Simple Summary
Periampullary adenocarcinomas are uncommon cancers that develop near the junction of the pancreas, bile duct, and small intestine. Although surgery is the main treatment, survival varies widely among patients, even when tumors appear similar. This study analyzed 120 patients who underwent curative surgery at a university hospital to explore whether microscopic tumor appearance could predict patient outcomes. We found that patients whose tumors showed an intestinal pattern lived much longer than those with pancreatobiliary or pancreatic patterns. Tumors with more lymph node involvement also had worse survival. These findings show that microscopic tumor subtype and lymph node burden provide valuable information for predicting prognosis after surgery. Incorporating these factors into clinical practice may help doctors better estimate survival, select adjuvant treatments, and design more precise studies in the future.
Abstract
Background: Morphological subclassification may refine prognosis after curative pancreaticoduodenectomy (PD) for periampullary cancers. Methods: We conducted a single-center retrospective cohort including 120 consecutive PDs performed between 2005 and 2022. Tumors were classified as intestinal (INT), pancreatobiliary (PB), or pancreatic ductal adenocarcinoma (PAN). Clinicopathologic variables included T stage, margin status, lymphovascular and perineural invasion, and lymph node ratio (LNR; cutoff 0.154 determined by ROC/Youden). Overall survival (OS) was the primary endpoint and was analyzed using Kaplan–Meier with log-rank tests and multivariable Cox regression. Results: INT tumors were associated with earlier T stage, fewer adverse histologic features, and higher R0 resection rates compared with PB and PAN. In multivariable analysis, mortality risk was higher for PB (HR 4.41; 95% CI 1.25–15.53) and PAN (HR 13.96; 95% CI 3.99–48.75) relative to INT. LNR ≥ 0.154 independently predicted worse OS (HR 1.93; 95% CI 1.11–3.35). Mean OS was 108.8 months for INT, 62.0 months for PB, and 22.7 months for PAN (log-rank p < 0.001). Conclusions: Morphological subtype and LNR are independent prognostic factors after PD for periampullary malignancies. Integrating morphology and nodal burden into risk models may improve postoperative stratification and guide adjuvant therapy.
1. Introduction
Periampullary adenocarcinomas comprise four adjacent anatomic sites: ampulla of Vater, distal common bile duct, duodenum, and pancreatic head ductal adenocarcinoma [1,2]. Surgical resection remains the principal therapeutic option to improve survival and offer a cure, most commonly via pancreaticoduodenectomy in resectable cases [3]. Traditionally, these tumors are staged by anatomic origin according to the American Joint Committee on Cancer (AJCC) [4]. Nonetheless, survival after resection varies substantially even among neoplasms of the same site [5,6], and anatomic proximity can hinder precise identification of the primary tumor, with implications for adjuvant treatment selection [7].
To address heterogeneity beyond anatomic site, Kimura et al. proposed two histomorphologic patterns in ampullary carcinoma—intestinal (INT) and pancreatobiliary (PB) [8]. On routine hematoxylin–eosin, INT tumors resemble gastric/colonic tubular adenocarcinoma with elongated, well-formed glands and basally oriented oval nuclei, whereas PB tumors exhibit papillary projections with delicate fibrovascular cores; immunophenotypically, INT commonly expresses CDX2 and rarely MUC1, while PB shows strong MUC1 with weak/absent CDX2 [6,9]. INT tumors tend to be less infiltrative and have better outcomes than PB, and morphological subclassification is increasingly considered when selecting postoperative systemic therapy, particularly for ampullary disease [8,10].
However, the prognostic value of morphology across periampullary sites remains uncertain. Histomorphologic subclassification has demonstrated prognostic value in other malignancies, such as gastric and colorectal adenocarcinomas, suggesting that similar morphological frameworks may also enhance prognostic evaluation in ampullary and pancreatic ductal adenocarcinoma (PDAC). PDAC’s biological heterogeneity and the lack of robust prognostic markers beyond TNM staging continue to challenge risk stratification and therapeutic decision-making [8,10].
Pancreatic ductal adenocarcinoma exhibits the poorest post-resection outcomes (≈5–20% five-year survival), whereas ampullary adenocarcinoma reaches ≈33–68%; [5,10,11] some studies report no survival difference between pancreatic adenocarcinoma (PAN) and ampullary PB subtype [6]. In this context, we aimed to determine whether morphological subtype (INT, PB, and PAN) is associated with distinct clinicopathologic features and overall survival in a single-center cohort of patients undergoing curative-intent pancreaticoduodenectomy between 2005 and 2022, and to evaluate whether lymph node ratio provides additional prognostic stratification.
