Simple Summary
Immune checkpoint inhibitor-based combinations have recently become the standard first-line therapy for unresectable hepatocellular carcinoma. However, some patients derive little or no benefit from the outset, while others who initially achieve disease control later experience early progression. Reliable markers to predict these outcomes remain scarce. This study focused on two widely used tumor markers in clinical practice: alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP). We analyzed patients treated with atezolizumab plus bevacizumab and found that early changes in these markers at weeks 3 and 9 accurately predicted both primary progression and early progression after initial disease control. These findings suggest that simple blood tests can support biomarker-based responder selection, providing actionable guidance on whether to continue atezolizumab plus bevacizumab, switch to alternative therapies, or integrate locoregional approaches. Such biomarker-driven adaptive strategies may help personalize treatment and improve outcomes in patients with unresectable hepatocellular carcinoma.
Abstract
Background/Objectives: Immune checkpoint inhibitor (ICI)-based combinations are the standard first-line therapy for unresectable hepatocellular carcinoma (HCC). A major challenge is the early identification of patients with primary progression (1st-PD) and those who experience early progression despite initial disease control (2nd-PD). This study evaluated whether very early treatment changes in alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) could serve as predictors of treatment response during atezolizumab plus bevacizumab (Atz + Bev) therapy. Methods: A total of 147 patients treated with Atz + Bev were retrospectively analyzed. Serum tumor markers were measured approximately every 3 weeks, and radiologic responses were assessed using the Response Evaluation Criteria in Solid Tumors version 1.1 at week 6 (first evaluation) and again at a median of 14.8 weeks (second evaluation). Results: At the first evaluation, 32 patients achieved a partial response, 81 showed stable disease, and 25 had progression. In the week 3 landmark analysis, early increases in AFP (ratio ≥ 1.4) or DCP (ratio ≥ 1.0) identified patients who would experience primary radiologic progression, with a clear separation in landmark progression-free survival (PFS) (3.4 vs. 13.1 months; p < 0.001). Among the 109 patients with disease control at week 6, 92 maintained control and 17 progressed at the second evaluation. In the week 9 landmark cohort, modest rises in AFP (ratio ≥ 1.1) or DCP (ratio ≥ 1.5) identified individuals at risk for early secondary progression, again showing marked differences in landmark PFS (3.8 vs. 14.0 months; p < 0.001). Conclusions: The dynamic monitoring of AFP and DCP provides a simple framework for biomarker-based responder selection and adaptive treatment optimization during Atz + Bev therapy. Clinically actionable thresholds at weeks 3 and 9 may support timely treatment switching and the integration of locoregional strategies, enabling personalized, biomarker-guided management to improve outcomes in unresectable HCC.
1. Introduction
Systemic therapy for unresectable hepatocellular carcinoma (HCC) has advanced markedly in recent years, with immune checkpoint inhibitor (ICI)-based combinations [1,2,3] now established as the standard first-line treatment [4,5,6]. A major clinical challenge, however, is the early identification of patients who show primary progression (1st-PD)—that is, disease progression at the first radiological assessment—so that ineffective therapy can be avoided and subsequent treatment introduced without delay. In this context, the concept of biomarker-based responder selection, using early on-treatment biomarkers to distinguish likely responders from non-responders, has gained increasing attention as a strategy to optimize treatment sequencing and preserve therapeutic opportunities [7].
In addition, a substantial subset of patients who initially achieve disease control (DC) at the first evaluation later display progression at the second evaluation, termed early progression among patients with initial disease control (2nd-PD). Current treatment strategies with ICI-based regimens are increasingly exploring the integration of locoregional therapy with systemic immunotherapy [5,8], even in patients with stable disease (SD), in order to augment antitumor immunity before progression occurs. Identifying patients at risk of 2nd-PD despite initial DC is, therefore, of growing clinical importance, particularly for optimizing treatment sequencing.
Atezolizumab plus bevacizumab (Atz + Bev) has emerged as the predominant first-line ICI-based regimen in contemporary real-world practice following landmark clinical trials [1] and constituted the therapeutic backbone of the present analysis. Concurrently, alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) continue to function as key serum biomarkers in the clinical evaluation and longitudinal monitoring of HCC [9,10], yet reliable biomarkers for predicting treatment response in systemic therapy remain undefined. Recent studies have demonstrated the usefulness of early on-treatment changes in AFP and DCP, particularly around the first radiological evaluation (approximately 6 weeks), for predicting primary progression (1st-PD) [11,12,13,14,15,16]. However, whether these tumor marker dynamics retain predictive value at subsequent evaluations—such as the second imaging assessment—remains unclear.
This study, therefore, aimed to evaluate the predictive value of dynamic changes in AFP and DCP ratios during Atz + Bev therapy in patients with unresectable HCC. Specifically, we investigated the ability of these markers to predict both 1st-PD and 2nd-PD to determine whether serial tumor marker monitoring could serve as a practical tool for biomarker-based responder selection and adaptive treatment optimization in real-world clinical practice.
2. Materials and Methods
2.1. Patients
Between October 2020 and April 2025, a total of 151 patients diagnosed with unresectable HCC and deemed unsuitable for surgical resection or locoregional therapy received Atz + Bev at our institution. Eligibility criteria were as follows: imaging- or biopsy-confirmed HCC; Barcelona Clinic Liver Cancer (BCLC) stage C or stage B unsuitable for surgery or locoregional therapy; Eastern Cooperative Oncology Group (ECOG) performance status 0–1 (or stable 2); and Child–Pugh score ≤ 7. Prior locoregional therapy, including TACE, and prior systemic therapy were allowed if hepatic function had recovered. Overall, 4 patients with less than six weeks of follow-up were excluded, leaving 147 patients for analysis.
