Simple Summary
The impact of COVID-19 vaccinations on the safety and efficacy of immune checkpoint inhibitors (ICIs) is still unclear. This research sought to determine whether periodic vaccine boosters could affect the immune-related adverse event (irAE) and survival rates in metastatic non-small cell lung cancer (NSCLC) patients receiving ICIs. The prospective assessment showed no increase in irAE rates or differences in survival outcomes between vaccinated and unvaccinated patients. Among patients with high PD-L1 expression levels who received ICIs alone, vaccine receipt correlated with improved overall survival. Recommended COVID-19 vaccine boosters can be safely administered in patients with advanced NSCLC undergoing immune checkpoint blockade.
Abstract
Background: Increasing evidence suggests that the immunogenicity of COVID-19 mRNA vaccines might influence the efficacy of immune checkpoint inhibitors (ICIs). Current studies have not considered the impact of additional vaccinations, which are now recommended as a preventive strategy against SARS-CoV-2 infection for cancer patients receiving active treatments. Consequently, we leveraged the prospective monitoring from the Vax-On-Third study to explore whether periodic mRNA vaccine boosters administered around the start of ICIs could influence the rates of immune-related adverse events (irAEs) and survival outcomes in patients with advanced non-small cell lung cancer (NSCLC). Methods: Our study included patients with a histological diagnosis of metastatic NSCLC and available PD-L1 tumor proportion score (TPS), who had undergone at least two cycles of upfront treatment with pembrolizumab, cemiplimab, or their combination with platinum-based chemotherapy. Patients who received any additional mRNA-based vaccine doses within 60 days before to 30 days after starting ICIs accounted for the exposed cohort. Those who declined further boosters formed the reference cohort. We analyzed differences in irAE frequencies, progression-free survival (PFS), and overall survival (OS) using univariate and multivariate analyses. Results: Between 27 November 2021 and 31 March 2024, we enrolled 226 eligible patients. The exposed cohort consisted of 112 patients who had received either a third or fourth dose of tozinameran or a bivalent booster. Based on PD-L1 expression levels, 93 (41%) and 133 (59%) patients received single-agent ICIs (PD-L1 TPS ≥ 50%) or combination regimens (PD-L1 TPS < 50%), respectively. Propensity-score matching using comprehensive criteria resulted in two cohorts of 102 patients each, with an optimal balance of prognostic factors. A thorough analysis of any grade irAEs showed no significant differences between the cohorts. A longitudinal survival assessment with a median follow-up of 22.8 (95% CI 19.2–26.0) months showed no difference between the cohorts. The median PFS for the reference and exposed cohorts was 7.5 (95% CI 5.9–9.1) and 8.2 (95% CI 6.2–10.2) months, respectively (p = 0.408; HR 0.88 [95% CI 0.66–1.18]). The median OS for the reference and exposed cohorts was 10.5 (95% CI 7.9–13.0) and 13.8 (95% CI 12.0–15.5) months, respectively (p = 0.170; HR 0.81 [95% CI 0.59–1.09]). Multivariate analysis confirmed that receiving additional mRNA vaccine boosters did not significantly affect the risk of disease progression or mortality. Univariate analysis within the subgroup of patients with high PD-L1 TPS who received single-agent ICIs showed a significant OS advantage for patients in the exposed cohort (9.7 [95% CI 8.1–11.2] vs. 18.6 [95% CI 13.5–23.6] months; p = 0.034; HR 0.59 [95% CI 0.36–0.96]). Conclusion: After optimally balancing prognostic factors, regular mRNA vaccine boosters at the onset of ICIs did not impact the safety and survival of patients with advanced NSCLC. The improved outcome observed in patients with high PD-L1 expression levels aligns with previous findings and warrants further investigation.
1. Introduction
The SARS-CoV-2 pandemic has triggered the most severe health emergency in recent history [1]. By the close of 2023, COVID-19 had affected over 770 million individuals and claimed approximately 7 million lives worldwide [2]. Pharmaceutical advancements facilitated the swift creation and authorization of vaccines targeting SARS-CoV-2 spike proteins, utilizing either adenoviral vectors or mRNA technology [3]. The latter has proven to be the most effective measure against the COVID-19 pandemic, curbing infection rates and fostering herd immunity development [4]. Despite the exclusion of frail individuals from mRNA vaccine clinical trials, this group now represents the population of greatest concern regarding COVID-19 risks [5]. Recently, the World Health Organization (WHO) reduced the overall alert status but continues to emphasize SARS-CoV-2 vaccination as a priority for vulnerable populations [6]. Cancer patients undergoing active treatment are of particular interest in this context. While booster doses of mRNA vaccines can mitigate COVID-19 complications, waning immunity and the emergence of new variants have left these patients susceptible to persistent breakthrough SARS-CoV-2 infections [7]. Consequently, expert committees advocate for the regular administration of updated COVID-19 vaccines to cancer patients receiving immunosuppressive therapies [8].
