidence ratio |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor beta |
| Th17 | T-helper 17 cells |
| TNF-α | Tumor necrosis factor alpha |
| TME | Tumor microenvironment |
| UTI | Urinary tract infection |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Aktary, M.L.; Ghebrial, M.; Wang, Q.; Shack, L.; Robson, P.J.; Kopciuk, K.A. Health-Related and Behavioral Factors Associated with Lung Cancer Stage at Diagnosis: Observations from Alberta’s Tomorrow Project. Cancer Control 2022, 29, 10732748221091678. [Google Scholar] [CrossRef]
- Dantas, A.A.G.; de Oliveira, N.P.D.; Costa, G.A.B.; Martins, L.F.L.; dos Santos, J.E.M.; Migowski, A.; Cancela, M.d.C.; de Souza, D.L.B. Multilevel analysis of social determinants of advanced stage colorectal cancer diagnosis. Sci. Rep. 2024, 14, 9667. [Google Scholar] [CrossRef] [PubMed]
- Wassie, L.A.; Mekonnen, C.K.; Tiruneh, Y.M.; Melkam, M.; Belachew, E.A.; Zegeye, A.F. Advanced-stage presentation of cancer at the time of diagnosis and its associated factors among adult cancer patients at Northwest Amhara comprehensive specialized hospitals, Northwest Ethiopia 2022. BMC Cancer 2024, 24, 68. [Google Scholar] [CrossRef]
- Mc, C.W.; Mason, J.M., 3rd. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J. Med. Assoc. State Ala. 1951, 21, 162–166. [Google Scholar]
- Klein, R.S.; Recco, R.A.; Catalano, M.T.; Edberg, S.C.; Casey, J.I.; Steigbigel, N.H. Association of Streptococcus bovis with Carcinoma of the Colon. N. Engl. J. Med. 1977, 297, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Corredoira, J.; Grau, I.; Garcia-Rodriguez, J.F.; Alonso-Garcia, P.; Garcia-Pais, M.; Rabuñal, R.; Garcia-Garrote, F.; Ardanuy, C.; Coira, A.; Lopez-Alvarez, M.; et al. The clinical epidemiology and malignancies associated with Streptococcus bovis biotypes in 506 cases of bloodstream infections. J. Infect. 2015, 71, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gotland, N.; Uhre, M.L.; Sandholdt, H.; Mejer, N.; Lundbo, L.F.; Petersen, A.; Larsen, A.R.; Benfield, T. Increased risk of incident primary cancer after Staphylococcus aureus bacteremia. Medicine 2020, 99, e19984. [Google Scholar] [CrossRef]
- Justesen, U.S.; Ellebæk, M.B.; Qvist, N.; Iachina, M.; Frimodt-Møller, N.; Søes, L.M.; Skovgaard, S.; Lemming, L.; Samulioniene, J.; Andersen, S.L.; et al. Colorectal cancer and association with anaerobic bacteraemia: A Danish nationwide population-based cohort study. J. Infect. 2024, 89, 106212. [Google Scholar] [CrossRef]
- Lai, H.; Lin, C.; Cheng, K.; Kao, J.; Chou, J.; Peng, C.; Lai, S.; Chen, P.; Sung, F. Increased Incidence of Gastrointestinal Cancers Among Patients with Pyogenic Liver Abscess: A Population-Based Cohort Study. Gastroenterology 2014, 146, 129–137.e1. [Google Scholar] [CrossRef]
- Mohan, B.P.; Aravamudan, V.M.; Khan, S.R.; Chandan, S.; Ponnada, S.; Asokkumar, R.; Navaneethan, U.; Adler, D.G. Prevalence of colorectal cancer in cryptogenic pyogenic liver abscess patients. Do they need screening colonoscopy? A systematic review and meta-analysis. Dig. Liver Dis. 2019, 51, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.K.; Farkas, D.K.; Pedersen, L.; Weiss, N.S.; Thomsen, R.W.; Sørensen, H. Pneumonia and the incidence of cancer: A Danish nationwide cohort study. J. Intern. Med. 2014, 277, 429–438. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Farkas, D.K.; Søgaard, M.; Schønheyder, H.C.; Thomsen, R.W.; Sørensen, H.T. Gram-negative bacteremia as a clinical marker of occult malignancy. J. Infect. 2017, 74, 153–162. [Google Scholar] [CrossRef]
