Neurofibromatosis Type 1: Genetic Mechanisms and Advances in Therapeutic Innovation
Simple Summary
Abstract
1. Introduction
2. Molecular Mechanisms of NF1 Pathologies: Genetics and Pathways
2.1. The NF1 Gene
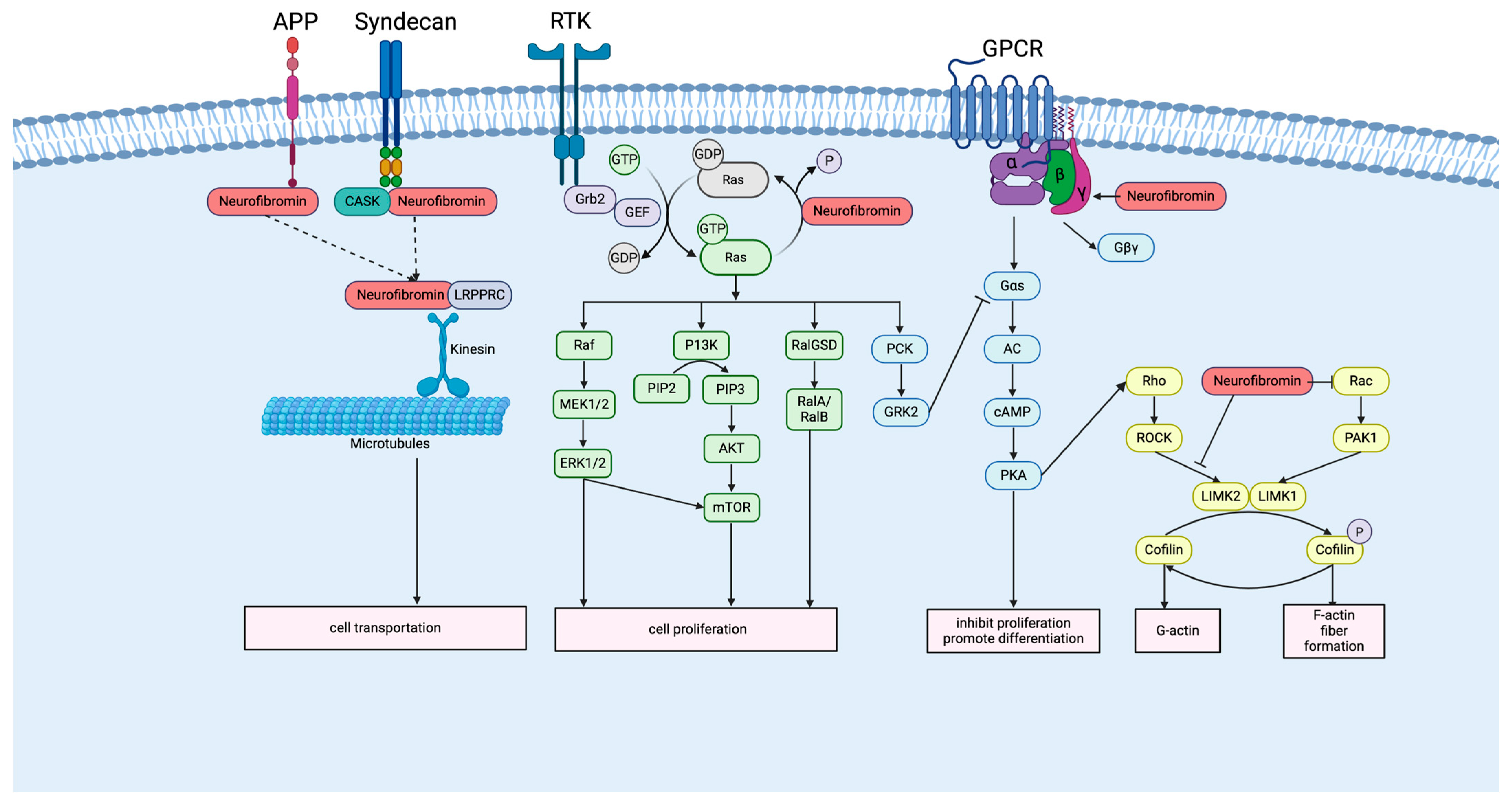
2.2. Neurofibromin and Signaling Pathway
3. Clinical Manifestations of NF1
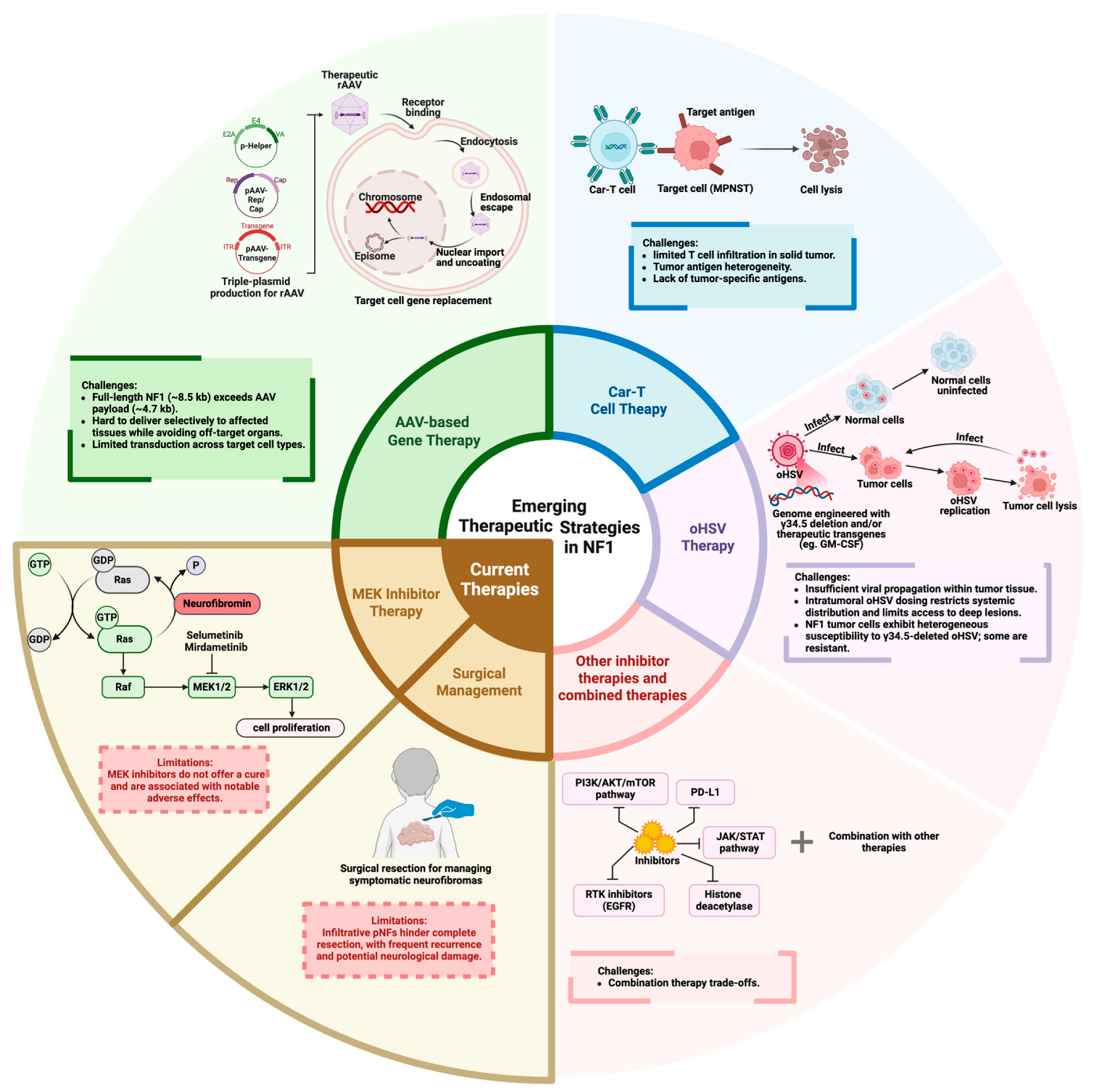
4. Current Therapy for NF1
4.1. Surgical Management
4.2. MEK Inhibitor Therapy
5. Emerging Therapeutic Strategies
5.1. AAV-Based Gene Therapy
5.2. CAR-T Cell Therapy
5.3. oHSV Therapy
5.4. Other Molecular Targets and Pathway Inhibitors
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NF1 | Neurofibromatosis Type 1 |
| cNFs | cutaneous neurofibromas |
| pNFs | plexiform neurofibromas |
| MPNSTs | malignant peripheral nerve sheath tumors |
| HGMD® | Human Gene Mutation Database |
| GAP | GTPase-activating protein |
| NLS | nuclear localization signal |
| CSRD | cysteine/serine-rich domain |
| TBD | tubulin-binding region |
| GRD | GAP-related domain |
| LRD | leucine-rich domain |
| PH | pleckstrin homology |
| CTD | C-terminal domain |
| SBR | syndecan-binding region |
| SynGAP | synaptic RasGAP |
| PAM | PI3K/AKT/mTOR |
| AC | adenylyl cyclase |
| GPCR | G protein-coupled receptors |
| 5-HT6r | 5-hydroxytryptamine receptor 6 |
| LRPPRC | leucine-rich pentatricopeptide-repeat-containing protein |
| APP | amyloid precursor protein |
| CALMs | café-au-lait macules |
| LOH | loss of heterozygosity |
| PRC2 | Polycomb Repressive Complex 2 |
| AAV | adeno-associated virus |
| HSV | herpes simplex virus |
| CAR-T | chimeric antigen receptor T cell |
| oHSV | oncolytic herpes simplex virus |
| T-VEC | Talimogene laherparepvec |
| DLT | dose-limiting toxicities |
| MTD | maximum tolerated dose |
| CART | Antigen-specific cytokine-activated T cells |
| CTLs | cytotoxic T lymphocytes |
| DCvac | dendritic cell vaccine |
| LGG | low-grade glioma |
| rAAVs | recombinant adeno-associated viruses |
| hSCs | human Schwann cells |
| C10 | C-terminal 10 AA of HRAS domain |
| C24 | C-terminal 24 AA of KRAS4B |
| IF | Immunofluorescence |
| hNu | human nuclear antigen |
| RTKs | receptor tyrosine kinases |
| HDACis | histone deacetylase inhibitors |
References
- Lammert, M.; Friedman, J.M.; Kluwe, L.; Mautner, V.F. Prevalence of Neurofibromatosis 1 in German Children at Elementary School Enrollment. Arch. Dermatol. 2005, 141, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Masocco, M.; Kodra, Y.; Vichi, M.; Conti, S.; Kanieff, M.; Pace, M.; Frova, L.; Taruscio, D. Mortality associated with neurofibromatosis type 1: A study based on Italian death certificates (1995–2006). Orphanet J. Rare Dis. 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, E.; Leppävirta, J.; Koffert, A.; Suominen, S.; Vahtera, J.; Vahlberg, T.; Pöyhönen, M.; Peltonen, J.; Peltonen, S. Incidence and Mortality of Neurofibromatosis: A Total Population Study in Finland. J. Investig. Dermatol. 2015, 135, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.N.; Gutmann, D.H.; Fletcher, J.A.; Glover, T.W.; Collins, F.S.; Downward, J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992, 356, 713–715. [Google Scholar] [CrossRef]
- Lidzba, K.; Granström, S.; Lindenau, J.; Mautner, V. The adverse influence of attention-deficit disorder with or without hyperactivity on cognition in neurofibromatosis type 1. Dev. Med. Child Neurol. 2012, 54, 892–897. [Google Scholar] [CrossRef]
- Friedman, J.M. Neurofibromatosis 1. In GeneReviews(®); Adam, M.P., Bick, S., Mirzaa, G.M., Eds.; University of Washington: Seattle, WA, USA, 1998; [Updated 2025]; ISSN 2372-0697. [Google Scholar]
- Markham, A.; Keam, S.J. Selumetinib: First Approval. Drugs 2020, 80, 931–937. [Google Scholar] [CrossRef]
- Hoy, S.M. Mirdametinib: First Approval. Drugs 2025, 85, 977–984. [Google Scholar] [CrossRef]
- Kun, E.; Tsang, Y.; Ng, C.; Gershenson, D.; Wong, K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021, 92, 102137. [Google Scholar] [CrossRef]
- Philpott, C.; Tovell, H.; Frayling, I.M.; Cooper, D.N.; Upadhyaya, M. The NF1 somatic mutational landscape in sporadic human cancers. Hum. Genom. 2017, 11, 13. [Google Scholar] [CrossRef]
- Trovó-Marqui, A.; Tajara, E. Neurofibromin: A general outlook. Clin. Genet. 2006, 70, 1–13. [Google Scholar] [CrossRef]
- Jett, K.; Friedman, J.M. Clinical and genetic aspects of neurofibromatosis 1. Genet. Med. 2010, 12, 1–11. [Google Scholar] [CrossRef]
- Anastasaki, C.; Le, L.Q.; Kesterson, R.A.; Gutmann, D.H. Updated nomenclature for human and mouse neurofibromatosis type 1 genes. Neurol. Genet. 2017, 3, e169. [Google Scholar] [CrossRef]
- Deininger, P.L. Alu elements: Know the SINEs. Genome Biol. 2011, 12, 236. [Google Scholar] [CrossRef]
- Alesi, V.; Genovese, S.; Lepri, F.R.; Catino, G.; Loddo, S.; Orlando, V.; Di Tommaso, S.; Morgia, A.; Martucci, L.; Di Donato, M.; et al. Deep Intronic LINE-1 Insertions in NF1: Expanding the Spectrum of Neurofibromatosis Type 1-Associated Rearrangements. Biomolecules 2023, 13, 725. [Google Scholar] [CrossRef]
- Abramowicz, A.; Gos, M. Neurofibromin in neurofibromatosis type 1—Mutations in NF1gene as a cause of disease. Dev. Period Med. 2014, 18, 297–306. [Google Scholar]
- Hino, O.; Kobayashi, T. Mourning Dr. Alfred G. Knudson: The two-hit hypothesis, tumor suppressor genes, and the tuberous sclerosis complex. Cancer Sci. 2017, 108, 5–11. [Google Scholar] [PubMed]
- Brems, H.; Beert, E.; de Ravel, T.; Legius, E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009, 10, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Bergoug, M.; Doudeau, M.; Godin, F.; Mosrin, C.; Vallée, B.; Bénédetti, H. Neurofibromin Structure, Functions and Regulation. Cells 2020, 9, 2365. [Google Scholar] [CrossRef]
- Hinman, M.N.; Sharma, A.; Luo, G.; Lou, H. Neurofibromatosis type 1 alternative splicing is a key regulator of Ras signaling in neurons. Mol. Cell Biol. 2014, 34, 2188–2197. [Google Scholar] [PubMed]
- Gutmann, D.H.; Geist, R.T.; Rose, K.; Wright, D.E. Expression of two new protein isoforms of the neurofibromatosis type 1 gene product, neurofibromin, in muscle tissues. Dev. Dyn. 1995, 202, 302–311. [Google Scholar] [CrossRef]
