Integrating PET and MRI Radiomics for Staging and Prognostic Stratification in Anal Canal Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
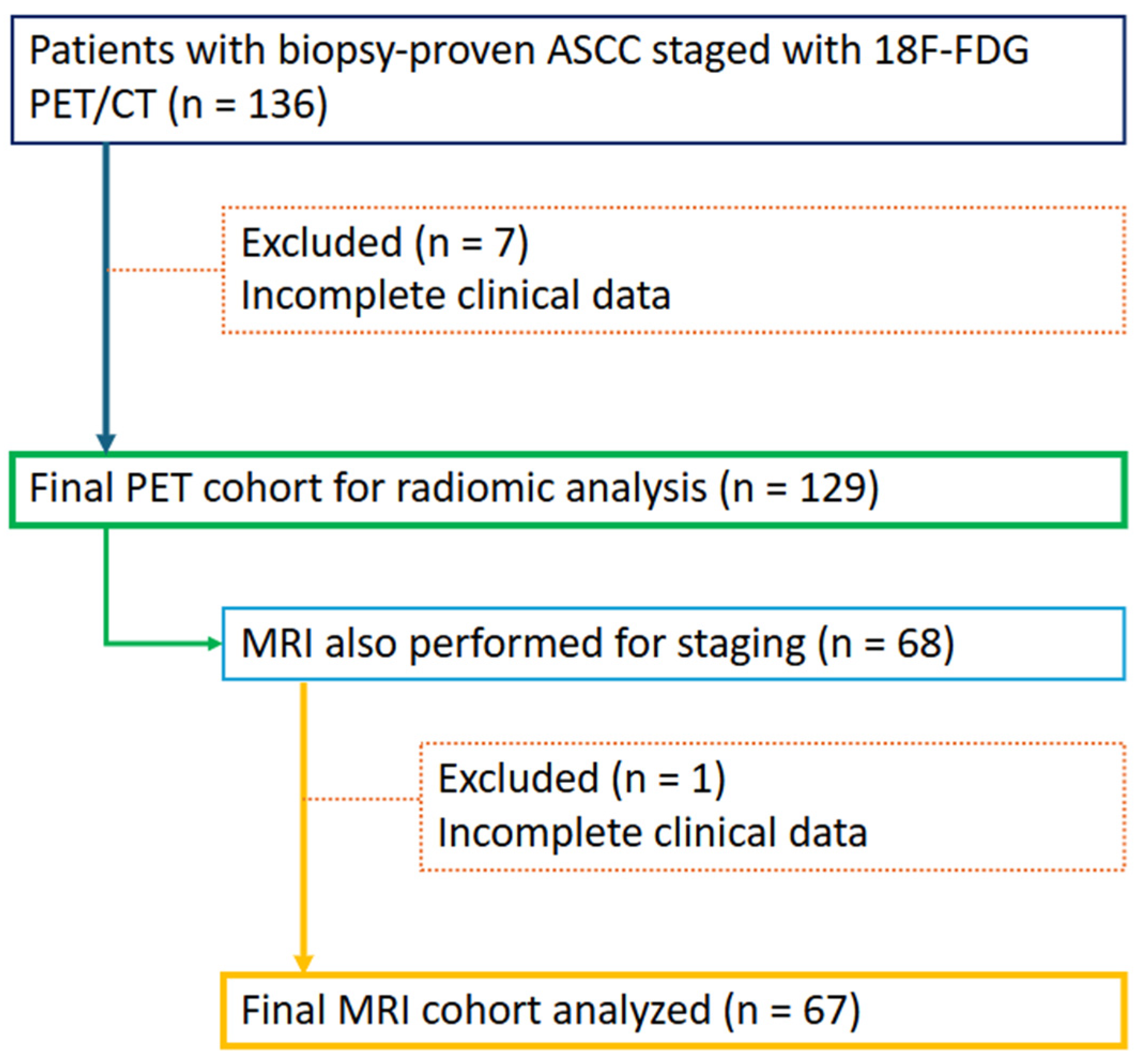
2.1. Study Sample
2.2. Image Acquisition
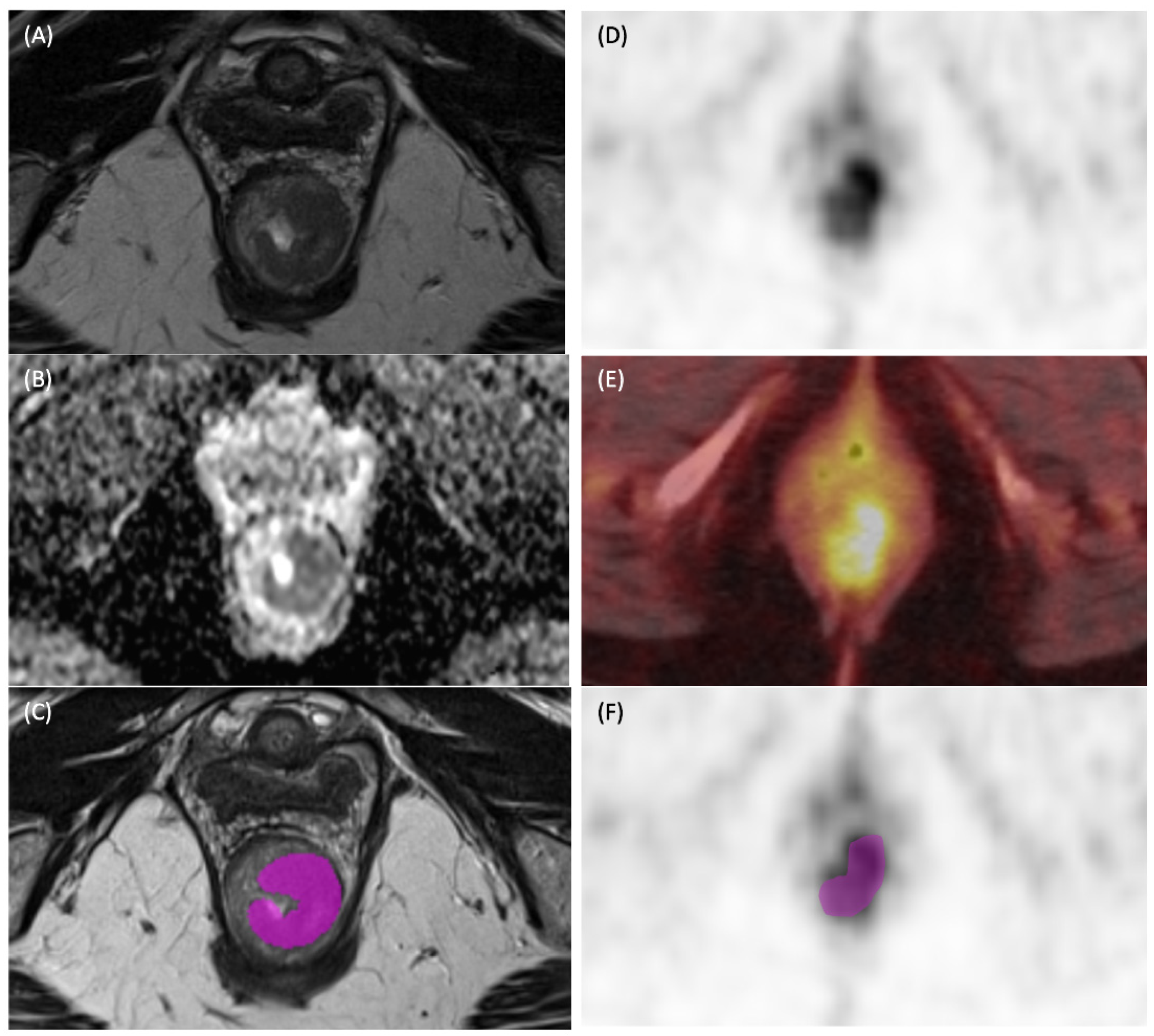
