Integrating Surgery and Ablative Therapies for the Management of Multiple Primary Lung Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis and Publication Bias
3. Results
3.1. Study Selection
3.2. The Characteristics of Included Studies
3.3. Quality and Risk of Bias Within Studies
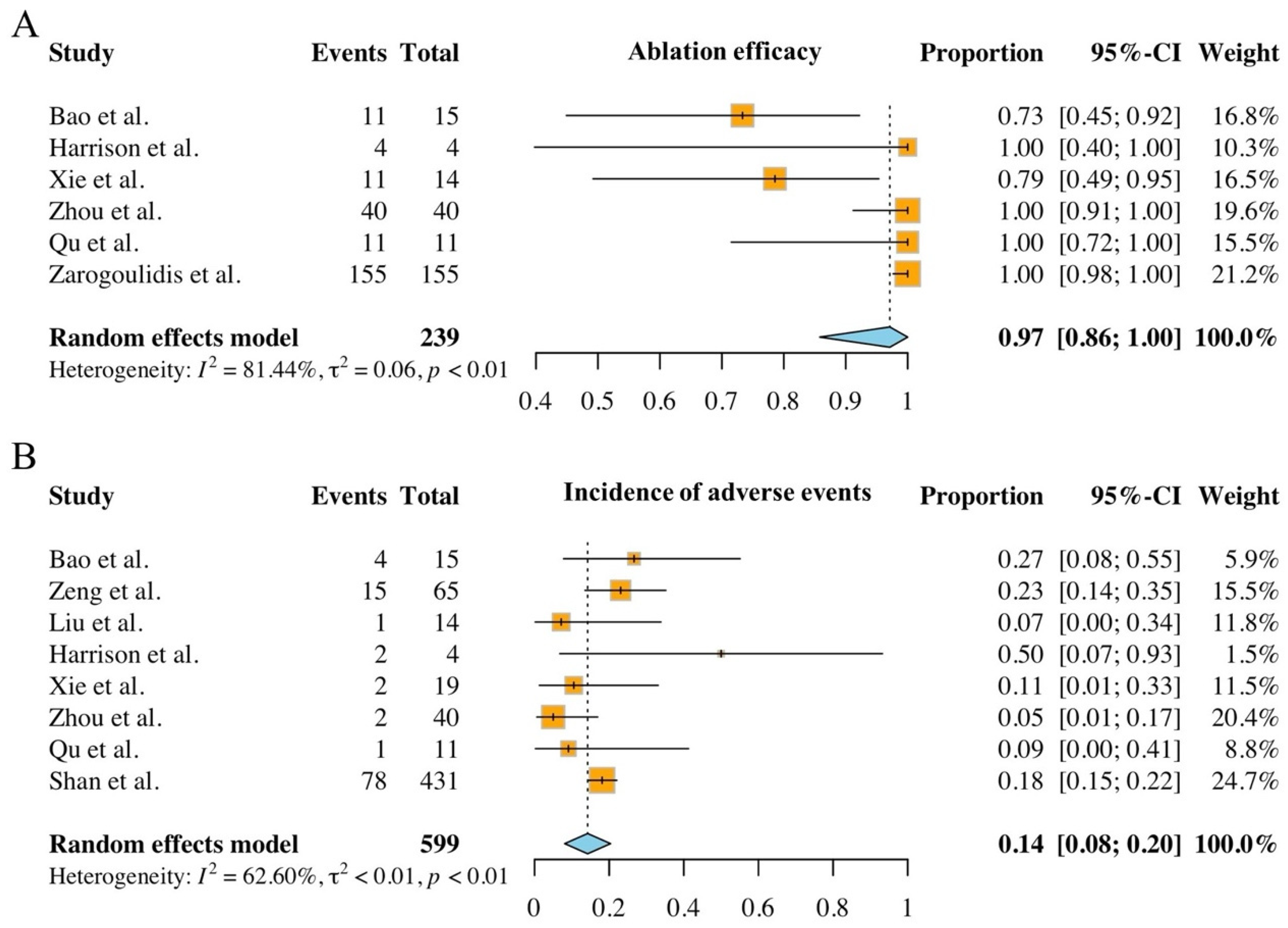
3.4. The Ablation Safety and Efficacy of Included Studies
3.5. Ablation-Related Adverse Events and Complications
3.6. Meta-Analysis
4. Case Report
4.1. Clinical History
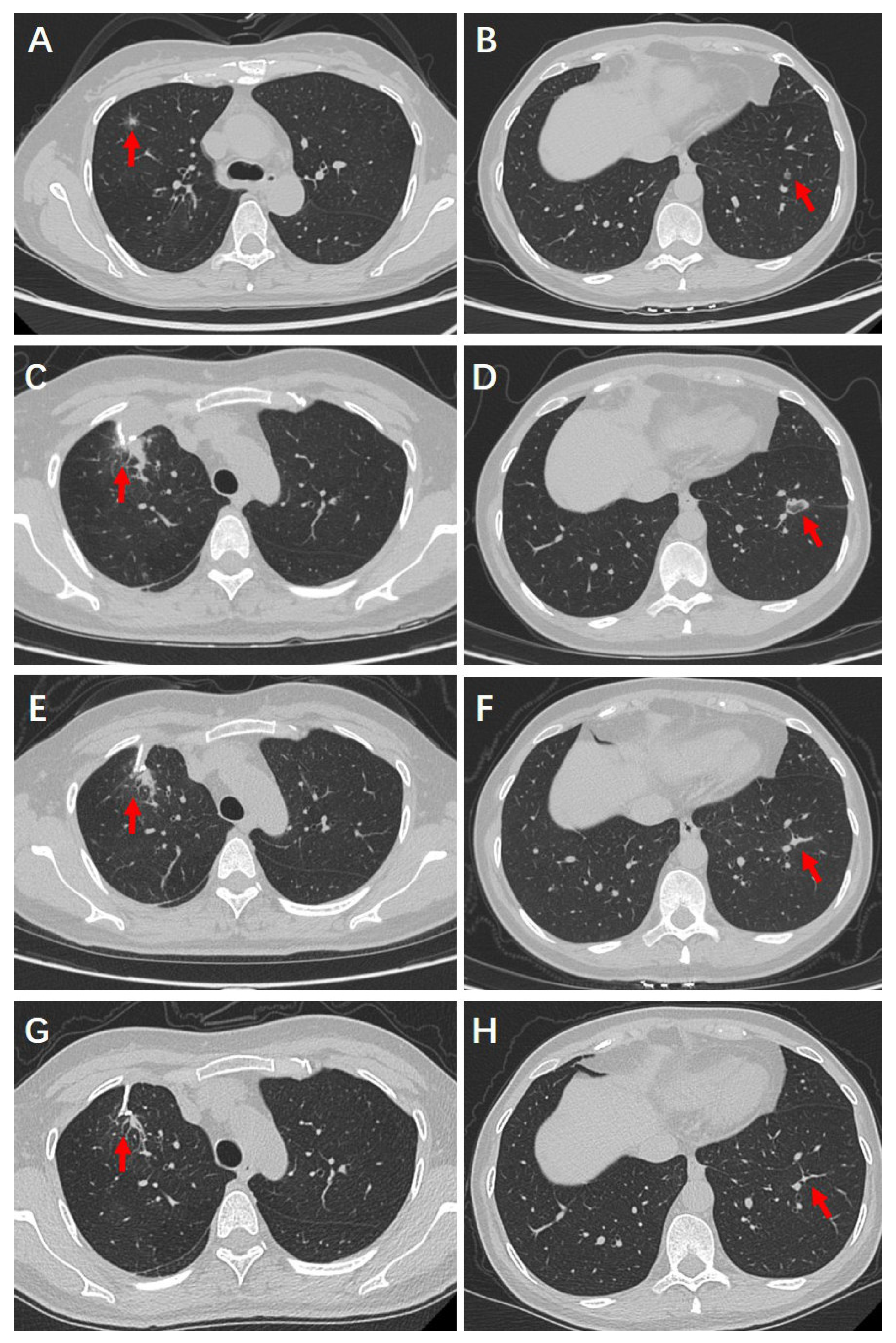
4.2. Preoperative Examinations
4.3. Surgical and Ablative Management
4.4. Postoperative Recovery and Follow-Up Outcomes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolgin, E. Oncogene-specific advocacy groups bring a patient-centric perspective to studies of lung cancer. Nature 2020, 587, S16–S17. [Google Scholar] [CrossRef]
- Healey, N. Better treatments for lung cancer that spreads to the brain. Nature 2020, 587, S14–S15. [Google Scholar] [CrossRef]
- Brody, H. Lung cancer. Nature 2020, 587, S7. [Google Scholar] [CrossRef]
- Chen, M.C.; Yang, H.S.; Dong, Z.; Li, L.J.; Li, X.M.; Luo, H.H.; Li, Q.; Zhu, Y. Immunogenomic features of radiologically distinctive nodules in multiple primary lung cancer. Cancer Immunol. Immunother. CII 2024, 73, 217. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Wang, C.; Herth, F.J.F.; Guo, Z.; Zhang, X. Multiple primary lung cancer: Updates and perspectives. Int. J. Cancer 2024, 155, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, B.; Guo, W.; Peng, Y.; Tian, H.; Xu, J.; Wang, S.; Chen, X.; Hu, B.; Liu, C.; et al. Genomic characteristics and immune landscape of super multiple primary lung cancer. EBioMedicine 2024, 101, 105019. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhou, B.; Bie, F.; Huai, Q.; Xue, X.; Guo, L.; Tan, F.; Xue, Q.; Zhao, L.; Gao, S. Single-cell RNA sequencing analysis reveals transcriptional heterogeneity of multiple primary lung cancer. Clin. Transl. Med. 2023, 13, e1453. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Zhou, L.; Zhao, J.; Ma, X.; Yang, F.; Qi, W. Sublobar resection versus lobectomy in the treatment of synchronous multiple primary lung cancer. World J. Surg. Oncol. 2023, 21, 135. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Gao, S.; Xue, Q.; Tan, F.; Li, Z.; Gao, Y. Plasma metabolomics study in screening and differential diagnosis of multiple primary lung cancer. Int. J. Surg. Lond. Engl. 2023, 109, 297–312. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Yang, Z.; Chen, P.; Xu, J.; Tian, Y.; Fan, T.; Xiao, C.; Bai, G.; Li, L.; et al. Genetic trajectory and clonal evolution of multiple primary lung cancer with lymph node metastasis. Cancer Gene Ther. 2023, 30, 507–520. [Google Scholar] [CrossRef]
- Cheng, H.; Guo, Z.; Zhang, X.; Wang, X.J.; Li, Z.; Huo, W.W.; Zhong, H.-C.; Li, X.-J.; Wu, X.-W.; Li, W.-H.; et al. Lack of evolutionary convergence in multiple primary lung cancer suggests insufficient specificity of personalized therapy. J. Genet. Genomics Yi Chuan Xue Bao 2023, 50, 330–340. [Google Scholar] [CrossRef]
- Huo, J.W.; Luo, T.Y.; He, X.Q.; Gong, J.W.; Lv, F.J.; Li, Q. Radiological classification, gene-mutation status, and surgical prognosis of synchronous multiple primary lung cancer. Eur. Radiol. 2022, 32, 4264–4274. [Google Scholar] [CrossRef]