- Geist, R.T.; Gutmann, D.H. Expression of a developmentally-regulated neuron-specific isoform of the neurofibromatosis 1 (NF1) gene. Neurosci. Lett. 1996, 211, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Müller, R.; Kenner, O.; Leistner, W.; Hein, C.; Vogel, W.; Bartelt, B. The N-terminal splice product NF1-10a-2 of the NF1 gene codes for a transmembrane segment. Biochem. Biophys. Res. Commun. 2002, 294, 496–503. [Google Scholar] [CrossRef]
- Vandenbroucke, I.; Van Oostveldt, P.; Coene, E.; De Paepe, A.; Messiaen, L. Neurofibromin is actively transported to the nucleus. FEBS Lett. 2004, 560, 98–102. [Google Scholar] [CrossRef]
- Sherekar, M.; Han, S.W.; Ghirlando, R.; Messing, S.; Drew, M.; Rabara, D.; Waybright, T.; Juneja, P.; O’Neill, H.; Stanley, C.B.; et al. Biochemical and structural analyses reveal that the tumor suppressor neurofibromin (NF1) forms a high-affinity dimer. J. Biol. Chem. 2020, 295, 1105–1119. [Google Scholar] [CrossRef]
- Lupton, C.J.; Bayly-Jones, C.; D’andrea, L.; Huang, C.; Schittenhelm, R.B.; Venugopal, H.; Whisstock, J.C.; Halls, M.L.; Ellisdon, A.M. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat. Struct. Mol. Biol. 2021, 28, 982–988. [Google Scholar] [CrossRef]
- Chaker-Margot, M.; Werten, S.; Dunzendorfer-Matt, T.; Lechner, S.; Ruepp, A.; Scheffzek, K.; Maier, T. Structural basis of activation of the tumor suppressor protein neurofibromin. Mol. Cell 2022, 82, 1288–1296.e5. [Google Scholar] [CrossRef]
- Napolitano, F.; Dell’aquila, M.; Terracciano, C.; Franzese, G.; Gentile, M.T.; Piluso, G.; Santoro, C.; Colavito, D.; Patanè, A.; De Blasiis, P.; et al. Genotype-Phenotype Correlations in Neurofibromatosis Type 1: Identification of Novel and Recurrent NF1 Gene Variants and Correlations with Neurocognitive Phenotype. Genes 2022, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Mangoura, D.; Sun, Y.; Li, C.; Singh, D.; Gutmann, D.H.; Flores, A.; Ahmed, M.; Vallianatos, G. Phosphorylation of neurofibromin by PKC is a possible molecular switch in EGF receptor signaling in neural cells. Oncogene 2005, 25, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Arun, V.; Wiley, J.C.; Kaur, H.; Kaplan, D.R.; Guha, A. A novel neurofibromin (NF1) interaction with the leucine-rich pentatricopeptide repeat motif-containing protein links neurofibromatosis type 1 and the french canadian variant of leigh’s syndrome in a common molecular complex. J. Neurosci. Res. 2013, 91, 494–505. [Google Scholar] [CrossRef]
- Ratner, N.; Miller, S.J. A RASopathy gene commonly mutated in cancer: The neurofibromatosis type 1 tumour suppressor. Nat. Rev. Cancer 2015, 15, 290–301. [Google Scholar] [CrossRef] [PubMed]
- King, P.D.; Lubeck, B.A.; Lapinski, P.E. Nonredundant Functions for Ras GTPase-Activating Proteins in Tissue Homeostasis. Sci. Signal. 2013, 6, re1. [Google Scholar] [CrossRef] [PubMed]
- Fadhlullah, S.F.B.; Halim, N.B.A.; Yeo, J.Y.T.; Ho, R.L.Y.; Um, P.; Ang, B.T.; Tang, C.; Ng, W.H.; Virshup, D.M.; Ho, I.A.W. Pathogenic mutations in neurofibromin identifies a leucine-rich domain regulating glioma cell invasiveness. Oncogene 2019, 38, 5367–5380. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Welti, S.; Bonneau, F.; Scheffzek, K. A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. Embo Rep. 2006, 7, 174–179. [Google Scholar] [CrossRef]
- Koliou, X.; Fedonidis, C.; Kalpachidou, T.; Mangoura, D. Nuclear import mechanism of neurofibromin for localization on the spindle and function in chromosome congression. J. Neurochem. 2015, 136, 78–91. [Google Scholar] [CrossRef]
- Afratis, N.A.; Nikitovic, D.; Multhaupt, H.A.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 539–551. [Google Scholar] [CrossRef]
- Richardson, D.S.; Spehar, J.M.; Han, D.T.; Chakravarthy, P.A.; Sizemore, S.T. The RAL Enigma: Distinct Roles of RALA and RALB in Cancer. Cells 2022, 11, 1645. [Google Scholar] [CrossRef]
- Stansfield, B.K.; Bessler, W.K.; Mali, R.; Mund, J.A.; Downing, B.D.; Kapur, R.; Ingram, D.A. Ras-Mek-Erk Signaling Regulates Nf1 Heterozygous Neointima Formation. Am. J. Pathol. 2014, 184, 79–85. [Google Scholar] [CrossRef]
- Scheffzek, K.; Shivalingaiah, G. Ras-Specific GTPase-Activating Proteins—Structures, Mechanisms, and Interactions. Cold Spring Harb. Perspect. Med. 2018, 9, a031500. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Yasuda, R. Neurofibromin Is the Major Ras Inactivator in Dendritic Spines. J. Neurosci. 2014, 34, 776–783. [Google Scholar] [CrossRef]
- Hannan, F.; Ho, I.; Tong, J.J.; Zhu, Y.; Nurnberg, P.; Zhong, Y. Effect of neurofibromatosis type I mutations on a novel pathway for adenylyl cyclase activation requiring neurofibromin and Ras. Hum. Mol. Genet. 2006, 15, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, C.; Gutmann, D.H. Neuronal NF1/RAS regulation of cyclic AMP requires atypical PKC activation. Hum. Mol. Genet. 2014, 23, 6712–6721. [Google Scholar] [CrossRef]
- A Pride, N.; Barton, B.; Hutchins, P.; Coghill, D.R.; Korgaonkar, M.S.; Hearps, S.J.C.; Rouel, M.; Malarbi, S.; North, K.N.; Payne, J.M. Effects of methylphenidate on cognition and behaviour in children with neurofibromatosis type 1: A study protocol for a randomised placebo-controlled crossover trial. BMJ Open 2018, 8, e021800. [Google Scholar] [CrossRef]
- Báez-Flores, J.; Rodríguez-Martín, M.; Lacal, J. The therapeutic potential of neurofibromin signaling pathways and binding partners. Commun. Biol. 2023, 6, 436. [Google Scholar] [CrossRef]
- Vallée, B.; Doudeau, M.; Godin, F.; Gombault, A.; Tchalikian, A.; de Tauzia, M.-L.; Bénédetti, H. Nf1 RasGAP Inhibition of LIMK2 Mediates a New Cross-Talk between Ras and Rho Pathways. PLoS ONE 2012, 7, e47283. [Google Scholar] [CrossRef]