- Laupland, K.B.; Edwards, F.; Furuya-Kanamori, L.; Paterson, D.L.; Harris, P.N. Bloodstream Infection and Colorectal Cancer Risk in Queensland Australia, 2000–2019. Am. J. Med. 2023, 136, 896–901. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Veres, K.; Vandenbroucke-Grauls, C.M.; Vandenbroucke, J.P.; Sørensen, H.T.; Schønheyder, H.C. Community-Acquired Escherichia coli Bacteremia after Age 50 and Subsequent Incidence of a Cancer Diagnosis: A Danish Population–Based Cohort Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2626–2632. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Barda, N.; Goldvaser, H.; Dagan, N.; Zer, A.; Diker-Cohen, T.; Balicer, R.; Gafter-Gvili, A. Incidence of lung cancer following pneumonia in smokers: A population-based study. QJM Int. J. Med. 2021, 115, 287–291. [Google Scholar] [CrossRef]
- Kwong, T.N.; Wang, X.; Nakatsu, G.; Chow, T.C.; Tipoe, T.; Dai, R.Z.; Tsoi, K.K.; Wong, M.C.; Tse, G.; Chan, M.T.; et al. Association Between Bacteremia from Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology 2018, 155, 383–390.e8. [Google Scholar] [CrossRef]
- Gaab, M.E.; Lozano, P.O.; Ibañez, D.; Manese, K.D.; Riego, F.M.; Tiongco, R.E.; Albano, P.M. A Meta-Analysis on the Association of Colibactin-Producing pks+ Escherichia coli with the Development of Colorectal Cancer. Lab. Med. 2022, 54, 75–82. [Google Scholar] [CrossRef]
- Abe, E.; Ishikawa, K.; Onishi, K.; Mori, N. Anaerobic gram-negative rod bacteremia as a marker of gastrointestinal cancer in Japanese patients: A single-center retrospective study. Chin. Clin. Oncol. 2024, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Farkas, D.K.; Friis, S.; Sværke, C.; Ording, A.G.; Nørgaard, M.; Sørensen, H.T. Endocarditis and Risk of Cancer: A Danish Nationwide Cohort Study. Am. J. Med. 2013, 126, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Chiu, C.; Rayner, C.K.; Wu, K.; Chiu, Y.; Hu, M.; Chuah, S.; Tai, W.; Liang, C.; Wang, H. Associated factors in Streptococcus bovis bacteremia and colorectal cancer. Kaohsiung J. Med. Sci. 2016, 32, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Ursi, M.P.; Bertolino, L.; Andini, R.; D’Amico, F.; Iossa, D.; Karruli, A.; D’Avenia, E.; Manduca, S.; Bernardo, M.; Zampino, R.; et al. Enterococcal infective endocarditis is a marker of current occult or future incident colorectal neoplasia. Eur. J. Intern. Med. 2021, 83, 68–73. [Google Scholar] [CrossRef]
- Østergaard, L.; Dahl, A.; Chamat-Hedemand, S.; Stahl, A.; Pries-Heje, M.; Moser, C.; Søgaard, K.; Agergaard, C.; Mortensen, K.; Iversen, K.; et al. Prevalence of colorectal cancer in patients with Enterococcus faecalis blood stream infection: A nationwide study. J. Infect. 2025, 91, 106644. [Google Scholar] [CrossRef]
- Huang, W.; Chang, J.W.; See, L.; Tu, H.; Chen, J.; Liaw, C.; Lin, Y.; Yang, T. Higher rate of colorectal cancer among patients with pyogenic liver abscess with Klebsiella pneumoniae than those without: An 11-year follow-up study. Color. Dis. 2012, 14, e794–e801. [Google Scholar] [CrossRef]
- Suzuki, H.; Kidder, I.; Tanaka, T.; Goto, M. Incidence of Colorectal Cancer in Patients Diagnosed with Pyogenic Liver Abscess. JAMA Netw. Open 2023, 6, e2348218. [Google Scholar] [CrossRef]
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef]