The unprecedented circumstances of the COVID-19 pandemic made it challenging to anticipate the strength of immune responses triggered by mRNA vaccines. Existing guidelines for vaccination, based on experience with cytotoxic therapies, were inadequate in addressing the complexities introduced by immune checkpoint blockade [9]. mRNA vaccines have proven highly effective in stimulating humoral immunity by promoting the production of spike-specific neutralizing antibodies and enhancing adaptive immunity through T cell activation and differentiation [10,11]. Immune checkpoint inhibitors (ICIs) counteract T cell exhaustion by interfering with immunosuppressive signaling between antigen-presenting cells and T cells, thus enhancing anti-tumor immune responses [12]. The immunomodulatory effects of both interventions may result in a complex interplay characterized by mutual enhancement of T cell-mediated responses [13]. These interactions raise concerns about potential clinical consequences, including the intensification of immune-related adverse events (irAEs) and the influence on vaccine efficacy and cancer treatment outcomes [14]. The theoretical synergy has prompted several clinical investigations into patient safety, particularly regarding irAEs [15]. Emerging evidence also suggests that the immunogenicity of COVID-19 mRNA vaccines may enhance the effectiveness of the immune checkpoint blockade across various solid tumors [16]. In addition, recent research has indicated that combining COVID-19 mRNA vaccination with ICIs leads to improved overall survival rates. These enhanced outcomes have been observed across several advanced disease settings, including mixed case series of metastatic cancers [17], melanoma [18], head and neck cancers [19,20], and most notably, non-small cell lung cancer (NSCLC) [20,21]. The available studies were retrospective and based their findings on exposure to the initial two-dose regimen, a vaccine schedule with a reduced immunogenic profile that is no longer recommended [22]. Furthermore, these investigations did not account for the effects of additional booster doses, which are currently the recommended preventive measures against SARS-CoV-2 infection in cancer patients undergoing active treatment [23]. Consequently, we leveraged prospective monitoring from the Vax-On-Third study to examine whether the periodic administration of COVID-19 mRNA vaccines near the initiation of immune checkpoint blockade could influence safety and improve survival outcomes in patients with advanced NSCLC.
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
The current investigation aimed to evaluate patients with advanced NSCLC who participated in the Vax-On-Third study (EudraCT identifier code: 2021-002611-54). This prospective study adhered to the STROBE guidelines for observational research [24] and received approval from the relevant Ethics Committee (registration code: 1407/CE Lazio1). The same institution also authorized the use of anonymized data for research purposes (protocol number: Oss-R-281; registration code: 855/CE Lazio1). Clinical data were gathered from the National Drug Agency registry, which provides the reimbursement for high-cost drugs through a prospective monitoring of safety and efficacy [25]. All participants gave written informed consent before any procedures were conducted. The primary eligibility criteria for this analysis included a histologically confirmed diagnosis of NSCLC, stage IV disease according to the eighth edition of the AJCC staging system for lung cancer [26], the availability of baseline PD-L1 tumor proportion score (TPS), an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–2, completion of at least two cycles of initial treatment with anti-PD-1 agents (pembrolizumab or cemiplimab) or their combination with platinum-based chemotherapy, absence of genetic mutations suitable for first-line targeted therapies, and a minimum of 12 months of prospective observation following the initiation of treatment. Additionally, patients with disease recurrence following thoracic surgery or definitive radiotherapy were eligible if progression occurred more than 24 weeks after the last perioperative chemotherapy. However, any prior treatment with anti-PD-(L)1 inhibitors was a disqualifying factor for this analysis. Patients with a metastatic involvement of the central nervous system (CNS) could participate if they were asymptomatic or neurologically stable following radiotherapy.
2.2. Data Collection and Outcome Assessments
The National Drug Agency was the source of demographic, clinical, pathological, and molecular features, along with treatment outcomes concerning safety, disease response, and survival rates. The agency’s pharmacovigilance database provided insights into the frequency and severity of irAEs [27]. An immunohistochemical evaluation of PD-L1 TPS was conducted using the 22C3 pharmDx anti-PD-L1 antibody and the Dako Omnis platform, following the manufacturer’s guidelines (Agilent Technologies, Inc., Santa Clara, CA, USA). The level of TPS was described as the percentage of at least 100 viable tumor cells showing positive membrane staining for PD-L1 expression [28]. The assessment of PD-L1 TPS required quality control procedures, including internal validation cohorts and confirmation of findings through inter-observer reliability. Before each treatment session, patients received a thorough physical examination and underwent laboratory tests, including standard blood work and assessments of thyroid, renal, hepatobiliary, pancreatic, adrenocortical, pituitary, and muscle functions. The attending physician utilized the Common Criteria for Toxicity (CTC)-AE version 5.0 to define and grade irAEs at each treatment cycle [29]. The evaluation of irAEs was conducted without blinding due to the necessity of reporting to the pharmacovigilance agency. According to the National Drug Agency’s guidelines, a baseline disease assessment was conducted within four weeks of starting treatment. These recommendations also call for an initial disease reassessment between 12 and 16 weeks after treatment commencement and every four to six months thereafter. A blinded radiologist evaluated patients’ records using the Response Evaluation Criteria in Solid Tumors for immunotherapy (iRECIST) [30]. Patients who received any additional mRNA vaccine doses between 60 days before and 30 days after starting anti-PD-1 therapy accounted for the exposed cohort, while those unwilling to receive further boosters constituted the reference cohort. The primary endpoint of this study involved examining the effects of periodic vaccine boosters on irAE, progression-free survival (PFS), and overall survival (OS) rates. The secondary endpoint was to assess these outcomes in relation to PD-L1 TPS levels and subsequent treatment choices (ICIs alone for patients with PD-L1 TPS ≥ 50% or a combination of ICIs and platinum-based chemotherapy for patients with PD-L1 TPS < 50%). PFS was measured from the first administration of ICIs to the date of radiologically ascertained disease progression. OS was calculated from the initial administration of ICIs until the date of certified death, irrespective of the cause. Patients who were free of progressive disease or were still alive by the last follow-up were censored (as of 31 March 2025).