2.3. Data Extraction
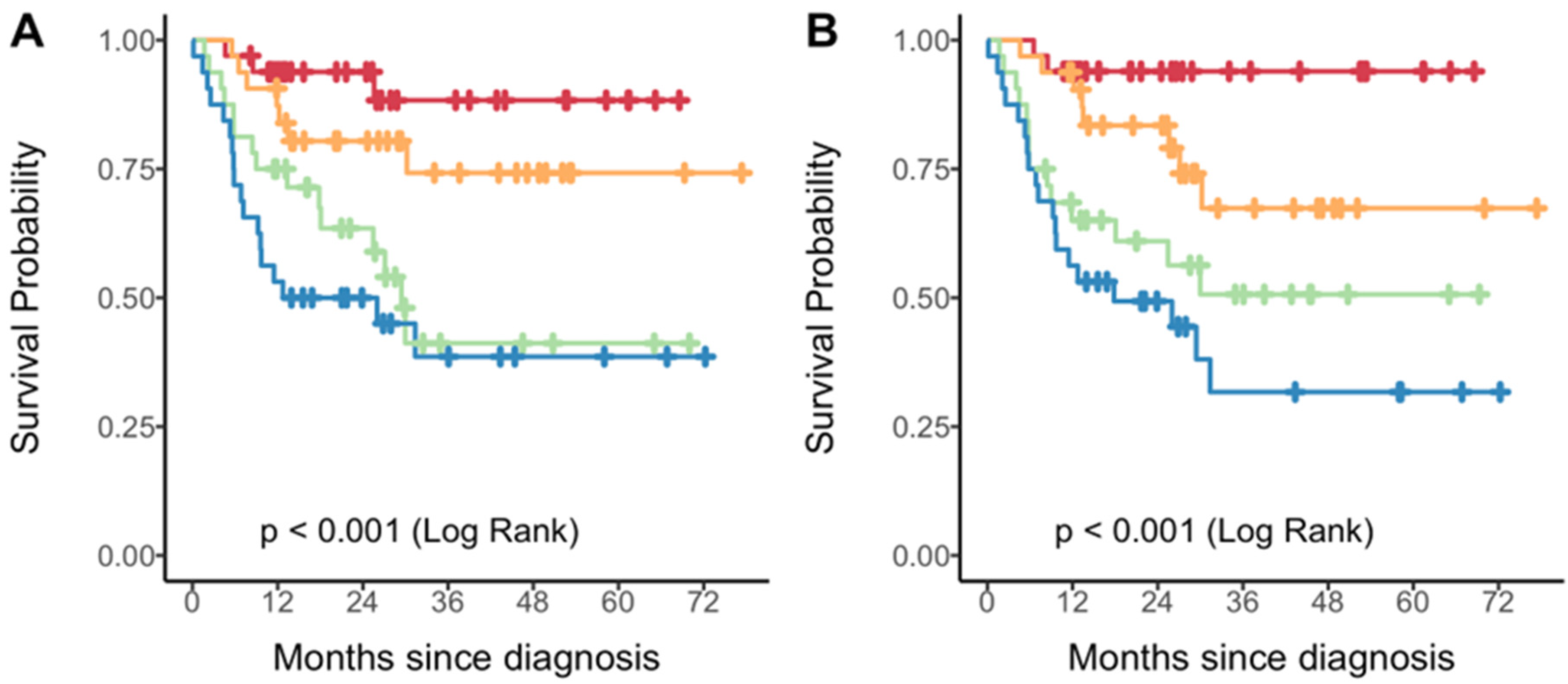
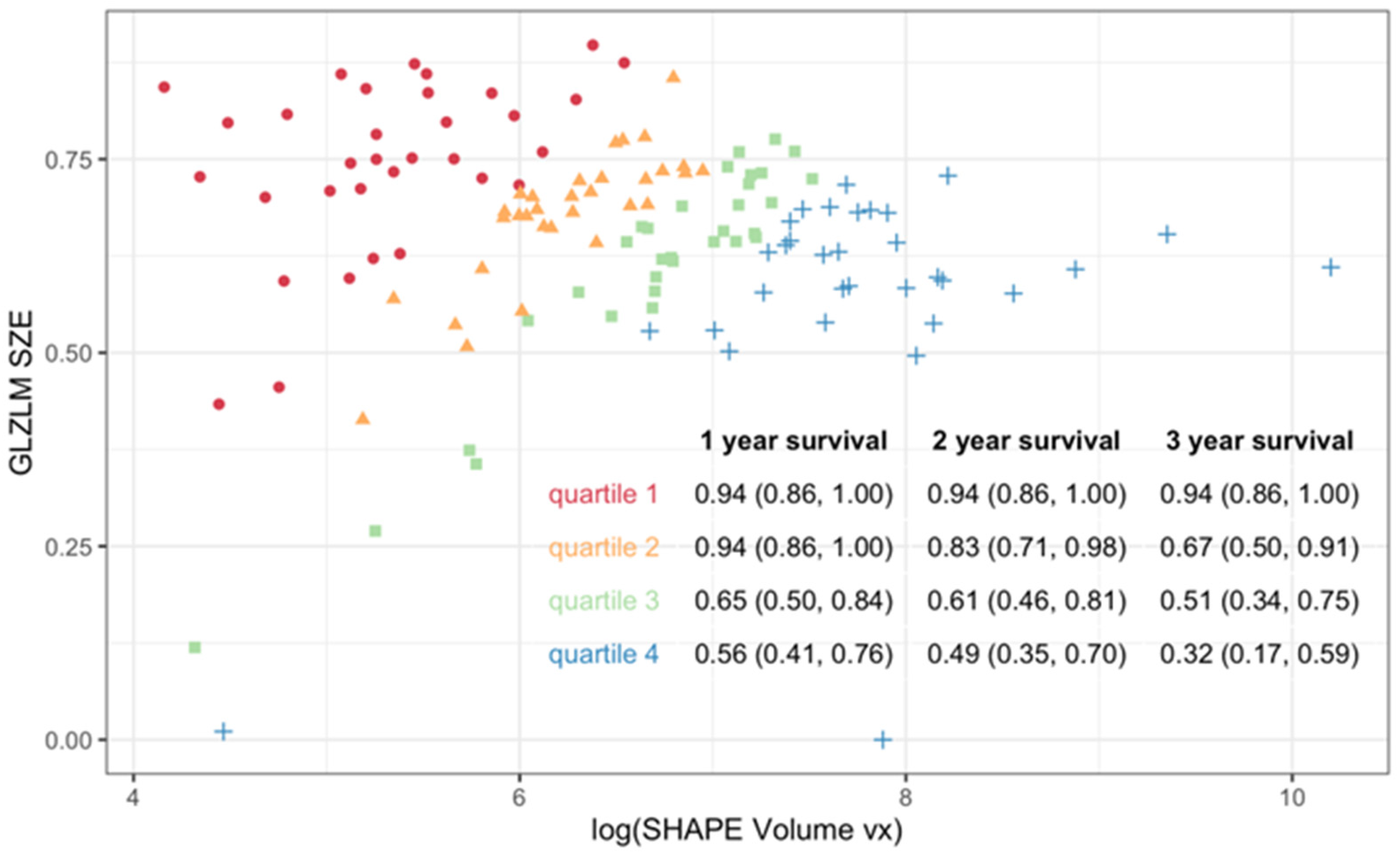
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Kang, Y.J.; Smith, M.; Canfell, K. Anal cancer in high-income countries: Increasing burden of disease. PLoS ONE 2018, 13, e0205105. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Salati, S.; Al Kadi, A.L. Anal cancer—A review. Int. J. Health Sci. 2012, 6, 133–140. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures. 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 12 March 2024).