- Li, D.; Jiang, S.-F.; Lei, N.Y.; Shah, S.C.; Corley, D.A. Effect of Helicobacter pylori Eradication Therapy on the Incidence of Noncardia Gastric Adenocarcinoma in a Large Diverse Population in the United States. Gastroenterology 2023, 165, 391–401.e2. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Liu, C.-J.; Chen, T.-J.; Chen, T.-L.; Yeh, Y.-C.; Wu, H.-S.; Tseng, C.-P.; Wang, F.-D.; Tzeng, C.-H.; Fung, C.-P. Pyogenic Liver Abscess as the Initial Manifestation of Underlying Hepatocellular Carcinoma. Am. J. Med. 2011, 124, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wozniak, A.; Cook, P.; Adaniel, C.; Acevedo, J.; Azócar, L.; Hsing, A.W.; Roa, J.C.; Pasetti, M.F.; Miquel, J.F.; et al. Salmonella enterica serovar Typhi and gallbladder cancer: A case–control study and meta-analysis. Cancer Med. 2016, 5, 3310–3325. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Zhan, P.; Suo, L.-J.; Qian, Q.; Shen, X.-K.; Qiu, L.-X.; Yu, L.-K.; Song, Y. Chlamydia pneumoniae infection and lung cancer risk: A meta-analysis. Eur. J. Cancer 2011, 47, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lin, C.; Liang, J.; Liu, S.; Sung, F.; Chang, Y.; Kao, C. Urinary tract infection increases subsequent urinary tract cancer risk: A population-based cohort study. Cancer Sci. 2013, 104, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.H.; Hanum, N.; Grotenhuis, A.J.; Castaño-Vinyals, G.; van der Heijden, A.G.; Aben, K.K.; Mysorekar, I.U.; Kiemeney, L.A. Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br. J. Cancer 2014, 112, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Talamini, R.; Negri, E.; Franceschi, S.; La Vecchia, C. Genital and urinary tract diseases and prostate cancer risk. Eur. J. Cancer Prev. 2006, 15, 254–257. [Google Scholar] [CrossRef]
- Jansåker, F.; Li, X.; Sundquist, K. Acute cystitis and subsequent risk of urogenital cancer: A national cohort study from Sweden. BMJ Public Health 2025, 3, e002495. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Huang, W.-Y.; Lin, K.-T.; Lin, C.-S.; Chao, H.-L.; Yang, J.-F.; Lin, C.-L.; Kao, C.-H. Lower Urinary Tract Infection and Subsequent Risk of Prostate Cancer: A Nationwide Population-Based Cohort Study. PLoS ONE 2017, 12, e0168254. [Google Scholar] [CrossRef]
- Caini, S.; Gandini, S.; Dudas, M.; Bremer, V.; Severi, E.; Gherasim, A. Sexually transmitted infections and prostate cancer risk: A systematic review and meta-analysis. Cancer Epidemiol. 2014, 38, 329–338. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Z.; Luo, H.; Zhang, W.; Zhu, X. Chlamydia Trachomatis Infection-Associated Risk of Cervical Cancer. Medicine 2016, 95, e3077. [Google Scholar] [CrossRef]
- McShane, C.M.; Murray, L.J.; Engels, E.A.; Landgren, O.; Anderson, L.A. Common community-acquired infections and subsequent risk of multiple myeloma: A population-based study. Int. J. Cancer 2013, 134, 1734–1740. [Google Scholar] [CrossRef]
- Rawla, P.; Vellipuram, A.R.; Bandaru, S.S.; Raj, J.P. Colon Carcinoma Presenting as Streptococcus anginosus Bacteremia and Liver Abscess. Gastroenterol. Res. 2017, 10, 376–379. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Su, S.-L.; Li, C.-W.; Tsai, C.-S.; Lo, C.-L.; Syue, L.-S.; Li, M.-C.; Lee, C.-C.; Lee, N.-Y.; Ko, W.-C.; et al. Pancreaticobiliary Cancers and Aeromonas Isolates Carrying Type III Secretion System Genes ascF-ascG Are Associated with Increased Mortality: An Analysis of 164 Aeromonas Infection Episodes in Southern Taiwan. Front. Cell. Infect. Microbiol. 2021, 11, 749269. [Google Scholar] [CrossRef]
