Simple Summary
Esophageal cancer is an aggressive malignancy associated with poor survival outcomes. The current standard for locally advanced disease combines chemotherapy and radiotherapy followed by surgery. In a subset of patients, this combined approach can achieve complete tumor regression on imaging and biopsies. For such patients, the necessity of proceeding with surgery remains uncertain, given the substantial risks and postoperative morbidity associated with esophagectomy. In our study, we compared patients who underwent surgery after achieving a complete response with those who were managed through structured surveillance. Our findings demonstrate that younger patients derived a significant survival advantage from surgery, whereas in older patients, surveillance was safe and sometimes associated with better outcomes. These results highlight the need for age-adapted treatment strategies in esophageal cancer, aiming to maximize survival benefits in younger patients while minimizing unnecessary risks in the elderly.
Abstract
Background/Objectives: Esophageal cancer (EC) remains highly lethal. The standard management of locally advanced disease includes neoadjuvant chemoradiotherapy (nCRT) followed by surgery. However, the role of esophagectomy in patients achieving clinical complete response (cCR) after nCRT remains uncertain. Methods: We conducted a retrospective study at the Davidoff Cancer Center, Rabin Medical Center (2013–2023). Patients with thoracic EC (adenocarcinoma and squamous cell carcinoma) stage cT2–4a, N+, M0 who received nCRT (cisplatin/5-FU or CROSS regimen with 41.4–50.4 Gy) were included. Patients with cCR, defined by negative biopsies, endoscopic ultrasound, and PET-CT, were managed with surgery or surveillance. Survival was analyzed using Kaplan–Meier and Cox regression. Results: Of 252 patients treated with nCRT, 118 achieved cCR. Seventy underwent surgery, with 47% (33 patients) achieving pathological complete response (pCR), and 48 were managed with surveillance. Five-year overall survival (OS) was 48% with surveillance and 49% with surgery; disease-free survival (DFS) was 36% vs. 43%. No significant differences were observed in OS (HR = 0.75, 95% CI 0.47–1.26) or DFS (HR = 0.88, 95% CI 0.55–1.41). In patients ≤70 years, surgery conferred an OS and DFS benefit (HR = 0.44, p = 0.03). No benefit was observed in patients >70 years, where outcomes trended against surgery. On multivariable analysis, older age (p = 0.005) and female sex (p = 0.007) were independent predictors of OS. Conclusions: In younger patients (≤70 years), surgery yielded significant survival benefit, supporting its role as the preferred treatment. In patients >70 years, surveillance produced comparable or superior outcomes, suggesting deferral of surgery may avoid morbidity without compromising survival. Age-specific tailoring of management is essential.
1. Introduction
Esophageal cancer (EC) is a highly lethal malignancy characterized by both local invasion and distant dissemination [1,2]. Globally, it ranks as the eighth most common cancer and the sixth leading cause of cancer-related mortality [3,4,5]. The standard treatment paradigm for locally advanced disease involves either neoadjuvant chemoradiotherapy (nCT) or perioperative chemotherapy, both followed by esophagectomy [6,7,8,9], resulting in 5-year overall survival (OS) rates of 36–60% [10,11,12,13,14,15]. Survival outcomes vary by histologic subtype, with patients with squamous cell carcinoma (SCC) achieving higher pathological complete response (pCR) rates and superior long-term survival—with 5-year OS rates approaching 50–60% following nCRT plus surgery, whereas adenocarcinoma demonstrates lower response rates and 5-year OS rates of roughly 35–45%, even with modern perioperative regimens such as FLOT [10,11,12,16,17,18]. The landmark CROSS trial established the superiority of nCT plus surgery over surgery alone, demonstrating improved 5-year OS (47% vs. 34%) and notable rates of pCR, particularly in SCC (49%) [10].
In recent years, several studies have questioned the necessity of esophagectomy in patients achieving a clinical complete response (cCR) following chemoradiotherapy, particularly among those with SCC [17,18]. A meta-analysis comparing chemoradiotherapy followed by active surveillance with standard esophagectomy demonstrated comparable OS [19]. Similarly, a recent prospective study reported no significant differences in survival or locoregional failure rates between surgery and active surveillance in patients achieving cCR after nCRT [20]. Consistent with these findings, a multicenter propensity-matched study observed no significant differences in 3-year OS and DFS between patients managed with active surveillance and those undergoing immediate surgery [21,22].
The SANO trial, which compared active surveillance with standard esophagectomy in patients achieving a cCR after nCRT, demonstrated non-inferior survival between the two groups [23]. Retrospective studies of clinically complete responders managed with either active surveillance or immediate surgery have similarly shown no association with higher rates of distant dissemination or more severe adverse outcomes when surgery was deferred [22,24,25]. Building on these findings, we aimed to conduct a broader real-world cohort analysis to complement the controlled setting of prospective trials. Accordingly, we performed a retrospective single-institution study comparing survival outcomes in patients with cCR following nCRT who either underwent surgery or were managed without surgical intervention.