2.3. Statistical Analysis
This study applied SPSS version 23.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA) for all statistical assessments and Prism version 9.0 (GraphPad Software Inc., San Diego, CA, USA) for graphical representations. The sample size calculator tailored for observational cohort studies was utilized to determine the number of patients needed for this analysis [31]. The research relied on a superiority design hypothesis. The selected design parameters P0 (the proportion of patients in the unexposed cohort with a PFS duration of at least one year) and P1 (the proportion of patients in the exposed cohort with a PFS duration of at least one year) were set at 0.40 and 0.60, respectively. Given an alpha and beta error probability of 0.05 and 0.80, respectively, a unexposed/exposed ratio of 1.0, and an expected drop rate of 3%, both cohorts required at least 100 cases. Descriptive statistics included a mean with standard deviation (SD) for continuous variables as well as absolute and relative frequencies with interquartile range (IQR) or 95% confidence interval (CI) for categorical variables. We implemented propensity score matching (PSM) to address a potential imbalance of baseline covariates between the cohorts. Propensity scores were estimated through a multivariate logistic regression analysis. This model accounted for the covariates with potential prognostic value, including age, sex, ECOG PS, histological subtype, number of metastatic sites, any specific metastatic involvement (bone, CNS, and/or liver), PD-L1 TPS, body mass index (BMI), smoking habits, previous thoracic radiotherapy, lung immune prognostic index (LIPI) score, treatment regimen, and concomitant medications (corticosteroids, acetaminophen [APAP], antibiotics, and/or proton pump inhibitors [PPIs]). In an effort to ensure adequate numbers of participants and an equal representation in both cohorts, we employed a one-to-one matching approach using the nearest-neighbor algorithm with a 0.2 caliber width. PSM required the application of R software version 4.1.2 and the MatchIt library [32]. Univariate comparisons were performed before and after PSM to demonstrate the balance of prognostic factors using Pearson’s χ2, Mann–Whitney’s U test, or the Kruskal–Wallis test, as appropriate. The balance of covariates between study cohorts was further assessed by calculating the standardized mean difference (SMD), with a value less than 0.1 indicating a well-balanced outcome [33]. The estimation and comparison of PFS and OS relied on the Kaplan–Meier method and the log-rank test, respectively. With regard to primary endpoints related to safety and survival outcomes, univariate and multivariate Cox regression models were applied to calculate hazard ratios (HRs) with 95% CIs and compare rates of immune-related toxicities, disease progression, and mortality. To mitigate alpha inflation from repeated univariate comparisons, the multivariate analysis included vaccine booster exposure as an experimental variable in addition to all covariates used to estimate propensity scores. The subgroup analysis concerning secondary endpoints involved univariate testing only. All tests were two-tailed, and a p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
Between 27 November 2021 and 31 March 2024, we successfully enrolled 226 patients who met the eligibility requirements in full. The median (SD) age was 68.9 (11.2) years, and 30.1% were female. All participants had metastatic disease, with the majority showing an ECOG PS of 0 or 1. Based on PD-L1 TPS levels, 93 patients (41.2%) were treated with single-agent anti-PD-1 therapy (either pembrolizumab or cemiplimab), while 133 patients (58.8%) received the combination with platinum-based chemotherapy. Every participant had completed the initial two-dose regimen of tozinameran at least six months prior to their histological diagnosis. The reference group consisted of 114 patients who had not received any booster doses, whereas the exposed group included 112 patients who had received at least one additional vaccination at various times. Following government guidelines, 109 patients (48.2%) received a third dose of tozinameran between 27 September 2021 and 30 June 2022; 20 patients (8.9%) received a fourth dose from 1 July 2022 to 13 September 2022; and 97 patients (42.9%) received the bivalent booster starting 14 September 2022. In univariate comparisons, we observed a significant imbalance in the distribution of ECOG PS scores and previous exposure to chest radiotherapy. PSM was used to achieve a balanced distribution of baseline covariates between the cohorts. After applying PSM, we analyzed a combined group of 102 patients from each cohort. Within the exposed cohort, 13 patients (12.7%) received the booster vaccination after starting ICI therapy, with a median delay of 2 days (ranging from 1 to 6 days). The remaining 89 patients (87.3%) were given the booster vaccination before initiation of treatment, with a median advance of 18 days (ranging from 1 to 50 days). Table 1 depicts the baseline characteristics of PSM-adjusted population that was relevant for all subsequent evaluations. Supplementary Table S1 provides the baseline characteristics of original unadjusted population.

Table 1.
Patient characteristics of PSM-adjusted population.
3.2. Safety Analysis
The median duration of anti-PD-1 therapy was 8.0 cycles (IQR 4.0–15.0) in the entire population adjusted for PSM. This statistic was consistent across the reference cohort (8.0 cycles [IQR 3.0–13.5]) and the exposed cohort (8.0 cycles [IQR 4.7–13.0]; p = 0.290). Over a median follow-up period of 5.6 months (IQR 2.8–10.3), we observed 72 irAEs, resulting in an overall incidence rate of 35.3% (95% CI 28.2–42.9). Immune-related toxicities involved 64 (31.4%, 95% CI 25.1–38.2) patients, with 8 (3.9%. 95% CI 3.1–4.8) experiencing two simultaneous or sequential adverse events. Immune-related thyroid dysfunctions, skin reactions, colitis, pneumonitis, arthritis, hepatitis, and pancreatitis were the most common toxicities of all grades, with an incidence rate exceeding 2% of cases. Gastrointestinal and pulmonary adverse events were the most frequently reported grade 3 irAEs. No grade 4 immune-related toxicities were observed (Table 2). With the exception of an increased incidence of mild to moderate liver toxicity in the reference subgroup, univariate analysis of any grade irAEs showed no further significant differences between the cohorts (Table 3). Multivariate analysis using comprehensive criteria confirmed that vaccine exposure did not consistently predict the likelihood of irAEs (Supplementary Table S2).

Table 2.
Immune-related adverse events in PSM-adjusted population (N = 204).

Table 3.
Immune-related adverse events by vaccine exposure cohorts in PSM-adjusted population (N = 204).