- Villalonga, E.; Mosrin, C.; Normand, T.; Girardin, C.; Serrano, A.; Žunar, B.; Doudeau, M.; Godin, F.; Bénédetti, H.; Vallée, B. LIM Kinases, LIMK1 and LIMK2, Are Crucial Node Actors of the Cell Fate: Molecular to Pathological Features. Cells 2023, 12, 805. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Roberts, A.M.; Volta, M.; Sheng, M.; Roberts, R.G. Bipartite interaction between neurofibromatosis type I protein (neurofibromin) and syndecan transmembrane heparan sulfate proteoglycans. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Donarum, E.A.; Halperin, R.F.; Stephan, D.A.; Narayanan, V. Cognitive dysfunction in NFI knock-out mice may result from altered vesicular trafficking of APP/DRD3 complex. BMC Neurosci. 2006, 7, 22. [Google Scholar] [CrossRef]
- Kiuru, M.; Busam, K.J. The NF1 gene in tumor syndromes and melanoma. Mod. Pathol. 2017, 97, 146–157. [Google Scholar] [CrossRef]
- Ozarslan, B.; Russo, T.; Argenziano, G.; Santoro, C.; Piccolo, V. Cutaneous Findings in Neurofibromatosis Type 1. Cancers 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Grit, J.L.; Johnson, B.K.; Dischinger, P.S.; Essenburg, C.J.; Adams, M.; Campbell, S.; Pollard, K.; Pratilas, C.A.; Triche, T.J.; Graveel, C.R.; et al. Distinctive epigenomic alterations in NF1-deficient cutaneous and plexiform neurofibromas drive differential MKK/p38 signaling. Epigenetics Chromatin 2021, 14, 7. [Google Scholar] [CrossRef]
- Stylianides, C.; Hadjigavriel, G.; Theotokis, P.; Vakirlis, E.; Meditskou, S.; Manthou, M.E.; Dermitzakis, I. Epigenetic Mechanisms in Neurofibromatosis Types 1 and 2. Epigenomes 2025, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Kumar, A.; Yu, Z.; Shipman, T.; Wang, Y.; McKay, R.M.; Xing, C.; Le, L.Q. Basement membrane proteins in extracellular matrix characterize NF1 neurofibroma development and response to MEK inhibitor. J. Clin. Investig. 2023, 133, e168227. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Arrazola, I.; Magallón-Lorenz, M.; Fernández-Rodríguez, J.; Zhang, J.; Lee, E.; Ortega-Bertran, S.; Creus-Bachiller, E.; Farrés-Casas, J.; Wilson, K.M.; McKnight, C.; et al. iPSC-derived NF1-CDKN2A-PRC2 deficient neural crest cells mimic glial-to-neuro-mesenchymal transition and form MPNST-like tumors in vivo. bioRxiv 2025. bioRxiv:2025.08.13.670072. [Google Scholar]
- Lee, W.; Teckie, S.; Wiesner, T.; Ran, L.; Granada, C.N.P.; Lin, M.; Zhu, S.; Cao, Z.; Liang, Y.; Sboner, A.; et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat. Genet. 2014, 46, 1227–1232. [Google Scholar] [CrossRef]
- Gutmann, D.H.; McLellan, M.D.; Hussain, I.; Wallis, J.W.; Fulton, L.L.; Fulton, R.S.; Magrini, V.; Demeter, R.; Wylie, T.; Kandoth, C.; et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2012, 23, 431–439. [Google Scholar] [CrossRef]
- Barton, B.; Wolters, P.L.; Walsh, K.S.; Ullrich, N.J.; Rosser, T.; Tonsgard, J.; Viskochil, D.; Schorry, E.; Klesse, L.J.; Fisher, M.J.; et al. Psychosocial functioning and determinants of the health-related quality of life in children with neurofibromatosis type 1 and cognitive impairments. J. Neuro-Oncol. 2025, 174, 65–76. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Kallionpää, R.A.; Clementi, M.; Trevisson, E.; Mautner, V.F.; Howell, S.J.; Lewis, L.; Zehou, O.; Peltonen, S.; Brunello, A.; et al. Breast cancer in neurofibromatosis 1: Survival and risk of contralateral breast cancer in a five country cohort study. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 398–406. [Google Scholar] [CrossRef]
- Crucis, A.; Richer, W.; Brugières, L.; Bergeron, C.; Marie-Cardine, A.; Stephan, J.-L.; Girard, P.; Corradini, N.; Munzer, M.; Lacour, B.; et al. Rhabdomyosarcomas in children with neurofibromatosis type I: A national historical cohort. Pediatr. Blood Cancer 2015, 62, 1733–1738. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Flotho, C. Juvenile myelomonocytic leukemia: Who’s the driver at the wheel? Blood 2019, 133, 1060–1070. [Google Scholar] [CrossRef]
- Chauvel-Picard, J.; Lion-Francois, L.; Beuriat, P.-A.; Paulus, C.; Szathmari, A.; Mottolese, C.; Gleizal, A.; Di Rocco, F. Craniofacial bone alterations in patients with neurofibromatosis type 1. Child’s Nerv. Syst. 2020, 36, 2391–2399. [Google Scholar] [CrossRef]
- Barreto-Duarte, B.; Andrade-Gomes, F.H.; Arriaga, M.B.; Araújo-Pereira, M.; Cubillos-Angulo, J.M.; Andrade, B.B. Association between neurofibromatosis type 1 and cerebrovascular diseases in children: A systematic review. PLoS ONE 2021, 16, e0241096. [Google Scholar] [CrossRef]
- Fisher, M.J.; O Blakeley, J.; Weiss, B.D.; Dombi, E.; Ahlawat, S.; Akshintala, S.; Belzberg, A.J.; Bornhorst, M.; A Bredella, M.; Cai, W.; et al. Management of neurofibromatosis type 1-associated plexiform neurofibromas. Neuro-Oncology 2022, 24, 1827–1844. [Google Scholar] [CrossRef]
- Chen, A.P.; Coyne, G.O.; Wolters, P.L.; Martin, S.; Farschtschi, S.; Blanco, I.; Chen, Z.; Darrigo, L.G.; Eoli, M.; Whittle, J.R.; et al. Efficacy and safety of selumetinib in adults with neurofibromatosis type 1 and symptomatic, inoperable plexiform neurofibromas (KOMET): A multicentre, international, randomised, placebo-controlled, parallel, double-blind, phase 3 study. Lancet 2025, 405, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Dombi, E.; Baldwin, A.; Marcus, L.J.; Fisher, M.J.; Weiss, B.; Kim, A.; Whitcomb, P.; Martin, S.; Aschbacher-Smith, L.E.; Rizvi, T.A.; et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N. Engl. J. Med. 2016, 375, 2550–2560. [Google Scholar] [CrossRef]
- Balagula, Y.; Huston, K.B.; Busam, K.J.; Lacouture, M.E.; Chapman, P.B.; Myskowski, P.L. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Investig. New Drugs 2010, 29, 1114–1121. [Google Scholar] [CrossRef]
- Staedtke, V.; Anstett, K.; Bedwell, D.; Giovannini, M.; Keeling, K.; Kesterson, R.; Kim, Y.; Korf, B.; Leier, A.; McManus, M.L.; et al. Gene-targeted therapy for neurofibromatosis and schwannomatosis: The path to clinical trials. Clin. Trials 2023, 21, 51–66. [Google Scholar] [CrossRef]
- Leier, A.; Moore, M.; Liu, H.; Daniel, M.; Hyde, A.M.; Messiaen, L.; Korf, B.R.; Selvakumaran, J.; Ciszewski, L.; Lambert, L.; et al. Targeted exon skipping of NF1 exon 17 as a therapeutic for neurofibromatosis type I. Mol. Ther.-Nucleic Acids 2022, 28, 261–278. [Google Scholar] [CrossRef]
- Moutal, A.; Yang, X.; Li, W.; Gilbraith, K.B.; Luo, S.; Cai, S.; François-Moutal, L.; Chew, L.A.; Yeon, S.K.; Bellampalli, S.S.; et al. CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain 2017, 158, 2301–2319. [Google Scholar] [CrossRef]