2. Materials and Methods
Data were retrospectively collected for patients treated with curative intent for locally advanced esophageal cancer at the Davidoff Cancer Center, Rabin Medical Center, between January 2013 and December 2023. The inclusion criteria were as follows: (1) age 18 years or older, (2) thoracic squamous cell carcinoma or adenocarcinoma, (3) locally advanced stages, defined as cT2-4aN+M0 according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM classification, (4) cCR, defined by chest computed tomography (CT), upper gastrointestinal (GI) endoscopy with biopsies, and positron emission tomography (PET) scan results after nCRT, (5) who were treated with neoadjuvant chemoradiotherapy (nCRT) followed either by esophagectomy or by active surveillance. This study was conducted at a high-volume tertiary medical center specializing in complex surgical procedures, including esophagectomy. The gastroenterologists involved in patient evaluation and follow-up are among the most experienced in the country, are recognized national leaders in advanced endoscopy, and utilize state-of-the-art endoscopic technologies. Approximately three expert gastroenterologists with dedicated expertise in this field were involved in the management of these patients.
Exclusion criteria comprised cervical esophageal cancer, missing medical records, and metastatic disease at diagnosis.
The nCRT protocol included two chemotherapy regimens. The first consisted of two intravenous cycles of cisplatin (100 mg/m2) on days 1 and 29, combined with continuous 24-h infusion of 5-FU (800–1000 mg/m2) on days 1–4 and 29–32 of a 35-day cycle. The second regimen followed the CROSS protocol, consisting of weekly carboplatin (AUC 2 mg/mL/min) and paclitaxel (50 mg/m2) administered on days 1, 8, 15, 22, and 29. In the nCRT setting, radiotherapy was delivered concurrently to a total dose of 41.4 Gy in 23 fractions of 1.8 Gy each, with five fractions per week as per the CROSS protocol. For definitive chemoradiotherapy, the total dose was 50.4 Gy.
Restaging was performed four weeks after completion of nCRT with PET-CT imaging. Criteria for cCR included: (1) radiographic tumor resolution on CT, (2) a reduction in maximum standardized uptake value (SUVmax) >35% on PET-CT, and (3) absence of residual tumor on endoscopy with negative biopsy. Treatment strategies were subsequently discussed in a multidisciplinary tumor board comprising medical oncologists, radiation oncologists, surgeons, and radiologists. Patients deemed medically unfit for surgery due to significant comorbidities were managed with definitive chemoradiotherapy (50.4 Gy concurrent with chemotherapy). Additional patients who declined surgery after achieving cCR were also managed with active surveillance.
Patients in the active surveillance cohort underwent PET-CT and endoscopy every three months. Endoscopic findings suspicious for recurrence were biopsied for histological confirmation. Patients in the surgical cohort underwent esophagectomy with curative intent, using either open or minimally invasive techniques. Postoperative surveillance included PET-CT every three months during the first two years, every six months during the subsequent three years, and annually thereafter, combined with routine endoscopy. Biopsies were obtained for suspicious endoscopic findings. The follow-up protocol was consistent across both surveillance and surgical groups.
Clinical and histopathological data were retrieved from institutional databases, including demographic characteristics, the date of diagnosis, follow-up details, and the most recent clinic visit. Tumor histology, anatomical location, and TNM staging were recorded based on endoscopic ultrasound (EUS) and PET-CT assessments. Data on initiation of chemoradiotherapy, chemotherapy regimen, radiation dose and fields, surgical details, and pathological reports were collected. Follow-up information included PET-CT findings, recurrence dates, survival status, and date of last clinic visit.
Statistical Analysis
Baseline characteristics and postoperative outcomes were summarized as proportions (percentages) for categorical variables and as medians with interquartile ranges (IQR) for continuous variables. Continuous variables were compared using Student’s t-test, while categorical variables were analyzed using the chi-square (χ2) test. Fisher’s exact test was employed when comparing two categorical variables or when expected frequencies were low.
Median follow-up duration was estimated using the reverse Kaplan–Meier method, accounting only for patients who remained alive. Survival outcomes were analyzed using Kaplan–Meier curves for both the active surveillance and immediate surgery groups. Comparisons between groups were performed using the log-rank test, and results were expressed as hazard ratios (HRs) with 95% confidence intervals (CI). All statistical tests were two-sided, and a p-value < 0.05 was considered statistically significant. Analyses were performed using SAS software, version 4.9 (SAS Institute Inc., Cary, NC, USA).
3. Results
Baseline Characteristics
During the study period, 252 patients with locally advanced esophageal cancer were treated with nCRT. Among them, 118 achieved a cCR, including 64 patients (54%) with SCC and 54 patients (45%) with adenocarcinoma (AC). Of these 118 patients, 70 underwent esophagectomy, while 48 continued on active surveillance without surgery, primarily due to medical comorbidities or refusal of surgical intervention. Among the surgical cohort, 33 patients (47%) achieved a pathological complete response (pCR).
