SCODA: A Low-Cost Prehabilitation Strategy to Improve Outcomes After Cytoreductive Surgery in a Low-Resource Setting
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Eligibility and Selection
2.3. Intervention: The SCODA Program
- Iron supplementation (ferrous sulfate 325 mg/day) aimed to correct preoperative anemia, a prevalent and modifiable risk factor for transfusions, particularly in LMIC contexts. The 90-day duration corresponded to the erythropoiesis cycle, allowing time for hematologic response.
- Food-based nutritional prehabilitation: (1.5–2 g of protein/kg/day) Nutritional counseling prioritized low-cost, locally available protein sources, including legumes (lentils, chickpeas), eggs, and sardines combined with caloric enhancers such as olive oil and nuts. While patients were encouraged to incorporate additional protein sources when feasible, the core dietary recommendations were designed to remain accessible in low-resource settings.
- Graded walking regimen, progressively increasing from 30 to 90 min daily over three weeks, was prescribed to improve cardiopulmonary fitness and reduce the risk of pulmonary complications.
2.4. ERAS Protocol and Pre-Anesthesia Optimization
2.4.1. Cardiopulmonary Optimization
2.4.2. Nutritional and Hematologic Preparation
2.4.3. Psychological Counseling
2.5. Intraoperative Management and Postoperative Phase
2.5.1. Hemodynamic Monitoring
2.5.2. Anesthetic Protocol
2.5.3. Surgical Safety Measures
2.5.4. Early Mobilization and Vigilance
2.6. Outcome Measures
2.7. Data Collection and Analysis
3. Results
3.1. Patient Demographics and Baseline Characteristics
3.2. Perioperative Outcomes
3.3. Multivariate Analysis
4. Discussion
4.1. A Pragmatic Prehabilitation Model for LMIC Settings
4.2. Physical Activity and Duration of Prehabilitation
4.3. Nutritional Support and Protein Intake
4.4. Iron Supplementation and Transfusion Avoidance
4.5. Psychological Support and Program Adherence
4.6. Combined Impact on Postoperative Outcomes
4.7. Reducing Pulmonary Complications and Mortality
4.8. Implementation and Sustainability
4.9. Policy Implication
4.10. Challenges and Lessons Learned
4.11. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souadka, A.; Habbat, H.; Makni, A.; Abid, M.; El Mouatassim, Z.; Daghfous, A.; Korjani, Z.; Rebai, W.; Ayadi, M.; Messai, W.H.; et al. Advancing Treatment Outcomes for Peritoneal Surface Malignancies in Low- and Middle-Income Countries: Insights from the First Multicenter Study in North Africa. Cancers 2025, 17, 2113. [Google Scholar] [CrossRef]