- Heidenreich, M.; Zhang, F. Applications of CRISPR–Cas systems in neuroscience. Nat. Rev. Neurosci. 2015, 17, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Leier, A.; Bedwell, D.M.; Chen, A.T.; Dickson, G.; Keeling, K.M.; Kesterson, R.A.; Korf, B.R.; Lago, T.T.M.; Müller, U.F.; Popplewell, L.; et al. Mutation-Directed Therapeutics for Neurofibromatosis Type I. Mol. Ther.-Nucleic Acids 2020, 20, 739–753. [Google Scholar] [CrossRef]
- Park, S.J.; Lukkes, J.L.; Chan, K.-K.; Drozd, H.P.; Burgin, C.B.; Qian, S.; Sullivan, M.M.; Guevara, C.G.; Cunningham, N.; Arenas, S.; et al. A haploinsufficiency restoration strategy corrects neurobehavioral deficits in Nf1+/– mice. J. Clin. Investig. 2025, 135, e188932. [Google Scholar] [CrossRef]
- De Haan, P.F.R.; Diemen, V.; Toscano, M.G. Viral gene delivery vectors: The next generation medicines for immune-related diseases. Hum. Vaccin. Immunother. 2021, 17, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Santana-Armas, M.L.; de Ilarduya, C.T. Strategies for cancer gene-delivery improvement by non-viral vectors. Int. J. Pharm. 2021, 596, 120291. [Google Scholar] [CrossRef]
- Santiago-Ortiz, J.L.; Schaffer, D.V. Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Release 2016, 240, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Drouyer, M.; Chu, T.-H.; Labit, E.; Haase, F.; Navarro, R.G.; Nazareth, D.; Rosin, N.; Merjane, J.; Scott, S.; Cabanes-Creus, M.; et al. Novel AAV variants with improved tropism for human Schwann cells. Mol. Ther.-Methods Clin. Dev. 2024, 32, 101234. [Google Scholar] [CrossRef] [PubMed]
- Haidar, E.A.; Prabhakar, S.; Cheah, P.S.; Hanlon, K.S.; Espinoza, P.; Crain, A.V.; Patel, N.; Radcliff, G.W.; Cheng, M.; Hernández, I.C.; et al. Engineered AAV capsids mediate transduction of murine neurofibroma and sciatic nerve. Gene Ther. 2025, 32, 385–397. [Google Scholar] [CrossRef]
- Bai, R.-Y.; Shi, J.; Liu, J.; Sun, N.; Lu, Y.; Chen, X.; Xu, M.; Lim, H.; Li, Y.; Xu, H.; et al. Development of an adeno-associated virus vector for gene replacement therapy of NF1-related tumors. Nat. Commun. 2025, 16, 8594. [Google Scholar] [CrossRef]
- Widemann, B.C.; Dombi, E.; Gillespie, A.; Wolters, P.L.; Belasco, J.; Goldman, S.; Korf, B.R.; Solomon, J.; Martin, S.; Salzer, W.; et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro-Oncology 2014, 16, 707–718. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Abdelkader, R.E.; Nada, A.H.; Younes, S.; Hanen, G.; Shahwan, G.; Hamad, M.; Meshref, M.; Nashwan, A.J. Effect of Everolimus on Prognosis of Neurofibromatosis Type 1 Lesions: A Systematic Review and Meta Analysis. Clin. Ther. 2024, 46, 865–869. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Prabhu, S.P.; Reddy, A.T.; Fisher, M.J.; Packer, R.; Goldman, S.; Robison, N.J.; Gutmann, D.H.; Viskochil, D.H.; Allen, J.C.; et al. A phase II study of continuous oral mTOR inhibitor everolimus for recurrent, radiographic-progressive neurofibromatosis type 1–associated pediatric low-grade glioma: A Neurofibromatosis Clinical Trials Consortium study. Neuro-Oncology 2020, 22, 1527–1535. [Google Scholar] [CrossRef]
- A Robertson, K.; Nalepa, G.; Yang, F.-C.; Bowers, D.C.; Ho, C.Y.; Hutchins, G.D.; Croop, J.M.; A Vik, T.; Denne, S.C.; Parada, L.F.; et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: A phase 2 trial. Lancet Oncol. 2012, 13, 1218–1224. [Google Scholar] [CrossRef]
- Kim, A.; Dombi, E.; Tepas, K.; Fox, E.; Martin, S.; Wolters, P.; Balis, F.M.; Jayaprakash, N.; Turkbey, B.; Muradyan, N.; et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr. Blood Cancer 2012, 60, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Grüllich, C. Cabozantinib: A MET, RET, and VEGFR2 Tyrosine Kinase Inhibitor. Recent Results Cancer Res. 2014, 201, 207–214. [Google Scholar] [PubMed]
- Batty, P.; Lillicrap, D. Adeno-associated viral vector integration: Implications for long-term efficacy and safety. J. Thromb. Haemost. 2024, 22, 2945–2960. [Google Scholar] [CrossRef] [PubMed]
- Henshey, B.; Carneiro, A.; Lei, K.; Schaffer, D.; Boulis, N.M. Adeno-associated viral vector targeted evolution for neurofibromatosis gene delivery. Trends Mol. Med. 2025, 31, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, K.K.; Ingram, D.A.; Zhang, Y.; Bollag, G.; Clapp, D. Neurofibromin GTPase-activating Protein-related Domains Restore Normal Growth in Nf1−/− Cells. J. Biol. Chem. 2001, 276, 7240–7245. [Google Scholar] [CrossRef]
- Bodempudi, V.; Yamoutpoor, F.; Pan, W.; Dudek, A.Z.; Esfandyari, T.; Piedra, M.; Babovick-Vuksanovic, D.; Woo, R.A.; Mautner, V.F.; Kluwe, L.; et al. Ral Overactivation in Malignant Peripheral Nerve Sheath Tumors. Mol. Cell. Biol. 2009, 29, 3964–3974. [Google Scholar] [CrossRef]
- Thomas, S.L.; Deadwyler, G.D.; Tang, J.; Stubbs, E.B.; Muir, D.; Hiatt, K.K.; Clapp, D.W.; De Vries, G.H. Reconstitution of the NF1 GAP-related domain in NF1-deficient human Schwann cells. Biochem. Biophys. Res. Commun. 2006, 348, 971–980. [Google Scholar] [CrossRef]
- Bai, R.-Y.; Esposito, D.; Tam, A.J.; McCormick, F.; Riggins, G.J.; Clapp, D.W.; Staedtke, V. Feasibility of using NF1-GRD and AAV for gene replacement therapy in NF1-associated tumors. Gene Ther. 2019, 26, 277–286. [Google Scholar] [CrossRef]
- Cui, X.-W.; Ren, J.-Y.; Gu, Y.-H.; Li, Q.-F.; Wang, Z.-C. NF1, Neurofibromin and Gene Therapy: Prospects of Next-Generation Therapy. Curr. Gene Ther. 2020, 20, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Young, L.C.; de Salazar, R.G.; Han, S.-W.; Huang, Z.Y.S.; Merk, A.; Drew, M.; Darling, J.; Wall, V.; Grisshammer, R.; Cheng, A.; et al. Destabilizing NF1 variants act in a dominant negative manner through neurofibromin dimerization. Proc. Natl. Acad. Sci. USA 2023, 120, e2208960120. [Google Scholar] [CrossRef]
