Simple Summary
While the elevated risk of atrial fibrillation (AF) in patients diagnosed with cancer is well established, the effect of cancer stage on the risk of AF is unclear. A nationwide cohort study in Korea revealed a progressive increase in AF risk according to the clinical stage of gastric cancer, especially in those without conventional risk factors for AF. This highlights the need for closer monitoring and management of AF to improve the survival of patients with advanced-stage gastric cancer.
Abstract
Background/Objectives: Patients with gastric cancer (GC) have an elevated risk of atrial fibrillation (AF) and cardiovascular mortality, compared with the general population. However, the effect of the cancer stage on the development of AF remains unclear. This study aimed to evaluate the relationship between the risk of AF and GC stage based on the Surveillance, Epidemiology, and End Results (SEER) stage classifications. Methods: This retrospective population-based cohort study enrolled patients diagnosed with GC between 2012 and 2019, using anonymized data from the Cancer Public Library Database of South Korea. Patients were followed up until 2020. The risk of AF was assessed in relation to the SEER stage of GC (localized, regional, distant) using adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs). Subgroup analyses were performed according to age, sex, year of diagnosis, and comorbidities. Results: Of the 211,500 patients enrolled in this study, 7266 were diagnosed with AF during follow-up. The risk of AF increased progressively with cancer stage, with aHRs of 2.00 (95% CI 1.81–2.22) for the distant stage and 1.32 (95% 1.25–1.41) for the regional stage, compared with the localized stage. Subgroup analyses showed a consistent association between advanced cancer stage and a higher AF risk; the association was stronger in the younger, female, and non-hypertensive subgroups. Conclusions: The risk of AF in patients with GC is associated with the initial stage, highlighting the need for the closer monitoring and management of AF to improve the survival of patients with advanced-stage GC.
1. Introduction
Gastric cancer (GC) is a leading malignancy worldwide and ranks fifth in terms of incidence and mortality []. In South Korea, gastric cancer is the most prevalent cancer and fourth most commonly diagnosed cancer, with an incidence rate of 57.2 per 100,000 in 2021 []. As survival rates among gastric cancer patients have improved over recent decades, there is growing interest in the long-term management of gastric cancer survivors []. Recent studies have shown that patients with gastric cancer have an increased risk of cardiovascular diseases, including atrial fibrillation (AF), and a higher cardiovascular mortality compared to the general population [,].
The association between cancer and AF has been demonstrated not only in gastric cancer but also in other cancer types, as shown in previous studies [,,,,,]. Numerous shared risk factors—such as advanced age, male sex, obesity, and smoking—as well as overlapping pathophysiological mechanisms, including heightened inflammation and neurohormonal dysregulation, may explain this relationship. Additionally, cancer treatment-related complications, such as those arising from chemotherapy or radiotherapy, may further contribute to the development of AF [,]. However, no data are currently available regarding the risk of AF in relation to gastric cancer stage at the time of diagnosis. Given that shared pathophysiological mechanisms and cancer-associated complications may promote AF’s development, we hypothesized that patients diagnosed with gastric cancer at an advanced stage may have a higher risk of AF. Therefore, this study aimed to investigate the risk of AF according to the clinical stage at the time of gastric cancer diagnosis.
2. Materials and Methods
2.1. Data Source and Study Population
This study utilized anonymized data from the Cancer Public Library Database of South Korea, a comprehensive database of patients newly diagnosed with cancer between 2012 and 2019 []. Patients diagnosed with GC were identified using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) code C16; subsequently, a cohort of 235,167 patients with GC was constructed. Those aged under 30 years (N = 673) and those with missing data for SEER stage (N = 11,769) and other covariates (N = 4234) were excluded. Among these patients, those who had been diagnosed with AF prior to cancer diagnosis, identified by the ICD-10 code I48, were excluded (N = 6991). Ultimately, a total of 211,500 patients were analyzed. (Figure 1) The index date was defined as the date of GC diagnosis, and the cohort was followed up until the diagnosis of AF, death, or the end of the study period. To ensure a minimum of one year of follow-up for all patients and to minimize follow-up bias, the study period was concluded on 31 December 2020.
