Simple Summary
Lung cancer is one of the most common and deadly cancers worldwide. Surgery has been the main treatment for patients whose tumors can be removed, but many patients still experience recurrence after surgery. Novel medicines called targeted therapies can greatly improve outcomes for patients whose tumors have specific genetic changes. In this narrative review, we discuss landmark clinical trials of targeted therapies in resectable non-small cell lung cancer. We highlight how these therapies, when given before or after surgery, can lower the chances of cancer returning or improve survival. We also emphasize the importance of genetic testing to guide treatment planning. By bringing together the latest research, this review shows how targeted therapies are changing the treatment of resectable non-small cell lung cancer.
Abstract
Background: Recent landmark clinical trials have introduced the role of targeted therapy with surgery for resectable non-small cell lung cancers (NSCLCs). Methods: This narrative review summarizes data from recent clinical trials and retrospective studies to highlight the evolving interplay between targeted therapy and resectable NSCLC. Results: For patients with epidermal growth factor receptor (EGFR) mutations, the ADAURA trial demonstrated significant improvements in disease-free and overall survival with adjuvant osimertinib after complete resection. The NeoADAURA trial expanded the role of osimertinib to neoadjuvant treatment as it showed benefit in major pathologic response rates when compared to chemotherapy alone. Neoadjuvant osimertinib may facilitate surgical resection, especially for patients with lymph node involvement. Furthermore, the ALINA trial established the role of adjuvant alectinib, another targeted therapy, for patients with anaplastic lymphoma kinase (ALK) positive resectable NSCLC. Given the evidence for use of these novel targeted therapies in patients with resectable lung cancer, early molecular profiling is critical for patients with NSCLC to help guide pre- and postoperative treatment. The use of targeted therapies may even expand to stage IV NSCLC as clinical trials are ongoing and could possibly redefine the role of surgery in advanced disease. Conclusions: While there are ongoing trials to clarify the optimal timing of targeted therapies and surgical resection, current data supports the use of targeted therapies as part of multimodality care in surgically resectable NSCLC.
1. Background
Lung cancer is the leading cause of cancer-related deaths worldwide, with over 2 million new cases and 1.8 million deaths annually [1]. The predominant histological subtype of lung cancer, non-small-cell lung cancer (NSCLC), has been historically managed according to disease extent as the key factor in treatment strategy. For the early stage of localized resectable NSCLC, surgery to remove the tumor is often first line. If the tumor size is large or has nodal involvement, then systemic therapy comes into play either in the neoadjuvant, adjuvant, or perioperative setting [2]. Chemotherapy has been associated with modest improvements to overall survival and disease-free survival; however, recurrence rates remain quite high. One-third to one-half of patients may recur after curative resection [3].
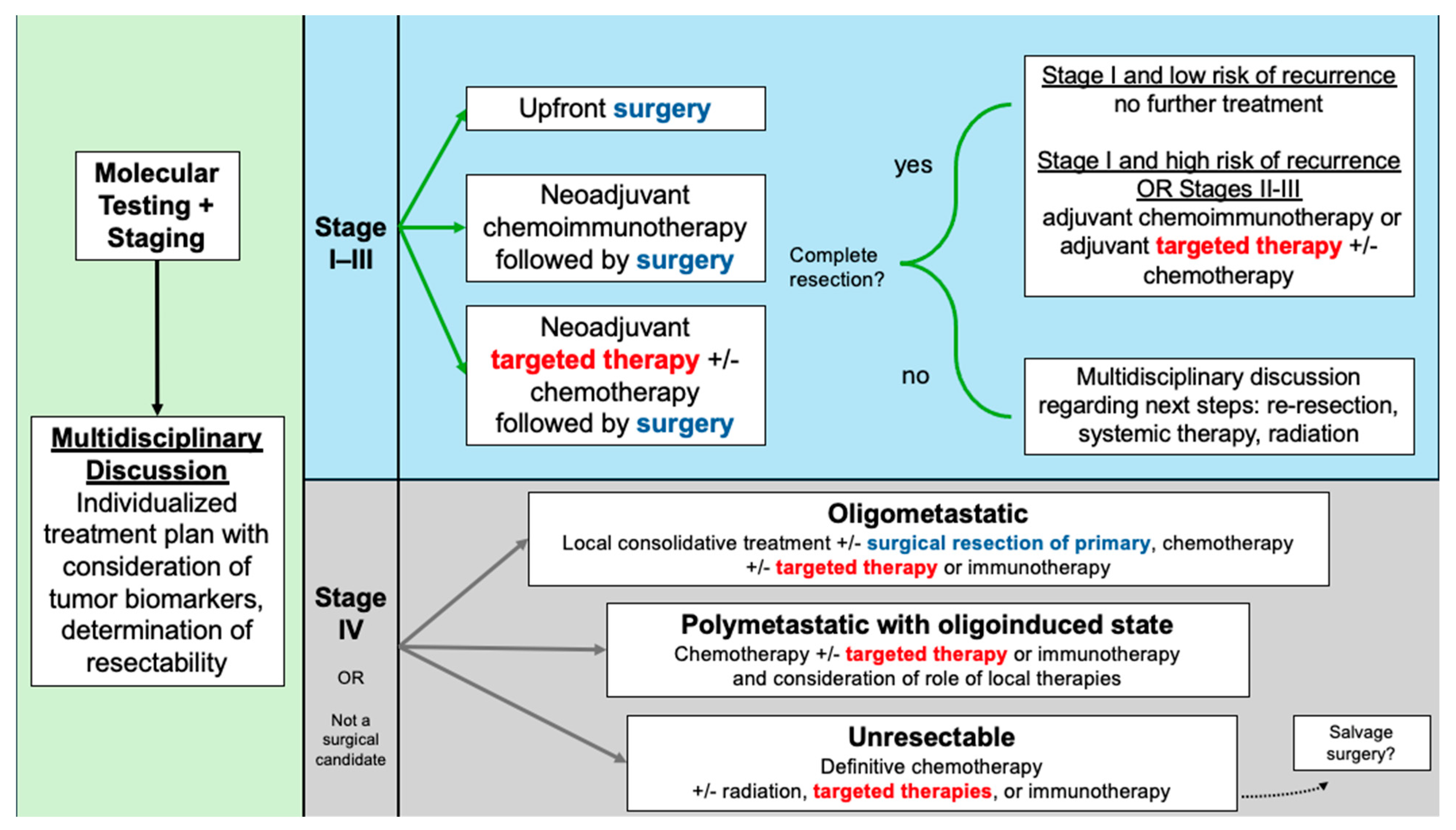