- Kagiava, A.; Richter, J.; Tryfonos, C.; Leal-Julià, M.; Sargiannidou, I.; Christodoulou, C.; Bosch, A.; Kleopa, K.A. Efficacy of AAV serotypes to target Schwann cells after intrathecal and intravenous delivery. Sci. Rep. 2021, 11, 23358. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, Y.; Biferi, M.G.; Besse, A.; Astord, S.; Cohen-Tannoudji, M.; Marais, T.; Barkats, M. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front. Mol. Neurosci. 2015, 8, 36. [Google Scholar] [CrossRef]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Mazuelas, H.; Uriarte-Arrazola, I.; Fernández-Rodríguez, J.; Magallón-Lorenz, M.; Villanueva, A.; Lázaro, C.; Gel, B.; Serra, E.; Carrió, M. Generation of human iPSC-derived neurofibromaspheres for in vitro and in vivo uses. STAR Protoc. 2023, 4, 102198. [Google Scholar] [CrossRef]
- Datta, P.; Rhee, K.-D.; Staudt, R.J.; Thompson, J.M.; Hsu, Y.; Hassan, S.; Drack, A.V.; Seo, S. Delivering large genes using adeno-associated virus and the CRE-lox DNA recombination system. Hum. Mol. Genet. 2024, 33, 2094–2110. [Google Scholar] [CrossRef]
- Maddalena, A.; Tornabene, P.; Tiberi, P.; Minopoli, R.; Manfredi, A.; Mutarelli, M.; Rossi, S.; Simonelli, F.; Naggert, J.K.; Cacchiarelli, D.; et al. Triple Vectors Expand AAV Transfer Capacity in the Retina. Mol. Ther. 2018, 26, 524–541. [Google Scholar] [CrossRef]
- Grieger, J.C.; Samulski, R.J. Packaging Capacity of Adeno-Associated Virus Serotypes: Impact of Larger Genomes on Infectivity and Postentry Steps. J. Virol. 2005, 79, 9933–9944. [Google Scholar] [CrossRef] [PubMed]
- Chohan, K.L.; Siegler, E.L.; Kenderian, S.S. CAR-T Cell Therapy: The Efficacy and Toxicity Balance. Curr. Hematol. Malign- Rep. 2023, 18, 9–18. [Google Scholar] [CrossRef]
- Albelda, S.M. CAR T cell therapy for patients with solid tumours: Key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 2023, 21, 47–66. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Gong, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial final results. Nat. Med. 2024, 30, 2224–2234. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Tang, N.; Cheng, L.; Hao, J.; Xu, B.; Pan, X.; Wei, X.; Wu, H.; Wang, H. Development of CAR-T cell therapy for NF1/SWN-related nerve sheath tumor treatment. Acta Neuropathol. Commun. 2025, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic Herpes Simplex Virus-Based Therapies for Cancer. Cells 2021, 10, 1541. [Google Scholar] [CrossRef]
- Grigg, C.; Blake, Z.; Gartrell, R.; Sacher, A.; Taback, B.; Saenger, Y. Talimogene laherparepvec (T-Vec) for the treatment of melanoma and other cancers. Semin. Oncol. 2016, 43, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Stewart, G.R.; Yeomans, J.; Linford, A.; Lath, D.; Conner, J.; Muthana, M.; Chantry, A.D.; Lawson, M.A. HSV1716 Prevents Myeloma Cell Regrowth When Combined with Bortezomib In Vitro and Significantly Reduces Systemic Tumor Growth in Mouse Models. Viruses 2023, 15, 603. [Google Scholar] [CrossRef]
- Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs 2022, 36, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 2016, 4, 53. [Google Scholar] [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.-Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Kangas, C.; Krawczyk, E.; He, B. Oncolytic HSV: Underpinnings of Tumor Susceptibility. Viruses 2021, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Mezhir, J.J.; Bickenbach, K.; Veerapong, J.; Charron, J.; Posner, M.C.; Roizman, B.; Weichselbaum, R.R. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J. Virol. 2006, 80, 1110–1120. [Google Scholar] [PubMed]
- Antoszczyk, S.; Spyra, M.; Mautner, V.F.; Kurtz, A.; Stemmer-Rachamimov, A.O.; Martuza, R.L.; Rabkin, S.D. Treatment of orthotopic malignant peripheral nerve sheath tumors with oncolytic herpes simplex virus. Neuro-Oncology 2014, 16, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Zhang, T.; Fukuhara, H.; Kuroda, T.; Todo, T.; Canron, X.; Bikfalvi, A.; Martuza, R.L.; Kurtz, A.; Rabkin, S.D. Dominant-Negative Fibroblast Growth Factor Receptor Expression Enhances Antitumoral Potency of Oncolytic Herpes Simplex Virus in Neural Tumors. Clin. Cancer Res. 2006, 12, 6791–6799. [Google Scholar] [CrossRef]
- Antoszczyk, S.; Rabkin, S.D. Prospects and progress of oncolytic viruses for treating peripheral nerve sheath tumors. Expert Opin. Orphan Drugs 2015, 4, 129–138. [Google Scholar] [CrossRef]
- Walker, J.A.; Upadhyaya, M. Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin. Ther. Targets 2018, 22, 419–437. [Google Scholar] [CrossRef]
- Bai, R.-Y.; Xu, M.; Lu, Y.; Liu, J.; Staedtke, V. Pan-RAS inhibitor RMC-7977 is efficacious in treating NF1-related tumors. Neuro-Oncol. Adv. 2025, 7, vdaf065. [Google Scholar] [CrossRef]
- Johansson, G.; Mahller, Y.Y.; Collins, M.H.; Kim, M.O.; Nobukuni, T.; Perentesis, J.; Cripe, T.P.; Lane, H.A.; Kozma, S.C.; Thomas, G.; et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol. Cancer Ther. 2008, 7, 1237–1245. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Springer, M.G.; Choi, K.; Jousma, E.; Rizvi, T.A.; Dombi, E.; Kim, M.-O.; Wu, J.; Ratner, N. STAT3 inhibition reduces macrophage number and tumor growth in neurofibroma. Oncogene 2018, 38, 2876–2884. [Google Scholar] [CrossRef]
- Brown, J.A.; Gianino, S.M.; Gutmann, D.H. Defective cAMP Generation Underlies the Sensitivity of CNS Neurons to Neurofibromatosis-1 Heterozygosity. J. Neurosci. 2010, 30, 5579–5589. [Google Scholar] [CrossRef]
- Ki, D.H.; He, S.; Rodig, S.; Look, A.T. Overexpression of PDGFRA cooperates with loss of NF1 and p53 to accelerate the molecular pathogenesis of malignant peripheral nerve sheath tumors. Oncogene 2016, 36, 1058–1068. [Google Scholar] [CrossRef]
- Peacock, J.D.; Pridgeon, M.G.; Tovar, E.A.; Essenburg, C.J.; Bowman, M.; Madaj, Z.; Koeman, J.; Boguslawski, E.A.; Grit, J.; Dodd, R.D.; et al. Genomic Status of MET Potentiates Sensitivity to MET and MEK Inhibition in NF1-Related Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2018, 78, 3672–3687. [Google Scholar] [CrossRef]
- Staedtke, V.; Gray-Bethke, T.; Riggins, G.J.; Bai, R.-Y. Preventative Effect of Mebendazole against Malignancies in Neurofibromatosis 1. Genes 2020, 11, 762. [Google Scholar] [CrossRef]