2. Methods
2.1. Study Design and Setting
This was a single-center, longitudinal, retrospective cohort study conducted at a tertiary academic hospital. Data were prospectively collected in institutional protocols and medical records from 2005 to 2022.
All procedures complied with institutional and national research ethics. The study was approved by the Research Ethics Committee (COEP/UFMG), protocol CAAE 23377113100005149; informed consent was waived due to the retrospective design.
2.2. Patients and Eligibility
We screened 162 consecutive patients considered for curative resection and obtained a final cohort of 120 after applying eligibility criteria (Figure 1). Inclusion criteria: ductal adenocarcinoma of the pancreatic head and ampullary adenocarcinoma treated with pancreaticoduodenectomy. Exclusion criteria: distal common bile duct or duodenal primary tumors; benign conditions; non-ductal pancreatic neoplasms (e.g., solid pseudopapillary tumor, neuroendocrine tumors, intraductal papillary mucinous neoplasm); and non-adenocarcinoma papillary tumors. Adjuvant chemotherapy was indicated and managed by the institutional Medical Oncology Service according to prevailing protocols during the study period.
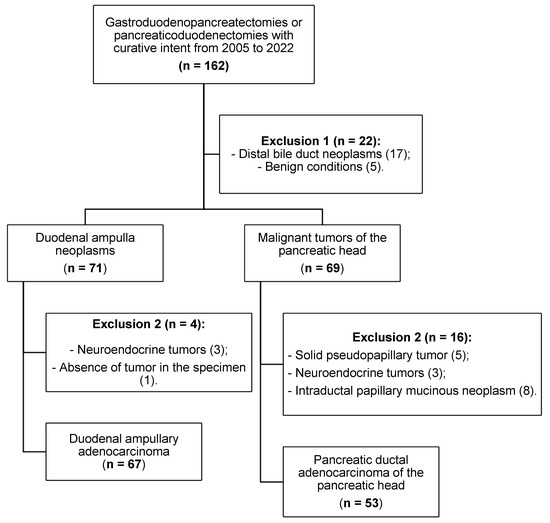
Figure 1.
Patient selection and application of inclusion/exclusion criteria.
2.3. Variables and Definitions
We collected clinical-demographic variables (age, sex, smoking, alcohol use), preoperative risk by ASA physical status classification [12], and postoperative complications graded by Clavien–Dindo (validated Portuguese translation) [13]. Preoperative laboratory data comprised CA 19-9, total and direct bilirubin, alkaline phosphatase, GGT, AST, ALT, albumin, hemoglobin, leukocyte and lymphocyte counts, and platelets. Histopathologic assessment followed AJCC 8th-edition TNM criteria for exocrine pancreatic and ampullary cancers [4], recording tumor grade, lymphovascular invasion (LVI), perineural invasion (PNI), and resection margin status (R0/R1). The lymph node ratio (LNR) was defined as the number of positive lymph nodes divided by the total retrieved and was analyzed categorically using the prespecified study cut-off.
2.4. Histopathologic Review and Morphological Classification
All slides were reviewed by an experienced pathologist at the institutional Pathology Department. Morphological subtype for ampullary tumors was assigned as intestinal (INT) or pancreatobiliary (PB) based primarily on hematoxylin–eosin (H&E) features, complemented by immunohistochemistry (MUC1, MUC2, MUC5AC, CK7, CK20, CDX2) when necessary; from the 33rd case onward, immunohistochemistry was reserved for equivocal H&E classifications (Figure 2). Pancreatic ductal adenocarcinoma (PAN) was analyzed as a distinct group. Staging followed the AJCC 8th edition [4].
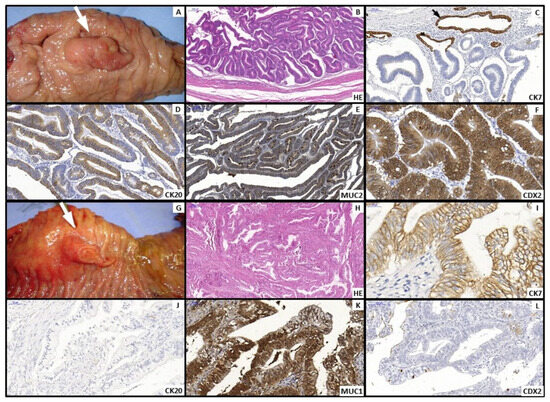
Figure 2.