- Shepherd, N.A.; Scholefield, J.H.; Love, S.B.; England, J.; Northover, J. Prognostic factors in anal squamous carcinoma: A multivariate analysis of clinical, pathological and flow cytometric parameters in 235 cases. Histopathology 1990, 16, 545–555. [Google Scholar] [CrossRef]
- Congedo, A.; Mallardi, D.; Danti, G.; De Muzio, F.; Granata, V.; Miele, V. An updated review on imaging and staging of anal cancer—Not just rectal cancer. Tomography 2023, 9, 1694–1710. [Google Scholar] [CrossRef]
- Durot, C.; Dohan, A.; Boudiaf, M.; Servois, V.; Soyer, P.; Hoeffel, C. Cancer of the anal canal: Diagnosis, staging and follow-up with MRI. Korean J. Radiol. 2017, 18, 946–956. [Google Scholar] [CrossRef]
- Stewart, D.B.; Gaertner, W.B.; Glasgow, S.C.; Herzig, D.O.; Feingold, D.; Steele, S.R. The American Society of Colon and Rectal Surgeons clinical practice guidelines for anal squamous cell cancers (Revised 2018). Dis. Colon Rectum 2018, 61, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Mirshahvalad, S.A.; Mesci, A.; Murad, V.; Kohan, A.; Ortega, C.; Veit-Haibach, P.; Metser, U. [18F]-FDG PET in anal canal cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 258–277. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Anal carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 653–677. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.; Alverbratt, C.; Liljegren, A.; Björkander, E.; Strandell, A.; Samuelsson, O.; Palm, S.; Hallqvist, A. PET/CT imaging for radiation therapy planning in anal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2018, 126, 6–12. [Google Scholar] [CrossRef]
- Jones, M.; Hruby, G.; Solomon, M.; Rutherford, N.; Martin, J. The role of FDG-PET in the initial staging and response assessment of anal cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 2015, 22, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.; Elsayed, N.; Theophanous, S.; Samuel, R.; Cooper, R.; Casanova, N.; Tolan, D.J.; Gilbert, A.; Scarsbrook, A.F. Combined PET-CT and MRI for response evaluation in patients with squamous cell anal carcinoma treated with curative-intent chemoradiotherapy. Eur. Radiol. 2022, 32, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Bao, J.; Xia, Y.; Zhang, J.; Zhang, L.; Huang, X.; Wang, J. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PLoS ONE 2013, 8, e79008. [Google Scholar] [CrossRef]
- Le Bihan, D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology 2013, 268, 318–322. [Google Scholar] [CrossRef]
- Temperley, H.C.; O’Sullivan, N.J.; Waters, C.; Corr, A.; Mehigan, B.J.; O’Kane, G.; McCormick, P.; Gillham, C.; Rausa, E.; Larkin, J.O.; et al. Radiomics: Contemporary applications in the management of anal cancer; a systematic review. Am. Surg. 2024, 90, 445–454. [Google Scholar] [CrossRef]
- Giraud, N.; Saut, O.; Aparicio, T.; Ronchin, P.; Bazire, L.-A.; Barbier, E.; Lemanski, C.; Mirabel, X.; Etienne, P.-L.; Lièvre, A.; et al. MRI-based radiomics input for prediction of 2-year disease recurrence in anal squamous cell carcinoma. Cancers 2021, 13, 193. [Google Scholar] [CrossRef]
- Spithoff, K.; Cummings, B.; Jonker, D.; Biagi, J. Chemoradiotherapy for squamous cell cancer of the anal canal: A systematic review. Clin. Oncol. 2014, 26, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Guren, M.G.; Khan, K.; Brown, G.; Renehan, A.; Steigen, S.; Deutsch, E.; Martinelli, E.; Arnold, D. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1087–1100. [Google Scholar] [CrossRef]
- Morris, V.K.; Dorth, J.A.; Kennedy, E.B.; Eng, C. Systemic therapy for stage I–III anal squamous cell carcinoma: ASCO guideline clinical insights. JCO Oncol. Pract. 2025, 21, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Lukovic, J.; Mesci, A.; MacCrostie, P.; Chan, R.; Mak, V.; Avery, L.; Singnurkar, A.; Langer, D.L.; Ly, K.; et al. [18F]-FDG PET/CT in the initial staging of squamous cell cancer of the anal canal: Results of a prospective multicenter registry. J. Nucl. Med. 2025, 66, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.J.; Beets, G.L.; Maas, M.; Curvo-Semedo, L.; Kessels, A.G.H.; Thywissen, T.; Beets-Tan, R.G.H. Tumour ADC measurements in rectal cancer: Effect of ROI methods on ADC values and interobserver variability. Eur. Radiol. 2011, 21, 2567–2574. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Buvat, I. Local Image Features Extraction—LIFEx. LIFEx Version 6.0n Manual; LITO: Orsay, France, 2024; pp. 1–49. [Google Scholar]
- Orlhac, F.; Frouin, F.; Nioche, C.; Ayache, N.; Buvat, I. Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology 2019, 291, 53–59. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. B 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Li, L.; Greene, T.; Hu, B. A simple method to estimate the time-dependent receiver operating characteristic curve and the area under the curve with right-censored data. Stat. Methods Med. Res. 2018, 27, 2264–2278. [Google Scholar] [CrossRef]
- Li, X.; Yin, Z.; Li, L. tdROC: Nonparametric Estimation of Time-Dependent ROC, Brier Score, and Survival Difference. Available online: https://cran.r-universe.dev/tdROC/doc/manual.html (accessed on 26 October 2025).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 22 March 2025).