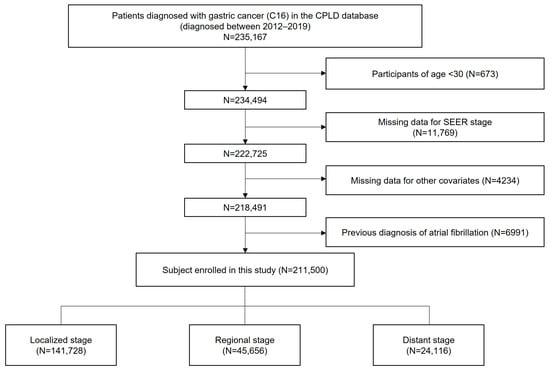
Figure 1.
Flowchart showing the enrollment process.
This study was exempted from review by the Institutional Review Board of Seoul National University, South Korea (IRB No. 2411-121-1590), and the requirement for informed consent was waived owing to the retrospective design of the study.
2.2. Definitions
GC staging was based on the Surveillance, Epidemiology, and End Results Program (SEER) system, classified as localized, regional, or distant. Data on the initial treatments performed within 4 months of diagnosis, including surgery, chemotherapy, radiotherapy, and immunotherapy, was collected. Comorbidities were identified as described in Supplementary Table S1. The primary outcome was a diagnosis of AF, identified using the International Classification of Diseases, 10th revision (ICD-10) code I48, either as a hospitalization diagnosis or confirmed on at least two occasions in outpatient clinics.
2.3. Statistical Analysis
Continuous variables were represented as mean ± standard deviation and compared using analysis of variance, whereas categorical variables were expressed as proportions and compared using the chi-square test. AF risk according to the GC SEER stage was calculated using five multivariable Cox proportional hazard models. Model 1 was unadjusted. Model 2 was adjusted for sex and age. Model 3 was additionally adjusted for income and residential area on top of Model 2. Model 4 was additionally adjusted for history of diabetes mellitus, hypertension, and dyslipidemia on top of Model 3. Model 5 was additionally adjusted for initial therapy on top of Model 4. The cumulative incidence of AF was illustrated using Kaplan–Meier curves and compared using the log-rank test. Subgroup analyses were performed based on age, sex, year of diagnosis, and comorbidities.
Statistical significance was set at a two-sided p-value < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R software 4.4.1 for Windows.
3. Results
3.1. Baseline Characteristics
The baseline characteristics of the study population according to the SEER summary stage at diagnosis are summarized in Table 1. Of the 211,500 patients analyzed, 141,728 were in the localized stage, 45,656 in the regional stage, and 24,116 in the distant stage at diagnosis. Compared to the advanced stages, patients in the localized stage showed a stronger male predominancy and tended to be younger, predominantly in the age between 40 and 64. Most patients in the localized and regional stage received surgery as an initial treatment, while most patients in the distant stage initially received chemotherapy. Patients in the localized stage showed a higher prevalence of hypertension and dyslipidemia compared to the regional and distant stage. The mean follow-up duration of the study population was 3.89 ± 2.58 years, with a significantly shorter follow-up duration in advanced stages (Table 1).

Table 1.
Baseline characteristics of the study population.
3.2. Risk of Atrial Fibrillation According to the SEER Stage at Diagnosis of Gastric Cancer
During the follow-up period, 4765 patients, 1754 patients, and 747 patients in the localized, regional, distant stages, respectively, were diagnosed with AF, with incidence rates of 7.47, 11.06 and 27.73 per 1000 person-years, respectively (Table 2).

Table 2.
Risk of atrial fibrillation according to the SEER stage at the time of diagnosis of gastric cancer.
In all five models, the risk of developing AF was progressively higher in more advanced stages. In Model 5 which was adjusted for age, sex, income, residence, comorbidities, and initial therapy, the adjusted hazard ratio was 1.32 (95% confidence interval [CI] 1.25–1.41) in the regional stage and 2.00 (95% CI 1.81–2.22) in the distant stage compared with the localized stage. (Table 2) The cumulative incidence of AF also increased progressively according to GC stage (Figure 2).