(A–F). Intestinal type: (A) Polypoid Vater’s ampulla tumor (White arrow). (B) Microscopy—Hematoxylin and Eosin (H&E) staining: irregular gland-forming adenocarcinoma composed of elongated cells with stratified nuclei and scattered goblet cells. Immunohistochemical profile: CK7 negative, black arrow shows CK7 positive control cells of peribiliary glands (C); CK20 (D), MUC2 (E), and CDX2 (F) positive. (G–L). Pancreatobiliary type: (G) Polypoid and eroded tumor of Vater’s ampulla (white arrow). (H) Microscopy—Hematoxylin and Eosin (H&E) staining: irregularly infiltrating glands with pale cytoplasm. Immunohistochemical profile: CK7 positive (I); CK20 negative (J), MUC1 positive (K), and CDX2 negative (L).
2.5. Outcomes
The primary outcome was overall survival (OS), measured in months from surgery to death from any cause or last follow-up. For survival analyses, deaths within 30 postoperative days were excluded. OS at 12, 36, and 60 months was also estimated.
2.6. Statistical Analysis
Descriptive statistics used means/SD or medians/IQR and frequencies, as appropriate. Group comparisons employed χ2 or Fisher’s exact tests (categorical), Kruskal–Wallis and Mann–Whitney (non-parametric continuous), or Student’s t-test (parametric). To assess the prognostic performance of LNR, we constructed ROC curves and selected the optimal cutoff by Youden’s index, identifying LNR ≥ 0.154 (sensitivity 51.8%, specificity 85.5%) as the best discriminator for death during follow-up. Survival was estimated by Kaplan–Meier with log-rank tests; covariates with p ≤ 0.20 in univariable analyses and/or clinical relevance entered a multivariable Cox proportional hazards model. Two-sided p < 0.05 defined statistical significance. Analyses were performed in IBM SPSS® v23 for Mac (Chicago, IL, USA).
3. Results
3.1. Cohort and Perioperative Outcomes
Of 162 screened cases, 120 patients met eligibility criteria and were included (Figure 1). Baseline clinical characteristics and postoperative outcomes by group are summarized in Table 1. The study cohort comprised 34 intestinal (INT), 33 pancreatobiliary (PB), and 53 pancreatic ductal adenocarcinoma (PAN) cases. The PB subgroup showed a female predominance (66.6%), whereas PAN had more males (62.3%; p = 0.019). Smoking and alcohol use were more frequent in PAN than PB (non-smokers: 45.3% vs. 69.7%; p = 0.027; alcohol use: 43.4% vs. 15.2%; p = 0.007). Most complications were minor (Clavien–Dindo I–II–IIIA); 30-day mortality was low and similar across groups (INT 11.8%, PB 6.1%, PAN 5.7%; p = 0.536).

Table 1.
Distribution of clinical and demographic variables, postoperative complications, and mortality, and comparison among groups according to different histopathological subtypes in patients with periampullary tumors undergoing curative-intent surgical resection.
3.2. Preoperative Laboratory Profile
Median CA 19-9 was lower in INT versus PAN (20 vs. 267.5 U/mL; p < 0.001), while PB was intermediate (29.5 U/mL; overall p = 0.001). Total and direct bilirubin were highest in PAN (medians 11.6 and 9.9 mg/dL, respectively) and lowest in INT (0.8 and 0.3 mg/dL; both overall p < 0.001). GGT, AST, and ALT were also higher in PAN/PB than INT (e.g., GGT medians 472.5/PB 365.5/INT 147.5 U/L; p = 0.022). Albumin, hemoglobin, leukocytes, lymphocytes, and platelets did not differ significantly between groups.
3.3. Pathologic Findings
Details are presented in Table 2. INT tumors were predominantly T1 (55.9%), whereas PAN cases were mainly T3–T4 (≥70%; p < 0.001). Nodal status differed substantially: N0 was more frequent in INT than PAN (76.5% vs. 30.2%; p < 0.001); overall node positivity was highest in PAN (69.8%) versus PB (45.5%) and INT (23.5%). Limited liver metastatic disease was observed intraoperatively (M1: PB 1 case; PAN 5 cases). Margins were more often positive in PAN (35.8%) versus INT (0%) and PB (3%; p < 0.001). Lymphovascular and perineural invasion were frequent in PAN (84.9% and 96.2%) and uncommon in INT (~12% each; both p < 0.001). Lymph node ratio (LNR) < 0.154 predominated in INT (91.2%) versus PB (69.7%) and PAN (64.2%; overall p = 0.018).

Table 2.
Distribution of histopathological variables and comparison among groups according to different histopathological subtypes in patients with periampullary tumors submitted to curative-intent surgical resection.