- Farschtschi, S.; Kluwe, L.; Park, S.-J.; Oh, S.-J.; Mah, N.; Mautner, V.-F.; Kurtz, A. Upregulated immuno-modulator PD-L1 in malignant peripheral nerve sheath tumors provides a potential biomarker and a therapeutic target. Cancer Immunol. Immunother. 2020, 69, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Nicholls, L.A.; Babiker, H.M.; Liau, J.; Mahadevan, D. PD-1 Inhibition Achieves a Complete Metabolic Response in a Patient with Malignant Peripheral Nerve Sheath Tumor. Cancer Immunol. Res. 2019, 7, 1396–1400. [Google Scholar] [CrossRef]
- Khan, B.; Qahwaji, R.M.; Alfaifi, M.S.; Mobashir, M. Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics 2024, 16, 732. [Google Scholar] [CrossRef]
- Huang, P.Y.; Shih, I.A.; Liao, Y.C.; You, H.L.; Lee, M.J. A novel HDAC11 inhibitor potentiates the tumoricidal effects of cordycepin against malignant peripheral nerve sheath tumor through the Hippo signaling pathway. Am. J. Cancer Res. 2022, 12, 873–892. [Google Scholar] [PubMed]
- Zhang, X.; Murray, B.; Mo, G.; Shern, J.F. The Role of Polycomb Repressive Complex in Malignant Peripheral Nerve Sheath Tumor. Genes 2020, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, J.-P.; Liao, C.-P.; Le, L.Q. Translating current basic research into future therapies for neurofibromatosis type 1. Br. J. Cancer 2020, 123, 178–186. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, Z. Advanced progress in the genetic modification of the oncolytic HSV-1 virus. Front. Oncol. 2025, 14, 1525940. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, Y.; Zhang, Z.; Meng, W.; Li, L. Barriers and solutions for CAR-T therapy in solid tumors. Cancer Gene Ther. 2025, 32, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 658399. [Google Scholar] [CrossRef] [PubMed]
- Ayele, K.; Wakimoto, H.; Nauwynck, H.J.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Understanding the interplay between oHSV and the host immune system: Implications for therapeutic oncolytic virus development. Mol. Ther. 2024, 33, 1327–1343. [Google Scholar] [CrossRef] [PubMed]
| Therapy | Specific Agent | Tumor Type | Status | Study Purpose | Treatment Plan | Reference |
|---|---|---|---|---|---|---|
| AAV Therapy | Pep2hSC1 and Pep2hSC2 capsids | pNF–derived Schwann cells (pNF01.3), C57BL/6J mice, hFRG mice (FRG mice engrafted with human hepatocytes) | Preclinical Study | This preclinical study investigates newly engineered AAV capsids, Pep2hSC1 and Pep2hSC2, to determine their capacity to efficiently and selectively transduce human Schwann cells, including those derived from NF1-associated pNF. Both vectors demonstrate substantially higher Schwann-cell tropism than existing AAV serotypes, with Pep2hSC2 showing exceptional specificity by avoiding fibroblast transduction. | 16-week-old male C57BL/6J mice underwent sciatic nerve exposure, bilateral 10-s crush injury, and immediate microinjection of 3 µL AAV (AAV-DJ, Pep2hSC1, or Pep2hSC2) both proximal and distal to the crush site. 6 to 8-week-old hFRG mice were injected intravenously with 2 × 1011 vg of each AAV variant via the tail vein. | Drouyer et al. [77] |
| AAV-SC3 and AAV-SC4 capsids | NF1 and Charcot-Marie Tooth disease involve SCs in C57BL/6 mice and Nf1flox/flox/Flucflox mice | Preclinical Study | This study is designed to develop and evaluate engineered AAV9 capsids—AAV-SC3 and AAV-SC—capable of efficiently targeting Schwann cells and NF1-associated neurofibromas after systemic delivery. | Different dosages of AAV were injected through the tail vein from 1010 vg to 1012 vg/mouse. | Haidar et al. [78] | |
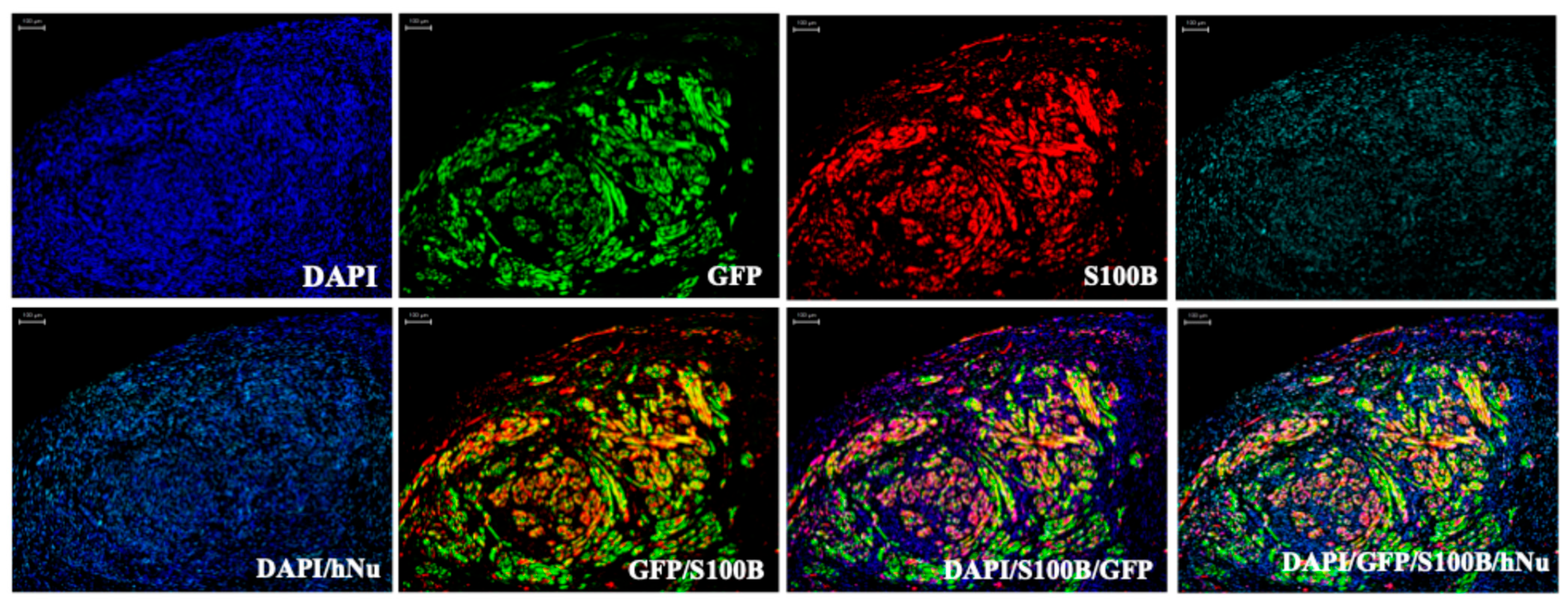
| AAV-K55 capsid with GRDC24 as payload | NF1-related MPNST, pNF, neurofibromas, glioma xenografted in NSG mice | Preclinical Study | This study is designed to develop and evaluate the AAV-K55 capsid, capable of delivering a functional truncated neurofibromin construct, GRDC24, to NF1-associated tumors, with the goal of inhibiting RAS signaling and restoring Schwann-cell function. | Two separate 1 × 1012 vg doses or a single 2 × 1012 vg dose of AAV-GRDC24 were administered by tail-vein injection to xenografted NSG mice. | Bai et al. [79] | |
| oHSV Therapy | IMLYGIC (Talimogene laherparepvec (T-VEC)) | cNF in adults (≥18 yrs) | Phase I (single-arm, open-label, interventional) | This study is designed to evaluate the feasibility, tolerability, and efficacy of IMLYGIC for treating cNF in adults with NF1. | The trial administers IMLYGIC as an intralesional monotherapy delivered over four 28-day treatment cycles, with clinical evaluation throughout to monitor response. | NCT07102394 |
| HSV1716 | Refractory non-CNS solid tumors, including NF1-associated MPNST in adolescents and young adults (7–30 yrs) | Phase I (single-arm, open-label, interventional, non-randomized) | This study is designed to assess the safety and dose-limiting toxicities (DLT) of HSV1716 administered either intratumorally or intravenously in patients with refractory solid tumors, including MPNST. | The trial delivers a single escalating dose with the option for additional dosing in a follow-up study phase, with toxicity assessments at 28 days and long-term immune monitoring for up to 15 years. | NCT00931931 | |
| T-VEC | Locally advanced unresectable soft tissue sarcomas (STS), including NF1-associated MPNST in adults (≥18 yrs) | Phase Ib/II (single arm, open-label, interventional) | This study is designed to evaluate the efficacy and safety of combining neoadjuvant T-VEC with preoperative radiation to improve pathological tumor response in unresectable STS. | The trial administers weekly intratumoral T-VEC starting at week 1, concurrent daily external-beam radiation during weeks 4–8, and continued weekly T-VEC through weeks 9–12, followed by surgical resection 4–6 weeks post-radiation. | NCT06660810 | |
| CAR-T cell Therapy | Arm A: second-generation 4-1BBζ EGFR806-EGFRt CAR-T cells Arm B: second-generation 4-1BBζ EGFR806-EGFRt plus second-generation 4 1BBζ CD19-Her2tG CAR-T cells | Recurrent or refractory malignant non-CNS solid tumors expressing EGFR, including NF1-associated MPNST, in pediatric and young adult patients (1–30 yrs) | Phase I (two-arm, open-label, interventional, non-randomized, parallel-assignment) | This study is designed to evaluate the safety, manufacturability, DLT, and early antitumor activity of autologous EGFR806-based CAR-T products in pediatric and young adult patients with relapsed or refractory EGFR-expressing non-CNS solid tumors, including MPNST. | Patients undergo leukapheresis and receive a single infusion of CD4/CD8 EGFR806 CAR-T cells alone (Arm A) or dual EGFR806xCD19 CAR-T cells (Arm B), followed by intensive monitoring for toxicity, CAR-T cell persistence in blood and bone marrow, and changes in tumor burden. | NCT03618381 |