2.2. Treatment and Adverse Events
Atezolizumab (1200 mg) and bevacizumab (15 mg/kg) were infused every three weeks. Safety was evaluated using the Common Terminology Criteria for Adverse Events, version 5.0 [17]. If grade ≥ 3 toxicity attributable to treatment developed, administration of the responsible agent(s) was paused and resumed only after improvement to grade 1 or 2. Therapy was maintained unless significant adverse events occurred, disease progression was confirmed, or patients elected to discontinue treatment.
2.3. Radiological Assessment
Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors, version 1.1 [18]. Contrast-enhanced computed tomography was performed at baseline, at approximately 6 weeks after initiation of therapy (first evaluation), and thereafter at intervals of 4–10 weeks based on clinical necessity. At the first evaluation (at week 6), patients with complete response (CR), partial response (PR), or SD were classified as the first disease control group (1st-DC), while those with progressive disease (PD) were classified as the 1st-PD group. The second evaluation was defined as the next imaging after the first evaluation and was analyzed using a landmark design restricted to the 1st-DC group.
Patients who were censored before the week 6 evaluation were included in the overall cohort but were not eligible for the week 6 landmark PFS analysis, as they had not reached the predefined landmark time point.
For the second landmark analysis (week 9), only patients who achieved disease control (CR/PR/SD) at the week 6 evaluation and had both AFP and DCP measurements available at approximately week 9 were included. Among the 1st-DC patients, four were excluded from the second evaluation because of insufficient biomarker data or loss to follow-up (including transfer to another institution). Therefore, the week 9 analysis was performed using the remaining patients with complete paired marker data.
2.4. Handling of Missing Data and Non-Evaluable (NE) Cases
Missing tumor marker values were not imputed. Patients with very low baseline AFP or DCP levels were excluded from ratio-based analyses because percentage changes cannot be reliably interpreted near the lower detection limit.
Patients who discontinued treatment before the first radiologic assessment due to adverse events or a deterioration of general condition and, therefore, lacked evaluable imaging were classified as non-evaluable (NE). These NE cases were excluded from response-based subgroup analyses (1st-DC and 1st-PD) but were included in the overall survival (OS) and progression-free survival (PFS) analyses until censoring.
2.5. Tumor Marker Measurement and Analysis
Serum AFP, DCP, and lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3) were measured at baseline and approximately every three weeks during treatment. Patients with baseline AFP ≥ 10 ng/mL, DCP ≥ 40 mAU/mL, or AFP-L3 ≥ 0.5% were included in tumor marker analyses. To evaluate longitudinal changes, each value was normalized to the baseline level (set as 1.0), and the ratio at each time point was calculated. These cutoff values (AFP ≥ 10 ng/mL and DCP ≥ 40 mAU/mL) are conventionally used thresholds indicating clinically meaningful elevations and have been adopted in prior studies evaluating early on-treatment biomarker dynamics in HCC [14]. Patients below these thresholds were excluded from ratio-based analyses to avoid disproportionate percentage changes near the detection limit.
2.6. Statistical Analysis
All statistical analyses were performed using EZR version 1.29 (built on R version 3.6.3; Saitama Medical Center, Jichi Medical University, Saitama, Japan) [19]. Progression-free survival (PFS), overall survival (OS), and treatment duration were estimated using the Kaplan–Meier method, and intergroup differences were compared with the log-rank test. In the overall cohort, PFS was defined as the time from initiation of Atz + Bev to radiologic progression or death, whichever occurred first. For landmark analyses, PFS was redefined from the corresponding landmark time points. Specifically, for the week 3 landmark analysis, PFS was measured from the date of the week 3 assessment among patients who remained on treatment and had evaluable AFP and DCP at week 3. For the week 9 landmark analysis, PFS was measured from the week 9 landmark time point among patients in the 1st-DC group who had complete AFP and DCP measurements at approximately week 9. These landmark approaches were adopted to avoid immortal time bias. Categorical variables were examined using Fisher’s exact test. A two-sided p-value < 0.05 was considered statistically significant.
The predictive performance of AFP and DCP ratios for identifying primary progression (1st-PD at week 6) and secondary progression (2nd-PD at the second evaluation) was evaluated using receiver operating characteristic curve analysis. The area under the curve was calculated, and optimal cutoff values were derived using the Youden index, along with sensitivity and specificity. For the second evaluation analysis, only patients who achieved 1st-DC at week 6 and had both AFP and DCP available at week 9 were included (landmark design). Kaplan–Meier’s analyses of PFS were performed from the landmark time point. Multivariate Cox’s proportional hazards models were constructed at both the week 3 and week 9 landmark time points to evaluate whether early AFP and DCP dynamics were independently associated with PFS. In these models, AFP and DCP ratios were entered as continuous covariates, and the analyses were adjusted for key clinical factors, including BCLC stage, ECOG performance status, the Child–Pugh score, and treatment line. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The proportional hazards assumption was checked using Schoenfeld’s residuals, and a p-value of <0.05 was considered statistically significant. Because AFP, DCP, and AFP-L3 were considered biologically independent markers, no multiplicity correction was applied to p-values. Follow-up was defined from the initiation of Atz + Bev therapy until death or last observation.
2.7. Ethical Considerations
This study was approved by the Ethics Committee of Fujita Health University (approval no. HM24-418) and conducted in accordance with the Declaration of Helsinki (1975, as revised in 2013) [20]. Written informed consent for Atz + Bev treatment was obtained from all patients; the requirement for additional consent for study participation was waived by the Ethics Committee due to the retrospective nature of the study.