3.4. Survival Analysis
No statistically significant difference in survival was observed for any of the clinical variables considered. Deaths within 30 postoperative days were excluded from survival analyses. Survival analysis according to the pathological variables studied is presented in Table 3.

Table 3.
Histopathological variables and mean survival of patients with periampullary tumors undergoing curative-intent surgical resection.
Both the anatomical location (papilla and pancreas) and the morphological classification (intestinal, pancreatobiliary, and pancreatic) were factors that impacted survival according to the univariate analysis (p < 0.001). The papilla group had a mean survival of 83.7 months, whereas the pancreas group showed a survival of 22.7 months (Figure 3a).
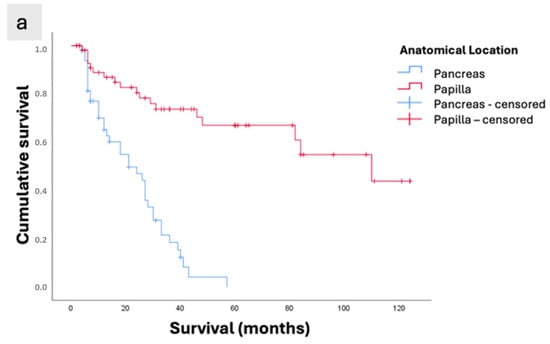
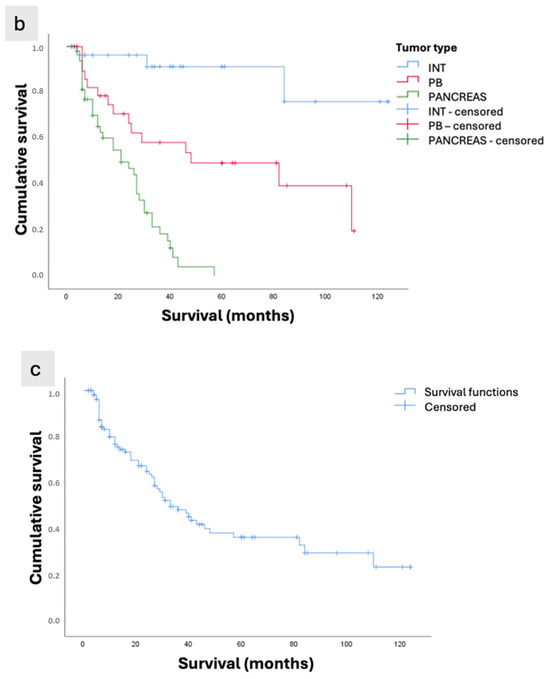
Figure 3.
Kaplan–Meier survival curves of patients with periampullary adenocarcinomas who underwent curative-intent surgical resection. (a) Survival according to anatomical location (Papilla vs. Pancreas; p < 0.001). (b) Survival according to morphological subtype (INT: intestinal subtype of ampullary tumors; PB: pancreatobiliary subtype of ampullary tumors; Pancreas: pancreatic ductal adenocarcinoma; p < 0.001). (c) Overall survival of the study population.
The analysis according to histopathological phenotype showed that tumors with the best mean survival were of the intestinal subtype (108.7 months), compared with 62 months for the pancreatobiliary subtype and only 22.7 months for pancreatic tumors (p < 0.001, Figure 3b).
The mean overall survival was 56.37 ± 5.43 months, with survival rates of 76.9%, 48.8%, and 37% at 12, 36, and 60 months, respectively (Figure 3c).
The intestinal subtype showed the longest survival (108.76 ± 8.04 months), followed by the pancreatobiliary subtype (62.04 ± 8.94 months), while patients with pancreatic ductal adenocarcinoma had the poorest survival (22.70 ± 2.24 months), p < 0.001 (Table 4). In the six cases in which metastatic disease was resected, liver metastases were identified intraoperatively and were located in regions favorable to resection. Among these, there was a single case of PB-type ampullary adenocarcinoma, with a postoperative survival of 8 months. In the remaining five cases of pancreatic adenocarcinoma, patient survival times were 41, 18, 7, 6, and 5 months, respectively.

Table 4.
Overall survival means and survival according to morphological classification, with survival rates at 12, 36, and 60 months in patients with periampullary tumors undergoing curative-intent surgical resection.
3.5. Multivariable Analysis
In Cox regression (Table 5), morphological subtype and LNR remained independent prognostic factors. Compared with INT, the hazard of death was higher for PB (HR 4.41; 95% CI 1.25–15.53; p = 0.021) and highest for PAN (HR 13.96; 95% CI 3.99–48.75; p < 0.001). LNR ≥ 0.154 independently predicted worse OS (HR 1.93; 95% CI 1.11–3.35; p = 0.018).