Among the 66 patients who underwent esophagectomy, postoperative complications were observed in several domains. Pulmonary complications occurred in 10 patients (15.1%), with pneumonia accounting for 15% of all reported complications. Respiratory failure was documented in 2 patients (3.0%). Anastomotic leakage was identified in 20% of surgical patients (13 of 66 patients). In addition, deep vein thrombosis (DVT) occurred in 3 patients (4.5%). These findings reflect the substantial morbidity associated with esophagectomy in this cohort.
Overall, 63 patients (53%) were male and 55 (46%) female (Table 1). Patients in the surveillance group were significantly older compared with those undergoing surgery (68% vs. 37.1%, p < 0.001). SCC was more frequent in the surveillance cohort (81.2% vs. 35.7%, p < 0.001). Advanced tumors (T3–T4) were common in both groups but more prevalent in the surgical group (90% vs. 75%, p = 0.04). The majority of patients in both groups had N1 nodal disease (66.6% surveillance vs. 68.5% surgery). A higher proportion of patients in the surveillance group received a definitive radiation dose, though this difference was not statistically significant (64.5% vs. 58.5%, p = 0.56).

Table 1.
Surgery versus surveillance.
With regard to neoadjuvant protocols, the CROSS regimen was administered in 47.9% of the surveillance group and 42.8% of the surgical group. Cisplatin plus 5-FU was administered in 41.6% of surveillance patients compared with 14.2% of surgical patients.
The 5-year OS rate was 48% in the surveillance group and 49% in the surgical group. 5-year DFS rates were 36% and 43%, respectively. Median OS was 2.57 years in the surveillance group and 2.63 years in the surgical group, and median DFS was 2.2 years in both groups.
During follow-up, 32 patients (68%) in the surveillance group died, 11 (23%) without evidence of disease (NED). In the surgical cohort, 37 patients (53%) died, including 5 (7%) NED.
No significant differences were observed in DFS (HR = 0.88, 95% CI 0.55–1.41, p = 0.25) or OS (HR = 0.75, 95% CI 0.47–1.26, p = 0.27) between the surgery and surveillance groups (Figure 1). Subgroup analyses by histology (AC vs. SCC) revealed no significant differences in either OS (p = 0.76 for AC, p = 0.35 for SCC) or DFS (p = 0.23 and p = 0.52, respectively) (Figure 2 and Figure 3).
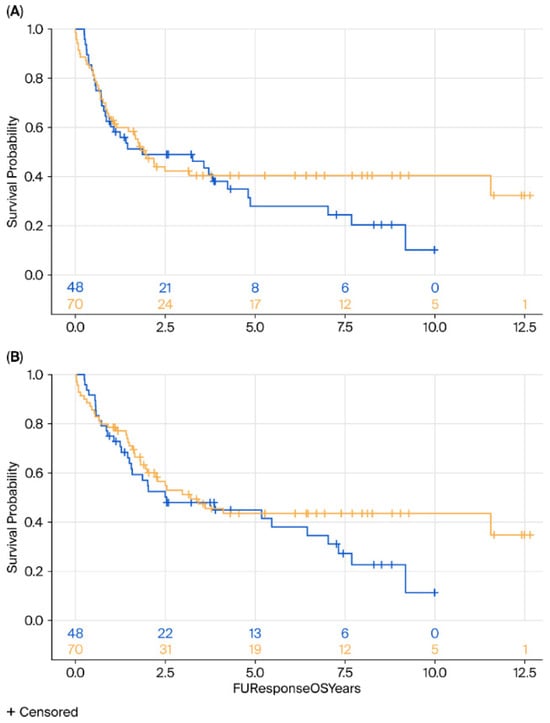
Figure 1.
Kaplan–Meier survival analysis of DFS (A) and OS (B) of surgery (yellow) vs. surveillance (blue) groups. DFS, disease-free survival; OS, overall survival.
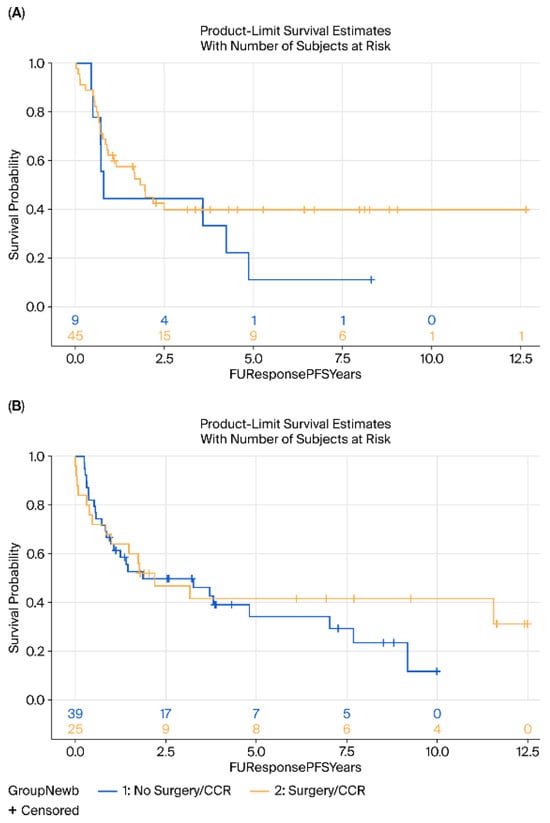
Figure 2.