While the results of staging studies have been the cornerstone of determining individualized NSCLC care plans, newer paradigms have emphasized the importance of incorporating molecular profiling into the decision-making process (Figure 1). Lung cancer prognoses have improved significantly with the use of tyrosine kinase inhibitors (TKIs) for patients with sensitizing mutations. Most commonly, these are epidermal growth factor receptor (EGFR) alterations, which occur in 10–15% of Caucasian patients and approximately 50% of Asian patients with NSCLC [4]. Another clinically relevant alteration in NSCLC involves anaplastic lymphoma kinase (ALK), an oncogenic tyrosine kinase, with ALK rearrangements occurring in 3–7% of cases [4]. ALK-rearranged NSCLC, like EGFR-mutated, tends to be associated with younger patients, diagnosis at more advanced stages, and greater risk of brain metastasis [5]. In addition to EGFR and ALK, a spectrum of less common oncogenic drivers—including ROS1, BRAF, MET, RET, NTRK, and others—has been identified, with targeted therapies either available or under active investigation, highlighting the growing complexity and precision of NSCLC management [5,6].
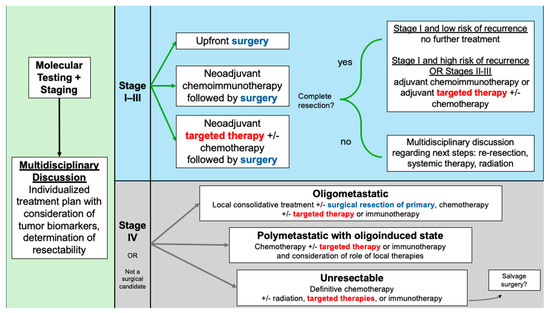
Figure 1.
Treatment algorithm for resectable NSCLC and considerations in advanced NSCLC.
2. Targeted Therapies for EGFR Mutations
2.1. ADAURA Trial
Targeted therapies were proven to be effective in patients with advanced stage NSCLC in clinical trials. The first generation TKI erlotinib was superior to chemotherapy in patients with stage IIIB or IV NSCLC harboring EGFR mutations [7,8]. This paved the groundwork for further investigations of erlotinib and gefitinib for EGFR mutations in stage III NSCLC. The EMERGING-CTONG 1103 trial was one of the first phase II randomized clinical trials of neoadjuvant and adjuvant erlotinib versus gemcitabine and cisplatin in resectable stage IIIA-N2 EGFR-mutated NSCLC and showed that erlotinib may improve progression-free survival and overall survival [9]. These studies demonstrated potential benefits in survival and responsiveness towards TKIs [7,8,9,10,11]. A third-generation TKI, osimertinib, was first approved by the U.S. Food and Drug Administration (FDA) in 2015, under an accelerated approval pathway for the treatment of patients with metastatic NSCLC harboring the EGFR T790M mutation, following progression on earlier generations of TKI [5,12]. The FLAURA trial demonstrated that osimertinib was associated with improved progression-free survival (PFS) and overall survival (OS) over gefitinib or erlotinib [13].
With promising data for osimertinib, investigators performed a double-blind, randomized controlled trial to explore the efficacy of the drug in resectable disease (ADAURA) [14]. This study population included patients with stage IB through IIIA NSLSC and Ex19del or L858R EGFR mutations. Randomization was assigned after surgery and chemotherapy in a 1:1 ratio according to stage, race, and mutation type. Patients received either 80 mg of oral osimertinib daily (N = 339) or placebo (N = 343) for three years. The primary outcome was disease-free survival (DFS) for patients with stage II to IIIA disease. At 24 months, DFS in the osimertinib group was 90% and 44% in the placebo group, indicating a significant reduction. In the overall population, DFS at 24 months was 89% in the osimertinib group and 52% in the placebo group. Amongst all subgroups (by sex, age, smoking history, race, stage, type of EGFR mutation, and adjuvant chemotherapy), osimertinib outperformed placebo in DFS. CNS-related recurrence was significantly reduced in the osimertinib group (2%) versus the placebo group (11%). Importantly, adverse events of grade 3 or higher were noted in 20% of osimertinib patients and 13% of placebo, indicating relative safety of osimertinib.