| Arm A: second-generation 4-1BBζ B7H3-EGFRt-DHFR CAR (B7H3-specific CAR-T cells). Arm B: second-generation 4-1BBζ B7H3-EGFRt-DHFR plus second-generation 4-1BBζ CD19-Her2tG (bispecific B7H3 × CD19 CAR-T cells). Arm C: same bispecific B7H3 × CD19 CAR-T cells as Arm B, given together with pembrolizumab. | Recurrent or refractory malignant non-primary CNS solid tumors expressing B7H3, including NF1-associated MPNST, in pediatric and young adult patients (0–26 yrs) | Phase I (three-arm, open-label, interventional, non-randomized, sequential-assignment) | This study is designed to evaluate the safety, feasibility, DLT, and maximum tolerated dose (MTD) of B7H3-directed CAR-T cell products, as well as their persistence, in children and young adults with relapsed or refractory non-CNS solid tumors, including MPNST. | The trial administers a single infusion of autologous B7H3 CAR-T cells alone (Arm A), bispecific B7H3 × CD19 CAR-T cells (Arm B), or bispecific B7H3 × CD19 CAR-T cells combined with pembrolizumab (Arm C), with serial assessments of toxicity, CAR-T persistence, and tumor response. | NCT04483778 | |
| B7-H3-CAR-T cells post-lymphodepleting chemotherapy | Relapsed or refractory B7-H3-positive non-CNS solid tumors, including NF1-associated MPNST, in pediatric and young adult patients (≤21 yrs) | Phase I (single-arm, open-label, interventional) | This study is designed to evaluate the safety, DLT, and MTD of autologous B7-H3 CAR-T cells in children and young adults with relapsed or refractory B7-H3–expressing solid tumors, including MPNST. | The trial delivers lymphodepleting fludarabine/cyclophosphamide followed by a single weight-based infusion of B7-H3 CAR-T cells, with a 6-week DLT evaluation period and ongoing follow-up through one year before transition to institutional long-term monitoring. | NCT04897321 | |
| Antigen-specific cytokine-activated T cells (CART)/cytotoxic T lymphocytes (CTLs) and dendritic cell vaccine (DCvac) | Neurofibromatosis (NF1, NF2) or schwannomatosis in patients (1–80 yrs) | Phase I/II (single-arm, open-label, interventional) | This study is designed to evaluate the safety and preliminary therapeutic activity of autologous CART/CTL plus DCvac immunotherapy in patients with neurofibromatosis or schwannomatosis who have progressing NF-related tumors. | The trial manufactures patient-specific antigen-reactive CART/CTL products and DCvac from autologous cells and delivers combined CART/CTL/DCvac infusions, with longitudinal monitoring of safety, tumor-associated markers, and radiographic response over 12–24 months. | NCT04085159 | |
| Small Molecule Inhibitors | Selumetinib (AZD6244 hyd sulfate) MEK Inhibitor | NF1-associated pNF patients aged 1 yr and older | FDA Approved | Selumetinib was first approved by the FDA on 10 April 2020, for pediatric NF1 PN patients aged 2 years and above based on the SPRINT Phase II Stratum I study (NCT01362803). On 10 September 2025, this approval was broadened to include patients as young as 1 year old, based on the SPRINKLE study (NCT05309668). | Selumetinib is recommended at 25 mg/m2 orally twice daily, given until progression or intolerable toxicity. | NCT01362803 NCT05309668 |
| Mirdametinib (PD-0325901) MEK Inhibitor | NF1-associated pNF patients aged 2 yrs and above | FDA Approved | Mirdametinib was approved by the FDA on 11 February 2025, for pediatric NF1 PN patients aged 2 years and above who are not amenable to complete surgical resection based on the ReNeu study (NCT03962543). | Mirdametinib is dosed at 2 mg/m2 orally twice daily for 21 days of each 28-day cycle, with or without food, and is continued until progression or intolerable toxicity. | NCT03962543 | |
| Tipifarnib (R115777) RAS Inhibitor | NF1-associated pNF in children and young adults (3–25 yrs) | Phase II (Interventional, randomized, flexible crossover, double-blinded, placebo-controlled trial) | This study is designed to determine whether the farnesyltransferase inhibitor tipifarnib can delay volumetric progression of pNF and to characterize its safety profile in children and young adults with NF1. Tipifarnib was well-tolerated but did not significantly prolong time to progression versus placebo [80]. | The patients receive oral tipifarnib or placebo at 200 mg/m2 twice daily on days 1–21 of repeated 28-day cycles, with clinical evaluation throughout to monitor response. | NCT00021541 | |
| Ulixertinib ERK Inhibitor | NF1-associated low-grade glioma (LGG) (Surgical ≥ 18 yrs; Non-surgical ≥ 12 yrs) | Early Phase I (Interventional, parallel assignment, open-label) | This study is designed to determine whether ulixertinib can cross the blood–brain barrier and to assess the safety and biological effects of preoperative ulixertinib in MAPK-driven gliomas, including NF1-associated LGG. | Participants will receive ulixertinib at the recommended phase II dose of 260 mg/m2 administered every 12 h on a continuous schedule in 28-day cycles, with clinical evaluation throughout to monitor response. | NCT05804227 | |
| Sirolimus (AY-22989) mTOR Inhibitor | NF1-associated pNF in children and young adults ≥ 3 yrs | Phase II (Interventional, single-arm per stratum, multi-cohort design) | This study is designed to assess whether sirolimus can extend time to progression in progressive PN or induce radiographic reduction in non-progressing PN, while evaluating feasibility, toxicity, and drug exposure characteristics in individuals with NF1. Sirolimus does not shrink pNF, but it consistently slows their growth and shows biologic activity with acceptable toxicity. | Treatment consists of continuous twice-daily sirolimus administered in 28-day cycles with individualized dosing to therapeutic trough targets, alongside scheduled MRI volumetrics and clinical assessments to guide ongoing therapy. | NCT00634270 | |