- Melnick, S.; Nazir, S.; Chwiecko, B.; Lloyd, B. There may be more than meets the eye with Clostridium perfringens bacteremia. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Kim, Y.S.; Park, J.M.; Kim, M.K.; Park, Y.G.; Chi, K.C.; Lee, J.H.; Lim, H.M. Omental Actinomycosis Coexisting with Colon Cancer. J. Korean Surg. Soc. 2009, 77, S17–S21. [Google Scholar] [CrossRef]
- Snellings, R.; Matsuo, T.; Wurster, S.; Jiang, Y.; Tarrand, J.; Cho, S.-Y.; Kontoyiannis, D.P. P-2294. Listeriosis in cancer patients: Uncommon and protean. Open Forum Infect. Dis. 2025, 12, ofae631.2447. [Google Scholar] [CrossRef]
- Masood, U.; Sharma, A.; Lowe, D.; Khan, R.; Manocha, D. Colorectal Cancer Associated with Streptococcus anginosus Bacteremia and Liver Abscesses. Case Rep. Gastroenterol. 2016, 10, 769–774. [Google Scholar] [CrossRef]
- Justesen, U.S.; Nielsen, S.L.; Jensen, T.G.; Dessau, R.B.; Møller, J.K.; Coia, J.E.; Andersen, S.L.; Pedersen, C.; Gradel, K.O. Bacteremia with Anaerobic Bacteria and Association with Colorectal Cancer: A Population-based Cohort Study. Clin. Infect. Dis. 2022, 75, 1747–1753. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.-J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Littman, A.J.; White, E.; Jackson, L.A.; Thornquist, M.D.; Gaydos, C.A.; Goodman, G.E.; Vaughan, T.L. Chlamydia pneumoniae Infection and Risk of Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1624–1630. [Google Scholar] [CrossRef]
- Goto, T. Airway Microbiota as a Modulator of Lung Cancer. Int. J. Mol. Sci. 2020, 21, 3044. [Google Scholar] [CrossRef]
- Lee, S.H.; Sung, J.Y.; Yong, D.; Chun, J.; Kim, S.Y.; Song, J.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 2016, 102, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, H.; Liu, K.; Pan, H.; Wang, B.; Zhu, M.; Wang, J.; Wang, H.; Chen, H.; Shen, D.; et al. Microbiota profiles in the saliva, cancerous tissues and its companion paracancerous tissues among Chinese patients with lung cancer. BMC Microbiol. 2023, 23, 237. [Google Scholar] [CrossRef] [PubMed]
- Mook, P.; O’bRien, S.J.; Gillespie, I.A. Concurrent Conditions and Human Listeriosis, England, 1999–2009. Emerg. Infect. Dis. 2011, 17, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, P.; Jiao, G.; Lv, J.; Ma, C.; Song, X.; Zhang, J.; Wu, C.; Li, R.; Zhu, H. Mimicking uterine malignancy: Pelvic actinomycosis with giant uterine leiomyoma. IDCases 2021, 23, e00878. [Google Scholar] [CrossRef]
- Saramago, S.M.; Cominho, J.C.; Proença, S.S.M.; Conde, P.J.C.; Nunes, F.M.P. Pelvic Actinomycosis Mimicking Pelvic Malignancy. Rev. Bras. Hematol. Hemoter. 2019, 41, 463–466. [Google Scholar] [CrossRef]
- Čelešnik, H.; Potočnik, U. Peripheral Blood Transcriptome in Breast Cancer Patients as a Source of Less Invasive Immune Biomarkers for Personalized Medicine, and Implications for Triple Negative Breast Cancer. Cancers 2022, 14, 591. [Google Scholar] [CrossRef]
- Martinez-Usatorre, A.; Ciarloni, L.; Angelino, P.; Wosika, V.; Conforte, A.J.; Costa, S.S.F.; Durandau, E.; Monnier-Benoit, S.; Satizabal, H.F.; Despraz, J.; et al. Human blood cell transcriptomics unveils dynamic systemic immune modulation along colorectal cancer progression. J. Immunother. Cancer 2024, 12, e009888. [Google Scholar] [CrossRef]