- Hatt, M.; Le Rest, C.C.; Antonorsi, N.; Tixier, F.; Tankyevych, O.; Jaouen, V.; Lucia, F.; Bourbonne, V.; Schick, U.; Badic, B.; et al. Radiomics in PET/CT: Current status and future AI-based evolutions. Semin. Nucl. Med. 2021, 51, 126–133. [Google Scholar] [CrossRef]
- Ha, S.; Choi, H.; Paeng, J.C.; Cheon, G.J. Radiomics in oncological PET/CT: A methodological overview. Nucl. Med. Mol. Imaging 2019, 53, 14–29. [Google Scholar] [CrossRef]
- Brown, P.J.; Zhong, J.; Frood, R.; Currie, S.; Gilbert, A.; Appelt, A.L.; Sebag-Montefiore, D.; Scarsbrook, A. Prediction of outcome in anal squamous cell carcinoma using radiomic feature analysis of pre-treatment FDG PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Chuong, M.; Latifi, K.; Tan, S.; Choi, W.; Hoffe, S.; Shridhar, R.; Lu, W. Prediction of anal cancer recurrence after chemoradiotherapy using quantitative image features extracted from serial 18F-FDG PET/CT. Front. Oncol. 2019, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk, K.; Prezzi, D.; Cascino, M.; Kozarski, R.; Gaya, A.; Siddique, M.; Cook, G.J.; Glynne-Jones, R.; Goh, V. MRI heterogeneity analysis for prediction of recurrence and disease-free survival in anal cancer. Radiother. Oncol. 2019, 134, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Hruby, G.; Coolens, C.; Driscoll, B.; Stanwell, P.; Kumar, M.; Capp, A.; Sridharan, S.; Arm, J.; Gallagher, S.; et al. A prospective, multi-centre trial of multiparametric MRI as a biomarker in anal carcinoma. Radiother. Oncol. 2020, 144, 7–12. [Google Scholar] [CrossRef] [PubMed]
| Covariate | Full Sample (n = 129) | MRI Available (n = 67) |
|---|---|---|
| Gender | ||
| Female | 67 (52) | 31 (46) |
| Male | 62 (48) | 36 (54) |
| Age at Dx | ||
| Mean (sd) | 61.7 (11.5) | 60.6 (11.3) |
| Median (Min, Max) | 61 (33, 92) | 60 (33, 87) |