- Selvam, M.; Chandrasekharan, A.; Sadanandan, A.; Anand, V.K.; Ramesh, S.; Murali, A.; Krishnamurthi, G. Radiomics analysis for distinctive identification of COVID-19 pulmonary nodules from other benign and malignant counterparts. Sci. Rep. 2024, 14, 7079. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, Y.; Vladzymyrskyy, A.; Arzamasov, K.; Omelyanskaya, O.; Shulkin, I.; Kozikhina, D.; Goncharova, I.; Reshetnikov, R.; Chetverikov, S.; Blokhin, I.; et al. Clinical application of radiological AI for pulmonary nodule evaluation: Replicability and susceptibility to the population shift caused by the COVID-19 pandemic. Int. J. Med. Inf. 2023, 178, 105190. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, M.; Rea, G.; Capitelli, L.; Lieto, R.; Bruzzese, D. Chest CT Lung Abnormalities 1 Year after COVID-19: A Systematic Review and Meta-Analysis. Radiology 2023, 308, e230535. [Google Scholar] [CrossRef]
- Hou, N.; Wang, L.; Li, M.; Xie, B.; He, L.; Guo, M.; Liu, S.; Wang, M.; Zhang, R.; Wang, K. Do COVID-19 CT features vary between patients from within and outside mainland China? Findings from a meta-analysis. Front. Public Health 2022, 10, 939095. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Z.; Nath, A.; Khan, A.; Neyaz, Z.; Marak, R.S.K.; Areekkara, P.; Tiwari, A.; Srivastava, S.; Agarwal, V.; Saxena, S.; et al. New insights into development and mortality of COVID-19-associated pulmonary aspergillosis in a homogenous cohort of 1161 intensive care patients. Mycoses 2022, 65, 1010–1023. [Google Scholar] [CrossRef]
- Shen, C.; Wang, X.; Tian, L.; Che, G. Microsatellite alteration in multiple primary lung cancer. J. Thorac. Dis. 2014, 6, 1499–1505. [Google Scholar]
- Shen, C.; Wang, X.; Tian, L.; Zhou, Y.; Chen, D.; Du, H.; Wang, W.; Liu, L.; Che, G. “Different trend” in multiple primary lung cancer and intrapulmonary metastasis. Eur. J. Med. Res. 2015, 20, 17. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Yao, F.; Yu, K.; Gu, H.; Han, B.; Zhao, H. Surgical Therapy for Bilateral Multiple Primary Lung Cancer. Ann. Thorac. Surg. 2016, 101, 1145–1152. [Google Scholar] [CrossRef]
- Shen, C.; Che, G. A Different Method in Diagnosis of Multiple Primary Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, e53–e54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Tang, Y.; Xue, Z.; Jin, X.; Ma, G.; Zhao, P.; Chu, X. SUVmax Ratio on PET/CT May Differentiate Between Lung Metastases and Synchronous Multiple Primary Lung Cancer. Acad. Radiol. 2020, 27, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, L.; Yin, G.; Zhang, J.; Zheng, S.; Cheung, H.; Wu, N.; Lu, N.; Mao, X.; et al. Genomic heterogeneity of multiple synchronous lung cancer. Nat. Commun. 2016, 7, 13200. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Fu, Y.; Cai, M.C.; Yan, Y.; Jing, Y.; Zhang, S.; Chen, M.; Wu, J.; Shen, Y.; Zhu, L.; et al. Simultaneous evolutionary expansion and constraint of genomic heterogeneity in multifocal lung cancer. Nat. Commun. 2017, 8, 823. [Google Scholar] [CrossRef]
- Goodwin, D.; Rathi, V.; Conron, M.; Wright, G.M. Genomic and Clinical Significance of Multiple Primary Lung Cancers as Determined by Next-Generation Sequencing. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1166–1175. [Google Scholar] [CrossRef]
- He, X.; Yang, Z.; Wu, F.; Liang, Q.; Liu, W.; Yu, F.; Chen, C. Confronting synchronous multiple primary lung cancers: Navigating the intersection of challenges and opportunities. Lung Cancer Amst. Neth. 2024, 197, 107994. [Google Scholar] [CrossRef]
- Chen, T.F.; Xie, C.Y.; Rao, B.Y.; Shan, S.C.; Zhang, X.; Zeng, B.; Lei, Y.Y.; Luo, H.H. Surgical treatment to multiple primary lung cancer patients: A systematic review and meta-analysis. BMC Surg. 2019, 19, 185. [Google Scholar] [CrossRef]