- Qi, F.; Gao, F.; Cai, Y.; Han, X.; Qi, Y.; Ni, J.; Sun, J.; Huang, S.; Chen, S.; Wu, C.; et al. Complex Age- and Cancer-Related Changes in Human Blood Transcriptome—Implications for Pan-Cancer Diagnostics. Front. Genet. 2021, 12, 746879. [Google Scholar] [CrossRef]
- Marx, S.; Wilken, F.; Miebach, L.; Ispirjan, M.; Kinnen, F.; Paul, S.; Bien-Möller, S.; Freund, E.; Baldauf, J.; Fleck, S.; et al. Immunophenotyping of Circulating and Intratumoral Myeloid and T Cells in Glioblastoma Patients. Cancers 2022, 14, 5751. [Google Scholar] [CrossRef]
- Casbon, A.-J.; Reynaud, D.; Park, C.; Khuc, E.; Gan, D.D.; Schepers, K.; Passegué, E.; Werb, Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl. Acad. Sci. USA 2015, 112, E566–E575. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Wang, L.; Jia, B.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; Mineishi, S.; Naik, S.; Khawaja, M.R.; Sivik, J.; Han, J.; et al. VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. OncoImmunology 2018, 7, e1469594. [Google Scholar] [CrossRef]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T Cells Recruited through CCL22/CCR4 Are Selectively Activated in Lymphoid Infiltrates Surrounding Primary Breast Tumors and Lead to an Adverse Clinical Outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10. [Google Scholar] [CrossRef]
- Chaudhary, B.; Elkord, E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Blaschitz, C.; Raffatellu, M. Th17 Cytokines and the Gut Mucosal Barrier. J. Clin. Immunol. 2010, 30, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Martins, L.M.S.; Dias, M.S.; Pereira, C.A.; Leite, J.A.; Gonçalves, E.C.S.; de Almeida, P.Z.; de Freitas, E.N.; Tostes, R.C.; Ramos, S.G.; et al. Interleukin-17/interleukin-17 receptor axis elicits intestinal neutrophil migration, restrains gut dysbiosis and lipopolysaccharide translocation in high-fat diet-induced metabolic syndrome model. Immunology 2018, 156, 339–355. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Meyer, M.A.; Baer, J.M.; Knolhoff, B.L.; Nywening, T.M.; Panni, R.Z.; Su, X.; Weilbaecher, K.N.; Hawkins, W.G.; Ma, C.; Fields, R.C.; et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Reid, C.M.; Evans, J.D.; Murgai, M.; Vicioso, Y.; Highfill, S.L.; Kasai, M.; Vahdat, L.; Mackall, C.L.; Lyden, D.; et al. Activation of Hematopoietic Stem/Progenitor Cells Promotes Immunosuppression Within the Pre–metastatic Niche. Cancer Res. 2016, 76, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Sun, H.-W.; Chen, H.-T.; Liang, J.; Yu, X.-J.; Wu, C.; Wang, Z.; Zheng, L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl. Acad. Sci. USA 2014, 111, 4221–4226. [Google Scholar] [CrossRef] [PubMed]
- Ciarloni, L.; Hosseinian, S.; Monnier-Benoit, S.; Imaizumi, N.; Dorta, G.; Ruegg, C. On behalf of the DGNP-COL-0310 Study Group. Discovery of a 29-Gene Panel in Peripheral Blood Mononuclear Cells for the Detection of Colorectal Cancer and Adenomas Using High Throughput Real-Time PCR. PLoS ONE 2015, 10, e0123904. [Google Scholar] [CrossRef]
- Khouzam, R.A.; Zaarour, R.F.; Brodaczewska, K.; Azakir, B.; Venkatesh, G.H.; Thiery, J.; Terry, S.; Chouaib, S. The Effect of Hypoxia and Hypoxia-Associated Pathways in the Regulation of Antitumor Response: Friends or Foes? Front. Immunol. 2022, 13, 828875. [Google Scholar] [CrossRef]