3.3. Survival Analysis
The median follow-up duration was 22.8 (95% CI 19.2–26.0) months in the PSM-adjusted population. By the cut-off date, 22 (10.8%) patients did not show any disease progression, while 34 (16.7%) patients were censored without experiencing any events relevant to survival. Across the whole population, the median PFS and OS were 7.6 (95% CI 6.5–8.8) months and 13.1 (95% CI 11.5–14.6) months, respectively. The median PFS in the reference and exposed cohorts was 7.5 (95% CI 5.9–9.1) months and 8.2 (95% CI 6.2–10.2) months, respectively (log-rank p = 0.408; HR 0.88 [95% CI 0.66–1.18]; Figure 1A). The median OS in the reference and exposed cohorts was 10.5 (95% CI 7.9–13.4) months and 13.8 (95% CI 12.0–15.5) months, respectively (log-rank p = 0.170 [HR 0.81 [95% CI 0.59–1.09]; Figure 1B). Univariate and multivariate analyses confirmed the inconsistency of booster vaccine exposure in predicting a different risk of disease progression (Table 4) and mortality (Table 5). Additional univariate comparison involved categorizing patients according to PD-L1 TPS levels. Among the 123 patients (60.3%) with PD-L1 TPS < 50% who received the combination of anti-PD-1 agents and platinum-based chemotherapy, PFS (Figure 2A) and OS (Figure 2B) did not differ significantly between the cohorts. Regarding the subgroup of 81 (39.7%) patients with PD-L1 TPS > 50% who were given anti-PD-1 therapy alone, the comparative assessment confirmed no difference in PFS (Figure 2C) but showed significantly improved OS for patients who received booster vaccination (9.7 [95% CI 8.1–11.2] vs. 18.6 [95% CI 13.5–23.6] months; p = 0.034; HR 0.59 [95% CI 0.36–0.96]; Figure 2D).
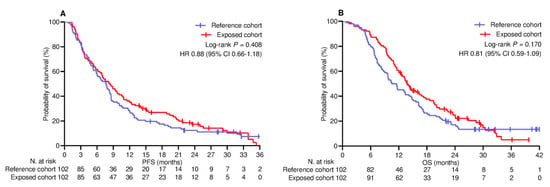
Figure 1.
Univariate comparison of survival in PSM−adjusted population (N = 204). (A) Progression-free survival; (B) Overall survival. PSM, propensity score matching; HR, hazard ratio; CI, confidence interval.

Table 4.
Analysis of progression-free survival in PSM-adjusted population (N = 204).

Table 5.
Analysis of overall survival in PSM-adjusted population (N = 204).
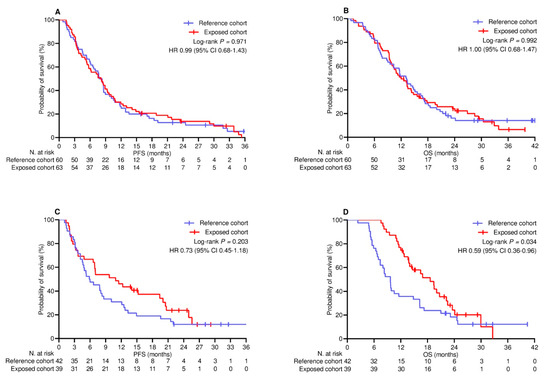
Figure 2.
Univariate comparison of survival by PD─L1 TPS. (A) PFS in patients with PD-L1 < 50% receiving ICIs and platinum-based chemotherapy; (B) OS in patients with PD-L1 < 50% receiving ICIs and platinum-based chemotherapy; (C) PFS in patients with PD-L1 ≥ 50% receiving ICIs alone; (D) OS in patients with PD-L1 ≥ 50% receiving ICIs alone. PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ICIs, immune checkpoint inhibitors.
4. Discussion
The simultaneous administration of COVID-19 mRNA vaccines and ICIs has attracted considerable attention due to the hypothetical potential for enhancing immune responses [34]. Initial analyses of the Vax-On-Third study yielded unprecedented results, suggesting increased survival and immune-related thyroid toxicity in patients receiving a third dose of tozinameran during immune checkpoint blockade [35]. However, these preliminary findings were considered tentative due to the limited sample size, heterogeneous case series, and significant variability in vaccination and treatment initiation timing. The present study builds upon these insights to more comprehensively examine the clinical implications of periodic boosters during ICI therapy. To this end, we conducted a longitudinal observation of the Vax-On-Third study, which primarily aimed to assess the safety and efficacy of additional vaccinations during active cancer treatment. Real-world data on clinical outcomes were obtained from the government registry, which monitors drug safety and efficacy prospectively for ICI reimbursement purposes [25]. In an effort to minimize population heterogeneity, we limited our analysis to the upfront treatment of advanced NSCLC, where ICIs are a recognized therapeutic approach [36]. Since the current study was observational using real-world data from current clinical practice, it was essential to achieve an optimal balance of baseline prognostic factors. In accordance with medical research best practices, we implemented a comprehensive weighting system based on relevant clinical, pathological, and pharmacological variables [37]. Furthermore, the most crucial methodological aspect involved precisely defining the interval between vaccination and the start of ICI therapy. Our predetermined time frame aligns with previous influenza vaccination studies [38]. This framework was also consistent with the kinetics of the immune response after the third dose of tozinameran. In this regard, the 90-day period following a vaccine injection is characterized by persistently high anti-spike antibody titers [39] and the emergence of clonal T-cell dominance specific for spike epitopes [40].