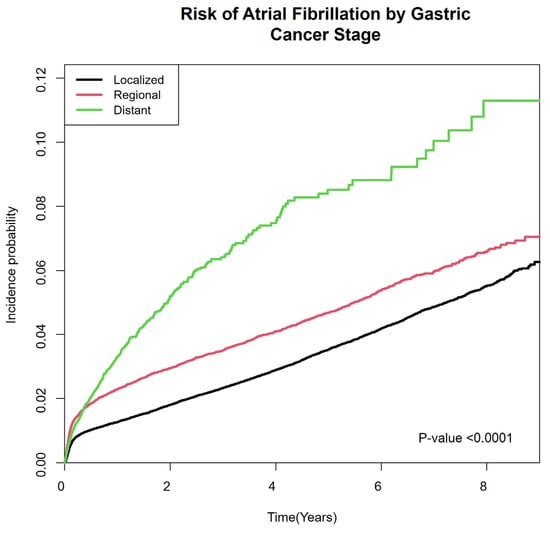
Figure 2.
Cumulative incidence of atrial fibrillation in gastric cancer patients according to SEER stage. SEER: Surveillance, Epidemiology, and End Results Program.
3.3. Subgroup Analysis
Subgroup analyses revealed that the risk of AF was consistently higher in patients diagnosed at a more advanced stage, regardless of age, sex, year of diagnosis, or comorbidities (Table 3). However, a significantly stronger correlation was observed between the GC stage and risk of AF in younger, female, and non-hypertensive subgroups. Additionally, patients diagnosed more recently (in 2016–2019) showed a significantly stronger association between GC stage and AF risk, compared with those diagnosed earlier (in 2012–2015).

Table 3.
Subgroup analysis.
4. Discussion
An elevated risk of AF in patients with various cancers has been demonstrated in multiple cohort studies and meta-analyses [,,,,]. With regard to cancer stage, a meta-analysis investigating the incidence of AF in breast cancer patients found no significant difference between early-stage and metastatic disease []. In contrast, a cohort study using the SEER–Medicare linked database reported that a higher SEER stage at diagnosis was associated with an increased risk of AF, showing conflicting results []. In the present study, the initial clinical stage of gastric cancer was significantly associated with a risk of AF. This finding suggests that an increased tumor burden or the systemic spread of cancer in advanced-stage gastric cancer is associated with the risk AF. This link between tumor burden and AF aligns with the proposed mechanistic pathway linking cancer and AF, in which heightened systemic inflammation driven by tumor-derived cytokines is thought to play a central role in the development of AF among patients with gastric cancer.
Previous studies have speculated that the elevated risk of AF in patients with cancer may be attributed to the shared risk factors between GC and AF. However, in this study, patients without conventional risk factors for AF, including younger, female, non-hypertensive patients, showed a stronger correlation between cancer stage and AF. One possible explanation is that individuals with conventional AF risk factors, including older, male, hypertensive populations, may already have an elevated baseline risk of AF, regardless of cancer stage. This could attenuate the relative impact of cancer stage on AF risk in this group. In terms of sex, prior studies have suggested that female sex is associated with a younger age and more aggressive tumor biology in advanced gastric cancer patients [,]. These sex-related biological differences of advanced gastric cancer, along with the pro-inflammatory effect of female sex hormones (e.g., estrogen), may lead to heightened systemic inflammation and, in turn, may elevate the risk of AF in the female subgroup. Regarding age, younger patients are more likely to receive intensive treatment regimens, including higher doses of chemotherapy or combination therapies. Although our multivariable model adjusted for initial treatment modalities, detailed information on specific chemotherapy agents and dosing was not available in the CPLD database. Such treatment-related differences may have contributed to the increased incidence of AF observed among younger patients with advanced-stage gastric cancer.