- Shimada, Y.; Saji, H.; Otani, K.; Maehara, S.; Maeda, J.; Yoshida, K.; Kato, Y.; Hagiwara, M.; Kakihana, M.; Kajiwara, N.; et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer Amst. Neth. 2015, 88, 174–180. [Google Scholar] [CrossRef]
- Leventakos, K.; Peikert, T.; Midthun, D.E.; Molina, J.R.; Blackmon, S.; Nichols, F.C.; Garces, Y.I.; Hallemeier, C.L.; Murphy, S.J.; Vasmatzis, G.; et al. Management of Multifocal Lung Cancer: Results of a Survey. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 1398–1402. [Google Scholar] [CrossRef]
- Moussa, A.M.; Ziv, E.; Solomon, S.B.; Camacho, J.C. Microwave Ablation in Primary Lung Malignancies. Semin. Interv. Radiol. 2019, 36, 326–333. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, X.; Ju, R.; Leng, J.; Tian, J.; Qu, S.; Tao, S.; Lyu, Y.; Zhang, N. Challenges in clinical practice, biological mechanism and prospects of physical ablation therapy for COPD. Life Sci. 2024, 349, 122718. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.E.; Issa, P.P.; Hussein, M.H.; Elshazli, R.M.; Haidari, M.; Errami, Y.; Shama, M.; Fawzy, M.S.; Kandil, E.; Toraih, E. Clinical outcomes and tumor microenvironment response to radiofrequency ablation therapy: A systematic review and meta-analysis. Gland Surg. 2024, 13, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qi, L.; Chen, J.; Lin, Q.; Yan, Y.; Chen, J.; Lin, Z. Microwave ablation therapy assisted by artificial pneumothorax and artificial hydrothorax for lung cancer adjacent to the vital organs. Front. Oncol. 2022, 12, 981789. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; He, Y.; Hu, Q.; Peng, M.; Zhang, Z.; Xie, S.; Yu, F. Survival benefit of thermal ablation therapy for patients with stage II-III non-small cell lung cancer: A propensity-matched analysis. Front. Oncol. 2022, 12, 984932. [Google Scholar] [CrossRef]
- Li, L.Z.; Wu, J.M.; Chen, T.; Zhao, L.C.; Zhuang, J.N.; Hong, H.S.; Zhang, A.; Zhang, H.T.; Fang, C.T. Ablation Therapy Combined with EGFR TKIs in the Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 6624429. [Google Scholar] [CrossRef]
- Li, M.; Qin, Y.; Mei, A.; Wang, C.; Fan, L. Effectiveness of radiofrequency ablation therapy for patients with unresected Stage IA non-small cell lung cancer. J. Cancer Res. Ther. 2020, 16, 1007–1013. [Google Scholar] [CrossRef]
- Halsey, K.; Wu, J.; Su, C.; Hsieh, B.; Yi, T.; Collins, S.A.; Kimia, B.; Zhang, P.J.; Healey, T.; Zhang, Z.; et al. Ablation Therapy for Advanced Stage Non-Small Cell Lung Cancer: A National Cancer Database Study. J. Vasc. Interv. Radiol. JVIR 2020, 31, 1210–1215.e4. [Google Scholar] [CrossRef]
- Qi, H.; Fan, W. Value of ablation therapy in the treatment of lung metastases. Thorac. Cancer 2018, 9, 199–207. [Google Scholar] [CrossRef]
- Dent, T.H.S. Microwave ablation therapy of pulmonary metastases. Radiology 2013, 266, 995–996. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. Lond. Engl. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Huffman, M.D.; Thomas, L.E. Tools for Evaluating and Improving Causal Inference: Introducing JAMA Cardiology Readers to the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) Tool. JAMA Cardiol. 2018, 3, 907. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Chalumeau, M.; Cohen, R.; Korevaar, D.A.; Khoshnood, B.; Bossuyt, P.M.M. Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J. Clin. Epidemiol. 2015, 68, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Bao, F.; Yu, F.; Wang, R.; Chen, C.; Zhang, Y.; Lin, B.; Wang, Y.; Hao, X.; Gu, Z.; Fang, W. Electromagnetic bronchoscopy guided microwave ablation for early stage lung cancer presenting as ground glass nodule. Transl. Lung Cancer Res. 2021, 10, 3759–3770. [Google Scholar] [CrossRef]