| Everolimus (RAD001) mTOR Inhibitor | NF1-associated pediatric LGG in children and young adults (3–22 yrs) | Phase II (Interventional, single-arm open-label) | This study is designed to evaluate whether daily everolimus can delay progression or induce shrinkage in NF1-associated low-grade gliomas while defining its safety profile and pharmacologic behavior. Across this and other studies, oral everolimus has not shown a significant reduction in lesion size [81]. | Treatment consists of once-daily oral everolimus administered in 28-day continuous cycles at a dose of 5 mg/m2 (maximum 10 mg), beginning on study day 1 and continued for up to 12 cycles or until progression, toxicity, or completion of 48 weeks of therapy [82]. | NCT01158651 | |
| Imatinib (STI-571) RTKs Inhibitor | NF1-associated pNF (3–65 yrs) | Phase II (Interventional, single-arm, open-label) | This study is designed to determine whether daily imatinib can produce radiographic or clinical responses in NF1-associated pNF while characterizing toxicity and biomarker changes. Objective responses occurred in 17% of the intention-to-treat population and in 26% of those receiving imatinib for 6 months or longer, each defined as ≥20% PN volume reduction. Most toxicities were mild, including rash and edema, while serious events such as neutropenia, hyperglycemia, and hepatic enzyme elevation were uncommon and reversible [83]. | Treatment consists of oral administration at 220 mg/m2 twice daily in children and 400 mg/m2 twice daily in adults, with dose reductions for toxicity. | NCT01673009 | |
| Sorafenib (BAY 43-9006, Nexavar) RTKs Inhibitor | Pediatric Ras-driven tumors, specifically NF1-associated inoperable pNF in children and young adults (3–18 yrs) | Phase I (Interventional, dose-escalation, single-arm, open-label) | This study is designed to establish the maximum tolerated dose and characterize the safety and biologic activity of sorafenib in NF1-associated pNF. | Treatment consists of continuous twice-daily sorafenib in 28-day cycles, with clinical evaluation throughout to monitor response. | NCT00727233 [84] | |
| Cabozantinib (XL l84) RTKs Inhibitor | NF1-associated pNF in children (3–15 yrs) | Phase II (Interventional, single-arm, open-label) | This study is designed to evaluate whether cabozantinib can achieve meaningful volumetric reduction in NF1-associated pNF, while establishing its tolerability and pharmacokinetic profile in pediatric and adult patients. Cabozantinib met its primary endpoint, achieving partial responses in 42% of evaluable patients, with a median 15.2% tumor-volume reduction and no on-treatment progression, while secondary analyses demonstrated consistent safety, pharmacokinetic profiles, and improvements in pain and quality-of-life measures [85]. | Cohort B (ages 3–15 yrs): 30 mg/m2 daily with escalation to 40 mg/m2 at cycle 3 if tolerated; reductions to 23–30 mg/m2 for toxicity. | NCT02101736 | |
| Pexidartinib (PLX3397) RTKs inhibitor | NF1-associated pNF and MPNST in children and adults (3–35 yrs) | Phase I (Interventional, single-arm, open-label) | This study is designed to define the safety profile and recommended phase II dose of pexidartinib in children and young adults with refractory malignancies, including NF1 pnF and MPNST. | Therapy consists of once-daily oral dosing (125 mg capsules) in continuous 28-day cycles with escalation based on MTD and expansion at RP2D to evaluate toxicity, PK, and early signals of clinical activity. | NCT02390752 | |
| Neoadjuvant nivolumab plus ipilimumab PD-1 inhibitor and CTLA-4 inhibitor | NF1-associated pre-malignant neurofibroma and MPNST in patients (12–100 yrs) | Phase I (Interventional, single-group, open-label, early-phase) | This study is designed to test the safety and feasibility of administering dual checkpoint inhibition before surgical resection of NF1-associated ANF or MPNST | Participants receive neoadjuvant combination immunotherapy with nivolumab and ipilimumab prior to standard-of-care management. Nivolumab is administered at 4.5 mg/kg intravenously every 3 weeks for 2 doses, together with ipilimumab 1 mg/kg intravenously every 3 weeks for 2 doses, with clinical evaluation throughout to monitor response. | NCT04465643 | |
| ASTX727 (INQOVI, combination of cedazuridine and decitabine) Cytidine deaminase (CDA) inhibitor; DNA methyltransferase (DNMT) inhibitor; | PRC2-loss MPNST in adults or adolescents | Phase II (Interventional, single-arm, open-label, single-group) | This study is designed to evaluate the therapeutic activity, safety, and tolerability of oral ASTX727 in patients meeting eligibility criteria for hypomethylating-agent-based therapy, with additional assessment of hematologic and clinical responses. | The trial administers oral cedazuridine/decitabine once daily on days 1–5 of each 21-day cycle with pegfilgrastim support on day 7, with clinical evaluation throughout to monitor response. | NCT04872543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Xu, M.; Chen, X.; Xu, H.; Sun, N.; Weisgerber, K.E.; Bai, R.-Y. Neurofibromatosis Type 1: Genetic Mechanisms and Advances in Therapeutic Innovation. Cancers 2025, 17, 3788. https://doi.org/10.3390/cancers17233788
Lu Y, Xu M, Chen X, Xu H, Sun N, Weisgerber KE, Bai R-Y. Neurofibromatosis Type 1: Genetic Mechanisms and Advances in Therapeutic Innovation. Cancers. 2025; 17(23):3788. https://doi.org/10.3390/cancers17233788
Chicago/Turabian StyleLu, Yuqing, Manzhu Xu, Xiaojun Chen, Huazhen Xu, Nihao Sun, Karis E. Weisgerber, and Ren-Yuan Bai. 2025. "Neurofibromatosis Type 1: Genetic Mechanisms and Advances in Therapeutic Innovation" Cancers 17, no. 23: 3788. https://doi.org/10.3390/cancers17233788
APA StyleLu, Y., Xu, M., Chen, X., Xu, H., Sun, N., Weisgerber, K. E., & Bai, R.-Y. (2025). Neurofibromatosis Type 1: Genetic Mechanisms and Advances in Therapeutic Innovation. Cancers, 17(23), 3788. https://doi.org/10.3390/cancers17233788