These encouraging results of osimertinib demonstrate the need for upfront genetic testing in NSCLC, especially for those who have had resected NSCLC. The ADAURA investigators later published results of five-year follow up data. They found the five-year overall survival in the osimertinib group to be 88% versus 78% in the placebo group, which was a statistically significant difference [15]. There was only one new serious adverse event with the additional data, which demonstrates the relative safety and tolerability of osimertinib that was shown in the original study [15]. While the ADAURA trial examined adjuvant osimertinib for three years, the ongoing TARGET trial is looking at a single arm of osimertinib for five years among patients with resected stage II to IIIB NSCLC to determine the optimal duration of adjuvant osimertinib [16]. Another ongoing trial similar to the ADAURA trial is ADAURA2, which examines patients with stage IA2 or IA3 EGFR-positive NSCLC on osimertinib versus placebo for 3 years [17]. Positive results from this trial would expand the role of adjuvant osimertinib after surgical resection in earlier stages of NSCLC than the ADAURA trial.
2.2. NeoADAURA Trial
Recently, the initial results of the first randomized trial with osimertinib in the neoadjuvant setting were released (NeoADAURA) [18]. Investigators enrolled patients with stage II to IIIB NSCLC with either Ex19del or L858R EGFR mutations who were deemed completely resectable by a multidisciplinary team. Patients were randomly assigned in a 1:1:1 ratio for ≥9 weeks of neoadjuvant therapy: 80 mg of oral osimertinib daily and platinum-based chemotherapy (N = 121); 80 mg of oral osimertinib daily monotherapy (N = 117); placebo- and platinum-based chemotherapy (N = 120). Randomization was stratified by disease stage, race, and EGFR mutation type. The primary outcome was major pathologic response (MPR). Following their neoadjuvant regimen, 98% patients went on to complete surgery, while the remaining patients did not due to progressive disease. There were no increases in postoperative complication rates with neoadjuvant osimertinib, demonstrating that neoadjuvant use does not impact perioperative outcomes and resectibility when compared to neoadjuvant chemotherapy. Patients who had neoadjuvant osimertinib and chemotherapy or osimertinib monotherapy had significantly higher rates of MPR than patients with placebo and neodjuvant chemotherapy, and this result was consistent among all subgroups. In addition, the rate of nodal downstaging at the time of surgery was higher for patients with osimertinib plus chemotherapy (53%) and osimertinib monotherapy (53%) compared to placebo and chemotherapy (21%). However, rates of pathological complete response (pCR) were quite low across all groups, with 4% for osimertinib plus chemotherapy, 9% for osimertinib monotherapy, and 0% for placebo plus chemotherapy, which may limit clinical applications and influence the results of longer-term overall survival which have not been published yet. It is important to note that this trial was not designed to determine whether neoadjuvant osimertinib would cause a previously borderline resectable tumor to be resectable, as all patients enrolled had resectable disease as a requirement [18]. As downstaging has been proposed to be the benefit of neoadjuvant therapies, further studies are necessary to determine if osimertinib could aid in resectability [19].
Multidisciplinary decision-making is essential when considering the optimal timing of surgery and neoadjuvant targeted therapy. While the NeoADAURA trial performed surgery after completion of at least 9 weeks of neoadjuvant osimertinib, further studies are necessary to determine the optimal duration and timing of neoadjuvant osimertinib with surgical resection [18]. Upfront molecular testing for sensitizing mutations should influence treatment planning as the NeoADAURA trial demonstrated superior results in patients who were taking osimertinib. In the absence of sensitizing mutations, the current standard of care is neoadjuvant chemotherapy in resectable NSCLC. However, chemotherapy is associated with higher rates of hematologic toxicities and adverse events when compared to osimertinib. Thus, it is crucial to offer patients osimertinib if they harbor sensitizing mutations as it has been shown to be better tolerated and have increased major pathologic response rates [18]. Standardization and reflexive testing protocols for molecular testing may need to be implemented nationwide as a recent study of the Medicare Surveillance, Epidemiology and End Results database found that there was substantial incongruence between patients receiving molecular testing and those receiving targeted therapy [20]. The authors found that only about half of the patients who were receiving targeted therapies had undergone prior molecular testing [20].
3. Targeted Therapies for ALK Mutations
ALINA Trial
Alectinib, an ALK-TKI, was first used in the phase 3 ALEX study for advanced stage ALK-positive NSCLC [21]. Its efficacy appeared promising against a first-generation ALK-TKI and was therefore investigated further under the ALINA trial. The ALINA trial is a phase 3 randomized trial which enrolled patients with completely resected stage IB, II, or IIIA NSCLC with ALK-rearranged disease [22]. Patients were randomly assigned to 600 mg of oral alectinib twice daily for 24 months (N = 130) or intravenous platinum-based chemotherapy for four 21-day cycles (N = 127) in a 1:1 ratio, stratified by disease stage and race. The primary outcome was DFS. At 2 years, DFS among patients taking alectinib was 93.6% versus 63.7% in the chemotherapy group. This differential remained profound at 3 years, with 88.7% DFS in the alectinib group versus 54.0% in the chemotherapy group. This was also consistent among all subgroups studied: age, race, sex, Eastern Cooperative Oncology Group (ECOG) performance status, disease stage, regional lymph node status, and smoking status [22].