Kaplan–Meier survival analysis of DFS (A) Adenocarcinoma and (B) Squamous cell carcinoma of surgery (yellow) vs. surveillance (blue) groups. DFS, disease-free survival.
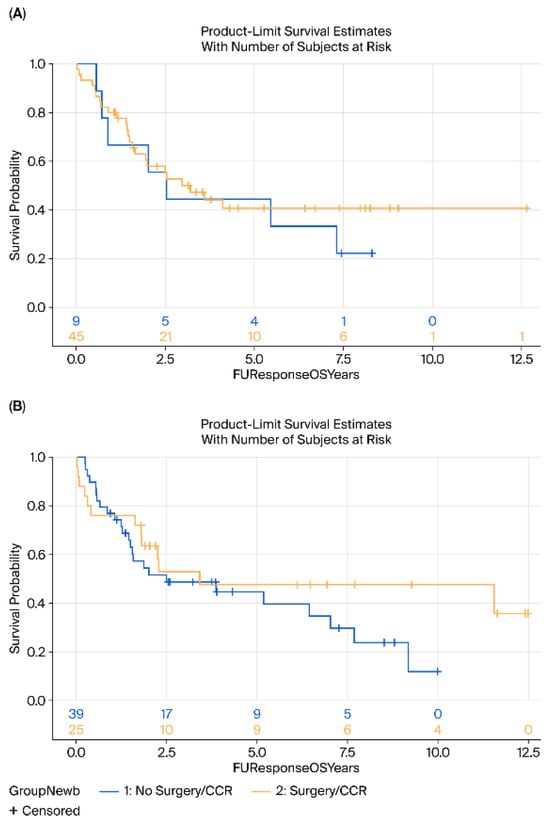
Figure 3.
Kaplan–Meier survival analysis of OS (A) Adenocarcinoma and (B) Squamous cell carcinoma of surgery (yellow) vs. surveillance (blue) groups. OS, Overall-survival.
When stratified by age, patients aged ≤70 years demonstrated a significant benefit from surgery compared with surveillance, with both improved disease-free survival DFS (HR = 0.44, 95% CI 0.20–0.90, p = 0.02) and OS (HR = 0.59, p = 0.032) (Figure 4).
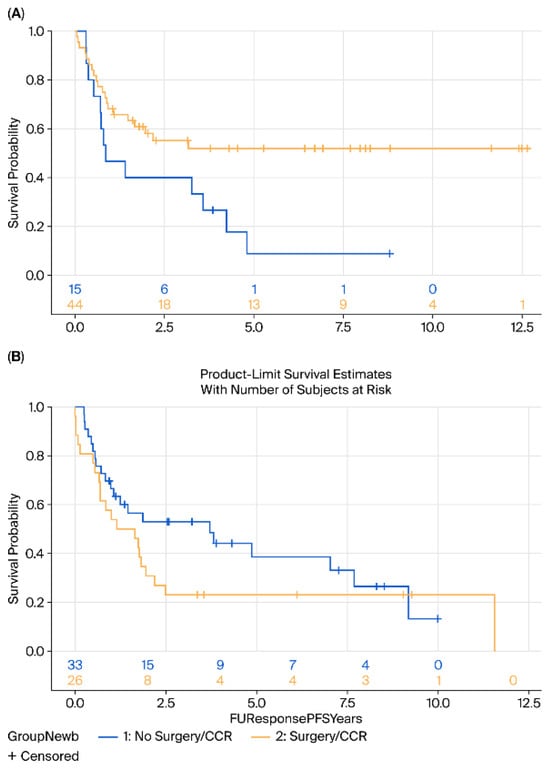
Figure 4.
Kaplan–Meier survival analysis of (A) DFS and (B) OS of patients aged 70 and younger comparing surgery (yellow) vs. surveillance (blue).
In contrast, among patients >70 years, no significant differences in OS (HR = 1.4, 95% CI 0.7–2.6, p = 0.25) or DFS (HR = 1.44, 95% CI 0.7–2.6, p = 0.20) were observed; indeed, a trend toward inferior outcomes with surgery was noted in this older cohort (Figure 5).