Regarding the primary purpose of this research, survival analysis in the general population revealed no differences between the cohorts in terms of PFS or OS. Even though the exposed cohort showed a numerically longer duration for both PFS and OS, the prolongation of survival remained far from reaching statistical significance. These findings do not support the experimental hypothesis of potential superiority associated with periodic exposure to COVID-19 mRNA vaccination and instead indicate a non-inferiority outcome. Likewise, we found no differences in the occurrence rates of irAEs. In this regard, our assessment was thorough in addressing immune-related toxicities during the course of treatment, with an observation period of approximately six months. Although they are less frequent, our safety monitoring may not have detected long-term irAEs as accurately. The latter consideration warrants caution and the need for further surveillance concerning late-onset adverse events. These findings suggest that recommended booster doses of COVID-19 mRNA vaccines, given shortly before or after beginning treatment, do not influence the efficacy and short-term safety of immune checkpoint blockade as upfront therapy for advanced NSCLC. While the results regarding immune-related toxicity rates are largely consistent with the available evidence [41], the survival outcomes of this study do not support previous claims. Earlier published studies on this subject have examined the effects of adenoviral vector vaccines, considered a mixed context of first-line and subsequent therapies, and were retrospective in nature, lacking a precise definition of the temporal relationship between vaccination and ICI therapy duration. Although these issues are relevant and may render even an indirect comparison inconsistent, at least two reasons could underlie the discrepancy with our results. The first explanation is methodological. We believe that prospective data gathering, stringent inclusion criteria, and prognostic balance at baseline mitigated selection bias [42]. This reduces the possibility that unestablished prognostic factors, such as COVID-19 vaccination, might affect survival and makes the analysis of results more reliable. Furthermore, we provided a precise definition of the timing between vaccination and the start of ICI therapy. The time frame was consistent with previous research on influenza vaccination [43] and allowed us to minimize the confounding effects of immortal-time bias [44,45]. An alternative explanation addresses the real extent of immune responses triggered by COVID-19 mRNA vaccines. The enhanced efficacy of immune checkpoint blockade relies on the assumption that administering mRNA vaccines simultaneously boosts T cell functionality, thus enhancing the potential for anticancer immune responses [46]. These vaccines work by delivering genetic instructions to the host for producing specific viral proteins. Upon injection, SARS-CoV-2 mRNA vaccines are detected by various innate sensors, initiating a primary cytokine response involving IFN-γ, IL-2, and IL-4. This response subsequently leads to the production of spike-specific antibodies and the activation and differentiation of T lymphocytes into spike-directed effector cells [11]. While concurrent ICI therapy has been demonstrated to amplify the intensity of the initial cytokine release [47], there is no evidence suggesting that ICI therapy can enhance subsequent T cell-mediated responses. Multiple studies investigating antigen-specific T cell responses have not found significant differences when comparing cancer patients receiving immune checkpoint blockade with healthy volunteers or unvaccinated individuals [48,49]. The most recent data available as preprints in the context of rapidly evolving evidence confirm the latter suggestions [50]. Given that the onset of irAEs may be contingent on the exacerbation of immune responses against self-tissue antigens, the lack of evidence demonstrating an increased risk of immune-related toxicities following COVID-19 mRNA vaccination supports the latter suggestions [51]. In this context, the hypothetical synergy between COVID-19 mRNA vaccines and immune checkpoint blockade is unlikely to result in a more effective anticancer response and improved outcomes.
The secondary aim of this study was to evaluate survival outcomes in relation to PD-L1 expression levels and subsequent treatment choices. Subgroup analysis of patients with low PD-L1 TPS receiving chemotherapy and ICIs confirmed previous results, showing no differences in PFS or OS. However, COVID-19 mRNA vaccine administration was associated with significant OS improvement in high PD-L1 TPS patients undergoing single-agent immune checkpoint blockade. Given the available evidence, the latter finding was not entirely unexpected. Earlier research has shown that SARS-CoV-2 infection can influence PD-1/PD-L1 axis functionality [52]. Patients with severe COVID-19 exhibit upregulation of the PD-1/PD-L1 pathway in several immune cell types, including monocytes, neutrophils, and T cells [53]. These checkpoint molecules may serve as prognostic indicators and potential therapeutic targets [54]. Further studies revealed that COVID-19 mRNA vaccination increases PD-L1 surface expression on peripheral blood granulocytes, monocytes, and intranodal or circulating T helper cells in both healthy and immunocompromised individuals [55,56]. Recent preclinical data have shown that intratumoral injections of tozinameran induce abundant tumor-infiltrating lymphocytes with enhanced local levels of proinflammatory markers and significantly increased expression of PD-L1 on tumor-associated immune cells. These effects would result in changing the tumor immune microenvironment to a state more favorable for the therapeutic efficacy of immune checkpoint blockade [57]. Assuming that mRNA vaccines targeting SARS-CoV-2 would similarly increase the PD-L1 TPS, more recent studies found that patients with advanced NSCLC were more likely to have elevated expression of this checkpoint molecule if they were vaccinated less than 100 days before diagnostic biopsy [58]. Notably, better OS rates were observed in recipients of COVID-19 mRNA vaccines within 100 days of starting ICI therapy than in unvaccinated patients [59]. Although the mechanisms underlying the modulation of the PD-1/PD-1 axis by these vaccines remain unclear, our subgroup analysis seems consistent with the latter findings. The current study’s results suggest that the survival benefit is confined to NSCLC patients with high PD-L1 TPS who received vaccination near the start of immune checkpoint blockade. However, caution is warranted due to significant differences in study conditions. Unlike the study by Grippin et al., where unvaccinated patients served as the reference group, all participants in our research had received an initial two-dose tozinameran series at least six months before diagnosis. Additionally, most recipients in the former study likely received initial COVID-19 mRNA vaccination priming, resulting in an immune response profile distinct from that induced by booster doses in our study cohort.
Of course, this study acknowledges several limitations, which may extend beyond the following issues. Firstly, despite the prospective nature of this research, the analysis relied on real-world data from consecutively enrolled patients without baseline stratification of prognostic factors. This methodology inherently carries confounding and selection bias, even with the use of strict inclusion criteria and prognostic balancing through an extensive PSM. Secondly, our assessments of treatment failure relied on radiological examinations that were blinded but not independent. Lack of external review of the disease response may lead to an overestimation of the efficacy of immune checkpoint blockade in terms of PFS. Thirdly, we considered patients who had received less than two cycles of treatment to be ineligible for safety and efficacy analysis. These patients generally represent a frail population with a poor prognosis [60]. Although the subgroup is small, their exclusion introduces a selection bias with a potential impact on the assessment of survival outcomes. The emerging evidence that a poor frailty score might predict an increased risk of irAE implies an additional limitation that affects the generalizability of our findings [61]. Fourthly, the current research relies on an accurate definition of the temporal relationship between vaccination and initiation of anti-PD-1 therapy. This experimental design minimizes the effects of immortal-time bias but implies that our findings may not be applicable to other vaccination schedules over the course of treatment. In addition, the interval between vaccination and the start of ICI therapy, which was noticeable among patients vaccinated in advance, may represent a residual confounding. Given the potential differences in immunogenicity among different vaccine booster types, a stratified analysis addressing this heterogeneity would be valuable. However, the numerical distribution of patients makes this subgroup statistic underpowered and therefore unreliable. Lastly, we must consider the risk of alpha inflation due to multiple univariate comparisons [62]. Although our multivariate analysis was thorough, incorporating all potential prognostic factors to reduce the risk of false-negative results in an unprecedented experimental context, the potential for false-positive results remains inherent in this methodology.