- Zeng, C.; Fu, X.; Yuan, Z.; Hu, S.; Wang, X.; Ping, W.; Cai, Y.; Wang, J. Application of electromagnetic navigation bronchoscopy-guided microwave ablation in multiple pulmonary nodules: A single-centre study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2022, 62, ezac071. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Su, L.; Wang, R. Treatment options for pulmonary multifocal ground glass opacity type adenocarcinoma: Surgery combine thermal ablation? J. Interv. Med. 2020, 3, 180–183. [Google Scholar] [CrossRef]
- Harrison, O.J.; Sarvananthan, S.; Tamburrini, A.; Peebles, C.; Alzetani, A. Image-guided combined ablation and resection in thoracic surgery for the treatment of multiple pulmonary metastases: A preliminary case series. JTCVS Tech. 2021, 9, 156–162. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.; Jiang, Y.; Sun, J.; Hogarth, D.K.; Herth, F.J.F. Microwave ablation via a flexible catheter for the treatment of nonsurgical peripheral lung cancer: A pilot study. Thorac. Cancer 2022, 13, 1014–1020. [Google Scholar] [CrossRef]
- Zhou, D.; Yao, T.; Huang, X.; Wu, F.; Jiang, Y.; Peng, M.; Qian, B.; Liu, W.; Yu, F.; Chen, C. Real-world comprehensive diagnosis and “Surgery + X” treatment strategy of early-stage synchronous multiple primary lung cancer. Cancer Med. 2023, 12, 12996–13006. [Google Scholar] [CrossRef]
- Qu, R.; Tu, D.; Hu, S.; Wang, Q.; Ping, W.; Hao, Z.; Cai, Y.; Zhang, N.; Wang, J.; Fu, X. Electromagnetic Navigation Bronchoscopy-Guided Microwave Ablation Combined With Uniportal Video-Assisted Thoracoscopic Surgery for Multiple Ground Glass Opacities. Ann. Thorac. Surg. 2022, 113, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Hohenforst-Schmidt, W.; Chen, W.; Porpodis, K.; Kosmidis, C.; Kotsakis, A.; Perdikouri, E.-I.; Tolis, C.; Ioannidis, A.; Sapalidis, K.; et al. Endobronchial Radiofrequency Ablation for pulmonary nodules with Radial-Ebus and Navigation: Pros and Cons. J. Cancer 2023, 14, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.-Q.; Wang, H.-Y.; He, X.-N.; Jiang, S.-S.; Wang, H.-H.; Lin, F.-X. Feasibility analysis of CT-guided thermal ablation of multiple pulmonary nodules combined with intraoperative biopsy. Front. Radiol. 2022, 2, 1036026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, J.; Shi, X.; Shen, J.; Liang, W.; Yang, C.; He, J. Prognosis of synchronous and metachronous multiple primary lung cancers: Systematic review and meta-analysis. Lung Cancer Amst. Neth. 2015, 87, 303–310. [Google Scholar] [CrossRef]
- Thakur, M.K.; Ruterbusch, J.J.; Schwartz, A.G.; Gadgeel, S.M.; Beebe-Dimmer, J.L.; Wozniak, A.J. Risk of Second Lung Cancer in Patients with Previously Treated Lung Cancer: Analysis of Surveillance, Epidemiology, and End Results (SEER) Data. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 46–53. [Google Scholar] [CrossRef]
- Lou, F.; Huang, J.; Sima, C.S.; Dycoco, J.; Rusch, V.; Bach, P.B. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J. Thorac. Cardiovasc. Surg. 2013, 145, 75–81, discussion 81–82. [Google Scholar] [CrossRef]
- Martini, N.; Melamed, M.R. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 1975, 70, 606–612. [Google Scholar] [CrossRef]
- Antakli, T.; Schaefer, R.F.; Rutherford, J.E.; Read, R.C. Second primary lung cancer. Ann. Thorac. Surg. 1995, 59, 863–866, discussion 867. [Google Scholar] [CrossRef]