3. Results
3.1. Baseline Characteristics
The baseline characteristics of the 147 patients with unresectable HCC treated with Atz + Bev are summarized in Table 1. The median age was 74 years (range, 38–90), and 119 patients (81.0%) were male. Underlying disease etiology included hepatitis B virus infection in 18 patients, hepatitis C virus infection in 29, and non-viral etiologies in 100. The Eastern Cooperative Oncology Group performance status was 0 in 112 patients, 1 in 29, and 2 in 6, while the Child–Pugh scores were 5 in 97 patients, 6 in 37, and 7 in 13.

Table 1.
Baseline characteristics of patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab (Atz + Bev).
According to the Barcelona Clinic Liver Cancer (BCLC) classification, 3 patients were stage A, 65 were stage B, and 79 were stage C. Regarding tumor markers, the median AFP level was 50.1 ng/mL (range, 1.8–2,037,310), and 96 patients (65.3%) had AFP ≥ 10 ng/mL. The median DCP level was 613 mAU/mL (range, 10–403,328), with 121 patients (82.3%) showing DCP ≥ 40 mAU/mL. The median AFP-L3 was 16.4% (range, <0.5–99.6), and 117 patients (79.6%) had AFP-L3 ≥ 0.5%.
Atz + Bev was administered as first-line therapy in 106 patients, as second-line in 38, and as third- or later-line therapy in 3. The median observation period was 14.5 months (range, 0.63–53.6).
3.2. Overall Treatment Outcomes
Of the 147 patients, the median OS was 21.0 months (95% confidence interval [CI] 17.0–24.6 months), the median PFS was 8.7 months (95% CI 6.7–10.6 months), and the median treatment duration was 6.6 months (95% CI 4.3–7.8 months). The best overall tumor response by RECIST v1.1 was CR in 2 patients (1.4%), PR in 47 (32.0%), SD in 65 (44.2%), PD in 25 (17.0%), and not evaluable (NE) in 9 (6.1%), yielding an overall response rate (ORR) of 33.3% and a disease control rate (DCR) of 77.6%.
3.2.1. Tumor Response at First Evaluation (6 Weeks)
After 6 weeks, radiological evaluation was performed with 138 patients, showing PR in 32 (21.8%), SD in 81 (55.1%), and PD in 25 (17.0%) (Figure 1). The remaining nine patients (6.1%) were NE, mainly due to treatment discontinuation following AEs or a deterioration of general condition, and were excluded from the analyses. Patients with PR or SD were classified as the 1st-DC group (n = 113), and those with PD as the 1st-PD group (n = 25).
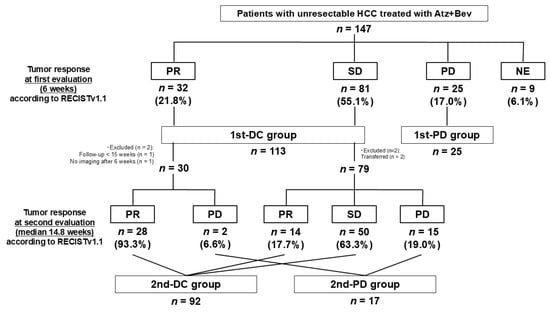
Figure 1.
Patient flow diagram and antitumor response during Atz + Bev therapy.
A total of 147 patients with unresectable HCC were enrolled. At the first radiologic evaluation (week 6), the responses were partial response (PR) in 32 patients, stable disease (SD) in 81, progressive disease (PD) in 25, and not evaluable (NE; mainly due to treatment discontinuation or deterioration) in 9. Patients with PR or SD were classified as the 1st-DC group (n = 113) and those with PD as the 1st-PD group (n = 25). Among the 1st-DC group, 109 proceeded to the second evaluation (median 14.8 weeks): 92 achieved disease control (2nd-DC) and 17 progressed (2nd-PD). Four patients were excluded due to short follow-up or missing data.
3.2.2. Tumor Response at Second Evaluation (Median 14.8 Weeks)
Among the 113 patients in the 1st-DC group, 4 were excluded from the second evaluation for the reasons shown in Figure 1, leaving 109 patients for assessment. The second evaluation was conducted at a median of 14.8 weeks (interquartile range, 14.7–15.7), and all patients were evaluable with no NE cases observed. Of the 30 patients who achieved PR at the first evaluation, 28 maintained PR and 2 experienced PD at the second evaluation. Among the 79 patients who achieved SD at the first evaluation, 14 converted to PR, 50 remained SD, and 15 developed PD. Patients with CR, PR, or SD at the second evaluation were classified as the 2nd-DC group, while those with PD were classified as the 2nd-PD group. As a result, 92 patients were categorized as 2nd-DC and 17 as 2nd-PD.
3.2.3. Tumor Marker Dynamics (1st-DC vs. 1st-PD)
As shown in Table 2 and Figure 2, serum AFP and DCP ratios began to diverge early between the 1st-DC and 1st-PD groups, with significant differences already evident at week 3 (AFP, p = 0.0006; DCP, p = 0.0046) and further accentuated at week 6. These results indicate that dynamic changes in the ratios at week 3 serve as a practical biomarker-guided tool for predicting primary non-response, while the differences at week 6 reflect consistency with radiological outcomes. In contrast, the AFP-L3 ratio showed no significant differences between the two groups.

Table 2.
Tumor marker ratios at weeks 3 and 6 (1st-DC vs. 1st-PD).