| Primary Tumor Location | ||
| Anal Canal | 129 (100) | 67 (100) |
| Histology | ||
| Squamous Cell Carcinoma | 129 (100) | 67 (100) |
| Differentiation | ||
| Moderate | 29 (26) | 12 (20) |
| Poor | 12 (11) | 7 (12) |
| Unknown | 57 (50) | 38 (63) |
| Well | 15 (13) | 3 (5) |
| Missing | 16 | 7 |
| LVSI Status | ||
| Negative | 3 (2) | 1 (1) |
| Positive | 2 (2) | |
| Unknown | 124 (96) | 66 (99) |
| TNM Staging | ||
| T Stage (Based on MRI) | ||
| T1 | 5 (7) | |
| T2 | 31 (46) | |
| T3 | 19 (28) | |
| T4 | 12 (18) | |
| N Stage (Based on MRI and PET/CT) | ||
| N0 | 26 (39) | |
| N1a | 24 (36) | |
| N1c | 17 (25) | |
| M Stage (Based on MRI and PET/CT) | ||
| M0 | 60 (90) | |
| M1 | 7 (10) | |
| PET Stage AJCC 9th Edition | ||
| I | 3 (2) | 2 (3) |
| IIA | 18 (15) | 15 (22) |
| IIB | 23 (19) | 13 (19) |
| IIIA | 48 (39) | 26 (39) |
| IIIB | 1 (1) | 1 (1) |
| IIIC | 6 (5) | 1 (1) |
| IV | 25 (20) | 9 (13) |
| Missing | 5 | |
| Follow-Up Months | ||
| Mean (sd) | 28.7 (18.4) | 32.2 (17.1) |
| Median (Min, Max) | 25.7 (1.7, 77.4) | 27.9 (1.7, 72.2) |
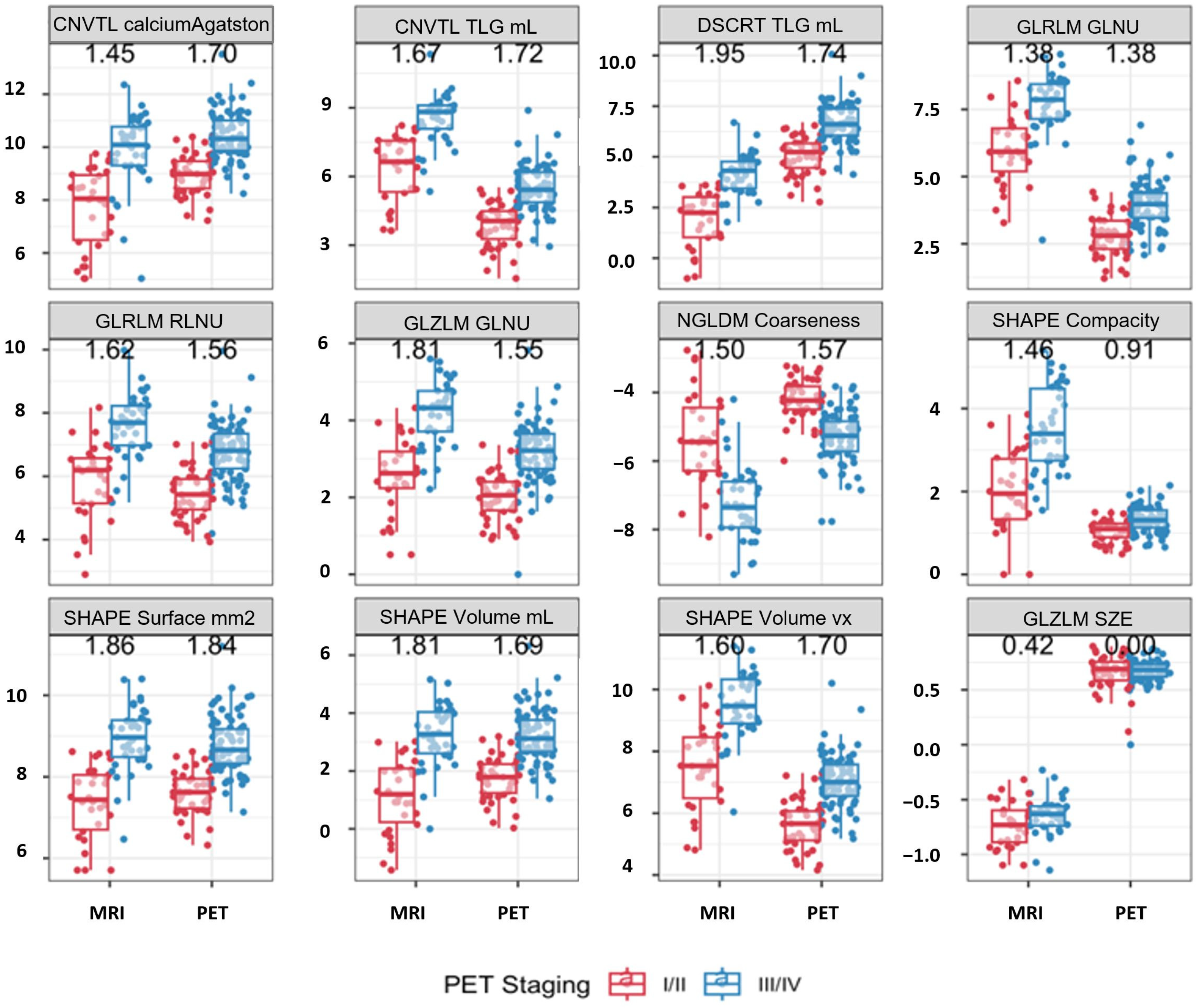
| Covariate | I/II (n = 44) | III/IV (n = 79) | Stage Difference p-Value | HR (95%CI) | Cox Model p-Value |
|---|---|---|---|---|---|
| CONVENTIONAL SUVbwcalciumAgatstonScore [onlyForCT] (log) | 9.0 (8.4–9.5) | 10.3 (9.9–11.0) | <0.001 | 1.86 (1.46, 2.37) | <0.001 |
| CONVENTIONAL TLG (mL) [onlyForPETorNM] (log) | 4.1 (3.3–4.5) | 5.4 (4.9–6.2) | <0.001 | 1.63 (1.29, 2.06) | <0.001 |
| DISCRETIZED TLG (mL) [onlyForPETorNM] (log) | 5.2 (4.5–5.7) | 6.6 (6.1–7.4) | <0.001 | 1.63 (1.29, 2.05) | <0.001 |
| SHAPE Volume (mL)(log) | 1.8 (1.2–2.2) | 3.1 (2.7–3.8) | <0.001 | 1.85 (1.46, 2.36) | <0.001 |
| SHAPE Volume (vx)(log) | 5.7 (5.1–6.1) | 7.0 (6.6–7.6) | <0.001 | 1.85 (1.45, 2.36) | <0.001 |