Differences in treatment according to cancer stage may also represent an important confounding factor. In particular, chemotherapeutic agents commonly used as first-line therapy in distant-stage gastric cancer, such as capecitabine and 5-fluorouracil, have been associated with cardiovascular complications []. While coronary vasospasm leading to myocardial ischemia is the most frequently reported cardiotoxicity, arrhythmias, including atrial fibrillation (AF), have also been observed with these agents [,,]. To account for such a cardiotoxicity of chemotherapeutic agents in advanced gastric cancer patients, this study utilized a multivariable Cox proportional hazards model adjusted for initial treatments within 4 months (Model 5), which still showed a significant relationship between cancer stage and AF risk. However, the extent of the increase was less prominent than that in Model 4, which did not adjust for the initial treatments. This suggests that the initial treatment may have partially contributed to the elevated risk of AF in advanced GC.
Some studies suggest that the risk of AF is highest early after cancer diagnosis and is no longer significant 5 years after diagnosis [,]. A meta-analysis revealed that AF rates in cancer survivals who have survived longer than 12 months were not significantly higher compared to the general population, suggesting a time-dependent association between cancer and AF []. In line with these results, in this study, patients diagnosed within 5 years showed a stronger correlation between cancer stage and AF risk. This can be explained by more pronounced systemic inflammation and cancer-associated complications shortly after diagnosis. Survival and surveillance biases may also be present, as patients with advanced-stage cancer in poor general condition, who are more likely to develop AF, are less likely to survive beyond 5 years, whereas healthy patients with early-stage cancer tend to have fewer visits to healthcare facilities after 5 years. Nevertheless, cancer stage remained a significant risk factor for AF even after 5 years, highlighting the fact that advanced cancer is a risk factor for AF, regardless of the time of diagnosis.
Currently, screening for AF is recommended by routine heart rhythm assessment during healthcare contact in individuals aged ≥ 65 [,]. Moreover, risk prediction models that incorporate conventional risk factors—such as age, smoking status, hypertension, and coronary artery disease—are commonly used for stratifying AF risk [,,]. However, there is limited evidence supporting AF screening specifically in cancer populations. The findings of our study underscore the potential importance of AF screening in patients with advanced gastric cancer, as they may be at increased risk for cardiovascular complications. Furthermore, our results suggest that cancer stage may serve as an independent risk factor for AF and thus could be considered as an additional parameter in future risk prediction models tailored for cancer patients.
There are several limitations to this study. One limitation of our study was that data on biomarkers related to systemic inflammation and metabolic dysregulation were not available. Longitudinal measurements of such markers during follow-up could have provided a more detailed mechanistic insight into the relationship between cancer progression and atrial fibrillation (AF). Moreover, the treatment data were limited to initial therapies administered within the first four months after diagnosis. As a result, any subsequent changes, delays, or additions to treatment could not be accounted for in the multivariable Cox proportional hazards models, potentially introducing unmeasured confounding. Moreover, the ICD-O-3 code C16 used to identify gastric cancer patients includes both gastric carcinoma and gastric neuroendocrine tumors (NETs), which may have introduced a potential source of bias. However, the majority of gastric cancers are carcinomas, and gastric NETs have been reported to account for only 0.3% to 1.8% of all gastric cancers []. Therefore, the impact of this subgroup on the overall findings is considered minimal. Additionally, given the high mortality associated with advanced gastric cancer, the cumulative incidence of AF may be influenced by competing risks, such as cancer-related death. This may have led to an underestimation of AF’s incidence. Finally, as the Korean population is predominantly of a single ethnic background, the generalizability of our findings may be limited. In particular, the epidemiology and biologic behavior of gastric cancer differs between Asian and non-Asian populations [,,], highlighting the need for the further validation of our results in more ethnically diverse cohorts.
5. Conclusions
This study utilized a comprehensive national database to demonstrate a significant association between GC stage and new-onset AF, with higher hazard ratios in advanced stages. This association was more pronounced in the younger, non-hypertensive, and female subgroups. These results highlight the potential clinical importance of vigilant monitoring for AF in patients with advanced-stage GC, particularly in those without traditional AF risk factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17122054/s1, Table S1: Operational definitions of comorbidities.