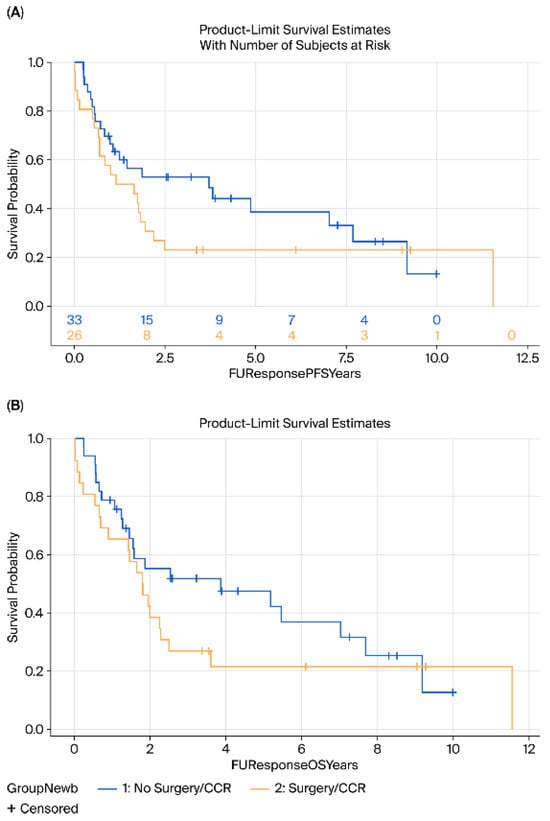
Figure 5.
Kaplan–Meier survival analysis of (A) DFS and (B) OS of patients above 70 years comparing surgery (yellow) vs. surveillance (blue).
In multivariable analysis, older age (p = 0.005) and female sex (p = 0.007) emerged as independent predictors of OS. No additional predictors were identified.
Recurrence rates were similar between groups, although local recurrences were more common in the surveillance cohort (12.5% vs. 8.5%). Overall recurrence rates were higher in the surveillance group (57.2% vs. 42.9%), though these differences did not reach statistical significance (p = 0.26).
4. Discussion
Neoadjuvant chemoradiotherapy (nCRT) has become the standard of care for treatment of locally advanced esophageal cancer, with landmark trials such as CROSS and NEOCRTEC5010 demonstrating improved overall and disease-free survival, as well as better local control, when nCRT is followed by surgery compared to surgery alone [10,26,27,28]. However, recent evidence has challenged the necessity of routine esophagectomy in all patients achieving a clinical complete response (cCR) after nCRT. Notably, retrospective studies and RCTs including the FFCD 9102 trial and analyses by van der Wilk et al. have shown no significant difference in OS between active surveillance and immediate surgery in selected patients with cCR, suggesting that non-operative management may be a viable alternative in appropriately selected individuals [19,29]. Furthermore, a meta-analysis evaluating the role of surgery in this setting indicated that nCRT alone was associated with superior survival outcomes compared with nCRT plus surgery, without significant differences in DFS [20].
This study aimed to assess whether active surveillance could be a safe alternative to surgery in patients with locally advanced esophageal cancer who achieved a cCR following chemoradiotherapy, without compromising survival outcomes. Our findings suggest that in patients with locally advanced esophageal cancer who achieve a cCR after chemoradiotherapy active surveillance may be a viable alternative to immediate surgery without compromising OS. Notably, in patients aged 70 years or younger, surgery was associated with a statistically significant improvement in DFS and OS compared with active surveillance.
The observation that the survival benefit associated with surgery in younger patients is linked with a corresponding improvement in OS, supports the consideration of surgery in younger patients. These findings underscore the importance of careful patient selection and clinical judgment when determining eligibility for non-operative management. Among patients older than 70 years, no differences in OS were observed between the surveillance and surgery groups. The benefit of surgery observed in younger patients may reflect selection bias, if younger individuals assigned to surveillance were, in fact, less fit for surgery—and their reduced fitness, rather than the lack of surgery, contributed to worse outcomes. Therefore, the observed effect may be attributable to confounding by indication rather than to the surgical intervention itself.
Our findings, showing no survival benefit from surgery in patients older than 70 years, are consistent with previous reports indicating that patients older than 75 years with SCC who achieved cCR did not derive additional benefit from surgery following chemoradiotherapy, whereas those with adenocarcinoma did demonstrate improved survival with surgical resection [30]. However, contrasting evidence from other studies suggests that trimodality therapy—combining nCRT with surgery—can significantly improve both OS and PFS compared with other treatment approaches, irrespective of patient age [31]. Therefore, this remains a controversial topic with no definitive conclusion. The key implication is that in an older patient population, a morbid surgical procedure can be avoided without compromising survival, ultimately preserving their quality of life. While several studies have compared nCRT followed by active surveillance vs. surgery, there is a lack of research specifically focusing on older patients. We believe that the findings of this study have significant implications for the management of this patient population, particularly for elderly patients, in whom surgery may potentially be avoided.
Among patients in the surgery group, a pathological complete response (pCR) was observed in 47%, indicating that current restaging modalities—such as CT, PET-CT, and endoscopic biopsy—underestimated residual disease in nearly half of cases. This discordance between cCR and pCR has been widely reported, with literature citing pCR rates among cCR patients ranging from 24% to 73%, likely due to heterogeneity in restaging approaches [12,17,18]. These findings highlight the need for standardized, rigorous restaging algorithms to more reliably identify true responders and guide treatment decisions. Recent evidence further suggests that even among patients achieving pCR, minimal residual disease may still be present when evaluated with advanced molecular or imaging-based techniques. A recent study demonstrated that circulating tumor DNA (ctDNA)-based monitoring can detect molecular residual disease in patients classified as pCR, identifying individuals at higher risk for recurrence despite histopathologic clearance. Such tools may complement conventional restaging and ultimately improve selection of candidates for active surveillance [32].