- Kozower, B.D.; Larner, J.M.; Detterbeck, F.C.; Jones, D.R. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e369S–e399S. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H. Multiple primary cancers or multiple metastases, that is the question. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 930–931. [Google Scholar] [CrossRef]
- Suh, Y.J.; Lee, H.-J.; Sung, P.; Yoen, H.; Kim, S.; Han, S.; Park, S.; Hong, J.H.; Kim, H.; Lim, J.; et al. A Novel Algorithm to Differentiate Between Multiple Primary Lung Cancers and Intrapulmonary Metastasis in Multiple Lung Cancers With Multiple Pulmonary Sites of Involvement. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, C.; Li, J.; Song, L.; Li, L.; Tian, P.; Li, W. A comprehensive algorithm to distinguish between MPLC and IPM in multiple lung tumors patients. Ann. Transl. Med. 2020, 8, 1137. [Google Scholar] [CrossRef]
- Girard, N.; Deshpande, C.; Lau, C.; Finley, D.; Rusch, V.; Pao, W.; Travis, W.D. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am. J. Surg. Pathol. 2009, 33, 1752–1764. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Franklin, W.A.; Nicholson, A.G.; Girard, N.; Arenberg, D.A.; Travis, W.D.; Mazzone, P.J.; Marom, E.M.; Donington, J.S.; Tanoue, L.T.; et al. The IASLC Lung Cancer Staging Project: Background Data and Proposed Criteria to Distinguish Separate Primary Lung Cancers from Metastatic Foci in Patients with Two Lung Tumors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 651–665. [Google Scholar] [CrossRef]
- Mansuet-Lupo, A.; Barritault, M.; Alifano, M.; Janet-Vendroux, A.; Zarmaev, M.; Biton, J.; Velut, Y.; Le Hay, C.; Cremer, I.; Régnard, J.-F.; et al. Proposal for a Combined Histomolecular Algorithm to Distinguish Multiple Primary Adenocarcinomas from Intrapulmonary Metastasis in Patients with Multiple Lung Tumors. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 844–856. [Google Scholar] [CrossRef]
- Naidich, D.P.; Bankier, A.A.; MacMahon, H.; Schaefer-Prokop, C.M.; Pistolesi, M.; Goo, J.M.; Macchiarini, P.; Crapo, J.D.; Herold, C.J.; Austin, J.H.; et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: A statement from the Fleischner Society. Radiology 2013, 266, 304–317. [Google Scholar] [CrossRef]
- Lee, D.S.; LaChapelle, C.; Taioli, E.; Kaufman, A.; Wolf, A.; Nicastri, D.; Flores, R.M. Second Primary Lung Cancers Demonstrate Similar Survival With Wedge Resection and Lobectomy. Ann. Thorac. Surg. 2019, 108, 1724–1728. [Google Scholar] [CrossRef]
- Lee, P.; Loo, B.W.; Biswas, T.; Ding, G.X.; El Naqa, I.M.; Jackson, A.; Kong, F.-M.; LaCouture, T.; Miften, M.; Solberg, T.; et al. Local Control following Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 160–171. [Google Scholar] [CrossRef]
- Csiki, E.; Simon, M.; Papp, J.; Barabás, M.; Mikáczó, J.; Gál, K.; Sipos, D.; Kovács, Á. Stereotactic body radiotherapy in lung cancer: A contemporary review. Pathol. Oncol. Res. 2024, 30, 1611709. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, K.; Liu, S.Y.; Yan, L.X.; Su, J.; Wu, Y.L.; Zhang, X.-C.; Zhong, W.Z.; Yang, X.N. Multiomics analysis reveals a distinct response mechanism in multiple primary lung adenocarcinoma after neoadjuvant immunotherapy. J. Immunother. Cancer 2021, 9, e002312. [Google Scholar] [CrossRef]
- Xu, L.; Shi, M.; Wang, S.; Li, M.; Yin, W.; Zhang, J.; Zhu, J.; Jiang, F.; Xia, W.; Qiu, N.; et al. Immunotherapy for bilateral multiple ground glass opacities: An exploratory study for synchronous multiple primary lung cancer. Front. Immunol. 2022, 13, 1009621. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, D.E.; Zagoria, R.J.; Akerley, W.; Mayo-Smith, W.W.; Kavanagh, P.V.; Safran, H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am. J. Roentgenol. 2000, 174, 57–59. [Google Scholar] [CrossRef]