The ALINA trial resulted in the approval of alectinib by the U.S. Food and Drug Administration (FDA) as an adjuvant therapy for patients with ALK-rearranged NSCLC [5]. Further studies are necessary to investigate the ideal treatment length of adjuvant alectinib. In addition, the ALNEO trial is currently investigating the role of alectinib as neoadjuvant therapy for patients with stage III resectable NSCLC [23]. A summary of key clinical trials with targeted therapies for EGFR and ALK can be found in Table 1.

Table 1.
Key clinical trials of EGFR- and ALK-targeted therapies in resectable NSCLC 1.
4. Targeted Therapies for Other Mutations
In addition to EGFR and ALK alterations, a spectrum of less common oncogenic drivers has been identified in NSCLC, including ROS1, BRAF V600E, MET exon 14 skipping, RET, NTRK, KRAS G12C, and HER2 mutations (Table 2) [24]. ROS1 rearrangements, present in approximately 1–2% of cases, are sensitive to crizotinib and entrectinib in advanced disease, though data in the adjuvant or neoadjuvant setting remain limited [25]. BRAF V600E mutations occur in 1–3% of NSCLC, and targeted therapy with dabrafenib plus trametinib demonstrates efficacy in metastatic disease, with early-phase trials now exploring their role after resection [26,27]. MET exon 14 skipping mutations, found in 3–4% of patients, and RET fusions, present in 1–2%, are both actionable with MET inhibitors (capmatinib, tepotinib) and RET inhibitors (selpercatinib, pralsetinib), though adjuvant investigations are still underway [28,29]. NTRK fusions are rare (<1%) but highly responsive to pan-TRK inhibitors such as larotrectinib and entrectinib, and ongoing basket trials continue to explore these agents in earlier-stage disease [5,30]. Emerging targets such as KRAS G12C (~13%) and HER2 (~2–3%) also have FDA-approved therapies in metastatic NSCLC, with early-phase studies evaluating their incorporation into resectable disease management [5,31]. Collectively, these developments underscore the rapidly evolving landscape of precision-targeted therapy in NSCLC, and ongoing and planned clinical trials will help define the role of specific agents for these less common mutations in both the adjuvant and neoadjuvant settings.

Table 2.
Other actionable mutations in NSCLC: prevalence, targeted therapies, and ongoing trials.
For patients without oncogenic driver mutations, immune checkpoint inhibitors (ICIs) have been changing the perioperative treatment of resectable NSCLC. These agents are antibodies against programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) and include nicolumab, pembrolizumab, atezolizumab, and durvalamab, which all currently have FDA approval in NSCLC. The CheckMate816 trial examined neoadjuvant nivolumab in patients with resectable stage IB–IIIA NSCLC without EGFR or ALK mutations and found that neoadjuvant nivolumab with chemotherapy resulted in improved pathologic complete response and event-free survival compared to chemotherapy alone [32]. More recent clinical trials have evaluated preoperative and postoperative chemoimmunotherapy regimens including the AEGEAN trial (durvalamab), the NEOTORCH trial (toripalimab), and the KEYNOTE-671 trial (pembrolizumab) which have consistently demonstrated improvement in event-free survival and pathologic complete response compared to conventional chemotherapy [33,34,35]. It is important to note that the efficacy of immunotherapy was most profound in patients with high PD-L1 expression or advanced disease. There are ongoing efforts to better elucidate patient selection and optimal neoadjuvant plus/minus adjuvant chemoimmunotherapy regimens [36].
5. Early Molecular Testing
The striking evidence for the use of targeted therapies suggests that patients with NSCLC should be routinely screened for certain mutations. There is a need for rapid testing for targetable mutations (EGFR, ALK, ROS1, BRAF, MET, RET, etc.) across all stages of NSCLC [37]. Previously, genetic testing for ALK was routine for advanced NSCLC only. However, given the benefit of targeted therapies in resectable NSCLC that have been highlighted by the ADAURA, NeoADAURA, and ALINA trials, the pool of patients undergoing genetic testing should be expanded [22]. The current Society of Thoracic Surgeons statement recommends patients with resectable, locally advanced NSCLC with targetable driver mutations to undergo induction chemotherapy, followed by resection, and adjuvant targeted therapy plus/minus chemotherapy [38]. Therefore, with guidelines shifting towards the use of targeted therapy for all resectable NSCLC, genetic testing is a crucial aspect of treatment planning.