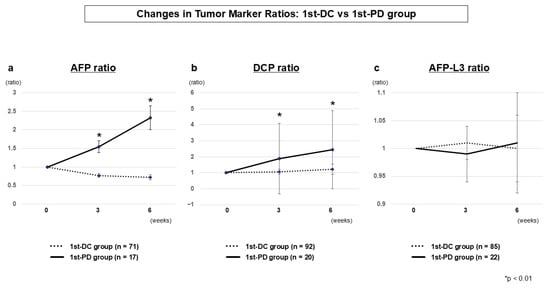
Figure 2.
Tumor marker dynamics at the first evaluation (1st-DC vs. 1st-PD) AFP ratio (a), DCP ratio (b), and AFP-L3 ratio (c). AFP and DCP ratios diverged significantly between groups as early as week 3, with clearer separation at week 6. AFP-L3 ratio showed no significant differences.
3.2.4. Tumor Marker Dynamics (2nd-DC vs. 2nd-PD)
Among patients achieving 1st-DC, AFP and DCP ratios clearly differentiated those who subsequently maintained disease control (2nd-DC) from those who experienced progression (2nd-PD). Significant differences were already evident at week 9 (AFP, p = 0.0035; DCP, p = 0.0010) and continued to widen at weeks 12 and 15 (Table 3 and Figure 3). From a clinical perspective, week 9 provides the most actionable time point to anticipate early progression despite initial disease control. The AFP-L3 ratio again showed no significant differences between the two groups.

Table 3.
Tumor marker ratios at weeks 3, 6, 9, 12, and 15 (2nd-DC vs. 2nd-PD).
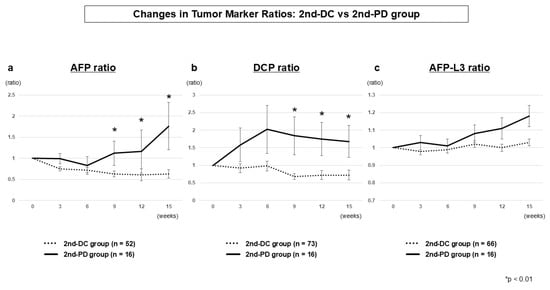
Figure 3.
Tumor marker dynamics at second evaluation (2nd-DC vs. 2nd-PD) AFP ratio (a), DCP ratio (b), and AFP-L3 ratio (c). AFP and DCP ratios diverged significantly from week 9 onward, whereas AFP-L3 ratio showed no significant differences.
3.3. Predictive Performance of Tumor Marker Ratios
We focused on week 3 as the earliest clinically relevant time point for predicting 1st-PD and on week 9 as a landmark time point for predicting 2nd-PD among patients who achieved 1st-DC. These time points were selected a priori to provide actionable clinical information between baseline/first evaluation and the second evaluation.
At the week 3 landmark, ROC analysis identified an AFP ratio ≥1.4 or a DCP ratio ≥ 1.0 as practical thresholds for predicting 1st-PD, with a high sensitivity (0.93) and a negative predictive value (0.97) (Table S1). In the landmark PFS analysis starting at week 3, patients meeting either cutoff showed significantly shorter PFS compared with those below both thresholds (median PFS from the week 3 landmark: 3.4 vs. 13.1 months; p < 0.001) (Figure 4a). For the week 9 landmark analysis in the 1st-DC cohort, an AFP ratio ≥ 1.1 or a DCP ratio ≥ 1.5 provided the best discrimination for predicting 2nd-PD, again with a high sensitivity (0.93) and a negative predictive value (0.97) (Table S2). Patients above either cutoff experienced markedly shorter PFS than those below both thresholds (median PFS from the week 9 landmark: 3.8 vs. 14.0 months; p < 0.001) (Figure 4b).
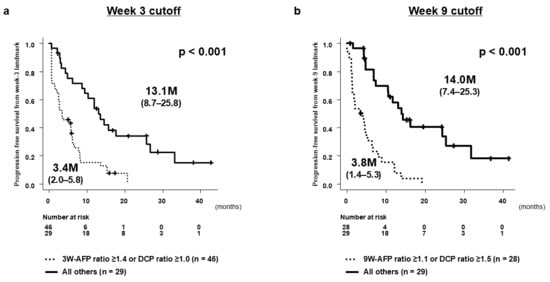
Figure 4.
Progression-free survival (PFS) according to early changes in AFP and DCP. (a) PFS from the week 3 landmark among patients who remained on treatment and had evaluable baseline and week 3 AFP/DCP measurements. PFS was measured from the week 3 assessment. Patients were stratified according to AFP ratio ≥ 1.4 and/or DCP ratio ≥ 1.0 at week 3. (b) PFS from the week 9 landmark among patients in the 1st-DC group who had complete AFP and DCP measurements at approximately week 9. PFS was measured from the week 9 landmark time point. Dashed horizontal lines indicate baseline, and asterisks highlight clinically relevant landmark time points. Statistical details for group comparisons are summarized in the tables.
Together, these findings highlight week 3 as a critical decision point for identifying primary non-responders and week 9 as the optimal landmark for detecting early secondary progression among patients who initially achieved disease control.
To determine whether early AFP and DCP dynamics provided independent predictive value beyond clinical background factors, multivariate Cox’s proportional hazards models were performed at both landmark time points. At the week 3 landmark, the AFP ratio remained a strong independent predictor of PFS (HR 3.822, 95% CI 2.198–6.644; p < 0.0001), whereas the DCP ratio did not retain statistical significance after adjustment (Table 4). In contrast, at the week 9 landmark within the 1st-DC cohort, both the AFP ratio (HR 1.725, 95% CI 1.251–2.378; p = 0.0009) and the DCP ratio (HR 1.140, 95% CI 1.022–1.273; p = 0.0188) remained independently associated with PFS (Table 5). These results indicate that AFP dynamics predominantly drive early prediction at week 3, while both AFP and DCP independently contribute to the identification of early secondary progression at week 9.