| SHAPE Surface (mm2) [onlyFor3DROI] (log) | 7.6 (7.2–7.9) | 8.7 (8.3–9.2) | <0.001 | 2.27 (1.68, 3.06) | <0.001 |
| SHAPE Compacity [onlyFor3DROI] (log) | 1.1 (0.9–1.2) | 1.3 (1.1–1.6) | <0.001 | 4.44 (1.69, 11.71) | 0.018 |
| GLRLM GLNU (log) | 2.8 (2.3–3.4) | 4.0 (3.5–4.4) | <0.001 | 1.98 (1.53, 2.55) | <0.001 |
| GLRLM RLNU (log) | 5.4 (5.0–5.9) | 6.8 (6.2–7.3) | <0.001 | 1.78 (1.39, 2.29) | <0.001 |
| NGLDM Coarseness (log) | −4.2 (−4.5–(−3.8)) | −5.3 (−5.7–(−4.8)) | <0.001 | 0.51 (0.38, 0.69) | <0.001 |
| NGLDM Busyness (log) | −1.8 (−2.4–(−1.2)) | −1.2 (−1.6–(−0.7)) | 0.001 | 1.89 (1.34, 2.66) | 0.002 |
| GLZLM GLNU (log) | 2.1 (1.7–2.4) | 3.2 (2.7–3.7) | <0.001 | 2.02 (1.51, 2.70) | <0.001 |
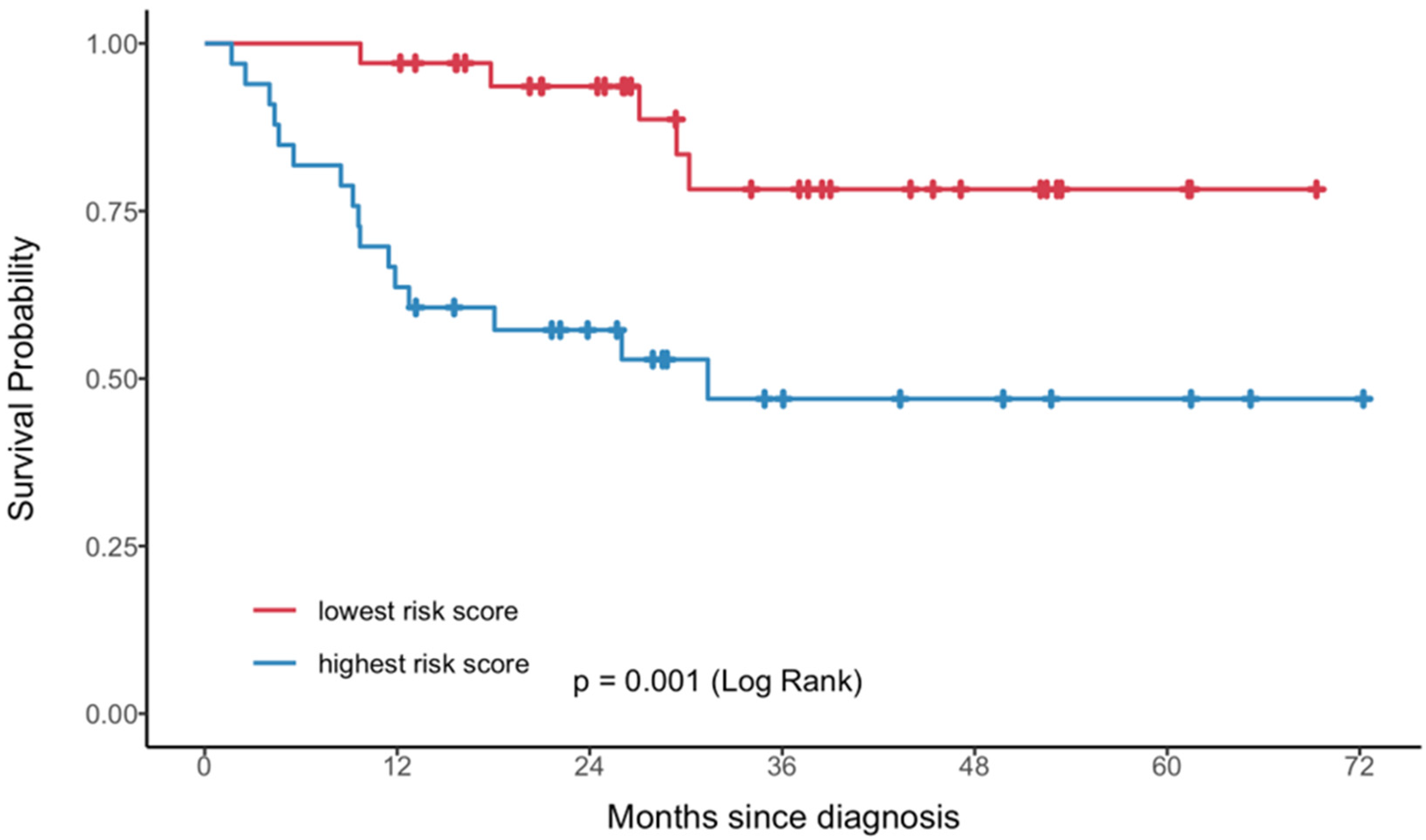
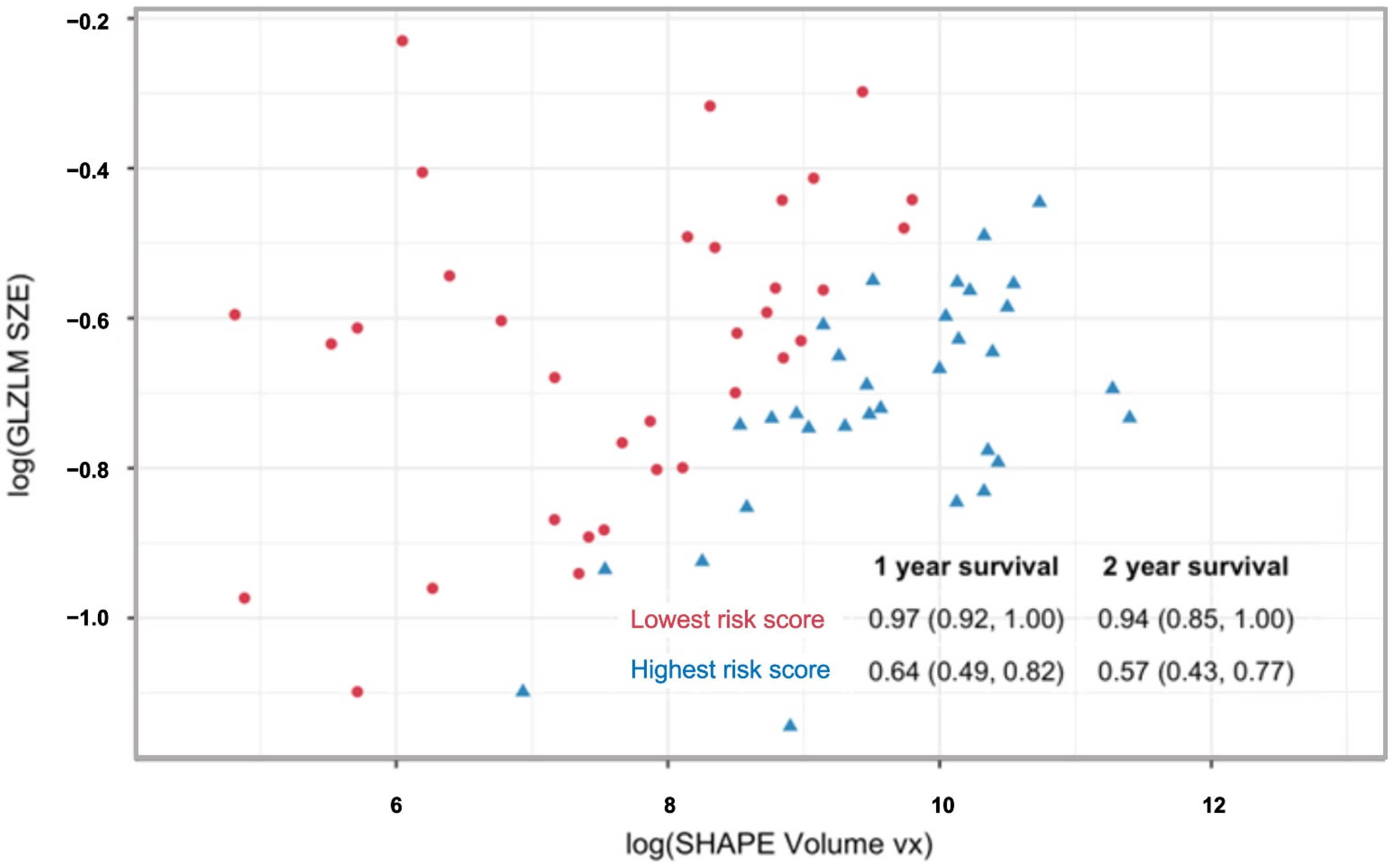
| Year | One-Feature Model | Two-Feature Model |
|---|---|---|
| 1 | 0.76 (0.69, 0.86) | 0.77 (0.71, 0.87) |
| 2 | 0.73 (0.66, 0.83) | 0.75 (0.68, 0.85) |
| 3 | 0.72 (0.61, 0.83) | 0.79 (0.71, 0.87) |
| 4 | 0.72 (0.61, 0.83) | 0.79 (0.71, 0.87) |
| 5 | 0.72 (0.61, 0.83) | 0.79 (0.71, 0.87) |
| Feature | I/II (n = 30) | III/IV (n = 37) | p-Value |
|---|---|---|---|
| Greater dimension cm | 3.0 (2.4–3.8) | 6.1 (4.5–7.3) | <0.001 |
| SHAPE Compacity onlyFor3DROI | 1.9 (1.3–2.8) | 3.4 (2.7–4.5) | <0.001 |
| CNVTL calciumAgatstonScore onlyForCT (log) | 8.0 (6.5–8.9) | 10.1 (9.3–10.8) | <0.001 |
| CNVTL TLG mL onlyForPETorNM (log) | 6.6 (5.3–7.6) | 8.8 (8.1–9.1) | <0.001 |
| DSCRT Kurtosis (log) | 1.1 (0.8–1.4) | 1.5 (1.4–1.7) | 0.049 |
| DSCRT ExcessKurtosis (log) | −4.0 (−4.0–0.1) | 0.5 (−0.1–1.0) | 0.044 |
| DSCRT TLG mL onlyForPETorNM (log) | 2.2 (1.0–3.0) | 4.3 (3.4–4.8) | <0.001 |
| SHAPE Volume mL (log) | 1.2 (0.2–2.1) | 3.3 (2.6–4.0) | <0.001 |
| SHAPE Volume vx (log) | 7.5 (6.5–8.5) | 9.5 (8.9–10.3) | <0.001 |