Local recurrence rates were higher in the active surveillance group compared with the surgery group, although this difference did not reach statistical significance. These findings are consistent with results from a systematic review and meta-analysis demonstrating no significant difference in distant metastatic recurrence between the two approaches, provided that cCR was confirmed and rigorous follow-up protocols were implemented [19]. Notably, the 5-year locoregional recurrence rate during active surveillance was approximately 40%, with 95% of patients undergoing successful R0 resection upon delayed surgery [19]. According to current literature, between 20 and 40% of patients who achieve a pCR following nCRT and surgery will experience disease recurrence, irrespective of histological subtype.
Current evidence suggests that while active surveillance may result in higher rates of locoregional recurrence compared to immediate surgery after chemoradiotherapy, the rates of distant metastatic relapse appear similar between groups [33]. Data from trials such as SANO and recent meta-analyses indicate that patients managed with active surveillance following a cCR experience local regrowth in approximately 40–50% of cases; however, most of these recurrences are amenable to timely salvage surgery, preserving overall survival outcomes [34,35]. In contrast, the rate of distant metastatic failure does not appear to differ significantly between active surveillance and immediate surgery cohorts, suggesting that surgery primarily affects local disease control rather than systemic progression. This suggests that there are three distinct patient groups that may benefit from active surveillance: (1) patients with aggressive tumors who ultimately experience distant metastatic failure and have therefore avoided unnecessary morbidity from surgery; (2) patients effectively cured by nCRT, eliminating the need for surgery entirely; and (3) patients who experience locoregional recurrence but benefit from an extended surgery-free interval compared to those undergoing immediate surgery. These findings underscore the importance of rigorous follow-up protocols in active surveillance strategies, allowing early detection and intervention for local recurrence while maintaining comparable rates of metastatic disease control relative to standard surgical approaches
Our study included patients with both adenocarcinoma and squamous cell carcinoma histologies, and we observed a substantial proportion of clinical complete responses across both groups. Notably, among patients with adenocarcinoma managed without surgery, survival outcomes were comparable to those of patients with squamous cell carcinoma undergoing active surveillance. These findings support the growing evidence that active surveillance may be a viable treatment strategy not only for squamous cell carcinoma—as is often assumed—but also for adenocarcinoma. It is important to note that current clinical practice for esophageal adenocarcinoma has shifted away from neoadjuvant chemoradiotherapy. Following the ESOPEC trial, perioperative chemotherapy with the FLOT regimen has emerged as the new standard of care, demonstrating superior overall and progression-free survival [16]. Consequently, this patient population is no longer the primary target for chemoradiotherapeutic strategies, as had been the case in the past. In the recently published SANO trial, two-thirds of patients with a cCR were found to have adenocarcinoma, with survival outcomes equivalent to those observed in the surgery arm. Our results therefore further support the consideration of active surveillance as a valid option in selected patients with adenocarcinoma.
Although our study is retrospective in nature, its findings are reinforced by a recently published prospective study, the SANO trial, that investigated the same clinical question and reached similar conclusions [23]. This concordance strengthens the overall validity of the observed association and highlights the robustness of the evidence. Furthermore, by including a broader, real-world cohort, the retrospective design enhances external validity and provides context to findings from randomized controlled trials.
This study has several important limitations. First, its retrospective, single-center, cohort study design introduces inherent methodological weaknesses, including selection bias, information bias, and limited internal validity. Known or unknown confounders may have influenced outcomes. Specifically, the study is subject to confounding by indication: patients managed with surveillance were not randomly assigned but were more likely to have baseline characteristics—such as comorbidities or frailty—that influenced both treatment allocation and outcomes. This inherent imbalance complicates causal interpretation of the observed outcomes, as patients who were older or had poorer baseline fitness were disproportionately assigned to the surveillance group, potentially influencing the results independently of the treatment strategy itself. Additionally, the relatively small sample size and limited follow-up duration reduce the statistical power to detect subtle but clinically meaningful differences and may also lead to unstable effect estimates, potentially exaggerating the true differences between treatment strategies. Finally, while this study adds to the growing body of evidence supporting active surveillance in selected patients, definitive conclusions require validation through prospective randomized controlled trials. The ongoing NEED trial is a phase III RCT assessing whether dCRT with salvage esophagectomy is non-inferior to nCRT with mandatory surgery for OS, and superior in HRQoL.
5. Conclusions
Surgery provides a survival benefit in younger patients with esophageal cancer who achieve clinical complete response after chemoradiotherapy, whereas surveillance is a safe and appropriate option for older patients, underscoring the need for age-adapted treatment strategies. Future prospective randomized studies are needed to validate age-adapted treatment strategies and to better define which patients may safely benefit from active surveillance. In addition, future research should incorporate standardized restaging criteria and advanced molecular or imaging biomarkers to more accurately identify true complete responders.