Table 4.
Multivariate Cox’s analysis for progression-free survival from the week 3 landmark.

Table 5.
Multivariate Cox’s analysis for progression-free survival from the week 9 landmark.
4. Discussion
This study demonstrated that early dynamic changes in AFP and DCP ratios provide clinically actionable signals for identifying primary progression (1st-PD) at the first evaluation (week 6) and early progression among patients with initial disease control (2nd-PD) at the second evaluation (median: 14.8 weeks). These findings support the application of biomarker-based responder selection to guide adaptive treatment decisions in advanced HCC. To our knowledge, this is the first study to systematically evaluate tumor marker dynamics at both the first and second radiological assessments during ICI-based therapy. Furthermore, we established clinically applicable thresholds at weeks 3 and 9, offering a simple and practical framework for individualized treatment decision-making in patients with unresectable HCC.
Our results confirm and extend previous evidence linking AFP and DCP kinetics with treatment efficacy under ICI-based regimens. At week 3, elevations in AFP and DCP ratios were strongly associated with primary progression. ROC-derived thresholds (AFP ratio 1.41, DCP ratio 0.92) were rounded to AFP ratio ≥ 1.4 or DCP ratio ≥ 1.0 to enhance their clinical usability and avoid misclassification near baseline values. With a high sensitivity (0.93) and a negative predictive value (0.97), this simple rule reliably identifies patients who are unlikely to benefit from continued Atz + Bev therapy. Primary progression under ICI-based therapy is often associated with an “immune-cold” tumor phenotype characterized by poor immune infiltration and limited responsiveness to checkpoint blockades [21,22,23]. For such patients, continued ICI-based therapy is unlikely to confer benefit, and early switching to molecular-targeted therapy—preferably lenvatinib, which uniquely inhibits both VEGFR and FGFR pathways—is recommended in current Japanese treatment algorithms [7,24,25]. Thus, week 3 AFP and DCP dynamics can serve as a cornerstone of biomarker-based responder selection, enabling timely transition to alternative mechanisms of action before irreversible disease progression occurs.
Among patients who achieved disease control at the first evaluation, increases in AFP and DCP ratios from week 9 onward were strongly associated with subsequent progression (2nd-PD). The week 9 thresholds of AFP ratio ≥ 1.1 or DCP ratio ≥ 1.5 serve as clinically actionable indicators to anticipate early progression even before radiographic confirmation; this provides a crucial therapeutic window during which treatment intensification or the integration of locoregional strategies can be implemented while liver function remains preserved. In this context, 2nd-PD may reflect acquired resistance rather than a primary immune-cold phenotype [26,27]. For such patients, early transition to molecular-targeted therapy—preferably lenvatinib—is a rational option [7,25,28]. Alternatively, on-demand locoregional therapy such as transarterial chemoembolization (TACE) targeting less-responsive intrahepatic lesions may enhance tumor antigen release and reinvigorate systemic antitumor immunity, allowing the continuation of the ICI-based regimen (e.g., Atz + Bev) [8,29,30,31]. Such a multimodal approach aims to convert acquired resistance into renewed immune responsiveness and prevent overt progression.
In the present study, multivariate analyses further reinforced the clinical relevance of these biomarker dynamics. At the week 3 landmark, the AFP ratio remained a strong and independent predictor of subsequent PFS after adjustment for BCLC stage, ECOG performance status, the Child–Pugh score, and treatment line, whereas the DCP ratio did not retain significance. This finding suggests that AFP dynamics predominantly capture the biological features underlying primary resistance to immune checkpoint inhibition. In contrast, at the week 9 landmark among patients who initially achieved disease control, both AFP and DCP ratios remained independently associated with PFS, indicating that the combination of these markers reflects emerging acquired resistance during ongoing therapy. These results support the complementary roles of AFP and DCP at different phases of treatment and highlight their utility as non-invasive, dynamic biomarkers for adaptive treatment sequencing.
These findings carry two major clinical implications. Firstly, identifying primary progression as early as week 3 can prevent the futile continuation of Atz + Bev and facilitate earlier sequencing to alternative systemic regimens. Secondly, detecting the risk of early progression at week 9 among 1st-DC patients may enable proactive multimodal strategies—such as on-demand TACE or radiofrequency ablation in combination with ongoing immunotherapy—to enhance antitumor immunity before overt progression occurs [30]. Together, these approaches represent a dual-track treatment framework—early switching for immune-cold primary resistance and immune-enhancing multimodal intervention for acquired resistance—that exemplifies the emerging paradigm of biomarker-based adaptive management in HCC.
Recently, combination strategies integrating systemic therapy and TACE have gained increasing attention, particularly for intermediate-stage HCC [5,8,29]. The integration of ICI-based or anti-VEGF/TKI therapy with TACE may compensate for the limitations of each modality and enhance antitumor immunity. The REPLACEMENT study [32] demonstrated that most patients with SD eventually experienced disease progression during atezolizumab plus bevacizumab therapy; therefore, adding TACE before radiological progression to enhance immune activation appears to be a reasonable strategy. Our findings, showing that early AFP and DCP elevations predict subsequent progression among patients with initial disease control, further support this evolving concept by identifying those who may benefit from timely multimodal intervention before radiological progression.