| SHAPE Sphericity onlyFor3DROI (log) | −0.5 (−0.5–(−0.4)) | −0.6 (−0.7–(−0.5)) | 0.031 |
| SHAPE Surface mm2 onlyFor3DROI (log) | 7.4 (6.7–8.0) | 9.0 (8.5–9.4) | <0.001 |
| GLRLM LRHGE (log) | 3.9 (3.1–4.5) | 4.7 (4.3–5.0) | 0.003 |
| GLRLM GLNU (log) | 5.9 (5.2–6.8) | 7.9 (7.1–8.4) | <0.001 |
| GLRLM RLNU (log) | 6.2 (5.1–6.6) | 7.7 (7.0–8.2) | <0.001 |
| NGLDM Coarseness (log) | −5.4 (−6.3–(−4.4)) | −7.4 (−7.9–(−6.6)) | <0.001 |
| NGLDM Contrast (log) | −4.0 (−4.5–(−3.5)) | −4.7 (−5.1–(−4.5)) | 0.021 |
| GLZLM LZE (log) | 10.9 (9.7–12.2) | 13.2 (12.2–14.1) | 0.007 |
| GLZLM LZHGE (log) | 13.0 (10.8–13.7) | 14.8 (14.0–15.7) | <0.001 |
| GLZLM GLNU (log) | 2.6 (2.2–3.2) | 4.3 (3.7–4.8) | <0.001 |
| GLZLM ZLNU (log) | 2.2 (1.3–2.8) | 4.0 (3.5–4.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murad, V.; Kohan, A.; Avery, L.; Ortega, C.; Mesci, A.; Veit-Haibach, P.; Metser, U. Integrating PET and MRI Radiomics for Staging and Prognostic Stratification in Anal Canal Cancer. Cancers 2025, 17, 3653. https://doi.org/10.3390/cancers17223653
Murad V, Kohan A, Avery L, Ortega C, Mesci A, Veit-Haibach P, Metser U. Integrating PET and MRI Radiomics for Staging and Prognostic Stratification in Anal Canal Cancer. Cancers. 2025; 17(22):3653. https://doi.org/10.3390/cancers17223653
Chicago/Turabian StyleMurad, Vanessa, Andres Kohan, Lisa Avery, Claudia Ortega, Aruz Mesci, Patrick Veit-Haibach, and Ur Metser. 2025. "Integrating PET and MRI Radiomics for Staging and Prognostic Stratification in Anal Canal Cancer" Cancers 17, no. 22: 3653. https://doi.org/10.3390/cancers17223653
APA StyleMurad, V., Kohan, A., Avery, L., Ortega, C., Mesci, A., Veit-Haibach, P., & Metser, U. (2025). Integrating PET and MRI Radiomics for Staging and Prognostic Stratification in Anal Canal Cancer. Cancers, 17(22), 3653. https://doi.org/10.3390/cancers17223653