- Ni, Y.; Xu, H.; Ye, X. Image-guided percutaneous microwave ablation of early-stage non-small cell lung cancer. Asia Pac. J. Clin. Oncol. 2020, 16, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Quirk, M.T.; Lee, S.; Murali, N.; Genshaft, S.; Abtin, F.; Suh, R. Alternatives to Surgery for Early-Stage Non-Small Cell Lung Cancer: Thermal Ablation. Clin. Chest Med. 2020, 41, 197–210. [Google Scholar] [CrossRef]
- Iguchi, T.; Hiraki, T.; Gobara, H.; Fujiwara, H.; Matsui, Y.; Soh, J.; Toyooka, S.; Kiura, K.; Kanazawa, S. Percutaneous radiofrequency ablation of lung cancer presenting as ground-glass opacity. Cardiovasc. Interv. Radiol. 2015, 38, 409–415. [Google Scholar] [CrossRef]
- Yang, X.; Ye, X.; Lin, Z.; Jin, Y.; Zhang, K.; Dong, Y.; Yu, G.; Ren, H.; Fan, W.; Chen, J.; et al. Computed tomography-guided percutaneous microwave ablation for treatment of peripheral ground-glass opacity-Lung adenocarcinoma: A pilot study. J. Cancer Res. Ther. 2018, 14, 764–771. [Google Scholar] [CrossRef]
- Kodama, H.; Yamakado, K.; Hasegawa, T.; Takao, M.; Taguchi, O.; Fukai, I.; Sakuma, H. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J. Vasc. Interv. Radiol. JVIR 2014, 25, 333–339. [Google Scholar] [CrossRef]
- Ye, X.; Fan, W.; Wang, H.; Wang, J.; Wang, Z.; Gu, S.; Feng, W.; Zhuang, Y.; Liu, B.; Li, X.; et al. Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J. Cancer Res. Ther. 2018, 14, 730–744. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Liu, S.; Liang, B.; Li, Y.; Xu, J.; Qian, W.; Lin, M.; Xu, M.; Niu, L. CT-Guided Percutaneous Cryoablation in Patients with Lung Nodules Mainly Composed of Ground-Glass Opacities. J. Vasc. Interv. Radiol. JVIR 2022, 33, 942–948. [Google Scholar] [CrossRef]
- Huang, G.; Yang, X.; Li, W.; Wang, J.; Han, X.; Wei, Z.; Meng, M.; Ni, Y.; Zou, Z.; Wen, Q.; et al. A feasibility and safety study of computed tomography-guided percutaneous microwave ablation: A novel therapy for multiple synchronous ground-glass opacities of the lung. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2020, 37, 414–422. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, X.; Qin, Z.; Xu, J.; Zeng, J.; Chen, J.; Niu, L.; Xu, M. Computed tomography-guided percutaneous cryoablation for lung ground-glass opacity: A pilot study. J. Cancer Res. Ther. 2019, 15, 370–374. [Google Scholar]
- Liu, B.; Ye, X. Management of pulmonary multifocal ground-glass nodules: How many options do we have? J. Cancer Res. Ther. 2020, 16, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Garg, S.K.; Cohen, P.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Downey, R.J.; Travis, W.D.; Solomon, S.B. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann. Surg. Oncol. 2013, 20 (Suppl. S3), S676–S683. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Country | Study Design | Study Period | Patients | Treatments | Procedure |
|---|---|---|---|---|---|---|---|
| Bao et al. [47] | 2021 | China | Prospective | June 2019–December 2020 | 5 solitary lung cancer and 10 MPLC. | Solitary lung cancer patients underwent MWA, and MPLC ones underwent VATS after MWA guided by ENB. | SuperDimensionTM navigation system; MWA: 40–80 W, 5–10 min; Surgery: VATS. |
| Zeng et al. [48] | 2022 | China | Retrospective | December 2019–June 2021 | 65 MPN patients. | 8 patients only underwent MWA and 57 patients underwent VATS combined with MWA guided by ENB. | SuperDimensionTM navigation system; Median ablation power: 45 (30–70) W; Median ablation time: 3 (3–5) min. |