Author Contributions
Conceptualization, Y.K., Y.L. and E.G.; methodology, Y.K. and O.I.; software, T.M.; validation, T.M. and Y.K.; formal analysis, T.M.; investigation, E.G., M.L. and N.A.H.; resources, Y.K.; data curation, E.G. and Y.K.; writing—original draft preparation, E.G.; writing—review and editing, E.G., T.M. and Y.K.; visualization, T.M.; supervision, B.B. and D.L.; project administration, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Rabin Medical Center (protocol code 0636-23, approval date 27 August 2023).
Informed Consent Statement
Informed consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data underlying this study consist of retrospective, patient-level clinical information and cannot be shared publicly due to institutional and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| cCr | Clinical complete response |
| CT | Computed tomography |
| DFS | Disease-free survival |
| EC | Esophageal cancer |
| nCRT | Neoadjuvant chemoradiotherapy |
| OS | Overall survival |
| pCR | Pathological complete response |
| PET-CT | Positron emission tomography |
| SCC | Squamous cell carcinoma |
| AC | Adenocarcinoma |
References
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef] [PubMed]
- DiSiena, M.; Perelman, A.; Birk, J.; Rezaizadeh, H. Esophageal cancer: An updated review. South. Med. J. 2021, 114, 161–168. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Li, J.; Ma, S. History and current situation of neoadjuvant treatment for locally advanced esophageal cancer. Thorac. Cancer 2021, 12, 2293–2299. [Google Scholar] [CrossRef]
- Turgeman, I.; Ben-Aharon, I. Evolving treatment paradigms in esophageal cancer. Ann. Transl. Med. 2021, 9, 903. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Ajani, J.A.; Barthel, J.S.; Bentrem, D.J.; D’Amico, T.A.; Das, P.; Denlinger, C.S.; Wright, C.D. Esophageal and Esophagogastric Junction Cancers. J. Natl. Compr. Cancer Netw. 2011, 9, 830–887. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, P.C.; Ubink, I.; van der Horst, S.; Boonstra, J.J.; Voest, E.E.; Ruurda, J.P.; Borel Rinkes, I.H.; Wiezer, M.J.; Schipper, M.E.; Siersema, P.D.; et al. Safety, efficacy, and long-term follow-up evaluation of perioperative epirubicin, Cisplatin, and capecitabine chemotherapy in esophageal resection for adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Bolger, J.C.; Donohoe, C.L.; Lowery, M.; Reynolds, J.V. Advances in the curative management of oesophageal cancer. Br. J. Cancer 2022, 126, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Dermanis, A.A.; Kamarajah, S.K.; Tan, B. The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal Junction Adenocarcinomas. Cancers 2023, 15, 4741. [Google Scholar] [CrossRef]
- Kingma, B.F.; Kooij, C.D.; Ruurda, J.P.; van Hillegersberg, R. An update on developments in curative treatment for locally advanced esophageal cancer: A narrative review. Ann. Esophagus 2025, 8. [Google Scholar] [CrossRef]
- Hoeppner, J.; Brunner, T.; Schmoor, C.; Bronsert, P.; Kulemann, B.; Claus, R.; Utzolino, S.; Izbicki, J.R.; Gockel, I.; Gerdes, B.; et al. Perioperative Chemotherapy or Preoperative Chemoradiotherapy in Esophageal Cancer. N. Engl. J. Med. 2025, 392, 323–335. [Google Scholar] [CrossRef]
- Agoston, A.T.; Zheng, Y.; Bueno, R.; Lauwers, G.Y.; Odze, R.D.; Srivastava, A. Predictors of disease recurrence and survival in esophageal adenocarcinomas with complete response to neoadjuvant therapy. Am. J. Surg. Pathol. 2015, 39, 1085–1092. [Google Scholar] [CrossRef]
- Alnaji, R.M.; Du, W.; Gabriel, E.; Singla, S.; Attwood, K.; Nava, H.; Malhotra, U.; Hochwald, S.N.; Kukar, M. Pathologic complete response is an independent predictor of improved survival following neoadjuvant chemoradiation for esophageal adenocarcinoma. J. Gastrointest. Surg. 2016, 20, 1541–1546. [Google Scholar] [CrossRef]
- van der Wilk, B.J.; Eyck, B.M.; Hofstetter, W.L.; Ajani, J.A.; Piessen, G.; Castoro, C.; Alfieri, R.; Kim, J.H.; Kim, S.B.; Furlong, H.; et al. Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: A systematic review and individual patient data meta-analysis. Ann. Surg. 2022, 275, 467–476. [Google Scholar] [CrossRef]