- Jo, J.W.; Suh, J.W.; Lee, S.C.; Namgung, H.; Park, D.-G. Current Status of Postoperative Morbidity Following Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer with Peritoneal Metastasis: A Prospective Single-Center Observational Study. Ann. Surg. Treat. Res. 2025, 108, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ma, Y.; Li, J.; Wen, L.; Zhang, G.; Huang, C.; Yao, X. Risk Factors for Postoperative Complications in Patients Undergoing Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy: A Meta-Analysis and Systematic Review. Int. J. Color. Dis. 2024, 39, 167. [Google Scholar] [CrossRef]
- Cortés-Guiral, D.; Mohamed, F.; Glehen, O.; Passot, G. Prehabilitation of Patients Undergoing Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Malignancy. Eur. J. Surg. Oncol. 2021, 47, 60–64. [Google Scholar]
- Strijker, D.; Meijerink, W.J.H.J.; van Heusden-Schotalbers, L.A.G.; van den Berg, M.G.A.; van Asseldonk, M.J.M.D.; Drager, L.D.; de Wilt, J.H.W.; van Laarhoven, K.J.H.M.; van den Heuvel, B. Multimodal Prehabilitation in Patients Undergoing Complex Colorectal Surgery, Liver Resection, and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Pilot Study on Feasibility and Potential Efficacy. Cancers 2023, 15, 1870. [Google Scholar] [CrossRef]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-Risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of Community-Based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2022, 275, e299–e306. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, M.; van Dalen, D.H.; Nahar-van Venrooij, L.M.W.; Meijerink, W.J.H.J.; Verdaasdonk, E.G.G. A Multimodal Prehabilitation Program in High-Risk Patients Undergoing Elective Resection for Colorectal Cancer: A Retrospective Cohort Study. Eur. J. Surg. Oncol. 2021, 47, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Stockley, C.; Bouchard-Fortier, A.; Mateshaytis, J.; Taqi, K.; Mack, L.; Nelson, G.; Chong, M.; Deban, M. Implementation of a Multidisciplinary Enhanced Recovery After Surgery (ERAS) Program for Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC). J. Surg. Oncol. 2024, 131, 527–534. [Google Scholar] [CrossRef]
- Bhandoria, G.; Solanki, S.L.; Bhavsar, M.; Balakrishnan, K.; Bapuji, C.; Bhorkar, N.; Bhandarkar, P.; Bhosale, S.; Divatia, J.V.; Ghosh, A.; et al. Enhanced Recovery after Surgery (ERAS) in Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Cross-Sectional Survey. Pleura Peritoneum 2021, 6, 99–111. [Google Scholar] [CrossRef]
- Boden, I. Appraisal of Clinical Practice Guideline: Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: 2018. J. Physiother. 2024, 70, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Carazza, M.; Souza, F.C.C.; de Lorenzo, A.R. Preoperative Cardiopulmonary Exercise Testing for Risk Assessment before Elective Coronary Artery Bypass Grafting Surgery. Heart Vessel. Transplant. 2021, 5, 172–176. [Google Scholar] [CrossRef]