| Liu et al. [49] | 2020 | China | Retrospective | March 2015–March 2019 | 48 multifocal adenocarcinoma patients presenting GGO. | 43 lesion wedge resections, 7 segmental resections and 17 lobectomy resections; 20 lesion ablations. | Thermal ablation: radiofrequency or microwave ablation. Ablation time: 30–120 min (mean: 43 min). |
| Harrison et al. [50] | 2021 | England | Retrospective | August 2018–January 2020 | 4 patients with multiple lung lesions | iCART | Neuwave percutaneous microwave ablation system; MWA: 60 W, 5 min; Surgery: uniportal VATS. |
| Xie et al. [51] | 2022 | China | Prospective | April 2018–July 2019 | 8 MPLC and 5 solitary lung cancer patients. | 19 lesions were treated with ENB-guided MWA. | ENB or BTPNA; MWV: 50–80 W, 3–10 min. |
| Zhou et al. [52] | 2023 | China | Prospective | April 2015–December 2020 | 582 synchronous MPLC patients. | 1198 lesion resections; CT-guided MWA treated 40 lesions in 37 patients; ENB-guided MWA treated 3 lesions in 3 patients. | Surgery: excision of primary and concurrent foci; “X”: including therapies like ablation and SBRT, management of high-risk residual, progressive, and new lesions. |
| Qu et al. [53] | 2021 | China | Retrospective | October 2015–December 2019 | 11 patients with multiple GGOs. | Surgical resection: primary lesion, invasive adenocarcinoma; Ablation: secondary, preinvasive or uncertain nature lesions. | ENB-guided MWA combined with single-port VATS; MWV: 60–80 W, 4–8 min. |
| Zarogoulidis et al. [54] | 2023 | Greece | Prospective | January 2019–July 2022 | 155 patients with single or multiple pulmonary nodules. | Using RFA to treat single or multiple nodules; All patients underwent pre-procedural PET-CT. | Radial-endobronchial ultrasound Fuji plus C-Arm (75 cases); Archemedes-Bronchus electromagnetic navigation system (80 cases). |
| Shan et al. [55] | 2022 | China | Retrospective | April 2022–July 2022 | 431 MPN patients. | The patients were divided into 4 groups: Group A (107 cases) underwent only CT-guided percutaneous biopsy; Group B (117 cases) was treated only with CT-guided thermal ablation; Groups C (103 cases) and D (104 cases) underwent CT-guided thermal ablation with immediate intraoperative biopsy. | CT-mediated percutaneous thermal ablation; MWV: 30–45 W, 2–5 min |
| Author | Complications | Safety of Ablation | Ablation Efficacy | Incidence of Adverse Events | Follow-Up Period | Progression | Postoperative LOS |
|---|---|---|---|---|---|---|---|
| Bao et al. [47] | 1 hemoptysis in patients with single lesion; 3 cases (air leakage, hemoptysis, lung infection) in patients with MPLC | 100.00% | 73.30% | 26.67% | NA | NA | NA |
| Zeng et al. [48] | 5 cases of pain, 2 of pneumothorax, 2 of subcutaneous emphysema, and 6 of persistent coughing. | 100.00% | NA | 23.08% | NA | No local recurrences or enlargement of pulmonary nodules. | Median: 8 days |
| Liu et al. [49] | 2 cases of air leakage, 1 of chylothorax, and 1 of massive pleural effusion occurred after resection; 1 case of air leak after ablation. | 100.00% | NA | 7.14% | Mean: 16 ± 13 months (range: 5–60 months) | LTP rate: 0.00% | Surgery: 3–25 days (mean: 6.2 days); Ablation: 1–10 days (mean: 3.5 days) |
| Harrison et al. [50] | 2 cases of intraoperative pneumothorax. | 100.00% | 100.00% | 50.00% | Median: 11 months (range: 0–24 months) | No recurrence | NA |
| Xie et al. [51] | 1 case of hemopneumothorax and 1 of pneumothorax. | 100.00% | 78.60% | 10.50% | Median: 33 (95% CI: 30.6–35.4) months | 2 year-LCR: 71.4%; Median PFS: 33 (95% CI: 15.0–51.0) months | NA |