In cases of resectable NSCLC, the molecular testing for genetic mutations can be performed on the specimen; however, the method of detection for each mutation varies based off mechanism of alteration and expression of the mutation. These tests range from mutation-specific polymerase chain reaction (PCR) assays to immunohistochemistry and fluorescence in situ hybridization (FISH) [39]. This makes molecular testing logistically complicated and often times unfeasible if a patient has advanced NSCLC and has undergone biopsies with limited tissue yield. The emergence of next generation sequencing (NGS) can help solve this problem as it can detect multiple targets simultaneously, potentially improving efficiency for detecting a wide range of mutations [40]. Studies have shown similar sensitivity between NGS and conventional technologies with advantages in detecting variants with low mutation rates or detecting non-hotspot mutations [41,42]. However, NGS requires a greater upfront cost for expensive and modern equipment which may not be accessible for all institutions. It is typically performed in centralized laboratories which may actually result in longer turnaround times than conventional methods which can be performed locally [39]. Other logistical or operational barriers to rapid molecular testing include tissue processing, testing algorithms, and choice of assay (i.e., single gene versus panel-based) [43]. Delays in biomarker test results may potentially lead to physicians choosing inferior therapy in cases where there is clinical urgency to begin treatment [43].
Obtaining adequate tissue samples can be difficult, especially when there is not a surgical specimen as is the case in advanced or unresectable NSCLC or in testing for neoadjuvant targeted therapy. This is because there may not be sufficient specimen to run the multitude of tests if NGS is not available; a single biopsy molecular profile may not reflect the heterogeneity of the tumor, and patients may not be able to tolerate an invasive procedure. Plasma-based NGS testing on circulating tumor DNA (ctDNA) or “liquid biopsies” have been developed and are currently being assessed to determine if they can address the limitations with tissue-based testing [40]. Some studies suggest that plasma-NGS may be able to detect clinically relevant mutations at similar rates when compared to tissue-NGS [44,45]. As these novel methods continue to advance, liquid biopsy may become an important complement or alternative to tissue-based testing, helping ensure timely and accurate detection of targetable mutations.
6. Advanced Disease and Targeted Therapies
Targeted therapies have created opportunities for surgical intervention in selected advanced stage NSCLC patients. Due to their high response rates in tumors with sensitizing mutations, targeted therapies have the potential to increase the pool of patients who could benefit from surgical resection [46]. Retrospective studies of patients with advanced NSCLC who underwent definitive systemic treatment with EGFR TKI followed by salvage surgery were able to be down-staged in approximately one-quarter of cases prior to surgery [47]. While salvage surgery did not improve recurrence rates, it did result in improved three-year overall survival rates at approximately 75% likely due to better local control of TKI-resistant tumor regions [47,48]. These findings suggest that targeted therapies may allow selected patients with advanced NSCLC to have surgery included in their multimodality treatment plans.
Another advanced stage of NSCLC in which surgical intervention may be indicated is oligometastatic disease, which refers to tumors with limited metastatic spread that could undergo aggressive local treatment with curative intent (however, there lacks a consensus definition) [46,49,50]. In a retrospective study of patients with oligometastatic NSCLC who underwent surgical resection for the primary lung tumor and either complete or partial treatment for metastatic sites, patients who had adjuvant TKI therapy had superior survival compared to the remainder of the adjuvant chemotherapy or no treatment cohorts [51]. This highlights how surgery can fit into multimodality treatment regimens, especially in patients with oligometastatic disease harboring TKI-sensitizing tumor biology. There are several ongoing clinical trials to determine if targeted therapy in combination with local consolidative therapy improves survival outcomes in patients with stage IV NSCLC, including oligometastatic and polymetastatic disease, when compared to targeted therapy alone [49,52]. The highly anticipated NORTHSTAR trial (NCT03410043) compared osimertinib with or without local consolidative therapy (surgery and/or radiation) in stage IV NSCLC, with stratification by extent of disease (i.e., oligometastatic, polymetastatic), and results are anticipated in late 2025 [53,54]. Similarly, the BRIGHTSTAR trial (NCT03707938) is investigating the role of brigatinib with local consolidative therapy in patients who have ALK-rearranged advanced oligometastatic or polymetastatic NSCLC, with promising early results presented at the 2020 American Society of Clinical Oncology Annual Meeting [55,56]. Together, these studies may help define the role of surgery within multimodality treatment strategies for stage IV NSCLC and clarify how best to integrate targeted therapy with local consolidative approaches.
7. Conclusions
Further clinical trials are necessary to clarify the optimal timing of targeted therapies and surgical resection. At present, results from recent landmark clinical trials support the use of targeted therapies with surgically resectable NSCLC in neoadjuvant and adjuvant settings. Therefore, routine and rapid genetic testing among early-stage NSCLC is necessary to help dictate optimal treatment plans with targeted therapies and surgical resection.