This concept is currently being prospectively tested in the IMPACT trial (jRCTs051230037) [33], a randomized phase 3 study in Japan that is evaluating the addition of intrahepatic control TACE to Atz + Bev in patients maintaining stable disease after 6–12 weeks of induction therapy. The IMPACT trial aims to determine whether this immune-boosting strategy can prolong overall survival by selectively targeting residual intrahepatic lesions while preserving hepatic function. Notably, early randomization at week 6 is permitted for patients showing tumor enlargement trends or rising AFP levels, reflecting the importance of biomarker dynamics in identifying high-risk SD patients. Our results provide real-world evidence supporting this rationale by demonstrating that increases in AFP or DCP at week 9 predict early secondary progression, indicating a population likely to benefit from such on-demand, immune-enhancing local interventions.
In Japan, recent systemic therapy strategies for unresectable HCC have increasingly emphasized early biomarker-based decision-making during ICI therapy [7,25]. Early changes in AFP and PIVKA-II have been shown to precede imaging-based assessments by 6–12 weeks and to distinguish responders from non-responders within 2–3 weeks after treatment initiation. Our findings expand this concept by demonstrating that dynamic AFP and DCP ratio changes at weeks 3 and 9 during Atz + Bev therapy cannot only identify primary progression but also predict early secondary progression among patients with initial disease control, reinforcing the clinical utility of biomarker-based responder selection in the immunotherapy era. These results complement and extend those of Tanabe et al., who examined early AFP and DCP kinetics primarily in relation to primary progression during Atezo/Bev therapy. While their study highlighted the predictive value of biomarker changes at the initial assessment, it did not evaluate the subsequent clinical course of patients who initially achieved disease control. In contrast, our analysis uniquely focuses on secondary progression (2nd-PD) by assessing biomarker dynamics at the second radiological evaluation using a predefined week 9 landmark. This approach provides new insight into early signals of acquired resistance among patients with initial disease control—an area of major clinical relevance that has not been systematically investigated previously. By integrating both week 3 and week 9 biomarker kinetics, our study proposes a dual-stage framework for identifying primary and secondary non-responders, thereby supporting more precise and timely treatment adaptation during Atezo/Bev therapy.
Although AFP and DCP may provide complementary information, the limited event numbers at the week 3 and week 9 landmark time points preclude the reliable construction of multivariable ROC models or nomograms without a substantial risk of overfitting. Therefore, we adopted simple dual-marker rules to maximize clinical applicability, while future multicenter studies will be needed to establish more complex integrated prediction models.
Although AFP and DCP are widely used tumor markers in HCC, the biological mechanisms linking their early elevation to resistance against immune checkpoint blockades remain incompletely understood. Primary resistance to ICI-based therapy is often associated with an “immune-cold” tumor microenvironment characterized by limited T-cell infiltration, impaired antigen presentation, and high intratumoral heterogeneity. Our findings are consistent with this concept; however, the present study did not include tumor tissue analyses, immune profiling, or circulating tumor DNA. Future studies integrating PD-L1 expression, T-cell infiltration, immune-related gene signatures, or ctDNA dynamics will be necessary to determine whether AFP/DCP kinetics reflect underlying immune phenotypes or molecular features associated with primary or acquired ICI resistance.
This study has several limitations. Firstly, it was a retrospective, single-center analysis with potential selection bias and a relatively small sample size. Secondly, the timing of the second evaluation varied among patients (median, 14.8 weeks), which may have influenced the assessment outcomes. Thirdly, the cutoff values of AFP and DCP ratios at weeks 3 and 9 were determined empirically in this cohort and, therefore, require external validation before clinical application. In addition, because the HBV- and HCV-related subgroups were relatively small, we were unable to reliably assess whether AFP or DCP dynamics differ by underlying disease etiology; larger multicenter cohorts will be needed to evaluate potential etiology-dependent differences in biomarker behavior. We did not perform formal time-dependent ROC analyses because our study was designed around two pre-specified, clinically meaningful landmark time points (weeks 3 and 9), and the number of events after each landmark was limited, making such analyses statistically unstable. Although OS is an important clinical endpoint, this study was specifically designed to evaluate early dynamic biomarker changes at predefined decision points in relation to primary and secondary progression. Because OS is strongly influenced by subsequent systemic or locoregional treatments after progression, it would not directly reflect the predictive value of AFP/DCP kinetics. Moreover, the limited number of OS events after each landmark rendered landmark OS analysis statistically unstable. Therefore, PFS from each landmark was selected as the most appropriate and clinically interpretable endpoint for assessing the utility of early AFP/DCP dynamics. Future studies with larger populations will be required to clarify the impact of these biomarker changes in OS. Finally, the increasing prevalence of tumor marker-negative HCC represents a practical limitation for biomarker-guided approaches. Thus, validation in larger, independent, multicenter cohorts is essential.
5. Conclusions
In patients with unresectable HCC treated with Atz + Bev, an AFP ratio ≥ 1.4 or DCP ratio ≥ 1.0 at week 3 reliably identifies primary progression, while an AFP ratio ≥ 1.1 or DCP ratio ≥ 1.5 at week 9 predicts early progression among those who initially achieved disease control. These simple, clinically applicable thresholds enable early treatment switching and the timely integration of locoregional strategies. Such biomarker-based responder selection and adaptive treatment approaches may ultimately improve survival outcomes by ensuring optimal sequencing and preserving future therapeutic opportunities in advanced HCC. Collectively, these findings highlight week 3 as the key time point for identifying primary progression and week 9 as the optimal landmark for predicting early progression, supporting the clinical utility of dynamic AFP and DCP monitoring as a non-invasive and practical biomarker-guided strategy for treatment optimization in unresectable HCC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17243891/s1, Table S1. Predictive performance of tumor marker ratios at week 3 for 1st-PD, Table S2. Predictive performance of tumor marker ratios at week 9 for 2nd-PD.