Table 5.
Multivariate analysis and Hazard Ratio (HR) of patients with periampullary tumors undergoing curative-intent surgical resection.
4. Discussion
In this single-center cohort of patients undergoing curative-intent pancreaticoduodenectomy (PD) for periampullary malignancies, morphological subtype emerged as an independent determinant of overall survival (OS): intestinal (INT) tumors had the most favorable outcomes, whereas pancreatobiliary (PB) and pancreatic ductal adenocarcinoma (PAN) displayed progressively worse survival. In multivariable analysis, mortality risk was higher for PB and highest for PAN when both were compared with INT, and lymph node ratio (LNR) ≥ 0.154 independently predicted inferior OS. Together, morphology and LNR refined risk beyond conventional clinicopathologic variables.
These findings support a phenotype-oriented approach to prognostication, complementing anatomic-site staging and addressing the heterogeneity observed within periampullary cancers. Originally described in ampullary carcinoma, the INT versus PB dichotomy is defined by distinct H&E architecture and immunophenotype (e.g., CDX2/MUC1 patterns) and has been linked to clinical behavior [6]. INT tumors in our series presented more favorable pathology (earlier T stage, more frequent N0, lower rates of lymphovascular/perineural invasion, and higher R0 resections), while PB tracked closer to the pancreatic phenotype, and PAN remained the worst-prognosis group—patterns that help explain the graded survival across INT → PB → PAN.
Nodal burden remains central in periampullary oncology. Beyond nodal positivity per se, the LNR provides a reproducible, pathology-based metric that incorporates both disease extent and the adequacy of nodal harvest. Using ROC/Youden methods, we identified 0.154 as the optimal cutoff; LNR ≥ 0.154 independently stratified mortality after adjustment for morphology and other covariates, underscoring its additive prognostic value to conventional N staging [14,15,16,17,18,19,20].
Tumor markers and cholestatic indices complemented the clinicopathologic profile. As expected, CA 19-9—a widely used but nonspecific biomarker subject to elevation in cholestasis—varied across groups, with higher levels in PAN; prior work from our group associated elevated preoperative CA 19-9 with an approximately fourfold increase in mortality risk among pancreatic tumors [21,22]. In this cohort, markers of cholestasis were more pronounced in PAN and, to a lesser extent, PB; GGT, in particular, was consistently higher in these two groups when compared with INT, a pattern concordant with its ductal origin and sensitivity to biliary obstruction [23].
From a pathobiologic perspective, the overlap between PB and PAN extended beyond survival to adverse histological features: both exhibited higher frequencies of lymphovascular and perineural invasion and more positive margins, whereas INT displayed a more favorable distribution of these factors. The greater perineural and lymphovascular infiltration in pancreatic primaries likely reflects later symptom onset and the rich sympathetic/parasympathetic innervation of the pancreas, contributing to their aggressive phenotype [6]. These co-segregating features plausibly mediate part of the morphology–survival relationship observed in our study.
Clinically, our results have two direct implications. First, routine reporting of morphological subtype (INT vs. PB) should accompany anatomic site and TNM in surgical pathology, given its independent prognostic value and potential to inform adjuvant therapy decisions. Second, integrating LNR with morphology offers pragmatic risk granularity for postoperative counseling and trial stratification, using data already available in standard workflows. Notably, the similarity between PB and PAN supports considering pancreatic-type adjuvant regimens for ampullary PB tumors, consistent with current pancreatic cancer guidelines [24,25]. In contrast, for INT tumors—which are closer to a colorectal-like biology—gemcitabine-based strategies may be less effective, suggesting that fluoropyrimidine-based regimens should be prioritized when chemotherapy is indicated [26,27,28,29].
Our data should be interpreted in light of several limitations. The retrospective design entails potential selection and information biases. Immunohistochemistry was applied selectively (reserved for equivocal cases after the 33rd case), which could introduce misclassification in borderline tumors. Adjuvant chemotherapy was not analyzed and may confound survival comparisons. Furthermore, we did not evaluate distal bile duct or duodenal primaries due to small numbers. Finally, while comorbidities were highly prevalent in our cohort (≈63–76%), their impact on OS was not significant here, in contrast to a prior study from our institution focusing solely on pancreatic head adenocarcinoma, which found worse survival with older age and comorbidities; differences from published prevalence (≈20–30%) likely reflect referral patterns and population differences [15,21,30]. A larger cohort of pancreatic ductal adenocarcinoma cases and an extended follow-up period would increase the statistical power and provide a more comprehensive understanding of long-term outcomes. However, additional cases with complete immunohistochemical profiling and verified clinical data are not yet available in our institutional database, and long-term recurrence data were not uniformly available for the entire study period. We plan to include more cases in future analyses, and multicenter collaborations are also envisioned to validate and expand upon our findings.