- Park, S.R.; Yoon, D.H.; Kim, J.H.; Kim, Y.H.; Kim, H.R.; Lee, H.J.; Jung, H.Y.; Lee, G.H.; Song, H.J.; Kim, D.H.; et al. A randomized phase III trial on the role of Esophagectomy in Complete Responders to Preoperative Chemoradiotherapy for Esophageal Squamous Cell Carcinoma (ESOPRESSO). Anticancer Res. 2019, 39, 5123–5133. [Google Scholar] [CrossRef]
- Park, J.; Yea, J.W.; Oh, S.A.; Park, J.W. Omitting surgery in esophageal cancer patients with complete response after neoadjuvant chemoradiotherapy: A systematic review and meta-analysis. Radiat. Oncol. 2021, 16, 219. [Google Scholar] [CrossRef]
- van der Wilk, B.J.; Noordman, B.J.; Neijenhuis, L.K.A.; Nieboer, D.; Nieuwenhuijzen, G.A.P.; Sosef, M.N.; van Berge Henegouwen, M.I.; Lagarde, S.M.; Spaander, M.C.W.; Valkema, R.; et al. Active Surveillance Versus Immediate Surgery in Clinically Complete Responders After Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A multicenter propensity matched study. Ann. Surg. 2021, 274, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Eyck, B.M.; van der Wilk, B.J.; Noordman, B.J.; Wijnhoven, B.P.L.; Lagarde, S.M.; Hartgrink, H.H.; Coene, P.P.L.O.; Dekker, J.W.T.; Doukas, M.; van der Gaast, A.; et al. Updated protocol of the SANO trial: A stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials 2021, 22, 345. [Google Scholar] [CrossRef] [PubMed]
- Castoro, C.; Scarpa, M.; Cagol, M.; Alfieri, R.; Ruol, A.; Cavallin, F.; Michieletto, S.; Zanchettin, G.; Chiarion-Sileni, V.; Corti, L.; et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: Is surgery always necessary? J. Gastrointest. Surg. 2013, 17, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Furlong, H.; Bass, G.; Breathnach, O.; O’Neill, B.; Leen, E.; Walsh, T.N. Targeting therapy for esophageal cancer in patients aged 70 and over. J. Geriatr. Oncol. 2013, 4, 107–113. [Google Scholar] [CrossRef]
- Tepper, J.; Krasna, M.J.; Niedzwiecki, D.; Hollis, D.; Reed, C.E.; Goldberg, R.; Kiel, K.; Willett, C.; Sugarbaker, D.; Mayer, R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 2008, 26, 1086–1092. [Google Scholar] [CrossRef]
- Stahl, M.; Stuschke, M.; Lehmann, N.; Meyer, H.J.; Walz, M.K.; Seeber, S.; Klump, B.; Budach, W.; Teichmann, R.; Schmitt, M.; et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 2005, 23, 2310–2317. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A phase III multicenter randomized open-label clinical trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef]
- Bedenne, L.; Michel, P.; Bouché, O.; Milan, C.; Mariette, C.; Conroy, T.; Pezet, D.; Roullet, B.; Seitz, J.F.; Herr, J.P.; et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 2007, 25, 1160–1168. [Google Scholar] [CrossRef]
- Koëter, M.; van Putten, M.; Verhoeven, R.H.A.; Lemmens, V.E.P.P.; Nieuwenhuijzen, G.A.P. Definitive chemoradiation or surgery in elderly patients with potentially curable esophageal cancer in the Netherlands: A nationwide population-based study on patterns of care and survival. Acta Oncol. 2018, 57, 1192–1200. [Google Scholar] [CrossRef]
- Linde, P.; Mallmann, M.; Adams, A.; Wegen, S.; Rosenbrock, J.; Trommer, M.; Marnitz, S.; Baues, C.; Celik, E. Chemoradiation for elderly patients (≥65 years) with esophageal cancer: A retrospective single-center analysis. Radiat. Oncol. 2022, 17, 187. [Google Scholar] [CrossRef]
- Panday, S.S.G.; van Klaveren, D.; Lagarde, S.M.; Lingsma, H.F.; Mostert, B.; Coene, P.-P.L.O.; Dekker, J.W.T.; Hartgrink, H.H.; Heisterkamp, J.; Hutteman, M.; et al. Accuracy of Predicting Residual Disease and Disease Progression During Active Surveillance for Esophageal Cancer. Ann. Surg. Oncol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Merritt, R.E.; Whyte, R.I.; D’Arcy, N.T.; Hoang, C.D.; Shrager, J.B. Morbidity and mortality after esophagectomy following neoadjuvant chemoradiation. Ann. Thorac. Surg. 2011, 92, 2034–2040. [Google Scholar] [CrossRef]
- D’Journo, X.B.; Boulate, D.; Fourdrain, A.; Loundou, A.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; O’Neill, J.R.; Hoelscher, A.; Piessen, G.; van Lanschot, J.; et al. Risk prediction model of 90-day mortality after esophagectomy for cancer. JAMA Surg. 2021, 156, 836–845. [Google Scholar] [CrossRef]
- van Nieuw Amerongen, M.P.; de Grooth, H.J.; Veerman, G.L.; Ziesemer, K.A.; van Berge Henegouwen, M.I.; Tuinman, P.R. Prediction of morbidity and mortality after esophagectomy: A systematic review. Ann. Surg. Oncol. 2024, 31, 3459–3470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).