- Souadka, A.; El Ahmadi, B. Beyond Surgical Excellence: Unveiling the Role of Intensive Rehabilitation in RIOT for CRS and HIPEC. J. Surg. Oncol. 2023, 128, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Govaerts, K.; Moran, B. Postoperative Complications. In A Practical Guide to Peritoneal Malignancy; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Robella, M.; Tonello, M.; Berchialla, P.; Sciannameo, V.; Ilari Civit, A.M.; Sommariva, A.; Sassaroli, C.; Di Giorgio, A.; Gelmini, R.; Ghirardi, V.; et al. Enhanced Recovery after Surgery (ERAS) Program for Patients with Peritoneal Surface Malignancies Undergoing Cytoreductive Surgery with or without HIPEC: A Systematic Review and a Meta-Analysis. Cancers 2023, 15, 570. [Google Scholar] [CrossRef]
- Pillinger, N.L.; Robson, J.L.; Kam, P. Nutritional Prehabilitation: Physiological Basis and Clinical Evidence. Anaesth. Intensive Care 2018, 46, 453–462. [Google Scholar] [CrossRef]
- Franssen, R.F.W.; Janssen-Heijnen, M.L.G.; Barberan-Garcia, A.; Vogelaar, F.J.; Van Meeteren, N.L.U.; Bongers, B.C. Moderate-Intensity Exercise Training or High-Intensity Interval Training to Improve Aerobic Fitness during Exercise Prehabilitation in Patients Planned for Elective Abdominal Cancer Surgery? Eur. J. Surg. Oncol. 2022, 48, 3–13. [Google Scholar] [CrossRef]
- Palma, S.; Hasenoehrl, T.; Jordakieva, G.; Ramazanova, D.; Crevenna, R. High-Intensity Interval Training in the Prehabilitation of Cancer Patients-a Systematic Review and Meta-Analysis. Support. Care Cancer 2021, 29, 1781–1794. [Google Scholar] [CrossRef]
- Smyth, E.; O’Connor, L.; Mockler, D.; Reynolds, J.V.; Hussey, J.; Guinan, E. Preoperative High Intensity Interval Training for Oncological Resections: A Systematic Review and Meta-Analysis. Surg. Oncol. 2021, 38, 101620. [Google Scholar] [CrossRef]
- Reece, L.; Dragicevich, H.; Lewis, C.; Rothwell, C.; Fisher, O.M.; Carey, S.; Alzahrani, N.A.; Liauw, W.; Morris, D.L. Preoperative Nutrition Status and Postoperative Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 2622–2630. [Google Scholar] [CrossRef]
- Jie, B.; Jiang, Z.-M.; Nolan, M.T.; Zhu, S.-N.; Yu, K.; Kondrup, J. Impact of Preoperative Nutritional Support on Clinical Outcome in Abdominal Surgical Patients at Nutritional Risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef]
- Shanahan, J.L.; Leissner, K.B. Prehabilitation for the Enhanced Recovery After Surgery Patient. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 880–882. [Google Scholar]
- Popescu, G.A.; Minca, D.G.; Jafal, N.M.; Toma, C.V.; Alexandrescu, S.T.; Costea, R.V.; Vasilescu, C. Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations. Medicina 2025, 61, 908. [Google Scholar] [CrossRef]