In summary, morphological subtype (INT, PB, PAN) and LNR independently stratify survival after curative-intent PD for periampullary adenocarcinoma. Systematic incorporation of both—alongside TNM—can sharpen postoperative risk assessment, inform adjuvant therapy selection (particularly aligning PB with pancreatic-type regimens), and improve stratification in clinical trials, addressing the persistent heterogeneity observed even within the same anatomic site [14,15,16,17,18,19,20,21,24,25,26,27,28,29]. Indeed, emerging evidence indicates that elucidating the complex crosstalk between cancer cells and the nervous system may pave the way for more effective and targeted therapies. Furthermore, advances in lipid-based nanomaterials have demonstrated remarkable potential in overcoming drug resistance, highlighting their promise for future therapeutic strategies aimed at reversing treatment resistance in these aggressive tumors [31,32,33].
5. Conclusions
In this single-center retrospective cohort, morphological subtype and lymph node ratio emerged as independent predictors of overall survival in patients undergoing curative-intent pancreaticoduodenectomy for periampullary adenocarcinomas. The intestinal subtype was associated with more favorable pathological features and significantly longer survival compared to pancreatobiliary and pancreatic types, which exhibited increasingly aggressive characteristics and poorer outcomes.
Integrating morphological classification and lymph node ratio into postoperative prognostic assessment enhances risk stratification beyond conventional TNM staging. These findings support the inclusion of morphological subtype reporting in routine pathology and suggest that pancreatobiliary-type ampullary tumors may benefit from adjuvant strategies similar to those used for pancreatic cancer. Future multicenter studies are needed to validate these results and further refine personalized treatment approaches for periampullary malignancies.
Author Contributions
Conceptualization, J.B.S. and V.R.; methodology, J.B.S., E.P.J. and V.R.; software, R.V.P.; validation, J.B.S., L.d.P.L. and V.R.; formal analysis, R.V.P., Y.P.H.Y., A.H.M., H.A.L. and P.F.d.L.e.S.; investigation, L.d.P.L. and J.B.S.; resources, V.R., E.P.J. and M.D.S.; data curation, R.V.P. and P.F.d.L.e.S.; writing—original draft preparation, J.B.S., Y.P.H.Y., A.H.M., H.A.L. and R.V.P.; writing—review and editing, J.B.S., E.P.J., Y.P.H.Y., A.H.M., H.A.L. and V.R.; visualization, R.V.P.; supervision, V.R.; project administration, J.B.S. and V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research protocol was approved by the Research Ethics Committee of the Federal University of Minas Gerais (UFMG), Brazil (CAAE: 69877517.7.0000.5149; Approval No. 2.237.075; Approval Date: 7 January 2014). Given the retrospective design of the study and the use of anonymized data, the requirement for informed consent was waived.
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Department of Pathology, School of Medicine, Federal University of Minas Gerais, for their assistance in reviewing the histological slides. We acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their institutional support in research and education. We are also grateful to our colleagues, mentors, professors, and all staff members of the Department of Digestive Surgery at the Hospital das Clínicas, Federal University of Minas Gerais—especially the Liver, Biliary Tract, Pancreas, and Spleen Group—for their collaboration and for the care provided to our patients.
Conflicts of Interest
The authors declare no conflicts of interest for this article.