Author Contributions
Conceptualization, V.Y. and M.B.A.; writing—original draft, V.Y. and M.B.A.; writing—review and editing, V.Y. and M.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NSCLC | Non-small cell lung cancer |
| EGFR | Epidermal growth factor receptor |
| TKI | Tyrosine kinase inhibitor |
| ALK | Anaplastic lymphoma kinase |
| PD-1 | Programmed death 1 |
| PD-L1 | Programmed death-ligand 1 |
| FDA | Food and Drug Administration |
| PFS | Progression-free survival |
| OS | Overall survival |
| DFS | Disease-free survival |
| MPR | Major pathologic response |
| ECOG | Eastern Cooperative Oncology Group |
| PCR | Polymerase chain reaction |
| NGS | Next generation sequencing |
| FISH | Fluorescence in situ hybridization |
| ctDNA | Circulating tumor DNA |
| CI | Confidence interval |
| EFS | Event-free survival |
| CNS | Central nervous system |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Spicer, J.D.; Cascone, T.; Wynes, M.W.; Ahn, M.-J.; Dacic, S.; Felip, E.; Forde, P.M.; Higgins, K.A.; Kris, M.G.; Mitsudomi, T.; et al. Neoadjuvant and Adjuvant Treatments for Early Stage Resectable NSCLC: Consensus Recommendations from the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2024, 19, 1373–1414. [Google Scholar] [CrossRef]
- Uramoto, H.; Tanaka, F. Recurrence after Surgery in Patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Blackhall, F.H.; Peters, S.; Bubendorf, L.; Dafni, U.; Kerr, K.M.; Hager, H.; Soltermann, A.; O’Byrne, K.J.; Dooms, C.; Sejda, A.; et al. Prevalence and Clinical Outcomes for Patients With ALK-Positive Resected Stage I to III Adenocarcinoma: Results from the European Thoracic Oncology Platform Lungscape Project. JCO J. Clin. Oncol. 2014, 32, 2780–2787. [Google Scholar] [CrossRef]
- Petrella, F.; Cara, A.; Cassina, E.M.; Degiovanni, S.; Libretti, L.; Lo Torto, S.; Pirondini, E.; Raveglia, F.; Spinelli, F.; Tuoro, A.; et al. Perioperative Strategies in Resectable Non-Squamous Non-Small Cell Lung Cancer with EGFR Mutations and ALK Rearrangement. Cancers 2025, 17, 1844. [Google Scholar] [CrossRef] [PubMed]
- Makarem, M.; Jänne, P.A. Top Advances of the Year: Targeted Therapy for Lung Cancer. Cancer 2024, 130, 3239–3250. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus Chemotherapy as First-Line Treatment for Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Zhong, W.-Z.; Yan, H.-H.; Chen, K.-N.; Chen, C.; Gu, C.-D.; Wang, J.; Yang, X.-N.; Mao, W.-M.; Wang, Q.; Qiao, G.-B.; et al. Erlotinib versus Gemcitabine plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer: Final Overall Survival Analysis of the EMERGING-CTONG 1103 Randomised Phase II Trial. Signal Transduct. Target. Ther. 2023, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Ostoros, G.; Cobo, M.; Ciuleanu, T.; McCormack, R.; Webster, A.; Milenkova, T. First-Line Gefitinib in Caucasian EGFR Mutation-Positive NSCLC Patients: A Phase-IV, Open-Label, Single-Arm Study. Br. J. Cancer 2014, 110, 55–62. [Google Scholar] [CrossRef]
- Casal Rubio, J.; Fírvida-Pérez, J.L.; Lázaro-Quintela, M.; Barón-Duarte, F.J.; Alonso-Jáudenes, G.; Santomé, L.; Afonso-Afonso, F.J.; Amenedo, M.; Huidobro, G.; Campos-Balea, B.; et al. A Phase II Trial of Erlotinib as Maintenance Treatment after Concurrent Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer (NSCLC): A Galician Lung Cancer Group (GGCP) Study. Cancer Chemother. Pharmacol. 2014, 73, 451–457. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. TAGRISSO © Highlights of Prescribing Information. 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208065s000lbl.pdf (accessed on 1 October 2025).
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Soo, R.A.; de Marinis, F.; Han, J.-Y.; Ho, J.C.-M.; Martin, E.; Servidio, L.; Sandelin, M.; Popat, S. TARGET: A Phase II, Open-Label, Single-Arm Study of 5-Year Adjuvant Osimertinib in Completely Resected EGFR-Mutated Stage II to IIIB NSCLC Post Complete Surgical Resection. Clin. Lung Cancer 2024, 25, 80–84. [Google Scholar] [CrossRef]
- Tsutani, Y.; Goldman, J.W.; Dacic, S.; Yatabe, Y.; Majem, M.; Huang, X.; Chen, A.; van der Gronde, T.; He, J. Adjuvant Osimertinib vs. Placebo in Completely Resected Stage IA2-IA3 EGFR-Mutated NSCLC: ADAURA2. Clin. Lung Cancer 2023, 24, 376–380. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tsuboi, M.; Weder, W.; Chen, K.-N.; Hochmair, M.J.; Shih, J.-Y.; Lee, S.Y.; Lee, K.-Y.; Nhung, N.V.; Saeteng, S.; et al. Neoadjuvant Osimertinib for Resectable EGFR-Mutated Non–Small Cell Lung Cancer. JCO 2025, 43, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Sanber, K.; Rosner, S.; Forde, P.M.; Marrone, K.A. Neoadjuvant Immunotherapy for Non-Small Cell Lung Cancer. BioDrugs 2023, 37, 775–791. [Google Scholar] [CrossRef]