Author Contributions
Conceptualization, T.K.; Methodology, T.K.; Validation, H.M. and Y.T.; Formal Analysis, T.K.; Investigation, T.K., H.M., Y.T., M.K., H.S., M.A., S.M., G.K., T.N., H.T., K.N., E.O., K.F., M.N. and R.M.; Data Curation, T.K., H.M., Y.T. and M.A.; Writing—Original Draft Preparation, T.K.; Writing—Review and Editing, H.M. and Y.T.; Visualization, T.K.; Supervision, Y.H.; Project Administration, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Fujita Health University School of Medicine (approval no. HM24-418; date of approval: 16 December 2024).
Informed Consent Statement
The requirement for additional consent for participation in this study was waived due to its retrospective design.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and institutional restrictions.
Acknowledgments
The authors would like to thank Akie Tsuda for her valuable assistance in collecting clinical data.
Conflicts of Interest
T.K. reports speaker fees from Eisai, Chugai, Takeda, Eli Lilly Japan, AstraZeneca, and Bristol Myers Squibb. All other authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| AFP | alpha-fetoprotein |
| AFP-L3 | lens culinaris agglutinin-reactive alpha-fetoprotein |
| AE | adverse event |
| Atz + Bev | atezolizumab plus bevacizumab |
| BCLC | Barcelona Clinic Liver Cancer |
| CI | confidence interval |
| CR | complete response |
| DCP | des-gamma-carboxy prothrombin |
| DC | disease control |
| DCR | disease control rate |
| ECOG | Eastern Cooperative Oncology Group |
| FGFR | fibroblast growth factor receptor |
| HCC | hepatocellular carcinoma |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| ICI | immune checkpoint inhibitor |
| IMPACT | Immunotherapy and TACE Combination Trial (jRCTs051230037) |
| NE | not evaluable |
| ORR | overall response rate |
| OS | overall survival |
| PD | progressive disease |
| PFS | progression-free survival |
| PR | partial response |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | stable disease |
| TACE | transarterial chemoembolization |
| TKI | tyrosine kinase inhibitor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Cutsem, E.; Sangro, B.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2022, 386, 1302–1314. [Google Scholar] [CrossRef]
- Yau, T.; Galle, P.R.; Decaens, T.; Sangro, B.; Qin, S.; da Fonseca, L.G.; Karachiwala, H.; Blanc, J.F.; Park, J.W.; Gane, E.; et al. Nivolumab plus Ipilimumab versus Lenvatinib or Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma (CheckMate 9DW): An Open-Label, Randomised, Phase 3 Trial. Lancet 2025, 405, 1851–1864. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.L.; Dawson, L.A.; Kelley, R.K.; Llovet, J.M.; Meyer, T.; Ricke, J.; Rimassa, L.; Sapisochin, G.; Vilgrain, V.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef]
- Lau, G.; Obi, S.; Zhou, J.; Tateishi, R.; Qin, S.; Zhao, H.; Otsuka, M.; Ogasawara, S.; George, J.; Chow, P.K.H.; et al. APASL Clinical Practice Guidelines on Systemic Therapy for Hepatocellular Carcinoma—2024. Hepatol. Int. 2024, 18, 1661–1683. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Treatment Decision-Making in Unresectable Hepatocellular Carcinoma: Importance of Understanding the Different Response Patterns between IO plus Anti-VEGF and IO plus IO Regimens. Liver Cancer 2025, 14, 119–126. [Google Scholar] [CrossRef]
- Akyildiz, A.; Yavuz, B.G.; Ismayilov, R.; Buyukaksoy, M.; Sozen, S.; Eid, J.R.; Avritscher, R.; Sunyoung, L.; Yalcin, S.; Kaseb, A.O. Comprehensive Review of Transarterial Chemoembolization Plus Systemic Therapy in Advanced Hepatocellular Carcinoma. J. Gastrointest. Cancer 2025, 56, 194. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Lee, Y.T.; Tseng, H.R.; Zhu, Y.; You, S.; Agopian, V.G.; Yang, J.D. Alpha-Fetoprotein: Past, Present, and Future. Hepatol. Commun. 2024, 8, e0422. [Google Scholar] [CrossRef]
- Yu, B.; Ma, W. Biomarker Discovery in Hepatocellular Carcinoma (HCC) for Personalized Treatment and Enhanced Prognosis. Cytokine Growth Factor Rev. 2024, 79, 29–38. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Muto, H.; Wada, Y.; Komura, G.; Nakano, T.; Tanaka, H.; Nakaoka, K.; Ohno, E.; Funasaka, K.; et al. Early Changes in Alpha-Fetoprotein and Des-γ-Carboxy Prothrombin Are Useful Predictors of Antitumor Response to Durvalumab plus Tremelimumab Therapy for Advanced Hepatocellular Carcinoma. Curr. Oncol. 2024, 31, 4225–4240. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Hashimoto, S.; Miyahara, R.; Sawaki, A.; Nakano, T.; Nakaoka, K.; Tanaka, H.; Miyachi, Y.; Mii, A.; et al. Early Changes in Alpha-Fetoprotein Are a Useful Predictor of Efficacy of Atezolizumab plus Bevacizumab Treatment in Patients with Advanced Hepatocellular Carcinoma. Oncology 2022, 100, 12–21. [Google Scholar] [CrossRef]