Abbreviations
The following abbreviations are used in this manuscript:
| AJCC | American Joint Committee on Cancer |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ASA | American Society of Anesthesiologists |
| CA 19-9 | Carbohydrate antigen 19-9 |
| CI | Confidence interval |
| GGT | Gamma-glutamyl transferase |
| H&E | Hematoxylin and eosin |
| HR | Hazard ratio |
| INT | Intestinal subtype |
| LNR | Lymph node ratio |
| OS | Overall survival |
| PB | Pancreatobiliary subtype |
| PAN | Pancreatic ductal adenocarcinoma |
| PNI | Perineural invasion |
| ROC | Receiver operating characteristic |
| R0/R1 | Margin status (negative/positive) |
| SD | Standard deviation |
| UFMG | Federal University of Minas Gerais |
References
- Sarmiento, J.M.; Nagomey, D.M.; Sarr, M.G.; Farnell, M.B. Periampullary cancers: Are there differences? Surg. Clin. N. Am. 2001, 81, 543–555. [Google Scholar] [CrossRef]
- Hester, C.A.; Dogeas, E.; Augustine, M.M.; Mansour, J.C.; Polanco, P.M.; Porembka, M.R.; Wang, S.C.; Zeh, H.J.; Yopp, A.C. Incidence and comparative outcomes of periampullary cancer: A population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J. Surg. Oncol. 2019, 119, 303–317. [Google Scholar] [CrossRef]
- Skórzewska, M.; Kurzawa, P.; Ciszewski, T.; Pelc, Z.; Polkowski, W.P. Controversies in the diagnosis and treatment of periampullary tumours. Surg. Oncol. 2022, 44, 101853. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Westgaard, A.; Tafjord, S.; Farstad, I.N.; Cvancarova, M.; Eide, T.J.; Mathisen, O.; Clausen, O.P.F.; Gladhaug, I.P. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Chan, C.K.; Toste, P.A.; Elliott, I.A.; Vasquez, C.R.; Sunjaya, D.B.; Swanson, E.A.; Koo, J.; Hines, O.J.; Reber, H.A.; et al. Association of Histopathologic Phenotype of Periampullary Adenocarcinomas with Survival. JAMA Surg. 2017, 152, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Pomianowska, E.; Grzyb, K.; Westgaard, A.; Clausen, O.P.F.; Gladhaug, I.P. Reclassification of tumour origin in resected periampullary adenocarcinomas reveals underestimation of distal bile duct cancer. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 1043–1050. [Google Scholar] [CrossRef][Green Version]
- Kimura, W.; Futakawa, N.; Yamagata, S.; Wada, Y.; Kuroda, A.; Muto, T.; Esaki, Y. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn. J. Cancer Res. Gann. 1994, 85, 161–166. [Google Scholar] [CrossRef]
- da Silveira Santos, J.P.L.; Machado, C.J.; Junior, E.P.; Rodrigues, J.B.S.R.; Vidigal, P.T.; Resende, V. Immunohistochemical Predictors for Intestinal and Pancreatobiliary Types of Adenocarcinoma of the Ampulla of Vater. J. Gastrointest. Surg. 2018, 22, 1171–1178. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Tang, Y.; Gao, R.; Bao, J.; Liang, B. Adjuvant therapy for periampullary carcinoma and the significance of histopathological typing: A systematic review. Transl. Oncol. 2022, 20, 101414. [Google Scholar] [CrossRef]
- Lin, P.W.; Lin, Y.J. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br. J. Surg. 1999, 86, 603–607. [Google Scholar] [CrossRef]
- Moreno, R.P.; Pearse, R.; Rhodes, A. European Surgical Outcomes Study (EuSOS) Group of the European Society of Intensive Care Medicine and European Society of Anaesthesiology Trials Groups. American Society of Anesthesiologists Score: Still useful after 60 years? Results of the EuSOS Study. Rev. Bras. Ter. Intensive 2015, 27, 105–112. [Google Scholar]
- Moreira, L.F.; Pessôa, M.C.M.; Mattana, D.S.; Schmitz, F.F.; Volkweis, B.S.; Antoniazzi, J.L.; Ribeiro, L. Cultural adaptation and the Clavien-Dindo surgical complications classification translated to Brazilian Portuguese. Rev. Col. Bras. Cir. 2016, 43, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Hruban, R.H.; Lillemoe, K.D.; Pitt, H.A. Periampullary adenocarcinoma: Analysis of 5-year survivors. Ann. Surg. 1998, 227, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Chien, M.W.; Earle, C.C. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann. Surg. 2003, 237, 74–85. [Google Scholar] [CrossRef]
- Delcore, R.; Rodriguez, F.J.; Forster, J.; Hermreck, A.S.; Thomas, J.H. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. Am. J. Surg. 1996, 172, 463–468; discussion 468–469. [Google Scholar] [CrossRef]
- Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Hruban, R.H.; Lillemoe, K.D.; Pitt, H.A. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann Surg. 1997, 226, 248–257; discussion 257–260. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Cameron, J.L.; Winter, J.M.; Assumpcao, L.; Lillemoe, K.D.; Wolfgang, C.; Hruban, R.H.; Schulick, R.D.; Yeo, C.J.; et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007, 141, 610–618. [Google Scholar] [CrossRef]
- Falconi, M.; Crippa, S.; Domínguez, I.; Barugola, G.; Capelli, P.; Marcucci, S.; Beghelli, S.; Scarpa, A.; Bassi, C.; Pederzoli, P. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann. Surg. Oncol. 2008, 15, 3178–3186. [Google Scholar] [CrossRef]
- Resende, V.; Endo, Y.; Munir, M.M.; Khalil, M.; Rashid, Z.; Lima, H.A.; Rawicz-Pruszyński, K.; Khan, M.M.M.; Katayama, E.; Tsilimigras, D.I.; et al. Prognostic value of nodal staging classification and number of examined lymph nodes among patients with ampullary cancer. J. Gastrointest. Surg. 2024, 28, 33–39. [Google Scholar] [CrossRef]
- Sancio, J.B.; Campanati, R.; Lima, L.D.P.; Rubião, F.; de-Freitas, J.C.; de-Melo, F.H.C.; Machado, C.J.; Sanches, M.D.; Resende, V. Preoperative prognostic factors in patients with ductal adenocarcinoma of the head of the pancreas. Rev. Colégio Bras. Cir. 2020, 47, e20202363. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Bosonnet, L.; Ghaneh, P.; Sutton, R.; Evans, J.; Healey, P.; Garvey, C.; Hughes, M.; Raraty, M.; Campbell, F.; et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery 2008, 143, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Q.; Zhang, Z.L.; Jia, Y.M.; Chen, R.X.; Peng, L. Preoperative CA19-9 and GGT ratio as a prognostic indicator in ampullary carcinoma. BMC Gastroenterol. 2023, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dadduzio, V.; Lombardi, L.; Ricci, A.D.; Gadaleta-Caldarola, G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Curr. Oncol. Tor. Ont. 2021, 28, 3393–3402. [Google Scholar] [CrossRef]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 603–605. [Google Scholar]
- Bolm, L.; Ohrner, K.; Nappo, G.; Rückert, F.; Zimmermann, C.; Rau, B.M.; Petrova, E.; Honselmann, K.C.; Lapshyn, H.; Bausch, D.; et al. Adjuvant therapy is associated with improved overall survival in patients with pancreatobiliary or mixed subtype ampullary cancer after pancreatoduodenectomy—A multicenter cohort study. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al. 2020, 20, 433–441. [Google Scholar] [CrossRef]
- Moore, D.F.; Pazdur, R.; Daugherty, K.; Tarassoff, P.; Abbruzzese, J.L. Phase II study of gemcitabine in advanced colorectal adenocarcinoma. Investig. New Drugs 1992, 10, 323–325. [Google Scholar] [CrossRef]
- Mani, S.; Kugler, J.W.; Knost, J.A.; Sciortino, D.F.; Gibbons, J.; Garcia, J.C.; Ansari, R.H.; Schilsky, R.L.; Vokes, E.E. Phase II trial of 150-minute weekly infusion of gemcitabine in advanced colorectal cancer: Minimal activity in colorectal cancer. Investig. New Drugs 1998, 16, 275–278. [Google Scholar] [CrossRef]
- Moekotte, A.L.; Malleo, G.; van Roessel, S.; Bonds, M.; Halimi, A.; Zarantonello, L.; Napoli, N.; Dreyer, S.B.; Wellner, U.F.; Bolm, L.; et al. Gemcitabine-based adjuvant chemotherapy in subtypes of ampullary adenocarcinoma: International propensity score-matched cohort study. Br. J. Surg. 2020, 107, 1171–1182. [Google Scholar] [CrossRef]
- Nathan, H.; Cameron, J.L.; Choti, M.A.; Schulick, R.D.; Pawlik, T.M. The volume-outcomes effect in hepato-pancreato-biliary surgery: Hospital versus surgeon contributions and specificity of the relationship. J. Am. Coll. Surg. 2009, 208, 528–538. [Google Scholar] [CrossRef]
- Li, J.; Gu, A.; Tang, N.; Zengin, G.; Li, M.; Liu, Y. Patient- derived xenograft models in pan-cancer: From bench to clinic. Interdiscip. Med. 2025, 3, e20250016. [Google Scholar] [CrossRef]
- Huang, M.; Gong, G.; Deng, Y.; Long, X.; Long, W.; Liu, Q.; Zhao, W.; Chen, R. Crosstalk between cancer cells and the nervous system. Med. Adv. 2023, 1, 173–189. [Google Scholar] [CrossRef]
- Dechbumroong, P.; Hu, R.; Keaswejjareansuk, W.; Namdee, K.; Liang, X.J. Recent advanced lipid-based nanomedicines for overcoming cancer resistance. Cancer Drug Resist. 2024, 7, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).