5. Conclusions
International consensus supports a regular mRNA vaccination as the most effective measure to prevent severe COVID-19 in cancer patients undergoing immunosuppressive treatments. This study provides initial evidence that recommended vaccine boosters are safe and do not influence expected survival rates in advanced NSCLC patients receiving ICIs, with or without chemotherapy. Consistent with the still preliminary data, the subgroup analysis even suggests a potential survival benefit for vaccinated patients with high PD-L1 expression who are treated exclusively with anti-PD-1 agents. However, due to study limitations and the lack of experimental evidence for reliable comparisons, these findings must be considered exploratory. Their value as hypothesis-generating results warrants prospective validation in independent series and, ideally, through randomized controlled trials with correlative biomarker analyses. Our findings are consistent with the recommendations of relevant guidelines for vaccination of cancer patients on active treatment and do not suggest a personalized immunization strategy based on different safety and efficacy profiles [63]. In agreement with the latest advances and recommendations, the primary rationale for advocating mRNA vaccination in advanced NSCLC patients eligible for immune checkpoint blockade is the prevention of severe COVID-19 [64].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17121948/s1, Table S1. Patient characteristics of PSM-unadjusted population. Table S2. Multivariate analysis of immune-related adverse events.
Author Contributions
Conceptualization, A.F. and F.N.; Data curation, A.F., A.V., C.S. and F.N.; Formal analysis, D.G.; Investigation, A.F., A.V., A.R., F.C., D.R., C.S. and F.N.; Methodology, F.N.; Supervision, E.M.R., C.S. and F.N.; Writing—review and editing, A.F., E.M.R. and F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from agencies in the public, commercial, or not-for-profit sectors and no sources of funding were used to assist in the preparation of this manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki. The Vax-On-Third study received approval by the referring Ethics Committee “Comitato Etico Lazio 1” (registration code: 1407/CE Lazio1; date of approval: 26 September 2021). Authorization to process clinical data was granted by the same institution (protocol number: Oss-R-281; registration code: 855/CE Lazio1; date of approval: 27 September 2022).
Informed Consent Statement
Written consent was obtained from all patients involved in this study, granting permission for the intended treatment and the utilization of anonymized clinical information for research purposes.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The Lancet Public Health. COVID-19 pandemic: What’s next for public health? Lancet Public Health 2022, 7, e391. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 31 March 2025).
- Ao, D.; He, X.; Liu, J.; Xu, L. Strategies for the development and approval of COVID-19 vaccines and therapeutics in the post-pandemic period. Signal Transduct. Target Ther. 2023, 8, 466. [Google Scholar] [CrossRef]
- Fontanet, A.; Cauchemez, S. COVID-19 herd immunity: Where are we? Nat. Rev. Immunol. 2020, 20, 583–584. [Google Scholar] [CrossRef]
- McAuley, H.J.C.; Evans, R.A.; Bolton, C.E.; Brightling, C.E.; Chalmers, J.D.; Docherty, A.B.; Elneima, O.; Greenhaff, P.L.; Gupta, A.; Harris, V.C.; et al. Prevalence of physical frailty, including risk factors, up to 1 year after hospitalisation for COVID-19 in the UK: A multicentre, longitudinal cohort study. eClinicalMedicine 2023, 57, 101896. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. 2023. Available online: https://www.who.int/europe/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 31 March 2025).
- Hosseini-Moghaddam, S.M.; Shepherd, F.A.; Swayze, S.; Kwong, J.C.; Chan, K.K.W. SARS-CoV-2 Infection, Hospitalization, and Mortality in Adults with and Without Cancer. JAMA Netw. Open. 2023, 6, 2331617. [Google Scholar] [CrossRef]
- COVID-19 Advice for the Public: Getting Vaccinated. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 31 March 2025).