- Turner, W.M.; Tuminello, S.; Untalan, M.; Flores, R.; Taioli, E. Incongruence Between Prerequisite Molecular Testing and Treatment with Personalized Therapies for Non-Small Cell Lung Cancer: A Surveillance, Epidemiology and End Results-Medicare Study. IJMS Int. J. Mol. Sci. 2025, 26, 4581. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated Overall Survival and Final Progression-Free Survival Data for Patients with Treatment-Naive Advanced ALK-Positive Non-Small-Cell Lung Cancer in the ALEX Study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.-S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef]
- Leonetti, A.; Boni, L.; Gnetti, L.; Cortinovis, D.L.; Pasello, G.; Mazzoni, F.; Bearz, A.; Gelsomino, F.; Passiglia, F.; Pilotto, S.; et al. Alectinib as Neoadjuvant Treatment in Potentially Resectable Stage III ALK-Positive NSCLC: Final Analysis of ALNEO Phase II Trial (GOIRC-01-2020-ML42316). JCO J. Clin. Oncol. 2025, 43, 8015. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non–Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764. [Google Scholar] [CrossRef]
- Remon, J.; Pignataro, D.; Novello, S.; Passiglia, F. Current Treatment and Future Challenges in ROS1- and ALK-Rearranged Advanced Non-Small Cell Lung Cancer. Cancer Treat. Rev. 2021, 95, 102178. [Google Scholar] [CrossRef]
- Tabbò, F.; Pisano, C.; Mazieres, J.; Mezquita, L.; Nadal, E.; Planchard, D.; Pradines, A.; Santamaria, D.; Swalduz, A.; Ambrogio, C.; et al. How Far We Have Come Targeting BRAF-Mutant Non-Small Cell Lung Cancer (NSCLC). Cancer Treat. Rev. 2022, 103, 102335. [Google Scholar] [CrossRef] [PubMed]
- Sforza, V.; Palumbo, G.; Cascetta, P.; Carillio, G.; Manzo, A.; Montanino, A.; Sandomenico, C.; Costanzo, R.; Esposito, G.; Laudato, F.; et al. BRAF Inhibitors in Non-Small Cell Lung Cancer. Cancers 2022, 14, 4863. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Smit, E.F.; Bauer, T.M. Capmatinib for Patients with Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutations: A Review of Preclinical and Clinical Studies. Cancer Treat. Rev. 2021, 95, 102173. [Google Scholar] [CrossRef]
- Hoe, H.J.; Solomon, B.J. Treatment of Non–Small Cell Lung Cancer with RET Rearrangements. Cancer 2025, 131, e35779. [Google Scholar] [CrossRef]
- Rohrberg, K.S.; Lassen, U. Detecting and Targeting NTRK Fusions in Cancer in the Era of Tumor Agnostic Oncology. Drugs 2021, 81, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Desai, A.; Boumber, Y. HER2 Alterations in Non-Small Cell Lung Cancer (NSCLC): From Biology and Testing to Advances in Treatment Modalities. Front. Oncol. 2025, 15, 1624124. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, W.; Wu, L.; Wang, W.; Zhang, P.; Fang, W.; Xing, W.; Chen, Q.; Yang, L.; Neotorch Investigators; et al. Perioperative Toripalimab Plus Chemotherapy for Patients with Resectable Non-Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial. JAMA 2024, 331, 201–211. [Google Scholar] [CrossRef]
- Spicer, J.D.; Garassino, M.C.; Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Chen, K.-N.; Dooms, C.; Majem, M.; et al. Neoadjuvant Pembrolizumab plus Chemotherapy Followed by Adjuvant Pembrolizumab Compared with Neoadjuvant Chemotherapy Alone in Patients with Early-Stage Non-Small-Cell Lung Cancer (KEYNOTE-671): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2024, 404, 1240–1252. [Google Scholar] [CrossRef]
- Dunne, E.G.; Fick, C.N.; Isbell, J.M.; Chaft, J.E.; Altorki, N.; Park, B.J.; Spicer, J.; Forde, P.M.; Gomez, D.; Iyengar, P.; et al. The Emerging Role of Immunotherapy in Resectable Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2024, 118, 119–129. [Google Scholar] [CrossRef] [PubMed]
- West, H.; Kim, J.Y. Rapid Advances in Resectable Non–Small Cell Lung Cancer: A Narrative Review. JAMA Oncol. 2024, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Cooke, D.T.; Kidane, B.; Tapias, L.F.; Lazar, J.F.; Awori Hayanga, J.W.; Patel, J.D.; Neal, J.W.; Abazeed, M.E.; Willers, H.; et al. The Society of Thoracic Surgeons Expert Consensus on the Multidisciplinary Management and Resectability of Locally Advanced Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2025, 119, 16–33. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular Testing and Targeted Therapy for Non-Small Cell Lung Cancer: Current Status and Perspectives. Crit. Rev. Oncol./Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Allison, D.H.R.; Feng, Y.; Jour, G.; Park, K.; Zhou, F.; Moreira, A.L.; Shen, G.; Feng, X.; Sabari, J.; et al. Comparison of Solid Tissue Sequencing and Liquid Biopsy Accuracy in Identification of Clinically Relevant Gene Mutations and Rearrangements in Lung Adenocarcinomas. Mod. Pathol. 2021, 34, 2168–2174. [Google Scholar] [CrossRef]