| Zhou et al. [52] | 2 cases of asymptomatic mild pneumothorax. | 100.00% | 100.00% | 5.00% | 3 months after ablation | LTP rate: 0.00% | NA |
| Qu et al. [53] | 1 case of postoperative pneumothorax. | 100.00% | 100.00% | 9.09% | Short-term | No local metastasis or recurrence | Mean: 6.2 ± 2.3 days |
| Zarogoulidis et al. [54] | Minor hemorrhage | 100.00% | 100.00% | NA | 1 year | No recurrences | Radial-ebus ablation 1.6 days; Bronchus ablation: 1.4 days. |
| Shan et al. [55] | Group A: 7 cases of hemoptysis, 8 of pneumothorax and 4 of pleural effusion; Group B: 14 of pneumothorax and 7 of pleural effusion; Group C: 11 of pneumothorax and 5 of pleural effusion; Group D: 13 of pneumothorax and 9 of pleural effusion | 100.00% | NA | 18.20% | NA | NA | A: 5.20 ± 0.88 days; B: 4.96 ± 1.06 days; C: 5.09 ± 1.20 day; D: 5.07 ± 1.01 days. |
| Type | Main Cause | Management | Ablation Types |
|---|---|---|---|
| Pain | Thermal/cold stimulation near pleura or tissue tension | Intra-operation: opioids, sedatives; Post-operation: NSAIDs | RFA, MWA, CA |
| Post-ablation syndrome | Inflammatory cytokine release and necrotic absorption | Symptomatic care; Short-term low-dose corticosteroids | RFA, MWA |
| Cough | Thermal irritation to alveoli, bronchial/pleural membrane | Pre-operation: codeine; Post-operation: antitussives, antibiotics | RFA, MWA |
| Pleural reaction | Vagal nerve stimulation | Pause ablation; Atropine and sedatives | RFA, MWA |
| Pneumothorax | Lung puncture; emphysema/multiple passes | Often self-limited; Chest drainage, Pleurodesis | RFA, MWA, CA |
| Pleural effusion | Thermal injury reaction, lesion close to the pleura | Observation for mild cases; Drainage when symptomatic or large | RFA, MWA |
| Hemorrhage | Vascular injury during puncture or ablation | Hemostatics, lateral positioning, embolization or surgery | RFA, MWA, CA |
| Infection | Underlying lung disease; Immunosuppression; Multiple ablations | Prophylactic antibiotics; Abscess drainage | RFA, MWA, CA |
| Cavity formation | Expected course following necrotic tissue expulsion | Generally self-absorbed; Antibiotics and drainage if infected; Monitor for fungal infection | RFA, MWA |
| Skin frostbite | Probe proximity to skin; Frost formation on needle | Wound care, infection prevention | CA |
| Cold shock | Hypothermia near large vessels | Rewarming; Fluid resuscitation; Vasopressors | CA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Shen, C.; Zhang, J.; Zhou, J.; Lin, X.; Wang, B.; Liao, H. Integrating Surgery and Ablative Therapies for the Management of Multiple Primary Lung Cancer: A Systematic Review. Cancers 2025, 17, 3699. https://doi.org/10.3390/cancers17223699
Dong Z, Shen C, Zhang J, Zhou J, Lin X, Wang B, Liao H. Integrating Surgery and Ablative Therapies for the Management of Multiple Primary Lung Cancer: A Systematic Review. Cancers. 2025; 17(22):3699. https://doi.org/10.3390/cancers17223699
Chicago/Turabian StyleDong, Zhenghao, Cheng Shen, Jingwen Zhang, Jian Zhou, Xiang Lin, Beinuo Wang, and Hu Liao. 2025. "Integrating Surgery and Ablative Therapies for the Management of Multiple Primary Lung Cancer: A Systematic Review" Cancers 17, no. 22: 3699. https://doi.org/10.3390/cancers17223699
APA StyleDong, Z., Shen, C., Zhang, J., Zhou, J., Lin, X., Wang, B., & Liao, H. (2025). Integrating Surgery and Ablative Therapies for the Management of Multiple Primary Lung Cancer: A Systematic Review. Cancers, 17(22), 3699. https://doi.org/10.3390/cancers17223699