- Kamboj, M.; Bohlke, K.; Baptiste, D.M.; Dunleavy, K.; Fueger, A.; Jones, L.; Kelkar, A.H.; Law, L.Y.; LeFebvre, K.B.; Ljungman, P.; et al. Vaccination of Adults with Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 2020, 20, 75–76. [Google Scholar] [CrossRef]
- Malek, A.E.; Cornejo, P.P.; Daoud, N.; Alam, M. The mRNA COVID-19 vaccine in patients with cancer receiving checkpoint inhibitor therapy: What we know and what we don’t. Immunotherapy 2022, 14, 91–94. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, G. SARS-CoV-2 infection and COVID-19 vaccination in cancer patients undergoing immune checkpoint inhibitors. Cell Death Dis. 2023, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, J.; Hou, X.; Yang, Q.; Zhou, Y.; Ye, J.; Wu, X.; Feng, Y.; Hu, T.; Xu, Z.; et al. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol. 2021, 17, 3477–3484. [Google Scholar] [CrossRef]
- New, J.; Shenton, L.; Ksayer, R.; Wang, J.; Zakharia, K.; Nicholson, L.J.; Pandey, A.C. Immune Checkpoint Inhibitors and Vaccination: Assessing Safety, Efficacy, and Synergistic Potential. Vaccines 2024, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liao, L.; Huang, W.; Feng, H.; Wang, W.; Huang, N.; Zhao, Z.; Shi, Y.; Ye, J.; Gu, K. Patients with advanced cancer were treated with immune checkpoint inhibitors and injected with COVID-19 vaccine to improve their prognosis without increasing pancreatic related adverse events. Hum. Vaccin. Immunother. 2024, 20, 2358575. [Google Scholar] [CrossRef]
- Niewolik, J.; Mikuteit, M.; Cossmann, A.; Vahldiek, K.; Gutzmer, R.; Müller, F.; Schröder, D.; Heinemann, S.; Behrens, G.M.N.; Dopfer-Jablonka, A.; et al. Immunogenicity of COVID-19 vaccination in melanoma patients under immune checkpoint blockade. Oncology 2022, 100, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.J.; Liu, Y.L.; Wen, K.; Kurts, C.; Wu, H.; Mei, Q.; Li, J. Potentially improved response of COVID-19 vaccinated nasopharyngeal cancer patients to combination therapy with anti-PD-1 blockade and chemotherapy. Ann Oncol. 2023, 34, 121–123. [Google Scholar] [CrossRef]
- Khaddour, K.; Kamel, J.M.; Meeder, N.; Xu, Z.; Sandra, C.; Pasquinelli, M.; Nguyen, R.H.-T.; Liu, L.C.; Gadi, V.K.; Feldman, L.E.; et al. Clinical outcomes in patients with metastatic non-small cell lung cancer and head and neck squamous cell cancer treated with immune checkpoint inhibitors according to COVID-19 vaccination status. J. Clin. Oncol. 2023, 41 (Suppl. 16), e14696. [Google Scholar] [CrossRef]
- Qian, Y.; Zhu, Z.; Mo, Y.Y.; Zhang, Z. COVID-19 vaccination is associated with enhanced efficacy of anti-PD-(L)1 immunotherapy in advanced NSCLC patients: A real-world study. Infect. Agent Cancer 2023, 18, 50. [Google Scholar] [CrossRef]
- Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 31 March 2025).
- Prevention and Treatment of Cancer-Related Infections. Version 3.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf (accessed on 31 March 2025).
- Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). Available online: https://www.strobe-statement.org (accessed on 31 March 2025).
- Registri Farmaci Sottoposti a Monitoraggio. Available online: https://www.aifa.gov.it/registri-farmaci-sottoposti-a-monitoraggio (accessed on 31 March 2024).
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Adverse Drug Reaction Monitoring. Available online: https://www.aifa.gov.it/web/guest/content/segnalazioni-reazioni-avverse (accessed on 31 March 2025).
- PD-L1 IHC 22C3 pharmDx. Available online: https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-22c3-pharmdx (accessed on 31 March 2025).
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 31 March 2025).
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. R Website. Available online: https://www.r-project.org (accessed on 31 March 2025).
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y.; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef]
- Brest, P.; Mograbi, B.; Hofman, P.; Milano, G. COVID-19 vaccination and cancer immunotherapy: Should they stick together? Br. J. Cancer 2022, 126, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nelli, F.; Giannarelli, D.; Fabbri, A.; Virtuoso, A.; Giron Berrios, J.R.; Marrucci, E.; Fiore, C.; Schirripa, M.; Signorelli, C.; Chilelli, M.G.; et al. Immune-related adverse events and disease outcomes after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 3217–3228. [Google Scholar] [CrossRef]
- Owen, D.H.; Ismaila, N.; Ahluwalia, A.; Feldman, J.; Gadgeel, S.; Mullane, M.; Naidoo, J.; Presley, C.J.; Reuss, J.E.; Singhi, E.K.; et al. Therapy for Stage IV Non-Small Cell Lung Cancer with Driver Alterations: ASCO Living Guideline, Version 2024.3. J. Clin. Oncol. 2025, 43, e2–e16. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Verzoni, E.; Cortellini, A.; Giusti, R.; Calvetti, L.; Ermacora, P.; Di Napoli, M.; Catino, A.; Guadalupi, V.; Guaitoli, G.; et al. Impact of influenza vaccination on survival of patients with advanced cancer receiving immune checkpoint inhibitors (INVIDIa-2): Final results of the multicentre, prospective, observational study. eClinicalMedicine 2023, 61, 102044. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, H.K.; Hennighausen, L.; Furth, P.A.; Sung, H.; Huh, J.W. Time-course analysis of antibody and cytokine response after the third SARS-CoV-2 vaccine dose. Vaccine X 2024, 20, 100565. [Google Scholar] [CrossRef]
- Aoki, H.; Kitabatake, M.; Abe, H.; Xu, P.; Tsunoda, M.; Shichino, S.; Hara, A.; Ouji-Sageshima, N.; Motozono, C.; Ito, T.; et al. CD8+ T cell memory induced by successive SARS-CoV-2 mRNA vaccinations is characterized by shifts in clonal dominance. Cell Rep. 2024, 43, 113887. [Google Scholar] [CrossRef]