Author Contributions
Conceptualization, M.J.O., Y.J.C., K.H. and S.-J.C.; Methodology, J.-H.J. and K.H.; Formal Analysis, J.-H.J.; Data Curation, M.J.O. and J.-H.J.; Visualization, M.J.O. and J.-H.J.; Resources, Y.J.C., J.-H.J. and K.H.; Writing—Original Draft, M.J.O.; Writing—Review and Editing, M.J.O., Y.J.C., J.-H.J., S.L. and S.-J.C.; Supervision, K.H. and S.-J.C.; Project administration, K.H. and S.-J.C.; Funding acquisition, S.-J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Research Foundation of Korea (RS-2025-00523468). The funding source of this study had no role in the study design, data collection, data analysis, data interpretation, the writing of the manuscript, or the decision of submission for publication.
Institutional Review Board Statement
This study was exempted from review by the Institutional Review Board of Seoul National University, South Korea (IRB No. 2411-121-1590).
Informed Consent Statement
The requirement for informed consent was waived owing to the retrospective design of this study.
Data Availability Statement
Data, analytic methods, and study materials will be available to other researchers upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Park, E.H.; Jung, K.-W.; Park, N.J.; Kang, M.J.; Yun, E.H.; Kim, H.-J.; Kim, J.-E.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2021. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2024, 56, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.L.; Kang, D.; Kim, H.; Cho, J.; Jeon, K.H.; Jung, W.; Shin, D.W.; Jeong, S.-M. Increased cardiovascular disease risk among adolescents and young adults with gastric cancer. Gastric Cancer 2024, 27, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Huang, J.; Chan, P.S.F.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.; Du, X.; Zhu, X.; Nie, C.; Han, X.; Tian, W.; Li, H.; Zhou, H. Risk factors associated with cardiovascular mortality among gastric cancer patients: A population-based analysis. Jpn. J. Clin. Oncol. 2022, 52, 1365–1374. [Google Scholar] [CrossRef]
- Yun, J.P.; Choi, E.-K.; Han, K.-D.; Park, S.H.; Jung, J.-H.; Park, S.H.; Ahn, H.-J.; Lim, J.-H.; Lee, S.-R.; Oh, S. Risk of atrial fibrillation according to cancer type: A nationwide population-based study. Cardio Oncol. 2021, 3, 221–232. [Google Scholar] [CrossRef]
- Jakobsen, C.B.; Lamberts, M.; Carlson, N.; Lock-Hansen, M.; Torp-Pedersen, C.; Gislason, G.H.; Schou, M. Incidence of atrial fibrillation in different major cancer subtypes: A Nationwide population-based 12 year follow up study. BMC Cancer 2019, 19, 1105. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Lakoski, S.G.; Qureshi, W.; Judd, S.E.; Howard, G.; Howard, V.J.; Cushman, M.; Soliman, E.Z. Relation Between Cancer and Atrial Fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am. J. Cardiol. 2015, 115, 1090–1094. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.-l.; Zhao, Q.-Q.; Peng, X.-d.; Wu, K.; Li, X.; Ruan, Y.-F.; Bai, R.; Liu, N.; Ma, C.S. The Association of New-Onset Atrial Fibrillation and Risk of Cancer: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2020, 2020, 2372067. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights Into Onco-Cardiology. JACC 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Rahman, F.; Ko, D.; Benjamin, E.J. Association of Atrial Fibrillation and Cancer. JAMA Cardiol. 2016, 1, 384–386. [Google Scholar] [CrossRef]
- Choi, D.W.; Guk, M.Y.; Kim, H.R.; Ryu, K.S.; Kong, H.J.; Cha, H.S.; Kim, H.J.; Chae, H.; Jeon, Y.S.; Kim, H.; et al. Data Resource Profile: The Cancer Public Library Database in South Korea. Cancer Res. Treat. 2024, 56, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Mauro, E.; Lucà, F.; Tetta, C.; Parise, O.; Parrini, I.; Parise, G.; Rao, C.M.; Matteucci, F.; Micali, L.R.; Gulizia, M.M.; et al. Breast Cancer and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1417. [Google Scholar] [CrossRef]