- Kelly, C.J.; Glover, L.E.; Campbell, E.L.; Kominsky, D.J.; Ehrentraut, S.F.; Bowers, B.E.; Bayless, A.J.; Saeedi, B.J.; Colgan, S.P. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 2013, 6, 1110–1118. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef]
- Sagar, P.M.; MacFie, J.; Sedman, P.; May, J.; Mancey-Jones, B.; Johnstone, D. Intestinal obstruction promotes gut translocation of bacteria. Dis. Colon Rectum 1995, 38, 640–644. [Google Scholar] [CrossRef]
- Valvani, A.; Martin, A.; Devarajan, A.; Chandy, D. Postobstructive pneumonia in lung cancer. Ann. Transl. Med. 2019, 7, 357. [Google Scholar] [CrossRef]
- Ciernikova, S.; Novisedlakova, M.; Cholujova, D.; Stevurkova, V.; Mego, M. The Emerging Role of Microbiota and Microbiome in Pancreatic Ductal Adenocarcinoma. Biomedicines 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Nougayrède, J.-P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli Induces DNA Double-Strand Breaks in Eukaryotic Cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; Van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Orberg, E.T.; Geis, A.L.; Chan, J.L.; Fu, K.; Shields, C.E.D.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 203–214.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-G.; Hwang, S.; Gwon, S.-Y.; Park, C.; Jo, M.; Hong, J.-E.; Rhee, K.-J. Bacteroides fragilis Toxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 by the E-Cadherin/β-Catenin/NF-κB Dependent Pathway. Biomedicines 2022, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.; Heinbockel, L.; Brandenburg, K.; Hornef, M.W. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes 2014, 5, 761–765. [Google Scholar] [CrossRef]
- Dougherty, M.W.; Jobin, C. Intestinal bacteria and colorectal cancer: Etiology and treatment. Gut Microbes 2023, 15, 2185028. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2020, 33, 127–148. [Google Scholar] [CrossRef]
- Li, T.-T.; Ogino, S.; Qian, Z.R. Toll-like receptor signaling in colorectal cancer: Carcinogenesis to cancer therapy. World J. Gastroenterol. 2014, 20, 17699–17708. [Google Scholar] [CrossRef]
- Prasad, S.V.; Fiedoruk, K.; Daniluk, T.; Piktel, E.; Bucki, R. Expression and Function of Host Defense Peptides at Inflammation Sites. Int. J. Mol. Sci. 2019, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, Y.; Lv, Y.; Huang, C.; Sheng, Q.; Zhao, P.; Ye, J.; Jiang, W.; Liu, L.; Song, X.; et al. Clostridium difficile colonization in preoperative colorectal cancer patients. Oncotarget 2017, 8, 11877–11886. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.L.; Packham, A.; Sharkey, A.R.; Cook, G.J. Advanced Imaging for Detection of Foci of Infection in Staphylococcus aureus Bacteremia- Can a Scan Save Lives? Semin. Nucl. Med. 2023, 53, 175–183. [Google Scholar] [CrossRef]
- Vos, F.J.; Bleeker-Rovers, C.P.; Sturm, P.D.; Krabbe, P.F.; van Dijk, A.P.; Cuijpers, M.L.; Adang, E.M.; Wanten, G.J.; Kullberg, B.-J.; Oyen, W.J. 18F-FDG PET/CT for Detection of Metastatic Infection in Gram-Positive Bacteremia. J. Nucl. Med. 2010, 51, 1234–1240. [Google Scholar] [CrossRef]
- Jeong, S.W.; Jang, J.Y.; Lee, T.H.; Kim, H.G.; Hong, S.W.; Park, S.H.; Kim, S.G.; Cheon, Y.K.; Kim, Y.S.; Cho, Y.D.; et al. Cryptogenic pyogenic liver abscess as the herald of colon cancer. J. Gastroenterol. Hepatol. 2012, 27, 248–255. [Google Scholar] [CrossRef]