- Borloni, B.; Huettner, H.; Schuerholz, T. Preoperative Nutritional Conditioning: Why, When and How. Visc. Med. 2019, 35, 299–304. [Google Scholar] [CrossRef]
- van Exter, S.H.; Drager, L.D.; van Asseldonk, M.J.M.D.; Strijker, D.; van der Schoot, N.D.; van den Heuvel, B.; Verlaan, S.; van den Berg, M.G.A. Adherence to and Efficacy of the Nutritional Intervention in Multimodal Prehabilitation in Colorectal and Esophageal Cancer Patients. Nutrients 2023, 15, 2133. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Feijoo, B.; Agusti-Garcia, N.; Sebio, R.; López-Hernández, A.; Sisó, M.; Glickman, A.; Carreras-Dieguez, N.; Fuste, P.; Marina, T.; Martínez-Egea, J.; et al. Feasibility of a Multimodal Prehabilitation Programme in Patients Undergoing Cytoreductive Surgery for Advanced Ovarian Cancer: A Pilot Study. Cancers 2022, 14, 1635. [Google Scholar] [CrossRef] [PubMed]
- El Bahaoui, N.; Lahnaoui, O.; Boubkri, N.; Benkabbou, A.; Majbar, A.; Mohsine, R.; Souadka, A. Do Cancer Diagnosis Announcements Impact Eating Habits in Cancer Patients: Pilot Study Describing the Moroccan Experience. J. Surg. 2023, 8, 1788. [Google Scholar] [CrossRef]
- McCool, K.W.; Sampene, E.; Polnaszek, B.; Connor, J.; Medlin, E.E.; Barroilhet, L. Neoadjuvant Chemotherapy Is Associated with a High Rate of Perioperative Blood Transfusion at the Time of Interval Cytoreductive Surgery. BMC Cancer 2018, 18, 1041. [Google Scholar] [CrossRef]
- Triphaus, C.; Judd, L.; Glaser, P.; Goehring, M.H.; Schmitt, E.; Westphal, S.; Füllenbach, C.; Lindau, S.; Zacharowski, K.; Meybohm, P.; et al. Effectiveness of Preoperative Iron Supplementation in Major Surgical Patients With Iron Deficiency: A Prospective Observational Study. Ann. Surg. 2021, 274, e212–e219. [Google Scholar]
- Lomnytska, M.; Karlsson, E.; Jonsdottir, B.; Lejon, A.-M.; Stålberg, K.; Poromaa, I.S.; Silins, I.; Graf, W. Peritoneal Cancer Index Predicts Severe Complications after Ovarian Cancer Surgery. Eur. J. Surg. Oncol. 2021, 47, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Paulo, J.; Oliveira, J.; Silva, M.; Silva, P.; Leite, F.; Valente, R.; Sousa, A.; Lobo, M. Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy: Analysis of Perioperative Risk Factors and Impact on Outcome. Cureus 2022, 14, e22937. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, M.K.; Gamboa, A.C.; Lee, R.M.; Zaidi, M.Y.; Kimbrough, C.; Grotz, T.; Fournier, K.; Powers, B.; Dineen, S.; Veerapong, J.; et al. The Intersection of Age and Tumor Biology with Postoperative Outcomes in Patients After Cytoreductive Surgery and HIPEC. Ann. Surg. Oncol. 2020, 27, 4894–4907. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Díaz, D.; Villanova Martínez, M.; Palencia Herrejón, E. Oncological Patients Admitted to an Intensive Care Unit. Analysis of Predictors of in-Hospital Mortality. Med. Intensiv. 2018, 42, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Waterland, J.L.; McCourt, O.; Edbrooke, L.; Granger, C.L.; Ismail, H.; Riedel, B.; Denehy, L. Efficacy of Prehabilitation Including Exercise on Postoperative Outcomes Following Abdominal Cancer Surgery: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 628848. [Google Scholar] [CrossRef]
- Miralpeix, E.; Mancebo, G.; Gayete, S.; Corcoy, M.; Solé-Sedeño, J.-M. Role and Impact of Multimodal Prehabilitation for Gynecologic Oncology Patients in an Enhanced Recovery After Surgery (ERAS) Program. Int. J. Gynecol. Cancer 2019, 29, 1235–1243. [Google Scholar] [CrossRef]
- Sehouli, J.; Mueller, K.; Richter, R.; Anker, M.; Woopen, H.; Rasch, J.; Grabowski, J.P.; Prinz-Theissing, E.; Inci, M.G. Effects of Sarcopenia and Malnutrition on Morbidity and Mortality in Gynecologic Cancer Surgery: Results of a Prospective Study. J. Cachexia Sarcopenia Muscle 2021, 12, 393–402. [Google Scholar] [CrossRef]

| Variable | Total (n = 169) | Pre-SCODA (n = 83) | SCODA (n = 86) | p-Value (Univariate Analysis) |
|---|---|---|---|---|
| Age, mean (SD) | 55.4 (9.9) | 54.4 (10.5) | 56.3 (9.1) | 0.170 |
| Sex (Male), n (%) | 73 (43.2) | 38 (45.8) | 35 (40.7%) | 0.480 |
| BMI *, mean (SD) | 22.7 (3.6) | 23.4 (3.8) | 22.0 (3.3) | 0.090 |
| PCI *, median (IQR) | 10 (8–14) | 10 (8–13) | 10 (9–14) | 0.410 |
| ASA * score ≥ 3, n (%) | 128 (75.7) | 56 (67) | 72 (84) | 0.010 |
| ECOG * 2–3, n (%) | 62 (36.7) | 28 (34) | 34 (40) | 0.390 |
| Primary tumor CRC * GC * OvC * PMP * | 81 (47) 7 (4) 42 (24) 39 (23) | 43 (51) 3 (3.6) 18 (21.6) 19 (22.9) | 38 (44) 4 (4.6) 24 (28) 20 (23) | 0.730 |
| Neoadjuvant therapy, n (%) | 51 (30.2%) | 22 (23%) | 29 (33%) | 0.170 |
| CC2 * resection | 10 | 6 (7.2) | 4 (4.5) | 0.530 |
| type of procedure CRS * only CRS + HIPEC * | 134 35 | 72 (86.7) 11 (13.3) | 62 (72) 24 (28) | 0.023 |
| Number of anastomoses > 2, n (%) | 41 (24.3%) | 21 (25.3%) | 20 (23.3%) | 0.780 |
| Preoperative hemoglobin, mean (SD) | 10.9 (1.5) | 10.2 (1.4) | 11.5 (1.2) | <0.001 |
| Preoperative albumin, mean (SD) | 3.5 (0.3) | 3.4 (0.4) | 3.5 (0.3) | 0.140 |
| Variable | Pre-SCODA (n = 83) | SCODA (n = 86) | p-Value |
|---|---|---|---|
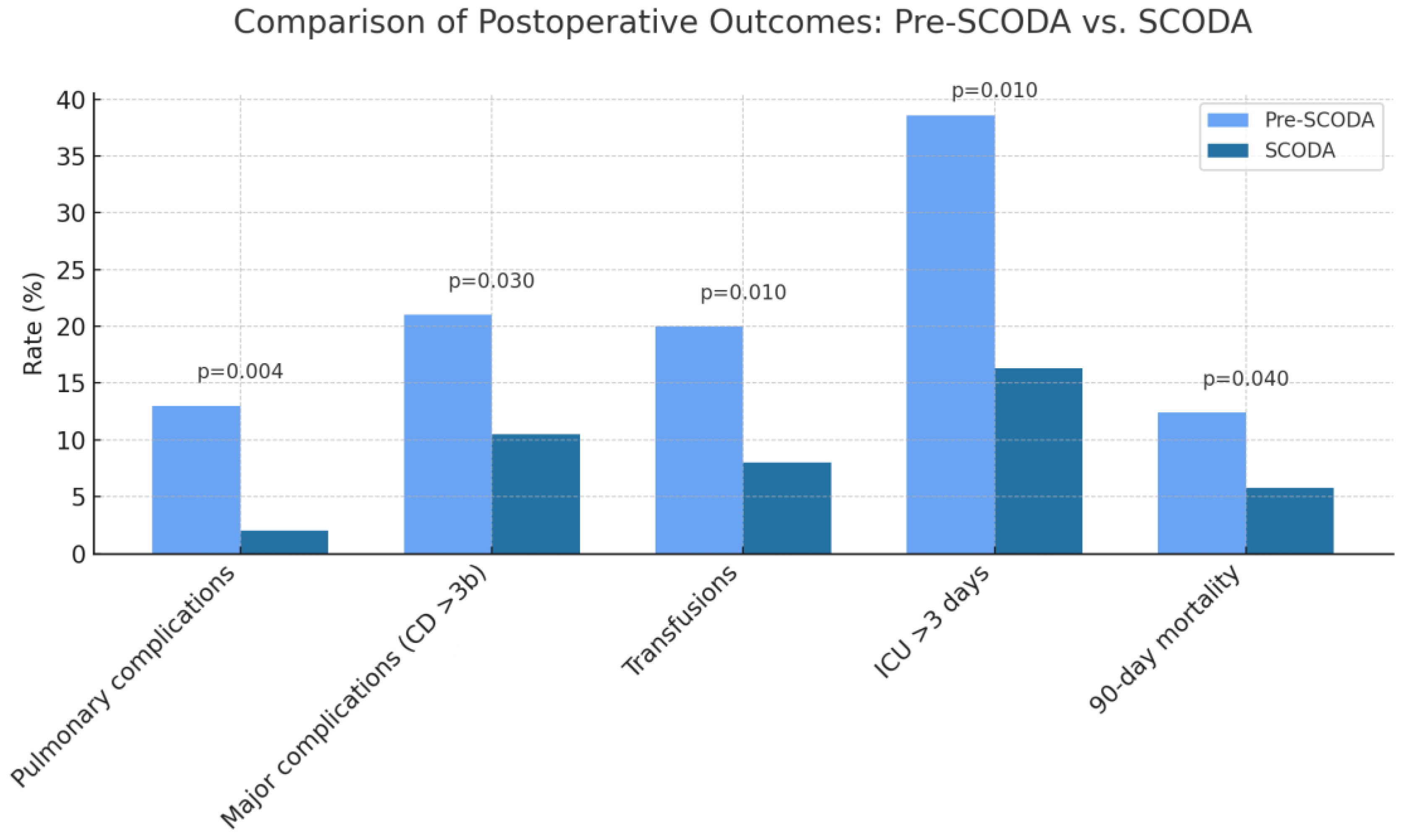
| Pulmonary complications, n (%) | 11 (13%) | 2 (2%) | 0.004 |
| Major complications (CD * ≥ 3b), n (%) | 17 (21%) | 9 (10.5%) | 0.030 |
| Post-op ICU * stay > 3 days, n (%) | 32 (38.6%) | 14 (16.3%) | 0.010 |
| Immediate post-op transfusion requirements, n (%) | 17 (20%) | 7 (8%) | 0.010 |
| 90-day mortality, n (%) | 10 (12.4%) | 5 (5.8%) | 0.040 |