- Gao, J.; Wu, H.; Shi, X.; Huo, Z.; Zhang, J.; Liang, Z. Comparison of Next-Generation Sequencing, Quantitative PCR, and Sanger Sequencing for Mutation Profiling of EGFR, KRAS, PIK3CA and BRAF in Clinical Lung Tumors. Clin. Lab. 2016, 62, 689–696. [Google Scholar] [CrossRef]
- Singh, R.R. Next-Generation Sequencing in High-Sensitive Detection of Mutations in Tumors: Challenges, Advances, and Applications. J. Mol. Diagn. 2020, 22, 994–1007. [Google Scholar] [CrossRef]
- Roy-Chowdhuri, S.; Mani, H.; Fox, A.H.; Tsao, A.; Sholl, L.M.; Farjah, F.; Johnson, B.E.; Osarogiagbon, R.U.; Rivera, M.P.; Silvestri, G.A.; et al. The American Cancer Society National Lung Cancer Roundtable Strategic Plan: Methods for Improving Turnaround Time of Comprehensive Biomarker Testing in Non–Small Cell Lung Cancer. Cancer 2024, 130, 4200–4212. [Google Scholar] [CrossRef]
- Mack, P.C.; Banks, K.C.; Espenschied, C.R.; Burich, R.A.; Zill, O.A.; Lee, C.E.; Riess, J.W.; Mortimer, S.A.; Talasaz, A.; Lanman, R.B.; et al. Spectrum of Driver Mutations and Clinical Impact of Circulating Tumor DNA Analysis in Non–Small Cell Lung Cancer: Analysis of over 8000 Cases. Cancer 2020, 126, 3219–3228. [Google Scholar] [CrossRef]
- Leighl, N.B.; Page, R.D.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; Lanman, R.B.; et al. Clinical Utility of Comprehensive Cell-Free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 4691–4700. [Google Scholar] [CrossRef]
- Suzuki, S.; Goto, T. Role of Surgical Intervention in Unresectable Non-Small Cell Lung Cancer. JCM J. Clin. Med. 2020, 9, 3881. [Google Scholar] [CrossRef]
- Lin, M.-W.; Yu, S.-L.; Hsu, Y.-C.; Chen, Y.-M.; Lee, Y.-H.; Hsiao, Y.-J.; Lin, J.-W.; Su, T.-J.; Jeffrey Yang, C.-F.; Chiang, X.-H.; et al. Salvage Surgery for Advanced Lung Adenocarcinoma After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment. Ann. Thorac. Surg. 2023, 116, 111–119. [Google Scholar] [CrossRef]
- Ohtaki, Y.; Shimizu, K.; Suzuki, H.; Suzuki, K.; Tsuboi, M.; Mitsudomi, T.; Takao, M.; Murakawa, T.; Ito, H.; Yoshimura, K.; et al. Salvage Surgery for Non-Small Cell Lung Cancer after Tyrosine Kinase Inhibitor Treatment. Lung Cancer 2021, 153, 108–116. [Google Scholar] [CrossRef]
- Fortich, S.; Piyadeoglu, D.; Celik, N.B.; Antonoff, M. Surgical Management of Oligometastatic Non-Small Cell Lung Cancer. Cancers 2025, 17, 2040. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Hendriks, L.E.L.; Berghmans, T.; Faivre-Finn, C.; GiajLevra, M.; GiajLevra, N.; Hasan, B.; Pochesci, A.; Girard, N.; Greillier, L.; et al. EORTC Lung Cancer Group Survey on the Definition of NSCLC Synchronous Oligometastatic Disease. Eur. J. Cancer 2019, 122, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Li, W.; Huang, Z.; Liu, W.; Bao, P.; Lai, Y.; Han, Y.; Li, X.; Zhao, J. Surgical Outcomes of Stage IV Non-Small Cell Lung Cancer: A Single-Center Experience. J. Thorac. Dis. 2019, 11, 5463–5473. [Google Scholar] [CrossRef] [PubMed]
- Na, K.J.; Kim, Y.T. The “New” Oligometastatic Disease State and Associated Therapies in Non-small Cell Lung Cancer: A Narrative Review. J. Surg. Oncol. 2023, 127, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.M.; Verbus, E.A.; Gandhi, S.; Heymach, J.V.; Hernandez, J.M.; Elamin, Y.Y. Osimertinib, Surgery, and Radiation Therapy in Treating Patients with Stage IIIB or IV Non-Small Cell Lung Cancer with EGFR Mutations (NORTHSTAR). Ann. Surg. Oncol. 2022, 29, 4688–4689. [Google Scholar] [CrossRef] [PubMed]
- Osimertinib, Surgery, and Radiation Therapy in Treating Patients with Stage IIIB or IV Non-Small Cell Lung Cancer with EGFR Mutations, NORTHSTAR Study; M.D. Anderson Cancer Center. Available online: https://clinicaltrials.gov/study/NCT03410043 (accessed on 1 October 2025).
- Elamin, Y.; Gandhi, S.; Antonoff, M.; Mott, F.; Gibbons, D.L.; Le, X.; Negrao, M.V.; Sepesi, B.; Karam, J.A.; Cascone, T.; et al. BRIGHTSTAR: A Pilot Trial of Local Consolidative Therapy (LCT) with Brigatinib in Tyrosine Kinase Inhibitor (TKI)-Naïve ALK-Rearranged Advanced NSCLC. JCO J. Clin. Oncol. 2020, 38, 9624. [Google Scholar] [CrossRef]
- Local Consolidative Therapy and Brigatinib in Treating Patients with Stage IV or Recurrent Non-Small Cell Lung Cancer; M.D. Anderson Cancer Center. Available online: https://clinicaltrials.gov/study/NCT03707938 (accessed on 1 October 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).