- Guha, A.; Fradley, M.G.; Dent, S.F.; Weintraub, N.L.; Lustberg, M.B.; Alonso, A.; Addison, D. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: A SEER-Medicare analysis. Eur. Heart J. 2021, 43, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.-H.; Lim, B.J.; Kim, H.; Kim, H.; Park, J.J.; Youn, Y.H.; Park, H.; Noh, S.H.; Kim, J.W. Sex disparity in gastric cancer: Female sex is a poor prognostic factor for advanced gastric cancer. Ann. Surg. Oncol. 2016, 23, 4344–4351. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Niu, P.; Wang, W.; Zhao, L.; Zhang, X.; Zhao, D.; Chen, Y. Sex Disparity in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. J. Oncol. 2022, 2022, 1269435. [Google Scholar] [CrossRef]
- Buza, V.; Rajagopalan, B.; Curtis, A.B. Cancer Treatment–Induced Arrhythmias. Circ. Arrhythmia Electrophysiol. 2017, 10, e005443. [Google Scholar] [CrossRef]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Jin, X.; Bai, Y.; Gao, L.; Wu, S. Incidence of and risk factors for cardiotoxicity after fluorouracil-based chemotherapy in locally advanced or metastatic gastric cancer patients. Cancer Chemother. Pharmacol. 2019, 84, 599–607. [Google Scholar] [CrossRef]
- Bao, Y.; Lee, J.; Thakur, U.; Ramkumar, S.; Marwick, T.H. Atrial fibrillation in cancer survivors—A systematic review and meta-analysis. Cardio-Oncol. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Joglar José, A.; Chung Mina, K.; Armbruster Anastasia, L.; Benjamin Emelia, J.; Chyou Janice, Y.; Cronin Edmond, M.; Deswal, A.; Eckhardt Lee, L.; Goldberger Zachary, D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation. JACC 2024, 83, 109–279. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Krijthe, B.P.; Aspelund, T.; Stepas, K.A.; Pencina, M.J.; Moser, C.B.; Sinner, M.F.; Sotoodehnia, N.; Fontes, J.D.; Janssens, A.C.; et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The CHARGE-AF consortium. J. Am. Heart Assoc. 2013, 2, e000102. [Google Scholar] [CrossRef]
- Hu, W.S.; Lin, C.L. Prediction of new-onset atrial fibrillation for general population in Asia: A comparison of C2HEST and HATCH scores. Int. J. Cardiol. 2020, 313, 60–63. [Google Scholar] [CrossRef]
- Himmelreich, J.C.; Veelers, L.; Lucassen, W.A.; Schnabel, R.B.; Rienstra, M.; van Weert, H.C.; Harskamp, R.E. Prediction models for atrial fibrillation applicable in the community: A systematic review and meta-analysis. EP Eur. 2020, 22, 684–694. [Google Scholar] [CrossRef]
- Hu, P.; Bai, J.a.; Liu, M.; Xue, J.; Chen, T.; Li, R.; Kuai, X.; Zhao, H.; Li, X.; Tian, Y.; et al. Trends of incidence and prognosis of gastric neuroendocrine neoplasms: A study based on SEER and our multicenter research. Gastric Cancer 2020, 23, 591–599. [Google Scholar] [CrossRef]
- Dong, E.; Duan, L.; Wu, B.U. Racial and Ethnic Minorities at Increased Risk for Gastric Cancer in a Regional US Population Study. Clin. Gastroenterol. Hepatol. 2017, 15, 511–517. [Google Scholar] [CrossRef]
- Gill, S.; Shah, A.; Le, N.; Cook, E.F.; Yoshida, E.M. Asian ethnicity–related differences in gastric cancer presentation and outcome among patients treated at a Canadian Cancer Center. J. Clin. Oncol. 2003, 21, 2070–2076. [Google Scholar] [CrossRef]
- Lui, F.H.; Tuan, B.; Swenson, S.L.; Wong, R.J. Ethnic Disparities in Gastric Cancer Incidence and Survival in the USA: An Updated Analysis of 1992–2009 SEER Data. Dig. Dis. Sci. 2014, 59, 3027–3034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).