| Postoperative hemoglobin, mean (SD *) | 9.2 (1.3) | 10.6 (1.1) | <0.001 |
| Postoperative albumin, mean (SD) | 2.9 (0.3) | 3.1 (0.4) | 0.090 |
| ECOG * 2–3 at discharge, n (%) | 30 (36%) | 19 (22%) | 0.030 |
| SCODA adherence to iron supplementation, n (%) | - | 73 (85%) | - |
| SCODA adherence to physical activity, n (%) | - | 69 (80%) | - |
| SCODA adherence to protein intake, n (%) | - | 76 (88%) | - |
| Outcome | Variable | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|
| Pulmonary Complications | SCODA Group (yes) | 0.26 (0.1–0.64) | 0.004 |
| PCI * (per unit) | 1.21 (1.08–1.36) | 0.001 | |
| Major Complications (CD3b *) | SCODA Group (yes) | 0.56 (0.28–1.13) | 0.106 |
| PCI (per unit) | 1.15 (1.05–1.26) | 0.002 | |
| Age (per year) | 1.04 (1–1.08) | 0.043 | |
| Transfusion Requirement | SCODA Group (yes) | 0.16 (0.07–0.38) | 0.005 |
| PCI (per unit) | 1.13 (1.03–1.25) | 0.014 | |
| Preop Hb * (per g/dL) | 1.1 (0.86–1.43) | 0.415 | |
| ICU * Stay > 3 Days | SCODA Group (yes) | 0.36 (0.16–0.81) | 0.014 |
| PCI (per unit) | 1.2 (1.08–1.33) | 0.001 | |
| ECOG 2–3 * | 1.95 (1.31–2.9) | 0.001 | |
| 90-Day Mortality | SCODA Group (yes) | 0.41 (0.20–0.84) | 0.014 |
| PCI (per unit) | 1.19 (1.09–1.31) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souadka, A.; Alami, L.; Elmouatassim, Z.; Lahnaoui, O.; El Bouazizi, Y.; Echiguer, S.; Ssouni, O.; El Fassi, A.; Ghannam, A.; Belkhadir, Z.H.; et al. SCODA: A Low-Cost Prehabilitation Strategy to Improve Outcomes After Cytoreductive Surgery in a Low-Resource Setting. Cancers 2025, 17, 3687. https://doi.org/10.3390/cancers17223687
Souadka A, Alami L, Elmouatassim Z, Lahnaoui O, El Bouazizi Y, Echiguer S, Ssouni O, El Fassi A, Ghannam A, Belkhadir ZH, et al. SCODA: A Low-Cost Prehabilitation Strategy to Improve Outcomes After Cytoreductive Surgery in a Low-Resource Setting. Cancers. 2025; 17(22):3687. https://doi.org/10.3390/cancers17223687
Chicago/Turabian StyleSouadka, Amine, Lina Alami, Zakaria Elmouatassim, Oumayma Lahnaoui, Yassine El Bouazizi, Sabrillah Echiguer, Oussama Ssouni, Ayman El Fassi, Abdelilah Ghannam, Zakaria Houssain Belkhadir, and et al. 2025. "SCODA: A Low-Cost Prehabilitation Strategy to Improve Outcomes After Cytoreductive Surgery in a Low-Resource Setting" Cancers 17, no. 22: 3687. https://doi.org/10.3390/cancers17223687
APA StyleSouadka, A., Alami, L., Elmouatassim, Z., Lahnaoui, O., El Bouazizi, Y., Echiguer, S., Ssouni, O., El Fassi, A., Ghannam, A., Belkhadir, Z. H., & El Ahmadi, B. (2025). SCODA: A Low-Cost Prehabilitation Strategy to Improve Outcomes After Cytoreductive Surgery in a Low-Resource Setting. Cancers, 17(22), 3687. https://doi.org/10.3390/cancers17223687