- Heo, N.-Y.; Hong, Y.M.; Kim, T.O.; Moon, Y.S.; Yang, S.Y.; Park, S.H.; Park, J.; Choi, J.H.; Kim, S.-M.; Yoon, K.T.; et al. The Prevalence of Colonic Neoplasm in Cryptogenic Pyogenic Liver Abscess: A Prospectively Enrolled Cross-sectional Study. Korean J. Gastroenterol. 2016, 68, 195–201. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Hafidh, R.R.; Abu Bakar, F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res. 2011, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Corredoira-Sanchez, J.; Garcia-Garrote, F.; Rabunal, R.; Lopez-Roses, L.; Garcia-Pais, M.J.; Castro, E.; Gonzalez-Soler, R.; Coira, A.; Pita, J.; Lopez-Alvarez, M.J.; et al. Association Between Bacteremia Due to Streptococcus gallolyticus subsp. gallolyticus (Streptococcus bovis I) and Colorectal Neoplasia: A Case-Control Study. Clin. Infect. Dis. 2012, 55, 491–496. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Mukherjee, S.; Nandi, S.; Bhuniya, S.; Tapadar, S.R.; Saha, M. A study on non-resolving pneumonia with special reference to role of fiberoptic bronchoscopy. Lung India 2013, 30, 27–32. [Google Scholar] [CrossRef]
- Lai, H.-C.; Lin, H.-C. Cryptogenic pyogenic liver abscess as a sign of colorectal cancer: A population-based 5-year follow-up study. Liver Int. 2010, 30, 1387–1393. [Google Scholar] [CrossRef]
- Bodilsen, J.; Søgaard, K.K.; Nielsen, H.; Omland, L.H. Brain Abscess and Risk of Cancer. Neurology 2022, 99, E835–E842. [Google Scholar] [CrossRef] [PubMed]
- You, M.S.; Lee, S.H.; Kang, J.; Choi, Y.H.; Choi, J.H.; Shin, B.-S.; Huh, G.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T.; et al. Natural Course and Risk of Cholangiocarcinoma in Patients with Recurrent Pyogenic Cholangitis: A Retrospective Cohort Study. Gut Liver 2019, 13, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Arab, T.; Malekzadegan, M.R.; Morante, J.; Cervellione, K.L.; Fein, A.M. Nonresolving pneumonia in the setting of malignancy. Curr. Opin. Pulm. Med. 2019, 25, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Venugopal, K.P.P.; Musthafa, S.; Narayan, K.V. Characterising aetiologies and clinical-radiological factors of non-resolving pneumonia in a tertiary care centre. Egypt. J. Bronchol. 2024, 18, 61. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Farkas, D.K.; Leisner, M.Z.; Schmidt, S.A.J.; Lash, T.L.; Sørensen, H.T. Fever of Unknown Origin and Incidence of Cancer. Clin. Infect. Dis. 2022, 75, 968–974. [Google Scholar] [CrossRef]
- Naito, T.; Tanei, M.; Ikeda, N.; Ishii, T.; Suzuki, T.; Morita, H.; Yamasaki, S.; Tamura, J.; Akazawa, K.; Yamamoto, K.; et al. Key diagnostic characteristics of fever of unknown origin in Japanese patients: A prospective multicentre study. BMJ Open 2019, 9, e032059. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, V.; Eslick, G.D. Systematic review with meta-analysis: The relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment. Pharmacol. Ther. 2014, 39, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wu, F.; Kwak, S.; Wang, C.; Usyk, M.; Freedman, N.D.; Huang, W.-Y.; Um, C.Y.; Gonda, T.A.; Oberstein, P.E.; et al. Oral Bacterial and Fungal Microbiome and Subsequent Risk for Pancreatic Cancer. JAMA Oncol. 2025, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Wang, C.; Usyk, M.; Wu, F.; Freedman, N.D.; Huang, W.-Y.; McCullough, M.L.; Um, C.Y.; Shrubsole, M.J.; Cai, Q.; et al. Oral Microbiome and Subsequent Risk of Head and Neck Squamous Cell Cancer. JAMA Oncol. 2024, 10, 1537–1547. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).