- Saeki, I.; Shimose, S.; Tomonari, T.; Ito, T.; Tani, J.; Takeuchi, Y.; Yoshioka, N.; Naito, T.; Takeuchi, M.; Kakizaki, S.; et al. Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin Can Predict the Objective Response of Patients with Hepatocellular Carcinoma Receiving Durvalumab plus Tremelimumab Therapy. PLoS ONE 2024, 19, e0311084. [Google Scholar] [CrossRef]
- Tanabe, N.; Saeki, I.; Aibe, Y.; Matsuda, T.; Hanazono, T.; Nishi, M.; Hidaka, I.; Kuwashiro, S.; Shiratsuki, S.; Matsuura, K.; et al. Early Prediction of Response Focused on Tumor Markers in Atezolizumab plus Bevacizumab Therapy for Hepatocellular Carcinoma. Cancers 2023, 15, 2927. [Google Scholar] [CrossRef]
- Tamaki, N.; Tada, T.; Kurosaki, M.; Yasui, Y.; Ochi, H.; Mashiba, T.; Sakamoto, A.; Marusawa, H.; Narita, R.; Uchida, Y.; et al. Optimal Threshold of Alpha-Fetoprotein Response in Patients with Unresectable Hepatocellular Carcinoma Treated with Atezolizumab and Bevacizumab. Investig. New Drugs 2022, 40, 1290–1297. [Google Scholar] [CrossRef]
- Uchikawa, S.; Kawaoka, T.; Murakami, S.; Miura, R.; Shirane, Y.; Johira, Y.; Kosaka, M.; Fujii, Y.; Fujino, H.; Ono, A.; et al. Significance of Changes in Tumor Markers in Patients Treated with Durvalumab plus Tremelimumab Combination Therapy as a Surrogate Marker for Tumor Response to Unresectable Hepatocellular Carcinoma. Hepatol. Res. 2024; in press. [Google Scholar]
- National Institutes of Health; National Cancer Institute, U.S.A.; Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5 × 11.pdf (accessed on 20 June 2020).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 64th General Assembly, Fortaleza, Brazil, October 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 20 June 2025).
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Liang, J.; Liu, Y.; Hou, X.; Zhang, M.; Li, Y.; Jiang, X. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front. Immunol. 2021, 12, 765101. [Google Scholar] [CrossRef]
- Salani, F.; Genovesi, V.; Vivaldi, C.; Massa, V.; Cesario, S.; Bernardini, L.; Caccese, M.; Graziani, J.; Berra, D.; Fornaro, L.; et al. Primary Resistance to Immunotherapy-Based Regimens in First Line Hepatocellular Carcinoma: Perspectives on Jumping the Hurdle. Cancers 2022, 14, 4896. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC Guidelines) 2019 Update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- The Japan Society of Hepatology (JSH). Manual for the Diagnosis and Treatment of Hepatocellular Carcinoma, 5th ed.; The Japan Society of Hepatology: Tokyo, Japan, 2025; Chapter 5; p. 249. (In Japanese) [Google Scholar]
- Xie, Q.; Zhang, P.; Wang, Y.; Mei, W.; Zeng, C. Overcoming Resistance to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Challenges and Opportunities. Front. Oncol. 2022, 12, 958720. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Trevisani, F. Mechanisms of Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Cancers 2022, 14, 4616. [Google Scholar] [CrossRef]
- Vogel, A.; Saborowski, A.; Rimassa, L.; El-Khoueiry, A. Navigating Second-Line Therapy after Immunotherapy in Advanced HCC. JHEP Rep. 2025, 101630. [Google Scholar] [CrossRef]
- Kudo, M. Immune Checkpoint Inhibitor plus Anti-VEGF/TKI Combined with Transarterial Chemoembolization in Locally Advanced Nonmetastatic Hepatocellular Carcinoma: Real-World Treatment Strategy Based on Phase 3 Clinical Trial Results. Liver Cancer 2025, 14, 241–247. [Google Scholar] [CrossRef]
- Kudo, M.; Aoki, T.; Ueshima, K.; Tsuchiya, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Achievement of Complete Response and Drug-Free Status by Atezolizumab plus Bevacizumab Combined with or without Curative Conversion in Patients with Transarterial Chemoembolization-Unsuitable, Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Proof-of-Concept Study. Liver Cancer 2023, 12, 321–338. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Muto, H.; Tachi, Y.; Ukai, T.; Wada, Y.; Komura, G.; Nakano, T.; Tanaka, H.; Nakaoka, K.; et al. Characteristics and Prognosis of Patients with Advanced Hepatocellular Carcinoma Treated with Atezolizumab/Bevacizumab Combination Therapy Who Achieved Complete Response. Curr. Oncol. 2024, 31, 6218–6231. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Tsuchiya, K.; Yamashita, T.; Shimose, S.; Numata, K.; Kodama, Y.; Itoh, S.; Tanaka, Y.; Kuroda, H.; et al. Primary Analysis of a Phase II Study of Atezolizumab plus Bevacizumab for TACE-Unsuitable Patients with Tumor Burden beyond Up-To-Seven Criteria in Intermediate-Stage Hepatocellular Carcinoma: REPLACEMENT Study. Liver Cancer, 2025; in press. [Google Scholar]
- Kodama, Y.; Ueshima, K.; Moriguchi, M.; Inaba, Y.; Yamashita, T.; Iwamoto, H.; Ueno, M.; Ogasawara, S.; Kuzuya, T.; Kodama, T.; et al. Protocol of the IMPACT Study: Randomized, Multicenter, Phase 3 Study Evaluating the Efficacy of Immunotherapy (Atezolizumab) plus Anti-VEGF Therapy (Bevacizumab) in Combination with Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma. BMC Cancer 2025, 25, 434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).