- Ruiz, J.I.; Lopez-Olivo, M.A.; Geng, Y.; Suarez-Almazor, M.E. COVID-19 vaccination in patients with cancer receiving immune checkpoint inhibitors: A systematic review and meta-analysis. J. Immunother. Cancer 2023, 11, e006246. [Google Scholar] [CrossRef]
- Wang, X.; Kattan, M.W. Cohort Studies: Design, Analysis, and Reporting. Chest 2020, 158, S72–S78. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Rosén, C.; Koliadi, A.; Digkas, E.; Gustavsson, A.; Nearchou, A.; Ullenhag, G.J. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. OncoImmunology 2021, 10, 1886725. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Lewis, R.J. Immortal Time Bias in Observational Studies. JAMA 2021, 325, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Sterne, J.A.C.; Higgins, J.P.T.; Shrier, I.; Hernández-Díaz, S. A Structural Description of Biases That Generate Immortal Time. Epidemiology 2025, 36, 107–114. [Google Scholar] [CrossRef]
- Gujar, S.; Pol, J.G.; Kim, Y.; Kroemer, G. Repurposing CD8+ T cell immunity against SARS-CoV-2 for cancer immunotherapy: A positive aspect of the COVID-19 pandemic? Oncoimmunology 2020, 9, 1794424. [Google Scholar] [CrossRef]
- Walle, T.; Bajaj, S.; Kraske, J.A.; Rösner, T.; Cussigh, C.S.; Kälber, K.A.; Müller, L.J.; Strobel, S.B.; Burghaus, J.; Kallenberger, S.M.; et al. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat. Cancer 2022, 3, 1039–1051. [Google Scholar] [CrossRef]
- Piening, A.; Ebert, E.; Khojandi, N.; Alspach, E.; Teague, R.M. Immune responses to SARS-CoV-2 in vaccinated patients receiving checkpoint blockade immunotherapy for cancer. Front. Immunol. 2022, 13, 1022732. [Google Scholar] [CrossRef]
- Song, N.J.; Chakravarthy, K.B.; Jeon, H.; Bolyard, C.; Reynolds, K.; Weller, K.P.; Reisinger, S.; Wang, Y.; Li, A.; Jiang, S.; et al. mRNA vaccines against SARS-CoV-2 induce divergent antigen-specific T-cell responses in patients with lung cancer. J. Immunother. Cancer 2024, 12, e007922. [Google Scholar] [CrossRef]
- Ravera, F.; Dameri, M.; Lombardo, I.; Stabile, M.; Fallani, N.; Scarsi, C.; Cigolini, B.; Gentilcore, G.; Domnich, A.; Zullo, L.; et al. Biological modifications of the immune response to COVID-19 vaccine in patients treated with anti-CD20 agents and immune-checkpoint inhibitors. BioRxiv 2024. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, S.H.; Fang, G.C.; Chou, W.C.; Liao, C.C.; Sun, C.P.; Jan, J.T.; Ma, H.H.; Ko, H.Y.; Ko, Y.A.; et al. Upregulation of PD-L1 by SARS-CoV-2 promotes immune evasion. J. Med. Virol. 2023, 95, e28478. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Conti, V.; Franci, G.; Sellitto, C.; Manzo, V.; Pagliano, P.; De Bellis, E.; Masullo, A.; Salzano, F.A.; Caputo, A.; et al. PD-L1 Dysregulation in COVID-19 Patients. Front. Immunol. 2021, 12, 695242. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Yin, Q.; Wu, Y.; Zhang, W. Potential protective role of the anti-PD-1 blockade against SARS-CoV-2 infection. Biomed. Pharmacother. 2021, 142, 111957. [Google Scholar] [CrossRef]
- Loacker, L.; Kimpel, J.; Bánki, Z.; Schmidt, C.Q.; Griesmacher, A.; Anliker, M. Increased PD-L1 surface expression on peripheral blood granulocytes and monocytes after vaccination with SARS-CoV-2 mRNA or vector vaccine. Clin. Chem. Lab. Med. 2022, 61, e17–e19. [Google Scholar] [CrossRef]
- MacManus, M.P.; Akhurst, T.; Lewin, S.R.; Hegi-Johnson, F. Response to COVID-19 vaccination imaged by PD-L1 PET scanning. EJNMMI Rep. 2024, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, J.C.; Rong, L.; He, Y.; Wang, X.; Lin, X.; Li, W.; Wu, Y.; Kuwentrai, C.; Su, C.; et al. The guided fire from within: Intratumoral administration of mRNA-based vaccines to mobilize memory immunity and direct immune responses against pathogen to target solid tumors. Cell Discov. 2025, 10, 127. [Google Scholar] [CrossRef]
- Grippin, A.J.; De, B.; Fink, K.; Swanson, D.; Young, C.; Chang, E.; Johns, A.; Cha, E.; Wei, X.; Dudzinski, S.; et al. MA15.04 Timing of Pre-Biopsy COVID-mRNA Vaccination and PD-L1 Expression in Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2023, 18, S153–S154. [Google Scholar] [CrossRef]
- Grippin, A.J.; Copling, S.; Kim, A.; Young, E.; Lohray, R.; Chang, E.; Kouzy, R.; Lewis, J.; Wei, X.; Rinsurongkawong, W.; et al. 995MO Association of SARS-CoV-2 mRNA vaccines with tumor PD-L1 expression and clinical responses to immune checkpoint blockade. Ann. Oncol. 2024, 35, S677–S678. [Google Scholar] [CrossRef]
- Yin, J.; Song, Y.; Tang, J.; Zhang, B. What is the optimal duration of immune checkpoint inhibitors in malignant tumors? Front. Immunol. 2022, 13, 983581. [Google Scholar] [CrossRef]
- Olsson Ladjevardi, C.; Koliadi, A.; Rydén, V.; Inan El-Naggar, A.; Digkas, E.; Valachis, A.; Ullenhag, G.J. Predicting immune-related adverse events using a simplified frailty score in cancer patients treated with checkpoint inhibitors: A retrospective cohort study. Cancer Med. 2023, 12, 13217–13224. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Buyse, M. Common pitfalls in statistical analysis: The perils of multiple testing. Perspect Clin. Res. 2016, 7, 106–107. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines in People with Cancer. Available online: https://www.cancer.org/cancer/managing-cancer/coronavirus-covid-19-and-cancer/covid-19-vaccines-in-people-with-cancer (accessed on 28 May 2025).
- Kamboj, M.; Kirkwood, M.K.; Bohlke, K.; Gralow, J.R.; Garrett-Mayer, E.; Aggarwal, C. More Frequent than Annual Administration of COVID-19 Vaccination May Be Appropriate for Patients with Cancer. JCO Oncol. Adv. 2025, 2, e2400107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).